Abstract
Objectives
Gingivitis is one of the most prevalent plaque-initiated dental diseases globally. It is challenging to maintain satisfactory plaque control without continuous professional advice. Artificial intelligence may be used to provide automated visual plaque control advice based on intraoral photographs.
Methods
Frontal view intraoral photographs fulfilling selection criteria were collected. Along the gingival margin, the gingival conditions of individual sites were labelled as healthy, diseased, or questionable. Photographs were randomly assigned as training or validation datasets. Training datasets were input into a novel artificial intelligence system and its accuracy in detection of gingivitis including sensitivity, specificity, and mean intersection-over-union were analysed using validation dataset. The accuracy was reported according to STARD-2015 statement.
Results
A total of 567 intraoral photographs were collected and labelled, of which 80% were used for training and 20% for validation. Regarding training datasets, there were total 113,745,208 pixels with 9,270,413; 5,711,027; and 4,596,612 pixels were labelled as healthy, diseased, and questionable respectively. Regarding validation datasets, there were 28,319,607 pixels with 1,732,031; 1,866,104; and 1,116,493 pixels were labelled as healthy, diseased, and questionable, respectively. AI correctly predicted 1,114,623 healthy and 1,183,718 diseased pixels with sensitivity of 0.92 and specificity of 0.94. The mean intersection-over-union of the system was 0.60 and above the commonly accepted threshold of 0.50.
Conclusions
Artificial intelligence could identify specific sites with and without gingival inflammation, with high sensitivity and high specificity that are on par with visual examination by human dentist. This system may be used for monitoring of the effectiveness of patients’ plaque control.
Key words: Gingivitis; Periodontal diseases; Community dentistry; Deep learning; Neural networks, computer; Artificial intelligence
Introduction
Periodontal disease is a chronic inflammatory disease that affects the periodontium and is categorised into gingivitis and periodontitis with reversible and irreversible tissue damages, respectively.1,2 It is one of the most prominent oral diseases, accounting for a significant amount of global public health burden every year, as well as 21% of global productivity loss, equivalent to USD 38.85 billion.[3], [4], [5] The prevalence of periodontal disease is estimated to be more than 50% worldwide, and nearly one-third of them are severe cases, that is, clinical attachment loss of more than 5 mm and bone loss of more than 30%, according to the World Health Organization.3,[6], [7], [8]
Periodontal disease is caused by accumulation of plaque biofilm along the gingival margin, resulting in localised gingival inflammation and host responses.[9], [10], [11], [12], [13] An early stage of periodontal disease, gingivitis, may be reversed by removal of plaque, and the progress to later stages of periodontitis may be halted.14
Furthermore, the development of periodontal disease is not consistent amongst all teeth and sites, and site predilections, i.e., site-specific, have been observed.15,16 For proper self-care or professional care, understanding and evaluations of clinical signs of individual sites are crucial.17 The clinical signs of gingivitis are inflammation-related and are a result of host response to dental plaque. As inflammation occurs at the gingival margin, redness (ie, change in colour); swelling (ie, change in volume); and loss of stippling appearance as loss of gingival fiber attachment (ie, change in surface characteristics) are observed, due to increase in blood flow (redness) and leakage of tissue fluid from blood vessels into the tissues (swelling).18,19 These changes are generally assessed visually by dentists, and patients may not be aware of the disease progression due to its chronic nature and lack of acute symptoms.16,20,21
Effective self-care plaque control measures such as tooth brushing and interdental cleaning are keys to periodontal disease prevention and control.22 Studies have revealed that frequent dental appointments are expensive yet ineffective in achieving sustained satisfactory plaque control at specific sites despite significant resources being committed to motivate and reinforce patients’ oral hygiene and plaque control measures.3,23,24
Artificial intelligence (AI) may provide a solution to this persistent clinical problem. The application of AI in various areas of dentistry has been gaining traction amongst the dental communities in recent years under name of automated digital dentistry and Dentistry 4.0.25 There are many clinical applications of AI in dentistry, from analysis of 2D radiography to 3D crown reconstruction, and AI has been utilised in detection of gingivitis from intraoral photographs.[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37] However, according to a recent literature review, there seems to be a lack of agreement in assessing the accuracy of prediction of gingivitis by AI systems, though 0.90 or above is regarded as excellent diagnostic accuracy for a general test.30,38,39 For an AI system to be used clinically for predicting gingivitis, it should have high sensitivity, that is, report diseased for any site where there is gingivitis, and high specificity, that is, report healthy for any site where there is no gingivitis. These parameters are one of the commonly used medical and dental diagnostic performance metrics40 and are proposed to measure the accuracy of AI prediction in this study.41
There are several network architectures that are currently used to detect gingivitis from intraoral photographs with accuracy ranging from 0.47 to 0.83, with 1.00 as the highest accuracy.30,[35], [36], [37],34,42,43 The accuracy of any diagnostic system for clinical use should be as high as possible, and accuracy of 0.90 or above should be targeted for clinical use.38,39,44,45
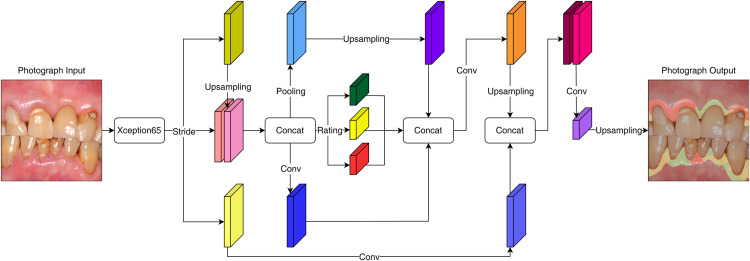
In this study, DeepLabv3+ built on Keras (v2.12, Google LLC) with TensorFlow 2 (v2.9, Google LLC) was adopted. This neural network was highly transferable and offered multiple pretrained checkpoints to facilitate learning of the datasets (Figure 1).[46], [47], [48], [49] Xception (v1.0, Google LLC) and MobileNetV2 (v1.0, Google LLC) were adopted as the backbone. Xception models used depth-wise separable convolutions with fewer connections and lighter model (ie, faster), and MobileNet models utilised the same convolutions with smaller model size and complexity, making it easier to construct.50,51
Fig. 1.
Illustration of architecture of DeepLabv3+ neural networks in this study.
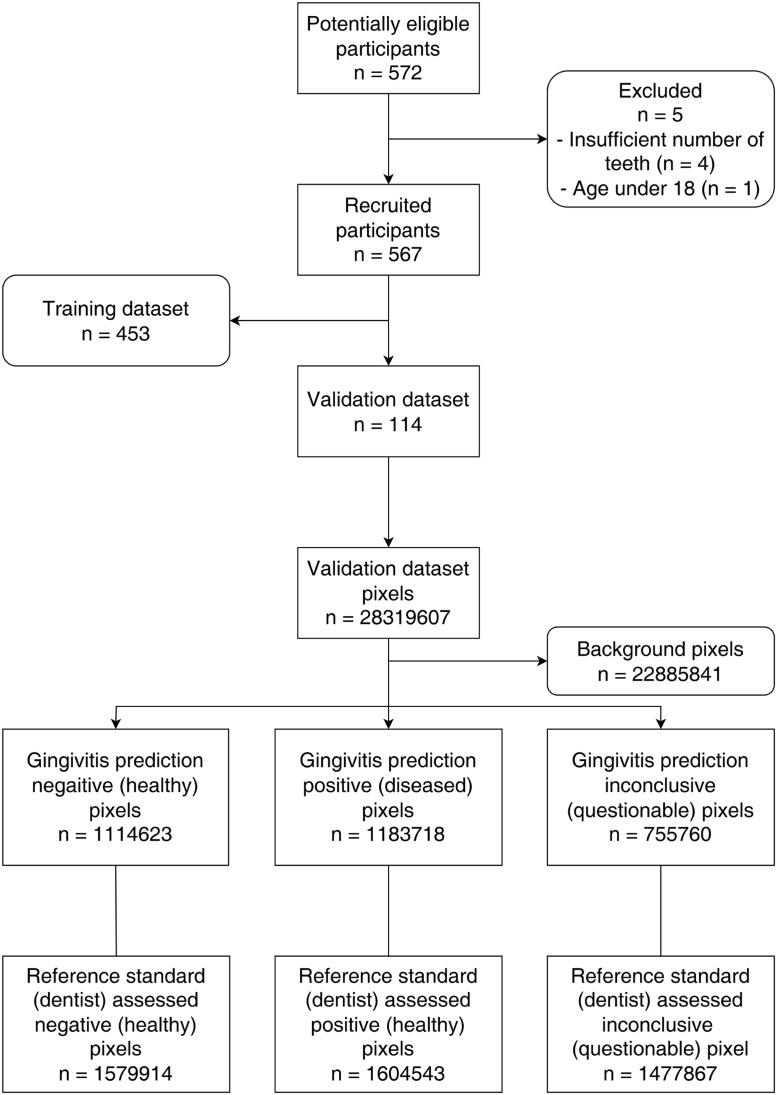
The objective of this study was to develop and to validate a novel AI system that can be used to diagnose gingivitis on intraoral photographs with accuracy at or above 0.90. The hypothesis of this study was that a novel AI system built with DeepLabv3+, after training with adequate number of intraoral photographs, would be able to predict the gingival health status with accuracy, in terms of sensitivity and specificity, at or above 0.90. This study was reported in format according to the Standards for Reporting Diagnostic Accuracy (STARD) 2015 statement (Figure 2).52
Fig. 2.
STARD-2015 flow diagram of this study.
Methods
This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB), Hong Kong Special Administrative Region, China (reference numbers: UW 20-230 and UW 21-447), and the Research, Ethics/Safety Sub-Committee (RESS) of Hong Kong Chu Hai College, Hong Kong Special Administrative Region, China (reference number: RESS/2022/06/006). This study was a prospective study, and data collection was planned before the execution of this study.
Data collection
Consecutive participants were recruited amongst patients attending the Comprehensive Dental Clinic of the University Dental Hospital from 2020 to 2022 according to the selection criteria (Table 1). Informed consent was obtained from all participants. Frontal-view intraoral photographs were taken using a digital single lens reflex (SLR) camera (EOS 700D, Canon) with a macro lens (EF 100mm f/2.8, Canon) and a ring flash (Marco Ring Lite MR-14EX, Canon). The sample size used for training the AI system was based on a recent study on using AI to detect periodontitis, which featured around 450 training datasets.53
Table 1.
Inclusion and exclusion criteria of study participants.
| Inclusion criteria | Participants who are Chinese and aged 18 or older |
| Participants who are able to give consent | |
| Participants who have 5 or more anterior teeth | |
| Participants who have adequate mouth opening for visualisation of at least 3 mm gingival tissue from maxillary and mandibular gingival margins | |
| Participants who are able to attend dental appointment and hold still during taking intraoral photograph | |
| Exclusion criteria | Participants who have non-plaque-related oral mucosal diseases that preclude the use of mirror retractors |
Data preparation
The gingival conditions of the collected intraoral photographs were labelled by a calibrated assessor, who was a dentist and based on visual assessment on a computer monitor (P2419H 23.8” W-LED monitor, Dell). The areas of interest within each frontal photograph were the gingival margin and around 3 mm gingival tissues apical to the margin. These areas were classified into 1 of 3 categories: healthy, diseased, or questionable, based on a screening instrument, Oral Health Assessment Tools (OHATs),[54], [55], [56] where the definitions were as follows:
-
-
Healthy: pink, smooth, no bleeding
-
-
Questionable: red, rough, swollen
-
-
Diseased: white/red patches, generalised redness, ulcers, swollen, bleeding
Unlabelled areas were classified in the system as background, making a total of 4 classifications. One week later, 10% of all photographs were labelled again by same assessor to measure the intra-assessor reliability in diagnosis of gingival conditions healthy, diseased, or questionable.57,58 The kappa value of the assessor was measured.
Around 450 photographs were randomly designated as training datasets by randomisation table, and the rest of the photographs were designated as validation datasets.
Photographs of the training datasets were augmented by cropping, rotating, or flipping randomly to enhance the training quality.59
Training and validation
Photographs from the training datasets were input into the AI system for training. After training, the AI system was then instructed to diagnose the gingival status of intraoral photographs of the validation datasets. Both the training and validation processes were performed on a Linux system powered by a graphic card of NVIDIA GeForce RTX 3090. The batch number was set as 4, which is the number of classifications, and the number of training iterations was set to be 30,000, a common iteration number to train 2-dimension AI systems.60,61
Measurements
The performance of the AI system was measured by true-positive rate, true-negative rate, false-positive rate, and false-negative rate. True-positive rate was the outcome where the AI correctly detected the diseased status, and true-negative rate was the outcome where the AI correctly detected the healthy status. False-positive rate and false-negative rate were the outcomes where AI treated healthy sites as diseased and diseased sites as healthy, respectively. Sensitivity and specificity were calculated based on the following formula:
Mean intersection-over-union, a ratio of true predictions (positive and negative) against the ground truth (actual health status), was a wide-adapted performance metric for segmentation models in field of artificial intelligence and was calculated by dividing the sum of 4 intersection-over-unions of healthy, diseased, questionable, and background by 4.62 Intersection-over-union of each category was calculated by the following formula:
where α was the dataset of diagnosis by dentist and β was the dataset of prediction by the AI system. In mathematics, the symbol ∪ represents the union of 2 sets, whilst ∩ represents the intersection of the sets.
Accuracy ranged from 0.00 to 1.00, and 1.00 was considered to be the maximum accuracy.63 The common threshold for acceptable prediction was 0.50.64
Results
In all, 572 potential participants were screened according to the study criteria. Four were rejected due to insufficient number of anterior teeth, and one was rejected due to age younger than 18 years. The number of recruited study participants was 567.
A total of 567 frontal-view intraoral photographs were taken from the study participants. Amongst the collected photographs, around 80% of the total (n = 453) were designated as training datasets, and the rest (n = 114) were designated as validation datasets.
The training datasets consisted of 113,745,208 pixels in total, with 9,270,413; 5,711,027; and 4,596,612 pixels labelled as healthy, diseased, and questionable, respectively. The validation datasets consisted of 28,319,607 pixels in total, with 1,579,914; 1,604,543; and 1,477,867 pixels labelled as healthy, diseased, and questionable, respectively. The assessor had a kappa value 0.92 over 2 attempts of labelling, which indicated high reliability.
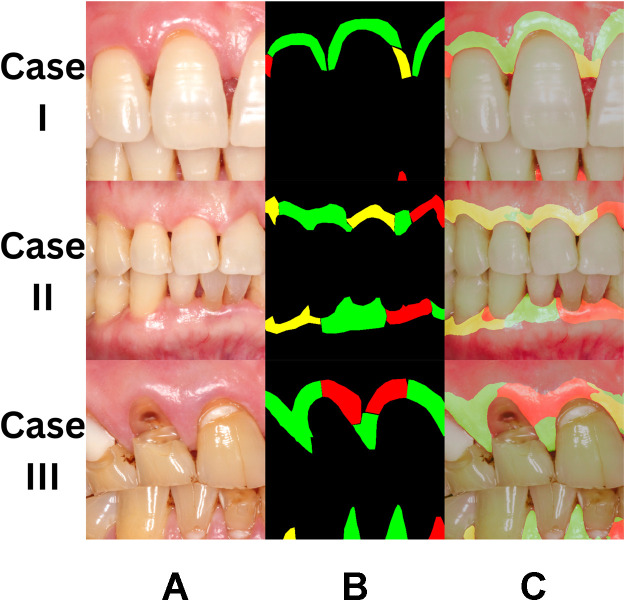
The AI system was then validated using intraoral photographs from the validation datasets, and results are presented in Figure 3. AI correctly predicted 1,114,623 healthy and 1,183,718 diseased pixels (Table 2), with a sensitivity of 0.92 and a specificity of 0.94. The mean intersection-over-union was 0.60.
Fig. 3.
Selected detection results of the validation set using the adopted segmentation model. A, Input intraoral photograph. B, Ground truth (health status) labelled by calibrated dentist. C, Detection results: green = healthy, red = diseased, yellow = questionable.
Table 2.
Predictions of the AI system compared to the diagnosis of a calibrated dentist.
| Predictions of the AI system (pixels) |
|||||
|---|---|---|---|---|---|
| Predicted as healthy | Predicted as diseased | Predicted as questionable | Predicted as background | ||
| Diagnosis of dentist (pixels) | Diagnosed healthy | 1,114,623 | 72,048 | 140,694 | 252,549 |
| Diagnosed diseased | 93,017 | 1,183,718 | 76,597 | 251,211 | |
| Diagnosed questionable | 248,694 | 258,035 | 755,760 | 215,378 | |
| Background | 275,697 | 352,303 | 143,442 | 22,885,841 | |
Discussion
The results of this study supported the hypothesis that a novel AI system built with DeepLabv3+, after training with adequate number of intraoral photographs, would be able to predict the gingival health status with accuracy, in terms of sensitivity and specificity, at or above 0.90. The novel AI system was able to identify specific sites with and without gingival inflammation with sensitivity and specificity that were almost on par with human dentists, which is one of the current methods used to detect gingival inflammation clinically.39,40,64 The result was encouraging and supported the use of AI in detection of gingivitis on intraoral photographs.
The AI system still had limitations and needed further development. Because the training was based on Chinese participants, the resulting system may work better on Chinese individuals compared with other ethnicities including White, Latino, and Black, though determining whether such a difference exists still needs further examination. Also, there was no evidence yet to suggest it would retain the same performance when it was applied to patients with various local and systemic modifying factors.14 Further studies into applications of this novel AI system in gingival inflammation detection would also be needed to further improve the accuracy of the system, with a goal of achieving superior performance as a periodontist. Moreover, clinical examination of the gingival conditions by probing might reduce the area with a questionable diagnosis and provide more robust gingival conditions for AI to learn. In addition, the performance of this system in clinical settings should be investigated with a longitudinal clinical trial design. Apart from the diagnosis of gingival conditions, the consistency of the outline of labeled areas should be addressed in the reliability test of the assessor.
When a population has a high prevalence of a particular disease such as gingivitis, it is expected that its diagnostic tests usually have high sensitivity, that is, a positive result when there is a disease, and low specificity, that is, a negative result when there is no disease. This is because it is easier for a diagnostic test to detect a disease when it has high prevalence and vice versa. However, gingivitis is a site-specific disease, and healthy sites may be found in patients with gingivitis. Therefore, similar numbers of healthy and diseased pixels as well as similar levels of sensitivity and specificity are found in this study.
With training datasets in larger quantities as well as in decreased diversity, the training outcomes may be further improved. However, room for improvement may be limited because the accuracy of this system was already above 0.90. Future studies would likely pave the way for applications of such AI systems in periodontology and, in a greater aspect, prevention and control of periodontal disease in communities.
Conclusions
AI is able to identify specific sites with and without gingival inflammation with high sensitivity and high specificity. Further investigation and training are required for possible improvements and clinical applications.
Conflict of interest
None disclosed.
Funding
The work described in this paper was fully supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (Grant number UGC/FDS13/E01/22).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.identj.2023.03.007.
Contributor Information
Richard Tai-Chiu Hsung, Email: richardhsung@chuhai.edu.hk.
Walter Yu Hang Lam, Email: retlaw@hku.hk.
Appendix. Supplementary materials
REFERENCES
- 1.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89:S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 3.Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontology. 2000;60(1):15–39. doi: 10.1111/j.1600-0757.2011.00425.x. 2012. [DOI] [PubMed] [Google Scholar]
- 4.Jamison DT, Alleyne G. Disease control priorities in developing countries. Herndon, VA: World Bank Publications; 2006. [PubMed]
- 5.Righolt AJ, Jevdjevic M, Marcenes W, Listl S. Global-, regional-, and country-level economic impacts of dental diseases in 2015. J Dent Res. 2018;97(5):501–507. doi: 10.1177/0022034517750572. [DOI] [PubMed] [Google Scholar]
- 6.Oral health: prevention is key. Lancet 2009;373(9657):1.https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(08)61933-9/fulltext. [DOI] [PubMed]
- 7.Oral health. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/oral-health#:∼:text=Periodontal%20(gum)%20disease&text=Severe%20periodontal%20diseases%20are%20estimated,oral%20hygiene%20and%20tobacco%20use. Accessed 15 March 2022.
- 8.Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions – Introduction and key changes from the 1999 classification. J Periodontol. 2018;89(S1):S1–S8. doi: 10.1002/JPER.18-0157. [DOI] [PubMed] [Google Scholar]
- 9.Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim) 2017;11(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Fenesy KE. Mt Sinai J Med; 1998. Periodontal disease: an overview for physicians; pp. 362–369. 65(5–6) [PubMed] [Google Scholar]
- 11.Clarke JK. On the bacterial factor in the ætiology of dental caries. Br J Exp Pathol. 1924;5(3):141–147. [Google Scholar]
- 12.Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63(4 Suppl):322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 13.Liljemark WF, Bloomquist C. Human oral microbial ecology and dental caries and periodontal diseases. Crit Rev Oral Biol Med. 1996;7(2):180–198. doi: 10.1177/10454411960070020601. [DOI] [PubMed] [Google Scholar]
- 14.Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. 1930. [DOI] [PubMed] [Google Scholar]
- 15.Breen HJ, Johnson NW, Rogers PA. Site-specific attachment level change detected by physical probing in untreated chronic adult periodontitis: review of studies 1982-1997. J Periodontol. 1999;70(3):312–328. doi: 10.1902/jop.1999.70.3.312. [DOI] [PubMed] [Google Scholar]
- 16.Kinane DF. Causation and pathogenesis of periodontal disease. Periodontol. 2000;25(1):8–20. doi: 10.1034/j.1600-0757.2001.22250102.x. 2001. [DOI] [PubMed] [Google Scholar]
- 17.Beck JD, Philips K, Moss K, Divaris K, Morelli T, Offenbacher S. Advances in precision oral health. Periodontol. 2000;82(1):268–285. doi: 10.1111/prd.12314. [DOI] [PubMed] [Google Scholar]
- 18.Immune response, in Medical Encyclopedia, I. A.D.A.M., Editor. National Institutes of Health.https://medlineplus.gov/ency/article/000821.htm#:~:text=The%20immune%20response%20is%20how,that%20appear%20foreign%20and%20harmful.
- 19.Reddy MS, Geurs NC, Jeffcoat RL, Proskin H, Jeffcoat MK. Periodontal disease progression. J Periodontol. 2000;71(10):1583–1590. doi: 10.1902/jop.2000.71.10.1583. [DOI] [PubMed] [Google Scholar]
- 20.Croxson LJ. Periodontal awareness: the key to periodontal health. Int Dent J. 1993;43(2 Suppl 1):167–177. [PubMed] [Google Scholar]
- 21.Brown LJ, Löe H. Prevalence, extent, severity and progression of periodontal disease. Periodontol. 2000;(2):57–71. doi: 10.1111/j.1600-0757.1993.tb00220.x. 1993. [DOI] [PubMed] [Google Scholar]
- 22.Löe H. Oral hygiene in the prevention of caries and periodontal disease. Int Dent J. 2000;50(3):129–139. doi: 10.1111/j.1875-595x.2000.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 23.Watt RG, Marinho VC. Does oral health promotion improve oral hygiene and gingival health? Periodontol. 2000;37:35–47. doi: 10.1111/j.1600-0757.2004.03796.x. 2005. [DOI] [PubMed] [Google Scholar]
- 24.Watt RG, Petersen PE. Periodontal health through public health - the case for oral health promotion. Periodontol. 2000;60(1):147–155. doi: 10.1111/j.1600-0757.2011.00426.x. 2012. [DOI] [PubMed] [Google Scholar]
- 25.Schwendicke F, Samek W, Krois J. Artificial intelligence in dentistry: chances and challenges. J Dent Res. 2020;99(7):769–774. doi: 10.1177/0022034520915714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrzański LA, Dobrzański LB. Dentistry 4.0 concept in the design and manufacturing of prosthetic dental restorations. Processes. 2020;8(5):525. [Google Scholar]
- 27.Revilla-León M, Gómez-Polo M, Vyas S, Barmak AB, Özcan M, Att W, et al. Artificial intelligence applications in restorative dentistry: a systematic review. J Prosthet Dent. 2021 doi: 10.1016/j.prosdent.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Revilla-León M, Gómez-Polo M, Vyas S, Barmak AB, Galluci GO, Att W, et al. Artificial intelligence applications in implant dentistry: a systematic review. J Prosthet Dent. 2021 doi: 10.1016/j.prosdent.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Chau RCW, Chong M, Thu KM, et al. Artificial intelligence-designed single molar dental prostheses: a protocol of prospective experimental study. PLOS One. 2022;17(6) doi: 10.1371/journal.pone.0268535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Revilla-León M, Gómez-Polo M, Barmak AB, Inam W, Kan JYK, Kois JC, et al. Artificial intelligence models for diagnosing gingivitis and periodontal disease: a systematic review. J Prosthet Dent 2022. doi: 10.1016/j.prosdent.2022.01.026. [DOI] [PubMed]
- 31.Li W, Jiang X, Sun W, Wang SH, Liu C, Zhang X, et al. Gingivitis identification via multichannel gray-level co-occurrence matrix and particle swarm optimization neural network. Int J Imaging Syst Technol. 2020;30(2):401–411. [Google Scholar]
- 32.Rana A, Yauney G, Wong LC, Gupta O, Muftu A, Shah P. Automated segmentation of gingival diseases from oral images. 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT). p. 144–7. doi: 10.1109/HIC.2017.8227605. [DOI]
- 33.Yauney G, Rana A, Wong LC, Javia P, Muftu A, Shah P. Automated process incorporating machine learning segmentation and correlation of oral diseases with systemic health. Engineering in Medicine and Biology Society (EMBC); 2019. [DOI] [PubMed]
- 34.Li W, Chen Y, Sun W, Brown M, Zhang X, Wang S, et al. A gingivitis identification method based on contrast-limited adaptive histogram equalization, gray-level co-occurrence matrix, and extreme learning machine. Int J Imaging Syst Technol. 2019;29(1):77–82. [Google Scholar]
- 35.Alalharith DM, Alharthi HM, Alghamdi WM, Alsenbel YM, Aslam N, Khan IU, et al. A deep learning-based approach for the detection of early signs of gingivitis in orthodontic patients using faster region-based convolutional neural networks. Int J Environ Res Public Health. 2020;17(22):8447. doi: 10.3390/ijerph17228447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Chen X. Springer; Singapore: 2020,. Gingivitis identification via GLCM and artificial neural network; pp. 95–106. [Google Scholar]
- 37.Sarkar A, Dey J, Chatterjee M, Bhowmik A, Karforma S. Neural soft computing based secured transmission of intraoral gingivitis image in e-health care. Indones J Electr Eng Comput Sci. 2019;14(1):178. [Google Scholar]
- 38.Šimundić AM. Measures of diagnostic accuracy: basic definitions. eJIFCC. 2009;19(4):203–211. [PMC free article] [PubMed] [Google Scholar]
- 39.Young Ho L. Overview of the process of conducting meta-analyses of the diagnostic test accuracy. J Rheum Dis. 2018;25(1):3–10. [Google Scholar]
- 40.Bader JD, Shugars DA, Bonito AJ. Systematic reviews of selected dental caries diagnostic and management methods. J Dent Educ. 2001;65(10):960–968. [PubMed] [Google Scholar]
- 41.Aggarwal R, et al. Diagnostic accuracy of deep learning in medical imaging: a systematic review and meta-analysis. NPJ Digit Med. 2021;4(1) doi: 10.1038/s41746-021-00438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yauney G, Rana A, Wong LC, Javia P, Muftu A, Shah P Automated process incorporating machine learning segmentation and correlation of oral diseases with systemic health. IEEE. doi: 10.48550/arXiv.1810.10664. [DOI] [PubMed]
- 43.Rana A, Yauney G, Wong LC, Gupta O, Muftu A, Shah P. Automated segmentation of gingival diseases from oral images. IEEE. doi: 10.1109/HIC.2017.8227605. [DOI]
- 44.Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3(1) doi: 10.1038/s41746-020-00324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Food and Drug Administration, Center for Devices and Radiological Health, Department of Health and Human Services. Statistical guidance on reporting results from studies evaluating diagnostic tests. 2018.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/statistical-guidance-reporting-results-studies-evaluating-diagnostic-tests-guidance-industry-and-fda.
- 46.Liang-Chieh Chen YZ, Papandreou G, Schroff F, Hartwig A. Encoder-decoder with atrous separable convolution for semantic image segmentation. European Conference on Computer Vision (ECCV) 2018; Munich, Germany; 2018. [Google Scholar]
- 47.Keras. Available from: https://keras.io/. Accessed 11 January 2023.
- 48.TensorFlow. Available from: https://www.tensorflow.org/. Accessed 11 January 2023.
- 49.Li G-H, Hsung TC, Ling WK, Lam WYH, Pelekos G, McGrath C. Automatic site-specific multiple level gum disease detection based on deep neural network. 2021. IEEE; 2021. [Google Scholar]
- 50.Dhillon A, Verma GK. Convolutional neural network: a review of models, methodologies and applications to object detection. Prog Artif Intell. 2020;9(2):85–112. [Google Scholar]
- 51.Sandler AHM, Zhu M, Zhmoginov A, Chen L-C. IEEE Conference on Computer Vision and Pattern Recognition. IEEE; 2018. MobileNetV2: inverted residuals and linear bottlenecks. [Google Scholar]
- 52.Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11) doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joo J, Jeong S, Jin H, Lee U, Yoon JY, Kim SC Periodontal disease detection using convolutional neural networks. IEEE.
- 54.Simpelaere IS, Nuffelen GV, Vanderwegen J, Wouters K, Bodt MD. Oral health screening: feasibility and reliability of the oral health assessment tool as used by speech pathologists. Int Dent J. 2016;66(3):178–189. doi: 10.1111/idj.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chalmers J, King PL, Spencer AJ, Wright FAC, Carter KD. The Oral Health Assessment Tool — validity and reliability. Aust Dent J. 2005;50(3):191–199. doi: 10.1111/j.1834-7819.2005.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 56.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25(4):229–235. [PubMed] [Google Scholar]
- 57.Zhang Yl, Le D, Hu WJ, Zhang H, Liang LZ, Chung KH, et al. Assessment of dynamic smile and gingival contour in young Chinese people. Int Dent J. 2015;65(4):182–187. doi: 10.1111/idj.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lajnert V, Pavicic DK, Pavlic A, Pokrajac-Bulian A, Spalj S. Smile Aesthetics Satisfaction Scale: development and validation of a new brief five-item measure of satisfaction with smile aesthetics in adults and the elderly. Int Dent J. 2018;68(3):162–170. doi: 10.1111/idj.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shorten C, Khoshgoftaar TM. A survey on image data augmentation for deep learning. J Big Data. 2019;6(1):60. doi: 10.1186/s40537-021-00492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nash W, Drummond T, Birbilis N A review of deep learning in the study of materials degradation. NPJ Mater Degrad. 2018;2(1):1–12. [Google Scholar]
- 61.Adeli H. Neural networks in civil engineering: 1989–2000. Comput-Aided Civ Infrastruct Eng. 2001;16(2):126–142. [Google Scholar]
- 62.Mirzaei K, Arashpour M, Asadi E, Masoumi H, Bai Y, Behnood A. 3D point cloud data processing with machine learning for construction and infrastructure applications: a comprehensive review. Adv Eng Informat. 2022;51 [Google Scholar]
- 63.Hoeser T, Kuenzer C. Object detection and image segmentation with deep learning on earth observation data: a review-part I: evolution and recent trends. Remote Sens. 2020;12(10):1667. [Google Scholar]
- 64.Usamentiaga R, Usamentiaga R, Lema DG, Pedrayes OD, Daniel G. Automated surface defect detection in metals: a comparative review of object detection and semantic segmentation using deep learning. IEEE Trans Ind Appl. 2022;58(3):4203–4213. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.