Abstract
Obesity and hypertension are intimately linked due to the various ways that the important cell types such as vascular smooth muscle cells (VSMC), endothelial cells (EC), immune cells, and adipocytes, communicate with one another to contribute to these two pathologies. Adipose tissue is a very dynamic organ comprised primarily of adipocytes, which are well known for their role in energy storage. More recently adipose tissue has been recognized as the largest endocrine organ because of its ability to produce a vast number of signaling molecules called adipokines. These signaling molecules stimulate specific types of cells or tissues with many adipokines acting as indicators of adipocyte healthy function, such as adiponectin, omentin, and FGF21, which show anti-inflammatory or cardioprotective effects, acting as regulators of healthy physiological function. Others, like visfatin, chemerin, resistin, and leptin are often altered during pathophysiological circumstances like obesity and lipodystrophy, demonstrating negative cardiovascular outcomes when produced in excess. This review aims to explore the role of adipocytes and their derived products as well as the impacts of these adipokines on blood pressure regulation and cardiovascular homeostasis.
Keywords: Adipose tissue, adipocytes, blood pressure, blood vessels
Graphical Abstract

1. INTRODUCTION:
The primary purpose of adipose fat tissue is derived from its capability as an energy storage silo in times of excess food intake and the ability to use these free fatty acids as energy in times of low nutrient availability. While the storage and mobilization of this lipid resource is a critical function of adipose tissue, it is far from its only function. The past several decades of research have revealed a plethora of important paracrine and endocrine functions which can affect nearby or distant tissues, many of which have measurable effects on blood pressure regulation and cardiovascular homeostasis.[1]
Many components of adipose tissue play important roles in the development of pathophysiological states which often lead to hypertension (HTN). Herein we will discuss the effect of adipose in different anatomical, hormonal, morphological, and cell signaling contexts and their typical end results. This review will highlight the fundamental discoveries which have enhanced our understanding of the influence of adipose on blood pressure homeostasis and how dysregulated adipose dependent signaling can lead to HTN and cardiovascular disease (CVD). We also postulate some future studies regarding adipose tissue and crosstalk with the cardiovascular system as well as novel or alternative targets for future therapeutic treatments.
The leading contributor of CVD events results from HTN, demonstrating that HTN constitutes the most modifiable cause of morbidity and mortality globally.[2],[3] With the new 2017 classification by the American Heart Association and the American College of Cardiology, systolic blood pressure measurements above 130 mmHg or diastolic blood pressure measurements above 80 mmHg indicate elevated arterial blood pressure that establishes a diagnosis of HTN, warranting early intervention to thwart CVD-related complications and death.[4] HTN is a complicated pathophysiological state which is thought to be the result of a variety of different conditions encompassing environmental, genetic, hemodynamic, endocrine, and neural influences in addition to many others (reviewed in[5]). This systems biology approach, described as a “Mosaic” by Dr. Irvine Page more than 60 years ago, has helped to inform our understanding of HTN as a multi-organ disease with many potential contributions.[6],[7]
As one of the many contributors to HTN, obesity is one of the most highly correlated pathophysiological conditions with HTN, and the Framingham Heart study suggests that nearly 80% of male patients with HTN and 65% of female patients with HTN cases were attributable directly to obesity.[8, 9].Obesity-related medical care costs constitute somewhere between $120–170 billion in annual direct and indirect costs.[10–14], On the other hand, lipodystrophy, a disease characterized by disturbances in fat distribution that can result in maldistribution or severe loss of adipose tissues,[15] shares a similar cardiovascular phenotype as obesity.[16] This indicates that the adipose tissue is an important regulator of cardiovascular physiology and that homeostasis requires balanced adipose quantity and quality in the right locations throughout the body. Additionally, CVD remains the most prevalent cause of death, with nearly 18 million deaths globally per annually attributable to CVD and nearly 700,000 CVD-related deaths in the US alone.,[17] Apart from the high rate of morbidity and mortality, CVD represents a tremendous economic cost across the globe. US spending on CVD-related hospitalizations alone was $109.1 billion in 2012,[18] and direct costs are projected to increase to more than $200 billion and $40 billion in indirect costs by the year 2030.[19] With the serious public health burden of both CVD and obesity, especially given their high correlation with one another, insight into the influence that obesity and adiposity play on the development of HTN is an increasingly important concern for public health.
2. Morphological and Structural Differences in Adipose Tissue Types:
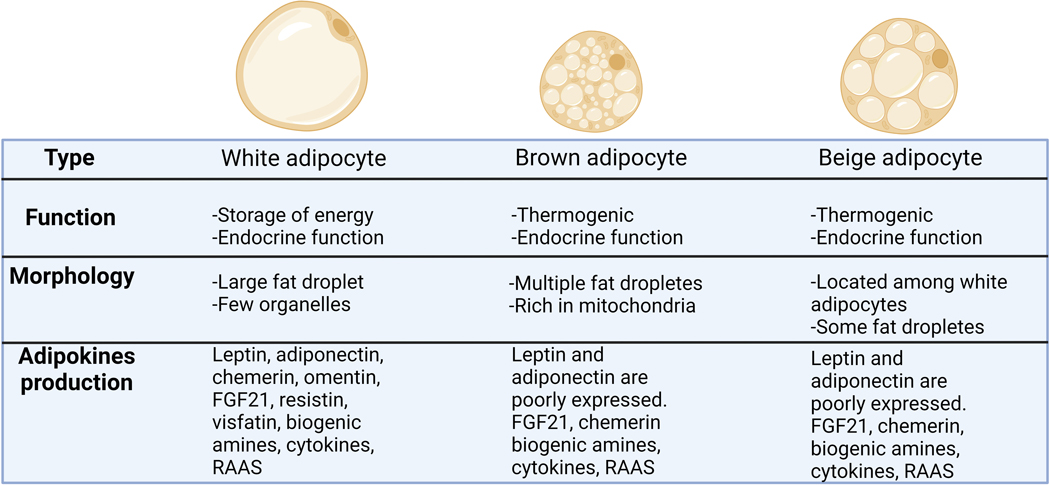
Three different groups of adipose tissue have been characterized by their distinct morphologies: White Adipose Tissue (WAT), Brown Adipose Tissue (BAT), and beige (or brite) adipose tissue (Figure 1). WAT is the most common form of adipose tissue within the body and is comprised of nearly 90% lipid content.[20] Several cell types are commonly found in WAT with adipocytes constituting approximately 1/3 of these, however, others include mesenchymal stromal cells, fibroblasts, macrophages, endothelial cells, pericytes, and pre-adipocytes.[21] Traditionally, WAT has been described as a tissue that passively stores excess energy as triglycerides.[22] However, recent studies have discovered that WAT is a highly active endocrine organ through its secretion of a vast number of molecules, therefore fine tuning the amount and quality of adipose tissue is essential for conferral of cardiovascular balance.[23] WAT is often found in predictable locations in healthy individuals, constituting 80% of total body fat,[24] with approximately 10–25% body weight for healthy males and 15–30% for healthy females.[25, 26] Males tend to accumulate more WAT in the visceral regions (mesenteric, retroperitoneal, and omental),[27, 28] while females typically have higher gluteofemoral deposition with less visceral adipose.[29, 30] Additionally, WAT is subject to significant age and/or sex hormone-dependent signaling which alters the rate and location of the adipose deposition.[31–33] Furthermore, deposition of epicardial and perivascular WAT will increase as total adiposity increases.[34] One of the most studied specific adipose tissue depots is the perivascular adipose tissue (PVAT), a unique adipose located surrounding the vasculature that secrets many vasoactive compounds which have been shown to contribute to both the physiological and pathophysiological function of the arteries which they surround.[35] We will discuss each of these components in more detail and the critical signaling mechanisms which occur from each type of WAT, as well as the signaling environment in which different WAT is often found.
Figure1. Adipocyte’s characterization.
White adipocytes store energy as a single large lipid droplet and have important endocrine functions. Brown adipocytes are smaller than white adipocytes and present multiple lipid droplets and a large number of mitochondria, [rich in Uncoupling Protein 1 (UCP1)], which is associated with thermogenesis. Brown adipocytes present a mild endocrine function. Beige adipocytes or brite adipocytes are an inducible form of thermogenic adipocytes that is found within WAT depots. They present several lipid droplets, but less than brown adipocytes, and a mild endocrine function.
BAT is named for the dark color of the tissue caused by the higher concentration of mitochondria within each adipocyte.[36] BAT consists of brown adipocytes, VSMC, endothelial cells, lymphocytes, fibroblasts, and stem cells. BAT was first found in the scapula of a groundhog in the 16th century by the Swiss naturalist Konrad Gessner who believed BAT might be a gland associated with hibernation. Many centuries later BAT was found to be a mature tissue,[37, 38] however it took many decades to elucidate its function. Today, BAT is well-known as a thermogenic organ due to its crucial role in the regulation of energy metabolism and systemic glucose balance and high expression of uncoupler proteins (i.e. UCP1, UCP2, and UCP3), which allow for heat generation through the loss of mitochondrial membrane potential.[39] The amount of BAT depends heavily on age group, as BAT in infants accounts for about 2–5% of body weight, whereas adult BAT accounts for less than 0.1% of total body mass. [40, 41] In some infants, this silo of BAT will continue to expand and develop into a tumor (known as a hibernoma) rather than transforming into other tissues or shrinking in size. It has been hypothesized that this condition is caused by abnormal mineralocorticoid signaling in brown adipose, which is caused by aldosterone.[42] Most BAT will change over the course of development to become similar in function and appearance to WAT, though some may be converted to skeletal muscle by signals such as PRDM16.[43] In adults, BAT is mainly found in cervical regions,[44] perirenal/adrenal area,[37] and surrounding the blood vessels as PVAT,[38] though it is not present in all arteries and is primarily located in regions of the thoracic aorta. While the PVAT found in the thoracic aorta is primarily comprised of BAT, adipocyte phenotypic switching, also known as a whitening or browning event, occurs when the composition of adipose tissue changes under particular circumstances, such as growth and development and pathological signaling linked to obesity.[45],[46] We will dedicate special attention to PVAT throughout this review article in light of its unique role in the regulatory control of vascular tone and vascular function.[47]
Interestingly, BAT mass and activity are greater in adult women than in adult men[33], however, it remains unclear why this sex difference is observed. Although adults do not have a large quantity of BAT, under certain environments, this amount can fluctuate significantly. For example, in northern Finland, BAT mass is greater in outdoor workers than in indoor workers [48], furthermore, the prevalence of detectable BAT is more pronounced in the winter and less in the summer,[41] indicating that low temperature may trigger an upregulation of BAT to generate heat. However, it is unclear whether there is a relationship between the quantity of BAT and blood pressure control.
Like WAT, BAT also is considered an endocrine organ and can secret diverse molecules including adiponectin, fibroblast growth factor 21 (FGF21), and neuregulin-4 (NRG4). Furthermore, in a very interesting study, Zhao et al 2022[49] showed that small extracellular vesicles secreted by BAT participate in exercise-induced cardioprotection by delivering micro-RNA molecules (miRNAs) to the heart during myocardial ischemia/reperfusion injury, indicating that not only bioactive molecules can be produced by adipose tissue, but vesicles, possibly adipo-vesicles, which have a very specific response for cardiovascular homeostasis. Moreover, a UCP-diphtheria toxin A (DTA) transgenic mouse, a very useful mouse to reduce BAT via targeted removal of UCP-DTA expressing cells, showed elevated body weight, cardiac abnormalities, and HTN, however it was not clear whether the lack of BAT causes HTN and cardiac dysfunction due to dysregulated metabolomics or whether these changes are due to other unforeseen effects of this model.[50] Thus the thermogenesis and endocrine functions of BAT might be crucial to regulate blood pressure by producing adipokines which will impact the cardiovascular function directly, or by regulating the metabolic profile, which in turn, can confer cardiovascular protection via a secondary mechanism.
Finally, a newly discovered type of tissue called “Beige” Adipose Tissue (or “brown in white” adipose tissue) describes a hybrid arrangement where a small proportion of brown adipocytes are located within a collection of white adipocytes.[51] The distribution of these various different tissues tends to be concentrated in specific regions of the body, contributing to the provision of a variety of functions in the anatomical locations in which they are found. Contrary to BAT, beige adipose tissue is often found in WAT tissue, where its primary function is the production of heat.
Our understanding of adipose has developed over the past few decades reveling many of the signaling process controlled by different types of adipose tissue. In addition to afferent responses to many organ systems, adipose tissue provides many of the necessary components for signaling throughout the body ranging from its function as an endocrine tissue, paracrine effects on surrounding tissue, and its self-regulatory autocrine effects. This review will focus on the adipose-derived “adipokine” signaling molecules which influence both local and distal tissues in important ways to regulate metabolic and cardiovascular function. We will also focus on how adipokines regulate, and are regulated by, the quality and function of adipose tissues.
3. Adipo-Receptors and adipokines
All different types of pharmacological receptors are expressed in adipocytes:
Ligand-gated ion channels: Transient receptors potential cation channel;[52] Nicotinic acetylcholine receptors (nAChRs);[53] γ-aminobutyric acid (GABA) receptors.[54]
Tyrosine kinase-coupled: Leptin receptors - OB-R; Insulin;[55, 56] Glucagon;[57] Insulin-like Growth Factor-I and II - IGF-I and II;[58] Growth hormone – GH;[59] Thyroid-stimulating hormone –THS;[60] Tumor necrosis factor-alpha - TNF-α;[61, 62] Interleukin-6 - IL-6;[63] Adenosine;[64] Gastric inhibitory peptide – GIP;[61, 65] Glucagon-like peptide-1 - GLP-1;[66] Neuropeptide Y-Y1 - NPY-Y1;[67] Atrial natriuretic peptide – ANP;[68] Epidermal, Platelet-derived, and fibroblast growth factors - (EGF, PDGF, FGF);[69–71] Transforming growth factor-beta TGF-β);[72, 73] receptors.
Intracellular steroid: Androgen;[74, 75] Estrogen;[74, 76] Progesterone;[77] Vitamin D;[78] Glucocorticoids receptors.[70]
G-protein-coupled (GPCR): Angiotensin II receptor;[79, 80] adiponectin receptors 1 and 2;[81] Catecholamine receptors (adrenergic receptors - α1, α2, β1, β2 and β3);[82, 83]
Trafficking receptors: Lipoprotein receptors (Very low density lipoproteins – (VLDL);[84] Low-density lipoproteins – (LDL);[85] High-density lipoproteins – (HDL)[86] and sortilin1.[87]
These receptors play crucial role on controlling the profile of adipocytes via function (endocrine, autocrine, and paracrine), differentiation (preadipocytes to adipocytes), mass (lipolysis and lipogenesis), and phenotype (browning and whitening processes).
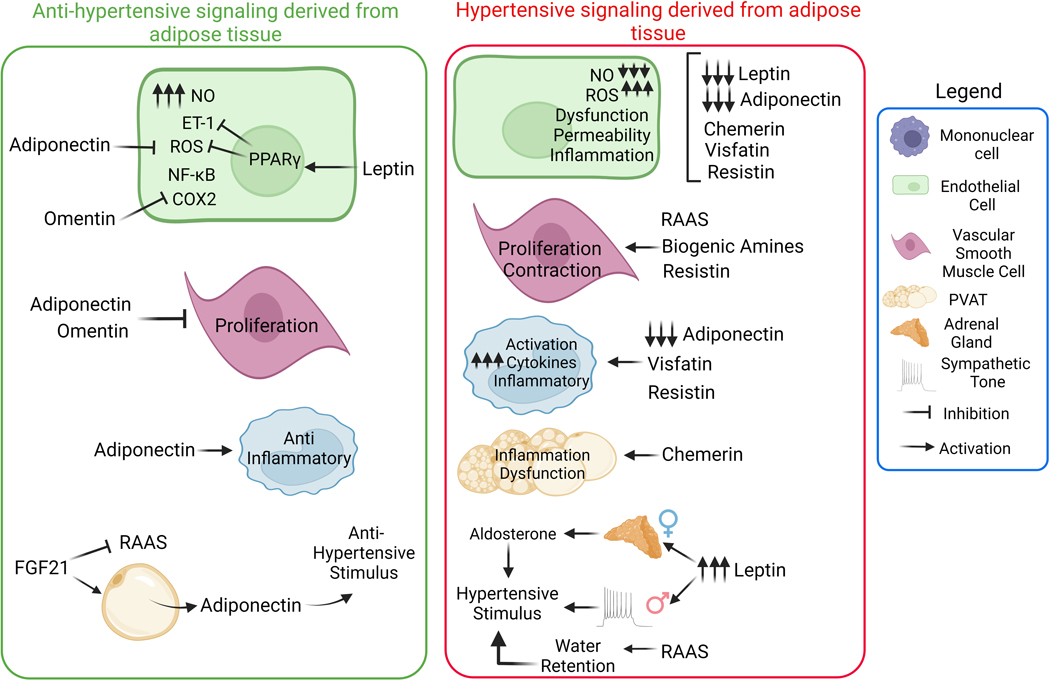
More than 600 bioactive molecules produced by adipose tissue are referred to as adipokines. Those molecules can act as autocrine, paracrine, and endocrine mediators, which play a crucial role in metabolic function, behavioral changes, immune responses, autonomic nervous system changes, cardiac performance, water balance, and vascular tone, which in turn will impact the regulation of blood pressure[88]. Leptin, adiponectin, chemerin, omentin, FGF21, resistin, visfatin, biogenic amines [norepinephrine, serotonin (5HT), histamine), and the anti-or pro-inflammatory cytokines interleukin-(IL-)10, IL-6, and tumor necrosis factor alpha (TNFα) are among the most studied adipokines, therefore these will be at the center of our focus (Figure 2).
Figure 2. Pro and anti-hypertensive stimulus caused by adipocytes-derived molecules.
Adipokines can interfere on cardiovascular function by diverse mechanisms: modulating endothelial and vascular function, maintaining the vascular phenotype, acting as an inflammatory regulator via direct mechanisms in the vasculature or immune cells. Furthermore, adipokines can regulate the secretion of other hormones such as aldosterone. Adipokines also regulate the cardiovascular system on a sex dependent manner (leptin induces hypertension in male, dependent on sympathetic activation, whereas in female it depends on aldosterone secretion. NO, nitric oxide; ROS, reactive oxygen species; ET-1, endothelin-1; PPARγ, peroxisome proliferator activated receptor gamma; FGF21, fibroblast growth factor 21; RAAS, renin–angiotensin–aldosterone system; NF-κB, Nuclear factor kappa B; COX2, cyclooxygenase-2.
3.1. Leptin:
The 16 kDa adipokine called Leptin, colloquially known as the satiety hormone, is most commonly synthesized in WAT with small levels produced from BAT, mammary tissue, skeletal muscle, bone marrow and stomach.[89] This hormone is subsequently secreted in response to food intake and interacts primarily with receptors in the hypothalamus to regulate appetite, energy expenditure, thermogenesis, immune function, insulin sensitivity and metabolism.[90–92] Leptin levels in serum plasma are often significantly elevated in individuals with obesity,[93, 94] because their levels are strictly associated with adipose fat mass. High levels of serum leptin often lead to “resistance” to leptin, wherein leptin fails to induce many of the metabolic effects, while still affecting the cardiovascular system.[95–97] which have been confirmed by a vast number of in vitro and in vivo models which demonstrate that leptin plays a significant role in the pathological development of HTN and cardiovascular disease.[98–100]
In obesity, leptin is described as a modulator of blood pressure through the regulation of sympathetic tone. Sprague-Dawley rats treated intravenously with recombinant leptin (1000 μg/kg) show elevated sympathetic nerve activity, in the kidneys, hindlimbs, and adrenal glands[101]. In contrast, Zucker rats, which possess a mutation in the gene for the leptin receptor, do not respond to leptin-induced sympathetic activity,[102] indicating that sympatho-activation of leptin requires the presence of an intact leptin receptor. Furthermore, deletion of protein tyrosine phosphatase 1B (Ptp1b), a key phosphatase for regulating leptin sensitivity, exacerbated leptin-induced HTN,[101] suggesting that Ptp1b is a master modulator and potential pharmacological target for the leptin-induced effects on cardiovascular physiology. Interestingly, the effects of Ptpb1b appear independent of proopiomelanocortin (POMC) neurons, as deletion of Ptp1b from POMC neurons, which have important effects on blood pressure, food intake, and energy expenditure[103], did not impact leptin-induced HTN[104]. Moreover, leptin exerts differential increases in blood pressure which have been found to be sex-dependent. In males leptin-induced HTN occurs through hyperactivation of sympathetic neurons[105, 106], whereas pre-menopausal females show leptin-induced HTN proceeds through elevated production of aldosterone due to a direct effect of leptin with its receptors located in their adrenal glands.[107] Findings of sex-dependent differences are a vital observation for proper identification of the ideal pharmacological tool to treat HTN-related cardiovascular risks in males and females. These findings imply that leptin-induced HTN and other related cardiovascular diseases may benefit from pharmaceutical interventions that target sympathetic activation in males and postmenopausal females or mineralocorticoid receptors in pre-menopausal females.
Interestingly, in congenital or acquired generalized lipodystrophy (CGL)-also known as Berardinelli-Seip Syndrome-nearly all lipocytes are absent, and leptin concentrations in both plasma and CSF are negligible, leading to aberrant metabolic function. These phenotypes commonly include high insulin resistance and type II diabetes mellitus, as well as high serum triglyceride concentrations correlated with the severity of lipodystrophy.[15, 16] Many of these conditions can lead to pancreatitis, non-alcoholic steatohepatitis, liver failure, and new studies are investigating the links between lipodystrophy and CVD-related disorders like HTN, atherosclerosis and hypertrophic cardiomyopathy.[108] In recent years leptin supplementation has been approved as an effective therapeutic treatment for many of the metabolic disorders associated with CGL.[107, 109] In mouse models of CGL, leptin supplementation has been sufficient to restore metabolic function, as well as improve cardiovascular physiology[110]. In this context, we observed that leptin treatment for 7 days improves the vascular function in a mouse model of congenital and acquired (induced by antiretroviral drug) lipodystrophies. Strikingly, this was mediated by the direct effect of leptin on endothelial cells, wherein activation of endothelial leptin receptors modulates oxidant status in the vasculature[111]. These data strongly suggest that basal activation of leptin signaling in endothelial cells is pivotal for vascular homeostasis. Aligned with the idea that leptin signaling controls vascular balance, we recently found that the ablation of leptin receptors in endothelial cells induces endothelial dysfunction and impairs vascular contractility per se[111, 112]. In addition, Hubert el al. 2017 observed that endothelial leptin signaling, but not leptin signaling in VSMC, facilitates neointima formation independent on body fat composition by regulating endothelin-1 production in endothelial cells via peroxisome proliferator-activated receptor γ (PPARγ)[113] Furthermore, large vessels such as aorta are still responsive to circulating leptin[114] which suggests that endothelial cells do not become resistant to leptin in obesity as a mechanism to counterbalance the HTN associated with obesity. Brown et al. 2015 showed that metreleptin (a recombinant analog of the human hormone leptin) does not affect the HTN in lipodystrophic patients after 6 months of treatment, but it significantly decreased the systolic and diastolic blood pressure after 12 months of supplementation indicating that leptin is a key regulator of blood pressure maintenance.[115] However, a key piece of this puzzle needs to be found: Why and how does leptin regulate blood pressure? Does it regulate metabolic profile and subsequently normalize the blood pressure, or it affects the vascular function directly?
Studies on leptin and its relationship to obesity and lipodystrophies are complicated by the hormone’s impact on metabolism. Future research on this model may employ a variety of techniques, such as conditional leptin receptor knockout mice in particular organs to ascertain the precise function of each cell type in the emergence of leptin-induced obesity. as it has been observed in obese ob/ob mice which lack functional leptin.[116] The precise involvement of cellular leptin signaling in the onset of adiposity and HTN may be clarified by using tissue specific knock out mouse model of the leptin receptor backcrossed with Bscl2, the gene that codes for the protein Seipin (related to congenital lipodystrophy type 2) or ob/ob mice, which will generate a deficient mice for circulating leptin with lack in leptin signaling in a specific cell population. The biggest obstacle to additional in-depth investigations continues to be the time and financial expenditures associated with constructing these models, despite their potential utility.
3.2. Adiponectin:
Adiponectin is a protein consisting of 244 amino acids with a molecular weight of approximately 28 kDa. This adipokine is produced and secreted almost exclusively from adipocytes[117], with three receptors discovered thus far: AdipoR1, AdipoR2, and T-cadherin (CDH13 or H-cadherin)[118]. AdipoR1 and R2 are broadly studied, whereas T-cadherin remains relatively understudied and remains somewhat controversial. Multiple studies have linked a deficiency in adiponectin to CVD and many related co-morbid factors such as insulin resistance,[119] type II diabetes, [120] obesity-related HTN,[121] hepatic fibrosis,[122] atherosclerosis [123] and ischemic heart disease[124]. A study that monitored a group of individuals over a 5-year period to identify potential early biomarkers of HTN identified hypoadiponectinemia in 1/3 of the patients who developed HTN by the end of the 5 years[125], suggesting that adiponectin may be an important molecule which helps to maintain healthy blood pressure. Interestingly, follow up studies reveal that individuals with hypoadiponectinemia had 400% higher rates of HTN-related morbidity than individuals with normal plasma adiponectin concentrations[126]. This has led to the identification of single nucleotide polymorphisms in the adiponectin gene (I164T and G276T substitutions) which cause a dominant negative hypoadiponectinemia-like phenotype which also strongly correlates with HTN, insulin resistance and type II diabetes.[121, 127, 128]
Adiponectin knockout mice display endothelial dysfunction, though these mice do not present with HTN at baseline. A high-salt diet, however, induces severe HTN in adiponectin null mice.[129] These mice also display impaired endothelial-dependent vasorelaxation in response to acetylcholine, express significantly less endothelial nitric oxide synthase (eNOS) mRNA in the aorta, demonstrate reduced nitric oxide (NO) metabolites in blood plasma, and less activation of eNOS following high-salt diet.[129] Several possible mechanistic explanations have been offered for the blood pressure-mediated effects of adiponectin through endothelial cell-regulated influences. The researchers also showed that replenishment of adiponectin expression via viral delivery raised NO metabolites, increased aortic eNOS expression, and restored normotensive blood pressure.[129] These changes were all prevented with co-treatment of L-nitro arginine methyl ester (L-NAME), indicating that the adiponectin-facilitated resistance to salt-induced HTN is mediated through eNOS. Similar studies have revealed that adiponectin plays a significant role in the development of salt-induced hypertension via working with cyclooxygenase (COX) enzymes.[130] COX-2 is an important player in endothelial cells which produces vasoactive prostanoids such as prostaglandin (PGI2),[131, 132] which have been shown to interact with adiponectin to ameliorate CVD-related co-morbidities.[124, 133, 134] One study showed that mice with endothelial cell-specific COX-2 knockout and adiponectin knockout show exacerbated HTN. Additionally, vascularization, which has previously been shown to be a COX-2-dependent,[135–137] was impaired in adiponectin knockout animals following ischemia[130]. These studies indicate important connections between adipose and endothelium via adiponectin signaling, however further studies are needed to define the direct connections which link these mechanisms.
In addition to effect on endothelial cells, adiponectin has been shown to influence HTN through immune cell function[138–142]In cultured macrophages, higher levels of adiponectin have been demonstrated to inhibit tumor necrosis factor α (TNFα)-mediated signaling and production[143, 144] and adiponectin overexpression suppressed TNFα-mediated hyperproliferation following retinal microvascular ischemia in mice.[145] Studies have also shown that adiponectin impairs foam cell formation,[146] and its overexpression protects against atherosclerosis in an atherogenic apolipoprotein E knockout mouse model[147]. These results suggest that adiponectin has a direct anti-inflammatory effect on macrophages, in line with recent research reviewing the role of immune cells in the development of systemic HTN[148]. Similarly, treatment of mouse peritoneal macrophages and stromal vascular fractions (vascular components and macrophages without adipocytes) upregulates interleukin-10, arginase-1, and lectin-1, which are classical M2 “resolving” macrophage markers, while suppressing classical M1 “inflammatory” macrophage markers TNFα, interleukin-6, and monocyte chemotactic protein-1[149]. All of these findings point to the possibility that adiponectin promotes the resolution of inflammatory signaling while suppressing pathological immune signaling and may lessen the severity of a number of vasculopathies, though the precise mechanisms by which it induces changes in macrophage behavior and function are still not fully understood.
Although this plausible connection between immune response and adiponectin has been indirectly described,[150] there is no evidence whether adiponectin-mediated immune signaling can control the genesis and progress of HTN. Conditional knockout mice for adiponectin receptors are available,[151] and they would be key to the confirmation of the connection between adiponectin, immune cells, and HTN. Adiponectin is a pleiotropic protein with several impacts on metabolic balance, much like leptin. The ideal method for analyzing the role of adiponectin in blood pressure regulation without influence from metabolic changes would be to create tissue-specific knockout mice for adiponectin receptors.
3.3. Chemerin
Chemerin, also known as retinoic acid receptor responder protein 2 (RARRES2) or tazarotene-induced gene-2 protein has a molecular weight of ~16 kDa and is best characterized by its production in adipose tissue[152], liver[153], and skin[154]. As an adipokine, circulating chemerin levels are associated with changes in body weight index, in other words, obese humans or animals display an increase in chemerin circulating levels[155], which in turn, is strongly correlated with obesity associated comorbidities including low grade inflammation[156], insulin resistance[157], PVAT dysfunction[158], endothelial and vascular dysfunction[159], and renal injury[160].
Chemerin is initially produced as a precursor, a 163 amino acid named prochemerin. The prochemerin undergoes processing by multiple proteases including cathepsins, elastase, and tryptase, which limits the concentration of prochemerin and increases chemerin levels[161, 162] though it is not clear yet whether the effects of prochemerin differs from chemerin. In addition, increases in protease activity plus the augmented fat mass in obesity[163, 164] could synergistically work to elevate the chemerin production and bioavailability. Thus, low grade inflammation in obesity could be attenuated by blocking the cleavage of prochemerin to chemerin.
Different receptors for chemerin have been identified so far, including chemokine-like receptor 1 (CMKLR1 or ChemR23), which is a Gi protein-linked receptor mainly expressed in immune cells, adipocytes, VSMC, and endothelial cells, chemokine receptor like 2 (CCRL2), and G-protein-coupled receptor 1 (GPR1)[165, 166]. ChemR23 is the most thoroughly studied receptor with a commercial antagonist available (CCX832) that can be used in both in vivo and in vitro experiments[160].
In rodent models of obesity, hypertension, and human mesenteric arteries, chemerin has demonstrated a negative impact on vascular function and blood pressure control. Chemerin seems to have a vasoconstriction action in aortae and mesenteric arteries[167] via modulating the endothelin-1 production, Erk1/2 activation[168], and NADPH oxidase-derived ROS[169], which perhaps can explain the reduced NO bioavailability and uncoupling eNOS[170] in aortae treated with chemerin, such pathway is dependent on CMKLR1 activation[167]. Nowadays, PVAT is considered a part of the blood vessel per se, and its presence is a very potent regulator of vascular tone[171] placing PVAT as an indispensable layer for vascular physiology. PVAT seems to produce a substantial amount of chemerin, which modulates adrenergic vascular constriction at naïve arteries (control arteries), whereas in pathophysiological environments[167], such as HTN and obesity, arteries are more susceptible to chemerin-induced vascular constriction[167]. All these findings support the idea that chemerin regulates vascular tone and that chemerin signaling is amplified in damaged endothelium.
PVAT is formed by adipocytes, other cells, and sympathetic nerve[171]. Interestingly, chemerin production by PVAT seems to be, at least in part, dependent on sympathetic activity. In a well-designed study, Darios et al 2016[172] discovered that electrical-field stimulation promotes vascular constriction in rat superior mesenteric arteries by leading to chemerin production from PVAT, this was confirmed by blocking the CMKLR1 receptor via pharmacological approach[172] or using chemerin deficient rat[173]. Chemerin-9, a chemerin analogue, can boost catecholamine synthesis in isolated adrenal glands when administered ex vivo because it binds to chemerin receptors in the adrenal[174]. Moreover, the main source of aldosterone is the adrenal gland[175, 176], it is unknown if chemerin can trigger aldosterone production by the adrenal gland. All of these studies suggest that chemerin receptors might make an attractive blood pressure target, particularly in obesity when chemerin levels are striking high.
In HTN, chemerin has been associated with inflammation[177] in humans and the CMKLR1 receptor is increased in the brain of spontaneously hypertensive rats (SHR), which contributes, at least partly to systemic hypertension development[178]. Furthermore, removal of the chemerin production by treating rats with antisense oligonucleotides (ASO) had a more pronounced drop in blood pressure in high-fat than high-salt treated Male Dahl S rats, interestingly a liver-specific ASO delivery resulted in a mild drop in blood pressure (less than whole body administration) indicating that chemerin derived from adipose tissue seems to be more important than hepatic production[179], similar findings were observed in healthy rats[180]. Perhaps the location surrounding the vessels and adrenals could make fat-derived chemerin more important for blood pressure control. Finally, a sex difference dependence was observed using chemerin knockout rats, where female chemerin knockout rats showed a reduction in DOCA-Salt-induced hypertension, but not male[181].
As previously described in this review, chemerin receptors are found in a vast cell population many of them are involved in blood pressure control including endothelial cells and VSMC. Thus, the CMKLR1 flox mouse[182] is a valuable tool to reveal the role of chemerin on specific cell population and molecular mechanisms involved in blood pressure maintenance and obesity associated HTN. Furthermore, CCRL2 flox is also available and could be useful to elucidate whether chemerin might be acting via another receptor than CMKLR1. Finally, GPR1 deficient mice is only available[183] as a global knockout model, which limits the cell specificity of chemerin.
Omentin:
Omentin (omentin-1, intelectin-1, intestinal lactoferrin receptor, endothelial lectin HL-1, galactofuranosebinding lectin) is an adipokine found primarily secreted by omental (visceral) adipose tissue into the bloodstream,[184, 185] with the capability to affect several important blood vessel functions.[186] Omentin is a secretory glycoprotein consisting of 295 amino acids and linked oligosaccharides, and its basic structure is a 120-kDa protein in which 40-kDa polypeptides are connected via disulfide bonds. In macrophages, omentin exerts its athero-protective effects via integrin receptors αvβ3 and αvβ5.
Omentin is inversely correlated to obesity, obese humans and rodents display a reduced circulating omentin,[186] such change could contribute to vascular injury and HTN associated with obesity. In fact, mice with overexpression of omentin are protected from obesity-induced adipose tissue inflammation,[187] it may place omentin as an interesting adipokine to treat comorbid obesity.
Recently, it has been reported that omentin, like adiponectin, may act as a marker of healthy adipose tissue throughout the body. In recent years, several studies have identified omentin as an important regulator of vascular function, both through direct and indirect mechanisms. One group has shown that pre-treatment of isolated rat aortas with omentin caused an increase in endothelium-dependent relaxation via acetylcholine, which is induced by the phosphorylation of eNOS at serine 1177 but not serine 473.[188]These authors also showed that concentration-dependent constriction of aortas and mesenteric arteries by norepinephrine was blunted by pre-treatment with omentin and that endothelium-dependent relaxation could be inhibited by L-NAME treatment. The proliferation of smooth muscle cells[189] is also inhibited by omentin, according to in vitro studies, and multiple inflammatory pathways, such as suppressed TNF signaling and reduced expression of ICAM-1 and VCAM-1 via interruption of nuclear factor kappa B (NF-kB) signaling in cultured endothelial cells, are also blunted by omentin treatment[158] Other in vitro studies identified omentin as a negative modulator of COX-2 expression, and showed that omentin-dependent suppression of TNFα signaling in human umbilical vein endothelial cells blocked inflammatory COX-2 activity and increased eNOS phosphorylation via AMP-activated protein kinase (AMPK).[190], [191] Another study showed similar findings where omentin treatment blunted COX-2 activity, and prevented c-Jun N-terminal kinase (JNK) activation,[158] likely as a result of increased eNOS/AMPK activity, though this hypothesis warrants further investigation. Activation of eNOS by omentin is likewise important for angiogenesis,[192] and omentin treatment helps with resistance to ischemia through activation of eNOS to stimulate endothelial cell proliferation and revascularization of damaged tissue.[193] Further in vivo studies using male Wistar-Kyoto rats showed that injection with omentin blunted increases in blood pressure following injections of the vasoconstrictors, norepinephrine, and angiotensin II (Ang II), or dimorpholamine.[194] While further mechanistic study of these animals was not performed, it is likely that endothelium-derived NO signaling is the main driver of this dilatory effect induced by omentin, especially given that L-NAME co-treatment abolished the omentin-induced differences between groups. There is speculation that omentin may be used as an indicator of endothelial cell function based upon its correlation with healthy endothelium-dependent vasodilatory function,[195] though many of the factors which were negatively correlated with omentin expression, such as high waist circumference, body mass index, adipocyte mass, and expression of inflammatory markers strongly suggest that omentin expression is heavily suppressed in conditions of hyper adiposity and hyperlipidemia. Interestingly, this study also identified a link between endothelium-independent vasodilation and omentin expression, though this connection requires further investigation. While it is likely that the increased adiposity and inflammation contribute more directly to the lack of vascular cell function, it is also possible that omentin affects NO binding in VSMC or the production and additional signaling factors. Future studies will be important to pinpoint the specific contributions of omentin and the therapeutic potential for omentin treatment in the clinic.
Most studies focus on the effects of circulating omentin, however omentin is in endothelial cells (which usually is one of the first cell population affected in the genesis of HTN and cardiovascular diseases[196]) and stromal-vascular cells from visceral adipose tissue,[195] Omentin may therefore control cellular activity through additional, as yet unidentified processes that act independently of these receptors. Therefore, more research is required to analyze the differences between circulating and intracellular omentin-dependent signaling systems.
FGF21:
While the primary source of fibroblast growth factor 21 (FGF21) production is from the liver,[197] an emerging body of research has identified BAT as an important physiologically relevant source of FGF21 production.[198] Moreover, FGF21 expression is cold-inducible,[199]and has been a target for novel therapeutic treatments such as “cool sculpting” procedures designed to elevate the metabolic activity of adipose to cold-exposed tissue in order to induce cryolipolysis.[200] Early research identified FGF21 as an important regulator of lipolysis in adipocytes,[201] and subsequent studies demonstrated that FGF21 treatment induced extracellular signal-related kinase (ERK) phosphorylation and ten-fold activation in WAT compared with liver tissue,[202] which suggests that adipose is highly sensitive to FGF21 signaling. Additionally, FGF21 treatment, which has been shown to have beneficial effects such as increased glucose tolerance and prevention of hyperinsulinemia,[203] failed to produce a significant reduction in insulin secretion or improved glucose tolerance in a transgenic lipodystrophic mouse model.[204] Though lipodystrophic models have demonstrated the importance of adipose-derived leptin,[205] Veniant et al. demonstrated that transplantation of wild-type WAT into lipodystrophic mice fully rescued FGF21-induced effects, suggesting that the adipose tissue, and not the liver, is the primary source of functional FGF21. Furthermore, this study shows that the physiological changes observed by treatment with FGF21 require healthy adipocytes. Similarly, adipose-specific conditional knockout of fibroblast growth factor receptor 1 (FGFR1), which is activated by FGF21,[206, 207] showed no FGF21-induced transcriptional changes in adipose tissue and prevented changes in hepatic expression of these markers. According to a recent study, FGF21 expression helps withstand changes in blood pressure and vascular function[207] brought on by Ang II. This is most likely accomplished by controlling the activation of ACE2. Moreover, inhibition of the MAS receptor, which is the primary target of the cleavage products of ACE2,[208] abolished the protection observed in blood vessel architecture, but failed to impact the blood pressure effects induced by Ang II treatment. These data demonstrate a critical function of FGF21 from healthy WAT and BAT in the regulation of the RAAS. Finally, crosstalk between FGF21 and adiponectin has been described before, where FGF21 stimulates the production of adiponectin at both transcriptional and post-translational levels likely by activation of PPARγ [209] Thus, FGF21 may exert beneficial effects on the cardiovascular system and blood pressure regulation via producing adiponectin, which was previously described in this review article as a key adipokine on vascular function and blood pressure homeostasis.
3.6. Resistin:
Resistin is a cysteine-rich hormone secreted from white adipocytes. This protein was initially identified and named for its association with insulin resistance.[210] Resistin is a pre-peptide with 108 amino acid residues in humans and 114 amino acid residues in mice, forming a ~12.5 kDa active peptide. Interestingly, the resistin peptide sequence in humans shares only 59% identity with mice and a noticeably dissimilar promoter region, both of which likely cause differential expression and operation of resistin in humans and mice.[211] Different receptors are capable of recognizing resistin. For example: resistin can induce production of cytokines in human peripheral blood mononuclear cells in a Tol-like Receptor 4 (TLR4)-dependent manner.[212] In addition, resistin also activates adenylyl cyclase-associated protein 1 receptor in human monocytes, which mediates inflammatory actions of this immune cell population[213]. It has also been demonstrated that resistin interacts with decorin lacking glycation, which functions as a resistin receptor, develops into adipocyte progenitors, and may control WAT expansion[214]. Multiple studies have shown a positive correlation between insulin resistance and higher levels of resistin both in humans and animal models[215–218]. As elevated resistin levels have been linked to higher levels of C-reactive protein in patients with asymptomatic coronary artery atherosclerosis and higher resistin has also been linked to restenosis after coronary artery stent as well as worse outcomes in patients with coronary artery disease, it has been proposed that resistin may function as a predictor of outcome and risk profile for cardiac disease[219]. Furthermore, resistin can increase the permeability of coronary rtery endothelial cells, increase coronary VSMC proliferation and promote vasoconstriction[220, 221]. Resistin expression in humans increases significantly in response to inflammatory markers such as IL-6, IL-1β, and TNFα [211, 222, 223], while mouse expression of resistin is strongly induced by high blood glucose and suppressed by TNFα[211]. TLR4 is activated when human resistin is exposed to rat hypothalamus, suggesting that resistin and immune activation may be related This hypothesis was supported by a study that established a mouse model that connects both high blood pressure and type II diabetes and demonstrates that TLR4 is an essential signaling component for the development of resistin-mediated high blood pressure and insulin resistance in mice while demonstrating that TLR4 null mice did not develop either pathology. Future study on resistin will be required to better understand the distinct biological responses in human cells and tissues as well as the reasons for the reported variations between human and mouse samples. Additionally, more research is necessary to understand the precise resistin-mediated alterations brought on by immune cell activation and WAT enlargement. These investigations will aid in defining the extent of resistin-induced genesis and development of HTN associated with obesity.
3.7. Visfatin:
Visfatin has a molecular weight of 52 KDa and its gene encodes a 491 amino acid peptide[224]. Visfatin binds and activates the insulin receptor causing a significant drop in glucose release from hepatocytes and stimulating glucose consumption in adipocytes[225]. Furthermore, visfatin can promote endothelial dysfunction by activating TLR4, NF-κB, and NLRP3[226]. As a result, visfatin can enhance metabolic profile, which appears to be extremely appealing for cardiovascular function; yet its direct effects on endothelial cells warn us of its side effects on cardiovascular homeostasis. The name visfatin is derived from the original hypothesis that this enzyme was primarily secreted from visceral fat. Recent studies have shown that the main source of visfatin is derived not from the adipocytes, but rather from the resident macrophages found surrounding the visceral adipocytes[227]. Fukhara et al. conducted a study of 101 patients and used computed tomography and identified a strong correlation between visceral fat and visfatin expression, while subcutaneous fat showed a weak correlation with visfatin expression[228]. In addition to white blood cells, lymphocytes and adipocytes, visfatin is produced from hepatocytes[229], and skeletal muscle[230, 231], however most evidence suggests that expression is induced in response to inflammatory stimuli[227, 232]. One such inflammatory mediator, oxidized LDL, has been shown to increase visfatin in monocytes in cell culture, which led to elevated matrix metalloproteinase expression[233], a common characteristic of unstable plaques in atherosclerosis. Another study identified that higher visfatin expression was associated with several adverse outcomes in patients with coronary artery pathologies, including elevated instability in coronary plaques, symptomatic carotid lesions, and higher reporting of unstable angina compared with individuals with lower visfatin expression. In addition, this study identified that higher visfatin was present in unstable nuclei than those of stable ones[234], suggesting that visfatin either influences plaque instability directly or is a late-stage marker of plaques that have been destabilized due to inflammation. Higher visfatin also increases the risk of acute coronary pathologies independently of traditionally associated risks such as hypercholesterolemia, hypertension, type 2 diabetes, obesity and smoking[235], suggesting that targeted suppression of visfatin may help to attenuate or prevent the development of coronary artery disease. Moreover, higher visfatin is also linked to pathological thickening of the carotid artery media and intima[236], and a subpopulation of Chinese patients who had a stroke showed elevated visfatin expression[237]. Together these data suggest that visfatin expression may act as a warning marker or causative contributor to atherosclerotic CVD. Further, targeted inhibition or suppression of visfatin activity under specific conditions may provide beneficial therapeutic outcomes against the development of ischemic coronary or cerebrovascular pathologies. These studies will likely require the use of drug eluting stents to determine the specific contribution of visfatin to morbidities associated directly with atherosclerosis.
4. Biogenic Amines:
Since the discovery in the 1990’s by Soltis and Cassis that PVAT directly affects agonist-induced constriction of blood vessels[238], many studies have identified both pro-contractile and pro-dilatory vaso-affective neurotransmitters, produced and metabolized by nearby adipose tissue, suggesting an important regulatory endocrine function for PVAT. Recent studies have shown that PVAT contains several catecholamine stores, namely norepinephrine, epinephrine and dopamine[239–241]. Treatment with tyramine, which shifts these intracellular reserves to release vesicular neurotransmitters[242], produces artery constriction without the involvement of sympathetic nerves[239]. Furthermore, removal of PVAT was sufficient to abolish this contractile response, indicating the presence of an “adipogenic” tone specifically in vascular beds such as the aorta and superior mesenteric artery which contain more PVAT than most arterial beds. These data are further supported by the finding that 5-HT is also found in PVAT and pharmacologically induced 5-HT release in PVAT elevates vascular contraction in both thoracic aorta and superior mesenteric arteries through norepinephrine secretion[243].
These investigations demonstrate a crucial link between the vascular endothelium and PVAT to strengthen their findings and provide a unique source for putative vasoactive amine neurotransmitters. As these responses have only recently been observed, more investigation is necessary both at the basic science and the clinical level, to identify the cellular mechanisms responsible for these observations and to scrutinize whether human tissue functions in the same manner. Sympatholytic therapeutics for the treatment of HTN may also exert their effects not only by acting in the nervous system, but also by blocking the synthesis of biogenic amines in adipose tissue, which in turn may modulate vascular tone. To date however, most studies have examined the impact of PVAT on ex-vivo animal tissues due to the difficulty in removal of vascular adipose from living organisms. As a result, most studies regarding vascular and metabolic function due to PVAT are speculative or correlative with regard to humans. Future non-invasive surgical procedures might provide insights into the specific function of PVAT in the human body.
5. Inflammatory Factors:
Classical cytokines are most commonly known to be secreted from white blood cells, primarily through intense upregulation and secretion of these signaling molecules following damage or exposure to pathogens[244]. Novel research over the past few decades have shown that adipose tissue is capable of expressing many inflammatory markers including IL-6, TNFα and a number of others[245]. While cytokine production is lower in adipose than in bone marrow or thymus, adipocyte and tissue-specific macrophage-derived production of these factors have significant physiological impacts on cardiovascular function[246].
Research by Hotamisligil et al. provided the first evidence that adipocytes could express TNFα, which was directly tied with increased adipose content and subsequent insulin resistance[247]. This marked an important discovery in the cardiovascular research field that increased adiposity is directly responsible for increased inflammatory signaling. TNFα levels are increased in overweight individuals[248]. and appears to be linked to the increased infiltration of adipose tissue which occurs in obese rodent models and in obese human subjects[249]. The levels of TNFα appear to increase prior to the increase observed in IL-6 or CRP expression, suggesting that TNFα acts as an earlier marker of adipose tissue dysfunction. It is important to note, however, that a meta-analysis of studies involving the use of TNFα inhibitors surprisingly showed that weight gain and increased BMI are observed in several studies as an apparent side effects of these treatments[250]. There are several mechanistic reasons which may lead to increase in body mass, including changes in nerve-mediated catabolism[251], increased appetite[252], and increased skeletal muscle lean mass in response to TNFα inhibitor therapy[253].
Further research shows that circulating IL-6 is elevated in venous blood flow from obese over lean individuals[254], suggesting that IL-6 secretion is influenced by amount and quality of adipose tissue[255]. IL-6 concentrations are significantly elevated in hepatic portal vein of morbidly obese individuals and these increase in IL-6 strongly led to elevated liver-derived C-reactive protein (CRP)[255], suggesting that the higher levels of inflammation in obese patients expedite to systemic inflammatory markers. While these data identify important correlative links between visceral adipose content and higher circulating IL-6 and CRP levels, more work is necessary to identify the specific influences of adipocytes from these patients and the degree to which immune cells from visceral or other adipose tissue sources impact circulating cytokine expression.
Inflammatory cytokines production is undoubtedly a critical component in the development of HTN, though lately we have begun to discover the impacts of other cell types in the production of these signaling molecules. Conditional deletion of these markers from adipocytes may help future researchers to identify the specific contributions of these markers from adipogenic sources. Further research will be necessary to identify the conditions which govern their production and distribution from not only classical immune sources but also from adipose tissues across the body.
6. Adipose Renin-Angiotensinogen-Aldosterone System:
The Renin-Angiotensinogen-Aldosterone-System (RAAS) is perhaps the most important pathway which regulates blood osmolarity and blood pressure throughout the body[256]. It produces critical changes in renal, neural, cardiac, and vascular tissues, as well as many others to increase water reabsorption and salt reabsorption, and cardiac and vascular contractile tone[256]. Most of the products in this pathway are regulated and produced outside of adipose tissue, however, there are several essential ways in which adipose tissue interacts with the RAAS to produce changes in its primary effectors. The RAAS works primarily through 3 molecules to mediate these processes: renin, Ang II, and aldosterone.
The angiotensinogen (AGT) production initiates the RAAS pathway, and further AGT cleavage via renin to form angiotensin I (Ang I). Subsequent cleavage of Ang I via angiotensin converting enzyme (ACE) creates the metabolically active Ang II. This AGT production is conducted primarily through production of liver derived AGT, though recent discoveries have found that adipose is the second largest producer of AGT. Despite the importance of liver-derived AGT, many studies have identified correlations between food intake and the expression of adipocyte-derived AGT[257]. Moreover, some studies have shown elevated adipocyte expression of AGT correlates with obesity and HTN in both animals and human patients[258], though some of these data remain controversial, and further meta-analyses of clinical data are necessary to identify the validity of these findings.
ATRs not only cause significant physiological changes in certain renal, cardiac, and vascular cell types, but they also enhance crucial downstream effects in adipose tissues. The actions of the Angiotensin II type 1 receptor (AT1) are known to lead functional adipocyte differentiation, increased fat mass, and higher insulin resistance[259]. AT1 signaling has likewise been shown to promote inflammatory signaling through upregulation of C-reactive protein, MCP-1, PAI-1, and TNFα[259]. Interestingly, adipocytes seem to have an active RAAS, for example: adipocytes express aldosterone synthase (CYP11B2) and produce aldosterone in an Ang II/AT1 /calcineurin/nuclear factor of activated T-cells–dependent manner. Such aldosterone production directly interferes with the vascular function in mesenteric arteries from type 2 diabetic db/db mice lacking functional leptin receptors[260]. More simply, type 2 diabetic mice have a high level of circulating Ang II, which activates the AT 1 in PVAT to produce aldosterone, and this mineralocorticoid production is known to induce impaired endothelial-dependent relaxation as a result.
Furthermore, as described by Dr. Belin de Chantemele’s group and mentioned previously in this review article, leptin, another physiologically relevant adipokine, produces aldosterone by directly activating the leptin receptor present in adrenal glands. This stimulation has been shown to impact the subsequent formation of cardiovascular lesions and HTN[107],[261]. This mechanism appears to have a strong sex-dependent component, and as such, represents an important consideration for treatment options. Together these studies demonstrate a functional and important adipose RAAS in addition to a unique signaling axis with the adrenal glands to govern the production of aldosterone.
Central nervous system (CNS), adipokines, and blood pressure mechanisms
Among the important roles of the CNS is the control of behavior and physiological systems including the cardiocirculatory[262]. The short (via baroreflex signaling) and long-term regulations of blood pressure by the brain have been extensively investigated mainly focusing on autonomic nervous system and its sympathetic branch[263], [264]Some adipokines can exert their effects on regulating blood pressure via CNS mechanisms[265],[266],[267]. In this section, we will briefly describe key findings showing how leptin and adiponectin can help maintaining or elevating blood pressure.
Compelling evidence place leptin as a critical link between obesity, sympathetic nervous overdrive, and hypertension[268]. Leptin is released into the bloodstream from adipocytes and circulates to the brain where it crosses the blood-brain barrier by a saturable system [269]. While mice and humans with loss-of-function mutations in leptin and the leptin receptor do not exhibit HTN, a mouse model of high fat diet-induced obesity does, and this is alleviated by blocking leptin signaling in the dorsomedial hypothalamus via different approaches[265] This set of findings suggest that leptin signaling in the dorsomedial hypothalamus triggers HTN in obesity.
Furthermore, leptin injections in the right lateral brain ventricle reduced the food intake in lean mice but failed to reduce in obese. However, the same treatment elevated the renal sympathetic nerve activation in lean and obese mice at the same scale, indicating that diet-induced obesity is associated with resistance to the metabolic actions of leptin, but preservation of its renal sympathetic nerve response and blood pressure [266]. Similar findings were observed in rabbit, where leptin treatment in intracerebroventricular triggered a further increase renal sympathetic nerve activity compared to the counterpart group[270]. In 2015, we demonstrated that increasing leptin signaling in proopiomelanocortin neurons (POMC) by deleting Ptp1b reduced baseline neurogenic control of blood pressure but increased the vascular adrenergic response[104]. In this controversial study, we did not evaluate whether Ptp1b might be regulating any additional trigger for sympathetic tone activation and blood pressure regulation or the direct effects of leptin in CNS.
Since adiponectin has been found in cerebrospinal fluid[271] its receptors (AdipoR1 and AdipoR2) are expressed in the brain [272]and injections of this adipokine in CNS affects the metabolism[273] and decreases sympathetic activation and blood pressure [274] [272], we can speculate that levels of adiponectin might be involved in the regulation of blood pressure dependent on central mechanisms. For example, both acute intravenous and lateral cerebral ventricular injections of adiponectin reduced renal sympathetic nerve activity and blood pressure[274], interestingly the intravenous effects of adiponectin disappeared post bilateral lesions of the hypothalamic suprachiasmatic nucleus (SCN). In addition, microinjection of adiponectin in the medial nucleus of the solitary tract in the area postrema decreased the blood pressure[272]. These data suggest that central adiponectin signaling can help maintaining the blood pressure and the striking increase in leptin levels plus a significant reduction of adiponectin in obesity condition might be working synergically to evoke changes in sympathetic tone and HTN. Finally, more research is required to determine whether a lack of adiponectin signaling in the brain might cause HTN or whether adiponectin centrally injected can lessen an already existing HTN.
7. Adipose tissue innervation and adipose tissue
Compelling evidence show a key role of adipose tissue innervation in physiological and pathophysiological conditions. The metabolic and secretory activities and plasticity of adipose tissue directly can affect the nervous system. Energy homeostasis, body fat composition, reproduction, immune response, cardiovascular function among others is, at least in parts, controlled by neural feedback between adipose tissue and CNS, and changes in adiposity (obesity and lipodystrophy) can negatively impact this crosstalk [275–278].
The neural innervation of adipose tissue was first described at the end of the 19th century [279], however its functional importance on modulating the adipose tissue functions bas been better examined more recently[280–282]. Through a bidirectional link between the brain and the adipose tissue depots, the neural communication of adipose tissue is crucial for the maintenance of energy homeostasis. Adipokines, which function as endocrine signals[283], and the autonomic nervous system facilitate this connection.
The autonomic nervous system is formed by two main branches, the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS), both innervate the adipose tissue. SNS regulates WAT and BAT functions by lipolysis, lipogenesis, adipocyte proliferation, thermogenesis, and adipokine secretion[284–286]. Under sympathetic stimulation, noradrenaline is produced, which acts directly on adipose tissue or CNS in order to control adipose tissue profile likely via β3-adrenoceptor[287]. The role of PNS on adipose tissue profile[281, 286, 288] is still questioned. However, evidence places PNS as a glucose uptake regulator and free fatty acid (FFA) metabolism inducer in adipose tissue[289]. In the next paragraphs, we will only describe very briefly the importance of adipose tissue innervation on controlling the cardiovascular outcomes.
The lipolysis-derived products or adipokines can promote a SNS feedback[290],[286, 291]. Leptin, TNF-α, and vascular endothelial growth factor (VEGF), which can be produced by adipose tissue, can modulate local sensory nerve fibers[292]. For example, in WAT, leptin can stimulate afferent nerve fibers and transmit functional signals to the CNS, which in turn stimulates the sympathetic impulse to adipose tissue[293, 294].
PVAT is well-innervated, and part of its anti-contractile effect is dependent on the sympathetic nerves[295]. PVAT in conductance arteries, where BAT is more prevalent, has sympathetic innervation that generates a pro-contractile effect, whereas sympathetic innervation of PVAT in resistance arteries (primarily composed by WAT) has an anti-contractile feature[296, 297]. Furthermore, sensory nerve fibers can promote the release of leptin and chemerin from the PVAT, which contributes to its vascular anti-contractile or contractile effect respectively[172, 298]. In addition, the sympathetic nerve-derived noradrenaline in PVAT can activate the β3-AR and promote the release of adiponectin, which acts as a vascular anticontractile molecule of PVAT[299]. In order to preserve the quality and bulk of adipose tissue, it is essential to consider the type and density of adipose tissue innervation.
7.1. Adjustment of adipose tissue mass, quality, and location
WAT depots are generally characterized as visceral or subcutaneous, visceral is the adipose tissue around the abdominal visceral organs, whereas subcutaneous is located in the dermis and fascia[300]. They can differ in term of inflammatory and metabolic profiles, for example visceral fat produces more vascular endothelial growth factor, IL-6, and plasminogen activator inhibitor 1 compared with subcutaneous tissue[1] (and has been associated with endothelial dysfunction and HTN[301]. Harmelen and colleagues studied the leptin profile in subcutaneous fat cells from non-obese and obese women and found that subcutaneous fat cells are larger and express and secrete more leptin than visceral fat cells, in both obese and non-obese women. Since women have more circulating leptin then men[302]. It would be interesting to determine whether the profile of fat depots also differ in men. Also, patients with a predominantly visceral fat distribution present higher levels of progranulin (an adipokine not explored in this review article) than obese patients with subcutaneous fat distribution[303]. Finally, larger adipocytes were related to metabolic dysfunction, endothelial dysfunction, and HTN indicating changes in adipocyte morphology/dimension can affect cardiometabolic and atherogenic profiles in obesity[301]. Therefore, mass, location, and quality of adipose tissue depots are key for a fine-tuning between adiposity and blood pressure regulation.
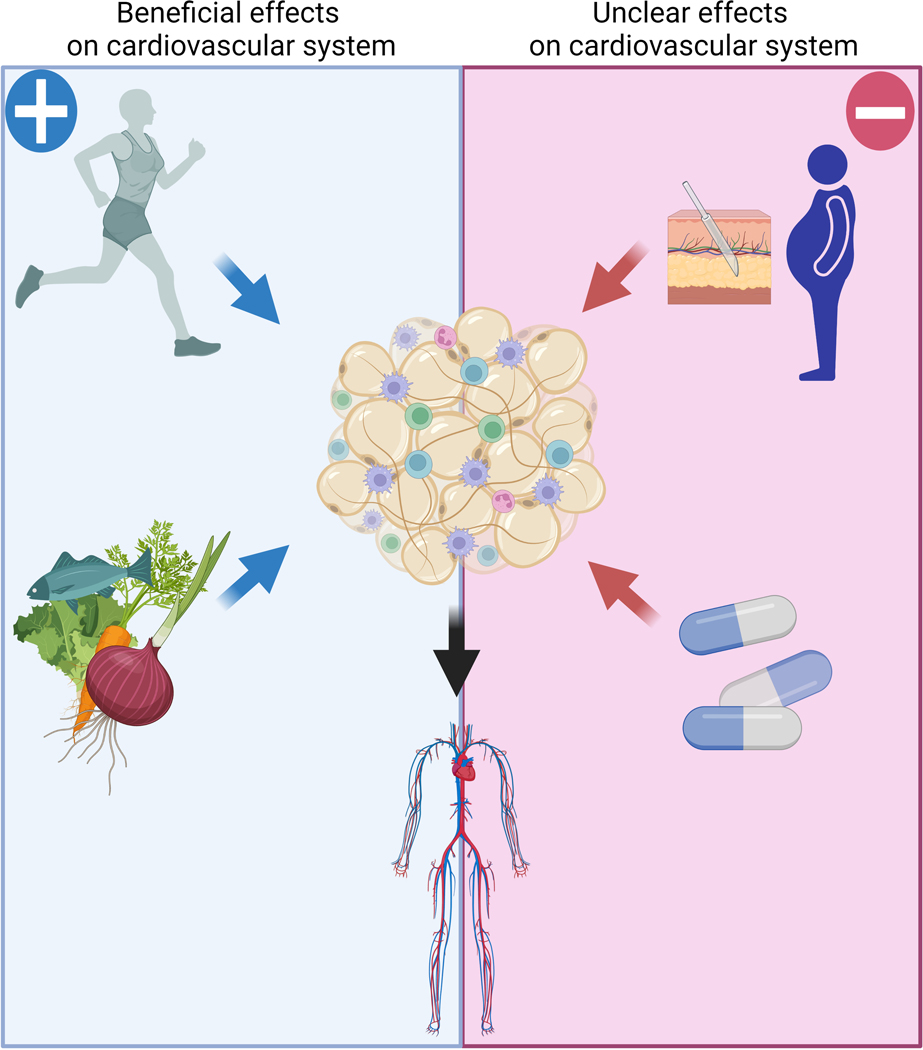
The lack of existing pharmaceutical strategies to directly target adipose tissue to prevent or accelerate formation of adipokines represents a substantial obstacle for the modulation of adipose tissue function. (Figure 3). Therefore, alternative interventions have been suggested including physical exercise, differential eating habits, liposuction, and pharmacological treatment to induce lipolysis or appetite suppression such as β−adrenergic-3 (β3) receptor agonists.
Figure 3. Interventions and body fat mass regulation.
In addition to the direct effects on cardiovascular system, physical exercise and balanced diet are known by diminishing the fat content, which is an additional protective mechanism on cardiovascular function. Combination of both is a powerful tool to reduce the cardiovascular risk. Surgical or pharmacological interventions are scientifically proven of reducing the fat mass, but both may have side effects on cardiovascular function including hypertension.
7.1.1. Physical exercise:
Physical activity is the most recommended non-pharmacological and non-surgical intervention to control the body fat mass and consequently improve metabolic and cardiovascular health[304]. In addition to improved insulin and leptin sensitivity, physical exercise attenuates the RAAS activity, modulates sympathetic tone, induces nitric oxide and prostacyclin production, augments endothelium-dependent vasodilation, promotes lipolysis, and inhibits several events involved in atherogenesis.[304] Finally, physical exercise has been shown to aid in the production of “good adipokines” like adiponectin and omentin for the cardiovascular system.[305]
7.1.2. Dietetics:
Similarly, to exercise, healthy dietary choices and portion control are key components for adipose mass management, and a combination of those two interventions is a powerful tool for a healthy life[306]. Reduction in sodium as well as high caloric and fat-containing foods can improve insulin sensitivity, furthermore, modulate the circulating levels of many adipokines, which in turn can positively affect many of the variables controlling blood pressure. Many of these changes include improved salt-sensitivity[307], reduced sympathetic outflow[308], improved vascular functions[309], and a healthier metabolic profile[310].
7.13. Surgical interventions:
Liposuction is the surgical removal of fat from the subcutaneous sources, which has a pivotal participation in nutritional and thermodynamic metabolic functions[311]. Liposuction remains one of the top 5 cosmetic surgical procedures in the US, with more than 200,000 surgeries performed in 2020[312]. Studies are controversial regarding the impact of liposuction on cardiovascular risk. It has been claimed that liposuction enhances metabolic response and cardiovascular health, suggesting that decreased subcutaneous adipose tissue is sufficient to trigger these benefits. However, the significance of changes in adipokine production has not yet been extensively analyzed. Some have reported that liposuction improves the metabolic response and cardiovascular health,[313],[314] indicating that reduced subcutaneous adipose tissue is sufficient to trigger metabolic and cardiovascular protection, however the role of changes in adipokine production has not been fully explored. Additional studies reported that liposuction does not confer any protection in terms of cardiovascular and metabolic function[315, 316], indicating that loss of subcutaneous adipose tissue mass per se will not achieve the metabolic benefits of weight loss. Finally, some studies have shown that liposuction of large amounts of subcutaneous fat can negatively affect metabolism and cardiovascular risk by increasing the proportion of visceral to subcutaneous adipose[317]. Due to the fact that these data are still debatable and that the amount of fat removed may have different effects on cardiovascular response as well as the state of the cardiovascular profile prior to surgery, these factors should be considered when interpreting the most recent research on this cosmetic procedure. Therefore, additional research is required to determine the significance of liposuction for controlling blood pressure and cardiovascular profile.
7.1.4. Pharmacological intervention:
Multiple pharmacological approaches have been used as modulators for adipose tissue mass including lipase inhibitors (Orlistat)[318], appetite and energy expenditure modulators (phentermine/topiramate, naltrexone-bupropion, liraglutide, and amphetamines)[319–322] β3-adrenoceptor agonists, among others. Particularly for this review, we are focusing only on β3-adrenoceptor class of drugs since this class of receptors are broadly expressed in cardiovascular cells including cardiomyocytes, VSMC, and endothelial cells [323],[324].
β3-adrenoceptor is abundantly expressed in adipose tissue. The discovery of the β3 receptors was met with significant doubt until it was first cloned in 1989[325], and it has quickly emerged as a desirable drug target. Agonists for this receptor increase lipolysis[326], induce increased energy expenditure[327], and sensitize to insulin action[328]. Such studies suggest that future drugs targeting the β3-adrenoceptor may provide a novel mechanism for the treatment of obesity and HTN, providing a potential new tool to treat these diseases.
Several studies have reported multiple intriguing findings regarding adipokine profiles, for example activation of β3-adrenoceptor suppresses leptin mRNA expression and circulating levels in mice[329]. In obese diabetic crossbred KK-Ay mice, administration of the β3-adrenoceptor agonist, CL-316,243, reduced the circulating levels of glucose, insulin, and TNF-α, and increased adiponectin expression[330]. These data strongly suggest that modulation of β3 receptor signaling is a promising target for the treatment of obesity.
In addition to have direct effect on visceral tissues, β3-adrenoceptor can mediate its anti-obesity effects via CNS. β3-adrenoceptor has been found in multiple areas of the brain[331], including brain stem and the hypothalamus[332], which provide multisynaptic innervation to brown and white adipose depots[333]. Therefore, we can predict that β3-adrenoceptor signaling may interfere with adipose tissue mass and quality. An interesting study conducted by Richard et al[334] demonstrated several beneficial effects caused by β3-adrenoceptor stimulation in the brain, central injection of β3-adrenoceptor agonist reduced food intake and body weight in rats, followed by increase in circulating insulin levels, browning response, and increased BAT mass. The same study also shows that β3-adrenoceptor stimulation reduced body weight, chow, and sucrose water intake and elevated browning in high-fat and high-sugar-fed rats. This study strongly indicates that the brain plays a major effect on β3-adrenoceptor stimulation-induced lipolysis and improved metabolic outcomes along the direct impact on adipocytes.
Furthermore, due to its role in lipolysis[335], vasodilation[336], CNS modulation[337],[338] and cardiac contractility,[339] β3-adrenoceptor agonists can confer cardiovascular protection and blood pressure maintenance by acting directly in cardiovascular tissues or CNS or by indirectly improving the adipose tissue mass and adipokines profile. β3AR cardioprotective and anti-hypertensive effects are partially dependent on CNS, whereas nerve-activated β3-adrenoceptor–mediated vasodilation is not observed in hypertensive rats, adrenaline-activated β3-adrenoceptor – mediated vasodilation is [337]. Also, β3-adrenoceptor agonist, SR58611, caused a decreased in blood pressure and activated baroreflex in normotensive dogs and in hypertensive dogs the drop in blood pressure is greater than normal dogs followed by a reflex-mediated tachycardia [338]. Overall, β3-adrenoceptor agonists are potent anti-obesity therapeutic drugs and safe for cardiovascular outcomes, including blood pressure.
However, one such β3-adrenoceptor agonist, mirabegron, which recently was approved for FDA clinical trials for the treatment of overactive bladder syndrome[340], caused a minor elevation in blood pressure amongst a small subgroup of patients undergoing treatment compared with patients typically treated with anti-muscarinics[341]. This discrepant observation may be due to the mild anti-hypertensive effects of some anti-muscarinic drugs. Furthermore, amibegron, an orally active and brain-penetrant β3-adrenoceptor agonist, has robust anxiolytic-like effects in rats[342], which might be followed by noradrenaline release via activation of central β3-AR[343]. This increase in noradrenaline might be associated with enhances in blood pressure since it is a very potent vasoconstrictor. However, this study did not evaluate any blood pressure changes. Thus, more studies are necessary to confirm the beneficial effects of this class of drug on cardiovascular system, as well as to test its safety in terms of blood pressure control.
8. Conclusions:
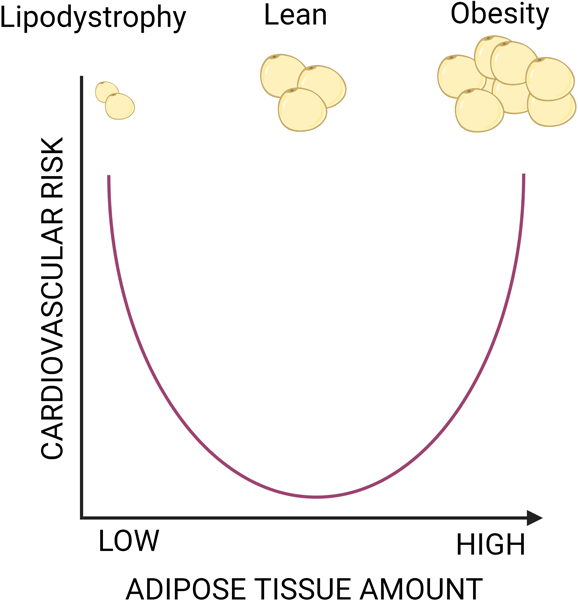
Adipose tissue is a vital organ for maintaining the cardiovascular system’s health and equilibrium. It is capable of responding to and producing a variety of signals that affect heart rate and blood pressure homeostasis. There are many clinical and animal studies that have started the process of identifying the pathways that affect how adipocytes communicate with nearby and distant organs, but there are still many unanswered questions about the precise contributing mechanisms at the cellular level. . Many clinical and animal studies have begun the process of establishing the networks which influence how adipocytes communicate with local and distant tissues, but many questions still remain regarding the specific contributory mechanisms involved at the cellular level. Future investigations are required to determine the extent and nature of adipogenic influence on HTN and CVD, in order to determine if these adipokine signaling molecules and adipocyte-derived factors can be repressed or exploited as potential therapeutic interventions or preventives. Adipose tissue is an essential organ that is capable of healthy and pathogenic signaling at both local and systemic levels, as several investigations over the past several decades have demonstrated. Location, mass, and quality of adipose tissue are therefore essential factors in the control of homeostatic blood pressure and cardiovascular function. (Figure 4).
Figure 4. Maintain the right amount and good quality of adipose tissue are the right thing to do.
High amount of adipose tissue (obesity) is associated with higher risk of cardiovascular disease, similarly, a drastic decrease in adipose tissue (lipodystrophy) increases the cardiovascular disease likely by alternating the levels of adipokines. Thus, keeping a healthy and good quantity of adipose mass can preserve the cardiovascular integrity.
9. Acknowledgment:
This work was supported by: NHLBI-R00 (R00HL14013903), AHA-CDA (CDA857268), Vascular Medicine Institute, the Hemophilia Center of Western Pennsylvania Vitalant, in part by Children’s Hospital of Pittsburgh of the UPMC Health System, and startup funds from University of Pittsburgh to TBN. All figures were designed using BioRender.
List of abbreviations
- 5HT
Serotonin
- β3
β−adrenergic-3
- ACE
Angiotensin Converting Enzyme
- ACE-2
Angiotensin Converting Enzyme-2
- AdipoR1
Adiponectin Receptor 1
- AdipoR2
Adiponectin Receptor 2
- AGT
Angiotensinogen
- AMPK
Adenosine Monophosphate-Activated Protein Kinase
- Ang I
Angiotensin I
- Ang II
Angiotensin II
- ATR’S
Angiotensin II Receptors
- AT1
Angiotensin II Receptor Type 1
- ASO
Antisense Oligonucleotides
- BAT
Brown Adipose Tissue
- CCRL2
Chemokine Receptor Like 2
- CDH13
cadherin 13
- CGL
Congenital or acquired Generalized Lipodystrophy
- CMKLR1
Chemokine-Like Receptor 1
- COX
Cyclooxygenase
- CRP
C-Reactive Protein
- CVD
Cardiovascular Disease
- CYP11B2
Cytochrome P450 Family 11 Subfamily B Member 2
- DOCA
Deoxycorticosterone Acetate
- DTA
Diphtheria Toxin A
- EC
Endothelial Cells
- eNOS
Endothelial Nitric Oxide Synthase
- ERK
Extracellular Signal-Related Kinase
- ET-1
Endothelin-1
- FDA
Food and Drug Administration
- FGF21
Fibroblast Growth Factor 21
- FGFR1
Fibroblast Growth Factor Receptor 1
- GPR1
G-Protein-Coupled Receptor 1
- H-Cadherin
Heart-Cadherin
- HTN
Hypertension
- ICAM-1
Intercellular Adhesion Molecule-1
- IL
Interleukin
- JNK
c-Jun N-terminal Kinase
- LDL
Low-Density Lipoprotein
- L-NAME
L-Nitro Arginine Methyl Ester
- MCP-1
Monocyte Chemoattractant Protein-1
- miRNAs
micro-RNA Molecules
- NADPH oxidase
Nicotinamide Adenine Dinucleotide Phosphate Oxidase
- NF-κB
Nuclear Factor Kappa B
- NLRP
Nucleotide-binding oligomerization domain Leucine rich Repeat and Pyrin domain containing
- NO
Nitric Oxide
- NRG4
Neuregulin-4
- PGI2
prostacyclin
- PPARγ
Peroxisome Proliferator-Activated Receptor γ
- PVAT
Perivascular Adipose Tissue
- POMC
Proopiomelanocortin
- PRDM16
PR domain-containing 16
- Ptp1b
Protein Tyrosine Phosphatase 1B
- PAI-1
Plasminogen Activator Inhibitor-1
- ROS
Reactive Oxygen Species
- RAAS
Renin-Angiotensinogen-Aldosterone-System
- RARRES2
Retinoic Acid Receptor Responder Protein 2
- SHR
Spontaneously Hypertensive Rats
- TLR4
Tol-Like Receptor 4
- TNFα
Tumor Necrosis Factor Alpha
- VCAM-1
Vascular Cell Adhesion Molecule-1
- VSMC
Vascular Smooth Muscle Cells
- UCP1
Uncoupling Protein 1
- UCP-2
Uncoupler Protein-2
- UCP-3
Uncoupling Protein 3
- US
United States
- WAT
White Adipose Tissue
10. References
- [1].Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW, Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans, Endocrinology 145(5) (2004) 2273–82. [DOI] [PubMed] [Google Scholar]
- [2].Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association, Circulation 139(10) (2019) e56–e528. [DOI] [PubMed] [Google Scholar]
- [3].Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr., 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Circulation 138(17) (2018) e426–e483. [DOI] [PubMed] [Google Scholar]
- [4].Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr., 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Journal of the American College of Cardiology 71(19) (2018) e127–e248. [DOI] [PubMed] [Google Scholar]
- [5].Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, Grassi G, Jordan J, Poulter NR, Rodgers A, Whelton PK, Hypertension, Nature reviews. Disease primers 4 (2018) 18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Page IH, Pathogenesis of arterial hypertension, Journal of the American Medical Association 140(5) (1949) 451–8. [DOI] [PubMed] [Google Scholar]
- [7].Harrison DG, Coffman TM, Wilcox CS, Pathophysiology of Hypertension: The Mosaic Theory and Beyond, Circulation research 128(7) (2021) 847–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Garrison RJ, Kannel WB, Stokes J 3rd, Castelli WP, Incidence and precursors of hypertension in young adults: the Framingham Offspring Study, Preventive medicine 16(2) (1987) 235–51. [DOI] [PubMed] [Google Scholar]
- [9].Dhaliwal SS, Welborn TA, Central obesity and multivariable cardiovascular risk as assessed by the Framingham prediction scores, The American journal of cardiology 103(10) (2009) 1403–7. [DOI] [PubMed] [Google Scholar]
- [10].Cawley J, Meyerhoefer C, The medical care costs of obesity: an instrumental variables approach, Journal of health economics 31(1) (2012) 219–30. [DOI] [PubMed] [Google Scholar]
- [11].An R, Health care expenses in relation to obesity and smoking among U.S. adults by gender, race/ethnicity, and age group: 1998–2011, Public health 129(1) (2015) 29–36. [DOI] [PubMed] [Google Scholar]
- [12].Andreyeva T, Luedicke J, Wang YC, State-level estimates of obesity-attributable costs of absenteeism, Journal of occupational and environmental medicine 56(11) (2014) 1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tsai AG, Williamson DF, Glick HA, Direct medical cost of overweight and obesity in the USA: a quantitative systematic review, Obesity reviews : an official journal of the International Association for the Study of Obesity 12(1) (2011) 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hammond RA, Levine R, The economic impact of obesity in the United States, Diabetes, metabolic syndrome and obesity : targets and therapy 3 (2010) 285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fiorenza CG, Chou SH, Mantzoros CS, Lipodystrophy: pathophysiology and advances in treatment, Nat Rev Endocrinol 7(3) (2011) 137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chehab FF, Obesity and lipodystrophy--where do the circles intersect?, Endocrinology 149(3) (2008) 925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L Jr., Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C, Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015, Journal of the American College of Cardiology 70(1) (2017) 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang D, Wang G, Zhang P, Fang J, Ayala C, Medical Expenditures Associated With Hypertension in the U.S., 2000–2013, American journal of preventive medicine 53(6s2) (2017) S164–s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ, Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association, Circulation 123(8) (2011) 933–44. [DOI] [PubMed] [Google Scholar]
- [20].Thomas LW, The chemical composition of adipose tissue of man and mice, Quarterly journal of experimental physiology and cognate medical sciences 47 (1962) 179–88. [DOI] [PubMed] [Google Scholar]
- [21].Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB, Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders, Frontiers in endocrinology 7 (2016) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hirsch J, Fried SK, Edens NK, Leibel RL, The fat cell, The Medical clinics of North America 73(1) (1989) 83–96. [DOI] [PubMed] [Google Scholar]
- [23].Oikonomou EK, Antoniades C, The role of adipose tissue in cardiovascular health and disease, Nature reviews. Cardiology 16(2) (2019) 83–99. [DOI] [PubMed] [Google Scholar]
- [24].Reddy P, Lent-Schochet D, Ramakrishnan N, McLaughlin M, Jialal I, Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes, Clinica chimica acta; international journal of clinical chemistry 496 (2019) 35–44. [DOI] [PubMed] [Google Scholar]
- [25].Womersley J, A comparison of the skinfold method with extent of ‘overweight’ and various weight-height relationships in the assessment of obesity, The British journal of nutrition 38(2) (1977) 271–84. [DOI] [PubMed] [Google Scholar]
- [26].Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C, Wilmore JH, The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study, International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 26(6) (2002) 789–96. [DOI] [PubMed] [Google Scholar]
- [27].Seidell JC, Oosterlee A, Deurenberg P, Hautvast JG, Ruijs JH, Abdominal fat depots measured with computed tomography: effects of degree of obesity, sex, and age, European journal of clinical nutrition 42(9) (1988) 805–15. [PubMed] [Google Scholar]
- [28].Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G, Bell JD, Magnetic resonance imaging of total body fat, Journal of applied physiology (Bethesda, Md. : 1985) 85(5) (1998) 1778–85. [DOI] [PubMed] [Google Scholar]
- [29].Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB, Obesity, regional body fat distribution, and the metabolic syndrome in older men and women, Archives of internal medicine 165(7) (2005) 777–83. [DOI] [PubMed] [Google Scholar]
- [30].Schreiner PJ, Terry JG, Evans GW, Hinson WH, Crouse JR 3rd, Heiss G, Sex-specific associations of magnetic resonance imaging-derived intra-abdominal and subcutaneous fat areas with conventional anthropometric indices. The Atherosclerosis Risk in Communities Study, American journal of epidemiology 144(4) (1996) 335–45. [DOI] [PubMed] [Google Scholar]
- [31].Nielsen TL, Hagen C, Wraae K, Brixen K, Petersen PH, Haug E, Larsen R, Andersen M, Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men, The Journal of clinical endocrinology and metabolism 92(7) (2007) 2696–705. [DOI] [PubMed] [Google Scholar]
- [32].Allan CA, McLachlan RI, Androgens and obesity, Current opinion in endocrinology, diabetes, and obesity 17(3) (2010) 224–32. [DOI] [PubMed] [Google Scholar]
- [33].Nedungadi TP, Clegg DJ, Sexual dimorphism in body fat distribution and risk for cardiovascular diseases, J Cardiovasc Transl Res 2(3) (2009) 321–7. [DOI] [PubMed] [Google Scholar]
- [34].Elsanhoury A, Nelki V, Kelle S, Van Linthout S, Tschöpe C, Epicardial Fat Expansion in Diabetic and Obese Patients With Heart Failure and Preserved Ejection Fraction-A Specific HFpEF Phenotype, Frontiers in cardiovascular medicine 8 (2021) 720690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Szasz T, Webb RC, Perivascular adipose tissue: more than just structural support, Clinical science (London, England : 1979) 122(1) (2012) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Enerbäck S, The origins of brown adipose tissue, The New England journal of medicine 360(19) (2009) 2021–3. [DOI] [PubMed] [Google Scholar]
- [37].Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C, Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease, Arteriosclerosis (Dallas, Tex.) 10(4) (1990) 497–511. [DOI] [PubMed] [Google Scholar]
- [38].Rosen ED, Spiegelman BM, Adipocytes as regulators of energy balance and glucose homeostasis, Nature 444(7121) (2006) 847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, Chacko AT, Deschamps LN, Herder LM, Truchan N, Glasgow AL, Holman AR, Gavrila A, Hasselgren PO, Mori MA, Molla M, Tseng YH, Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat, Nature medicine 19(5) (2013) 635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Heaton JM, The distribution of brown adipose tissue in the human, Journal of anatomy 112(Pt 1) (1972) 35–9. [PMC free article] [PubMed] [Google Scholar]
- [41].Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR, Identification and importance of brown adipose tissue in adult humans, The New England journal of medicine 360(15) (2009) 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zennaro MC, Le Menuet D, Viengchareun S, Walker F, Ricquier D, Lombès M, Hibernoma development in transgenic mice identifies brown adipose tissue as a novel target of aldosterone action, J Clin Invest 101(6) (1998) 1254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM, PRDM16 controls a brown fat/skeletal muscle switch, Nature 454(7207) (2008) 961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL, Health Effects of Overweight and Obesity in 195 Countries over 25 Years, The New England journal of medicine 377(1) (2017) 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bartelt A, Heeren J, Adipose tissue browning and metabolic health, Nature reviews. Endocrinology 10(1) (2014) 24–36. [DOI] [PubMed] [Google Scholar]
- [46].Kotzbeck P, Giordano A, Mondini E, Murano I, Severi I, Venema W, Cecchini MP, Kershaw EE, Barbatelli G, Haemmerle G, Zechner R, Cinti S, Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation, Journal of lipid research 59(5) (2018) 784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shimizu I, Walsh K, The Whitening of Brown Fat and Its Implications for Weight Management in Obesity, Current obesity reports 4(2) (2015) 224–9. [DOI] [PubMed] [Google Scholar]
- [48].Huttunen P, Hirvonen J, Kinnula V, The occurrence of brown adipose tissue in outdoor workers, European journal of applied physiology and occupational physiology 46(4) (1981) 339–45. [DOI] [PubMed] [Google Scholar]
- [49].Zhao H, Chen X, Hu G, Li C, Guo L, Zhang L, Sun F, Xia Y, Yan W, Cui Z, Guo Y, Guo X, Huang C, Fan M, Wang S, Zhang F, Tao L, Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection, Circulation research 130(10) (2022) 1490–1506. [DOI] [PubMed] [Google Scholar]
- [50].Cittadini A, Mantzoros CS, Hampton TG, Travers KE, Katz SE, Morgan JP, Flier JS, Douglas PS, Cardiovascular abnormalities in transgenic mice with reduced brown fat: an animal model of human obesity, Circulation 100(21) (1999) 2177–83. [DOI] [PubMed] [Google Scholar]
- [51].Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM, Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human, Cell 150(2) (2012) 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sánchez JC, Valencia-Vásquez A, García AM, Role of TRPV4 Channel in Human White Adipocytes Metabolic Activity, Endocrinology and metabolism (Seoul, Korea) 36(5) (2021) 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu RH, Mizuta M, Matsukura S, The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes, The Journal of pharmacology and experimental therapeutics 310(1) (2004) 52–8. [DOI] [PubMed] [Google Scholar]
- [54].Ikegami R, Shimizu I, Sato T, Yoshida Y, Hayashi Y, Suda M, Katsuumi G, Li J, Wakasugi T, Minokoshi Y, Okamoto S, Hinoi E, Nielsen S, Jespersen NZ, Scheele C, Soga T, Minamino T, Gamma-Aminobutyric Acid Signaling in Brown Adipose Tissue Promotes Systemic Metabolic Derangement in Obesity, Cell reports 24(11) (2018) 2827–2837.e5. [DOI] [PubMed] [Google Scholar]
- [55].Ahima RS, Flier JS, Leptin, Annual review of physiology 62 (2000) 413–37. [DOI] [PubMed] [Google Scholar]
- [56].Baile CA, Della-Fera MA, Martin RJ, Regulation of metabolism and body fat mass by leptin, Annual review of nutrition 20 (2000) 105–27. [DOI] [PubMed] [Google Scholar]
- [57].Iwanij V, Amos TM, Billington CJ, Identification and characterization of the glucagon receptor from adipose tissue, Molecular and cellular endocrinology 101(1–2) (1994) 257–61. [DOI] [PubMed] [Google Scholar]
- [58].Taylor JE, Scott CD, Baxter RC, Comparison of receptors for insulin-like growth factor II from various rat tissues, The Journal of endocrinology 115(1) (1987) 35–41. [DOI] [PubMed] [Google Scholar]
- [59].Vikman K, Carlsson B, Billig H, Edén S, Expression and regulation of growth hormone (GH) receptor messenger ribonucleic acid (mRNA) in rat adipose tissue, adipocytes, and adipocyte precursor cells: GH regulation of GH receptor mRNA, Endocrinology 129(3) (1991) 1155–61. [DOI] [PubMed] [Google Scholar]
- [60].Endo T, Ohta K, Haraguchi K, Onaya T, Cloning and functional expression of a thyrotropin receptor cDNA from rat fat cells, J Biol Chem 270(18) (1995) 10833–7. [DOI] [PubMed] [Google Scholar]
- [61].Hauner H, Petruschke T, Russ M, Röhrig K, Eckel J, Effects of tumour necrosis factor alpha (TNF alpha) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture, Diabetologia 38(7) (1995) 764–71. [DOI] [PubMed] [Google Scholar]
- [62].Hube F, Birgel M, Lee YM, Hauner H, Expression pattern of tumour necrosis factor receptors in subcutaneous and omental human adipose tissue: role of obesity and non-insulin-dependent diabetes mellitus, European journal of clinical investigation 29(8) (1999) 672–8. [DOI] [PubMed] [Google Scholar]
- [63].Mohamed-Ali V, Pinkney JH, Coppack SW, Adipose tissue as an endocrine and paracrine organ, International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 22(12) (1998) 1145–58. [DOI] [PubMed] [Google Scholar]
- [64].Vassaux G, Gaillard D, Mari B, Ailhaud G, Negrel R, Differential expression of adenosine A1 and A2 receptors in preadipocytes and adipocytes, Biochemical and biophysical research communications 193(3) (1993) 1123–30. [DOI] [PubMed] [Google Scholar]
- [65].Yip RG, Wolfe MM, GIP biology and fat metabolism, Life sciences 66(2) (2000) 91–103. [DOI] [PubMed] [Google Scholar]
- [66].Drucker DJ, Glucagon-like peptides, Diabetes 47(2) (1998) 159–69. [DOI] [PubMed] [Google Scholar]
- [67].Dyer CJ, Simmons JM, Matteri RL, Keisler DH, Effects of an intravenous injection of NPY on leptin and NPY-Y1 receptor mRNA expression in ovine adipose tissue, Domestic animal endocrinology 14(5) (1997) 325–33. [DOI] [PubMed] [Google Scholar]
- [68].Sarzani R, Paci VM, Zingaretti CM, Pierleoni C, Cinti S, Cola G, Rappelli A, Dessì-Fulgheri P, Fasting inhibits natriuretic peptides clearance receptor expression in rat adipose tissue, Journal of hypertension 13(11) (1995) 1241–6. [DOI] [PubMed] [Google Scholar]
- [69].Hauner H, Röhrig K, Petruschke T, Effects of epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) on human adipocyte development and function, European journal of clinical investigation 25(2) (1995) 90–6. [DOI] [PubMed] [Google Scholar]
- [70].Schmidt W, Pöll-Jordan G, Löffler G, Adipose conversion of 3T3-L1 cells in a serum-free culture system depends on epidermal growth factor, insulin-like growth factor I, corticosterone, and cyclic AMP, J Biol Chem 265(26) (1990) 15489–95. [PubMed] [Google Scholar]
- [71].Navre M, Ringold GM, Differential effects of fibroblast growth factor and tumor promoters on the initiation and maintenance of adipocyte differentiation, J Cell Biol 109(4 Pt 1) (1989) 1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Samad F, Yamamoto K, Pandey M, Loskutoff DJ, Elevated expression of transforming growth factor-beta in adipose tissue from obese mice, Molecular medicine (Cambridge, Mass.) 3(1) (1997) 37–48. [PMC free article] [PubMed] [Google Scholar]
- [73].Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B, Some recent advances in the chemistry and biology of transforming growth factor-beta, J Cell Biol 105(3) (1987) 1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y, Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2, Endocrinology 141(2) (2000) 649–56. [DOI] [PubMed] [Google Scholar]
- [75].Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y, In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones, Endocrinology 140(4) (1999) 1567–74. [DOI] [PubMed] [Google Scholar]
- [76].Björntorp P, The regulation of adipose tissue distribution in humans, International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 20(4) (1996) 291–302. [PubMed] [Google Scholar]
- [77].O’Brien SN, Welter BH, Mantzke KA, Price TM, Identification of progesterone receptor in human subcutaneous adipose tissue, The Journal of clinical endocrinology and metabolism 83(2) (1998) 509–13. [DOI] [PubMed] [Google Scholar]
- [78].Kamei Y, Kawada T, Kazuki R, Ono T, Kato S, Sugimoto E, Vitamin D receptor gene expression is up-regulated by 1, 25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes, Biochemical and biophysical research communications 193(3) (1993) 948–55. [DOI] [PubMed] [Google Scholar]
- [79].Jones BH, Standridge MK, Taylor JW, Moustaïd N, Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control, The American journal of physiology 273(1 Pt 2) (1997) R236–42. [DOI] [PubMed] [Google Scholar]
- [80].Zorad S, Fickova M, Zelezna B, Macho L, Kral JG, The role of angiotensin II and its receptors in regulation of adipose tissue metabolism and cellularity, General physiology and biophysics 14(5) (1995) 383–91. [PubMed] [Google Scholar]
- [81].Rasmussen MS, Lihn AS, Pedersen SB, Bruun JM, Rasmussen M, Richelsen B, Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots, Obesity (Silver Spring, Md.) 14(1) (2006) 28–35. [DOI] [PubMed] [Google Scholar]
- [82].Lafontan M, Berlan M, Fat cell adrenergic receptors and the control of white and brown fat cell function, Journal of lipid research 34(7) (1993) 1057–91. [PubMed] [Google Scholar]
- [83].Torres-Márquez ME, Romero-Avila MT, González-Espinosa C, García-Sáinz JA, Characterization of rat white fat cell alpha 1B-adrenoceptors, Molecular pharmacology 42(3) (1992) 403–6. [PubMed] [Google Scholar]
- [84].Tiebel O, Oka K, Robinson K, Sullivan M, Martinez J, Nakamuta M, Ishimura-Oka K, Chan L, Mouse very low-density lipoprotein receptor (VLDLR): gene structure, tissue-specific expression and dietary and developmental regulation, Atherosclerosis 145(2) (1999) 239–51. [DOI] [PubMed] [Google Scholar]
- [85].Kraemer FB, Laane C, Park B, Sztalryd C, Low-density lipoprotein receptors in rat adipocytes: regulation with fasting, The American journal of physiology 266(1 Pt 1) (1994) E26–32. [DOI] [PubMed] [Google Scholar]
- [86].Salter AM, Fong BS, Jimenez J, Rotstein L, Angel A, Regional variation in high-density lipoprotein binding to human adipocyte plasma membranes of massively obese subjects, European journal of clinical investigation 17(1) (1987) 16–22. [DOI] [PubMed] [Google Scholar]
- [87].Li J, Matye DJ, Wang Y, Li T, Sortilin 1 knockout alters basal adipose glucose metabolism but not diet-induced obesity in mice, FEBS Lett 591(7) (2017) 1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yiannikouris F, Gupte M, Putnam K, Cassis L, Adipokines and blood pressure control, Curr Opin Nephrol Hypertens 19(2) (2010) 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Friedman J, 20 years of leptin: leptin at 20: an overview, The Journal of endocrinology 223(1) (2014) T1–8. [DOI] [PubMed] [Google Scholar]
- [90].Park HK, Ahima RS, Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism, Metabolism 64(1) (2015) 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS, Role of leptin in the neuroendocrine response to fasting, Nature 382(6588) (1996) 250–2. [DOI] [PubMed] [Google Scholar]
- [92].Wada N, Hirako S, Takenoya F, Kageyama H, Okabe M, Shioda S, Leptin and its receptors, Journal of chemical neuroanatomy 61–62 (2014) 191–9. [DOI] [PubMed] [Google Scholar]
- [93].Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. , Serum immunoreactive-leptin concentrations in normal-weight and obese humans, The New England journal of medicine 334(5) (1996) 292–5. [DOI] [PubMed] [Google Scholar]
- [94].Ren J, Leptin and hyperleptinemia - from friend to foe for cardiovascular function, The Journal of endocrinology 181(1) (2004) 1–10. [DOI] [PubMed] [Google Scholar]
- [95].Rahmouni K, Haynes WG, Mark AL, Cardiovascular and sympathetic effects of leptin, Curr Hypertens Rep 4(2) (2002) 119–25. [DOI] [PubMed] [Google Scholar]
- [96].Pan H, Guo J, Su Z, Advances in understanding the interrelations between leptin resistance and obesity, Physiology & behavior 130 (2014) 157–69. [DOI] [PubMed] [Google Scholar]
- [97].Balland E, Cowley MA, New insights in leptin resistance mechanisms in mice, Frontiers in neuroendocrinology 39 (2015) 59–65. [DOI] [PubMed] [Google Scholar]
- [98].Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA, Leptin resistance and diet-induced obesity: central and peripheral actions of leptin, Metabolism: clinical and experimental 64(1) (2015) 35–46. [DOI] [PubMed] [Google Scholar]
- [99].Shek EW, Brands MW, Hall JE, Chronic leptin infusion increases arterial pressure, Hypertension (Dallas, Tex. : 1979) 31(1 Pt 2) (1998) 409–14. [DOI] [PubMed] [Google Scholar]
- [100].Kuo JJ, Jones OB, Hall JE, Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin, Hypertension (Dallas, Tex. : 1979) 37(2 Pt 2) (2001) 670–6. [DOI] [PubMed] [Google Scholar]
- [101].Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI, Receptor-mediated regional sympathetic nerve activation by leptin, J Clin Invest 100(2) (1997) 270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K, Pathophysiological role of leptin in obesity-related hypertension, J Clin Invest 105(9) (2000) 1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK, PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice, J Clin Invest 120(3) (2010) 720–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Bruder-Nascimento T, Butler BR, Herren DJ, Brands MW, Bence KK, Belin de Chantemèle EJ, Deletion of protein tyrosine phosphatase 1b in proopiomelanocortin neurons reduces neurogenic control of blood pressure and protects mice from leptin- and sympatho-mediated hypertension, Pharmacological research 102 (2015) 235–44. [DOI] [PubMed] [Google Scholar]
- [105].Belin de Chantemele EJ, Muta K, Mintz J, Tremblay ML, Marrero MB, Fulton DJ, Stepp DW, Protein tyrosine phosphatase 1B, a major regulator of leptin-mediated control of cardiovascular function, Circulation 120(9) (2009) 753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME, Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms, Circ Res 116(6) (2015) 991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemele EJ, Adipocyte-Derived Hormone Leptin Is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis, Circulation 132(22) (2015) 2134–45. [DOI] [PubMed] [Google Scholar]
- [108].Garg A, Clinical review#: Lipodystrophies: genetic and acquired body fat disorders, The Journal of clinical endocrinology and metabolism 96(11) (2011) 3313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Belin de Chantemèle EJ, Ali MI, Mintz JD, Rainey WE, Tremblay ML, Fulton DJ, Stepp DW, Increasing peripheral insulin sensitivity by protein tyrosine phosphatase 1B deletion improves control of blood pressure in obesity, Hypertension (Dallas, Tex. : 1979) 60(5) (2012) 1273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bruder-Nascimento T, Faulkner JL, Haigh S, Kennard S, Antonova G, Patel VS, Fulton DJR, Chen W, Belin de Chantemèle EJ, Leptin Restores Endothelial Function via Endothelial PPARγ-Nox1-Mediated Mechanisms in a Mouse Model of Congenital Generalized Lipodystrophy, Hypertension 74(6) (2019) 1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bruder-Nascimento T, Kress TC, Kennard S, Belin de Chantemèle EJ, HIV Protease Inhibitor Ritonavir Impairs Endothelial Function Via Reduction in Adipose Mass and Endothelial Leptin Receptor-Dependent Increases in NADPH Oxidase 1 (Nox1), C-C Chemokine Receptor Type 5 (CCR5), and Inflammation, J Am Heart Assoc 9(19) (2020) e018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Bruder-Nascimento T, Kress TC, Pearson M, Chen W, Kennard S, Belin de Chantemèle EJ, Reduced Endothelial Leptin Signaling Increases Vascular Adrenergic Reactivity in a Mouse Model of Congenital Generalized Lipodystrophy, Int J Mol Sci 22(19) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hubert A, Bochenek ML, Schutz E, Gogiraju R, Munzel T, Schafer K, Selective Deletion of Leptin Signaling in Endothelial Cells Enhances Neointima Formation and Phenocopies the Vascular Effects of Diet-Induced Obesity in Mice, Arterioscler Thromb Vasc Biol 37(9) (2017) 1683–1697. [DOI] [PubMed] [Google Scholar]
- [114].Rocha VDS, Claudio ERG, da Silva VL, Cordeiro JP, Domingos LF, da Cunha MRH, Mauad H, do Nascimento TB, Lima-Leopoldo AP, Leopoldo AS, High-Fat Diet-Induced Obesity Model Does Not Promote Endothelial Dysfunction via Increasing Leptin/Akt/eNOS Signaling, Front Physiol 10 (2019) 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Brown RJ, Meehan CA, Gorden P, Leptin Does Not Mediate Hypertension Associated With Human Obesity, Cell 162(3) (2015) 465–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW, Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice, Hypertension 58(2) (2011) 271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Khoramipour K, Chamari K, Hekmatikar AA, Ziyaiyan A, Taherkhani S, Elguindy NM, Bragazzi NL, Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition, Nutrients 13(4) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Polito R, Nigro E, Pecoraro A, Monaco ML, Perna F, Sanduzzi A, Genovese A, Spadaro G, Daniele A, Adiponectin Receptors and Pro-inflammatory Cytokines Are Modulated in Common Variable Immunodeficiency Patients: Correlation With Ig Replacement Therapy, Front Immunol 10 (2019) 2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y, Diet-induced insulin resistance in mice lacking adiponectin/ACRP30, Nature medicine 8(7) (2002) 731–7. [DOI] [PubMed] [Google Scholar]
- [120].Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y, Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys, Diabetes 50(5) (2001) 1126–33. [DOI] [PubMed] [Google Scholar]
- [121].Ohashi K, Ouchi N, Kihara S, Funahashi T, Nakamura T, Sumitsuji S, Kawamoto T, Matsumoto S, Nagaretani H, Kumada M, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Hiraoka H, Iwashima Y, Ishikawa K, Ohishi M, Katsuya T, Rakugi H, Ogihara T, Matsuzawa Y, Adiponectin I164T mutation is associated with the metabolic syndrome and coronary artery disease, J Am Coll Cardiol 43(7) (2004) 1195–200. [DOI] [PubMed] [Google Scholar]
- [122].Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, Okamoto Y, Kihara S, Miyagawa J, Shinomura Y, Funahashi T, Matsuzawa Y, Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin, Gastroenterology 125(6) (2003) 1796–807. [DOI] [PubMed] [Google Scholar]
- [123].Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y, Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice, Circulation 106(22) (2002) 2767–70. [DOI] [PubMed] [Google Scholar]
- [124].Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K, Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms, Nat Med 11(10) (2005) 1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chow W-S, Cheung BMY, Tso AWK, Xu A, Wat NMS, Fong CHY, Ong LHY, Tam S, Tan KCB, Janus ED, Lam T-H, Lam KSL, Hypoadiponectinemia as a Predictor for the Development of Hypertension, Hypertension 49(6) (2007) 1455–1461. [DOI] [PubMed] [Google Scholar]
- [126].Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T, Hypoadiponectinemia is an independent risk factor for hypertension, Hypertension 43(6) (2004) 1318–23. [DOI] [PubMed] [Google Scholar]
- [127].Kondo H, Shimomura I, Matsukawa Y, Kumada M, Takahashi M, Matsuda M, Ouchi N, Kihara S, Kawamoto T, Sumitsuji S, Funahashi T, Matsuzawa Y, Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome, Diabetes 51(7) (2002) 2325–8. [DOI] [PubMed] [Google Scholar]
- [128].Kishida K, Nagaretani H, Kondo H, Kobayashi H, Tanaka S, Maeda N, Nagasawa A, Hibuse T, Ohashi K, Kumada M, Nishizawa H, Okamoto Y, Ouchi N, Maeda K, Kihara S, Funahashi T, Matsuzawa Y, Disturbed secretion of mutant adiponectin associated with the metabolic syndrome, Biochem Biophys Res Commun 306(1) (2003) 286–92. [DOI] [PubMed] [Google Scholar]
- [129].Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I, Adiponectin replenishment ameliorates obesity-related hypertension, Hypertension 47(6) (2006) 1108–16. [DOI] [PubMed] [Google Scholar]
- [130].Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, Herschman HR, Kihara S, Walsh K, Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism, Mol Cell Biol 29(13) (2009) 3487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Bulut D, Liaghat S, Hanefeld C, Koll R, Miebach T, Mügge A, Selective cyclo-oxygenase-2 inhibition with parecoxib acutely impairs endothelium-dependent vasodilatation in patients with essential hypertension, J Hypertens 21(9) (2003) 1663–7. [DOI] [PubMed] [Google Scholar]
- [132].Sun D, Liu H, Yan C, Jacobson A, Ojaimi C, Huang A, Kaley G, COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice, Am J Physiol Heart Circ Physiol 291(3) (2006) H1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Lappas M, Permezel M, Rice GE, Leptin and adiponectin stimulate the release of proinflammatory cytokines and prostaglandins from human placenta and maternal adipose tissue via nuclear factor-kappaB, peroxisomal proliferator-activated receptor-gamma and extracellularly regulated kinase 1/2, Endocrinology 146(8) (2005) 3334–42. [DOI] [PubMed] [Google Scholar]
- [134].Yokota T, Meka CS, Medina KL, Igarashi H, Comp PC, Takahashi M, Nishida M, Oritani K, Miyagawa J, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW, Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins, J Clin Invest 109(10) (2002) 1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ, Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis, Cancer Res 59(18) (1999) 4574–7. [PubMed] [Google Scholar]
- [136].Yoshida S, Amano H, Hayashi I, Kitasato H, Kamata M, Inukai M, Yoshimura H, Majima M, COX-2/VEGF-dependent facilitation of tumor-associated angiogenesis and tumor growth in vivo, Lab Invest 83(10) (2003) 1385–94. [DOI] [PubMed] [Google Scholar]
- [137].Wu G, Luo J, Rana JS, Laham R, Sellke FW, Li J, Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells, Cardiovasc Res 69(2) (2006) 512–9. [DOI] [PubMed] [Google Scholar]
- [138].Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K, Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies, J Clin Invest 117(2) (2007) 375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y, Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue, Circulation 107(5) (2003) 671–4. [DOI] [PubMed] [Google Scholar]
- [140].Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, Möhlig M, Pfeiffer AF, Luft FC, Sharma AM, Association between adiponectin and mediators of inflammation in obese women, Diabetes 52(4) (2003) 942–7. [DOI] [PubMed] [Google Scholar]
- [141].Krakoff J, Funahashi T, Stehouwer CD, Schalkwijk CG, Tanaka S, Matsuzawa Y, Kobes S, Tataranni PA, Hanson RL, Knowler WC, Lindsay RS, Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian, Diabetes Care 26(6) (2003) 1745–51. [DOI] [PubMed] [Google Scholar]
- [142].Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D, Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial, Jama 289(14) (2003) 1799–804. [DOI] [PubMed] [Google Scholar]
- [143].Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y, Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages, Blood 96(5) (2000) 1723–32. [PubMed] [Google Scholar]
- [144].Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, Hanazawa S, Yamashita Y, Adiponectin inhibits Toll-like receptor family-induced signaling, FEBS letters 579(30) (2005) 6821–6. [DOI] [PubMed] [Google Scholar]
- [145].Higuchi A, Ohashi K, Kihara S, Walsh K, Ouchi N, Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor-alpha expression, Circ Res 104(9) (2009) 1058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y, Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages, Circulation 103(8) (2001) 1057–63. [DOI] [PubMed] [Google Scholar]
- [147].Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P, Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis, Circ Res 102(2) (2008) 218–25. [DOI] [PubMed] [Google Scholar]
- [148].Harrison DG, The mosaic theory revisited: common molecular mechanisms coordinating diverse organ and cellular events in hypertension, Journal of the American Society of Hypertension : JASH 7(1) (2013) 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K, Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype, The Journal of biological chemistry 285(9) (2010) 6153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Luo Y, Liu M, Adiponectin: a versatile player of innate immunity, Journal of molecular cell biology 8(2) (2016) 120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, Lindén D, Opposing effects of adiponectin receptors 1 and 2 on energy metabolism, Diabetes 56(3) (2007) 583–93. [DOI] [PubMed] [Google Scholar]
- [152].Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, Mahaney MC, Rainwater DL, VandeBerg JL, MacCluer JW, Collier G, Blangero J, Walder K, Jowett JB, Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population, The Journal of clinical endocrinology and metabolism 94(8) (2009) 3085–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Krautbauer S, Wanninger J, Eisinger K, Hader Y, Beck M, Kopp A, Schmid A, Weiss TS, Dorn C, Buechler C, Chemerin is highly expressed in hepatocytes and is induced in non-alcoholic steatohepatitis liver, Experimental and molecular pathology 95(2) (2013) 199–205. [DOI] [PubMed] [Google Scholar]
- [154].Nagpal S, Patel S, Jacobe H, DiSepio D, Ghosn C, Malhotra M, Teng M, Duvic M, Chandraratna RA, Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin, The Journal of investigative dermatology 109(1) (1997) 91–5. [DOI] [PubMed] [Google Scholar]
- [155].Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D, Chemerin is a novel adipokine associated with obesity and metabolic syndrome, Endocrinology 148(10) (2007) 4687–94. [DOI] [PubMed] [Google Scholar]
- [156].Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, Farkas S, Scherer MN, Schäffler A, Aslanidis C, Schölmerich J, Buechler C, Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes, Clinical endocrinology 72(3) (2010) 342–8. [DOI] [PubMed] [Google Scholar]
- [157].Li X, Zhu Q, Wang W, Qi J, He Y, Wang Y, Lu Y, Wu H, Ding Y, Sun Y, Elevated chemerin induces insulin resistance in human granulosa-lutein cells from polycystic ovary syndrome patients, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 33(10) (2019) 11303–11313. [DOI] [PubMed] [Google Scholar]
- [158].Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y, Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells, Biochemical and biophysical research communications 408(2) (2011) 339–43. [DOI] [PubMed] [Google Scholar]
- [159].Neves KB, Nguyen Dinh Cat A, Alves-Lopes R, Harvey KY, Costa RMD, Lobato NS, Montezano AC, Oliveira AM, Touyz RM, Tostes RC, Chemerin receptor blockade improves vascular function in diabetic obese mice via redox-sensitive and Akt-dependent pathways, American journal of physiology. Heart and circulatory physiology 315(6) (2018) H1851–h1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Neves KB, Montezano AC, Alves-Lopes R, Bruder-Nascimento T, Costa RM, Costa RS, Touyz RM, Tostes RC, Upregulation of Nrf2 and Decreased Redox Signaling Contribute to Renoprotective Effects of Chemerin Receptor Blockade in Diabetic Mice, Int J Mol Sci 19(8) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Ge X, Yamaguchi Y, Zhao L, Bury L, Gresele P, Berube C, Leung LL, Morser J, Prochemerin cleavage by factor XIa links coagulation and inflammation, Blood 131(3) (2018) 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Du XY, Leung LL, Proteolytic regulatory mechanism of chemerin bioactivity, Acta biochimica et biophysica Sinica 41(12) (2009) 973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Mirea AM, Toonen EJM, van den Munckhof I, Munsterman ID, Tjwa E, Jaeger M, Oosting M, Schraa K, Rutten JHW, van der Graaf M, Riksen NP, de Graaf J, Netea MG, Tack CJ, Chavakis T, Joosten LAB, Increased proteinase 3 and neutrophil elastase plasma concentrations are associated with non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes, Molecular medicine (Cambridge, Mass.) 25(1) (2019) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Trostler N, Amin R, Shafrir E, Increased protease activity in muscles of obese- (ob/ob) mice, International journal of obesity 6(6) (1982) 557–66. [PubMed] [Google Scholar]
- [165].Ernst MC, Sinal CJ, Chemerin: at the crossroads of inflammation and obesity, Trends in endocrinology and metabolism: TEM 21(11) (2010) 660–7. [DOI] [PubMed] [Google Scholar]
- [166].Kaur J, Adya R, Tan BK, Chen J, Randeva HS, Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis, Biochemical and biophysical research communications 391(4) (2010) 1762–8. [DOI] [PubMed] [Google Scholar]
- [167].Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R, Fink GD, Chemerin connects fat to arterial contraction, Arteriosclerosis, thrombosis, and vascular biology 33(6) (2013) 1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Lobato NS, Neves KB, Filgueira FP, Fortes ZB, Carvalho MH, Webb RC, Oliveira AM, Tostes RC, The adipokine chemerin augments vascular reactivity to contractile stimuli via activation of the MEK-ERK1/2 pathway, Life sciences 91(13–14) (2012) 600–6. [DOI] [PubMed] [Google Scholar]
- [169].Neves KB, Nguyen Dinh Cat A, Lopes RA, Rios FJ, Anagnostopoulou A, Lobato NS, de Oliveira AM, Tostes RC, Montezano AC, Touyz RM, Chemerin Regulates Crosstalk Between Adipocytes and Vascular Cells Through Nox, Hypertension (Dallas, Tex. : 1979) 66(3) (2015) 657–66. [DOI] [PubMed] [Google Scholar]
- [170].Neves KB, Lobato NS, Lopes RA, Filgueira FP, Zanotto CZ, Oliveira AM, Tostes RC, Chemerin reduces vascular nitric oxide/cGMP signalling in rat aorta: a link to vascular dysfunction in obesity?, Clinical science (London, England : 1979) 127(2) (2014) 111–22. [DOI] [PubMed] [Google Scholar]
- [171].Ferland DJ, Mullick AE, Watts SW, Chemerin as a Driver of Hypertension: A Consideration, American journal of hypertension 33(11) (2020) 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Darios ES, Winner BM, Charvat T, Krasinksi A, Punna S, Watts SW, The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery, American journal of physiology. Heart and circulatory physiology 311(2) (2016) H498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Flood ED, Watts SW, Endogenous Chemerin from PVAT Amplifies Electrical Field-Stimulated Arterial Contraction: Use of the Chemerin Knockout Rat, International journal of molecular sciences 21(17) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Tu J, Yang Y, Zhang J, Lu G, Ke L, Tong Z, Kasimu M, Hu D, Xu Q, Li W, Regulatory effect of chemerin and therapeutic efficacy of chemerin‑9 in pancreatogenic diabetes mellitus, Molecular medicine reports 21(3) (2020) 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Faulkner JL, Bruder-Nascimento T, Belin de Chantemèle EJ, The regulation of aldosterone secretion by leptin: implications in obesity-related cardiovascular disease, Current opinion in nephrology and hypertension 27(2) (2018) 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Bruder-Nascimento T, da Silva MA, Tostes RC, The involvement of aldosterone on vascular insulin resistance: implications in obesity and type 2 diabetes, Diabetol Metab Syndr 6(1) (2014) 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Gu P, Jiang W, Lu B, Shi Z, Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients, Clinical and experimental hypertension (New York, N.Y. : 1993) 36(5) (2014) 326–32. [DOI] [PubMed] [Google Scholar]
- [178].Yamamoto A, Otani K, Okada M, Yamawaki H, Chemokine-like Receptor 1 in Brain of Spontaneously Hypertensive Rats Mediates Systemic Hypertension, International journal of molecular sciences 22(21) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Ferland DJ, Flood ED, Garver H, Yeh ST, Riney S, Mullick AE, Fink GD, Watts SW, Different blood pressure responses in hypertensive rats following chemerin mRNA inhibition in dietary high fat compared to dietary high-salt conditions, Physiological genomics 51(11) (2019) 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Ferland DJ, Seitz B, Darios ES, Thompson JM, Yeh ST, Mullick AE, Watts SW, Whole-Body but Not Hepatic Knockdown of Chemerin by Antisense Oligonucleotide Decreases Blood Pressure in Rats, The Journal of pharmacology and experimental therapeutics 365(2) (2018) 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Watts SW, Darios ES, Mullick AE, Garver H, Saunders TL, Hughes ED, Filipiak WE, Zeidler MG, McMullen N, Sinal CJ, Kumar RK, Ferland DJ, Fink GD, The chemerin knockout rat reveals chemerin dependence in female, but not male, experimental hypertension, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 32(12) (2018) fj201800479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Lin Y, Cai Q, Luo Y, Li B, Chen Y, Yang X, Xuan Y, Yang H, He R, Epithelial chemerin-CMKLR1 signaling restricts microbiota-driven colonic neutrophilia and tumorigenesis by up-regulating lactoperoxidase, Proceedings of the National Academy of Sciences of the United States of America 119(29) (2022) e2205574119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Rourke JL, Muruganandan S, Dranse HJ, McMullen NM, Sinal CJ, Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice, The Journal of endocrinology 222(2) (2014) 201–15. [DOI] [PubMed] [Google Scholar]
- [184].Schäffler A, Neumeier M, Herfarth H, Fürst A, Schölmerich J, Büchler C, Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue, Biochimica et biophysica acta 1732(1–3) (2005) 96–102. [DOI] [PubMed] [Google Scholar]
- [185].Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW, Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action, American journal of physiology. Endocrinology and metabolism 290(6) (2006) E1253–61. [DOI] [PubMed] [Google Scholar]
- [186].Çelik M, Nar R, Nar G, Sökmen E, Günver G, Serum omentin-1 levels in hypertensive patients, Journal of human hypertension 35(3) (2021) 290–295. [DOI] [PubMed] [Google Scholar]
- [187].Zhou H, Zhang Z, Qian G, Zhou J, Omentin-1 attenuates adipose tissue inflammation via restoration of TXNIP/NLRP3 signaling in high-fat diet-induced obese mice, Fundamental & clinical pharmacology 34(6) (2020) 721–735. [DOI] [PubMed] [Google Scholar]
- [188].Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y, Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels, Biochemical and biophysical research communications 393(4) (2010) 668–72. [DOI] [PubMed] [Google Scholar]
- [189].Kazama K, Okada M, Yamawaki H, A novel adipocytokine, omentin, inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell migration through antioxidative mechanism, American journal of physiology. Heart and circulatory physiology 306(12) (2014) H1714–9. [DOI] [PubMed] [Google Scholar]
- [190].Yamawaki H, Vascular effects of novel adipocytokines: focus on vascular contractility and inflammatory responses, Biological & pharmaceutical bulletin 34(3) (2011) 307–10. [DOI] [PubMed] [Google Scholar]
- [191].Wang Z, Nakayama T, Inflammation, a link between obesity and cardiovascular disease, Mediators of inflammation 2010 (2010) 535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK, Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability, Proceedings of the National Academy of Sciences of the United States of America 98(5) (2001) 2604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Maruyama S, Shibata R, Kikuchi R, Izumiya Y, Rokutanda T, Araki S, Kataoka Y, Ohashi K, Daida H, Kihara S, Ogawa H, Murohara T, Ouchi N, Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism, The Journal of biological chemistry 287(1) (2012) 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Kazama K, Okada M, Hara Y, Yamawaki H, A novel adipocytokine, omentin, inhibits agonists-induced increases of blood pressure in rats, The Journal of veterinary medical science 75(8) (2013) 1029–34. [DOI] [PubMed] [Google Scholar]
- [195].Moreno-Navarrete JM, Ortega F, Castro A, Sabater M, Ricart W, Fernández-Real JM , Circulating omentin as a novel biomarker of endothelial dysfunction, Obesity (Silver Spring, Md.) 19(8) (2011) 1552–9. [DOI] [PubMed] [Google Scholar]
- [196].Bolad I, Delafontaine P, Endothelial dysfunction: its role in hypertensive coronary disease, Current opinion in cardiology 20(4) (2005) 270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Woo YC, Xu A, Wang Y, Lam KS, Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives, Clinical endocrinology 78(4) (2013) 489–96. [DOI] [PubMed] [Google Scholar]
- [198].Cuevas-Ramos D, Mehta R, Aguilar-Salinas CA, Fibroblast Growth Factor 21 and Browning of White Adipose Tissue, Front Physiol 10 (2019) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS, Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans, Cell metabolism 19(2) (2014) 302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Loap S, Lathe R, Mechanism Underlying Tissue Cryotherapy to Combat Obesity/Overweight: Triggering Thermogenesis, Journal of obesity 2018 (2018) 5789647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA, Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21, Cell metabolism 5(6) (2007) 415–25. [DOI] [PubMed] [Google Scholar]
- [202].Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, Busby J, Hecht R, Li YS, Li Y, Lindberg R, Véniant MM, Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects, American journal of physiology. Endocrinology and metabolism 297(5) (2009) E1105–14. [DOI] [PubMed] [Google Scholar]
- [203].Li H, Wu G, Fang Q, Zhang M, Hui X, Sheng B, Wu L, Bao Y, Li P, Xu A, Jia W, Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat, Nat Commun 9(1) (2018) 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Véniant MM, Hale C, Helmering J, Chen MM, Stanislaus S, Busby J, Vonderfecht S, Xu J, Lloyd DJ, FGF21 promotes metabolic homeostasis via white adipose and leptin in mice, PLoS One 7(7) (2012) e40164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A, Leptin-replacement therapy for lipodystrophy, The New England journal of medicine 346(8) (2002) 570–8. [DOI] [PubMed] [Google Scholar]
- [206].Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro OM, Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21, The Journal of biological chemistry 282(37) (2007) 26687–26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M, BetaKlotho is required for metabolic activity of fibroblast growth factor 21, Proceedings of the National Academy of Sciences of the United States of America 104(18) (2007) 7432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Santos RA, Ferreira AJ, Pinheiro SV, Sampaio WO, Touyz R, Campagnole-Santos MJ, Angiotensin-(1–7) and its receptor as a potential targets for new cardiovascular drugs, Expert opinion on investigational drugs 14(8) (2005) 1019–31. [DOI] [PubMed] [Google Scholar]
- [209].Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X, Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice, Cell metabolism 17(5) (2013) 779–89. [DOI] [PubMed] [Google Scholar]
- [210].Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA, The hormone resistin links obesity to diabetes, Nature 409(6818) (2001) 307–12. [DOI] [PubMed] [Google Scholar]
- [211].Schwartz DR, Lazar MA, Human resistin: found in translation from mouse to man, Trends in endocrinology and metabolism: TEM 22(7) (2011) 259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI, Resistin competes with lipopolysaccharide for binding to toll-like receptor 4, Journal of cellular and molecular medicine 14(6b) (2010) 1419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, Lee S, Kim JY, Lee J, Yang HM, Mook-Jung I, Nam KY, Chung J, Lazar MA, Kim HS, Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes, Cell Metab 19(3) (2014) 484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG, An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells, Cell stem cell 9(1) (2011) 74–86. [DOI] [PubMed] [Google Scholar]
- [215].McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S, Resistin, central obesity, and type 2 diabetes, Lancet (London, England) 359(9300) (2002) 46–7. [DOI] [PubMed] [Google Scholar]
- [216].Levy JR, Davenport B, Clore JN, Stevens W, Lipid metabolism and resistin gene expression in insulin-resistant Fischer 344 rats, American journal of physiology. Endocrinology and metabolism 282(3) (2002) E626–33. [DOI] [PubMed] [Google Scholar]
- [217].Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N, Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process?, Diabetes 51(10) (2002) 2951–8. [DOI] [PubMed] [Google Scholar]
- [218].Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV, Serum resistin (FIZZ3) protein is increased in obese humans, The Journal of clinical endocrinology and metabolism 88(11) (2003) 5452–5. [DOI] [PubMed] [Google Scholar]
- [219].Park HK, Ahima RS, Resistin in rodents and humans, Diabetes & metabolism journal 37(6) (2013) 404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Jamaluddin MS, Yan S, Lü J, Liang Z, Yao Q, Chen C, Resistin increases monolayer permeability of human coronary artery endothelial cells, PLoS One 8(12) (2013) e84576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Chen C, Jiang J, Lü JM, Chai H, Wang X, Lin PH, Yao Q, Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells, American journal of physiology. Heart and circulatory physiology 299(1) (2010) H193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, Zbinden S, Clavijo LC, Jang GJ, Andrews JA, Zhu J, Epstein SE, The potential role of resistin in atherogenesis, Atherosclerosis 182(2) (2005) 241–8. [DOI] [PubMed] [Google Scholar]
- [223].Qi Q, Wang J, Li H, Yu Z, Ye X, Hu FB, Franco OH, Pan A, Liu Y, Lin X, Associations of resistin with inflammatory and fibrinolytic markers, insulin resistance, and metabolic syndrome in middle-aged and older Chinese, European journal of endocrinology 159(5) (2008) 585–93. [DOI] [PubMed] [Google Scholar]
- [224].Saddi-Rosa P, Oliveira CS, Giuffrida FM, Reis AF, Visfatin, glucose metabolism and vascular disease: a review of evidence, Diabetology & metabolic syndrome 2 (2010) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Brown JE, Onyango DJ, Ramanjaneya M, Conner AC, Patel ST, Dunmore SJ, Randeva HS, Visfatin regulates insulin secretion, insulin receptor signalling and mRNA expression of diabetes-related genes in mouse pancreatic beta-cells, Journal of molecular endocrinology 44(3) (2010) 171–8. [DOI] [PubMed] [Google Scholar]
- [226].Romacho T, Valencia I, Ramos-González M, Vallejo S, López-Esteban M, Lorenzo O, Cannata P, Romero A, San Hipólito-Luengo A, Gómez-Cerezo JF, Peiró C, Sánchez-Ferrer CF, Visfatin/eNampt induces endothelial dysfunction in vivo: a role for Toll-Like Receptor 4 and NLRP3 inflammasome, Scientific reports 10(1) (2020) 5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Curat CA, Wegner V, Sengenès C, Miranville A, Tonus C, Busse R, Bouloumié A, Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin, Diabetologia 49(4) (2006) 744–7. [DOI] [PubMed] [Google Scholar]
- [228].Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I, Visfatin: a protein secreted by visceral fat that mimics the effects of insulin, Science 307(5708) (2005) 426–30. [DOI] [PubMed] [Google Scholar]
- [229].Garten A, Petzold S, Barnikol-Oettler A, Körner A, Thasler WE, Kratzsch J, Kiess W, Gebhardt R, Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes, Biochemical and biophysical research communications 391(1) (2010) 376–81. [DOI] [PubMed] [Google Scholar]
- [230].Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR, Skeletal muscle NAMPT is induced by exercise in humans, American journal of physiology. Endocrinology and metabolism 298(1) (2010) E117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Krzysik-Walker SM, Ocón-Grove OM, Maddineni SR, Hendricks GL 3rd, Ramachandran R, Is visfatin an adipokine or myokine? Evidence for greater visfatin expression in skeletal muscle than visceral fat in chickens, Endocrinology 149(4) (2008) 1543–50. [DOI] [PubMed] [Google Scholar]
- [232].Varma V, Yao-Borengasser A, Rasouli N, Bodles AM, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer HJ 3rd, McGehee RE Jr., Fried SK, Kern PA, Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation, The Journal of clinical endocrinology and metabolism 92(2) (2007) 666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Dahl TB, Yndestad A, Skjelland M, Øie E, Dahl A, Michelsen A, Damås JK, Tunheim SH, Ueland T, Smith C, Bendz B, Tonstad S, Gullestad L, Frøland SS, Krohg-Sørensen K, Russell D, Aukrust P, Halvorsen B, Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization, Circulation 115(8) (2007) 972–80. [DOI] [PubMed] [Google Scholar]
- [234].Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T, Association between plasma visfatin and vascular endothelial function in patients with type 2 diabetes mellitus, Metabolism: clinical and experimental 56(4) (2007) 451–8. [DOI] [PubMed] [Google Scholar]
- [235].Liu SW, Qiao SB, Yuan JS, Liu DQ, Association of plasma visfatin levels with inflammation, atherosclerosis and acute coronary syndromes (ACS) in humans, Clinical endocrinology 71(2) (2009) 202–7. [DOI] [PubMed] [Google Scholar]
- [236].Zhong M, Tan HW, Gong HP, Wang SF, Zhang Y, Zhang W, Increased serum visfatin in patients with metabolic syndrome and carotid atherosclerosis, Clinical endocrinology 69(6) (2008) 878–84. [DOI] [PubMed] [Google Scholar]
- [237].Lu LF, Yang SS, Wang CP, Hung WC, Yu TH, Chiu CA, Chung FM, Shin SJ, Lee YJ, Elevated visfatin/pre-B-cell colony-enhancing factor plasma concentration in ischemic stroke, Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 18(5) (2009) 354–9. [DOI] [PubMed] [Google Scholar]
- [238].Soltis EE, Cassis LA, Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness, Clinical and experimental hypertension. Part A, Theory and practice 13(2) (1991) 277–96. [DOI] [PubMed] [Google Scholar]
- [239].Ayala-Lopez N, Martini M, Jackson WF, Darios E, Burnett R, Seitz B, Fink GD, Watts SW, Perivascular adipose tissue contains functional catecholamines, Pharmacology research & perspectives 2(3) (2014) e00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Ayala-Lopez N, Jackson WF, Burnett R, Wilson JN, Thompson JM, Watts SW, Organic cation transporter 3 contributes to norepinephrine uptake into perivascular adipose tissue, American journal of physiology. Heart and circulatory physiology 309(11) (2015) H1904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Török J, Zemančíková A, Kocianová Z, Interaction of perivascular adipose tissue and sympathetic nerves in arteries from normotensive and hypertensive rats, Physiological research 65(Suppl 3) (2016) S391–s399. [DOI] [PubMed] [Google Scholar]
- [242].Diaz J, Ni W, Thompson J, King A, Fink GD, Watts SW, 5-Hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats, The Journal of pharmacology and experimental therapeutics 325(3) (2008) 1031–8. [DOI] [PubMed] [Google Scholar]
- [243].Kumar RK, Darios ES, Burnett R, Thompson JM, Watts SW, Fenfluramine-induced PVAT-dependent contraction depends on norepinephrine and not serotonin, Pharmacological research 140 (2019) 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Dinarello CA, Historical insights into cytokines, European journal of immunology 37 Suppl 1(Suppl 1) (2007) S34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Ouchi N, Parker JL, Lugus JJ, Walsh K, Adipokines in inflammation and metabolic disease, Nature reviews. Immunology 11(2) (2011) 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Surmi BK, Hasty AH, Macrophage infiltration into adipose tissue: initiation, propagation and remodeling, Future lipidology 3(5) (2008) 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Hotamisligil GS, Shargill NS, Spiegelman BM, Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance, Science 259(5091) (1993) 87–91. [DOI] [PubMed] [Google Scholar]
- [248].Badawi A, Klip A, Haddad P, Cole DE, Bailo BG, El-Sohemy A, Karmali M, Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention, Diabetes, metabolic syndrome and obesity : targets and therapy 3 (2010) 173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Bilan PJ, Samokhvalov V, Koshkina A, Schertzer JD, Samaan MC, Klip A, Direct and macrophage-mediated actions of fatty acids causing insulin resistance in muscle cells, Archives of physiology and biochemistry 115(4) (2009) 176–90. [DOI] [PubMed] [Google Scholar]
- [250].Patsalos O, Dalton B, Leppanen J, Ibrahim MAA, Himmerich H, Impact of TNF-α Inhibitors on Body Weight and BMI: A Systematic Review and Meta-Analysis, Frontiers in pharmacology 11 (2020) 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr., NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia, Science 289(5488) (2000) 2363–6. [DOI] [PubMed] [Google Scholar]
- [252].Hosoi T, Okuma Y, Nomura Y, The mechanisms of immune-to-brain communication in inflammation as a drug target, Current drug targets. Inflammation and allergy 1(3) (2002) 257–62. [DOI] [PubMed] [Google Scholar]
- [253].Metsios GS, Stavropoulos-Kalinoglou A, Douglas KM, Koutedakis Y, Nevill AM, Panoulas VF, Kita M, Kitas GD, Blockade of tumour necrosis factor-alpha in rheumatoid arthritis: effects on components of rheumatoid cachexia, Rheumatology (Oxford, England) 46(12) (2007) 1824–7. [DOI] [PubMed] [Google Scholar]
- [254].Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW, Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo, The Journal of clinical endocrinology and metabolism 82(12) (1997) 4196–200. [DOI] [PubMed] [Google Scholar]
- [255].Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S, Visceral fat adipokine secretion is associated with systemic inflammation in obese humans, Diabetes 56(4) (2007) 1010–3. [DOI] [PubMed] [Google Scholar]
- [256].Patel S, Rauf A, Khan H, Abu-Izneid T, Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 94 (2017) 317–325. [DOI] [PubMed] [Google Scholar]
- [257].Yasue S, Masuzaki H, Okada S, Ishii T, Kozuka C, Tanaka T, Fujikura J, Ebihara K, Hosoda K, Katsurada A, Ohashi N, Urushihara M, Kobori H, Morimoto N, Kawazoe T, Naitoh M, Okada M, Sakaue H, Suzuki S, Nakao K, Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy, American journal of hypertension 23(4) (2010) 425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA, Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice, Hypertension (Dallas, Tex. : 1979) 60(6) (2012) 1524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Dandona P, Dhindsa S, Ghanim H, Chaudhuri A, Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade, Journal of human hypertension 21(1) (2007) 20–7. [DOI] [PubMed] [Google Scholar]
- [260].Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Corrêa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM, Adipocytes produce aldosterone through calcineurin-dependent ignaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction, Hypertension (Dallas, Tex. : 1979) 59(5) (2012) 1069–78. [DOI] [PubMed] [Google Scholar]
- [261].Huby AC, Otvos L Jr., Belin de Chantemèle EJ, Leptin Induces Hypertension and Endothelial Dysfunction via Aldosterone-Dependent Mechanisms in Obese Female Mice, Hypertension (Dallas, Tex. : 1979) 67(5) (2016) 1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Johnson AK, Xue B, Central nervous system neuroplasticity and the sensitization of hypertension, Nature reviews. Nephrology 14(12) (2018) 750–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Joyner MJ, Charkoudian N, Wallin BG, Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications, Hypertension (Dallas, Tex. : 1979) 56(1) (2010) 10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Ma D, Feitosa MF, Wilk JB, Laramie JM, Yu K, Leiendecker-Foster C, Myers RH, Province MA, Borecki IB, Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study, Hypertension (Dallas, Tex. : 1979) 53(3) (2009) 473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG Jr., Licinio J, Brown RD, Enriori PJ, O’Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA, Leptin mediates the increase in blood pressure associated with obesity, Cell 159(6) (2014) 1404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG, Role of selective leptin resistance in diet-induced obesity hypertension, Diabetes 54(7) (2005) 2012–8. [DOI] [PubMed] [Google Scholar]
- [267].Kosari S, Rathner JA, Badoer E, Central resistin enhances renal sympathetic nerve activity via phosphatidylinositol 3-kinase but reduces the activity to brown adipose tissue via extracellular signal-regulated kinase 1/2, Journal of neuroendocrinology 24(11) (2012) 1432–9. [DOI] [PubMed] [Google Scholar]
- [268].Rahmouni K, Obesity, sympathetic overdrive, and hypertension: the leptin connection, Hypertension (Dallas, Tex. : 1979) 55(4) (2010) 844–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM, Leptin enters the brain by a saturable system independent of insulin, Peptides 17(2) (1996) 305–11. [DOI] [PubMed] [Google Scholar]
- [270].Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA, Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits, Hypertension (Dallas, Tex. : 1979) 55(4) (2010) 862–8. [DOI] [PubMed] [Google Scholar]
- [271].Kusminski CM, McTernan PG, Schraw T, Kos K, O’Hare JP, Ahima R, Kumar S, Scherer PE, Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum, Diabetologia 50(3) (2007) 634–42. [DOI] [PubMed] [Google Scholar]
- [272].Hoyda TD, Smith PM, Ferguson AV, Adiponectin acts in the nucleus of the solitary tract to decrease blood pressure by modulating the excitability of neuropeptide Y neurons, Brain research 1256 (2009) 76–84. [DOI] [PubMed] [Google Scholar]
- [273].Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE, Brain adipocytokine action and metabolic regulation, Diabetes 55 Suppl 2 (2006) S145–54. [DOI] [PubMed] [Google Scholar]
- [274].Tanida M, Shen J, Horii Y, Matsuda M, Kihara S, Funahashi T, Shimomura I, Sawai H, Fukuda Y, Matsuzawa Y, Nagai K, Effects of adiponectin on the renal sympathetic nerve activity and blood pressure in rats, Experimental biology and medicine (Maywood, N.J.) 232(3) (2007) 390–7. [PubMed] [Google Scholar]
- [275].Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM, Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients, Circulation 119(12) (2009) 1661–70. [DOI] [PubMed] [Google Scholar]
- [276].Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z, Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome, Nature medicine 13(7) (2007) 803–11. [DOI] [PubMed] [Google Scholar]
- [277].Pénicaud L, Cousin B, Leloup C, Atef N, Casteilla L, Ktorza A, Changes in autonomic nervous system activity and consecutive hyperinsulinaemia: respective roles in the development of obesity in rodents, Diabetes & metabolism 22(1) (1996) 15–24. [PubMed] [Google Scholar]
- [278].Alvarez GE, Beske SD, Ballard TP, Davy KP, Sympathetic neural activation in visceral obesity, Circulation 106(20) (2002) 2533–6. [DOI] [PubMed] [Google Scholar]
- [279].Dogiel AS, Die sensiblen Nervenendigungen im Herzen und in den Blutgefässen der Säugethiere, Archiv für mikroskopische Anatomie volume 52 (1898) 44–70. [Google Scholar]
- [280].Blaszkiewicz M, Willows JW, Johnson CP, Townsend KL, The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance, Biology 8(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Wang Y, Leung VH, Zhang Y, Nudell VS, Loud M, Servin-Vences MR, Yang D, Wang K, Moya-Garzon MD, Li VL, Long JZ, Patapoutian A, Ye L, The role of somatosensory innervation of adipose tissues, Nature 609(7927) (2022) 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Blaszkiewicz M, Gunsch G, Willows JW, Gardner ML, Sepeda JA, Sas AR, Townsend KL, Adipose Tissue Myeloid-Lineage Neuroimmune Cells Express Genes Important for Neural Plasticity and Regulate Adipose Innervation, Frontiers in endocrinology 13 (2022) 864925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Zhu Q, Glazier BJ, Hinkel BC, Cao J, Liu L, Liang C, Shi H, Neuroendocrine Regulation of Energy Metabolism Involving Different Types of Adipose Tissues, International journal of molecular sciences 20(11) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Scherer PE, Adipose tissue: from lipid storage compartment to endocrine organ, Diabetes 55(6) (2006) 1537–45. [DOI] [PubMed] [Google Scholar]
- [285].Ahima RS, Flier JS, Adipose tissue as an endocrine organ, Trends in endocrinology and metabolism: TEM 11(8) (2000) 327–32. [DOI] [PubMed] [Google Scholar]
- [286].Bartness TJ, Bamshad M, Innervation of mammalian white adipose tissue: implications for the regulation of total body fat, The American journal of physiology 275(5) (1998) R1399–411. [DOI] [PubMed] [Google Scholar]
- [287].Morrison SF, Madden CJ, Central nervous system regulation of brown adipose tissue, Comprehensive Physiology 4(4) (2014) 1677–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ, White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation, American journal of physiology. Regulatory, integrative and comparative physiology 291(5) (2006) R1243–55. [DOI] [PubMed] [Google Scholar]
- [289].Messina G, Valenzano A, Moscatelli F, Salerno M, Lonigro A, Esposito T, Monda V, Corso G, Messina A, Viggiano A, Triggiani AI, Chieffi S, Guglielmi G, Monda M, Cibelli G, Role of Autonomic Nervous System and Orexinergic System on Adipose Tissue, Frontiers in physiology 8 (2017) 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Song CK, Schwartz GJ, Bartness TJ, Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue, American journal of physiology. Regulatory, integrative and comparative physiology 296(3) (2009) R501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK, Sensory and sympathetic nervous system control of white adipose tissue lipolysis, Molecular and cellular endocrinology 318(1–2) (2010) 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Guilherme A, Henriques F, Bedard AH, Czech MP, Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus, Nature reviews. Endocrinology 15(4) (2019) 207–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Rahmouni K, Leptin-Induced Sympathetic Nerve Activation: Signaling Mechanisms and Cardiovascular Consequences in Obesity, Current hypertension reviews 6(2) (2010) 104–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Shiuchi T, Toda C, Okamoto S, Coutinho EA, Saito K, Miura S, Ezaki O, Minokoshi Y, Induction of glucose uptake in skeletal muscle by central leptin is mediated by muscle β(2)-adrenergic receptor but not by AMPK, Scientific reports 7(1) (2017) 15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Saxton SN, Withers SB, Heagerty AM, Emerging Roles of Sympathetic Nerves and Inflammation in Perivascular Adipose Tissue, Cardiovascular drugs and therapy 33(2) (2019) 245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, Heagerty AM, Role of Sympathetic Nerves and Adipocyte Catecholamine Uptake in the Vasorelaxant Function of Perivascular Adipose Tissue, Arteriosclerosis, thrombosis, and vascular biology 38(4) (2018) 880–891. [DOI] [PubMed] [Google Scholar]
- [297].Saxton SN, Clark BJ, Withers SB, Eringa EC, Heagerty AM, Mechanistic Links Between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue, Physiological reviews 99(4) (2019) 1701–1763. [DOI] [PubMed] [Google Scholar]
- [298].Abu Bakar H, Robert Dunn W, Daly C, Ralevic V, Sensory innervation of perivascular adipose tissue: a crucial role in artery vasodilatation and leptin release, Cardiovascular research 113(8) (2017) 962–972. [DOI] [PubMed] [Google Scholar]
- [299].Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, Heagerty AM, Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses, American journal of physiology. Heart and circulatory physiology 304(6) (2013) H786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O, Localization of fat depots and cardiovascular risk, Lipids in health and disease 17(1) (2018) 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Suárez-Cuenca JA, De La Peña-Sosa G, De La Vega-Moreno K, Banderas-Lares DZ, Salamanca-García M, Martínez-Hernández JE, Vera-Gómez E, Hernández-Patricio A, Zamora-Alemán CR, Domínguez-Pérez GA, Ruíz-Hernández AS, Gutiérrez-Buendía JA, Melchor-López A, Ortíz-Fernández M, Montoya-Ramírez J, Gaytán-Fuentes OF, Toríz-Ortíz A, Osorio-Valero M, Orozco-Vázquez J, Alcaráz-Estrada SL, Rodríguez-Arellano ME, Maldonado-Arriaga B, Pérez-Cabeza de Vaca R, Escamilla-Tilch M, Pineda-Juárez JA, Téllez-González MA, García S, Mondragón-Terán P, Enlarged adipocytes from subcutaneous vs. visceral adipose tissue differentially contribute to metabolic dysfunction and atherogenic risk of patients with obesity, Scientific reports 11(1) (2021) 1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Couillard C, Mauriège P, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Després JP, Plasma leptin concentrations: gender differences and associations with metabolic risk factors for cardiovascular disease, Diabetologia 40(10) (1997) 1178–84. [DOI] [PubMed] [Google Scholar]
- [303].Youn BS, Bang SI, Klöting N, Park JW, Lee N, Oh JE, Pi KB, Lee TH, Ruschke K, Fasshauer M, Stumvoll M, Blüher M, Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue, Diabetes 58(3) (2009) 627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Pescatello LS, Buchner DM, Jakicic JM, Powell KE, Kraus WE, Bloodgood B, Campbell WW, Dietz S, Dipietro L, George SM, Macko RF, McTiernan A, Pate RR, Piercy KL, Physical Activity to Prevent and Treat Hypertension: A Systematic Review, Medicine and science in sports and exercise 51(6) (2019) 1314–1323. [DOI] [PubMed] [Google Scholar]
- [305].Golbidi S, Laher I, Exercise induced adipokine changes and the metabolic syndrome, Journal of diabetes research 2014 (2014) 726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Atakan MM, Koşar Ş N, Güzel Y, Tin HT, Yan X, The Role of Exercise, Diet, and Cytokines in Preventing Obesity and Improving Adipose Tissue, Nutrients 13(5) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Pilic L, Pedlar CR, Mavrommatis Y, Salt-sensitive hypertension: mechanisms and effects of dietary and other lifestyle factors, Nutrition reviews 74(10) (2016) 645–58. [DOI] [PubMed] [Google Scholar]
- [308].Young JB, Landsberg L, Diet-induced changes in sympathetic nervous system activity: possible implications for obesity and hypertension, Journal of chronic diseases 35(12) (1982) 879–86. [DOI] [PubMed] [Google Scholar]
- [309].Man AWC, Li H, Xia N, Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function, Oxidative medicine and cellular longevity 2020 (2020) 1496462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Welly RJ, Liu TW, Zidon TM, Rowles JL 3rd, Park YM, Smith TN, Swanson KS, Padilla J, Vieira-Potter VJ, Comparison of Diet versus Exercise on Metabolic Function and Gut Microbiota in Obese Rats, Medicine and science in sports and exercise 48(9) (2016) 1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Ibrahim MM, Subcutaneous and visceral adipose tissue: structural and functional differences, Obesity reviews : an official journal of the International Association for the Study of Obesity 11(1) (2010) 11–8. [DOI] [PubMed] [Google Scholar]
- [312].Rohrich RJ, Savetsky IL, Avashia YJ, Assessing Cosmetic Surgery Safety: The Evolving Data, Plastic and reconstructive surgery. Global open 8(5) (2020) e2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Busetto L, Bassetto F, Zocchi M, Zuliani F, Nolli ML, Pigozzo S, Coin A, Mazza M, Sergi G, Mazzoleni F, Enzi G, The effects of the surgical removal of subcutaneous adipose tissue on energy expenditure and adipocytokine concentrations in obese women, Nutrition, metabolism, and cardiovascular diseases : NMCD 18(2) (2008) 112–20. [DOI] [PubMed] [Google Scholar]
- [314].Giugliano G, Nicoletti G, Grella E, Giugliano F, Esposito K, Scuderi N, D’Andrea F, Effect of liposuction on insulin resistance and vascular inflammatory markers in obese women, British journal of plastic surgery 57(3) (2004) 190–4. [DOI] [PubMed] [Google Scholar]
- [315].Mohammed BS, Cohen S, Reeds D, Young VL, Klein S, Long-term effects of large-volume liposuction on metabolic risk factors for coronary heart disease, Obesity (Silver Spring, Md.) 16(12) (2008) 2648–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS, Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease, The New England journal of medicine 350(25) (2004) 2549–57. [DOI] [PubMed] [Google Scholar]
- [317].Benatti F, Solis M, Artioli G, Montag E, Painelli V, Saito F, Baptista L, Costa LA, Neves R, Seelaender M, Ferriolli E, Pfrimer K, Lima F, Roschel H, Gualano B, Lancha A Jr., Liposuction induces a compensatory increase of visceral fat which is effectively counteracted by physical activity: a randomized trial, The Journal of clinical endocrinology and metabolism 97(7) (2012) 2388–95. [DOI] [PubMed] [Google Scholar]
- [318].Holmbäck U, Grudén S, Litorp H, Willhems D, Kuusk S, Alderborn G, Söderhäll A, Forslund A, Effects of a novel weight-loss combination product containing orlistat and acarbose on obesity: A randomized, placebo-controlled trial, Obesity (Silver Spring, Md.) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Lonneman DJ Jr., Rey JA, McKee BD, Phentermine/Topiramate extended-release capsules (qsymia) for weight loss, P & T : a peer-reviewed journal for formulary management 38(8) (2013) 446–52. [PMC free article] [PubMed] [Google Scholar]
- [320].Billes SK, Sinnayah P, Cowley MA, Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss, Pharmacological research 84 (2014) 1–11. [DOI] [PubMed] [Google Scholar]
- [321].Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L, The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss, J Clin Invest 124(10) (2014) 4473–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Poulton AS, Hibbert EJ, Champion BL, Nanan RK, Stimulants for the Control of Hedonic Appetite, Frontiers in pharmacology 7 (2016) 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Cannavo A, Koch WJ, Targeting β3-Adrenergic Receptors in the Heart: Selective Agonism and β-Blockade, Journal of cardiovascular pharmacology 69(2) (2017) 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Chang Y, Dagat LA, Yusuf A, Zahriya Y, Staputyte K, Worley E, Holt A, Canuteson N, Messieha V, Halila K, Regulation of bFGF-induced effects on rat aortic smooth muscle cells by β(3)-adrenergic receptors, Current research in pharmacology and drug discovery 3 (2022) 100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, Strosberg AD, Molecular characterization of the human beta 3-adrenergic receptor, Science 245(4922) (1989) 1118–21. [DOI] [PubMed] [Google Scholar]
- [326].Decara J, Rivera P, Arrabal S, Vargas A, Serrano A, Pavón FJ, Dieguez C, Nogueiras R, Rodríguez de Fonseca F, Suárez J, Cooperative role of the glucagon-like peptide-1 receptor and β3-adrenergic-mediated signalling on fat mass reduction through the downregulation of PKA/AKT/AMPK signalling in the adipose tissue and muscle of rats, Acta physiologica (Oxford, England) 222(4) (2018) e13008. [DOI] [PubMed] [Google Scholar]
- [327].Dubois-Deruy E, Gelinas R, Beauloye C, Esfahani H, Michel LYM, Dessy C, Bertrand L, Balligand JL, Beta 3 adrenoreceptors protect from hypertrophic remodelling through AMP-activated protein kinase and autophagy, ESC heart failure 7(3) (2020) 920–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].García-Rubi E, Calles-Escandón J, Insulin resistance and type 2 diabetes mellitus: its relationship with the beta 3-adrenergic receptor, Archives of medical research 30(6) (1999) 459–64. [DOI] [PubMed] [Google Scholar]
- [329].Mantzoros CS, Qu D, Frederich RC, Susulic VS, Lowell BB, Maratos-Flier E, Flier JS, Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice, Diabetes 45(7) (1996) 909–14. [DOI] [PubMed] [Google Scholar]
- [330].Fu L, Isobe K, Zeng Q, Suzukawa K, Takekoshi K, Kawakami Y, The effects of beta(3)-adrenoceptor agonist CL-316,243 on adiponectin, adiponectin receptors and tumor necrosis factor-alpha expressions in adipose tissues of obese diabetic KKAy mice, European journal of pharmacology 584(1) (2008) 202–6. [DOI] [PubMed] [Google Scholar]
- [331].Rodriguez M, Carillon C, Coquerel A, Le Fur G, Ferrara P, Caput D, Shire D, Evidence for the presence of beta 3-adrenergic receptor mRNA in the human brain, Brain research. Molecular brain research 29(2) (1995) 369–75. [DOI] [PubMed] [Google Scholar]
- [332].Summers RJ, Papaioannou M, Harris S, Evans BA, Expression of beta 3-adrenoceptor mRNA in rat brain, British journal of pharmacology 116(6) (1995) 2547–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Castillo-Meléndez M, McKinley MJ, Summers RJ, Intracerebroventricular administration of the beta(3)-adrenoceptor agonist CL 316243 causes Fos immunoreactivity in discrete regions of rat hypothalamus, Neuroscience letters 290(3) (2000) 161–4. [DOI] [PubMed] [Google Scholar]
- [334].Richard JE, López-Ferreras L, Chanclón B, Eerola K, Micallef P, Skibicka KP, Wernstedt Asterholm I, CNS β(3)-adrenergic receptor activation regulates feeding behavior, white fat browning, and body weight, American journal of physiology. Endocrinology and metabolism 313(3) (2017) E344–e358. [DOI] [PubMed] [Google Scholar]
- [335].Louis SN, Jackman GP, Nero TL, Iakovidis D, Louis WJ, Role of beta-adrenergic receptor subtypes in lipolysis, Cardiovascular drugs and therapy 14(6) (2000) 565–77. [DOI] [PubMed] [Google Scholar]
- [336].Gauthier C, Sèze-Goismier C, Rozec B, Beta 3-adrenoceptors in the cardiovascular system, Clinical hemorheology and microcirculation 37(1–2) (2007) 193–204. [PubMed] [Google Scholar]
- [337].Berg T, Piercey BW, Jensen J, Role of beta1–3-adrenoceptors in blood pressure control at rest and during tyramine-induced norepinephrine release in spontaneously hypertensive rats, Hypertension (Dallas, Tex. : 1979) 55(5) (2010) 1224–30. [DOI] [PubMed] [Google Scholar]
- [338].Donckier JE, Massart PE, Van Mechelen H, Heyndrickx GR, Gauthier C, Balligand JL, Cardiovascular effects of beta 3-adrenoceptor stimulation in perinephritic hypertension, European journal of clinical investigation 31(8) (2001) 681–9. [DOI] [PubMed] [Google Scholar]
- [339].Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, Ellis CL, Miller KL, Vandegaer K, Bedja D, Gabrielson KL, Paolocci N, Kass DA, Barouch LA, Cardioprotective effect of beta-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase, Journal of the American College of Cardiology 59(22) (2012) 1979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Ouslander JG, Management of overactive bladder, The New England journal of medicine 350(8) (2004) 786–99. [DOI] [PubMed] [Google Scholar]
- [341].Bragg R, Hebel D, Vouri SM, Pitlick JM, Mirabegron: a Beta-3 agonist for overactive bladder, The Consultant pharmacist : the journal of the American Society of Consultant Pharmacists 29(12) (2014) 823–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Stemmelin J, Cohen C, Terranova JP, Lopez-Grancha M, Pichat P, Bergis O, Decobert M, Santucci V, Françon D, Alonso R, Stahl SM, Keane P, Avenet P, Scatton B, le Fur G, Griebel G, Stimulation of the beta3-Adrenoceptor as a novel treatment strategy for anxiety and depressive disorders, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 33(3) (2008) 574–87. [DOI] [PubMed] [Google Scholar]
- [343].Claustre Y, Leonetti M, Santucci V, Bougault I, Desvignes C, Rouquier L, Aubin N, Keane P, Busch S, Chen Y, Palejwala V, Tocci M, Yamdagni P, Didier M, Avenet P, Le Fur G, Oury-Donat F, Scatton B, Steinberg R, Effects of the beta3-adrenoceptor (Adrb3) agonist SR58611A (amibegron) on serotonergic and noradrenergic transmission in the rodent: relevance to its antidepressant/anxiolytic-like profile, Neuroscience 156(2) (2008) 353–64. [DOI] [PubMed] [Google Scholar]