ABSTRACT
Coronavirus disease 2019 (COVID-19) cases in China has grown rapidly after adjustment of the dynamic zero-COVID-19 strategy. However, how different vaccination states affect symptoms, severity and post COVID conditions was unclear. Here, we used an online questionnaire to investigate the infection status of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among 11,897 participants, with 55.55% positive and 28.42% negative. The common COVID-19 symptoms were fatigue (73.31%), cough (70.02%), fever (65.25%) and overall soreness (58.64%); self-reported asymptomatic infection accounted for 0.7% of participants. The persistent symptoms at 1 month after infection included fatigue (48.7%), drowsiness (34.3%), cough (30.1%), decreased exercise ability (23.1%) and pharyngeal discomfort (19.4%), which was reduced by more than 200% at 2 months. Participants with complications such as chronic obstructive pulmonary disease, respiratory diseases, diabetes, hypertension, etc. have a higher proportion of hospitalization and longer recovery time (p < = 0.01). Multiple vaccination statuses reduced the infection (p < 0.001) and severity rates (p = 0.022) by varying degrees as well as reduced the risk of high fever (>39.1 °C), chills, diarrhea and ageusia/anosmia, respectively (p < 0.05). Vaccination may enhance some upper respiratory symptoms, including sore throat, nasal congestion and runny nose, respectively (p < 0.05). Participants who had been vaccinated within 3 months were better protected by helping reduce their risk of overall soreness, chills and ageusia/anosmia, respectively (p < 0.05). In conclusion, our work has updated the epidemic characteristics of the breakthrough infection (BTI) wave after the dynamic zero-COVID-19 strategy, providing data and insights on how different vaccination statuses affect COVID-19 symptoms and disease prognosis.
KEYWORDS: COVID-19, Breakthrough infection, Omicron sub-variant, Vaccination, Post-COVID conditions
1. Introduction
Over 693 millions of infected cases and approximately 6.9 million deaths, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to challenge and breakthrough host immunity in its unique mutational way [1–3]. Since China adjusted its dynamic zero-coronavirus disease 2019 (zero-COVID-19) strategy in December 2022, the number of infections in China has experienced grown rapidly [4–6]. Most infected Chinese people complain that the symptoms of COVID-19 are much more uncomfortable than those of common viruses with colds, leading to widespread panic. The widespread symptoms of torture have raised concerns among infected individuals in China about the previously reported contradictory asymptomatic COVID-19 infection rate [7–11]. In addition, many infected people personally realize that mild symptoms are not actually easy and bearable. Some explanations suggest that infection symptoms are related to one’s infection period, virus variants and immunity statuses [12–14].
The Chinese infection peak dominated by BF.7 and BA.5.2 may provide a good research model for BTI [15], because the vast majority of them have not been infected before, with clean immune background. Although there were reports on the symptoms after infection with BF.7 or BA.5.2 [16,17], few were associated with symptoms and vaccination statuses. Thus, the characteristics of BTI remain unclear, particularly after the adjustment of the zero-COVID-19 strategy. Moreover, the symptoms and severity of people with different vaccine immunity statuses and the specific impact on human life safety need to be investigated.
Driving factors for the adjustment of domestic epidemic strategies comprise 1) the immune barrier against SARS-CoV-2 established by universal vaccination and 2) variants with gradually weakened pathogenicity [6,18]. Nonetheless, the performance of the protective barrier established by previous vaccinations remains to be supported by real-world data in the widespread BTI following the implementation of the zero-COVID-19 strategy. On the one hand, the vaccination times of infected people are highly heterogeneous and their vaccinations’ protective effects against SARS-CoV-2 may differ; these factors may lead to asynchronous symptoms and varying severity [19]. On the other hand, the association between different vaccination statuses and acute symptoms after infection needs further investigation. Importantly, the proportion and patterns of persistent post-COVID-19 conditions (long COVID) after BTI caused by new SARS-CoV-2 sub-variants also require investigation and evaluation. It has been reported that long COVID caused by Omicron variant is characterized by cardiovascular system, respiratory system, nervous system and so on [20–22] reported that the main long-term symptoms after BA.2 BTI were neuropsychiatric problems, such as pain, fatigue and sleep difficulties [22]. In addition, some residents have received a 4th or even 5th vaccine dose as a booster shot prior to infection. Whether these booster shots offer better protection is worth investigating epidemiologically, which will inform subsequent scientific prevention and control measures and vaccination management protocols.
Here, we conducted a survey on SARS-CoV-2 infection soon after the adjustment of China’s dynamic zero-COVID-19 strategy. Our goal was to characterize the relationship among different vaccination statuses and infection rates, improvement rates, acute symptoms and post-infection symptoms.
2. Methods
2.1. Research objective
An online questionnaire survey was conducted in all provinces of China from January 22 to February 21, 2023. The respondent voluntarily filled out the “Questionnaire on SARS-CoV-2 infection among Chinese residents.” At the end of the survey, 12,806 questionnaires were collected. We filtered out potential duplicate samples according to IP address, sex, age. Abnormal questionnaires were also filtered according to the notes and the completion times of SARS-CoV-2–negative and SARS-CoV-2–positive personnel. A small number of participants who were located abroad, infected too early (before 2022) or could not determine their own infection status were also filtered out. Finally, a total of 909 samples were filtered and 11,897 samples were included in the subsequent analysis. All participants voluntarily participated in the online survey and gave their informed consent. This study was approved by the Institute of Microbiology, Chinese Academy of Sciences of Research Ethics Committee (No.: APIMCAS2023006-1)
2.2. Research methods
The questionnaire survey included the following questions: location, sex, age, number of vaccinations, SARS-CoV-2 infection status, underlying medical conditions, smoking and drinking habits, etc. (Table S1). SARS-CoV-2–positive participants were further asked about the date of infection, infection symptoms, timing of significant symptom improvement, medical treatments and family vaccination history and symptom similarity. According to the date of infection in the positive participants, the symptoms at 1–4 months after infection were collected. All participants can fill in basic information such as age, region, gender, vaccination history and underlying disease. In order to exclude the impact of SARS-CoV-2 negative and uncertain infection status on symptom statistics, we have set question associations and jumps to ensure that only participants with nucleic acid and/or antigen confirmed SARS-CoV-2 positive can fill in infection symptom related questions. As of the end of questionnaire collection, due to the small number of participants who had been infected for more than 4 months, this data was not included in the analysis results. The “Questionnaire Star” platform (https://www.wjx.cn) was used to distribute the questionnaire links. The survey platform automatically recorded the IP address (city) and completion times of the respondents when they submitted their information.
2.3. Statistical analysis
R software (Version 4) was used for data quality control and statistical analysis. Logistic regression analysis was used to calculate the contribution or impact of each grouping factor on prognosis compared to the reference group (odds ratio: OR). Compared with reference factors, the larger the OR value, the greater the impact and contribution of grouping factors and the stronger the correlation between grouping factors and prognosis results. OR = 1 means that there is no correlation between grouping factors and disease prognosis. OR > 1 indicates a positive correlation between grouping factors and disease prognosis. OR < 1 means that grouping factors are negatively correlated with infection or symptoms (protective factors). The chi-square (χ2) test was used to calculate the significance of the frequency differences between the groups and p-values ≤ 0.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics of participants
The survey included 11,897 participants from seven regions across China (p < 0.001) (Table 1 and Figure 1A). A total of 6728 (55.55%) participants reported positive results for SARS-CoV-2 antigen and/or nucleic acid tests and 1788 (15.03%) were self-suspected to be positive (Table 1 and Figure S1A). This was consistent with the public statistical data that positive rate of SARS-CoV-2 between about 3%−60% from December 9, 2022 to February 25, 2023 and reached its peak around December 25 (https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/). The proportion of female participants was significantly higher than that of male participants (7074 versus 4823, p < 0.001) (Table 1 and Figure S1B). Participants were mainly between 18 and 29 years old, 30–39 years old and 40–49 years old, with significant differences in age (p < 0.001) (Table 1 and Figure S1C). Among the participants, 8479 were vaccinated with 3 doses of the COVID-19 vaccine, 2111 were vaccinated with 2 doses, 749 were vaccinated with 4 doses and only 350 were unvaccinated (Figure S1D), indicating that most of the participants had an immunity status established by the vaccine. The overall smoking rate in the SARS-CoV-2–positive group was only less than 1% higher than that in the SARS-CoV-2–negative group (Table 1 and Figure S1E) indicating a slight association between smoking and SARS-CoV-2 infection. An association between drinking and SARS-CoV-2 infection was observed, as the proportion of non-drinkers in the SARS-CoV-2–negative group was higher than that in the SARS-CoV-2–positive group (53.18% vs. 47.9%) (p < 0.001) (Table 1 and Figure S1F). Finally, the common underlying medical conditions of the participants mainly included hypertension and diabetes (Table 1). The SARS-CoV-2–positive participants have a higher proportion of respiratory diseases (asthma, chronic bronchitis, etc.), hypertension and pregnancy (p < 0.001) (Table 1).
Table 1.
Baseline characteristics of the questionnaire participants.
| Baseline category | SARS-CoV-2–negative | SARS-CoV-2–positive | Suspected positive | p-value |
|---|---|---|---|---|
| Region | ||||
| East China | 781 (23.1%) | 1953 (29.03%) | 318 (17.79%) | p < 0.001 |
| South China | 267 (7.9%) | 461 (6.85%) | 120 (6.71%) | |
| Central China | 1279 (37.83%) | 1864 (27.71%) | 852 (47.65%) | |
| North China | 608 (17.98%) | 1565 (23.26%) | 252 (14.09%) | |
| Northeast China | 61 (1.8%) | 169 (2.51%) | 36 (2.01%) | |
| Northwest China | 120 (3.55%) | 283 (4.21%) | 77 (4.31%) | |
| Southwest China | 265 (7.84%) | 433 (6.44%) | 133 (7.44%) | |
| Sex | ||||
| Male | 1486 (43.95%) | 2736 (40.67%) | 601 (33.61%) | p < 0.001 |
| Female | 1895 (56.05%) | 3992 (59.33%) | 1187 (66.39%) | |
| Age | ||||
| Under 18 years | 53 (1.57%) | 58 (0.86%) | 23 (1.29%) | p < 0.001 |
| 18–29 years | 1878 (55.55%) | 2899 (43.09%) | 1161 (64.93%) | |
| 30–39 years | 398 (11.77%) | 1162 (17.27%) | 145 (8.11%) | |
| 40–49 years | 338 (10%) | 1099 (16.33%) | 161 (9%) | |
| 50–59 years | 343 (10.14%) | 839 (12.47%) | 153 (8.56%) | |
| 60–69 years | 259 (7.66%) | 491 (7.3%) | 94 (5.26%) | |
| 70–80 years | 82 (2.43%) | 142 (2.11%) | 44 (2.46%) | |
| Over 80 years | 30 (0.89%) | 38 (0.56%) | 7 (0.39%) | |
| Smoking | ||||
| Non-smoker | 3037 (89.83%) | 6147 (91.36%) | 1677 (93.79%) | p < 0.001 |
| 1–5 cigarettes every month | 41 (1.21%) | 120 (1.78%) | 17 (0.95%) | |
| 1–5 cigarettes per day | 63 (1.86%) | 134 (1.99%) | 23 (1.29%) | |
| 6–10 cigarettes per day | 64 (1.89%) | 130 (1.93%) | 29 (1.62%) | |
| 11–20 cigarettes per day | 112 (3.31%) | 134 (1.99%) | 22 (1.23%) | |
| More than cigarettes 20 per day | 64 (1.89%) | 63 (0.94%) | 20 (1.12%) | |
| Drinking | ||||
| Non-drinker | 1798 (53.18%) | 3223 (47.9%) | 1007 (56.32%) | p < 0.001 |
| 1–3 times a year | 1021 (30.2%) | 2175 (32.33%) | 560 (31.32%) | |
| 1–3 times a month | 363 (10.74%) | 925 (13.75%) | 156 (8.72%) | |
| 1–3 times a week | 144 (4.26%) | 338 (5.02%) | 38 (2.13%) | |
| Once a day or more | 55 (1.63%) | 67 (1%) | 27 (1.51%) | |
| Medical condition | ||||
| Chronic obstructive pulmonary disease | 18 (0.53%) | 46 (0.68%) | 14 (0.78%) | 0.518 |
| Emphysema | 7 (0.21%) | 33 (0.49%) | 10 (0.56%) | 0.071 |
| Respiratory diseases (asthma, chronic bronchitis, etc.) | 93 (2.75%) | 304 (4.52%) | 72 (4.03%) | p < 0.001 |
| Diabetes | 87 (2.57%) | 207 (3.08%) | 47 (2.63%) | 0.29 |
| Hypertension | 235 (6.95%) | 576 (8.56%) | 103 (5.76%) | p < 0.001 |
| Cardio-cerebrovascular disease | 67 (1.98%) | 164 (2.44%) | 34 (1.9%) | 0.205 |
| Chronic nephrosis | 12 (0.35%) | 37 (0.55%) | 8 (0.45%) | 0.399 |
| Chronic liver disease | 23 (0.68%) | 47 (0.7%) | 11 (0.62%) | 0.93 |
| Pregnancy | 13 (0.38%) | 70 (1.04%) | 1 (0.06%) | p < 0.001 |
| Malignant tumor in treatment | 8 (0.24%) | 18 (0.27%) | 2 (0.11%) | 0.483 |
| Malignant tumor after treatment | 22 (0.65%) | 69 (1.03%) | 12 (0.67%) | 0.1 |
| Benign tumor | 17 (0.5%) | 57 (0.85%) | 15 (0.84%) | 0.147 |
| Autoimmune disease | 46 (1.36%) | 113 (1.68%) | 19 (1.06%) | 0.12 |
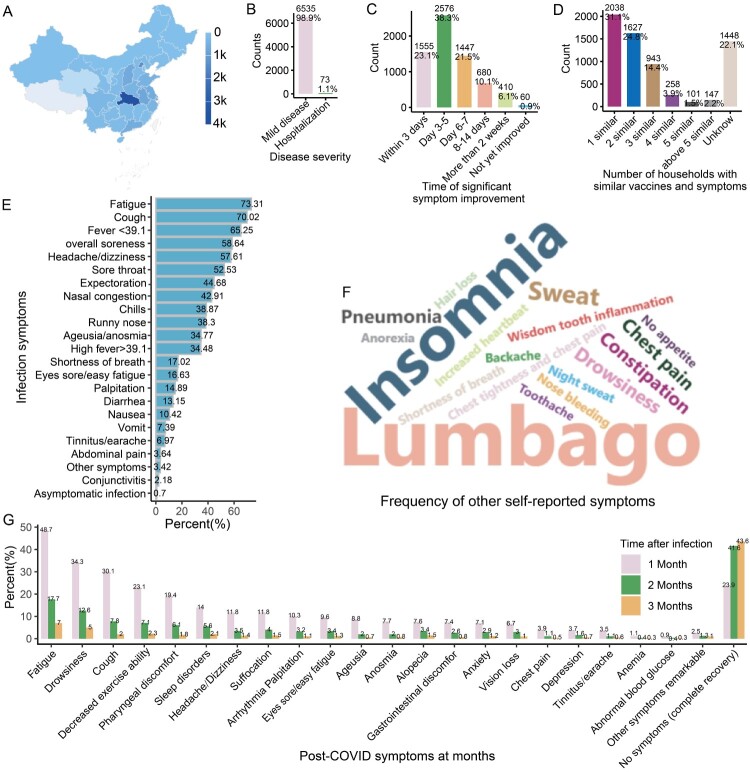
Figure 1.
Symptom distribution of positive participants. A: Map showing the provincial distribution of the participants in China. The top five provinces with the most participants were Hubei, Beijing, Anhui, Shanxi and Guangdong. B: Diagram showing the proportion of hospitalizations (severe COVID-19) of infected participants. C: Diagram showing the timing of when participants’ symptoms improved significantly. D: Diagram illustrating the proportion of infected individuals reporting similarities in symptoms and vaccination status with at least one family member, indicating the potential role of close family contact in contributing to the spread of the virus. E: Bar chart showing the symptom frequencies of infected subjects. F: Word frequency map showing the other symptoms most commonly reported by the infected participants. The data comes from symptom frequency statistics filled out by participants themselves. When participants feel that the symptom options in the questionnaire do not match their own situation, they can choose other options and supplement their own symptoms. G: Bar chart showing participants’ symptom frequencies at 1–3 months.
Furthermore, we compared the impact of different comorbidities on the prognosis of COVID-19 participants. The statistical results showed that multiple comorbidities were significantly associated with the severity of COVID-19 (Table S2, p < 0.001), including chronic obstructive pulmonary disease (1.05% versus 8.7%), respiratory diseases (1% versus 3.44%), diabetes (1% versus 4.46%), hypertension (0.79% versus 4.46%), cardio-cerebrovascular disease (0.79% versus 13.75%). In addition, the statistical results also showed that comorbidities had significantly delayed improvement time in symptoms, such as chronic obstructive pulmonary disease, respiratory diseases, hypertension, cardio-cerebrovascular disease, chronic nephrosis, tumor and autoimmune (Table S3, p < = 0.01). Participants with these comorbidities had a lower proportion of significant improvement in symptoms within a week (Table S3). These data suggest that COVID-19 patients with comorbidities may need more recovery time and medical monitoring.
3.2. Common symptoms and persistent symptoms of SARS-CoV-2–positive participants
A total of 6728 participants tested positive for SARS-CoV-2, of whom 98.47% were infected after October 2022 (Figure S1G) and 73 people required hospitalization (1.1%), while the rest were considered to have mild symptoms (Figure 1B). After infection, about 83% of participants reported significant symptom improvement within 1 week and about 6% improved significantly after more than two weeks (Figure 1C). Interestingly, about 78% reported that at least one family member had a vaccination status and symptoms that were similar to their own (Figure 1D), indicating a high incidence of contact infection among family members.
The most common infectious symptoms among COVID-19 participants were fatigue (73.31%), cough (70.02%), fever (65.25%), overall soreness (58.64%), headache/dizziness (57.61%), sore throat (52.53%), expectoration (44.68%), nasal congestion (42.91%), chills (38.87%), runny nose (38.83%), ageusia/anosmia (34.77%) and high fever > 39.1 °C (34.48%) (Figure 1E). Uncommon symptoms included conjunctivitis (2.18%) and abdominal pain (3.64%) (Figure 1E). In addition, the other commonly self-reported symptoms were mainly lumbago and insomnia (Figure 1F). It is worth noting that only 0.7% of the positive participants self-reported themselves as asymptomatically infected, (Figure 1E); however, this proportion may have been an underestimate due to low-frequency testing of asymptomatic individuals.
Next, we analyzed the symptoms of positive participants during the 1–3-month period after infection to assess persistent symptoms (medium and long-term symptoms). The results showed that many participants reported the following symptoms one month after infection: fatigue (48.7%), drowsiness (34.3%), cough (30.1%), decreased exercise ability (23.1%), pharyngeal discomfort (19.4%), sleep disorders (14%), headache/dizziness (11.8%), arrhythmia/palpitation (10.4%) and so on (Figure 1G). Notably, most long-term symptoms were significantly reduced by more than 200% within 2 months after infection, leaving only a higher proportion of fatigue (17.7%) and drowsiness (12.6%) (Figure 1F). By 3 months post-infection, the proportion of fatigue (7%) and drowsiness (5%) symptoms further decreased to less than 10% or 5%, respectively (Figure 1G). The data illustrates the prevalence of long COVID triggered by SARS-CoV-2 infection, with many people requiring 1–2 months to recover and a small portion requiring more time for full recovery.
3.3. Effect of vaccine dose on breakthrough infection
We analyzed the relationship between vaccine doses and infection rates, the time of significant symptom improvement and disease severity. Significant differences between these factors (p < 0.001, p < 0.001, p = 0.006) were observed (Figure 2A–C). Among these, the positive rate of SARS-CoV-2 in participants with one vaccine dose was comparable to that in unvaccinated participants; however, the positive rate of participants with 2–3 doses was reduced (p < 0.001) (Figure 2A). Notably, the positive rate of SARS-CoV-2 was the lowest in participants vaccinated with 4–5 doses (Figure 2A). Participants who received 1–5 doses of vaccine had a higher proportion of significant improvement in symptoms within 1 week than unvaccinated ones who had a higher proportion of improvement for more than 2 weeks and had not improved (p < 0.001) (Figure 2B). This indicates that vaccination accelerates the improvement of COVID-19 symptoms by varying degrees. Finally, the results showed that unvaccinated participants had a higher proportion of hospitalizations (p = 0.006) (Figure 2C).
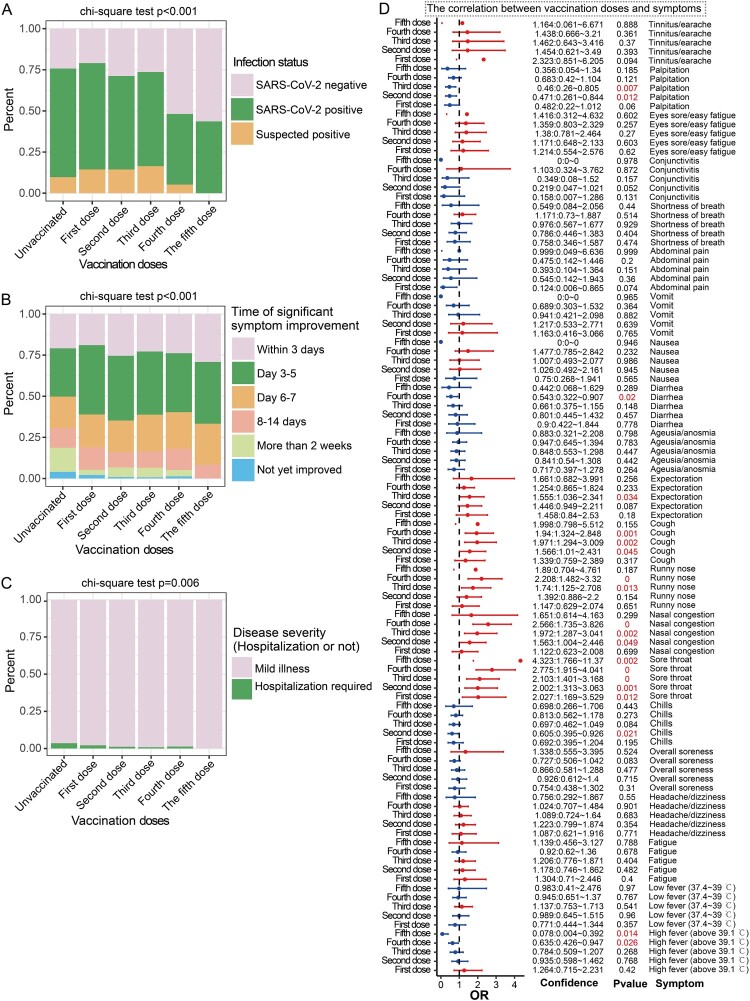
Figure 2.
Breakthrough infections in participants with different vaccine doses. A: Stacked bar chart showing the proportion of SARS-CoV-2–positive participants in the unvaccinated group and different vaccine dose groups. B: Stacked bar chart showing the proportion of participants demonstrating significant symptom improvement within one week in the unvaccinated group and the different vaccine dose groups. C: Stacked bar chart showing the proportion of hospitalized participants in the unvaccinated group and the different vaccine dose groups. D: Forest plot showing the effects of different vaccine doses on symptom frequencies. Unvaccinated participants were used as control variables. OR > 1 represents an increased risk of symptoms, OR < 1 represents a reduced risk of symptoms, which is a potential protective factor and OR = 1 represents no correlation with symptoms.
In subsequent analyses, we employed multivariate logistic regression to assess the relationship between vaccination doses and infection symptoms. After adjusting for age, sex, medical condition, smoking and drinking habits, region and recent date of vaccination, the results showed that different vaccine doses were associated with different symptoms. Specifically, compared to unvaccinated status, 2–3 doses of vaccine significantly reduced the occurrence of palpitations (OR = 0.46 p = 0.007 and OR = 0.471 p = 0.012) (Figure 2D). Additionally, the OR of diarrhea in the 4-dose vaccine group was significantly reduced (OR = 0.543, p = 0.02) and the 2-dose vaccine group had a significantly reduced proportion of chills (OR = 0.605 p = 0.021) (Figure 2D). Moreover, 4–5 doses of vaccine significantly decreased the incidence of high fever (>39.1 °C; OR = 0.635 p = 0.026; OR = 0.078 p = 0.014) (Figure 2D). However, vaccination also increased the ORs of some symptoms. For example, the OR for sore throat in participants who received 1–5 doses of vaccine was significantly high (OR = 2.027, OR = 2.002, OR = 2.103, OR = 2.775, OR = 4.323, respectively; p < 0.05) and the ORs of nasal congestion (OR = 1.563, OR = 1.972, OR = 2.566, respectively; p < 0.05) and cough (OR = 1.566, OR = 1.971, OR = 1.94, respectively; p < 0.05) in the 2–4-dose group were significantly increased (Figure 2D). In addition, in participants who received 3–4 doses of vaccine, the ORs of runny nose (OR = 1.74, p = 0.013, OR = 2.208, p < 0.001) and expectoration (OR = 1.555, p = 0.034) were significantly increased in participants (Figure 2D).
3.4. Impact of recent date of vaccination on breakthrough infection
We analyzed the relationship between the recent date of vaccination of the participants and their infection rates, as well as their symptom improvement time and symptom severity data. The results showed that the longer the time since vaccination, the higher the infection rate (p < 0.001). Participants who had been vaccinated within 3 months had the lowest positive rate of SARS-CoV-2 (Figure 3A). No overall significant difference in the time of significant symptom improvement between the different groups based on their recent vaccination times (p = 0.094) was observed; however, it was still observed that a higher proportion of participants who had been vaccinated over 6 months prior required over 2 weeks to alleviate their symptoms (Figure 3B). No overall significant difference in the proportion of severe cases among the different groups based on their recent vaccination timings (p = 0.257) was observed (Figure 3C), which may be due to the limited number of severe cases in the dataset.
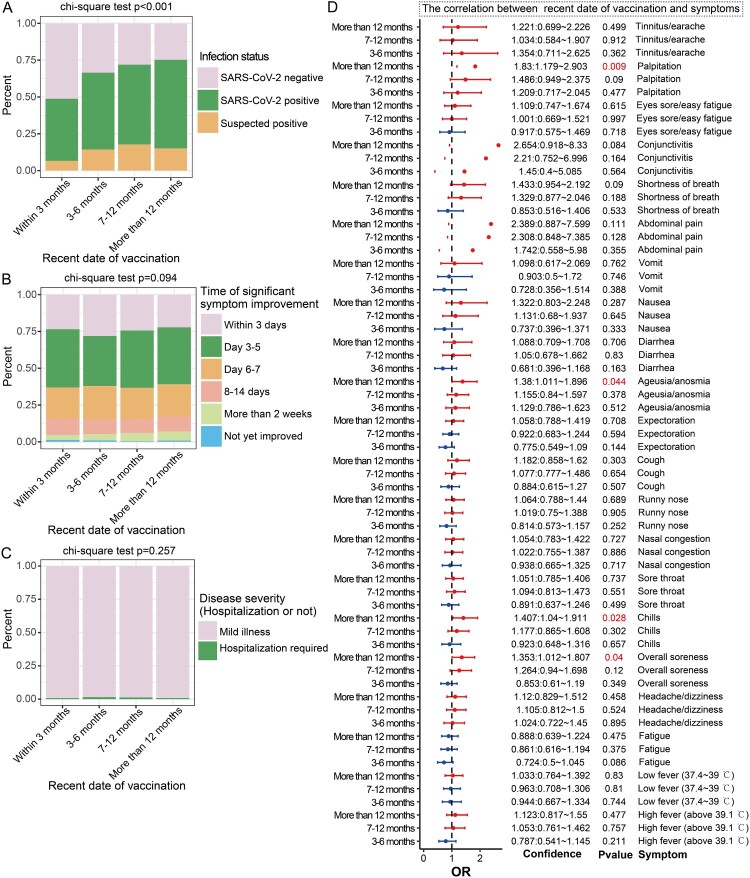
Figure 3.
Vaccination timing is associated with breakthrough infection. A: Stacked bar chart showing the proportion of SARS-CoV-2–positive cases among the different vaccination timing groups. B: Stacked bar chart showing the timing of significant improvement of symptoms in the different vaccination timing groups. C: Stacked bar chart showing the proportion of hospitalization among the different vaccination timing groups. D: Forest plot showing the effect of vaccination timing on the prevalence of COVID-19 symptoms across different groups. The group of participants who received vaccinations within 3 months served as the control group in this analysis. OR > 1 represents an increased odds ratio of symptoms, OR < 1 represents a reduced risk of symptoms, which is a potential protective factor, and OR = 1 represents no correlation with symptoms.
We used multivariate logistic regression to adjust for vaccine doses and other factors to assess the relationship between vaccination time and symptoms. The results showed that in participants who had been vaccinated over 12 months previously, compared with participants who had been vaccinated within 3 months, the ORs of various symptoms increased significantly, including palpitation (OR = 1.83, p = 0.009), ageusia/anosmia (OR = 1.38, p = 0.044), chills (OR = 1.407, p = 0.028) and overall soreness (OR = 1.353, p = 0.04) (Figure 3D).
3.5. Effects of different COVID-19 vaccination statuses on infection
We found that there were significant overall differences in infection rates among groups with different vaccination combinations and unvaccinated groups (p < 0.001) (Figure 4A). In detail, compared to unvaccinated participants, individuals who received a 3-dose protein subunit vaccine or a combination of a 2-dose inactivated vaccine and 1 protein subunit vaccine had a lower infection rate compared to unvaccinated participants. Importantly, the infection rate was lowest among participants who received 4-dose COVID-19 vaccinations (4-dose protein subunit vaccines or 3-dose inactivated + 1 protein subunit vaccines or 3-dose inactivated + 1-adenovirus-vectored vaccine) (Figure 4A). Overall, significant differences in the improvement time between participants with different vaccine combinations and unvaccinated participants were observed (p < 0.001) (Figure 4B). For instance, the proportion of participants who received more than 2 doses of vaccines and those who improved within 1 week was higher than that of unvaccinated participants. In contrast, the proportion of slow improvement (persistent symptoms for more than 2 weeks or no improvement) was higher in the unvaccinated group (Figure 4B). Participants vaccinated with 2 doses of adenovirus-vectored vaccines had the highest proportion of rapid improvement within 3 days (Figure 4B).
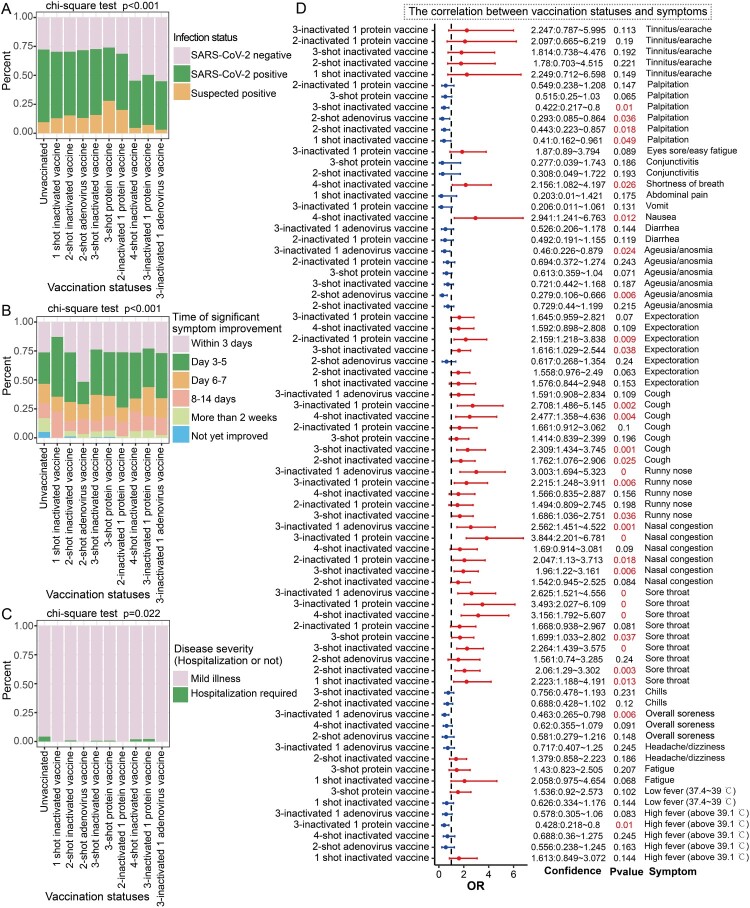
Figure 4.
Correlation between vaccination status and breakthrough infection. A: Stacked bar chart showing the proportion of SARS-CoV-2–positive cases in the unvaccinated group and among the different vaccination status groups. B: Stacked bar chart showing the proportion of time for significant improvement of symptoms in both the unvaccinated group and in groups with different vaccination statuses. C: Stacked bar chart showing the proportion of hospitalizations in the unvaccinated group and the different vaccination status groups. D: Forest plot showing the effects of different vaccination statuses on different symptoms compared to unvaccinated individuals, who were used as controls. OR > 1 represents an increased risk (odds ratio) of symptoms, OR < 1 represents a reduced risk of symptoms, which is a potential protective factor, and OR = 1 represents no association with symptoms. Only results with p < 0.25 are shown in the figure, complete results and significance are shown in Figures S2.
We found that participant vaccination status was significantly associated with COVID-19 severity (p = 0.022) (Figure 4C), as the unvaccinated population had a higher proportion of hospitalizations. Next, after adjusting for multiple covariate factors and recent vaccination timing, the correlation between vaccination status and symptoms was revealed (Figure 4D and Figure S2). The results showed that compared with being unvaccinated, the combination of 2 or more doses of vaccine reduced the OR of high fever (>39.1 °C) (OR < 1, p < 0.25), especially in the 3-inactivated + 1 protein subunit vaccine group (OR = 0.428, p = 0.01) (Figure 4D). In addition, receipt of 3-inactivated + 1 adenovirus-vectored vaccines significantly reduced the OR of overall soreness (OR = 0.463, p = 0.006) and ageusia/anosmia (OR = 0.46, p = 0.024), which was also significantly negatively correlated with two-shot adenovirus-vectored vaccines (OR = 0.279, p = 0.006) (Figure 4D). The results also demonstrated that multiple vaccine combinations reduced the OR of palpitation overall, including 1-shot inactivated vaccines (OR = 0.41, p = 0.049), 2-shot inactivated vaccines (OR = 0.443, p = 0.018), 2-shot adenovirus-vectored vaccines (OR = 0.293, p = 0.036) and 3-shot inactivated vaccines (OR = 0.422, p = 0.01) (Figure 4D).
Different vaccine combinations were also positively correlated with certain symptoms. Among these, the risk of sore throat in participants with different vaccine combinations was significantly higher than that in unvaccinated participants (OR > 1.5, p < 0.05). At the same time, some vaccine combinations increased the risk of nasal congestion, runny nose, cough and expectoration (OR > 1, p < 0.05), such as 3-inactivated vaccines, 2-inactivated + 1 protein subunit vaccines and 3-inactivated + 1 protein subunit vaccines (Figure 4D). The results also demonstrated that participants who received 4 shots of inactivated vaccines had higher ORs for nausea (OR = 2.941, p < 0.012) and shortness of breath (OR = 2.156, p = 0.026) (Figure 4D). Interestingly, these symptoms appear to be primarily upper respiratory symptoms.
3.6. Association between different vaccination statuses and medium- to long COVID-19 symptoms
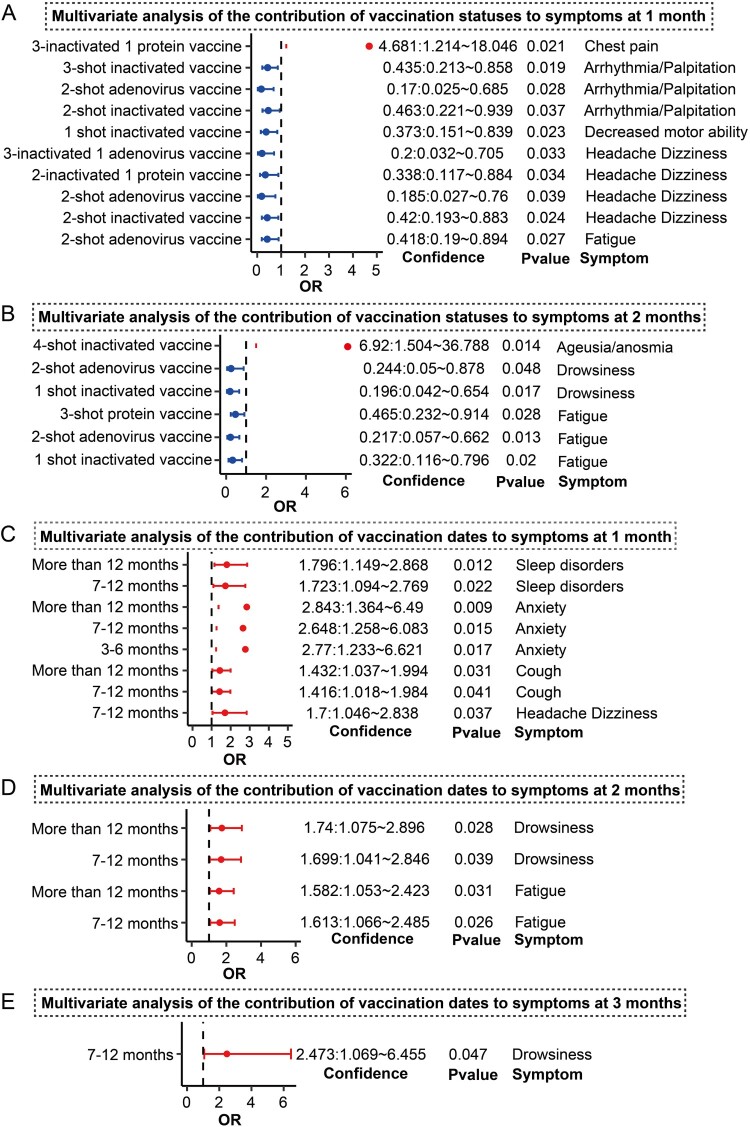
We calculated the relationship between different vaccination statuses and medium- to long-term symptoms after infection. The results showed that 2-shot adenovirus-vectored vaccines significantly reduced the OR of fatigue 1 month after infection (OR = 0.418, p = 0.027). In addition, 2-shot inactivated vaccines (OR = 0.42, p = 0.024), 2-shot adenovirus-vectored vaccines (OR = 0.185, p = 0.039), 2-inactivated + 1 protein subunit vaccines (OR = 0.338, p = 0.034) and 3-inactivated + 1 adenovirus-vectored vaccines (OR = 0.2, p = 0.033) led to significantly reduced headache/dizziness symptoms 1 month after infection (Figure 5A and Figure S3). In addition, 2-shot inactivated vaccines (OR = 0.463, p = 0.037), 2-shot adenovirus-vectored vaccines (OR = 0.17, p = 0.028) and 3-shot inactivated vaccines (OR = 0.435, p = 0.019) significantly reduced the OR of arrhythmia/palpitation at 1 month (Figure 5A). In contrast, we found that 3-inactivated + 1 protein subunit vaccines increased the OR of chest pain symptoms at 1 month (OR = 4.681, p = 0.021). Further results showed that 1-shot inactivated vaccines, 2-shot adenovirus-vectored vaccines and 3-shot protein subunit vaccines significantly reduced the risk of fatigue at 2 months (OR < 0.5, p < 0.05) and 1-shot inactivated vaccines and 2-shot adenovirus-vectored vaccines significantly reduced drowsiness (OR < 0.5, p < 0.05) (Figure 5B and Figure S3). Furthermore, participants that received 4-shot inactivated vaccines had a significant risk of ageusia/anosmia 2 months after infection (OR = 6.92, p = 0.014).
Figure 5.
Effect of vaccination background and vaccination time on medium- to long-term symptoms. A: Forest plot showing the effects of unvaccinated status and different vaccine combinations on symptoms at 1 month. B: Forest plot showing the effects of unvaccinated status and different vaccine combinations on symptoms at 2 months. C–E: Forest plot showing the effects of different vaccination timings on symptoms at 1–3 months. Those with vaccine combinations were compared to unvaccinated subjects. The recent vaccination time was compared with vaccination within 3 months. OR > 1 represents an increased risk of symptoms, OR < 1 represents a reduced risk of symptoms, which is a potential protective factor, and OR = 1 represents no association with symptoms. Only results with p < 0.05 are shown in the figure, complete results and significance are shown in Figures S3–S6.
Overall, symptoms tended to be reduced within 1–2 months after infection. In addition, there was no significant relationship between symptoms and vaccine status at 3 months (Figure S4). The effect of vaccination time on medium and long-term symptoms was also analyzed (Figure S5–S6). The results showed that compared with recent vaccination timings within 3 months, participants whose last vaccination shot was 7–12 months prior had a significantly higher risk of headache/dizziness (OR = 1.7, p = 0.037), cough (OR = 1.416, p = 0.041), anxiety (OR = 2.648, p = 0.015) and sleep disorders (OR = 1.723, p = 0.022) 1 month after infection (Figure 5C and Figure S5). Additionally, participants vaccinated over 12 months previously had a significantly increased risk of cough (OR = 1.432, p = 0.031), anxiety (OR = 2.843, p = 0.009) and sleep disorders (OR = 1.796, p = 0.012) 1 month after infection (Figure 5C) and the same was true for anxiety (OR = 2.77, p = 0.017) in those vaccinated 3–6 months prior. Further results showed that COVID-19 positive participants vaccinated 7–12 months prior and greater than 12 months prior had a significantly increased risk of fatigue and drowsiness (OR > 1, p < 0.05) at 2 months and these symptoms persisted until 3 months after infection (Figure 5D–E and Figure S6). Taken together, these results suggest that the interval between one’s most recent vaccine dose and the time of infection may contribute to the persistence of symptoms (long COVID).
4. Discussion
Extensive SARS-CoV-2 infections in China mainly occurred after the adjustment of the dynamic zero-COVID-19 strategy. Previous studies reported that the immune barrier established by COVID-19 vaccination reduces the proportion of severe cases [23–25] and even the positive rate of SARS-CoV-2 to a certain extent [26,27], while the evolution of the virus into new variants rarely causes lower respiratory symptoms [7,8]. Nevertheless, during the most recent infection wave in China, the rapid spread of infection has caused panic in the public and led to an excessive strain on medical resources. As a result, reevaluating whether the epidemiological characteristics of COVID-19 after the adjustment of the dynamic zero-COVID-19 strategy are the same as those observed previously is crucial. Currently, a large proportion of residents in China have been vaccinated with inactivated vaccines and some have been vaccinated with adenovirus-vectored vaccines and/or protein subunit vaccines. Prior to this, there was also heterogeneity in the time and frequency of vaccination in the Chinese population. Studies on the relationship between different vaccination statuses and BTI in the real world after the zero-COVID-19 strategy are still lacking.
This study determined a key correlation between vaccination statuses, BTI symptoms and symptom persistence. Following our statistical analysis, only 1.1% of the positive participants reported that they required hospitalization (Figure 1), which was a significant reduction from the proportion of severe cases in the early stages of COVID-19 [28,29]. Consistent with previous reports, comorbidities related to COVID-19 severity include hypertension, diabetes, chronic obstructive pulmonary disease, respiratory diseases, cardiovascular disease and tumors, etc. (Table S2) [22,30]. The proportion of self-reported asymptomatic participants in positive participants was only 0.7%, which was significantly lower than previous reports [7–11]. We speculate that this proportion may be underestimated as it is not directly a statistical result of detecting SARS-CoV-2 in healthy individuals and the detection rate is usually lower in asymptomatic infected individuals [7–11]. In addition, questionnaire participants may be vulnerable to psychological and environmental influences when choosing certain symptoms, such as fatigue and drowsiness. Although about 83% of participants with BTI exhibit significant improvements in their symptoms within a week, a large proportion of subjects still have a variety of medium and long-term symptoms at 1–2 months, especially fatigue, drowsiness, cough, decreased exercise ability, pharyngeal discomfort, sleep disorders and headache/dizziness (Figure 1). These medium to long-term symptoms are basically consistent with the previously reported high frequency of long COVID symptoms [22], but the proportion of persistent symptoms caused by BA. 5 and BF. 7 is lower than that caused by previous variants, ranging from 8.89% to 20% three months after infection [31–33]. Overall, complete physical recovery still takes longer than expected, which is significantly different from the time required to recuperate from the common viral cold [34]. Symptom recovery after SARS-CoV-2 infection and its mechanism requires further study and this research may provide insights into social life and work management strategies to promote more humane care and sick leave procedures after infection.
Our data support some hypothesized conclusions, including that vaccination can significantly reduce the positive rate of SARS-CoV-2, the proportion of severe cases and the time to significant symptom improvement and these protective effects are enhanced when the number of vaccine doses increases [35,36]. In general, our data show that four or five vaccine doses exhibit the best protective effects. Although the sample size for this analysis was small, it may provide evidence to support subsequent booster vaccines, particularly for elderly and immunocompromised individuals. In addition, our results showed that different vaccine doses and vaccine combinations could significantly reduce the risk of extreme fever (>39.1 °C), chills, diarrhea, ageusia/anosmia and other symptoms (Figures 2 and 4). On the other hand, vaccination may increase the frequency of symptoms such as sore throat, nasal congestion, runny nose and expectoration (Figures 2 and 4). It is noteworthy that these increased symptoms were mainly discomforts of the nasal cavity and throat, which are parts of the upper respiratory tract, while the reduced symptoms were mainly lower respiratory symptoms or other symptoms (such as extreme high fever and palpitation). We speculate that increased upper respiratory symptoms may be necessary to activate respiratory immunity to eliminate the virus. Overall, our results also supported the fact that different vaccination combinations significantly reduced mid- and long-term symptoms such as fatigue, drowsiness and headache (Figure 5).
In addition to vaccination doses and vaccine combinations, our findings suggest that the interval between one’s recent vaccine shot and BTI contributes to the mitigation of COVID-19. This is evidenced by the fact that the group of participants who were vaccinated within 3 months had a lower positive rate than the group vaccinated 7–12 months prior and the time to improvement in the former group was also shorter (Figure 3). In addition, participants who had been vaccinated for 3 months before infection also had a significantly lower risk of overall soreness, chills and ageusia/anosmia (Figure 3), as well as long-term symptoms including fatigue, drowsiness, headache, cough, anxiety and sleep disorders at 1–3 months (Figure 5). These results indicate that timely booster shots may reduce the disease severity and duration of symptoms.
Overall, this study offers important insights and data regarding the relationship between vaccination statuses and BTI after zero-COVID-19 strategy. Nevertheless, this research has several limitations. First, our questionnaire survey focused on the infection wave after China’s dynamic zero-COVID-19 strategy adjustment and the conclusion was mainly aimed at the prevailing BA.5 and BF.7. Second, the effectiveness of the information in online survey depends on the participants’ self-perception, so some participants may have some deviations in symptom recall and we cannot give a real clinical objective assessment. Third, a large number of participants were mainly vaccinated with inactivated vaccines, while the population samples vaccinated with protein subunit vaccine, adenovirus-vectored vaccine and mixed vaccination were limited. Fourth, although our survey covered the whole China to assess BTI across the country, the number of participants in different provinces was not balanced. Fifth, the investigation timing was not long enough to assess long COVID longer than three months, six months or even more post infection. In the future, we hope that more diverse samples and more comprehensive and systematic investigations using more diverse samples will be carried out to gain a better understanding of the relationship between COVID-19 vaccination and SARS-CoV-2 BTI.
Author contributions
GFG, XZ, SJQ and YHL conceived the questionnaire questions. SJQ and YHL created the questionnaire link. SHQ obtained and analyzed the data. SHQ wrote the first draft. YHL, XZ and GFG revised the manuscript. XPM and GFG supervised the project. LKW helped distribute the questionnaire. All authors have agreed to publish the manuscript.
Supplementary Material
Acknowledgements
We thank William J. Liu (NHC Key Laboratory of Biosafety, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention), Kefang Liu (Institute of Microbiology, CAS) and Shuguang Tan (Institute of Microbiology, CAS) for their help in question design and questionnaire dissemination. Additionally, our gratitude goes to the participants who completed the survey and the “Questionnaire Star” platform.
Funding Statement
This work was supported by China Postdoctoral Science Foundation: [Grant Number 2022M723344]; Bill & Melinda Gates Foundation: [Grant Number INV-027420].
Data and code availability statement
The original questionnaire data in this study can be accessed upon reasonable request by contacting the corresponding author. The analysis code for the manuscript results can be obtained on github https://github.com/Shijie0825/COVID-19-online-survey.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Liu S, Liang Z, Nie J, et al. . Sera from breakthrough infections with SARS-CoV-2 BA.5 or BF.7 showed lower neutralization activity against XBB.1.5 and CH.1.1 [published online ahead of print, 2023 Jun 14]. Emerg Microbes Infect. 2023;12(2):2225638. doi: 10.1080/22221751.2023.2225638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu S, Luo S, Bai B, et al. . Omicron breakthrough infections in wild-type SARS-CoV-2 vaccinees elicit high levels of neutralizing antibodies against pangolin coronavirus GX_P2V. J Med Virol. 2023;95(8):e29031. doi: 10.1002/jmv.29031 [DOI] [PubMed] [Google Scholar]
- 3.Abbasi J. What to know about EG.5, the latest SARS-CoV-2 “variant of interest” [published online ahead of print, 2023 Aug 18]. JAMA. 2023. doi: 10.1001/jama.2023.16498 [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, Wang L, Feng Z, et al. . Characterisation of SARS-CoV-2 variants in Beijing during 2022: an epidemiological and phylogenetic analysis. Lancet. 2023;401(10377):664–672. doi: 10.1016/S0140-6736(23)00129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Liu M, Liang W.. The dynamic COVID-zero strategy in China. China CDC Wkly. 2022;4(4):74–75. doi: 10.46234/ccdcw2022.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Zhang F, Liu Y, et al. . Clinical Characteristics of Mild Patients with Breakthrough Infection of Omicron Variant in China after Relaxing the Dynamic Zero COVID-19 Policy. Vaccines (Basel). 2023;11(5):968. doi: 10.3390/vaccines11050968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Liu Z, Wang Z, et al. . Clinical characteristics of 1139 mild cases of the SARS-CoV-2 omicron variant infected patients in Shanghai. J Med Virol. 2023;95(1):e28224. doi: 10.1002/jmv.28224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrett N, Tapley A, Andriesen J, et al. . High asymptomatic carriage With the omicron variant in South Africa. Clin Infect Dis. 2022;75(1):e289–e292. doi: 10.1093/cid/ciac237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu W, Guo Y, Zhang S, et al. . Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 omicron variant: a systematic review and analysis. J Med Virol. 2022;94(12):5790–5801. doi: 10.1002/jmv.28066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blom K, Havervall S, Marking U, et al. . Infection rate of SARS-CoV-2 in asymptomatic healthcare workers, Sweden, June 2022. Emerg Infect Dis. 2022;28(10):2119–2121. doi: 10.3201/eid2810.221093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graydon EK, Malloy AMW, Machmach K, et al. . High baseline frequencies of natural killer cells are associated with asymptomatic SARS-CoV-2 infection. Curr Res Immunol. 2023;4:100064. Published 2023 Jul 15. doi: 10.1016/j.crimmu.2023.100064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durstenfeld MS, Peluso MJ, Peyser ND, et al. . Factors associated With long COVID symptoms in an online cohort study. Open Forum Infect Dis. 2023;10(2):ofad047. doi: 10.1093/ofid/ofad047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman ML, Oyebanji OA, Moisi D, et al. . Association of cytomegalovirus serostatus With severe acute respiratory syndrome coronavirus 2 vaccine responsiveness in nursing home residents and healthcare workers. Open Forum Infect Dis. 2023;10(2):ofad063. doi: 10.1093/ofid/ofad063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortellini A, Tabernero J, Mukherjee U, et al. . SARS-CoV-2 omicron (B.1.1.529)-related COVID-19 sequelae in vaccinated and unvaccinated patients with cancer: results from the OnCovid registry. Lancet Oncol. 2023;24(4):335–346. doi: 10.1016/S1470-2045(23)00056-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Gao GF.. No novel prevalent mutations detected in the circulating strains of BF.7, BA.5.2, DY, and XBB - China, November 2022 to June 2023. China CDC Wkly. 2023;5(30):672–673. doi: 10.46234/ccdcw2023.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman MM, Akash S, Islam MR.. SARS-CoV-2 new variant BF.7: a new public threat globally, symptoms, precautions, transmission rate, and futures perspective - correspondence. Int J Surg. 2023;109(2):181–183. doi: 10.1097/JS9.0000000000000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huo D, Yu T, Shen Y, et al. . A comparison of clinical characteristics of infections with SARS-CoV-2 omicron subvariants BF.7.14 and BA.5.2.48 – China, October-December 2022. China CDC Wkly. 2023;5(23):511–515. doi: 10.46234/ccdcw2023.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Wang Y, Zhang T, et al. . Status of and perspectives on COVID-19 vaccination after lifting of the dynamic zero-COVID policy in China. Glob Health Med. 2023;5(2):112–117. doi: 10.35772/ghm.2022.01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai L, Duan H, Liu X, et al. . Omicron neutralisation: RBD-dimer booster versus BF.7 and BA.5.2 breakthrough infection [published online ahead of print, 2023 Aug 14]. Lancet. 2023;S0140-6736(23):01367–3. doi: 10.1016/S0140-6736(23)01367-3 [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Zang C, Xu Z, et al. . Data-driven identification of post-acute SARS-CoV-2 infection subphenotypes. Nat Med. 2023;29(1):226–235. doi: 10.1038/s41591-022-02116-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis HE, McCorkell L, Vogel JM, et al. . Long COVID: major findings, mechanisms and recommendations [published correction appears in Nat Rev microbiol. 2023 Jun;21(6):408]. Nat Rev Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai J, Lin K, Zhang H, et al. . A one-year follow-up study of systematic impact of long COVID symptoms among patients post SARS-CoV-2 omicron variants infection in Shanghai, China. Emerg Microbes Infect. 2023;12(2):2220578. doi: 10.1080/22221751.2023.2220578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai L, Gao L, Tao L, et al. . Efficacy and safety of the RBD-dimer-based COVID-19 vaccine ZF2001 in adults. N Engl J Med. 2022;386(22):2097–2111. doi: 10.1056/NEJMoa2202261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng H, Cao Y, Chen X, et al. . Disease profile and plasma neutralizing activity of post-vaccination omicron BA.1 infection in Tianjin, China: a retrospective study. Cell Res. 2022;32(8):781–784. doi: 10.1038/s41422-022-00674-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu FC, Li YH, Guan XH, et al. . Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel AB, Kanevsky I, Che Y, et al. . BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283–289. doi: 10.1038/s41586-021-03275-y [DOI] [PubMed] [Google Scholar]
- 27.Tao K, Tzou PL, Nouhin J, et al. . SARS-CoV-2 antiviral therapy. Clin Microbiol Rev. 2021;34(4):e0010921. doi: 10.1128/CMR.00109-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin S, Li W, Shi X, et al. . Cases reveal important prognosis signatures of COVID-19 patients. Comput Struct Biotechnol J. 3044;2021(19):1163–1175. doi: 10.1016/j.csbj.2021.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen N, Zhou M, Dong X, et al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheong HH, Sio FI, Chan CC, et al. . Clinical characteristics of COVID-19 patients infected by the omicron variants in Macao, China: a cross-sectional study. Health Sci Rep. 2023;6(7):e1361. doi: 10.1002/hsr2.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Li Y, Shao TR, et al. . Some characteristics of clinical sequelae of COVID-19 survivors from Wuhan, China: a multi-center longitudinal study. Influenza Other Respir Viruses. 2022;16(3):395–401. doi: 10.1111/irv.12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Huang L, Wang Y, et al. . 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2023;401(10393):e21–e33. doi: 10.1016/S0140-6736(23)00810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Q, Wang C, Jing Q, et al. . Follow-up of patients with COVID-19 by the delta variant after hospital discharge in Guangzhou, Guandong, China. Rev Inst Med Trop Sao Paulo. 2022;64:e31. doi: 10.1590/s1678-9946202264031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedberg P, Karlsson Valik J, van der Werff S, et al. . Clinical phenotypes and outcomes of SARS-CoV-2, influenza, RSV and seven other respiratory viruses: a retrospective study using complete hospital data. Thorax. 2022;77(2):154–163. doi: 10.1136/thoraxjnl-2021-216949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan M, Duan H, An Y, et al. . A booster of delta-omicron RBD-dimer protein subunit vaccine augments sera neutralization of omicron sub-variants BA.1/BA.2/BA.2.12.1/BA.4/BA.5. Emerg Microbes Infect. 2023;12(1):e2179357. doi: 10.1080/22221751.2023.2179357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang T, Zhang S, Dai DF, et al. . Safety and immunogenicity of heterologous boosting with orally aerosolised or intramuscular Ad5-nCoV vaccine and homologous boosting with inactivated vaccines (BBIBP-CorV or CoronaVac) in children and adolescents: a randomised, open-label, parallel-controlled, non-inferiority, single-centre study. Lancet Respir Med. 2023;11(8):698–708. doi: 10.1016/S2213-2600(23)00129-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original questionnaire data in this study can be accessed upon reasonable request by contacting the corresponding author. The analysis code for the manuscript results can be obtained on github https://github.com/Shijie0825/COVID-19-online-survey.