Abstract
Introduction
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is an increasingly recognized cause of chronic kidney disease. ADTKD pregnancy outcomes have not previously been described.
Methods
A cross-sectional survey was sent to women from ADTKD families.
Results
Information was obtained from 85 afffected women (164 term pregnancies) and 23 controls (50 pregnancies). Only 16.5% of genetically affected women knew they had ADTKD during pregnancy. Eighteen percent of ADTKD mothers had hypertension during pregnancy versus 12% in controls (p = 0.54) and >40% in comparative studies of chronic kidney disease in pregnancy. Eleven percent of births of ADTKD mothers were <37 weeks versus 0 in controls (p < 0.0001). Cesarean section occurred in 19% of pregnancies in affected women versus 38% of unaffected individuals (p = 0.06). Only 12% of babies required a neonatal intensive care unit stay.
Conclusions
ADTKD pregnancies had lower rates of hypertension during pregnancy versus other forms of chronic kidney disease, which may have contributed to good maternal and fetal outcomes.
Keywords: Maternal health, autosomal dominant tubulointerstitial kidney disease, UMOD, MUC1, pregnancy, epidemiology
Introduction
Chronic kidney disease (CKD) in pregnancy places both the mother and child at risk. 1 The mother is at risk of hypertension, eclampsia, and worsening CKD. 2 The child is at risk of prematurity and intrauterine growth restriction. 3 There have been several studies examining pregnancy outcomes in women with different forms of CKD.1,4 These studies have demonstrated high rates of premature delivery, cesarean section, neonatal intensive care unit (NICU) stay1,4 and an accelerated decline in estimated glomerular filtration rate (eGFR). 1 In a study by Wiles and colleagues, 1 delivery occurred before 37 weeks in 41%, cesarean section in 51%, and a NICU stay in 24% of 79 mothers with CKD Stage 3a. These investigators found pre-pregnancy hypertension to be a significant risk factor for poor outcomes. There have also been similar studies of autosomal dominant polycystic kidney disease (ADPKD),3,5 diabetic nephropathy, 6 cystinosis, 7 and Alport syndrome. 8 A meta-analysis of pregnancy in IgA nephropathy showed an increased rate of cesarean section (49%), preterm delivery (14%) and preeclampsia (9%), though there was not an increased risk of loss of kidney function. 9 In the IgA nephropathy study only 13% of individuals had an eGFR less than 60 ml/min/1.73 m2.
The purpose of this investigation was to improve our understanding of pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease (ADTKD) in order to better inform affected women regarding future pregnancies. ADTKD is the fifth most common genetic cause of end-stage kidney disease (ESKD), 10 constituting about 1% of ESKD patients. 11 The two most common causes of ADTKD are mutations in the UMOD 12 and MUC1 gene. 13 The median age of ESKD for ADTKD-UMOD is 54 years and 46 years for ADTKD-MUC1, 14 though the age of ESKD varies according to mutation and other unknown factors. 15 In contrast to uromodulin, which is expressed exclusively in the thick ascending limb, 16 mucin-1 is expressed throughout the body, including in the breast and uterus. 17 Mucin-1 production increases during pregnancy. 18 Thus, we hypothesized that patients with ADTKD-MUC1 could be at increased risk of complications during pregnancy.
The genetic cause of ADTKD-UMOD was identified in 2001 19 and the genetic cause of ADTKD-MUC1 in 2013. 13 Currently, there are more than 500 families diagnosed with ADTKD compared with less than 20 families described in the literature prior to 2000. Thus, until recently, many women did not know that they were affected by ADTKD at the time of pregnancy and did not have their kidney function monitored. As more younger women are being diagnosed with ADTKD, they are interested to know the effects this condition will have on their health during pregnancy and the health of their baby. Thus, we are at a crossroads where knowledge of pregnancy outcomes would be helpful to women who now have a genetic diagnosis, but where limited information is available because previously women did not know they had this condition when they were pregnant.
To better understand pregnancy outcomes in ADTKD, we performed a cross-sectional survey of women from ADTKD families, including genetically affected individuals and family members who were genetically unaffected and did not have kidney disease. In addition, we performed an analysis of the association of the number of pregnancies with the age of ESKD. As most affected women had not been diagnosed with ADTKD prior to their pregnancy due to the genetic cause not being known at the time, serum creatinine values prior to pregnancy were unavailable in the majority of participants. We therefore estimated the eGFR at the age of pregnancy using the most recent eGFR, the mean rate of decline for all women in our ADTKD registry, and the time interval between the age of pregnancy and the age of the most recent serum creatinine measurement.
Materials and methods
The research protocol (IRB00000352) was approved by the Wake Forest School of Medicine Institutional Review Board.
Registry characteristics
The Wake Forest ADTKD registry includes 1454 individuals and 270 families, 20 with 348 consented individuals from 105 families with ADTKD-MUC1 and 500 individuals from 165 families with ADTKD-UMOD who have undergone genotyping as previously described.19,21,22 The registry also includes 671 individuals from families with ADTKD in whom historical data has been obtained, and 515 individuals who were genotyped and found not to have the familial ADTKD mutation (genetically unaffected). Information available on historical individuals includes a clinical diagnosis of kidney disease, number of children, and age of ESKD. Serum creatinine values on study participants were obtained through review of patient medical records or when serum creatinine was measured as part of the study protocol.
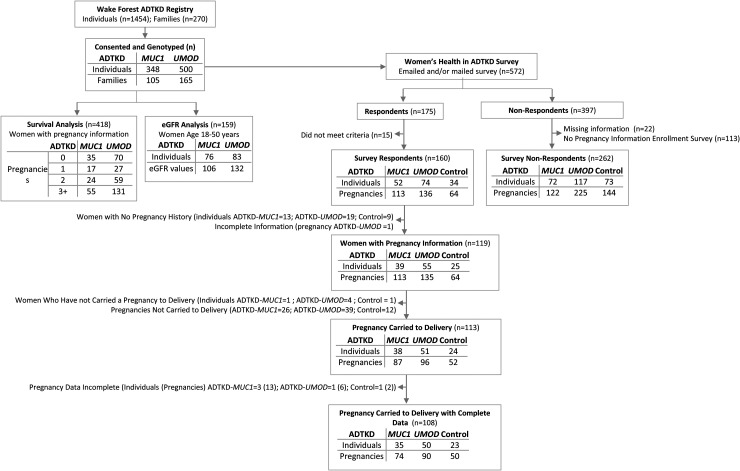
All serum creatinine values for women with genetically proven ADTKD-MUC1 or ADTKD-UMOD between the ages of 18 and 50 were obtained from the registry, and the eGFR was determined with the CKD-EPI equation 23 (see flow diagram in Figure 1). Survival curves to age of ESKD were constructed using Kaplan-Meier estimates of survivor functions. Groups were compared within disease type by performing the Type 3 test of main effects. We calculated the mean rate of annual decline of eGFR of women aged over 18 years in our registry in whom there was a time interval of at least 5 years between serum creatinine measurements, according to disease type. For patients in whom we did not have an eGFR measurement prior to pregnancy, we estimated an eGFR at the time of pregnancy by multiplying the years from pregnancy to the patient's most recent serum creatinine measurement by the mean annual decline of our cohort (according to disease) and subtracting this from the most recent eGFR. If patients had reached ESKD (started dialysis or received a kidney transplant) without a prior serum creatinine value available, we estimated an eGFR of 10 ml/min/1.73 m2 at the age of ESKD as the most recent eGFR determination. We then calculated the estimated CKD stage from this eGFR: Stage 1 (eGFR > 90 ml/min/1.73 m2), Stage 2 (eGFR 60 to <90 ml/min/1.73 m2), Stage 3 (eGFR 30 to <60 ml/min/1.73 m2), Stage 4 (eGFR 15 to <30 ml/min/1.73 m2).
Figure 1.
Flow diagram showing different subsets used in the analyses.
A standardized questionnaire (see Supplementary Material) was created and entered into the NIH-sponsored REDCap program, 24 which immediately captures all data responses entered into the database. The survey was sent to all individuals in our registry who had a genetic diagnosis of ADTKD-UMOD or ADTKD-MUC1.19,21 The survey was also sent to genetically unaffected females who also did not have any other known kidney disease.
Statistical analysis
Responses were analyzed based on whether variables were summarized at the person-level or pregnancy-level, considering some individuals had more than one pregnancy. Descriptive statistics of person-level characteristics were computed and tests between groups performed using Chi-squared tests for categorical variables and t-tests for continuous variables. Associations between continuous variables and disease status at the pregnancy-level were assessed using a general linear mixed model with compound-symmetry covariance structure to account for correlation between an individual's pregnancies. Likewise, associations between binary variables and disease status at the pregnancy-level were assessed with generalized estimating equation models with exchangeable correlation structure. In order to compare cells in which some entries were equal to zero, 100,000 resamples were performed to approximate a permutation test.
Results
For the 418 women in our registry with information on past pregnancies and a genetic diagnosis of ADTKD-MUC1 or ADTKD-UMOD (Figure 1), we performed a survival analysis with ESKD being the outcome and number of pregnancies being the independent variable. There was no statistical relationship between the age of ESKD and number of pregnancies for ADTKD-MUC1 (p = 0.16) or ADTKD-UMOD (p = 0.43) (Supplementary Figure 1). There was no stepwise change in age of ESKD according to number of pregnancies for either condition.
Using data of women over 18 years of age with eGFR determinations greater than 5 years apart from our ADTKD registry, we calculated a mean annual rate of eGFR decline of 2.0 ± 3.2 ml/min/1.73 m2 in 41 women with ADTKD-MUC1 and 1.3 ± 2.0 ml/min/1.73 m2 in 88 women with ADTKD-UMOD. We then calculated an estimated stage of CKD for each pregnancy for all except 2 study participants (5 pregnancies) without a serum creatinine determination. Of 159 women, 22 (14%) had an eGFR value within 2 years prior to pregnancy. There were 29 patients (18%) with eGFR measurements ≤5 years after pregnancy, 28 (18%) 5 to ≤10 years, and 57 (36%) with an eGFR measurement more than 10 years after pregnancy. Of these 57 women, 14 (25%) had an eGFR >60 ml/min/1.73 m2. There were 23 (14%) patients with no eGFR measurements post-pregnancy who developed ESKD between 2 and 39 years after pregnancy, with 8 women within 10 years after pregnancy.
We then analyzed the results of our cross-sectional survey (Figure 1). Of 572 women in the registry, 175 responded and 397 women did not respond. Of these non-respondents, 375 have recorded genetic status, age, and ESKD status, 262 completed a health survey upon initial enrollment in our study that included information about number of pregnancies. Of the 175 women who completed the survey, 15 women did not meet entry criteria and were excluded because of having a different disease or not being part of an ADTKD family. There remained 52 women with ADTKD-MUC1, 74 women with ADTKD-UMOD and 34 genetically unaffected family members. Of 262 non-respondents with information available about pregnancies, 189 were affected with ADTKD and 73 were controls. The respondents and non-respondents were quite similar, with no significant differences regarding age, genetic diagnosis, number of pregnancies, and age of ESKD (Supplementary Table 1).
Supplementary Table 2 shows the characteristic of survey respondents. The mean age of affected women completing the survey was 48.5 ± 14.7 years in the affected group and 47.2 ± 13.0 years in the controls (p = 0.56). There was a mean interval of approximately 20 years between pregnancy data collection. The control respondents were better educated, with 97% having a college or professional degrees versus 72% in the affected group (p = 0.001). Approximately 25% of the women in each group had 0 pregnancies. The mean age at first pregnancy was 25.4 ± 4.9 years in the affected group versus 27.5 ± 5.8 years in the controls (p = 0.07). The number of pregnancies was similar between groups.
After removing responses from women who had no pregnancies and one pregnancy with incomplete information (see Figure 1), there were 39 women with ADTKD-MUC1 with 113 pregnancies, 55 women with ADTKD-UMOD with 135 pregnancies, and 25 geneticaly unaffected women with 64 pregnancies who served as controls. Of 248 ADTKD pregnancies with outcome information provided, 183 pregnancies were carried to delivery (74%), versus 52 of 64 control pregnancies(81%, p = 0.22) (see Supplementary Table 3). The mean time to miscarriage was approximately 9 weeks for all groups, with approximately 12% of individuals in all groups having a miscarriage at >14 weeks (see Supplementary Table 3).
After removing individuals with incomplete survey responses and pregnancy losses, there remained 35 ADTKD-MUC1 mothers with 74 pregnancies carried to delivery, 50 ADTKD-UMOD mothers with 90 pregnancies, and 23 unaffected mothers with 50 pregnancies.
Table 1 shows characteristics prior to pregnancy limited to women who carried to delivery. The age of pregnancy was lower in the genetically affected (27.6 ± 4.8 vs. 29.3 ± 4.1 (p = 0.03)). Anemia was significantly more common in affected individuals (24% vs. 2%, p = 0.04). Thirteen percent of affected individuals smoked during pregnancy versus 2% of controls (p = 0.1), and 23% of controls and 40% of unaffected consumed more than one drink containing alcohol each week (p = 0.16). Only 49% were aware that inherited kidney disease was present in their family prior to pregnancy, and only 16% knew that they had kidney disease prior to pregnancy. Of 43 patients who knew they had inherited kidney disease in their family at the time of pregnancy but did not know if they had inherited kidney disease, 9% discussed family planning with their doctor. In 0 cases the doctor discouraged pregnancy or recommended terminating pregnancy. Of 12 patients who knew they had inherited kidney disease at the time of pregnancy, 67% discussed family planning with their doctor. In 3 cases the doctor discouraged pregnancy, and in 2 cases the doctor recommended terminating pregnancy because of the health of the mother. Of 104 respondents with ADTKD, 20% stated that having a family history of ADKTD influenced their decision to have children. Of 103 respondents with ADTKD, 28% stated that decreasing kidney function influenced their decision to have children
Table 1.
Patient characteristics prior to pregnancy in women who carried to delivery.
| Characteristic | ADTKD-MUC1 | ADTKD-UMOD | ADTKD-MUC1 and ADTKD-UMOD | Genetically unaffected | p-Value with person as cluster |
|---|---|---|---|---|---|
| Individuals (n) | 35 | 50 | 85 | 23 | |
| Pregnancies (n) | 74 | 90 | 164 | 50 | |
| Age during pregnancy (years, mean ± SD) | 28.27 ± 4.79 | 26.97 ± 4.79 | 27.56 ± 4.82 | 29.34 ± 4.09 | 0.032 |
| Total responses | 73 | 88 | 161 | 50 | |
| BMI (kg/m2 mean ± SD) | 22.05 ± 2.91 | 22.77 ± 4.45 | 22.48 ± 3.89 | 22.67 ± 3.83 | 0.85 |
| Total responses | 25 | 37 | 62 | 19 | |
| Diabetes (n (%)) | 0 | 3 (3%) | 3 (2%) | 0 | 0.0094 a |
| Gout (n (%)) | 0 | 5 (6%) | 5 (3%) | 0 | 0.0020 a |
| Hypertension (n (%)) | 3 (4%) | 9 (10%) | 12 (7%) | 0 | <0.0001 a |
| Anemia (n (%)) | 20 (27%) | 20 (22%) | 40 (24%) | 1 (2%) | 0.035 |
| In vitro fertilization used (n (%)) | 1 (1%) | 1 (1%) | 2 (1%) | 1 (2%) | 0.69 |
| Other disease (n (%)) | 4 (5%) | 2 (2%) | 6 (4%) | 1 (2%) | 0.87 |
| Urine protein present (n (%)) | 0 | 3 (3%) | 3 (2%) | 0 | 0.0095 a |
| Smoked (n (%)) | 7 (9%) | 15 (17%) | 22 (13%) | 1 (2%) | 0.10 |
| Drank > 1 drink/week (n (%)) | 23 (31%) | 15 (17%) | 38 (23%) | 20 (40%) | 0.16 |
| Used marijuana or cocaine (n (%)) | 2 (3%) | 2 (2%) | 4 (2%) | 0 | 0.0020 a |
| Did not drink, smoke, or use drugs (n (%)) | 46 (62%) | 62 (69%) | 108 (66%) | 29 (58%) | 0.46 |
| Took allopurinol during pregnancy (n) | 0 | 0 | 0 | 0 | |
| Took febuxostat during pregnancy (n) | 0 | 0 | 0 | 0 | |
| Took an angiotensin converting enzyme inhibitor during pregnancy (n (%)) | 1 (1%) | 2 (2%) | 3 (2%) | 0 | |
| Took an angiotensin receptor blocker during pregnancy (n) | 0 | 0 | 0 | 0 | |
| Responses to questions about family planning | |||||
| “I knew there was kidney disease in my family prior to pregnancy.” (n (%)) | 36 (49%) | 44 (49%) | 80 (49%) | 37 (74%) | 0.060 |
| “I knew I had inherited kidney disease prior to pregnancy.” (n (%)) | 5 (7%) | 22 (24%) | 27 (16%) | NA | |
| “I told my doctor I had inherited kidney disease.” (n (%)) | 3 (4%) | 18 (20%) | 21 (13%) | NA | |
| “I discussed family planning.” (n (%)) | 8 (11%) | 9 (10%) | 17 (10%) | 1 (2%) | 0.14 |
| “My doctor discouraged pregnancy.” (n (%)) | 0 | 2 (2%) | 2 (1%) | NA | |
| “My doctor recommended pregnancy termination.” (n (%)) | 0 | 3 (3%) | 3 (2%) | NA | |
Due to null values, a permutation test was performed with 100,000 resamples to determine statistical significance.
Table 2 shows maternal outcomes from pregnancies carried to delivery according to ADTKD subtype and estimated CKD stage, with five pregnancies not included due to no eGFR information. Fifty-seven percent of pregnancies were estimated to have Stage 1 or 2 CKD, 34% Stage 3 CKD, and 9% having Stage 4 CKD. An important finding were the low rates of hypertension, an important contributor to pregnancy morbidity in CKD patients. Less than 13% of patients in all CKD stages had been diagnosed with hypertension prior to pregnancy. In total, 16% of Stage 2, 31% of Stage 3, and 27% of Stage 4 CKD pregnancies had baseline hypertension or developed hypertension during pregnancy. In general, maternal outcomes were excellent, with higher complication rates with worsening CKD stage. Only 15% of Stage 3 CKD pregnancies were hospitalized due to hypertension. Swelling developed in 34% of Stage 2, 30% of Stage 3 and 47% of Stage 4 CKD pregnancies (each with p < 0.05 when compared to Stage 1). Importantly, 17% of Stage 3 and 27% of Stage 4 CKD pregnancies reported worsening kidney function during pregnancy (p < 0.01). Complications were similar between pregnancies with ADTKD-UMOD and ADTKD-MUC1.
Table 2.
Maternal complications in pregnancies carried to delivery in 35 women with ADTKD-MUC1, 50 women with ADTKD-UMOD and 23 unaffected family members.
| Characteristic | ADTKD-MUC1 | ADTKD-UMOD | ADTKD-MUC1 and ADTKD-UMOD | Unaffected | CKD Stage a | |||
|---|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | |||||
| Pregnancies (n) | 74 | 90 | 164 | 50 | 32 (20%) | 58 (37%) | 54 (34%) | 15 (9%) |
| Twin or greater (n (%)) | 2 (3%) | 0 | 2 (1%) | 0 | 0 | 1 (2%) | 1 (2%) | 0 |
| Prior hypertension | 3 (4%) | 9 (10%) | 12 (7%) | 0 | 1 (3%) | 3 (5%) | 6 (11%) | 2 (13%) |
| Diagnosed with new onset high blood pressure during pregnancy (n/pregnancies without prior hypertension (%)) b | 6/71 (8%) | 17/81 (21%) | 23/152 (15%) | 6/50 (12%) | 4/31 (13%) | 6/55 (11%) | 11/48 (23%) | 2/13 (17%) |
| High blood pressure during pregnancy (n (%)) | 9 (12%) | 26 (29%) | 35 (21%) | 6 (12%) | 5 (16%) | 9 (16%) | 17 (31%) | 4 (27%) |
| Hospitalized for high blood pressure (n (%)) | 3 (4%) | 9 (10%) | 12 (7%) | 1 (2%) | 1 (3%) | 3 (5%) | 8 (15%) | 0 |
| Diabetes (n (%)) | 1 (1%) | 1 (1%) | 2 (1%) | 4 (8%) | 1 (3%) | 1 (2%) | 0 | 0 |
| Gout (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proteinuria (n (%)) | 4 (5%) | 5 (6%) | 9 (5%) | 1 (2%) | 1 (3%) | 2 (3%) | 6 (11%) | 0 |
| Seizures (n (%)) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia (n (%)) | 10 (14%) | 9 (10%) | 19 (12%) | 1 (2%) | 6 (19%) | 3 (5%) | 5 (9%) | 5 (33%) |
| New anemia (n (%)) | 8 (11%) | 4 (4%) | 12 (7%) | 1 (2%) | 5 (20%) | 0 | 3 (8%) | 4 (31%) |
| Receive erythropoietin or darbopoietin or similar agent to increase hemoglobin (n (%)) | 3 (4%) | 0 | 3 (2%) | 0 | 0 | 0 | 1 (2%) | 2 (13%)* |
| Swelling in feet or hands (n (%)) | 18 (24%) | 27 (30%) | 45 (27%) | 12 (24%) | 2 (6%) | 20 (34%) ** | 16 (30%) * | 7 (47%) ** |
| Maintained follow up during pregnancy (n (%)) | 47 (64%) | 70 (78%) | 117 (71%) | 36 (72%) | 20 (63%) | 44 (76%) | 37 (69%) | 14 (93%) |
| Other health complications (n (%)) | 11 (15%) | 9 (10%) | 20 (12%) | 5 (10%) | 4 (13%) | 8 (14%) | 4 (7%) | 4 (27%) |
| Pregnancies (n) | 74 | 90 | 164 | 32 | 55 | 42 | 7 | |
| Worsening kidney function (n (%)) | 7 (9%) | 10 (11%) | 17 (10%) | NA | 0 | 4 (7%)* | 9 (17%)** | 4 (27%)** |
| Dialysis (n (%)) | 0 | 1 (1%) | 1 (1%) | NA | 0 | 1 (2%) | 0 | 0 |
Five pregnancies were not able to be categorized for CKD stage.
The denominator for this row is the number of patients who did not have high blood pressure prior to pregnancy.
*Significantly different than Stage 1 at p < 0.05, **significantly different than Stage 1 at p < 0.01.
Regarding pregnancy outcomes, 21% of Stage 3, and 7% of Stage 4 CKD pregnancies underwent delivery prior to 37 weeks, which is significantly better than at least 41% early delivery for pregnancies with CKD Stage 3 of other causes.1,4 The cesarean section rate was 24% for CKD Stage 3 versus >50% in two other studies of CKD Stage 3.1,4 Similarly, NICU stay was 20% in Stage 3 CKD compared with 24% in CKD Stage 3a and 41% CKD Stage 3b and 44% CKD Stage 3 in two other studies.1,4 Comparing pregnancies in affected mothers with hypertension prior to pregnancy (12) versus those without pre-pregnancy hypertension (149), 25% had NICU admission versus 10% (p = 0.17), 25% delivered prior to 37 weeks versus 9% (p = 0.12), and the birth weights were 2875.8 ± 852.8 g versus 3311.2 ± 644.1 g (p = 0.09).
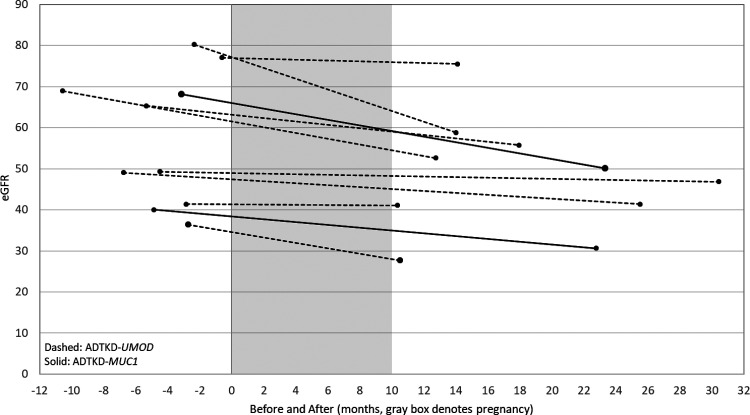
Figure 2 and Supplementary Table 4 provide data on women with eGFR values at the time of pregnancy. There were 10 women with eGFR measurements before and within a year of completing pregnancy. Five of these women had Stage 2 CKD (mean eGFR 70.89 ± 5.99 ml/min/1.73 m2), and five had Stage 3 CKD (mean eGFR 37.53 ± 8.03 ml/min/1.73 m2). In the CKD Stage 3 group, 60% had anemia prior to pregnancy, one was hospitalized for hypertension, two had premature births and three had cesarean sections. Birth weights were significantly lower in Stage 3 versus Stage 2 CKD (2580.9 ± 508 (n = 5) versus 3120.7 ± 113.4 (n = 5), p = 0.009). The mean eGFR change was −3.42 ± 3.42 ml/min/1.73 m2/year in CKD Stage 3 and −3.87 ± 14.09 ml/min/1.73m2/year in CKD Stage 2. We compared these values to women in our registry of similar age, who had their first determination of eGFR at a mean age of 28.3 years and their second determination at a mean age of 30.4 years. The mean change of eGFR was −3.40 ml/min/1.73 m2 over 2.13 years which translated to a mean eGFR change of −1.2 ml/min/1.73 m2/year.
Figure 2.
Estimated glomerular filtration rate (eGFR) values before and after pregnancy for 10 women in whom data was available.
Discussion
In this cross-sectional analysis, we found that women with ADTKD and their children generally had very good pregnancy outcomes.
We first showed that there was no statistical difference in the age of ESKD according to number of pregnancies. Unfortunately, there was significant bias in this analysis for which we could not account, as women with better kidney function may have been more likely to have more children. While we could not account for this bias, there was no stepwise association between increasing number of pregnancies and earlier age of ESKD.
We compared pregnancy outcomes in ADTKD with other CKD registries (see Table 3). The Torino-Calgliari Obesrvational Study reported on 504 pregnancies in women with CKD of all causes, including 38% with tubulointerstitial disease, 16% glomerular disease, 10% systemic lupus, and 6% diabetic nephropathy. 4 Wiles et al. reported on 178 pregnancies, 1 with 28% having glomerulonephritis, 26% reflux nephropathy, 9% diabetic nephropathy, 8% congenital/rare kidney disease, and 4% polycystic kidney disease. According to CKD stage, the outcomes for ADTKD patients and their children were better than in studies of CKD of all causes (see Table 3). In patients with estimated CKD Stage 3, only 31% suffered from hypertension during pregnancy, versus >50% in the comparison cohorts. Premature deliveries occurred in 21% of CKD Stage 3 versus >40% in other studies, and cesarean sections were performed in 24% versus >50% in other studies.1,4 Low levels of hypertension in the cohort may have been an important factor in the low levels of adverse outcomes. In a multicenter study of CKD 1 and a study of ADPKD, 5 adverse pregnancy outcomes in CKD were highly correlated with hypertension, and in our study, mothers with ADTKD were found to have a relatively low prevalence of hypertension. The lower prevalence of hypertension in ADTKD may have led to better outcomes. In one study of ADTKD-UMOD, hypertension was present in 0% of individuals with an eGFR > 80 ml/min/1.73 m2, and only 44% of patients with an eGFR between 50 and 80 ml/min/1.73 m2. 12 ADTKD-MUC1 is also associated with lower levels of hypertension, with only 33% of women noted to have hypertension in one study. 25
Table 3.
Comparison of pregnancy studies in CKD.
| Wu et al. 3 | Chapman et al. 5 | Piccoli et al. 4 | Wiles | ADTKD Cohort Study | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | ADPKD | ADPKD | CKD Stage 2 | CKD Stage 3 | 3a | 3b | Unaffected | Estimated CKD Stage 1 | Estimated CKD Stage 2 | Estimated CKD Stage 3 | Estimated CKD Stage 4 |
| Mothers (n) | 54 | 235 | 23 | 35 women with ADTKD-MUC1 and 50 with ADTKD-UMOD | |||||||
| Pregnancies (n) | 92 | 485 | 87 | 37 | 79 | 38 | 50 | 32 | 58 | 54 | 15 |
| BMI (kg/m2 mean ± SD) | 26.4 | 24.9 | 23.0 ± 4.5 | 22.21 ± 3.30 | 23.58 ± 5.35 | 21.59 ± 2.18 | 22.96 ± 4.99 | ||||
| Mean age (years; mean ± SD) | 29 ± 6.2 | 33.8 ± 4.5 | 33.5 ± 4.1 | 33.2 | 33.1 | 29.3 ± 4.1 | 27.41 ± 5.02 | 28.69 ± 4.79 | 26.40 ± 4.78 | 27.14 ± 4.52 | |
| Hypertension prior to pregnancy (n or % as given in paper) | ≥20% | 16% | 41% | 54% | 71% | 87% | 0 | 1 (3%) | 3 (5%) | 6 (11%) | 2 (13%) |
| Hypertension during pregnancy (baseline HTN + new onset HTN) | ≥32% | 32% | 52% | 76% | ≥71% | ≥87% | 6 (12%) | 5 (16%) | 9 (16%) | 17 (31%) | 4 (27%) |
| New onset hypertension (n) | 12% | 16% | 18% | 47% | 6 (12%) | 4 (13%) | 6 (11%) | 11 (23%) | 2 (17%) | ||
| Edema (n) | 11% | 25% | 12 (24%) | 2 (6%) | 20 (34%) | 16 (30%) | 7 (47%) | ||||
| Preeclampsia (n) | 9% | 11% | |||||||||
| Premature delivery (<37 weeks) (n or % as given in paper) | 11% | 9% | 51% | 78% | 41% | 62% | 0 | 1 (3%) | 2 (3%) | 12 (21%) | 1 (7%) |
| Cesarean section (n or % as given in paper) | 14% | 7% | 70% | 78% | 51% | 65% | 19 (38%) | 6 (19%) | 8 (14%) | 13 (24%) | 4 (27%) |
| NICU stay | 28 | 44 | 24% | 41% | 3 (6%) | 2 (6%) | 5 (9%) | 11 (20%) | 1 (7%) | ||
| Birth weight (g) (mean ± SD) | 3289 ± 746 | 2484 ± 707 | 2226 ± 582 | 2750 | 2490 | 3760 ± 581 | 3352 ± 621 | 3515 ± 540 | 2993 ± 721 | 3293 ± 744 | |
| Decline in eGFR (ml/min/1.73 m2/year) | 2.9 | 4.6 | |||||||||
There were several weaknesses in our comparison. A significant obstacle in our analysis was the lack of a diagnosis of ADTKD prior to pregnancy in many participants, which resulted in limited eGFR measurements. We therefore estimated eGFR for cohort participants. There was an increasing rate of complications with worsening estimated kidney disease stage, suggesting that our estimations were in general successful. We found that 58/159 (36%) of pregnancies were in estimated Stage 2 CKD and that 54/159 (34%) pregnancies were in estimated Stage 3 CKD. In our ADTKD registry in women with available serum creatinine measurements, we found a median eGFR at age 25 years (the median age of pregnancy in our cohort) to be 56.7 ml/min/1.73 m2 for 159 women and that 30% of patients had Stage 3 CKD and 32% had Stage 3 CKD, very similar to what was estimated in our pregnancy cohort. Also, temporal and geographic trends in NICU admissions and cesarean section rates could have contributed to differences. Another weakness of our study was that hypertension was self-reported, and the definition of hypertension has changed over time and may differ by country. However, the low rate of hypertension is consistent with a low rate of hypertension found in patients with ADTKD. 26 Another weakness of this study was recall bias, as many of the births occurred a number of years ago. Fortunately, maternal recall of distant pregnancy events has been shown to be quite accurate. 27 Our study also included few Black individuals. Black individuals are less likely to be referred and undergo genetic testing, and we are trying to improve our outreach in this area. 20 While these are notable shortcomings that affected our ability to compare with other registries, our cohort in general showed very favorable outcomes for maternal and fetal health.
Given that mucin-1 is expressed in the uterus and breast and that uromodulin is expressed only in the kidney tissues, it is important to note that there were no statistical differences in pregnancy outcomes between patients with ADTKD-MUC1 and ADTKD-UMOD.
The results of our study suggest that pregnancies in women with ADTKD were associated with good outcomes. It is important to note that there appeared to be increasing loss of kidney function with worsening baseline kidney function, and there were more complications with a more advanced stage of CKD or in patients with hypertension.
We believe that the following recommendations are important for women with ADTKD: (1) If possible, pregnancy should not be delayed and considered earlier in life, when kidney function is better and when maternal and child outcomes will be improved. (2) Angiotensin converting enzyme inhibitors and allopurinol should be stopped prior to pregnancy or if the possibility of becoming pregnant exists. (3) Overall, patients are likely to have good outcomes with pregnancy especially if normotensive at time of pregnancy, though further information in the change in eGFR during pregnancy is needed.
Supplemental Material
Supplemental material, sj-docx-1-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine
Supplemental material, sj-pptx-2-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine
Supplemental material, sj-pdf-3-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine
Supplemental material, sj-pdf-4-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine
Supplemental material, sj-pdf-5-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine
Acknowledgements
The authors thank all patients and families who participated in this study. The National Center for Medical Genomics (LM2018132) kindly provided sequencing and genotyping.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SK and colleagues were supported by the Ministry of Health of the Czech Republic (grant NV17-29786A, NU21-07-00033), the Ministry of Education of the Czech Republic (grant LTAUSA19068) and by institutional programs of Charles University in Prague (UNCE/MED/007 and PROGRES-Q26/LF1). AJB was funded by NIH-NIDDK R21 DK106584, CKD Biomarkers Consortium Pilot and Feasibility Studies Program funded by the NIH-NIDDK (U01 DK103225), the Slim Health Foundation, the Black-Brogan Foundation, Soli Deo Gloria.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Ethical approval: The ethics committee of Wake Forest School of Medicine approved this study (IRB00000352).
Guarantor: AJB
Contributorship: AJB, KOK, AHW, and VR drafted the survey for participants. KOK wrote the sub-study protocol for WFUHS IRB approval of the project and created the project in REDCap. AHW, EJ, CDL, and KOK performed statistical analysis and creation of tables and figures. VR, AT, LM, and AK contacted patients to complete the survey. DMC, IB, and MZ contributed substantially to the design of the study. SK and AJB came up with the initial concept for the manuscript, oversaw all aspects of analysis and developed the first drafts of the manuscript. All authors contributed to manuscript revisions.
ORCID iD: Anthony J Bleyer https://orcid.org/0000-0002-2804-5273
Supplemental material: Supplemental material for this article is available online.
References
- 1.Wiles K, Webster P, Seed PT, et al. The impact of chronic kidney disease Stages 3-5 on pregnancy outcomes. Nephrol Dial Transplant 2021; 36: 2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumakura S, Okamoto K, Takeuchi S, et al. Kidney function, blood pressure and proteinuria were associated with pregnancy outcomes of pregnant women with chronic kidney disease: a single-center, retrospective study in the Asian population. Clin Exp Nephrol 2020; 24: 547–556. [DOI] [PubMed] [Google Scholar]
- 3.Wu M, Wang D, Zand L, et al. Pregnancy outcomes in autosomal dominant polycystic kidney disease: a case-control study. J Matern Fetal Neonatal Med 2016; 29: 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccoli GB, Cabiddu G, Attini R, et al. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol 2015; 26: 2011–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman AB, Johnson AM, Gabow PA. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1994; 5: 1178–1185. [DOI] [PubMed] [Google Scholar]
- 6.Seah JM, Kam NM, Wong L, et al. The association between maternal renal function and pregnancy outcomes in type 1 and type 2 diabetes. Diabetes Res Clin Pract 2020; 165: 108225. [DOI] [PubMed] [Google Scholar]
- 7.Blakey H, Proudfoot-Jones J, Knox E, et al. Pregnancy in women with cystinosis. Clin Kidney J 2019; 12: 855–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunini F, Zaina B, Gianfreda D, et al. Alport syndrome and pregnancy: a case series and literature review. Arch Gynecol Obstet 2018; 297: 1421–1431. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Lu JD, Zhu Y, et al. Renal outcomes of pregnant patients with immunoglobulin A nephropathy: a systematic review and meta-analysis. Am J Nephrol 2019; 49: 214–224. [DOI] [PubMed] [Google Scholar]
- 10.Groopman EE, Marasa M, Cameron-Christie S, et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 2019; 380: 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devuyst O, Olinger E, Weber S, et al. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primers 2019; 5: 60. [DOI] [PubMed] [Google Scholar]
- 12.Bleyer AJ, Woodard AS, Shihabi Z, et al. Clinical characterization of a family with a mutation in the uromodulin (Tamm-Horsfall glycoprotein) gene. Kidney Int 2003; 64: 36–42. [DOI] [PubMed] [Google Scholar]
- 13.Kirby A, Gnirke A, Jaffe DB, et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet 2013; 45: 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olinger E, Hofmann P, Kidd K, et al. Clinical and genetic spectra of autosomal dominant tubulointerstitial kidney disease due to mutations in UMOD and MUC1. Kidney Int 2020; 98: 717–731. [DOI] [PubMed] [Google Scholar]
- 15.Bleyer AJ, Kmoch S, Antignac C, et al. Variable clinical presentation of an MUC1 mutation causing medullary cystic kidney disease type 1. Clin J Am Soc Nephrol 2014; 9: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rampoldi L, Scolari F, Amoroso A, et al. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 2011; 80: 338–347. [DOI] [PubMed] [Google Scholar]
- 17.Apostolopoulos V, Stojanovska L, Gargosky SE. MUC1 (CD227): a multi-tasked molecule. Cell Mol Life Sci 2015; 72: 4475–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheli CD, Morris DL, Neaman IE, et al. Measurement of four tumor marker antigens in the sera of pregnant women. J Clin Lab Anal 1999; 13: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart TC, Gorry MC, Hart PS, et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 2002; 39: 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleyer AJ, Kidd K, Robins V, et al. Outcomes of patient self-referral for the diagnosis of several rare inherited kidney diseases. Genet Med 2020; 22: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumenstiel B, Defelice M, Birsoy O, et al. Development and validation of a mass spectrometry-based assay for the molecular diagnosis of Mucin-1 kidney disease. J Mol Diagn 2016; 18: 566–571. [DOI] [PubMed] [Google Scholar]
- 22.Zivna M, Kidd K, Pristoupilova A, et al. Noninvasive immunohistochemical diagnosis and novel MUC1 mutations causing autosomal dominant tubulointerstitial kidney disease. J Am Soc Nephrol 2018; 29: 2418–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stavrou C, Koptides M, Tombazos C, et al. Autosomal-dominant medullary cystic kidney disease type 1: clinical and molecular findings in six large Cypriot families. Kidney Int 2002; 62: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 26.Bleyer AJ, Kidd K, Zivna M, et al. Autosomal dominant tubulointerstitial kidney disease. Adv Chronic Kidney Dis 2017; 24: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yawn BP, Suman VJ, Jacobsen SJ. Maternal recall of distant pregnancy events. J Clin Epidemiol 1998; 51: 399–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine
Supplemental material, sj-pptx-2-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine
Supplemental material, sj-pdf-3-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine
Supplemental material, sj-pdf-4-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine
Supplemental material, sj-pdf-5-obm-10.1177_1753495X221133150 for Maternal health and pregnancy outcomes in autosomal dominant tubulointerstitial kidney disease by Anthony J Bleyer, Kendrah O Kidd, Adrienne H Williams, Emily Johnson, Victoria Robins, Lauren Martin, Abbigail Taylor, Alice Kim, Isai Bowline, Dervla M Connaughton, Carl D Langefeld, Martina Zivna and Stanislav Kmoch in Obstetric Medicine