Abstract
Background
Observational studies have indicated an association between polyunsaturated fatty acids (PUFAs) and chronic pain, but the potential causal link remains controversial. Here, we aimed to investigate whether a causal relationship exists between the concentration of circulating PUFAs and chronic pain as well as the direction of this association.
Methods
We collected statistical data from relevant genome-wide association studies to explore the causal link between four PUFAs, along with the ratio of omega-6 fatty acids (FAs) to omega-3 FAs (omega-6:3 ratio), and chronic pain in eight specific body parts. We used the inverse-variance weighting (IVW) method for two-sample Mendelian randomization (MR) analysis and conducted supplementary analyses using four other methods (MR-Egger, weighted median, weighted mode, and simple mode). To verify the robustness of the MR study, we performed multiple sensitivity analyses.
Results
The results revealed a negative correlation between omega-3 FAs [IVW, OR 95% CI: 0.952 (0.914, 0.991), p = 0.017] and docosahexaenoic acid (DHA) [IVW, OR 95% CI: 0.935 (0.893, 0.978), p = 0.003] with abnormal and pelvic pain. Furthermore, a positive correlation was observed between the omega-6:3 ratio [IVW, OR 95% CI: 1.057 (1.014, 1.101), p = 0.009] with abdominal and pelvic pain. Additionally, we found a negative correlation between omega-3 FAs [IVW, OR 95% CI: 0.947 (0.902, 0.994), p = 0.028] and lower back pain or sciatica. However, no causal relationship was found between the concentration of circulating PUFAs and pain in other body parts, including the face, throat and chest, joints, limbs, lower back, and gynecological parts. The robustness of these MR results was verified through multi-validity and retention method analyses.
Conclusion
Our analysis suggests that higher circulating concentrations of omega-3 FAs and DHA and a lower omega-6:3 ratio are associated with a reduced risk of abdominal and pelvic pain. Additionally, a higher concentration of circulating omega-3 FAs is linked to a reduced risk of lower back pain and/or sciatica. These findings have major implications for the targeted prevention and treatment of chronic pain using PUFAs.
Keywords: chronic pain, polyunsaturated fatty acid, Mendelian randomization, causal inference, genetics
1. Introduction
Chronic pain is defined as a pain sensation that persists or recurs for over 3 months (1). Over 30% of the global population currently suffers from chronic pain, making it a major public health issue (2). Unlike acute pain, chronic pain is long-lasting and can persist for years or even longer, imposing a substantial burden on individuals and the economy (3, 4). Therefore, identifying risk and protective factors for chronic pain is crucial for its prevention and treatment.
Polyunsaturated fatty acids (PUFAs) are essential fatty acids (FAs) that cannot be synthesized by the body and must be obtained through dietary intake (5). PUFAs are key components of cell membranes and play a crucial role in cell structure and function (6). Additionally, PUFAs are involved in regulating inflammatory response, antithrombotic processes, lipid metabolism, and other physiological functions (7–9). Observational studies have shown an association between PUFA intake and the occurrence and severity of chronic pain (10–13). Omega-3 FAs can reduce inflammatory markers in chondrocytes, exerting anti-inflammatory and analgesic effects, whereas omega-6 FAs can promote inflammation and induce pain (14). However, a five-year randomized controlled study conducted in the United States found that omega-3 FA supplementation did not improve knee pain caused by osteoarthritis (15). Therefore, the causal relationship between PUFAs and chronic pain remains unclear.
Mendelian randomization (MR) utilizes genetic variation as an instrumental variable to infer causal relationships between exposure and disease outcome (16). By following the Mendelian inheritance law of “random distribution of parental alleles to offspring” during gamete formation, genetic variation is not influenced by traditional confounding factors such as socioeconomic status, environmental exposure, and behavioral factors. Thus, MR overcomes the limitations of traditional epidemiological studies, which can be affected by confounding factors and reverse causality (17). In this study, we aimed to investigate the causal relationship and its directionality between circulating PUFA concentrations and pain in different body sites using a two-sample MR analysis with large-scale open-access genome-wide association study (GWAS) pooled data.
2. Materials and methods
2.1. Study design
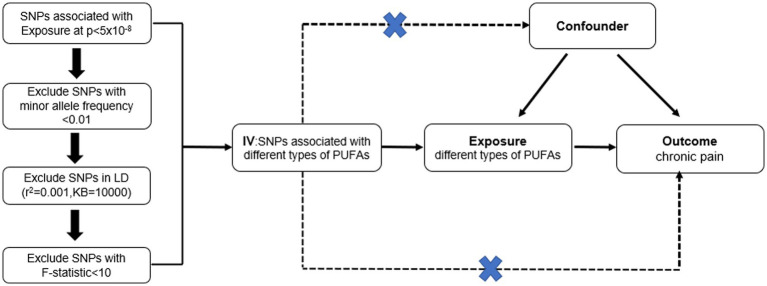
In this study, MR was used to analyze the causal relationship between concentrations of circulating PUFAs, including omega-3 FAs, omega-6 FAs, linoleic acid, and docosahexaenoic acid (DHA), along with the ratio of omega-6 FAs to omega-3 FAs (omega-6:3 ratio), and pain in eight sites (Figure 1). The pain locations included the face, throat and chest, abdomen and pelvis, lower back and/or sciatica, joints, limbs, low back, and gynecological parts.
Figure 1.
Mendelian randomized study design between different types of polyunsaturated fatty acid and chronic pain in different body parts.
MR is a method used to infer a causal relationship between exposure factors and outcomes by analyzing the association between genetic variation and exposure factors, as well as genetic variation and outcomes. Therefore, MR studies require instrumental variables that meet three core assumptions: (1) strong and robust correlation between instrumental variables and exposure factors (association assumption); (2) independence of instrumental variables from confounding factors that affect the exposure–outcome relationship (independence hypothesis); (3) genetic variation affecting outcomes only through exposure factors and not through other pathways (exclusivity hypothesis) (18–20).
2.2. Data sources
All data from this study are publicly available GWAS summary statistics; therefore, no additional ethical approval or informed consent was required. Pooled data on circulating PUFA concentrations and pain in different sites were obtained from the IEU OPEN GWAS PROJECT database.1 To avoid the bias of population heterogeneity, only pooled data from European populations were used. Detailed information on the GWAS datasets is provided in Table 1.
Table 1.
The list of Genome-wide summary association studies (GWAS) included in the Mendelian randomization study.
| Exposures | GWAS ID | Population | Year | Consortium | Participants | No. SNP |
|---|---|---|---|---|---|---|
| Omega-3 FAs | met-d-Omega_3 | European | 2020 | UK biobank | 114,999 | 12,321,875 |
| Omega-6 FAs | met-d-Omega_6 | European | 2020 | UK biobank | 114,999 | 12,321,875 |
| Linoleic acid | met-d-LA | European | 2020 | UK biobank | 114,999 | 12,321,875 |
| DHA | met-d-DHA | European | 2020 | UK biobank | 114,999 | 12,321,875 |
| RO63 | met-d-Omega_6_by_ Omega_3 | European | 2020 | UK biobank | 114,999 | 12,321,875 |
| Outcome | GWAS ID | Population | Year | Consortium | Participants | No. SNP |
|---|---|---|---|---|---|---|
| Atypical facial pain | finn-b-G6_ATYFAC | European | 2021 | Finn Gen | 701 cases/195,047 controls | 16,380,408 |
| Pain in throat and chest | finn-b-R18_PAIN_ THROAT_CHEST | European | 2021 | Finn Gen | 24,609 cases/ 163,123 controls | 16,380,345 |
| Abdominal and pelvic pain | finn-b-R18_ABDOMI_ PELVIC_PAIN | European | 2021 | Finn Gen | 49,416cases/ 161,968 controls | 16,380,396 |
| Lower back pain or/and sciatica | finn-b-M13_LOWBACK PAINORANDSCIATICA | European | 2021 | Finn Gen | 19,509 cases/ 199,283 controls | 16,380,466 |
| Pain in joint | finn-b-JOINTPAIN | European | 2021 | Finn Gen | 13,419 cases/ 131,550 controls | 16,380,073 |
| Pain in limb | finn-b-M13_LIMBPAIN | European | 2021 | Finn Gen | 12,606 cases/ 167,641 controls | 16,380,354 |
| Low back pain | finn-b-M13_LOWBACK PAIN | European | 2021 | Finn Gen | 13,178 cases/ 164,682 controls | 16,380,287 |
| Gynecological related pain | finn-b-N14_FEMGEN PAIN | European | 2021 | Finn Gen | 3,316 cases/ 68,969 controls | 16,376,838 |
FAs, fatty acids; DHA, Docosahexaenoic acid; No. SNP, number of SNPs included in the analysis; RO63, ratio of omega-6 fatty acids to omega-3 fatty acids; Gynecological related pain, pain and other conditions associated with female genital organs and menstrual cycle.
GWAS data on circulating PUFA concentrations were obtained from the UK biobank and included 114,999 European participants. The GWAS data for pain in eight body parts were obtained from the Finland database. The detailed sample sizes were as follows: atypical facial pain (701 cases and 195,047 controls), throat and chest pain (24,609 cases and 163,123 controls), abdominal and pelvic pain (49,416 cases and 161,968 controls), lower back pain or sciatica (19,509 cases and 199,283 controls), joint pain (13,419 cases and 131,550 controls), limb pain (12,606 cases and 167,641 controls), low back pain (13,178 cases and 164,682 controls), and gynecological-related pain (pain and other conditions associated with female genital organs and menstrual cycle; 3,316 cases and 68,969 controls). We performed an MR analysis using single nucleotide polymorphisms (SNPs) associated with circulating PUFA concentrations as exposure factors and pain in different body sites as outcome factors.
2.3. Selection of IVs
To fulfill the first MR hypothesis that SNPs must be strongly associated with circulating PUFA concentrations, SNPs significantly associated with circulating PUFA concentrations (p < 5 × 10−8, r2 < 0.001, genetic distance = 10,000 kb) were selected at the genome-wide level. Thereafter, to satisfy the second MR hypothesis that genetic variation is not associated with potential confounders, we queried the Phenoscanner database2 to ensure that the selected SNPs were not associated with known confounders. Finally, the F statistic was calculated to evaluate the presence of weak instrumental variable bias for the selected instrumental variables. F > 10 indicates the absence of weak instrumental variable bias, further supporting the association hypothesis. The calculation formula for F was as follows: F = [R2/(1 – R2)] × [(N-K-1)/K], where N is the sample size of exposure factors, K is the number of instrumental variables, and R2 is the proportion of variation of exposure factors explained by instrumental variables (21).
2.4. MR statistical analysis
We used two-sample MR to examine the causal relationship and its directionality between circulating PUFA concentrations and pain in different body sites. Inverse variance weighting (IVW) was used as the primary analysis method, whereas weighted median, MR-Egger, weighted mode, and simple mode were used as supplementary analysis methods. Cochrane Q value and MR-Egger intercept were used to assess heterogeneity and horizontal pleiotropy, respectively (22). The MR-Pleiotropy RESidual Sum and Outlier (MR-PRESSO) method was employed to detect and adjust for potential pleiotropy (23). Additionally, a leave-one-out analysis was conducted by deleting one SNP at a time to examine the influence of SNPs with potential horizontal pleiotropy on MR estimates (24). Sensitivity analyses were visually illustrated using scatter, funnel, and forest plots to demonstrate the robustness of the MR study. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to present the causal effect between circulating PUFA concentrations and pain in different body sites. All statistical analyses were performed using R software (version 4.2.1) with the “TwoSampleMR” (25) and “MR-PRESSO” (26) packages. A significance level of p < 0.05 was considered to indicate a causal relationship.
3. Results
3.1. GWAS of PUFAs and pain in different body parts
We extracted 29–50 strongly associated SNPs from the GWAS dataset of circulating PUFA concentrations. The SNPs corresponding to different types of circulating PUFA concentrations are presented as specific features of IVs in Supplementary Tables 1–6. F-values were calculated for all SNPs with a value greater than 10.
3.2. Primary MR analysis
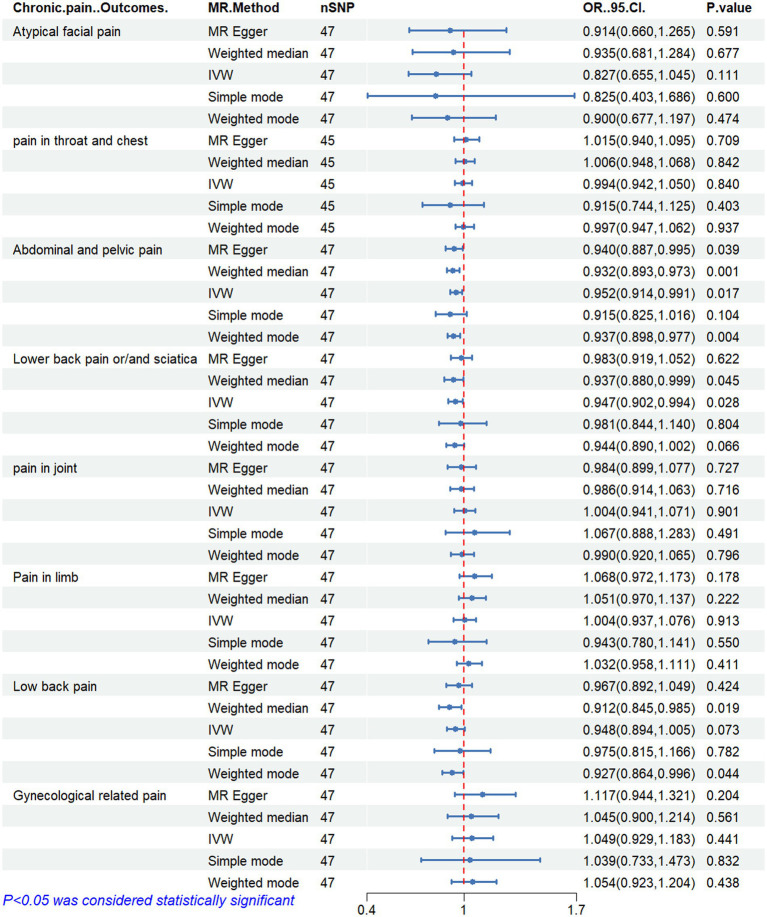
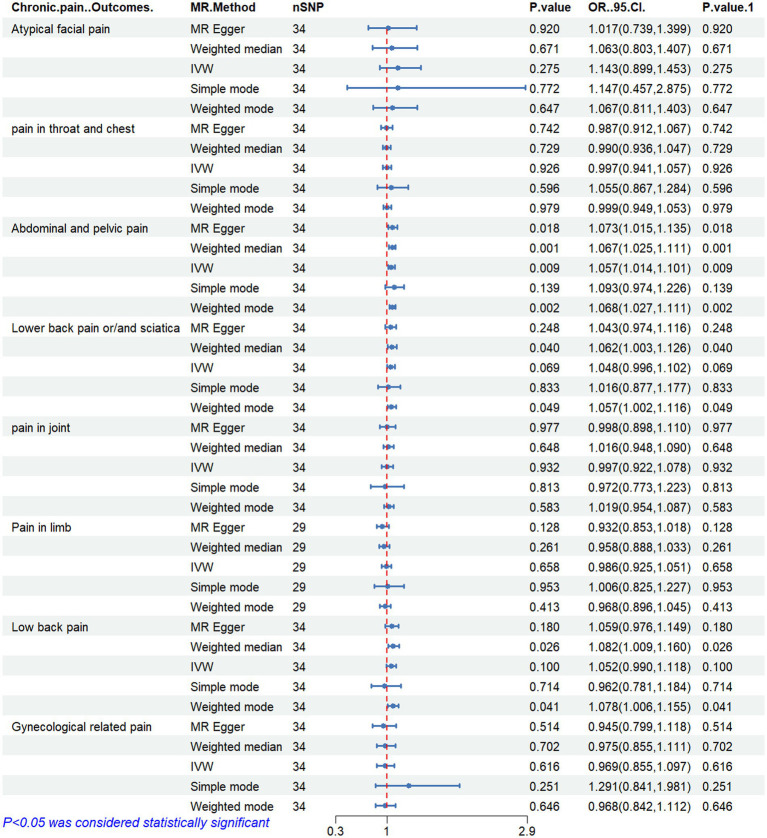
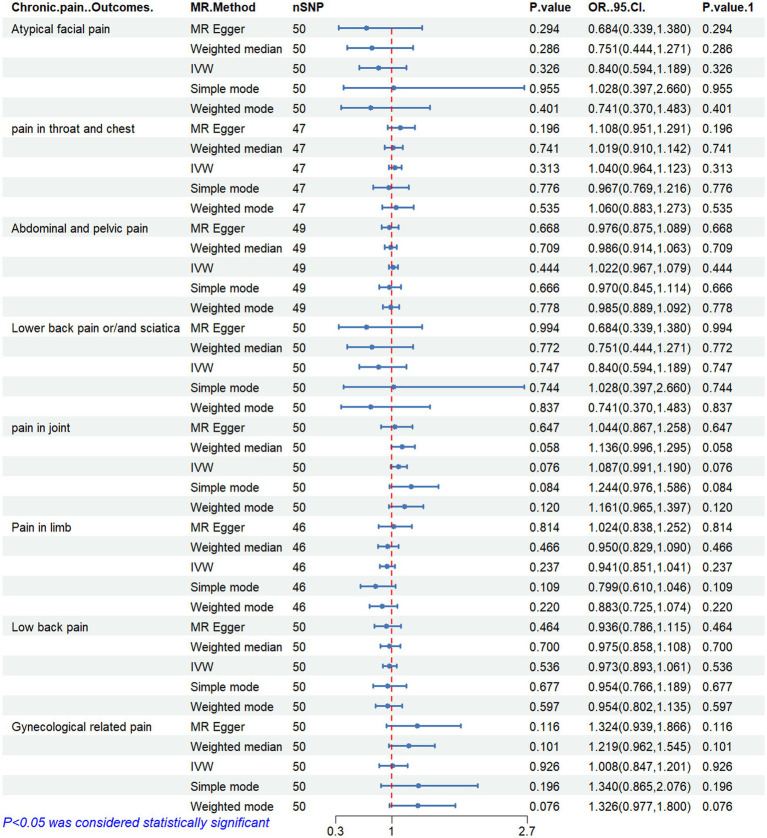
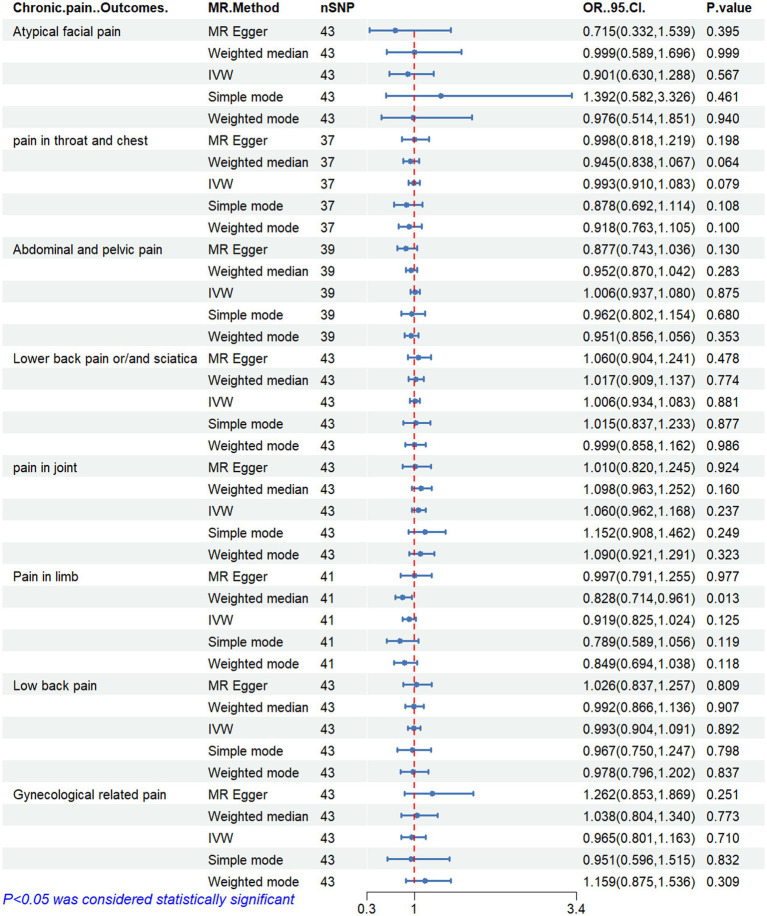
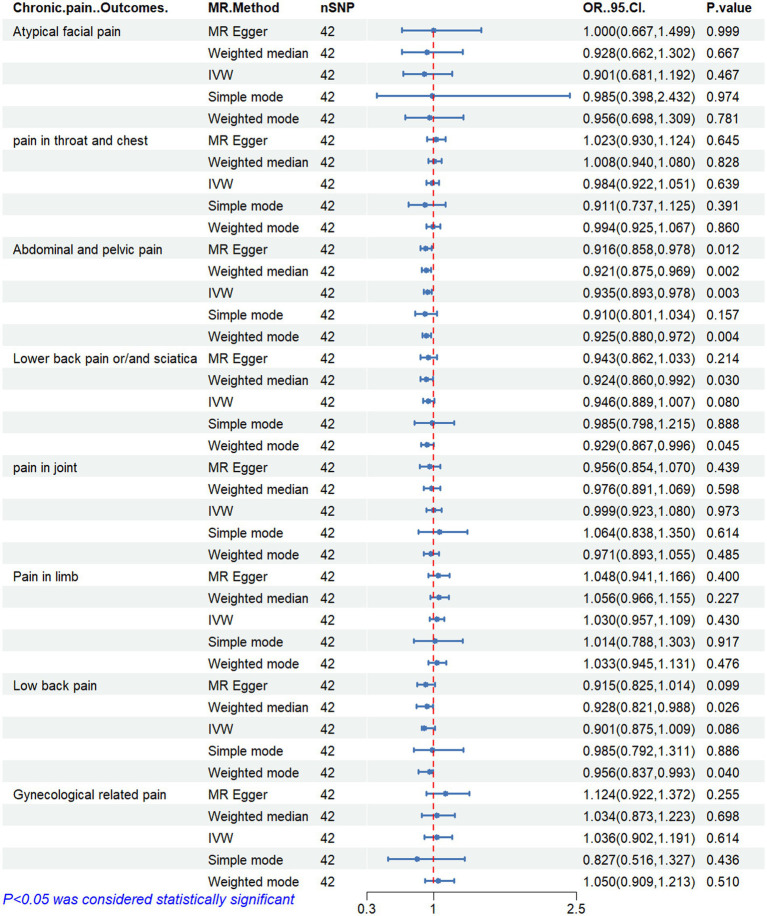
Figures 2–6 display the results of MR analysis examining the association between circulating PUFA concentrations and pain in different anatomical sites. In this MR study, we observed a negative correlation between omega-3 FAs [IVW, OR 95% CI: 0.952 (0.914, 0.991), p = 0.017] and DHA [IVW, OR 95% CI: 0.935 (0.893, 0.978), p = 0.003] with abdominal and pelvic pain. In contrast, the omega-6:3 ratio [IVW, OR 95% CI: 1.057 (1.014, 1.101), p = 0.009] showed a positive correlation with abdominal and pelvic pain. Additionally, we found that omega-3 FAs [IVW, OR 95% CI: 0.947 (0.902, 0.994), p = 0.028] were negatively correlated with lower back pain and/or sciatica. However, no causal link was observed between circulating PUFA concentrations and pain in other body sites, as measured using IVW (p > 0.05). Scatter plots (Supplementary Figures 1–5) were used to visualize the effect size for each MR analysis.
Figure 2.
MR analysis results of Omega-3 fatty acids and pain in different body parts. No. SNP, number of SNPs included in the analysis; OR, Odds ratio; CI, confidence interval; IVW, Inverse-variance-weighted.
Figure 6.
MR analysis results of ratio of omega-6 fatty acids to omega-3 fatty acids and pain in different body parts. No. SNP, number of SNPs included in the analysis; OR, Odds ratio; CI, confidence interval; IVW, Inverse-variance-weighted.
Figure 3.
MR analysis results of Omega-6 fatty acids and pain in different body parts. No. SNP, number of SNPs included in the analysis; OR, Odds ratio; CI, confidence interval; IVW, Inverse-variance-weighted.
Figure 4.
MR analysis results of linoleic acid and pain in different body parts. No. SNP, number of SNPs included in the analysis; OR, Odds ratio; CI, confidence interval; IVW, Inverse-variance-weighted.
Figure 5.
MR analysis results of docosahexaenoic acid and pain in different body parts. No. SNP, number of SNPs included in the analysis; OR, Odds ratio; CI, confidence interval; IVW, Inverse-variance-weighted.
3.3. Supplementary and sensitivity analyses
In this MR study, we used additional analysis methods, namely weighted median, MR-Egger, weighted mode, and simple mode, as supplementary analyses to complement the primary IVW method. The Q and p values of MR-Egger and IVW analyses were used to assess heterogeneity, while the MR-Egger intercept and its p-value were used to detect pleiotropy. In cases where heterogeneity was present, causality was determined using a random effects model. The MR-PRESSO analysis was used to reduce heterogeneity and pleiotropy in the estimates of causal effects by removing outliers and reassessing causality. Notably, the MR-Egger intercept method did not detect any potential horizontal pleiotropy (p > 0.05), indicating the robustness of the study results. The outcomes of the heterogeneity and pleiotropy analyses are presented in Table 2. Additionally, a leave-one-out sensitivity analysis was conducted to verify the effect of each SNP site on the overall causal relationship. The results demonstrated that systematically removing individual SNPs and repeating the MR analysis did not yield significant differences in the aforementioned causal relationships (Supplementary Figures 6–10). Funnel plots depict the balanced distribution of each SNP (Supplementary Figures 11–15), and forest plots display estimates of individual SNP results (Supplementary Figures 16–20).
Table 2.
Sensitivity analysis of Mendelian randomization studies of polyunsaturated fatty acids and different body parts.
| Traits (Outcome) | Fatty acids (Exposure) | Heterogeneity test | MR-Egger pleiotropy test | MR-PRESSO | F statistics | ||
|---|---|---|---|---|---|---|---|
| p-value | |||||||
| Q-value | p-value | Intercept | p-value | ||||
| Atypical facial pain | Omega-3 FAs | 42.665 | 0.613 | −0.012 | 0.391 | 0.637 | 264.64 |
| Omega-6 FAs | 50.003 | 0.433 | 0.013 | 0.511 | 0.385 | 123.56 | |
| Linoleic Acid | 41.797 | 0.480 | 0.015 | 0.507 | 0.475 | 129.93 | |
| DHA RO63 | 40.887 30.124 | 0.476 0.611 | −0.011 0.018 | 0.487 0.284 | 0.561 0.655 | 210.76336.64 | |
| Pain in throat and chest | Omega-3 FAs | 60.663 | 0.048 | −0.002 | 0.454 | 0.066 | 264.54 |
| Omega-6 FAs | 52.953 | 0.224 | −0.004 | 0.356 | 0.255 | 118.22 | |
| Linoleic Acid | 41.770 | 0.234 | −0.0003 | 0.952 | 0.246 | 118.17 | |
| DHA RO63 | 59.616 50.852 | 0.030 0.024 | −0.004 0.002 | 0.277 0.692 | 0.051 0.085 | 210.89338.73 | |
| Abdominal and pelvic pain | Omega-3 FAs | 66.439 | 0.026 | 0.002 | 0.538 | 0.05 | 267.12 |
| Omega-6 FAs | 56.939 | 0.177 | 0.003 | 0.354 | 0.179 | 125.39 | |
| Linoleic Acid | 53.823 | 0.046 | 0.008 | 0.084 | 0.061 | 110.86 | |
| DHA RO63 | 50.547 46.758 | 0.146 0.057 | 0.002–0.002 | 0.415 0.404 | 0.2 0.136 | 210.95338.81 | |
| Lower back pain or/and sciatica | Omega-3 FAs | 37.939 | 0.795 | −0.004 | 0.126 | 0.805 | 264.39 |
| Omega-6 FAs | 47.124 | 0.549 | −0.001 | 0.861 | 0.621 | 123.48 | |
| Linoleic Acid | 40.128 | 0.553 | −0.003 | 0.469 | 0.682 | 129.86 | |
| DHA RO63 | 46.656 33.791 | 0.251 0.429 | 0.0003 0.004 | 0.933 0.829 | 0.306 0.496 | 209.51336.41 | |
| Pain in joint | Omega-3 FAs | 54.863 | 0.173 | 0.002 | 0.530 | 0.201 | 270.57 |
| Omega-6 FAs | 54.434 | 0.275 | 0.002 | 0.637 | 0.366 | 125.38 | |
| Linoleic Acid | 47.913 | 0.245 | 0.003 | 0.611 | 0.366 | 131.66 | |
| DHA RO63 | 50.414 54.827 | 0.1490.009 | 0.004–0.0003 | 0.297 0.959 | 0.208 0.06 | 212.76341.84 | |
| Pain in limb | Omega-3 FAs | 63.067 | 0.048 | −0.008 | 0.071 | 0.066 | 266.62 |
| Omega-6 FAs | 57.535 | 0.100 | −0.006 | 0.343 | 0.102 | 129.32 | |
| Linoleic Acid | 55.155 | 0.056 | −0.005 | 0.441 | 0.058 | 133.56 | |
| DHA RO63 | 44.484 28.356 | 0.327 0.446 | −0.002 0.008 | 0.671 0.083 | 0.38 0.482 | 210.69363.87 | |
| Low back pain | Omega-3 FAs | 43.202 | 0.590 | −0.002 | 0.491 | 0.575 | 266.87 |
| Omega-6 FAs | 49.298 | 0.461 | 0.002 | 0.620 | 0.519 | 124.25 | |
| Linoleic Acid | 46.868 | 0.280 | −0.002 | 0.730 | 0.438 | 130.59 | |
| DHA RO63 | 42.586 33.900 | 0.403 0.424 | 0.002 0.004 | 0.490 0.822 | 0.412 0.416 | 210.82338.60 | |
| Gynecological related pain | Omega-3 FAs | 54.807 | 0.175 | −0.008 | 0.297 | 0.234 | 288.78 |
| Omega-6 FAs | 56.404 | 0.218 | −0.018 | 0.080 | 0.146 | 130.77 | |
| Linoleic Acid | 51.101 | 0.158 | −0.018 | 0.136 | 0.15 | 143.53 | |
| DHA RO63 | 44.903 39.586 | 0.312 0.200 | −0.009 0.009 | 0.271 0.666 | 0.382 0.311 | 222.02357.52 | |
DHA, Docosahexaenoic acid; RO63, ratio of omega-6 FAs to omega-3 FAs; Gynecological related pain, pain and other conditions associated with female genital organs and menstrual cycle; p < 0.05 is set as the significant threshold. p < 0.05 was considered statistically significant.
4. Discussion
We used MR to systematically assess the causal relationship and its direction between four PUFAs and omega-6:3 ratios and pain in eight body parts. The results revealed a negative correlation between the concentration of omega-3 FAs and DHA and the occurrence of abdominal and pelvic pain. Conversely, a positive correlation was observed between the omega-6:3 ratio and abdominal and pelvic pain. Furthermore, we observed a negative association between omega-3 FA concentration and lower back pain and/or sciatica. However, no causal relationship was found between circulating PUFA concentrations and pain in other body sites.
Omega-3 fatty acids have been found to have anti-inflammatory and analgesic effects, while Omega-6 fatty acids promote inflammation (27). Research has demonstrated that omega-3 fatty acids can influence cellular signaling pathways by altering the composition and function of cell membranes, thus reducing inflammatory responses (28). Specifically, DHA, one of the Omega-3 fatty acids, can reduce inflammation by inhibiting the synthesis of the inflammatory mediator prostaglandin E2 and combating oxidative stress (29, 30). Additionally, studies have shown that higher ratios of Omega-6 to Omega-3 fatty acids are associated with an increased risk of cardiovascular disease, autoimmune diseases, and cancer (31, 32). Therefore, maintaining a proper balance of omega-3 and Omega-6 fatty acid intake is crucial for maintaining good health. In theory, this evidence supports that higher circulating concentrations of omega-3 FAs and DHA, along with lower levels of the omega-6 to omega-3 ratio, may reduce the risk of abdominal and pelvic pain.
Several observational studies have explored the association between PUFAs and chronic pain (33–37). In a randomized controlled study, the infusion of omega-3 FAs demonstrated efficacy in alleviating pain caused by rheumatoid arthritis (38). Another study indicated that dietary supplementation with omega-3 FAs and DHA could reduce the incidence and progression of pain in the older population (39). However, omega-6 FAs may contribute to inflammation, as significantly elevated levels of omega-6 FA metabolites were reported in women with irritable bowel syndrome (40). Similarly, another study found a higher omega-6:3 ratio to be associated with orofacial pain, headache, and low back pain (41). In a randomized controlled study, joint pain in patients with inflammatory bowel disease was effectively ameliorated by correcting the omega-6:3 ratio (42). Our MR study produced consistent results, demonstrating that higher omega-6:3 ratios elevate the risk of abdominal pain, whereas higher circulating omega-3 FAs and DHA concentrations alleviate abdominal pain. However, no causal link was observed with joint pain.
Although observational studies have shown an association between PUFAs and chronic pain, only a few MR studies have been conducted. To the best of our knowledge, only one MR study has investigated the relationship between omega-3 FAs and low back pain risk, revealing that genetically increasing circulating omega-3 FA concentrations can reduce this risk (43). However, our findings do not support this conclusion. In our MR study, only the weighted median and weighted mode methods indicated a causal relationship between circulating omega-3 FA concentrations and low back pain, as measured using the IVW method [OR 95% CI: 0.948 (0.894, 1.005), p = 0.073], but no causal relationship was found between them. Notably, the sample size of our study was nearly twice that of the aforementioned MR study. Moreover, our study data was derived from different patient populations, sourced from the UK biobank and Finland genome project (Finn Gen), effectively avoiding sample duplication. Additionally, our findings remained robust across multiple analytical approaches, exhibiting no heterogeneity or pleiotropy. Although this MR study represents the largest and most comprehensive investigation to date in terms of sample size, FA types, and pain locations, future studies will require larger datasets from genomic association studies to obtain more accurate and reliable results.
This study possesses several advantages. First, this is the most comprehensive MR study to date exploring the relationship between PUFAs and pain. We included four different types of PUFAs and the omega-6:3 ratio while investigating causal associations with pain in eight body parts. This comprehensive approach enhances the coverage and credibility of our results. Second, compared with observational studies, MR studies overcome the effects of confounding variables and reverse causality. Third, we selected SNPs that satisfied the MR hypothesis as IVs, all of which exhibited strong associations with PUFAs. Lastly, all SNPs were derived from European populations, thereby minimizing the possibility of population stratification bias.
Nonetheless, this study has some limitations. First, it relied on publicly available pooled data from GWASs, which restricted our ability to analyze other subtypes of PUFAs (e.g., alpha-linolenic, eicosapentaenoic, and arachidonic acids) in investigating the relationship between PUFAs and pain. Second, despite the utilization of a large GWAS database, our study might have been unable to detect small causal relationships. Third, this study can only establish the relationship between genes and phenotypes but cannot elucidate the biological mechanism of these relationships. Therefore, the occurrence and development of complex phenotypes cannot be fully explained. Lastly, the study population primarily consisted of individuals of European ancestry, limiting the generalizability to other ethnic populations.
5. Conclusion
This MR study suggests that higher circulating omega-3 FA and DHA concentrations and lower omega-6:3 ratios are associated with a decreased risk of abdominal and pelvic pain. Maintaining an appropriate omega-6:3 ratio is crucial for the prevention and management of abdominal and pelvic pain. Furthermore, higher circulating omega-3 FA concentrations may reduce the risk of lower back pain and/or sciatica. However, no causal relationship was found between circulating PUFA concentrations and pain in other body parts, including pain related to the face, throat and chest, joints, limbs, low back pain, and gynecological sites. These findings contribute to the targeted prevention and treatment of chronic pain through the use of PUFAs.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found in the article/Supplementary material.
Author contributions
YD: Writing – review & editing, Conceptualization, Writing – original draft. YC: Conceptualization, Writing – review & editing, Data curation, Software. RG: Writing – review & editing, Supervision, Validation. RJ: Writing – review & editing, Project administration, Resources, Visualization. CZ: Data curation, Software, Writing – review & editing, Visualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This research was facilitated by the generous sharing of GWAS summary statistics. We thank the participants, researchers, and staff involved in the numerous other studies from which we obtained data for this report. We would like to thank Bullet Edits Limited for English language editing and proofreading.
Footnotes
1https://gwas.mrcieu.ac.uk, accessed on June 10, 2023
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1265928/full#supplementary-material
References
- 1.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. (2019) 160:19–27. doi: 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 2.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7 [DOI] [PubMed] [Google Scholar]
- 3.Fillingim RB, Loeser JD, Baron R, Edwards RR. Assessment of chronic pain: domains, methods, and mechanisms. J Pain. (2016) 17:T10–20. doi: 10.1016/j.jpain.2015.08.010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. (2019) 160:53–9. doi: 10.1097/j.pain.0000000000001365, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health and disease--a review. Part 1: classification, dietary sources and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2011) 155:117–30. doi: 10.5507/bp.2011.038, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Kremmyda LS, Tvrzicka E, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health and disease: a review. Part 2: fatty acid physiological roles and applications in human health and disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2011) 155:195–218. doi: 10.5507/bp.2011.052, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Papackova Z, Cahova M. Fatty acid signaling: the new function of intracellular lipases. Int J Mol Sci. (2015) 16:3831–55. doi: 10.3390/ijms16023831, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Carvalho CCCR, Caramujo MJ. The various roles of fatty acids. Molecules. (2018) 23:2583. doi: 10.3390/molecules23102583, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rombaldova M, Janovska P, Kopecky J, Kuda O. Omega-3 fatty acids promote fatty acid utilization and production of pro-resolving lipid mediators in alternatively activated adipose tissue macrophages. Biochem Biophys Res Commun. (2017) 490:1080–5. doi: 10.1016/j.bbrc.2017.06.170, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. (2007) 129:210–23. doi: 10.1016/j.pain.2007.01.020, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Sanders AE, Weatherspoon ED, Ehrmann BM, Soma PS, Shaikh SR, Preisser JS, et al. Circulating Omega-6 and Omega-3 polyunsaturated fatty acids in painful temporomandibular disorder and low back pain. J Pain. (2022) 23:1724–36. doi: 10.1016/j.jpain.2022.05.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mustonen AM, Nieminen P. Fatty acids and oxylipins in osteoarthritis and rheumatoid arthritis-a complex field with significant potential for future treatments. Curr Rheumatol Rep. (2021) 23:41. doi: 10.1007/s11926-021-01007-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shapiro H, Singer P, Ariel A. Beyond the classic eicosanoids: peripherally-acting oxygenated metabolites of polyunsaturated fatty acids mediate pain associated with tissue injury and inflammation. Prostaglandins Leukot Essent Fatty Acids. (2016) 111:45–61. doi: 10.1016/j.plefa.2016.03.001, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Loef M, Schoones JW, Kloppenburg M, Ioan-Facsinay A. Fatty acids and osteoarthritis: different types, different effects. Joint Bone Spine. (2019) 86:451–8. doi: 10.1016/j.jbspin.2018.07.005, PMID: [DOI] [PubMed] [Google Scholar]
- 15.MacFarlane LA, Cook NR, Kim E, Lee IM, Iversen MD, Gordon D, et al. The effects of vitamin D and marine omega-3 fatty acid supplementation on chronic knee pain in older US adults: results from a randomized trial. Arthritis Rheumatol. (2020) 72:1836–44. doi: 10.1002/art.41416, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219 [DOI] [PubMed] [Google Scholar]
- 17.Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. (2020) 11:376. doi: 10.1038/s41467-019-14156-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess S, Foley CN, Zuber V. Inferring causal relationships between risk factors and outcomes from genome-wide association study data. Annu Rev Genomics Hum Genet. (2018) 19:303–27. doi: 10.1146/annurev-genom-083117-021731, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. (2019) 10:486–96. doi: 10.1002/jrsm.1346, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boef AGC, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. (2015) 44:496–511. doi: 10.1093/ije/dyv071, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Burgess S, Thompson SG. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat Med. (2011) 30:1312–23. doi: 10.1002/sim.4197 [DOI] [PubMed] [Google Scholar]
- 22.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. elife. (2018) 7:e34408. doi: 10.7554/eLife.34408, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saini RK, Keum YS. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance – a review. Life Sci. (2018) 203:255–67. doi: 10.1016/j.lfs.2018.04.049, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. (2013) 38:1154–63. doi: 10.1016/j.immuni.2013.05.015, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Yang P, Jiang Y, Fischer SM. Prostaglandin E3 metabolism and cancer. Cancer Lett. (2014) 348:1–11. doi: 10.1016/j.canlet.2014.03.010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Wang D, Zong Y, Yang X. DHA protects hepatocytes from oxidative injury through GPR120/ERK-mediated Mitophagy. Int J Mol Sci. (2021) 22:5675. doi: 10.3390/ijms22115675, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. (2002) 56:365–79. doi: 10.1016/s0753-3322(02)00253-6 [DOI] [PubMed] [Google Scholar]
- 32.Sanders AE, Weatherspoon ED, Ehrmann BM, Soma PS, Shaikh SR, Preisser JS, et al. Circulating polyunsaturated fatty acids, pressure pain thresholds, and nociplastic pain conditions. Prostaglandins Leukot Essent Fatty Acids. (2022) 184:102476. doi: 10.1016/j.plefa.2022.102476, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen S, Unger JM, Crew KD, Till C, Greenlee H, Gralow J, et al. Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Res Treat. (2018) 172:603–10. doi: 10.1007/s10549-018-4946-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galán-Arriero I, Serrano-Muñoz D, Gómez-Soriano J, Goicoechea C, Taylor J, Velasco A, et al. The role of Omega-3 and Omega-9 fatty acids for the treatment of neuropathic pain after neurotrauma. Biochim Biophys Acta Biomembr. (2017) 1859:1629–35. doi: 10.1016/j.bbamem.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 35.Cardia L, Calapai F, Mondello C, Quattrone D, Elisa Sorbara E, Mannucci C, et al. Clinical use of omega-3 fatty acids in migraine: a narrative review. Medicine (Baltimore). (2020) 99:e22253. doi: 10.1097/MD.0000000000022253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unda SR, Villegas EA, Toledo ME, Asis Onell G, Laino CH. Beneficial effects of fish oil enriched in omega-3 fatty acids on the development and maintenance of neuropathic pain. J Pharm Pharmacol. (2020) 72:437–47. doi: 10.1111/jphp.13213, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Georgieva M, Wei Y, Dumitrascuta M, Pertwee R, Finnerup NB, Huang W. Fatty acid suppression of glial activation prevents central neuropathic pain after spinal cord injury. Pain. (2019) 160:2724–42. doi: 10.1097/j.pain.0000000000001670, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Bahadori B, Uitz E, Thonhofer R, Trummer M, Pestemer-Lach I, McCarty M, et al. Omega-3 fatty acids infusions as adjuvant therapy in rheumatoid arthritis. JPEN J Parenter Enteral Nutr. (2010) 34:151–5. doi: 10.1177/0148607109342130, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Carballo-Casla A, García-Esquinas E, Banegas JR, Rodríguez-Artalejo F, Ortolá R. Fish consumption, omega-3 fatty acid intake, and risk of pain: the seniors-ENRICA-1 cohort. Clin Nutr. (2022) 41:2587–95. doi: 10.1016/j.clnu.2022.09.007, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Clarke G, Fitzgerald P, Hennessy AA, Cassidy EM, Quigley EM, Ross P, et al. Marked elevations in pro-inflammatory polyunsaturated fatty acid metabolites in females with irritable bowel syndrome. J Lipid Res. (2010) 51:1186–92. doi: 10.1194/jlr.P000695, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders AE, Weatherspoon ED, Ehrmann BM, Soma PS, Shaikh SR, Preisser JS, et al. Circulating polyunsaturated fatty acids and pain intensity in five chronic pain conditions. J Pain. (2023) 24:478–89. doi: 10.1016/j.jpain.2022.10.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjørkkjaer T, Brun JG, Valen M, Arslan G, Lind R, Brunborg LA, et al. Short-term duodenal seal oil administration normalised n-6 to n-3 fatty acid ratio in rectal mucosa and ameliorated bodily pain in patients with inflammatory bowel disease. Lipids Health Dis. (2006) 5:6. doi: 10.1186/1476-511X-5-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou S, Zhu G, Xu Y, Gao R, Li H, Han G, et al. Mendelian randomization study on the putative causal effects of Omega-3 fatty acids on low Back pain. Front Nutr. (2022) 9:819635. doi: 10.3389/fnut.2022.819635, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found in the article/Supplementary material.