ABSTRACT
Viruses can utilize host splicing machinery to enable the expression of multiple genes from a limited-sized genome. Orthobornaviruses use alternative splicing to regulate the expression level of viral proteins and achieve efficient viral replication in the nucleus. Although more than 20 orthobornaviruses have been identified belonging to eight different viral species, virus-specific splicing has not been demonstrated. Here, we demonstrate that the glycoprotein (G) transcript of parrot bornavirus 4 (PaBV-4; species Orthobornavirus alphapsittaciforme), a highly virulent virus in psittacines, undergoes mRNA splicing and expresses a soluble isoform termed sGP. Interestingly, the splicing donor for sGP is not conserved in other orthobornaviruses, including those belonging to the same orthobornavirus species, suggesting that this splicing has evolved as a PaBV-4-specific event. We have also shown that exogenous expression of sGP does not affect PaBV-4 replication or de novo virion infectivity. In this study, to investigate the role of sGP in viral replication, we established a reverse genetics system for PaBV-4 by using avian cell lines and generated a recombinant virus lacking the spliced mRNA for sGP. Using the recombinant viruses, we show that the replication of the sGP-deficient virus is significantly slower than that of the wild-type virus and that the exogenous expression of sGP cannot restore its propagation efficiency. These results suggest that autologous or controlled expression of sGP by splicing may be important for PaBV-4 propagation. The reverse genetics system for avian bornaviruses developed here will be a powerful tool for understanding the replication strategies and pathogenesis of avian orthobornaviruses.
IMPORTANCE
Parrot bornavirus 4 (PaBV-4) is the dominant cause of proventricular dilatation disease, a severe gastrointestinal and central nervous system disease among avian bornaviruses. In this study, we discovered that PaBV-4 expresses a soluble isoform of glycoprotein (G), called sGP, through alternative splicing of the G mRNA, which is unique to this virus. To understand the role of sGP in viral replication, we generated recombinant PaBV-4 lacking the newly identified splicing donor site for sGP using a reverse genetics system and found that its propagation was significantly slower than that of the wild-type virus, suggesting that sGP plays an essential role in PaBV-4 infection. Our results provide important insights not only into the replication strategy but also into the pathogenesis of PaBV-4, which is the most prevalent bornavirus in captive psittacines worldwide.
KEYWORDS: bornavirus, avian virus, proventricular dilatation disease, RNA splicing, reverse genetic analysis
INTRODUCTION
Avian bornaviruses belonging to the family Bornaviridae, genus Orthobornavirus, are nonsegmented, negative-strand RNA viruses that infect a wide range of avian species worldwide (1, 2). There are 20 avian bornaviruses belonging to five different species, some of which cause severe gastrointestinal and central nervous system symptoms known as proventricular dilatation disease (PDD) in psittacines. Parrot bornavirus 2 (PaBV-2) and 4 (PaBV-4) within the species Orthobornavirus alphapsittaciforme are the dominant viruses causing PDD in captive psittacines worldwide (3 - 7). Despite the increasing importance of parrot bornaviruses as etiological agents of this fatal disease, the details of their transmission and pathogenicity are not well understood.
The genome of orthobornaviruses, including avian bornaviruses, consists of approximately 8.9 kb encoding six open reading frames: nucleoprotein (N), phosphoprotein (P), accessory protein X, matrix protein (M), glycoprotein (G), and large protein (L) in the 3´ to 5´ order (8). Similar to other mononegaviruses, orthobornaviruses are thought to follow a gradient of genome transcription, in which the 3´ proximal genes are transcribed at higher rates than the 5´ proximal genes (9 - 12). On the other hand, unlike other RNA viruses, orthobornaviruses replicate and transcribe in the cell nucleus (13). In addition, several studies on Borna disease virus 1 (BoDV-1; species Orthobornavirus bornaense), the prototype of the family Bornaviridae, which causes fatal encephalitis in many mammalian species, including humans, have shown that BoDV-1 rigorously regulates gene expression to control viral particle production, leading to the establishment of a persistent infection in the nucleus (14 - 16). These features suggest that orthobornaviruses may have evolved unique mechanisms for gene expression, even at the posttranscription level.
Viruses often use the host splicing machinery to produce multiple proteins from the limited length of the viral genome and to control gene expression levels through alternative splicing. As intranuclear replicating viruses, orthobornaviruses use the host splicing machinery to control the expression levels of several different proteins encoded in a single transcription unit. BoDV-1 is known to have at least four splicing donor and five splicing acceptor sites in the genome (17 - 19). The transcripts encoding the M, G, and L genes are polycistronic and undergo alternative splicing to produce appropriate levels of each viral protein (17, 18). In addition, it has recently been shown that there are two short introns in the BoDV-1 N transcript and that alternative splicing of the BoDV-1 N expresses some isoforms of the N that negatively regulate viral polymerase activity in infected cells (20). These observations revealed that BoDV-1 posttranscriptionally regulates the expression levels of viral proteins using the host splicing machinery and maintains infection in the nucleus (20). Since orthobornaviruses share similar genome structures and signal sequences, it is likely that there is a common regulatory mechanism for gene expression; however, species- or genotype-specific regulation of gene expression in orthobornaviruses has not yet been demonstrated.
In this study, we identified a novel splicing intron within the G gene of PaBV-4 isolated from the brains of birds with PDD by transcriptome analysis of infected quail fibrosarcoma cells. Interestingly, the comparison of genome sequences among orthobornaviruses revealed that the newly identified splicing donor in the G gene was not conserved in the species Orthobornavirus alphapsittaciforme but was specific to PaBV-4 strains. We found that the novel splicing of the G intron produced an extracellular secreted isoform of G, termed sGP, which lacked the transmembrane (TM) sequence regions but contained a signal peptide (SP) at the N-terminus. Functional analyses of sGP showed that exogenous expression of this isoform did not affect PaBV-4 replication or de novo virion infectivity. To better understand the role of sGP in viral replication, we established a reverse genetics system for PaBV-4 and generated recombinant PaBV-4 (rPaBV-4) mutated at the splicing donor site for sGP expression. Experimental infection with rPaBV-4 revealed that intrinsic expression of sGP from the viral genome is required for proper viral replication in cultured quail cells. These observations demonstrate that there is a virus-specific mechanism of gene expression that controls viral replication of a dominant pathogen among avian bornaviruses.
RESULTS
Identification of a novel splicing of the PaBV-4 glycoprotein gene
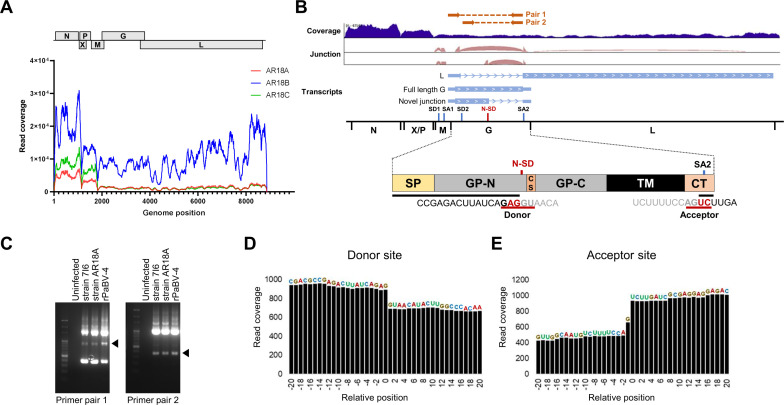
During epidemiological studies of avian bornaviruses in Japan, we detected PaBV-4 in the brains of three blue and yellow macaws (Ara ararauna, animal IDs AR18A, 18B, and 18C) that died of PDD-like disease (21). To understand the replication profiles of PaBV-4 in the infected brains, we performed transcriptome analysis using total RNA isolated from the brains by next-generation sequencing (NGS), and the sequence reads were mapped to PaBV-4 reference genomes. As shown in Fig. 1A, the sequence reads were mapped to the entire viral genome in all samples. In addition, high read coverage was observed in the N and P genes located in the 3´ region of the viral genome. Integrative Genomics Viewer showed the coverage and splicing junctions of the mapped RNA reads from an AR18A-infected macaw brain (Fig. 1B). Interestingly, in addition to the previously reported splicing patterns of polycistronic transcripts encoding the M, G, and L genes, a novel spliced intron (position 2,966 nt to 3,674 nt) within the G gene was detected, suggesting that a spliced variant of the PaBV-4 G gene is produced in infected brains (Fig. 1B and C). Note that due to the low viral load in the brain tissue, we did not obtain sufficient read coverage for the AR18B and AR18C samples to reliably detect the spliced variant. To verify whether the newly identified splicing event of the G gene is also found in cultured cells infected with PaBV-4, we performed the reverse transcription polymerase chain reaction (RT-PCR) on RNA extracted from QT6 quail fibroblast cultures persistently infected with PaBV-4 isolates 7I6 or AR18A (21, 22). As shown in Fig. 1D, we were able to detect the new splicing of the G gene in the infected QT6 cells using two different primer sets. This result indicates that the newly identified G intron is used for PaBV-4 replication in both in vivo and in vitro infection.
Fig 1.
Identification of an alternative splicing event in the PaBV-4 G gene. (A) Read coverage of viral RNA in brain tissue of three PaBV-4-infected macaws. Viral reads acquired by RNA-seq were mapped to the PaBV-4 reference genome (accession number JX065209). (B) Viral transcripts and splicing junction in the G gene of PaBV-4. Splicing junctions were identified by STAR and visualized by Integrative Genomics Viewer (IGV). A schematic diagram of PaBV-4 G protein is indicated. SP, the signal peptide; GP-N, ectodomain subunit region (gp27); CS, the cleavage site by furin; GP-C, the transmembrane subunit region (gp29); TM, the transmembrane region; CT, the cytoplasmic tail; N-SD, newly identified splicing donor site; SA2, splicing acceptor 2. (C) Detection of novel G splicing in PaBV-4-infected cells by RT-PCR using different primer pairs. Agarose gel images of RT-PCR are shown. Two different primer pairs (primer pair 1 and 2; see Materials and Methods) were used. The directions and positions of each primer are shown in Fig. 1B. The bands marked with a triangle indicate amplicons of the identified G splice. (D and E) Read coverage of newly identified splicing junction in the G gene in PaBV-4 strain AR18A-infected QT6 cells. The splicing donor (G) and acceptor (T) sites were defined as position 0, and the relative sequence positions are shown.
The newly identified splicing event of the G gene used canonical eukaryotic splicing signals (AG/GU-AG) and branch point sequences, strongly suggesting the involvement of the host splicing machinery. The acceptor site of G splicing was common to that of L mRNA (Fig. 1B). To estimate the expression levels of the variants of the G transcript, Mixture of Isoforms (MISO) software, which quantifies the expression level of alternatively spliced genes from RNA-seq data, was used (23). As shown in Fig. 1E, deleted read coverage was detected between the predicted donor and acceptor sites, and the splicing of the G intron was estimated to be approximately 22.5% of the total transcripts containing the G sequence in PaBV-4 strain AR18A-infected QT6 cells (Fig. 1D). This suggests that G splicing occurs at a relatively high rate in PaBV-4-infected cells.
To determine whether the newly identified splicing of the G gene is conserved in the genus Orthobornavirus, especially in avian bornaviruses, we reconstructed a phylogenetic tree of known orthobornaviruses based on the alignment of full-length G gene coding sequences. While the splicing signals are conserved among all PaBV-4 G sequences in the NCBI database, interestingly, other orthobornaviruses, even PaBV-1, -2, -3, and -7, which belong to the same species as PaBV-4, namely Orthobornavirus alphapsittaciforme, and are also associated with PDD, do not have the canonical splicing signals in this region (Fig. 2A). RNA-seq analyses of cell cultures persistently infected with orthobornaviruses available in our lab confirmed that the splicing event does not occur in cells infected with PaBV-2, PaBV-7, munia bornavirus 1 (MuBV-1; species Orthobornavirus serini), and BoDV-1 (Fig. 2B to F). These results suggested that the splicing signals were acquired only in PaBV-4 during its evolution.
Fig 2.
The newly identified splicing is found only in PaBV-4. (A) Phylogenetic analysis of orthobornavirus G genes using the neighbor-joining method. NCBI accession numbers are shown next to each taxon. The surrounding nucleotide sequences of the splicing donor and acceptor sites are shown for each isolate. (B–F) Read coverage of the relative splicing donor site achieved from cell cultures persistently infected with different orthobornaviruses: (B) PaBV-4 strain 7I6, (C) PaBV-2 strain KOKO, (D) PaBV-7 strain KU2020, (E) MuBV-1, and (F) BoDV-1 strain He/80.
The spliced GP transcript allows expression of a soluble extracellular isoform
The newly identified splicing of the PaBV-4 G gene revealed that the spliced transcript retains the protein coding sequence and that this variant may produce a truncated isoform of G, consisting of three-quarters of the N-terminal subunit of glycosylated polypeptide (GP-N) region from the N-terminus and half of the cytoplasmic tail (CT) region to the C-terminus (Fig. 3A). The predicted structure of the isoform suggested that this variant might be a secreted protein because it lacks the TM sequences but contains an SP, a short amino acid sequence that directs the protein to the secretory pathway (24, 25). To analyze the characteristics of the predicted G isoform, named sGP, of PaBV-4, we constructed expression plasmids of sGP. As shown in Fig. 3B, while anti-BoDV-1 G and FLAG antibodies detected approximately 90 kDa bands in the 293T cells transfected with full-length G expression plasmids of PaBV-4 (FullG and FullG-FLAG), expression plasmids of the truncated isoforms (sGP, FLAG-sGP, and sGP-FLAG) produced approximately 40 kDa bands (Fig. 3B). Although immunoblotting detected several signals in close proximity, which may be due to differences in glycosylation of the G protein, we did not further confirm their glycosylation in this study. In addition, the FullG- and FullG-FLAG-transfected cells induced fainter bands of the same size as sGP. These observations revealed the translation and expression potential of sGP. To investigate the subcellular localization of sGP, QT6 cells expressing SV40 large T antigen (QT6-T) were transfected with mCherry or FLAG-tagged sGP and visualized by immunofluorescence assay (IFA). Full-length G was localized to the Golgi complex and around the nuclear membrane, similar to BoDV-1 G, and sGP was found to be distributed from the Golgi complex to the cytoplasm in transfected cells (Fig. 3C and D). We also investigated whether sGP is secreted by transfected cells. The expression plasmid coding for sGP tagged with HiBiT small peptide tag at the C-terminus (sGP-HiBiT) was transfected into PaBV-4-infected or uninfected QT6-T cells, and sGP expression in the intracellular and extracellular fractions was detected by the HiBiT-based luciferase system, which is the high sensitive method to enable detection of very low amounts of tagged protein by a luminescence assay. As shown in Fig. 3E and F, while intracellular expression of sGP-HiBiT and mCherry-HiBiT (a non-secreting negative control) was detected in the cells, only the culture supernatant from the sGP-HiBiT-transfected cells showed luciferase activity, suggesting that sGP is secreted from the transfected cells. Intriguingly, a higher level of secreted sGP was detected in the infected cells compared to the uninfected cells, indicating that viral replication or viral products may influence the secretion of sGP.
Fig 3.
The newly identified splicing event produces an extracellular isoform of G. (A) Schematic representation of the full-length PaBV-4 G gene and the spliced isoform of G, sGP. While the full-length gene encodes for the signal peptide sequence (SP), the GP-N ectodomain subunit region (gp27), the cleavage site by furin (CS), the GP-C transmembrane subunit region (gp29), the transmembrane region (TM), and the cytoplasmic tail (CT), the spliced product encodes only for SP, GP-N, and the N-terminal part of CT. (B) Detection of recombinant G isoforms: Western blot analysis was performed on HEK293T cells transfected with full-length G or sGP expression plasmids using anti-BoDV-1 G or anti-FLAG antibodies. (C) The mCherry-fused signal peptide (SP-mCherry), full-length G (G-mCherry), and sGP (sGP-mCherry) were observed in transfected QT6-T cells. (D) Subcellular localization of FLAG-fused full-length G and sGP in transfected QT6-T cells was analyzed by IFA using anti-BoDV-1 G or anti-FLAG antibodies. Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Intracellular (E) and extracellular (F) expression of HiBit-labeled sGP. HiBit expression was detected by chemiluminescence assay from cell lysate (E, intracellular) or cell culture supernatant (F, extracellular) at 48 h after the transfection of each construct into uninfected (white column) or PaBV-4-infected (gray column) QT6-T cells. Individual dots represent three independent experiments. Bars indicate the arithmetic mean ± standard error (SE).
Exogenous expression of sGP does not affect the viral transcription and infectivity of de novo virions
Previous studies of other RNA viruses, such as Ebola virus (EBOV), have reported that the expression of soluble glycoproteins has a significant effect on viral replication and virulence (26 - 28). Therefore, we next investigated the role of sGP in PaBV-4 replication. First, to investigate the effect of sGP on viral polymerase activity, various amounts of the expression plasmid of sGP were transfected into DF-1 cells expressing SV40 large T antigen (DF-1T), together with the minigenome plasmids of PaBV-4 (29, 30). As shown in Fig. 4A, additional expression of sGP did not affect the polymerase activity of PaBV-4 in avian cells. Furthermore, we transfected the sGP expression plasmid into PaBV-4-infected QT6 cells and examined the effect of sGP overexpression on viral replication through the quantification of viral RNAs. The results showed that sGP expression did not alter the amount of viral mRNA or genomic RNA in the transfected cells at any dose of transfected plasmid (Fig. 4B). In addition, we titrated virus particles recovered from the supernatant of sGP-transfected PaBV-4-infected QT6 cells on fresh QT6 cells to determine whether additional expression of the G isoform affects the infectivity of the de novo virus particle. However, no change was observed in the levels of either the de novo viral titer (Fig. 4C). These results suggested that sGP does not have a direct effect on viral transcription or infectivity.
Fig 4.
The overexpression of sGP does not affect viral replication. (A) Effect of sGP expression on viral polymerase activity. PaBV-4 minigenome assays were performed in sGP-overexpressing DF-1T cells. The empty plasmid as a control (white column) or sGP expression plasmid (gray column) was transfected with minigenome and helper plasmids. Luciferase activity was measured 72 h after transfection. The amount of transfected plasmid is indicated. Results are shown as relative polymerase activity normalized to the sample with the lowest concentration of the empty vector (Control, 50 ng). (B–D) Effects of sGP expression on viral transcription, replication, and particle production. (B) PaBV-4-infected QT6 cells were transfected with empty plasmid as a control (white column) or sGP expression plasmid (gray column), and qRT-PCR was performed to quantify viral mRNA or genomic RNA at 48 h post transfection. (C) The supernatant of sGP-transfected PaBV-4-infected QT6 cells was collected at 48 h post transfection and dilution series of the supernatant were inoculated into uninfected QT6 cells to determine viral titers by IFA using an anti-PaBV-4 P antibody 3 days after inoculation. Dots indicate three independent experiments. Bars represent arithmetic mean ± standard error (SE).
Development of a reverse genetics system for PaBV-4
To better understand the role of the splicing event of the PaBV-4 G transcript, we developed a reverse genetics system for PaBV-4. To generate full-length cDNA clones of the PaBV-4 genome, we extracted total RNA from PaBV-4 strain #6758-infected QM7 cells (31). The amplified PaBV-4 cDNA was introduced into an expression plasmid for BoDV-1 reverse genetics (32) to replace the BoDV-1 coding sequence. Briefly, the full-length cDNA clone of the PaBV-4 genome was cloned between the hammerhead ribozyme (HamRz) and hepatitis delta ribozyme (HdRz) sequences downstream of the RNA Pol II promoter. The PaBV-4 N, P, and L expression plasmids as helper plasmids have been described previously (29, 30). A cDNA plasmid containing an extra viral transcription cassette was constructed by inserting the transcription initiation signal (S3) and termination signal (T2) between the P and M genes to allow for the insertion of reporter genes, such as the green fluorescent protein (GFP) gene (Fig. 5A). In addition, we constructed PaBV-4 cDNA plasmids with an additional ACAA sequence at the 3´ end of the transcribed RNA because previous reports have shown that the addition of four nucleotides at the 3´ end of the viral genome RNA significantly increases the rescue efficiency of recombinant BoDV-1 in reverse genetics (33, 34).
Fig 5.
Development of a PaBV-4 reverse genetics system and rescue of sGP-deficient PaBV-4. Schematic representation of the genome structure of rPaBV-4 p/m GFP (A) and the PaBV-4 reverse genetics procedure (B). (C and D) QT6 or DF-1 cells were transfected with expression plasmids encoding for different variants of rPaBV-4 p/m GFP together with helper plasmids. (−)AACA and (+)AACA indicate the modification of the terminal sequence of the PaBV-4 cDNA plasmid. At 96 h after transfection, the transfected cells were cocultured with fresh blasticidin-resistant QT6 cells and blasticidin treatment was started to remove the transfected cells. The rescue efficiency was assessed by determining the number of GFP-positive cells at 6 days (C) or 30 days (D) after transfection. Individual dots represent six independent experiments. Bars indicate the arithmetic mean ± standard error (SE). Statistical analysis was performed by Tukey’s multiple comparison test. **, P < 0.01. (E) Detection of PaBV-4 P and GFPs in QT6 cells infected with the indicated viruses by Western blot analysis. Antibodies used in this analysis are indicated on the left side of the panels. (F) Representative bright field image (top) and GFP fluorescence (bottom) from rPaBV-4 p/m GFP persistently infected cells 60 days after transfection. Scale bar: 100 µm.
In a previous study, we showed that bornaviruses belonging to the species Orthobornavirus alphapsittaciforme, including PaBV-4, cannot replicate in mammalian-derived cell lines (30). Therefore, in the reverse genetics system of PaBV-4, we used avian-derived cell lines, such as the QT6 quail and DF-1 chicken cell lines. In addition, we used QT6-T and DF-1T cell, allowing sustained replication of transfected plasmids in transduced cells (30). The rescue of recombinant PaBV-4 was performed according to the rBoDV-1 reverse genetics system (Fig. 5B). The expression plasmids for PaBV-4 genome RNA and N, P, and L were cotransfected into avian cell lines in an appropriate ratio and then cocultured with blasticidin-resistant QT6 cells to recover the recombinant viruses. The rPaBV-4 encoding GFP (rPaBV-4 p/m GFP) was more efficiently recovered from QT6 quail cells than from DF-1 chicken cells. Furthermore, the use of SV40 large T antigen-expressing cells as well as the addition of AACA to the 3´ end of the genome increased the rescue efficiency (Fig. 5C and D). We also showed that rPaBV-4 p/m GFP replicates, albeit slowly, and maintains a persistent infection in QT6 cells for at least 90 days (Fig. 5E and F).
A mutant in the G splicing donor site reduces the propagation efficiency of rPaBV-4
To understand the effect of newly identified splicing of the G gene on PaBV-4 replication, we introduced a mutation in the splicing donor site of the PaBV-4 genome (Fig. 6A). We successfully rescued the rPaBV-4 p/m GFP carrying the mutant rPaBV-4 (rPaBV-4 MutΔsGP). RNA-seq analysis of QT6 cells infected with rPaBV-4 wild-type (WT) and rPaBV-4 MutΔsGP revealed that the sequence change in the splicing donor efficiently eliminated the splicing of the G gene (Fig. 6B). Immunofluorescence assays using anti-PaBV-4 N and P antibodies did not reveal any changes in the intracellular localization of these proteins in rPaBV-4 MutΔsGP-infected cells (Fig. 6C). Furthermore, we did not observe any differences in the size or number of intranuclear viral factories between WT and MutΔsGP-infected cells (Fig. 6C).
Fig 6.
rPaBV-4 without the G splicing donor reduces viral propagation. (A) Schematic diagram of the PaBV-4 G and the sequence design of the G splicing donor mutant for MutΔsGP. The nucleotide sequences of the splicing donor and acceptor sites in the G gene are shown in red letters. SP, signal peptide; GP-N, ectodomain subunit region (gp27); CS, the cleavage site by furin; GP-C, transmembrane subunit region (gp29); TM, transmembrane region; CT, cytoplasmic region. (B) Read coverage of RNA-seq data around the G splicing donor site. The splicing donor (G) was defined as 0, and the relative sequence positions are shown with the read coverage in WT (left) and MutΔsGP-infected QT6 cells (right). (C) Localization of N and P proteins in MutΔsGP-infected QT6 cells. IFA was performed using anti-BoDV-1 N and anti-BoDV-1 P antibodies. Nuclei were counterstained with DAPI. Scale bars: 10 µm. (D) Growth kinetics of rPaBV-4 MutΔsGP. QT6 cells were inoculated with WT and MutΔsGP viruses at a multiplicity of infection (MOI) of 0.05, and the percentage of GFP-positive cells was measured every 3 days. **, P < 0.01. (E) Competition assay between WT and MutΔsGP viruses. rPaBV-4 WT- and MutΔsGP-infected QT6 cells were cocultured with uninfected QT6 cells at a ratio of 1:1:8, and the population percentages of WT and MutΔsGP viruses in the culture were determined by sequencing of 25 cloned RT-PCR products every 3 days. D, days after coculture. Three independent experiments were performed and the respective mean values are shown. (F) The propagation of MutΔsGP virus in sGP-expressing QT6 cells. Parental (empty) and sGP-expressing (sGP) QT6 cells were inoculated with rPaBV-4 WT or MutΔsGP at an MOI of 0.05. Levels of viral genomic RNA (left) and P mRNA (right) were measured by qRT-PCR on the indicated days. dpi, day after inoculation. Means ± standard error (SE) of relative viral RNA levels normalized to GAPDH of three independent experiments are shown. The amount of viral RNA at 3 dpi was defined as 1.0, and the values for each time point (dpi) are shown as relative ratios. Significance was analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Different letters (a, b, c) indicate statistically significant differences at P < 0.05.
To investigate the propagation of rPaBV-4 MutΔsGP in vitro, we next infected QT6 cells with the mutant virus. As shown in Fig. 6D, rPaBV-4 MutΔsGP replicated more slowly than the WT virus in the QT6 cells. To further understand the role of sGP in virus replication, we quantified growth efficiency using the competition assay. QT6 cells infected with rPaBV-4 MutΔsGP or rPaBV-4 WT were cocultured with uninfected QT6 cells at the same ratio (Fig. 6E). As a result, cells infected with rPaBV-4 WT gradually increased in proportion among the cultured cells, finally reaching approximately 80% at day 18 after coculture. These results suggested that the intrinsic expression of sGP from viral genome RNA affects the spread of rPaBV-4 in cultured cells.
Finally, we generated QT6 cells stably expressing recombinant sGP and infected them with rPaBV-4 MutΔsGP and rPaBV-4 WT to investigate whether the engineered cells could rescue the phenotype of the MutΔsGP virus. However, the stable expression of sGP did not enhance the propagation of rPaBV-4 MutΔsGP, as estimated by the replication and transcription efficiencies observed in QT6 cells (Fig. 6F). Together with the observations in Fig. 3, these results suggested that the sGP isoform does not affect viral propagation as an extrinsic factor and that autologous or regulated expression may be critical to exert the function of sGP in PaBV-4 propagation.
DISCUSSION
In this study, we identified a novel splicing intron within the G gene of PaBV-4, a member of the species Orthobornavirus alphapsittaciforme, and demonstrated that this splicing event produces a secreted isoform of G, sGP. Using a newly developed reverse genetics system for PaBV-4, we showed that intrinsic expression of the transcript for sGP from the viral genome, but not exogenous expression from a plasmid, is required for proper viral replication in vitro, suggesting that regulated or appropriate levels of sGP are important to elicit the effect on viral propagation. Further studies will be necessary to understand the role of sGP in the replication and pathogenesis of PaBV-4. In particularly, experimental infection of psittacines would be of interest to elucidate the role of the PaBV-4 sGP on pathogenesis and possibly immune escape in vivo.
Interestingly, the newly identified intron was conserved in PaBV-4 but not in other members of the species Orthobornavirus alphapsittaciforme (namely PaBV-1, -2, -3, and -7) (3 - 5). Subtype-specific gene expression has been demonstrated in several viruses. For example, it has been shown that different genotypes of hepatitis B virus exhibit different splicing patterns of their transcripts, although the mechanism and significance of this is still unclear (35 - 37). In addition, the splicing efficiency of H1N1 influenza A virus segment 8 mRNAs is reported to be lower than that of H3N2 viruses (38, 39). The genotype-dependent gene expression pattern has also been reported in respiratory syncytial virus infection (40). Considering that viral splicing depends on host machinery, cell type- or host-specific factors would have determined its efficiency. Thus, the type-specific splicing event may indicate an adaptation to the host-specific environment during the evolution of the genotype. This may be related to host-specific evolution in viral virulence or pathogenesis. It may be of interest to introduce splice sites for sGP into the genomes of other avian bornaviruses to understand their phenotypes in spreading and pathogenesis.
Orthobornavirus G is an envelope glycoprotein that plays a role in cell entry, including receptor attachment and membrane fusion of the viral particle (25, 41, 42). Although the critical functions of envelope G in viral replication and virion production have been demonstrated in many RNA viruses, the roles of orthobornavirus G are not well understood. In a previous study, we showed that the overexpression of BoDV-1 G in infected cells resulted in increased levels of the precursor form of G and the production of immature particles containing the uncleaved G, leading to a reduction in viral infectivity (43). Although the cleavage and intracellular dynamics of the G of avian bornaviruses have not yet been elucidated, an appropriate level of G may also be required for efficient maturation of viral particles in infected cells. Therefore, it is conceivable that sGP or the splicing event of PaBV-4 G may control the expression level of the precursor and mature G in the infected cells. In contrast, as observed in the HiBiT luciferase system, the secretion level of sGP appears to be influenced by PaBV-4 infection. It would be of interest to investigate the interplay between the maturation of G and sGP production in infected cells in the future.
Some RNA viruses are known to produce soluble glycoproteins to control the effect on viral replication and virulence in infected hosts. It has been reported that EBOV abundantly expresses soluble GP during infection, but its expression is cell type dependent (26 - 28). Recombinant EBOV lacking soluble GP expression was more cytotoxic but less pathogenic in animals, suggesting that soluble GP serves as a virulence factor. Furthermore, EBOV soluble GP has been shown to have anti-inflammatory activities and to act as a decoy for EBOV GP antibodies (44, 45). These may contribute to systemic viral replication in infected hosts. Similar soluble forms of envelope glycoproteins have been identified in other RNA viruses, including Lassa virus, respiratory syncytial virus, and rabies virus (46 - 49). Although the roles of these soluble forms have not been studied in detail, it is likely that such isoforms have immunomodulatory and decoy functions similar to those of EBOV soluble GP (44, 45, 50, 51). In this study, we did not observe a direct effect of sGP expression on viral transcription or infectivity (Fig. 4); therefore, it is conceivable that PaBV-4 sGP may also play a role in decoying immune targets to support viral replication and spread in vivo.
In this study, we developed a reverse genetics system for PaBV-4, which is the first report of a reverse genetics system for an avian bornavirus. We compared two avian cell lines, chicken DF-1 and quail QT6 cells, as well as those expressing SV40 T antigen, for the transfection of the full-length genome cDNA plasmid and helper plasmids encoding the N, P, and L of PaBV-4. In addition, we added an ACAA sequence at the 3´ end of the genome terminus to improve the rescue efficiency of the recombinant virus. In this study, we successfully rescued replication-competent rPaBV-4 expressing GFP from the region between the P and M genes. The reverse genetics system developed here not only will accelerate research on PaBV-4 replication and pathogenesis but also will facilitate the antiviral drug screening and vaccine development for parrot bornavirus infection to prevent and treat PDD in psittacines. We are currently developing reverse genetics systems for other avian bornaviruses that will help us better understand their replication and virulence in host species.
We identified a soluble form of the G envelope glycoprotein of PaBV-4. Although the detailed role of this shed isoform has not been elucidated, further studies with recombinant avian bornaviruses will elucidate the significance of this unique isoform in the infection and pathogenesis of PaBV-4, which is the most widely distributed bornavirus in psittacines worldwide.
MATERIALS AND METHODS
Cell lines and viruses
QT6 (quail fibroblast-like cells, ATCC CRL-1708) cell lines were maintained in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F12; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% heat-inactivated fetal calf serum (FCS; MP Biomedical, Santa Ana, CA, USA). HEK293T and DF-1 (chicken embryonic fibroblasts, ATCC CRL-12203) cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) with 10% heat-inactivated FCS. The SV40 large T antigen-expressing QT6 (QT6-T) or DF-1 (DF-1T) cell lines were generated using the PiggyBac transposon vector and Super PiggyBac transposase expression vector system (System Biosciences, LLC). To generate cell lines stably expressing sGP, sGP gene was inserted into the cloning site of the CSII-CMV plasmid for lentiviral vector (RIKEN; RDB04377), and the resultant lentiviral vectors were inoculated into the cell lines. To produce blasticidin-resistant QT6 cells, lentiviral vector encoding the blasticidin resistance gene was produced and introduced into the cells.
Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. rBoDV-1 used in this study was generated by a reverse genetics system using a cDNA plasmid from strain He/80 (32). PaBV-2 strain KOKO (22), PaBV-4 strain AR18A (21), PaBV-4 strain 7I6 (22), and MuBV-1 (30) were reported previously. PaBV-7 strain KU2020 was isolated from brain samples of a PaBV-7- positive Goffin’s cockatoo (Cacatua goffiniana) suffering from PDD in Japan (R. Komorizono, A. Makino, et al., unpublished data).
Plasmid construction
The full-length cDNA of the PaBV-4 clone was constructed according to the protocol described previously (32). To construct a plasmid expressing the full-length PaBV-4 antigenomic RNA, pPaBV-4, a cloned full-length cDNA of PaBV-4 strain #6758 (accession number JX065209), was inserted into the Xho I and Not I sites of pCAG-HRSV3 with HamRz at the 5´ region and HdRz at the 3´ region. A plasmid containing an additional transcription cassette, pPaBV-4 p/m, was generated by inserting transcription initiation (S3) and termination (T2) signal sequences between the P gene and the M gene with Bst BI and Pac I sites. To generate GFP-encoding vectors, the GFP gene was amplified from peGFP-N1 (Clontech) and inserted into pPaBV-4 p/m with the above Bst BI and Pac I sites.
Reverse genetics of PaBV-4
QT6, QT6-T, DF-1, and DF-1T cells were seeded in 6-well plates and transfected with 2 µg of pPaBV-4, 0.5 µg of pCAGGS-PaBV-4 N, 0.025 µg of pCAGGS-PaBV-4 P, and 0.25 µg of pCAGGS-PaBV-4 L gene expression plasmids using the TransIT-X2 Dynamic Delivery System (Mirus Bio, Madison, WI, USA). The transfected cells were trypsinized and seeded on 10 cm dishes 4 days after transfection. After 24 h, the transfected cells were cocultured with approximately 2 × 105 blasticidin-resistant QT6 cells. Cocultured cells were passaged every 4 days. The rescue efficiency of recombinant PaBV-4 was evaluated by determining the number of GFP-positive foci per well.
Cell-free virus preparation
Cell-free virus solutions were prepared by sonication as previously described (52). Briefly, cells infected with BoDV-1, PaBV-2 strain KOKO, PaBV-4 strain AR18A, PaBV-4 strain 7I6, PaBV-7 strain KU2020, or MuBV-1 were suspended in OptiMEM (Invitrogen) and subjected to sonication using a BIORUPTOR II (Sonic Bio, Kanagawa, Japan). After the centrifugation of the sonicated cell suspensions at 1,200 × g for 25 min at 4°C, the supernatant was collected and stored at −80°C as a cell-free virus solution.
RT-PCR
Total RNA was extracted from cells using the RNeasy Mini Kit (Qiagen) and reverse transcribed using the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) with random hexamer or oligo dT primers, according to the manufacturer’s instructions. RT-PCR was performed with PrimeStar MAX polymerase (TaKaRa Bio, Shiga, Japan) according to the manufacturer’s instructions. Primers for the M gene to L gene region (primer pair 1) and GP1 to L gene region (primer pair 2) were used to detect alternative splicing events, as shown in Fig. 1B. The sequences of primers were as follows: pair 1 forward: 5´-tacacgacttcctggtcttaactc-3´, pair 2 forward: 5´-acactgagtgatggaagcctac-3´, pair 1/pair 2 reverse: 5´-cgaacatgatgcggcaatgat-3´.
qRT-PCR analysis
Total RNA was extracted using the RNeasy mini kit (Qiagen). Reverse transcription was performed using the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) with oligo dT primers for viral mRNA or viral genome-specific primers for viral genomic RNA. qRT-PCR analyses were performed using Thunderbird SYBR qPCR mix (Toyobo). The sequences of primer pairs used were as follows: PaBV-4 forward: 5´-atgaattcaaaacacacctacgt-3´, reverse: 5´-ttaagggccggaatggcgtatgt-3´, quail GAPDH forward: 5´-gcaaccgtgttgtggacttg-3´, reverse: 5´-gggaacagaactggcctctc-3´, PaBV-4 genomic RNA-specific RT primer: 5´-gttgcggtaacaaccaaccagcaac-3´.
Minigenome assay
The PaBV-4 minigenome assay was performed according to the protocol described previously (29, 30). Briefly, DF-1T cells were transfected with a Pol II-driven PaBV-4 minigenome plasmid carrying the Gaussia luciferase gene and helper plasmids expressing the PaBV-4 N, P, and L genes using Lipofectamine 2000 (Thermo Fisher Scientific). Seventy-two hours after transfection, Gaussia luciferase activity was measured with a BioLux luciferase assay kit (New England BioLabs, Ipswich, MA, USA) and normalized to the corresponding WST-1 activity (TaKaRa), according to the manufacturer’s instructions.
Western blotting
Cultured cells were lysed with SDS sample buffer. Cell lysates were subjected to SDS-PAGE on Any kD Mini-PROTEAN TGX Precast Protein Gels (Bio-Rad, Hercules, CA, USA) after sonication using a Bioruptor II (Sonic Bio, Kanagawa, Japan). Proteins were transferred by a Trans-Blot Turbo PVDF Transfer Pack (Bio-Rad). The membranes were blocked with Blocking One (Nacalai Tesque, Kyoto, Japan) and then reacted with anti-BoDV-1 N (rabbit polyclonal HB01), anti-BoDV-1 P (rabbit polyclonal HB03) (53), anti-BoDV-1 G (rabbit polyclonal HBG), anti-M2 FLAG tag (Merck, Darmstadt, Germany), and anti-Tubulin (B-5-1-2; Sigma-Aldrich) antibodies, followed by a reaction with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch). The bound antibodies were detected using ECL Plus Western blotting detection reagents (GE Healthcare, Chicago, IL, USA).
Immunofluorescence analysis
Cells were fixed with 4% paraformaldehyde after the removal of the culture medium and permeabilized by incubation in phosphate-buffered saline (PBS) containing 0.25% Triton X-100 for 10 min. After permeabilization, fixed cells were incubated with anti-BoDV-1 N, anti-BoDV-1 P, and anti-M2 FLAG tag antibodies. This incubation step was followed by incubation with the appropriate Alexa Fluor-conjugated secondary antibodies (Thermo Fisher Scientific) and DAPI (4′,6-diamidino-2-phenylindole; Merck). An ECLIPSE Ti confocal laser scanning microscope (Nikon, Tokyo, Japan) was used for imaging and data collection.
RNA-seq and data processing
Total RNA from the brain tissue of PaBV-4-infected individuals and bornavirus-infected QT6 cells or OL cells was extracted using TRIzol reagent (Invitrogen), according to the manufacturer’s instructions. NGS libraries were prepared using a TruSeq stranded mRNA Library kit (Illumina), according to the manufacturer’s instructions. NGS was performed on the NovaSeq 6000 Illumina platform. Paired-end raw data were processed using prinseq plus-plus 0.20v1.2.4, trimgalore v4.1, and MultiQC v1.13 to check read quality (54 - 56). The RNA sequencing reads were mapped to each viral reference genome sequence using STAR v2.7.10a and visualized by Integrative Genomics Viewer (57, 58). Alternative splicing junctions were analyzed by MISO software v0.5.4, and read coverages were quantified by the pysamstats tool (23). The respective reference viral genome sequences used for read mapping were as follows: BoDV-1: AJ311522; PaBV-2: EU781967; PaBV-4: JX065209; PaBV-7: JX065210; MuBV-1: OQ550279.
Reconstruction of the phylogenetic tree
Nucleotide sequences of orthobornavirus G genes were derived from GenBank and aligned by the MAFFT program (59). Phylogenetic analysis was conducted using the neighbor-joining tree build method with the Juke-Cantor model and 10,000 bootstrap replicates by MEGA X (60). The accession numbers and genotypes of each sequence are shown in the figure.
HiBit detection assay
To quantify the expression level of intracellular or extracellular sGP, a HiBit tag-fused sGP expression plasmid was generated by PCR extension. Forty-eight hours after transfection into QT6-T cells, luciferase activity was measured with a Nano Glo HiBiT Lytic Detection System (Promega) and NanoGlo HiBiT Extracellular Detection System (Promega) according to the manufacturer’s instructions. As a control, a HiBit tag fused to a 2× mCherry expression plasmid was used.
Competition assay
Equal numbers (1 × 103) of QT6 cells persistently infected with rPaBV-4 WT or rPaBV-4 MutΔsGP were seeded with 8 × 103 uninfected QT6 cells in the same well of the culture plate. Cocultured cells were passaged, and total RNA was harvested every 3 days. RNA was reverse transcribed using a Verso cDNA synthesis kit (Thermo Fisher Scientific) and amplified by PCR using PrimeStar MAX polymerase (TaKaRa). The PCR products of the splice junction region of the G gene were cloned into the pCR4Blunt-TOPO vector (Thermo Fisher Scientific), according to the manufacturer’s guidelines. Twenty-five colonies per PCR product were picked and sequenced to estimate the percentage of each recombinant virus in the population.
ACKNOWLEDGMENTS
We thank Dr. Yukiko Sassa (Tokyo University of Agriculture and Technology, Japan) and Dr. Reiko Soga (Grow-Wing Animal Hospital, Kanagawa, Japan) for providing MuBV-1 isolates and fecal samples of PDD-affected macaws, respectively.
This work was supported in part by JSPS KAKENHI, grant numbers JP19K22530 (K.T.), JP20H00662 (K.T.), JP21K19909 (K.T.), and 18K05991 (A.M.); MEXT KAKENHI, grant number 19H04834 (A.M.); AMED, grant number JP20wm0325011h0001 (A.M.); JST START Project Promotion Type (commercialization support), grant number JPMJST2113 (K.T.); the JSPS Core-to-Core Program, JPJSCCA20190008 (K.T.); a 2021 Kaketsuken Research Grant (K.T.); the Joint Usage/Research Center Program of the Institute for Life and Medical Sciences, Kyoto University; and the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG), grant number RU-1923/2-1 (D.R.).
Contributor Information
Keizo Tomonaga, Email: tomonaga@infront.kyoto-u.ac.jp.
Anice C. Lowen, Emory University School of Medicine, Atlanta, Georgia, USA
REFERENCES
- 1. Kistler AL, Gancz A, Clubb S, Skewes-Cox P, Fischer K, Sorber K, Chiu CY, Lublin A, Mechani S, Farnoushi Y, Greninger A, Wen CC, Karlene SB, Ganem D, DeRisi JL. 2008. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol J 5:88. doi: 10.1186/1743-422X-5-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Honkavuori KS, Shivaprasad HL, Williams BL, Quan PL, Hornig M, Street C, Palacios G, Hutchison SK, Franca M, Egholm M, Briese T, Lipkin WI. 2008. Novel borna virus in psittacine birds with proventricular dilatation disease. Emerg Infect Dis 14:1883–1886. doi: 10.3201/eid1412.080984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sassa Y, Horie M, Fujino K, Nishiura N, Okazaki S, Furuya T, Nagai M, Omatsu T, Kojima A, Mizugami M, Ueda K, Iki H, Ebisawa K, Tomonaga K, Mizutani T. 2013. Molecular epidemiology of avian bornavirus from pet birds in Japan. Virus Genes 47:173–177. doi: 10.1007/s11262-013-0913-3 [DOI] [PubMed] [Google Scholar]
- 4. Heffels-Redmann U, Enderlein D, Herzog S, Herden C, Piepenbring A, Neumann D, Müller H, Capelli S, Müller H, Oberhäuser K, Gerlach H, Kaleta EF, Lierz M. 2011. Occurrence of avian bornavirus infection in captive psittacines in various European countries and its association with proventricular dilatation disease. Avian Pathol 40:419–426. doi: 10.1080/03079457.2011.589825 [DOI] [PubMed] [Google Scholar]
- 5. Weissenböck H, Bakonyi T, Sekulin K, Ehrensperger F, Doneley RJT, Dürrwald R, Hoop R, Erdélyi K, Gál J, Kolodziejek J, Nowotny N. 2009. Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease. Emerg Infect Dis 15:1453–1459. doi: 10.3201/eid1509.090353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubbenstroth D, Schmidt V, Rinder M, Legler M, Twietmeyer S, Schwemmer P, Corman VM. 2016. Phylogenetic analysis supports horizontal transmission as a driving force of the spread of avian bornaviruses. PLoS One 11:e0160936. doi: 10.1371/journal.pone.0160936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubbenstroth D. 2022. Avian bornavirus research-A comprehensive review. Viruses 14:1513. doi: 10.3390/v14071513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cubitt B, Oldstone C, de la Torre JC. 1994. Sequence and genome organization of borna disease virus. J Virol 68:1382–1396. doi: 10.1128/JVI.68.3.1382-1396.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abraham G, Banerjee AK. 1976. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A 73:1504–1508. doi: 10.1073/pnas.73.5.1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Testa D, Chanda PK, Banerjee AK. 1980. Unique mode of transcription in vitro by vesicular Stomatitis virus. Cell 21:267–275. doi: 10.1016/0092-8674(80)90134-8 [DOI] [PubMed] [Google Scholar]
- 11. Iverson LE, Rose JK. 1981. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell 23:477–484. doi: 10.1016/0092-8674(81)90143-4 [DOI] [PubMed] [Google Scholar]
- 12. Liang B. 2020. Structures of the mononegavirales polymerases. J Virol 94:e00175–20. doi: 10.1128/JVI.00175-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsumoto Y, Hayashi Y, Omori H, Honda T, Daito T, Horie M, Ikuta K, Fujino K, Nakamura S, Schneider U, Chase G, Yoshimori T, Schwemmle M, Tomonaga K. 2012. Bornavirus closely associates and segregates with host chromosomes to ensure persistent Intranuclear infection. Cell Host Microbe 11:492–503. doi: 10.1016/j.chom.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 14. Korn K, Coras R, Bobinger T, Herzog SM, Lücking H, Stöhr R, Huttner HB, Hartmann A, Ensser A. 2018. Fatal encephalitis associated with borna disease virus 1. N Engl J Med 379:1375–1377. doi: 10.1056/NEJMc1800724 [DOI] [PubMed] [Google Scholar]
- 15. Schlottau K, Forth L, Angstwurm K, Höper D, Zecher D, Liesche F, Hoffmann B, Kegel V, Seehofer D, Platen S, Salzberger B, Liebert UG, Niller HH, Schmidt B, Matiasek K, Riemenschneider MJ, Brochhausen C, Banas B, Renders L, Moog P, Wunderlich S, Seifert CL, Barreiros A, Rahmel A, Weiss J, Tappe D, Herden C, Schmidt-Chanasit J, Schwemmle M, Rubbenstroth D, Schlegel J, Pietsch C, Hoffmann D, Jantsch J, Beer M. 2018. Fatal encephalitic borna disease virus 1 in solid-organ transplant recipients. N Engl J Med 379:1377–1379. doi: 10.1056/NEJMc1803115 [DOI] [PubMed] [Google Scholar]
- 16. Niller HH, Angstwurm K, Rubbenstroth D, Schlottau K, Ebinger A, Giese S, Wunderlich S, Banas B, Forth LF, Hoffmann D, Höper D, Schwemmle M, Tappe D, Schmidt-Chanasit J, Nobach D, Herden C, Brochhausen C, Velez-Char N, Mamilos A, Utpatel K, Evert M, Zoubaa S, Riemenschneider MJ, Ruf V, Herms J, Rieder G, Errath M, Matiasek K, Schlegel J, Liesche-Starnecker F, Neumann B, Fuchs K, Linker RA, Salzberger B, Freilinger T, Gartner L, Wenzel JJ, Reischl U, Jilg W, Gessner A, Jantsch J, Beer M, Schmidt B. 2020. Zoonotic spillover infections with borna disease virus 1 leading to fatal human encephalitis, 1999-2019: an epidemiological investigation. Lancet Infect Dis 20:467–477. doi: 10.1016/S1473-3099(19)30546-8 [DOI] [PubMed] [Google Scholar]
- 17. Cubitt B, Oldstone C, Valcarcel J, Carlos de la Torre J. 1994. RNA splicing contributes to the generation of mature mRNAs of borna disease virus, a non-segmented negative strand RNA virus. Virus Res 34:69–79. doi: 10.1016/0168-1702(94)90120-1 [DOI] [PubMed] [Google Scholar]
- 18. Schneider PA, Schneemann A, Lipkin WI. 1994. RNA splicing in borna disease virus, a nonsegmented, negative-strand RNA virus. J Virol 68:5007–5012. doi: 10.1128/JVI.68.8.5007-5012.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomonaga K, Kobayashi T, Lee BJ, Watanabe M, Kamitani W, Ikuta K. 2000. Identification of alternative splicing and negative splicing activity of a nonsegmented negative-strand RNA virus, borna disease virus. Proc Natl Acad Sci U S A 97:12788–12793. doi: 10.1073/pnas.97.23.12788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kojima S, Sato R, Yanai M, Komatsu Y, Horie M, Igarashi M, Tomonaga K. 2019. Splicing-dependent subcellular targeting of borna disease virus nucleoprotein Isoforms. J Virol 93:e01621-18. doi: 10.1128/JVI.01621-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Komorizono R, Tomonaga K, Makino A. 2020. Development of a reverse transcription-loop-mediated isothermal amplification assay for the detection of parrot bornavirus 4. J Virol Methods 275:113749. doi: 10.1016/j.jviromet.2019.113749 [DOI] [PubMed] [Google Scholar]
- 22. Horie M, Sassa Y, Iki H, Ebisawa K, Fukushi H, Yanai T, Tomonaga K. 2016. Isolation of avian bornaviruses from psittacine birds using QT6 quail cells in Japan. J Vet Med Sci 78:305–308. doi: 10.1292/jvms.15-0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katz Y, Wang ET, Airoldi EM, Burge CB. 2010. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nat Methods 7:1009–1015. doi: 10.1038/nmeth.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garry CE, Garry RF. 2009. Proteomics computational analyses suggest that the bornavirus glycoprotein is a class III viral fusion protein (gamma penetrene). Virol J 6:145. doi: 10.1186/1743-422X-6-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gonzalez-Dunia D, Cubitt B, Grasser FA, de la Torre JC. 1997. Characterization of borna disease virus P56 protein, a surface glycoprotein involved in virus entry. J Virol 71:3208–3218. doi: 10.1128/JVI.71.4.3208-3218.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de La Vega M-A, Wong G, Kobinger GP, Qiu X. 2015. The multiple roles of sGP in Ebola pathogenesis. Viral Immunology 28:3–9. doi: 10.1089/vim.2014.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Volchkova VA, Klenk HD, Volchkov VE. 1999. Delta-peptide is the carboxy-terminal cleavage fragment of the nonstructural small glycoprotein sGP of Ebola virus. Virology 265:164–171. doi: 10.1006/viro.1999.0034 [DOI] [PubMed] [Google Scholar]
- 28. Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci U S A 93:3602–3607. doi: 10.1073/pnas.93.8.3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reuter A, Horie M, Höper D, Ohnemus A, Narr A, Rinder M, Beer M, Staeheli P, Rubbenstroth D. 2016. Synergistic antiviral activity of ribavirin and interferon-α against parrot bornaviruses in avian cells. J Gen Virol 97:2096–2103. doi: 10.1099/jgv.0.000555 [DOI] [PubMed] [Google Scholar]
- 30. Komorizono R, Sassa Y, Horie M, Makino A, Tomonaga K. 2020. Evolutionary selection of the nuclear localization signal in the viral nucleoprotein leads to host adaptation of the genus orthobornavirus Viruses 12:1291. doi: 10.3390/v12111291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubbenstroth D, Rinder M, Kaspers B, Staeheli P. 2012. Efficient isolation of avian bornaviruses (ABV) from naturally infected psittacine birds and identification of a new ABV genotype from a salmon-crested cockatoo (Cacatua Moluccensis). Vet Microbiol 161:36–42. doi: 10.1016/j.vetmic.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 32. Daito T, Fujino K, Honda T, Matsumoto Y, Watanabe Y, Tomonaga K. 2011. A novel borna disease virus vector system that stably expresses foreign proteins from an Intercistronic noncoding region. J Virol 85:12170–12178. doi: 10.1128/JVI.05554-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin A, Hoefs N, Tadewaldt J, Staeheli P, Schneider U. 2011. Genomic RNAs of borna disease virus are elongated on internal template motifs after realignment of the 3' termini. Proc Natl Acad Sci U S A 108:7206–7211. doi: 10.1073/pnas.1016759108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schneider U, Schwemmle M, Staeheli P. 2005. Genome trimming: a unique strategy for replication control employed by borna disease virus. Proc Natl Acad Sci U S A 102:3441–3446. doi: 10.1073/pnas.0405965102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ito N, Nakashima K, Sun S, Ito M, Suzuki T. 2019. Cell type diversity in hepatitis B virus RNA splicing and its regulation. Front Microbiol 10:207. doi: 10.3389/fmicb.2019.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J, Wu M, Wang F, Zhang W, Wang W, Zhang X, Zhang J, Liu Y, Liu Y, Feng Y, Zheng Y, Hu Y, Yuan Z. 2015. Hepatitis B virus spliced variants are associated with an impaired response to interferon therapy. Sci Rep 5:16459. doi: 10.1038/srep16459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taha TY, Anirudhan V, Limothai U, Loeb DD, Petukhov PA, McLachlan A. 2020. Modulation of hepatitis B virus pregenomic RNA stability and splicing by histone deacetylase 5 enhances viral biosynthesis. PLoS Pathog 16:e1008802. doi: 10.1371/journal.ppat.1008802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nilsson K, Abdurahman S, Schwartz S. 2020. Influenza virus natural sequence heterogeneity in segment 8 affects interactions with cellular RNA-binding proteins and splicing efficiency. Virology 549:39–50. doi: 10.1016/j.virol.2020.08.005 [DOI] [PubMed] [Google Scholar]
- 39. Dubois J, Terrier O, Rosa-Calatrava M, Krug R, Palese P. 2014. Influenza viruses and mRNA splicing: Doing more with less. mBio 5. doi: 10.1128/mBio.00070-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Piedra FA, Qiu X, Teng MN, Avadhanula V, Machado AA, Kim DK, Hixson J, Bahl J, Piedra PA. 2020. Non-gradient and genotype-dependent patterns of RSV gene expression. PLoS One 15:e0227558. doi: 10.1371/journal.pone.0227558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clemente R, de la Torre JC. 2007. Cell-to-cell spread of borna disease virus proceeds in the absence of the virus primary receptor and furin-mediated processing of the virus surface glycoprotein. J Virol 81:5968–5977. doi: 10.1128/JVI.02426-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lennartz F, Bayer K, Czerwonka N, Lu Y, Kehr K, Hirz M, Steinmetzer T, Garten W, Herden C. 2016. Surface glycoprotein of borna disease virus mediates virus spread from cell to cell. Cell Microbiol 18:340–354. doi: 10.1111/cmi.12515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sakai M, Fujita Y, Komorizono R, Kanda T, Komatsu Y, Noda T, Tomonaga K, Makino A. 2021. Optimal expression of the envelope glycoprotein of orthobornaviruses determines the production of mature virus particles. J Virol 95:e02221-20. doi: 10.1128/JVI.02221-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu W, Banadyga L, Emeterio K, Wong G, Qiu X. 2019. The roles of Ebola virus soluble glycoprotein in replication, pathogenesis, and countermeasure development. Viruses 11:999. doi: 10.3390/v11110999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Basler CF. 2013. A novel mechanism of immune evasion mediated by Ebola virus soluble glycoprotein. Expert Rev Anti Infect Ther 11:475–478. doi: 10.1586/eri.13.30 [DOI] [PubMed] [Google Scholar]
- 46. Branco LM, Grove JN, Moses LM, Goba A, Fullah M, Momoh M, Schoepp RJ, Bausch DG, Garry RF. 2010. Shedding of soluble glycoprotein 1 detected during acute Lassa virus infection in human subjects. Virol J 7:306. doi: 10.1186/1743-422X-7-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arnold R, König B, Werchau H, König W. 2004. Respiratory syncytial virus deficient in soluble G protein induced an increased proinflammatory response in human lung epithelial cells. Virology 330:384–397. doi: 10.1016/j.virol.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 48. Shingai M, Azuma M, Ebihara T, Sasai M, Funami K, Ayata M, Ogura H, Tsutsumi H, Matsumoto M, Seya T. 2008. Soluble G protein of respiratory syncytial virus inhibits toll-like receptor 3/4-mediated IFN-beta induction. Int Immunol 20:1169–1180. doi: 10.1093/intimm/dxn074 [DOI] [PubMed] [Google Scholar]
- 49. Gaudin Y, Moreira S, B N Jean J, Blondel D, Flamand A, Tuffereau C. 1999. Soluble ectodomain of rabies virus glycoprotein expressed in eukaryotic cells folds in a monomeric conformation that is antigenically distinct from the native state of the complete, membrane-anchored glycoprotein. J Gen Virol 80 ( Pt 7):1647–1656. doi: 10.1099/0022-1317-80-7-1647 [DOI] [PubMed] [Google Scholar]
- 50. Furuyama W, Shifflett K, Feldmann H, Marzi A. 2021. The Ebola virus soluble glycoprotein contributes to viral pathogenesis by activating the MAP kinase signaling pathway. PLoS Pathog 17:e1009937. doi: 10.1371/journal.ppat.1009937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nehls J, Businger R, Hoffmann M, Brinkmann C, Fehrenbacher B, Schaller M, Maurer B, Schönfeld C, Kramer D, Hailfinger S, Pöhlmann S, Schindler M. 2019. Release of immunomodulatory Ebola virus glycoprotein-containing microvesicles is suppressed by tetherin in a species-specific manner. Cell Rep 26:1841–1853. doi: 10.1016/j.celrep.2019.01.065 [DOI] [PubMed] [Google Scholar]
- 52. Fujino K, Yamamoto Y, Daito T, Makino A, Honda T, Tomonaga K. 2017. Generation of a non-transmissive borna disease virus vector lacking both matrix and glycoprotein genes. Microbiol Immunol 61:380–386. doi: 10.1111/1348-0421.12505 [DOI] [PubMed] [Google Scholar]
- 53. Watanabe M, Zhong Q, Kobayashi T, Kamitani W, Tomonaga K, Ikuta K. 2000. Molecular ratio between borna disease viral-p40 and -p24 proteins in infected cells determined by quantitative antigen capture ELISA. Microbiol Immunol 44:765–772. doi: 10.1111/j.1348-0421.2000.tb02561.x [DOI] [PubMed] [Google Scholar]
- 54. Schmieder R, Edwards R. 2011. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864. doi: 10.1093/bioinformatics/btr026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trim Galore software. n.d. Available from: https://github.com/FelixKrueger/TrimGalore
- 56. Ewels P, Magnusson M, Lundin S, Käller M. 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32:3047–3048. doi: 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li H, Durbin R. 2010. Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics 26:589–595. doi: 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. doi: 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. doi: 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kumar S, Stecher G, Li M, Knyaz C, Tamura K, Battistuzzi FU. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]