Abstract
Background:
Repeated implantation failure (RIF) is considered one of the major challenges facing clinician in assisted reproduction technologies (ART) despite the significant advances that have been made in this field. Platelet rich plasma (PRP), also known as autologous conditioned plasma, is a protein concentrate with anti-inflammatory and pro-regenerative characteristics. The use of PRP in women undergoing ART has been studied in the past, with varying degrees of success. The goal of this trial was to see if injecting PRP into the uterus improves pregnancy outcomes in women receiving ART.
Methods:
PubMed, Embase, Scopus, Web of Science, and the Cochrane Database of Clinical Trials were among the databases searched (CENTRAL), from 2015 to 2021. The pooled estimates were calculated using a meta-analysis with a random-effects model. There were 14 studies with a total of 1081 individuals (549 cases and 532 controls).
Results:
There was no difference in miscarriage rates between women who got PRP and those who received placebo (P≤0.90). Chemical pregnancy (P≤0.00), clinical pregnancy (P ≤0.001), and implantation rate (P≤ 0.001) were all significantly higher in women. Endometrial thickness increased in women who got PRP vs women who received placebo after the intervention (P ≤0.001).
Conclusion:
PRP may be an alternate therapeutic approach for individuals with thin endometrium and RIF, according to the findings of this comprehensive study. To determine the subgroup that would benefit the most from PRP, more prospective, big, and high-quality randomized controlled trials (RCTs) are needed.
Keywords: Clinical pregnancy, Implantation rate, Platelet rich plasma, Randomized controlled trials
Introduction
A worldwide problem in terms of public health, infertility affects 13% of all couples. Approximately 5% of women will experience two consecutive miscarriages, over 75% of which are due to an implantation failure and are never diagnosed as clinical pregnancies. Implantation is a complicated process that needs both the mother as a host and the embryo to work well. The recurring failure of embryos to implant following their transfer can be referred to as repeated implantation failure (RIF) (1,2). RIF can occur at any one of the three stages of the implantation process: apposition, adhesion, or invasion (3). Failure to implant a fertilized egg into the uterine lining can be caused by a number of factors, but the majority of cases fall into one of these four categories: abnormal embryonic defects; abnormal embryo-endometrial cross-talk; impairment in the regulation of immunologic mediators; and altered endometrial receptivity (4).
In order to combat RIF and to achieve pregnancy, both male and female gametes are employed in assisted reproductive technology (ART), a range of medical techniques for treating infertile people. Approximately 5 million children have been born through ART to date (5). In ART, implantation process is influenced by a variety of variables, including immunological variables, endometrial receptivity, and embryo quality (6). The various techniques such as thin endometrium treatment (7), endometrial stimulation (8–9), blastocyst transfer (10), cytoplasmic transfer (11) and intrauterine administration of autologous peripheral blood mononuclear have all been used in ART to increase the implantation rate and subsequently the chance of a live birth in couples with RIF (12–13). However, many patients still have RIF despite these ART modalities. As a result, patients with a history of treatment failure need an alternative therapy that has a higher success rate.
Platelet-rich plasma (PRP) has recently been infused intrauterine to encourage endometrial development and receptivity. With the help of PRP, some success has recently been made in the treatment of RIF and thin endometrium (14–16). The PRP, also known as autologous conditioned plasma, is a protein concentrate with anti-inflammatory and pro-regenerative properties that is made from fresh whole blood and processed to eliminate red blood cells. It is a concentrate of platelet-rich plasma protein with any platelet concentration over that of baseline whole blood, which ranges between 150000 and 450 000 per microliter. Because of its autologous origin, PRP injectate may vary based on the specific manufacturer and procedure used to administer it (17). The primary rationale for employing PRP in patients who have experienced prior ET failures is based on the control of growth factor and cytokine expression in the endometrium, which was first introduced by Chang et al., 2015 (18). Studies have shown contradictory outcomes when it comes to the use of PRP in women undergoing ART (19–24).
The purpose of this analysis was to determine if intrauterine infusion of PRP improves pregnancy end points in women taking ART.
Methods
Types of studies
This systematic review and meta-analysis assessed the efficacy of intrauterine (IU) PRP infusion with control (placebo or other therapy) in improving clinical outcome following assisted reproduction in subfertile women. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards and the Cochrane Handbook for Systematic Reviews of Interventions were followed.
Criteria for considering studies for this review
Studies were considered for inclusion in our evaluation if they met the following requirements: It was an RCT, quasi-experimental, and cohort study in which the objectives were medically verified pregnancy outcomes (live birth, clinical pregnancy, and miscarriage), and it was a randomised controlled trial. ii) The intervention consisted of an intrauterine infusion of platelet-rich plasma (PRP) around the time of embryo transfer (ET), iii) the control group consisted of any other active intervention, no intervention, or a placebo, and iv) the population of interest consisted of subfertile women undergoing assisted reproduction with any ovarian stimulation protocol, including no ovarian stimulation protocol. The following types of research were excluded: case-control studies, case series studies, cross-sectional studies, and animal or cell culture studies. In addition, research were eliminated if we were unable to gather sufficient information about the study methodology or outcomes.
Search methods for identification of studies
A systematic search was conducted using Medline (through PubMed), Embase, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials to identify potential studies (from inception to august 2021). In addition, the reference and citation lists of published publications were hand-searched to find additional research that met the criteria. We also looked for unpublished and in-press research in grey literature (clinical trials registries, conference proceedings). (“In Vitro Fertilization” OR “IVF” OR “Intracytoplasmic sperm injection” OR “ICSI” OR “Embryo transfer” AND “Platelet-rich plasma” OR “PRP” OR “Autologous platelet-rich plasma” OR “Platelet rich plasma gel”) were among the search phrases used.
Electronic searches
Databases searched included PubMed, Embase, Scopus, Web of Science and the Cochrane Database of Clinical Trials (CENTRAL). As the number of studies are large enough to support generalization inference beyond the included studies, meta-analysis using random-effects model was performed to calculate the pooled estimates. This model was appropriate for undertaking uncertainty resulting from heterogeneity among studies.
Data synthesis
We collected pregnancy outcomes from each of the included trials by treatment stratum and computed the RR with 95 percent CI for each endpoint in PRP vs control women. Review Manager 5.3 was used to conduct statistical analysis (Cochrane Collaboration, 2014). Subgroup analyses were used to determine the effect of IU PRP infusion on pregnancy outcomes, taking into account relevant study characteristics such as embryo stage at transfer (cleavage versus blastocyst), PRP dosage (0.5 ml, 1 ml, and 1ml), study population (RIF versus thin endometrium), and study design (RCT versus cohort).
Description of studies
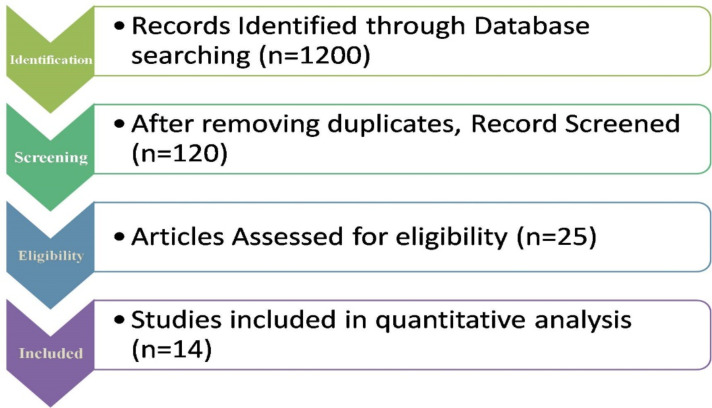
A preliminary search of the electronic literature returned 1200 results (From PubMed, Embase, Scopus, Web of Science, Cochrane Library and other sources). Reference management software was used to save all of the citations (Mendeley). These citations’ titles and abstracts were examined to exclude extraneous publications, yielding 25 possibly qualifying research. After reviewing the complete content of the articles listed above, 11 were eliminated. There were 14 case series, one case report, and one that didn’t offer enough data to be included in the meta-analysis. Fig.1 depicts the flow diagram of the literature search and study selection process.
Fig.1:
Flow diagram of the literature search and study selection process
Results
Table 1 outlines the main characteristics of all included studies. Studies were conducted between 2015 and 2021, of which 13 studies were published after 2016. The studies were conducted in Iran (9 studies) (21, 22, 23, 25, 26, 27, 28, 16, 29)., Russia (1study) (30)., India (1 study) (31)., Turkey (1 study), (32)., UK (1 study) (33) and one in China, (24). Seven studies were RCTs (16, 22, 23, 25, 28, 29, 33) and seven were cohort (21, 24, 26, 27, 30, 31, 32). The population in all studies except two were patients with RIF. All studies compared PRP versus placebo except one that compared PRP versus Granulocyte Colony Stimulating Factor (GCSF) (22). The sample size ranged from 24 to 123 participants. Three studies administered the PRPwith dose ≤ 0.5 mL (21, 23, 29), two studies with dose 0.5 to 1 mL (16, 28) and two studies with dose ≥ 1 mL (22, 26). Three studies transferred the embryo in cleavage stage and the remaining studies in blastocyst stage. All studies transferred the embryo in freeze condition.
Table 1:
Study characteristics
| Study (Ref) | Country | Study design | Population | Sample size | Intervention(s) | Control | Transfer type | Outcome Measures | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Case | Control | ||||||||
| Allahveisi et al., 2020 (16) | Iran | Randomized Clinical trial | Women with repeated failed implantation | 25 | 25 | Intrauterine infusion of 0.5 ml of PRP was performed 48 h before embryo transfer | Intrauterine infusion of 0.5 ml of Ringer serum was done 48 h before embryo transfer | Frozen embryo transfer | The intrauterine infusion of PRP before frozen embryo transfer in infertile women with a history of Failed implantation will not make any significant effect on the result of pregnancy. |
| Zamaniyan et al., 2020 (28) | Iran | Randomized controlled trial | Women with recurrent implantation failure | 55 | 43 | The 0.5 ml of intrauterine infusion of platelet-rich plasma at 4–6 times higher concentration than peripheral blood infused intrauterine 48 h before embryo transfer. | Standard protocol | Frozen embryo transfer | Intrauterine infusion of platelet-rich plasma 48 h before freeze thawed embryo transfer may have more effectiveness in in vitro fertilization (IVF) outcomes in recurrent implantation failure. |
| Zargar et al., 2021 (29) | Iran | Prospective randomized Trial | Infertile women with at least two IVF failures | 40 | 40 | Intrauterine infusion of PRP | Patients without PRP injection | Frozen embryo transfer | Endometrial injection of platelet-rich plasma for IVF failure patients did not significantly improve the IVF process, pregnancy, and live birth rates. |
| Aghajanzadeh et al., 2020 (27) | Iran | Women suffering from repeated implantation failure | 30 | 0 | Intrauterine infusions of autologous purified platelet preparations | Standard protocol | Frozen embryo transfer | Platelet-rich plasma might potentially yield beneficial effects as a safe therapeutic option offered alongside other treatments designed to improve the reproductive outcomes of women with repeated implantation failure. | |
| Frantz et al., 2020 (26) | Iran | Retrospective study | Patients with and without a history of having undergone frozen embryo transfers. | 24 | 0 | Platelet-Rich plasma | Standard protocol | Frozen embryo transfer | PRP improves intrauterine receptivity to embryo implantation, regardless Of whether the endometrium reached the appropriate growth for embryo transfer. |
| Tehraninejadet al., 2020 (25) | Iran | Patients suffering from repeated implantation failure | 42 | 43 | 1 ml of PRP was extracted from 10 cc of whole blood via two rounds of centrifugation and infused 2 days before the embryo transfer | Standard protocol | Frozen embryo transfer | PRP is not an effective adjuvant treatment for in vitro fertilization of patients with repeated implantation failure and normal endometrial thickness undergoing embryo transfer. | |
| Melo et al., 2019 (33) | UK | Non-randomized interventional study | Women with low ovarian reserve undergoing fertility Treatment | 46 | 37 | 3-month course of intracortical injections of autologous platelet-rich plasma (PRP) upon ovarian reserve markers | No Intervention | Frozen embryo transfer | PRP injections are effective and safe to improve markers of low ovarian reserve prior to assisted reproductive technology |
| Eftekhar et al., 2018 (21) | Iran | Randomized Clinical trial | Women with thin endometrium (endometrium thickness < 7 mm) | 40 | 43 | HRT+intrauterine infusion of 0.5 to 1 ml PRP on the 13th day of HRT cycle | HRT without intrauterine infusion of PRP | Frozen embryo transfer | Chemical pregnancy, Clinical pregnancy, Miscarriage, Endometrial thickness |
| Obidniak et al., 2017 (30) | Russia | Randomized Clinical trial | RIF, normal karyotype, absence of uterine factors of infertility, absence of chromosomal abnormalities in previous pregnancy | 45 | 45 | Underwent ET with Intrauterine infusion of 2.0 ml of autologous PRP | Underwent ET without intrauterine administration | Frozen embryo transfer | Implantation Rate, Clinical pregnancy |
| Nazari et al., 2019 (23) | Iran | Randomized Clinical trial | RIF: three or more failures of IVF-ET therapy without poor ovarian reserve | 49 | 48 | Intrauterine infusion of 1 ml of platelet-rich plasma 48 hrs. Before blastocyst transfer | Underwent ET without intrauterine administration | Frozen embryo transfer | Chemical pregnancy, Clinical pregnancy |
| Madhavan, et al., 2015 (31) | India | Cohort | RIF: two or more failures of IVF-ET therapy without poor ovarian reserve | 42 | 56 | Patients who received intrauterine infusion of 0.3–0.4 ml PRP on Day 8/9 of the HRT | Underwent ET without intrauterine administration | Frozen embryo transfer | Clinical Pregnancy |
| Chang et al., 2019 (34) | China | Cohort | Patients with thin endometrium | 34 | 30 | HRT+intrauterine infusion of 0.5 to 1 ml PRP on the 13th day of HRT cycle | Underwent ET Without intrauterine administration | Frozen embryo transfer | Implantation Rate, Clinical pregnancy, Miscarriage, Endometrial thickness |
| Mehrafza et al., 2019 (22) | Iran | Cohort | Patients with history of more than 2 repeated failed embryo transfer cycles | 67 | 56 | Intrauterine infusion of 1 ml of PRP | Underwent ET without intrauterine administration | Frozen embryo transfer | Implantation Rate, Chemical pregnancy, Clinical pregnancy |
| Coksueret al., 2019 (32) | Turkey | Cohort | RIF: one or more failures of IVF-ET therapy without poor ovarian reserve | 34 | 36 | Intrauterine infusion of 1 ml of PRP | Underwent ET without intrauterine administration | Frozen embryo transfer | Clinical pregnancy, Miscarriage, Live Birth |
Risk of bias assessment
For random allocation, all of the trials were found to have a minimal risk of bias. For one experiment, allocation concealment was shown to have a significant risk of bias. Half of the studies were found to have an uncertain risk of performance bias, while one research had a high risk of participant and personnel blinding. All except one of the studies were found to have a minimal probability of attrition bias. The exposed and non-exposed subjects in all cohort studies were drawn from the same community sample. All of the studies had sufficient diagnostic criteria for the outcomes of interest, as well as a detailed description of how the outcomes were measured. Only one research used an acceptable approach to account for confounding.
Clinical pregnancy
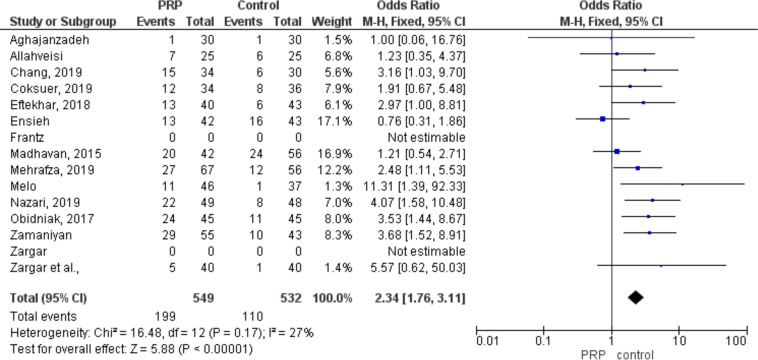
Pooling results from 14 studies, which compared clinical pregnancy between PRP and control (placebo or other active intervention), including 1081 participants (549 cases and 532 controls), showed a significantly higher probability of clinical pregnancy in PRP group (RR: 2.34, 95% CI 1.76 to 3.11; P< 0.001. (Fig. 2).
Fig. 2:
Forest plot detailed odds ratio and 95% CI for clinical pregnancy
Chemical pregnancy
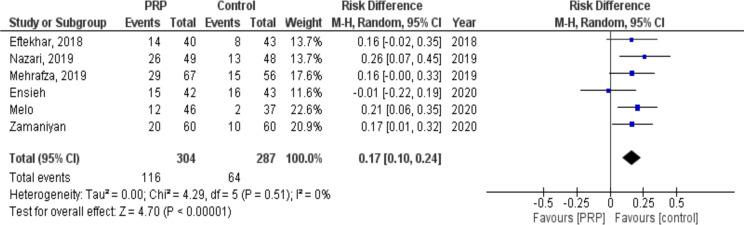
Six studies with 591 participants (304 cases and 287 controls) compared chemical pregnancy between PRP and control (placebo or other active intervention) groups. The probability of chemical pregnancy was significantly higher in women who received PRP compared with control (RR: 0.17, 95% CI: 0.10 to 0.24; P< 0.00001, I2= 0%. The results of the I2 statistics showed that there was no heterogeneity among the investigations (Fig. 3).
Fig. 3:
Forest plot showing individual and combined effect size estimates and 95% CI in studies that evaluated the risk of chemical pregnancy in women who received intrauterine platelet rich plasma versus control
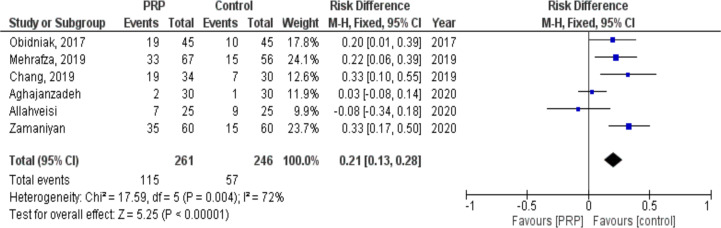
Implantation rate
The effect of PRP on implantation rate was evaluated in six studies involving 507 subjects (261 cases and 246 controls). Following the intervention, implantation rate significantly increased in patients who received PRP compared to controls (RR: 0.21, 95% CI: 0.13 to 0.28; P< 0.00001, I2= 72%. There was a heterogeneity of 72% among the studies Fig. 4).
Fig. 4:
Forest plot showing individual and combined effect size estimates and 95% CI in studies that evaluated the risk of implantation rate in women who received intrauterine platelet rich plasma versus control
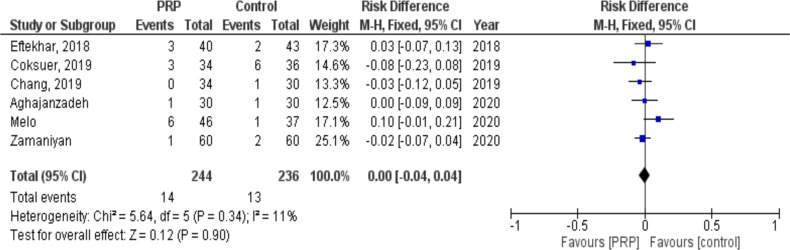
Miscarriage
We retrieved six studies with 480 subjects (244 cases and 236 controls) in which miscarriage was compared between PRP and placebo groups. There was no difference between women who received PRP compared with placebo regarding miscarriage (RR: 0.00, 95% CI: −0.04 to 0.04; P= 0.90, I2= 11%. It was shown that there was a heterogeneity of 11% among the studies (Fig. 5).
Fig. 5:
Forest plot showing individual and combined effect size estimates and 95% CI in studies that evaluated the risk of miscarriage in women who received intrauterine platelet rich plasma versus control
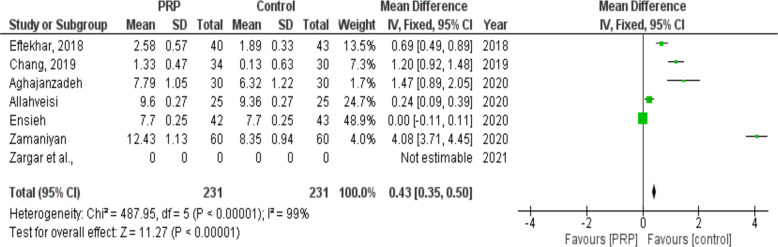
Endometrial thickness
Changes in endometrial thickness following PRP infusion were examined in six studies (231 cases and 231 controls). Following the intervention, endometrial thickness increased in women who received PRP compared to women who received placebo (SMD: 0.43, 95% CI: 0.35 to 0.50; P< 0.00001, I2= 99%. There was a heterogeneity of 99 % among the studies Fig. 6).
Fig. 6:
Forest plot showing individual and combined effect size estimates and 95% CI in studies that evaluated the standardized mean difference of endometrial thickness in women who received intrauterine platelet rich plasma versus control
Discussion
In this analysis, we examined 14 trials that investigated the impact of intrauterine infusion of PRP for 1075 women (573 cases and 502 controls) who were having a frozen-thawed ET cycle and had thin endometrium (two studies) or recurrent implantation failure (five studies). Patients in the PRP group had better beneficial effects than those in the placebo group in terms of clinical pregnancy, chemical pregnancy, implantation rate, and endometrial thickness, and the advantages persisted after subgroup analysis took into account PRP dosage (0.5 ml, 1 ml, and 0.5 ml), study population (RIF versus thin endometrium), and study design (RCT versus cohort). Five studies evaluated the live birth rate between PRP and controls, which is the most significant main outcome of assisted reproduction. However, the majority of these studies did not disclose implantation rates, miscarriage rates, or endometrial thickness measurements.
Several studies reporting these results found that the implantation rate, chemical pregnancy rate, and endometrial thickness were considerably greater in women who got PRP as compared to those who did not get PRP. The statistical measure of homogeneity, heterogeneity, was low across all pregnancy outcomes evaluated, indicating that the effects were consistent throughout the trials. A subgroup analysis based on research design revealed that PRP had a greater impact on clinical trial outcomes than on cohort studies, as demonstrated by the findings of the study. In addition, following subgroup analysis, the heterogeneity across studies was decreased, with nil reported in clinical trials and 11 percent recorded in cohort studies after subgroup analysis.
An evidence-based medical system built on a hierarchical system of classification of evidence, often known as the levels of evidence, is a cornerstone of evidence-based medicine (EBM). The study’s design, as well as the outcomes measured, have an impact on the strength of the evidence. The Oxford CEBM Levels of Evidence recommendations classify individual randomized controlled trials with tight CI as level 1b of evidence, but cohort studies are classed three levels below and in the 2b level of evidence, according to the CEBM Levels of Evidence guidelines (34). As a result, we may draw more certain conclusions about the effectiveness of PRP in women who have had a frozen-thawed ET cycle. The subgroup of research that transmitted the embryo at the cleavage stage did not show a statistically significant difference, while the subset of studies that sent the embryo at the blastocyst stage did show a statistically significant rise in the number of clinical pregnancies.
A number of factors, including greater physiological synchronization, improved embryo selection for transfer, increased live birth rates, and complete chromosomal screening, have contributed to the current trend toward moving from cleavage stage to blastocyst stage embryo transfer (ET). The blastocyst stage ET, on the other hand, has been associated with more unfavourable pregnancy outcomes, including as premature delivery and congenital abnormalities, according to many studies (35). In a review by Maheshvari, the evidence presented clearly demonstrates that the available data at this time are weak and do not justify discontinuing blastocyst stage embryo transfer. However, in the interest of long-term outcomes, a larger RCT of day 3 versus day 5 embryo transfer, involving highly experienced laboratories and including longer term follow-up data on offspring outcome are required to provide conclusive evidence for discontinuing blastocyst stage embryo transfer (36).
Intra-uterine injection of platelet-rich plasma (PRP), regardless of research design or study population, enhances the clinical pregnancy rate in women who have had a frozen-thawed embryo transfer cycle (24). The researchers discovered that the impact of PRP in a subgroup of trials that gave PRP at a dosage of 0.5 to 1mL was greater than the effect of PRP in studies that delivered PRP at doses of less than 0.5 mL and less than 1 mL. The interpretation of this discovery is complicated by the existence of a significant issue. None of the studies considered provided sufficient information on the procedure used for PRP preparation, the processing equipment utilized, the spinning parameters used, the platelet concentration, the WBC count, the growth factor analysis performed, or the activation of PRP employed.
It is recommended in a systematic study that a formula be used to compare the efficacy of PRP. There are four components to this formula: PRP volume, the platelet rich plasma platelet concentration, the whole blood volume, and the whole blood platelet concentration (37–38). It is difficult to determine the precise function of PRP in the implantation process. However, numerous potential processes have been postulated, including the ones listed below: The movement of human primary endometrial epithelial cells, endometrial stromal fibroblasts, and endometrial mesenchymal stem cells (MSC) as well as bone marrow-derived MSC was enhanced by activated PRP, according to the findings (2). as a result of their regulatory effects on proliferation, apoptosis, inflammation, cell adhesion, chemotaxis, and immunological responses during blastocyst implantation The growth factors VEGF, TGF-β, PDGF, IGF1, EGF, and HGF are some of the growth factors that stimulate cell regeneration, proliferation, and vascularization in the body (4). Cell migration by chemo attraction, mesenchymal to epithelial trans-differentiation, and inflammation, which may be the most essential. 5. It has a stimulatory impact on the production of numerous proinflammatory cytokines (IL1A, IL1B, IL1R2), chemokines (CCL5, CCL7, CXCL13), and matrix-metalloproteins (MMPs) (MMP3, MMP7, MMP26) (39–45).
Women who have had an ET cycle that has been frozen and thawed had higher clinical pregnancy rates, according to our systematic review and meta-analysis. This is true regardless of the research design or the study population. Three trials have shown that endometrial injection of platelet-rich plasma for IVF failure patients did not result in a substantial improvement in the IVF procedure, pregnancy rates, or live birth rates compared to standard treatment. These results is reassuring the previous study done by Hijiagha et al (46). We believe that PRP increases intrauterine responsiveness to embryo implantation, regardless of whether or not the endometrium has grown to the necessary size to allow for embryo transfer to take place. More randomised clinical trials with higher sample sizes are required, even if the findings of this research did not demonstrate substantial heterogeneity as a consequence of the study design, study population, or embryo stage at the time of the transfer. Furthermore, since complete data on pregnant problems and poor pregnancy outcomes was not available, we were unable to offer definitive findings in this study.
Conclusion
In this analysis of 14 trials involving 1075 women undergoing frozen-thawed embryo transfer with thin endometrium or recurrent implantation failure, intrauterine infusion of PRP showed significant improvements in clinical pregnancy, chemical pregnancy, implantation rate, and endometrial thickness compared to the placebo group. The advantages persisted across different PRP dosages and study designs. However, more research with larger sample sizes and comprehensive data on pregnancy outcomes is needed to draw definitive conclusions about the effectiveness of PRP in assisted reproduction.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The study was self-funded.
Footnotes
Conflict of interest
None declared.
References
- 1.Simon A, Laufer N. (2012). Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet,29(11):1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinehart J. (2007). Recurrent implantation failure: definition. J Assist Reprod Genet, 24(7):284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoozemans DA, Schats R, Lambalk CB, et al. (2004). Human embryo implantation: current knowledge and clinical implications in assisted reproductive technology. Reprod Biomed Online, 9(6):692–715. [DOI] [PubMed] [Google Scholar]
- 4.Diedrich K, Fauser BCJM, Devroey P, et al. (2007). The role of the endometrium and embryo in human implantation. Hum Reprod Update, 13(4):365–377. [DOI] [PubMed] [Google Scholar]
- 5.Kissin DM, Jamieson DJ, Barfield WD. (2014). Monitoring health outcomes of assisted reproductive technology. N Engl J Med, 371: 91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen VM, Wilson RD, Cheung A. (2006). Genetics Committee, Reproductive Endocrinology and Infertility Committee. Pregnancy outcomes after assisted reproductive technology. J Obstet Gynaecol Can,28(3):220–233. [DOI] [PubMed] [Google Scholar]
- 7.Lebovitz O, Orvieto R. (2014). Treating patients with “thin” endometrium - an ongoing challenge. Gynecol Endocrinol, 30(6):409–414. [DOI] [PubMed] [Google Scholar]
- 8.Paulson RJ. (2011). Hormonal induction of endometrial receptivity. Fertil Steril,96(3):530–535. [DOI] [PubMed] [Google Scholar]
- 9.Bourgain C, Devroey P. (2003). The endometrium in stimulated cycles for IVF. Hum Reprod Update,9(6):515–22. [DOI] [PubMed] [Google Scholar]
- 10.lujovsky D, Farquhar C, Quinteiro Retamar AM, et al. (2016). Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev, 6: CD002118. [DOI] [PubMed] [Google Scholar]
- 11.Sobek A, Tkadlec E, Klaskova E, et al. (2020). Cytoplasmic Transfer Improves Human Egg Fertilization and Embryo Quality: an Evaluation of Sibling Oocytes in Women with Low Oocyte Quality. Reprod Sci,28(5):1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshioka S, Fujiwara H, Nakayama T, et al. (2006). Intrauterine administration of autologous peripheral blood mononuclear cells promotes implantation rates in patients with repeated failure of IVF-embryo transfer. Hum Reprod,21(12):3290–4. [DOI] [PubMed] [Google Scholar]
- 13.Yu N, Zhang B, Xu M, et al. (2016). Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) activated by HCG improves the implantation and pregnancy rates in patients with repeated implantation failure: a prospective randomized study. Am J Reprod Immunol,76(3):212–6. [DOI] [PubMed] [Google Scholar]
- 14.Zadehmodarres S, Salehpour S, Saharkhiz N, et al. (2017). Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod,21(1):54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell SJ, Kwok YSS, Nguyen TTTN, et al. (2022). Autologous platelet-rich plasma improves the endometrial thickness and live birth rate in patients with recurrent implantation failure and thin endometrium. J Assist Reprod Genet 39: 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allahveisi A, Seyedoshohadaei F, Rezaei M, et al. (2020). The effect of platelet-rich plasma on the achievement of pregnancy during frozen embryo transfer in women with a history of failed implantation. Heliyon, 6(3): e03577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos-Mikich A, de Oliveira R, Frantz N. (2018). Platelet-rich plasma therapy and reproductive medicine. J Assist Reprod Genet, 35(5): 753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Y, Li J, Chen Y, et al. (2015). Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med,8(1):1286–1290. [PMC free article] [PubMed] [Google Scholar]
- 19.Eftekhar M, Neghab N, Naghshineh E, et al. (2018). Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan J Obstet Gynecol, 57(6):810–813. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Li J, Wei L N, et al. (2019). Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine, 98(3):e14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GKim H, Shin JE, Koo HS, et al. (2019). Effect of Autologous Platelet-Rich Plasma Treatment on Refractory Thin Endometrium During the Frozen Embryo Transfer Cycle. A Pilot Study. Front Endocrinol (Lausanne),10: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehrafza M, Kabodmehri R, Nikpouri Z, et al. (2019). Comparing the Impact of Autologous Platelet-rich Plasma and Granulocyte Colony Stimulating Factor on Pregnancy Outcome in Patients with Repeated Implantation Failure. J Reprod Infertil, 20(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- 23.Nazari L, Salehpour S, Hosseini M S, et al. (2020). The effects of autologous platelet-rich plasma in repeated implantation failure: a randomized controlled trial. Hum Fertil (Camb), 23(3):209–213. [DOI] [PubMed] [Google Scholar]
- 24.Maleki-Hajiagha A, Razavi M, Rezaeinejad M, et al. (2019). Intrauterine administration of autologous peripheral blood mononuclear cells in patients with recurrent implantation failure: A systematic review and meta-analysis. J Reprod Immunol, 131: 50–56. [DOI] [PubMed] [Google Scholar]
- 25.Tehraninejad E S, Kashani NG, Hosseini A, et al. (2021). Autologous platelet-rich plasma infusion does not improve pregnancy outcomes in frozen embryo transfer cycles in women with history of repeated implantation failure without thin endometrium. J Obstet Gynaecol Res, 47(1):147–151. [DOI] [PubMed] [Google Scholar]
- 26.Frantz N, Ferreira M, Kulmann M I, et al. (2020). Platelet-Rich plasma as an effective alternative approach for improving endometrial receptivity - a clinical retrospective study. JBRA Assist Reprod, 24(4):442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aghajanzadeh F, Esmaeilzadeh S, Basirat Z, et al. (2020). Using autologous intrauterine platelet-rich plasma to improve the reproductive outcomes of women with recurrent implantation failure. JBRA Assist Reprod, 24(1):30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamaniyan M, Peyvandi S, Heidaryan Gorji, et al. (2021). Effect of platelet-rich plasma on pregnancy outcomes in infertile women with recurrent implantation failure: a randomized controlled trial. Gynecol Endocrinol, 37(2):141–145. [DOI] [PubMed] [Google Scholar]
- 29.Zargar M, Pazhouhanfar P, Najafian M, et al. (2021). Effects of intrauterine autologous platelet-rich plasma infusions on outcomes in women with repetitive in vitro fertilization failures: a prospective randomized study. Clin Exp Obstet Gynecol, 48(1):179–184). [Google Scholar]
- 30.Obidniak D, Gzgzyan A, Feoktistov, et al. (2017). Randomized controlled trial evaluating efficacy of autologous platelet-rich plasma therapy for patients with recurrent implantation failure. Fertil Steril, 108 (3): E370. [Google Scholar]
- 31.Madhavan A, Naidu P, Rani K Kaur, et al. (2018). Intrauterine autologous platelet-rich plasma therapy to improve implantation rates in patients undergoing frozen embryo transfer: A pilot study. The Onco Fertility Journal, 1(2):81–85. [Google Scholar]
- 32.Coksuer H, Akdemir Y, Ulas Barut M. (2019). Improved in vitro fertilization success and pregnancy outcome with autologous platelet-rich plasma treatment in unexplained infertility patients that had repeated implantation failure history. Gynecol Endocrinol,35(9):815–818. [DOI] [PubMed] [Google Scholar]
- 33.Melo P, Navarro C, Jones C, et al. (2020). The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. J Assist Reprod Genet, 37(4):855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eldredge JD. (2000). Evidence-based librarianship: an overview. Bull Med Libr Assoc, 88(4):289–302. [PMC free article] [PubMed] [Google Scholar]
- 35.Glujovsky D, Farquhar C. (2016). Cleavage-stage or blastocyst transfer: what are the benefits and harms? Fertil Steril, 106(2):244–250. [DOI] [PubMed] [Google Scholar]
- 36.Maheshwari A, Hamilton M, Bhattacharya S. (2016). Should we be promoting embryo transfer at blastocyst stage? Reprod Biomed Online, 32(2):142–146. [DOI] [PubMed] [Google Scholar]
- 37.Alves R, Grimalt R. (2018). A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord, 4(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fadadu PP, Mazzola AJ, Hunter CW, et al. (2019). Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: a call for PRP standardization. Reg Anesth Pain Med, 2018–100356. [DOI] [PubMed] [Google Scholar]
- 39.Sánchez AR, Sheridan PJ, Kupp LI, et al. (2003). Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants, 18(1):93–103. [PubMed] [Google Scholar]
- 40.Bendinelli P, Matteucci E, Dogliotti G, et al. (2010). Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol, 225(3):757–766. [DOI] [PubMed] [Google Scholar]
- 41.Filardo G, Kon E, Roffi A, et al. (2015). Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc, 23(9):2459–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Hicks JJ, Wang L, et al. (2016). Customized platelet-rich plasma with transforming growth factor β1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials, 87: 147–156. [DOI] [PubMed] [Google Scholar]
- 43.Meheux CJ, McCulloch PC, Lintner DM, et al. (2016). Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthroscopy, 32(3):495–505. [DOI] [PubMed] [Google Scholar]
- 44.Dai WL, Zhou A G, Zhang H, et al. (2017). Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy, 33(3):659–670.e1. [DOI] [PubMed] [Google Scholar]
- 45.Gibreel A, El-Adawi N, Elgindy E, et al. (2015). Endometrial scratching for women with previous IVF failure undergoing IVF treatment. Gynecol Endocrinol, 31(4):313–316. [DOI] [PubMed] [Google Scholar]
- 46.Maleki-Hajiagha A, Razavi M, Rouholamin S, et al. (2020). Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: A systematic review and meta-analysis. J Reprod Immunol,137:103078. [DOI] [PubMed] [Google Scholar]