Abstract
Coral reef ecosystems are being fundamentally restructured by local human impacts and climate-driven marine heatwaves that trigger mass coral bleaching and mortality1. Reducing local impacts can increase reef resistance to and recovery from bleaching2. However, resource managers lack clear advice on targeted actions that best support coral reefs under climate change3 and sector-based governance means most land- and sea-based management efforts remain siloed4. Here we combine surveys of reef change with a unique 20-year time series of land–sea human impacts that encompassed an unprecedented marine heatwave in Hawai‘i. Reefs with increased herbivorous fish populations and reduced land-based impacts, such as wastewater pollution and urban runoff, had positive coral cover trajectories predisturbance. These reefs also experienced a modest reduction in coral mortality following severe heat stress compared to reefs with reduced fish populations and enhanced land-based impacts. Scenario modelling indicated that simultaneously reducing land–sea human impacts results in a three- to sixfold greater probability of a reef having high reef-builder cover four years postdisturbance than if either occurred in isolation. International efforts to protect 30% of Earth’s land and ocean ecosystems by 2030 are underway5. Our results reveal that integrated land–sea management could help achieve coastal ocean conservation goals and provide coral reefs with the best opportunity to persist in our changing climate.
Subject terms: Climate-change ecology, Community ecology
Surveys of reef change are combined with a unique 20-year time series of land–sea human impacts and the results show that integrated land–sea management could help achieve coastal ocean conservation goals and provide coral reefs with the best opportunity to persist in our changing climate.
Main
Coastal areas contain some of the most biologically diverse and productive marine ecosystems on Earth6. But with four times the population density living within 20 km of the ocean compared to the rest of the world7, direct human impacts on local scales are fundamentally restructuring these important marine communities8. Coastal areas are also affected by stronger and more frequent disturbances fuelled by human-induced climate change9. These human stressors are especially acute on tropical coral reefs where up to 90% of the local population live along the shoreline10. Land-based stressors, such as wastewater pollution, combine with sea-based stressors, such as overfishing, to disrupt natural ecological feedbacks on reefs11. Corals are further stressed by prolonged periods of anomalously warm ocean temperatures, known as marine heatwaves12, that can cause mass coral bleaching13 and mortality and fundamentally transform reef assemblages14,15.
Reducing human impacts on local scales to maintain ecosystem integrity has been the guiding model of coral reef conservation for decades3. Its importance was established in the indigenous stewardship of island ecosystems, which used a decentralized and integrated resource management strategy that extended from the mountains to the sea16,17. By contrast, contemporary centralized governance means most terrestrial and ocean management efforts remain siloed4,17,18. As a result, whereas local resource managers have aspired to an integrated land–sea approach19, evidence of its efficacy above either approach in isolation remains wanting and difficult to test. Detecting conservation benefits in highly dynamic ecosystems is challenging20, but recent studies have identified salient connections between local conditions and coral reef resistance to and recovery potential following mass bleaching2,11,21–23. Managers therefore require unambiguous targets for the combination of land–sea human impacts they should mitigate to support coral reef persistence under climate change. Hampering these efforts are a lack of spatially resolved data on local drivers of coral reef ecosystems over time. Researchers are often forced to use proxies such as population density24,25 and reef accessibility26, or composite indices such as ‘water quality’11 that can be affected by anything from deforestation27 to aquaculture28. Such proxies do not identify the policy levers local resource managers can pull and are less likely to result in management actions or successful conservation outcomes.
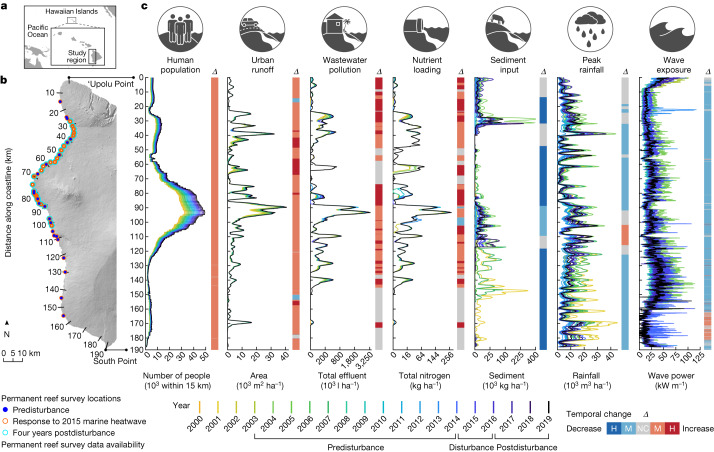
Here we present a unique 20-year time series of land–sea human impacts and environmental factors known to affect coral reef ecosystem processes across our study region in the Hawaiian Islands (Fig. 1a). Human factors include urban runoff, wastewater pollution, nutrient loading, sediment input and local restrictions on types of fishing gear. Environmental factors include peak and annual rainfall, wave exposure, variability in ocean temperatures and heat stress, irradiance and phytoplankton biomass. We also incorporate multiple fish biomass metrics that represent the critical role reef fish play in maintaining coral reef ecosystem function29–31 (see Extended Data Table 1 for a full list of factors). We combined this dataset with recurring, permanently marked and site-specific underwater survey data on coral reef benthic communities (Fig. 1b). Our study reefs spanned large spatiotemporal gradients in land–sea human impacts and environmental factors (Fig. 1c) that are comparable to coral reef ecosystems globally (Extended Data Fig. 1), and which experienced the most severe marine heatwave on record in the Hawaiian Islands (Extended Data Fig. 2). We quantified drivers of coral reef benthic change at the scale of individual reefs over 12 years before disturbance (2003–2014), during and immediately following the marine heatwave (2014–2016) and four years postdisturbance (2016–2019). Our findings show that simultaneously mitigating local human impacts on both land and sea supports positive coral cover trajectories in the absence of periodic acute disturbance, reduces coral loss during a marine heatwave and promotes coral reef persistence following severe heat stress.
Fig. 1. Select local land–sea human impacts and environmental factors on coral reefs in our study region in Hawai‘i.
a, Geographic location of the Hawaiian Islands. b, Study region with reef surveys shown for the following: reef trajectories predisturbance (n = 23; Fig. 2), coral response to the 2015 marine heatwave (n = 80; Fig. 3) and coral reefs four years postdisturbance (n = 55; Fig. 4). c, Spatial distribution in annual, high-resolution (100 m) data on local human impacts and environmental factors from 2000 to 2019 (coloured lines). The y axis represents distance along the coastline in kilometres from north to south along the study region in b. Vertical bar represents the change over time (Δ) for each 100 m section along the coast. A change over time is high (H, Δ ≥ 50%), moderate (M, 0 > Δ < 50%) or there is no change (NC, grey), with blue hues indicating decreases and red hues indicating increases. Change is based on the mean difference between the first 5 years (2000–2004) and the most recent 5 years (2015–2019) in the time series. This accounted for year-to-year variability in the episodic nature of factors such as wave exposure, rainfall and sediment input. A subset of factors is shown in c owing to space constraints. Additional factors (not shown) include annual rainfall, phytoplankton biomass, ocean temperature (mean and variability), heat stress, irradiance, fishing gear restrictions, depth and metrics of fish biomass. The distribution, change over time and variability of all factors are shown in Supplementary Fig. 1. See Extended Data Table 1 and Supplementary Information for detailed information on local land–sea human impacts and environmental factors.
Extended Data Table 1.
Local land-sea human impacts and environmental factors considered for our analyses
Extended Data Fig. 1. Comparison of human, environmental, and climate factors for reefs in Hawai‘i with coral reef ecosystems globally.
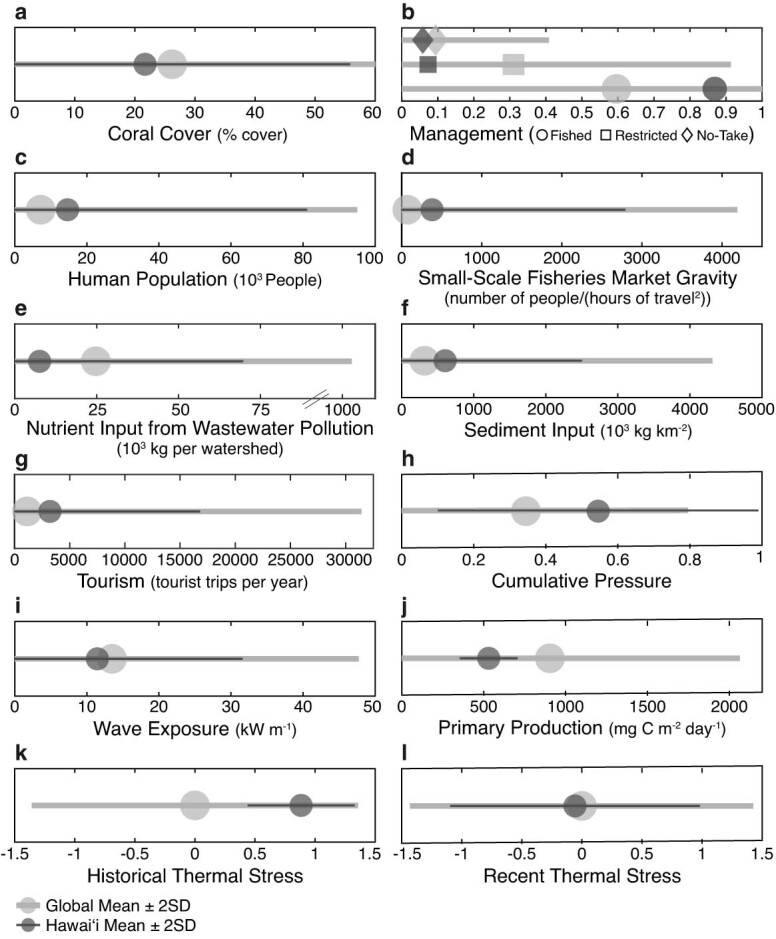
Dots represent global (light grey) and Hawai‘i (dark grey) mean values. Error bars represent the mean ± 2 standard deviation (SD). Factors presented are: a, Coral cover (per cent hard coral; global n = 2,584; Hawai‘i n = 137); b, Proportional reef area by country that is open (fished), gear restricted (restricted), or fully restricted (no-take) to fishing (sample number same as in a); c, Human population within 5 km in 2018 (global n = 54,596; Hawai‘i n = 199); d, Small-scale fisheries market gravity (number of people/(hours of travel)2 represents human use and fishing pressure related to the size and accessibility of coral reefs to nearby human settlements and markets59 (n = same as in c); e, Annual input of nitrogen (103 kg) per watershed from wastewater pollution on coral reefs (global n = 38,033; Hawai‘i n = 324); f, Sediment input (103 kg km−2) to coral reefs (n = same as in c); g, Annual number of tourist visits driven by coral reefs combining on-reef (e.g., recreational diving and snorkelling) and reef-adjacent (e.g., provision of calm waters, sand beaches, views, and seafood) aspects (sample number same as in c); h, Cumulative pressure score from stressors to coral reefs per 5 km reef containing pixel (unitless); i, Mean wave energy, or wave power (kW m−1), from 1979–2009 (n = same as in a); j, Mean primary productivity (mg C m−2 day−1) between 2003–2013 (n = same as in a); k-l, Unitless metric of (k) historical (1985–2017) and (l) recent (2014–2017) thermal stress on coral reefs, whereby positive values represent more desirable (i.e., less thermal stress) over the respective time frames105 (n = same as in c). The mean for reefs in Hawai‘i falls within 2 SD of the global mean for all factors. Data are from the following sources: a,b,i,j from106 e from48; c,d,f,g,h,k, from107. Factors presented here were obtained from global datasets and will differ from those presented within our present study owing to the methodological differences as well as differences in their spatiotemporal extent and resolution.
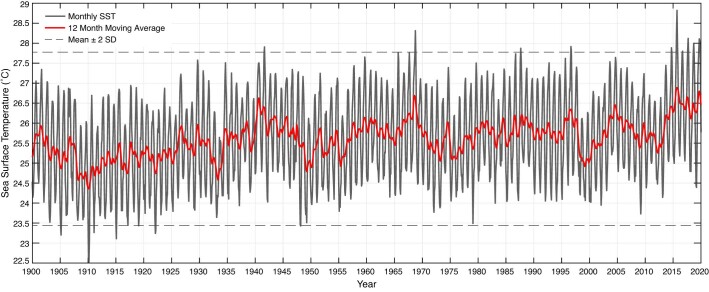
Extended Data Fig. 2. Long-term ocean temperature record for Hawai‘i.
Monthly sea surface temperature (SST) for the main Hawaiian Islands from 1900–2020. Dashed lines represent ± 2 standard deviations (SD) above and below the long-term mean. Red line is the 12-month moving average. The 2015 marine heatwave is represented by the highest SST values over the 120-year time series. Data are from NOAA’s Extended Reconstructed SST v5 (https://www.ncei.noaa.gov/products/land-based-station/noaa-global-temp) and values shown are the 90th percentile of monthly SST from within the vicinity of the main Hawaiian Islands (18.5 to 22.5°N; −160.5 to −154.5°W).
Reef trajectories predisturbance
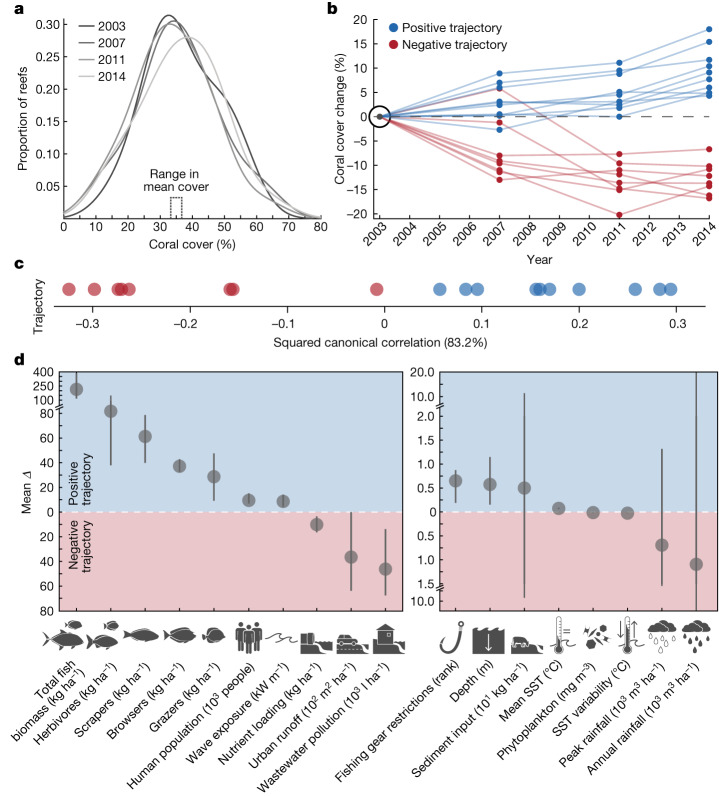
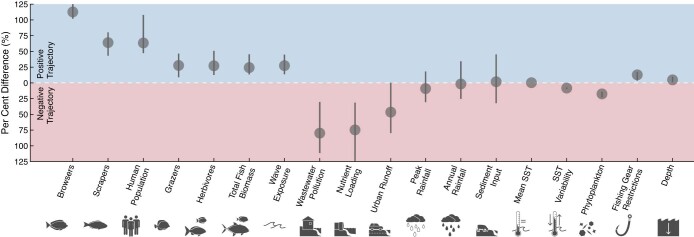
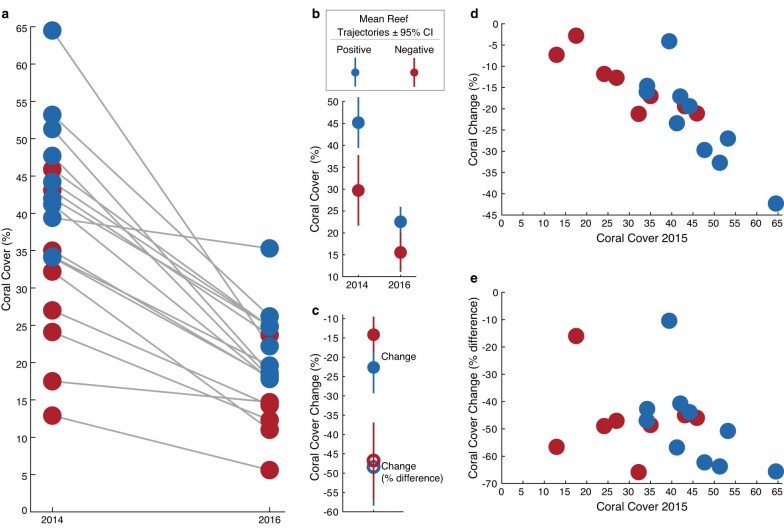
Coral cover among reefs surveyed in 2003 was 36.9 ± 2.3% (mean ± s.e.; n = 23) and changed by less than 3% in the subsequent years leading up to the 2015 marine heatwave (Fig. 2a). However, coral cover trajectories on individual reefs varied considerably over this time period: 44% of reefs showed a positive trajectory (that is, increased coral cover), 35% of reefs showed a negative trajectory (that is, decreased coral cover) and the remaining reefs showed no change (Fig. 2b). To the best of our knowledge, no acute disturbance occurred that can explain these divergent trajectories. Yet, we did find distinct differences in local conditions between positive and negative trajectory reefs in the years before and inclusive of this time frame (Fig. 2c). For example, the average biomass of all fishes, all herbivorous fishes and groups of herbivorous fishes that fill important ecological roles such as scrapers, grazers and browsers30 were 24–113% (29–214 kg ha−1) greater on reefs with positive trajectories compared to those with negative trajectories (Fig. 2d and Extended Data Fig. 3). These patterns probably reflect positive feedbacks, whereby increasing coral cover promotes habitat suitability for reef fishes, with herbivorous fishes then facilitating coral growth by reducing competitive exclusion by fleshy algae32. By contrast, wastewater pollution, nutrient loading and urban runoff were 46–80% greater on reefs with negative trajectories compared to those with positive trajectories. Despite these land-based human stressors being comparatively higher on reefs with negative trajectories, reefs with positive trajectories had 63% greater human population density (the number of people within a 15 km radius). This finding supports the notion that human population density is a poor indicator of human-driven land–sea impacts at local scales33. We observed minimal differences between positive and negative trajectory reefs relative to fishing gear restrictions, depth, sediment input, ocean temperatures, phytoplankton biomass and rainfall. Wave exposure was slightly higher (8.6 kW m−1) on reefs with positive trajectories, but the difference is minor because the entire study region is generally protected from large wave events34.
Fig. 2. Reef trajectories predisturbance and associated local land–sea human impacts and environmental factors.
a, Coral cover distributions among surveyed reefs between 2003 and 2014 (n = 23). b, Coral cover trajectories of individual reefs. A reef was considered on a positive trajectory (blue; n = 10) or negative trajectory (red; n = 8) if coral cover between 2003 and 2014 changed by more than 3%. This cut-off was based on mean coral cover range among all 23 reefs for the 12-year predisturbance period (range 2.8%; min 34.1%; max 36.9%). Reefs with no coral cover change (within ±3%) are not shown. c, Difference in local conditions between positive versus negative trajectory reefs (PERMANOVA, pseudo-F1,17 = 3.38, P = 0.001) visualized along a single multivariate axis (capturing the multidimensional and correlated nature of the data, Supplementary Fig. 2) using a canonical analysis of principal coordinates (n = same as in b). Allocation success equalled 90 and 87.5% for positive and negative trajectory reefs, respectively (more than 50% indicates an increasingly more distinct set of conditions than expected by chance alone). d, Mean difference (dots) in drop-one jackknife values with upper and lower bars representing the respective maximum and minimum differences in local human impacts and environmental factors between positive and negative trajectory reefs (n = same as in b). Blue and red shaded regions indicate factors that were greater on reefs that had positive and negative trajectories, respectively. Zero line represents equal values. See Extended Data Fig. 3 for the percentage difference in local conditions between positive and negative trajectory reefs. We included all local human impacts and environmental factors in d to provide a general comparison of local conditions between reefs with divergent trajectories. See Fig. 1b for reef locations and Supplementary Fig. 3 for predictor variable distributions. See Methods, Extended Data Table 1 and Supplementary Information for detailed information on local land–sea human impacts and environmental factors.
Extended Data Fig. 3. Per cent difference in mean drop-one jackknife values of local human impacts and environmental factors between positive and negative trajectory reefs.
The per cent difference ((V1−V2)/[(V1+V2)/2]; dots) was quantified by taking the ratio of the mean in drop-one jackknife values between positive (n = 10) and negative (n = 8) trajectory reefs (sensu)88. Upper and lower bars represent the respective maximum and minimum per cent differences. Blue and red shaded regions indicate factors that were greater on reefs that had positive and negative trajectories, respectively. Zero line represents equal values. Outliers that fell outside a threshold of ±2 standard deviations of the median were removed prior to analysis. See Fig. 1b for reef locations and Fig. 2d for mean absolute differences in factor values. See Methods, Extended Data Table 1, and Supplementary Information for detailed information on local land-sea human impacts and environmental factors.
Coral response to the marine heatwave
In 2015, the Hawaiian Islands experienced the strongest marine heatwave on record over the past 120 years (Extended Data Fig. 2). Ocean temperatures across our study region were 2.2 °C above normal and peaked at 29.4 °C (Fig. 3a). Degree heating weeks (DHWs), a widely used heat stress metric for coral reefs, averaged 12 DHWs among surveyed reefs (Fig. 3b), far exceeding the eight DHW threshold expected to cause severe and widespread coral bleaching and mortality35. Reef surveys performed one year following the marine heatwave showed that nearly one-quarter of reefs (19 out of 80) lost more than 20% coral cover whereas the hardest-hit reef lost 49% (Fig. 3c). But not all reefs experienced such catastrophic change. Coral cover remained unchanged or increased on 18% (14 out of 80) of reefs surveyed. This divergent ecological response was unexpected given that all reefs were exposed to similarly extreme levels of heat stress (Fig. 3b).
Fig. 3. Local land–sea human impacts and environmental factors that modified coral response to the 2015 marine heatwave.
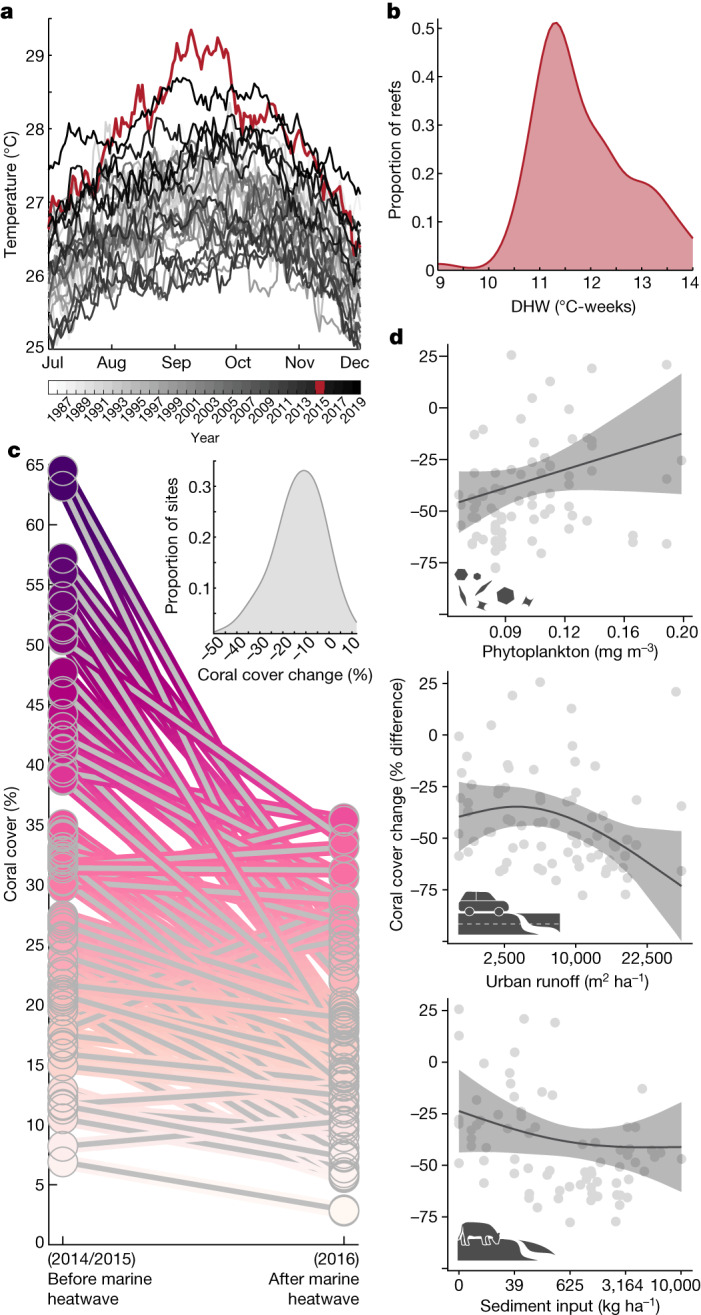
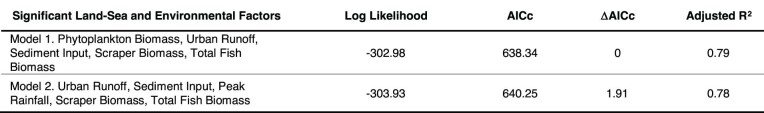
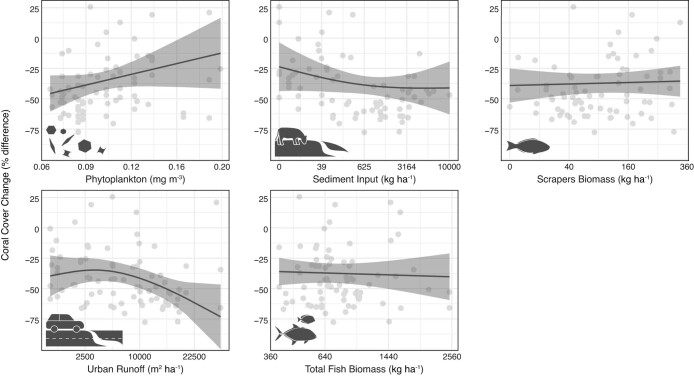
a, Historical (1986–2019) SSTs during the seasonal peak (July–December) averaged across the study region; 2015 marine heatwave shown in red. b, Maximum DHW exposure in 2015, a common heat stress metric, among surveyed reefs. All reefs exceeded the eight DHW threshold expected to produce severe and widespread coral bleaching and mortality. c, Coral cover before (2014–2015) and one year following (2016) the marine heatwave among surveyed reefs (n = 80, Fig. 1b). The inset represents the distribution of absolute coral cover change. d, The GAMM results (R2 = 0.79) showing key factors explaining coral response to the marine heatwave. Change accounts for starting condition, defined as: percentage difference = ((Aa,i − Ab,i)/Ab,i) × 100, where Ab and Aa are the mean coral cover values at each reef in 2014 or 2015, and 2016, respectively (Methods and Supplementary Fig. 4). Positive and negative relationships reduce or increase coral loss, respectively. Shaded regions represent 80% confidence intervals. Factors with the strongest model averaged slopes are shown. Total fish biomass and scraper biomass were also important factors in our models but had weak slopes (representing less than 5% change; Extended Data Fig. 4). Relative importance of factors among all models (that is, sum of AICc model weights across all models containing each factor) were: sediment input (0.99), scraper biomass (0.99), total fish biomass (0.90), urban runoff (0.60), phytoplankton biomass (0.38), wastewater pollution (0.28), peak rainfall (0.20), nutrient loading (0.19), grazer biomass (0.16), DHW (0.08), wave power (0.07), depth (0.06) and fishing gear restrictions (0.05). See Extended Data Table 1 for full list of factors included in the analysis, including those removed that were highly correlated (r > 0.7, see Methods and Supplementary Fig. 5). See Supplementary Fig. 6 for predictor variable distributions.
Interactions between heat stress and local conditions such as a high abundance of competitive macroalgae can exacerbate coral bleaching and mortality22. However, we lack a detailed understanding of the land- and sea-based factors that mediate coral response to marine heatwaves. Using a generalized additive mixed-modelling framework, we identified the land–sea factors that best explained variations in coral cover change (accounting for starting cover) among reefs one year after the 2015 marine heatwave in Hawai‘i (Fig. 3d and Extended Data Table 2).
Extended Data Table 2.
Summary of generalized additive mixed effects models (GAMM) relating coral response to the 2015 marine heatwave with local land-sea human impacts and environmental factors
The top two candidate models are shown. AICc, Akaike’s information criterion corrected for small sample size; ΔAICc, change in AICc across the candidate models (ΔAICc ≤ 2 of the top model are shown); Adjusted R2, proportion of variation in the response variable explained by the candidate model.
Coral bleaching involves the breakdown of the mutualistic relationship between the coral animal and its algal endosymbionts36. A prolonged breakdown in this relationship often results in coral starvation and death, as much of the energetic demands of corals are met by the photosynthetic activity of its endosymbionts36. We found that reefs with the highest levels of water column phytoplankton biomass (that is, chlorophyll-a) during the marine heatwave showed reduced coral mortality (Fig. 3d). Productivity increases nearshore to tropical islands such as Hawai‘i37 and is further concentrated by small-scale ocean processes that attract dense aggregations of plankton38. The increase in nutritional subsidies to the coral animal may have helped to reduce coral starvation during the heatwave or provided higher energetic reserves that promoted their recovery39. In other regions (for example, Great Barrier Reef), high levels of chlorophyll-a are an indicator of poor water quality that drives negative outcomes for corals40. Here, chlorophyll-a was uncorrelated to land-based human impacts (Supplementary Fig. 6) and probably reflective of natural gradients in energetic subsidies that facilitated coral survival. Working towards locally relevant management strategies requires understanding how human impacts superimpose on natural biophysical drivers, such as phytoplankton biomass24, to influence reef ecosystem response to acute disturbance.
Coastal runoff can deliver a broad spectrum of land-based contaminants that degrade nearshore water quality, with cascading effects on coral health41. We found that reefs exposed to the lowest levels of urban runoff, and to a lesser extent sediment input, experienced a modest reduction in coral mortality from the marine heatwave (Fig. 3d). Urban runoff often contains heavy metals and petrochemicals that cause coral tissue death42 and sediment input can impede the photosynthetic capacity of corals and reduce growth by burying coral colonies41. Together, these stressors can undermine the natural defence abilities of corals and increase the likelihood of mortality from heat stress40. Although turbid waters may shade corals from excessive sunlight that can exacerbate coral bleaching, high levels of heat stress can override any protective benefits decreased light may provide43. Existing but underused local and national policies such as the Clean Water Act in the United States provide actionable pathways for marine management interventions of land-based stressors44. Management strategies that leverage such policies to help mitigate coastal runoff, particularly in urban areas, may support increased coral survival during severe marine heatwaves.
We also found that total fish biomass and scraper biomass were important factors in our models (Extended Data Table 2). Healthy fish populations provide numerous reef-scale ecosystem functions29, including some species releasing beneficial nutrient subsidies that increase coral thermal tolerance45. Scrapers remove fast-growing algal turfs that could otherwise outcompete and overgrow stress-compromised corals30. By comparison to phytoplankton biomass and coastal runoff, the slopes of the relationships between total fish biomass and scraper biomass with heat-driven coral loss were weak (Extended Data Fig. 4). Intense marine heatwaves can cause severe coral mortality even on highly protected, uninhabited reefs with intact fish populations46, suggesting that extreme heat stress may simply overwhelm the functional roles of reef fish over short time scales. However, abundant fish populations, in particular herbivores, can support coral reef recovery potential following disturbance2. Understanding whether this positive relationship holds across gradients in land-based impacts is key for supporting targeted fisheries management in coastal marine ecosystems.
Extended Data Fig. 4. Generalized Additive Mixed Model (GAMM) results (R2 = 0.79) showing key local land-sea human impacts and environmental factors that modified coral response to the 2015 marine heatwave.
Positive and negative relationships reduce or increase coral loss, respectively. Because changes in coral cover following disturbance can be affected by variations in starting condition (reefs with higher initial cover have greater scope for loss, and vice versa)91 we modelled relative coral cover change following ref. 92 to ensure comparability across reefs (see Methods). Median values with shaded region representing the 80% confidence interval. The relative importance of factors among all models (i.e., sum of AICc model weights across all models containing each factor) was as follows: sediment input (0.99), scraper biomass (0.99), total fish biomass (0.90), urban runoff (0.60), phytoplankton biomass (0.38), wastewater pollution (0.28), peak rainfall (0.20), nutrient loading (0.19), grazer biomass (0.16), DHW (0.08), wave power (0.07), depth (0.06), and fishing gear restrictions (0.05). See Extended Data Table 1 for full list of local land-sea human impacts and environmental factors included in the analysis, including those removed that were highly correlated (Fig. S5). See Fig. S6 for predictor variable distributions.
Coral reefs four years postdisturbance
The dominant reef-builders in tropical coral reef ecosystems are hard corals and crustose coralline algae25. Crustose coralline algae are encrusting calcifying algae that fuse the reef framework together and promote coral growth by serving as a successional prerequisite for coral recruitment and suppressing competitive fleshy algae25. Given that coral cover can take a decade or more to recover to prebleaching levels47, assessing the total cover of reef-building organisms (hard coral + crustose coralline algae) is more indicative of coral reef recovery potential following disturbance. Our surveys four years following the 2015 marine heatwave found that reef-builder cover ranged from 3.4 to 51.9% (mean of 24.3% ± 1.7 s.e.; n = 55; Fig. 4a). Critically, there were different reefs with high (more than or equal to the 75th percentile) and low (less than or equal to the 25th percentile) reef-builder cover before and after the marine heatwave. Nearly two-thirds of reefs with high reef-builder cover in 2019 did not support such levels of cover before the marine heatwave. Similarly, we observed a more than 40% change in the location of reefs with low reef-builder cover between 2015 and 2019. This reshuffling of reefs in terms of relative reef-builder cover suggested differential coral reef persistence in the years following severe heat stress.
Fig. 4. Local management scenarios that support coral reef persistence four years postdisturbance.
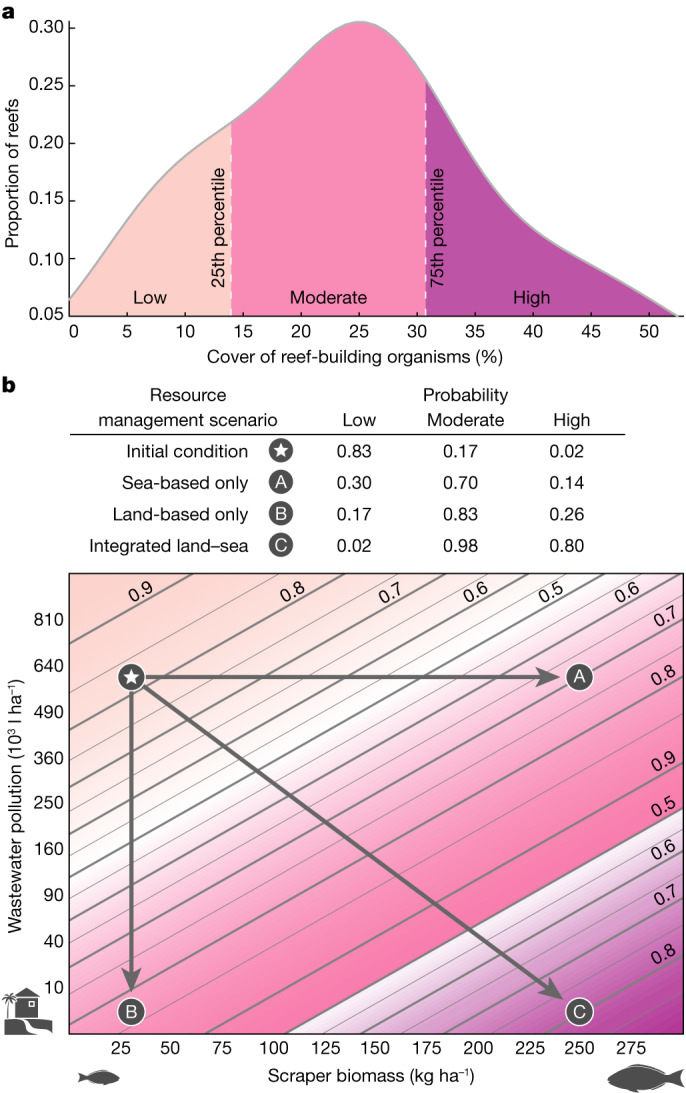
a, Percentage cover of reef-building organisms (hard coral + crustose coralline algae) among reefs surveyed (n = 55) in 2019, four years following the marine heatwave. Colours represent low (≤25th percentile), moderate (>25th and <75th percentile) or high (≥75th percentile) cover. b, Probability of low, moderate or high cover of reef-builders shown in relation to variations in scraper biomass and wastewater pollution. Example scenarios show that simultaneously decreasing wastewater pollution and increasing scraper biomass results in a far greater probability of high reef-builder cover (scenario ‘C’) than achieving either management scenario in isolation (scenarios ‘A’ and ‘B’). The upper (250 kg ha−1) and lower (30 kg ha−1) management scenarios for scraper biomass represented the 92nd and 36th percentiles, respectively. We specifically chose 250 kg ha−1 as it approximates the long-term mean (2003–2019; n = 17) scraper biomass in Kealakekua Bay, a marine protected area in our study region where no fishing has been allowed since 1969 (Supplementary Fig. 11). Similarly, the upper (600,000 l ha−1) and lower (2,500 l h−1) management scenarios chosen for wastewater pollution represented the 95th and 36th percentiles of the 2019 distribution, respectively (Supplementary Fig. 12). Probability values and lines were derived from the top model from ordinal logistic regression modelling (Extended Data Table 3, Methods and Supplementary Information). Colours for low, moderate and high in b are the same as those in a. See Extended Data Table 1 for full list of local land–sea human impacts and environmental factors included in the analysis, including those removed that were highly correlated (r > 0.7, Methods and Supplementary Fig. 8). See Supplementary Fig. 9 for predictor variable distributions.
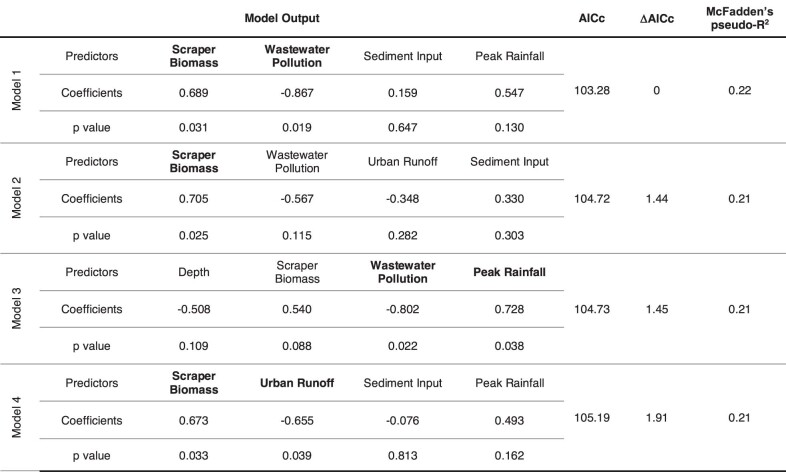
We used an ordinal logistic regression framework to identify the local land–sea human impacts and environmental factors that best supported coral reef persistence in the years following the 2015 marine heatwave. Decreased wastewater pollution and increased scraper biomass were the most important and significant (P < 0.05) in predicting whether a reef had relatively higher reef-builder cover four years postdisturbance (Extended Data Table 3). Pollution from human waste affects coastal marine ecosystems globally48 and is especially harmful to corals from untreated sources, such as septic tanks and cesspools, which are both common in Hawai‘i49. Consequently, high concentrations of toxins and pathogens leach into coastal waters that increase coral disease, reduce coral growth and reproduction, and increase coral susceptibility to bleaching42. These negative impacts on coral persistence are therefore much reduced in areas of decreased wastewater pollution. Scrapers reveal bare substrate as they feed and facilitate the settlement, growth and survival of crustose coralline algae and corals following acute disturbance30. Beyond these top-down effects on benthic condition, bottom-up effects of improved habitat quality could be contributing to the positive relationship we observed between scraper biomass and higher reef-builder cover. Parrotfish are the dominant scrapers in Hawai‘i, and typically have home ranges of less than 1 km (ref. 50). Furthermore, our scraper biomass estimates were derived from multiple observations across several time points following the marine heatwave, rather than a single snapshot estimate. Such strong site-based fidelity, combined with our recurring surveys, suggests that resident scrapers played a key role in promoting higher reef-builder cover rather than the association driven purely by an influx of individuals seeking more favourable habitat postdisturbance.
Extended Data Table 3.
Summary of ordinal logistic regression (OLR) models relating the per cent cover of reef-builders (hard coral + crustose coralline algae) four years following the 2015 marine heatwave to local land-sea human impacts and environmental factors
AICc, Akaike’s information criterion corrected for small sample size; ΔAICc, change in AICc across the candidate models (ΔAICc ≤ 2 of the top model are shown); McFadden’s pseudo-R2, proportion of variation explained by candidate model (0.2–0.4 represent an excellent fit)98. Significant predictors at p < 0.05 are in bold.
Sea-based management efforts are often disconnected from those occurring on land17,18. We generated management scenarios of how varying scraper biomass (sea-based management) and wastewater pollution (land-based management) influenced the probability of being in a low, moderate (more than the 25th and less than the 75th percentile) or high reef-builder cover category. Our findings indicate that an integrated management approach can result in a positive synergistic outcome for coral reefs (Fig. 4b). For example, four years following the marine heatwave, a reef across our study region with low scraper biomass (for example, 30 kg ha−1) and relatively high wastewater pollution (for example, 600,000 l ha−1) is most likely to have low reef-builder cover (83% probability) (Fig. 4b, ‘initial condition’). Where scraper biomass is higher (for example, 250 kg ha−1) but wastewater pollution remains high, there is a 70% probability of moderate reef-builder cover (scenario A). Conversely, where wastewater pollution is lower (for example, 2,500 l ha−1), but scraper biomass remains low, there is an 83% probability of moderate reef-builder cover (scenario B). However, if both land and sea management scenarios occur, there is an 80% probability of high reef-builder cover (scenario C). Combining land and sea management resulted in a three- to sixfold increase in the probability of high reef-builder cover four years following severe heat stress than if land or sea were managed in isolation.
Conclusion
Here we show that simultaneously mitigating local land- and sea-based human impacts promotes coral reef persistence before, during and in the years following a historically unprecedented marine heatwave in Hawai‘i. Our unique spatially and temporally resolved data highlighted the specific impacts that best correlated with coral reef persistence in each of these temporal periods. For example, the biomass of all reef-fish groups was associated with positive reef trajectories over the 12 years leading up to the marine heatwave. By contrast, scraper biomass was the only fish group associated with positive outcomes for reefs four years following severe heat stress. This suggests that reef fish play essential functional roles at different points in time and that particular feeding and behaviours are probably critical for reef persistence following acute distrubance30. Similarly, land-based impacts consistently emerged as driving negative coral reef outcomes, but the combination of stressors changed depending on the observational time window in question. Highly resolved data on the local human impacts that drive reef ecosystem trajectories over time are unlikely to be available in most regions. However, our overarching finding that integrated land–sea management benefits coral reefs under ocean warming, is applicable to populated reefs globally.
The local human impacts we identify here represent the direct or proximate drivers of reef condition in our study. These in turn are dictated by an array of distal socioeconomic and cultural factors such as human migration and urbanization, finance, trade and tourism that indirectly affect how people interact with coral reefs1,51. Distal human drivers also underpin climate change that is driving severe marine heatwaves that trigger mass coral bleaching at global scales. Increases in future ocean temperatures and the frequency and severity of coral bleaching events52 could simply overwhelm the positive effects of local management actions on coral reefs. However, there is substantial variation in the projected rates of ocean warming within and among countries under reduced emissions scenarios52. Actions that support coral reef persistence locally alongside global reductions in greenhouse gas emissions may buy reefs more time to adapt and persist into the future. Contemporary governance must therefore shift towards an integrated approach to align management strategies with reef ecosystem processes and the coincident multiscale human drivers that affect them1.
An ambitious effort is underway to protect 30% of Earth’s land and sea areas by 2030 as part of the recently adopted Kunming-Montreal Global Biodiversity Framework5. The motivation behind the ‘30 by 30’ is to support ecological resilience, conserve biodiversity and preserve ecosystem services that underpin human well-being53. The 30 by 30 has broad participation and is being incorporated into conservation efforts by nations globally. However, our results reveal that sea-based management alone is insufficient to mitigate the full spectrum of local human effects on coastal ecosystems such as coral reefs. These efforts must therefore explicitly couple the respective 30% land–sea targets to realize coastal ocean conservation goals. But in most coastal geographies, 30% protection is impractical and unethical given the high proportion of people that live near and depend on these ecosystems54. Instead, mitigating land-based impacts such as wastewater pollution must occur together with fisheries governance for successful conservation outcomes, akin to long-standing indigenous stewardship practices of island ecosystems16. Only by adopting coupled land–sea policy measures, alongside global emissions reductions, will coral reef ecosystems and the human communities they support have the best opportunity for persistence in our changing climate.
Methods
Study site
Hawai‘i Island (19.55° N, 155.66° W) is the southeastern most island of the Hawaiian Archipelago, located in the northern central Pacific (Fig. 1). The western section has roughly 200 km of coastline predominantly oriented north to south. The coastline contains the longest contiguous reef ecosystem in the main Hawaiian Islands55 and large gradients in human population, local land–sea impacts and environmental factors that are comparable to reef ecosystems globally (Extended Data Fig. 1). The region represents an ideal study location for resolving the land–sea human impacts driving reef ecosystem change and coral trajectories following acute climate-driven disturbance.
Reef surveys
Full details related to sampling design, site selection and survey frequency for benthic and reef-fish data collection across our study region are in the Supplementary Information. In brief, underwater visual surveys of benthic assemblages were collated from three monitoring programmes for the following years (number of reefs surveyed are in parentheses): 2003 (23), 2007 (23), 2011 (23), 2014 (40), 2015 (40), 2016 (80), 2017 (80), 2018 (15) and 2019 (55). All benthic surveys used permanently marked pins to ensure the same area of reef was surveyed over time. High-resolution photographs were collected by using photoquadrats at 1 m intervals along 25 m belt-transects (n = 26 photographs per transect). Between 30 and 50 random points were overlaid on each photograph and the benthic component under each point was identified to the lowest possible taxonomic level. Percentage cover of the major functional groups at each reef were used in this analysis, namely hard coral and crustose coralline algae. Surveys of reef-fish assemblages were performed along the same permanently marked 25 m transects concurrently with benthic surveys. However, reef-fish surveys were performed more frequently (one to six times per year from 2003 to 2019) than benthic surveys, depending on the reef location and monitoring programme performing the surveys. In all surveys, fishes were identified to species, sized and enumerated. To account for differences among programmes in how researchers surveyed reef fish, counts were calibrated using species and method specific adjustments56.
Local land–sea human impacts and environmental factors
Fish biomass
The biomass of fishes at a given reef was measured as total fish biomass, herbivore fish biomass and the biomass of browsers, grazers and scrapers56. Total fish biomass is an indicator of the overall state of the fish assemblage57 and is reduced in areas that have increased fishing pressure58,59. In Hawai‘i, non-commercial nearshore fisheries dominate, with people fishing for recreational, subsistence and cultural purposes60,61. However, the dominant harvesting modes and magnitude of fishing activities are largely unknown at spatial or temporal scales relevant to this study62. As such, we include total fish biomass in part to represent fishing effort on reefs but recognize its shortcomings in capturing reef- and species-specific differences in fishing pressure across our study region. We also include herbivores and subdivisions by feeding guilds that represent important indicators of resilience on coral reefs30,63,64. Browsers are defined as herbivores that feed on macroalgae and associated epiphytic material, and are important for reducing the cover of larger, more established macroalgae. Grazers are herbivores that feed largely on small algal turfs, helping to prevent their succession into larger macroalgae, and scrapers are herbivores that closely crop the substrate and open up new space to promote the settlement, growth and survival of crustose coralline algae and corals30.
We followed established methods for calculating fish biomass56. The biomass of individual fishes was estimated using the allometric length–weight conversion: W = aTLb, where parameters a and b are species-specific constants, TL is total length (cm) and W is weight (g). Length–weight fitting parameters were obtained from a comprehensive assessment of Hawai‘i specific parameters56 and FishBase65. Fish species were excluded from fish biomass calculations according to life history characteristics that are not well captured with visual surveys, including cryptic benthic species, nocturnal species, pelagic schooling species and manta rays.
Human population
We quantified human population density using NASA Gridded Population of the World v.4 (ref. 66). The dataset is available at 1 km resolution at 5-year intervals. Linear interpolation was used to fill in the missing years and produce annual time steps of human population within 15 km of each 100 m grid cell across our study region (Supplementary Fig. 12).
Wastewater pollution
We calculated wastewater effluent (l ha−1 yr−1) and nitrogen input (kg ha−1 yr−1) from onsite sewage disposal systems (for example, cesspools and septic tanks) and injection wells (collectively OSDS) in coastal waters at 100 m resolution. Only OSDS located within a modelled one-year groundwater travel time of the coast were included in the analysis and nutrients from OSDS were assumed to flow to the nearest point on the shoreline. Wastewater effluent and nutrient input were estimated on the basis of ref. 67 and discharge rates and nutrient loading according to ref. 68. A Gaussian decay function was used to estimate dispersal offshore, approaching zero at 2 km (Supplementary Figs. 13–15). This same dispersal function was also used for nutrient input, urban runoff, sediment input and rainfall, which are each described below.
Nutrient input
We calculated nutrient input (kg ha−1 yr−1) at 100 m resolution as the combination of total nitrogen from OSDS (Wastewater pollution section above) and golf courses. The total golf course area per watershed was derived from NOAA Coastal Change Analysis Program (CCAP) land-use and land-cover data and Landsat cloud-free composite images created with Google Earth Engine. The golf course area was multiplied by an annual nitrogen application rate of 585 kg ha−1 (refs. 69,70) and then by a leaching rate of 32%71–73 to estimate nitrogen that either runs off or reaches the groundwater. We also imposed a reduction in nitrogen that reached the ocean on the basis of distance inland and used subwatershed catchment data74 to estimate nutrient transport from golf courses to the coastline (Supplementary Figs. 16–18).
Urban runoff
We quantified the total area of impervious surfaces (that is, paved roads, parking lots, sidewalks and roofs) within 10 km of the coastline at 100 m resolution for each year from 2000 to 2017 (Supplementary Figs. 19 and 20). Data were extracted from NOAA CCAP land-use land-cover data from 1992, 2001, 2005 and 2010. We also digitized 2017 impervious surface cover from a single cloud-free Landsat 8 image (courtesy of the United States Geological Survey, USGS) (15 m resolution pan-sharpened). Years in between data availability were filled in by linear interpolation.
Rainfall
We quantified annual rainfall (m3 ha−1 ) and peak rainfall (maximum 3-day rainfall total, m3 ha−1) at 100 m resolution. Daily rainfall data were generated following refs. 75,76. Rainfall from each rain station was used to derive interpolated surfaces at annual time steps using Empirical Bayesian Kriging in ArcGIS. Subwatershed catchment data74 were clipped to 0–10 km from the coast and used to calculate rainfall per drainage area (Supplementary Figs. 21 and 22).
Sediment input
The Integrated Valuation of Ecosystem Services and Tradeoffs sediment delivery model was used to derive long-term annual average sediment input (kg ha−1) reaching the coast77–80 at 100 m resolution. We then modulated the long-term annual average sediment over time by watershed on the basis of discharge calculated from peak rainfall data (Rainfall section above). Discharge by watershed was calculated following ref. 81. Sediment load was assumed to scale with discharge according to a approximate ratings curve following ref. 82 (Supplementary Figs. 23 and 24).
Fishing gear restrictions
We created a categorical value for local fishing gear restrictions using regulation information and marine managed area boundary designations updated from ref. 80. All regulations were evaluated for prohibition of gear categories in relation to fishing for reef finfish species over time: line fishing, lay nets, spear fishing and aquarium collection. Ranked fishing gear categories are as follows: (1) full no-take, (2) no lay net, spear or aquarium, (3) no lay net or aquarium, (4) no lay net, (5) no aquarium and (6) open to all gear types (Supplementary Table 1 and Supplementary Fig. 25).
Sea surface temperature and heat stress
The mean and variability (that is, standard deviation) in summertime sea surface temperature (SST) was calculated over a 90-day window centred on the maximum value of a 7-day moving window average for each SST pixel (Supplementary Fig. 26). Mean regional temperature (Fig. 3a) was calculated by taking the 7-day running mean of daily values and then averaging across all coastal pixels within our study region. Heat stress on reefs during the 2015 marine heatwave was assessed using DHW35, a widely used metric by coral reef scientists across the world. All data were NOAA’s Coral Reef Watch v.3.1, available daily at 5 km resolution35.
Phytoplankton biomass and irradiance
We used satellite derived chlorophyll-a (mg m−3; a proxy for phytoplankton biomass) and irradiance (E m−2 d−1) from two sources. The long-term mean (2002–2013) in 8-day, 4 km data were obtained from ref. 80 and shown in Fig. 2d and Extended Data Fig. 3. All subsequent analysis used the visible-infrared imaging/radiometer suite, which has high spatial (750 m) and temporal (daily) resolution data starting in 2014 (provided by NOAA’s Coral Reef Watch). All data were quality controlled and masked to account for cloud cover (Supplementary Information) and optically shallow waters following ref. 83 (Supplementary Fig. 27).
Wave exposure
Wave power (kW m−1) combines wave height and period and provides a more representative metric of wave exposure than wave height alone84. A series of nested grids (from global to 50 m) using WAVEWATCH III85 and Simulating Waves Nearshore86 were used to quantify wave transformation over the reef environment at 50 m, at hourly intervals across our study region from ref. 87 and updated for this study. Annual data were then generated for each 50 m grid cell by taking the mean of the top 97.5% in daily maximum wave power (Supplementary Fig. 28).
Depth
Depth of the reef floor (m) was measured using diver depth gauges during the in-water reef surveys.
Statistical analyses
Coral reef trajectories predisturbance
We quantified the change in coral cover at 23 reefs from 2003 to 2014. A reef was considered to have a positive trajectory or negative trajectory if coral cover from the 2003 survey to the 2014 survey increased or decreased by greater than 3%, respectively (Fig. 2b). This cut-off was based on the range in mean coral cover among all 23 reefs across the 12-year period (range 2.8%; minimum 34.1%; maximum of 36.9%). We then quantified local human impacts and environmental factors at each reef as follows: fish biomass metrics were from the mean of all annual surveys for each year from 2003 to 2014; human population, wastewater pollution, nutrient loading, urban runoff, annual rainfall, peak rainfall, SST mean and SST variability from the mean of all data from 2000 to 2014. Phytoplankton biomass and irradiance were from the maximum monthly climatology from 2002 to 2013. Sediment and wave exposure came from the mean of the top five events from each year spanning 2000–2014. Fishing gear restrictions were from marine managed area designation at the onset of reef surveys and the depth came from in-water diver-assessed values.
The difference in local human impacts and environmental factors between positive and negative trajectory reefs were then calculated as the difference in the mean drop-one jackknife values for each impact or factor88. Upper and lower bars in Fig. 2d represent the respective maximum and minimum differences in drop-one jackknife values between positive and negative trajectory reefs. Before calculating the drop-one jackknife values, we identified and removed outliers that fell outside a threshold of ±2 standard deviations of the median. We formally tested for a difference in the local conditions of positive versus negative trajectory reefs using a multivariate permutational analysis of variance (PERMANOVA)89 based on a Euclidean distance similarity matrix, type III (partial) sums-of-squares and unrestricted permutations of the normalized data. We visualized the results in Fig. 2c using a constrained analysis of principal coordinates90 and calculated the cross-validation allocation success (a measure of group distinctness) from the leave-one-out procedure of the constrained analysis of principal coordinates analysis.
Coral response to the 2015 marine heatwave
Our goal was to assess the local land–sea human impacts and environmental factors that best explained changes in coral cover as a consequence of the 2015 marine heatwave. Any potential to observe change, however, could be influenced by variations in starting condition. Reefs with higher initial cover (such as those on positive coral cover trajectories predisturbance, Fig. 2b) had greater scope for loss and vice versa91 (Extended Data Fig. 5). To account for this and ensure comparability across reefs (Supplementary Fig. 4) we calculated coral cover change following ref. 92 as:
where Ab and Aa are the mean coral cover values at each reef in 2014 or 2015, and 2016, respectively.
Extended Data Fig. 5. Coral cover change following the 2015 marine heatwave on positive versus negative coral cover trajectory reefs.
a, Coral cover on positive (blue; n = 10) and negative (red; n = 8) trajectory reefs surveyed (see Fig. 2b in main manuscript) prior to (2014) and 1-year following (2016) the marine heatwave. b, Positive trajectory reefs have a higher mean coral cover both prior to, and to a lesser extent, following the marine heatwave compared to negative trajectory reefs. c, Positive trajectory reefs experience increased absolute coral cover loss following the marine heatwave (underlying relationship shown in panel d), but this difference is largely absent once starting coral cover condition is accounted for (underlying relationship shown in panel e).
We then calculated the following predictors based on current literature and our hypotheses of the principal factors that drive changes in coral cover owing to severe heat stress (Extended Data Table 1). Fish biomass metrics included the mean of fish data that were coupled with benthic surveys: 2014 (n = 40) or 2015 (n = 40) and 2016 (n = 80); human population, wastewater pollution, nutrient loading, urban runoff, annual rainfall, peak rainfall and wave exposure were taken from the mean of all data from 2012 to 2016, sediment was measured from the mean of the top three events from 2006 to 2016; SST mean and SST variability were taken from the mean from 2000 to 2014; DHW was the maximum value for 2015; phytoplankton biomass and irradiance was the mean from June to November 2015, representing the time inclusive of the marine heatwave; fishing gear restrictions was the marine managed area designation before the marine heatwave (2014 or 2015, depending on the reef surveyed) and depth came from in-water diver-assessed values.
We then tested for correlations between coral loss and our suite of predictor variables using a generalized additive mixed-effects modelling (GAMM) framework24 with the gamm4 (ref. 93) package for R (www.r-project.org) v.4.0.2. Before model fitting, we identified the presence of outliers in our predictor variables as any point that fell outside a threshold of ±2 standard deviations of the median. We then applied an additional step to retain any point above this threshold that was within 25% of the maximum predictor value below the threshold. This ensured that no data points were unnecessarily discarded from our formal model-fitting process because of applying an arbitrary threshold cut-off for data inclusion. The following predictors were square-root transformed to down-weight the influence of values at the extreme ends of their distributions: all fish biomass metrics, wastewater pollution, urban runoff, nutrient loading, phytoplankton biomass and peak rainfall. A fourth-root transformation was applied to sediment.
To reduce model overfitting, Pearson’s correlation coefficients were calculated among all predictors (Supplementary Fig. 5), removing one of each pair of highly correlated (r > 0.7) predictors. To further strive for model parsimony, we a priori excluded human population density from the model-fitting process as it was a poor indicator of human-driven land-to-sea impacts on local scales (Figs. 1c and 2d and Extended Data Fig. 3). We also excluded browser biomass as they represented less than 10% on average of total herbivore biomass across all reefs before, during and postdisturbance. This resulted in the following predictors included in the models (correlated predictors in parentheses were removed): total fish biomass, biomass of scrapers, biomass of grazers (total herbivore biomass), DHW (SST mean and variability), wastewater pollution, nutrient input, urban runoff, sediment input and peak rainfall (annual rainfall correlated with both), wave power, phytoplankton biomass (irradiance), fishing gear restrictions and depth. The decision of which correlated predictors to retain was based on a hypothesis-driven approach, in part whether the given predictor had the potential to directly (for example, sediment input) rather than indirectly (for example, annual rainfall driving sediment input) affect heat-driven coral loss.
We incorporated a random spatial factor to account for the possible influence of a change in an underlying variable along the coastline not quantified in this study. This was done by breaking the coastline up into discrete 10 km sections running north to south. Section size was determined using hierarchical clustering based on pairwise Euclidean distances between reefs and identifying an inflection point in the intragroup variance24 (Supplementary Fig. 7). We fitted GAMMs for all possible candidate models (unique combinations of the predictor variables) using the UGamm wrapper function, in combination with the dredge function in the MuMIn package94. Nonlinear smoothness in the models was determined using penalized cubic regression splines, with the number of knots (limited to four to reduce overfitting) spread evenly throughout each covariate. All possible candidate models were computed (unique combinations of the predictor variables) but limiting the total number of predictors in any given candidate model to five to reduce overfitting. We used Akaike’s information criterion with a bias correction for small sample sizes95 (AICc) for model comparison and all models within ΔAICc ≤ 2 of the top model (ΔAICc = 0) are presented in Extended Data Table 2. To visualize the effect of predictor terms on coral cover change, we averaged the coefficients from the top models (that is, ΔAICc ≤ 2) to generate a predicted dataset and set all other predictor terms to their median value. Finally, we calculated a measure of predictor variable relative importance within each candidate model by calculating the sum of AICc model weights for each predictor (that is, the sum of model weights across all models containing each predictor; Fig. 3).
Coral reefs four years postdisturbance
Our goal was to assess the local land–sea human impacts and environmental factors that best explained variations in the cover of reef-building organisms four years following the marine heatwave. The cover of reef-building organisms for reefs surveyed in 2019 (n = 55) were parsed into three categories on the basis of the following percentiles: low, less than or equal to the 25th; moderate, more than 25th and less than 75th; and high, more than or equal to the 75th. We then performed ordinal logistic regression96 to determine the probability of a given reef having high, moderate or low cover of reef-building organisms on the basis of the prevailing local human impacts and environmental factors (that is, predictor variables; Extended Data Table 1). Logit models are multivariate extensions of generalized linear regression models that provide parameter estimates by means of maximum likelihood estimation (MLE) to model the relative log odds of observing a reef-builder cover category or less versus observing the remaining higher categories:
Here, i indexes each of N observations, with categories yi, and the left-hand side of the equation is the logit of the probability of a reef-builder category of j or lower, for j = 1 (high) or 2 (moderate). Reefs with low reef-builder cover contributed to the regression through calculation of the log odds. Each Cj is an MLE-computed model intercept, and each Bk is the MLE coefficient corresponding to the standardized independent variable zik, for k = 1 through n, where n is the variable number of predictors used in a given candidate model, hence the ellipsis (...). A fundamental component of this model is the assumption of proportional odds, or parallel regression, which indicates that Bk values are independent of the logit level j. The validity of this parallel regression assumption was ascertained using Brant’s Wald test97, as well as a likelihood ratio test (α = 0.05).
We then calculated the following predictors based on current literature and our hypotheses of the principal factors that drive changes in reef-builder cover across space and time following a major thermal disturbance: fish biomass metrics, wastewater pollution, nutrient loading, urban runoff, annual rainfall, peak rainfall, wave exposure, phytoplankton biomass and irradiance: the mean of all data from 2016 to 2019; sediment was measured as the mean of top three events over the 2006–2019 time period; SST mean and SST variability: mean of all data from 2000 to 2018. Note that 2019 was excluded in SST mean and SST variability owing to the marine heatwave that affected Hawai‘i21, but occurred after our 2019 fish and benthic surveys; fishing gear restrictions involved the marine managed area designation in 2016 and depth was assessed by in-water diver-assessed values.
We used the same process as in the GAMM analysis to remove outliers in our predictor variables (above). We then square-root transformed the following predictors to down-weight the influence of values at the extreme ends of their distributions: total fish biomass, wastewater pollution, sediment input and nutrient loading. Pearson’s correlation coefficients were calculated among all predictors (Supplementary Fig. 8), removing highly correlated (r > 0.7) predictors. For the reasons outlined in our GAMM analysis and for continuity, we a priori excluded human population density and the biomass of browsers from the model-fitting process. This resulted in the following predictors included in the models (correlated predictors in parentheses were removed): total fish biomass, biomass of scrapers, biomass of grazers (total herbivore biomass), wastewater pollution, nutrient input, sediment input, urban runoff (phytoplankton biomass), wave exposure, fishing gear restrictions and depth. The decision of which correlated predictors to retain followed the same logic as our GAMM analysis. The mean and variability in SST were excluded given the negligible range of values among reefs (0.1 and 0.025 °C, respectively). All possible candidate models were computed while limiting the total number of predictors in any given candidate model to four (to reduce overfitting and to account for the lower response variable replication compared to our GAMM analysis). Models were computed using the multinomial logistic regression function mnrfit in MATLAB. We again used AICc for model comparison and all models within ΔAICc ≤ 2 of the top model (ΔAICc = 0) are presented in Extended Data Table 3. McFadden’s pseudo-R2 was computed for the highest ranked models and ranged from 0.21 to 0.22. Unlike traditional R2 values, McFadden’s pseudo-R2 of more than 0.2 represents an excellent fit98. Models within ΔAICc ≤ 2 of model 1 in Extended Data Table 3 demonstrated comparable levels of goodness of fit and parsimony99,100. Many of the parameter coefficients within these models were sensitive to the underlying variability in the data and their estimates did not differ significantly from zero (P < 0.05). The top model contained parameters with covariate estimates significantly different from zero, namely scraper biomass and wastewater pollution. Using model 1, we examined changes in the probability of a given reef having high (more than or equal to the 75th percentile), moderate (more than the 25th and less than the 75th percentile) or low (less than or equal to the 25th percentile) reef-builder cover (Fig. 4a) on the basis of variations in these two land–sea predictors (Fig. 4b). Probability curves for high, moderate and low were calculated on the basis of changing scraper biomass and wastewater pollution and holding all other predictors at their mean.
Resource management scenarios
The resource management scenarios presented in Fig. 4b were selected on the basis of the following rationale. We chose 250 kg ha−1 as the management target for scraper biomass as this value approximates the long-term mean (2003–2019; n = 17) biomass of scrapers within Kealakekua Bay, a marine protected area where no fishing has been allowed since 1969 (Supplementary Fig. 10). Kealakekua Bay is also exposed to numerous land-based stressors, including high levels of wastewater pollution (258,000 l h−1 in 2019). As such, our value of 250 kg ha−1 represents an estimate of scraper biomass on a reef with strong fisheries protection but with land-based stressors present. In addition, we compared our upper (250 kg ha−1) and lower (30 kg ha−1) scraper biomass values to the distribution of scraper biomass among all reefs (n = 80) in 2019, the most recent time point in which all reefs were surveyed within the same year (Supplementary Fig. 10). The upper and lower limits represent the 92nd and 36th percentiles, respectively. For wastewater pollution, we used our 2019, 100 m grid cell values that fell along the 10 m isobath (same as Fig. 1c) but constrained the latitudinal extent to be consistent with the northern- and southern-most locations of the 2019 reef surveys. This approach provided far greater replication and a more representative assessment of wastewater pollution along the coastline for which to assess our management scenarios. The upper (600,000 l ha−1) and lower (2,500 l h−1) values chosen for wastewater pollution represented the 95th and 36th percentiles of the 2019 distribution, respectively (Supplementary Fig. 11).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-023-06394-w.
Supplementary information
Acknowledgements
We thank A. Dillon of Aline Designs for graphics support, the numerous divers, boat drivers and support staff from the Hawai‘i Division of Aquatic Resources, National Park Service and The Nature Conservancy for logistical and data collection support, individuals from the Golf Course Superintendents Association of America, M. Johnson and R. Doog for their input in determining the golf course nitrogen application rates, R. Longman for contributing updated rainfall data, E. Darling for providing data layers from ref. 57, P. Neubauer and Y. Eynaud for analytical advice, W. Walsh for being an early supporter of this effort and J. Link and J. Samhouri for their review of an early version of the manuscript. This research was supported by NOAA’s Integrated Ecosystem Assessment Program (contribution no. 2022_7), NOAA’s Fisheries and The Environment Program, NOAA’s Pacific Islands Fisheries Science Center, and grants from the NOAA Coral Reef Conservation Program (grant no. NA16NOS4820059) and National Marine Fisheries Service, Office of Habitat Conservation (grant nos. EA133F17SE1203 and NA17NMF4630301) and the Harold K.L. Castle Foundation.
Extended data figures and tables
Author contributions
J.M.G., G.J.W. and J.L. conceived the study. J.M.G., G.J.W. and J.L. developed and implemented the analyses. J.M.G. and G.J.W. led the writing of the manuscript with G.P.A. and J.L. All other authors made substantive contributions to the manuscript and contributed data that were central to this effort.
Peer review
Peer review information
Nature thanks Joshua Cinner, Nicholas Graham, Peter Mumby and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All data that support the findings of this study are available at https://github.com/jamisongove/Coral-Reef-Persistence. Reef fish length–weight parameters were obtained from FishBase (https://fishbase.org) and ref. 56, human population data from NASA Gridded Population of the World v.4 (https://sedac.ciesin.columbia.edu/data/set/gpw-v4-population-count-rev11), land-use and land-cover data from the NOAA Coastal Change Analysis Program (https://www.coast.noaa.gov/htdata/raster1/landcover/bulkdownload/), soils data from USDA Gridded Soil Survey Geographic Database (gSSURGO; https://www.nrcs.usda.gov/resources/data-and-reports/gridded-soil-survey-geographic-gssurgo-database), subwatershed catchment data from USGS Stream Stats (https://water.usgs.gov/GIS/metadata/usgswrd/XML/ds680_archydrohucs.xml)74, watershed and digital elevation model data from USGS National Hydrography Dataset (https://www.usgs.gov/national-hydrography/national-hydrography-dataset), rainfall data from refs. 75,76, Landsat 8 satellite image from USGS (https://earthexplorer.usgs.gov/), Landsat 7 and 8 cloud-free composites derived using Google Earth Engine (https://earthengine.google.com/), individual wastewater systems for Hawai‘i from refs. 101,102, marine managed area designation from ref. 80 and downloadable from the State of Hawai‘i (https://planning.hawaii.gov/gis), fishing regulations from the State of Hawai‘i (https://dlnr.hawaii.gov/dar/fishing/fishing-regulations/), SST and DHW data from NOAA Coral Reef Watch (https://coralreefwatch.noaa.gov/product/5km), ocean colour (chlorophyll-a and irradiance) data from NOAA Coral Reef Watch (https://coralreefwatch.noaa.gov/product/oc/index.php) and ref. 80. See Methods and Supplementary Information for more detailed information on the data used to support the findings of this study.
Code availability
Statistical analyses were performed using the software packages R (www.r-project.org) v.4.0.2 (using libraries gamm4, MuMIn, foreach, doMC, ggplot2, gmt, tidyverse, zoo and lubridate)103, MATLAB (www.mathworks.com) using v.2021a (using Statistics and Machine Learning toolbox), ArcGIS Desktop (www.esri.com) v.10.6 with Advanced licensing and extensions Spatial Analyst and Geostatistical Analyst, Integrated Valuation of Ecosystem Services and Tradeoffs Sediment Delivery Ratio model (https://naturalcapitalproject.stanford.edu/software/invest) and the PERMANOVA+ (ref. 89) add-on for Primer v.7 (ref. 104). Code is available for download at https://github.com/jamisongove/Coral-Reef-Persistence.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jamison M. Gove, Gareth J. Williams
Contributor Information
Jamison M. Gove, Email: jamison.gove@noaa.gov
Gareth J. Williams, Email: g.j.williams@bangor.ac.uk
Extended data
is available for this paper at 10.1038/s41586-023-06394-w.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-023-06394-w.
References
- 1.Hughes TP, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 2.Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature. 2015;518:94–97. doi: 10.1038/nature14140. [DOI] [PubMed] [Google Scholar]
- 3.McLeod E, et al. The future of resilience-based management in coral reef ecosystems. J. Environ. Manage. 2019;233:291–301. doi: 10.1016/j.jenvman.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Taljaard S, et al. Implementing integrated coastal management in a sector-based governance system. Ocean Coast. Manage. 2012;67:39–53. doi: 10.1016/j.ocecoaman.2012.06.003. [DOI] [Google Scholar]
- 5.CBD. Kunming-Montreal Global Biodiversity Framework. In Proc. Conference of Parties to the Convention on Biological Diversity Fifteenth Meeting CBD/COP/15/L.25 (2022).
- 6.Tittensor DP, et al. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466:1098–1101. doi: 10.1038/nature09329. [DOI] [PubMed] [Google Scholar]
- 7.Kummu M, et al. Over the hills and further away from coast: global geospatial patterns of human and environment over the 20th–21st centuries. Environ. Res. Lett. 2016;11:034010. doi: 10.1088/1748-9326/11/3/034010. [DOI] [Google Scholar]
- 8.He Q, Silliman BR. Climate change, human impacts, and coastal ecosystems in the Anthropocene. Curr. Biol. 2019;29:R1021–R1035. doi: 10.1016/j.cub.2019.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Doney SC, et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012;4:11–37. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- 10.Andrew NL, Bright P, de la Rua L, Teoh SJ, Vickers M. Coastal proximity of populations in 22 Pacific Island countries and territories. PLoS ONE. 2019;14:e0223249. doi: 10.1371/journal.pone.0223249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacNeil MA, et al. Water quality mediates resilience on the Great Barrier Reef. Nat. Ecol. Evol. 2019;3:620–627. doi: 10.1038/s41559-019-0832-3. [DOI] [PubMed] [Google Scholar]
- 12.Oliver ECJ, et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018;9:1324. doi: 10.1038/s41467-018-03732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes TP, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 14.Hughes TP, et al. Global warming transforms coral reef assemblages. Nature. 2018;556:492–496. doi: 10.1038/s41586-018-0041-2. [DOI] [PubMed] [Google Scholar]
- 15.Edgar GJ, et al. Continent-wide declines in shallow reef life over a decade of ocean warming. Nature. 2023;615:858–865. doi: 10.1038/s41586-023-05833-y. [DOI] [PubMed] [Google Scholar]
- 16.Winter, K. B. et al. Indigenous stewardship through novel approaches to collaborative management in Hawai’i. Ecol. Soc.28, 26 (2023).
- 17.Sandin SA, et al. Harnessing island–ocean connections to maximize marine benefits of island conservation. Proc. Natl Acad. Sci. USA. 2022;119:e2122354119. doi: 10.1073/pnas.2122354119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpern BS, Lester SE, McLeod KL. Placing marine protected areas onto the ecosystem-based management seascape. Proc. Natl Acad. Sci. USA. 2010;107:18312–18317. doi: 10.1073/pnas.0908503107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall, P. A., Schuttenberg, H. Z. & West, J. M. A Reef Manager’s Guide to Coral Bleaching (Great Barrier Reef Marine Park Authority, 2006).
- 20.Mumby PJ, Chaloupka M, Bozec Y-M, Steneck RS, Montero-Serra I. Revisiting the evidentiary basis for ecological cascades with conservation impacts. Conserv. Lett. 2022;15:e12847. doi: 10.1111/conl.12847. [DOI] [Google Scholar]
- 21.Asner GP, et al. Mapped coral mortality and refugia in an archipelago-scale marine heat wave. Proc. Natl Acad. Sci. USA. 2022;119:e2123331119. doi: 10.1073/pnas.2123331119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donovan MK, et al. Local conditions magnify coral loss after marine heatwaves. Science. 2021;372:977–980. doi: 10.1126/science.abd9464. [DOI] [PubMed] [Google Scholar]
- 23.Baum JK, et al. Transformation of coral communities subjected to an unprecedented heatwave is modulated by local disturbance. Sci. Adv. 2023;9:eabq5615. doi: 10.1126/sciadv.abq5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams GJ, Gove JM, Eynaud Y, Zgliczynski BJ, Sandin SA. Local human impacts decouple natural biophysical relationships on Pacific coral reefs. Ecography. 2015;38:751–761. doi: 10.1111/ecog.01353. [DOI] [Google Scholar]
- 25.Smith JE, et al. Re-evaluating the health of coral reef communities: baselines and evidence for human impacts across the central Pacific. Proc. R. Soc. B. 2016;283:20151985. doi: 10.1098/rspb.2015.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maire E, et al. How accessible are coral reefs to people? A global assessment based on travel time. Ecol. Lett. 2016;19:351–360. doi: 10.1111/ele.12577. [DOI] [PubMed] [Google Scholar]
- 27.Maina J, et al. Human deforestation outweighs future climate change impacts of sedimentation on coral reefs. Nat. Commun. 2013;4:1986. doi: 10.1038/ncomms2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hozumi A, Hong PY, Kaartvedt S, Røstad A, Jones BH. Water quality, seasonality, and trajectory of an aquaculture-wastewater plume in the Red Sea. Aquacult. Environ. Inter. 2018;10:61–77. doi: 10.3354/aei00254. [DOI] [Google Scholar]
- 29.Brandl SJ, et al. Coral reef ecosystem functioning: eight core processes and the role of biodiversity. Front. Ecol. Environ. 2019;17:445–454. doi: 10.1002/fee.2088. [DOI] [Google Scholar]
- 30.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 31.Cinner JE, et al. Meeting fisheries, ecosystem function, and biodiversity goals in a human-dominated world. Science. 2020;368:307–311. doi: 10.1126/science.aax9412. [DOI] [PubMed] [Google Scholar]
- 32.Bozec Y-M, Yakob L, Bejarano S, Mumby PJ. Reciprocal facilitation and non-linearity maintain habitat engineering on coral reefs. Oikos. 2013;122:428–440. doi: 10.1111/j.1600-0706.2012.20576.x. [DOI] [Google Scholar]
- 33.Cinner JE, Graham NAJ, Huchery C, MacNeil MA. Global effects of local human population density and distance to markets on the condition of coral reef fisheries. Conserv. Biol. 2013;27:453–458. doi: 10.1111/j.1523-1739.2012.01933.x. [DOI] [PubMed] [Google Scholar]
- 34.Stopa JE, et al. Wave energy resources along the Hawaiian Island chain. Renew. Energy. 2013;55:305–321. doi: 10.1016/j.renene.2012.12.030. [DOI] [Google Scholar]
- 35.Skirving W, et al. CoralTemp and the Coral Reef Watch Coral Bleaching Heat Stress Product Suite version 3.1. Remote Sens. 2020;12:3856. doi: 10.3390/rs12233856. [DOI] [Google Scholar]
- 36.Glynn PW. Coral-reef bleaching - ecological perspectives. Coral Reefs. 1993;12:1–17. doi: 10.1007/BF00303779. [DOI] [Google Scholar]
- 37.Gove JM, et al. Near-island biological hotspots in barren ocean basins. Nat. Commun. 2016;7:10581. doi: 10.1038/ncomms10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitney JL, et al. Surface slicks are pelagic nurseries for diverse ocean fauna. Sci. Rep. 2021;11:3197. doi: 10.1038/s41598-021-81407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–1189. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- 40.Wooldridge SA. Water quality and coral bleaching thresholds: formalising the linkage for the inshore reefs of the Great Barrier Reef, Australia. Mar. Pollut. Bull. 2009;58:745–751. doi: 10.1016/j.marpolbul.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Fabricius KE. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 2005;50:125–146. doi: 10.1016/j.marpolbul.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 42.Nalley EM, et al. Water quality thresholds for coastal contaminant impacts on corals: a systematic review and meta-analysis. Sci. Total Environ. 2021;794:148632. doi: 10.1016/j.scitotenv.2021.148632. [DOI] [PubMed] [Google Scholar]
- 43.Carlson, R. R., Li, J., Crowder, L. B. & Asner, G. P. Large-scale effects of turbidity on coral bleaching in the Hawaiian islands. Front. Mar. Sci.9, 969472 (2022).
- 44.Carlson RR, Foo SA, Burns JHR, Asner GP. Untapped policy avenues to protect coral reef ecosystems. Proc. Natl Acad. Sci. USA. 2022;119:e2117562119. doi: 10.1073/pnas.2117562119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shantz AA, et al. Positive interactions between corals and damselfish increase coral resistance to temperature stress. Glob. Change Biol. 2023;29:417–431. doi: 10.1111/gcb.16480. [DOI] [PubMed] [Google Scholar]
- 46.Vargas-Ángel B, et al. El Niño-associated catastrophic coral mortality at Jarvis Island, central Equatorial Pacific. Coral Reefs. 2019;38:731–741. doi: 10.1007/s00338-019-01838-0. [DOI] [Google Scholar]
- 47.Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS. Recovery of an isolated coral reef system following severe disturbance. Science. 2013;340:69–71. doi: 10.1126/science.1232310. [DOI] [PubMed] [Google Scholar]
- 48.Tuholske C, et al. Mapping global inputs and impacts from of human sewage in coastal ecosystems. PLoS ONE. 2021;16:e0258898. doi: 10.1371/journal.pone.0258898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mezzacapo M, et al. Review article: Hawai’i’s cesspool problem: review and recommendations for water resources and human health. J. Cont. Wat. Res. Ed. 2020;170:35–75. [Google Scholar]
- 50.Meyer CG, Papastamatiou YP, Clark TB. Differential movement patterns and site fidelity among trophic groups of reef fishes in a Hawaiian marine protected area. Mar. Biol. 2010;157:1499–1511. doi: 10.1007/s00227-010-1424-6. [DOI] [Google Scholar]
- 51.Cinner, J. E. & Kittinger, J. N. in Ecology of Fishes on Coral Reefs (ed. Mora, C.) 215–220 (Cambridge Univ. Press, 2015).
- 52.van Hooidonk R, et al. Local-scale projections of coral reef futures and implications of the Paris Agreement. Sci. Rep. 2016;6:39666. doi: 10.1038/srep39666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinerstein E, et al. A global deal for nature: guiding principles, milestones, and targets. Sci. Adv. 2019;5:eaaw2869. doi: 10.1126/sciadv.aaw2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obura DO, et al. Achieving a nature- and people-positive future. One Earth. 2023;6:105–117. doi: 10.1016/j.oneear.2022.11.013. [DOI] [Google Scholar]
- 55.Jokiel PL, Brown EK, Friedlander A, Rodgers SKU, Smith WR. Hawai’i coral reef assessment and monitoring program: spatial patterns and temporal dynamics in reef coral communities. Pac. Sci. 2004;58:159–174. doi: 10.1353/psc.2004.0018. [DOI] [Google Scholar]
- 56.Donovan MK, et al. Combining fish and benthic communities into multiple regimes reveals complex reef dynamics. Sci. Rep. 2018;8:16943. doi: 10.1038/s41598-018-35057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClanahan TR, et al. Prioritizing key resilience indicators to support coral reef management in a changing climate. PLoS ONE. 2012;7:e42884. doi: 10.1371/journal.pone.0042884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cinner JE, et al. Bright spots among the world’s coral reefs. Nature. 2016;535:416–419. doi: 10.1038/nature18607. [DOI] [PubMed] [Google Scholar]
- 59.Cinner JE, et al. Gravity of human impacts mediates coral reef conservation gains. Proc. Natl Acad. Sci. USA. 2018;115:E6116–E6125. doi: 10.1073/pnas.1708001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kittinger JN, et al. From reef to table: social and ecological factors affecting coral reef fisheries, artisanal seafood supply chains, and seafood security. PLoS ONE. 2015;10:e0123856. doi: 10.1371/journal.pone.0123856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grafeld S, Oleson KLL, Teneva L, Kittinger JN. Follow that fish: uncovering the hidden blue economy in coral reef fisheries. PLoS ONE. 2017;12:e0182104. doi: 10.1371/journal.pone.0182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delaney DG, et al. Patterns in artisanal coral reef fisheries revealed through local monitoring efforts. PeerJ. 2017;5:e4089. doi: 10.7717/peerj.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heenan A, Williams ID. Monitoring herbivorous fishes as indicators of coral reef resilience in American Samoa. PLoS ONE. 2013;8:e79604. doi: 10.1371/journal.pone.0079604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green, A. L. & Bellwood, D. R. Monitoring Functional Groups of Herbivorous Reef Fishes as Indicators of Coral Reef Resilience: A Practical Guide for Coral Reef Managers in the Asia Pacific Region (IUCN, 2009).
- 65.Froese, R. & Pauly, D. FishBase: a global information system on fishes. FishBasewww.fishbase.org (2002).
- 66.CIESIN. Gridded population of the World, version 4 (GPWv4). NASA Socioeconomic Data and Applications Center (SEDAC)https://sedac.ciesin.columbia.edu/data/collection/gpw-v4 (2016).
- 67.Whittier, R. B. & El-Kadi, A. I. Human Health and Environmental Risk of Onsite Sewage Disposal Systems for the Hawaiian Islands of Kauai, Maui, Molokai, and Hawaii (Hawaii Department of Health, 2014).
- 68.Delevaux JMS, et al. A linked land-sea modeling framework to inform ridge-to-reef management in high oceanic islands. PLoS ONE. 2018;13:e0193230. doi: 10.1371/journal.pone.0193230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.GCSAA. Nutrient Use and Management Practices on U.S. Golf Courses. Golf Course Environmental Profile Volume II (GCSAA, 2016).
- 70.Brosnan, J. T. & Deputy, J. Bermudagrass. Turf Managementhttps://scholarspace.manoa.hawaii.edu/server/api/core/bitstreams/a09df1ab-e604-42b6-bd81-4f97cad6d060/content (2008).
- 71.Kunimatsu T, Sudo M, Kawachi T. Loading rates of nutrients discharging from a golf course and a neighboring forested basin. Water Sci. Technol. 1999;39:99–107. doi: 10.2166/wst.1999.0535. [DOI] [Google Scholar]
- 72.Wong JWC, Chan CWY, Cheung KC. Nitrogen and phosphorus leaching from fertilizer applied on golf course: lysimeter study. Water, Air, Soil Pollut. 1998;107:335–345. doi: 10.1023/A:1005096122921. [DOI] [Google Scholar]
- 73.Shuman LM. Phosphate and nitrate movement through simulated golf greens. Water, Air, Soil Pollut. 2001;129:305–318. doi: 10.1023/A:1010303025998. [DOI] [Google Scholar]
- 74.Rea A, Skinner KD. Geospatial datasets for watershed delineation and characterization used in the Hawai’i StreamStats web application. US Geol. Surv. Data Ser. 2012;680:12. [Google Scholar]
- 75.Longman RJ, et al. Compilation of climate data from heterogeneous networks across the Hawaiian Islands. Sci. Data. 2018;5:180012. doi: 10.1038/sdata.2018.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Longman RJ, Newman AJ, Giambelluca TW, Lucas M. Characterizing the uncertainty and assessing the value of gap-filled daily rainfall data in Hawai’i. J. Appl. Met. Clim. 2020;59:1261–1276. doi: 10.1175/JAMC-D-20-0007.1. [DOI] [Google Scholar]
- 77.Sharp, R. et al. InVEST Version 3.2.0 User’s Guide (The Natural Capital Project, The Nature Conservancy and World Wildlife Fund, 2015).
- 78.Hamel P, Chaplin-Kramer R, Sim S, Mueller C. A new approach to modeling the sediment retention service (InVEST 3.0): case study of the Cape Fear catchment, North Carolina, USA. Sci. Total Environ. 2015;524–525:166–177. doi: 10.1016/j.scitotenv.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 79.Falinski, K. A. Predicting Sediment Export into Tropical Coastal Ecosystems to Support Ridge to Reef Management. Thesis, Univ. Hawai’i at Manoa (2016).
- 80.Wedding LM, et al. Advancing the integration of spatial data to map human and natural drivers on coral reefs. PLoS ONE. 2018;13:e0189792. doi: 10.1371/journal.pone.0189792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Natural Resources Conservation Service. National Engineering Handbook–Part 630 Hydrology Ch. 10 (US Department of Agriculture, 2004).
- 82.Glysson, G. D. Sediment-Tansport Curves Report No. 87–218 (USGS, 1987).
- 83.Gove JM, et al. Quantifying climatological ranges and anomalies for Pacific coral reef ecosystems. PLoS ONE. 2013;8:e61974. doi: 10.1371/journal.pone.0061974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gove JM, et al. Coral reef benthic regimes exhibit non-linear threshold responses to natural physical drivers. Mar. Ecol. Prog. Ser. 2015;522:33–48. doi: 10.3354/meps11118. [DOI] [Google Scholar]
- 85.Tolman HL. A mosaic approach to wind wave modeling. Ocean Model. Online. 2008;25:35–47. doi: 10.1016/j.ocemod.2008.06.005. [DOI] [Google Scholar]
- 86.Booij N, Ris RC, Holthuijsen LH. A third-generation wave model for coastal regions: 1. model description and validation. J. Geophys. Res. (Oceans) 1999;104:7649–7666. doi: 10.1029/98JC02622. [DOI] [Google Scholar]
- 87.Li N, et al. Thirty-four years of Hawai’i wave hindcast from downscaling of climate forecast system reanalysis. Ocean Model. Online. 2016;100:78–95. doi: 10.1016/j.ocemod.2016.02.001. [DOI] [Google Scholar]
- 88.Graham NAJ, et al. Changing role of coral reef marine reserves in a warming climate. Nat. Commun. 2020;11:2000. doi: 10.1038/s41467-020-15863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anderson, M., Gorley, R. N. & Clarke, R. K. Permanova+ for Primer: Guide to Software and Statistical Methods (Primer-E Limited, 2008).
- 90.Anderson MJ, Willis TJ. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology. 2003;84:511–525. doi: 10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2. [DOI] [Google Scholar]
- 91.Côté IM, Gill JA, Gardner TA, Watkinson AR. Measuring coral reef decline through meta-analyses. Philos. Trans. R. Soc. Ser. B: Biol. Sci. 2005;360:385–395. doi: 10.1098/rstb.2004.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Graham NAJ, et al. Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems. PLoS ONE. 2008;3:e3039. doi: 10.1371/journal.pone.0003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wood S, Scheipl F, Wood MS. Package ‘gamm4’. Am. Stat. 2017;45:0.2–5. [Google Scholar]
- 94.R Core Team. MuMln: Multi-Model Inference R package v.1.13.4 (R Foundation for Statistical Computing, 2015).
- 95.Hurvich CM, Tsai C-L. Regression and time series model selection in small samples. Biometrika. 1989;76:297–307. doi: 10.1093/biomet/76.2.297. [DOI] [Google Scholar]
- 96.Safaie A, et al. High frequency temperature variability reduces the risk of coral bleaching. Nat. Commun. 2018;9:1671. doi: 10.1038/s41467-018-04074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brant R. Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics. 1990;46:1171–1178. doi: 10.2307/2532457. [DOI] [PubMed] [Google Scholar]
- 98.McFadden, D. Conditional Logit Analysis of Qualitative Choice Behavior (Academic Press, 1974).
- 99.Burnham, K. P. & Anderson, D. R. (eds) Model selection and multimodel inference. A practical information-theoretic approach (Springer, 1998).
- 100.Richards SA. Testing ecological theory using the information-theoretical approach: examples and cautionary results. Ecology. 2005;86:2805–2814. doi: 10.1890/05-0074. [DOI] [Google Scholar]
- 101.Individual Wastewater System Database (Hawai’i Department of Health, 2017).
- 102.Underground Injection Control Permit Application Files (Hawai’i Department of Health, 2017).
- 103.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing https://www.R-project.org/ (2021).
- 104.Clarke, K. & Gorley, R. Getting started with PRIMER v.7. PRIMER-E: Plymouth (Plymouth Marine Laboratory, 2015).
- 105.Beyer HL, et al. Risk-sensitive planning for conserving coral reefs under rapid climate change. Conserv. Lett. 2018;11:e12587. doi: 10.1111/conl.12587. [DOI] [Google Scholar]
- 106.Darling ES, et al. Social–environmental drivers inform strategic management of coral reefs in the Anthropocene. Nat. Ecol. Evol. 2019;3:1341–1350. doi: 10.1038/s41559-019-0953-8. [DOI] [PubMed] [Google Scholar]
- 107.Andrello M, et al. A global map of human pressures on tropical coral reefs. Conserv. Lett. 2022;15:e12858. doi: 10.1111/conl.12858. [DOI] [Google Scholar]
- 108.Adam TC, Burkepile DE, Ruttenberg BI, Paddack MJ. Herbivory and the resilience of Caribbean coral reefs: knowledge gaps and implications for management. Mar. Ecol. Prog. Ser. 2015;520:1–20. doi: 10.3354/meps11170. [DOI] [Google Scholar]
- 109.Williams ID, et al. Human, oceanographic and habitat drivers of Central and Western Pacific coral reef fish assemblages. PLoS ONE. 2015;10:e0120516. doi: 10.1371/journal.pone.0120516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnson JV, Dick JTA, Pincheira-Donoso D. Local anthropogenic stress does not exacerbate coral bleaching under global climate change. Global Ecol. Biogeogr. 2022;31:1228–1236. doi: 10.1111/geb.13506. [DOI] [Google Scholar]
- 111.Wear SL, Thurber RV. Sewage pollution: mitigation is key for coral reef stewardship. Ann. N. Y. Acad. Sci. 2015;1355:15–30. doi: 10.1111/nyas.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jokiel PL, Hunter CL, Taguchi S, Watarai L. Ecological impact of a fresh-water ‘reef kill’ in Kaneohe Bay, Oahu, Hawai’i. Coral Reefs. 1993;12:177–184. doi: 10.1007/BF00334477. [DOI] [Google Scholar]
- 113.Rodgers KuS, et al. Impact to coral reef populations at Hā‘ena and Pila‘a, Kaua‘i, following a record 2018 freshwater flood event. Diversity. 2021;13:66. doi: 10.3390/d13020066. [DOI] [Google Scholar]
- 114.Dollar SJ. Wave stress and coral community structure in Hawai’i. Coral Reefs. 1982;1:71–81. doi: 10.1007/BF00301688. [DOI] [Google Scholar]
- 115.Storlazzi CD, Brown EK, Field ME, Rodgers K, Jokiel PL. A model for wave control on coral breakage and species distribution in the Hawaiian Islands. Coral Reefs. 2005;24:43–55. doi: 10.1007/s00338-004-0430-x. [DOI] [Google Scholar]
- 116.Chassot E, et al. Global marine primary production constrains fisheries catches. Ecol. Lett. 2010;13:495–505. doi: 10.1111/j.1461-0248.2010.01443.x. [DOI] [PubMed] [Google Scholar]
- 117.Duarte C, Cebrian J. The fate of marine autotrophic production. Limnol. Oceanogr. 1996;41:1758–1766. doi: 10.4319/lo.1996.41.8.1758. [DOI] [Google Scholar]
- 118.Weis VM. Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J. Exp. Biol. 2008;211:3059–3066. doi: 10.1242/jeb.009597. [DOI] [PubMed] [Google Scholar]
- 119.Gonzalez-Espinosa PC, Donner SD. Cloudiness reduces the bleaching response of coral reefs exposed to heat stress. Glob. Change Biol. 2021;27:3474–3486. doi: 10.1111/gcb.15676. [DOI] [PubMed] [Google Scholar]
- 120.MacNeil MA, et al. Recovery potential of the world’s coral reef fishes. Nature. 2015;520:341–344. doi: 10.1038/nature14358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are available at https://github.com/jamisongove/Coral-Reef-Persistence. Reef fish length–weight parameters were obtained from FishBase (https://fishbase.org) and ref. 56, human population data from NASA Gridded Population of the World v.4 (https://sedac.ciesin.columbia.edu/data/set/gpw-v4-population-count-rev11), land-use and land-cover data from the NOAA Coastal Change Analysis Program (https://www.coast.noaa.gov/htdata/raster1/landcover/bulkdownload/), soils data from USDA Gridded Soil Survey Geographic Database (gSSURGO; https://www.nrcs.usda.gov/resources/data-and-reports/gridded-soil-survey-geographic-gssurgo-database), subwatershed catchment data from USGS Stream Stats (https://water.usgs.gov/GIS/metadata/usgswrd/XML/ds680_archydrohucs.xml)74, watershed and digital elevation model data from USGS National Hydrography Dataset (https://www.usgs.gov/national-hydrography/national-hydrography-dataset), rainfall data from refs. 75,76, Landsat 8 satellite image from USGS (https://earthexplorer.usgs.gov/), Landsat 7 and 8 cloud-free composites derived using Google Earth Engine (https://earthengine.google.com/), individual wastewater systems for Hawai‘i from refs. 101,102, marine managed area designation from ref. 80 and downloadable from the State of Hawai‘i (https://planning.hawaii.gov/gis), fishing regulations from the State of Hawai‘i (https://dlnr.hawaii.gov/dar/fishing/fishing-regulations/), SST and DHW data from NOAA Coral Reef Watch (https://coralreefwatch.noaa.gov/product/5km), ocean colour (chlorophyll-a and irradiance) data from NOAA Coral Reef Watch (https://coralreefwatch.noaa.gov/product/oc/index.php) and ref. 80. See Methods and Supplementary Information for more detailed information on the data used to support the findings of this study.
Statistical analyses were performed using the software packages R (www.r-project.org) v.4.0.2 (using libraries gamm4, MuMIn, foreach, doMC, ggplot2, gmt, tidyverse, zoo and lubridate)103, MATLAB (www.mathworks.com) using v.2021a (using Statistics and Machine Learning toolbox), ArcGIS Desktop (www.esri.com) v.10.6 with Advanced licensing and extensions Spatial Analyst and Geostatistical Analyst, Integrated Valuation of Ecosystem Services and Tradeoffs Sediment Delivery Ratio model (https://naturalcapitalproject.stanford.edu/software/invest) and the PERMANOVA+ (ref. 89) add-on for Primer v.7 (ref. 104). Code is available for download at https://github.com/jamisongove/Coral-Reef-Persistence.