Abstract
Breast cancer in men is rare, but a relevant public health issue, yielding a 25% higher risk of mortality comparing to female counterparts. The representation of males in clinical trials has been scarce and treatment decisions are based mainly on extrapolations from data in females. In the setting of estrogen-dependent metastatic disease, the use of everolimus has been seldom reported, although the PI3K/AKT/mTOR pathway seems to be a critical oncogenic driver. This paper dissects hallmark biological features of ER+/HER2-advanced male breast cancer, setting a comprehensive basis to promote personalized care, focusing on the potential of targeting the PI3K/AKT/mTOR pathway.
Keywords: Male breast cancer, mTOR, PIK3CA, Everolimus
Highlights
-
•
Evidence on optimal treatment sequencing for male breast cancer is scarce, due to its rarity.
-
•
In this brief communication, we review the role of the PI3k/AKT/mTOR pathway as an oncogenic driver for male breast cancer, and the rationale for the use of targeted agents in this patient population.
-
•
We propose a rationale for treatment choice towards individualized care for male patients with advanced breast cancer.
1. Introduction
Male Breast Cancer (BC) accounts for less than 1% of breast cancer diagnosis every year worldwide [1]. Lack of awareness and stigma associated with male BC often lead to delayed diagnosis, explaining the high rates of advanced disease at presentation (∼42% stage III/IV), endowing a worse survival compared to women [2]. Despite improvements in the last years regarding BC-specific survival for male patients, outcomes are still suboptimal compared to female counterparts [3].
Due to its rarity, data regarding Male BC come mostly from case series, although in the last years, joint efforts as the International Male BC Program allowed the collection of retrospective and prospective data, building on existing evidence regarding this malignancy [4]. Male BC is predominantly an hormone dependent disease, with more than 90% patients presenting with estrogen receptor (ER) positive tumours [2,5,6] and less than 15% with human epidermal growth factor receptor 2 (HER2) positive disease [2,7]. In large retrospective series, ER, progesterone receptor (PR), androgen receptor (AR) and risk gene-signatures were found to be prognostic, while grade and ki-67 did not, contrasting with data in female BC [2,8,9].
Males have historically been underrepresented in BC randomized clinical trials (RCTs). Additionally, the very few studies conducted in this particular population have been closed due to lack of accrual [2,10]. This situation led to restrained approved options for males, being treatment strategies mostly supported by retrospective data, with paucity of evidence coming from prospective, randomised controlled trials. Hence, clinical management of male BC is mostly extrapolated from female BC [11]. To overcome this problem the United States Food and Drug Administration (FDA) issued important recommendations in 2020, advising against excluding male patients from RCT's in BC [12].
Endocrine therapy (ET) with tamoxifen is the preferred adjuvant systemic therapy for ER + male BC, with demonstrated survival benefit [13]. The effectiveness of aromatase inhibitors (AIs) in this setting is less clear and this ET should not be prescribed without a gonadotropin-releasing hormone (GnRH) analogue [14,15]. Abemaciclib, the only approved cyclin dependent kinase 4/6 (CDK4/6) inhibitor in the adjuvant setting for ER + BC, has improved event-free survival in patients with high risk of recurrence, as per the landmark results of the MonarchE trial [16]. Males with BC were also included in this trial, accounting for 21 (0.7%) patients randomized in the experimental arm and 15 (0.5%) in the control arm.
In the metastatic setting, ET is the preferred option for ER + disease [4]. Like for early disease, tamoxifen has shown to be effective even in the presence of predominant visceral or soft tissue disease [17]. Regarding AIs, which should be offered together with a GnRH analogue, case reports series showed activity of this combination, although at the expense of worst quality of life [18,19]. Also, some data for the use of the selective estrogen receptor degrader (SERD) fulvestrant in male BC have been published [20], showing response rates similar to the one observed in female BC patients, either in first or later lines of treatment. More recently, CDK4/6 inhibitors have become standard of care for the treatment of metastatic BC in first and second lines. Despite male patients have been mostly not included in the registry trials, real world data show similar efficacy and tolerability [[21], [22], [23], [24], [25], [26], [27]]. In endocrine-unresponsive and/or significantly symptomatic disease, chemotherapy should be also considered similarly to the guideline-supported recommendations made for female BC. The use of everolimus in male BC has only been reported in three cases in the literature [[28], [29], [30]], added to either tamoxifen, exemestane or a phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) inhibitor, with 2 patients experiencing a prolonged response, without significant toxicity. Indeed, accumulated knowledge brought from molecular profiling, unravels the importance of the phosphatidylinositol-3-kinase (PI3K)/serine-threonine protein kinase AKT/mammalian target of rapamycin (mTOR) pathway in Male BC progression [31,32]. Here we report a case of a male BC patient who underwent treatment with everolimus and an AI in the metastatic setting and further dissect hallmark biological features of this disease, setting a comprehensive basis to promote personalized care of male BC, focusing on the opportunities arising from targeting the Pi3K/AKT/mTOR pathways.
2. Clinical presentation
After several years of feeling a breast lump, a 73-year-old man was diagnosed with BC on his right breast already with skin ulceration at presentation, in 2007. The diagnosis and the first lines of treatment were carried out in another institution. Of note, he had a family history of a father with prostate cancer, mother with colorectal cancer and cousin with lung cancer, all of them diagnosed at an older age. After appropriate staging showing no distant metastasis, he underwent radical mastectomy and axillary lymph-node dissection for locally advanced breast cancer. The pathology report confirmed a 3 cm, grade 2, ductal invasive carcinoma, with skin ulceration and 6 positive lymph-nodes out of 12 resected (pT4bpN2). Immunohistochemistry (IHC) analysis revealed a “triple-positive” disease (ER, PR, HER2 positive). The patient then underwent adjuvant chemotherapy with 6 cycles of docetaxel and epirubicine, followed by adjuvant ET with tamoxifen, adjuvant radiotherapy (50Gy + 10Gy) and one year of adjuvant Trastuzumab. He then remained under follow-up.
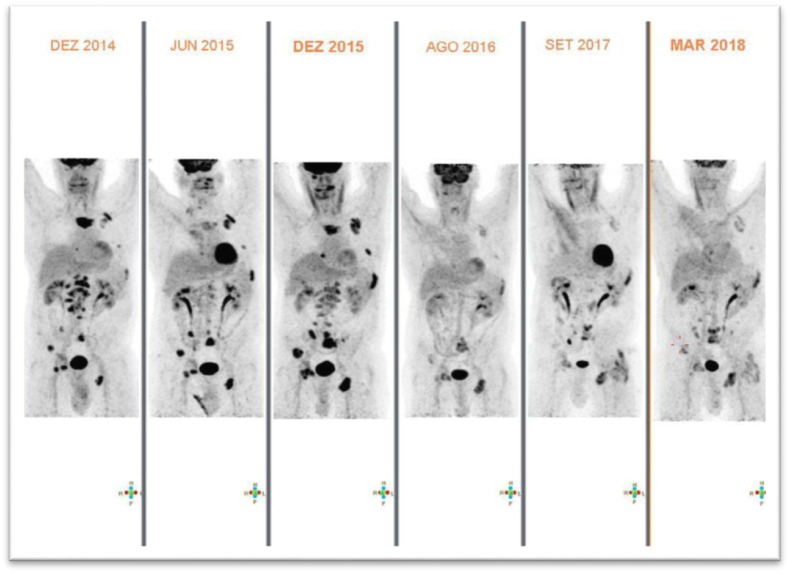
In January 2013, after a disease-free interval of 5 years and 6 months, a rise in tumour markers led to disease restaging with the identification of bone metastases in a bone scan. First line treatment for metastatic disease was then initiated with letrozole, goserelin and zoledronic acid as bone modifying agent. No explanation was provided in the medical files to justify not using any anti-HER2 therapy. In April 2014, after a progression free-survival (PFS) of 15 months, bone progressive disease (PD) was documented. Docetaxel plus trastuzumab was initiated as second line treatment. The patient discontinued docetaxel after five cycles due to grade 3 (CTCAE v5.0) nail toxicity. Anticancer treatment was then maintained with tamoxifen, trastuzumab and zoledronic acid. In December 2014, pleural and bone PD was documented. The patient underwent palliative antalgic radiation therapy on sternum and lumbar regions, but kept the same systemic anticancer treatment. At this timepoint, the patient requested a second opinion at our Institution. Review of the available tumor biopsies confirmed the diagnosis of breast cancer but with different IHC profile: ER-positive, PR-negative and HER2-negative (using two different testing methods). A baseline positron emission tomography-computed tomography (PET-CT) was performed. Germline testing for pathogenic variants in the BRCA 1/2 genes resulted negative. As bone PD was documented in PET-CT in december 2015, anti-HER2 therapy was stopped and a third line of cancer-directed treatment was initiated with everolimus 5 mg (reduced dose due to patient's frailty) together with exemestane, goserelin and zoledronic acid. First assessment of response with PET-CT after 12 weeks showed marked response and the patient remained with disease in response with the ongoing treatment until march 2018, when bone progression was documented (PFS 28 months) (Fig. 1).
Fig. 1.
Evolution of imaging in PET-CT with before and after therapy with Everolimus. Everolimus was initiated in dec 2015 and kept until march 2018.
As the patient had good quality of life and an Eastern Cooperative Oncology Group (ECOG) Performance status of 1, fourth line of treatment with palbociclib, fulvestrant and goserelin was then pursued. The bone-targeted agent was switched to monthly denosumab. The patient achieved clinical and imagological response with no major toxicities and good quality of life. In april 2020 (PFS of 25 months), PET CT documented bone progression and “de novo” disease in the liver. Next-generation sequencing analysis (Oncomine™ Focus Assay) was performed at the initial biopsy specimen, and a PIK3CA mutation (c.3140A > G p.(His1047Arg), in exon 21) was found, thus eliciting the start of a fifth line of therapy with alpelisib together with anastrozole and goserelin. However, due to regulatory constraints, the patient did not initiate this treatment and also declined further chemotherapy. The patient was kept in best supportive care and died in january 2021.
3. Discussion
Evidence is mounting regarding biological hallmark differences between male and female BC and few papers have been published in the last decade in this regard [[31], [32], [33], [34], [35]]. This knowledge allows us to consider that treating male BC based on the experience in female BC may likely be suboptimal. As an example, Johansson and her group [35,36] showed, with genomic profiling and hierarchical clustering, a different genomic subtype present in male BC which was not observed in female BC. Through investigation of potential candidate driver genes, THY1, a gene involved in invasion and related to epithelial to mesenchymal transition, was associated with significant inferior survival in male BC. Furthermore, Callari et al. [32] studied the transcriptomic landscape of male BC compared with female BC and identified a differential overexpression of mTOR and EIF4E, both essential in the regulation of the PI3K/AKT/mTOR signalling pathway, generating the hypothesis of increased susceptibility to treatments targeting this lane in Male BC. Likewise, Piscuoglio et al. [31], using multigene sequencing panels, showed that male BC harboured frequently mutations in PIK3CA (20%) and GATA3 (15%) genes. In a case report of a long-term male responder with metastatic BC (18 months of stable disease) whole-exome sequencing of the primary tumour showed moderate expression of the mTORC1-specific phosphorylation ribosomal protein S6, suggesting that this pathway may have been active [29]. Additionally, the tumour was relatively stable at chromosome level with a very quiet copy-number profile (copy-number-neutral loss of heterozygosity in Chromosome 1p, gain of Chromosome 16p, and loss of Chromosome 16q), which has been associated with improved prognosis [37]. Genetic analysis using NGS of tumour samples from patients included in the BOLERO-2 trial showed that lower chromosomal instability was associated with increased PFS gain derived from treatment with everolimus, in advanced ER+/HER2-female BC [38]. No somatic mutations or copy-number variants were found in the PI3K/mTOR pathway genes, such as PIK3CA, PTEN, MTOR, TSC1 or 2. However, it is proved that PI3K alterations are not necessary for response to mTOR inhibitors, since these act downstream of PI3K [38].
The mTOR kinase represents a key coordinator of cell growth and metabolism, lying both upstream and downstream of the PI3K pathway, being the main cross-talked pathway to the ER and identified as the main mechanism of resistance to ET [39]. Regulators of the PIK3CA/mTOR pathway include, other than gain of function mutations in PIK3CA which lead to hyperactivation of downstream pro-survival signalling pathways, AKT1 and the RAS/RAF/mitogen-activated protein kinase (MAPK) pathway, intersecting at multiple points [40,41].
The first published deoxyribonucleic acid (DNA) sequencing analysis series studying biomarkers and mutations in the PIK3CA/mTOR pathway in familiar male BC showed that PIK3CA mutation are less common in patients with familial disease, further elucidating regarding the different basis of male BC in BRCA2 mutation carriers and the low benefit that these patients may derive from PIK3CA inhibitors [42]. Indeed, the oncogenic drive of PIK3CA may be more important in non-BRCA2 carriers, where estrogenic effects may play a more significant role. However, in vitro studies show that, in cancers with defects in DNA homologous recombination, targeting the PIK3CA increases sensitization to PARP inhibition [43] and these findings may serve as ground for in vivo research in the field.
Many trials showed promising results concerning the role of mTOR inhibitors in reversing the hormone resistance in metastatic BC [44]. Interestingly, the TAMRAD study showed that everolimus combined with tamoxifen after first line treatment with AI was associated with improved clinical benefit [45]. Likewise, the BOLERO 2 trial showed that the addition of everolimus to exemestane was associated with improved PFS, more expressed in patients whose tumours revealed primary endocrine resistance [44]. Our case report adds to the scarce evidence in the literature reporting the benefit of inhibiting the PI3K/AKT/mTOR pathway to reverse endocrine resistance in male BC, summarized in Table 1 [[28], [29], [30]]. In our reported case, the addition of everolimus to exemestane was the therapeutic option that yielded the biggest benefit in terms of disease control.
Table 1.
Clinical Cases reporting use of inhibitors of PIK3CA/AKT/mTOR pathway in male breast cancer.
| First Author (Reference) | Country (Year of Publication) | Sites of metastasis at start of Everolimus | Line in which Everolimus was used | Endocrine treatment Combined with Everolimus | Best imagiologic response to Everolimus |
|---|---|---|---|---|---|
| Kattan, J [28] | Lebanon (2014) | Liver | 3rd | Tamoxifen | Partial Response |
| Brannon, A. Rose [29] | USA (2016) | Lymph nodes, Lung | 3rd | PI3K/mTOR inhibitor BEZ235 | Partial Response |
| Ballatore, R [30]. | Italy (2016) | Lung, subcutaneous nodules | 13th | Exemestane | Partial Response |
In the reported clinical situation, 4th line treatment with palbociclib and fulvestrant was then pursued, as per the pivotal PALOMA-3 clinical trial design [46]. Although this treatment showed efficacy and is currently recommended as first or second-line for the treatment of ER+/HER2-advanced BC, by the time the patient was first treated, this therapeutic option was still not available in Portugal in first and second-lines. Nevertheless, the yielded PFS reassures the meaningful clinical benefit obtained with the exposure to CDK4/6 inhibitors in conjunction with fulvestrant, even in later lines.
Upon disease progression and in the setting of an emergent PIK3CA mutation found using NGS techniques, the proposal of using the PIK3CA inhibitor alpelisib also warrants further scrutiny. Mutations in the PIK3CA gene, encoding the p110α subunit of phosphatidylinositol-3-kinase (PI3K) are associated with resistance to chemotherapy and poor prognosis [47]. The SOLAR-1 trial findings demonstrated the role of PIK3CA as a predictive biomarker for alpelisib clinical efficacy, significantly improving PFS in ER+, HER2-, metastatic BC following progression on or after an endocrine-based regimen [48]. However, the SOLAR-1 trial excluded patients with prior exposure to mTOR inhibitors and therefore, the benefit of the sequential use of everolimus and alpelisib in different treatment lines is not clear and also deserves clinical investigation, at least in patients with PIK3CA-mutated disease. Indeed, in the proof-of-concept BELLE-3 trial, the pan-class I PI3K inhibitor buparlisib improved PFS when compared to placebo in patients with disease progression after prior treatment with everolimus, with an hazard ratio of 0.5 in the subgroup of PIK3CA-mutated metastatic BC [49]. Furthermore, at a biological level, it is important to note that, in BC, mTOR activation results, via negative feedback, in the inhibition of PI3K signalling. Accordingly, when mTOR is pharmacologically inhibited, the loss of negative feedback results in increased activity of PI3K and its effector, the AKT/PKB kinase, thereby dampening the antiproliferative effects of mTOR inhibition. This compromised feedback will likely allow cancer cells to acquire the ability to signalling through this pathway. Moreover, disruption of such normally self-attenuating signals can contribute to the development of adaptive resistance towards therapeutic drugs targeting mitogenic pathways [50].
Current international guidelines issued to guide management of male BC, consider targeted therapy with mTOR inhibitors as well as options guided by PIK3CA mutations in the setting of advanced disease, using the same indications and combinations that are offered to women [3,27,51]. Unfortunately, after the BOLERO-2, other prospective trials investigating the efficacy and tolerability of everolimus have also not included male patients [[52], [53], [54]]. Likewise, even though SOLAR-1 allowed for enrolment of men, the final population only gathered female patients [48]. Changing the paradigm of male representation in trials in BC, the recently published CAPITELLO-291 [55] showed the added benefit of the AKT inhibitor capivasertib, when combined with fulvestrant in patients with ER+/HER2-advanced BC in whom disease progressed during or after AI, with or without CDK4/6i. Noteworthy, 3 male patients out of a total 355 in the experimental arm and 4 out of 353 in the placebo arm were included in the final analysis.
Unfortunately, male exclusion from clinical trials still represent a public health issue that has to be called of. Despite the recent FDA guidance recommendations, only a few trials of the currently ongoing phase I-IV trials evaluating the inhibition of the PI3K/AKT/MTOR pathway in ER+/HER2-is allowing for the inclusion of male patients. These trials and their treatment arms are summarized in Table 2.
Table 2.
Ongoing trials in metastatic BC targeting the PI3K/AKT/MTOR pathway.
| Trial | Phase | Population | Arms |
|---|---|---|---|
| NCT03959891 | I | HR+/HER2-Female | Fulvestrant + Ipatasertib vs. Aromatase Inhibitor + Ipatasertib vs. Fulvestrant + Ipatasertib + Palbociclib |
| NCT04060862 | IB-III | HR+/HER2-Female and Male | Stage 3: ipatasertib + palbociclib + fulvestrant vs. placebo + palbociclib + fulvestrant |
| NCT03337724 | III | TNBC Female and Male |
ipatasertib + paclitaxel vs. placebo + paclitaxel |
| NCT03280563 | IB-II | HR+/HER2− Female |
Stage 1: Atezolizumab + Ipatasertib + Fulvestrant vs. Atezolizumab + Ipatasertib vs. Atezolizumab + Fulvestrant vs. Atezolizumab + Entinostat vs. Fulvestrant (placebo)Stage 2: Atezolizumab + Bevacizumab + Endocrine Therapy |
| NCT03800836 | I | TNBC Female and Male |
In Cohort 1: ipatasertib + atezolizumab + paclitaxel (nab-paclit) ± antra |
| NCT03424005 | IB-II | TNBC Female and Male |
Stage 1: Atezolizumab + Nab-Paclitaxel ± Tocilizumab vs. Nab-Paclitaxel vs. Atezolizumab + Sacituzumab GovitecanStage 2: Capecitabine vs. Atezolizumab + Ipatasertib vs. Atezolizumab + SGN-LIV1A vs. Atezolizumab + Selicrelumab + Bevacizumab vs. tezolizumab + Chemo (Gemcitabine + Carboplatin or Eribulin) |
| NCT03395899 | II | HR+/HER2− Female |
Atezolizumab vs. Atezolizumab + Cobimetinib vs. Atezolizumab + Ipatasertib vs. Atezolizumab + Ipatasertib + Bevacizumab |
| NCT02390427 | I | HER2+ Female and Male |
Taselisib + Pertuzumab + Trastuzumab + Paclitaxel vs. Taselisib + Pertuzumab + Trastuzumab vs. Taselisib + Trastuzumab emtansine + Pertuzumab vs. Taselisib + Trastuzumab emtansine |
| NCT02167854 | I | HER2+ Female |
Study Evaluating the Safety and Tolerability of LJM716, BYL719 and Trastuzumab in Patients with Metastatic HER2+ Breast Cancer |
| NCT04208178 | III | HER2+ Female and Male |
Study of Alpelisib (BYL719) in Combination with Trastuzumab and Pertuzumab as Maintenance Therapy in Patients With HER2-positive Advanced Breast Cancer With a PIK3CA Mutation (EPIK-B2 |
4. Conclusions
While advances in the approach and treatment of female BC have been notable in the last years and knowledge on this disease continues to evolve, data on male BC remains limited. Lack of understanding regarding biology and prognosis of this disease leads often to severe distress and little support to men facing this diagnosis. Certain impediments, like inability to perform randomised trials in male BC due to low incidence of the disease and per protocol inclusion constrains should prompt efforts at setting up large multi-institutional, worldwide observational structured studies must be conducted in order to achieve high-quality real-world data capable of enable the emergence of meaningful guideline of therapies to treat and improve survival of male BC patients. It is also essential that future BC clinical trials do not exclude male patients, allowing for data collection and striving for a gender-neutral regulatory approval.
Funding
This research received no external funding.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
None.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer Statistics. 2018;68(1):7–30. doi: 10.3322/caac.21442. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F., Bartlett J.M.S., Slaets L., van Deurzen C.H.M., van Leeuwen-Stok E., Porter P., et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer Program. Ann Oncol. 2018;29(2):405–417. doi: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giordano S.H. Breast cancer in men. NEJM. 2018;(78):2311–2320. doi: 10.1056/NEJMra1707939. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., André F., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano S.H., Cohen D.S., Buzdar A.U., Perkins G., Hortobagyi G.N. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 6.Leone J.P., Leone J., Zwenger A.O., Iturbe J., Vallejo C.T., Leone B.A. Prognostic significance of tumor subtypes in male breast cancer: a population-based study. Breast Cancer Res Treat. 2015 Aug;152(3):601–609. doi: 10.1007/s10549-015-3488-y. [DOI] [PubMed] [Google Scholar]
- 7.Leach I.H., Ellis I.O., Elston C.W. c-erb-B-2 expression in male breast carcinoma. J Clin Pathol. 1992;45:942. doi: 10.1136/jcp.45.10.942-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massarweh S.A., Sledge G.W., Miller D.P., McCullough D., Petkov V.I., Shak S. Molecular Characterization and mortality from breast cancer in men. J Clin Oncol. 2018;36(14):1396–1404. doi: 10.1200/JCO.2017.76.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayani J., Poncet C., Crozier C., Neven A., Piper T., Cunningham C., et al. Evaluation of multiple transcriptomic gene risk signatures in male breast cancer. npj Breast Cancer. 2021;7(1) doi: 10.1038/s41523-021-00301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madden N.A., Macdonald O.K., Call J.A., Schomas D.A., Lee C.M., Patel S. Radiotherapy and male breast cancer: a population-based registry analysis. Am J Clin Oncol. 2016 Oct;39(5):458–462. doi: 10.1097/COC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 11.Kiluk J.V., Lee M.C., Park C.K., Meade T., Minton S., Harris E., et al. Male breast cancer: management and follow-up recommendations. Breast J. 2011;17(5):503–509. doi: 10.1111/j.1524-4741.2011.01148.x. [DOI] [PubMed] [Google Scholar]
- 12.Male Breast Cancer Fda. 2020. Developing drugs for treatment guidance for industry.https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugsand/or [Google Scholar]
- 13.Eggemann H., Brucker C., Schrauder M., Thill M., Flock F., Reinisch M., et al. Survival benefit of tamoxifen in male breast cancer: prospective cohort analysis. Br J Cancer [Internet. 2020;123(1):33–37. doi: 10.1038/s41416-020-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutuli B., Le-Nir C.C.-S., Serin D., Kirova Y., Gaci Z., Lemanski C., et al. Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases. Crit Rev Oncol Hematol. 2010 Mar;73(3):246–254. doi: 10.1016/j.critrevonc.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Bernard-Marty C., Azambuja E., Dal Lago L., Piccart M.J., Cardoso F. In: Male breast cancer BT - breast cancer and molecular medicine. Piccart M.J., Hung M.-C., Solin L.J., Cardoso F., Wood W.C., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2006. pp. 903–923. [DOI] [Google Scholar]
- 16.Johnston S.R.D., Harbeck N., Hegg R., Toi M., Martin M., Shao Z.M., et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol Off J Am Soc Clin Oncol. 2020 Dec;38(34):3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi S., Haibe-Kains B., Desmedt C., Wirapati P., Lallemand F., Tutt A.M., et al. Predicting prognosis using molecular profiling in estrogen receptor-positive breast cancer treated with tamoxifen. BMC Genomics [Internet] 2008;9(1):239. doi: 10.1186/1471-2164-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyen J., Italiano A., Largillier R., Ferrero J.M., Fontana X., Thyss A. Aromatase inhibition in male breast cancer patients: biological and clinical implications. Ann Oncol. 2009;21(6):1243–1245. doi: 10.1093/annonc/mdp450. [DOI] [PubMed] [Google Scholar]
- 19.Zagouri F., Sergentanis T.N., Azim H.A., Chrysikos D., Dimopoulos M.A., Psaltopoulou T. Aromatase inhibitors in male breast cancer: a pooled analysis. Breast Cancer Res Treat. 2015;151(1):141–147. doi: 10.1007/s10549-015-3356-9. [DOI] [PubMed] [Google Scholar]
- 20.Zagouri F., Sergentanis T.N., Chrysikos D., Dimopoulos M.A., Psaltopoulou T. Fulvestrant and male breast cancer: a pooled analysis. Breast Cancer Res Treat. 2015;149(1):269–275. doi: 10.1007/s10549-014-3240-z. [DOI] [PubMed] [Google Scholar]
- 21.Tripathy D., Im S.-A., Colleoni M., Franke F., Bardia A., Harbeck N., et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol [Internet. 2018 Jul 1;19(7) doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 22.Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.-A., et al. Phase III randomized study of Ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: monaleesa-3. J Clin Oncol Off J Am Soc Clin Oncol. 2018 Aug;36(24):2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 23.Rugo H.S., Finn R.S., Diéras V., Ettl J., Lipatov O., Joy A.A., et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019 Apr;174(3):719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetz M.P., Toi M., Campone M., Sohn J., Paluch-Shimon S., Huober J., et al. Monarch 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 25.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.-A., Masuda N., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phas. Lancet Oncol [Internet] 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 26.Sledge G.W.J., Toi M., Neven P., Sohn J., Inoue K., Pivot X., et al. Monarch 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2017 Sep;35(25):2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 27.Hassett M.J., Somerfield M.R., Baker E.R., Cardoso F., Kansal K.J., Kwait D.C., et al. Management of male breast cancer: ASCO guideline. J Clin Oncol. 2020;38(16):1849–1863. doi: 10.1200/JCO.19.03120. [DOI] [PubMed] [Google Scholar]
- 28.Kattan J., Kourie H.R. The use of everolimus to reverse tamoxifen resistance in men with metastatic breast cancer: a case report. Invest New Drugs. 2014;32(5):1046–1047. doi: 10.1007/s10637-014-0133-2. [DOI] [PubMed] [Google Scholar]
- 29.Brannon A.R., Frizziero M., Chen D., Hummel J., Gallo J., Riester M., et al. Molecular analysis of a male breast cancer patient with prolonged stable disease under mTOR/PI3K inhibitors BEZ235/everolimus. Mol Case Stud. 2016;2(2) doi: 10.1101/mcs.a000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballatore Z., Pistelli M., Battelli N., Pagliacci A., De Lisa M., Berardi R., et al. Everolimus and exemestane in long survival hormone receptor positive male breast cancer: case report. BMC Res Notes. 2016;9(1):1–6. doi: 10.1186/s13104-016-2301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piscuoglio S., Ng C.K.Y., Murray M.P., Guerini-Rocco E., Martelotto L.G., Geyer F.C., et al. The genomic landscape of male breast cancers. Clin Cancer Res. 2016;22(16):4045–4056. doi: 10.1158/1078-0432.CCR-15-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callari M., Cappelletti V., De Cecco L., Musella V., Miodini P., Veneroni S., et al. Gene expression analysis reveals a different transcriptomic landscape in female and male breast cancer. Breast Cancer Res Treat. 2011;127(3):601–610. doi: 10.1007/s10549-010-1015-8. [DOI] [PubMed] [Google Scholar]
- 33.Shaaban A.M., Ball G.R., Brannan R.A., Cserni G., Di Benedetto A., Dent J., et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat. 2012;133(3):949–958. doi: 10.1007/s10549-011-1856-9. [DOI] [PubMed] [Google Scholar]
- 34.Johansson I., Nilsson C., Berglund P., Lauss M., Ringnér M., Olsson H., et al. Gene expression profiling of primary male breast cancers reveals two unique subgroups and identifies N-acetyltransferase-1 (NAT1) as a novel prognostic biomarker. Breast Cancer Res [Internet. 2012;14(1):R31. doi: 10.1186/bcr3116. http://breast-cancer-research.com/content/14/1/R31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson I., Ringnér M., Hedenfalk I. The landscape of candidate driver genes differs between male and female breast cancer. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0078299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson I., Nilsson C., Berglund P., Strand C., Jönsson G., Staaf J., et al. High-resolution genomic profiling of male breast cancer reveals differences hidden behind the similarities with female breast cancer. Breast Cancer Res Treat. 2011;129(3):747–760. doi: 10.1007/s10549-010-1262-8. [DOI] [PubMed] [Google Scholar]
- 37.A'Hern R.P., Jamal-Hanjani M., Szász A.M., Johnston S.R.D., Reis-Filho J.S., Roylance R., et al. Taxane benefit in breast cancer - a role for grade and chromosomal stability. Nat Rev Clin Oncol. 2013;10:357–364. doi: 10.1038/nrclinonc.2013.67. [DOI] [PubMed] [Google Scholar]
- 38.Hortobagyi G.N., Chen D., Piccart M., Rugo H.S., Burris H.A., Pritchard K.I., et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from BOLERO-2. J Clin Oncol [Internet. 2015;34(5):419–426. doi: 10.1200/JCO.2014.60.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciruelos Gil E.M. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014 Aug;40(7):862–871. doi: 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003 Jan;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 41.Junttila M.R., Li S.-P., Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J Off Publ Fed Am Soc Exp Biol. 2008 Apr;22(4):954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 42.Deb S., Do H., Byrne D., Jene N., Dobrovic A., Fox S.B. PIK3CA mutations are frequently observed in BRCAX but not BRCA2 -associated male breast cancer. Breast Cancer Res [Internet. 2013;15(4):R69. doi: 10.1186/bcr3463. http://breast-cancer-research.com/content/15/4/R69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benvenuti S., Frattini M., Arena S., Zanon C., Cappelletti V., Coradini D., et al. PIK3CA cancer mutations display gender and tissue specificity patterns. Hum Mutat. 2008 Feb;29(2):284–288. doi: 10.1002/humu.20648. [DOI] [PubMed] [Google Scholar]
- 44.Yardley D.A., Noguchi S., Pritchard K.I., Burris H.A., 3rd, Baselga J., Gnant M., et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013 Oct;30(10):870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bachelot T., Bourgier C., Cropet C., Ray-Coquard I., Ferrero J.-M., Freyer G., et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol Off J Am Soc Clin Oncol. 2012 Aug;30(22):2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 46.Turner N.C., Slamon D.J., Ro J., Bondarenko I., Im S.-A., Masuda N., et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018 Nov;379(20):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 47.Mosele F., Stefanovska B., Lusque A., Tran Dien A., Garberis I., Droin N., et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol Off J Eur Soc Med Oncol. 2020 Mar;31(3):377–386. doi: 10.1016/j.annonc.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Rugo H.S., Lerebours F., Ciruelos E., Drullinsky P., Ruiz Borrego M., Neven P., et al. Alpelisib (ALP) + fulvestrant (FUL) in patients (pts) with PIK3CA-mutated (mut) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inh. J Clin Oncol. 2020;38(15_suppl):1006. [Google Scholar]
- 49.Di Leo A., Johnston S., Lee K.S., Ciruelos E., Lønning P.E., Janni W., et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018 Jan;19(1):87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 50.Sudarsanam S., Johnson D.E. Functional consequences of mTOR inhibition. Curr Opin Drug Discov Devel. 2010 Jan;13(1):31–40. [PubMed] [Google Scholar]
- 51.Macdonald S., Oncology R., General M. Breast cancer breast cancer. J R Soc Med. 2016;70(8):515–517. https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf [Google Scholar]
- 52.Kornblum N., Zhao F., Manola J., Klein P., Ramaswamy B., Brufsky A., et al. Randomized phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer resistant to aromatase inhibitor therapy: results of Pr. J Clin Oncol Off J Am Soc Clin Oncol. 2018 Jun;36(16):1556–1563. doi: 10.1200/JCO.2017.76.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Generali D., Montemurro F., Bordonaro R., Mafodda A., Romito S., Michelotti A., et al. Everolimus plus exemestane in advanced breast cancer: safety results of the BALLET study on patients previously treated without and with chemotherapy in the metastatic setting. Oncol. 2017;22(6):648–654. doi: 10.1634/theoncologist.2016-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Im Y.-H., Karabulut B., Lee K.S., Park B.-W., Adhav A., Cinkir H.Y., et al. Safety and efficacy of everolimus (EVE) plus exemestane (EXE) in postmenopausal women with locally advanced or metastatic breast cancer: final results from EVEREXES. Breast Cancer Res Treat. 2021 Jul;188(1):77–89. doi: 10.1007/s10549-021-06173-z. [DOI] [PubMed] [Google Scholar]
- 55.Turner N., Howell S., Jhaveri K., Gomez H., Toi M., Hu X., et al. 2020. 350TiP A phase III trial of capivasertib and fulvestrant versus placebo and fulvestrant in patients with HR+/HER2− breast cancer (CAPItello-291) [DOI] [Google Scholar]