ABSTRACT
Importance
Preserving skin health is crucial for atopic dermatitis control as well as for the thriving of children. However, a well‐developed and validated tool that measures the knowledge, attitude, and practice of skin care is lacking.
Objective
To develop and validate the atopic dermatitis and infant skincare knowledge, attitude, and practice (ADISKAP 1.0) scale that measures parental health literacy on atopic dermatitis and skin care.
Methods
We conducted a review of the literature, a focus group (two dermatologists and 12 parents), and a panel discussion in order to generate the ADISKAP prototype. Two samples of parents with knowingly superior (dermatologists, n = 59) and inferior (general population, n = 395) knowledge traits participated in the validation of ADISKAP. Cronbach's alpha was reported as a measure of internal consistency, and the intraclass correlation coefficient (ICC) was calculated to assess the test‐retest validity. The known‐groups technique was used to evaluate construct validity.
Results
The ADISKAP scale contained 17 items after content and face validity validation. After removing items that displayed poor test‐retest reliability (n = 4) and construct validity (n = 3), 12 items were retained in the ADISKAP 1.0.
Interpretation
ADISKAP 1.0 is a reliable and valid tool for assessing parental knowledge, attitude, and practice on infantile atopic dermatitis and skin care.
Keywords: Atopic dermatitis, Attitude, Health literacy, Knowledge, Practice, Scale, Skin care
Atopic dermatitis and infant skincare knowledge, attitude, and practice (ADISKAP 1.0) is a reliable and valid scale for assessing parental health literacy on atopic dermatitis and skincare. This tool is a 12‐item scale that measures the knowledge, attitude, and practices of bathing, the use of emollients, and topical corticosteroid. ADISKAP was proven to be a reliable and valid tool that clearly separates two groups of knowingly superior and inferior knowledge traits. The use of ADISKAP can aid in the evaluation of parental health literacy and the implementation of educational campaigns.
INTRODUCTION
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease, affecting up to 38.71% of Chinese children in the infantile phase. 1 About 80% of cases develop AD in infancy or childhood. A significant health burden is inflicted by the severe pruritus, prolonged disease course, and compromised quality of life. Skin serves essential homeostatic functions, including (1) providing a barrier to water loss, light, and irritants, (2) infection control and immunosurveillance, (3) resilience to mechanical trauma, (4) sensation and tactile discrimination, (5) thermal regulation, and (6) acid mantle formation. 2 Structurally, infants have smaller keratinocytes, thinner epidermis, lower natural moisturizing factors and sebum levels, and underdeveloped immune resilience to Staphylococcus aureus. 3 , 4 , 5 These structural vulnerabilities render the skin of infants highly susceptible to AD. While the skin of infants is critical for the thriving of a child, suboptimal skin care knowledge, attitude, and practice (KAP) may compromise the integrity and function of the skin.
Skin care is a broad term that encompasses proper cleansing, the use of emollients, and the care of the skin under disease states, et al. Skincare was regarded as the cornerstone of AD treatment. It has been recommended that moisturizers be used liberally and frequently to minimize xerosis, which is a cardinal feature of AD. 6 Bathing is relevant in maintaining the skin barrier function. Studies have shown that prolonged exposure to water disrupts the stratum corneum intercellular lamellar bilayers and enhances skin permeability and susceptibility to irritants. 7 , 8 , 9 Consensus guidelines of Korea and the European Academy of Dermatology and Venereology endorsed short periods of bathing of 5–10 min and less than 5 min respectively. 10 , 11 Topical corticosteroids remain to be the first‐line therapy in AD during disease flares, however, fear of steroids can critically hurdle treatment adherence, resulting in persistent disease and escalation of treatment to systemic agents. 12 Topical corticosteroid phobia is a global issue, with the prevalence ranging from 31% to 95.7% across different countries. In the current study, we set out to quantify the KAP of caregivers anchoring on the use of emollients, bathing practices, and topical steroid phobia.
In 1998, therapeutic patient education was recognized as beneficial in improving chronic disease patient self‐efficacy. 13 Since then, scales on health literacy have been developed and validated, aiding the quantification of patient KAP. 14 , 15 Measuring health knowledge and attitude is crucial because it is the knowledge people acquire that leads to the development of an attitude, which in turn leads to practice changes. 16 A number of studies have explored disease‐specific patient and caregiver KAP. 17 , 18 , 19 However, skin care awareness under normal and pathological conditions has not been investigated. Despite growing evidence showing the unique functions and needs of infant skin, less is known about the translation of clinical research into the skincare KAP of parents. Moreover, there is no tool available for assessing parental knowledge competence of infant skin care. By measuring parents’ KAP, skin health campaigns, and educational efforts could be directed more specifically. In this study, we endeavored to develop and validate a new scaling instrument, the AD and infant skincare KAP (ADISKAP) scale, to measure the skin care literacy of parents with children aged 0–2 years.
METHODS
Ethical approval
This study was approved by the Ethics Committee of Beijing Children's Hospital (2019‐k‐270). All participants signed written informed consent.
Item generation and content validity
A literature review was first conducted by three dermatologists to generate focus group topics, which included bathing practices, emollient application, and topical corticosteroid phobia. Next, two experienced dermatologists held three focus groups, each consisting of four parents of children aged 0–2 years. Discussions were recorded and independently analyzed by two investigators (Mutong Zhao and Qiong Wu). Sun protection was added as the fourth topic during the focus group discussion. We then used the themes from the focus group and the literature review to form items of the prototype ADISKAP, resulting in a 20‐item scale ADISKAP‐20 with a five‐point Likert‐type rating scale (Sheet S1). To establish content validity, a panel of experts consisting of five dermatologists with attending or more advanced levels of competence, one general practitioner, and one epidemiologist then reevaluated the ADISKAP‐20. Face validity was established by seeking feedback from both parents (n = 13) and medical practitioners (pediatricians, n = 2; general practitioners, n = 3; obstetricians, n = 2).
Participants
A total of 417 parents, consecutively selected from the obstetric and pediatric clinic of a tertiary general hospital in Beijing (Capital Medical University Daxing Teaching Hospital), China, were approached, and 395 agreed to participate in the validation. To be included in the study, parents should fulfill the following inclusion criteria: parents of children aged 0–24 months old who were at the clinic for routine postnatal checkups or routine checkups of the child. They must be able to communicate in written and spoken Chinese. Either the mother or the father whoever is the primary caregiver, but not both, was eligible to be included. Written consent was obtained from all participants before the scales were sent out. An additional convenient sample of dermatologists (n = 59) from Beijing, who fulfill the aforementioned criteria, were invited to participate in the study to demonstrate construct validity.
Construct validity
The known‐groups method was employed to assess the construct validity. 20 Briefly, one group (i.e., dermatologists) with a knowingly superior KAP as could be measured by the scale under validation is compared to another group (i.e., the general population) with a knowingly inferior KAP, 20 and construct validity can be demonstrated by a successful separation of the two groups.
Test‐retest reliability
Participants were sent an electronic version of the ADISKAP‐17 scale four weeks after completing the initial assessment. And the intra‐class correlation coefficient (ICC) was calculated using data from the initial and repeated tests to assess the test‐retest reliability. Items with an ICC of < 0.5 indicate poor test‐retest reliability and should be excluded.
Statistical analysis
Data analyses were conducted using STATA 14.0 (Stata Statistical Software: College Station, TX: Stata Corp LP). Cronbach's α coefficient was calculated to assess the internal reliability of the scale. Coefficient scores of > 0.8 generally indicate good internal reliability. 21 Mann‐Whitney U‐test was used to compare scores from the two groups with knowingly superior (dermatologists) and inferior knowledge (general population). All tests were two‐tailed with a significance level set at P < 0.05.
RESULTS
Item generation
Figure 1 displays the flow of items through the developmental and validation stages of ADISKAP 1.0. A 20‐item prototype of ADISKAP was generated from the focus groups (Sheet S1). Four items on sun protection and one item on diaper rash were removed at the specialist panel discussion so that the instrument deals with the chosen topic of AD and related skin care. Three items were rephrased as they represent double‐barreled items. Double‐barreled items can convey two or more ideas, and an endorsement of the item might refer to either or both ideas and should thus be avoided. 20 An additional two items were rephrased (one at the panel discussion and one at the focus group) for clarity, which led to the ADISKAP‐17 (Sheet 1) being evaluated.
FIGURE 1.
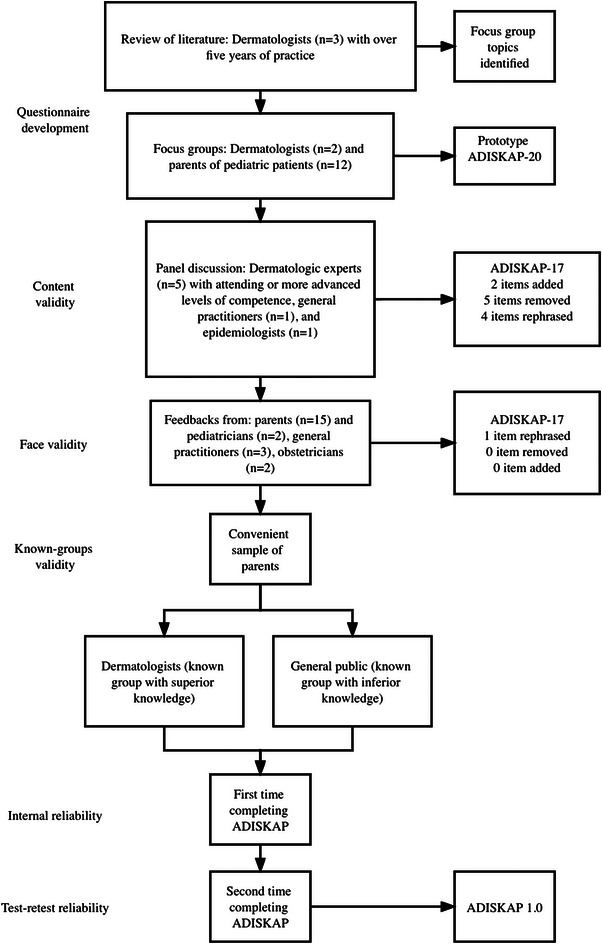
The flow of scale development and validation. ADISKAP, atopic dermatitis and infant skincare knowledge, attitude, and practice.
SHEET 1.
The 17‐item atopic dermatitis and infant skincare knowledge, attitude and practice scale (ADISKAP‐17)
| 1. I should apply body wash every time I bathe my baby. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 2. Moisturizer should be applied all over my baby's body. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 3. Moisturizer should be used no more than once a day. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 4. I only use moisturizer after I bathe my baby. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 5. Massage oil is an adequate supplement for baby moisturizer. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 6. Moisturizer is not necessary for my baby at summer time. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 7. Sweat is an irritant to the baby's skin. † | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 8. I'm concerned to use topical corticosteroids on my baby. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 9. Topical corticosteroids will make babies fat. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 10. Topical corticosteroids will induce premature puberty in babies. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 11. If I had to use topical corticosteroids on my baby, I'd be concerned that my baby will become addicted to the drug. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 12. If I had to use topical corticosteroids on my baby, I'd be concerned that my baby will become resistant to the drug. | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 13. When bathing my baby, I set the temperature at above 40°C. ‡ | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 14. My baby should be scrubbed regularly when bathing. ‡ | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 15. I dry my baby off naturally at a warm place after bathing. § | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 16. My baby's bath time should be kept within 10 minutes. ‡ , § | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
| 17. A moisturizer a baby uses should be fragrance‐free. ‡ , § , ¶ | ||||
| □Strongly Agree | □Agree | □Undecided | □Disagree | □Strongly Disagree |
Added at specialist panel discussion.
Removed due to poor test‐retest reliability.
Removed due to poor construct validity.
Added at face group discussion.
Test‐retest reliability and internal reliability
A total of 454 parents completed the scale at the initial assessment, and 228 completed the retest scale. The characteristics of the participants are presented in Table 1. The ICCs for each item are displayed in Table 2. Of the items included in the prototype of ADISKAP‐17, four were removed as they showed ICCs of less than 0.5, which indicated poor reliability. 22 The ADISKAP‐17 and the final 12‐item ADISKAP 1.0 had good test‐retest reliability with ICCs of 0.83 (95% CI, 0.79–0.87) and 0.83 (95% CI, 0.79–0.87), respectively.
TABLE 1.
Demographics of the participating parents
| Test‐retest reliability (n = 228) | Known groups validity (n = 454) | |||
|---|---|---|---|---|
| Items | Dermatologist (n = 48) | General public (n = 180) | Dermatologist (n = 59) | General public (n = 395) |
| Gender | ||||
| Male | 11 (22.9) | 24 (13.3) | 13 (22.0) | 53 (13.4) |
| Female | 37 (77.1) | 156 (86.7) | 46 (78.0) | 342 (85.6) |
| Age (years) | 31.8 ± 3.7 | 30.8 ± 4.3 | 31.0 ± 4.0 | 31.0 ± 4.5 |
| Education | ||||
| High school or lower | 0 (0.0) | 39 (21.7) | 0 (0.0) | 97 (24.6) |
| College | 3 (6.3) | 124 (68.9) | 4 (6.8) | 259 (65.6) |
| Graduate school or higher | 45 (93.8) | 17 (9.4) | 55 (93.2) | 39 (9.9) |
| Income (CNY per month) | ||||
| <5000 | 10 (20.8) | 62 (34.4) | 12 (20.3) | 133 (33.7) |
| 5000–9999 | 23 (47.9) | 91 (50.6) | 28 (47.5) | 201 (50.9) |
| 10 000–20 000 | 12 (25.0) | 24 (13.3) | 16 (27.1) | 52 (13.2) |
| >20 000 | 3 (6.3) | 3 (1.7) | 3 (5.1) | 9 (2.3) |
| History of atopic dermatitis of the child | 13 (27.1) | 60 (33.3) | 14 (23.7) | 128 (32.4) |
| Family history of atopic dermatitis | ||||
| First degree relatives | 11 (22.9) | 18 (10.0) | 14 (23.7) | 46 (11.7) |
| Other relatives | 3 (6.3) | 1 (0.6) | 4 (6.8) | 2 (0.5) |
| None | 34 (70.8) | 161 (89.4) | 41 (69.5) | 347 (87.9) |
Data are shown as mean ± standard deviation or n (%).
TABLE 2.
Intraclass correlation coefficient (ICC) and mean difference for atopic dermatitis and infant skincare knowledge, attitude, and practice (ADISKAP) test‐retest reliability
| Questionnaire item | ICC (95% CI) | P‐value | Mean difference (test‐retest) | SD |
|---|---|---|---|---|
| 1 | 0.69 (0.61–0.75) | <0.001 | −0.02 | 0.92 |
| 2 | 0.72 (0.64–0.79) | <0.001 | −0.01 | 0.95 |
| 3 | 0.63 (0.54–0.70) | <0.001 | −0.14 | 1.04 |
| 4 | 0.62 (0.53–0.69) | <0.001 | −0.04 | 0.96 |
| 5 | 0.64 (0.56–0.71) | <0.001 | −0.08 | 1.01 |
| 6 | 0.56 (0.47–0.65) | <0.001 | 0.10 | 0.92 |
| 7 | 0.61 (0.49–0.70) | <0.001 | −0.04 | 1.20 |
| 8 | 0.66 (0.58–0.73) | <0.001 | 0.05 | 1.09 |
| 9 | 0.62 (0.53–0.69) | <0.001 | 0.14 | 1.10 |
| 10 | 0.59 (0.50–0.67) | <0.001 | −0.04 | 1.24 |
| 11 | 0.68 (0.60–0.74) | <0.001 | 0.08 | 1.09 |
| 12 | 0.64 (0.56–0.72) | <0.001 | −0.11 | 1.13 |
| 13† | 0.38 (0.27–0.49) | <0.001 | 0.00 | 1.26 |
| 14† | 0.48 (0.37–0.57) | <0.001 | 0.06 | 1.28 |
| 15 | 0.56 (0.46–0.64) | <0.001 | 0.01 | 1.18 |
| 16† | 0.46 (0.33–0.57) | <0.001 | 0.03 | 0.84 |
| 17 † | 0.47 (0.34–0.58) | <0.001 | −0.09 | 1.17 |
| ADISKAP‐17 | 0.83 (0.79–0.87) | <0.001 | −0.05 | 6.45 |
| ADISKAP 1.0 | 0.83 (0.79–0.87) | <0.001 | −0.25 | 5.17 |
Abbreviations: CI, confidence interval; SD, standard deviation.
Removed due to poor test‐retest reliability.
Internal reliability was established using data from the initial assessment (n = 454). The ADISKAP‐17 and the final ADISKAP 1.0 (12 items) displayed acceptable internal reliability with Cronbach's α of 0.78 and 0.79, respectively. 21
Construct validity
We employed the known‐groups method to evaluate construct validity. Data from dermatologists (n = 59) were compared with parents representing the general population (n = 395) using the initial assessment. The known superior group scored significantly higher than the known inferior group in 14 items (Table 3). Three items were removed as they failed to separate the two groups.
TABLE 3.
Participant responses and construct validity of atopic dermatitis and infant skincare knowledge, attitude, and practice (ADISKAP)‐17 using the known‐groups method
| Questionnaire item | Score | Dermatologist (known group with superior knowledge, n = 59) | General public (known group with inferior knowledge, n = 395) | P‐value |
|---|---|---|---|---|
| 1 | 1 | 2 (3.39) | 17 (4.30) | 0.002 |
| 2 | 3 (5.08) | 61 (15.44) | ||
| 3 | 3 (5.08) | 61 (15.44) | ||
| 4 | 22 (37.29) | 124 (31.39) | ||
| 5 | 29 (49.15) | 132 (33.42) | ||
| 2 | 1 | 2 (3.39) | 27 (6.84) | <0.001 |
| 2 | 0 (0.00) | 86 (21.77) | ||
| 3 | 1 (1.69) | 76 (19.24) | ||
| 4 | 10 (16.95) | 128 (32.41) | ||
| 5 | 46 (77.97) | 78 (19.75) | ||
| 3 | 1 | 1 (1.69) | 55 (13.92) | <0.001 |
| 2 | 4 (6.78) | 109 (27.59) | ||
| 3 | 5 (8.47) | 121 (30.63) | ||
| 4 | 23 (38.98) | 91 (23.04) | ||
| 5 | 26 (44.07) | 19 (4.81) | ||
| 4 | 1 | 1 (1.69) | 25 (6.33) | <0.001 |
| 2 | 0 (0.00) | 65 (16.46) | ||
| 3 | 0 (0.00) | 79 (20.00) | ||
| 4 | 26 (44.07) | 170 (43.04) | ||
| 5 | 32 (54.24) | 56 (14.18) | ||
| 5 | 1 | 1 (1.69) | 32 (8.10) | <0.001 |
| 2 | 3 (5.08) | 96 (24.30) | ||
| 3 | 9 (15.25) | 112 (28.35) | ||
| 4 | 22 (37.29) | 112 (28.35) | ||
| 5 | 24 (40.68) | 43 (10.89) | ||
| 6 | 1 | 0 (0.00) | 10 (2.53) | <0.001 |
| 2 | 3 (5.08) | 36 (9.11) | ||
| 3 | 3 (5.08) | 72 (18.23) | ||
| 4 | 17 (28.81) | 160 (40.51) | ||
| 5 | 36 (61.02) | 117 (29.62) | ||
| 7 | 1 | 5 (8.47) | 39 (9.87) | 0.004 |
| 2 | 5 (8.47) | 54 (13.67) | ||
| 3 | 6 (10.17) | 95 (24.5) | ||
| 4 | 17 (28.81) | 105 (26.58) | ||
| 5 | 26 (44.07) | 102 (25.82) | ||
| 8 | 1 | 1 (1.69) | 103 (26.08) | <0.001 |
| 2 | 3 (5.08) | 81 (20.51) | ||
| 3 | 14 (23.73) | 149 (37.72) | ||
| 4 | 12 (20.34) | 37 (9.37) | ||
| 5 | 29 (49.15) | 25 (6.33) | ||
| 9 | 1 | 1 (1.69) | 43 (10.89) | <0.001 |
| 2 | 1 (1.69) | 53 (13.42) | ||
| 3 | 3 (5.08) | 124 (31.39) | ||
| 4 | 9 (15.25) | 105 (26.58) | ||
| 5 | 45 (76.27) | 70 (17.72) | ||
| 10 | 1 | 1 (1.69) | 69 (17.47) | <0.001 |
| 2 | 1 (1.69) | 47 (11.9) | ||
| 3 | 4 (6.78) | 138 (34.94) | ||
| 4 | 14 (23.73) | 88 (22.28) | ||
| 5 | 39 (66.1) | 53 (13.42) | ||
| 11 | 1 | 1 (1.69) | 93 (23.54) | <0.001 |
| 2 | 2 (3.39) | 67 (16.96) | ||
| 3 | 7 (11.86) | 140 (35.44) | ||
| 4 | 16 (27.12) | 59 (14.94) | ||
| 5 | 33 (55.93) | 36 (9.11) | ||
| 12 | 1 | 1 (1.69) | 88 (22.28) | <0.001 |
| 2 | 3 (5.08) | 89 (22.53) | ||
| 3 | 9 (15.25) | 135 (34.18) | ||
| 4 | 16 (27.12) | 57 (14.43) | ||
| 5 | 30 (50.85) | 26 (6.58) | ||
| 13 | 1 | 2 (3.39) | 20 (5.06) | 0.005 |
| 2 | 1 (1.69) | 25 (6.33) | ||
| 3 | 1 (1.69) | 52 (13.16) | ||
| 4 | 14 (23.73) | 91 (23.04) | ||
| 5 | 41 (69.49) | 207 (52.41) | ||
| 14 | 1 | 1 (1.69) | 36 (9.11) | <0.001 |
| 2 | 3 (5.08) | 49 (12.41) | ||
| 3 | 4 (6.78) | 58 (14.68) | ||
| 4 | 9 (15.25) | 99 (25.06) | ||
| 5 | 42 (71.19) | 153 (38.73) | ||
| 15 † | 1 | 4 (6.78) | 34 (8.61) | 0.333 |
| 2 | 5 (8.47) | 49 (12.41) | ||
| 3 | 7 (11.86) | 54 (13.67) | ||
| 4 | 20 (33.9) | 118 (29.87) | ||
| 5 | 23 (38.98) | 140 (35.44) | ||
| 16 † | 1 | 0 (0.00) | 4 (1.01) | 0.830 |
| 2 | 4 (6.78) | 23 (5.82) | ||
| 3 | 9 (15.25) | 66 (16.71) | ||
| 4 | 20 (33.9) | 137 (34.68) | ||
| 5 | 26 (44.07) | 165 (41.77) | ||
| 17 † | 1 | 1 (1.69) | 13 (3.29) | 0.442 |
| 2 | 10 (16.95) | 40 (10.13) | ||
| 3 | 4 (6.78) | 62 (15.70) | ||
| 4 | 25 (42.37) | 117 (29.62) | ||
| 5 | 19 (32.20) | 163 (41.27) |
Data are shown as n (%).
Removed due to poor construct validity.
At this stage, a total of five items were removed from the ADISKAP‐17 due to either poor reliability or poor separation of the known groups or both, resulting in a 12‐item scale ADISKAP 1.0 (English and Chinese version, see Sheets S2 and S3 respectively). The respective scores of ADISKAP 1.0 items are depicted as a heatmap (Figure 2).
FIGURE 2.
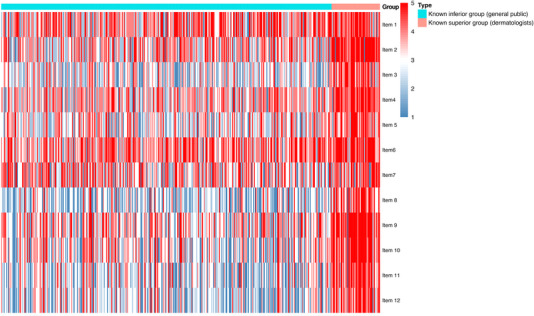
Heatmap of the knowledge, attitude, and practice traits of parents. The columns of the heatmap are ordered by groups.
DISCUSSION
The ADISKAP 1.0 (Sheet S2) is the first scale developed to measure parental KAP of AD and skin care for infants. We employed comprehensive procedures such as a literature review, focus groups, test‐retest reliability, and construct validity during the development and validation of the scale. The final ADISKAP 1.0 scale demonstrated acceptable internal reliability and good test‐retest reliability with a clear separation of the two groups with distinct KAP traits. This is crucial for identifying the target population of low AD skin health literacy for educational campaigns. Another strength of this study is the sampling of participants. By consecutively including all eligible parents, the resulting sample is regarded as more likely to represent the target population than simple convenience sampling. 23
There are a number of limitations. Firstly, although we had a large sample size, there lacks an adequate representation of parents from non‐Chinese ethnic origins. As KAP are developed within a cultural context, further validation is required to allow cultural adaptations. Secondly, this instrument is developed based on misconceptions of skin care practices of infants aged under 2 years old and among whom the validation process took place, thereby, generalizability to a broader age group is limited. Finally, further validation is required to establish criterion validity. Criterion validity refers to the extent to which a measure is related to an outcome. Data collection for assessing the ability of the ADISKAP 1.0 instrument to predict the development and quality of life of AD in parent‐child dyads is being undertaken by our team.
In conclusion, ADISKAP 1.0 is a novel tool designed to measure the AD and skin care KAP of parents to guide educational initiatives. ADISKAP 1.0 was developed following rigorous procedures and was shown to be reliable and valid. Future endeavors could be directed at using this instrument to improve parental health literacy for pediatric AD patient education purposes.
CONFLICT OF INTEREST
Dr. Lin Ma is a member of Pediatric Investigation editorial board.
Supporting information
Supporting Information
Sun X, Zhao M, Wu Q, Tian J, Shen C, Liang Y, et al. Development and validation of the atopic dermatitis and infant skincare knowledge, attitude, and practice (ADISKAP 1.0) scale. Pediatr Investig. 2023;7:153–162. 10.1002/ped4.12374
Contributor Information
Lin Ma, Email: bch_maleen@aliyun.com.
Xiuhua Ma, Email: mxhdxqyy@126.com.
REFERENCES
- 1. Guo Y, Li P, Tang J, Han X, Zou X, Xu G, et al. Prevalence of atopic dermatitis in Chinese children aged 1‐7 ys. Sci Rep. 2016;6:29751. DOI: 10.1038/srep29751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Visscher MO, Adam R, Brink S, Odio M. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33:271‐280. DOI: 10.1016/j.clindermatol.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 3. Stamatas GN, Nikolovski J, Luedtke MA, Kollias N, Wiegand BC. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr Dermatol. 2010;27:125‐131. DOI: 10.1111/j.1525-1470.2009.00973.x [DOI] [PubMed] [Google Scholar]
- 4. Yuan C, Zou Y, Xueqiu Y, Shima K, Miyauchi Y, Naoe A, et al. Properties of skin in Chinese infants: developmental changes in ceramides and in protein secondary structure of the stratum corneum. Biomed Res Int. 2017;2017:3594629. DOI: 10.1155/2017/3594629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McAleer MA, Jakasa I, Raj N, O'Donell CPF, Lane ME, Rawlings AV, et al. Early‐life regional and temporal variation in filaggrin‐derived natural moisturizing factor, filaggrin‐processing enzyme activity, corneocyte phenotypes and plasmin activity: implications for atopic dermatitis. Br J Dermatol. 2018;179:431‐441. DOI: 10.1111/bjd.16691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116‐132. DOI: 10.1016/j.jaad.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warner RR, Stone KJ, Boissy YL. Hydration disrupts human stratum corneum ultrastructure. J Invest Dermatol. 2003;120:275‐284. DOI: 10.1046/j.1523-1747.2003.12046.x [DOI] [PubMed] [Google Scholar]
- 8. Tsai TF, Maibach HI. How irritant is water? An overview. Contact Dermatitis. 1999;41:311‐314. DOI: 10.1111/j.1600-0536.1999.tb06990.x [DOI] [PubMed] [Google Scholar]
- 9. Gittler JK, Wang JF, Orlow SJ. Bathing and associated treatments in atopic dermatitis. Am J Clin Dermatol. 2017;18:45‐57. DOI: 10.1007/s40257-016-0240-2 [DOI] [PubMed] [Google Scholar]
- 10. Kim JE, Kim HJ, Lew BL, Lee KH, Hong SP, Jang YH, et al. Consensus guidelines for the treatment of atopic dermatitis in Korea (Part I): general management and topical treatment. Ann Dermatol. 2015;27:563‐577. DOI: 10.5021/ad.2015.27.5.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wollenberg A, Barbarot S, Bieber T, Christen‐Zaech S, Deleuran M, Fink‐Wagner A, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657‐682. DOI: 10.1111/jdv.14891 [DOI] [PubMed] [Google Scholar]
- 12. Contento M, Cline A, Russo M. Steroid phobia: a review of prevalence, risk factors, and interventions. Am J Clin Dermatol. 2021;22:837‐851. DOI: 10.1007/s40257-021-00623-6 [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization . Regional Office for Europe. Therapeutic patient education: continuing education programmes for health care providers in the field of prevention of chronic diseases: report of a WHO working group. Accessed January 12, 2023. https://apps.who.int/iris/handle/10665/108151
- 14. Holley S, Knibb R, Latter S, Liossi C, Mitchell F, Radley R, et al. Development and validation of the Adolescent Asthma Self‐Efficacy Questionnaire (AASEQ). Eur Respir J. 2019;54:1801375. DOI: 10.1183/13993003.01375-2018 [DOI] [PubMed] [Google Scholar]
- 15. Simon AE, Juszczyk D, Smyth N, Power E, Hiom S, Peake MD, et al. Knowledge of lung cancer symptoms and risk factors in the U.K.: development of a measure and results from a population‐based survey. Thorax. 2012;67:426‐432. DOI: 10.1136/thoraxjnl-2011-200898 [DOI] [PubMed] [Google Scholar]
- 16. Bettinghaus EP. Health promotion and the knowledge‐attitude‐behavior continuum. Prev Med. 1986;15:475‐491. DOI: 10.1016/0091-7435(86)90025-3 [DOI] [PubMed] [Google Scholar]
- 17. Ersser SJ, Farasat H, Jackson K, Gardiner E, Sheppard ZA, Cowdell F. Parental self‐efficacy and the management of childhood atopic eczema: development and testing of a new clinical outcome measure. Br J Dermatol. 2015;173:1479‐1485. DOI: 10.1111/bjd.14175 [DOI] [PubMed] [Google Scholar]
- 18. Cheng NS, Chau JPC, Hon KLE, Chow CM, Choi KC, Lo SHS, et al. Translation and validation of a Chinese version of the parental self‐efficacy with eczema care index. Dermatology. 2020;236:361‐368. DOI: 10.1159/000505450 [DOI] [PubMed] [Google Scholar]
- 19. Mitchell AE, Morawska A, Fraser JA, Sillar K. Child behaviour problems and childhood illness: development of the eczema behaviour checklist. Child Care Health Dev. 2017;43:67‐74. DOI: 10.1111/cch.12412 [DOI] [PubMed] [Google Scholar]
- 20. DeVellis RF. Scale Development: Theory and Applications. SAGE Publications; 2012. [Google Scholar]
- 21. Gliem JA, Gliem RR. Calculating, interpreting, and reporting Cronbach's Alpha reliability coefficient for Likert‐type scales. Accessed January 12, 2023. https://scholarworks.iupui.edu/bitstream/handle/1805/344/Gliem+&+Gliem.pdf?sequence=1
- 22. Koo TK, Li MY. A Guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155‐163. DOI: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence‐Based Medicine: How to Practice and Teach EBM. 2nd Ed. Churchill Livingstone; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


