Abstract
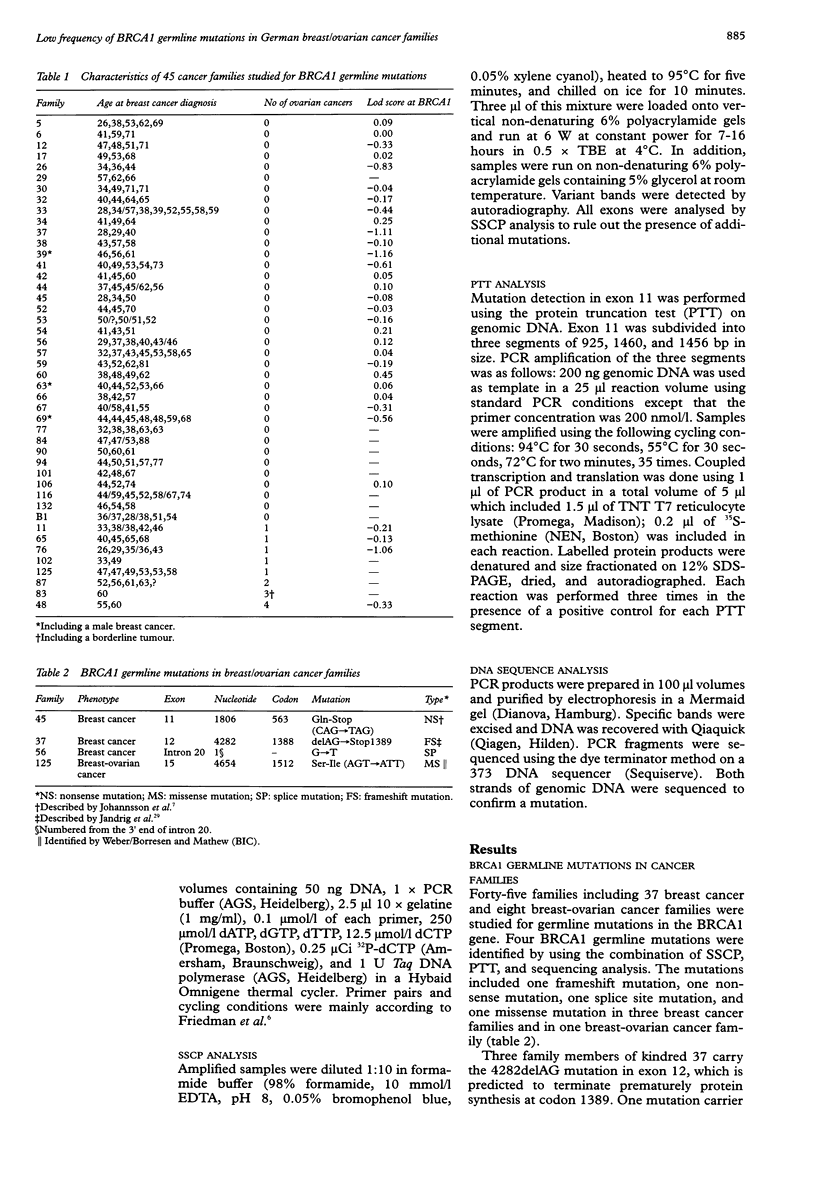
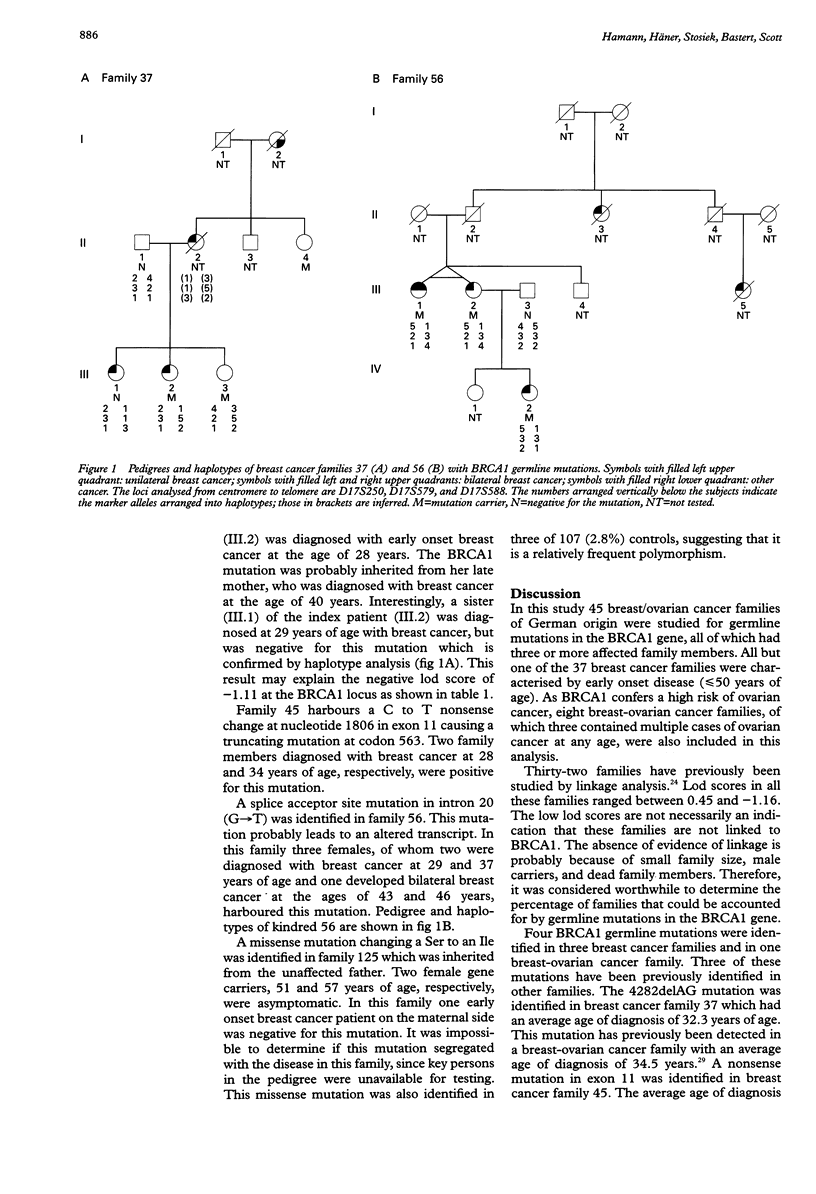
In this study we investigated 45 German breast/ovarian cancer families for germline mutations in the BRCA1 gene. We identified four germline mutations in three breast cancer families and in one breast-ovarian cancer family. among these were one frameshift mutation, one nonsense mutation, one novel splice site mutation, and one missense mutation. The missense mutation was also found in 2.8% of the general population, suggesting that it is not disease associated. The average age of disease onset in those families harbouring causative mutations was between 32.3 and 37.4 years, whereas the family harbouring the missense mutation had an average age of onset of 51.2 years. These findings show that BRCA1 is implicated in a small fraction of breast/ovarian cancer families suggesting the involvement of another susceptibility gene(s).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castilla L. H., Couch F. J., Erdos M. R., Hoskins K. F., Calzone K., Garber J. E., Boyd J., Lubin M. B., Deshano M. L., Brody L. C. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat Genet. 1994 Dec;8(4):387–391. doi: 10.1038/ng1294-387. [DOI] [PubMed] [Google Scholar]
- Cornelis R. S., Neuhausen S. L., Johansson O., Arason A., Kelsell D., Ponder B. A., Tonin P., Hamann U., Lindblom A., Lalle P. High allele loss rates at 17q12-q21 in breast and ovarian tumors from BRCAl-linked families. The Breast Cancer Linkage Consortium. Genes Chromosomes Cancer. 1995 Jul;13(3):203–210. doi: 10.1002/gcc.2870130310. [DOI] [PubMed] [Google Scholar]
- Easton D. F., Bishop D. T., Ford D., Crockford G. P. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993 Apr;52(4):678–701. [PMC free article] [PubMed] [Google Scholar]
- Ford D., Easton D. F., Bishop D. T., Narod S. A., Goldgar D. E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994 Mar 19;343(8899):692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Friedman L. S., Ostermeyer E. A., Szabo C. I., Dowd P., Lynch E. D., Rowell S. E., King M. C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994 Dec;8(4):399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- Futreal P. A., Liu Q., Shattuck-Eidens D., Cochran C., Harshman K., Tavtigian S., Bennett L. M., Haugen-Strano A., Swensen J., Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994 Oct 7;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Goldgar D. E., Fields P., Lewis C. M., Tran T. D., Cannon-Albright L. A., Ward J. H., Swensen J., Skolnick M. H. A large kindred with 17q-linked breast and ovarian cancer: genetic, phenotypic, and genealogical analysis. J Natl Cancer Inst. 1994 Feb 2;86(3):200–209. doi: 10.1093/jnci/86.3.200. [DOI] [PubMed] [Google Scholar]
- Gudas J. M., Nguyen H., Li T., Cowan K. H. Hormone-dependent regulation of BRCA1 in human breast cancer cells. Cancer Res. 1995 Oct 15;55(20):4561–4565. [PubMed] [Google Scholar]
- Hall J. M., Friedman L., Guenther C., Lee M. K., Weber J. L., Black D. M., King M. C. Closing in on a breast cancer gene on chromosome 17q. Am J Hum Genet. 1992 Jun;50(6):1235–1242. [PMC free article] [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Hamann U., Becher H., Zimmermann T., Pella K., Bastert G., Chang-Claude J. German family study on hereditary breast-ovarian cancer. J Med Genet. 1996 Aug;33(8):633–635. doi: 10.1136/jmg.33.8.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann U., Brauch H., Garvin A. M., Bastert G., Scott R. J. German family study on hereditary breast and/or ovarian cancer: germline mutation analysis of the BRCA1 gene. Genes Chromosomes Cancer. 1997 Feb;18(2):126–132. [PubMed] [Google Scholar]
- Hogervorst F. B., Cornelis R. S., Bout M., van Vliet M., Oosterwijk J. C., Olmer R., Bakker B., Klijn J. G., Vasen H. F., Meijers-Heijboer H. Rapid detection of BRCA1 mutations by the protein truncation test. Nat Genet. 1995 Jun;10(2):208–212. doi: 10.1038/ng0695-208. [DOI] [PubMed] [Google Scholar]
- Hosking L., Trowsdale J., Nicolai H., Solomon E., Foulkes W., Stamp G., Signer E., Jeffreys A. A somatic BRCA1 mutation in an ovarian tumour. Nat Genet. 1995 Apr;9(4):343–344. doi: 10.1038/ng0495-343. [DOI] [PubMed] [Google Scholar]
- Jandrig B., Grade K., Seitz S., Waindzoch B., Müller M., Bender E., Nothnagel A., Rohde K., Schlag P. M., Kath R. BRCA1 mutations in German breast-cancer families. Int J Cancer. 1996 Oct 9;68(2):188–192. doi: 10.1002/(SICI)1097-0215(19961009)68:2<188::AID-IJC8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Johannsson O., Ostermeyer E. A., Håkansson S., Friedman L. S., Johansson U., Sellberg G., Brøndum-Nielsen K., Sele V., Olsson H., King M. C. Founding BRCA1 mutations in hereditary breast and ovarian cancer in southern Sweden. Am J Hum Genet. 1996 Mar;58(3):441–450. [PMC free article] [PubMed] [Google Scholar]
- Lane T. F., Deng C., Elson A., Lyu M. S., Kozak C. A., Leder P. Expression of Brca1 is associated with terminal differentiation of ectodermally and mesodermally derived tissues in mice. Genes Dev. 1995 Nov 1;9(21):2712–2722. doi: 10.1101/gad.9.21.2712. [DOI] [PubMed] [Google Scholar]
- Marquis S. T., Rajan J. V., Wynshaw-Boris A., Xu J., Yin G. Y., Abel K. J., Weber B. L., Chodosh L. A. The developmental pattern of Brca1 expression implies a role in differentiation of the breast and other tissues. Nat Genet. 1995 Sep;11(1):17–26. doi: 10.1038/ng0995-17. [DOI] [PubMed] [Google Scholar]
- Merajver S. D., Pham T. M., Caduff R. F., Chen M., Poy E. L., Cooney K. A., Weber B. L., Collins F. S., Johnston C., Frank T. S. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995 Apr;9(4):439–443. doi: 10.1038/ng0495-439. [DOI] [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Plummer S. J., Anton-Culver H., Webster L., Noble B., Liao S., Kennedy A., Belinson J., Casey G. Detection of BRCA1 mutations by the protein truncation test. Hum Mol Genet. 1995 Oct;4(10):1989–1991. doi: 10.1093/hmg/4.10.1989. [DOI] [PubMed] [Google Scholar]
- Serova O., Montagna M., Torchard D., Narod S. A., Tonin P., Sylla B., Lynch H. T., Feunteun J., Lenoir G. M. A high incidence of BRCA1 mutations in 20 breast-ovarian cancer families. Am J Hum Genet. 1996 Jan;58(1):42–51. [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Eidens D., McClure M., Simard J., Labrie F., Narod S., Couch F., Hoskins K., Weber B., Castilla L., Erdos M. A collaborative survey of 80 mutations in the BRCA1 breast and ovarian cancer susceptibility gene. Implications for presymptomatic testing and screening. JAMA. 1995 Feb 15;273(7):535–541. [PubMed] [Google Scholar]
- Simard J., Tonin P., Durocher F., Morgan K., Rommens J., Gingras S., Samson C., Leblanc J. F., Bélanger C., Dion F. Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet. 1994 Dec;8(4):392–398. doi: 10.1038/ng1294-392. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Easton D. F., Evans D. G., Ponder B. A. Allele losses in the region 17q12-21 in familial breast and ovarian cancer involve the wild-type chromosome. Nat Genet. 1992 Oct;2(2):128–131. doi: 10.1038/ng1092-128. [DOI] [PubMed] [Google Scholar]
- Szabo C. I., Wagner L. A., Francisco L. V., Roach J. C., Argonza R., King M. C., Ostrander E. A. Human, canine and murine BRCA1 genes: sequence comparison among species. Hum Mol Genet. 1996 Sep;5(9):1289–1298. doi: 10.1093/hmg/5.9.1289. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Kwitek A. E., May P. E., Wallace M. R., Collins F. S., Ledbetter D. H. Dinucleotide repeat polymorphisms at the D17S250 and D17S261 loci. Nucleic Acids Res. 1990 Aug 11;18(15):4640–4640. [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Wu L. C., Wang Z. W., Tsan J. T., Spillman M. A., Phung A., Xu X. L., Yang M. C., Hwang L. Y., Bowcock A. M., Baer R. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996 Dec;14(4):430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]


