Abstract
Background
The Janssen-Ad26.COV2.S vaccine is authorized for use in several countries, with more than 30 million doses administered. Mild and severe allergic adverse events following immunization (AEFI) have been reported.
Objective
We sought to detail allergic reactions reported during the Sisonke phase 3B study in South Africa.
Methods
A single dose of the Ad26.COV2.S vaccine was administered to 4,77,234 South African health care workers between February 17 and May 17, 2021. Monitoring of adverse events used a combination of passive reporting and active case finding. Telephonic contact was attempted for all adverse events reported as “allergy.” Anaphylaxis adjudication was performed using the Brighton Collaboration and National Institute of Allergy and Infectious Disease case definitions.
Results
Only 251 (0.052%) patients reported any allergic-type reaction (<1 in 2000), with 4 cases of adjudicated anaphylaxis (Brighton Collaboration level 1, n = 3) (prevalence of 8.4 per million doses). All anaphylaxis cases had a previous history of drug or vaccine-associated anaphylaxis. Cutaneous allergic reactions were the commonest nonanaphylatic reactions and included self-limiting, transient/localized rashes requiring no health care contact (n = 92) or isolated urticaria and/or angioedema (n = 70; median onset, 48 [interquartile range, 11.5-120] hours postvaccination) that necessitated health care contact (81%), antihistamine (63%), and/or systemic/topical corticosteroid (16%). All immediate (including adjudicated anaphylaxis) and most delayed AEFI (65 of 69) cases resolved completely.
Conclusions
Allergic AEFI are rare following a single dose of Ad26.COV, with complete resolution in all cases of anaphylaxis. Although rare, isolated, delayed-onset urticaria and/or angioedema was the commonest allergic AEFI requiring treatment, with nearly half occurring in participants without known atopic disease.
Key words: Allergic reaction, anaphylaxis, Janssen-Ad26.COV2.S vaccine, urticaria
The ongoing global effort to vaccinate an estimated 60% of the human population started in December 2020. In just 6 months, mass vaccine campaigns have seen approximately 5 billion doses of coronavirus disease 2019 (COVID-19) vaccines administered and more than a quarter of the world population having received at least 1 dose.1 There are currently 13 different vaccines in use, with 3 major emergency authorized platforms: inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Sinopharm and Sinovac-CoronaVac), adenoviral-vectored (ChadOx/AstraZeneca and Ad26.COV2.S), and the mRNA vaccines (Moderna mRNA 1273 and Pfizer/BionNtech Comirnaty)—the latter 2 technologies with either little or no previous large-scale human use in other infections. The mass use of these novel vaccine technologies has meant increased reporting of uncommon and rare adverse events following immunization (AEFI), including immune-mediated events such as Guillain-Barré syndrome and anaphylaxis.2 In this publication, the Brighton Collaboration case definition of anaphylaxis was used. All other allergic reactions were classified on the basis of symptoms and signs in keeping with an allergic etiology, but without documented allergic sensitization. Large cohort studies such as Sisonke, a phase 3B study of the Ad26.COV2.S in South African (SA) health care workers (HCWs), are invaluable to provide more detailed information about these uncommon adverse events of special interest.
Allergic AEFI are well known and reported with almost all registered vaccines, with the prevalence of anaphylaxis in most vaccine safety surveillance systems approximately 1 in a million doses.3,4 Soon after the emergency authorization of mRNA COVID-19 vaccines, an increased prevalence of anaphylaxis was noted with this novel platform, with prevalence estimates from 2 to 100 per million doses,2,5,6 and self-reported allergic reactions in approximately 2% of vaccine recipients.2 Few reports of anaphylaxis following ChAdOx1-S adenovirus vaccine are available, with prevalence estimates of 0.3 to 33 per million doses.5 No anaphylactic events occurred in the ENSEMBLE trials of the Ad26.COV2.S,7,8 with only 1 postmarketing surveillance study reported to date.9 There are several reports of self-limiting, delayed, large local allergic reactions surrounding the injection site following the Moderna mRNA 1273 vaccine,10 but the prevalence, spectrum, and outcomes of delayed allergic reactions to other COVID-19 vaccines have not been reported. Thus, we aimed to detail the spectrum of immediate and delayed allergic AEFIs reported following Ad26.COV2.S vaccination in a large cohort of SA HCWs.
Methods
The Sisonke (“Together” in isiZulu) phase 3B study is an open-label, single-arm implementation study of the Ad26.COV2.S COVID-19 vaccine among adult (>18 years) SA HCWs. The Ad26.COV2.S vaccine consists of the active adenovirus 26 replication-incompetent viral vector with SARS-COV-2 spike insert, and excipients including sucrose, sodium chloride, magnesium chloride, polysorbate 80, edetate disodium, and ethanol, at pH 6.6.11 The trial is sponsored by the SA Medical Research Council, with vaccines provided by Janssen Vaccines & Prevention B.V, a pharmaceutical company of Johnson & Johnson (NCT04838795) (http://sisonkestudy.samrc.ac.za/). The institutional review boards/ethics committees of participating clinical research sites approved the study, which was conducted under the oversight of the South African Health Products Regulatory Authority. At the time of publication, the Sisonke Study is in the process of administering booster doses of the Ad26.COV2.S vaccine and the allergy-related outcomes of the second/booster doses are not yet known.
Vaccinations were conducted in collaboration with the routine Provincial Department of Health public and private vaccination centers across all 9 provinces of South Africa and overseen by Good Clinical Practice–trained personnel linked to each of the ENSEMBLE trial research sites. Participants underwent informed consent before receiving a single intramuscular injection of Ad26.COV2.S at a dose level of 5 × 1010 virus particles. Participants with a previous history of allergic reactions to vaccinations were observed for 30 minutes postvaccination, whereas the rest of the participants were observed for 15 minutes postvaccination.
Safety monitoring was conducted through a combination of passive reporting and active case finding. An electronic adverse event reporting link was sent via text message on days 1, 7, and 14 postvaccination. Adverse events could also be reported either by calling a toll-free 24-hour safety line or through the completion of an adverse event report form, which was available at vaccination sites and hospitals. The safety team reviewed serious adverse events and adverse events of special interest reports daily. Full details of the Sisonke pharmacovigilance and safety reporting processes are detailed elsewhere.12
The Sisonke safety database was searched for allergic AEFI using a comprehensive list of possible allergy-related search terms (see Online Repository Appendix at www.jaci-global.org). Duplicates and clear nonallergic entries were removed, and then all reports were screened by an allergist. In addition to the initial reporting, telephonic contact was attempted with all participants reporting a possible allergy AEFI where details were missing. The event details were clarified, past medical and allergy history collected, as well as details of treatment, and outcomes of mild/moderate allergic AEFI requiring treatment and health care contact. Suspected anaphylaxis cases were adjudicated by 2 physicians using the Brighton Collaboration and National Institute of Allergy and Infectious Disease case definition, with cases needing to meet both definitions to be considered confirmed cases.13, 14, 15 Immediate reactions were restricted to those occurring within 6 hours postvaccination.16
Descriptive statistical analyses were performed using counts and proportions for categorical data, and medians and interquartile ranges for continuous variables. All statistical analyses were conducted using STATA version 14 (STATA Corp, College Station, Tex).
Results
From February 17 to May 17, 2021, a total of 4,77,234 (female n = 3,57,481 [74.9%]) SA HCWs received a single, open-label dose of the Ad26.COV2.S vaccine in the Sisonke phase 3B study. A total of 10,279 (2.2%) HCWs reported AEFI, of which 139 (1.4%) were severe adverse events. Searching of these AEFI identified 569 possible allergic reactions for screening, and 318 could be excluded as nonallergic AEFI (Fig 1), leaving 251 (0.052%) probable allergic AEFI for an overall prevalence of 1 in 2000 doses. Thereafter, more detailed review including telephonic contact was attempted for the 251 participants with probable allergic AEFI, of which 38 of 251 (15%) were uncontactable. Of the 251, 139 did not require/seek medical attention. Many patients (92 of 251 [36%]) reported a localized, transient rash (morphology undocumented) or puritis that was self-limiting (lasting <24 hours), responsive to over-the-counter medications, and not requiring contact with either a family physician or emergency medical services. Six patients (2%) noted isolated worsening of existing symptoms of allergic rhinoconjuncitivitis in the 24 hours following vaccination, necessitating use of existing allergic medications. Four participants (see exclusions Fig 1) reported delayed onset of non–urticarial-type rashes (1 blistering, 2 purpuric, and 1 eczematous), all of which were referred to specialist dermatologists. One of these cases had a confirmed cutaneous vasculitis (on the basis of skin biopsy), and another case was known with underlying systemic lupus erythematous.
Fig 1.
Overall summary of all allergy AEFI with Janssen Ad26COV2.S reported during the Sisonke phase 3B study. HSV, Herpes simplex virus; NIAID, National Institute of Allergy and Infectious Diseases. ∗Nonallergy exclusion definitions: Injection-site reactions included swelling, redness, and localized itching around the area of vaccination; immunization stress response included all symptoms related to anxiety including fainting and vasovagal episodes within a few minutes postvaccination; reactogenicity included participants who reported 1 or more of fever, rigors, muscle aches, nausea/vomiting, atypical chest pains, sore throat, and/or cough within the first 72 hours postvaccination; Infections included SARS-CoV-2 RT-PCR positivity, tonsillitis, upper and lower respiratory tract infection, and tooth abscess. Other diagnoses included uncontrolled hypertension, thromboembolism, neuralgia, trauma, shingles/HSV1 reactivation, uncontrolled hypertension, arthralgia, and respiratory symptoms occurring more than 28 days postvaccination. †Uncontactable or inadequate information meant that despite 3 separate phone call attempts, we were unable to get sufficient information about the adverse reaction to classify it as allergy or nonallergy. ‡Localized/transient rash or pruritis needed to be a reported rash of any morphology that was self-limiting (lasting <24 hours), and responsive to over-the-counter medications with no requirement for any contact with either a family physician or an emergency medical service. §Worsening of existing allergic rhinoconjunctivitis: Patients with previously diagnosed allergic rhinitis reported worsened symptoms after receiving the vaccine.
Four cases of immediate allergic AEFI were adjudicated as anaphylaxis, accounting for an overall prevalence of 8.4 per million doses. Table I details the 4 adjudicated cases of anaphylaxis that met both Brighton Collaboration case definition (n = 3 of 4 level 1 and 1 of 4 level 3) of anaphylaxis and National Institute of Allergy and Infectious Disease criteria. The median (interquartile range [IQR]) age of patients with anaphylaxis was 50 (45-53) years, and all cases were female. The median (IQR) time to onset was 10 (9-52) minutes, with 3 of 4 cases having an onset of reaction within the 15-minute protocol-recommended observation time. All patients had a background of atopic disease, with all 4 giving a history of previous anaphylaxis to medication. One patient had a history of vaccine-associated anaphylaxis to a yellow fever vaccine. One of these patients had prior confirmed SARS-CoV-2 infection. All patients required emergency room treatment, with 3 being admitted to hospital. All patients except 1 received epinephrine, antihistamines, intravenous corticosteroids, and beta-agonist therapies. All patients recovered completely. Unfortunately, only 1 of 4 patients had a postreaction tryptase measurement; this single normal tryptase measurement (4.99 μg/L [1.0-15.0]) was performed 6 hours postvaccine in a 56-year-old woman, known with uncontrolled asthma, who presented with facial angioedema and bronchospasm 10 minutes after vaccination (Table I).
Table I.
Characteristics of 4 cases of adjudicated anaphylaxis meeting both Brighton Collaboration and NIAID case definition
| Age (y) | Sex | Time to reaction | General symptoms | Brighton score and allergy symptoms | NIAID score | Atopy history | Anaphylaxis history | Past medical history | Previous COVID | Hospitalized | Adrenaline used | MC tryptase <6 h after reaction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 | F | 10 min | Nil | Level 1 Major:
|
Yes | Asthma Penicillin allergy |
Yes (penicillin) | Systemic lupus Fibromyalgia Hypothyroidism Anemia |
No | Yes | Yes | ND |
| 56 | F | 10 min | Headache | Level 1 Major:
|
Yes | Asthma/allergic rhinitis Eczema Sensitized to HDM, tree pollen Polysorbate 80 allergy |
Yes (yellow fever vaccine) | Hypertension Glaucoma |
Yes | Yes | No | 4.99 μg/L (1.0-15.0) |
| 35 | F | 6 h | Fever | Level 1 Major:
|
Yes | Beta-lactam allergy, NSAID allergy | Yes (BLA, NSAID) | Nil | No | Yes | Yes | ND (elevated IgE and CRP) |
| 48 | F | 5 min | Nil | Level 3 Minor
|
Yes | Allergy to latex, sodium benzoate, local anesthetic | Yes (local anesthetic) | Nil | No | Emergency room only | Yes | ND |
BLA, Beta-lactam antibiotic; CRP, C-reactive protein; GIT, gastrointestinal; HDM, house dust mite; NSAID, nonsteroidal anti-inflammatory drug; MC, mast cell; ND, not done; NIAID, National Institute of Allergy and Infectious Diseases.
Mild/moderate allergic AEFI that necessitated treatment and contact with the medical services occurred in 90 of 251 (35.8%). The commonest allergic AEFI were delayed (>6 hours) reactions (n = 69 of 107 [64%]), with isolated urticaria and/or angioedema (n = 70 of 107 [65%]) the most frequently reported individual allergic phenotype (Table II).
Table II.
Characteristics and management of nonanaphylaxis allergic AEFI stratified by timing of onset (immediate vs delayed)
| Characteristic | All cases, (n = 107) | Immediate reaction (≤6 h; n = 24∗) | Delayed reaction (>6 h; n = 69∗) | Isolated urticaria and/or angioedema (n = 70) |
|---|---|---|---|---|
| Sex: female, n (%) | 91 (85) | 21 (88) | 57 (83) | 60 (86) |
| Age (y), median (IQR) | 38 (28-45) | 38 (29-52) | 37 (27-43) | 39 (28-46) |
| Time to reaction (h), median (IQR) | 24 (6-96) | 0.75 (0.1-3) | 48 (22-120) | 48 (11.5-120) |
| Allergy symptoms, n (%) | ||||
| Angioedema | 26 (24) | 6 (25) | 17 (25) | 16 (27) |
| Urticaria | 73 (68) | 12 (50) | 54 (78) | 61 (87) |
| Generalized pruritis | 30 (28) | 7 (29) | 22 (32) | 18 (26) |
| Respiratory symptoms† | 35 (33) | 12 (50) | 17 (25) | Excluded |
| Gastrointestinal symptoms† | 11 (10) | 3 (13) | 5 (7) | 3 (4) |
| Cardiovascular symptoms† | 7 (7) | 3 (13) | 2 (3) | Excluded |
| Patients with reactogenic effects‡ | 67 (63) | 14 (58) | 46 (67) | 41 (56) |
| Local injection-site reaction | 34 (32) | 8 (33) | 22 (32) | 19 (27) |
| Allergy history, n (%) | ||||
| None | 35 (33) | 7 (29) | 26 (38) | 30 (43) |
| Unknown | 19 (18) | 0 | 8 (12) | 15 (21) |
| Any atopic disease, n (%) | ||||
| Previous any anaphylaxis | 6 (6)§ | 3 (13) | 3 (4) | 3 (4) |
| Drug allergy | 11 (1) | 4 (17) | 7 (10) | 5 (7) |
| Asthma | 26 (24) | 9 (38) | 16 (23) | 8 (11) |
| Atopic dermatitis | 15 (14) | 2 (8) | 13 (12) | 8 (11) |
| Past medical history, n (%) | ||||
| Unknown | 17 (15) | 0 | 8 (12) | 14 (20) |
| None | 54 (50) | 10 (42) | 42 (60) | 32 (45) |
| HIV on ART | 3 (3) | 1 (4) | 2 (3) | 3 (4) |
| Patients with noncommunicable diseases | 24 (22)‖ | 14 (58) | 20 (29) | 24 (34) |
| COVID-19 before vaccination | 6 (6) | 4 (17) | 2 (2) | 4 (6) |
| Management, n (%) | ||||
| Unknown | 14 (13) | 0 (0) | 5 (7) | 13 (19) |
| Required treatment | 90 (84) | 24 (100) | 62 (90) | 57 (81) |
| Hospitalized: Admitted | 8 (8) | 3 (13) | 5 (7) | 1 (1) |
| Emergency room visit | 11 (10) | 4 (17) | 7 (10) | 4 (6) |
| Treatment(s) received, n (%) | ||||
| Adrenaline¶ | 5 (5) | 4 (17) | 1 (1) | 1 (1) |
| Systemic steroids | 30 (28) | 12 (50) | 18 (26) | 11 (16) |
| Antihistamines | 62 (58) | 18 (75) | 41 (59) | 44 (63) |
| Inhalational treatment# | 36 (33) | 9 (38) | 10 (14) | 0 |
| Other∗∗ | 16 (14) | 3 (13) | 13 (19) | 13 (19) |
ART, Antiretrovirals; HPT, hypertension; GORD, gastrooesphageal reflux disease; PCOS, polycystic ovary syndrome.
There were 14 participants in whom the time of reaction onset was unknown.
Respiratory symptoms included 1 or more of bronchospasm, upper airway swelling, subjective dyspnea without wheeze or stridor, persistent dry cough, hoarse voice, sensation of throat closure. Gastrointestinal symptoms included 1 or more of nausea and vomiting, diarrhea, abdominal pain. Cardiovascular symptoms included 1 or more of hypotension, tachycardia, and decreased level of consciousness or loss of consciousness.
Common nonallergic vaccine adverse events include 1 or more of fever (n = 49), headache (n = 53), or myalgia (n = 43).
Anaphylaxis to latex (n = 1), anaphylaxis to beta-lactam (n = 1), anaphylaxis to bee venom (n = 1), unknown (n = 3); however, no patients reported previous vaccine anaphylaxis.
Noncommunicable diseases include HPT (n = 11), diabetes (n = 5), dyslipidemia (n = 3), hypothyroidism (n = 5), GORD (n = 3), PCOS (n = 2), Gilberts disease (n = 1), congenital heart disease (n = 1), osteoporosis (n = 2), breast cancer in remission (n = 2), rheumatoid arthritis (n = 1), endometriosis (n = 1), and osteoarthritis (n = 1).
Received adrenaline due to severe bronchospasm or angioedema of the face and tongue.
Nebulization (n = 12), inhaled corticosteroids (n = 13), long-acting β-agonist/inhaled corticosteroid (n = 2), long-acting β-agonist (n = 2), short-acting β-agonist (n = 1).
Antibiotics (n = 3), paracetamol (n = 5), thiamine (n = 1), intravenous fluids (n = 6).
Table II presents demographic details, past medical and allergy history, clinical features, and management of nonanaphylactic allergy AEFI needing medical attention, comparing immediate with delayed reactions, and isolated urticaria and/or angioedema. Similar to anaphylaxis cases, most (91 of 107 [85%]) nonanaphylactic allergy AEFI occurred in females with a median (IQR) age of 37 (27-43) years. Vaccine “reactogenicity” (common side effects associated with adenoviral vector infection and immune activation such as fever, headache, or myalgia occurring and resolving in the first 24-72 hours) was common, occurring in 67 of 107(63%) participants. Overall, 53 of 88 (60%) participants with nonanaphylactic allergy AEFI had a history of previous anaphylaxis or atopic disease. Of the 24 immediate nonanaphylactic allergy AEFI, 10 occurred within 15 minutes, 7 between 1 and 3 hours, and 5 between 4 and 6 hours postdosing (median [IQR], 0.75 [1-3] hours). Among delayed reactions, 24 of 69 (35%) started within 24 hours postdosing, with 29 of 69 (42%) starting between 3 and 21 days postvaccination. Only 8 cases of nonanaphylactic allergy AEFI required admission to hospital, with most successfully treated with antihistamines (62 of 107 [58%]) and oral corticosteroids (30 of 107 [28%]) only. Five patients in this group received epinephrine treatment for either severe bronchospasm, or angioedema of the upper respiratory tract due to concern around airway swelling.
Isolated urticaria and/or angioedema was the commonest single allergic reaction phenotype. Most (39 of 70 [56%]) occurred 24 hours or more after dosing, with a median (IQR) of 48 (11.5-120) hours. More than half of these patients had no history of atopy. Only 1 patient in this group needed hospitalization, and only 4 needed to receive emergency treatment.
Data on number of days of symptoms were not available; however, all patients, except 4, reported complete resolution of their AEFI at the time of telephonic contact (5-7 months postvaccination), with most indicating that symptoms resolved within a week of onset. Four patients reported urticaria and/or angioedema that was ongoing at the time of contact, and these patients have been referred for further allergy care and management as chronic urticaria.
Discussion
The tremendous global scale of COVID-19 vaccination means that the cumulative number of uncommon or rare AEFI, such as anaphylaxis, can be expected to occur in larger numbers than usual in the coming 12 to 18 months when compared with usual background rates. In addition, national vaccine safety groups and clinicians need published data on registered COVID-19 vaccines to inform the public, create awareness of patients at risk of AEFI, and correctly manage mild and severe AEFI. No severe (>grade 3) allergic AEFI were noted in the phase I to III studies of Janssen Ad26.COV2.S,7,8 and only 1 postmarketing surveillance study from the Vaccine Adverse Events Reporting System reported on “rash” as a nonanaphylactic allergic AEFI.9 Thus, this study provides the largest cohort with detailed allergic AEFI reporting following vaccination with the Janssen Ad26.COV2.S vaccine.
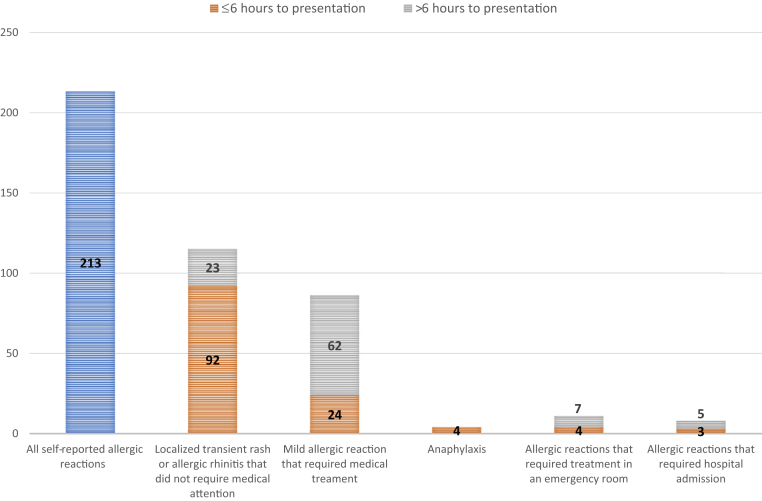
Allergic AEFI with the Ad26.COV2.S vaccine are uncommon in this large cohort, with an estimated prevalence for any allergic AEFI including anaphylaxis of 1 in 2000 doses (0.052%) and rate of anaphylaxis of 8.4 cases per million doses (0.0008%). This rate for anaphylaxis is higher than the approximately 1 case per million doses reported for most approved vaccines,3,4 and considerably higher than the less than 0.5 per million dose rate reported after investigation of 79 reports to the US Vaccine Adverse Events Reporting Systems following 7.98 million doses of Ad26.COV2.S administered in the United States.9 The anaphylaxis rate is closest to the recent meta-analysis–suggested rate of 7.91 cases per million doses.5 Considering vaccine-specific rates among the meta-analysis data sets, anaphylaxis to mRNA COVID-19 vaccines is estimated at more than 20 anaphylaxis cases per million doses,5 whereas regulatory data sets for the ChadOx/AstraZeneca adenoviral-vectored vaccine estimate rates between 0.32 and 33.4 per million doses. The wide range in reported rates may be due to overreporting in large pharmacovigilance reporting systems with inclusion of nonallergic reactions, because many reports do not meet criteria when reviewed and subjected to a more detailed allergy workup.5 Our large data set, adjudication of cases, and robust reporting systems mean that this reported anaphylaxis rate for the Ad26.COV2.S vaccine is likely accurate, and closer to the mRNA COVID-19 vaccine rates than previously reported. Nevertheless, the overall rates for any allergic AEFI and anaphylaxis are rare, and lower than the rates of up to 1 in 50 for nonanaphylactic reactions reported for mRNA COVID-19 vaccines.2 Thus, overall, the Ad26.COV2.S vaccine appears to have a lower risk of inducing allergic reactions when compared with the mRNA vaccines. Furthermore, from an overall vaccine safety perspective, no patients died or suffered circulatory collapse with anaphylaxtic events, and most allergic reactions were mild self-limiting urticaria/angioedema events not associated with an increased risk of subsequent anaphylaxis (Fig 2). None of the anaphylaxis patients received premedication, and our study does not provide data on reactions to the second dose of Ad26.COV2.S vaccine. Similar to increasing experience with mRNA COVID-19 vaccines, allergic reactions to second doses of Ad26.COV2.S vaccine may be less severe, and offset by premedication with antihistamines and leukotriene receptor antagonists.17,18
Fig 2.
Allergic reactions to the Janssen Ad26COV2.S vaccine stratified by timing, severity, and treatment requirements.
We did not determine allergic sensitization and identify the trigger for either anaphylactic or mild allergic-like reactions in this study. Several possible mechanisms of hypersensitivity to COVID-19 vaccines have emerged including IgG against excipients, for example, polyethylene glycol19 or complement activation-related pseudoallergy.20 The similar rates of anaphylaxis in this study compared with those reporting for mRNA vaccines, with different excipients, favor non–IgE-mediated mechanisms or cross-reactivity between polysorbate 80 and polyethylene glycol. Non–IgE-mediated reactions in mRNA COVID-19 vaccines are also supported by low skin prick versus basophil activation test positivity,19 the lack of anaphylaxis-related mortality (globally), and the lack of postreaction mast cell tryptase elevation (when performed) (Table I). A female predominance in all anaphylaxis cases and nonanaphylatic allergic reactions was noted in this cohort, consistent with hypersensitivity to the mRNA COVID-19 vaccines.21 Female sex as a risk factor has been noted in several atopic disease including drug allergy. This is thought to be multifactorial, including hormonal influences on mast cell receptors, shared exposure to sensitizing agents, differing perceptions of risk, and medication use.22,23
The commonest allergic AEFI in this cohort was a delayed urticarial rash and generalized puritis with or without angioedema, with onset usually a day or up to 21 days following vaccination (Fig 2). In the ENSEMBLE study, 8 versus 5 cases of urticaria were reported in active versus placebo arms of the phase III study; unfortunately, timing of onset postdosing was not reported.7 Urticaria and angioedema have been well reported with several registered vaccines, for example, influenza and toxoid vaccines,24,25 as well as COVID-19 vaccines.26 Catala et al26 reported 405 cutaneous reactions following COVID-19 vaccines in a Spanish population, with the commonest being urticarial, followed by morbilliform and papulovesicular rashes. Interestingly, the only adenoviral-vectored vaccine included was the ChadOx/AstraZeneca vaccine, and urticaria accounted for a fifth of all cutaneous reactions reported to this vaccine.26 Urticaria is also associated with several viral infections, including adenovirus and more recently SARS-CoV-2 infections.27,28 Furthermore, unlike the immediate allergic AEFI, patients experiencing delayed urticarial AEFI less commonly had a background of atopic disease. Patients experiencing this reaction are not at increased risk for anaphylaxis. This also suggests that the mechanisms underlying these reactions relate to non-IgE pathways and the immune interaction—both innate and adaptive—with viral vector–expressed viral proteins, vaccine ingredients, or combinations of these.
Most reactions could be managed symptomatically with antihistamines/corticosteroids and were self-limiting, not requiring hospitalization or emergency treatment. However, in 4 patients, vaccine-induced urticaria has not resolved, now lasting more than 6 weeks postvaccination. Vaccines can rarely trigger chronic spontaneous urticaria24 and induce urticarias such as cold urticaria.29 Some infections, including SARS-CoV-2, have also been shown to exacerbate chronic spontaneous urticaria.30 Patients developing new or exacerbated chronic spontaneous urticaria following COVID-19 vaccination should be reviewed by an allergist, with treatment focusing on the use of high doses of antihistamines, rather than unnecessary corticosteroids, which may interfere with the development of a protective vaccine response.31 Further research is now required to examine the effects of COVID-19 vaccines on cohorts of chronic urticaria.30,32
The major strength of this study was the large cohort size and the robust passive and active safety surveillance systems that allowed for comprehensive AEFI reporting for allergic events. However, because not all allergic AEFI were followed up at prespecified time points, and events were managed at hospitals across the country by nonstudy staff, a small amount of data could not be captured.
In conclusion, this study is the first to detail allergic AEFI following use of the novel Ad26.COV2.S vaccine. Reassuringly, allergic AEFI were very rare, with complete resolution of all cases of anaphylaxis. Self-limiting delayed urticaria was the commonest allergic AEFI, and clinicians should be aware that these can occur several days after vaccination. Most allergic reactions were self-limiting and could be managed with antihistamines and corticosteroids without prolonged morbidity.
Acknowledgments
J. Peter, S. Takuva, A. Takalani, I. Engelbrecht, N. Garrett, A. Goga, V. Louw, J. Opie, B. Jacobson, I. Sanne, L. Gail-Bekker, and G. Gray are on the Sisonke Safety Committee. G. Gray is the primary investigator in the Sisonke Study.
Footnotes
The Sinsonke Study is funded by the South African Medical Research Council. J.P.’s research is supported by a career development award (grant no. K43TW011178-04) and financial support from the National Institutes of Health (award no. K43TW011178-02); the European Developing Clinical Trials Partnership (EDCTP2 Program supported by the European Union grant no. TMA2017SF-1981); and the SA Medical Research Council and National Research Foundation.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B., et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su J.R., Moro P.L., Ng C.S., Lewis P.W., Said M.A., Cano M.V. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990-2016. J Allergy Clin Immunol. 2019;143:1465–1473. doi: 10.1016/j.jaci.2018.12.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeil M.M., Weintraub E.S., Duffy J., Sukumaran L., Jacobsen S.J., Klein N.P., et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenhawt M., Abrams E.M., Shaker M., Chu D.K., Khan D., Akin C., et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9:3546–3567. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325:780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadoff J., Gray G., Vandebosch A., Cardenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shay D.K., Gee J., Su J.R., Myers T.R., Marquez P., Liu R., et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine—United States, March-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:680–684. doi: 10.15585/mmwr.mm7018e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal K.G., Freeman E.E., Saff R.R., Robinson L.B., Wolfson A.R., Foreman R.K., et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384:1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takuva S., Takalani A., Garrett N., Goga A., Peter J., Louw V., et al. Thromboembolic events in the South African Ad26.COV2.S Vaccine Study. N Engl J Med. 2021;385:570–571. doi: 10.1056/NEJMc2107920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panel N.I.-S.E., Boyce J.A., Assa’ad A., Burks A.W., Jones S.M., Sampson H.A., et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loprinzi Brauer C.E., Motosue M.S., Li J.T., Hagan J.B., Bellolio M.F., Lee S., et al. Prospective validation of the NIAID/FAAN criteria for emergency department diagnosis of anaphylaxis. J Allergy Clin Immunol Pract. 2016;4:1220–1226. doi: 10.1016/j.jaip.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Ruggeberg J.U., Gold M.S., Bayas J.M., Blum M.D., Bonhoeffer J., Friedlander S., et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 16.Demoly P., Adkinson N.F., Brockow K., Castells M., Chiriac A.M., Greenberger P.A., et al. International Consensus on drug allergy. Allergy. 2014;69:420–437. doi: 10.1111/all.12350. [DOI] [PubMed] [Google Scholar]
- 17.Krantz M.S., Kwah J.H., Stone C.A., Jr., Phillips E.J., Ortega G., Banerji A., et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181:1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krantz M.S., Bruusgaard-Mouritsen M.A., Koo G., Phillips E.J., Stone C.A., Jr., Garvey L.H. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don’t give up on the second dose! Allergy. 2021;76:2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren C.M., Snow T.T., Lee A.S., Shah M.M., Heider A., Blomkalns A., et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B., Li Q., Shi C., Zhang X. Drug-induced pseudoallergy: a review of the causes and mechanisms. Pharmacology. 2018;101:104–110. doi: 10.1159/000479878. [DOI] [PubMed] [Google Scholar]
- 21.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kvedariene V., Sitkauskiene B., Tamasauskiene L., Rudzeviciene O., Kasiulevicius V., Nekrosyte G., et al. Prevalence of self-reported drug hypersensitivity reactions among Lithuanian children and adults. Allergol Immunopathol (Madr) 2019;47:32–37. doi: 10.1016/j.aller.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Afify S.M., Pali-Scholl I. Adverse reactions to food: the female dominance—A secondary publication and update. World Allergy Organ J. 2017;10:43. doi: 10.1186/s40413-017-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magen E., Shalom G., Waitman D., Kahan N. Chronic spontaneous urticaria following vaccination. Int J Adv Res. 2018;6:1434–1439. [Google Scholar]
- 25.Halsey N.A., Griffioen M., Dreskin S.C., Dekker C.L., Wood R., Sharma D., et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine. 2013;31:6107–6112. doi: 10.1016/j.vaccine.2013.09.066. [DOI] [PubMed] [Google Scholar]
- 26.Catala A., Munoz-Santos C., Galvan-Casas C., Roncero Riesco M., Revilla Nebreda D., Sola-Truyols A., et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142–152. doi: 10.1111/bjd.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genovese G., Moltrasio C., Berti E., Marzano A.V. Skin manifestations associated with COVID-19: current knowledge and future perspectives. Dermatology. 2021;237:1–12. doi: 10.1159/000512932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedi B., Raap U., Wieczorek D., Kapp A. Urticaria and infections. Allergy Asthma Clin Immunol. 2009;5:10. doi: 10.1186/1710-1492-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raison-Peyron N., Philibert C., Bernard N., Du-Thanh A., Barbaud A., Bessis D. Cold contact urticaria following vaccination: four cases. Acta Derm Venereol. 2016;96:852–853. doi: 10.2340/00015555-2358. [DOI] [PubMed] [Google Scholar]
- 30.Kocaturk E., Salman A., Cherrez-Ojeda I., Criado P.R., Peter J., Comert-Ozer E., et al. The global impact of the COVID-19 pandemic on the management and course of chronic urticaria. Allergy. 2021;76:816–830. doi: 10.1111/all.14687. [DOI] [PubMed] [Google Scholar]
- 31.Bermingham W.H., Ardern-Jones M.R., Huissoon A.P., Krishna M.T. Forewarned is forearmed: chronic spontaneous urticaria as a potential risk to effective SARS-CoV-2 vaccine uptake and global public health. Br J Dermatol. 2021;185:838–839. doi: 10.1111/bjd.20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goncalo M., Gimenez-Arnau A., Al-Ahmad M., Ben-Shoshan M., Bernstein J.A., Ensina L.F., et al. The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184:226–236. doi: 10.1111/bjd.19561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.