Abstract
Objective
Novel disease-specific and mechanism-based treatments sharing good evidence of efficacy for migraine have been recently marketed. However, reimbursement by insurers depends on treatment failure with classic anti-migraine drugs. In this systematic review and meta-analysis, we aimed to identify and rate the evidence for efficacy of flunarizine, a repurposed, first- or second-line treatment for migraine prophylaxis.
Methods
A systematic search in MEDLINE, Cochrane CENTRAL, and ClinicalTrials.gov was performed for trials of pharmacological treatment in migraine prophylaxis, following the Preferred Reporting Items for Systematic Reviews (PRISMA). Eligible trials for meta-analysis were randomized, placebo–controlled studies comparing flunarizine with placebo. Outcomes of interest according to the Outcome Set for preventive intervention trials in chronic and episodic migraine (COSMIG) were the proportion of patients reaching a 50% or more reduction in monthly migraine days, the change in monthly migraine days (MMDs), and Adverse Events (AEs) leading to discontinuation.
Results
Five trials were eligible for narrative description and three for data synthesis and analysis. No studies reported the predefined outcomes, but one study assessed the 50% reduction in monthly migraine attacks with flunarizine as compared to placebo showing a benefit from flunarizine with a low or probably low risk of bias. We found that flunarizine may increase the proportion of patients who discontinue due to adverse events compared to placebo (risk difference: 0.02; 95% CI -0.03 to 0.06).
Conclusions
Published flunarizine trials predate the recommended endpoints for evaluating migraine prophylaxis drugs, hence the lack of an adequate assessment for these endpoints. Further, modern-day, large‐scale studies would be valuable in re-evaluating the efficacy of flunarizine for the treatment of migraines, offering additional insights into its potential benefits.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-023-01657-3.
Keywords: Migraine, Prophylactic treatment, Flunarizine, Meta-analysis
Introduction
Until recently, migraine preventive treatment was limited to a variety of drugs that have been primarily developed to treat other conditions but were later found to be also effective in migraine prevention. Within one of these classes, calcium channel blockers have been studied for the prevention of migraine, of which flunarizine is the most widely used. Flunarizine is a mixed sodium and calcium channel blocker whose preventive effect in migraine might at least in part be attributed to block P/Q-type channels in the brain. The P/Q-type calcium channel is a presynaptic high-voltage-gated calcium channel contributing to vesicle release at synaptic terminals. A number of neurological diseases have been attributed to the malfunctioning of P/Q channels, including migraine.
Calcium channel antagonists prevent calcium from entering cells, resulting in relaxation of heart and vascular smooth muscle, thereby decreasing blood pressure. However, the therapeutic dose of flunarizine was shown to be unlikely to exert calcium-antagonistic effects on cerebral vessels [1]. In contrast, the calcium channel antagonist nimodipine could exert an effect on cerebral arteries at a therapeutic dose, but nimodipine was not better than placebo in migraine prophylaxis [1, 2]. These data suggest that the prophylactic effect of the calcium channel blockers might not be mediated by its direct vasodilatory effect on arteries.
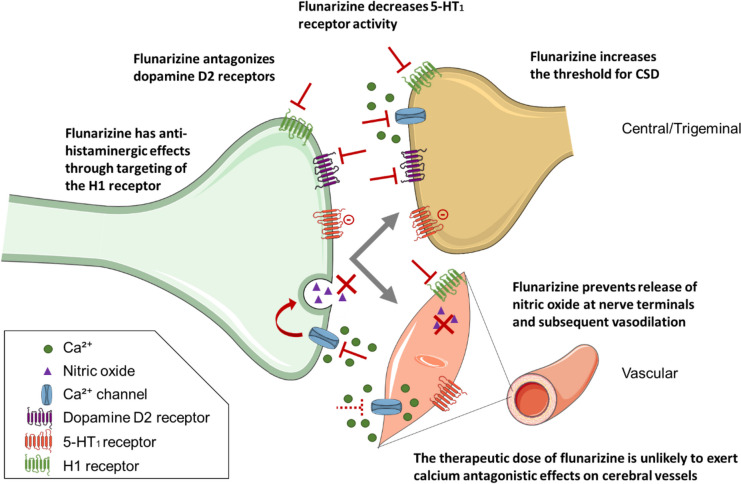
Flunarizine was shown to affect the production and release of nitric oxide in canine cerebral arteries by blocking the influx of Ca2+ induced by action potentials at nerve terminals [3] and increases the threshold for cortical spreading depression [4, 5], which is thought to underlie migraine aura. Moreover, flunarizine antagonizes the dopamine D2 receptor [6], and has antihistaminergic effects, through targeting of the H1 receptor, whereas the calcium channel blocker verapamil affects the H2 receptor [7]. Another calcium channel blocker—nifedipine -does not target either of these histamine receptors yet it has one clinical study which demonstrated antimigraine effects [8]. Long-term administration of verapamil, flunarizine and nifedipine has also been demonstrated to lead to a reduction in the activity of the 5-HT1 receptors in the hippocampus and cerebral cortex of rats [9]. Verapamil, nifedipine and diltiazem inhibit 5-HT2 receptors in human brain tissue, while verapamil could also target 5-HT1A receptors, albeit with a lower potency [10]. Given the established efficacy of triptans, which are 5-HT1 receptor agonists, in the treatment of migraine, it is worth considering that the anti-migraine effect of calcium channel blockers may be mediated at least in part through their impact on the serotonergic system (Fig. 1).
Fig. 1.
Potential mechanisms of action for the anti-migraine effect of the Ca2+ channel blocker flunarizine. Flunarizine affects the production and release of nitric oxide in canine cerebral arteries by blocking the influx of Ca.2+ induced by action potentials at nerve terminals [3] and increases the threshold for cortical spreading depression [4, 5]. Moreover, flunarizine antagonizes the dopamine D2 receptor [6], and has antihistaminergic effects through targeting of the H1 receptor [7]. Calcium channel blockers can affect the serotonergic system [8, 9] and prolonged treatment with flunarizine results in decreased activity of 5-HT1 receptors in the hippocampus and cerebral cortex of rats [8]. The therapeutic dose of flunarizine for the treatment of migraine is unlikely to exert calcium antagonistic effects on cerebral vessels [1]
Flunarizine has been introduced for the management of migraine in the 1980s [11, 12]. Reports on its protective effects against brain hypoxia via the reduction of intracellular calcium overload and inhibitor effects on the contractility of cranial arteries in animal models led to the investigation of its possible prophylactic role in the management of migraine [11, 12]. Flunarizine is suggested in several national treatment guidelines as a drug with level A evidence for migraine prophylaxis, with a recommended dose of 5–10 mg [13]. However, it is important to note that the availability of flunarizine varies among European countries (https://www.ema.europa.eu/en/documents/psusa/flunarizine-list-nationally-authorised-medicinal-products-psusa/00001416/201505_en.pdf) and that it is not marketed in the United States.
The purpose of this study was to re-appraise critically the published trials that evaluated the possible benefits of flunarizine versus placebo in migraine in a systematic approach and illuminate the role of flunarizine on the prophylactic management of migraine through a meta-analysis.
Methods
This work is the second study of the series aiming to re-appraise different types of classic migraine preventive medications. We conducted a systematic review and meta-analysis and report our results according to the PRISMA statement [14]. We have previously described the methods for this review in detail in the systematic review and meta-analysis focused on the prophylactic role of amitriptyline in migraine [15].
In consultation with a research librarian, we searched MEDLINE, EMBASE, Cochrane CENTRAL, and ClinicalTrials.gov from inception to August 13, 2022 for randomized trials of drug treatments for migraine prophylaxis, without language restrictions (Supplement 1).
Pairs of reviewers, working independently and in duplicate to reduce the potential for errors, screened titles and abstracts of search records and subsequently the full texts of records deemed eligible at the title and abstract screening stage. We included randomized trials that compared flunarizine with placebo for migraine prevention in adults. We excluded trials investigating children or adolescents; or those that randomized a study sample fewer than 25 patients in each treatment arm from data synthesis and analysis [15].
We collected data on trial and patient characteristics (e.g., country of recruitment or severity of migraine), interventions, and outcomes of interest. Our outcomes of interest were pre-specified according to the Outcome Set for preventive intervention trials in chronic and episodic migraine (COSMIG) [16]. We included the proportion of patients with a 50% or more reduction in migraine days per month, change in migraine days per month, and adverse events leading to discontinuation. Monthly headache days or monthly migraine attacks were extracted when monthly migraine days were not reported. We assessed the risk of bias using a modified Cochrane RoB 2.0 tool [17, 18].
For all outcomes, we performed a frequentist random-effects meta-analysis using the restricted maximum likelihood (REML) estimator. We analyzed 50% or more reduction in monthly migraine days as relative risks, monthly migraine days as mean differences, and adverse events leading to discontinuation as risk differences, since we expected many studies to report no or few events with placebo. To facilitate interpretation, we report dichotomous outcomes as number of events per 1,000 patients.
We assessed the certainty of evidence using the GRADE approach [19] and reported using GRADE simple language summaries [15, 20].
Results
Our systematic literature review yielded 10,826 records, 1276 records proved to be potentially eligible after title and abstract screening, of which five trials were eligible for the narrative description [21–25] and three for data synthesis and analysis [21, 24, 25]. Details of the study selection are shown on the PRISMA flowchart diagram (Fig. 2).
Fig. 2.
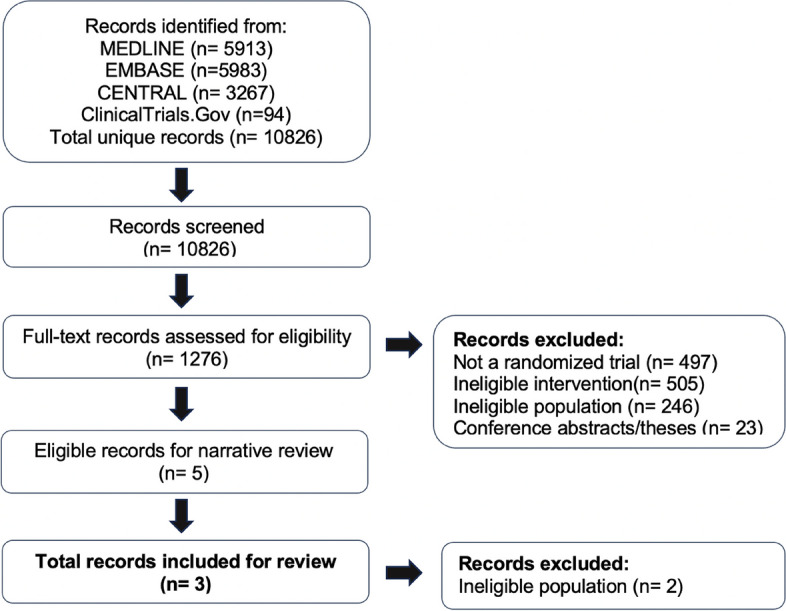
PRISMA flowchart of the study. The systematic search yielded a total of 10,826 unique records. Title and abstract screening resulted in 1276 records potentially eligible, and after full‐text review 5 records proved eligible. Records that did not describe full‐text peer‐reviewed reports of randomized placebo-controlled trials in participants ≥ 18 years old of flunarizine in migraine prevention were excluded
Narrative description of flunarizine in placebo-controlled trials
The first conducted study [21] published in 1981 reported a median attack frequency reduction of 57% in 29 patients treated with flunarizine 10 mg/day versus 14% in 29 patients treated with placebo. In this study, the duration and severity of attacks did not change; mild sedation and dry mouth were reported as main adverse events (AEs). In a second study flunarizine was judged beneficial in 59% of a sample of 17 participants treated with flunarizine 10 mg/day compared to 18 treated with placebo, with a mean monthly attack reduction at month three of -2.5 in the flunarizine group versus -1.2 in the placebo group. Additionally, flunarizine was associated with sedation at the beginning of the treatment [22]. A year later a small cross-over study (n = 9) reported a ~ 50% reduction of migraine attacks with flunarizine 10 mg/day in the third month, interestingly no AEs were reported [23]. In another cross-over placebo-controlled study including 29 patients with a duration of 16 weeks for each treatment period and a 4-week baseline/wash-out period [24], the authors reported that the median number of migraine attacks measured per four-week period was 3.5 during baseline/washout period, 3.2 during placebo treatment, and 2.0 while on flunarizine. In this study, migraine attack frequency, hours with migraine, and migraine index were statistically significantly reduced by 50% or more in the last four-week period on flunarizine. In the last referred study, including 50 patients treated with flunarizine, flunarizine showed a statistically significant 50% reduction in attack frequency compared to 40% in the placebo group [25].
Overall, the design of these older studies is not in line with the current international guidelines for controlled drug trials in migraine, which were first published after 1991 [26]. The definition of migraine and the criteria used for its classification varied between the referred trials. Headaches were classified as common migraine or classical migraine based on the Ad Hoc Committee on Classification of Headache of the NIH [27] in three trials [21–23] and the modified criteria of the Ad Hoc Committee on the Classification of Headache by Olesen et al. [28] in another one [24]. One trial performed in 1991 [25] used the first edition of the International Headache Classification (ICHD-1) [29] and recruited migraine individuals with or without aura (Table 1).
Table 1.
Placebo controlled randomized clinical trials of flunarizine. trial characteristics
| Study name | Study design | Funding | Country | Sample size | Age, median (range) | % Male | Types of migraine | Drug dosage (daily) | Active Tx period (weeks) | Outcome measures | Tolerability |
|---|---|---|---|---|---|---|---|---|---|---|---|
| dLouis 1981 [21] | PCS | NR | Belgium |
58 (29 in each arm) |
29 (20–47) |
50 |
Common migraine (31%) or Classical migraine (69%)a |
10 mg | 12 |
Reduction in: • Monthly migraine attacks (at least one attack at 3rd mo: 17.2% vs 55.2%, p = 0.006) • Duration and severity of migraine attacks, did not differ between two interventions |
Mild day-time sedation in 2 patients on flunarizine (without withdrawal) Dry mouth; in 2 controls (without withdrawal) |
| dSørensen 1986 [24] | PCS, Cross-over | NR | Denmark | 29e |
40 (19–63) |
20.7 | Common migraineb | 10 mg | 16 |
Reduction in: • Monthly migraine attacks (reduction in number of attacks at 4th mo: 50% vs 15%, p = 0.02) • Duration and severity of migraine attacks (p = NS, for both) |
Mild day-time sedation in 3 patients on flunarizine (1 withdrawal) |
| dFreitag 1991 [25] | PCS | NR | US |
101 (50 in flunarizine arm) |
NR | 25.7 |
MwA (32.7%) or MwoA (67.3%)c |
10 mg | 16 |
Reduction in: • Number of migraine attacks from baseline to the end (50% vs 39.9%, p = 0.018) • Duration and severity of migraine attacks (reduced in flunarizine arm, but p = NS for both) |
5 withdrawals among patients on flunarizine†† 3 withdrawals among controls |
| Frenken 1984 [22] | PCS | NR | Netherlands |
35 (17 in flunarizine arm) |
NR | 17.1 |
Common migraine (60%) or Classical migraine (40%)a |
10 mg | 12 |
Reduction in: • Monthly migraine attacks (significant reduction in flunarizine arm; p = 0.029 at 1st mo) • Duration of migraine attacks (p value is not reported) • Severity of migraine attacks (p = NS) |
Day-time sedation in 7, weight gain in 3 and other AEs in 2 patients on flunarizine Day-time sedation in 3, stomach complaints in 4, and other AEs in 1 of controls |
| Mendenopoulos 1985 [23] | PCS | NR | Greece |
20 (9 in flunarizine arm) |
20–65 | 20 | Classical migraine a | 10 mg | 12–16 |
Reduction in: • Monthly Migraine attacks (50% reduction in attacks after 3 mo; p = 0.033) • Duration and severity of migraine attacks (significant reduction in flunarizine arm; p = 0.037 and p = 0.006, respectively, after 3rd month) |
No AEs |
AEs Adverse events, FU Follow-up, MwA Migraine with aura, MwA Migraine without aura, NR Not reported, NS not significant, PCS Prospective cohort study, Tx Treatment
adefined according to the Ad Hoc Committee on Classification of Headache of the NIH [25]
bdefined according to the modified criteria of the Ad Hoc Committee on the Classification of Headache by Olesen et al. [26]
cdefined according to the first edition of the International Headache Classification [27]
dStudies included in the meta-analysis
eOne patient withdrew on placebo because of increasing number of migraine attacks
fDetails of the adverse events leading to withrawal were not given in the article
Three trials [21, 24, 25] randomized more than 25 patients in each treatment arm and only one of them had a larger sample size of overall 101 patients [25]. Age groups were not reported in two records [22, 25], middle-aged patients were mostly reported to be recruited in the remaining trials [21, 23, 24]. Most of the patients were females. None of the RCTs comparing flunarizine versus placebo reported information on funding to support the study. Research methods were overall not described in detail. Patients who were assigned following the withdrawal of anti-migraine medications [21–23, 25] or those taking drugs for migraine prevention were excluded [24]. However, the baseline medication-free period was reported in only one record [23]. Only two trials stated that patients were asked to complete a diary documenting headache frequency, characteristics and AEs [21, 24].
All RCTs reported a significant reduction in frequency of migraine attacks for flunarizine 10 mg/day over placebo. The duration and severity of migraine attacks were also assessed and tended to decrease in the flunarizine-treated patients in comparison with the placebo-treated ones in only two trials [23, 25] reaching statistical significance in one of these trials [23].
Data synthesis and analysis
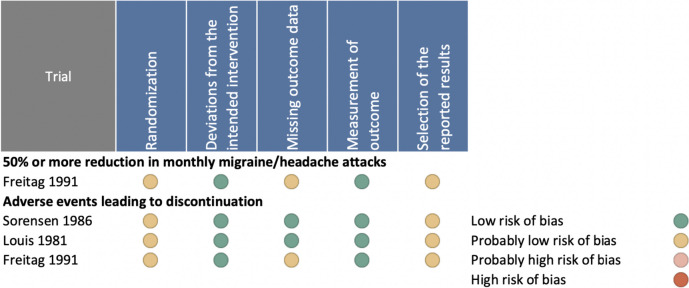
Three trials with a total of 188 participants were eligible and included for the quantitative analysis [21, 24, 25]. The other two trials were excluded since they assigned less than 25 individuals in each group [22, 23]. Two of the included trials were performed in Europe [21, 24] and one in the USA [25]. Characteristics of the five trials, eligible for narrative description, including study design, sample size, flunarizine dosage, period of active treatment, median age, sex distribution, type of migraine, outcome measures, and tolerability issues are presented in Table 1, while the risk of bias of eligible trials is presented in Fig. 3.
Fig. 3.
Risk of bias assessment
50% responder rate
The outcome of 50% or more reduction in migraine days per month was not reported. One study reported on the 50% reduction in migraine attacks in favor of flunarizine with a low or probably low risk of bias [25] (Fig. 3).
Monthly migraine days
No available data.
Adverse events leading to discontinuation
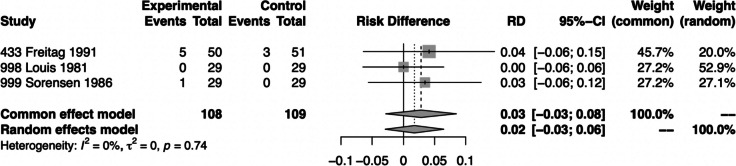
We could only perform a quantitative analysis on AEs leading to discontinuation showing that significantly more participants treated with flunarizine discontinued treatment than those treated with placebo (Fig. 4). In the pooled analysis, ten participants treated with flunarizine reported AEs but six withdrew from the treatment [21, 24, 25]. In the placebo arm, five participants reported AEs and three withdrew. This outcome was rated as low or probably low risk of bias for all three RCTs and of high certainty according to the GRADE approach (Fig. 3, Table 2). Mild daytime sedation and weight gain were the most common AEs leading to discontinuation [21, 22, 24, 25]. However, several AEs such as dry mouth and stomach complaints as well as daytime sedation were also reported by patients treated with placebo [21, 22].
Fig. 4.
Forest plot of analysis comparing flunarizine with placebo for adverse events leading to discontinuation
Table 2.
Flunarizine compared to placebo for migraine prophylaxis
| Patient or population: migraine Intervention: prophylaxis with flunarizine Comparison: placebo | |||||
|---|---|---|---|---|---|
| Outcomes |
№ of participants (studies) Follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
| Risk with placebo | Risk difference with flunarizine | ||||
| 50% or more reduction in monthly migraine days | No data | ||||
| Monthly migraine days | No data | ||||
| Adverse events leading to discontinuation |
188 (3 RCTs) |
High |
RD 0.02 (-0.03 to 0.06) |
0 per 1,000 |
20 more per 1,000 (30 fewer to 60 more) |
CI Confidence interval, RD Risk difference
GRADE Working Group grades of evidence: High certainty; we are very confident that the true effect lies close to that of the estimate of the effect
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
Discussion
Flunarizine is one of the first oral, repurposed drugs used for migraine prevention. Although RCTs comparing flunarizine with placebo share several important methodological limitations, flunarizine still stands as a drug of choice in Europe, with class A evidence [30]. That is the reason why flunarizine is among the list of first-line treatments that migraine patients must fail to be eligible for specific novel migraine preventives in many countries. The present study revealed that the evidence on this medication efficacy is limited to studies mostly conducted more than a quarter of a century ago and empiric knowledge in clinical practice. Available trial data are not of enough quality to recommend its use as a migraine preventive treatment with class A level of evidence.
Although our findings are generally in line with a recently published comprehensive meta-analysis [31], with different methodological approaches though, we are coming in contrast in supporting the attribution of a Level A evidence for efficacy of flunarizine, on which also the authors raise concerns on the quality of data from older studies. The results of trials done in the past following different quality criteria should be evaluated with modern quality criteria data and scored. Currently, a grade A recommendation should be a result of large, RCTs showing consistent, impressive benefits with few AEs and minimal inconvenience and cost [19].
Our aim was to explore the existing evidence following the best methodological boundaries, rather than to reach clinically relevant conclusions by applying sensitivity analyses and risk of bias assessments. Our search concluded that the existing evidence for the efficacy of flunarizine in migraine prevention is of very low quality, in contrast to modern preventives, which are additionally disease-specific and mechanism-based.
So far, all five trials yielded by our systematic search showed a significant reduction in migraine attacks over placebo in the limited observational period that each study was conducted. Current international guidelines on migraine prevention trials recommend as primary outcome the change of migraine days from baseline and, alternatively, the change from baseline in moderate/severe headache days or 50% responder rate for the reduction of migraine days [32]. The outcome measured in the referred trials included reduction in monthly migraine attacks, duration, and severity of migraine attacks. Therefore, the recommended outcome measures [32] could not be analyzed in this reappraisal study. Additionally, the study populations in all trials were very small, which raises questions regarding the strength of study results. Available studies had an additional number of limitations [21–25]: a) none of the studies mentioned sample size calculation, b) lack of detailed methodology description (inclusion and exclusion criteria, definition of migraine with or without aura, statistical analysis plan, and randomization methods), c) active treatment periods were short and varied between 12–16 weeks, d) details regarding baseline observation period were lacking, e) blinding procedure was not described in most of them, f) drop-out rates and reasons, except withdrawals due to AEs were not clearly stated, g) comorbidity of other primary headaches.
We could only analyze the AEs leading to discontinuation. Day-time sedation and weight gain were the most commonly reported AEs of flunarizine. We found a significant difference between placebo and 10 mg flunarizine, regarding comparison of AEs leading to discontinuation, which is in line with experts’ clinical experience. Accordingly, flunarizine seems to be a safe and well-tolerated drug. On the other hand, our findings should be interpreted cautiously, as I2 value had an absolute homogeneity (I2 = 0%) (Fig. 4), which may also be criticized to show the low validity of this latter finding. Additionally, one study reports no AEs in total [23], which could be due to poor and incomplete recording during the trial and/or the small numbers of participants (n = 9).
According to the GRADE approach the certainty of evidence is rated high for the outcome of AEs leading to discontinuation (Fig. 4). As mentioned, there is a noted mismatch in the design of these older studies compared to current international guidelines for controlled drug trials in migraine. There is also a variance in the migraine definition and classification criteria used among the flunarizine trials. This issue is classified as "indirectness", which pertains to the difference between the queries addressed in individual studies and the question that the systematic review aims to answer.
As per the GRADE guidance, we could downgrade the rating for indirectness if the effect reported in the studies did not accurately represent the effect concerning our question. Despite these issues, we did not downgrade the rating since the safety and tolerability of drugs is unlikely to be affected by diagnostic criteria. Nevertheless, we considered downgrading the certainty of evidence to moderate due to imprecision, based on the Minimum Important Difference (MID). Since the effect estimate meets the MID, we are rating the certainty that flunarizine increases the proportion of patients that experience AEs leading to discontinuation. Nevertheless, the confidence interval crosses the MID, which is indicative for imprecision. Hence, we conclude that flunarizine probably increases the proportion of patients that experience AEs leading to discontinuation.
Furthermore, results from post-marketing cohort studies regarding discontinuation due to AEs are provided. One study including 838 participants (aged 7–93 years) treated with flunarizine 5–20 mg (> 60% in 10 mg flunarizine group) up to 8 months, reports a percentage of 6% of subjects who discontinued due to AEs [33]. In another study, which was published in a Journal supplement only 14 out of 1435 participants, treated up to 6 months, with a follow-up period of an additional 6 months, discontinued due to AEs [34]. Overall, the most common adverse events reported were weight gain, fatigue and drowsiness.
Two head-to-head studies comparing flunarizine with propranolol showed an almost equal efficacy and tolerability profile [35, 36]. One study of 808 subjects treated for 16 weeks reported that flunarizine 10 mg was at least as effective as 160 mg propranolol concerning all evaluated parameters, and both drugs were well tolerated [35]. Another double-blind study comparing flunarizine 10 mg and propranolol 160 mg in more than 400 patients diagnosed with “classical migraine”, reported similar efficacy results (number, duration, and severity of attacks) [36]. Sedation or fatigue, gastric pain, vertigo and nausea were reported as the most important AEs in both groups [36].
Flunarizine could be beneficial as a treatment choice in relation to specific comorbidities. Considering its inhibitory effects on the calcium-related contraction of vascular smooth muscle, flunarizine may be a good option for prophylaxis in migraine individuals with cardiovascular diseases such as arrythmias [37]. Additionally, the vestibular depressive effect of this drug was also demonstrated in animals and humans [11], and recent trials reported the benefit of flunarizine on vestibular symptoms in patients with vestibular migraine [38, 39].
Conclusions
Five trials of flunarizine as prophylactic agent yielded by our systematic search showed a significant reduction in migraine attacks over placebo in limited observational periods, in small population groups and selected outcome parameters. Based on these results, flunarizine has been used as a first or second line prophylactic treatment for migraine in many countries. The present critical and systematic reappraisal of these trials, in accordance with current international guidelines for controlled trials on migraine prevention reveals an insufficiency of data to support the attribution of a Level A evidence for efficacy. The introduction of novel migraine-specific drugs with good evidence of efficacy and excellent tolerability challenges the role of traditional oral preventatives such as flunarizine. Further large-scale studies, head-to-head trials or real-world evidence would be useful to support the role and efficacy of flunarizine in migraine prevention.
Supplementary Information
Acknowledgements
Figure 1 was designed using Servier Medical Art by Servier (smart.servier.com), licensed under a Creative Commons Attribution 3.0 Unported License. We also thank Sarah Kirsh, Muizz Hussain, and Jordan Pepper for their valuable contribution in the statistical analysis.
Abbreviations
- Aes
Adverse events
- Ca2+
Calcium cations
- CGRP
Calcitonin gene-related peptide
- FDA
Food and Drug Administration
- COSMIG
Outcome Set for preventive intervention trials in chronic and episodic migraine
- CSD
Cortical spreading depression
- PRISMA
Preferred Reporting Items for Systematic Reviews
- MMDs
Monthly migraine days
- RCT
Randomised Controlled Trial
Authors’ contributions
CD, EE, and DU initiated the review drafting and revision of the article. CL, AMvdB, RGG, MSdR, UR, JV, TdV, RO and SS contributed with a critical review of the article. Statistical analyses were performed by DZ and MH. EE, AMvdB, TdV, and DZ prepared figures and tables. All authors read and approved the final manuscript.
Funding
This paper was funded by the European Headache Federation (EHF).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christina I. Deligianni, Esme Ekizoglu, Derya Uluduz, Christian Lampl, Antoinette MaassenVanDenBrink, Tessa de Vries, Dena Zeraatkar, and Simona Sacco contributed equally to this work.
References
- 1.Jansen I, Tfelt-Hansen P, Edvinsson L. Comparison of the calcium entry blockers nimodipine and flunarizine on human cerebral and temporal arteries: role in cerebrovascular disorders. Eur J Clin Pharmacol. 1991;40:7–15. doi: 10.1007/BF00315132. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JL, Cogbill E, Santana-Davila R, Eldredge C, Collier W, Gradall A, Sehgal N, Kuester J. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS ONE. 2015;10:e0130733. doi: 10.1371/journal.pone.0130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayajiki K, Okamura T, Toda N. Flunarizine, an anti-migraine agent, impairs nitroxidergic nerve function in cerebral arteries. Eur J Pharmacol. 1997;329:49–53. doi: 10.1016/S0014-2999(97)10103-0. [DOI] [PubMed] [Google Scholar]
- 4.Shimazawa M, Hara H, Watano T, Sukamoto T. Effects of Ca2+ channel blockers on cortical hypoperfusion and expression of c-Fos-like immunoreactivity after cortical spreading depression in rats. Br J Pharmacol. 1995;115:1359–1368. doi: 10.1111/j.1476-5381.1995.tb16624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wauquier A, Ashton D, Marrannes R. The effects of flunarizine in experimental models related to the pathogenesis of migraine. Cephalalgia. 1985;5(2):119–123. doi: 10.1177/03331024850050S222. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosio C, Stefanini E. Interaction of flunarizine with dopamine D2 and D1 receptors. Eur J Pharmacol. 1991;197:221–223. doi: 10.1016/0014-2999(91)90526-V. [DOI] [PubMed] [Google Scholar]
- 7.Taylor JE, Defeudis FV. Interactions of verapamil, D 600, flunarizine and nifedipine with cerebral histamine-receptors. Neurochem Int. 1986;9:379–381. doi: 10.1016/0197-0186(86)90079-3. [DOI] [PubMed] [Google Scholar]
- 8.Shukla R, Garg RK, Nag D, Ahuja RC. Nifedipine in migraine and tension headache: a randomised double blind crossover study. J Assoc Physicians India. 1995;43(11):770–772. [PubMed] [Google Scholar]
- 9.Popova J, Staneva-Stoytcheva D, Ivanova E, Tosheva T. The long-term treatment with the Ca(2+)-antagonists nifedipine, verapamil, flunarizine and with the calmodulin antagonist trifluoperazine decreases the activity of 5-HT1 receptors in rat cerebral cortex and hippocampus. Gen Pharmacol. 1991;22:1147–1149. doi: 10.1016/0306-3623(91)90593-U. [DOI] [PubMed] [Google Scholar]
- 10.Peroutka SJ. Antimigraine drug interactions with serotonin receptor subtypes in human brain. Ann Neurol. 1988;23:500–504. doi: 10.1002/ana.410230512. [DOI] [PubMed] [Google Scholar]
- 11.Holmes B, Brogden RN, Heel RC, Speight TC, Avery GS. Flunarizine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use. Drugs. 1984;27:6–44. doi: 10.2165/00003495-198427010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Amery WK, Caers LI, Aerts TJ. Flunarizine, a calcium entry blocker in migraine prophylaxis. Headache. 1985;25:249–254. doi: 10.1111/j.1526-4610.1985.hed2505249.x. [DOI] [PubMed] [Google Scholar]
- 13.Evers S, Afra J, Frse A, Goadsby PJ, Linde M, May A, Sándor PS. EFNS guideline on the drug treatment of migraine–revised report of an EFNS task force. Eur J Neurol. 2009;16:968–981. doi: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 15.Lampl C, Jan Versijpt J, Amin FM, Deligianni CI, Raquel Gil-Gouveia R, Jassal T, et al. European Headache Federation (EHF) critical re-appraisal and meta-analysis of oral drugs in migraine prevention—part 1: amitriptyline. J Headache Pain. 2023;24:39. doi: 10.1186/s10194-023-01573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haywood K, Potter R, Froud R, Pearce G, Box B, Muldoon L, et al. Core outcome set for preventive intervention trials in chronic and episodic migraine (COSMIG): an international, consensus-derived and multistakeholder initiative. BMJ Open. 2021;11(11):e043242. doi: 10.1136/bmjopen-2020-043242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitre T, Mah J, Helmeczi W, Khalid MF, Cui S, Zhang M, et al. Medical treatments for idiopathic pulmonary fibrosis: a systematic review and network meta-analysis. Thorax. 2022;77:1243–1250. doi: 10.1136/thoraxjnl-2021-217976. [DOI] [PubMed] [Google Scholar]
- 18.Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Louis PA. Double-blind Placebo-controlled Prophylactic Study of Flunarizine (Sibelium) in Migraine. Headache. 1981;21:235–239. doi: 10.1111/j.1526-4610.1981.hed2106235.x. [DOI] [PubMed] [Google Scholar]
- 22.Frenken CWGM, Nuijten STM. Flunarizine, a new preventive approach to migraine. A double-blind comparison with placebo. Clin Neurol Neurosurg. 1984;86:17–20. doi: 10.1016/0303-8467(84)90273-7. [DOI] [PubMed] [Google Scholar]
- 23.Mendenopoulos G, Manafi Th, Logothetis I, Bostantjopoulou S. Flunarizine in the prevention of classical migraine: a placebo-controlled evaluation. Cephalalgia. 1985;5:31–37. doi: 10.1046/j.1468-2982.1985.0501031.x. [DOI] [PubMed] [Google Scholar]
- 24.Sørensen PS, Hansen K, Olesen J. A placebo-controlled, double-blind, cross-over trial of flunarizine in common migraine. Cephalalgia. 1986;6:7–14. doi: 10.1046/j.1468-2982.1986.0601007.x. [DOI] [PubMed] [Google Scholar]
- 25.Freitag FG, Diamond S, Diamond M. A placebo controlled trial of flunarizine in migraine prophylaxis. Cephalalgia 11(suppl 11): 157–158.
- 26.International Headache Society Committee on Clinical Trials in Migraine [No authors listed] (1991) Guidelines for controlled trials of drugs in migraine. First edition Cephalalgia 11:1–12. https://www.ncbi.nlm.nih.gov/books/NBK45458. [PubMed]
- 27.AdHoc and Committee Classification of Headache. JAMA. 1962;179:717–718. [Google Scholar]
- 28.Olesen J, Krabbe AÆ, Tfelt-Hansen P. Methodological aspects of prophylactic drug trials in migraine. Cephalalgia. 1981;1:127–141. doi: 10.1046/j.1468-2982.1981.0103127.x. [DOI] [PubMed] [Google Scholar]
- 29.Headache Classification Committee of the International Headache Society. [No authors listed] Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(7):1–96. [PubMed] [Google Scholar]
- 30.Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, Sándor PS. European Federation of Neurological Societies. EFNS guideline on the drug treatment of migraine--revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–81. 10.1111/j.1468-1331.2009.02748.x. PMID: 19708964. [DOI] [PubMed]
- 31.Stubberuda A, Flaaena NM, McCrory DC, Pedersene SA, Linde M. Flunarizine as prophylaxis for episodic migraine: a systematic review with meta-analysis. Pain. 2019;160:762–772. doi: 10.1097/j.pain.0000000000001456. [DOI] [PubMed] [Google Scholar]
- 32.Diener HC, Tassorelli C, Dodick DW, Silberstein SD, Lipton RB, Ashina M, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of migraine attacks in episodic migraine in adults. International Headache Society Clinical Trials Committee. Cephalalgia. 2020;40:1026–1044. doi: 10.1177/0333102420941839. [DOI] [PubMed] [Google Scholar]
- 33.Verspeelt J, De Locht P, Amery WK. Post-marketing cohort study comparing the safety and efficacy of flunarizine and propranolol in the prophylaxis of migraine. Cephalalgia. 1996;16(5):328–36. doi: 10.1046/j.1468-2982.1996.1605328.x. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Lage JM. Flunarizine (Sibelium) in the prophylaxis of migraine. An open, long-term, multicenter trial. Cephalalgia. 1988;8(8):15–20. doi: 10.1177/03331024880080S804. [DOI] [PubMed] [Google Scholar]
- 35.Diener HC, Matias-Guiu J, Hartung E, Pfaffenrath V, Ludin HP, Nappi G, De Beukelaar F. Efficacy and tolerability in migraine prophylaxis of flunarizine in reduced doses: a comparison with propranolol 160 mg daily. Cephalalgia. 2002;22:209–221. doi: 10.1046/j.1468-2982.2002.t01-1-00309.x. [DOI] [PubMed] [Google Scholar]
- 36.Lücking CH, Oestreich W, Schmidt R, Soyka D. Flunarizine vs. propranolol in the prophylaxis of migraine: two double-blind comparative studies in more than 400 patients. Cephalalgia. 1988;8(8):21–26. doi: 10.1177/03331024880080S805. [DOI] [PubMed] [Google Scholar]
- 37.Billman GE. The antiarrhythmic and antifibrillatory effects of calcium antagonists. J Cardiovasc Pharmacol. 1991;18(10):S107–S117. doi: 10.1097/00005344-199118101-00019. [DOI] [PubMed] [Google Scholar]
- 38.Liu F, Ma T, Che X, Wang Q. Yu S (2017) The Efficacy of Venlafaxine, Flunarizine, and Valproic Acid in the Prophylaxis of Vestibular Migraine. Front Neurol. 2017;8:524. doi: 10.3389/fneur.2017.00524.eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lepcha A, Amalanathan S, Augustine AM, Tyagi AK, Balraj A. Flunarizine in the prophylaxis of migrainous vertigo: A randomized controlled trial. Eur Arch Otorhinolaryngol. 2014;271:2931–2936. doi: 10.1007/s00405-013-2786-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.