Abstract
Purpose:
While current rehabilitation practice for improving arm and hand function relies on physical/occupational therapy, a growing body of research evaluates the effects of technology-enhanced rehabilitation. We review interventions that combine a brain-computer interface (BCI) with electrical stimulation (ES) for upper limb movement rehabilitation to summarize the evidence on (1) populations of study participants, (2) BCI-ES interventions, and (3) the BCI-ES systems.
Method:
After searching seven databases, two reviewers identified 23 eligible studies. We consolidated information on the study participants, interventions, and approaches used to develop integrated BCI-ES systems. The included studies investigated the use of BCI-ES interventions with stroke and spinal cord injury (SCI) populations. All studies used electroencephalography to collect brain signals for the BCI, and functional electrical stimulation was the most common type of ES. The BCI-ES interventions were typically conducted without a therapist, with sessions varying in both frequency and duration.
Results:
Of the 23 eligible studies, only 3 studies involved the SCI population, compared to 20 involving individuals with stroke.
Conclusions:
Future BCI-ES interventional studies could address this gap. Additionally, standardization of device and rehabilitation modalities, and study-appropriate involvement with therapists, can be considered to advance this intervention towards clinical implementation.
Key Words: brain-computer interfaces, electric stimulation, electric stimulation therapy, neurological rehabilitation, upper extremity
Résumé
Objectif :
les pratiques de réadaptation actuelles pour améliorer le fonctionnement de la main et du bras reposent sur la physiothérapie et l’ergothérapie, mais de plus en plus de recherches évaluent les effets de la réadaptation améliorée par la technologie. Les chercheurs analysent les interventions qui combinent une interface cerveau-ordinateur (ICO) à la stimulation électrique (SÉ) en réadaptation des mouvements des membres supérieurs pour résumer les données probantes sur 1) les populations de participants aux études, 2) les interventions d’ICO-SÉ et 3) les systèmes d’ICO-SÉ.
Méthodologie :
après avoir fouillé sept bases de données, deux analystes ont extrait 23 études admissibles. Les chercheurs ont regroupé l’information sur les participants aux études, de même que sur les interventions et les approches utilisées pour mettre au point des systèmes d’ICO-SÉ intégrés. Les études portaient sur l’utilisation des interventions d’ICO-SÉ auprès des populations victimes d’un accident vasculaire cérébral ou d’une lésion médullaire. Toutes faisaient appel à l’électroencéphalographie pour obtenir les signaux cérébraux de l’ICO, et la SÉ fonctionnelle était la SÉ la plus courante. Les interventions d’ICO-SÉ se déroulaient généralement sans thérapeute, et la fréquence et la durée des séances étaient variables.
Résultats :
sur les 23 études admissibles, seulement trois traitaient de la population victime d’une lésion médullaire, par rapport à 20 de personnes victimes d’un accident vasculaire cérébral.
Conclusions :
les futures études d’interventions d’ICO-SÉ pourraient corriger cette lacune. De plus, on peut envisager de standardiser les modalités des appareils et de la réadaptation et de prévoir une participation avec les thérapeutes adaptée à l’étude pour faire progresser cette intervention vers la mise en œuvre clinique.
Mots-clés : interfaces cerveau-ordinateur, membre supérieur, réadaptation neurologique, stimulation électrique, traitement de stimulation électrique
Upper limb impairments
Upper limb impairments, highly prevalent in individuals who experience stroke, are among the most common disabling deficits.1 Approximately 50% of stroke survivors experience persistent upper limb impairments.2 A similar trend is observed among individuals who experience spinal cord injuries (SCIs).3 More specifically, cervical SCIs often result in tetraplegia, in which motor and sensory functions of the lower limbs, trunk, and upper limbs are affected. Estimates from Canada indicate that tetraplegia occurs in 44% of SCI cases.5 Several studies have revealed that individuals with tetraplegia consider regaining arm and hand function a priority for improving their quality of life.5–8
Upper limb impairments, such as weakness and spasticity, are also associated with upper motor neuron (UMN) syndrome. UMNs are in the central nervous system and carry movement-related activity.6 Lesions in the UMNs occur if there is damage to the motor neurons above nuclei of cranial nerves or the cells in the anterior horn of the spinal cord.
Activity-based therapies
The most common approach for the treatment of impaired motor function following a neurological injury is rehabilitation interventions involving physical or occupational therapy. More recently, the fundamental elements of therapeutic interventions have targeted direct involvement of the central nervous system and task-specific movement practice.9 The interventions incorporating these elements are referred to as activity-based therapies (ABTs).9 ABTs stimulate neuroplastic changes by involving parts of the body affected by the injury in intensive and repetitive task-oriented movements.10 Repetition and training intensity, considered examples of dosage, must be sufficient to stimulate neuroplastic change.11 For example, ABT requires thousands of movement repetitions.12,13 Technology, and robotic systems in particular, have been investigated as tools to increase intensity during upper limb rehabilitation.14 Lum and colleagues concluded that the primary benefit of robotic-assisted rehabilitation is to enable a greater number of repetitions, thus increasing the dosage of the intervention.15
Electrical stimulation
Another example of a widely used technology in rehabilitation is electrical stimulation (ES), which artificially activates nerves and muscles using electrical pulses. Two common types of ES in physical medicine are neuromuscular electrical stimulation (NMES) and functional electrical stimulation (FES). NMES refers to the application of electrical charge to produce contractions in a single muscle. For example, clinically, NMES has been used to reduce pain and shoulder subluxation post-stroke.16 In comparison, FES applies stimulation to muscle groups to facilitate functional movements. In clinical practice, FES is used to support practicing tasks of daily living, such as holding and lifting commonly used objects like a water bottle. The combination of FES technology and physical/occupational therapy has resulted in FES therapy (FEST), which can be used for unilateral and bilateral rehabilitation programmes. Several studies have successfully demonstrated that FEST can improve upper limb motor function in individuals with stroke and SCI.17–21
Brain-computer interface
An emerging technology increasingly investigated to support rehabilitation is a brain-computer interface (BCI).22 A BCI enables the user to interact with the environment without movement, using only brain activity to control an external device (e.g., computer). In the rehabilitation of voluntary motor function, BCIs are commonly used to control devices that facilitate movement during therapy. In such applications, a BCI can also serve as a tool to verify the patient's engagement during therapy. For example, BCI and ES integration has resulted in a new rehabilitation strategy referred to as a BCI-controlled ES (BCI-ES) intervention. In BCI-ES interventions, the BCI activates the ES when it detects a patient's imagined or attempted movements practiced during therapy. From a neurological perspective, the descending motor command detected by the BCI is subsequently converted into an executed movement supported by ES. The BCI-ES technology may enhance ABT by engaging the central nervous system and involving affected limbs in a large number of therapy sessions.23
Study objectives
The feasibility and efficacy of BCI-ES for upper limb rehabilitation has been reported in several studies, including randomized controlled trials. As these studies were conducted with various neurological populations, we aimed to identify gaps in the populations that have been exposed to BCI-ES interventions but could nonetheless benefit from BCI-ES. We also aimed to consolidate the information on proposed BCI and ES systems, strategies to integrate BCI and ES systems, and intervention durations and intensities. In addition, we generated a brief overview of the clinical outcome measures used to assess BCI-ES interventions.
This review examines the available literature on BCI-ES interventions targeting upper limb motor rehabilitation in order to answer the following questions:
-
1.
Which populations of individuals with neurological injuries have participated in studies investigating BCI-ES interventions for upper limb rehabilitation?
-
2.
What have been the durations and intensities among the reported BCI-ES interventions for upper limb motor rehabilitation?
-
3.
What approaches have been used to develop BCI and ES systems and BCI-ES interventions for upper limb motor rehabilitation?
-
4.
Which complementary technologies were used alongside BCI and ES for upper limb motor rehabilitation?
Methods
The present scoping review was conducted systematically, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines with the scoping review extension (PRISMA-ScR; see PRISMA-ScR checklist in Supplemental Material). We used the framework proposed by Arksey and O’Malley.24
Data sources and search strategy
The contents of seven electronic databases were searched, from each database's inception to 5 February 2021: Cumulative Index of Nursing & Allied Health Literature (CINAHL), Embase, Emcare, Medline (Ovid), PubMed (non-Medline), Scopus, and Web of Science Core Collection. The search strategies were developed in collaboration with an Information Specialist, utilizing the PICO framework,25 with subject headings as appropriate for each database, and free text terms relevant to the topical concepts.
Our search strategy was as follows. The population we focused on was individuals with upper limb impairments, and the intervention was a brain-computer interface. The comparator, or more precisely the intervention qualifier, was electrical stimulation, to retrieve materials discussing BCI in combination with ES. No outcomes were stipulated to keep the results as comprehensive as possible. The results were limited to humans, but no date or language limits were applied at this point. The complete Medline search strategy is available in Appendix 1.
Study selection
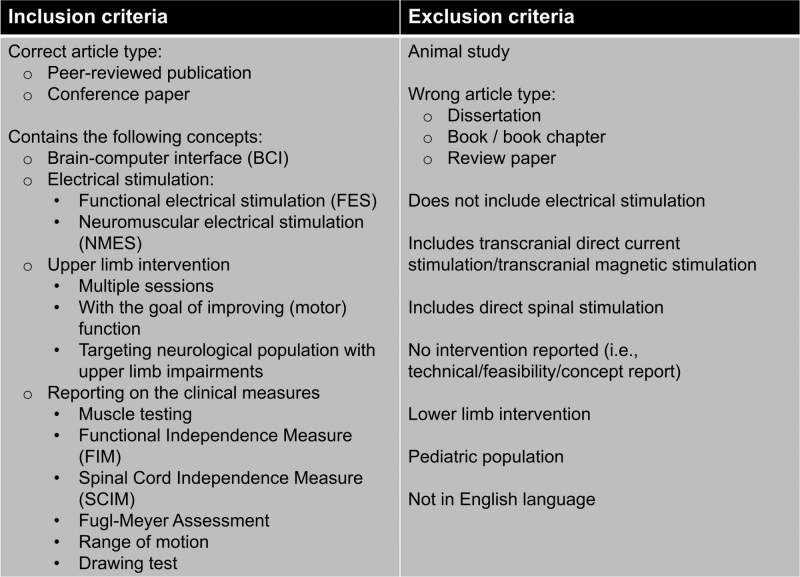
After removing duplicates, two reviewers independently conducted the screening of titles and abstracts based on the inclusion criteria. First, only peer-reviewed journal publications and conference proceedings were included. Second, the work described had to include the following four concepts: (1) brain-computer interface, (2) electrical stimulation, (3) upper limb intervention, and (4) assessments of motor function. We excluded dissertations, books, book chapters, other review papers, and reports written in languages other than English. Furthermore, we excluded animal studies and studies done in paediatric populations (i.e., less than 18 years of age). We also excluded reports missing any of the four inclusion concepts, such as reports exclusively describing BCI-ES technology. One reviewer fully extracted data relevant to the objectives, with a second reviewer checking the extracted data afterwards. The inclusion and exclusion criteria are shown in Figure 1.
Figure 1.
The inclusion and exclusion criteria.
Study descriptors
Study types
We distinguished between controlled studies (i.e., studies including a control group), single-arm interventional studies, and case series and case studies grouped together as the last category.
Population
Neurological conditions: To characterize study participants based on neurological conditions, we recorded general neurological descriptors reported in the studies as the underlying cause for the upper limb motor impairments.
Sex: For reporting on sex, we recorded the number of male and female participants reported in each study we analyzed.
Chronicity and time post injury: To characterize chronicity, we relied on the time post-injury values reported in the included studies. These values referred to the time difference between the occurrence of a neurological injury and the start of the intervention, expressed in days, months, or years. For the stroke population, we used the definitions of sub-acute (7 days to <6 months) and chronic stages (≥6 months) introduced by Bernhardt and colleagues.26 We could not find such definitions in the SCI population, so we used Fawcett and colleagues’ suggestion of subacute (3 days to <12 months) and chronic (≥12 months) stages of rehabilitation.27
Severity of upper limb impairments: To describe the severity of upper limb impairments among the study participants, we relied primarily on baseline scores of clinical assessments, when available, and secondly on the descriptions provided by the authors. Specifically, in the stroke population we relied on the Fugl-Meyer Assessment-Upper Extremity (FMA-UE) score.28 The FMA-UE measures impairment after stroke, with scores ranging from 0 to 66 points, with lower scores indicating greater impairment. It is one of the most used assessments in upper limb stroke rehabilitation research. Using the FMA-UE score, Woytowicz and colleagues29 defined four levels of severity among individuals with stroke:
-
•
Severe: zero to 15 points
-
•
Severe–Moderate: 16 to 34 points
-
•
Moderate–Mild: 35 to 53 points
-
•
Mild: 54 to 66 points
In studies in which the modified FMA-UE (mFMA-UE) was used (without the coordination, speed, and reflexes subsections), with the score ranging from 0 to 54 points, the authors referred to the range between 0 and 25 points as severe upper limb impairment.30
In individuals with SCI, we relied on the American Spinal Injury Association Impairment Scale (AIS), a clinical assessment with five grades, from grade A to grade E, ranging from the most severe impairment to the least.31
Interventions and clinical outcome measures
Interventions: To characterize the BCI-ES interventions, we recorded the number of sessions, frequency (i.e., number of sessions per week), and the length of a single session in minutes. We also recorded if the studies reported having a therapist present during the intervention.
Clinical outcome measures: We recorded the pre- and post-intervention scores on the clinical outcome measures (e.g., FMA-UE, Action Research Arm Test) to provide an overview of the clinical efficacy of the BCI-ES for upper limb motor rehabilitation. When possible, we also recorded the average changes between pre- and post-intervention scores. We focused on the studies that reported statistical descriptions of the clinical results in numerical values (e.g., mean, median, standard deviation). We extracted information on the significance of the statistical differences in the clinical scores within groups and the differences in score changes between groups, when available.
Technology
Brain-computer interface: We characterized the BCI systems on three levels. The first level referred to the modality used to acquire brain activity (e.g., EEG), indicating the invasiveness and accessibility of the system. The second level referred to the number of channels used to develop the BCI system used during BCI-ES intervention. The number of channels used for the BCI during a therapy session could indicate the setup time. With the same type of EEG setup (i.e., wired or wireless), fewer channels allow for a shorter setup time and longer treatment within a single session. Lastly, the third level of the description referred to the calibration of the BCI system or the training requirements for using the system. This characteristic also affects the clinical feasibility, where multiple long training sessions may represent a barrier in clinical adoption of BCI technology.
Electrical stimulation: We also characterized the ES systems on three levels. The first level referred to the distinction between FES and NMES, as described in the reports. The second level referred to the number of channels used for delivering the stimulation. The number of channels indicates the versatility of an ES system, suggesting that a system with more channels could support a variety of functional tasks involving multiple muscle groups required for both reaching and grasping, regardless of how it was used in the reported study. Finally, the third level referred to the upper limb muscle groups targeted during the intervention; we distinguished between movements focused on the hand (fingers and/or wrist) and arm (elbow and/or shoulder). The movements focused on the hand targeted muscles of the forearm, such as flexor digitorum and extensor digitorum; while the movements focused on the arm targeted biceps brachii, triceps brachii, and/or deltoid muscles.
Additional technology: Lastly, we recorded any additional technology used alongside the BCI and ES for upper limb motor rehabilitation strategies.
Results
Synthesis of results
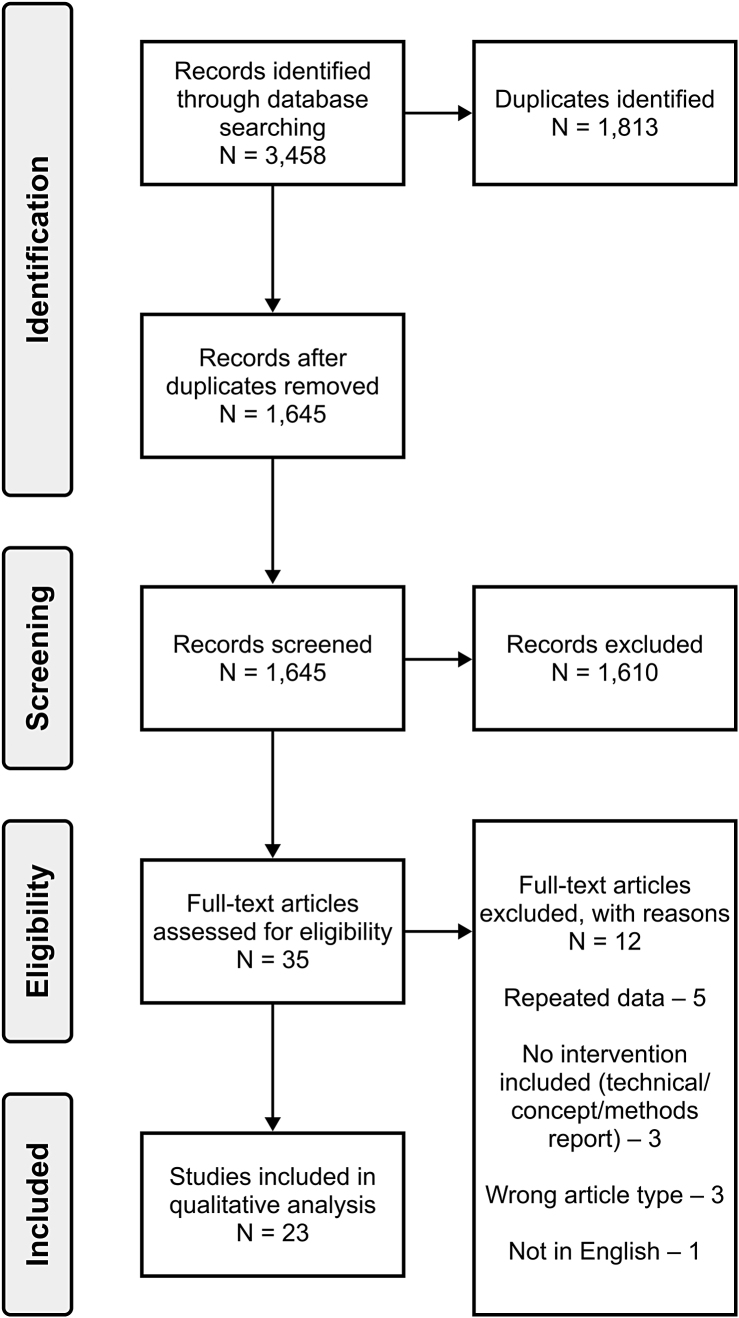
The search conducted on 5 February 2021 resulted in 3,458 records. The breakdown of search results by the specific database is included in Appendix 2. We identified and removed 1,813 duplicates, leaving 1,645 records for screening. At the title and abstract screening stage, there was an agreement between the two reviewers in 98.66% of the observations, with k = 0.704 (i.e., substantial agreement).32 Any disagreements were discussed between the two reviewers at the end of the title and abstract screening stage, and the decision to include or exclude the article was made jointly. Any articles for which the inclusion-exclusion decision was unclear based on the title and abstract were included for the full-text screening. Based on the title and abstract screening, we excluded 1,610 records, and the remaining 35 records were assessed by full-text screening, conducted in the same way as the title and abstract screening by two reviewers. Overall, we identified 23 records for qualitative synthesis.23,30–53 The breakdown of the screening process via a PRISMA flow diagram is shown in Figure 2.54
Figure 2.
The PRISMA flow diagram
Study descriptors
All records included in the final analysis were journal articles.
Study types
Seven out of 23 identified records had control groups and included between 12 and 30 participants. Eight of 23 records, with the number of participants ranging from 4 to 51, were labelled as single-arm interventional studies. The remaining eight records were case studies or case series, with one or two participants, respectively.
Population
The breakdown of population and intervention details reported in the analyzed studies is presented in Table 1.
Table 1.
Detailed Breakdown of the Population of Study Participants and Clinical Interventions in 23 Studies Included in the Review
| Population | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Authors (Year) n = 23 | Study type | Condition | Chronicity | Intervention, n | Control, n | Total no. of sessions | Frequency (sessions/wk) | Session length, min | Presence of therapist |
| Biasiucci et al. (2018) | Controlled study | Stroke | Chronic | 14 | 13 | 10 | 0–2 | 60 | Yes |
| Daly et al. (2009) | Case study | Stroke | Chronic | 1 | N/A | 9 | 3 | 45 | No |
| Grimm et al. (2016) | SAI study | Stroke | Chronic | 7 | N/A | 2 | N/A | — | No |
| Hashimoto et al. (2020) | SAI study | Stroke | Sub-acute | 4 | N/A | 2–4 | — | 40–50 | No |
| Irimia et al. (2017) | Case series | Stroke | Chronic | 2 | N/A | 10 | — | — | No |
| Irimia et al. (2018) | SAI study | Stroke | Sub-acute and chronic | 5 | N/A | 10–24 | — | 60 | No |
| Jang et al. (2016) | RCT | Stroke | Sub-acute | 10 | 10 | 30 | 5 | 30 | Yes |
| Jovanovic et al. (2020) | Case study | Stroke | Chronic | 1 | N/A | 80 | 3 | 60 | Yes |
| Kim et al. (2016) | RCT | Stroke | Sub-acute | 15 | 15 | 12 | 3 | 30 | Yes |
| Lee t al. (2020) | RCT | Stroke | Sub-acute | 13 | 13 | 20 | 5 | 30 | Yes |
| Li et al. (2014) | RCT | Stroke | Sub-acute | 7 | 7 | 24 | 3 | 60–90* | No |
| Marquez-Chin et al. (2016) | Case study | Stroke | Chronic | 1 | N/A | 40 | 3 | 90 | No |
| Miao et al. (2020) | RCT | Stroke | Sub-acute and chronic | 8 | 8 | 12 | 3 | N/A | No |
| Mukaino et al. (2014) | Case study | Stroke | Chronic | 1 | N/A | 25 | 5 | 60 | No |
| Osuagwu et al. (2016) | RPS | SCI | Sub-acute | 7 | 5 | 20 | 3–5 | 60 | No |
| Remsik et al. (2019) | SAI study | Stroke | Sub-acute and chronic | 21 | N/A | 9–15 | 2–3 | 120 | No |
| Sebastian-Romagosa et al. (2020) | SAI study | Stroke | Sub-acute and chronic | 51 | N/A | 25 | 2 | 60 | No |
| Tabernig et al. (2018) | SAI study | Stroke | Chronic | 8 | N/A | 20 | 4 | 60 | Yes |
| Trincado-Alonso et al. (2018) | SAI study | SCI | Sub-acute | 4 | N/A | 5 | — | 60 | Yes |
| Vuckovic et al. (2015) | Case series | SCI | Sub-acute | 2 | N/A | 4–10 | 2–3 | 60 | No |
| Young et al. (2014) | Case study | Stroke | Sub-acute | 1 | N/A | 13 | 2–3 | 120 | No |
| Young et al. (2015) | SAI study | Stroke | Sub-acute and chronic | 16 | N/A | 9–15 | 0–3 | 120 | No |
| Zhang et al. (2018) | Case study | Stroke | Chronic | 1 | N/A | 18 | 3 | 90 | No |
The session length of 60–90 minutes included brain-computer interface–controlled electrical stimulation as well as conventional therapy.
SAI = single-arm interventional; RCT = randomized-controlled trial; RPS = randomized pilot study; SCI = spinal cord injury.
Neurological conditions: The majority (20 of 23) of the reviewed studies included participants with stroke, and the remaining reports included individuals with cervical SCI. There were 272 participants across the 23 studies: 254 individuals with stroke and 18 individuals with SCI.
The breakdown of neurological conditions and study types are summarized in Table 2.
Table 2.
The Summary of Study Types and the Distribution of Chronicity Based on Neurological Condition
| Study type | ||||
|---|---|---|---|---|
| Condition | Controlled study | Single-arm study | Case series/study | Total |
| Stroke | 6 | 7 | 7 | 20 |
| SCI | 1 | 1 | 1 | 3 |
| All | 7 | 8 | 8 | 23 |
| Chronicity | ||||
|---|---|---|---|---|
| Chronic | Sub-acute | Both | Total | |
| Stroke | 10 | 4 | 6 | 20 |
| Spinal cord injury | 0 | 3 | 0 | 3 |
| All | 10 | 7 | 6 | 23 |
Sex: There were 111 female and 161 male participants. All of the female participants were stroke survivors. The 18 participants in the three studies involving individuals with SCI were males.
Chronicity and time post injury: The breakdown of chronicity and neurological conditions is presented in Table 2. Ten studies exclusively included individuals in the chronic stage of rehabilitation. In contrast, seven studies investigated the use of BCI-ES with individuals at the sub-acute stage. The remaining six studies included individuals in both stages of rehabilitation (i.e., chronic and sub-acute).
Severity of upper limb impairments: Of the 20 studies with the stroke population included in the final analysis, 12 of them used standard or modified FMA-UE for clinical assessment of the participants’ upper limb motor functions at baseline. Eight of those 12 studies included multiple participants, and seven reported mean and standard deviations (SDs) or raw FMA-UE scores. In a single study, the authors reported the median and inter-quartile range (0.25 and 0.75 quantiles) values. Based on the available (m)FMA-UE values, we concluded that most participants would be classified as severe and severe-moderate, based on the clustering from Woytowicz and colleagues.29 In two studies, (m)FMA-UE values reached the moderate-mild classification. The remaining four studies reporting on (m) FMA-UE baseline values were case studies, where three studies included participants from the severe-moderate group and one from the severe group. The graphical representation of these findings is presented in online Figure 1A, included in the supplemental material.
The three studies focused on the SCI population included 18 participants. AIS was used to describe the level of injury in all three studies. Seventeen participants were classified as incomplete (AIS grades B, C, D), and a single participant was classified with a complete injury (AIS A). The distribution of participants based on the AIS classification is shown in online Figure 1B, included in the supplemental material.
Interventions and clinical outcome measures
Interventions: The number of reported sessions varied considerably within the reviewed studies. They ranged from two sessions (1 FES + 1 BCI-FES) delivered to determine the effect of therapy on wrist range of motion (ROM),30 to 80 (73 BCI-FES + 7 FES) therapy sessions conducted in a case study for general upper limb motor rehabilitation after chronic stroke.23 Similarly, the frequency of the intervention ranged from 1 to 5 sessions per week. The session length was reported in 19 out of the 23 records, lasting between 30 to 120 minutes. Sixty minutes was the most common length reported in 8 out of 19 records. The presence of a therapist (i.e., involvement in the BCI-ES portion of the study) was reported in seven studies. In the remaining 15 studies, we concluded that the BCI-ES interventions were conducted without a therapist.
Clinical outcome measures: The clinical outcome measure scores from 10 studies (9 studies in stroke and 1 in SCI) are summarized in Table 3. In nine out of the ten studies, the extracted metrics were mean and standard deviation, and in the single remaining study, we extracted median (and interquartile range).
Table 3.
The Overview of the Clinical Outcome Measures in 10 Studies That Reported on Statistical Descriptions
| Authors (Year) n = 10 | Population, Condition / chronicity | Clinical outcome measures | Scores, mean (SD) | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Pre | Post | Change (Pre-Post) | Pre | Post | Change (Pre-Post) | |||
| Biasiucci et al. (2018) | Stroke / Chronic | FMA-UE | 21.60(10.80)* | 28.30(14.50)* | 6.60(5.60)† | 19.90(11.20) | 22.00(12.20) | 2.10(3.00)† |
| ASH | 1.60(1.20) | 1.90(0.90) | — | 2.10(1.40) | 1.70(1.20) | — | ||
| wrist flexor | ||||||||
| ASH | 1.10(1.20) | 0.90(1.10) | — | 1.20(1.40) | 0.60(0.70) | — | ||
| wrist extensor | ||||||||
| MRC | 1.40(0.90) | 2.60(1.20) | — | 1.30(1.00) | 1.60(1.20) | — | ||
| wrist extensor | ||||||||
| ESS | 66.20(12.60) | 69.60(14.40) | — | 63.80(11.20) | 64.60(11.00) | — | ||
| Grimm et al. (2016) | Stroke / Chronic | ROM | 18.00(6.00) | 26.00(8.00) | — | — | — | — |
| wrist | ||||||||
| Jang et al. (2016) | Stroke / Sub-acute | MFT | 8.10(9.20) | 15.90(6.90) | 7.80(4.30) | 8.20(9.90) | 13.10(8.30) | 4.90(4.50) |
| MAS shoulder | 0.60(0.70) | 1.00(0.80) | 0.40(0.70) | 0.60(0.70) | 1.10(0.70) | 0.50(0.70) | ||
| Kim et al. (2016) | Stroke / Sub-acute | FMA-UE | 26.80(7.22)* | 34.67(9.31)* | 7.87(2.42)† | 21.87(8.22)* | 24.80(9.51)* | 2.93(2.74)† |
| MAL-AOU | 42.67(22.81)* | 55.73(28.57)* | 13.07(7.56)† | 38.60(23.89)* | 44.40(26.83)* | 5.80(5.51)† | ||
| MAL-QOM | 19.93(7.43)* | 29.33(14.69)* | 9.40(8.70)† | 6.87(7.29)* | 9.00(9.49)* | 2.13(3.23)† | ||
| MBI | 87.67(3.85)* | 90.87(4.03)* | 3.20(1.70)† | 70.93(13.77)* | 72.60(14.12)* | 1.67(1.50)† | ||
| ROM | 25.84(11.37)* | 38.90(16.93)* | 13.06(8.64)† | 9.91(13.89)* | 14.06(18.91)* | 4.15(6.63)† | ||
| wrist flexion | ||||||||
| Lee et al. (2020) | Stroke / Sub-acute | FMA-UE | 30.53(5.05)* | 33.84(7.45)* | 3.30(3.44)† | 29.52(3.64) | 29.84(3.53) | 0.30(0.63)† |
| WMFT | 21.30(5.05)* | 24.76(7.92)* | 3.46(1.72)† | 20.15(3.80) | 20.53(3.82) | 0.38(0.65)† | ||
| MAL | 50.07(13.98)* | 58.61(17.76)* | 8.53(7.41)† | 51.23(7.71)* | 52.38(7.68)* | 1.15(0.98)† | ||
| MBI | 52.84(11.05)* | 59.92(13.96)* | 7.07(6.31)† | 50.76(6.77)* | 51.76(7.58)* | 1.07(1.25)† | ||
| Li et al. (2014) | Stroke / Sub-acute | FMA-UE | 13.57(4.72)* | 26.29(11.97)* | 12.71(12.16) | 11.71(2.63)* | 18.43(4.89)* | 6.71(4.46) |
| ARAT | 0.29(0.76)* | 18.29(9.46)* | 18.00(9.46)† | 0.00(0.00)* | 7.57(8.20)* | 7.57(9.46)† | ||
| Miao et al. (2020) | Stroke / Sub-acute and chronic | FMA-UE | 19.50(9.90) | 23.00(11.40) | 3.50(5.18) | 20.60(9.70) | 21.50(10.00) | 0.87(1.25) |
| Osuagwu et al. (2016)‡ | SCI / Sub-acute | ROM | 8.30 | 24.35 | — | 21.90 | 32.80 | — |
| wrist | ||||||||
| Remsik et al. (2019)§ | Stroke / Sub-acute and chronic | ARAT | 16.90(23.00)* | 18.30(23.40)* | 1.30 | — | — | — |
| SIS | 33.60(38.10) | 39.00(37.50) | 5.40 | — | — | — | ||
| hand function | ||||||||
| SIS | 50.10(23.70) | 53.40(24.90) | 3.30 | — | — | — | ||
| recovery | ||||||||
| NIHSS | 3.80(3.50) | 3.80(3.10) | 0.00 | — | — | — | ||
| BI | 91.40(14.00) | 92.00(13.90) | 0.60 | — | — | — | ||
| Grip strength (lbs) | 18.80(21.50)* | 22.60(23.50)* | 3.80 | — | — | — | ||
| 9-HPT (s) | 17.70(22.80) | 15.00(19.10) | −2.50 | — | — | — | ||
| MMSE | 27.20(3.80) | 27.80(2.70) | 0.60 | — | — | — | ||
| CES-D | 7.60(5.80) | 7.80(9.90) | 0.20 | — | — | — | ||
| Scores, (median IQR) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change (Pre-Post) | ||||||
| Sebastian-Romagosa et al. (2020) | Stroke / Sub-acute and chronic | FMA-UE | 19 (9.63–33.88)* | 22 (12.00–41.75)* | 4.68(4.92) | — | — | — |
| BI | 90 (70.00–95.00) | 95 (67.50–100.00) | 2.62(5.82) | — | — | — | ||
| MAS | 2.50 (0.63–3.50)* | 1 (0.00–3.00)* | −0.72(0.83) | — | — | — | ||
| Wrist | ||||||||
| MAS | 2.50 (1.00–3.50)* | 2 (1.00–3.00)* | –0.63(0.82) | — | — | — | ||
| Fingers | ||||||||
Within group significant difference in pre-post scores
Between groups significant difference in mean score changes
Only the mean was available in this study.
The scores reported in this study are based on the 9 out of 21 individuals who realized improved ARAT scores following the intervention.
FMA-UE = Fugl-Meyer Assessment – Upper Extremity score; ASH = Ashworth Scale; MAS = Modified Ashworth Scale; MRC = Medical Research Council; ESS = European Stroke Scale; ROM = range of motion; MFT = Manual Function Test; MAL = Motor Activity Log; MAL-AOU = Motor Activity Log Activity of Use; MAL- QOM = Motor Activity Log Quality of Movement; BI = Barthel Index; MBI = Modified Barthel Index; WMFT = Wolf Motor Function Test; ARAT = Action Research Arm Test; SIS = Stroke Impact Scale; SCI = spinal cord injury; NIHSS = National Institutes of Health Stroke Scale; 9-HPT = Nine-Hole Peg Test; MMSE = Mini Mental State Examination; CES-D = Center for Epidemiologic Studies - Depression; IQR = interquartile range.
The studies used a variety of clinical outcome measures, with studies often using multiple measures. The most common assessment was the FMA-UE, used in six out of nine studies with individuals with stroke. Other notable assessments in stroke rehabilitation studies included the Barthel Index,55 (Modified) Ashworth Scale,56 and Motor Activity Log.57 The single study including participants with SCI reported on the wrist ROM.
The pre- and post-intervention scores showed a trend of improved upper limb motor function. Six out of nine studies in the stroke population recorded a statistically significant difference within the BCI-ES intervention groups in at least one outcome measure. Similarly, among five studies with control groups, three reported a statistically significant difference in the average pre-post changes between the BCI-ES and the control groups.
Technology
A detailed breakdown of technology details reported in the analyzed studies is presented in Table 4.
Table 4.
Overview of the brain-computer interface–controlled electrical stimulation (BCI-ES) Systems and Any Additional Technology Used in the 23 Studies Included in the Review
| BCI | ES | ||||||
|---|---|---|---|---|---|---|---|
| Authors (Year) n = 23 | Signal | Number of channels | Calibration type | Type | Number of channels | Stimulation target | Additional technology |
| Biasiucci et al. (2018) | mEEG | 16 | 1 session | FES | 1 | Hand | — |
| Daly et al. (2009) | mEEG | 1 | Inconclusive | FES | Inconclusive | Finger | Monitor |
| Grimm et al. (2016) | mEEG | 3 | Inconclusive | Subthreshold- | 2 | Hand | Arm exoskeleton |
| NMES | EMG classifier | ||||||
| Hashimoto et al. (2020) | bEEG | 2 | Continuously | NMES | 1 | Hand | Head mounted display |
| Irimia et al. (2017) | mEEG | 45 | Continuously | FES | Inconclusive | Hand | Monitor |
| Irimia et al. (2018) | mEEG | 64 | Continuously | FES | 1 per hand | Hand | Monitor |
| Jang et al. (2016) | mEEG | 1 | Continuously | FES | 1 | Arm | — |
| Jovanovic et al. (2020) | mEEG | 1 | 1 session | FES | 4 | Arm and hand | — |
| Kim et al. (2016) | mEEG | 2 | Inconclusive | FES | 1 | Hand | Monitor |
| Lee et al. (2020) | mEEG | 2 | Continuously | FES | 1 | Hand | Monitor |
| Li et al. (2014) | mEEG | 16 | FES | 1 | Hand | Monitor | |
| Marquez-Chin et al. (2016) | mEEG | 1 | 1 session | FES | 4 | Arm | — |
| Miao et al. (2020) | mEEG | 16 | 1 session | FES | 1 per hand | Hand | Monitor |
| Mukaino et al. (2014) | mEEG | 10 | Inconclusive | NMES | 1 | Hand | Monitor |
| Osuagwu et al. (2016) | bEEG | 3 | Continuously | FES | 4 | Hand | Monitor |
| Remsik et al. (2019) | mEEG | 2 | Inconclusive | FES | 1 | Hand | Monitor |
| TDU for tongue stimulation | |||||||
| Sebastian-Romagosa et al. (2020) | mEEG | 16 | 1 session | FES | 1 per hand | Hand | Monitor |
| Tabernig et al. (2018) | mEEG | 14* | Continuously | FES | 1 | Hand | — |
| Trincado-Alonso et al. (2018) | mEEG | 15 | Continuously | FES | 2 | Hand | Monitor |
| Vuckovic et al. (2015) | bEEG | 2 | Continuously | FES | 1 | Hand | Monitor |
| Young et al. (2014) | mEEG | 5 | Continuously | FES | 1 | Hand | Monitor |
| TDU for tongue stimulation | |||||||
| Young et al. (2015) | mEEG | 16 | Continuously | FES | Inconclusive | Hand | Monitor |
| TDU for tongue stimulation | |||||||
| Zhang et al. (2018) | mEEG | 32 | 3 “warm-up” sessions | FES | 1 | Hand | Elbow orthosis |
Note: Inconclusive indicates that the data point was unobtainable from the descriptions provided in the report.
We could not confirm whether all available 14 channels were used, or only a subset. However, based on the description provided in the article, it is more likely that 14 channels were used.
mEEG = monopolar electroencephalography; bEEG = bipolar electroencephalography; FES = functional electrical stimulation; NMES = neuromuscular electrical stimulation; EMG = electromyography; TDU = tongue display unit.
Brain-computer interface: In terms of the modality used to acquire brain activity, EEG was used in all 23 studies. The number of channels that was used, however, ranged from one to 64. A single monopolar EEG (mEEG) channel was used in three studies; two mEEG channels were used in three more studies. Four studies reported using systems with 16 mEEG channels, and three studies reported systems with 32 or more mEEG channels, with 64 monopolar channels used in just one study.37 Three more systems were described using bipolar EEG (bEEG) configurations with two or three channels.
Regarding the BCI system calibration, five systems required a single BCI calibration session to determine optimal BCI parameters, primarily the EEG electrode location(s) and frequency bands. In 11 studies, the calibration involved continuous reconfiguration of BCI parameters with shorter training intervals before each therapy (feedback) session. In other studies, the calibration was separately done before starting the intervention, with one report describing an approach in which the first week of the six-week training programme was used as a ‘warm-up week’ to familiarize the participants with the technology.
Electrical stimulation: FES was used in 20 studies, while NMES was used in three studies. A single study reported using “subthreshold NMES,” which does not produce muscle contractions during movement practice but is believed to produce overall activation of sensorimotor neuronal networks.30 Studies that targeted functional tasks used FES to stimulate muscle groups. Other studies used NMES to focus on single muscles (i.e., extensor carpi radialis,35 extensor digitorum communis).44
The number of channels used for stimulation was reported in 20 out of 23 reports. In the remaining three studies, the number of stimulation channels was inconclusive. Single-channel stimulation was reported in 15 studies, including three studies that used one channel per hand (two channels in total). Two-channel stimulation was reported in two studies, and four-channel stimulation was reported in three studies. A single study described a stimulation protocol to assist with index finger movements. Stimulation protocols targeting the hand (wrist with or without finger movements) were more common and reported in 19 studies. In three studies, the stimulation targeted the arm, involving the shoulder with or without elbow movements. Finally, in a single study, both the arm and hand were stimulated simultaneously.
Additional technology: Regarding additional technologies, we considered the patient-facing technology used in addition to BCI and ES as a part of the intervention. Five studies did not use any additional technology. In the remaining 18 studies, the most common additional technology included computer monitors used to display commands (i.e., cues) during BCI-ES training and provide visual feedback. The visual feedback was typically presented in the form of a first-person view of the arms and hands of a virtual reality avatar mimicking the practiced movements. A single study used a head-mounted display for visual feedback instead of a monitor.35 In two studies,39,40 the monitor was used to display videos of movements as a part of action-observation training (AOT), completed separately prior to the BCI-ES therapy. Other notable additional technologies included an exoskeleton for anti-gravity arm support,30 an active elbow orthosis,53 and a Tongue Display Unit for feedback via tongue stimulation.46,51,52
Discussion
In this scoping review, we aimed to consolidate the existing information on the growing field of research evaluating the combined use of BCI and ES technologies as an intervention for the recovery of motor functions in individuals with upper limb impairments. The included studies were similarly distributed among controlled studies (i.e., studies including a control group), single-arm interventional studies, and case studies/series. From our findings, BCI-ES interventions for upper limb motor rehabilitation in adults have been evaluated in stroke and SCI populations. However, there is a large discrepancy in the representation of these two populations in BCI-ES interventional studies; research has focused predominantly on the stroke population (86.9% of the records, or 20 out of 23 studies).
In terms of participants’ sex, there were significant differences between the studies focusing on stroke and SCI populations. In 20 studies in the stroke population, 43.7% of the participants were women, which is comparable to the global average of 48% estimated for 2016.58 On the other hand, in three studies with the SCI population, there were no female individuals among the 18 participants. Globally, male individuals are three times more likely to be affected by traumatic SCI than females, while the proportion between the two sexes is relatively equal for non-traumatic SCI.59 The discrepancies in the number of male and female participants, observed in analysed SCI studies, were not the intentions of the respective authors. Nonetheless, given the sexual dimorphism in response to therapy and recovery from SCI,60,61 it is vital to have representation of both male and female participants in motor rehabilitation studies.
As part of the approach to the BCI-ES intervention, only seven articles reported on a therapist's involvement during clinical assessments, planning, or delivery of the intervention. While technology can enhance a rehabilitation strategy, therapists critically observe and guide patients’ movements, and modify therapy based on their clinical reasoning. Currently, therapists’ clinical experience is used to direct the implementation of ABT for neurorehabilitation while the development of specific guidelines (e.g., dosage, parameters, timing of intervention) are under investigation.62 This means that therapists should be included when technology, such as BCI-ES, is integrated into therapy, especially in the early stages. However, the extent of a therapist's involvement can be modified depending on the intervention.
In some cases, the therapist's presence might only be required for the assessments and to plan the treatment – a scenario that is especially applicable for BCI-ES use at home.63 In these situations, BCI-ES is designed to be used by the patient themselves or with the help of caregivers. However, in the context of in- or outpatient rehabilitation programmes in clinical environments, therapists are typically present, with their impact stretching beyond the movement support. For example, patients recovering from stroke have reported that physiotherapists can not only be a valuable source of information and advice about functional exercise, but also provide a source of faith and hope.64 Nonetheless, including a therapist in the research team can facilitate collaboration and accelerate research into clinical practice.9
Regarding chronicity of the participants, studies in the stroke population have been conducted in both chronic and sub-acute groups, with an increased number of studies in the chronic group. Earlier research has suggested that recovery of motor function (including upper limb) plateaus in the chronic stage of stroke rehabilitation (> 6 months post-injury),65–67 especially in individuals with severe impairments.68 However, the notion of plateaued recovery is presently being challenged, potentially due to the rise in therapeutic strategies using technology to engage with sensorimotor neuronal networks, such as BCI-ES interventions.
The brief overview of the clinical efficacy, which included ten studies that reported statistical results, demonstrated the capacity of the BCI-ES interventions to facilitate improvement in upper limb motor function. Across the ten studies, we observed a trend of improved scores on one or more assessments following the interventions. Moreover, five out of ten studies included control groups and compared the effects of BCI-ES therapy to other forms of ES (e.g., randomly-triggered FES, conventional FES). These studies compared the pre-post changes in clinical outcome measures between groups, and in three occurrences, the differences were statistically significant. For example, Kim and colleagues39 reported that the mean change in the FMA-UE scores of 7.87 (SD 2.42) in the AOT + BCI-FES group was significantly greater than the mean change of 2.93 (SD 2.74) in the FES group. The same applied to the mean changes in the FMA-UE scores between the intervention (ΔFMA-UE = 6.6 [SD 5.6]) and the control group (ΔFMA-UE = 2.1 [SD 3.0]) in the study by Biasiucci and colleagues.33 Both of these studies were conducted in the stroke population, and the latter included individuals with chronic stroke.
In contrast, there were no studies with individuals with chronic SCI. Nonetheless, a study by Osuagwu and colleagues,45 involving 12 individuals with sub-acute SCI, observed a greater mean change in the wrist ROM in the BCI-FES group (ΔROM = 16.05°) compared to the FES group (ΔROM = 10.9°). These early promising results warrant further BCI-ES interventional studies in both sub-acute and chronic SCI populations.
Regarding technology, we observed an array of BCI and ES systems. The diversity of implementations is expected due to the relatively young age of the field. However, we also observed some common trends. EEG was used for BCI development in all studies, which was not surprising considering the short-term nature of the BCI application in therapy. EEG systems are non-invasive, and they can be donned and doffed with little effort. However, we observed diversity in the number of EEG channels – an aspect of the BCI systems that can affect the setup time. In a clinical application where sessions are often one hour in duration, there is a significant difference between a 30-minute and 10-minute setup. Therefore, BCI systems with fewer channels are more likely to be considered for clinical application.
In contrast, a greater number of stimulation channels might be more beneficial for clinical practice. More FES channels can support a wide range of complex upper limb movement, giving the system flexibility to meet the needs of individuals with different types of upper limb impairments and varying degrees of severity. However, among the reviewed studies that reported the number of stimulation channels (20 of 23), single-channel FES was more common than any other type of ES. Overall, three studies reported on the BCI-ES systems with BCIs using three or fewer EEG channels and four FES channels. The chart illustrating all of the BCI-ES systems based on the number of BCI and FES channels is shown in online Figure 2, included in the supplemental material.
Across the reviewed studies, hand movements were the most common stimulation target. All but three out of 23 records targeted movements involving the wrist and/or fingers. In the remaining three studies involving arm movements, only one reported on a FES system that supported both hand and arm movements (i.e., isolated as well as simultaneous reaching and grasping). Currently, FES is considered to be one of the components of ABT.69,70 With continued research that produces successful findings, it is foreseeable that BCI-FES could be considered as another technological modality to support ABT.
In two studies that involved practicing arm movements, the FES was used to support the hand while a robotic device supported the arm. Grimm and colleagues30 used an anti-gravity arm exoskeleton alongside subthreshold NMES to elicit sensory feedback. Zhang and colleagues53 used an active orthosis to enable elbow extension and flexion.
While robotic devices were used as additional technology in two studies, computer monitors were overwhelmingly included in 15 studies. The monitors were typically used to cue participants to imagine movements and provide visual feedback. In two studies, the monitors were used for displaying movements as a part of AOT before the BCI-ES sessions. Depending on the participants’ preference and learning approach, monitors could be used to provide visual feedback.
Our study has two major limitations. The primary limitation is that we did not include a critical appraisal of the included sources of evidence, thus limiting the ability to evaluate study quality and risk of bias. A significant number of the studies analyzed were case studies and case series. These results can inform preliminary judgments about study quality, suggesting a lower quality of evidence relative to randomized controlled trials.
The second limitation is related to the brief analysis of clinical outcome measures, which included 10 out of 23 reviewed research articles. While we observed a trend of improved motor function following BCI-ES interventions in these preliminary results, there is significant diversity in the outcome measures used in the 10 analyzed studies, which limits the strength of the evidence.
Future directions
This scoping review provides a platform for future research related to population characteristics and experimental design. Considering that there are sex-based differences surrounding SCI4,60,61 and stroke,71 sex sub-analyses should be completed once more data aggregates. Many stroke studies included in this review used the modified or standard version of the FMA-UE. Further standardization of outcome measures both in stroke and SCI populations should be considered.
There was an assortment of experimental designs across studies, making comparisons difficult. Research studies should develop a protocol for BCI-ES setup and therapy. The number and frequency of therapy sessions should be documented along with the number of repetitions of a functional task. By documenting this information, it will be easier to determine whether the therapy meets the criteria for ABT. Furthermore, the standardization of BCI-ES calibration and the number of channels used could lead to minimal setup time while maintaining reliability. Following study designs precisely, standardizing instruments (i.e., device and therapy), and taking repeated outcome measurements at specified intervals can provide a better platform to determine the patient's prognosis.72
Additionally, the number of stimulation channels could be increased to support a wider range of ES-assisted movements. Studies could be conducted with or without feedback using a computer monitor. If included, the feedback content should be optimized (e.g., avatar or realtime recording). Once the technology and therapeutic intervention are better understood under similar research conditions, researchers can determine whether device settings and therapeutic interventions should be tailored to the individual.
Conclusion
We conducted the presented scoping review to understand current knowledge and knowledge gaps around using BCI-ES technology to enhance therapeutic interventions for upper limb motor functions. We identified that more studies are needed to investigate the effects of BCI-ES interventions following SCI, specifically in chronic and female populations. Moreover, measuring the setup time and standardization in BCI-ES interventions should be considered to evaluate BCI-ES rehabilitation strategies in clinical environments. Finally, we believe that a clinician (e.g., therapist) trained in neurorehabilitation should be a part of future studies investigating BCI-ES interventions.
Key Messages
(1) What is already known on this topic
Both BCI and ES technologies are increasingly being investigated, and in rare cases used, for upper limb motor rehabilitation after neurological injuries, like stroke or SCI. These technologies are significant in relation to activity-based therapies, which aim to engage individuals with impairments in high-intensity task-specific training that targets the nervous system.
(2) What this study adds
Our scoping review identified a great diversity in the reported BCI-ES interventions for arm and hand therapy in two neurological populations: individuals living with stroke and those with SCI. However, we found a low number of BCI-ES interventional studies involving individuals with SCI (3 out of 23 reviewed articles). Additionally, these three studies showed a lack of representation of female participants in BCI-ES interventional studies in the SCI population. Across the 23 articles included in the review, we also observed various designs for BCI and ES systems and strategies for integrating these two technologies into upper limb rehabilitation. We discussed elements of these systems, particularly the number of BCI and ES channels, that can impact the clinical application of BCI-ES interventions.
Supplemental Material
References
- 1.Faria-Fortini I, Michaelsen SM, Cassiano JG, et al. Upper extremity function in stroke subjects: relationships between the international classification of functioning, disability, and health domains. J Hand Ther. 2011;24(3):257–65. 10.1016/j.jht.2011.01.002. Medline: [DOI] [PubMed] [Google Scholar]
- 2.Broeks JG, Lankhorst GJ, Rumping K, et al. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21(8):357–64. 10.1080/096382899297459. Medline: [DOI] [PubMed] [Google Scholar]
- 3.Dimbwadyo-Terrer I, Trincado-Alonso F, de los Reyes-Guzmán A, et al. Upper limb rehabilitation after spinal cord injury: a treatment based on a data glove and an immersive virtual reality environment. Disabil Rehabil Assist Technol. 2016;11(6):462–7. 10.3109/17483107.2015.1027293. Medline: [DOI] [PubMed] [Google Scholar]
- 4.Noonan VK, Fingas M, Farry A, et al. Incidence and prevalence of spinal cord injury in Canada: a national perspective. Neuroepidemiology. 2012;38(4):219–26. 10.1159/000336014. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–83. 10.1089/neu.2004.21.1371. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Snoek GJ, IJzerman MJ, Hermens HJ, et al. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42(9):526–32. 10.1038/sj.sc.3101638. Medline: [DOI] [PubMed] [Google Scholar]
- 7.Simpson LA, Eng JJ, Hsieh JTC, et al. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 2012;29(8):1548–55. 10.1089/neu.2011.2226. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo C, Tran Y, Anderson K, et al. Functional priorities in persons with spinal cord injury: using discrete choice experiments to determine preferences. J Neurotrauma. 2016;33(21):1958–68. 10.1089/neu.2016.4423. Medline: [DOI] [PubMed] [Google Scholar]
- 9.Behrman AL, Ardolino EM, Harkema SJ. Activity-based therapy: from basic science to clinical application for recovery after spinal cord injury. J Neurol Phys Ther. 2017;41(Suppl 3 IV STEP Spec Iss):S39–45. 10.1097/npt.0000000000000184. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musselman KE, Shah M, Zariffa J. Rehabilitation technologies and interventions for individuals with spinal cord injury: translational potential of current trends. J NeuroEng Rehabil. 2018;15(1):40. 10.1186/s12984-018-0386-7. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–39. 10.1044/1092-4388(2008/018) [DOI] [PubMed] [Google Scholar]
- 12.Sawaki L, Butler AJ, Leng X, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22(5):505–13. 10.1177/1545968308317531. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathian K, Buxbaum LJ, Cohen LG, et al. Neurological principles and rehabilitation of action disorders: common clinical deficits. Neurorehabil Neural Repair. 2011;25(5_suppl):21S–32S. 10.1177/1545968311410941. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang VS, Krakauer JW. Robotic neurorehabilitation: a computational motor learning perspective. J NeuroEng Rehabil. 2009;6(1):5. 10.1186/1743-0003-6-5. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lum P, Reinkensmeyer D, Mahoney R, et al. Robotic devices for movement therapy after stroke: current status and challenges to clinical acceptance. Top Stroke Rehabil. 2002;8(4):40–53. 10.1310/9kfm-kf81-p9a4-5ww0. Medline: [DOI] [PubMed] [Google Scholar]
- 16.Chae J, Sheffler L, Knutson J. Neuromuscular electrical stimulation for motor restoration in Hemiplegia. Top Stroke Rehabil. 2008;15(5):412–26. 10.1310/tsr1505-412. Medline: [DOI] [PubMed] [Google Scholar]
- 17.Popovic MB, Popovic DB, Schwirtlich L, et al. Functional Electrical Therapy (FET): clinical trial in chronic hemiplegic subjects. Neuromodulation. 2004;7(2):133–40. 10.1111/j.1094-7159.2004.04017.x. Medline: [DOI] [PubMed] [Google Scholar]
- 18.Mangold S, Keller T, Curt A, et al. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord. 2005;43(1):1–13. 10.1038/sj.sc.3101644. Medline: [DOI] [PubMed] [Google Scholar]
- 19.Thrasher TA, Zivanovic V, McIlroy W, et al. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. 2008;22(6):706–14. 10.1177/1545968308317436. Medline: [DOI] [PubMed] [Google Scholar]
- 20.Hebert DA, Bowen JM, Ho C, et al. Examining a new functional electrical stimulation therapy with people with severe upper extremity hemiparesis and chronic stroke: a feasibility study. Br J Occup Ther. 2017;80(11):651–9. 10.1177/0308022617719807 [DOI] [Google Scholar]
- 21.Popovic MR, Kapadia N, Zivanovic V, et al. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair. 2011;25(5):433–42. 10.1177/1545968310392924. Medline: [DOI] [PubMed] [Google Scholar]
- 22.Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7(11):1032–43. 10.1016/s1474-4422(08)70223-0. Medline: [DOI] [PubMed] [Google Scholar]
- 23.Jovanovic LI, Kapadia N, Lo LN, et al. Restoration of upper limb function after chronic severe hemiplegia: a case report on the feasibility of a brain-computer interface-triggered functional electrical stimulation therapy. Am J Phys Med Rehabil. 2020;99(3):E35–40. 10.1097/phm.0000000000001163. Medline: [DOI] [PubMed] [Google Scholar]
- 24.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. 10.1080/1364557032000119616. Medline:34647832 [DOI] [Google Scholar]
- 25.Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7(1):16. 10.1186/1472-6947-7-16. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. 2017;12(5):444–50. 10.1177/1747493017711816. Medline: [DOI] [PubMed] [Google Scholar]
- 27.Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45(3):190–205. 10.1038/sj.sc.3102007. Medline: [DOI] [PubMed] [Google Scholar]
- 28.Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. Medline: [PubMed] [Google Scholar]
- 29.Woytowicz EJ, Rietschel JC, Goodman RN, et al. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch Phys Med Rehabil. 2017;98(3):456–62. 10.1016/j.apmr.2016.06.023. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimm F, Walter A, Spuler M, et al. Hybrid neuroprosthesis for the upper limb: combining brain-controlled neuromuscular stimulation with a multi-joint arm exoskeleton. Front. 2016;10:367. 10.3389/fnins.2016.00367. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts TT, Leonard GR, Cepela DJ. Classifications in brief: American Spinal Injury Association (ASIA) impairment scale. Clin Orthop Relat Res. 2017;475(5):1499–504. 10.1007/s11999-016-5133-4. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. 10.2307/2529310. Medline: [DOI] [PubMed] [Google Scholar]
- 33.Biasiucci A, Leeb R, Iturrate I, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9(1):2421. 10.1038/s41467-018-04673-z. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daly JJ, Cheng R, Rogers J, et al. Feasibility of a new application of noninvasive Brain Computer Interface (BCI): a case study of training for recovery of volitional motor control after stroke. J Neurol Phys Ther. 2009;33(4):203–11. 10.1097/npt.0b013e3181c1fc0b. Medline: [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto Y, Kakui T, Ushiba J, et al. Portable rehabilitation system with brain-computer interface for inpatients with acute and subacute stroke: a feasibility study. Assist Technol. 2020;21:1–9. 10.1080/10400435.2020.1836067. Medline: [DOI] [PubMed] [Google Scholar]
- 36.Irimia DC, Cho W, Ortner R, et al. Brain-computer interfaces with multi-sensory feedback for stroke rehabilitation: a case study. Artif Organs. 2017;41(11):E178–84. 10.1111/aor.13054. Medline: [DOI] [PubMed] [Google Scholar]
- 37.Irimia DC, Oliner R, Poboroniuc MS, et al. High classification accuracy of a motor imagery based brain-computer interface for stroke rehabilitation training. Front Robot AI. 2018;5:9. 10.3389/frobt.2018.00130. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang YY, Kim TH, Lee BH. Effects of brain-computer interface-controlled functional electrical stimulation training on shoulder subluxation for patients with stroke: a randomized controlled trial. Occup Ther Int. 2016;23(2):175–85. 10.1002/oti.1422. Medline: [DOI] [PubMed] [Google Scholar]
- 39.Kim T, Kim S, Lee B. Effects of action observational training plus brain-computer interface-based functional electrical stimulation on paretic arm motor recovery in patient with stroke: a randomized controlled trial. Occup Ther Int. 2016;23(1):39–47. 10.1002/oti.1403. Medline: [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Kim SS, Lee BH. Action observation training and brain-computer interface controlled functional electrical stimulation enhance upper extremity performance and cortical activation in patients with stroke: a randomized controlled trial. Physiother. 2020; 1–9. 10.1080/09593985.2020.1831114. Medline: [DOI] [PubMed] [Google Scholar]
- 41.Li M, Liu Y, Wu Y, et al. Neurophysiological substrates of stroke patients with motor imagery-based brain-computer interface training. Int J Neurosci. 2014;124(6):403–15. 10.3109/00207454.2013.850082. Medline: [DOI] [PubMed] [Google Scholar]
- 42.Marquez-Chin C, Marquis A, Popovic MR. EEG-triggered functional electrical stimulation therapy for restoring upper limb function in chronic stroke with severe hemiplegia. Case Report. 2016;2016:9146213. 10.1155/2016/9146213. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miao Y, Chen S, Zhang X, et al. BCI-based rehabilitation on the stroke in sequela stage. Neural Plast. 2020;2020:8882764. 10.1155/2020/8882764. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukaino M, Ono T, Shindo K, et al. Efficacy of brain-computer interface-driven neuromuscular electrical stimulation for chronic paresis after stroke. J Rehabil Med. 2014;46(4):378–82. 10.2340/16501977-1785. Medline: [DOI] [PubMed] [Google Scholar]
- 45.Osuagwu BC, Wallace L, Fraser M, et al. Rehabilitation of hand in subacute tetraplegic patients based on brain computer interface and functional electrical stimulation: a randomised pilot study. J Neural Eng. 2016;13(6):065002. 10.1088/1741-2560/13/6/065002. Medline: [DOI] [PubMed] [Google Scholar]
- 46.Remsik AB, Williams L, Gjini K, et al. Ipsilesional mu rhythm desynchronization and changes in motor behavior following post stroke BCI intervention for motor rehabilitation. Front. 2019;13:18. 10.3389/fnins.2019.00053. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sebastian-Romagosa M, Cho W, Ortner R, et al. Brain computer interface treatment for motor rehabilitation of upper extremity of stroke patients–a feasibility study. Front. 2020;14:591435. 10.3389/fnins.2020.591435. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabernig CB, Lopez CA, Carrere LC, et al. Neurorehabilitation therapy of patients with severe stroke based on functional electrical stimulation commanded by a brain computer interface. J Rehabil Assist Technol Eng. 2018;5:12. 10.1177/2055668318789280. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trincado-Alonso F, Lopez-Larraz E, Resquin F, et al. A pilot study of brain-triggered electrical stimulation with visual feedback in patients with incomplete spinal cord injury. J Med Biol Eng. 2018;38(5):790–803. 10.1007/s40846-017-0343-0 [DOI] [Google Scholar]
- 50.Vuckovic A, Wallace L, Allan DB. Hybrid brain-computer interface and functional electrical stimulation for sensorimotor training in participants with tetraplegia: a proof-of-concept study. J Neurol Phys Ther. 2015;39(1):3–14. 10.1097/npt.0000000000000063. Medline: [DOI] [PubMed] [Google Scholar]
- 51.Young BM, Nigogosyan Z, Nair VA, et al. Case report: post-stroke interventional BCI rehabilitation in an individual with preexisting sensorineural disability. Front Neuroeng. 2014;7:18. 10.3389/fneng.2014.00018. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young BM, Nigogosyan Z, Walton LM, et al. Dose-response relationships using brain–computer interface technology impact stroke rehabilitation. Front Hum Neurosci. 2015;9:361. 10.3389/fnhum.2015.00361. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Elnady AM, Randhawa BK, et al. Combining mental training and physical training with goal-oriented protocols in stroke rehabilitation: a feasibility case study. Front Hum Neurosci. 2018;12:125. 10.3389/fnhum.2018.00125. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–9. 10.1016/0895-4356(89)90065-6 [DOI] [PubMed] [Google Scholar]
- 56.Meseguer-Henarejos A-B, Sánchez-Meca J, López-Pina J-A, et al. Inter- and intra-rater reliability of the modified ashworth scale: a systematic review and meta-analysis. Eur J Phys Rehabil Med. 2018;54(4):576–90. 10.23736/s1973-9087.17.04796-7. Medline: [DOI] [PubMed] [Google Scholar]
- 57.Uswatte G, Taub E, Morris D, et al. The motor activity log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67(7):1189–94. 10.1212/01.wnl.0000238164.90657.c2. Medline: [DOI] [PubMed] [Google Scholar]
- 58.Lindsay MP, Norrving B, Sacco RL, et al. World Stroke Organization (WSO): global stroke fact sheet 2019. Int J Stroke. 2019;14(8):806–17. 10.1177/1747493019881353. Medline: [DOI] [PubMed] [Google Scholar]
- 59.Thietje R, Hirschfeld S. Epidemiology of spinal cord injury. In: Weidner N, Rupp R, Tansey KE, editors. Neurological aspects of spinal cord injury [Internet]. Cham: Springer International Publishing; 2017. [cited 2020 Oct 30]. p. 3–17. 10.1007/978-3-319-46293-6_1. [DOI] [Google Scholar]
- 60.Ghnenis AB, Burns DT, Osimanjiang W, et al. A long-term pilot study on sex and spinal cord injury shows sexual dimorphism in functional recovery and cardio-metabolic responses. Sci Rep. 2020;10(1):2762. 10.1038/s41598-020-59628-6. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart AN, MacLean SM, Stromberg AJ, et al. Considerations for studying sex as a biological variable in spinal cord injury. Front Neurol. 2020;11:802. 10.3389/fneur.2020.597689. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dromerick AW, Lum PS, Hidler J. Activity-based therapies. NeuroRx. 2006;3(4):428–38. 10.1016/j.nurx.2006.07.004. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zulauf-Czaja A, Al-Taleb MKH, Purcell M, et al. On the way home: a BCI-FES hand therapy self-managed by sub-acute SCI participants and their caregivers: a usability study. J NeuroEng Rehabil. 2021;18(1):44. 10.1186/s12984-021-00838-y. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pound P, Bury M, Gompertz P, et al. Views of survivors of stroke on benefits of physiotherapy. Qual Health Care. 1994;3(2):69–74. 10.1136/qshc.3.2.69. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duncan PW, Goldstein LB, Matchar D, et al. Measurement of motor recovery after stroke: outcome assessment and sample size requirements. Stroke. 1992. Aug 1;23(8):1084–9. 10.1161/01.str.23.8.1084. Medline: [DOI] [PubMed] [Google Scholar]
- 66.Duncan PW, Goldstein LB, Horner RD, et al. Similar motor recovery of upper and lower extremities after stroke. Stroke. 1994;25(6):1181–8. 10.1161/01.str.25.6.1181. Medline: [DOI] [PubMed] [Google Scholar]
- 67.Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke. 2006;37(9):2348–53. 10.1161/01.str.0000238594.91938.1e. Medline: [DOI] [PubMed] [Google Scholar]
- 68.Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb. Stroke. 2003;34(9):2181–6. 10.1161/01.str.0000087172.16305.cd. Medline: [DOI] [PubMed] [Google Scholar]
- 69.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86(10):1406–25. 10.2522/ptj.20050212. Medline: [DOI] [PubMed] [Google Scholar]
- 70.McDonald JW, Sadowsky CL, Stampas A. Chapter 20 - the changing field of rehabilitation: optimizing spontaneous regeneration and functional recovery. In: Verhaagen J, McDonald JW, editors. Handbook of clinical neurology [Internet]. Elsevier; 2012. p. 317–36. Medline: [DOI] [PubMed] [Google Scholar]
- 71.Petrea RE, Beiser AS, Seshadri S, et al. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40(4):1032–7. 10.1161/strokeaha.108.542894. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kollen B, Kwakkel G, Lindeman E. Functional recovery after stroke: a review of current developments in stroke rehabilitation research. Rev Recent Clin Trials. 2006;1(1):75–80. 10.2174/157488706775246111. Medline: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.