Abstract
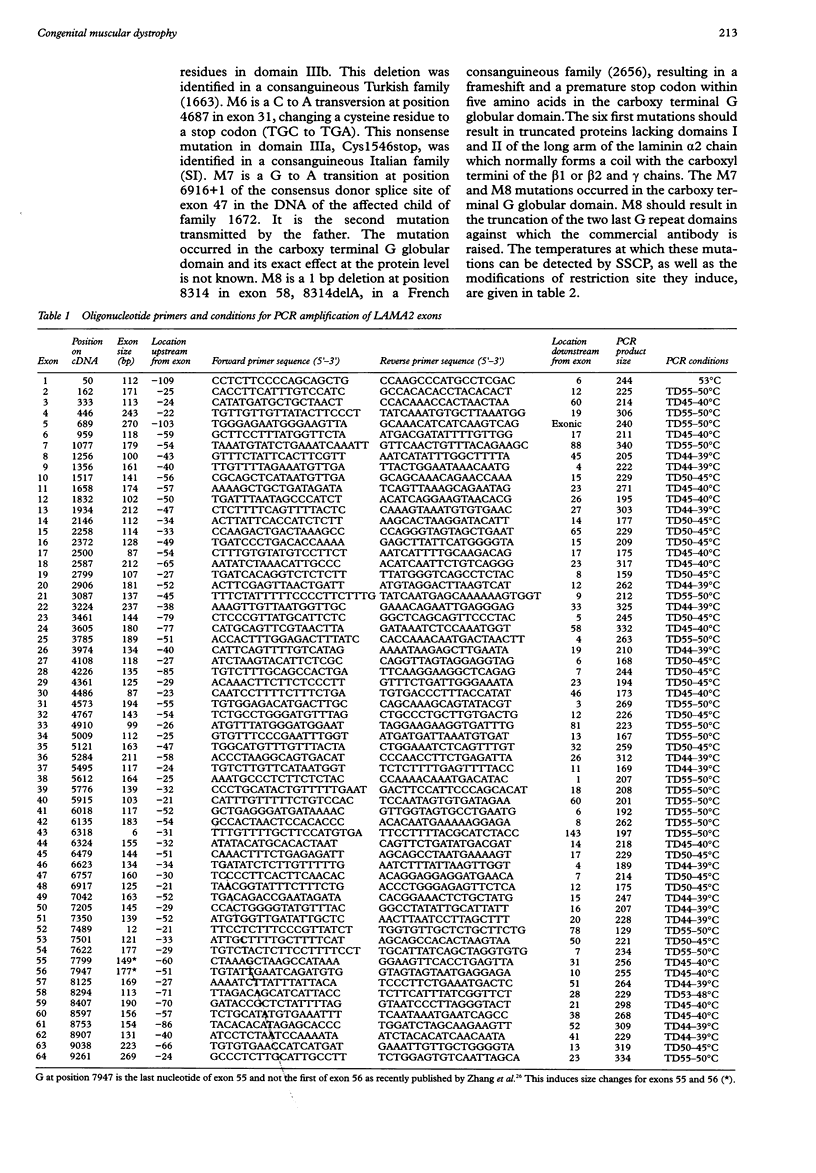
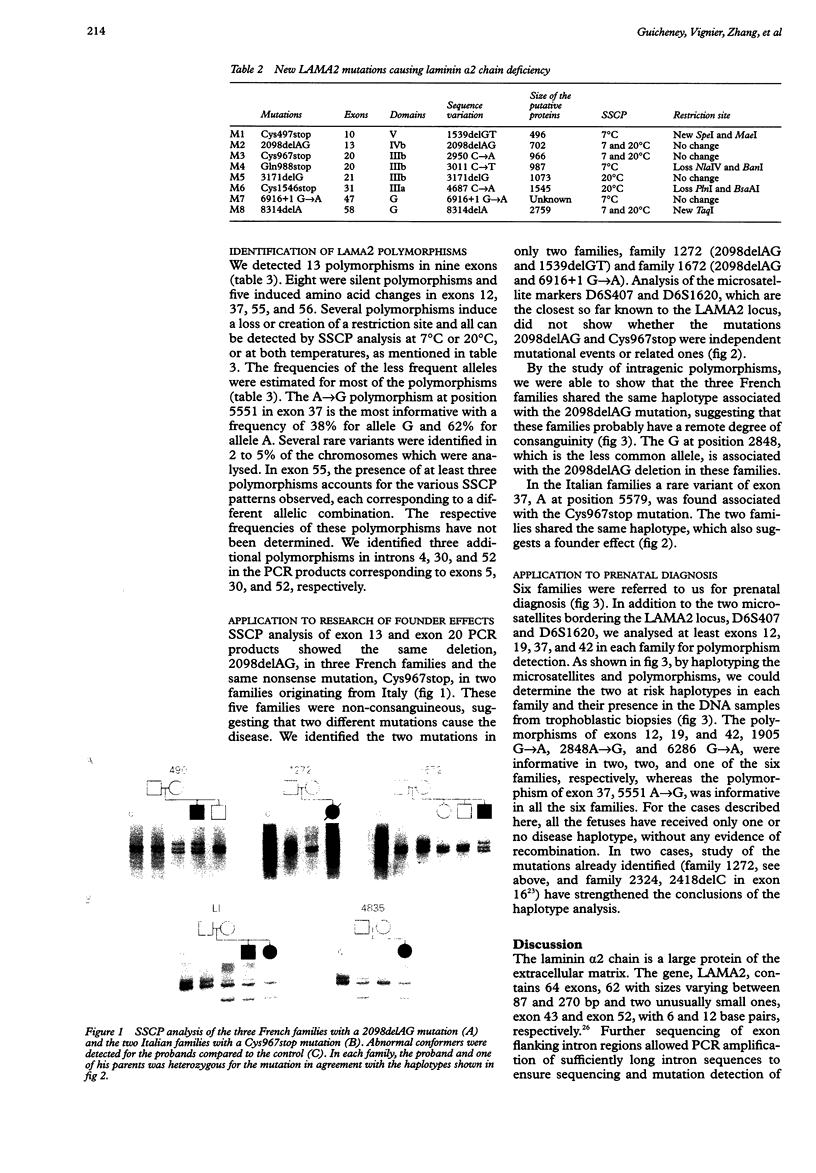
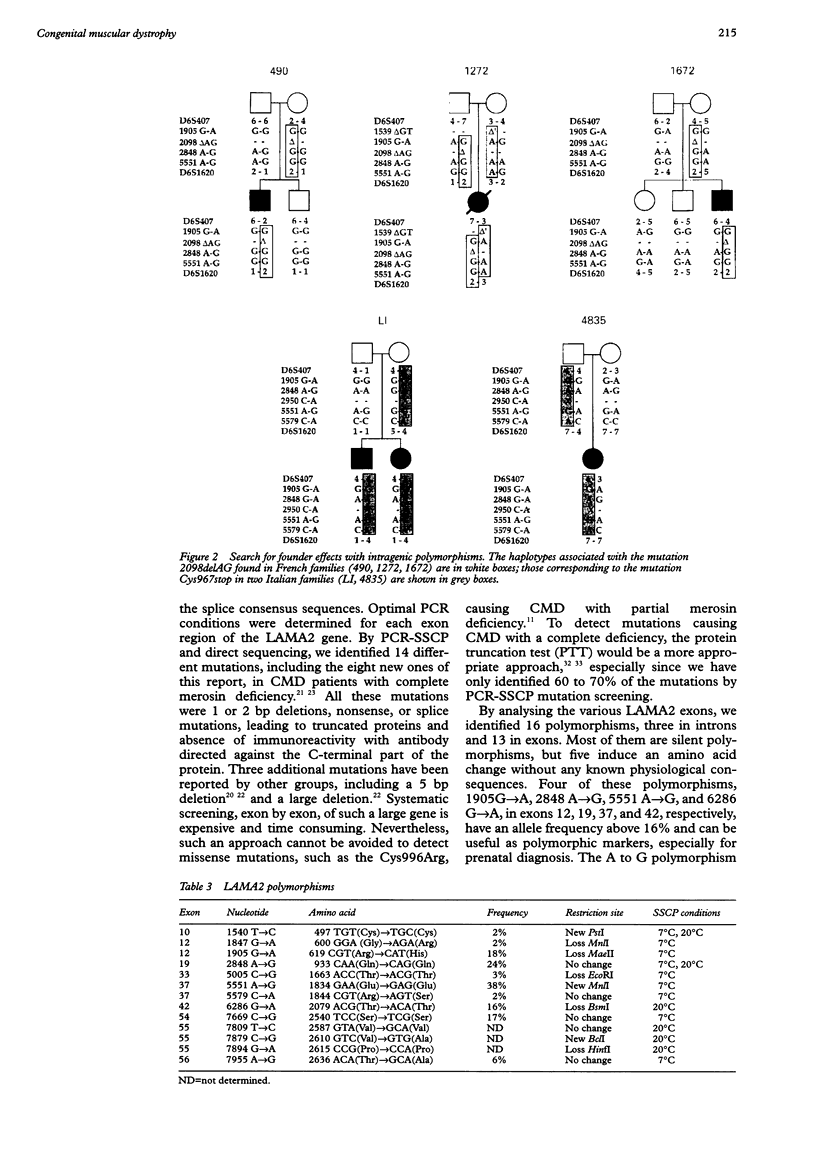
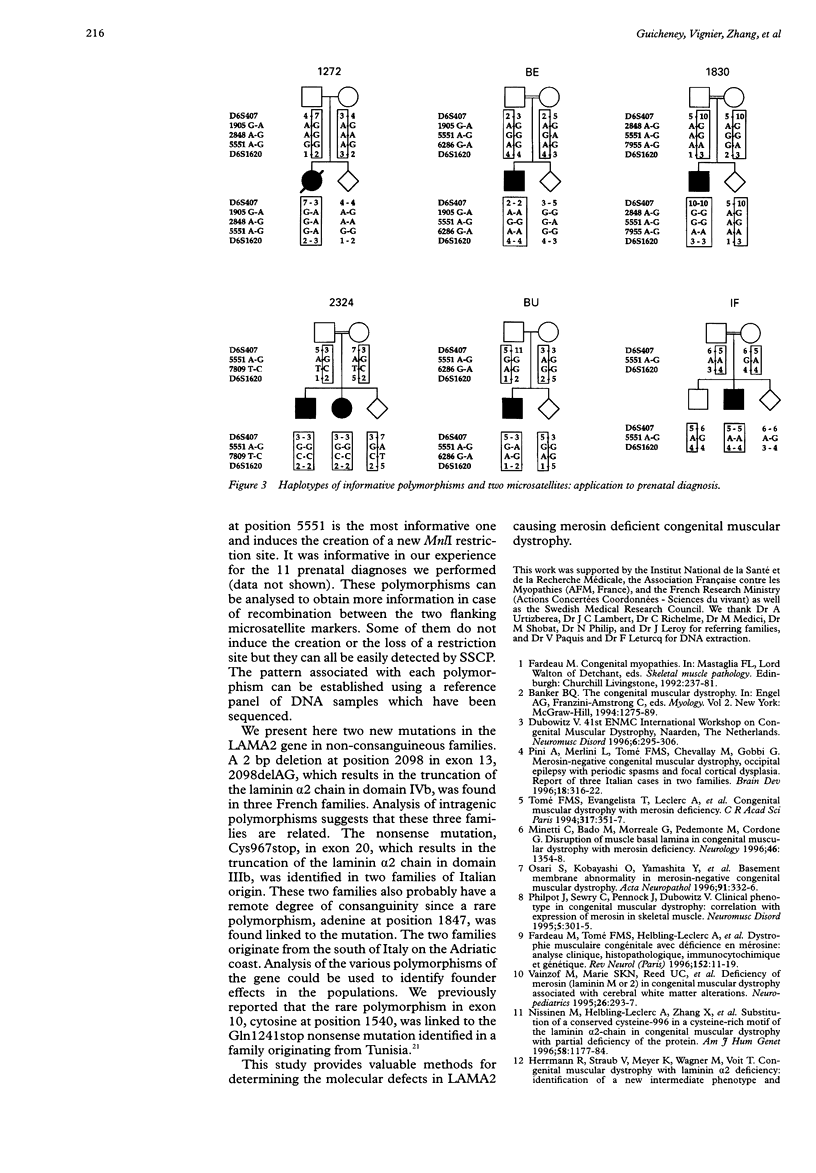
Classical congenital muscular dystrophy with merosin deficiency is caused by mutations in the laminin alpha2 chain gene (LAMA2). Extended sequencing of the introns flanking the 64 LAMA2 exons was carried out and, based on these sequences, oligonucleotide primers were designed to amplify the coding region of each exon separately. By PCR-SSCP analysis, we identified eight new mutations in nine families originating from various countries. All induced a premature truncation of the protein, either in the short arm or in the globular C-terminal domain. A 2 bp deletion in exon 13, 2098delAG, was found in three French non-consanguineous families and a nonsense mutation of exon 20, Cys967stop, in two other non-consanguineous families originating from Italy. Determination of rare intragenic polymorphisms permitted us to show evidence of founder effects for these two mutations suggesting a remote degree of consanguinity between the families. Other, more frequent polymorphisms, G to A 1905 (exon 12), A to G 2848 (exon 19), A to G 5551 (exon 37), and G to A 6286 (exon 42), were used as intragenic markers for prenatal diagnosis. This study provides valuable methods for determining the molecular defects in LAMA2 causing merosin deficient congenital muscular dystrophy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allamand V., Sunada Y., Salih M. A., Straub V., Ozo C. O., Al-Turaiki M. H., Akbar M., Kolo T., Colognato H., Zhang X. Mild congenital muscular dystrophy in two patients with an internally deleted laminin alpha2-chain. Hum Mol Genet. 1997 May;6(5):747–752. doi: 10.1093/hmg/6.5.747. [DOI] [PubMed] [Google Scholar]
- Dib C., Fauré S., Fizames C., Samson D., Drouot N., Vignal A., Millasseau P., Marc S., Hazan J., Seboun E. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature. 1996 Mar 14;380(6570):152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. 22nd ENMC sponsored workshop on congenital muscular dystrophy held in Baarn, The Netherlands, 14-16 May 1993. Neuromuscul Disord. 1994 Jan;4(1):75–81. doi: 10.1016/0960-8966(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. 41st ENMC International Workshop on Congenital Muscular Dystrophy 8-10 March 1996, Naarden, The Netherlands. Neuromuscul Disord. 1996 Aug;6(4):295–306. doi: 10.1016/0960-8966(96)00358-6. [DOI] [PubMed] [Google Scholar]
- Echenne B., Rivier F., Jellali A. J., Azais M., Mornet D., Pons F. Merosin positive congenital muscular dystrophy with mental deficiency, epilepsy and MRI changes in the cerebral white matter. Neuromuscul Disord. 1997 May;7(3):187–190. doi: 10.1016/s0960-8966(97)00452-5. [DOI] [PubMed] [Google Scholar]
- Fardeau M., Tomé F. M., Helbling-Leclerc A., Evangelista T., Ottolini A., Chevallay M., Barois A., Estournet B., Harpey J. P., Fauré S. Dystrophie musculaire congénitale avec déficience en mérosine: analyse clinique, histopathologique, immunocytochimique et génétique. Rev Neurol (Paris) 1996 Jan;152(1):11–19. [PubMed] [Google Scholar]
- Hayashi Y. K., Ishihara T., Domen K., Hori H., Arahata K. A benign allelic form of laminin alpha 2 chain deficient muscular dystrophy. Lancet. 1997 Apr 19;349(9059):1147–1147. doi: 10.1016/S0140-6736(05)63023-1. [DOI] [PubMed] [Google Scholar]
- Helbling-Leclerc A., Topaloglu H., Tomé F. M., Sewry C., Gyapay G., Naom I., Muntoni F., Dubowitz V., Barois A., Estournet B. Readjusting the localization of merosin (laminin alpha 2-chain) deficient congenital muscular dystrophy locus on chromosome 6q2. C R Acad Sci III. 1995 Dec;318(12):1245–1252. [PubMed] [Google Scholar]
- Helbling-Leclerc A., Zhang X., Topaloglu H., Cruaud C., Tesson F., Weissenbach J., Tomé F. M., Schwartz K., Fardeau M., Tryggvason K. Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995 Oct;11(2):216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- Mercuri E., Muntoni F., Berardinelli A., Pennock J., Sewry C., Philpot J., Dubowitz V. Somatosensory and visual evoked potentials in congenital muscular dystrophy: correlation with MRI changes and muscle merosin status. Neuropediatrics. 1995 Feb;26(1):3–7. doi: 10.1055/s-2007-979711. [DOI] [PubMed] [Google Scholar]
- Mercuri E., Pennock J., Goodwin F., Sewry C., Cowan F., Dubowitz L., Dubowitz V., Muntoni F. Sequential study of central and peripheral nervous system involvement in an infant with merosin-deficient congenital muscular dystrophy. Neuromuscul Disord. 1996 Dec;6(6):425–429. doi: 10.1016/s0960-8966(96)00383-5. [DOI] [PubMed] [Google Scholar]
- Minetti C., Bado M., Morreale G., Pedemonte M., Cordone G. Disruption of muscle basal lamina in congenital muscular dystrophy with merosin deficiency. Neurology. 1996 May;46(5):1354–1358. doi: 10.1212/wnl.46.5.1354. [DOI] [PubMed] [Google Scholar]
- Mora M., Moroni I., Uziel G., di Blasi C., Barresi R., Farina L., Morandi L. Mild clinical phenotype in a 12-year-old boy with partial merosin deficiency and central and peripheral nervous system abnormalities. Neuromuscul Disord. 1996 Oct;6(5):377–381. doi: 10.1016/0960-8966(96)00359-8. [DOI] [PubMed] [Google Scholar]
- Osari S., Kobayashi O., Yamashita Y., Matsuishi T., Goto M., Tanabe Y., Migita T., Nonaka I. Basement membrane abnormality in merosin-negative congenital muscular dystrophy. Acta Neuropathol. 1996;91(4):332–336. doi: 10.1007/s004010050433. [DOI] [PubMed] [Google Scholar]
- Pegoraro E., Mancias P., Swerdlow S. H., Raikow R. B., Garcia C., Marks H., Crawford T., Carver V., Di Cianno B., Hoffman E. P. Congenital muscular dystrophy with primary laminin alpha2 (merosin) deficiency presenting as inflammatory myopathy. Ann Neurol. 1996 Nov;40(5):782–791. doi: 10.1002/ana.410400515. [DOI] [PubMed] [Google Scholar]
- Philpot J., Sewry C., Pennock J., Dubowitz V. Clinical phenotype in congenital muscular dystrophy: correlation with expression of merosin in skeletal muscle. Neuromuscul Disord. 1995 Jul;5(4):301–305. doi: 10.1016/0960-8966(94)00069-l. [DOI] [PubMed] [Google Scholar]
- Pini A., Merlini L., Tomé F. M., Chevallay M., Gobbi G. Merosin-negative congenital muscular dystrophy, occipital epilepsy with periodic spasms and focal cortical dysplasia. Report of three Italian cases in two families. Brain Dev. 1996 Jul-Aug;18(4):316–322. doi: 10.1016/0387-7604(96)00028-9. [DOI] [PubMed] [Google Scholar]
- Roest P. A., Roberts R. G., Sugino S., van Ommen G. J., den Dunnen J. T. Protein truncation test (PTT) for rapid detection of translation-terminating mutations. Hum Mol Genet. 1993 Oct;2(10):1719–1721. doi: 10.1093/hmg/2.10.1719. [DOI] [PubMed] [Google Scholar]
- Roest P. A., Roberts R. G., van der Tuijn A. C., Heikoop J. C., van Ommen G. J., den Dunnen J. T. Protein truncation test (PTT) to rapidly screen the DMD gene for translation terminating mutations. Neuromuscul Disord. 1993 Sep-Nov;3(5-6):391–394. doi: 10.1016/0960-8966(93)90083-v. [DOI] [PubMed] [Google Scholar]
- Shorer Z., Philpot J., Muntoni F., Sewry C., Dubowitz V. Demyelinating peripheral neuropathy in merosin-deficient congenital muscular dystrophy. J Child Neurol. 1995 Nov;10(6):472–475. doi: 10.1177/088307389501000610. [DOI] [PubMed] [Google Scholar]
- Sunada Y., Edgar T. S., Lotz B. P., Rust R. S., Campbell K. P. Merosin-negative congenital muscular dystrophy associated with extensive brain abnormalities. Neurology. 1995 Nov;45(11):2084–2089. doi: 10.1212/wnl.45.11.2084. [DOI] [PubMed] [Google Scholar]
- Tomé F. M., Evangelista T., Leclerc A., Sunada Y., Manole E., Estournet B., Barois A., Campbell K. P., Fardeau M. Congenital muscular dystrophy with merosin deficiency. C R Acad Sci III. 1994 Apr;317(4):351–357. [PubMed] [Google Scholar]
- Vainzof M., Marie S. K., Reed U. C., Schwartzman J. S., Pavanello R. C., Passos-Bueno M. R., Zatz M. Deficiency of merosin (laminin M or alpha 2) in congenital muscular dystrophy associated with cerebral white matter alterations. Neuropediatrics. 1995 Dec;26(6):293–297. doi: 10.1055/s-2007-979777. [DOI] [PubMed] [Google Scholar]
- Vuolteenaho R., Nissinen M., Sainio K., Byers M., Eddy R., Hirvonen H., Shows T. B., Sariola H., Engvall E., Tryggvason K. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994 Feb;124(3):381–394. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer U. M., Engvall E. Merosin/laminin-2 and muscular dystrophy. Neuromuscul Disord. 1996 Dec;6(6):409–418. doi: 10.1016/s0960-8966(96)00384-7. [DOI] [PubMed] [Google Scholar]
- Zhang X., Vuolteenaho R., Tryggvason K. Structure of the human laminin alpha2-chain gene (LAMA2), which is affected in congenital muscular dystrophy. J Biol Chem. 1996 Nov 1;271(44):27664–27669. doi: 10.1074/jbc.271.44.27664. [DOI] [PubMed] [Google Scholar]
- van der Knaap M. S., Smit L. M., Barth P. G., Catsman-Berrevoets C. E., Brouwer O. F., Begeer J. H., de Coo I. F., Valk J. Magnetic resonance imaging in classification of congenital muscular dystrophies with brain abnormalities. Ann Neurol. 1997 Jul;42(1):50–59. doi: 10.1002/ana.410420110. [DOI] [PubMed] [Google Scholar]




