Since 1997, the overall uterine cancer mortality rate has increased; from 2001 to 2018, the uterine cancer mortality rate increased among non-Hispanic Black women, with the largest increase observed in younger non-Hispanic Black and Hispanic women.
Abstract
OBJECTIVE:
To analyze mortality trends in uterine cancer in the United States over 50 years with an emphasis on age and race and ethnicity.
METHODS:
Data on uterine cancer deaths from 1969 to 2018 were obtained from the National Center for Health Statistics. Trends were examined by age and race and ethnicity after adjustment for the hysterectomy rate and pregnancy.
RESULTS:
Uterine cancer mortality decreased between 1969 and 1997 (from 6.03 to 4.00/100,000) but increased between 1997 and 2018 (from 4.00 to 5.02/100,000). From 2001 to 2018, mortality rates increased by 1.25-fold across all age groups. In 2018, the mortality rate from uterine cancer for patients aged 70 years or older and 60–69 years was sixfold and threefold higher, respectively, than in younger patients (aged 50–59 years) (54.87/100,000 vs 27.80/100,000 vs 8.70/100,000). The mortality rate for non-Hispanic Black women was 2.2-fold higher than for non-Hispanic White, Hispanic, and non-Hispanic Asian or Pacific Islander women (17.6/100,000 vs 7.82/100,000, 6.54/100,000, and 4.24/100,000, respectively). On an intersection analysis of age and race, non-Hispanic Black women aged older than 60 years had a threefold higher mortality rate than non-Hispanic White women (72/100,000 vs 24/100,000). A notable finding was that young non-Hispanic Black and Hispanic women (30–39 years) had the highest annual increases in mortality at 3.3% and 3.8% per year compared with 2.2% in non-Hispanic White women.
CONCLUSION:
Since 2001, the uterine cancer mortality rate has increased across all four racial and ethnic groups examined, with the highest increase seen among non-Hispanic Black women. The largest increase in mortality was observed among younger non-Hispanic Black and Hispanic women.
Uterine cancer is the fourth most common cancer and the sixth most common cause of death among women in the United States.1,2 It is the most frequent gynecologic cancer in the United States, with an estimated 66,200 new cases and 13,030 deaths in 2023.2 Despite advances in cancer research, uterine cancer incidence in the United States continues to rise. Uterine cancer is currently one of the only cancers with increasing incidence and mortality in the United States.1 Although it affects predominantly postmenopausal women, this rise in incidence has been reported across all age groups.3
Significant racial disparities also exist regarding uterine cancer. From 2010 to 2019, the mortality rate for uterine cancer increased by 1.9% per year among non-Hispanic Black women compared with 1.6% per year among White women.4 In 2019, the mortality rate in non-Hispanic Black women was nearly double that in White women (9.0/100,000 vs 4.6/100,000), reflecting the largest Black–White disparity in 5-year relative survival of all cancers (63% vs 84%).4 Obesity is a significant risk factor for uterine cancer; it is suggested that the uptrend in uterine cancer incidence can be attributed to increasing rates of obesity and decreasing rates of hysterectomy for benign pathology.3,5 Although this may explain the increasing incidence of uterine cancer, the factors behind the rise in uterine cancer mortality rates are likely multifactorial and poorly understood.6
From 1999 to 2016, the Centers for Disease Control and Prevention (CDC) observed an average annual increase of 1.1% in the uterine cancer mortality rate.7 In addition, stark racial disparities in uterine cancer mortality rates were reported, consistent with prior literature.4,7,8 Between 1999 and 2016, the annual increase in mortality rates among non-Hispanic Black, Hispanic, and non-Hispanic Asian or Pacific Islander women was higher than in non-Hispanic White women (1.5%, 1.7%, and 2.5%, respectively, vs 1.0%).7 Moreover, non-Hispanic Black women experience uterine cancer mortality at a twofold greater rate compared with any other racial or ethnic group.8
Although uterine cancer mortality rates by race and ethnicity are well documented, the effect of the intersection of race and ethnicity and age on uterine cancer mortality remains unclear. These prior studies are also limited by a lack of analysis of trends over an extended period. In this report, we analyzed data over a 50-year period to explore the disparities in uterine cancer mortality in the United States with an emphasis on the intersection of age and race and ethnicity.
METHODS
Data on mortality attributable to uterine cancer from 1969 to 2018 were obtained from the National Center for Health Statistics (NCHS). The NCHS, part of the CDC, is a U.S. federal statistical agency that creates a publicly accessible database containing an extensive array of deidentified health data and statistical information. The NCHS collects data from birth certificates, death certificates, patient medical records, health care facilities, standardized physicals, laboratory tests, and patient interviews. These data collected over 50 years allow the examination of variations in trends over time. Without this extensive time period, it would not be possible to evaluate the extensive positive and negative trends over time. The cohort was divided by six mutually exclusive age groups and four mutually exclusive racial and ethnic groups. Age groups were 0–29 years, 30–39, 40–49, 50–59, 60–69, and 70 or older. Age and race data were self-reported. Racial and ethnic groups were non-Hispanic Black, non-Hispanic White, Hispanic, and non-Hispanic Asian Pacific Islander. Race and age are included because we wanted to be comprehensive regarding the demographic variables that were included in the National Cancer Database and BRFSS (Behavioral Risk Factor Surveillance System) data sets.
BRFSS data from 2001 to 2018 were used to estimate hysterectomy and pregnancy prevalence within the cohort.9 We incorporated the pregnancy prevalence into our analysis to be comprehensive and to allow our data to accurately reflect clinical trial enrollment criteria and data. BRFSS is a publicly accessible national database sponsored primarily by the CDC that contains self-reported health data ranging from chronic conditions to risk behaviors and preventive service utilization. BRFSS collects data through random digit dialing telephone surveys and completes more than 400,000 interviews annually. The hysterectomy rate was assumed to be zero for women aged younger than 18 years. Pregnancy corrections were done only among women aged 20–45 years because BRFSS records pregnancy data only among that age group. Data from the National Cancer Database were additionally used to account for uterine cancer treatment–related hysterectomies by year and race and ethnicity. The National Cancer Database is a nationwide clinical oncology database supervised jointly by the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The National Cancer Database collects oncology hospital registry data from more than 1,500 facilities accredited by the Commission on Cancer. These estimates were used to adjust 2001–2018 mortality rates by hysterectomy and pregnancy, thereby correcting the specific population at risk. Because all data used in this study were deidentified, this study was exempt from IRB approval.
Overall uterine cancer mortality trends were determined with data from 1969 to 2018. Uterine cancer mortality trends by age and race were determined with data from 2001 to 2018 because data before 2001 did not report information on age or ethnicity. Average annual percent changes were used to quantify changes in mortality rates from 1969 to 2018 and were calculated with Joinpoint regression (Joinpoint 4.9.0.0), which allowed different slopes for four periods (1969–1989, 1989–1997, 1997–2008, and 2008–2018). To determine whether the average annual percent change was statistically significant, we used a two-sided t test for zero Joinpoints and a two-sided z test for one or more Joinpoints. Annual mortality rates per 100,000 women were age-adjusted to the 2000 U.S. standard population. Changes in uterine cancer mortality rates were considered increased if the average annual percent change was greater than 0 and decreased if the average annual percent change was less than 0. P<.05 was considered statistically significant using R 4.2.1.
RESULTS
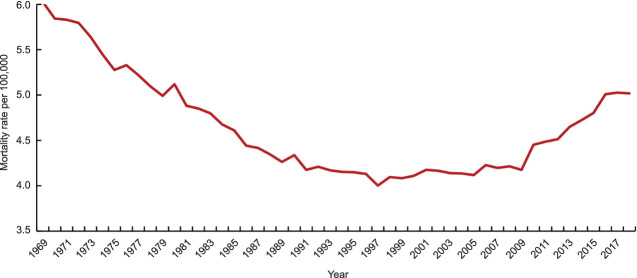
From 1969 to 1997, the mortality rate from uterine cancer decreased by 1.5-fold (6.03–4.00/100,000). The largest average annual decrease was seen between 1969 and 1989 at 1.66% (P<.001). However, the mortality rate subsequently increased by 1.25-fold from 1997 to 2018 (4.00–5.02/100,000). The highest increase rate began in 2008, with an average annual increase of 1.94% (P<.001) from 2008 to 2018 (Fig. 1).
Fig. 1. Uterine cancer mortality trend over time, 1969–2018.
Somasegar. 50-Year Trends in U.S. Uterine Cancer Mortality. Obstet Gynecol 2023.
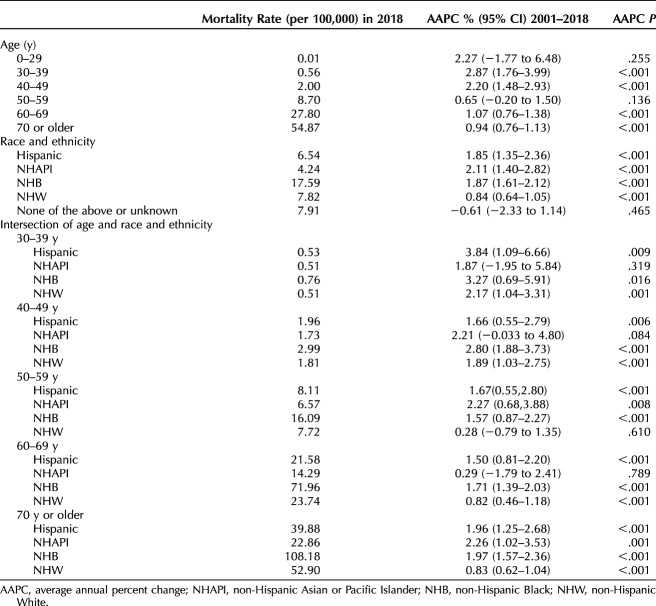
From 2001 to 2018, data on race and ethnicity and age were available for 843,118 women (Table 1). The mortality rate was directly proportional to age, with older women having higher rates of uterine cancer mortality than younger women. Compared with women aged 50–59 years, those aged 60–69 years and 70 years or older had threefold and sixfold higher mortality rates in 2018, respectively (8.7/100,000 vs 27.8/100,000 vs 54.87/100,000). Women aged 50–59 years, 60–69, and 70 or older demonstrated annual increases in mortality of 0.65% (P=.136), 1.07% (P<.001), and 0.94% (P<.001), respectively. The uterine cancer mortality rate rose from 2001 to 2018 across all age groups after adjustment for hysterectomy and pregnancy. However, the highest average annual percent increase in mortality was seen among women aged younger than 50 years. Women aged 0–29 years, 30–39, and 40–49 had annual increases in mortality of 2.27% (P=.255), 2.87% (P<.001), and 2.20% (P<.001), respectively (Table 2).
Table 1.
Demographic Characteristics, 2001–2018, National Center for Health Statistics
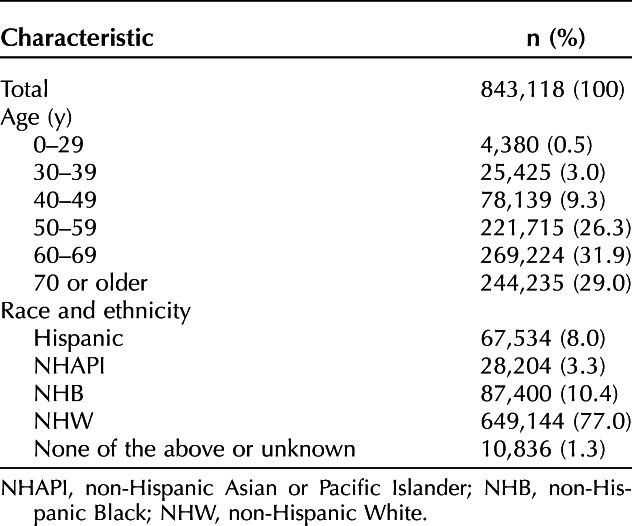
Table 2.
Uterine Cancer Mortality Rate in 2018 and Average Annual Percent Change by Age and Race from 2001 to 2018
From 2001 to 2018, uterine cancer mortality rates increased across all four racial and ethnic groups. Compared with non-Hispanic White, Hispanic, and non-Hispanic Asian or Pacific Islander women, non-Hispanic Black women had twofold, threefold, and fourfold higher mortality rates, respectively (7.82/100,000 vs 6.54/100,000 vs 4.24/100,000 vs 17.59/100,000) (Fig. 2). Although uterine cancer mortality rates were lowest among non-Hispanic Asian or Pacific Islander women in 2018, this group had the highest average annual percent increase from 2001 to 2018. Specifically, non-Hispanic Asian or Pacific Islander women had an annual increase of 2.11% (P<.001) from 2001 to 2008 compared with 1.85% (P<.001), 1.87% (P<.001), and 0.84% (P<.001) for Hispanic, non-Hispanic Black, and non-Hispanic White women, respectively (Table 2).
Fig. 2. Uterine cancer mortality rate by race and ethnicity, 2001–2018.
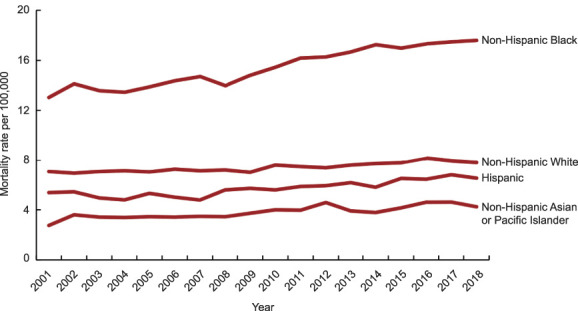
Somasegar. 50-Year Trends in U.S. Uterine Cancer Mortality. Obstet Gynecol 2023.
In an intersectionality analysis by age and race and ethnicity, uterine cancer mortality was highest in older non-Hispanic Black women (Fig. 3). In 2018, the highest mortality rate was observed in non-Hispanic Black women aged 70 years or older (108.18/100,000), twofold that of non-Hispanic White women (52.90/100,000) of that age. The second-highest mortality rate was observed in non-Hispanic Black women aged 60–69 years, who had a threefold higher mortality rate than non-Hispanic White women of the same age (71.96/100,000 vs 23.74/100,000) (Table 2). Although overall uterine cancer mortality rates were lower among women aged younger than 50 years, non-Hispanic Black and Hispanic women aged 30–39 years had greater increases in uterine cancer mortality compared with non-Hispanic White women. From 2001 to 2018, 30–39-year-old non-Hispanic Black and Hispanic women had annual increases of 3.27% (P=.016) and 3.84% (P=.009) compared with 2.17% (P=.001) among non-Hispanic White women (Fig. 4).
Fig. 3. Uterine cancer mortality rate by age and race and ethnicity, 2018. NHAPI, non-Hispanic Asian or Pacific Islander; NHB, non-Hispanic Black; NHW, non-Hispanic White.
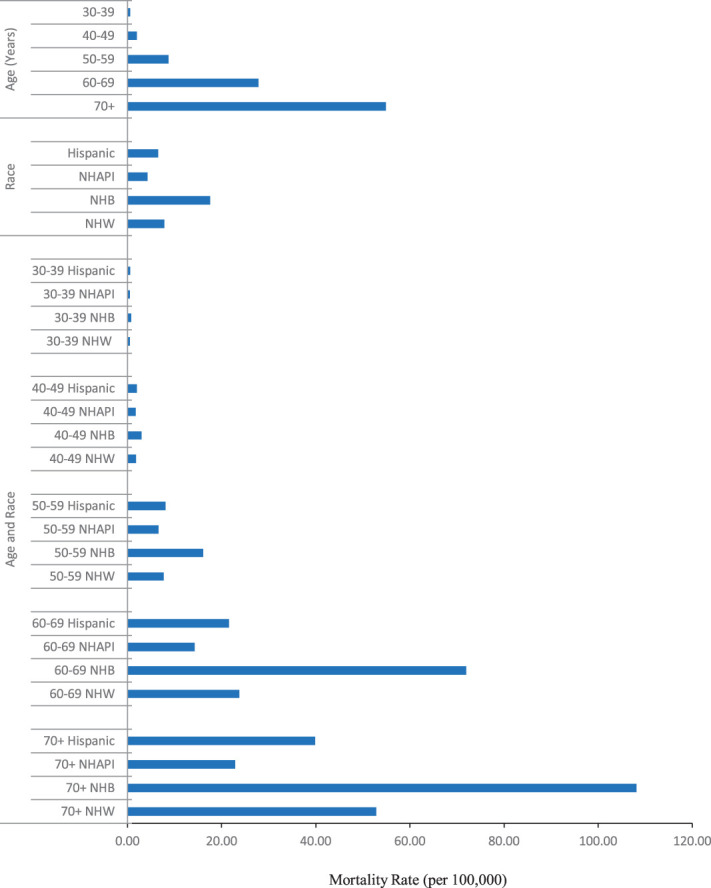
Somasegar. 50-Year Trends in U.S. Uterine Cancer Mortality. Obstet Gynecol 2023.
Fig. 4. Uterine cancer average annual percent change (AAPC) by age and race and ethnicity, 2001–2018. *P<.001. NHAPI, non-Hispanic Asian or Pacific Islander; NHB, non-Hispanic Black; NHW, non-Hispanic White.
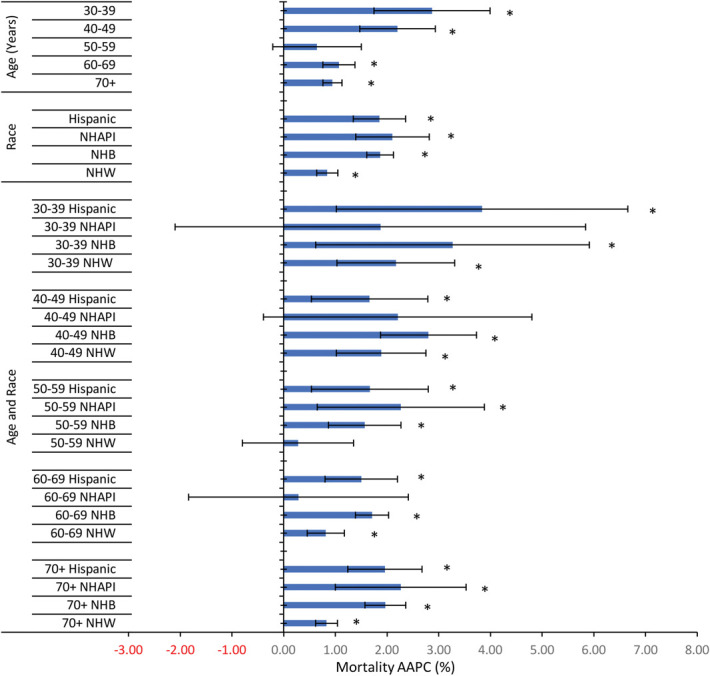
Somasegar. 50-Year Trends in U.S. Uterine Cancer Mortality. Obstet Gynecol 2023.
DISCUSSION
In this analysis, we found that uterine cancer mortality rates increased from 1997 to 2018, with mortality rates being highest among older non-Hispanic Black women aged 70 years or older. In addition, mortality rates are increasing most rapidly among younger racial and ethnic minority groups, specifically non-Hispanic Black and Hispanic women, compared with non-Hispanic White women. Although previous data have shown an average annual percent change of 1.1% for uterine cancer mortality among non-Hispanic Black, our analysis over a longer period shows a greater average annual percent change of 1.87%.7 This rise in uterine cancer mortality and the racial disparities enmeshed in it have been similarly documented in other studies.3–5,8 These trends are in direct contrast to the decreases in mortality rates that have been observed in many other cancer types, including lung cancer and colorectal cancers.1
Uterine cancer mortality is higher in older women compared with younger women. Several reasons contribute to this. First, older women are more likely to present with more aggressive pathologic features compared with younger women. Specifically, older women (70 years or older) are about two times more likely to present with advanced disease compared with younger patients (39% vs 19%).10 Older women are also less likely to receive comprehensive surgical care for their cancer and are more likely have treatment-related complications. Physician bias is also thought to contribute to lower rates of surgical care among older patients.11
Women aged younger than 50 years had the highest average annual percent increase in mortality. Several studies have found a similar increase in the incidence of early-onset uterine cancer, particularly among non-Hispanic Black and Hispanic women.12–15 Guo et al15 reported a notable annual percent increase in uterine cancer in younger age groups between 2001 and 2017: 3.6% among 20–29-year-olds and 3% among 30–39-year-olds. This increase in early-onset uterine cancer is also seen in other obesity-related cancers, including colorectal, multiple myeloma, gallbladder, kidney, and pancreatic cancers.16 This points to a possible association between these trends and the rapid increase in the prevalence of obesity in younger age groups. In 2017–2018, the highest obesity rates in the country were seen among patients aged 40–59 years (11.5%), followed by those aged 20–39 years (9.1%) and then those aged 60 years or older (5.8%).17
The racial disparities in uterine cancer mortality that we found in our analysis have been similarly documented in the Surveillance, Epidemiology, and End Results database.9 One analysis showed overall significantly lower survival rates among non-Hispanic Black women compared with non-Hispanic White, Hispanic, and non-Hispanic Asian or Pacific Islander women, regardless of histology and stage (5-year relative survival 63.2% vs 86.1%, 81.4%, and 83.7%, respectively). Proposed factors that contribute to such disproportionately elevated mortality rates in racial and ethnic minority groups (particularly non-Hispanic Black women) fall into systemic, practitioner, and patient categories.8 Systemic factors relating to structural racism create differences in racialized individuals' ability to receive quality health care services. Practitioner-level factors involve issues with interpersonal communication, bias, and discrimination that can affect clinical decision making and reduce the likelihood of receiving guideline-concordant care.8 Those barriers to optimal care are thought to contribute to the well-documented differences in endometrial cancer treatment that non-Hispanic Black and Hispanic women receive. Several studies have documented non-Hispanic Black and Hispanic women's reduced likelihood of receiving definitive surgical treatment, optimal surgical procedures, sufficient lymph node sampling, and lymphadenectomy in the setting of their uterine cancer.18–20 In addition, even though non-Hispanic Black women are more likely than non-Hispanic White women to receive care at high-volume surgical centers, they continue to experience worse outcomes than White women in those facilities.21
Among several considerations, patient-level factors including molecular or genetic, cultural, educational, and socioeconomic differences. Her2/neu expression and p53 mutations are found more frequently in non-Hispanic Black patients with endometrial cancer and are associated with poor prognosis. Moreover, somatic mutations in DNA mismatch repair genes are reported more frequently in non-Hispanic Asian or Pacific Islander women.22 In addition, socioeconomic deprivation and having no or publicly funded insurance, both of which are more prevalent in racial and ethnic minority groups, are associated with worse survival from uterine cancer.8 Non-Hispanic Black women also have higher exposure to toxic chemicals found in hair-straightening products.
The highest mortality rate seen among older non-Hispanic Black patients sheds light on the complex interplay between different social systems that exaggerate inequities and health disparities. For example, an older non-Hispanic Black patient's age and race put her at higher risk of not receiving definitive surgical treatment for uterine cancer. The actual combined effect of the interactions between race and ethnicity and age-associated risk factors described previously remain poorly understood. In addition, part of the cause of higher uterine cancer mortality rates among older non-Hispanic Black women and younger minority women could be attributed in part to the higher rates of obesity in these populations.12–15,23 In 2017–2018, 39.8% of non-Hispanic White women, 43.7% of Hispanic women, 56.9% of non-Hispanic Black women, and 17.2% of non-Hispanic Asian or Pacific Islander women had obesity.24 The higher levels of obesity seen among racial and ethnic minorities have been attributed to a wide variety of social and structural factors.25 Those include an increased likelihood of being surrounded by food deserts and higher levels of food insecurity, poor neighborhood walkability scores, and high crime rates, as well as elevated chronic stress levels.25
The increase in uterine cancer mortality rates following a preceding period of declining rates from 1969 to 1997 might be attributed to the lower use of estrogen plus progesterone hormonal therapy, the decrease in the rates of hysterectomies, and the rise in obesity levels.26–28 In addition, alternative treatments of common benign gynecologic conditions that have previously been treated with hysterectomy led to a decline in the hysterectomy rate that started in the 1980s.27 Moreover, endometrial cancers make up most uterine cancers, and as previously mentioned, the main risk factor for endometrial cancers is elevated body weight.23 Women who have overweight or obesity are two to four times as likely to develop endometrial cancer as women with lower body mass index.28 Data from the National Cancer Institute and CDC attributed 60.3% of corpus uteri tumors to obesity, further supporting uterine cancer being one of the cancers most strongly associated with obesity.28,29
In addition, given the strong association between obesity and uterine cancer, interventions aimed at weight reduction are critical. Several studies point to a reduction in cancer risk and mortality associated with weight loss.28–31 For example, bariatric surgery has been shown to significantly decrease the risk of uterine cancer (odds ratio 0.43, 95% CI 0.26–0.71, P=.010,).30,31 Equitable implementation of programs aimed at risk reduction through physical activity and access to healthy foods is essential and warrants national attention. Public policy efforts aimed at regulating access to processed foods and sugar-sweetened beverages can also help address the obesity epidemic.32 Such interventions, specifically when implemented among the target populations identified in this analysis, can result in reducing the disparities among uterine cancer mortality by age and race and ethnicity.
This is a large, nationally representative cohort study that evaluated uterine cancer trends by age and race and ethnicity, as well as the intersection of both age and race and ethnicity over a 50-year period. Despite our large sample size and correction for hysterectomy and pregnancy, there are important limitations to our analysis to consider. Reporting of race and ethnicity in the NCHS is based on data from medical records and death certificates, which may not be fully accurate. In addition, all histologic tumor types were grouped into a broad category, which can mask varying trends by tumor type because it is known that certain uterine cancers, such as sarcomas, are far more aggressive and associated with a worse prognosis than others, such as endometrioid adenocarcinomas. In our analysis, we did not adjust for potential changes in rates of hysterectomy and pregnancy during the study period because these changes were minor and we did not speculate that the results would significantly change. Finally, our data did not have information on age and race before 2001. Given this limitation of our database, we elected to use the Census data from 2000, which is roughly the median time point in our study, as a standard for comparison.
In conclusion, uterine cancer mortality has increased across all racial and ethnic and age groups from 2001 to 2018. This increase, however, has disproportionately affected non-Hispanic Black women across age groups. Here, we explored some of the complex systemic, social, environmental, and molecular factors that might be contributing to these disparities. However, additional studies are needed to further explore the complex interplay of the multifactorial reasons behind these widening gaps to inform targeted prevention efforts.
Footnotes
Financial Disclosure John Chan reports receiving funding from AstraZeneca, Glaxosmithkline, Immunogen, and Myriad for his efforts in this study. He also reports receiving direct payments from AstraZeneca, Eisai, Glaxosmithkline, Genmab/Seagen, Agenus, Immunogen, Merck, Mersana, MTTI, Myriad, and Roche. He has also received research funding from the Denis Cobb Hale Chair, the Angela Wang Johnson Fund, and the Fisher Family Funds. The authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D350.
REFERENCES
- 1.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer 2018;124:2785–800. doi: 10.1002/cncr.31551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. doi: 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 3.Temkin SM, Kohn EC, Penberthy L, Cronin KA, Rubinsak L, Dickie LA, et al. Hysterectomy-corrected rates of endometrial cancer among women younger than age 50 in the United States. Cancer Causes Control 2018;29:427–33. doi: 10.1007/s10552-018-1018-z [DOI] [PubMed] [Google Scholar]
- 4.Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/Black people 2022. CA Cancer J Clin 2022;72:202–29. doi: 10.3322/caac.21718 [DOI] [PubMed] [Google Scholar]
- 5.Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med 2020;383:2053–64. doi: 10.1056/nejmra1514010 [DOI] [PubMed] [Google Scholar]
- 6.Eakin CM, Liao CI, Salani R, Cohen JG, Kapp DS, Chan JK. The association of obesity with type I uterine cancer: is this an oversimplification? Am J Obstet Gynecol 2022;227:538–9. doi: 10.1016/j.ajog.2022.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henley SJ, Miller JW, Dowling NF, Benard VB, Richardson LC. Uterine cancer incidence and mortality–United States, 1999-2016. MMWR Morb Mortal Wkly Rep 2018;67:1333–8. doi: 10.15585/mmwr.mm6748a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whetstone S, Burke W, Sheth SS, Brooks R, Cavens A, Huber-Keener K, et al. Health disparities in uterine cancer: report from the Uterine Cancer Evidence Review Conference. Obstet Gynecol 2022;139:645–59. doi: 10.1097/AOG.0000000000004710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-corrected uterine corpus cancer incidence trends and differences in relative survival reveal racial disparities and rising rates of nonendometrioid cancers. J Clin Oncol 2019;37:1895–908. doi: 10.1200/JCO.19.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaknin Z, Perri T, Lau S, Deland C, Drummond N, Rosberger Z, et al. Outcome and quality of life in a prospective cohort of the first 100 robotic surgeries for endometrial cancer, with focus on elderly patients. Int J Gynecol Cancer 2010;20:1367–73. doi: 10.1111/IGC.0b013e3181f2950a [DOI] [PubMed] [Google Scholar]
- 11.Duska L, Shahrokni A, Powell M. Treatment of older women with endometrial cancer: improving outcomes with personalized care. Am Soc Clin Oncol Educ Book 2016;35:164–74. doi: 10.1200/edbk_158668 [DOI] [PubMed] [Google Scholar]
- 12.Ugai T, Sasamoto N, Lee HY, Ando M, Song M, Tamimi RM, et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol 2022;19:656–73. doi: 10.1038/s41571-022-00672-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Habeshian TS, Zhang J, Peeri NC, Du M, De Vivo I, et al. Differential trends in rising endometrial cancer incidence by age, race, and ethnicity. JNCI Cancer Spectr 2023;7:pkad001. doi: 10.1093/jncics/pkad001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott AR, Stoltzfus KC, Tchelebi LT, Trifiletti DM, Lehrer EJ, Rao P, et al. Trends in cancer incidence in US adolescents and young adults, 1973-2015. JAMA Netw Open 2020;3:e2027738. doi: 10.1001/jamanetworkopen.2020.27738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo F, Levine L, Berenson A. Trends in the incidence of endometrial cancer among young women in the United States, 2001 to 2017. J Clin Oncol 2021;39:5578. doi: 10.1200/jco.2021.39.15_suppl.5578 [DOI] [Google Scholar]
- 16.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 2019;4:e137–47. doi: 10.1016/S2468-2667(18)30267-6 [DOI] [PubMed] [Google Scholar]
- 17.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief 2020:1–8. [PubMed] [Google Scholar]
- 18.Baskovic M, Lichtensztajn DY, Nguyen T, Karam A, English DP. Racial disparities in outcomes for high-grade uterine cancer: a California cancer registry study. Cancer Med 2018;7:4485–95. doi: 10.1002/cam4.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol Oncol 2013;130:652–9. doi: 10.1016/j.ygyno.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bregar AJ, Alejandro Rauh-Hain J, Spencer R, Clemmer JT, Schorge JO, Rice LW, et al. Disparities in receipt of care for high-grade endometrial cancer: a National Cancer Data Base analysis. Gynecol Oncol 2017;145:114–21. doi: 10.1016/j.ygyno.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 21.Armstrong K, Randall TC, Polsky D, Moye E, Silber JH. Racial differences in surgeons and hospitals for endometrial cancer treatment. Med Care 2011;49:207–14. doi: 10.1097/MLR.0b013e3182019123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guttery DS, Blighe K, Polymeros K, Symonds RP, Macip S, Moss EL. Racial differences in endometrial cancer molecular portraits in the Cancer Genome Atlas. Oncotarget 2018;9:17093–103. doi: 10.18632/oncotarget.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Liu H, Yang S, Zhang J, Qian L, Chen X. Overweight, obesity and endometrial cancer risk: results from a systematic review and meta-analysis. Int J Biol Markers 2014;29:e21–9. doi: 10.5301/jbm.5000047 [DOI] [PubMed] [Google Scholar]
- 24.Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. Accessed February 17, 2023. https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm
- 25.Lee A, Cardel M, Donahoo WT. Social and environmental factors influencing obesity In: Endotext. MDText.com, Inc; 2000. [Google Scholar]
- 26.Constantine GD, Kessler G, Graham S, Goldstein SR. Increased incidence of endometrial cancer following the Women's Health Initiative: an assessment of risk factors. J Womens Health 2019;28:237–43. doi: 10.1089/jwh.2018.6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol 2016;6:89. doi: 10.3389/fonc.2016.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol 2011;205:518–25. doi: 10.1016/j.ajog.2011.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31–54. doi: 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Luo Y, Dai H, Deng Z. Effects of bariatric surgery on cancer risk: evidence from meta-analysis. Obes Surg 2020;30:1265–72. doi: 10.1007/s11695-019-04368-4 [DOI] [PubMed] [Google Scholar]
- 31.Tsui ST, Yang J, Zhang X, Spaniolas K, Kim S, Griffin T, et al. The risk of female-specific cancer after bariatric surgery in the state of New York. Surg Endosc 2021;35:4267–74. doi: 10.1007/s00464-020-07915-8 [DOI] [PubMed] [Google Scholar]
- 32.Rogers NT, Cummins S, Forde H, Jones CP, Mytton O, Rutter H, et al. Associations between trajectories of obesity prevalence in English primary school children and the UK soft drinks industry levy: an interrupted time series analysis of surveillance data. PLoS Med 2023;20:e1004160. doi: 10.1371/journal.pmed.1004160 [DOI] [PMC free article] [PubMed] [Google Scholar]