Abstract
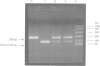
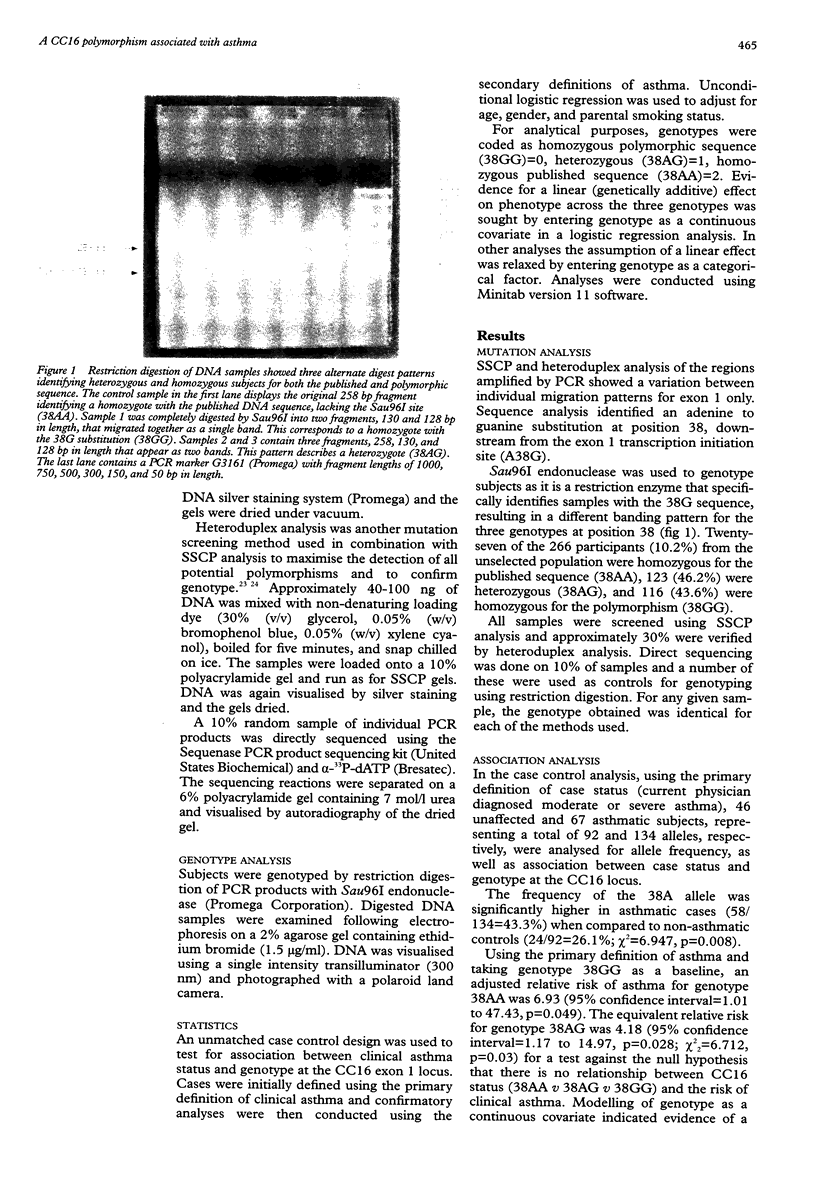
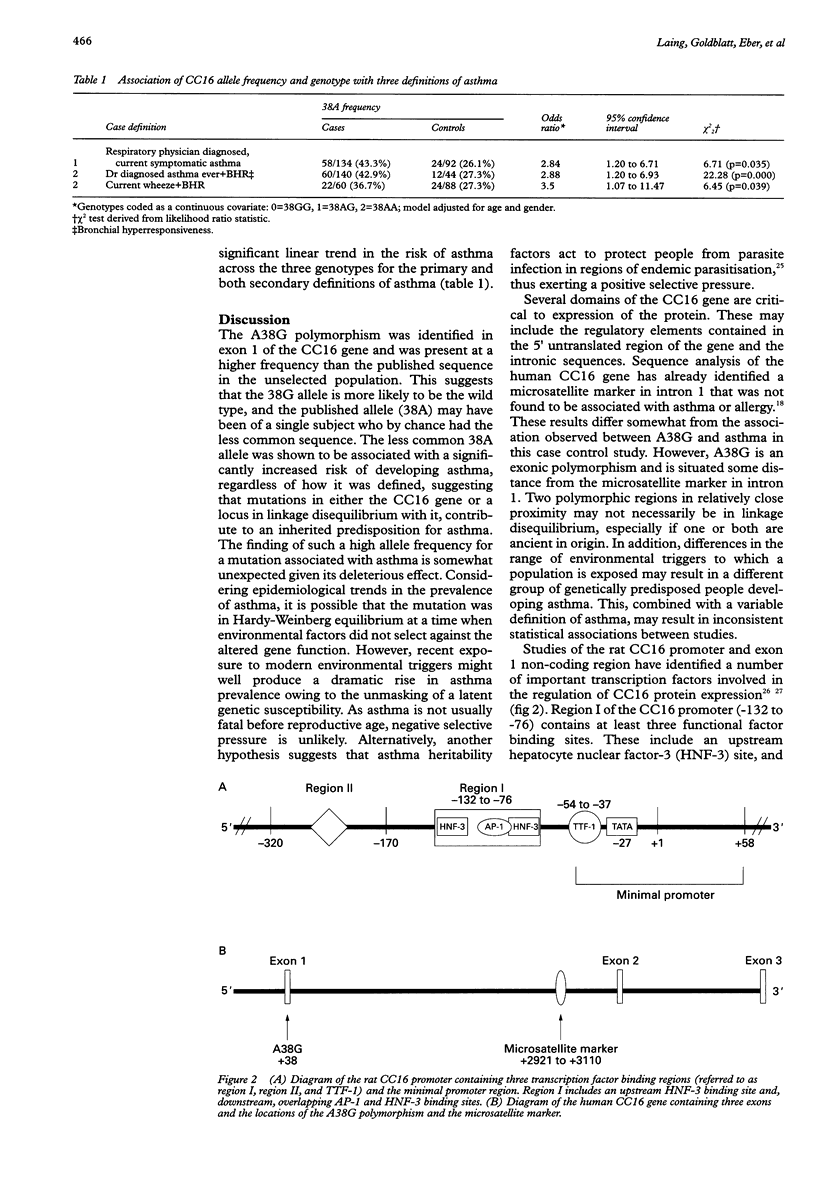
Several quantitative traits associated with the asthma phenotype have been linked to markers on chromosome 11q13, although the gene responsible has yet to be well established. The gene for Clara cell secretory protein (CC16) is an ideal candidate for involvement in an inherited predisposition to asthma because of its chromosomal location, the role of the CC16 protein in controlling airway inflammation, and differences in levels of the protein between asthmatics and healthy controls. All three CC16 exons were screened in an unselected population of 266 subjects from 76 families and a cohort of 52 severely asthmatic children. A combination of single strand conformational polymorphism (SSCP) analysis, heteroduplex analysis, DNA sequencing, and restriction digestion was used. Mutation detection methods identified an adenine to guanine substitution in the CC16 gene at position 38 (A38G) downstream from the transcription initiation site within the non-coding region of exon 1. In the unselected population, 43.6% were homozygous for the polymorphic sequence (38GG) and 46.2% were heterozygous (38AG). All the asthmatic and unaffected children from both populations were selected for an unmatched case control analysis consisting of 67 asthmatic and 46 unaffected subjects. Those homozygous for the published sequence (38AA) had a 6.9-fold increased risk of developing asthma (p=0.049) and heterozygotes (38AG) a 4.2-fold increased risk (p=0.028). Modelling of genotype as a continuous covariate indicated evidence of a significant linear trend across the three genotypes (odds ratio=2.84 per unit increase in genotype code, p=0.018). These associations were independent of age, gender, and tobacco smoke exposure. These data and the known anti-inflammatory role of CC16 in the respiratory tract suggest that alteration to the gene at position 38 may contribute to asthma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard A., Marchandise F. X., Depelchin S., Lauwerys R., Sibille Y. Clara cell protein in serum and bronchoalveolar lavage. Eur Respir J. 1992 Nov;5(10):1231–1238. [PubMed] [Google Scholar]
- Cookson W. O., Sharp P. A., Faux J. A., Hopkin J. M. Linkage between immunoglobulin E responses underlying asthma and rhinitis and chromosome 11q. Lancet. 1989 Jun 10;1(8650):1292–1295. doi: 10.1016/s0140-6736(89)92687-1. [DOI] [PubMed] [Google Scholar]
- Cookson W. O., Young R. P., Sandford A. J., Moffatt M. F., Shirakawa T., Sharp P. A., Faux J. A., Julier C., Nakumuura Y., Nakumura Y. Maternal inheritance of atopic IgE responsiveness on chromosome 11q. Lancet. 1992 Aug 15;340(8816):381–384. doi: 10.1016/0140-6736(92)91468-n. [DOI] [PubMed] [Google Scholar]
- Cotton R. G. Current methods of mutation detection. Mutat Res. 1993 Jan;285(1):125–144. doi: 10.1016/0027-5107(93)90060-s. [DOI] [PubMed] [Google Scholar]
- Daniels S. E., Bhattacharrya S., James A., Leaves N. I., Young A., Hill M. R., Faux J. A., Ryan G. F., le Söuef P. N., Lathrop G. M. A genome-wide search for quantitative trait loci underlying asthma. Nature. 1996 Sep 19;383(6597):247–250. doi: 10.1038/383247a0. [DOI] [PubMed] [Google Scholar]
- Dierynck I., Bernard A., Roels H., De Ley M. Potent inhibition of both human interferon-gamma production and biologic activity by the Clara cell protein CC16. Am J Respir Cell Mol Biol. 1995 Feb;12(2):205–210. doi: 10.1165/ajrcmb.12.2.7865218. [DOI] [PubMed] [Google Scholar]
- Hayward A. Who's to blame for asthma? Lancet. 1995 Nov 11;346(8985):1243–1243. doi: 10.1016/s0140-6736(95)91855-8. [DOI] [PubMed] [Google Scholar]
- Hill M. R., Cookson W. O. A new variant of the beta subunit of the high-affinity receptor for immunoglobulin E (Fc epsilon RI-beta E237G): associations with measures of atopy and bronchial hyper-responsiveness. Hum Mol Genet. 1996 Jul;5(7):959–962. doi: 10.1093/hmg/5.7.959. [DOI] [PubMed] [Google Scholar]
- Hill M. R., James A. L., Faux J. A., Ryan G., Hopkin J. M., le Söuef P., Musk A. W., Cookson W. O. Fc epsilon RI-beta polymorphism and risk of atopy in a general population sample. BMJ. 1995 Sep 23;311(7008):776–779. doi: 10.1136/bmj.311.7008.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesur O., Bernard A., Arsalane K., Lauwerys R., Bégin R., Cantin A., Lane D. Clara cell protein (CC-16) induces a phospholipase A2-mediated inhibition of fibroblast migration in vitro. Am J Respir Crit Care Med. 1995 Jul;152(1):290–297. doi: 10.1164/ajrccm.152.1.7541278. [DOI] [PubMed] [Google Scholar]
- Mukherjee D. C., Agrawal A. K., Manjunath R., Mukherjee A. B. Suppression of epididymal sperm antigenicity in the rabbit by uteroglobin and transglutaminase in vitro. Science. 1983 Feb 25;219(4587):989–991. doi: 10.1126/science.6130601. [DOI] [PubMed] [Google Scholar]
- Palmer L. J., Paré P. D., Faux J. A., Moffatt M. F., Daniels S. E., LeSouëf P. N., Bremner P. R., Mockford E., Gracey M., Spargo R. Fc epsilon R1-beta polymorphism and total serum IgE levels in endemically parasitized Australian aborigines. Am J Hum Genet. 1997 Jul;61(1):182–188. doi: 10.1086/513888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnik-Glavac M., Glavac D., Dean M. Sensitivity of single-strand conformation polymorphism and heteroduplex method for mutation detection in the cystic fibrosis gene. Hum Mol Genet. 1994 May;3(5):801–807. doi: 10.1093/hmg/3.5.801. [DOI] [PubMed] [Google Scholar]
- Sandford A. J., Shirakawa T., Moffatt M. F., Daniels S. E., Ra C., Faux J. A., Young R. P., Nakamura Y., Lathrop G. M., Cookson W. O. Localisation of atopy and beta subunit of high-affinity IgE receptor (Fc epsilon RI) on chromosome 11q. Lancet. 1993 Feb 6;341(8841):332–334. doi: 10.1016/0140-6736(93)90136-5. [DOI] [PubMed] [Google Scholar]
- Sawaya P. L., Luse D. S. Two members of the HNF-3 family have opposite effects on a lung transcriptional element; HNF-3 alpha stimulates and HNF-3 beta inhibits activity of region I from the Clara cell secretory protein (CCSP) promoter. J Biol Chem. 1994 Sep 2;269(35):22211–22216. [PubMed] [Google Scholar]
- Schiffmann E., Geetha V., Pencev D., Warabi H., Mato J., Hirata F., Brownstein M., Manjunath R., Mukherjee A., Liotta L. Adherence and regulation of leukotaxis. Agents Actions Suppl. 1983;12:106–120. doi: 10.1007/978-3-0348-9352-7_6. [DOI] [PubMed] [Google Scholar]
- Shirakawa T., Li A., Dubowitz M., Dekker J. W., Shaw A. E., Faux J. A., Ra C., Cookson W. O., Hopkin J. M. Association between atopy and variants of the beta subunit of the high-affinity immunoglobulin E receptor. Nat Genet. 1994 Jun;7(2):125–129. doi: 10.1038/ng0694-125. [DOI] [PubMed] [Google Scholar]
- Thomas M. J., Umayahara Y., Shu H., Centrella M., Rotwein P., McCarthy T. L. Identification of the cAMP response element that controls transcriptional activation of the insulin-like growth factor-I gene by prostaglandin E2 in osteoblasts. J Biol Chem. 1996 Sep 6;271(36):21835–21841. doi: 10.1074/jbc.271.36.21835. [DOI] [PubMed] [Google Scholar]
- Toonen R. F., Gowan S., Bingle C. D. The lung enriched transcription factor TTF-1 and the ubiquitously expressed proteins Sp1 and Sp3 interact with elements located in the minimal promoter of the rat Clara cell secretory protein gene. Biochem J. 1996 Jun 1;316(Pt 2):467–473. doi: 10.1042/bj3160467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas P., Pruzanski W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab Invest. 1986 Oct;55(4):391–404. [PubMed] [Google Scholar]
- Van Vyve T., Chanez P., Bernard A., Bousquet J., Godard P., Lauwerijs R., Sibille Y. Protein content in bronchoalveolar lavage fluid of patients with asthma and control subjects. J Allergy Clin Immunol. 1995 Jan;95(1 Pt 1):60–68. doi: 10.1016/s0091-6749(95)70153-2. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Korfhagen T. R. Regulation of gene transcription in respiratory epithelial cells. Am J Respir Cell Mol Biol. 1996 Feb;14(2):118–120. doi: 10.1165/ajrcmb.14.2.8630260. [DOI] [PubMed] [Google Scholar]
- Yan K., Salome C., Woolcock A. J. Rapid method for measurement of bronchial responsiveness. Thorax. 1983 Oct;38(10):760–765. doi: 10.1136/thx.38.10.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Le Souëf P. N., Geelhoed G. C., Stick S. M., Turner K. J., Landau L. I. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med. 1991 Apr 25;324(17):1168–1173. doi: 10.1056/NEJM199104253241704. [DOI] [PubMed] [Google Scholar]
- van Herwerden L., Harrap S. B., Wong Z. Y., Abramson M. J., Kutin J. J., Forbes A. B., Raven J., Lanigan A., Walters E. H. Linkage of high-affinity IgE receptor gene with bronchial hyperreactivity, even in absence of atopy. Lancet. 1995 Nov 11;346(8985):1262–1265. doi: 10.1016/s0140-6736(95)91863-9. [DOI] [PubMed] [Google Scholar]