Abstract
Sleep problems are prevalent in children with neurodevelopmental disabilities and are associated with the expression of restricted and repetitive behaviors (RRBs). Children (n = 57) with autism spectrum disorder (ASD, n = 38) or developmental delay (DD, n = 19) participated in multiple assessments of intellectual ability, ASD symptoms, and RRBs (3 timepoints for ASD, 2 for DD). Sleep problems assessed at age 4 via parent report were associated with trajectories of higher-order RRBs (sameness/ritualistic/compulsive behaviors) from age 2–6 in the ASD group, and from age 2–4 in the DD group, even after controlling for intellectual ability, social-affective symptoms, and anxiety. Trajectories of stereotyped/restricted behaviors were unrelated to sleep problems. Sleep problems were associated with trajectories of higher-order (but not lower-order) RRBs in a transdiagnostic sample.
Keywords: Sleep, Restricted behaviors, Repetitive behaviors, Anxiety, Autism spectrum disorder, Developmental delay, Neurodevelopmental disability
Children with neurodevelopmental disabilities suffer from sleep problems at much higher rates than typically-developing children (Liu et al. 2006; Mannion et al. 2013; Maskey et al. 2013; Meltzer and Mindell 2008; Reynolds et al. 2019).
Common sleep problems in this population include delayed sleep onset, early morning waking, repeated night awakenings, parasomnias (e.g., night terrors and sleepwalking), and reduced overall sleep duration (Cortesi et al. 2010; Kotagal and Broomall 2012; Reynolds and Malow 2011; Singh and Zimmerman 2015). If left untreated, these problems (alone or in combination) could impair a child’s ability to benefit from behavioral interventions and adversely affect developmental outcomes (Abel et al. 2018; Schreck et al. 2004). Sleep problems have been directly associated with the severity of symptoms in children with neurodevelopmental disabilities (Mayes and Calhoun 2009; Owens 2005), suggesting that treating sleep problems could lead to generalized improvements in core symptomatology and child functioning (e.g., Loring et al. 2018; Malow et al. 2012, 2014).
The current investigation focuses on sleep problems in relation to restricted and repetitive behavior (RRB), a core symptom of autism spectrum disorder (ASD) that is also often present in children with other neurodevelopmental disabilities (Bishop et al. 2007b; Bodfish et al. 2000; Leekam et al. 2011). This broad category of behaviors includes stereotyped motor movements, sensory-seeking, repetitive or ritualistic behavior, insistence on sameness, and restricted interests. Superordinate categories of RRBs—namely “higher-order” (insistence on sameness, circumscribed interests, compulsive, ritualistic, and restricted behaviors), and “lower-order” (stereotyped motor movements)—have been identified (Bishop et al. 2013; Georgiades et al. 2010; Mirenda et al. 2010), and may have different genetic or neurobiological underpinnings (Hollander et al. 2005; Lam et al. 2008; Langen et al. 2014; Szatmari et al. 2006; Wolff et al. 2013).
RRBs are associated with critical aspects of child functioning (e.g., executive function; Mosconi et al. 2009) and can interfere with family functioning due to increased parent stress (Bishop et al. 2007a; Johnson et al. 2018). Interventions have been developed that specifically target RRBs (Boyd et al. 2011), yet the natural history of RRBs across development remains unclear. For example, it has been reported that some subtypes of RRBs increase over development, while others decrease or remain steady. Observed patterns of RRB subtypes differ across studies (Richler et al. 2010; South et al. 2005) and in samples with typical vs. atypical development (Leekam et al. 2011). Individual differences in the extent and severity of RRBs have been attributed to neurobiological (Estes et al. 2011; Langen et al. 2014; Wolff et al. 2013), genetic (Lewis and Kim 2009), and psychological factors (e.g., a child’s ability to regulate arousal; Lidstone et al. 2014). Considering the relationship between sleep problems and specific subtypes of RRBs could potentially explain some of the inconsistent findings regarding the developmental course of RRBs in children with neurodevelopmental disabilities.
Sleep problems have been associated with increased RRBs in multiple associational studies (Cohen et al. 2014; Gabriels et al. 2005; Goldman et al. 2009; Hoffman et al. 2005; Hundley et al. 2016; Mayes and Calhoun 2009; Park et al. 2012; Schreck et al. 2004; Tudor et al. 2012). Actigraphy-derived sleep measures have been associated with ritualistic, compulsive, sameness, and restricted behavior scores on the Repetitive Behavior Scale (RBS-R; Goldman et al. 2009), as well as clinician-observed daytime repetitive behaviors (Abel et al. 2018). Parent-reported sleep problems have been associated with self-injurious and compulsive behavior (Goldman et al. 2011), stereotyped behavior (Hundley et al. 2016; Tudor et al. 2012), and repetitive behavior scores from the Autism Diagnostic Interview (ADI; Hundley et al. 2016; Park et al. 2012). Although prior studies make a compelling case for a relationship between sleep and RRBs, it is possible that variation in a third variable (e.g., non-verbal IQ or anxiety) may account for these associations (Gabriels et al. 2005; Hundley et al. 2016). Children with high anxiety show increased higher-order RRBs (Rodgers et al. 2012; Lidstone et al. 2014) and have greater difficulty sleeping (Alfano et al. 2007) than non-anxious children. Thus, it is possible that anxiety explains some or all of the association between sleep problems and higher-order RRBs (see Hundley et al. 2016). Further work is clearly needed to understand how sleep difficulties—in the context of other aspects of child functioning—relate to the expression of RRBs in children with ASD and other neurodevelopmental disabilities.
The current study aims to contribute new knowledge about the association between sleep and RRBs in a sample of children diagnosed with neurodevelopmental disabilities (ASD or developmental delay; DD) who were followed with multiple behavioral assessments between 2 and 6 years of age (3 timepoints for ASD, 2 for DD). Repetitive behaviors are common to all neurodevelopmental disabilities, yet the majority of literature exploring sleep-RRB associations has focused solely on children with ASD. We included the DD group as a comparison sample to test whether relationships found in the ASD group were disorder-specific. Our transdiagnostic sample had the additional advantage of being assessed with gold-standard diagnostic and developmental tests across multiple timepoints, supplemented with parent reports of RRBs, sleep problems, and anxiety symptoms. We conducted our analyses on pre-existing data; the original study did not assess sleep and anxiety at every timepoint, thus precluding our ability to conduct a full longitudinal analysis. Taking into account the structure of our data, the goals of the current study were to: (1) examine whether sleep problems at age 4 were associated with trajectories of specific RRB subtypes over early development,1 (2) test whether associations between sleep problems and RRB subtypes at age 4 persist when controlling for additional aspects of child functioning (i.e., intellectual ability, social symptom severity) and (3) explore whether anxiety mediates sleep-RRB associations. Based on prior literature, we predicted that children with more severe sleep problems at age 4 would show higher levels of repetitive behavior across early development; analyses of RRB subtypes in relation to sleep and other child characteristics (including anxiety) were exploratory.
Methods
Participants
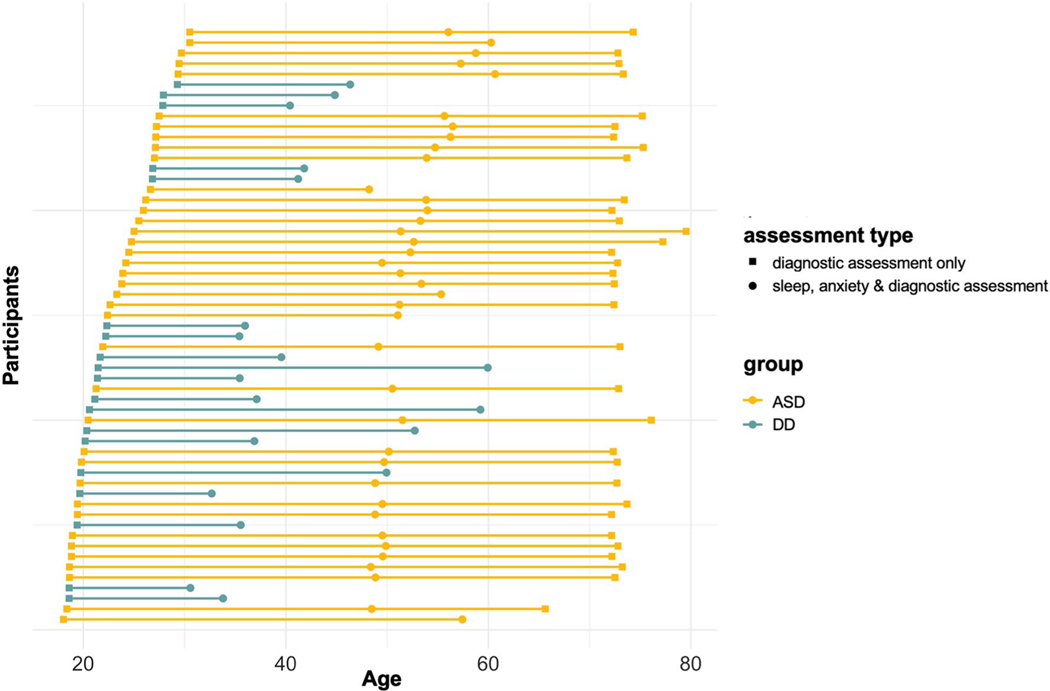
Participants were a cohort of children with diagnoses of ASD (n = 38) or developmental delay (n = 19), initially recruited between 18–20 months of age (Dawson et al. 2010). Diagnostic assessments were repeated at T2 (~ age 4, n = 57) and T3 (~ age 6, ASD only, n = 33). Figure 1 depicts the age of participants at each assessment. Clinical diagnosis of ASD was based on direct assessment with the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al. 2003), the Autism Diagnostic Observation Scale (ADOS; Lord et al. 1999) and meeting DSM-IV criteria according to two independent clinicians (Dawson et al. 2010). Children were recruited for the idiopathic DD group based on parent concern about developmental delay (not ASD). Children were included in the DD group if they scored below cutoff on the Mullen Scales of Early Learning (Mullen 1995; overall score < 1 standard deviation below mean and/or at least one subscale score < 1.5 standard deviations below the mean), did not meet criteria for ASD, and did not have any other known syndromes associated with developmental delay (e.g., Down Syndrome). Informed consent was obtained from parents of children included in the study, and all study procedures were approved by the University of Washington Institutional Review Board.
Fig. 1.
Age distribution. The distribution of participant ages at each assessment shown by group (ASD vs. DD). ASD symptoms and intellectual ability were assessed in both groups at every timepoint. Sleep and anxiety were assessed at the T2 only
Participants were included in current analyses if they had data available from at least 2 timepoints and were assessed for sleep problems at T2. Table 1 depicts the sample characteristics at the T1 (baseline) assessment. The ASD and DD groups did not differ on age, sex, race, ethnicity, or maternal education. The ASD group had significantly lower intellectual ability scores, and significantly higher autism symptoms and restricted/repetitive behavior scores compared to the DD group at baseline. As can be seen in Fig. 1, there was a broader range in age at the time of the T2 assessment for the DD group (range 31–60 months) compared to the ASD group (range 48–61 months). All models included age as a continuous variable to minimize the influence the observed age discrepancies at some timepoints.
Table 1.
Baseline characteristics of sample
| Autism spectrum disorder (ASD; n = 38) (%) | Developmental delay (DD; n = 19) (%) | Group comparison | |
|---|---|---|---|
| % Male | 76 | 68 | n.s. |
| % White | 74 | 63 | n.s. |
| % Non-Hispanic | 84 | 84 | n.s. |
| % Mother graduated college | 71 | 84 | n.s. |
|
| |||
| Mean (SD) | Mean (SD) | ||
|
| |||
| Age | 23.6 (3.9) | 22.4 (3.5) | n.s. |
| ADOS | 7.3 (1.6) | 2.3 (2.1) | *** |
| MSEL | 60.5 (8.9) | 73.1 (11.6) | *** |
| RBS-R: sameness/ritualistic/compulsive | 6.6 (5.8) | 0.8 (1.3) | *** |
| RBS-R: Self-injurious | 1.1 (1.2) | 0.7 (1.5) | n.s. |
| RBS-R: Stereotyped/restricted | 5.3 (2.9) | 1.1 (1.7) | *** |
p < .05
p < .01
p < .001
p < .1
Between T1 and T2, 36 children in the ASD group participated in a randomized control trial of an intensive behavioral intervention; 19 received the experimental treatment (Early Start Denver Model; ESDM) and 17 received community-based treatment as usual (COM). The remaining children with ASD (n = 2) and DD (n = 19) were not randomized to intervention groups but did participate in available community-based services during the same interval. The intervention trial ended at T2, but children with ASD were followed longitudinally through T3. In the current analyses, children were combined across all intervention groups (randomized ESDM, COM, and non-randomized). Treatment effects among the ESDM and COM groups have been previously reported (Dawson et al. 2010; Estes et al. 2015). There were no significant differences post-intervention in sameness/ritualistic/compulsive or self-injurious behavior by treatment group; the ESDM group had lower stereotyped/restricted behavior scores by T3, two years after the intervention ended (t(24) = 2.4, p = 0.03). We did not observe any intervention effects on sleep problems and intervention effects are not the focus of the current report. See Table S1 (Online Resource) for characteristics of the ASD sample by treatment group.
No children in the sample were diagnosed with a medically-based sleep problem. One child in the ASD group was taking an anxiolytic, sedative, and melatonin for anxiety and insomnia, and two others were taking melatonin. One child in the DD group was medicated for seizures. No use of stimulant medications was reported. Results did not differ including and excluding these four participants.
Measures
Sleep
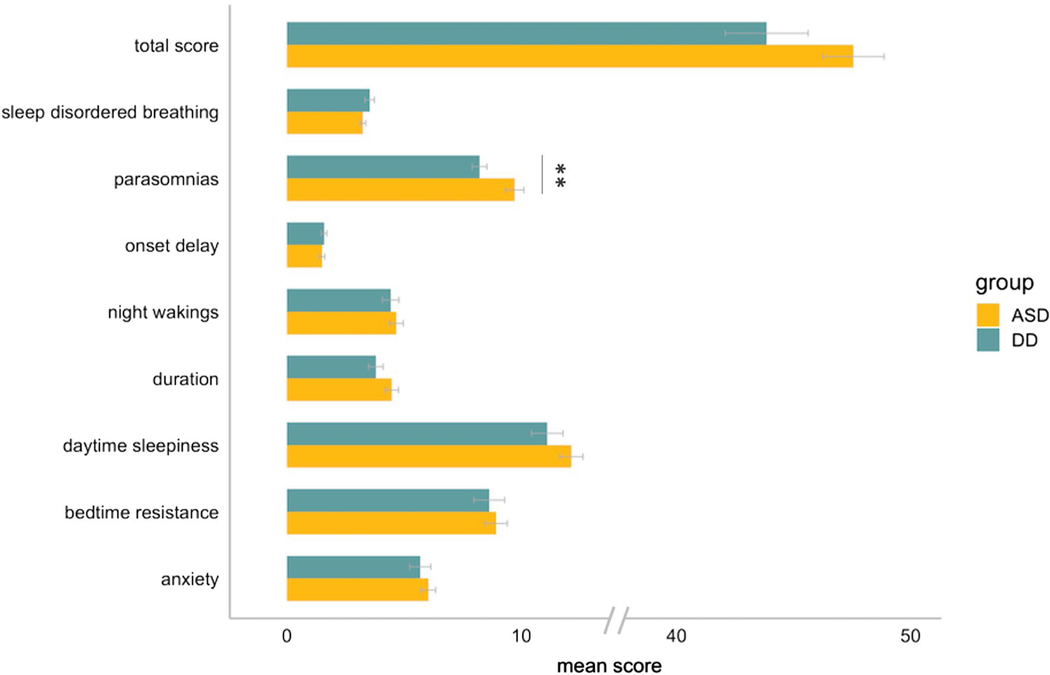
The Children’s Sleep Habits Questionnaire (Owens et al. 2000) is a 33-item parent/caregiver rated scale that was developed to screen for both behavioral and medically-based sleep problems in children ages 4–10. It yields a total score (with cut-off of ≥ 41 indicative of clinically-significant sleep problems) as well as scores on eight subscales: (1) bedtime resistance, (2) sleep onset delay, (3) sleep duration, (4) sleep anxiety, (5) night wakings, (6) parasomnias, (7) sleep-disordered breathing, and (8) daytime sleepiness. Two recent investigations proposed that a 5-factor (Johnson et al. 2016) or 4-factor (Katz et al. 2018) structure for the CSHQ may be more appropriate for assessing sleep in children with ASD. We elected to present results (see Fig. 2) using the original subscale structure due to the transdiagnostic nature of our sample and to facilitate comparison of our results with prior work. Total CSHQ score was used for all analyses.
Fig. 2.
CSHQ subscale scores. Average scores across each CSHQ subscale by group (ASD vs. DD). For visualization purposes, average subscale scores were computed so that scores on subscales containing different numbers of items could be more easily compared. Error bars depict standard error of the mean. Asterix denotes significant group difference (p < .01)
Restricted/Repetitive Behavior
The Repetitive Behavior Scale-Revised (Bodfish et al. 2000) is a 43-item parent/caregiver-rated scale that measures a range of restricted and repetitive behaviors observed over the previous month. The original scale yields scores on six subscales: compulsive (e.g., arranging/checking), self-injurious (e.g., hit/scratch self), ritualistic (e.g., perform activities in a certain order), restricted (e.g., preoccupation with one subject or activity), sameness (e.g., resistance to change), and stereotypical behaviors (e.g., body rocking, hand/finger mannerisms). Subsequent factor analyses of the RBS-R have supported a 5 factor or 3 factor structure (Lam and Aman 2007; Mirenda et al. 2010). We report analyses using the 3 factor structure (Mirenda et al. 2010), which combines scores across the subscales to form a composite sameness/ritualistic/compulsive factor, a composite stereotyped/restricted factor, and a third self-injurious factor. Analyses were also conducted using the 5 factor structure (Mirenda et al. 2010) and are available from the first author by request. The RBS-R produces a “count” score, based on the number of items endorsed by parents, as well as a weighted “severity” score that reflects the parents’ judgement about whether a given behavior is mild, moderate, or severe. To reduce the potential for rater bias to influence our results, we elected to use RBS-R count scores for current analyses consistent with previous work (Wolff et al. 2014).
Autism Symptoms
The ADOS (Lord et al. 1999) is a semi-structured, standardized measure of social relatedness, communication, play, and repetitive behaviors administered by an examiner trained to research standards. The overall severity score was used to describe sample characteristics (Table 1). Because the overall severity score includes repetitive behavior items, our analyses used the ADOS social affect (ADOS SA) score (Hus et al. 2014) to avoid overlap between repetitive behavior items on the ADOS and our primary outcome variable (RBS-R count score).
Intellectual Ability
The Mullen Scales of Early Learning (Mullen 1995)—a standardized developmental test for children ages birth to 68 months—was used to measure child intellectual ability at the T1 & T2 assessments. Standard scores on the Early Learning Composite (M 100; SD 15), which combines scores across 4 subscales (Fine Motor, Visual Reception, Expressive Language, and Receptive Language), were utilized for these analyses.
The Differential Ability Scales (DAS; Elliott et al. 1990) School Age Level—a standardized cognitive assessment tool for children ages 2 years 6 months to 17 years 11 months— was used to measure child intellectual ability at the T3 assessment. Standard scores on General Conceptual Ability (M 100; SD 15), which reflects conceptual and reasoning ability in verbal and nonverbal domains, were included in subsequent analyses.
Anxiety Symptoms
The Pervasive Developmental Disorder Behavior Inventory (PDD-BI; Cohen et al. 2003) is an 124-item, parent-report questionnaire that assesses a wide range of symptoms related to neurodevelopmental disabilities. The Specific Fears subscale score assesses fears and anxieties associated with encountering new situations, stimuli, or with parental separation, and was used in our analyses as a measure of anxiety at T2 (scores on the PDD-BI were not collected at T1).
Data Analysis
Data analyses were conducted in R (R Core Team 2017). Two-tailed significance tests were used throughout with an alpha level of 0.05. Spearman correlation coefficients were reported for associations with RBS-R count scores. The R code used to generate all analyses is publicly available at http://faculty.washington.edu/kmacd/TAP_analysis.html.
Trajectories of Restricted/Repetitive Behaviors
We examined sleep at T2 in relation to developmental trajectories of RRBs from T1–T3. An advantage of this approach is that it allowed us to take advantage of existing longitudinal data on RRBs and other child characteristics from approximately age 2–6. A disadvantage of this approach is that it limits our ability to draw conclusions about the relationship of sleep to RRBs at individual timepoints across development.
Linear mixed-effect models were used to examine the relationship between child characteristics (including sleep at T2) and trajectories of RRB subtypes. Three separate models were used for each of the RRB subtypes (stereo-typed/restricted, sameness/ritualistic/compulsive, and self-injurious). This analytic approach for repeated measures data allowed for use of all available data for each child (permitting adjustment of confounding variables at multiple timepoints), yet was robust to missing data and did not require equal intervals between visits. We assumed a Poisson distribution given that the outcome variables (RRB subtypes) were counts. Each model contained three time-varying regressors (i.e., measured at each timepoint along with RRBs): age, ADOS SA score, and intellectual ability (Mullen or DAS), and T2 sleep problems as a time-invariant regressor. T2 sleep problems was added to each model in a second analysis step and assessed for improvement in model fit. All continuous regressors were standardized prior to analysis. Separate models were run for the ASD and DD groups due to the different number of timepoints available (3 vs. 2). Models for the ASD group included a random intercept and random slope for age; models for the DD group included only random intercept, as data were available for this group at only two timepoints. For the sake of parsimony, additional demographic variables (sex, race, ethnicity and maternal education) were not included in models as they were not significantly associated with sleep problems or RRBs and did not differ between the groups. Linear mixed-effect model analyses were conducted with the lme4 package in R (Bates et al. 2015).
Exploratory Mediation Analyses with Anxiety
PDD-BI Specific Fears scores were used to explore whether anxiety mediated the relationship between sleep problems and specific RRBs at T2. PDD-BI data were available on subset of participants at T2 (33 ASD; 18 DD). Mediation tests were only conducted when the minimum conditions for mediation were met (Baron and Kenny 1986); in this case, the IV (sleep problems) was significantly associated with the mediator (anxiety) and both IV and mediator were associated with the DV (repetitive behavior). Suitability for mediation was determined for each group (ASD vs. DD) and each of the three RRB subtypes (stereotyped/restricted, sameness/ritualistic/compulsive, and self-injurious) at T2 via separate Poisson regression models that corrected for age, intellectual ability, and ADOS SA score. When suitable, tests for significance of mediation were conducted using the mediation package in R (Tingley et al. 2014).
Results
Frequency of T2 Sleep Problems and Relation to Child Characteristics
At T2, there was no difference in sleep problems between the ASD and DD groups (t(37.6) = 1.7, p = 0.10), and no difference in the proportion of scores above the clinical cutoff (ASD = 74%, DD = 53%; x2(1) = 1.7, p = 0.20). CSHQ subscale scores between groups are depicted in Fig. 2. The ASD group had higher scores on the parasomnia subscale than the DD group (t(54) = 2.9, p < 0.01); none of the other subscale scores were significantly different between the groups. At T2 in both the ASD and DD groups, sleep problems were not significantly related to age (ASD: rs = −0.02, p = 0.90; DD: rs = 0.33, p = 0.17), intellectual ability (ASD: rs = 0.15, p = 0.37; DD: rs = −0.11, p = 0.65), social-affective symptom severity (ASD: rs = −0.09, p = 0.59; DD: rs = 0.11, p = 0.64), or anxiety (ASD: rs = 0.26, p = 0.15; DD: rs = 0.30, p = 0.23). Full description of child characteristics at T2 are available in Table S2 (Online Resource).
Relationship Between T2 Sleep Problems and Trajectories of RRBs from T1–T3
Table 2 summarizes results of the full models in the ASD and DD groups for each of the three RRB subtypes. For children in the ASD group from T1–T3, sameness/ritualistic/compulsive behaviors increased with age from T1–T3 and were higher for children with more social-affective symptoms. There was also a trend-level association between lower intellectual ability and increased sameness/ritualistic/compulsive behaviors. The model for sameness/ritualistic/compulsive behaviors was significantly improved after addition of T2 sleep problems, χ2(1) = 5.59, p = 0.02, indicating that children with more sleep problems at T2 showed higher trajectories of sameness/ritualistic/compulsive behaviors over the study period.
Table 2.
Parameter estimates from linear mixed-effects model analysis
| Sameness/ritualistic/compulsive |
Self-injurious |
Stereotyped/restricted |
||||
|---|---|---|---|---|---|---|
| Estimate (SE) | Z | Estimate (SE) | Z | Estimate (SE) | Z | |
| ASD group Age |
0.24 (.07) | 3.70*** | − 0.45 (.24) | − 1.86† | 0.03 (.06) | 0.56 |
| ADOS (SA) | 0.29 (.08) | 3.48*** | 0.04 (.23) | 0.16 | 0.15 (.09) | 1.72† |
| IQ/DQ | − 0.15 (.08) | − 1.95† | − 0.28 (.20) | − 1.40 | − 0.22 (.06) | − 3.50*** |
| T2 Sleep problems | 0.03 (.01) | 2.47* | 0.02 (.02) | 1.09 | 0.01 (.01) | 1.75† |
| DD group Age |
1.31 (.27) | 4.85*** | − 0.36 (.46) | 0.78 | 0.77 (.27) | 2.82** |
| ADOS (SA) | 0.54 (.21) | 2.51* | 0.83 (.47) | 1.76† | 0.45 (.21) | 2.12* |
| IQ/DQ | 0.29 (.26) | 1.11 | − 0.04 (.63) | − .007 | − 0.15 (.29) | − 0.53 |
| T2 sleep problems | 0.07 (.02) | 2.79** | 0.11 (.06) | 1.96† | − 0.01 (.03) | − 0.35 |
p < .05
p < .01
p < .001
p < .1
Trajectories of self-injurious behavior showed a trend-level decrease with age, and no improvement with the addition of T2 sleep problems, χ2(1) = 1.12, p = 0.29. Finally, trajectories of stereotyped/restricted behaviors were higher for those with lower intellectual abilities. There were also trend-level associations between higher stereotyped/ restricted behaviors and higher social-affective symptoms on the ADOS, and there was a trend towards improvement in model fit with the addition of T2 sleep problems (χ2(1) = 2.93, p = 0.09), suggesting that sleep problems at T2 were weakly associated with higher trajectories of stereotyped/restricted behaviors across the study period.
Similar results were found for the DD group. Of the three RRB subtypes, only the sameness/ritualistic/compulsive model was significantly improved by the addition of T2 sleep problems (χ2(1) = 7.2, p = 0.007). Similar to the ASD group, children with DD who had more sleep problems at T2 showed higher trajectories of sameness/ritualistic/compulsive behaviors from T1–T2. Adding sleep to the models for self-injurious (χ2(1) = 2.9, p = 0.09) and stereotyped/restricted (χ2(1) = 0.12, p = 0.72) behaviors did not significantly improve model fit, though the improvement for the self-injurious model was at trend-level.
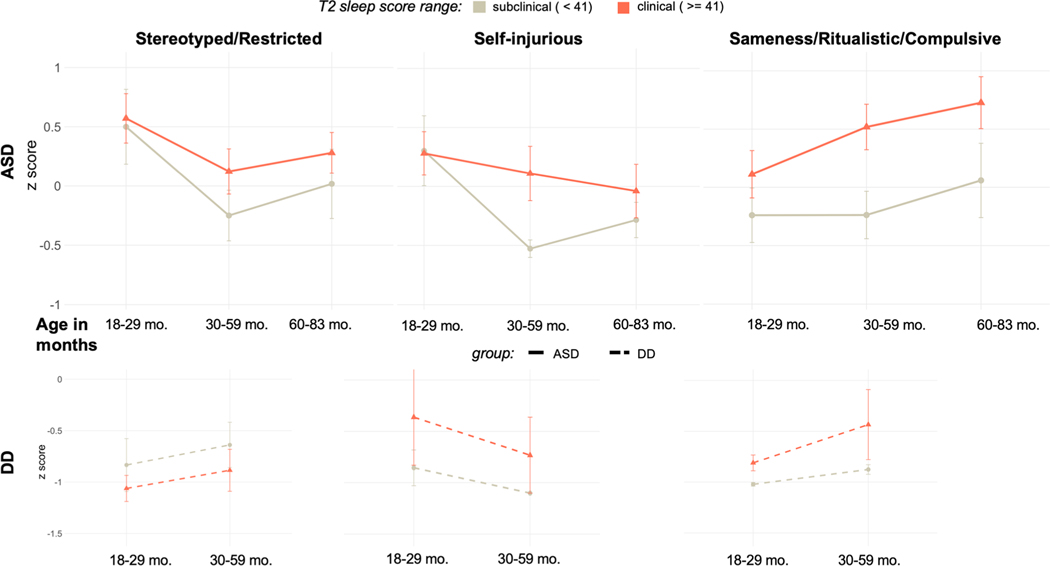
To visualize the relationship between sleep at T2 and RRB trajectories from T1–T3 in the ASD group (and from T1–T2 in the DD group), participants were split into two groups—“subclinical” (n = 10) and “clinical” (n = 28)—based upon the established CSHQ clinical cutoff score of 41. Mean count of RRB subtypes at T1, T2, and T3 are depicted for the subclinical and clinical groups in Fig. 3.
Fig. 3.
RRBs over time. RRB count score for each subtype over time (the horizontal axis depicts age ranges in months). For visualization purposes, participants were split into groups based on the established clinical threshold for the CSHQ (ASD: 10 subclinical, 28 clinical; DD: 9 subclinical, 10 clinical) and mean Z scores were plotted for each group at each timepoint. Note that this has been done for visualization only; LMM analyses in the text treated both age and T2 sleep problems as continuous variables
Anxiety Symptoms and Sleep Problems
We conducted exploratory analyses to examine whether anxiety (measured with the PDD-BI-Specific Fears subscale) mediated the relationship between sleep problems and specific RRB subtypes across the whole sample at T2. Sleep problems were significantly related to anxiety scores for the ASD group only; however, stereotyped/restricted behaviors were not related to sleep problems and thus the stereotyped/ restricted model was excluded from mediation analyses. This left two models to test for mediation in the ASD group: self-injurious and sameness/ritualistic/compulsive behavior.
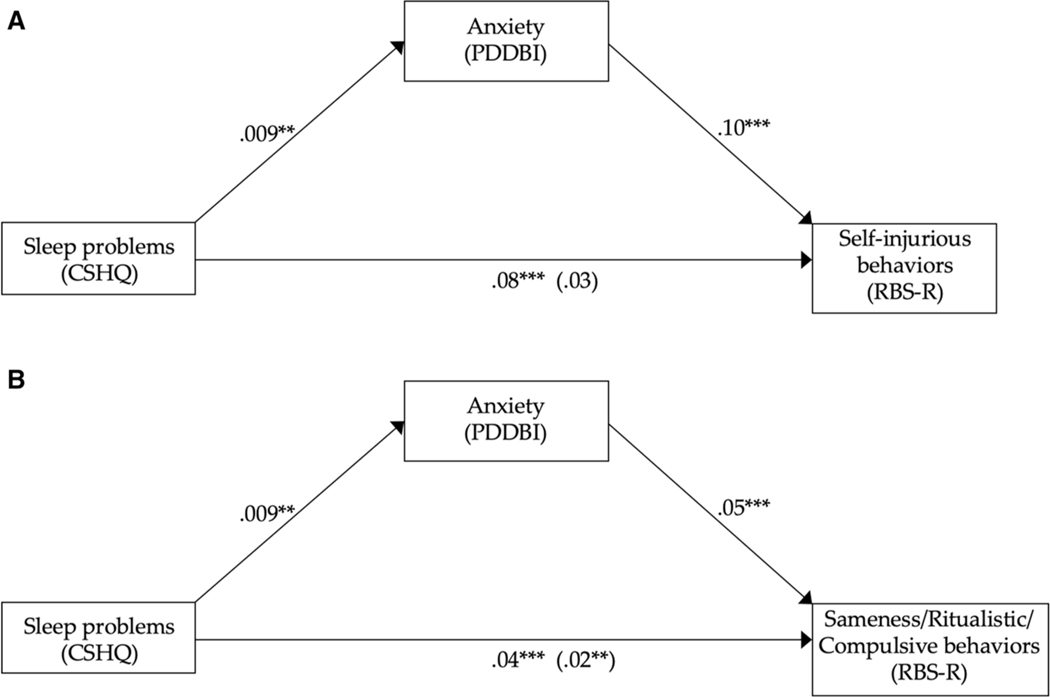
At T2, sleep problems were significantly associated with anxiety scores (β = 0.009 SE = 0.003, p = 0.005), and anxiety scores were significantly associated with self-injurious (β = 0.10, SE = 0.03, p < 0.001) and sameness/ ritualistic/compulsive behaviors (β = 0.05, SE = 0.007, p < 0.001). When the mediator, anxiety scores, was added to the model, sleep scores were no longer associated with self-injurious behaviors (β = 0.03 SE = 0.03, p = 0.3), suggesting full mediation (Fig. 4a). However, sleep problems remained significantly associated with sameness/ritualistic/ compulsive behavior even after controlling for the mediator, anxiety symptoms, (β = 0.02, SE = 0.009, p = 0.007). In other words, both sleep problems and anxiety symptoms were significantly associated with sameness/ritualistic/compulsive behaviors (Fig. 4b). The indirect effect of sleep problems on sameness/ritualistic/compulsive behavior via anxiety was formally tested for significance using a bootstrap estimation approach with 1000 samples. These results indicated that the indirect coefficient was not significant (β = 1.3, p = 0.87) and therefore it appears that anxiety did not mediate the effect of sleep on sameness/ritualistic/compulsive behavior; rather, both sleep and anxiety appear to contribute independently to sameness/ritualistic/compulsive behaviors at T2.
Fig. 4.
Exploratory mediation analysis. Regression coefficients for the relationship between sleep problems and a self-injurious behaviors or b sameness/ ritualistic/compulsive behaviors, mediated by anxiety, in the ASD group at age 4. The regression coefficient between sleep problems and RRB subtype, controlling for anxiety, is in parenthesis. Anxiety mediated the relationship between sleep problems and self-injurious behaviors (a), but not sameness/ ritualistic/compulsive behaviors (b). *p < .05, **p < .01, ***p < .001
Discussion
This study investigated trajectories of restricted/repetitive behaviors through early development in relation to parent-reported sleep problems at age 4 in a sample of children with neurodevelopmental disabilities (ASD or developmental delay). We found that trajectories of sameness/ritualistic/ compulsive behaviors were significantly higher in children who had sleep problems at age 4; in both the ASD and DD groups, children with clinically-significant sleep problems at age 4 showed higher levels of sameness/ritualistic/compulsive behaviors across early development than children without sleep problems. In contrast, trajectories of self-injurious and stereotyped/restricted behaviors were not significantly related to age 4 sleep problems in either group. Follow-up exploratory analyses revealed that in the ASD group, sleep problems and anxiety were significantly and independently associated with sameness/ritualistic/compulsive at age 4.
The reported association between sleep and RRBs is consistent with prior work (Gabriels et al. 2005; Goldman et al. 2009; Hoffman et al. 2005; Hundley et al. 2016; Mayes and Calhoun 2009; Park et al. 2012; Schreck et al. 2004; Tudor et al. 2012); we extend this previous literature by evaluating sleep at age 4 (a single timepoint) in relation to trajectories of repetitive behavior subtypes across early childhood (age 2–6). The relationship between age 4 sleep problems and trajectories of sameness/ritualistic/compulsive behaviors persisted even after controlling for social-affective ASD symptoms, intellectual ability, and age. In contrast to prior research suggesting that associations between sleep and higher-order RRBs are explained by anxiety (Hundley et al. 2016), we found that anxiety did not mediate the relationship between sleep problems and higher-order RRBs in our sample. Instead, we found that, at age 4, anxiety symptoms and sleep problems independently contributed to sameness/ ritualistic/compulsive behaviors. Differences in study methodology (use of the RBS-R vs. ADI-R to measure RRBs), population (transdiagnostic vs. ASD-only), age range, or design (longitudinal vs. cross-sectional) may help explain the discrepancy between our results and those of Hundley et al. (2016); however, further research to clarify the relationship between RRB subtypes and sleep problems is warranted.
These results make several novel contributions to the literature on sleep problems in children with neurodevelopmental disabilities. First, we report that children with sleep difficulties at age 4 showed different trajectories of RRBs in early childhood. The majority of prior work showing sleep-RRB associations has been based on cross-sectional data collected at a single timepoint for each child (Gabriels et al. 2005; Goldman et al. 2009; Hoffman et al. 2005; Hundley et al. 2016; Schreck et al. 2004; Tudor et al. 2012). An exception can be found in a recent report by Abel et al. (2018), in which the authors analyzed 5 nights of actigraphy data in relation to daytime behaviors and found that average (but not night-to-night) sleep patterns were related to higher rates of repetitive behavior. The authors suggest that cumulative sleep debt may contribute to the expression of repetitive behaviors. Although our data cannot provide evidence of directionality due to the fact that sleep was not measured longitudinally, our findings provide new evidence that children with sleep problems at age 4 show a pattern of increased expression of higher-order RRBs across early development, even after controlling for other child characteristics (i.e., social-affective ASD symptoms and intellectual ability).
Second, our sample included children with both ASD and DD, assessed directly by research clinicians using gold-standard diagnostic instruments, providing a well-characterized and diagnostically broader population than has been previously reported in the literature (Gabriels et al. 2005; Goldman et al. 2009; Hoffman et al. 2005; Park et al. 2012; Schreck et al. 2004; Tudor et al. 2012). The similar pattern of results we observed for the ASD and DD groups suggests that the relationship between sleep and RRBs extends beyond an ASD-specific phenomenon to children with neurodevelopmental disabilities more broadly.
Finally, our results revealed an association between sleep and a specific subtype of RRBs—sameness/ritualistic/compulsive behavior—that persisted even after controlling for intellectual ability, social-affective ASD symptoms, and anxiety. Prior studies have highlighted this subtype of RRB as one which tends to increase in frequency across development (Richler et al. 2010; Wolff et al. 2014), is independent of gender, race, diagnosis, and IQ (Hus et al. 2007), and is under more familial (presumably, genetic) control than the repetitive sensorimotor subtype (Lam et al. 2008; Szatmari et al. 2006). In addition, neuroimaging studies have reported associations between higher- (but not lower-) order RRBs and striatal morphometry in children with ASD; specifically, rate of growth (Langen et al. 2014) and total volume of dorsal striatal structures (Wolff et al. 2013). Our finding that sleep was selectively associated with higher-order RRBs (and to a lesser extent, self-injurious behaviors), but not lower-order RRBs provides an additional piece of evidence that higher-order RRBs are a biologically-relevant subset of behaviors that may prove useful for identifying homogenous subgroups of children to investigate shared etiology (Bishop et al. 2013).
This study has important limitations that warrant discussion. First, sleep was not measured at each timepoint, and while the relationship between sleep and RRBs may have some stability across development (Humphreys et al. 2014), a full understanding of the longitudinal relationship will require simultaneous measurements of sleep and RRBs across a greater developmental time span. Second, sleep was measured via parent-report, rather than with objective measures (e.g., actigraphy or polysomnography). RRBs and anxiety were also measured via parent-report; thus, correlations between multiple parent-report measures may be artificially inflated due to common-method variance. Use of the RBS-R count score limited our potential to detect differences in the severity of different repetitive behaviors over time. Finally, the sample was relatively small, and consisted of children of more highly educated parents. This likely reduced our power to detect effects that may be revealed in larger samples. For example, a recent study of almost 2000 children found that children with ASD had significantly higher CSHQ scores than children with non-ASD developmental delays (Reynolds et al. 2019), whereas we did not find a significant difference in CSHQ scores between our ASD and DD groups. Our DD group was only followed at two of the three timepoints, which further limits our ability to draw definitive conclusions about trajectories of RRBs in this group. Given these limitations, it is important to replicate these findings in larger, more representative and transdiagnostic samples.
The relationship we report between sleep problems and higher-order RRBs is likely bidirectional. Children who must follow rigid or compulsive bedtime routines may fall asleep later, or have a harder time sleeping when their routine is disrupted, than children who are more flexible at bedtime (Patzold et al. 1998). Similarly, anxiety can negatively impact sleep (Alfano et al. 2007), and inadequate sleep can lead to increased anxiety symptoms in childhood (Astill et al. 2012; Berger et al. 2012; Johnson et al. 2000; Sadeh 2007; Sivertsen et al. 2015; Vriend et al. 2013). The relationship between anxiety and RRBs could also be bidirectional, as RRBs themselves may serve an emotion regulation function (Gabriels et al. 2013; Spiker et al. 2012). In this case, increased anxiety may be associated with increased RRBs not because RRBs are an expression of anxiety, but because RRBs represent a child’s attempt to regulate his or her arousal, which may be a more frequent requirement under conditions of sleep deprivation. Our data and prior literature also suggest that the nature of these bidirectional relationships may also differ depending on the subtype of RRB considered (Gotham et al. 2013).
Why might disrupted sleep cause or exacerbate the expression of higher-order RRBs in children with neurodevelopmental disabilities? Insights into potential mechanisms can be gleaned from the sleep deprivation and animal literature. Chronic sleep deprivation has been shown to affect cognitive and brain function in ways that could contribute to RRBs. For example, mice under sleep deprivation shift from using hippocampally-dependent to striatum-dependent learning strategies (Hagewoud et al. 2010). Higher-order RRBs have been associated with striatal morphometry in ASD (Hollander et al. 2005; Langen et al. 2014; Wolff et al. 2013), so it is possible that sleep deprivation could shift children from hippocampal to striatally-mediated learning systems, which favor repetition and tend to be less flexible. Evidence from the human sleep literature is consistent with this assertion; cognitive rigidity and a tendency towards perseveration have repeatedly been reported as consequences of sleep deprivation in adults (Durmer and Dinges 2005). Additional evidence that sleep problems may play a causal role in exacerbating RRBs comes from intervention studies. Both behavioral and pharmacological treatments (e.g., melatonin) have been shown to improve sleep and also reduce RRBs in children and adolescents with ASD (Loring et al. 2018; Malow et al. 2012, 2014), suggesting that inadequate or disrupted sleep may contribute to the expression of RRBs.
An alternative possibility is that the relationship between RRBs and sleep problems is not causal, but rather a consequence of unmeasured neurobiological factors that affect both RRBs and sleep but not other aspects of disorder severity. For example, cortical-basal ganglia pathways that play a role in maintaining vigilant wakefulness are associated with the expression of repetitive behavior in animal models (Lazarus et al. 2012; Whitehouse and Lewis 2015). Sleep and RRBs may result from dysfunction in a shared neural pathway, and over time the affective and cognitive consequences of sleep deprivation may further exacerbate RRBs. Investigating sleep-by-age interactions in samples where both sleep and RRBs are measured longitudinally could help delineate the timecourse of this relationship.
Elucidation of a possible causal mechanism between sleep problems and RRBs in neurodevelopmental disabilities will require additional research. The current findings, in combination with prior evidence, make a strong case that sleep is independently associated with higher-order RRBs above and beyond other measures of disorder severity or developmental functioning. The relationship exists across diagnoses (ASD and DD) and in a sample of children with ASD who were exposed to intensive early intervention, suggesting that sleep-specific interventions may be required to improve sleep (and perhaps, decrease higher-order RRBs) in children with ASD and other neurodevelopmental disabilities.
Supplementary Material
Acknowledgments
The authors thank the children and parents who participated in this research. We additionally thank Dr. Brian Flaherty and Kevin Donovan for their input on statistical analyses and Dr. Jason Wolff and for his comments on an earlier version of the manuscript. Some of these data were collected as part of a clinical trial, supported by a National Institute of Mental Health grant U54MH066399 (Dawson, PI), and registered at www.clinicaltrials.gov with identifier NCT00090415. The follow-up study was supported by the National Institute of Mental Health and the National Institute of Child Health and Human Development (U19HD34565, P50HD066782, R01HD-55741).
Funding
Some of these data were collected as part of a clinical trial, supported by National Institute of Mental Health grant U54MH066399, and registered at www.clinicaltrials.gov with identifier NCT00090415. The follow-up study was supported by the National Institute of Mental Health and the National Institute of Child Health and Human Development (U19HD34565, P50HD066782, R01HD-55741).
Compliance with Ethical Standards
Drs. Rogers and Dawson receive royalties from Guilford Press Early Start Denver Model materials. Dr. Dawson is on the Scientific Advisory Boards of Janssen Research and Development, Akili, Inc., and Roche Pharmaceutical Company, has received grant funding from Janssen Research and Development, L.L.C. and PerkinElmer, speaker fees from ViaCord, and receives royalties from Guilford Press and Oxford University Press.
Footnotes
Conflict of interest
Drs. MacDuffie, Munson, Greenson, Ward and Estes report no potential conflicts of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from parents of all participants included in the study.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10803-020-04438-y ) contains supplementary material, which is available to authorized users.
We use the term trajectory throughout given our interest in patterns of RRBs over time. The DD group only had data at 2 timepoints, which is not the typical structure for a trajectory analysis; however, for ease of communication we have elected to use the term trajectory to describe the results for both groups.
References
- Abel EA, Schwichtenberg AJ, Brodhead MT, & Christ SL (2018). Sleep and challenging behaviors in the context of intensive behavioral intervention for children with autism. Journal of Autism and Developmental Disorders, 48(11), 3871–3884. 10.1007/s10803-018-3648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CA, Ginsburg GS, & Kingery JN (2007). Sleep-related problems among children and adolescents with anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 46(2), 224–232. 10.1097/01.chi.0000242233.06011.8e. [DOI] [PubMed] [Google Scholar]
- Astill RG, Van der Heijden KB, & van IJzendoorn MH, & Van Someren, E. J. W., (2012). Sleep, cognition, and behavioral problems in school-age children: A century of research metaanalyzed. Psychological Bulletin, 138(6), 1109–1138. 10.1037/a0028204. [DOI] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173–1182. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, & Walker SC (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Berger RH, Miller AL, Seifer R, Cares SR, & LeBourgeois MK (2012). Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. Journal of Sleep Research, 21(3), 235–246. 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S, Gahagan S, & Lord C (2007b). Re-examining the core features of autism: A comparison of autism spectrum disorder and fetal alcohol spectrum disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines, 48(11), 1111–1121. 10.1111/j.1469-7610.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Hus V, Duncan A, Huerta M, Gotham K, Pickles A, et al. (2013). Subcategories of restricted and repetitive behaviors in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(6), 1287–1297. 10.1007/s10803-012-1671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Cain AC, & Lord C. (2007a). Predictors of perceived negative impact in mothers of children with autism spectrum disorder. American Journal of Mental Retardation: AJMR, 112(6), 450–461. 10.1016/j.scitotenv.2020.140540. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, & Lewis MH (2000). Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders, 30(3), 237–243. [DOI] [PubMed] [Google Scholar]
- Boyd BA, McDonough SG, Rupp B, Khan F, & Bodfish JW (2011). Effects of a family-implemented treatment on the repetitive behaviors of children with autism. Journal of Autism and Developmental Disorders, 41(10), 1330–1341. 10.1007/s10803-010-1156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL, Schmidt-Lackner S, Romanczyk R, & Sudhalter V. (2003). The PDD Behavior Inventory: A rating scale for assessing response to intervention in children with pervasive developmental disorder. Journal of Autism and Developmental Disorders, 33(1), 31–45. [DOI] [PubMed] [Google Scholar]
- Cohen S, Conduit R, Lockley SW, Rajaratnam SM, & Cornish KM (2014). The relationship between sleep and behavior in autism spectrum disorder (ASD): A review. Journal of Neurodevelopmental Disorders, 6(1), 44. 10.1186/1866-1955-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesi F, Giannotti F, Ivanenko A, & Johnson K. (2010). Sleep in children with autistic spectrum disorder. Sleep Medicine, 11(7), 659–664. 10.1016/j.sleep.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. (2010). Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics, 125(1), e17–23. 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmer JS, & Dinges DF (2005). Neurocognitive consequences of sleep deprivation. Seminars in Neurology, 25(1), 117–129. 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Elliott CD, Murray GJ, & Pearson LS (1990). Differential ability scales. Texas: San Antonio. [Google Scholar]
- Estes A, Munson J, Rogers SJ, Greenson J, Winter J, & Dawson G. (2015). Long-term outcomes of early intervention in 6-year-old children with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 54(7), 580–587. 10.1016/j.jaac.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Shaw DWW, Sparks BF, Friedman S, Giedd JN, Dawson G, et al. (2011). Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Research: Official Journal of the International Society for Autism Research, 4(3), 212–220. 10.1002/aur.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriels RL, Agnew JA, Pan Z, Holt KD, Reynolds A, & Laudenslager ML (2013). Elevated repetitive behaviors are associated with lower diurnal salivary cortisol levels in autism spectrum disorder. Biological Psychology, 93(2), 262–268. 10.1016/j.biopsycho.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Gabriels RL, Cuccaro ML, Hill DE, Ivers BJ, & Goldson E. (2005). Repetitive behaviors in autism: Relationships with associated clinical features. Research in Developmental Disabilities, 26(2), 169–181. 10.1016/j.ridd.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Georgiades S, Papageorgiou V, & Anagnostou E. (2010). Brief report: Repetitive behaviours in Greek individuals with autism spectrum disorder. Journal of Autism and Developmental Disorders, 40(7), 903–906. 10.1007/s10803-009-0927-9. [DOI] [PubMed] [Google Scholar]
- Goldman SE, McGrew S, Johnson KP, Richdale AL, Clemons T, & Malow BA (2011). Sleep is associated with problem behaviors in children and adolescents with Autism Spectrum Disorders. Research in Autism Spectrum Disorders, 5(3), 1223–1229. [Google Scholar]
- Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, & Malow BA (2009). Defining the sleep phenotype in children with autism. Developmental Neuropsychology, 34(5), 560–573. 10.1080/87565640903133509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Bishop SL, Hus V, Huerta M, Lund S, Buja A, et al. (2013). Exploring the relationship between anxiety and insistence on sameness in autism spectrum disorders. Autism Research: Official Journal of the International Society for Autism Research, 6(1), 33–41. 10.1002/aur.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Tiba PA, Novati A, Hogenelst K, Weinreder P, et al. (2010). Coping with sleep deprivation: Shifts in regional brain activity and learning strategy. Sleep, 33(11), 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CD, Sweeney DP, Gilliam JE, Apodaca DD, Lopez-Wagner MC, & Castillo MM (2005). Sleep problems and symptomology in children with autism. Focus on Autism and Other Developmental Disabilities, 20(4), 194–200. 10.1177/10883576050200040101. [DOI] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, et al. (2005). Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry, 58(3), 226–232. 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Humphreys JS, Gringras P, Blair PS, Scott N, Henderson J, Fleming PJ, et al. (2014). Sleep patterns in children with autistic spectrum disorders: A prospective cohort study. Archives of Disease in Childhood, 99(2), 114–118. 10.1136/archdischild-2013-304083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley RJ, Shui A, & Malow BA (2016). Relationship between subtypes of restricted and repetitive behaviors and sleep disturbance in autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(11), 3448–3457. 10.1007/s10803-016-2884-4. [DOI] [PubMed] [Google Scholar]
- Hus V, Gotham K, & Lord C. (2014). Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44(10), 2400–2412. 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Pickles A, Cook EH, Risi S, & Lord C. (2007). Using the autism diagnostic interview-revised to increase phenotypic homogeneity in genetic studies of autism. Biological Psychiatry, 61(4), 438–448. 10.1016/j.biopsych.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Johnson CR, DeMand A, Lecavalier L, Smith T, Aman M, Foldes E, et al. (2016). Psychometric properties of the children’s sleep habits questionnaire in children with autism spectrum disorder. Sleep Medicine, 20, 5–11. 10.1016/j.sleep.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Johnson CR, Smith T, DeMand A, Lecavalier L, Evans V, Gurka M, et al. (2018). Exploring sleep quality of young children with autism spectrum disorder and disruptive behaviors. Sleep Medicine, 44, 61–66. 10.1016/j.sleep.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Chilcoat HD, & Breslau N. (2000). Trouble sleeping and anxiety/depression in childhood. Psychiatry Research, 94(2), 93–102. [DOI] [PubMed] [Google Scholar]
- Katz T, Shui AM, Johnson CR, Richdale AL, Reynolds AM, Scahill L, et al. (2018). Modification of the Children’s Sleep Habits Questionnaire for Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 48(8), 2629–2641. 10.1007/s10803-018-3520-2. [DOI] [PubMed] [Google Scholar]
- Kotagal S, & Broomall E. (2012). Sleep in children with autism spectrum disorder. Pediatric Neurology, 47(4), 242–251. 10.1016/j.pediatrneurol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Lam KSL, & Aman MG (2007). The Repetitive Behavior Scale-Revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(5), 855–866. 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lam KSL, Bodfish JW, & Piven J. (2008). Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. Journal of Child Psychology and Psychiatry, 49(11), 1193–1200. 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Bos D, Noordermeer SDS, Nederveen H, van Engeland H, & Durston S. (2014). Changes in the development of striatum are involved in repetitive behavior in autism. Biological Psychiatry, 76(5), 405–411. 10.1016/j.biopsych.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Huang Z-L, Lu J, Urade Y, & Chen J-F (2012). How do the basal ganglia regulate sleep–wake behavior? Trends in Neurosciences, 35(12), 723–732. 10.1016/j.tins.2012.07.001 . [DOI] [PubMed] [Google Scholar]
- Leekam SR, Prior MR, & Uljarevic M. (2011). Restricted and repetitive behaviors in autism spectrum disorders: A review of research in the last decade. Psychological Bulletin, 137(4), 562–593. 10.1037/a0023341. [DOI] [PubMed] [Google Scholar]
- Lewis M, & Kim S-J (2009). The pathophysiology of restricted repetitive behavior. Journal of Neurodevelopmental Disorders, 1(2), 114–132. 10.1007/s11689-009-9019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidstone J, Uljarevic M, Sullivan J, Rodgers J, McConachie H, Freeston M, et al. (2014). Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorders. Research in Autism Spectrum Disorders, 8(2), 82–92. 10.1016/j.rasd.2013.10.001. [DOI] [Google Scholar]
- Liu X, Hubbard JA, Fabes RA, & Adam JB (2006). Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry and Human Development, 37(2), 179–191. 10.1007/s10578-006-0028-3. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, & Risi S. (1999). Autism diagnostic observation schedule (ADOS). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Loring WA, Johnston RL, Shui AM, & Malow BA, (2018). Impact of a brief behavioral intervention for insomnia on daytime behaviors in adolescents with autism spectrum disorders. Journal of Contemporary Psychotherapy, 89(6), 485. 10.1007/s10879-018-9381-3. [DOI] [Google Scholar]
- Malow B, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes D, & Burnette C. (2012). Melatonin for sleep in children with autism: A controlled trial examining dose, tolerability, and outcomes. Journal of Autism and Developmental Disorders, 42(8), 1729–37–author reply 1738. 10.1007/s10803-011-1418-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow BA, Adkins KW, Reynolds A, Weiss SK, Loh A, Fawkes D, et al. (2014). Parent-based sleep education for children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 44(1), 216–228. 10.1007/s10803-013-1866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion A, Leader G, & Healy O. (2013). An investigation of comorbid psychological disorders, sleep problems, gastrointestinal symptoms and epilepsy in children and adolescents with Autism Spectrum Disorder. Research in Autism Spectrum Disorders, 7(1), 35–42. [Google Scholar]
- Maskey M, Warnell F, Parr JR, Le Couteur A, & McConachie H. (2013). Emotional and behavioural problems in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 43(4), 851–859. 10.1007/s10803-012-1622-9. [DOI] [PubMed] [Google Scholar]
- Mayes SD, & Calhoun SL (2009). Variables related to sleep problems in children with autism. Research in Autism Spectrum Disorders, 3(4), 931–941. [Google Scholar]
- Meltzer LJ, & Mindell JA (2008). Behavioral sleep disorders in children and adolescents. The Psychiatric Dimensions of Sleep Medicine, 3(2), 269–279. [Google Scholar]
- Mirenda P, Smith IM, Vaillancourt T, Georgiades S, Duku E, Szatmari P, et al. (2010). Validating the repetitive behavior scalerevised in young children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 40(12), 1521–1530. 10.1007/s10803-010-1012-0. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz A-M, Seidenfeld A, Guter S, Stanford LD, et al. (2009). Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychological Medicine, 39(9), 1559–1566. 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Owens JA (2005). The ADHD and sleep conundrum: A review. Journal of Developmental & Behavioral Pediatrics, 26(4), 312–322. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, & McGuinn M. (2000). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, 23(8), 1043–1051. [PubMed] [Google Scholar]
- Park S, Cho S-C, Cho IH, Kim B-N, Kim J-W, Shin M-S, et al. (2012). Sleep problems and their correlates and comorbid psychopathology of children with autism spectrum disorders. Research in Autism Spectrum Disorders, 6(3), 1068–1072. [Google Scholar]
- Patzold LM, Richdale AL, & Tonge BJ (1998). An investigation into sleep characteristics of children with autism and Asperger’s Disorder. Journal of Paediatrics and Child Health, 34(6), 528–533. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. Retrieved July 17, 2018, from https://www.R-project.org/. [Google Scholar]
- Reynolds AM, & Malow BA (2011). Sleep and autism spectrum disorders. Pediatric Sleep Medicine Update, 58(3), 685–698. 10.1016/j.pcl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Reynolds AM, Soke GN, Sabourin KR, Hepburn S, Katz T, Wiggins LD, et al. (2019). Sleep problems in 2- to 5-yearolds with autism spectrum disorder and other developmental delays. Pediatrics, 143(3), e20180492. 10.1542/peds.2018-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler J, Huerta M, Bishop SL, & Lord C. (2010). Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Development and Psychopathology, 22(1), 55–69. 10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J, Glod M, Connolly B, & McConachie H. (2012). The relationship between anxiety and repetitive behaviours in autism spectrum disorder. Journal of Autism and Developmental Disorders, 42(11), 2404–2409. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, & Lord C. (2003). Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services, 29, 30. [Google Scholar]
- Sadeh A. (2007). Consequences of sleep loss or sleep disruption in children. Sleep Medicine Clinics, 2(3), 513–520. 10.1016/j.jsmc.2007.05.012. [DOI] [Google Scholar]
- Schreck KA, Mulick JA, & Smith AF (2004). Sleep problems as possible predictors of intensified symptoms of autism. Research in Developmental Disabilities, 25(1), 57–66. [DOI] [PubMed] [Google Scholar]
- Singh K, & Zimmerman AW (2015). Sleep in autism spectrum disorder and attention deficit hyperactivity disorder. Neurologic and Psychiatric Disorders and Sleep, 22(2), 113–125. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Harvey AG, Reichborn-Kjennerud T, Torgersen L, Ystrom E, & Hysing M. (2015). Later emotional and behavioral problems associated with sleep problems in toddlers: A longitudinal study. JAMA Pediatrics, 169(6), 575–582. 10.1001/jamapediatrics.2015.0187. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, & McMahon WM (2005). Repetitive behavior profiles in Asperger syndrome and high-functioning autism. Journal of Autism and Developmental Disorders, 35(2), 145–158. [DOI] [PubMed] [Google Scholar]
- Spiker MA, Lin CE, Van Dyke M, & Wood JJ (2012). Restricted interests and anxiety in children with autism. Autism, 16(3), 306–320. 10.1177/1362361311401763. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, et al. (2006). Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. Journal of Child Psychology and Psychiatry and Allied Disciplines, 47(6), 582–590. 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, & Imai K. (2014). Mediation: R package for causal mediation analysis. Journal of Statistical Software, 59(5), 1–38. 10.18637/jss.v059.i05.26917999 [DOI] [Google Scholar]
- Tudor ME, Hoffman CD, & Sweeney DP (2012). Children with autism: Sleep problems and symptom severity. Focus on Autism and Other Developmental Disabilities, 27(4), 254–262. 10.1177/1088357612457989. [DOI] [Google Scholar]
- Vriend JL, Davidson FD, Corkum PV, Rusak B, Chambers CT, & McLaughlin EN (2013). Manipulating sleep duration alters emotional functioning and cognitive performance in children. Journal of Pediatric Psychology, 38(10), 1058–1069. 10.1093/jpepsy/jst033 [DOI] [PubMed] [Google Scholar]
- Whitehouse CM, & Lewis MH (2015). Repetitive behavior in neurodevelopmental disorders: Clinical and translational findings. The Behavior Analyst, 38(2), 163–178. 10.1007/s40614-015-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Botteron KN, Dager SR, Elison JT, Estes AM, Gu H, et al. (2014). Longitudinal patterns of repetitive behavior in toddlers with autism. Journal of Child Psychology and Psychiatry, 55(8), 945–953. 10.1111/jcpp.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Hazlett HC, Lightbody AA, Reiss AL, & Piven J. (2013). Repetitive and self-injurious behaviors: Associations with caudate volume in autism and fragile X syndrome. Journal of Neurodevelopmental Disorders, 5(1), 12. 10.1186/1866-1955-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.