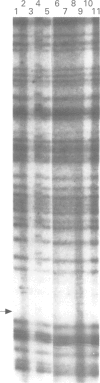
Abstract
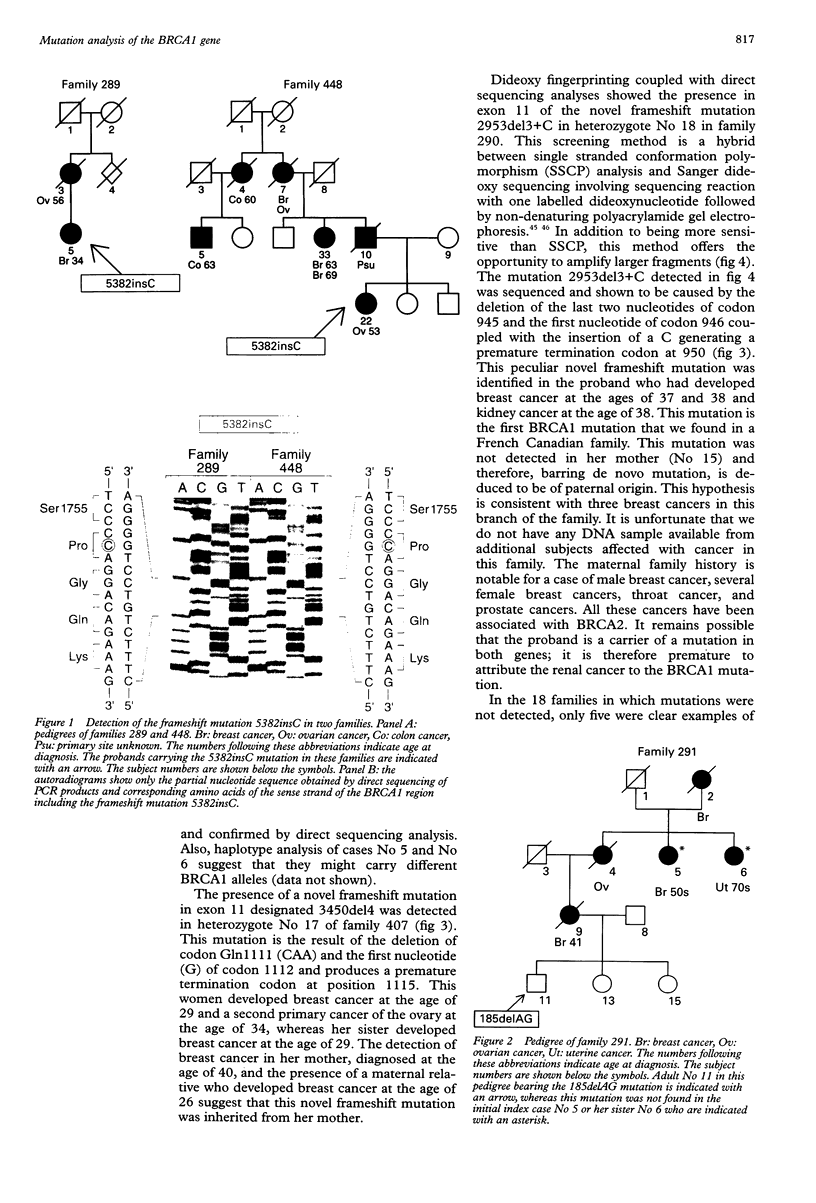
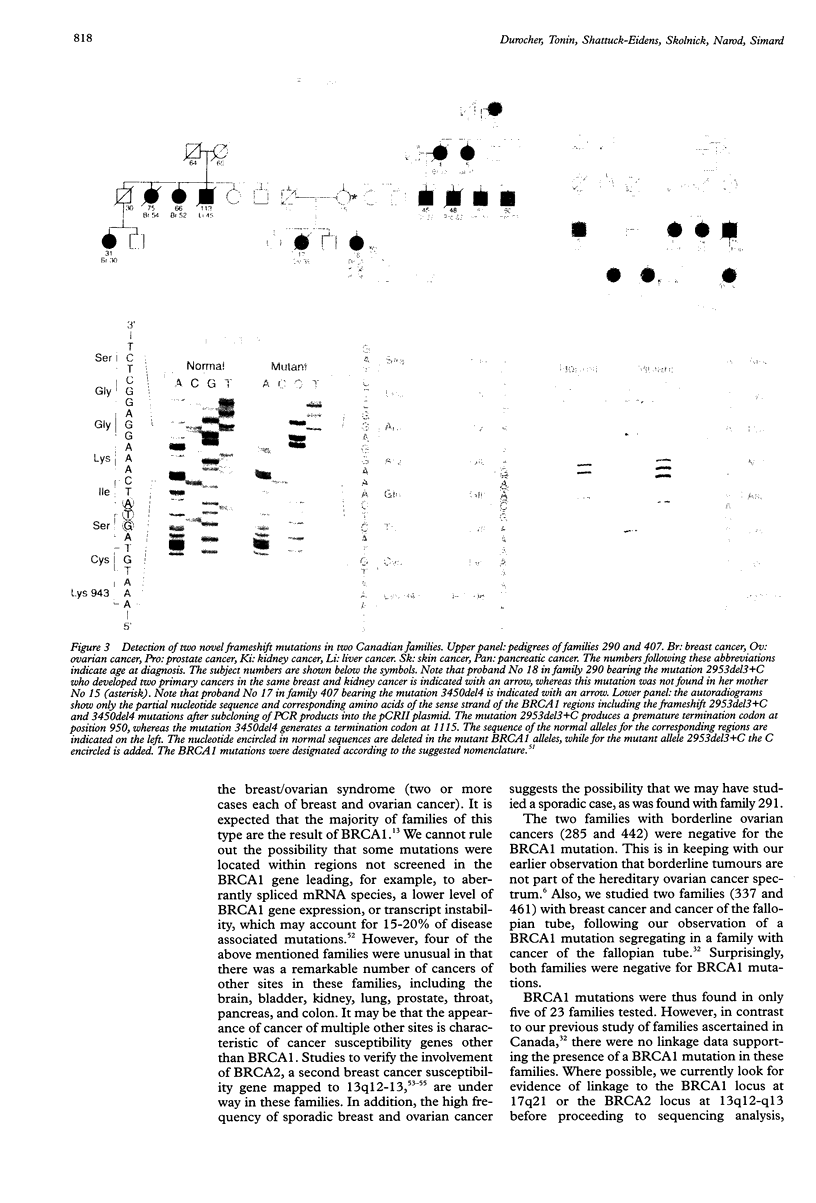
Germline mutations in the BRCA1 tumour suppressor gene on chromosome 17q21 are responsible for approximately half of the cases of hereditary breast cancer, including the majority of familial breast/ovarian cancers. To increase our knowledge of the spectrum of BRCA1 mutations, we have extended our analysis to include patients with varied family histories of cancer of the breast, ovary, and at multiple other sites. We have analysed 23 unrelated familial cases using direct sequencing or a combination of dideoxy fingerprinting and sequencing procedures. Twenty one of these families contained three or more cases of breast or ovarian cancer and two families had one case of breast cancer diagnosed before the age of 40 and one case of ovarian cancer. The common frameshift mutation 5382insC was detected in two patients, and the 185delAG mutation was found in a family of Ashkenazi Jewish descent. The novel frameshift mutation 3450del4 (CAAG) was detected in a patient who developed breast cancer at the age of 28 and ovarian cancer at the age of 34. Three other women in this family were diagnosed with breast cancer at the ages of 26, 29, and 40. The novel framshift mutation 2953del3+C was found in a French Canadian woman who had developed two primary cancers of the breast at the age of 37 and 38 and renal cancer at the age of 38.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet A. L., Tsui L. C. A suggested nomenclature for designating mutations. Hum Mutat. 1993;2(4):245–248. doi: 10.1002/humu.1380020402. [DOI] [PubMed] [Google Scholar]
- Blaszyk H., Hartmann A., Schroeder J. J., McGovern R. M., Sommer S. S., Kovach J. S. Rapid and efficient screening for p53 gene mutations by dideoxy fingerprinting. Biotechniques. 1995 Feb;18(2):256–260. [PubMed] [Google Scholar]
- Boyd M., Harris F., McFarlane R., Davidson H. R., Black D. M. A human BRCA1 gene knockout. Nature. 1995 Jun 15;375(6532):541–542. doi: 10.1038/375541b0. [DOI] [PubMed] [Google Scholar]
- Castilla L. H., Couch F. J., Erdos M. R., Hoskins K. F., Calzone K., Garber J. E., Boyd J., Lubin M. B., Deshano M. L., Brody L. C. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat Genet. 1994 Dec;8(4):387–391. doi: 10.1038/ng1294-387. [DOI] [PubMed] [Google Scholar]
- Claus E. B., Risch N., Thompson W. D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991 Feb;48(2):232–242. [PMC free article] [PubMed] [Google Scholar]
- Durocher F., Shattuck-Eidens D., McClure M., Labrie F., Skolnick M. H., Goldgar D. E., Simard J. Comparison of BRCA1 polymorphisms, rare sequence variants and/or missense mutations in unaffected and breast/ovarian cancer populations. Hum Mol Genet. 1996 Jun;5(6):835–842. doi: 10.1093/hmg/5.6.835. [DOI] [PubMed] [Google Scholar]
- Easton D. F., Bishop D. T., Ford D., Crockford G. P. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993 Apr;52(4):678–701. [PMC free article] [PubMed] [Google Scholar]
- Easton D. F., Ford D., Bishop D. T. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995 Jan;56(1):265–271. [PMC free article] [PubMed] [Google Scholar]
- Easton D. F., Matthews F. E., Ford D., Swerdlow A. J., Peto J. Cancer mortality in relatives of women with ovarian cancer: the OPCS Study. Office of Population Censuses and Surveys. Int J Cancer. 1996 Jan 26;65(3):284–294. doi: 10.1002/(SICI)1097-0215(19960126)65:3<284::AID-IJC2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ford D., Easton D. F., Bishop D. T., Narod S. A., Goldgar D. E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994 Mar 19;343(8899):692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Ford D., Easton D. F., Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet. 1995 Dec;57(6):1457–1462. [PMC free article] [PubMed] [Google Scholar]
- Foulkes W. D., Narod S. A. Hereditary breast and ovarian cancer: epidemiology, genetics, screening and predictive testing. Clin Invest Med. 1995 Dec;18(6):473–483. [PubMed] [Google Scholar]
- Friedman L. S., Ostermeyer E. A., Szabo C. I., Dowd P., Lynch E. D., Rowell S. E., King M. C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994 Dec;8(4):399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- Friedman L. S., Szabo C. I., Ostermeyer E. A., Dowd P., Butler L., Park T., Lee M. K., Goode E. L., Rowell S. E., King M. C. Novel inherited mutations and variable expressivity of BRCA1 alleles, including the founder mutation 185delAG in Ashkenazi Jewish families. Am J Hum Genet. 1995 Dec;57(6):1284–1297. [PMC free article] [PubMed] [Google Scholar]
- Futreal P. A., Liu Q., Shattuck-Eidens D., Cochran C., Harshman K., Tavtigian S., Bennett L. M., Haugen-Strano A., Swensen J., Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994 Oct 7;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Gayther S. A., Harrington P., Russell P., Kharkevich G., Garkavtseva R. F., Ponder B. A. Rapid detection of regionally clustered germ-line BRCA1 mutations by multiplex heteroduplex analysis. UKCCCR Familial Ovarian Cancer Study Group. Am J Hum Genet. 1996 Mar;58(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Gayther S. A., Warren W., Mazoyer S., Russell P. A., Harrington P. A., Chiano M., Seal S., Hamoudi R., van Rensburg E. J., Dunning A. M. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995 Dec;11(4):428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- Gudas J. M., Nguyen H., Li T., Cowan K. H. Hormone-dependent regulation of BRCA1 in human breast cancer cells. Cancer Res. 1995 Oct 15;55(20):4561–4565. [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Hogervorst F. B., Cornelis R. S., Bout M., van Vliet M., Oosterwijk J. C., Olmer R., Bakker B., Klijn J. G., Vasen H. F., Meijers-Heijboer H. Rapid detection of BRCA1 mutations by the protein truncation test. Nat Genet. 1995 Jun;10(2):208–212. doi: 10.1038/ng0695-208. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Thompson M. E., Szabo C., Robinson-Benion C., Arteaga C. L., King M. C., Jensen R. A. Growth retardation and tumour inhibition by BRCA1. Nat Genet. 1996 Mar;12(3):298–302. doi: 10.1038/ng0396-298. [DOI] [PubMed] [Google Scholar]
- Hoskins K. F., Stopfer J. E., Calzone K. A., Merajver S. D., Rebbeck T. R., Garber J. E., Weber B. L. Assessment and counseling for women with a family history of breast cancer. A guide for clinicians. JAMA. 1995 Feb 15;273(7):577–585. [PubMed] [Google Scholar]
- Inoue R., Fukutomi T., Ushijima T., Matsumoto Y., Sugimura T., Nagao M. Germline mutation of BRCA1 in Japanese breast cancer families. Cancer Res. 1995 Aug 15;55(16):3521–3524. [PubMed] [Google Scholar]
- Lane T. F., Deng C., Elson A., Lyu M. S., Kozak C. A., Leder P. Expression of Brca1 is associated with terminal differentiation of ectodermally and mesodermally derived tissues in mice. Genes Dev. 1995 Nov 1;9(21):2712–2722. doi: 10.1101/gad.9.21.2712. [DOI] [PubMed] [Google Scholar]
- Marquis S. T., Rajan J. V., Wynshaw-Boris A., Xu J., Yin G. Y., Abel K. J., Weber B. L., Chodosh L. A. The developmental pattern of Brca1 expression implies a role in differentiation of the breast and other tissues. Nat Genet. 1995 Sep;11(1):17–26. doi: 10.1038/ng0995-17. [DOI] [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Narod S. A., Feunteun J., Lynch H. T., Watson P., Conway T., Lynch J., Lenoir G. M. Familial breast-ovarian cancer locus on chromosome 17q12-q23. Lancet. 1991 Jul 13;338(8759):82–83. doi: 10.1016/0140-6736(91)90076-2. [DOI] [PubMed] [Google Scholar]
- Narod S. A., Ford D., Devilee P., Barkardottir R. B., Lynch H. T., Smith S. A., Ponder B. A., Weber B. L., Garber J. E., Birch J. M. An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995 Jan;56(1):254–264. [PMC free article] [PubMed] [Google Scholar]
- Narod S. A., Madlensky L., Bradley L., Cole D., Tonin P., Rosen B., Risch H. A. Hereditary and familial ovarian cancer in southern Ontario. Cancer. 1994 Oct 15;74(8):2341–2346. doi: 10.1002/1097-0142(19941015)74:8<2341::aid-cncr2820740819>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Neuhausen S. L., Marshall C. J. Loss of heterozygosity in familial tumors from three BRCA1-linked kindreds. Cancer Res. 1994 Dec 1;54(23):6069–6072. [PubMed] [Google Scholar]
- Neuhausen S. L., Swensen J., Miki Y., Liu Q., Tavtigian S., Shattuck-Eidens D., Kamb A., Hobbs M. R., Gingrich J., Shizuya H. A P1-based physical map of the region from D17S776 to D17S78 containing the breast cancer susceptibility gene BRCA1. Hum Mol Genet. 1994 Nov;3(11):1919–1926. doi: 10.1093/hmg/3.11.1919. [DOI] [PubMed] [Google Scholar]
- Peto J., Easton D. F., Matthews F. E., Ford D., Swerdlow A. J. Cancer mortality in relatives of women with breast cancer: the OPCS Study. Office of Population Censuses and Surveys. Int J Cancer. 1996 Jan 26;65(3):275–283. doi: 10.1002/(SICI)1097-0215(19960126)65:3<275::AID-IJC1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Phelan C. M., Rebbeck T. R., Weber B. L., Devilee P., Ruttledge M. H., Lynch H. T., Lenoir G. M., Stratton M. R., Easton D. F., Ponder B. A. Ovarian cancer risk in BRCA1 carriers is modified by the HRAS1 variable number of tandem repeat (VNTR) locus. Nat Genet. 1996 Mar;12(3):309–311. doi: 10.1038/ng0396-309. [DOI] [PubMed] [Google Scholar]
- Plummer S. J., Anton-Culver H., Webster L., Noble B., Liao S., Kennedy A., Belinson J., Casey G. Detection of BRCA1 mutations by the protein truncation test. Hum Mol Genet. 1995 Oct;4(10):1989–1991. doi: 10.1093/hmg/4.10.1989. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Yoon H. S., Sommer S. S. Dideoxy fingerprinting (ddE): a rapid and efficient screen for the presence of mutations. Genomics. 1992 Jun;13(2):441–443. doi: 10.1016/0888-7543(92)90266-u. [DOI] [PubMed] [Google Scholar]
- Serova O., Montagna M., Torchard D., Narod S. A., Tonin P., Sylla B., Lynch H. T., Feunteun J., Lenoir G. M. A high incidence of BRCA1 mutations in 20 breast-ovarian cancer families. Am J Hum Genet. 1996 Jan;58(1):42–51. [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Eidens D., McClure M., Simard J., Labrie F., Narod S., Couch F., Hoskins K., Weber B., Castilla L., Erdos M. A collaborative survey of 80 mutations in the BRCA1 breast and ovarian cancer susceptibility gene. Implications for presymptomatic testing and screening. JAMA. 1995 Feb 15;273(7):535–541. [PubMed] [Google Scholar]
- Simard J., Feunteun J., Lenoir G., Tonin P., Normand T., Luu The V., Vivier A., Lasko D., Morgan K., Rouleau G. A. Genetic mapping of the breast-ovarian cancer syndrome to a small interval on chromosome 17q12-21: exclusion of candidate genes EDH17B2 and RARA. Hum Mol Genet. 1993 Aug;2(8):1193–1199. doi: 10.1093/hmg/2.8.1193. [DOI] [PubMed] [Google Scholar]
- Simard J., Tonin P., Durocher F., Morgan K., Rommens J., Gingras S., Samson C., Leblanc J. F., Bélanger C., Dion F. Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet. 1994 Dec;8(4):392–398. doi: 10.1038/ng1294-392. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Easton D. F., Evans D. G., Ponder B. A. Allele losses in the region 17q12-21 in familial breast and ovarian cancer involve the wild-type chromosome. Nat Genet. 1992 Oct;2(2):128–131. doi: 10.1038/ng1092-128. [DOI] [PubMed] [Google Scholar]
- Struewing J. P., Abeliovich D., Peretz T., Avishai N., Kaback M. M., Collins F. S., Brody L. C. The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet. 1995 Oct;11(2):198–200. doi: 10.1038/ng1095-198. [DOI] [PubMed] [Google Scholar]
- Struewing J. P., Brody L. C., Erdos M. R., Kase R. G., Giambarresi T. R., Smith S. A., Collins F. S., Tucker M. A. Detection of eight BRCA1 mutations in 10 breast/ovarian cancer families, including 1 family with male breast cancer. Am J Hum Genet. 1995 Jul;57(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Szabo C. I., King M. C. Inherited breast and ovarian cancer. Hum Mol Genet. 1995;4(Spec No):1811–1817. doi: 10.1093/hmg/4.suppl_1.1811. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Behbakht K., McGovern P. E., Chiu H. C., Couch F. J., Weber B. L., Friedman L. S., King M. C., Furusato M., LiVolsi V. A. Mutation analysis of the BRCA1 gene in ovarian cancers. Cancer Res. 1995 Jul 15;55(14):2998–3002. [PubMed] [Google Scholar]
- Tavtigian S. V., Simard J., Rommens J., Couch F., Shattuck-Eidens D., Neuhausen S., Merajver S., Thorlacius S., Offit K., Stoppa-Lyonnet D. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996 Mar;12(3):333–337. doi: 10.1038/ng0396-333. [DOI] [PubMed] [Google Scholar]
- Thompson M. E., Jensen R. A., Obermiller P. S., Page D. L., Holt J. T. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995 Apr;9(4):444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- Tonin P., Serova O., Lenoir G., Lynch H., Durocher F., Simard J., Morgan K., Narod S. BRCA1 mutations in Ashkenazi Jewish women. Am J Hum Genet. 1995 Jul;57(1):189–189. [PMC free article] [PubMed] [Google Scholar]
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995 Dec 21;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- Wooster R., Neuhausen S. L., Mangion J., Quirk Y., Ford D., Collins N., Nguyen K., Seal S., Tran T., Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994 Sep 30;265(5181):2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]