Abstract
Cardiovascular disease (CVD) is the leading cause of death globally. Habitual consumption of tree nuts and peanuts is associated with cardioprotective benefits. Food-based dietary guidelines globally recommend nuts as a key component of a healthy diet. This systematic review and meta-analysis were conducted to examine the relationship between tree nut and peanut consumption and risk factors for CVD in randomized controlled trials (RCTs) (PROSPERO: CRD42022309156). MEDLINE, PubMed, CINAHL, and Cochrane Central databases were searched up to 26 September, 2021. All RCT studies that assessed the effects of tree nut or peanut consumption of any dose on CVD risk factors were included. Review Manager software was used to conduct a random effect meta-analysis for CVD outcomes from RCTs. Forest plots were generated for each outcome, between-study heterogeneity was estimated using the I2 test statistic and funnel plots and Egger’s test for outcomes with ≥10 strata. The quality assessment used the Health Canada Quality Appraisal Tool, and the certainty of the evidence was assessed using grading of recommendations assessment, development, and evaluation (GRADE). A total of 153 articles describing 139 studies (81 parallel design and 58 cross-over design) were included in the systematic review, with 129 studies in the meta-analysis. The meta-analysis showed a significant decrease for low-density lipoprotein (LDL) cholesterol, total cholesterol (TC), triglycerides (TG), TC:high-density lipoprotein (HDL) cholesterol, LDL cholesterol:HDL cholesterol, and apolipoprotein B (apoB) following nut consumption. However, the quality of evidence was “low” for only 18 intervention studies. The certainty of the body of evidence for TC:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apoB were “moderate” because of inconsistency, for TG were “low,” and for LDL cholesterol and TC were “very low” because of inconsistency and the likelihood of publication bias. The findings of this review provide evidence of a combined effect of tree nuts and peanuts on a range of biomarkers to create an overall CVD risk reduction.
Keywords: nuts, peanuts, CVD, cholesterol, lipids, apo, blood pressure, meta-analysis
Statements of Significance.
This is the first systematic review and meta-analysis of randomized controlled trials to provide a comprehensive picture of whether all or some types of tree nuts and peanuts are preferential for improving CVD risk and if there is a dose response to the effect.
Introduction
Cardiovascular disease (CVD) is the leading cause of death globally, accounting for 17.9 million lives lost each year (32% of all global deaths) [1]. The high prevalence and mortality rate of CVD is not unique to high-income countries but also to low- and middle-income countries. Low- and middle-income countries account for ∼80% of all CVD deaths, with ∼40% of these deaths defined as premature [2]. Contributing to an increased risk of CVD is the consumption of poor diets. As part of a healthy diet, food-based dietary guidelines globally recommend nuts as a key component, with a typical serving of 15–30 g/d [3,4]. Nuts are rich in fats, making them energy-dense, and concern regarding the impact of nuts on body weight has been reported in the literature [[5], [6], [7], [8]]. A recent review has demonstrated that energy compensation may occur following a meal with nuts, which mitigates this concern [9]. However, nuts are considered to be good sources of unsaturated fatty acids, vitamin E, minerals (for example, magnesium and potassium), plant sterols, polyphenols, and dietary fibers [10]. Tree nuts are defined as dry fruits that contain a seed within the ovary wall that becomes hard at maturity. Tree nuts include almonds, Brazil nuts, cashews, chestnuts, hazelnuts, macadamias, pecans, pine nuts, pistachios, and walnuts. Although peanuts, also known as ground nuts, are botanically classified as a legume rather than a nut, they appear in cuisines in a similar way to that of tree nuts and have a similar nutrient composition. This review offers the advantage of including whole tree nuts, peanuts, and mixed nuts in dietary patterns.
Habitually consuming tree nuts and peanuts has been associated with cardioprotective benefits [11]. The effects of nuts have been shown via improvements to lipid profiles, glucose regulation, and antioxidant effects [[12], [13], [14]] and their ability to mediate inflammation, hyperglycemia, and oxidative stress [15]. In addition, a considerable amount of evidence has been reported from meta-analyses of prospective cohort studies that higher nut consumption is associated with lower CVD incidence and/or CVD mortality [[16], [17], [18], [19], [20], [21]]. However, to date, only 1 meta-analysis of 61 randomized and nonrandomized controlled intervention trials has reported the effects of all tree nuts and dose response on CVD risk factors [22]. Since 2015, the consumption of all nuts has remained largely under investigation within randomized controlled trials (RCTs). Most meta-analyses of RCTs have focused on only 1 type of nut – almonds [23,24], cashews [25,26], peanuts [27], pistachios [28], and walnuts [29] on key CVD risk factors (for example, blood lipids, apolipoproteins, blood pressure, and inflammation). Therefore, the effect of combined tree nut and peanut consumption remains unclear. This review provides an opportunity to advance the understanding of whether all or some types of tree nuts and peanuts are preferential for improving CVD risk and if there is a dose response to the effect.
Despite the known health benefits associated with tree nut and peanut consumption and the promotion of nut intakes through dietary guidance messages, no health claim has been authorized globally for a cause-and-effect relationship between nuts and CVD. However, in 2012 the European Food Safety Authority panel substantiated a health claim related to 30 g/d of walnuts having an improvement in endothelium-dependent vasodilation [30]. Further, in 2014, Food Standards Australia New Zealand considered the relationship between walnuts and endothelium-dependent vasodilation [31] not to be assessable because of the small number of studies and high risk of bias. More recently, the United States Food and Drug Administration (FDA) 2017 approved a qualified health claim for 1.5 oz (42.5 g/d) of macadamia nuts and reduced risk of coronary heart disease (CHD) [32]. The FDA has also approved qualified health claims for nuts (2003) [33] and walnuts (2004) [34]. However, these 2 health claims were approved based on supportive but not conclusive evidence that consuming nuts and walnuts may reduce risk of CHD. The above-mentioned regulatory applications were constructed from the available scientific literature at the time. Since these applications were assessed, new studies have become available. An update of the literature is warranted to determine if the findings from the observational evidence are also demonstrated in experimental studies of nut consumption on CVD outcomes. Therefore, we performed a systematic review and meta-analysis of randomized controlled trials (RCT) studies to quantify the relation between nut consumption and risk of CVD.
Methods
This systematic review and meta-analysis are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [35] and checklist (Supplemental Table 1). The protocol was registered in PROSPERO (the international prospective register of systematic reviews, registration no. CRD42022309156).
Search strategy and study selection
A systematic search was undertaken in MEDLINE (EBSCO), PubMed, CINAHL (EBSCO), and Cochrane Central databases from inception to 26 September, 2021. The search strategy used a combination of keywords and Medical Subject Headings in addition to free-text search terms related to nuts and the cardiovascular outcomes of interest (that is, blood lipids, apolipoproteins, and blood pressure). Both MEDLINE and PubMed databases were searched to ensure that recent studies were identified [36,37]. An example of the detailed search strategy for the MEDLINE database is presented in Supplemental Table 2. Studies were restricted to those published in the English language only.
The records identified from the search strategy were imported to Covidence software (Covidence Systematic Review software; Veritas Health Innovation), and duplicates were removed. Records were screened by title and abstract by 1 reviewer (LH), and subsequent full-text candidate articles were assessed by 2 independent reviewers (LH and EPN). Where a study protocol was retrieved by the search (for example, a clinicaltrials.gov listing), a search was conducted to determine if a relevant article had been published from the study. Any discrepancies with full-text screening were resolved by the research team (LH, EPN, YCP) by discussion to consensus. Reference lists of the included articles were manually searched to identify additional articles.
In the case that the results from 1 study were reported in multiple articles, all articles were checked (LH, EPN, YCP) to avoid duplication of study populations in the analysis or oversight of new information on included outcomes. Where different information for included outcomes was reported across the articles, all relevant articles were included. Where the same outcomes from a single study were reported across multiple articles, decisions relating to article inclusion were based firstly on the length of the follow-up for the outcome and then on the total sample size, with articles including larger sample sizes prioritized.
Study eligibility criteria
Studies were included if they: 1) were RCTs (parallel or cross-over); 2) assessed the effects of tree nut or peanut consumption [whole tree nuts (almonds, Brazil nuts, cashew, chestnut, hazelnut, macadamia, pecan, pine nut, pistachio, or walnut), mixed nuts, peanuts, or their related products (for example, nut oils, nut powders, nut flours, and new genetic varieties of nuts)] of any dose on CVD risk factors [total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides (TG), apolipoprotein A-I (apoA-I), apolipoprotein B (apoB), systolic blood pressure (SBP), and diastolic blood pressure (DBP)]; 3) with a control or comparator group (an intervention with a lower amount of nuts or a usual diet without the addition of nuts); 4) in adults or children (>2 y of age) and healthy individuals or with chronic noncommunicable diseases such as diabetes, hyperlipidemia, or hypertension; 5) a study duration of ≥3 wk; and 6) outcomes available as a change from baseline or at the end of the intervention as final values.
Studies were excluded if they were: 1) animal studies, observational studies, nonrandomized study design, reviews, commentaries, or clinical trials without a control group; 2) in children <2 y or acutely ill individuals; 3) targeting coconut and coconut products, as their nutrient profiles differ from the aforementioned “nuts” [38]; or 4) unable to isolate the effect of nut consumption on the health outcome.
Data extraction
Outcome data were extracted by the first researcher (LH) and confirmed by a second researcher (MCS). A third researcher (ES) also performed quality checking on a 10% random sample of the data extracted for inclusion in the meta-analysis using source data verification [39]. The following data were extracted from each study: study details, country, study design, type of nut, nut dose, study duration, sample size and loss to follow-up, participant details [age, sex, body mass index (BMI, in kg/m2), and health status], intervention, background diet, a method to measure health effect and method to measure food consumption, results, and quality appraisal.
Mean changes in relevant outcomes were extracted where possible, and in the case that these data were not available, mean final values were retrieved as recommended by the Cochrane Handbook [40]. Study authors were contacted for additional information if the published article did not provide sufficient information. Where a study involved >1 intervention group meeting the inclusion criteria, data for the 2 intervention groups were combined as recommended by the Cochrane Handbook [40], with the sample size divided across the 2 groups in the case of cross-over studies to avoid a unit-of-analysis error. In the case of the Prevention with Mediterranean Diet (PREDIMED) study [41], which included 2 intervention arms featuring a Mediterranean diet supplemented with either nuts or olive oil and a low-fat control arm, data from the arm receiving the Mediterranean diet with olive oil was treated as the comparator group, and included studies were required to compare the nut group with the olive oil group. This decision was made to ensure that the outcomes were not confounded by differences in the background diet of the 2 groups. Data were converted to common units, for example, mg/dL was converted to mmol/L by multiplying by 0.0259 for LDL cholesterol, HDL cholesterol, and TC and by multiplying by 0.0113 for TG. Standard deviations (SDs) were imputed from standard errors (SEs) or 95% confidence intervals (CIs) using formulas in the Cochrane Handbook [42]. Where studies reported medians and 25th and 75th percentiles, means and SDs were imputed using the formulas developed by Wan et al. [43]. Where imputation was not possible (for example, where geometric means were reported or where a median was reported with 95% CIs), the values were used in place of the means. Where studies did not report sufficient data to be included in the meta-analysis (for example, if they did not report either SDs, SEs, 95% CIs, or interquartile range), the results were reported descriptively.
Data synthesis and analysis
Review Manager software version 5.3 (RevMan; Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014) was used to conduct the random-effects meta-analyses to determine the weighted mean differences (with 95% CIs) in change or final mean values for each measure. Forest plots were generated for each outcome. Cross-over studies were treated in the same way as parallel studies by comparing measurements from the intervention periods with the control periods. Although this approach results in a unit-of-analysis error, it is considered a conservative approach [40] and was therefore deemed appropriate.
The proportion of total variation attributable to between-study heterogeneity was estimated using the I2 test statistic [44] to provide an indication of the consistency of the results. An I2 value of ≥75% was deemed to indicate a high level of inconsistency, 50%–75% substantial inconsistency, 36%–60% moderate inconsistency, and 0%–35% low inconsistency; based on the recommendations by Higgins et al. [44] I2 values were generated for each analysis. The presence of small study effects (which may be the result of publication bias) was explored via funnel plots, which were conducted for all outcomes with ≥10 strata. Egger’s test was then conducted in Stata IC software version 15.1 (StataCorp LLC) to test for funnel plot asymmetry.
Prespecified subgroup analyses were conducted based on study design, nut group, and nut dose (≤30 g/d, 31–60 g/d, >60 g/d, based on ∼30 g typically recommended as 1 serving of nuts in dietary guidelines globally [3]), study duration (<12 wk compared with ≥12 wk, aligning with the approaches used in previous meta-analyses of nut consumption [[45], [46], [47]]) and participant health status to explore differences in the magnitude of effects between subgroups. For the studies that reported nut dose as a percentage of energy intake, we multiplied the reported percentage by the reported mean energy intake of the trial participants to recalculate the dosage in grams per day. Subgroup analyses were conducted where there were ≥10 effect sizes per outcome in total [40], although the number of effect sizes per individual subgroup was not restricted. As part of the sub-analyses based on nut dose, sensitivity analyses were also conducted to explore the effect of whole nuts by removing individual studies that assessed the effect of nut oils, nut powders, and/or nut flours from the meta-analysis.
Quality assessment
All included studies were assessed using the Health Canada Quality Appraisal Tool [48]. Quality assessment was completed by 1 researcher (EPN), and the presence or absence of a clearly stated hypothesis, statistical power, study duration, and the background diet was taken into account when evaluating the quality of each study.
The certainty of the body of evidence for interventions included in the meta-analyses was determined using the grading of recommendations assessment, development, and evaluation (GRADE) [49]. GRADE evaluates the study design, risk of bias, inconsistency, indirectness, imprecision, and other considerations such as publication bias. GRADEpro GDT software [GRADEpro Guideline Development Tool (software). McMaster University and Evidence Prime, 2022. Available from gradepro.org] was used to conduct the certainty of evidence appraisal.
Results
A total of 56,965 articles were identified from the systematic search, including the review of relevant reference lists. After applying the exclusion criteria, 153 articles describing 139 studies were included in the systematic review and 129 studies in the meta-analysis. Ten studies (11 strata) [[50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60]] were excluded from the meta-analysis because of insufficient data reported; therefore, the results of the 11 strata are reported descriptively below for each study outcome. The process of study inclusion and exclusion is shown in Figure 1.
FIGURE 1.
PRISMA [35] flow diagram of study selection.
Abbreviations: CINAHL, Cumulated Index to Nursing and Allied Health Literature; MEDLINE, Medical Literature Analysis and Retrieval System Online.
Characteristics of 139 included studies (81 parallel designs and 58 cross-over designs), including 9099 participants in the meta-analysis, are shown in Supplemental Table 3. Study duration ranged from 3–260 wk (or 5 y); only 8 [[61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72]] out of 139 studies (6%) had a duration of >1 y.) Studies were conducted in 25 countries across 6 continents. The mean participant age was 48.3 y, and 116 studies (83%) included both males and females. Studies included participants who were healthy [51,53,56,58,[73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105]], had risk factors for chronic disease such as overweight or obesity [55,57,63,68,69,72,[106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132]], hypercholesterolemia/hyperlipidemia [59,60,71,[133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160]], prediabetes [61,[161], [162], [163], [164]], at risk of CVD or had diagnosed CVD [52,[165], [166], [167]], at risk of metabolic syndrome or met the criteria for metabolic syndrome [64,70,[168], [169], [170], [171], [172], [173], [174], [175], [176]], had type 2 diabetes mellitus [54,62,[177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193]], had diagnosed coronary artery disease [[194], [195], [196], [197], [198]], or included a mixture of health conditions [50,[65], [66], [67],[199], [200], [201], [202]]. Included studies examined the effects of consumption of a range of tree nuts, including walnuts [52,56,59,60,62,63,[68], [69], [70],75,76,81,83,84,86,[96], [97], [98],102,104,111,116,119,125,129,134,139,146,151,152,[157], [158], [159],162,167,170,176,184,186,187,192,202], almonds [50,51,53,57,58,61,72,80,85,89,90,94,95,103,106,110,[112], [113], [114], 117,118,120,[131], [132], [133],143,153,155,156,160,164,165,177,[181], [182], [183],191,195,198], pistachios [54,87,121,126,138,141,142,144,145,149,154,161,169,174,[188], [189], [190]], hazelnuts [55,105,128,136,150,178,201], mixed nuts [[64], [65], [66], [67],73,77,99,107,115,130,171,180,196,197], cashews [74,91,179,185], macadamias [78,79,82,127,140,147], pecans [92,93,123,137,166,194], Brazil nuts [122,168,199,200], and peanuts [71,88,100,101,108,109,124,148,163,175,193] as well as comparing 2 different types of nuts [135,172,173] (Table 1). Nuts were consumed in either prescribed doses, ranging from ∼3 [59] to 88 [124] g/d for nut oils, flour, and butter and for whole nuts from 8 [116] to 168 [85] g/d or were included to provide a proportion of dietary energy, for example, 10% [178,179] to 30% [100,101] of total energy (equating to ∼21–76 g/d).
TABLE 1.
Included studies and the type of nut analyzed (n = 139)
| Type of nut | Number of studies n | Participants n | Female % | Mean age (y) | Mean duration (wk) | Mean nut dose (g/d) | Participant health status |
|---|---|---|---|---|---|---|---|
| Almond | 36 | 2276 | 58 | 47 | 12 | 58 | 1 CVD or at CVD risk, 1 multiple health conditions, 2 CAD, 2 prediabetic, 4 T2DM, 6 hypercholesterolemia/hyperlipidemia, 8 healthy, and 12 overweight/obese |
| Brazil nut | 4 | 216 | 69 | 49 | 11 | 14 | 1 MetS or at risk of MetS, 1 overweight/obese, and 2 multiple health conditions |
| Cashew nut | 4 | 394 | 59 | 54 | 7 | 37 | 2 healthy and 2 T2DM |
| hazelnut | 7 | 393 | 56 | 41 | 8 | 37 | 1 healthy, 1 multiple health conditions, 1 T2DM, 2 hypercholesterolemia/hyperlipidemia, and 2 overweight/obese |
| Macadamia | 6 | 237 | 57 | 40 | 5 | 49 | 1 overweight/obese, 2 hypercholesterolemia/hyperlipidemia, and 3 healthy |
| Peanut | 10 | 895 | 46 | 45 | 14 | 58 | 1 prediabetic, 1 MetS or at risk of MetS, 1 T2DM, 2 healthy, 2 hypercholesterolemia/hyperlipidemia, and 3 overweight/obesity |
| Pecan | 6 | 272 | 51 | 50 | 8 | 58 | 1 CAD, 1 CVD or at CVD risk, 1 hypercholesterolemia/hyperlipidemia, 1 overweight/obese, and 2 healthy |
| Pistachio | 14 | 652 | 56 | 49 | 12 | 62 | 1 healthy, 1 prediabetic, 2 MetS or at risk of MetS, 2 overweight/obese, 3 T2DM, and 5 hypercholesterolemia/hyperlipidemia |
| Walnut | 39 | 2824 | 52 | 52 | 16 | 38 | 1 prediabetic, 1 multiple health conditions, 2 CVD or CVD risk, 3 MetS or at risk of MetS, 5 T2DM, 7 overweight/obese, 9 hypercholesterolemia/hyperlipidemia, and 11 healthy |
| Mixed nuts | 11 | 858 | 49 | 47 | 35 | 45 | 1 CAD, 1 multiple health conditions, 1 T2DM, 2 MetS, 3 healthy, and 3 overweight/obese |
| Different study arms compared different nuts (for example, group 1 consumed almonds, and group 2 consumed walnuts) | 2 | 82 | 52.5 | 51 | 6 | 69 | 1 hypercholesterolemia/hyperlipidemia and 1 MetS or at risk of MetS |
| Total (all nuts) | 139 | 9099 | 54 | 48 | 14 | 48 |
Abbreviations: CAD, coronary artery disease; CVD, cardiovascular disease; MetS, metabolic syndrome; T2DM, type 2 diabetes mellitus.
Effect of nut consumption on study outcomes
LDL cholesterol
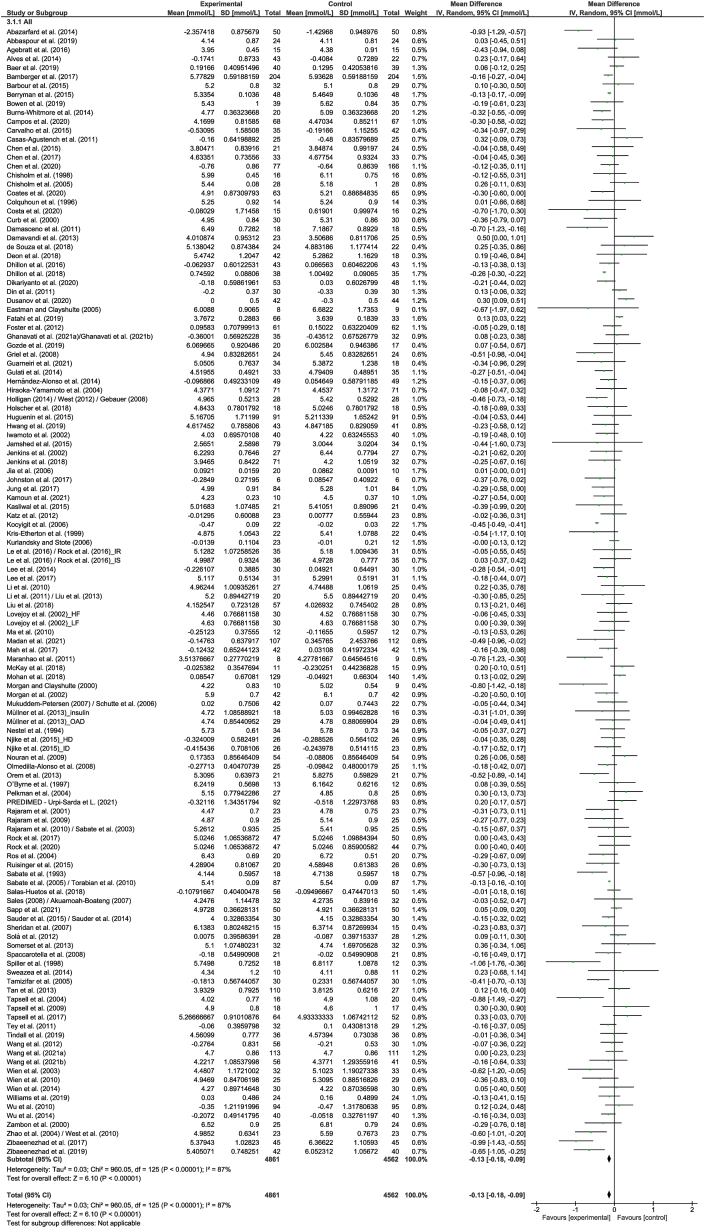
A total of 126 strata from 122 studies explored the effect of nut consumption on LDL cholesterol. The meta-analysis showed that nut consumption was associated with a significant decrease in LDL cholesterol (Table 2 and Figure 2). Of the 126 strata, 35 explored the effect of walnut consumption, and 101 had a duration of ≤3 mo. Eleven strata from 10 RCTs [50,[52], [53], [54], [55], [56], [57], [58], [59], [60]] were removed from the meta-analysis, of which 2 [56,60] reported a significant reduction in LDL cholesterol with the consumption of nuts. Subgroup analysis indicated significant differences, including those related to study design, nut type, and health status (Supplemental Table 4). Larger reductions in LDL cholesterol were found in cross-over studies, compared to studies with a parallel design, although the magnitude of effects in the different subgroups is not likely to be clinically significant. Regarding nut type and health status, it should be noted that the significance of the effects should be interpreted with caution because of the uneven distribution of studies in the subgroups. When studies assessing the effect of nut oils, nut powders, and/or nut flours were removed from the dose subgroup analysis, similar results were found in the overall analysis (Supplemental Table 5).
TABLE 2.
Change in outcomes following nut intervention compared to a control
| Outcome | Number of strata | Number of studies | Number of participants | Effect estimate | Inconsistency (I2)% |
|---|---|---|---|---|---|
| LDL cholesterol, mmol/L | 126 | 122 | 9487 | −0.11 (−0.14, −0.07), P < 0.00001 | 80 |
| HDL cholesterol, mmol/L | 126 | 122 | 10,102 | 0.00 (−0.01, 0.02), P = 0.59 | 81 |
| TC, mmol/L | 126 | 122 | 9423 | −0.13 (−0.18, −0.09), P < 0.00001 | 87 |
| TG, mmol/L | 122 | 118 | 9744 | −0.06 (−0.08, −0.03), P < 0.00001 | 61 |
| TG:HDL cholesterol | 5 | 5 | 331 | −0.12 (−0.40, 0.15), P = 0.37 | 0 |
| TC:HDL cholesterol | 51 | 49 | 3837 | −0.15 (−0.24, −0.07), P = 0.0003 | 68 |
| HDL cholesterol:LDL cholesterol | 3 | 3 | 209 | −0.06 (−0.23, 0.11), P = 0.46 | 53 |
| LDL cholesterol:HDL cholesterol | 39 | 38 | 2539 | −0.14 (−0.20, 0.08), P < 0.00001 | 75 |
| ApoB, mg/dL | 39 | 39 | 3069 | −3.01 (−4.44, −1.58), P < 0.00001 | 44 |
| ApoA-I, mg/dL | 33 | 33 | 2259 | −1.02 (−2.53, 0.49), P = 0.19 | 0 |
| SBP, mmHg | 71 | 70 | 6521 | −0.05 (−0.83, 0.72), P = 0.89 | 47 |
| DBP, mmHg | 67 | 66 | 6198 | −0.10 (−0.53, 0.33), P = 0.65 | 24 |
Abbreviations: ApoA-I, apolipprotein A-1; ApoB, apolipoprotein B; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
FIGURE 2.
Difference in LDL cholesterol (mmol/L) between nut consumption and control. The diamond indicates a weighted mean difference with 95% CIs.
Abbreviation: IV, inverse variance.
TC
A total of 126 strata from 122 studies explored the effect of nut consumption on TC. The meta-analysis showed that nut consumption was associated with a significant decrease in TC (Table 2 and Figure 3). Walnuts (n = 35) and almonds (n = 33) were the most common nut types in this analysis. Eleven strata from 10 RCTs [50,[52], [53], [54], [55], [56], [57], [58], [59], [60]] were removed from the meta-analysis, of which 3 [54,56,60] reported a significant reduction in TC with the consumption of nuts. Variation in the magnitude of the effect was observed for nut type, although these results should be interpreted with caution because of the uneven distribution of studies in the subgroups (Supplemental Table 6). For the nut dose, a significant dose response was found. Stronger effects were observed for >60 g/d; however, there was substantial heterogeneity that should be considered when interpreting results. Findings were similar when studies assessing the effect of nut oils, nut powders, and/or nut flours were removed (Supplemental Table 5).
FIGURE 3.
Difference in total cholesterol (mmol/L) between nut consumption and control. The diamond indicates a weighted mean difference with 95% CIs.
Abbreviation: IV, inverse variance.
TG
A total of 122 strata from 118 studies explored the effect of nut consumption on TG. The meta-analysis showed that nut consumption was associated with a significant decrease in TG (Table 2 and Figure 4). Eleven strata from 10 RCTs [50,[52], [53], [54], [55], [56], [57], [58], [59], [60]] were removed from the meta-analysis, of which 3 [56,59,60] reported a significant reduction in TG with the consumption of nuts. Subgroup analysis indicated differences related to nut type (Supplemental Table 7). However, this significant effect should be interpreted with caution because of the uneven distribution of studies included in the subgroups. When studies assessing the effect of nut oils, nut powders, and/or nut flours were removed from the dose subgroup analysis, results were similar to the overall analysis (Supplemental Table 5).
FIGURE 4.
Difference in TGs (mmol/L) between nut consumption and control. The diamond indicates a weighted mean difference with 95% CIs.
Abbreviation: IV, inverse variance.
TC to HDL cholesterol ratio
A total of 51 strata from 49 studies explored the effect of nut consumption on TC:HDL cholesterol. The meta-analysis showed that nut consumption was associated with a significant decrease in TC:HDL cholesterol (Table 2 and Figure 5). Six strata from 6 RCTs [53,54,56,57,60,105] were removed from the meta-analysis, of which 3 [53,54,60] reported a significant reduction in TC:HDL cholesterol with the consumption of nuts. The subgroup analyses indicated that a similar magnitude of the effect was found across the different subgroups (Supplemental Table 8). After removing studies assessing the effect of nut oils, nut powders, and/or nut flours from the dose subgroup analysis, results were similar to the overall analysis (Supplemental Table 5).
FIGURE 5.
Difference in total cholesterol to HDL cholesterol ratio between nut consumption and control. The diamond indicates a weighted mean difference with 95% CIs.
Abbreviation: IV, inverse variance.
LDL cholesterol to HDL cholesterol ratio
A total of 39 strata from 38 studies explored the effect of nut consumption on LDL cholesterol:HDL cholesterol. The meta-analysis showed that nut consumption was associated with a significant decrease in LDL cholesterol:HDL cholesterol (Table 2 and Figure 6). Two strata from 2 RCTs were removed from the meta-analysis, of which both [53,54] reported a significant reduction in LDL cholesterol:HDL cholesterol with the consumption of nuts. Variation in the magnitude of the effect was observed for nut type, study duration, and health status (Supplemental Table 9). Although studies with a shorter duration (<12 wk) had a larger decrease in LDL cholesterol:HDL cholesterol than found for studies of ≥12 wk, the magnitude of the effects in the subgroups is not likely to be clinically significant. For nut type and health status, these results should be interpreted with caution because of the small and/or uneven distribution of studies included in the subgroups. When studies assessing the effect of nut oils, nut powders, and/or nut flours were removed from the dose subgroup analysis, similar results were found in the overall analysis (Supplemental Table 5).
FIGURE 6.
Difference in LDL cholesterol to HDL cholesterol ratio between nut consumption and control. The diamond indicates a weighted mean difference with 95% CIs.
Abbreviation: IV, inverse variance.
ApoB
A total of 39 strata from 39 studies explored the effect of nut consumption on apoB. The meta-analysis showed that nut consumption was associated with a significant decrease in apoB (Table 2 and Figure 7). One stratum from 1 RCT [57] was removed from the meta-analysis, though no significant finding in relation to apoB and the consumption of nuts was reported. Subgroup analysis indicated significant subgroup differences related to study design, nut type, and health status (Supplemental Table 10). Larger decreases in apoB were observed in cross-over studies, compared to parallel studies, although the magnitude of the effects in the different subgroups is not likely to be clinically significant. Other significant effects for nut type and health status should be interpreted with caution because of the small and/or uneven distribution of studies included in the subgroups. When studies assessing the effect of nut oils, nut powders, and/or nut flours were removed from the dose subgroup analysis, similar results were found in the overall analysis (Supplemental Table 5).
FIGURE 7.
Difference in apoB (mg/dL) between nut consumption and control. The diamond indicates a weighted mean difference with 95% CIs.
Abbreviation: IV, inverse variance.
HDL cholesterol, TG to HDL cholesterol ratio, HDL cholesterol to LDL cholesterol ratio, apoA-I, SBP, and DBP
The meta-analysis showed that consumption of nuts had no significant effect on HDL cholesterol and did not result in significant differences in TG:HDL cholesterol, HDL cholesterol:LDL cholesterol, apoA-I, SBP, and DBP (Table 2 and Supplemental Figures 1–6). In the case of the subgroup analyses, a similar magnitude of the effect was found across the different subgroups for apoA-I (Supplemental Table 11) and SBP (Supplemental Table 12). Although there were significant subgroup differences found for HDL cholesterol (Supplemental Table 13) and DBP (Supplemental Table 14) according to health status, these differences should be interpreted with caution because of the uneven distribution in the number of participants and studies included in the subgroups. Variation in the magnitude of the effect was also observed for DBP and study duration (Supplemental Table 14). Although a reduction in DBP was only observed in studies with a duration of 12 or more weeks, it should be noted that the magnitude of this reduction was small and not likely to be clinically significant. Similar results to the original analysis were found when studies assessing the effect of nut oils, nut powders, and/or nut flours were removed from the dose subgroup analysis for HDL cholesterol, TG:HDL cholesterol, HDL cholesterol:LDL cholesterol, apoA-I, SBP, and DBP (Supplemental Table 5).
Heterogeneity
Considerable heterogeneity (I2 >75%) was observed for LDL cholesterol, HDL cholesterol, and TC (see Table 2). In addition, substantial heterogeneity (I2: 50%–75%) was observed for 4 measures (TG, TC:HDL cholesterol, LDL cholesterol:HDL cholesterol, and HDL cholesterol:LDL cholesterol), moderate heterogeneity (I2: 36%–60%) for 2 measures (apoB and SBP), whereas low heterogeneity (I2: 0%–35%) was observed for 3 measures (TG:HDL cholesterol, apoA-I, and DBP).
Small study effects
Funnel plots were generated for outcomes with 10 or more strata (LDL cholesterol, HDL cholesterol, TC, TG, TC:HDL cholesterol, LDL cholesterol:HDL cholesterol, apoA-I, apoB, SBP, and DBP) (see Supplemental Figures 7–16). Egger’s test outcomes indicated funnel plot asymmetry for LDL cholesterol (bias: −0.69; 95% CI: −1.10, −0.28; P = 0.001), HDL cholesterol (bias: 0.58; 95% CI: 0.07, 1.09; P = 0.027), TC (bias: −0.82; 95% CI: −1.33, −0.31; P = 0.002), TG (bias: −0.58; 95% CI: −0.86, −0.30; P = 0.000), SBP (bias: −0.61; 95% CI: −1.11, −0.12; P = 0.016), and DBP (bias: −0.57; 95% CI: −0.99, −0.14; P = 0.010). These results indicate the presence of small study effects, which may be caused by publication bias. By comparison, funnel plot asymmetry was not detected for TC:HDL cholesterol (bias: 0.42; 95% CI: −0.33, 1.16; P = 0.266), LDL cholesterol:HDL cholesterol (bias: 0.14; 95% CI: −0.63, 0.91; P = 0.713), apoB (bias: 0.35; 95% CI: −0.20, 0.90; P = 0.205), and apoA-I (bias: 0.42; 95% CI: −0.34, 1.19; P = 0.268).
Quality assessment
Quality assessment is presented for each study in Supplemental Table 3, with full quality assessment available in Supplemental Table 15. The quality of evidence was “low” for n = 18 intervention studies [50,51,53,55,[58], [59], [60],77,78,82,85,90,100,101,122,124,127,146,149,150].
The certainty of the body of evidence was assessed for studies included in meta-analyses using GRADE, with results presented in Supplemental Table 16. The certainty of the body of evidence for apoA-I was “high.” The body of evidence for TC:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apoB was downgraded to “moderate” because of inconsistency and for DBP because of the likelihood of publication bias. The certainty for TG and SBP was downgraded to “low” because of inconsistency and the likelihood of publication bias, and the certainty for TG:HDL cholesterol was downgraded to “low” because of imprecision and the likelihood of publication bias. Certainty for LDL cholesterol, HDL cholesterol, TC, was downgraded to “very low” because of the inconsistency and likelihood of publication bias, and HDL cholesterol:LDL cholesterol because of inconsistency, imprecision, and the likelihood of publication bias.
Discussion
This systematic review and meta-analysis showed favorable effects of tree nut and peanut consumption on lowering LDL cholesterol, TC, TG, TC:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apoB from RCTs including 9099 participants. These findings align with a review conducted in 2015, which explored the effects of all tree nuts on CVD risk factors [22]. The present meta-analysis builds on these findings with the addition of studies published since the 2015 review [22] and focuses solely on RCTs. It also includes studies investigating other nut forms, including nut oils [55,59,71,88,100,101,124,147,[158], [159], [160],168,186,187,202,203], nut butter [88,124], and nut flour [199] and adolescents (aged 13 y or older) [136]. Subgroup analyses found differences across the types of nuts for outcomes, LDL cholesterol, TC, TG, LDL cholesterol:HDL cholesterol, and apoB. Having fewer studies within the Brazil nut, cashew nut, hazelnut, macadamia, and pecan subgroups may have played a role in the significant effects observed. A dose response and stronger effects were also observed for >60 g/d for TC. Despite the nut dose response, a reduction in LDL cholesterol, TC, TG, TC:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apoB, and an increase in HDL were found in ≤30 g/d of nuts. Taken together, this meta-analysis provides a timely effect of tree nut and peanut intake on overall CVD risk reduction, including dose-response relations.
The scientific evidence indicates that the consumption of nuts is beneficial to positive changes in many biomarkers of CVD. Although biomarkers were assessed in this review, the population effect for LDL cholesterol, HDL cholesterol, TC, and TG was based on >4500 interventions and >4500 control participants, and SBP and DBP were based on >3000 interventions and >3000 control participants. This substantial population size for high-quality study designs indicates significant reductions in LDL cholesterol, TC, and TG and no change in HDL cholesterol. Despite the heterogeneity, this combined effect suggests that nuts do not simply display positive changes to 1 biomarker of CVD risk but a range of biomarkers to create an overall risk reduction. Although the findings of the SBP and DBP studies indicated a nonsignificant reduction, there was less heterogeneity which is of clinical relevance. The effects on HDL cholesterol were also not indicative of risk reduction, though the meta-analysis suggested no change, which is also a favorable outcome. Both blood lipids and blood pressure are major risk factors for CVD and are included in the Framingham risk equation [204], used globally by clinicians. Therefore, this review provides evidence of a causal link between nut intake and lower CVD risk.
In this review, we observed that there were significant differences in outcomes (LDL cholesterol, TC, TG, LDL cholesterol:HDL cholesterol, and apoB) across different types of nuts. Consideration is needed when interpreting the findings of this review as it is unclear whether all or some types of nuts are preferential for improving CVD risk. This review suggests stronger evidence for almonds and walnuts because of the greater number of studies (n > 30), and therefore more studies are needed for other nut types.
Our findings suggest a dose response was observed for tree nut and peanut consumption of >60 g/d for TC. Similar findings were reported in a recent meta-analysis of RCTs and non-RCTs in this field [22], which showed the relationship between tree nut intake and TC and LDL cholesterol was nonlinear, with stronger effects at >60 g/d. However, the scientific evidence suggests that consumers typically do not meet recommended serving sizes of 15–30 g. In Australia, for example, nut intake was estimated at an average of 4.61 g/d, with only 5.6% of the population consuming the recommended daily amount of nuts [205]. Public health messaging needs to support reasons for consumers to increase their intake of nuts. However, nuts, as a food group because of their high-fat content, have been related to consumer concerns about weight gain, a risk factor for CVD. While this review did not look at body weight, a recent meta-analysis of 106 RCTs and 6 prospective cohorts showed that higher nut intake was associated with reductions in body weight and body fat [206]. Establishing health messages about the regular consumption of nuts to reduce CVD risk may shift consumer opinion and add to the acknowledgment of core foods for positive lifestyle change related to CVD risk.
Consideration should be given when interpreting findings from this review, which included only RCTs when compared to real-life consumer settings, as they are substantially different. RCTs aiming to explore the influence of specific foods must consider many methodological challenges, including the design of the dietary intervention and control arms to avoid increases in total energy intake, which could skew results [207]. Although a dose response was reported for TC, several studies incorporated nuts as a proportion of a participant’s total energy, resulting in substantial variation between individual nut doses. Whether the energy value of nuts was adjusted for in the total diet may also influence the results. We did not consider in our subgroup analysis whether the energy provided by nuts was accounted for by dietary modeling or advice to substitute other foods or nuts, though there is evidence suggesting that energy-restricted diets are effective in improving the blood lipid profile [208] and blood pressure [209]. This highlights the importance of total energy intake and should be considered in future meta-analyses. Further, energy-restricted diet studies that were designed for weight loss were included in this analysis. As weight loss is a significant contributor to improving CVD risk factors, it is challenging to determine whether the effects observed from this review were from nuts alone or if further benefits were obtained from the additional weight loss. The design of the control arm may have also impacted our results; for example, Agebratt et al. [73] compared nut intake with a control intervention (that is, fruit), which may have potentially influenced the control effects. A previous meta-analysis has highlighted the potential impact of control groups on underestimating intervention effects in weight-loss RCTs [210]. Thus, RCTs that aim to explore the influence of specific foods should carefully design the dietary intervention and control arms to avoid a total energy increase to skew results. Despite these challenges, the most recent meta-analyses of prospective cohort studies in this field [21] have shown an inverse association between nut consumption and CVD incidence and mortality. Taken together, this review provides the most recent evidence that the findings from RCTs conducted in highly controlled conditions support the findings from observational studies that reflect free-living populations.
This review has several strengths and limitations. A notable strength was that the processes followed current guidelines in review conduct, reporting, and analysis. The review is grounded in the evidence with findings aligned to previous reviews of observational studies and 1 review of randomized and nonrandomized trials. We considered a range of outcomes associated with CVD, including blood lipids, apolpoproteins,and blood pressure. In a recent review by the authors, the effects of nuts and inflammation (c-reactive protein, adiponectin, tumor necrosis factor alpha, interleukin-6, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1) reported nonsignificant changes because of a lack of consistent available evidence, and as a result, such variables were not included in this review [211]. However, we recognize that the outcomes explored in this review are not interchangeable with disease endpoints such as CVD mortality and morbidity. The range of population groups addressed in the included studies of this review may be considered both a strength and a limitation. The populations included some groups who had existing risk factors for CVD, such as overweight and type 2 diabetes mellitus, whereas others were considered “healthy” populations. Because of the existing comorbidities, the effect size of some studies may appear greater than if all studies targeted a healthy population. For cross-over trials, there were no additional exclusion criteria applied, which meant studies using this design were not required to meet a “sufficient” wash-out period for inclusion in this review. This may have resulted in potential carryover effects, though the potential impact on the results was considered in the quality appraisal. These above-mentioned points need to be considered when interpreting the findings.
The heterogeneity of the evidence included can also be considered a limitation. Variation existed because of study design and duration, participant health status, nut type, and dose, although these factors were explored in subgroup analyses. In particular, relatively few studies in some nut types (Brazil nut, cashew nut, hazelnut, macadamia, and pecan) limit the statistical power to detect a potential interaction that needs to be considered in relation to the whole body of evidence. Of the different tree nut types, all tree nuts except chestnuts were addressed in the body of evidence that was reviewed. The lower fat profile of chestnuts suggests that they could mechanistically work differently from other nut types [212]. In addition, pine nuts were only included in 1 study as part of mixed nuts consumed by participants [171]. Thought should be given to the variability of nuts as a food group, as there are a number of methodological challenges relating to the study of dietary patterns and CVD. One major challenge is related to the way food groups are formed and the limitations of the different food composition databases used to analyze the outcomes, especially if energy is adjusted [213]. Background diets also varied between studies, with some prescribing dietary guidelines, whereas others advised participants to follow their habitual diet, which may have varied considerably between individuals. For 1 study, the PREDIMED study [[65], [66], [67]], we considered the olive oil arm to be the control arm when compared with the other Mediterranean diet arms. The authors acknowledge that olive oil provides unsaturated fats and has been associated with lipid-lowering properties. This comparison, however, eliminates the confounding nature of the overall Mediterranean diet, allowing for the comparison between nut and olive oil consumption. Similarly, 1 study [176] also included seeds in their control diet. Although linseeds have a similar nutrient profile to nuts, a meta-analysis reported particular doses and participant characteristics to influence the lipid-lowering effect [214]. Analysis of funnel plots suggested the results for LDL cholesterol, HDL cholesterol, TC, TG, SBP, and DBP may have been influenced by small study effects, which may be caused by publication bias. This resulted in downgrading the quality of evidence for these outcomes. Finally, although we were unable to explore the distribution of the published data included in this meta-analysis, several studies reported median values rather than means, which may suggest that some of the data was skewed and may have impacted our analyses.
In conclusion, this systematic review and meta-analysis of the effects of tree nut and peanut consumption on improving risk of CVD finds evidence of significantly favorable effects on LDL cholesterol, TC, TG, TC:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apoB. The nonsignificant differences in SBP and DBP suggest a lack of consistent evidence for the effects of tree nuts and peanuts on blood pressure. The results should also be interpreted with caution because of the large variation in the included studies. The findings of this review provide further insight into the combined effect that tree nuts and peanuts do not simply display positive changes to 1 biomarker of CVD risk but a range of biomarkers to create an overall risk reduction. The findings of this review also build on previous observational and intervention meta-analyses, which have shown an inverse association between nut consumption and risk of CVD. Although stronger effects are observed for tree nut and peanut consumption of >60 g/d for LDL cholesterol and TC, most dietary guidelines recommend the consumption of 15–30 g as a serving size. Importantly, this review shows a reduction in LDL cholesterol, TC, TG, TC:HDL cholesterol, LDL cholesterol:HDL cholesterol, and apoB, and an increase in HDL with the consumption of ≤30 g/d of nuts, supporting dietary guidelines. As consumers are not currently meeting these recommendations, public health messaging is needed to support reasons for consumers to increase their intake of nuts, which also have favorable effects on risk of CVD.
Acknowledgments
We thank Dr. Vivienne Guan for her contribution to the scoping review, which set the foundations for this systematic review. We also thank Ms. Elizabeth Sherry for her help with quality checking (source data verification).
The authors’ responsibilities were as follows—LH: designed and conducted the research, performed the literature search, wrote the initial draft, and had primary responsibility for final content; LH, EPN, and YCP: performed data screening; LH and MCS: performed data extraction; EPN: performed quality assessment; and all authors: read and approved the final manuscript.
Data availability
The data used in this review are available from the corresponding author upon reasonable request.
Funding
This research was funded by the Australian Nut Industry Council Ltd, which did not provide any input into the design, collection, analysis, interpretation of the data, or manuscript writing.
Author disclosures
EPN has previously received funding from the International Nut and Dried Fruit Council. In addition, EPN and YCP have previously received funding from the California Walnut Commission. LH and MCS, no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.05.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cardiovascular diseases (CVDs) key facts. World Health Organization. 2021 https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds [Internet] [cited 21 April, 2022]. Available from: [Google Scholar]
- 2.Anand S., Bradshaw C., Prabhakaran D. Prevention and management of CVD in LMICs: why do ethnicity, culture, and context matter? BMC Med. 2020;18(1):7. doi: 10.1186/s12916-019-1480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neale E.P., Tapsell L.C. In: Health Benefits of Nuts and Dried Fruits. Alasalvar C., Salas-Salvado J., Ros E., Sabate J., editors. Taylor & Francis Group; Boca Raton, FL: 2020. Nuts in Healthy dietary Patterns and Dietary Guidelines. [Google Scholar]
- 4.Herforth A., Arimond M., Álvarez-Sánchez C., Coates J., Christianson K., Muehlhoff E. A global review of food-based dietary guidelines. Adv. Nutr. 2019;10(4):590–605. doi: 10.1093/advances/nmy130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown R.C., Gray A.R., Yong L.C., Chisholm A., Leong S.L., Tey S.L. A comparison of perceptions of nuts between the general public, dietitians, general practitioners, and nurses. PeerJ. 2018;6 doi: 10.7717/peerj.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yong L.C., Gray A.R., Chisholm A., Leong S.L., Tey S.L., Brown R.C. Barriers to and facilitators and perceptions of nut consumption among the general population in New Zealand. Public Health Nutr. 2017;20(17):3166–3182. doi: 10.1017/S1368980017002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlak R., Colby S., Herring J. Beliefs, benefits, barriers, attitude, intake and knowledge about peanuts and tree nuts among WIC participants in eastern North Carolina. Nutr. Res. Pract. 2009;3(3):220–225. doi: 10.4162/nrp.2009.3.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlak R., London H., Colby S., WallBassett E., Sira N. Perception of nut intake among individuals with or at risk for heart disease and/or diabetes. J. Behav. Health. 2012;1(3):185–188. doi: 10.5455/jbh.20120501060143. [DOI] [Google Scholar]
- 9.Nikodijevic C.J., Probst Y.C., Tan S.Y., Neale E.P. The effects of tree nut and peanut consumption on energy compensation and energy expenditure: a systematic review and meta-analysis. Adv. Nutr. 2023;14(1):77–98. doi: 10.1016/j.advnut.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Probst Y.C., Guan V.X., Kent K. Dietary phytochemical intake from foods and health outcomes: a systematic review protocol and preliminary scoping. BMJ Open. 2017;7(2) doi: 10.1136/bmjopen-2016-013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitok E., Sabaté J. Nuts and cardiovascular disease. Prog. Cardiovasc. Dis. 2018;61(1):33–37. doi: 10.1016/j.pcad.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Sabaté J., Oda K., Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch. Intern. Med. 2010;170(9):821–827. doi: 10.1001/archinternmed.2010.79. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins D.J., Kendall C.W., Josse A.R., Salvatore S., Brighenti F., Augustin L.S., et al. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J. Nutr. 2006;136(12):2987–2992. doi: 10.1093/jn/136.12.2987. [DOI] [PubMed] [Google Scholar]
- 14.Downs S.M., Fanzo J. Is a cardio-protective diet sustainable? A review of the synergies and tensions between foods that promote the health of the heart and the planet. Curr. Nutr. Rep. 2015;4(4):313–322. doi: 10.1007/s13668-015-0142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Souza R.G.M., Schincaglia R.M., Pimentel G.D., Mota J.F. Nuts and human health outcomes: a systematic review. Nutrients. 2017;9(12):1311. doi: 10.3390/nu9121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayhew A.J., de Souza R.J., Meyre D., Anand S.S., Mente A. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br. J. Nutr. 2016;115(2):212–225. doi: 10.1017/S0007114515004316. [DOI] [PubMed] [Google Scholar]
- 17.Aune D., Keum N., Giovannucci E., Fadnes L.T., Boffetta P., Greenwood D.C., et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14(1):207. doi: 10.1186/s12916-016-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo C., Zhang Y., Ding Y., Shan Z., Chen S., Yu M., et al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2014;100(1):256–269. doi: 10.3945/ajcn.113.076109. [DOI] [PubMed] [Google Scholar]
- 19.Zhou D., Yu H., He F., Reilly K.H., Zhang J., Li S., et al. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2014;100(1):270–277. doi: 10.3945/ajcn.113.079152. [DOI] [PubMed] [Google Scholar]
- 20.Grosso G., Yang J., Marventano S., Micek A., Galvano F., Kales S.N. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2015;101(4):783–793. doi: 10.3945/ajcn.114.099515. [DOI] [PubMed] [Google Scholar]
- 21.Becerra-Tomás N., Paz-Graniel I., Kendall C.W.C., Kahleova H., Rahelić D., Sievenpiper J.L., et al. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. Nutr. Rev. 2019;77(10):691–709. doi: 10.1093/nutrit/nuz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Gobbo L.C., Falk M.C., Feldman R., Lewis K., Mozaffarian D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015;102(6):1347–1356. doi: 10.3945/ajcn.115.110965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phung O.J., Makanji S.S., White C.M., Coleman C.I. Almonds have a neutral effect on serum lipid profiles: a meta-analysis of randomized trials. J. Am. Diet. Assoc. 2009;109(5):865–873. doi: 10.1016/j.jada.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Lee-Bravatti M.A., Wang J., Avendano E.E., King L., Johnson E.J., Raman G. Almond consumption and risk factors for cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2019;10(6):1076–1088. doi: 10.1093/advances/nmz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalali M., Karamizadeh M., Ferns G.A., Zare M., Moosavian S.P., Akbarzadeh M. The effects of cashew nut intake on lipid profile and blood pressure: a systematic review and meta-analysis of randomized controlled trials, Complement. Ther. Med. 2020;50:102387. doi: 10.1016/j.ctim.2020.102387. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadifard N., Haghighatdoost F., Mansourian M., Hassannejhad R., Sadeghi M., Roohafza H., et al. Long-term association of nut consumption and cardiometabolic risk factors. Nutr. Metab. Cardiovasc. Dis. 2019;29(9):972–982. doi: 10.1016/j.numecd.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Jafari Azad B., Daneshzad E., Azadbakht L. Peanut and cardiovascular disease risk factors: a systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020;60(7):1123–1140. doi: 10.1080/10408398.2018.1558395. [DOI] [PubMed] [Google Scholar]
- 28.Hadi A., Asbaghi O., Kazemi M., Haghighian H.K., Pantovic A., Ghaedi E., et al. Consumption of pistachio nuts positively affects lipid profiles: a systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021:1–14. doi: 10.1080/10408398.2021.2018569. [DOI] [PubMed] [Google Scholar]
- 29.Banel D.K., Hu F.B. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am. J. Clin. Nutr. 2009;90(1):56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific opinion on the substantiation of health claims related to walnuts and maintenance of normal blood LDL-cholesterol concentrations (ID 1156, 1158) and improvement of endothelium-dependent vasodilation (ID 1155, 1157) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011;9(4):2074. [Google Scholar]
- 31.Food Standards Australia and New Zealand (FSANZ) FSANZ; 2014. Systematic Review of the Evidence for a Relationship Between Walnuts and Endothelium-Dependent Vasodilation. [Google Scholar]
- 32.United States Food and Drug Administration (FDA) 2016. Petition for a Qualified Health Claim for Macadamia Nuts and Reduced Risk of Coronary Heart Disease. (Docket No. FDA-2015-Q-4850) [Google Scholar]
- 33.United States Food and Drug Administration (FDA) Docket No 02P-0505); 2003. Qualified Health Claims: Letter of Enforcement Discretion –Nuts and Coronary Heart Disease. [Google Scholar]
- 34.United States Food and Drug Administration (FDA) 2004. Qualified Health Claims: Letter of Enforcement Discretion –Walnuts and Coronary Heart Disease. Docket No 02P-0292) [Google Scholar]
- 35.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen L., Suhami R. The art and science of study identification: a comparative analysis of two systematic reviews. BMC Med. Res. Methodol. 2016;16(1):24. doi: 10.1186/s12874-016-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bramer W.M., Rethlefsen M.L., Kleijnen J., Franco O.H. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst. Rev. 2017;6(1):245. doi: 10.1186/s13643-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown R.C., Tey S.L., Gray A.R., Chisholm A., Smith C., Fleming E., et al. Association of nut consumption with cardiometabolic risk factors in the 2008/2009 New Zealand Adult Nutrition Survey. Nutrients. 2015;7(9):7523–7542. doi: 10.3390/nu7095351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houston L., Probst Y., Humphries A. Measuring data quality through a source data verification audit in a clinical research setting. Stud. Health Technol. Inform. 2015;214:107–113. [PubMed] [Google Scholar]
- 40.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 41.Estruch R., Ros E., Salas-Salvadó J., Covas M.I., Corella D., Arós F., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 42.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al. Transformations and Skewed Data. 2021. Section 6-5-2-4. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. (updated February 2021) Cochrane. [Google Scholar]
- 43.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viguiliouk E., Kendall C.W., Blanco Mejia S., Cozma A.I., Ha V., Mirrahimi A., et al. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLOS ONE. 2014;9(7) doi: 10.1371/journal.pone.0103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanco Mejia S., Kendall C.W.C., Viguiliouk E., Augustin L.S., Ha V., Cozma A.I., et al. Effect of tree nuts on metabolic syndrome criteria: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2014;4(7) doi: 10.1136/bmjopen-2013-004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neale E.P., Guan V., Tapsell L.C., Probst Y.C. Effect of walnut consumption on markers of blood glucose control: a systematic review and meta-analysis. Br. J. Nutr. 2020;124(7):641–653. doi: 10.1017/S0007114520001415. [DOI] [PubMed] [Google Scholar]
- 48.Health Canada . Bureau of Nutritional Sciences FD, Health Products and Food Branch; 2009. Guidance Document for Preparing a Submission for Food Health Claims. [Google Scholar]
- 49.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choudhury K., Clark J., Griffiths H.R. An almond-enriched diet increases plasma α-tocopherol and improves vascular function but does not affect oxidative stress markers or lipid levels. Free Radic. Res. 2014;48(5):599–606. doi: 10.3109/10715762.2014.896458. [DOI] [PubMed] [Google Scholar]
- 51.Fraser G.E., Bennett H.W., Jaceldo K.B., Sabaté J. Effect on body weight of a free 76 kilojoule (320 calorie) daily supplement of almonds for six months. J. Am. Coll. Nutr. 2002;21(3):275–283. doi: 10.1080/07315724.2002.10719221. [DOI] [PubMed] [Google Scholar]
- 52.Holt R.R., Yim S.J., Shearer G.C., Hackman R.M., Djurica D., Newman J.W., et al. Effects of short-term walnut consumption on human microvascular function and its relationship to plasma epoxide content. J. Nutr. Biochem. 2015;26(12):1458–1466. doi: 10.1016/j.jnutbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Jaceldo-Siegl K., Sabaté J., Batech M., Fraser G.E. Influence of body mass index and serum lipids on the cholesterol-lowering effects of almonds in free-living individuals. Nutr. Metab. Cardiovasc. Dis. 2011;21(suppl 1):S7–S13. doi: 10.1016/j.numecd.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Khorramirad A., Heidari S., Parham M., Jang S.A., Hozoori M. Pistachio nut effects on the blood lipid profile in patients with type II diabetes mellitus: a single-blind randomized crossover controlled clinical trial, Iran. Heart J. 2021;22(3):23–32. [Google Scholar]
- 55.Lima R.P.A., do Nascimento R.A.F., Luna R.C.P., Persuhn D.C., da Silva A.S., da Conceição Rodrigues Gonçalves M., et al. Effect of a diet containing folate and hazelnut oil capsule on the methylation level of the ADRB3 gene, lipid profile and oxidative stress in overweight or obese women. Clin. Epigenet. 2017;9:110. doi: 10.1186/s13148-017-0407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McKay D.L., Chen C.Y., Yeum K.J., Matthan N.R., Lichtenstein A.H., Blumberg J.B. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study. Nutr. J. 2010;9:21. doi: 10.1186/1475-2891-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palacios O.M., Maki K.C., Xiao D., Wilcox M.L., Dicklin M.R., Kramer M., et al. Effects of consuming almonds on insulin sensitivity and other cardiometabolic health markers in adults with prediabetes. J. Am. Coll. Nutr. 2020;39(5):397–406. doi: 10.1080/07315724.2019.1660929. [DOI] [PubMed] [Google Scholar]
- 58.Zaveri S., Drummond S. The effect of including a conventional snack (cereal bar) and a nonconventional snack (almonds) on hunger, eating frequency, dietary intake and body weight. J. Hum. Nutr. Diet. 2009;22(5):461–468. doi: 10.1111/j.1365-277X.2009.00983.x. [DOI] [PubMed] [Google Scholar]
- 59.Zibaeenezhad M.J., Rezaiezadeh M., Mowla A., Ayatollahi S.M., Panjehshahin M.R. Antihypertriglyceridemic effect of walnut oil. Angiology. 2003;54(4):411–414. doi: 10.1177/000331970305400404. [DOI] [PubMed] [Google Scholar]
- 60.Zibaeenezhad M.J., Shamsnia S.J., Khorasani M. Walnut consumption in hyperlipidemic patients. Angiology. 2005;56(5):581–583. doi: 10.1177/000331970505600509. [DOI] [PubMed] [Google Scholar]
- 61.Madan J., Desai S., Moitra P., Salis S., Agashe S., Battalwar R., et al. Effect of almond consumption on metabolic risk factors–glucose metabolism, hyperinsulinemia, selected markers of inflammation: a randomized controlled trial in adolescents and young adults. Front. Nutr. 2021;8(363):668622. doi: 10.3389/fnut.2021.668622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tapsell L.C., Batterham M.J., Teuss G., Tan S.Y., Dalton S., Quick C.J., et al. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur. J. Clin. Nutr. 2009;63(8):1008–1015. doi: 10.1038/ejcn.2009.19. [DOI] [PubMed] [Google Scholar]
- 63.Tapsell L.C., Lonergan M., Batterham M.J., Neale E.P., Martin A., Thorne R., et al. Effect of interdisciplinary care on weight loss: a randomised controlled trial. BMJ Open. 2017;7(7) doi: 10.1136/bmjopen-2016-014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casas-Agustench P., López-Uriarte P., Bulló M., Ros E., Cabré-Vila J.J., Salas-Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011;21(2):126–135. doi: 10.1016/j.numecd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Urpi-Sarda M., Casas R., Chiva-Blanch G., Romero-Mamani E.S., Valderas-Martínez P., Salas-Salvadó J., et al. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J. Nutr. 2012;142(6):1019–1025. doi: 10.3945/jn.111.148726. [DOI] [PubMed] [Google Scholar]
- 66.Casas R., Urpi-Sardà M., Sacanella E., Arranz S., Corella D., Castañer O., et al. Anti-inflammatory effects of the Mediterranean diet in the early and late stages of atheroma plaque development. Mediators Inflamm. 2017:3674390. doi: 10.1155/2017/3674390. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urpi-Sarda M., Casas R., Sacanella E., Corella D., Andrés-Lacueva C., Llorach R., et al. The 3-year effect of the Mediterranean diet intervention on inflammatory biomarkers related to cardiovascular disease. Biomedicines. 2021;9(8) doi: 10.3390/biomedicines9080862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le T., Flatt S.W., Natarajan L., Pakiz B., Quintana E.L., Heath D.D., et al. Effects of diet composition and insulin resistance status on plasma lipid levels in a weight loss intervention in women. J. Am. Heart Assoc. 2016;5(1) doi: 10.1161/JAHA.115.002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rock C.L., Flatt S.W., Pakiz B., Quintana E.L., Heath D.D., Rana B.K., et al. Effects of diet composition on weight loss, metabolic factors and biomarkers in a 1-year weight loss intervention in obese women examined by baseline insulin resistance status. Metabolism. 2016;65(11):1605–1613. doi: 10.1016/j.metabol.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al Abdrabalnabi A., Rajaram S., Bitok E., Oda K., Beeson W.L., Kaur A., et al. Effects of supplementing the usual diet with a daily dose of walnuts for two years on metabolic syndrome and its components in an elderly cohort. Nutrients. 2020;12(2):451. doi: 10.3390/nu12020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen C.G., Wang P., Zhang Z.Q., Ye Y.B., Zhuo S.Y., Zhou Q., et al. Effects of plant oils with different fatty acid composition on cardiovascular risk factors in moderately hypercholesteremic Chinese adults: a randomized, double-blinded, parallel-designed trial. Food Funct. 2020;11(8):7164–7174. doi: 10.1039/d0fo00875c. [DOI] [PubMed] [Google Scholar]
- 72.Foster G.D., Shantz K.L., Vander Veur S.S., Oliver T.L., Lent M.R., Virus A., et al. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am. J. Clin. Nutr. 2012;96(2):249–254. doi: 10.3945/ajcn.112.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agebratt C., Ström E., Romu T., Dahlqvist-Leinhard O., Borga M., Leandersson P., et al. A randomized study of the effects of additional fruit and nuts consumption on hepatic fat content, cardiovascular risk factors and basal metabolic rate. PLOS ONE. 2016;11(1) doi: 10.1371/journal.pone.0147149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baer D.J., Novotny J.A. Consumption of cashew nuts does not influence blood lipids or other markers of cardiovascular disease in humans: a randomized controlled trial. Am. J. Clin. Nutr. 2019;109(2):269–275. doi: 10.1093/ajcn/nqy242. [DOI] [PubMed] [Google Scholar]
- 75.Bamberger C., Rossmeier A., Lechner K., Wu L., Waldmann E., Stark R.G., et al. A walnut-enriched diet reduces lipids in healthy Caucasian subjects, independent of recommended macronutrient replacement and time point of consumption: a prospective, randomized, controlled trial. Nutrients. 2017;9(10) doi: 10.3390/nu9101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burns-Whitmore B., Haddad E., Sabaté J., Rajaram S. Effects of supplementing n-3 fatty acid enriched eggs and walnuts on cardiovascular disease risk markers in healthy free-living lacto-ovo-vegetarians: a randomized, crossover, free-living intervention study. Nutr. J. 2014;13:29. doi: 10.1186/1475-2891-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chisholm A., Mc Auley K., Mann J., Williams S., Skeaff M. Cholesterol lowering effects of nuts compared with a Canola oil enriched cereal of similar fat composition. Nutr. Metab. Cardiovasc. Dis. 2005;15(4):284–292. doi: 10.1016/j.numecd.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 78.Colquhoun D.M., Humphries J.A., Moores D., Somerset S.M. Effects of a macadamia nut enriched diet on serum lipids and lipoproteins compared to a low fat diet. Food Australia. 1996;48(5):216–221. [Google Scholar]
- 79.Curb J.D., Wergowske G., Dobbs J.C., Abbott R.D., Huang B. Serum lipid effects of a high-monounsaturated fat diet based on macadamia nuts. Arch. Intern. Med. 2000;160(8):1154–1158. doi: 10.1001/archinte.160.8.1154. [DOI] [PubMed] [Google Scholar]
- 80.Dhillon J., Thorwald M., De La Cruz N., Vu E., Asghar S.A., Kuse Q., et al. Glucoregulatory and cardiometabolic profiles of almond vs. cracker snacking for 8 weeks in young adults: a randomized controlled trial. Nutrients. 2018;10(8) doi: 10.3390/nu10080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Din J.N., Aftab S.M., Jubb A.W., Carnegy F.H., Lyall K., Sarma J., et al. Effect of moderate walnut consumption on lipid profile, arterial stiffness and platelet activation in humans. Eur. J. Clin. Nutr. 2011;65(2):234–239. doi: 10.1038/ejcn.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hiraoka-Yamamoto J., Ikeda K., Negishi H., Mori M., Hirose A., Sawada S., et al. Serum lipid effects of a monounsaturated (palmitoleic) fatty acid-rich diet based on macadamia nuts in healthy, young Japanese women. Clin. Exp. Pharmacol. Physiol. 2004;31(suppl 2):S37–S38. doi: 10.1111/j.1440-1681.2004.04121.x. [DOI] [PubMed] [Google Scholar]
- 83.Holscher H.D., Guetterman H.M., Swanson K.S., An R., Matthan N.R., Lichtenstein A.H., et al. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: a randomized controlled trial. J. Nutr. 2018;148(6):861–867. doi: 10.1093/jn/nxy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iwamoto M., Imaizumi K., Sato M., Hirooka Y., Sakai K., Takeshita A., et al. Serum lipid profiles in Japanese women and men during consumption of walnuts. Eur. J. Clin. Nutr. 2002;56(7):629–637. doi: 10.1038/sj.ejcn.1601400. [DOI] [PubMed] [Google Scholar]
- 85.Jia X., Li N., Zhang W., Zhang X., Lapsley K., Huang G., et al. A pilot study on the effects of almond consumption on DNA damage and oxidative stress in smokers. Nutr. Cancer. 2006;54(2):179–183. doi: 10.1207/s15327914nc5402_4. [DOI] [PubMed] [Google Scholar]
- 86.Kamoun A., Hammouda O., Turki M., Maaloul R., Chtourou M., Bouaziz M., et al. Moderate walnut consumption improved lipid profile, steroid hormones and inflammation in trained elderly men: a pilot study with a randomized controlled trial. Biol. Sport. 2021;38(2):245–252. doi: 10.5114/biolsport.2020.97676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kocyigit A., Koylu A.A., Keles H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2006;16(3):202–209. doi: 10.1016/j.numecd.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 88.Kris-Etherton P.M., Pearson T.A., Wan Y., Hargrove R.L., Moriarty K., Fishell V., et al. High-monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am. J. Clin. Nutr. 1999;70(6):1009–1015. doi: 10.1093/ajcn/70.6.1009. [DOI] [PubMed] [Google Scholar]
- 89.Kurlandsky S.B., Stote K.S. Cardioprotective effects of chocolate and almond consumption in healthy women. Nutr. Res. 2006;26(10):509–516. doi: 10.1016/j.nutres.2006.08.007. [DOI] [Google Scholar]
- 90.Liu Y., Hwang H.J., Kim H.S., Park H. Time and intervention effects of daily almond intake on the changes of lipid profile and body composition among free-living healthy adults. J. Med. Food. 2018;21(4):340–347. doi: 10.1089/jmf.2017.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mah E., Schulz J.A., Kaden V.N., Lawless A.L., Rotor J., Mantilla L.B., et al. Cashew consumption reduces total and LDL cholesterol: a randomized, crossover, controlled-feeding trial. Am. J. Clin. Nutr. 2017;105(5):1070–1078. doi: 10.3945/ajcn.116.150037. [DOI] [PubMed] [Google Scholar]
- 92.Morgan W.A., Clayshulte B.J. Pecans lower low-density lipoprotein cholesterol in people with normal lipid levels. J. Am. Diet Assoc. 2000;100(3):312–318. doi: 10.1016/S0002-8223(00)00097-3. [DOI] [PubMed] [Google Scholar]
- 93.Rajaram S., Burke K., Connell B., Myint T., Sabaté J. A monounsaturated fatty acid-rich pecan-enriched diet favorably alters the serum lipid profile of healthy men and women. J. Nutr. 2001;131(9):2275–2279. doi: 10.1093/jn/131.9.2275. [DOI] [PubMed] [Google Scholar]
- 94.Rajaram S., Connell K.M., Sabaté J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. Br. J. Nutr. 2010;103(6):907–912. doi: 10.1017/S0007114509992480. [DOI] [PubMed] [Google Scholar]
- 95.Sabaté J., Haddad E., Tanzman J.S., Jambazian P., Rajaram S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: a randomized feeding trial. Am. J. Clin. Nutr. 2003;77(6):1379–1384. doi: 10.1093/ajcn/77.6.1379. [DOI] [PubMed] [Google Scholar]
- 96.Sabaté J., Cordero-Macintyre Z., Siapco G., Torabian S., Haddad E. Does regular walnut consumption lead to weight gain? Br. J. Nutr. 2005;94(5):859–864. doi: 10.1079/bjn20051567. [DOI] [PubMed] [Google Scholar]
- 97.Sabaté J., Fraser G.E., Burke K., Knutsen S.F., Bennett H., Lindsted K.D. Effects of walnuts on serum lipid levels and blood pressure in normal men. N. Engl. J. Med. 1993;328(9):603–607. doi: 10.1056/NEJM199303043280902. [DOI] [PubMed] [Google Scholar]
- 98.Torabian S., Haddad E., Cordero-MacIntyre Z., Tanzman J., Fernandez M.L., Sabate J. Long-term walnut supplementation without dietary advice induces favorable serum lipid changes in free-living individuals. Eur. J. Clin. Nutr. 2010;64(3):274–279. doi: 10.1038/ejcn.2009.152. [DOI] [PubMed] [Google Scholar]
- 99.Salas-Huetos A., Moraleda R., Giardina S., Anton E., Blanco J., Salas-Salvado J., et al. Effect of nut consumption on semen quality and functionality in healthy males: a randomized controlled trial. Am. J. Clin. Nutr. 2018;108(5):953–962. doi: 10.1093/ajcn/nqy181. [DOI] [PubMed] [Google Scholar]
- 100.Akuamoah-Boateng L., Iyer S.S., Sales R.L., Lokko P., Lartey A., Monteiro J.B.R., et al. Effect of peanut oil consumption on energy balance. J. Appl. Res. 2007;2:185. [Google Scholar]
- 101.Sales R.L., Coehlo S.B., Costa N.M.B., Bressan J., Iyer S., Boateng L.A., et al. The effects of peanut oil on lipid profile of normolipidemic adults: a three-country collaborative study. J. Appl. Res. 2008;8(2):216–225. [Google Scholar]
- 102.Spaccarotella K.J., Kris-Etherton P.M., Stone W.L., Bagshaw D.M., Fishell V.K., West S.G., et al. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr. J. 2008;7:13. doi: 10.1186/1475-2891-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan S.Y., Mattes R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur. J. Clin. Nutr. 2013;67(11):1205–1214. doi: 10.1038/ejcn.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu L., Piotrowski K., Rau T., Waldmann E., Broedl U.C., Demmelmair H., et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. Metabolism. 2014;63(3):382–391. doi: 10.1016/j.metabol.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 105.Tey S.L., Brown R., Gray A., Chisholm A., Delahunty C. Nuts improve diet quality compared to other energy-dense snacks while maintaining body weight. J. Nutr. Metab. 2011;2011:357350. doi: 10.1155/2011/357350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abazarfard Z., Salehi M., Keshavarzi S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: a randomized controlled clinical trial. J. Res. Med. Sci. 2014;19(5):457–464. [PMC free article] [PubMed] [Google Scholar]
- 107.Abbaspour N., Roberts T., Hooshmand S., Kern M., Hong M.Y. Mixed nut consumption may improve cardiovascular disease risk factors in overweight and obese adults. Nutrients. 2019;11(7) doi: 10.3390/nu11071488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moreira Alves R.D., Boroni Moreira A.P., Macedo V.S., Bressan J., de Cássia Gonçalves Alfenas R., Mattes R., et al. High-oleic peanuts: new perspective to attenuate glucose homeostasis disruption and inflammation related obesity. Obesity (Silver Spring). 2014;22(9):1981–1988. doi: 10.1002/oby.20825. [DOI] [PubMed] [Google Scholar]
- 109.Barbour J.A., Howe P.R., Buckley J.D., Bryan J., Coates A.M. Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients. 2015;7(9):7381–7398. doi: 10.3390/nu7095343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bowen J., Luscombe-Marsh N.D., Stonehouse W., Tran C., Rogers G.B., Johnson N., et al. Effects of almond consumption on metabolic function and liver fat in overweight and obese adults with elevated fasting blood glucose: a randomised controlled trial. Clin. Nutr. ESPEN. 2019;30:10–18. doi: 10.1016/j.clnesp.2018.12.088. [DOI] [PubMed] [Google Scholar]
- 111.Canales A., Benedí J., Nus M., Librelotto J., Sánchez-Montero J.M., Sánchez-Muniz F.J. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra CHD-risk factor. J. Am. Coll. Nutr. 2007;26(3):225–232. doi: 10.1080/07315724.2007.10719605. [DOI] [PubMed] [Google Scholar]
- 112.Coates A.M., Morgillo S., Yandell C., Scholey A., Buckley J.D., Dyer K.A., et al. Effect of a 12-week almond-enriched diet on biomarkers of cognitive performance, mood, and cardiometabolic health in older overweight adults. Nutrients. 2020;12(4) doi: 10.3390/nu12041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Souza R.G.M., Gomes A.C., de Castro I.A., Mota J.F. A baru almond-enriched diet reduces abdominal adiposity and improves high-density lipoprotein concentrations: a randomized, placebo-controlled trial. Nutrition. 2018;55-56:154–160. doi: 10.1016/j.nut.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 114.Dhillon J., Tan S.Y., Mattes R.D. Almond consumption during energy restriction lowers truncal fat and blood pressure in compliant overweight or obese adults. J. Nutr. 2016;146(12):2513–2519. doi: 10.3945/jn.116.238444. [DOI] [PubMed] [Google Scholar]
- 115.Dusanov S., Svendsen M., Ruzzin J., Kiviranta H., Gulseth H.L., Klemsdal T.O., et al. Effect of fatty fish or nut consumption on concentrations of persistent organic pollutants in overweight or obese men and women: a randomized controlled clinical trial. Nutr. Metab. Cardiovasc. Dis. 2020;30(3):448–458. doi: 10.1016/j.numecd.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 116.Fatahi S., Haghighatdoost F., Larijani B., Azadbakht L. Effect of weight reduction diets containing fish, walnut or fish plus walnut on cardiovascular risk factors in overweight and obese women. Arch. Iran. Med. 2019;22(10):574–583. [PubMed] [Google Scholar]
- 117.Johnston C.S., Sweazea K.L., Schwab E., McElaney E.A. Almond ingestion contributes to improved cardiovascular health in sedentary older adults participating in a walking intervention: a pilot study. J. Funct. Foods. 2017;39:58–62. doi: 10.1016/j.jff.2017.10.010. [DOI] [Google Scholar]
- 118.Jung H., Chen C.Y.O., Blumberg J.B., Kwak H.-K. The effect of almonds on vitamin E status and cardiovascular risk factors in Korean adults: a randomized clinical trial. Eur. J. Nutr. 2018;57:2069–2079. doi: 10.1007/s00394-017-1480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Katz D.L., Davidhi A., Ma Y., Kavak Y., Bifulco L., Njike V.Y. Effects of walnuts on endothelial function in overweight adults with visceral obesity: a randomized, controlled, crossover trial. J. Am. Coll. Nutr. 2012;31(6):415–423. doi: 10.1080/07315724.2012.10720468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee Y., Berryman C.E., West S.G., Chen C.O., Blumberg J.B., Lapsley K.G., et al. Effects of dark chocolate and almonds on cardiovascular risk factors in overweight and obese individuals: a randomized controlled-feeding trial. J. Am. Heart Assoc. 2017;6(12) doi: 10.1161/JAHA.116.005162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Z., Song R., Nguyen C., Zerlin A., Karp H., Naowamondhol K., et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J. Am. Coll. Nutr. 2010;29(3):198–203. doi: 10.1080/07315724.2010.10719834. [DOI] [PubMed] [Google Scholar]
- 122.Maranhão P.A., Kraemer-Aguiar L.G., de Oliveira C.L., Kuschnir M.C., Vieira Y.R., Souza M.G., et al. Brazil nuts intake improves lipid profile, oxidative stress and microvascular function in obese adolescents: a randomized controlled trial. Nutr. Metab. (Lond). 2011;8(1):32. doi: 10.1186/1743-7075-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McKay D.L., Eliasziw M., Chen C.-Y.O., Blumberg J.B. A pecan-rich diet improves cardiometabolic risk factors in overweight and obese adults: a randomized controlled trial. Nutrients. 2018;10(3) doi: 10.3390/nu10030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pelkman C.L., Fishell V.K., Maddox D.H., Pearson T.A., Mauger D.T., Kris-Etherton P.M. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am. J. Clin. Nutr. 2004;79(2):204–212. doi: 10.1093/ajcn/79.2.204. [DOI] [PubMed] [Google Scholar]
- 125.Rock C.L., Flatt S.W., Barkai H.S., Pakiz B., Heath D.D. Walnut consumption in a weight reduction intervention: effects on body weight, biological measures, blood pressure and satiety. Nutr. J. 2017;16(1):76. doi: 10.1186/s12937-017-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rock C.L., Zunshine E., Nguyen H.T., Perez A.O., Zoumas C., Pakiz B., et al. Effects of pistachio consumption in a behavioral weight loss intervention on weight change, cardiometabolic factors, and dietary intake. Nutrients. 2020;12(7) doi: 10.3390/nu12072155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Somerset S., Graham L., Markwell K., Colquhoun D. Isoenergetic replacement of dietary saturated with monounsaturated fat via macadamia nuts can affect coronary risk in overweight subjects. e-SPEN J. 2013;12(1):143–144. [Google Scholar]
- 128.Tey S.L., Gray A.R., Chisholm A.W., Delahunty C.M., Brown R.C. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. J. Nutr. 2013;143(8):1254–1262. doi: 10.3945/jn.113.174714. [DOI] [PubMed] [Google Scholar]
- 129.Tindall A.M., Petersen K.S., Skulas-Ray A.C., Richter C.K., Proctor D.N., Kris-Etherton P.M. Replacing saturated fat with walnuts or vegetable oils improves central blood pressure and serum lipids in adults at risk for cardiovascular disease: a randomized controlled-feeding trial. J. Am. Heart Assoc. 2019;8(9) doi: 10.1161/JAHA.118.011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang J., Wang S., Henning S.M., Qin T., Pan Y., Yang J., et al. Mixed tree nut snacks compared to refined carbohydrate snacks resulted in weight loss and increased satiety during both weight loss and weight maintenance: a 24-week randomized controlled trial. Nutrients. 2021;13(5):1512. doi: 10.3390/nu13051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wien M.A., Sabaté J.M., Iklé D.N., Cole S.E., Kandeel F.R. Almonds vs complex carbohydrates in a weight reduction program. Int. J. Obes. Relat. Metab. Disord. 2003;27(11):1365–1372. doi: 10.1038/sj.ijo.0802411. [DOI] [PubMed] [Google Scholar]
- 132.Williams P.T., Bergeron N., Chiu S., Krauss R.M. A randomized, controlled trial on the effects of almonds on lipoprotein response to a higher carbohydrate, lower fat diet in men and women with abdominal adiposity. Lipids Health Dis. 2019;18(1):83. doi: 10.1186/s12944-019-1025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berryman C.E., West S.G., Fleming J.A., Bordi P.L., Kris-Etherton P.M. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: a randomized controlled trial. J. Am. Heart Assoc. 2015;4(1) doi: 10.1161/JAHA.114.000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chisholm A., Mann J., Skeaff M., Frampton C., Sutherland W., Duncan A., et al. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur. J. Clin. Nutr. 1998;52(1):12–16. doi: 10.1038/sj.ejcn.1600507. [DOI] [PubMed] [Google Scholar]
- 135.Damasceno N.R., Pérez-Heras A., Serra M., Cofán M., Sala-Vila A., Salas-Salvadó J., et al. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr. Metab. Cardiovasc. Dis. 2011;21(suppl 1):S14–S20. doi: 10.1016/j.numecd.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 136.Deon V., Del Bo C., Guaraldi F., Abello F., Belviso S., Porrini M., et al. Effect of hazelnut on serum lipid profile and fatty acid composition of erythrocyte phospholipids in children and adolescents with primary hyperlipidemia: a randomized controlled trial. Clin. Nutr. 2018;37(4):1193–1201. doi: 10.1016/j.clnu.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 137.Eastman W.A., Clayshulte B.J. Pecan effects on serum lipoproteins and dietary intakes of hyperlipidemic individuals consuming self-selected diets. Fam. Con. Sci. Res. J. 2005;33(3):197–207. [Google Scholar]
- 138.Gebauer S.K., West S.G., Kay C.D., Alaupovic P., Bagshaw D., Kris-Etherton P.M. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose-response study. Am. J. Clin. Nutr. 2008;88(3):651–659. doi: 10.1093/ajcn/88.3.651. [DOI] [PubMed] [Google Scholar]
- 139.Gozde O., Mercanligil S.M., Kabaran S., Ogmen S. Effects of walnut-enriched diet on blood lipids and glucose profiles in hyperlipidemic subjects: a randomized controlled trial. Prog. Nutr. 2019;21(4):825–835. doi: 10.23751/pn.v21i4.7852. [DOI] [Google Scholar]
- 140.Griel A.E., Cao Y., Bagshaw D.D., Cifelli A.M., Holub B., Kris-Etherton P.M. A macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women. J. Nutr. 2008;138(4):761–767. doi: 10.1093/jn/138.4.761. [DOI] [PubMed] [Google Scholar]
- 141.Holligan S.D., West S.G., Gebauer S.K., Kay C.D., Kris-Etherton P.M. A moderate-fat diet containing pistachios improves emerging markers of cardiometabolic syndrome in healthy adults with elevated LDL levels. Br. J. Nutr. 2014;112(5):744–752. doi: 10.1017/S0007114514001561. [DOI] [PubMed] [Google Scholar]
- 142.West S.G., Gebauer S.K., Kay C.D., Bagshaw D.M., Savastano D.M., Diefenbach C., et al. Diets containing pistachios reduce systolic blood pressure and peripheral vascular responses to stress in adults with dyslipidemia. Hypertension. 2012;60(1):58–63. doi: 10.1161/HYPERTENSIONAHA.111.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jenkins D.J., Kendall C.W., Marchie A., Parker T.L., Connelly P.W., Qian W., et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. 2002;106(11):1327–1332. doi: 10.1161/01.cir.0000028421.91733.20. [DOI] [PubMed] [Google Scholar]
- 144.Kasliwal R.R., Bansal M., Mehrotra R., Yeptho K.P., Trehan N. Effect of pistachio nut consumption on endothelial function and arterial stiffness. Nutrition. 2015;31(5):678–685. doi: 10.1016/j.nut.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 145.Kay C.D., Gebauer S.K., West S.G., Kris-Etherton P.M. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J. Nutr. 2010;140(6):1093–1098. doi: 10.3945/jn.109.117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morgan J.M., Horton K., Reese D., Carey C., Walker K., Capuzzi D.M. Effects of walnut consumption as part of a low-fat, low-cholesterol diet on serum cardiovascular risk factors. Int. J. Vitam. Nutr. Res. 2002;72(5):341–347. doi: 10.1024/0300-9831.72.5.341. [DOI] [PubMed] [Google Scholar]
- 147.Nestel P., Clifton P., Noakes M. Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J. Lipid. Res. 1994;35(4):656–662. doi: 10.1016/S0022-2275(20)41179-4. [DOI] [PubMed] [Google Scholar]
- 148.Ghadimi Nouran M., Kimiagar M., Abadi A., Mirzazadeh M., Harrison G. Peanut consumption and cardiovascular risk. Public Health Nutr. 2010;13(10):1581–1586. doi: 10.1017/S1368980009992837. [DOI] [PubMed] [Google Scholar]
- 149.O’Byrne D.J., Knauft D.A., Shireman R.B. Low fat-monounsaturated rich diets containing high-oleic peanuts improve serum lipoprotein profiles. Lipids. 1997;32(7):687–695. doi: 10.1007/s11745-997-0088-y. [DOI] [PubMed] [Google Scholar]
- 150.Orem A., Yucesan F.B., Orem C., Akcan B., Kural B.V., Alasalvar C., et al. Hazelnut-enriched diet improves cardiovascular risk biomarkers beyond a lipid-lowering effect in hypercholesterolemic subjects. J. Clin. Lipidol. 2013;7(2):123–131. doi: 10.1016/j.jacl.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 151.Rajaram S., Haddad E.H., Mejia A., Sabaté J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am. J. Clin. Nutr. 2009;89(5):1657S–1663S. doi: 10.3945/ajcn.2009.26736S. [DOI] [PubMed] [Google Scholar]
- 152.Ros E., Núñez I., Pérez-Heras A., Serra M., Gilabert R., Casals E., et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109(13):1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 153.Ruisinger J.F., Gibson C.A., Backes J.M., Smith B.K., Sullivan D.K., Moriarty P.M., et al. Statins and almonds to lower lipoproteins (the STALL Study) J. Clin. Lipidol. 2015;9(1):58–64. doi: 10.1016/j.jacl.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 154.Sheridan M.J., Cooper J.N., Erario M., Cheifetz C.E. Pistachio nut consumption and serum lipid levels. J. Am. Coll. Nutr. 2007;26(2):141–148. doi: 10.1080/07315724.2007.10719595. [DOI] [PubMed] [Google Scholar]
- 155.Spiller G.A., Jenkins D.A., Bosello O., Gates J.E., Cragen L.N., Bruce B. Nuts and plasma lipids: an almond-based diet lowers LDL-C while preserving HDL-C. J. Am. Coll. Nutr. 1998;17(3):285–290. doi: 10.1080/07315724.1998.10718761. [DOI] [PubMed] [Google Scholar]
- 156.Tamizifar B., Rismankarzadeh M., Vosoughi A.A., Rafieeyan M., Aminzade A. A low-dose almond-based diet decreases LDL-C while preserving. HDL-C. 2005;8(1):45–51. [Google Scholar]
- 157.Zambón D., Sabaté J., Muñoz S., Campero B B., Casals E., Merlos M., et al. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann. Intern. Med. 2000;132(7):538–546. doi: 10.7326/0003-4819-132-7-200004040-00005. [DOI] [PubMed] [Google Scholar]
- 158.Zhao G., Etherton T.D., Martin K.R., West S.G., Gillies P.J., Kris-Etherton P.M. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J. Nutr. 2004;134(11):2991–2997. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 159.West S.G., Krick A.L., Klein L.C., Zhao G., Wojtowicz T.F., McGuiness M., et al. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J. Am. Coll. Nutr. 2010;29(6):595–603. doi: 10.1080/07315724.2010.10719898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zibaeenezhad M.J., Ostovan P., Mosavat S.H., Zamirian M., Attar A. Almond oil for patients with hyperlipidemia: a randomized open-label controlled clinical trial, Complement. Ther. Med. 2019;42:33–36. doi: 10.1016/j.ctim.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 161.Hernández-Alonso P., Salas-Salvadó J., Baldrich-Mora M., Juanola-Falgarona M., Bulló M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care. 2014;37(11):3098–3105. doi: 10.2337/dc14-1431. [DOI] [PubMed] [Google Scholar]
- 162.Njike V.Y., Ayettey R., Petraro P., Treu J.A., Katz D.L. Walnut ingestion in adults at risk for diabetes: effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res. Care. 2015;3(1) doi: 10.1136/bmjdrc-2015-000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sapp P.A., Kris-Etherton P.M., Petersen K.S. Peanuts or an isocaloric lower fat, higher carbohydrate nighttime snack have similar effects on fasting glucose in adults with elevated fasting glucose concentrations: a 6-week randomized crossover trial. J. Nutr. 2022;152(1):153–162. doi: 10.1093/jn/nxab347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wien M., Bleich D., Raghuwanshi M., Gould-Forgerite S., Gomes J., Monahan-Couch L., et al. Almond consumption and cardiovascular risk factors in adults with prediabetes. J. Am. Coll. Nutr. 2010;29(3):189–197. doi: 10.1080/07315724.2010.10719833. [DOI] [PubMed] [Google Scholar]
- 165.Dikariyanto V., Smith L., Francis L., Robertson M., Kusaslan E., O’Callaghan-Latham M., et al. Snacking on whole almonds for 6 weeks improves endothelial function and lowers LDL cholesterol but does not affect liver fat and other cardiometabolic risk factors in healthy adults: the ATTIS study, a randomized controlled trial. Am. J. Clin. Nutr. 2020;111(6):1178–1189. doi: 10.1093/ajcn/nqaa100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guarneiri L.L., Paton C.M., Cooper J.A. Pecan-enriched diets alter cholesterol profiles and triglycerides in adults at risk for cardiovascular disease in a randomized, controlled trial. J. Nutr. 2021;151(10):3091–3101. doi: 10.1093/jn/nxab248. [DOI] [PubMed] [Google Scholar]
- 167.Olmedilla-Alonso B., Granado-Lorencio F., Herrero-Barbudo C., Blanco-Navarro I., Blázquez-García S., Pérez-Sacristán B. Consumption of restructured meat products with added walnuts has a cholesterol-lowering effect in subjects at high cardiovascular risk: a randomised, crossover, placebo-controlled study. J. Am. Coll. Nutr. 2008;27(2):342–348. doi: 10.1080/07315724.2008.10719710. [DOI] [PubMed] [Google Scholar]
- 168.Costa E.S.L.M., Pereira de Melo M.L., Faro Reis F.V., Monteiro M.C., Dos Santos S.M., Quadros Gomes B.A., et al. Comparison of the effects of Brazil nut oil and soybean oil on the cardiometabolic parameters of patients with metabolic syndrome: a randomized trial. Nutrients. 2019;12(1) doi: 10.3390/nu12010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gulati S., Misra A., Pandey R.M., Bhatt S.P., Saluja S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition. 2014;30(2):192–197. doi: 10.1016/j.nut.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 170.Hwang H.J., Liu Y., Kim H.S., Lee H., Lim Y., Park H. Daily walnut intake improves metabolic syndrome status and increases circulating adiponectin levels: randomized controlled crossover trial. Nutr. Res. Pract. 2019;13(2):105–114. doi: 10.4162/nrp.2019.13.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee Y.J., Nam G.E., Seo J.A., Yoon T., Seo I., Lee J.H., et al. Nut consumption has favorable effects on lipid profiles of Korean women with metabolic syndrome. Nutr. Res. 2014;34(9):814–820. doi: 10.1016/j.nutres.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 172.Mukuddem-Petersen J., Stonehouse Oosthuizen W., Jerling J.C., Hanekom S.M., White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. Br. J. Nutr. 2007;97(6):1144–1153. doi: 10.1017/S0007114507682944. [DOI] [PubMed] [Google Scholar]
- 173.Schutte A.E., Van Rooyen J.M., Huisman H.W., Mukuddem-Petersen J., Oosthuizen W., Hanekom S.M., et al. Modulation of baroreflex sensitivity by walnuts versus cashew nuts in subjects with metabolic syndrome. Am. J. Hypertens. 2006;19(6):629–636. doi: 10.1016/j.amjhyper.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 174.Wang X., Li Z., Liu Y., Lv X., Yang W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr. J. 2012;11:20. doi: 10.1186/1475-2891-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang D., Sun L., Liu X., Niu Z., Chen S., Tang L., et al. Replacing white rice bars with peanuts as snacks in the habitual diet improves metabolic syndrome risk among Chinese adults: a randomized controlled trial. Am. J. Clin. Nutr. 2021;113(1):28–35. doi: 10.1093/ajcn/nqaa307. [DOI] [PubMed] [Google Scholar]
- 176.Wu H., Pan A., Yu Z., Qi Q., Lu L., Zhang G., et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J. Nutr. 2010;140(11):1937–1942. doi: 10.3945/jn.110.126300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen C.M., Liu J.F., Li S.C., Huang C.L., Hsirh A.T., Weng S.F., et al. Almonds ameliorate glycemic control in Chinese patients with better controlled type 2 diabetes: a randomized, crossover, controlled feeding trial. Nutr. Metab. (Lond). 2017;14(1):51. doi: 10.1186/s12986-017-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Damavandi R.D., Eghtesadi S., Shidfar F., Heydari I., Foroushani A.R. Effects of hazelnuts consumption on fasting blood sugar and lipoproteins in patients with type 2 diabetes. J. Res. Med. Sci. 2013;18(4):314–321. [PMC free article] [PubMed] [Google Scholar]
- 179.Damavandi R.D., Mousavi S.N., Shidfar F., Mohammadi V., Rajab A., Hosseini S., et al. Effects of daily consumption of cashews on oxidative stress and atherogenic indices in patients with type 2 diabetes: a randomized, controlled-feeding trial. Int. J. Endocrinol. Metab. 2019;17(1) doi: 10.5812/ijem.70744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jenkins D.J.A., Kendall C.W.C., Lamarche B., Banach M.S., Srichaikul K., Vidgen E., et al. Nuts as a replacement for carbohydrates in the diabetic diet: a reanalysis of a randomised controlled trial. Diabetologia. 2018;61(8):1734–1747. doi: 10.1007/s00125-018-4628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li S.C., Liu Y.H., Liu J.F., Chang W.H., Chen C.M., Chen C.Y. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism. 2011;60(4):474–479. doi: 10.1016/j.metabol.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 182.Liu J.F., Liu Y.H., Chen C.M., Chang W.H., Chen C.Y. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: a randomized crossover controlled feeding trial. Eur. J. Nutr. 2013;52(3):927–935. doi: 10.1007/s00394-012-0400-y. [DOI] [PubMed] [Google Scholar]
- 183.Lovejoy J.C., Most M.M., Lefevre M., Greenway F.L., Rood J.C. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am. J. Clin. Nutr. 2002;76(5):1000–1006. doi: 10.1093/ajcn/76.5.1000. [DOI] [PubMed] [Google Scholar]
- 184.Ma Y., Njike V.Y., Millet J., Dutta S., Doughty K., Treu J.A., et al. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care. 2010;33(2):227–232. doi: 10.2337/dc09-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mohan V., Gayathri R., Jaacks L.M., Lakshmipriya N., Anjana R.M., Spiegelman D., et al. Cashew nut consumption increases HDL cholesterol and reduces systolic blood pressure in Asian indians with Type 2 diabetes: a 12-week randomized controlled trial. J. Nutr. 2018;148(1):63–69. doi: 10.1093/jn/nxx001. [DOI] [PubMed] [Google Scholar]
- 186.Müllner E., Plasser E., Brath H., Waldschütz W., Forster E., Kundi M., et al. Impact of polyunsaturated vegetable oils on adiponectin levels, glycaemia and blood lipids in individuals with type 2 diabetes: a randomised, double-blind intervention study. J. Hum. Nutr. Diet. 2014;27(5):468–478. doi: 10.1111/jhn.12168. [DOI] [PubMed] [Google Scholar]
- 187.Zibaeenezhad M.J.Z., Aghasadeghi K., Hakimi H., Yarmohammadi H., Nikaein F. The effect of walnut oil consumption on blood sugar in patients with diabetes mellitus type 2. Int. J. Endocrinol. Metab. 2016;14(3) doi: 10.5812/ijem.34889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Parham M., Heidari S., Khorramirad A., Hozoori M., Hosseinzadeh F., Bakhtyari L., et al. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: a randomized crossover trial. Rev. Diabet. Stud. 2014;11(2):190–196. doi: 10.1900/RDS.2014.11.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sauder K.A., McCrea C.E., Ulbrecht J.S., Kris-Etherton P.M., West S.G. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes: a randomized trial. Metabolism. 2015;64(11):1521–1529. doi: 10.1016/j.metabol.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sauder K.A., McCrea C.E., Ulbrecht J.S., Kris-Etherton P.M., West S.G. Pistachio nut consumption modifies systemic hemodynamics, increases heart rate variability, and reduces ambulatory blood pressure in well-controlled type 2 diabetes: a randomized trial. J. Am. Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sweazea K.L., Johnston C.S., Ricklefs K.D., Petersen K.N. Almond supplementation in the absence of dietary advice significantly reduces C-reactive protein in subjects with type 2 diabetes. J. Funct. Foods. 2014;10:252–259. doi: 10.1016/j.jff.2014.06.024. [DOI] [Google Scholar]
- 192.Tapsell L.C., Gillen L.J., Patch C.S., Batterham M., Owen A., Baré M., et al. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. 2004;27(12):2777–2783. doi: 10.2337/diacare.27.12.2777. [DOI] [PubMed] [Google Scholar]
- 193.Wien M., Oda K., Sabaté J. A randomized controlled trial to evaluate the effect of incorporating peanuts into an American Diabetes Association meal plan on the nutrient profile of the total diet and cardiometabolic parameters of adults with type 2 diabetes. Nutr. J. 2014;13:10. doi: 10.1186/1475-2891-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Campos V.P., Portal V.L., Markoski M.M., Quadros A.S., Bersch-Ferreira Â.C., Garavaglia J., et al. Effects of a healthy diet enriched or not with pecan nuts or extra-virgin olive oil on the lipid profile of patients with stable coronary artery disease: a randomised clinical trial. J. Hum. Nutr. Diet. 2020;33(3):439–450. doi: 10.1111/jhn.12727. [DOI] [PubMed] [Google Scholar]
- 195.Chen C.Y., Holbrook M., Duess M.A., Dohadwala M.M., Hamburg N.M., Asztalos B.F., et al. Effect of almond consumption on vascular function in patients with coronary artery disease: a randomized, controlled, cross-over trial. Nutr. J. 2015;14:61. doi: 10.1186/s12937-015-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ghanavati M., Alipour Parsa S., Nasrollahzadeh J. A calorie-restricted diet with nuts favourably raises plasma high-density lipoprotein-cholesterol in overweight and obese patients with stable coronary heart disease: a randomised controlled trial. Int. J. Clin. Pract. 2021;75(9) doi: 10.1111/ijcp.14431. [DOI] [PubMed] [Google Scholar]
- 197.Ghanavati M., Hosseinabadi S.M., Parsa S.A., Safi M., Emamat H., Nasrollahzadeh J. Effect of a nut-enriched low-calorie diet on body weight and selected markers of inflammation in overweight and obese stable coronary artery disease patients: a randomized controlled study. Eur. J. Clin. Nutr. 2021;75(7):1099–1108. doi: 10.1038/s41430-020-00819-9. [DOI] [PubMed] [Google Scholar]
- 198.Jamshed H., Sultan F.A., Iqbal R., Gilani A.H. Dietary almonds increase serum HDL cholesterol in coronary artery disease patients in a randomized controlled trial. J. Nutr. 2015;145(10):2287–2292. doi: 10.3945/jn.114.207944. [DOI] [PubMed] [Google Scholar]
- 199.Carvalho R.F., Huguenin G.V., Luiz R.R., Moreira A.S., Oliveira G.M., Rosa G. Intake of partially defatted Brazil nut flour reduces serum cholesterol in hypercholesterolemic patients—a randomized controlled trial. Nutr. J. 2015;14:59. doi: 10.1186/s12937-015-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huguenin G.V., Moreira A.S., Siant’Pierre T.D., Gonçalves R.A., Rosa G., Oliveira G.M., et al. Effects of dietary supplementation with brazil nuts on microvascular endothelial function in hypertensive and dyslipidemic patients: a randomized crossover placebo-controlled trial. Microcirculation. 2015;22(8):687–699. doi: 10.1111/micc.12225. [DOI] [PubMed] [Google Scholar]
- 201.Solà R., Valls R.M., Godàs G., Perez-Busquets G., Ribalta J., Girona J., et al. Cocoa, hazelnuts, sterols and soluble fiber cream reduces lipids and inflammation biomarkers in hypertensive patients: a randomized controlled trial. PLOS ONE. 2012;7(2) doi: 10.1371/journal.pone.0031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zibaeenezhad M.J., Farhadi P., Attar A., Mosleh A., Amirmoezi F., Azimi A. Effects of walnut oil on lipid profiles in hyperlipidemic type 2 diabetic patients: a randomized, double-blind, placebo-controlled trial. Nutr. Diabetes. 2017;7(4):e259. doi: 10.1038/nutd.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kaseb F., Rashidi M., Afkhami-Ardekani M., Fallahzadeh H. Effect of olive, almond and walnut oil on cardiovascular risk factors in type 2 diabetic patients. Int. J. Diabetes Dev. Ctries. 2013;33(2):115–119. doi: 10.1007/s13410-012-0108-9. [DOI] [Google Scholar]
- 204.Wilson P.W., D’Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 205.Nikodijevic C.J., Probst Y.C., Batterham M.J., Tapsell L.C., Neale E.P. Nut consumption in a representative survey of Australians: a secondary analysis of the 2011–2012 National Nutrition and Physical Activity Survey. Public Health Nutr. 2020;23(18):3368–3378. doi: 10.1017/S1368980019004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nishi S.K., Viguiliouk E., Blanco Mejia S., Kendall C.W.C., Bazinet R.P., Hanley A.J., et al. Are fatty nuts a weighty concern? A systematic review and meta-analysis and dose-response meta-regression of prospective cohorts and randomized controlled trials. Obes. Rev. 2021;22(11) doi: 10.1111/obr.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hébert J.R., Frongillo E.A., Adams S.A., Turner-McGrievy G.M., Hurley T.G., Miller D.R., et al. Perspective: randomized controlled trials are not a panacea for diet-related research. Adv. Nutr. 2016;7(3):423–432. doi: 10.3945/an.115.011023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Meng H., Zhu L., Kord-Varkaneh H., O Santos H., Tinsley G.M., Fu P. Effects of intermittent fasting and energy-restricted diets on lipid profile: a systematic review and meta-analysis. Nutrition. 2020;77:110801. doi: 10.1016/j.nut.2020.110801. [DOI] [PubMed] [Google Scholar]
- 209.Bes-Rastrollo M., Wedick N.M., Martinez-Gonzalez M.A., Li T.Y., Sampson L., Hu F.B. Prospective study of nut consumption, long-term weight change, and obesity risk in women. Am. J. Clin. Nutr. 2009;89(6):1913–1919. doi: 10.3945/ajcn.2008.27276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dawson J.A., Kaiser K.A., Affuso O., Cutter G.R., Allison D.B. Rigorous control conditions diminish treatment effects in weight loss-randomized controlled trials. Int. J. Obes. (Lond). 2016;40(6):895–898. doi: 10.1038/ijo.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Neale E.P., Tapsell L.C., Guan V., Batterham M.J. The effect of nut consumption on markers of inflammation and endothelial function: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7(11) doi: 10.1136/bmjopen-2017-016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ros E. Health benefits of nut consumption. Nutrients. 2010;2(7):652–682. doi: 10.3390/nu2070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tapsell L.C., Neale E.P., Probst Y. Dietary patterns and cardiovascular disease: insights and challenges for considering food groups and nutrient sources. Curr. Atheroscler. Rep. 2019;21(3):9. doi: 10.1007/s11883-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yang C., Xia H., Wan M., Lu Y., Xu D., Yang X., et al. Comparisons of the effects of different flaxseed products consumption on lipid profiles, inflammatory cytokines and anthropometric indices in patients with dyslipidemia related diseases: systematic review and a dose-response meta-analysis of randomized controlled trials. Nutr. Metab. (Lond). 2021;18(1):91. doi: 10.1186/s12986-021-00619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this review are available from the corresponding author upon reasonable request.