Abstract
Introduction
The aim of the study was to evaluate the preventive effect of 13 drugs on colorectal cancer (CRC) and guide the clinical application of these drugs.
Material and methods
PubMed, Web of Science, Embase, Cochrane Library, and China National Knowledge Infrastructure were searched for randomized controlled trials (RCTs) and cohort studies. The Cochrane bias risk assessment tool and the Newcastle-Ottawa Scale quality evaluation tool were used to evaluate the quality of the included RCTs and cohort studies. The funnel plot was used to analyze publication bias. A network meta-analysis of the extracted data was conducted using Stata16.0 software.
Results
A total of 57 studies (34 RCTs and 23 cohort studies) involving 82719 participants were included. The network meta-analysis revealed that the quality of the included studies was good; the funnel plot showed no obvious publication bias. The network meta-analysis showed that the preventive effect of 13 drugs on CRC was better than that of the placebo. Allopurinol (SUCRA: 97.2%) was found to have the best effect, followed by berberine (SUCRA: 89.9%), non-aspirin NSAIDs (SUCRA: 84.5%), statins (SUCRA: 66.5%), metformin (SUCRA: 66.3%), calcium (SUCRA: 48.9%), mesalazine (SUCRA: 44.5%), ursodeoxycholic acid (SUCRA: 42.6%), vitamin D (SUCRA: 41.4%), mercaptopurine (SUCRA: 39.4%), aspirin (SUCRA: 30.4%), folic acid (SUCRA: 24.9%), and eicosapentaenoic acid (SUCRA: 16.3%).
Conclusions
The preventive effect of allopurinol on CRC was better than that of the other 13 drugs. These results can help doctors and patients understand the preventative effects of these drugs more intuitively and provide an evidence-based basis for the clinical application of these drugs.
Keywords: colorectal cancer, chemical prevention, network meta-analysis, drug intervention
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death worldwide. About 1 in 10 cancer patients aged < 50 years old is diagnosed with CRC [1, 2]. The 5-year survival rate is < 15%, which causes a heavy burden on human health [3]. CRC prevention strategies include the following four aspects: endoscopy, population screening, chemoprophylaxis, and lifestyle changes [4]. Unfortunately, patient compliance, popularity, and high-cost limit population screening [5]. At the same time, missed CRCs are frequent in those who underwent endoscopy [6], and population screening is not enough to fully filtrate patients with CRC [7]. Therefore, exploring chemoprevention strategies to reduce the incidence and mortality of CRC has attracted the attention of researchers and has become a crucial research hotspot.
Some meta-analyses show that non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) [8], aspirin [9], metformin [10], calcium [11], berberine [12], mesalazine [13], statins [14], ursodeoxycholic acid [15], folic acid [16], and other drugs can effectively reduce the incidence of CRC. Yet, clinical studies have proved the uneven efficacy of these drugs. Still, some studies only compared the chemoprophylaxis of one drug with a controlled drug in patients with CRC, and there is still a lack of studies that compared multiple drugs. Therefore, finding a more suitable drug is of essential importance, especially when doctors consider using chemoprophylaxis strategies to prevent CRC.
Traditional meta-analyses can judge the effect of different intervention measures by direct head-to-head comparison; however, the research on direct head-to-head comparison is limited. When there is a lack of direct comparison data, indirect comparison of different intervention measures was used [17]. Network meta-analysis can be used to make direct and indirect comparisons according to logical reasoning [18]. At the same time, network meta-analysis can rank different interventions, selecting the best and worst interventions [19]. In this study, we used Stata16.0 software to compare the preventive effect of 13 drugs (allopurinol, aspirin, berberine, calcium, eicosapentaenoic acid, folic acid, mercaptopurine, mesalazine, metformin, non-aspirin NSAIDs, statins, ursodeoxycholic acid, vitamin D) on CRC.
Material and methods
Registration
This study was registered on PROSPERO with registration number CRD42022333172.
Search strategy
The following five databases were searched: PubMed, Web of Science, Embase, Cochrane Library, and China National Knowledge Infrastructure. The search strategy was built using the PICOS tool: P (Population): patients with previous CRC; I (Intervention): 13 drugs; C (Comparison): the control group was given a placebo or one or more drugs (only including 13 drugs mentioned above); O (Outcomes): the number of patients with recurrent CRC; S (Study type): RCTs or cohort studies. Connect PICOS through Boolean logic operators. The study was published between 1 January 2000, and 1 July 2022, with no language restrictions. Table I shows the detailed search strategy (take PubMed as an example).
Table I.
Search strategy on PubMed
| Search | Query |
|---|---|
| #1 | “Colorectal Neoplasms”[Mesh] |
| #2 | (((((((((((((((Colorectal Neoplasm) OR (Neoplasm, Colorectal)) OR (Colorectal Neoplasm)) OR (Neoplasms, Colorectal)) OR (Colorectal Tumors)) OR (Colorectal Tumor)) OR (Tumor, Colorectal)) OR (Tumors, Colorectal)) OR (Colorectal Cancer)) OR (Cancer, Colorectal)) OR (Cancers, Colorectal)) OR (Colorectal Cancers)) OR (Colorectal Carcinoma)) OR (Carcinoma, Colorectal)) OR (Carcinomas, Colorectal)) OR (Colorectal Carcinomas) |
| #3 | #1 OR #2 |
| #4 | “Chemoprevention”[Mesh] |
| #5 | (((((Chemoprophylaxis) OR (Precaution))OR (Precautions, Universal)OR (Precaution, Universal)OR (Universal Precaution) |
| #6 | #4 OR #5 |
| #7 | (Cohort studies) OR (Randomized controlled trials)[Publication Type] |
| #8 | #3 AND #6 AND #7 |
Study selection
Inclusion criteria were as follows: (1) patients with previous CRC; (2) clinical RCTs or cohort studies; (3) experimental groups were treated with one of the following drugs: allopurinol, aspirin, berberine, calcium, eicosapentaenoic acid, folic acid, mercaptopurine, mesalazine, metformin, non-aspirin NSAIDs, statins, ursodeoxycholic acid, vitamin D; (4) controls were treated with a placebo or one or more drugs (only including 13 drugs mentioned above); (5) methods for CRC detection were colonoscopy, biopsy or tumor markers in colorectal mucosa; (6) the results were the number of patients with recurrent CRC in the experimental and control groups.
Exclusion criteria were as follows: (1) duplicate literature/studies from different databases; (2) studies with incomplete data; (3) patients with other tumors; (4) cellular or animal studies; (5) reviews, conference abstracts, case-control studies, case reports, and letters.
We used the document management software Endnote to screen the articles. The two researchers made a preliminary screening by reading the titles and abstracts of the articles. Then, the preliminarily selected articles were carefully read by two researchers. In case of disagreements, a third researcher was invited.
Data extraction
A standardized, six-item, pre-designed data extraction form was used to record study data. Data included the following: (1) the name of the first author; (2) year of publication; (3) country; (4) age; (5) drug and sample size of the experimental and control groups; and (6) methods for CRC detection.
Quality assessment
The included RCTs were evaluated by the risk of bias tool recommended by the Cochrane Handbook for systematic reviews (version 5.1) [20]. The assessment included the following seven items: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other bias. Each item includes three options: low risk, unclear risk, and high risk.
The Newcastle-Ottawa Scale (NOS) quality evaluation tool was used to evaluate the quality of cohort studies [21]. The NOS quality evaluation tool includes three dimensions: Selection, Comparability, and Exposure, with a total score of 9. Selection includes 4 items and 1 point for each item: (1) adequate definition of the case; (2) representativeness of the cases; (3) selection of controls; and (4) definition of controls. Comparability contains a 2-point item: comparability of cases and controls based on the design or analysis. Finally, exposure includes 3 items and 1 point for each item: (1) ascertainment of exposure, (2) same method of ascertainment for cases and controls, (3) non-response rate. Articles with a total score of ≥ 6 were considered good quality.
Data analysis
A frequentist framework was used to perform a network meta-analysis of the outcome using Stata software (version 16.0). In this network meta-analysis with drug intervention, all variables were dichotomous variables. Odds ratio (OR) was used as the effective index in the counting data, and 95% CI was used as the effective index in interval estimation. The preventive effects of two and more groups of drugs were analyzed by direct and indirect comparison. We used the node-splitting method to analyze each node, and the consistency of the results was evaluated by comparing the statistical differences between direct and indirect evidence. In the network meta-analysis map, each node represents a different intervention drug. The larger the sample size, the larger the node; the line of the connecting node represents the direct comparison between different drugs and the more related studies, the thicker the line.
The intervention hierarchy was summarized and reported as a P score. P score is the frequency simulation of the surface under the cumulative ranking curve (SUCRA) value, which can measure the degree of certainty that one intervention is better. The range of the P score is 0-1, where 1 indicates the best intervention with certainty, 0 suggests the worst intervention with certainty, and the higher the p-value, the higher the degree of advantage. We made a funnel chart and observed its symmetry to determine the presence of any publication bias.
Results
Search process
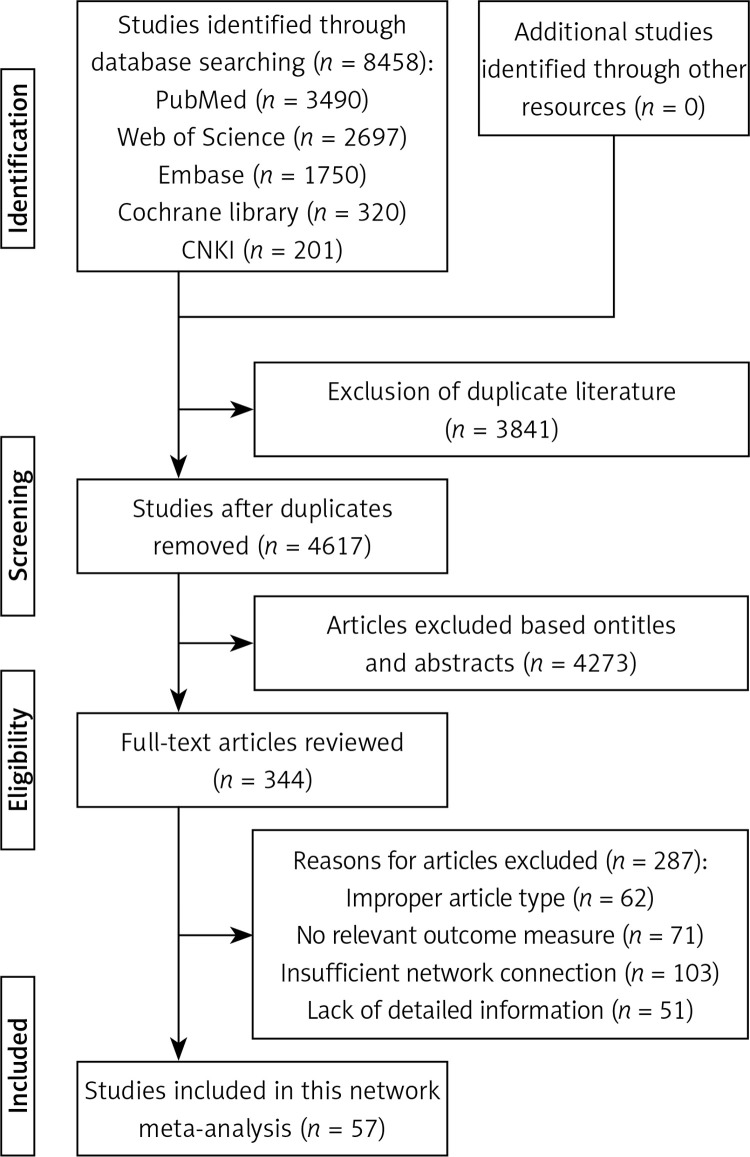
The search process is shown in Figure 1. According to the search strategy, 8458 articles were searched from PubMed, Web of Science, Embase, Cochrane Library, and China National Knowledge Infrastructure databases. A total of 3841 repeated articles were excluded. Then, titles and abstracts of the remaining 4671 articles were analyzed, after which 4273 articles were excluded. Consequently, 287 articles were excluded after reading the full text (reasons were the following: improper article type, no relevant outcome measure, insufficient network connection, and lack of detailed information). Finally, 57 articles [22–78] were included in this network meta-analysis.
Figure 1.
Search process of this network meta-analysis
Characteristics of included studies
Characteristics of included studies are shown in Table II. Among the 57 included articles, 34 were RCTs and 23 were cohort studies. Thirty studies were from North America, 15 were from Europe, 10 were from Asia, and 2 were from Australia. A total of 34 articles included elderly, 12 young and middle-aged subjects, and 11 all age groups. The intervention measures of 22 studies involved aspirin, 1 article involved eicosapentaenoic acid, 2 involved allopurinol, 13 involved non-aspirin NSAIDs, 8 involved metformin, 5 involved statins, 8 involved mesalazine, 6 involved ursodeoxycholic acid, 3 involved folic acid, 6 involved vitamin D, 9 involved calcium, and 39 involved placeboes. There were 40 double-arm studies and 17 multi-arm studies. Three studies used tumor markers in colorectal mucosa as the detection method, 9 used biopsy, and 45 used colonoscopy.
Table II.
Characteristics of 57 included studies in this network meta-analysis
| Author | Country | Year | Age | Treatment | Detection | Study design | ||
|---|---|---|---|---|---|---|---|---|
| Case | Control | Case (Total/relapse) | Control (Total/relapse) | |||||
| Mark A. Hull | UK | 2018 | 62–69 | 62–69 | Eicosapentaenoic acid (153/97) | Aspirin (163/100) | Colonoscopy | RCT |
| Placebo (163/100) | ||||||||
| Fan-Gen Hsu | China | 2021 | 66.5 ±13.7 | 66.5 ±13.8 | Allopurinol (1111/500) | Non-aspirin NSAIDs (8618/4310) | Colonoscopy | Cohort study |
| Matteo Puntoni | Italy | 2012 | 61 ±8 | 61 ±8 | Allopurinol (49/21) | Placebo (24/11) | Biopsy | RCT |
| Laura Reyes-Uribe | USA | 2020 | 20–70 | 20–70 | Non-aspirin NSAIDs (31/2) | Placebo (23/21) | Tumor markers | RCT |
| Randall E. Harris | USA | 2008 | 63.2 ±0.7 | 63.5 ±0.6 | Non-aspirin NSAIDs (13/4) | Aspirin (22/7) | Biopsy | Cohort study |
| John A. Baron | USA | 2006 | 40–86 | 40–86 | Non-aspirin NSAIDs (1158/460) | Placebo (1218/646) | Colonoscopy | RCT |
| Francis M. Giardiello | USA | 2002 | 8–25 | 8–25 | Non-aspirin NSAIDs (21/9) | Placebo (20/11) | Tumor markers | RCT |
| Takuma Higurashi | Japan | 2016 | 40–78 | 40–79 | Metformin (71/27) | Placebo (62/35) | Colonoscopy | RCT |
| Jin Ha Lee | Korea | 2011 | 33–87 | 30–88 | Metformin (258/55) | Aspirin (106/90) | Tumor markers | Cohort study |
| CR Garrett | USA | 2012 | 18–80 | 18–80 | Metformin (208/125) | Aspirin (119/95) | Biopsy | Cohort study |
| Susan Spillane | Ireland | 2013 | 71–80 | 71–79 | Metformin (207/58) | Placebo (108/35) | Biopsy | Cohort study |
| Brielan Smiechowski | Canada | 2013 | 72.8 ±8.7 | 72.5 ±8.5 | Metformin (4850/444) | Placebo (1594/163) | Colonoscopy | Cohort study |
| Majken Cardel | Denmark | 2014 | 66–80 | 61–77 | Metformin (6451/1161) | Aspirin (5125/976) | Biopsy | Cohort study |
| Non-aspirin NSAIDs (1005/149) | ||||||||
| Statins (3669/673) | ||||||||
| Amikar Sehdev | USA | 2014 | 26–64 | 23–64 | Metformin (3042/983) | Statins (3083/992) | Biopsy | Cohort study |
| Non-aspirin NSAIDs (1237/372) | ||||||||
| Bruce Y. Tung | USA | 2016 | 36.4 ±2.6 | 27.4 ±3.1 | Ursodeoxycholic acid (41/13) | Mesalazine (12/5) | Colonoscopy | Cohort study |
| Urban Sjöqvist | Sweden | 2004 | 27–73 | 32–54 | Ursodeoxycholic acid (10/5) | Placebo (9/7) | Colonoscopy | RCT |
| David S.Alberts | USA | 2005 | 66.1 ±8.7 | 66.4 ±8.3 | Ursodeoxycholic acid (613/251) | Placebo (579/254) | Colonoscopy | RCT |
| J. M. Wolf | USA | 2005 | 31 ±13 | 26 ±12 | Ursodeoxycholic acid (28/8) | Placebo (92/27) | Biopsy | Cohort study |
| Darrell S. Pardi | USA | 2003 | 22–65 | 21–68 | Ursodeoxycholic acid (29/3) | Placebo (23/8) | Biopsy | RCT |
| Folic acid (11/6) | ||||||||
| L. Lindström | Sweden | 2012 | 18–67 | 22–67 | Ursodeoxycholic acid (48/13) | Mesalazine (86/26) | Colonoscopy | RCT |
| Placebo (50/15) | ||||||||
| Hideki Ishikawa | Japan | 2021 | 31–47 | 25–43 | Mesalazine (52/20) | Non-aspirin NSAIDs (40/12) | Colonoscopy | RCT |
| Placebo (39/17) | ||||||||
| F. Carrat | France | 2016 | 52.6 ±15.8 | 52.5 ±15.7 | Mesalazine (324/103) | Folic acid (23/3) | Colonoscopy | Cohort study |
| Mercaptopurine (11/4) | ||||||||
| Jeffrey Tang | USA | 2009 | 36.6 ±16.2 | 34.7 ±14 | Mesalazine (44/15) | Folic acid (21/3) | Colonoscopy | Cohort study |
| Mercaptopurine (5/1) | ||||||||
| Non-aspirin NSAIDs (6/1) | ||||||||
| David T. Rurin | USA | 2006 | 36–62 | 34–56 | Mesalazine (111/22) | Folic acid (122/26) | Colonoscopy | Cohort study |
| J. Eaden | UK | 2000 | 41.3 ±16.8 | 43.6 ±16.1 | Mesalazine (135/51) | Non-aspirin NSAIDs (9/4) | Colonoscopy | Cohort study |
| Jonathan P. Terdiman | USA | 2007 | 50–80 | 50–80 | Mesalazine (671/157) | Non-aspirin NSAIDs (377/88) | Colonoscopy | Cohort study |
| Sierra Matula | USA | 2005 | 28.6 ±13.3 | 28.5 ±12 | Mercaptopurine (96/15) | Placebo (219/39) | Colonoscopy | Cohort study |
| Robert Benamouzig | France | 2009 | 56.5 ±8.6 | 58.5 ±10.2 | Aspirin (79/38) | Placebo (57/34) | Colonoscopy | RCT |
| Elizabeth L. Barry | USA | 2009 | 57.6 ±9.6 | 59.1 ±9.3 | Aspirin (650/278) | Placebo (329/155) | Colonoscopy | RCT |
| Folic acid (452/205) | ||||||||
| Harsh Sheth | UK | 2018 | 45–80 | 45–80 | Aspirin (580/324) | Non-aspirin NSAIDs (887/467) | Colonoscopy | Cohort study |
| Jane C. Figueiredo | USA | 2008 | 59.0 ±9.3 | 56.2 ±9.6 | Aspirin (721/300) | Folic acid (501/221) | Colonoscopy | RCT |
| Maria V. Grau | USA | 2009 | 57.6 ±9.1 | 57.5 ±9.3 | Aspirin (565/256) | Placebo (285/143) | Colonoscopy | RCT |
| John Burn | UK | 2011 | 20–80 | 20–85 | Aspirin (427/18) | Placebo (434/30) | Colonoscopy | RCT |
| Hideki Ishikawa | Japan | 2012 | 39.7 ±12.8 | 36.7 ±13.9 | Aspirin (17/10) | Placebo (17/14) | Colonoscopy | RCT |
| John A. Baron | USA | 2003 | 57.3 ±9.9 | 57.4 ±9.9 | Aspirin (721/300) | Placebo (363/171) | Colonoscopy | RCT |
| Hideki Ishikawa | Japan | 2014 | 60.0 ±7.3 | 60.5 ±6.6 | Aspirin (152/56) | Placebo (159/73) | Colonoscopy | RCT |
| Jane C. Figueiredo | USA | 2010 | 59.3 ±9.4 | 59.7 ±9.5 | Folic acid (1324/343) | Placebo (1308/339) | Colonoscopy | RCT |
| Qin-Yan Gao | China | 2013 | 60.8 ±7.5 | 60.2 ±7.1 | Folic acid (430/64) | Placebo (430/132) | Colonoscopy | RCT |
| Richard F. A. Logan | UK | 2008 | 58.3 ±9.4 | 57.9 ±9.7 | Folic acid (215/65) | Non-aspirin NSAIDs (217/49) | Colonoscopy | RCT |
| Placebo (204/56) | ||||||||
| Bernard F. Cole | USA | 2007 | 57.0 ±9.6 | 57.0 ±9.5 | Folic acid (501/221) | Placebo (486/206) | Colonoscopy | RCT |
| John A. Baron | USA | 2015 | 58.7 ±7.0 | 58.2 ±7.0 | Calcium (762/345) | Vitamin D (1024/438) | Colonoscopy | RCT |
| Placebo (380/183) | ||||||||
| Claire Bonithon-Kopp | Belgium | 2000 | 58.8 ±8.8 | 59.3 ±8.4 | Calcium (176/28) | Placebo (178/36) | Colonoscopy | RCT |
| Veronika Fedirko | USA | 2010 | 58.2 ±9.7 | 52.7 ±11.0 | Calcium (616/314) | Placebo (770/382) | Colonoscopy | Cohort study |
| Non-aspirin NSAIDs (616/250) | ||||||||
| Elizabeth L. Barry | USA | 2019 | 59.0 ±7.0 | 58.0 ±7.0 | Calcium (434/193) | Placebo (448/228) | Colonoscopy | RCT |
| Vitamin D (380/183) | ||||||||
| Aspirin (577/277) | ||||||||
| Non-aspirin NSAIDs (490/208) | ||||||||
| Marıá Elena Martı´nez | USA | 2002 | 64.3 ±9.3 | 64.5 ±9.0 | Calcium (514/240) | Placebo (790/399) | Colonoscopy | RCT |
| Vitamin D (452/207) | ||||||||
| Maria V. Grau | USA | 2005 | 60.8 ±8.9 | 57.5 ±9.6 | Calcium (409/127) | Placebo (423/159) | Colonoscopy | RCT |
| Aspirin (721/300) | ||||||||
| Elizabeth T. Jacobs | USA | 2007 | 66.0 ±8.5 | 66.2 ±8.5 | Calcium (778/210) | Vitamin D (1649/504) | Colonoscopy | RCT |
| Rowena Chau | Australia | 2016 | 41.5 ±13.1 | 42.4 ±10.5 | Calcium (178/48) | Folic acid (154/37) | Biopsy | Cohort study |
| Placebo (1730/685) | ||||||||
| Maria V.Grau | USA | 2007 | 60.5 ±8.9 | 60.6 ±9.1 | Calcium (405/162) | Placebo (417/185) | Colonoscopy | RCT |
| E. Aigner | Australia | 2014 | 30.1 ±11.3 | 23.5 ±11.3 | Vitamin D (80/14) | Placebo (719/196) | Colonoscopy | Cohort study |
| Cari Lewis | USA | 2015 | 65.9 ±9.4 | 62.6 ±10.5 | Vitamin D (95/23) | Placebo (358/89) | Colonoscopy | Cohort study |
| Yingxuan Chen | China | 2020 | 52–64 | 51–64 | Berberine (429/116) | Placebo (426/168) | Colonoscopy | RCT |
| Weiqiang Wang | China | 2020 | 61.7 ±11.0 | 61.5 ±10.7 | Berberine (42/4) | Placebo (42/12) | Colonoscopy | RCT |
| Lili Liu | China | 2018 | 18–70 | 18–70 | Berberine (47/7) | Placebo (44/15) | Colonoscopy | RCT |
| D Mansouri | UK | 2013 | 50–74 | 50–75 | Statins (621/295) | Aspirin (451/216) | Colonoscopy | Cohort study |
| Ali A. Siddiqui | USA | 2009 | 62.2 ±10.9 | 64.0 ±10.0 | Statins (1688/583) | Aspirin (1632/668) | Colonoscopy | Cohort study |
| Thomas Broughton | UK | 2013 | 63.8 ±12.2 | 64.2 ±11.5 | Statins (68/21) | Aspirin (43/23) | Colonoscopy | RCT |
| Non-aspirin NSAIDs (16/13) | ||||||||
| Metformin (18/8) | ||||||||
Results of quality assessment
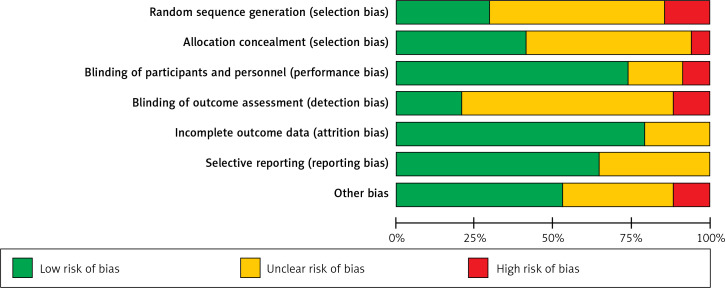
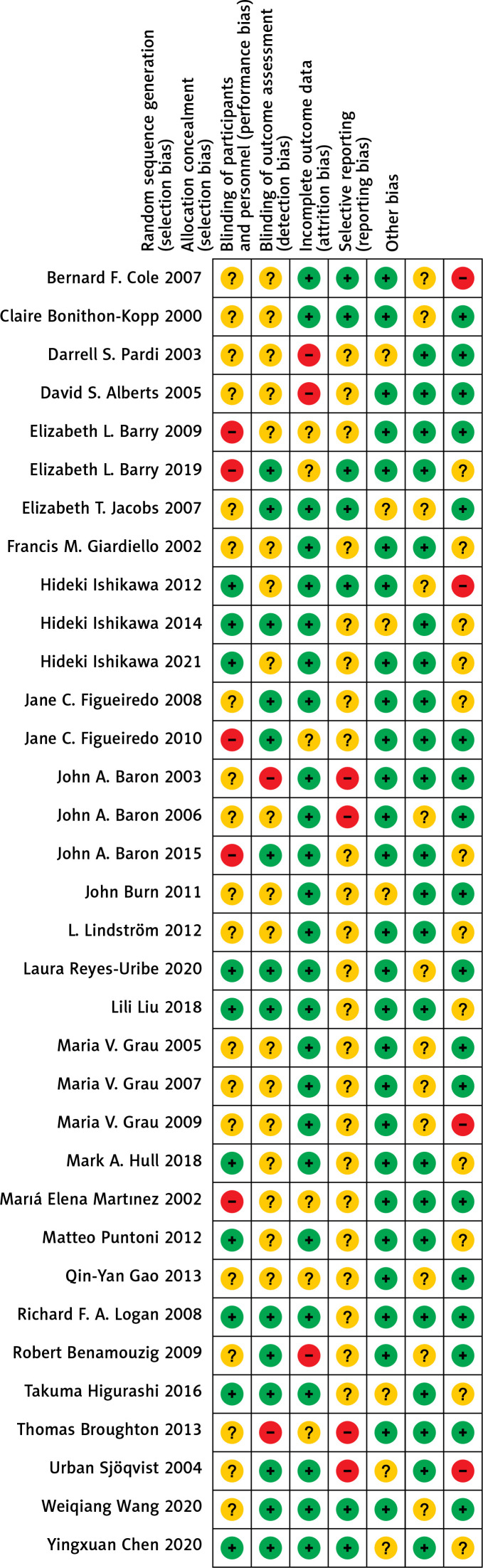
The quality assessment results of 34 RCTs are as follows: (1) Ten studies that were related to the random sequence generation were judged to have a low risk; 5 studies did not involve the random sequence generation and were considered to have a high risk; 19 studies only mentioned random while did not mention the sequences, so they were judged to have an unknown risk. (2) Fourteen studies used the method of allocation concealment and were judged to have a low risk; 2 studies did not adopt the allocation concealment method and were evaluated to have a high risk. The remaining 18 studies did not mention allocation concealment and were considered to have an unknown risk. (3) In terms of blinding of participants and personnel, 25 studies that used the blind method were considered to have a low risk; 3 studies without the blind method were judged to have a high risk; 6 studies were evaluated to have an unknown risk without the blind method. (4) Regarding blinding of outcome assessment, 7 studies used the blind method and were judged to have a low risk; 4 studies did not use a blind approach and were considered to have a high risk; 23 studies did not mention the blinding, so they were evaluated to have an unknown risk. (5) Regarding incomplete outcome data, 27 studies with a low risk did not report on lost cases or described cases lost to follow-up, while 7 did not describe cases lost to follow-up and were judged to have an unknown risk. (6) Regarding selective reporting, 22 studies described the outcome indicators and were defined to have a low risk, while 12 studies described only the main indicators and were defined to have an unknown risk. (7) In other biases, 18 studies with low risks reported no adverse reactions; 4 studies with high risks reported adverse reactions; 12 articles did not mention adverse reactions and were defined to have an unknown risk. The details are shown in Figures 2 and 3.
Figure 2.
Risk of bias summary graph of 34 included randomized controlled trials
Figure 3.
Risk of bias of 34 included randomized controlled trials (Review authors’ judgments about each risk of bias item for each included study. +, low risk; −, high risk;– unclear risk.)
The quality assessment results of 23 cohort studies are as follows: 3 studies had 6 points, 11 had 7 points, and 9 had 8 points. The scores of 23 cohort studies were all ≥ 6, indicating good quality (Table III).
Table III.
Quality assessment of 23 cohort studies by using NOS quality evaluation tool
| Author | Year | Selection | Comparability | Exposure | Total points |
|---|---|---|---|---|---|
| Fan-Gen Hsu | 2021 | ★★★ | ★ | ★★★ | 7 |
| Randall E. Harris | 2008 | ★★★★ | ★ | ★★★ | 8 |
| Jin Ha Lee | 2011 | ★★★ | ★★ | ★★★ | 8 |
| CR Garrett | 2012 | ★★★★ | ★★ | ★★ | 8 |
| Susan Spillane | 2013 | ★★★ | ★ | ★★★ | 7 |
| Brielan Smiechowski | 2013 | ★★★ | ★ | ★★ | 6 |
| Majken Cardel | 2014 | ★★★ | ★★ | ★★ | 7 |
| Amikar Sehdev | 2014 | ★★★★ | ★ | ★★★ | 8 |
| Bruce Y. Tung | 2016 | ★★★ | ★ | ★★ | 6 |
| J. M. Wolf | 2005 | ★★★ | ★ | ★★★ | 7 |
| F. Carrat | 2016 | ★★★★ | ★★ | ★★ | 8 |
| Jeffrey Tang | 2009 | ★★★ | ★ | ★★★ | 7 |
| David T. Rurin | 2006 | ★★★ | ★★ | ★★★ | 8 |
| J. Eaden | 2000 | ★★★ | ★ | ★★★ | 7 |
| Jonathan P. Terdiman | 2007 | ★★★★ | ★ | ★★ | 7 |
| Sierra Matula | 2005 | ★★★ | ★ | ★★★ | 7 |
| Harsh Sheth | 2018 | ★★★★ | ★ | ★★★ | 8 |
| Veronika Fedirko | 2010 | ★★★ | ★★ | ★★★ | 8 |
| Rowena Chau | 2016 | ★★★★ | ★ | ★★ | 7 |
| E. Aigner | 2014 | ★★★ | ★★ | ★★ | 7 |
| Cari Lewis | 2015 | ★★★ | ★★ | ★★ | 7 |
| D Mansouri | 2013 | ★★★ | ★ | ★★ | 6 |
| Ali A. Siddiqui | 2009 | ★★★★ | ★★ | ★★ | 8 |
Network meta-analysis
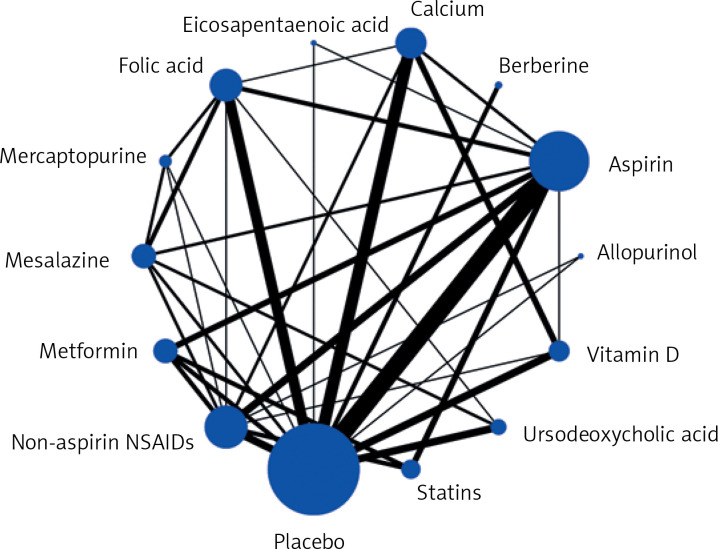
Figure 4 shows a network map of 13 drugs and the placebo. Each node represents a drug, and the node’s size is proportional to the sample size. The connecting lines express the comparison between drugs, and the thickness of the lines is proportional to the number of studies compared.
Figure 4.
Network map of randomized controlled trials and cohort studies comparing preventive effects of 13 different drugs on colorectal cancer
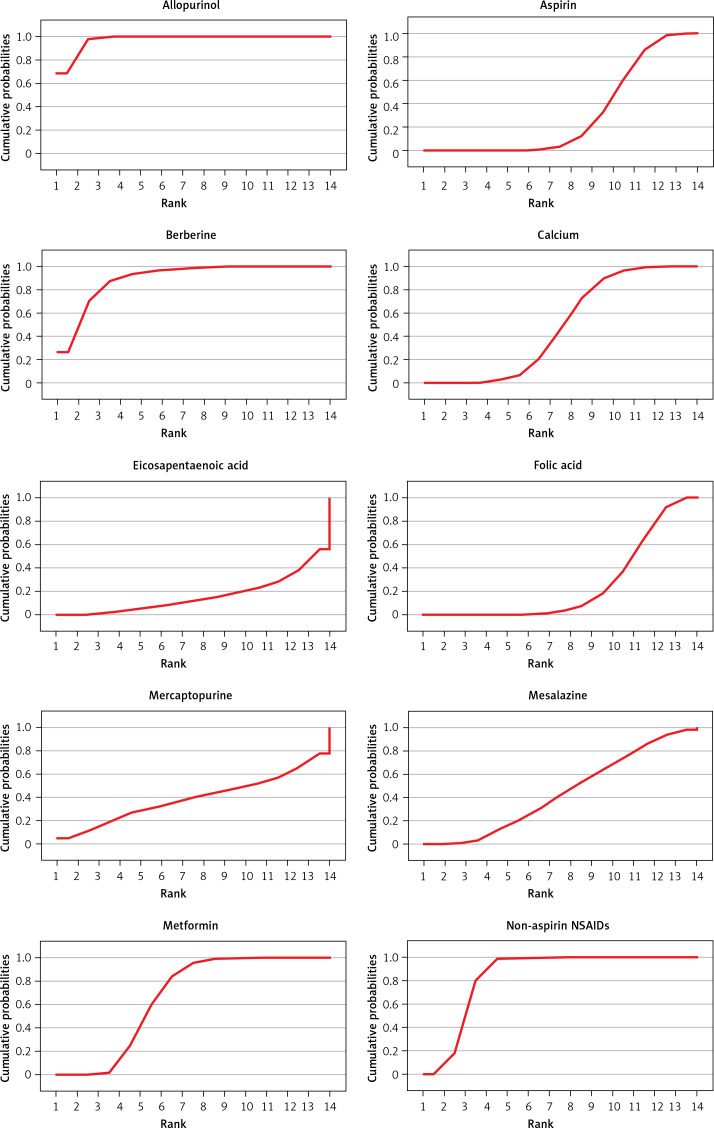
The rank of the preventive effect of all drugs on CRC is shown in Figure 5. The area under the red curve represents the surface under the cumulative ranking curve (SUCRA). The value of SUCRA is expressed as a percentage, and the higher the value, the better the drug’s effect. The best effect was seen for allopurinol (97.2%), followed by berberine (89.9%), non-aspirin NSAIDs (84.5%), statins (66.5%), metformin (66.3%), calcium (48.9%), mesalazine (44.5%), ursodeoxycholic acid (42.6%), vitamin D (41.4%), mercaptopurine (39.4%), aspirin (30.4%), folic acid (24.9%), and eicosapentaenoic acid (16.3%).
Figure 5.
Surface under cumulative ranking curve _SUCRA
The league table is shown in Table IV. The data in the table are the results of direct and indirect comparisons of each group of drugs. The effect size is expressed by OR (95% CI). OR < 1 indicates that the horizontal intervention is better than the longitudinal intervention, and OR > 1 suggests that the longitudinal intervention is better than the horizontal intervention. The comparison between the following drugs is statistically significant: compared to placebo: allopurinol (OR = 0.54, 95% CI: 0.47–0.63), berberine (OR = 0.59, 95% CI: 0.45–0.78), non-aspirin NSAIDs (OR = 0.66, 95% CI: 0.61–0.71), statins (OR = 0.74, 95% CI: 0.68–0.82), metformin (OR = 0.74, 95% CI: 0.68– 0.81), calcium (OR = 0.81, 95% CI: 0.74–0.88), vitamin D (OR = 0.83, 95% CI: 0.74–0.92), aspirin (OR = 0.86, 95% CI: 0.80–0.93), folic acid (OR = 0.89, 95% CI: 0.81–0.98); compared to allopurinol: non-aspirin NSAIDs (OR = 0.82, 95% CI: 0.73–0.93), statins (OR = 0.73, 95% CI: 0.63–0.85), metformin (OR = 0.73, 95% CI: 0.63–0.85), calcium (OR = 0.67, 95% CI: 0.57–0.79), mesalazine (OR = 0.66, 95% CI: 0.51–0.85), ursodeoxycholic acid (OR = 0.66, 95% CI: 0.51–0.85), vitamin D (OR = 0.66, 95% CI: 0.55– 0.78), aspirin (OR = 0.63, 95% CI: 0.54–0.73), folic acid (OR = 0.61, 95% CI: 0.51–0.72), eicosapentaenoic acid (OR = 0.53, 95% CI: 0.35–0.82); compared to berberine: calcium (OR = 0.74, 95% CI: 0.56–0.98), vitamin D (OR = 0.72, 95% CI: 0.54–0.96), aspirin (OR = 0.69, 95% CI: 0.52–0.91), folic acid (OR = 0.67, 95% CI: 0.50–0.89), eicosapentaenoic acid (OR = 0.59, 95% CI: 0.36–0.95); compared to non-aspirin: statins (OR = 0.88, 95% CI: 0.81–0.97), metformin (OR = 0.88, 95% CI: 0.81–0.96), calcium (OR = 0.82, 95% CI: 0.73–0.91), vitamin D (OR = 0.80, 95% CI: 0.70–0.90), aspirin (OR = 0.76, 95% CI: 0.70–0.83), folic acid (OR = 0.74, 95% CI: 0.66–0.83), eicosapentaenoic acid (OR = 0.65, 95% CI: 0.43–0.97); compared to statins: aspirin (OR = 0.86, 95% CI: 0.80–0.92), folic acid (OR = 0.84, 95% CI: 0.74–0.95); compared to metformin: aspirin (OR = 0.86, 95% CI: 0.80–0.92), folic acid (OR = 0.84, 95% CI: 0.74–0.94).
Table IV.
League table of preventive effects of 13 different drugs on colorectal cancer (Statistically significant values are marked in red)
| Allopurinol | Berberine | Non-aspirin NSAIDs | Statins | Metformin | Calcium | Mesalazine | Ursodeoxycholic acid | Vitamin D | Mercaptopurine | Aspirin | Folic acid | Eicosapentaenoic acid | Placebo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allopurinol | 1.09 (0.81–1.49) | 1.21 (1.07–1.37) | 1.37 (1.18–1.60) | 1.37 (1.18–1.60) | 1.48 (1.26–1.75) | 1.51 (1.17–1.95) | 1.52 (1.18–1.96) | 1.52 (1.28–1.82) | 1.59 (0.89–2.84) | 1.59 (1.37–1.85) | 1.64 (1.38–1.95) | 1.87 (1.23–2.85) | 1.84 (1.59–2.14) |
| 0.91 (0.67–1.24) | Berberine | 1.11 (0.84–1.47) | 1.25 (0.94–1.66) | 1.25 (0.95–1.66) | 1.36 (1.02–1.80) | 1.38 (0.97–1.96) | 1.39 (0.99–1.95) | 1.39 (1.04–1.86) | 1.45 (0.78–2.71) | 1.46 (1.10–1.92) | 1.50 (1.13–1.99) | 1.71 (1.06–2.76) | 1.68 (1.29–2.20) |
| 0.82 (0.73–0.93) | 0.90 (0.68–1.19) | Non-aspirin NSAIDs | 1.13 (1.03–1.24) | 1.13 (1.04–1.23) | 1.22 (1.10–1.36) | 1.24 (1.00–1.55) | 1.25 (1.00–1.57) | 1.26 (1.11–1.43) | 1.31 (0.75–2.31) | 1.31 (1.21–1.43) | 1.35 (1.20–1.52) | 1.54 (1.03–2.31) | 1.52 (1.40–1.65) |
| 0.73 (0.63–0.85) | 0.80 (0.60–1.06) | 0.88 (0.81–0.97) | Statins | 1.00 (0.93–1.07) | 1.08 (0.96–1.21) | 1.10 (0.87–1.39) | 1.11 (0.88–1.39) | 1.11 (0.97–1.27) | 1.16 (0.66–2.05) | 1.16 (1.08–1.25) | 1.19 (1.06–1.35) | 1.36 (0.91–2.04) | 1.34 (1.23–1.47) |
| 0.73 (0.63–0.85) | 0.80 (0.60–1.06) | 0.88 (0.81–0.96) | 1.00 (0.93–1.07) | Metformin | 1.08 (0.96–1.21) | 1.10 (0.87–1.38) | 1.11 (0.89–1.39) | 1.11 (0.97–1.27) | 1.16 (0.66–2.05) | 1.16 (1.08–1.25) | 1.19 (1.06–1.34) | 1.36 (0.91–2.04) | 1.34 (1.23–1.46) |
| 0.67 (0.57–0.79) | 0.74 (0.56–0.98) | 0.82 (0.73–0.91) | 0.92 (0.82–1.04) | 0.93 (0.83–1.04) | Calcium | 1.02 (0.80–1.29) | 1.03 (0.82–1.28) | 1.03 (0.93–1.14) | 1.07 (0.61–1.89) | 1.07 (0.97–1.19) | 1.10 (0.97–1.25) | 1.26 (0.84–1.89) | 1.24 (1.14–1.36) |
| 0.66 (0.51–0.85) | 0.73 (0.51–1.03) | 0.80 (0.64–1.00) | 0.91 (0.72–1.15) | 0.91 (0.72–1.15) | 0.98 (0.78–1.25) | Mesalazine | 1.01 (0.75–1.35) | 1.01 (0.79–1.29) | 1.06 (0.59–1.89) | 1.06 (0.84–1.33) | 1.09 (0.86–1.37) | 1.24 (0.79–1.96) | 1.22 (0.98–1.53) |
| 0.66 (0.51–0.85) | 0.72 (0.51–1.01) | 0.80 (0.64–1.00) | 0.90 (0.72–1.13) | 0.90 (0.72–1.13) | 0.98 (0.78–1.22) | 0.99 (0.74–1.33) | Ursodeoxycholic acid | 1.00 (0.79–1.27) | 1.05 (0.58–1.90) | 1.05 (0.84–1.30) | 1.08 (0.86–1.35) | 1.23 (0.78–1.93) | 1.21 (0.98–1.49) |
| 0.66 (0.55–0.78) | 0.72 (0.54–0.96) | 0.80 (0.70–0.90) | 0.90 (0.79–1.03) | 0.90 (0.79–1.03) | 0.97 (0.88–1.08) | 0.99 (0.77–1.27) | 1.00 (0.79–1.26) | Vitamin D | 1.04 (0.59–1.85) | 1.05 (0.93–1.18) | 1.07 (0.93–1.24) | 1.23 (0.81–1.85) | 1.21 (1.09–1.35) |
| 0.63 (0.35–1.12) | 0.69 (0.37–1.28) | 0.76 (0.43–1.34) | 0.86 (0.49–1.52) | 0.86 (0.49–1.52) | 0.93 (0.53–1.64) | 0.95 (0.53–1.70) | 0.96 (0.53–1.74) | 0.96 (0.54–1.70) | Mercaptopurine | 1.00 (0.57–1.76) | 1.03 (0.58–1.82) | 1.17 (0.59–2.34) | 1.16 (0.66–2.03) |
| 0.63 (0.54–0.73) | 0.69 (0.52–0.91) | 0.76 (0.70–0.83) | 0.86 (0.80–0.92) | 0.86 (0.80–0.92) | 0.93 (0.84–1.03) | 0.95 (0.75–1.19) | 0.95 (0.77–1.19) | 0.96 (0.85–1.08) | 1.00 (0.57–1.76) | Aspirin | 1.03 (0.92–1.14) | 1.17 (0.79–1.75) | 1.16 (1.07–1.25) |
| 0.61 (0.51–0.72) | 0.67 (0.50–0.89) | 0.74 (0.66–0.83) | 0.84 (0.74–0.95) | 0.84 (0.74–0.94) | 0.91 (0.80–1.03) | 0.92 (0.73–1.17) | 0.93 (0.74–1.17) | 0.93 (0.81–1.07) | 0.97 (0.55–1.72) | 0.97 (0.87–1.08) | Folic acid | 1.14 (0.76–1.72) | 1.13 (1.02–1.24) |
| 0.53 (0.35–0.82) | 0.59 (0.36–0.95) | 0.65 (0.43–0.97) | 0.73 (0.49–1.10) | 0.73 (0.49–1.10) | 0.79 (0.53–1.19) | 0.81 (0.51–1.27) | 0.81 (0.52–1.28) | 0.82 (0.54–1.23) | 0.85 (0.43–1.70) | 0.85 (0.57–1.27) | 0.88 (0.58–1.32) | Eicosapentaenoic acid | 0.99 (0.66–1.47) |
| 0.54 (0.47–0.63) | 0.59 (0.45–0.78) | 0.66 (0.61–0.71) | 0.74 (0.68–0.82) | 0.74 (0.68–0.81) | 0.81 (0.74–0.88) | 0.82 (0.65–1.02) | 0.83 (0.67–1.02) | 0.83 (0.74–0.92) | 0.86 (0.49–1.52) | 0.86 (0.80–0.93) | 0.89 (0.81–0.98) | 1.01 (0.68–1.51) | Placebo |
Results of publication bias
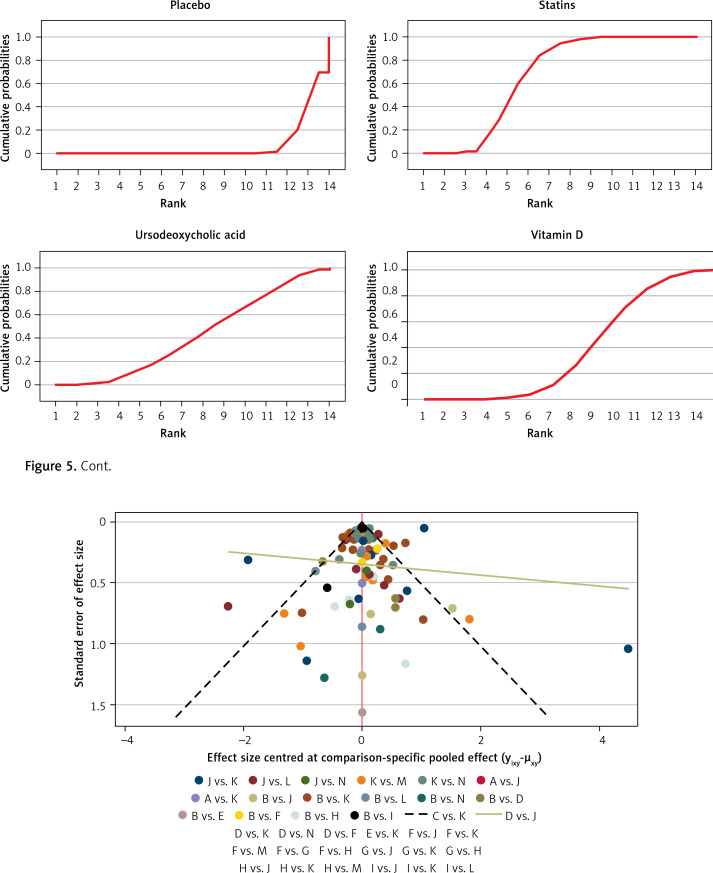
Figure 6 shows the funnel plot; the red line suggests the null hypothesis that study-specific effect sizes do not differ from respective comparison-specific pooled effect estimates. The dots of different colors represent the comparisons of different drugs. All the dots are evenly distributed on both sides of the funnel plot and roughly symmetrical, indicating no potential publication bias in this network meta-analysis. (Abbreviations: T – Total, R – Relapse, A – allopurinol, B – berberine, C – non-aspirin NSAIDs, D – statins, E – metformin, F – calcium, G – mesalazine, H – ursodeoxycholic acid, I – vitamin D, J – mercaptopurine, K – aspirin, L – folic acid, M – eicosapentaenoic acid, N – placebo).
Figure 6.
Funnel plot for network meta-analysis
Discussion
In this network meta-analysis, we compared the preventive effects of different drugs on CRC. Fifty-seven clinical studies were included, and the quality assessment confirmed that these studies were of good quality and had no significant publication bias. The effects of thirteen drugs were compared, and 82719 patients were included in the final analysis. The results revealed that allopurinol had the best preventive effect on CRC, followed by berberine, non-aspirin NSAIDs, statins, metformin, calcium, mesalazine, ursodeoxycholic acid, vitamin D, mercaptopurine, aspirin, folic acid, and eicosapentaenoic acid. This network meta-analysis overcame the limitations of the traditional meta-analysis, which can only directly compare two drugs. Herein, we performed direct and indirect comparisons of 13 different drugs, ranking the preventive effects of various drugs and providing more explicit guidance for clinicians.
CRC pathogenesis is not fully understood. Some of the factors affecting CRC are oxidative stress, inflammation, heredity, intestinal microflora structure, and living environment [79, 80]. Therefore, chemical drugs are an essential strategy for preventing CRC. Allopurinol, a structural analog of hypoxanthine, is widely used in the treatment of gout [81]. It can reduce uric acid levels by inhibiting xanthine oxidase [82]. Considering that xanthine oxidase is abundant in cancer tissues, clinical studies have suggested that allopurinol may inhibit the development of CRC by inhibiting xanthine oxidase [83]. Also, as allopurinol has been used for decades, there are solid clinical data. However, further elucidating its preventive effect on CRC constitutes a significant breakthrough. Allopurinol is more widely accepted than conventional chemotherapy drugs because of its low price and fewer side effects. Yet, more clinical studies are needed to further verify the preventive effect of allopurinol on CRC.
Berberine was first extracted from Coptis Chinensis and used in enteritis treatment. The anti-tumor effect of berberine was first reported in animal experiments, which showed that berberine could regulate the structure of intestinal microorganisms and inhibit tumor-related pathways to inhibit cancer [84]. In recent years, the preventive effect of berberine on CRC has been confirmed in clinical trials [73].
NSAIDs are considered one of the most effective drugs for preventing CRC, but their chemical protective mechanism remains unclear [85]. NSAIDs can change the cell cycle by reducing the expression of cyclin B1 and E and inducing apoptosis of cells by promoting the expression of Bax (an apoptotic protein) [86, 87]. In addition, NSAIDs can significantly inhibit the expression of cyclooxygenase-2 (cox-2), which is often upregulated in CRC [88].
Aspirin is one of the NSAIDs. Yet, some results showed that non-aspirin NSAIDs (sulindac, naproxen) are more effective in preventing CRC than aspirin. Statin is a cholesterol-lowering drug for the treatment of cardiovascular diseases. However, some studies have discovered that statins may also prevent CRC [89] by inducing tumor cell apoptosis, changing tumor cell adhesion, and preventing tumor cell cycle progression by inhibiting 3-hydroxy-3-methyl glutaryl coenzyme A reductase [90, 91].
Metformin is a first-line hypoglycemic drug for type 2 diabetes. Interestingly, some observational and preclinical studies showed that metformin has a potential anti-tumor effect [92, 93]. Metformin can inhibit the proliferation of cancer cells by activating tumor suppressors, inhibiting the mammalian target of rapamycin, and activating the liver kinase B1-dependent activation of AMPK [94].
Vitamin D and calcium are essential nutrients for bone mineralization that can prevent CRC [95, 96]. The anti-tumor mechanisms of vitamin D include affecting cell adhesion, regulating the cell cycle, affecting growth factor signal transduction, regulating tumor suppressor genes, and so on [97, 98]. In addition, calcium can bind to secondary bile acids and free fatty acids to inhibit the proliferation of cancer cells [99].
Mesalazine is widely used in the treatment of inflammatory bowel disease. However, inflammatory bowel disease is considered a significant risk factor for CRC. The oxidative stress caused by inflammation can damage DNA, activating oncogenes and suppressing tumor suppressor genes [100]. Mesalazine may exert a preventive role in CRC by reversing intestinal inflammation.
Ursodeoxycholic acid is a synthetic bile acid used to treat primary sclerosing cholangitis. Early clinical studies have shown that using ursodeoxycholic acid can reduce the incidence of CRC [101] by inhibiting the proliferation of cancer cells and mitotic signal transduction [102].
Folic acid is an indispensable nutrient in the human body, which has been confirmed to be involved in nucleotide synthesis and DNA methylation repair [103]. There is a large amount of epidemiological evidence proving that folic acid deficiency is closely related to CRC [104]. Folic acid can assist serine hydroxymethyltransferase (SHMT) in producing 5-century-10-methylenetetrahydrofolate, which can be used as a critical carrier of a single carbon group in the process of DNA methylation repair [105]. The repair of DNA methylation can restore tumor suppressor gene expression and inhibit tumor cell proliferation [106]. In addition, folic acid has been proven to inhibit oxidative stress and angiogenesis in CRC [107].
Eicosapentaenoic acid, an Omega (u)-3 polyunsaturated fatty acid (PUFA), was first found in cold-water fish oil [108]. It is often used in the treatment of cardiovascular diseases [109]. In recent years, its preventive effect on CRC has aroused great interest among scholars. However, the inhibitory mechanism of eicosapentaenoic acid on CRC is not completely clear. It has been recognized that eicosapentaenoic acid can inhibit cyclooxygenases (especially COX-2), reduce the conversion of arachidonic acid to prostaglandin E2, and eventually lead to the apoptosis of cancer cells [110].
This NMA included 82719 participants. The huge sample size can represent the whole of the patients and ensure the reliability of the results. The quality of the 57 included articles is good, and there was no obvious publication bias, which also ensures the reliability of the results. This study analyzed as many chemoprophylaxis drugs for CRC as possible, and the protective effects of the 13 drugs can provide better guidance for their clinical applications.
However, this study also has a few limitations. First, although we have tried our best to control the heterogeneity of the original research, some factors, such as different countries, gender, and age, may lead to inevitable heterogeneity. Second, some of the included RCTs had certain deficiencies in design, such as failure to use and explain randomization, blinding, and allocation methods. Third, we only compared the efficacy of the drugs; yet, we also found that these drugs had some side effects, such as nausea, vomiting, and dizziness. Future studies should compare the side effects of these drugs. Although this study has a specific clinical guidance value, more high-quality clinical studies are still needed to further verify these results.
In conclusion, compared with the placebo, 13 drugs in this study showed preventive effects on CRC, with allopurinol showing the best effect and eicosapentaenoic acid being the least effective.
Acknowledgments
The financial support of the Qinghai Science and Technology Department fund project (NO.2023-ZJ-788); Kunlun talents, Highend innovation and entrepreneurship talents are gratefully acknowledged.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W, Lin S, He Y, Wang J, Pan Y. Deep learning survival model for colorectal cancer patients (DeepCRC) with Asian clinical data compared with different theories. Arch Med Sci 2023; 19: 264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milano AF, Singer RB. The Cancer Mortality Risk Project - Cancer Mortality Risks by Anatomic Site: Part 1 - Introductory Overview; Part II - Carcinoma of the Colon: 20-Year Mortality Follow-up Derived from 1973-2013 (NCI) SEER*Stat Survival Database. J Insur Med 2017; 47: 65-94. [DOI] [PubMed] [Google Scholar]
- 4.Boursi B, Arber N: Current and future clinical strategies in colon cancer prevention and the emerging role of chemoprevention. Curr Pharm Des 2007, 13: 2274–328. [DOI] [PubMed] [Google Scholar]
- 5.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015; 64: 1637-49. [DOI] [PubMed] [Google Scholar]
- 6.Laurent E, Hussain H, Poon TKC, Ayantunde AA. The incidence, distribution and clinicopathology of missed colorectal cancer after diagnostic colonoscopy. Turk J Gastroenterol 2021; 32: 988-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domènech X, Garcia M, Benito L, et al. Cánceres de intervalo y sensibilidad de los programas poblacionales de cribado de cáncer colorrectal [Interval cancers and episode sensitivity in population-based screening programmes for colorectal cancer: a systematic review]. Gac Sanit 2015; 29: 464-71. [DOI] [PubMed] [Google Scholar]
- 8.Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiol 2019; 58: 52-62. [DOI] [PubMed] [Google Scholar]
- 9.Ghaddaf AA, Aziz M, Alomari MS, et al. Influence of aspirin on prevention of colorectal cancer: an updated systematic review and meta-analysis of randomized controlled trials. Int J Colorectal Dis 2021; 36: 1711-22. [DOI] [PubMed] [Google Scholar]
- 10.Harewood R, Disney R, Kinross J, von Wagner C, Cross AJ. Medication use and risk of proximal colon cancer: a systematic review of prospective studies with narrative synthesis and meta-analysis. Cancer Causes Control 2021; 32: 1047-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang D, Lei S, Wu Y, et al. Additively protective effects of vitamin D and calcium against colorectal adenoma incidence, malignant transformation and progression: a systematic review and meta-analysis. Clin Nutr 2020; 39: 2525-38. [DOI] [PubMed] [Google Scholar]
- 12.Fang S, Guo S, Du S, et al. Efficacy and safety of berberine in preventing recurrence of colorectal adenomas: a systematic review and meta-analysis. J Ethnopharmacol 2022; 282: 114617. [DOI] [PubMed] [Google Scholar]
- 13.Bonovas S, Fiorino G, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017; 45: 1179-92. [DOI] [PubMed] [Google Scholar]
- 14.Lytras T, Nikolopoulos G, Bonovas S. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World J Gastroenterol 2014; 20: 1858-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Khanna S, Pardi DS, Loftus EV Jr, Talwalkar JA. Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2013; 19: 1631-8. [DOI] [PubMed] [Google Scholar]
- 16.Carroll C, Cooper K, Papaioannou D, et al. Meta-analysis: folic acid in the chemoprevention of colorectal adenomas and colorectal cancer. Aliment Pharmacol Ther 2010; 31: 708-18. [DOI] [PubMed] [Google Scholar]
- 17.Donegan S, Williamson P, D’Alessandro U, Tudur Smith C. Assessing key assumptions of network meta-analysis: a review of methods. Res Synth Methods 2013; 4: 291-323. [DOI] [PubMed] [Google Scholar]
- 18.Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013; 159: 130-7. [DOI] [PubMed] [Google Scholar]
- 19.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med 2017; 12: 103-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, et al.; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014; 14: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hull MA, Sprange K, Hepburn T, et al.; seAFOod Collaborative Group . Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): a multicentre, randomised, double-blind, placebo-controlled, 2 × 2 factorial trial. Lancet 2018; 392: 2583-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu FG, Lai JN, Huang CY, Lin MC, Hsieh YW. Exploring the relationship between colorectal cancer and allopurinol: a Taiwanese population-based propensity-matched case-control study. J Clin Pharmacol 2021; 61: 1131-7. [DOI] [PubMed] [Google Scholar]
- 24.Puntoni M, Branchi D, Argusti A, et al. A randomized, placebo-controlled, preoperative trial of allopurinol in subjects with colorectal adenoma. Cancer Prev Res (Phila) 2013; 6: 74-81. [DOI] [PubMed] [Google Scholar]
- 25.Reyes-Uribe L, Wu W, Gelincik O, et al. Naproxen chemoprevention promotes immune activation in Lynch syndrome colorectal mucosa. Gut 2021; 70: 555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris RE, Beebe-Donk J, Alshafie GA. Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer 2008; 8: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baron JA, Sandler RS, Bresalier RS, et al.; APPROVe Trial Investigators . A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology 2006; 131: 1674-82. [DOI] [PubMed] [Google Scholar]
- 28.Giardiello FM, Yang VW, Hylind LM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med 2002; 346: 1054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higurashi T, Hosono K, Takahashi H, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol 2016; 17: 475-83. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH, Kim WH. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer 2012; 131: 752-9. [DOI] [PubMed] [Google Scholar]
- 31.Garrett CR, Hassabo HM, Bhadkamkar NA, et al. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer 2012; 106: 1374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spillane S, Bennett K, Sharp L, Barron TI. A cohort study of metformin exposure and survival in patients with stage I-III colorectal cancer. Cancer Epidemiol Biomarkers Prev 2013; 22: 1364-73. [DOI] [PubMed] [Google Scholar]
- 33.Smiechowski B, Azoulay L, Yin H, Pollak MN, Suissa S. The use of metformin and colorectal cancer incidence in patients with type II diabetes mellitus. Cancer Epidemiol Biomarkers Prev 2013; 22: 1877-83. [DOI] [PubMed] [Google Scholar]
- 34.Cardel M, Jensen SM, Pottegård A, Jørgensen TL, Hallas J. Long-term use of metformin and colorectal cancer risk in type II diabetics: a population-based case-control study. Cancer Med 2014; 3: 1458-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sehdev A, Shih YC, Vekhter B, Bissonnette MB, Olopade OI, Polite BN. Metformin for primary colorectal cancer prevention in patients with diabetes: a case-control study in a US population. Cancer 2015; 121: 1071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tung BY, Emond MJ, Haggitt RC, et al. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med 2001; 134: 89-95. [DOI] [PubMed] [Google Scholar]
- 37.Sjöqvist U, Tribukait B, Ost A, Einarsson C, Oxelmark L, Löfberg R. Ursodeoxycholic acid treatment in IBD-patients with colorectal dysplasia and/or DNA-aneuploidy: a prospective, double-blind, randomized controlled pilot study. Anticancer Res 2004; 24: 3121-7. [PubMed] [Google Scholar]
- 38.Alberts DS, Martínez ME, Hess LM, et al.; Phoenix and Tucson Gastroenterologist Networks . Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst 2005; 97: 846-53. [DOI] [PubMed] [Google Scholar]
- 39.Wolf JM, Rybicki LA, Lashner BA. The impact of ursodeoxycholic acid on cancer, dysplasia and mortality in ulcerative colitis patients with primary sclerosing cholangitis. Aliment Pharmacol Ther 2005; 22: 783-8. [DOI] [PubMed] [Google Scholar]
- 40.Pardi DS, Loftus EV Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology 2003; 124: 889-93. [DOI] [PubMed] [Google Scholar]
- 41.Lindström L, Boberg KM, Wikman O, et al. High dose ursodeoxycholic acid in primary sclerosing cholangitis does not prevent colorectal neoplasia. Aliment Pharmacol Ther 2012; 35: 451-7. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa H, Mutoh M, Sato Y, et al. Chemoprevention with low-dose aspirin, mesalazine, or both in patients with familial adenomatous polyposis without previous colectomy (J-FAPP Study IV): a multicentre, double-blind, randomised, two-by-two factorial design trial. Lancet Gastroenterol Hepatol 2021; 6: 474-81. [DOI] [PubMed] [Google Scholar]
- 43.Carrat F, Seksik P, Colombel JF, Peyrin-Biroulet L, Beaugerie L; CESAME Study Group. The effects of aminosalicylates or thiopurines on the risk of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther 2017; 45: 533-41. [DOI] [PubMed] [Google Scholar]
- 44.Tang J, Sharif O, Pai C, Silverman AL. Mesalamine protects against colorectal cancer in inflammatory bowel disease. Dig Dis Sci 2010; 55: 1696-703. [DOI] [PubMed] [Google Scholar]
- 45.Rubin DT, LoSavio A, Yadron N, Huo D, Hanauer SB. Aminosalicylate therapy in the prevention of dysplasia and colorectal cancer in ulcerative colitis. Clin Gastroenterol Hepatol 2006; 4: 1346-50. [DOI] [PubMed] [Google Scholar]
- 46.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther 2000; 14: 145-53. [DOI] [PubMed] [Google Scholar]
- 47.Terdiman JP, Steinbuch M, Blumentals WA, Ullman TA, Rubin DT. 5-Aminosalicylic acid therapy and the risk of colorectal cancer among patients with inflammatory bowel disease. Inflamm Bowel Dis 2007; 13: 367-71. [DOI] [PubMed] [Google Scholar]
- 48.Matula S, Croog V, Itzkowitz S, et al. Chemoprevention of colorectal neoplasia in ulcerative colitis: the effect of 6-mercaptopurine. Clin Gastroenterol Hepatol 2005; 3: 1015-21. [DOI] [PubMed] [Google Scholar]
- 49.Benamouzig R, Uzzan B, Martin A, et al.; APACC Study Group . Cyclooxygenase-2 expression and recurrence of colorectal adenomas: effect of aspirin chemoprevention. Gut 2010; 59: 622-9. [DOI] [PubMed] [Google Scholar]
- 50.Barry EL, Sansbury LB, Grau MV, et al. Cyclooxygenase-2 polymorphisms, aspirin treatment, and risk for colorectal adenoma recurrence--data from a randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2009; 18: 2726-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheth H, Northwood E, Ulrich CM, et al. Interaction between polymorphisms in aspirin metabolic pathways, regular aspirin use and colorectal cancer risk: a case-control study in unselected white European populations. PLoS One 2018; 13: e0192223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueiredo JC, Levine AJ, Grau MV, et al. Vitamins B2, B6, and B12 and risk of new colorectal adenomas in a randomized trial of aspirin use and folic acid supplementation. Cancer Epidemiol Biomarkers Prev 2008; 17: 2136-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grau MV, Sandler RS, McKeown-Eyssen G, et al. Nonsteroidal anti-inflammatory drug use after 3 years of aspirin use and colorectal adenoma risk: observational follow-up of a randomized study. J Natl Cancer Inst 2009; 101: 267-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burn J, Gerdes AM, Macrae F, et al.; CAPP2 Investigators . Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378: 2081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishikawa H, Wakabayashi K, Suzuki S, et al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial. Cancer Med 2013; 2: 50-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 2003; 348: 891-9. [DOI] [PubMed] [Google Scholar]
- 57.Ishikawa H, Mutoh M, Suzuki S, et al. The preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut 2014; 63: 1755-9. [DOI] [PubMed] [Google Scholar]
- 58.Figueiredo JC, Mott LA, Giovannucci E, et al. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int J Cancer 2011; 129: 192-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao QY, Chen HM, Chen YX, et al. Folic acid prevents the initial occurrence of sporadic colorectal adenoma in Chinese older than 50 years of age: a randomized clinical trial. Cancer Prev Res (Phila) 2013; 6: 744-52. [DOI] [PubMed] [Google Scholar]
- 60.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR; ukCAP Trial Group. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology 2008; 134: 29-38. [DOI] [PubMed] [Google Scholar]
- 61.Cole BF, Baron JA, Sandler RS, et al.; Polyp Prevention Study Group . Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007; 297: 2351-9. [DOI] [PubMed] [Google Scholar]
- 62.Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin d for the prevention of colorectal adenomas. N Engl J Med 2015; 373: 1519-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonithon-Kopp C, Kronborg O, Giacosa A, Räth U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet 2000; 356: 1300-6. [DOI] [PubMed] [Google Scholar]
- 64.Fedirko V, Bostick RM, Goodman M, Flanders WD, Gross MD. Blood 25-hydroxyvitamin D3 concentrations and incident sporadic colorectal adenoma risk: a pooled case-control study. Am J Epidemiol 2010; 172: 489-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barry EL, Lund JL, Westreich D, et al. Body mass index, calcium supplementation and risk of colorectal adenomas. Int J Cancer 2019; 144: 448-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martínez ME, Marshall JR, Sampliner R, Wilkinson J, Alberts DS. Calcium, vitamin D, and risk of adenoma recurrence (United States). Cancer Causes Control 2002; 13: 213-20. [DOI] [PubMed] [Google Scholar]
- 67.Grau MV, Baron JA, Barry EL, et al. Interaction of calcium supplementation and nonsteroidal anti-inflammatory drugs and the risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 2005; 14: 2353-8. [DOI] [PubMed] [Google Scholar]
- 68.Jacobs ET, Alberts DS, Benuzillo J, Hollis BW, Thompson PA, Martínez ME. Serum 25(OH)D levels, dietary intake of vitamin D, and colorectal adenoma recurrence. J Steroid Biochem Mol Biol 2007; 103: 752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chau R, Dashti SG, Ait Ouakrim D, et al. Multivitamin, calcium and folic acid supplements and the risk of colorectal cancer in Lynch syndrome. Int J Epidemiol 2016; 45: 940-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grau MV, Baron JA, Sandler RS, et al. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J Natl Cancer Inst 2007; 99: 129-36. [DOI] [PubMed] [Google Scholar]
- 71.Aigner E, Stadlmayr A, Huber-Schönauer U, et al. Gender- and site-specific differences of colorectal neoplasia relate to vitamin D. Aliment Pharmacol Ther 2014; 40: 1341-8. [DOI] [PubMed] [Google Scholar]
- 72.Lewis C, Xun P, He K. Vitamin D supplementation and quality of life following diagnosis in stage II colorectal cancer patients: a 24-month prospective study. Support Care Cancer 2016; 24: 1655-61. [DOI] [PubMed] [Google Scholar]
- 73.Chen YX, Gao QY, Zou TH, et al. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol Hepatol 2020; 5: 267-75. [DOI] [PubMed] [Google Scholar]
- 74.Wang WQ, Li X, Chen YH, Liu SP, Tang GM. Preventive effects of Berberine in the recurrence of colorectal adenoma after endoscopic resection. Chin J Gastroenterol Hepatol 2020; 29: 46-9. [Google Scholar]
- 75.Liu LL. A Randomized Controlled Trial of Berberine in the Prevention of Recurrence after Endoscopic Resection of Coloretal Adenomas. Xiamen University, 2018. [Google Scholar]
- 76.Mansouri D, McMillan DC, Roxburgh CS, Crighton EM, Horgan PG. The impact of aspirin, statins and ACE-inhibitors on the presentation of colorectal neoplasia in a colorectal cancer screening programme. Br J Cancer 2013; 109: 249-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siddiqui AA, Nazario H, Mahgoub A, Pandove S, Cipher D, Spechler SJ. The long-term use of statins is associated with a decreased incidence of adenomatous colon polyps. Digestion 2009; 79: 17-22. [DOI] [PubMed] [Google Scholar]
- 78.Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal adenomatous polyps. Int J Colorectal Dis 2013; 28: 469-76. [DOI] [PubMed] [Google Scholar]
- 79.Bożyk A, Krawczyk P, Reszka K, et al. Correlation between KRAS, NRAS and BRAF mutations and tumor localizations in patients with primary and metastatic colorectal cancer. Arch Med Sci 2022; 18: 1221-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients 2019; 11: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rakieh C, Conaghan PG. Diagnosis and treatment of gout in primary care. Practitioner 2011; 255: 17-20, 2-3. [PubMed] [Google Scholar]
- 82.Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 2006; 58: 87-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samra ZQ, Pervaiz S, Shaheen S, Dar N, Athar MA. Determination of oxygen derived free radicals producer (xanthine oxidase) and scavenger (paraoxonase1) enzymes and lipid parameters in different cancer patients. Clin Lab 2011; 57: 741-7. [PubMed] [Google Scholar]
- 84.Yu YN, Yu TC, Zhao HJ, et al. Berberine may rescue Fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget 2015; 6: 32013-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsioulias GJ, Go MF, Rigas B. NSAIDs and colorectal cancer control: promise and challenges. Curr Pharmacol Rep 2015; 1: 295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shiff SJ, Koutsos MI, Qiao L, Rigas B. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis. Exp Cell Res 1996; 222: 179-88. [DOI] [PubMed] [Google Scholar]
- 87.Poole JC, Thain A, Perkins ND, Roninson IB. Induction of transcription by p21Waf1/Cip1/Sdi1: role of NFkappaB and effect of non-steroidal anti-inflammatory drugs. Cell Cycle 2004; 3: 931-40. [PubMed] [Google Scholar]
- 88.Benelli R, Venè R, Ferrari N. Prostaglandin-endoperoxide synthase 2 (cyclooxygenase-2), a complex target for colorectal cancer prevention and therapy. Transl Res 2018; 196: 42-61. [DOI] [PubMed] [Google Scholar]
- 89.Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev 2012; 64: 102-46. [DOI] [PubMed] [Google Scholar]
- 90.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002; 16: 508-19. [DOI] [PubMed] [Google Scholar]
- 91.Jakobisiak M, Golab J. Potential antitumor effects of statins (Review). Int J Oncol 2003; 23: 1055-69. [PubMed] [Google Scholar]
- 92.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330: 1304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 2010; 33: 322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li M, Li X, Zhang H, Lu Y. Molecular mechanisms of metformin for diabetes and cancer treatment. Front Physiol 2018; 9: 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014; 14: 342-57. [DOI] [PubMed] [Google Scholar]
- 96.Zhang X, Keum N, Wu K, et al. Calcium intake and colorectal cancer risk: results from the nurses’ health study and health professionals follow-up study. Int J Cancer 2016; 139: 2232-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 2003; 3: 601-14. [DOI] [PubMed] [Google Scholar]
- 98.Ebert R, Schütze N, Adamski J, Jakob F. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol 2006; 248: 149-59. [DOI] [PubMed] [Google Scholar]
- 99.Keum N, Liu L, Hamada T, et al. Calcium intake and colon cancer risk subtypes by tumor molecular characteristics. Cancer Causes Control 2019; 30: 637-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 2004; 287: G7-17. [DOI] [PubMed] [Google Scholar]
- 101.Tung BY, Emond MJ, Haggitt RC, et al. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med 2001; 134: 89-95. [DOI] [PubMed] [Google Scholar]
- 102.Im E, Akare S, Powell A, Martinez JD. Ursodeoxycholic acid can suppress deoxycholic acid-induced apoptosis by stimulating Akt/PKB-dependent survival signaling. Nutr Cancer 2005; 51: 110-6. [DOI] [PubMed] [Google Scholar]
- 103.Duthie SJ. Folate and cancer: how DNA damage, repair and methylation impact on colon carcinogenesis. J Inherit Metab Dis 2011; 34: 101-9. [DOI] [PubMed] [Google Scholar]
- 104.Eichholzer M, Lüthy J, Moser U, Fowler B. Folate and the risk of colorectal, breast and cervix cancer: the epidemiological evidence. Swiss Med Wkly 2001; 131: 539-49. [DOI] [PubMed] [Google Scholar]
- 105.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr 2012; 3: 21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007; 128: 683-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsubara K, Komatsu S, Oka T, Kato N. Vitamin B6-mediated suppression of colon tumorigenesis, cell proliferation, and angiogenesis (review). J Nutr Biochem 2003; 14: 246-50. [DOI] [PubMed] [Google Scholar]
- 108.Calder PC, Yaqoob P. Omega-3 polyunsaturated fatty acids and human health outcomes. Biofactors 2009; 35: 266-72. [DOI] [PubMed] [Google Scholar]
- 109.Elagizi A, Lavie CJ, O’Keefe E, Marshall K, O’Keefe JH, Milani RV. An update on omega-3 polyunsaturated fatty acids and cardiovascular health. Nutrients 2021; 13: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Courtney ED, Matthews S, Finlayson C, et al. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis 2007; 22: 765-76. [DOI] [PubMed] [Google Scholar]