Abstract
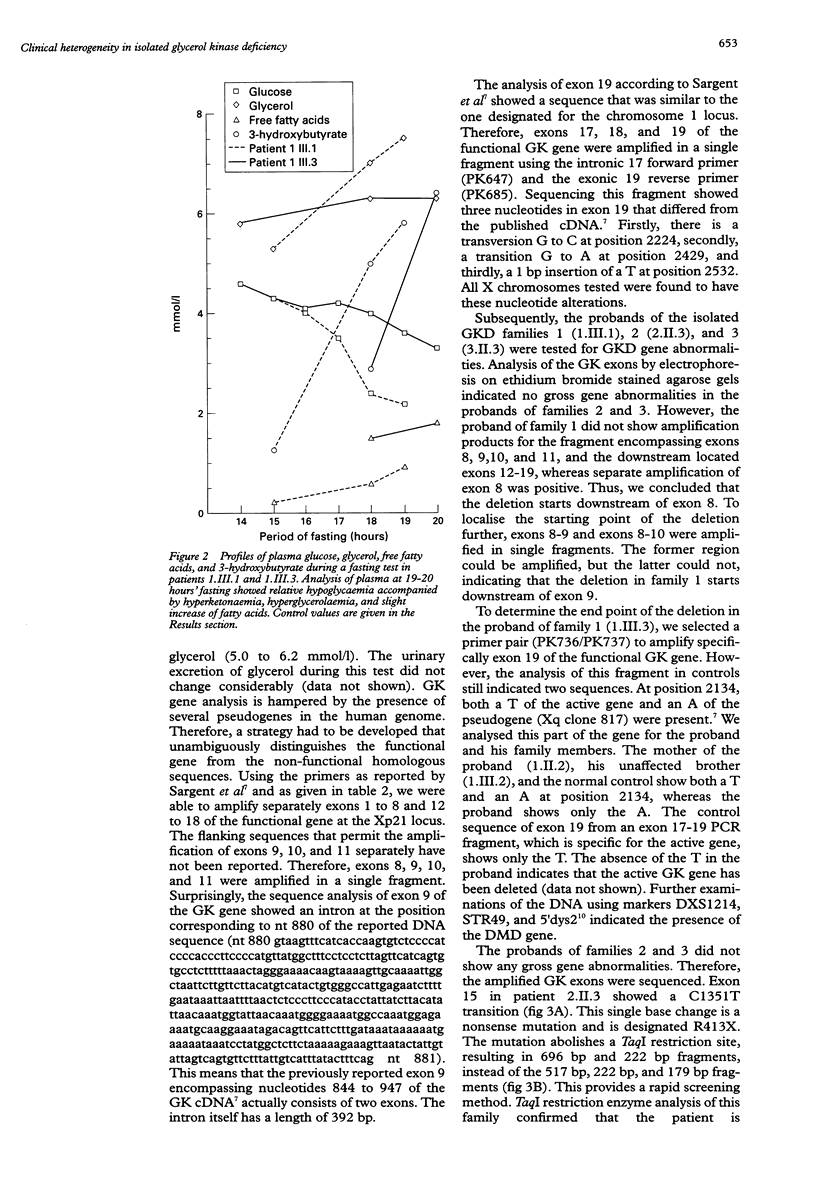
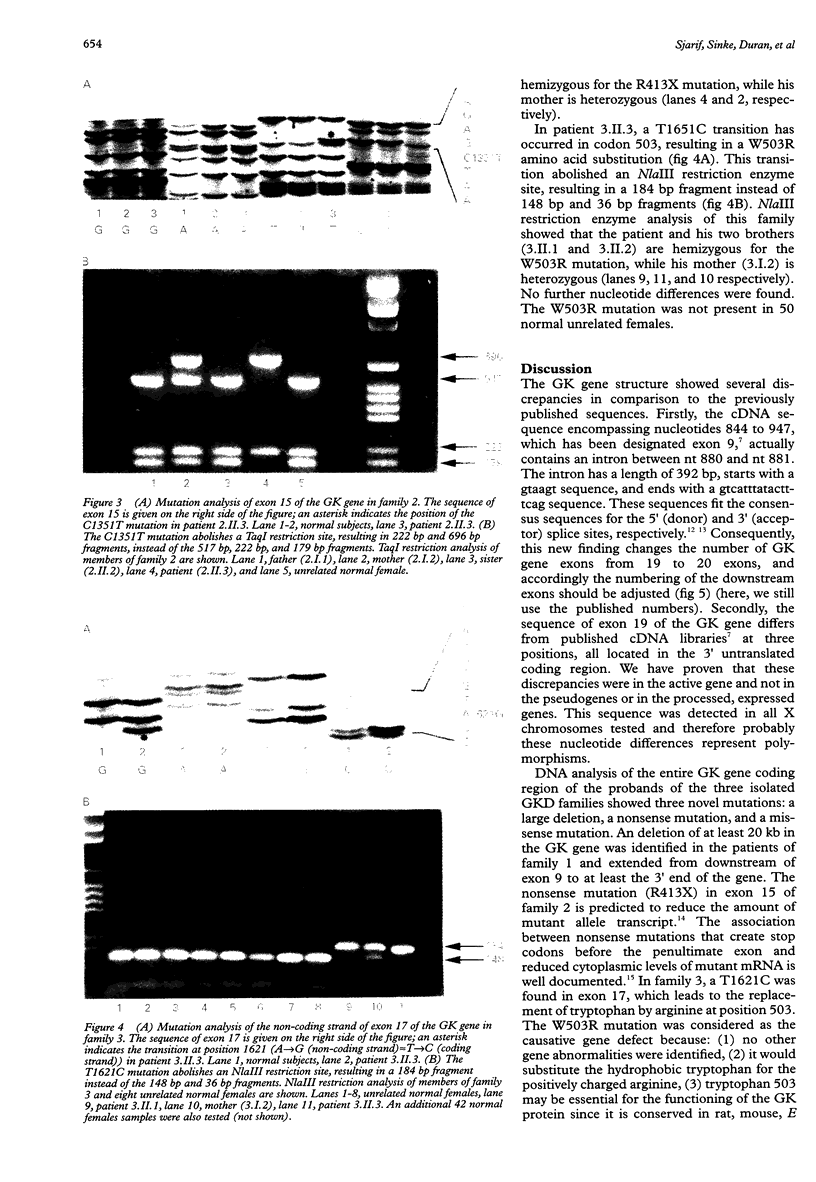
Isolated glycerol kinase deficiency (GKD) is an X linked recessive disorder. The clinical and biochemical picture may vary from a childhood metabolic crisis to asymptomatic adult "pseudohypertriglyceridaemia", the result of hyperglycerolaemia. We performed glycerol kinase (GK) gene analysis to study the molecular heterogeneity and genotype-phenotype correlation in eight males from three families with isolated GKD. All patients had hyperglycerolaemia and glyceroluria. Four patients from two families were essentially free of symptoms. Three patients had gastrointestinal symptoms with ketoacidosis or hypoglycaemia or both. One patient had recurrent convulsions as the only acute sign, without evidence that it was correlated with a catabolic state. Fasting tests in two symptomatic patients of family 1 showed hyperketotic states, together with a tendency to hypoglycaemia. The diagnosis was confirmed by a defective 14C-glycerol incorporation into trichloroacetic acid precipitable macromolecules in intact skin fibroblasts. Mutation screening of the GK gene was performed by amplification and direct sequencing of exons using PCR. Three novel mutations were identified: (1) a deletion starting downstream of exon 9, extending to the 3' end of the gene; (2) a nonsense mutation R413X caused by a C1351T transition; and (3) a missense mutation W503R caused by a T1651C transition. In addition, we found differences from the reported sequence: (1) exon 9 actually consists of two exons, which consequently will change the number of GK gene exons from 19 to 20 exons, and (2) nucleotide differences in exon 19. So far, no genotype-phenotype correlation can be established in these GKD families.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba H., Zhang X. J., Wolfe R. R. Glycerol gluconeogenesis in fasting humans. Nutrition. 1995 Mar-Apr;11(2):149–153. [PubMed] [Google Scholar]
- Blomquist H. K., Dahl N., Gustafsson L., Hellerud C., Holme E., Holmgren G., Matsson L., von Zweigbergk M. Glycerol kinase deficiency in two brothers with and without clinical manifestations. Clin Genet. 1996 Nov;50(5):375–379. doi: 10.1111/j.1399-0004.1996.tb02391.x. [DOI] [PubMed] [Google Scholar]
- Bonnefont J. P., Specola N. B., Vassault A., Lombes A., Ogier H., de Klerk J. B., Munnich A., Coude M., Paturneau-Jouas M., Saudubray J. M. The fasting test in paediatrics: application to the diagnosis of pathological hypo- and hyperketotic states. Eur J Pediatr. 1990 Dec;150(2):80–85. doi: 10.1007/BF02072043. [DOI] [PubMed] [Google Scholar]
- Deary I. J., Hepburn D. A., MacLeod K. M., Frier B. M. Partitioning the symptoms of hypoglycaemia using multi-sample confirmatory factor analysis. Diabetologia. 1993 Aug;36(8):771–777. doi: 10.1007/BF00401150. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., McIntosh I., Sakai L. Y., Corson G. M., Chalberg S. C., Pyeritz R. E., Francomano C. A. Four novel FBN1 mutations: significance for mutant transcript level and EGF-like domain calcium binding in the pathogenesis of Marfan syndrome. Genomics. 1993 Aug;17(2):468–475. doi: 10.1006/geno.1993.1349. [DOI] [PubMed] [Google Scholar]
- Guo W., Worley K., Adams V., Mason J., Sylvester-Jackson D., Zhang Y. H., Towbin J. A., Fogt D. D., Madu S., Wheeler D. A. Genomic scanning for expressed sequences in Xp21 identifies the glycerol kinase gene. Nat Genet. 1993 Aug;4(4):367–372. doi: 10.1038/ng0893-367. [DOI] [PubMed] [Google Scholar]
- Guo W., Worley K., Adams V., Mason J., Sylvester-Jackson D., Zhang Y. H., Towbin J. A., Fogt D. D., Madu S., Wheeler D. A. Genomic scanning for expressed sequences in Xp21 identifies the glycerol kinase gene. Nat Genet. 1993 Aug;4(4):367–372. doi: 10.1038/ng0893-367. [DOI] [PubMed] [Google Scholar]
- Huq A. H., Lovell R. S., Ou C. N., Beaudet A. L., Craigen W. J. X-linked glycerol kinase deficiency in the mouse leads to growth retardation, altered fat metabolism, autonomous glucocorticoid secretion and neonatal death. Hum Mol Genet. 1997 Oct;6(11):1803–1809. doi: 10.1093/hmg/6.11.1803. [DOI] [PubMed] [Google Scholar]
- Huq A. H., Lovell R. S., Sampson M. J., Decker W. K., Dinulos M. B., Disteche C. M., Craigen W. J. Isolation, mapping, and functional expression of the mouse X chromosome glycerol kinase gene. Genomics. 1996 Sep 15;36(3):530–534. doi: 10.1006/geno.1996.0500. [DOI] [PubMed] [Google Scholar]
- Jahoor F., Peters E. J., Wolfe R. R. The relationship between gluconeogenic substrate supply and glucose production in humans. Am J Physiol. 1990 Feb;258(2 Pt 1):E288–E296. doi: 10.1152/ajpendo.1990.258.2.E288. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- McCabe E. R., Sadava D., Bullen W. W., McKelvey H. A., Seltzer W. K., Rose C. I. Human glycerol kinase deficiency: enzyme kinetics and fibroblast hybridization. J Inherit Metab Dis. 1982;5(4):177–182. doi: 10.1007/BF02179133. [DOI] [PubMed] [Google Scholar]
- McIntosh I., Hamosh A., Dietz H. C. Nonsense mutations and diminished mRNA levels. Nat Genet. 1993 Jul;4(3):219–219. doi: 10.1038/ng0793-219. [DOI] [PubMed] [Google Scholar]
- Patel D., Kalhan S. Glycerol metabolism and triglyceride-fatty acid cycling in the human newborn: effect of maternal diabetes and intrauterine growth retardation. Pediatr Res. 1992 Jan;31(1):52–58. doi: 10.1203/00006450-199201000-00010. [DOI] [PubMed] [Google Scholar]
- Sargent C. A., Young C., Marsh S., Ferguson-Smith M. A., Affara N. A. The glycerol kinase gene family: structure of the Xp gene, and related intronless retroposons. Hum Mol Genet. 1994 Aug;3(8):1317–1324. doi: 10.1093/hmg/3.8.1317. [DOI] [PubMed] [Google Scholar]
- Scheuerle A., Greenberg F., McCabe E. R. Dysmorphic features in patients with complex glycerol kinase deficiency. J Pediatr. 1995 May;126(5 Pt 1):764–767. doi: 10.1016/s0022-3476(95)70409-4. [DOI] [PubMed] [Google Scholar]
- Walker A. P., Muscatelli F., Monaco A. P. Isolation of the human Xp21 glycerol kinase gene by positional cloning. Hum Mol Genet. 1993 Feb;2(2):107–114. doi: 10.1093/hmg/2.2.107. [DOI] [PubMed] [Google Scholar]
- Walker A. P., Muscatelli F., Stafford A. N., Chelly J., Dahl N., Blomquist H. K., Delanghe J., Willems P. J., Steinmann B., Monaco A. P. Mutations and phenotype in isolated glycerol kinase deficiency. Am J Hum Genet. 1996 Jun;58(6):1205–1211. [PMC free article] [PubMed] [Google Scholar]
- Wapnir R. A., Stiel L. Regulation of gluconeogenesis by glycerol and its phosphorylated derivatives. Biochem Med. 1985 Apr;33(2):141–148. doi: 10.1016/0006-2944(85)90022-5. [DOI] [PubMed] [Google Scholar]
- Wise J. E., Matalon R., Morgan A. M., McCabe E. R. Phenotypic features of patients with congenital adrenal hypoplasia and glycerol kinase deficiency. Am J Dis Child. 1987 Jul;141(7):744–747. doi: 10.1001/archpedi.1987.04460070046020. [DOI] [PubMed] [Google Scholar]