Abstract
Purpose
The hypothalamic–pituitary–adrenal (HPA) axis exerts many actions on the central nervous system (CNS) aside from stress regulation. Glucocorticoids (GCs) play an important role in affecting several cognitive functions through the effects on both glucocorticoid (GR) and mineralocorticoid receptors (MR). In this review, we aim to unravel the spectrum of cognitive dysfunction secondary to derangement of circulating levels of endogenous and exogenous glucocorticoids.
Methods
All relevant human prospective and retrospective studies published up to 2022 in PubMed reporting information on HPA disorders, GCs, and cognition were included.
Results
Cognitive impairment is commonly found in GC-related disorders. The main brain areas affected are the hippocampus and pre-frontal cortex, with memory being the most affected domain. Disease duration, circadian rhythm disruption, circulating GCs levels, and unbalanced MR/GR activation are all risk factors for cognitive decline in these patients, albeit with conflicting data among different conditions. Lack of normalization of cognitive dysfunction after treatment is potentially attributable to GC-dependent structural brain alterations, which can persist even after long-term remission.
Conclusion
The recognition of cognitive deficits in patients with GC-related disorders is challenging, often delayed, or mistaken. Prompt recognition and treatment of underlying disease may be important to avoid a long-lasting impact on GC-sensitive areas of the brain. However, the resolution of hormonal imbalance is not always followed by complete recovery, suggesting irreversible adverse effects on the CNS, for which there are no specific treatments. Further studies are needed to find the mechanisms involved, which may eventually be targeted for treatment strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40618-023-02091-7.
Keywords: Cognition, Brain, Mineralocorticoid receptor, Glucocorticoid receptor, Cushing syndrome, Adrenal insufficiency
Introduction
The hypothalamic–pituitary–adrenal (HPA) axis exerts many actions on the central nervous system (CNS) aside from stress regulation. Indeed, corticotropin-releasing hormone (CRH) fibers in the paraventricular nucleus of the hypothalamus also project to the brain stem and non-hypophysiotropic CRH neurons are abundant elsewhere, primarily in brain areas involved in sensory information processing (i.e., insulate cortex, parabrachial and solitary tract nuclei), emotional processing (i.e., amygdala, substantia nigra, and cingulate cortex), autonomic nervous system regulation (i.e., locus coeruleus), motor control (i.e., insulate cortex, substantia nigra), and cognitive functioning (i.e., pre-frontal cortex, substantia nigra) [1]. CRH also modulates behavioral activities concerning anxiety, mood, arousal, locomotion, reward, and feeding [2, 3], and increases sympathetic activation. Many of the non-hypophysiotropic behavioral and autonomic functions of these peptides can be viewed as complementary to activation of the HPA axis in the maintenance of homeostasis under exposure to stress (e.g., immune, cardiac, gastrointestinal, and reproduction effects) [4]. Hyperactivity of the HPA axis is a common neuroendocrine finding in affective disorders [2, 5], and the activation of central CRH pathways is a critical neurobiological substrate of anxiety and depressive states [3, 6]. The normalization of HPA regulation is highly predictive of successful treatment for these conditions.
Glucocorticoid (GC) secretion is regulated via a negative feedback mechanism, similar to the other hormonal axes. However, severe neurogenic stress and a large amount of CRH secreted in response to various stimuli can break through the feedback inhibition mediated by GCs. A higher level of feedback control is exerted by GC-responsive neurons in the hippocampus that project in the hypothalamus, determining the set point of pituitary responsiveness to GCs [7].
In this review, we describe the physiology governing the interaction between GCs, mineralocorticoids (MCs), and cognitive function, with the aim of unraveling the spectrum of cognitive dysfunction in different HPA-axis derangements involving endogenous and exogenous GCs’ secretion patterns, such as Cushing’s syndrome (CS), Adrenal insufficiency (AI), Congenital Adrenal Hyperplasia (CAH), and exogenous GCs (eGCs) treatment (see Table 1).
Table 1.
Summary of affected cognitive domains and related brain areas, along with MRI findings, during HPA-axis derangements
| Disease | Affected cognitive domains | Affected brain areas | MRI findings | Details/comments |
|---|---|---|---|---|
| Adrenal insufficiency (AI) |
Verbal memory and learning Executive functions (mental flexibility, attention, working memory) |
Hippocampus and limbic system Pre-frontal cortex |
Reduction in brain and intracranial volume Reduction in the surface area of the inferior parietal and cingulate cortex Reduction in the right lateral orbitofrontal cortex volume |
Ultradian cortisol fluctuation, disruption of circadian rhythm, unbalanced MR/GR activation and sleep disturbances, can all affect cognition in AI The role of disease duration, as well as that of replacement dosing, is still a matter of debate |
| Cushing Syndrome (CS) |
Verbal and visual intellectual skills, including memory and learning Executive functions (visuo-spatial abilities, attention, concentration, working memory, processing speed) |
Hippocampus and limbic system Neo-frontal cortex Pre-frontal cortex |
Hippocampal atrophy Global brain volume loss Cerebellar cortex and pre-frontal regions atrophy Microstructural changes in white/gray brain matter |
A full recovery does not always occur and might be influenced by several parameters |
| Congenital adrenal hyperplasia (CAH) |
Lower general intelligence and IQ Visual intellectual abilities (memory, perception) Executive functions (working memory, visuo-spatial abilities, selective attention) |
Frontal and pre-frontal cortex Hippocampus |
Reduction in whole brain volume (hippocampus, amygdala, subcortical structures and cerebellum) Microstructural changes in white/gray brain matter Increased CSF volume |
Cognitive impairment often associates with disease severity Adrenal crises, hyperandrogenaemia, cortisol deficiency, and GC dose regimens are possible risk factors for cognitive decline |
| Exogenous glucocorticoids (eGCs) |
Declarative memory, processing speed (Steroid dementia syndrome) Working memory |
Hippocampus Pre-frontal cortex |
Hippocampal and amygdala atrophy Reduced brain volume Decreased neuronal vitality Reduced blood flow and metabolism in memory-related brain regions |
Age, type and timing of eGCs administration, as well as duration of eGCs exposure likely act as influencing factors Acute/chronic effects of eGCs on cognitive function and brain structures is still a matter of debate GWS might affect cognitive function’ recovery |
CSF Cerebral Spinal Fluid, eGCs Exogenous Glucocorticoids, GR Glucocorticoid Receptor, IQ Intelligence Quotient, MR Mineralocorticoid Receptor, HPA hypothalamic–pituitary–adrenal, GWS glucocorticoid withdrawal syndrome
Physiology of glucocorticoids in the brain
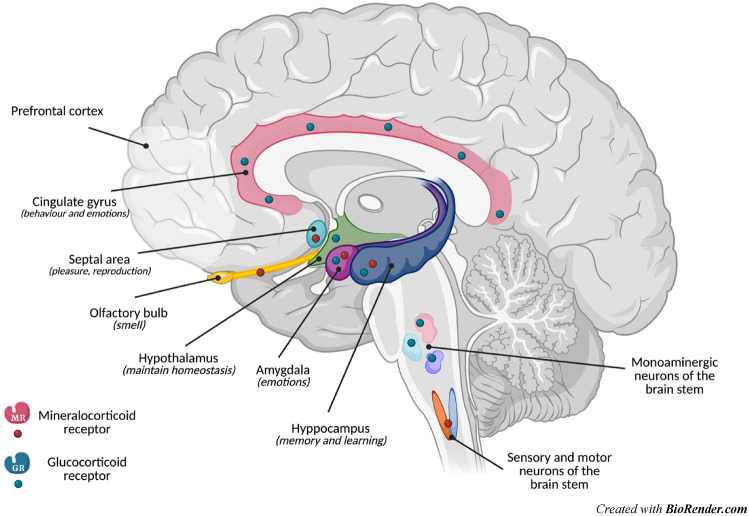
The kinetics of GC secretion follows two levels of control: a circadian rhythm, represented by extensive and pulsatile oscillations, on to which an ultradian rhythm is superimposed, interspersed within the daily kinetics with a recurrence of approximately one peak per hour, whose average amplitude influences the size of the major circadian fluctuations. Fluctuations in circulating GC correlate with the effects of hormones on target tissues. This pattern of GC secretion (rhythmic binding and dissociation of hormones from their receptors and pulsatility) is defined by the ultradian rhythm [8, 9]. Other than endogenous, eGCs, and other steroids can access the brain through the blood–brain barrier [10]. In the brain, GCs bind two different receptors: type I (the mineralocorticoid receptor—MR, so named because it binds aldosterone and GCs with high affinity) and type II (glucocorticoid receptor—GR, which has low affinity for MCs) [7, 11]. GRs are widely expressed throughout the brain, in neurons and glial cells, with high densities in limbic areas, monoaminergic neurons of the brain stem, and paraventricular and supraoptic nuclei of the hypothalamus, where they regulate the biosynthesis and release of vasopressin and CRH. The distribution of MRs is restricted to neurons of fewer brain areas: the limbic system (hippocampal formation, septal area, amygdala, and olfactory nucleus), sensory and motor neurons of brain stem and brain cortex [12]. The spatial distribution of GRs and MRs in the brain is summarized in Fig. 1.
Fig. 1.
The spatial distribution of glucocorticoid and mineralocorticoid receptors in the brain. Created with BioRender.com
While the GR has a high selectivity for GCs, MR is a promiscuous receptor capable of interacting with multiple ligands. Despite its high affinity for cortisol (tenfold higher compared to GRs [13]) and aldosterone, it can also bind progesterone, deoxycortisol, and deoxycorticosterone [13]. MR activation within the brain has been shown to mediate the stress-induced adaptive shift from a “cognitive” memory (mediated by hippocampus) to a more rigid, “habit-like” memory (mediated by striatum) [14, 15], reflecting the limited-resources condition of the “fight or flight” paradigm, in which the system is conceived to rapidly recall and enable simple stimulus responses to face a stressful situation.
The complex interaction between steroid hormones and the areas responsible for several cognitive domains within the brain is coordinated by specific enzymatic activities and enzyme/receptor zonation.
The ligand specificity of the MR, which determines its activation by either GCs or aldosterone in various tissues, is mediated by the 11-beta-hydroxysteroid dehydrogenase (11β-HSD) enzymes [16]. There are two different isoforms of 11β-HSD: type 1 and type 2. The type 1 enzyme is widely expressed in key GC target organs (adipose tissue, skeletal muscle, and liver), including the brain [17, 18]. It predominantly converts inactive cortisone to active cortisol [19], thus amplifying local GC bioavailability. Conversely, 11β-HSD type 2 inactivates cortisol to cortisone [20]. The primary role of 11β-HSD type 2 is to protect the MR from inappropriate activation by GCs [21] in mineralocorticoid sensitive tissue, such as the kidney, placenta, colon, and salivary glands by converting them in their 11-oxo metabolites, allowing aldosterone to bind to MR despite its 100–1000-fold lower concentration in the bloodstream, compared to GCs [22].
The different expression of these two isoforms within the brain areas impacts the activation of both MR and GR. Indeed, only 11β-HSD type 1 is expressed in hippocampal cells and limbic structures and, therefore, MRs are usually saturated by physiological cortisol concentrations in these areas, making them crucial for the emotional and memory processes [23]. Some brain areas also express 11β-HSD type 2, making them sensitive to aldosterone, such as the nucleus of solitary tract, which is implicated in modulating the behavioral response (including appetite, mood, and arousal) to fluctuations of sodium concentrations [24].
The regional distribution of MR and GR within the brain adds further complexity, suggesting that the brain response to GCs is extremely tightly regulated with a delicate between substrate availability and MR and/or GR activation. Specifically, during physiological basal and low pulsatile GCs conditions, GCs preferentially bind and activate the MR, resulting in an increased MR/GR activation ratio. This dynamic has a predominantly neuro-projective role. In fact, basal MR signaling contributes to the stabilization of excitatory postsynaptic currents, generating a negative feedback signal directed to the hypothalamus, limiting detrimental GC effects and mediating behavioral adaptation by proactively regulating the sensitivity of the neuroendocrine stress-response system [25]. Indeed, blocking MR activity with spironolactone has been shown to impair selective attention and visuo-spatial memory in healthy men [26], whereas MR stimulation with fludrocortisone improved spatial memory [27]. In contrast, when GC bioavailability increases beyond the saturating capacity of the MR receptors, as happens during the stress response, GCs bind to the otherwise inactive low-affinity GR, causing an inversion in the MR/GR ratio (i.e., a reduction of the MR/GR activation ratio) which allows the gradual recovery from stress during reactive mode [12]. However, chronic and prolonged stress may promote an excessive reduction of the MR:GR ratio [28, 29]. This results in the progressive loss of hippocampal ability to exert negative feedback on the HPA axis and phenomena of maladaptive synaptic plasticity. The effects of GR receptor activation on neurogenesis and the reduced neuroprotective MR signaling affect the reversibility of the functional disorder, precipitating the appearance of the pathological phenotype [30, 31].
In the CNS, gene expression regulation by GCs is most significant in the areas where neurogenesis occurs, even in adulthood. Neuronal proliferation and differentiation correlate inversely with the central bioavailability of GCs: in the presence of reduced concentrations of cortisol and, therefore, in conditions of activation of the MR receptors alone, higher growth and differentiation rates can be documented. However, excess central bioavailability of cortisol leads to GR activation and precipitates a reduction in neurogenesis [32]. Numerous pathological and para-physiological conditions can, by increasing cortisol levels, affect adult neurogenesis: among these, all the endocrine diseases causing alteration in GC secretion patterns. These might be responsible for an imbalance between GR and MR activation in the brain, leading to detrimental effects on cognitive function.
Cushing’s syndrome and cognition
Cushing’s syndrome (CS) is a severe, chronic, and life-threatening disease caused by prolonged hypercortisolism, which can be endogenous or exogenous. Endogenous hypercortisolism can result from ACTH-secreting tumors—either pituitary (Cushing’s Disease, CD) or extra-pituitary (ectopic CS) [33]—or ACTH-independent increase in adrenal production due to bilateral gland hyperplasia or tumoral lesions [34]. Of note, moderate hyperactivity of the HPA axis can also derive from adrenal incidentalomas presenting with mild autonomous cortisol secretion (MACS) and non-neoplastic hypercortisolism (NNH) states (including chronic alcoholism, polycystic ovary syndrome, anorexia nervosa, and psychiatric disorders) [35], resulting in partial clinical and/or biochemical overlap with overt CS [36]. Long-standing exposure to GC excess cause multiple medical comorbidities, most notably metabolic syndrome, increased cardiovascular risk, immune and musculoskeletal disorders, subfertility, and dermatological manifestations [37]. Neuropsychiatric disorders are common in patients with CS, the most frequent being major depression (50–81%) and, to a lesser extent, anxiety (66%) and bipolar disorder (30%) [38]. Cognitive impairment (sometimes also called “steroid dementia syndrome”) is another common finding in patients with CS, which significantly impairs patients’ quality of life [39]. Its prevalence is extremely variable, ranging from 15 to 83% of cases [40, 41].
Cognitive decline and mood disorders often overlap in patients with CS [40]. A recent prospective study [42] demonstrated that the improvement in the cognitive impairment in patients with CD after trans-sphenoidal surgery parallels (and perhaps depends on) the improvement in depressive scores, thus highlighting the close relationship between mood disorders and cognition [42]. Indeed, 4 weeks of antidepressant therapy (such as serotonin re-uptake inhibitors) proved effective in improving cognitive function (e.g., memory, orientation, spatial navigation, and verbal fluency) in adolescents with CD [43].
However, cognitive impairment can often occur as a separate neurological disorder in CS [38, 44]. Memory is the domain that is most frequently affected (86% of cases) [45] due to the density in GR and lack of 11β-HSD type 2 activity in hippocampal neurons, making them highly sensitive to HPA-axis hyperactivity. Indeed, moderate memory impairment has been reported in patients with CD [46], regardless of associated neuropsychiatric comorbidities. Up to two-thirds of patients with active CS report difficulties with the registration of new information, forgetfulness for appointments and locations of objects, as well as shortened attention span, reduced concentration ability, and impaired comprehension abilities [45, 47, 48]. In addition, worse performance on visual and spatial information [49, 50], attention, executive functioning, and non-verbal aspects of memory [50] have also been described. Although the data are not all consistent [46], a direct relationship between ACTH and cortisol levels with the severity of neuropsychiatric impairment has been described [44, 51].
A recent meta-analysis [47] including 294 patients with CS also confirmed cognitive decline in seven out of eight cognitive domains. Memory and learning-related functions (both visual and verbal) were the most impaired, along with the general intelligence and language skills domains. Other deficits concerned executive and visuo-spatial functions, as well as attention and processing speed [47].
Recently, the possibility that even mild hypercortisolism might associate with impaired cognitive function has been questioned. A small multicentre study evaluating cognitive function in 23 patients with MACS found differences in some (verbal fluency, symbol coding, and executive function) but not all (verbal and working memory) cognitive domains [52]. However, very recently, a prospective study on 63 patients with adrenal incidentalomas described worse performances regarding working memory, visuo-spatial domains, and overall cognitive function in patients with MACS compared to those with non-functioning adrenal adenomas. Multivariate linear regression also showed post-DST cortisol as a risk factor for cognitive impairment [53]. Albeit conflicting, these preliminary findings suggest that mild hypercortisolism might affect mental health and cognitive status, although its impact has yet to be clearly investigated. Moreover, a direct comparison between patients with MACS and overt CS has never been performed, underlining the urgent need for further prospective studies to elucidate the contribution of hypercortisolism degree on cognitive impairment.
Interestingly, the pattern of cognitive deficits in CS is similar to that described with aging [54], with impairment in general intellective capacity and poorer performances in executive functions, spatial memory, and attention tasks. This suggests that cortisol excess might exhibit an “aging-like” effect, further exacerbating the cognitive impairment typical of older age [54]. Similarly, higher plasma cortisol levels have been linked to faster cognitive deterioration in patients with Alzheimer’s Disease, and researchers are questioning whether alterations in genes involved in the regulation of the GC system may influence the risk for this condition. The study showed that patients carrying the apoE4 allele (a known risk factor for Alzheimer’s Disease) have elevated cerebrospinal fluid cortisol levels [55], reinforcing the hypothesis that hypercortisolism accelerates hippocampal damage and leads to a dementia-like cognitive phenotype [56]. Moreover, a rare haplotype in the region of the gene encoding 11β-HSD has been found to confer a sixfold higher risk for sporadic Alzheimer’s Disease [57], likely increasing neuronal GC-associated neurotoxicity.
From a morpho-structural point of view, early magnetic resonance imaging (MRI) studies linked hypercortisolism and hippocampal atrophy in patients with CS [58]. Since then, hippocampal atrophy has been reported as the most common finding in patients with active CS [47, 59–61], although global brain volume loss [62] and atrophy [48, 59, 63], smaller volumes in the cerebellar cortex [64], and the pre-frontal regions have also been observed [65]. Recently, decreased hippocampal volume (HV) was described only in patients whose memory scores were impaired [48], suggesting that hippocampal atrophy likely reflects higher disease severity and a more advanced state of cognitive dysfunction. Therefore, MRI assessment of HV may underestimate the neurocognitive consequences of CS [66].
Aside from HV, a negative correlation between urinary-free cortisol and subcortical gray matter and cerebral white matter MRI intensity has been reported [67]. Changes in white/gray matter (consistency, intensity, and homogeneity), as well as axonal and myelin damage at MRI, might precede detectable changes in brain volume [67] in CS, as demonstrated in patients with both active and cured CS [68, 69]. Interestingly, these alterations associate with worse information processing speed [70] and overall cognitive performance scores [69]. Recently, the use of advanced MRI sequences [71] revealed specific microstructural changes involving hippocampus/parahippocampal areas, which correlates with the clinical severity of CD and the degree of cognitive impairments [71].
Notably, functional and structural alterations similar to those found in CS have also been identified in states of NNH. HPA-axis hyperactivation has been hypothesized to play a role in the “alcohol dementia”, the cognitive deficits observed during and after chronic alcohol consumption withdrawal [72]. The underlying mechanisms are not entirely clear, but, considering the global brain volume alterations frequently described during alcohol intake (mainly involving white frontal matter [72], white matter microstructure [73], and hippocampal volume [74]), a glucocorticoid-mediated toxicity at the hippocampal level has been suggested [75]. Among the main causes of NNH, major depressive disorder often presents cognitive dysfunction as a core feature of the clinical spectrum [76]: explicit memory and executive functions are the most affected cognitive domains, with the hippocampus being the main impaired brain area [77]. Similarly, anorexia nervosa has been linked with impaired cognition [78], decreased HV [79], and altered functional connectivity, especially in the corticolimbic circuit, which is deeply involved in cognitive control [80]. Notably, in these patients, serum cortisol levels are inversely related to hippocampal and gray matter volumes [79].
In patients with CS, a prompt diagnosis of cognitive dysfunction is crucial as clinical manifestations often precede brain anomalies detected by imaging [81]. Longer disease duration and older age are associated with limited recovery of brain functioning, whereas earlier diagnosis and rapid normalization of hypercortisolism appear to reduce the progression of brain damage and functional impairments [81]. According to a recent meta-analysis [47], the majority of impaired cognitive domains undergo a significant improvement following surgery. Similarly, medical treatment of hypercortisolism, either with GC-receptor antagonists or steroidogenesis inhibitors, can improve psychiatric symptoms within weeks of therapy [82–84].
The timing and degree of the reversibility of cognitive dysfunction in CS are still a matter of debate. Some studies have reported both cognitive and brain morphological improvement following treatment [85–89]. A recovery in verbal fluency/recall and HV increase has been documented 18 months after medical treatment, with younger age being a predictor for functional recovery [88]. Endorsing these data, middle-aged (< 60 years), comorbidity-free patients in long-term (10 years) remission from CD exhibited the same hippocampus and pre-frontal cortex-dependent memory functioning when compared to healthy controls. While these results should be interpreted with caution due to a potential selection bias, it could be argued that the persistence of cognitive impairment after CD remission could be partially attributable to other comorbidities that might potentially affect cognition (i.e., diabetes, age, cardiovascular diseases, hormonal disbalance, and psychiatric disorders), further highlighting the importance of shortening the exposure to hypercortisolism and its comorbidities to preserve cognitive functioning [90].
Nevertheless, several studies have suggested that cognitive function impairment might not completely resolve following surgical treatment of CS [91]. Persistent cognitive impairment (attention, spatial orienting, alerting, working memory, verbal fluency, reading speed [92], and trail-making [93]) has been described after long-term remission in CD [92, 94] and adrenal CS [92], without differences between different etiologies [92]. To date, no accurate predictors of cognitive impairment recovery following remission have been identified.
The extent of the reversibility of structural brain abnormalities in patients with CS is still a matter of debate. The reduction of brain and HV in patients with active CD has been described as partially reversible after cure in some [95], but not all studies [48], and the amelioration of hippocampal morphology has been associated with symptom improvements [96].
Similarly, in patients with NNH, the resolution of the primary noxa usually associates with partial improvement of cognitive functioning, although this is not often mirrored by restoration of physiological brain morphology [73, 79, 97].
Decreased cortical thickness [98] and smaller volumes in the anterior cingulate cortex [99] can persist after long-term CS remission, but an inverse correlation with disease duration suggests a direct link between the prolonged exposure to GC excess and the alterations of brain structures involved in emotional and cognitive processes [100]. Indeed, a recent study including young patients (< 32 years) with short disease duration (< 3 years) has demonstrated a rapid and complete recovery (within 3 months of surgical treatment) of brain volume loss observed in the active phase of the disease [101]. New research approaches using functional MRI spectroscopy [102] are being used to explore neuronal vitality markers within the hippocampal area and their possible role in reversing hippocampal alterations.
It must be noted that successful treatment of CS can be followed by iatrogenic adrenal insufficiency, which is often transitory and promptly replaced with GC therapy. Current literature assessing the reversibility of cognitive and brain structure abnormalities following CS treatment is rather heterogeneous, with studies including hypocortisolemic [91], eucortisolemic [65], or mixed [88] cohort of patients. Whether the post-treatment serum cortisol fluctuations might shape cognitive recovery remains largely underexplored.
In conclusion, cognitive impairment is a common finding in patients with active CS and is directly related to cortisol levels and the duration of hypercortisolism. Long-term exposure to elevated cortisol levels affects several cognitive domains, including memory, verbal intellectual skills, and learning, reflecting a solid hippocampus and neo-frontal cortex involvement. Lack of complete normalization of cognitive functioning after treatment is likely attributable to GC-dependent structural brain alterations, which, if present at diagnosis, generally persist even after long-term remission.
A summary of the current evidence for cognitive function in CS is shown in Table 1 and Supplemental Table 1.
Adrenal insufficiency and cognition
Adrenal insufficiency (AI) is a relatively rare endocrine disorder with multiple causes that can be divided into primary (adrenal), secondary (pituitary), and tertiary (hypothalamus or eGC treatment) forms. Each form of AI has distinctive causes with implications for treatment and follow-up. Patients with primary AI require GC and MC replacement therapy, whereas individuals with secondary and tertiary AI usually necessitate only GC replacement [103–105]. If left untreated, AI is a life-threatening condition.
Patients with AI exhibit a broad spectrum of non-specific symptoms (e.g., fatigue, weakness, mental straining, and malaise) [106–108], and therefore, the diagnosis of psychiatric conditions is challenging, often delayed or mistaken [109].
Cortisol deficiency is known to induce many neuropsychiatric alterations, such as depression, delirium, and delusional ideas [110], that generally reverse after appropriate GC treatment. However, a few studies investigated cognitive function in patients with AI.
Compared to matched healthy controls, impaired declarative memory [111–114], poor performances in episodic memory [111], and verbal memory and learning [114] have been reported in patients with AI, despite stable replacement therapy. Worse performances in verbal and visual memory tasks [115], as well as some executive functions (including attention-related tasks [116] and processing speed) have also been observed, although some data are conflicting [113, 115, 117]. Conversely, no significant differences have been found with respect to concentration, working memory, and visuo-spatial functioning [111, 114].
The cognitive impairment found in AI patients is thought to be due to multiple cooperating pathogenic factors. A recent meta-analysis including more than 500 patients reported that both increased and decreased GCs levels might be responsible for impaired hippocampal-dependent memory and cognitive function [118]. In support of this hypothesis, the inhibition of cortisol production via metyrapone administration caused memory impairment in healthy patients, which was reversed after hydrocortisone treatment [119]. Furthermore, hypoadrenal patients who have experienced a long diagnostic delay and consequent prolonged exposure to cortisol deficiency have worse cognitive performance, notably declarative memory and processing speed [111]. Although there is some compelling evidence suggesting that circulating serum cortisol levels are an important contributor, the actual impact of cortisol deficiency has been questioned in a recent study, in which patients who omitted their morning hydrocortisone replacement doses did not report cognitive dysfunction [115].
In addition to absolute cortisol levels, both the circadian and ultradian rhythms have an important role to play [120]. Indeed, patients with AI generally experience an altered circadian profile of cortisol levels, ranging from low basal concentrations to supraphysiological peaks following acute GC administration. As already discussed, physiological cortisol levels influence cognitive function through a fine regulation of the dynamic balance between MR and GR activation within the brain. However, excessive cortisol fluctuations during replacement regimens might affect MR/GR activation ratio, leading to impaired cognition in patients with AI [121–123]. MR activation has been linked to the ability to learn new information, whereas GR activation is generally associated with memory storage and retrieval [121]. Albeit dedicated pharmacodynamics studies aiming to find the dose of MC able to elicit effects on cognitive functions have never been performed, there are multiple evidences (in healthy subjects as well as in patients with AI) demonstrating that fludrocortisone administration at doses equal or higher than those employed in clinical practice (0.1–0.4 mg per day) causes high MR occupation and improves different domains of memory tasks (verbal [124–126], working [126, 127], and visuo-spatial memory [128]). However, attention and executive functions were impaired during low MR occupation, even after a single omission of a fludrocortisone daily dose [124].
In this context, taking into account the impact of GCs on brain regions, such as the hippocampus and the pre-frontal cortex, the importance of using physiological total daily GC dose in a circadian fashion is crucial. This has to be taken into account both from a functional and structural point of view [129, 130], as degeneration of hippocampal neurons [131], and related impaired performance on declarative memory tasks [132] have been described in AI patients. Moreover, higher hydrocortisone replacement doses have been associated with worse cognitive function, mainly impacting attention, executive function, visual and motor tasks [133], as well as short-term memory [134]. Notably, these cognitive domains are closely linked to the hippocampus, a region exhibiting the highest density of GRs and MRs, and therefore particularly vulnerable to fluctuations in cortisol levels.
The role of disease duration has been proposed as a potential risk factor for cognitive dysfunction in AI, but studies have reported conflicting results and should be interpreted with caution due to the small sample size and follow-up duration. Longer disease duration that was associated with impaired verbal learning [115] and processing speed [111] and prolonged hydrocortisone treatment was found to negatively affect hippocampal structure and function [131]. However, these findings were not confirmed in later studies [133, 134] and require additional investigation.
A recent review of the literature underlined the concept that cognitive impairment is closely associated with sleep disturbances in patients with AI [122]. Hypoadrenalism is associated with poor sleep quality [135, 136], and sleep disturbances are reported in up to 34% of patients [135]. Sleep plays critical roles in memory consolidation [137, 138], a process starting during slow wave sleep, in which HPA-axis suppression allows the retainment of memories acquired throughout the day [139]. Cortisol ensures initiation and transition between different sleep stages [140–142]. Its physiological nadir occurs during the first hours of nocturnal sleep and allows predominant MR activation in the brain, which is crucial for the consolidation of declarative memories. It would be anticipated that any impairment in the normal circadian rhythm would be detrimental to this process [143]. For instance, night cortisol levels might be too low to activate the MR in patients with AI. Accordingly, impaired declarative memory retention associated with poor sleep quality has been described in patients with AI compared to healthy controls [144]. Despite stable replacement therapy, patients with AI often report reduced quality-of-life outcomes, notably related to sleep disturbances and other cognitive dysfunction (memory impairment and affective disorders among the others) [112, 135, 145].
Several studies have investigated the impact of different replacement regimens on sleep and cognitive functioning in this context. Albeit it generally fails to mimic normal circadian rhythm [146], HC replacement therapy ensures a more consolidated sleep pattern [147], as opposed to GC deprivation which results in poor quality of sleep [136, 147]. Higher HC doses at night, multiple daily HC administrations, and circadian rhythm disruption [117, 148] all lead to HPA-axis dysregulation and sub-optimal MR/GR activation [121], which in turn leads to poor sleep quality and cognitive impairment [149].
In conclusion, mild cognitive impairment is common in patients with AI, mainly affecting declarative memory, verbal learning, and processing speed. From a pathogenic perspective, there are multiple factors that have significant contributions to its development. Ultradian cortisol fluctuation, disruption of circadian rhythm, unbalanced MR/GR activation, and sleep disturbances can all affect cognition in AI [60]. The role of disease duration, as well as that of replacement dosing, is still a matter of debate.
A summary of the current evidence for cognitive function in AI is shown in Table 1 and Supplemental Table 2.
Congenital adrenal hyperplasia and cognition
Congenital adrenal hyperplasia (CAH) includes a group of autosomal recessive disorders characterized by enzymatic defects in adrenal steroidogenesis [150]. Mutations involving the 21-hydroxylase gene account for 95% of cases [151]. According to the enzymatic defect, the impairment in GC production results in increased ACTH secretion, which leads to a shift of the steroidogenic pathway toward sex steroid production, with different clinical pictures [152].
CAH can be classified into different forms according to residual enzyme activity. The classic CAH is generally associated with a more severe phenotype. In the most severe Salt-Wasting (SW-CAH) form, there is little or no residual enzyme activity, whereas patients with the Simple-Virilizing (SV-CAH) form still retain 1–5% enzyme activity [153]. The non-classic form of CAH is associated with various degrees of enzyme activity and is characterized by a late onset and milder symptoms, such as female virilization, menstrual irregularities, and subfertility [154]. Treatment generally consists of GC with or without MC replacement therapy, aiming to decrease androgen secretion, correct cortisol deficiency, reduce virilization, and restore fertility [155].
Several studies have investigated cognitive function in patients with CAH describing lower Intelligence Quotient (IQ) with worse overall intelligence [156–160]. A recent study also reported worse performances in visual perception, visual memory, and executive functioning in patients with CAH compared to age-matched, healthy controls [161].
Interestingly, a more severe disease phenotype also associates with greater cognitive impairment. A Swedish epidemiological study reported SW-CAH patients as less prone to complete primary education, exhibiting a higher frequency of disability pensions and sick day leaves compared to controls [162]. Other studies demonstrated worse performance in several cognitive domains (visual memory, fluid intelligence, and non-verbal reasoning tasks) in SW [157, 163, 164] compared to SV-CAH patients [158].
Various factors have been implicated in the development of cognitive impairment in SW-CAH patients. Brain injury related to hyponatraemic episodes secondary to salt-wasting crises has been proposed among the possible underlying mechanisms for lower IQ [157, 165]. Later studies have confirmed this finding in patients with CAH with a positive history of adrenal crises [163, 164]. The contribution of androgen excess to cognitive impairment may also be significant. Patients with CAH (both male and female) are typically exposed to increased androgen levels in utero [166]. However, CAH boys compensate for this higher exposure by reducing testicular androgens, maintaining higher, but still acceptable testosterone levels [166]. On the contrary, CAH females are more susceptible to gestational androgen excess [167]. Increased pre- and post-natal androgen levels affect neuronal development and brain functional connectivity [168, 169], and impair sex-specific cognitive dimorphic abilities, finally leading to an increased risk of learning disabilities [159]. Neuronal myelinization [170, 171] and brain hemispheres maturation [164] are among the alteration described.
Of note, increased levels of precursors (17-OH progesterone, 21-deoxycortisol, and 21-deoxycorticosterone, as well as their metabolites) are commonly observed in CAH patients. These molecules bind to the MR with different affinities and influence their activity [172], possibly interfering with the MR/GR balance in the brain. However, their impact on cognitive functioning in CAH patients has yet to be studied.
Androgens excess can affect cognitive domains differentially in women with CAH. Better performances on tasks involving cognitive domains which typically favor males [173] (mental rotation, spatial perception [174], and fine motor skills [175]) have been described: female patients with CAH and severe disease (and therefore the highest level of in-utero androgen exposure) perform similarly to both healthy males and male patients with CAH regarding spatial cognition [176]. On the other hand, not all cognitive domains benefit from androgen over-exposure and several studies have reported worse short-term memory than in controls [157, 167, 177].
Interestingly, some authors described a lack of cognitive impairment in children and adolescents with CAH, as opposed to their older counterparts [156, 177, 178]. While this observation might reflect a genuine age-related difference exerted by the underlying disease, it is plausible that this discrepancy might relate to the long-term therapeutic (perhaps supraphysiological) exposure to GCs. As discussed above, GC treatment often fails to replicate physiological circadian rhythms [179], resulting in times of under- or over-treatment, both of which can negatively impact cognitive function [177], especially memory [180]. Recent observations have also reported significantly lower IQ in poorly controlled patients affected by SW-CAH, with multivariate analysis showing that in addition to androgen levels and hyponatraemic episodes, higher GC doses were associated with cognitive impairment [163]. Indeed, GC over-treatment is known to affect hippocampal development and function by altering neuronal structure [59], with hippocampal subfield CA1 (closely associated with learning and memory processes) [181, 182] displaying a notable, dose-dependent responsiveness to GCs [183, 184].
As with CS, brain structure alterations are also described in patients with CAH. MRI studies have confirmed a significant reduction in whole brain volume and notably in the hippocampus, amygdala [185], thalamus, cerebellum, and brain stem [186], as well as alterations in areas closely associated with visuo-spatial and working memory (pre-frontal, parietal, and superior occipital cortex) [187]. Similarly, white matter [188–190] as well as gray matter [191] abnormalities in regions closely associated with cognitive functioning (hippocampus, hippocampal subiculum, and CA1 subregions) were described in patients with CAH [192–194]. Importantly, these alterations were not always related to GC dose, suggesting that over-treatment might not be the only factor involved in determining structural brain alterations. In fact, these alterations have also been recently linked with cortisol deficiency in patients with CAH [195]. The amygdala and hippocampus exhibit a high GR density [196] and are known to exert negative feedback on the HPA axis during the stress response [197]. Where HPA-axis function is dysregulated, feedback circuit disruption results in a lack of proliferation, cell death, and, consequently, smaller volumes in these areas [198], resulting in cognitive impairment.
It is likely that multiple factors, including prolonged exposure to androgen excess, cortisol deficiency, and GC-induced deterioration of brain regions, shape the cognitive impairment in patients with CAH [199, 200]. Other than pre-natal androgen excess, the role of pre-natal dexamethasone (DEX) therapy on cognitive function in CAH patients has been explored during the last decade. DEX treatment has been traditionally employed to prevent genital virilization in female fetuses at risk of CAH. The results are conflicting, and current guidelines refer to pre-natal DEX treatment as an experimental therapy [155], since the fetal risks from pre-natal DEX exposure outweigh the potential consequences of genital virilization [201]. Prenatal GC exposure can disrupt the HPA axis, enhancing the cortisol response to stress [202], with negative long-term consequences regarding mental health in childhood and adolescence [203]. Notably, DEX is not metabolized by 11β-HSD type 2 [103] and retains minimal (if not negligible) MC activity [204], which can be even more detrimental to the MR/GR activation ratio in MC-sensitive brain areas.
In utero, GC over-exposure increases the risk of affective, cognitive, and motor behavior impairment [205] and children treated prenatally with DEX have been reported to be less sociable, more emotional [206], and socially anxious [207] [206], albeit this was not confirmed in a recent meta-analysis [208] (probably due to the observational nature and the small sample size of the available studies). Similarly, the current evidence regarding cognitive function has yielded conflicting data. Several investigations reported no differences in general intelligence, long-term memory, and learning capabilities between children with CAH prenatally exposed to DEX versus those who were not. However, verbal working memory, self-perception for scholastic competence [207], and verbal intelligence [178] were found to be significantly reduced in the former group. Interestingly, a single study reported sex-specific long-term cognitive effects (slower mental processing) of pre-natal DEX in girls with CAH, but not boys [209]. The underpinning reasons are currently unknown.
In conclusion, patients with CAH have worse general intelligence and lower IQ compared to healthy controls, and cognitive impairment is often associated with disease severity. Adrenal crises, hyperandrogenaemia, cortisol deficiency, and GC dose regimens are all risk factors for cognitive decline in patients with CAH. This parallels with significant brain alterations seen at MRI, possibly secondary to abnormal brain development due to a mixture of in-utero hormonal imbalance and post-natal GC excess.
A summary of the current evidence for cognitive function in CS is shown in Table 1 and Supplemental Table 3.
Exogenous glucocorticoids’ treatment and cognition
eGCs are among the most prescribed drugs in clinical practice, being a mainstay for the treatment of several autoimmune and inflammatory disorders [210]. In recent years, a notable increase in prescription rates [211, 212] and, subsequently, in eGC-associated side effects has been observed [212–215]. For instance, adverse psychiatric side effects (APSEs) frequently occur during eGC treatment, with a prevalence ranging from 3 to 60% of cases [216]. While psychosis, mania/hypomania, depression, and anxiety are the most common findings, long-lasting cognitive impairment is also described during eGC therapy, with a prevalence ranging from 0.4 [217] to 7% of cases [218].
Overall, prolonged eGC treatment is known to cause cognitive deficits [219–221], in a pattern of neurocognitive decline known as “steroid dementia” [217], typically characterized by deficits in declarative memory, mental processing speed, and concentration [222].
Interestingly, different eGCs might exert varying effects on cognitive functioning due to their specific impact on the MR/GR balance in the brain. Short- and intermediate-acting compounds (SIAGCs), such as cortisone, hydrocortisone, and prednisone (PRED), can activate both MR and GR, whereas longer-acting eGCs (LAGCs), like DEX and methylprednisolone (MP), preferentially bind to GR, suppressing endogenous cortisol production via negative feedback on the HPA and causing a decrease in MR occupation.
Indeed, the administration of hydrocortisone has been shown to partially improve memory deficits produced by the chronic administration of DEX [223]. Similarly, in a randomized-controlled trial conducted on 50 children treated with DEX (6 mg/m2/day for two 5-day courses) with acute lymphoblastic leukemia, the administration of 10 mg/m2/day of thrice-daily hydrocortisone in a circadian fashion (higher dosage given in the morning) markedly improved the DEX-induced APSEs [224]. These beneficial effects have been ascribed to the refill of unoccupied brain MRs, which is typically associated with SIAGCs, but not LAGCs, resulting in a restoration of the correct MR/GR activation balance. Intriguingly, the fludrocortisone-mediated activation of the MR has been demonstrated to improve memory in healthy individuals [126].
The direct comparison between the neurocognitive effects of DEX and PRED has yielded unexpected results: in pediatric patients with lymphoblastic leukemia, no differences were found in cognitive functioning [225], except for worse fluid reasoning, higher likelihood of enrolling in special education services [226] and word reading [227] in DEX-treated patients. Similarly, the available findings regarding the effects of SIAGCs on cognition are controversial. Mild, acute rises in cortisol, following the administration of low-to-moderate doses of hydrocortisone (< 25 mg), are known to enhance memory consolidation [228], and emotional and habit learning [229, 230] in healthy individuals. However, the short-term administration of SIAGCs has also been shown to adversely affect memory performance in both adults [217, 231] and children [232]. Several studies in healthy subjects have reported poor long-term memory retrieval [233, 234] (mainly impairments in autobiographical memory [235, 236] and recall performance of verbal material) after acute challenge with HC [118]. On the other hand, attention, vigilance [237], working memory, and verbal executive functions [234, 238] seem to be less affected by eGC administration. The discrepancy between these findings might reflect different designs, small sample sizes, and an overall heterogeneity of the studies.
In this regard, a meta-analysis with a total of 563 healthy volunteers identified two possible factors influencing the relationship between eGC administration and memory. First, administering eGCs before learning was not found to have a significant effect on memory, whereas when given prior to information recall, a significant mnemonic impairment was seen [118], mainly affecting the retrieval of declarative memory [233, 239].
Second, the time of the day in which eGCs are administered appears to be an important determinant of their cognitive effects. Specifically, administration of modest doses (i.e., hydrocortisone 20–40 mg) before cognitive testing in the afternoon results in mild memory enhancement [240], whereas a morning administration (a time in which GRs are already partially saturated by higher endogenous cortisol levels) is associated with memory impairment [118], likely due to the oversaturation of GRs [241].
The cognitive alterations in patients receiving eGCs resemble those observed in patients with CS (impaired memory and verbal learning) [45]. However, in the latter group, cognitive impairment is typically persistent, whereas the extent of the reversibility of mnemonic impairments in patients receiving eGCs is still a matter of debate. While several reports have documented complete cognitive recovery within weeks of eGCs’ discontinuation [242, 243], others observed a long-term persistence of mild cognitive decrement up to 1 year following suspension [217, 244], a divergence that may be attributable to the different duration and fluctuations in eGC exposure [45].
Another concern is whether eGCs’ administration might affect brain structure. In agreement with older evidence [245, 246], recent investigations have documented the incidence of hippocampal and cerebral atrophy following eGC treatment. Notably, brain atrophy has been reported to occur shortly after the acute administration of high-dose methylprednisolone (1 g daily for 3 consecutive days) in patients with multiple sclerosis, with reduced brain volume being observed up to 2 months following intravenous infusion [247, 248]. However, current evidence is still limited and often inconsistent [219, 245, 249], with early studies suggesting a potential correlation between the degree of brain atrophy with eGC dose [245]. Moreover, a clear recovery of brain architecture following treatment discontinuation has been documented in some, but not all patients [245, 250]. To date, no reliable predictors for structural brain recovery have been identified.
A correlation between treatment duration and degree of morphological alteration has been suggested in an MRI and proton magnetic resonance spectroscopy study on 17 patients on long-term prescription with PRED therapy (≥ 10 mg/day for ≥ 6 months). Compared to matched controls, patients with longer treatment durations had smaller HVs, atrophy of the amygdala, and decreased neuronal vitality [219]. In line with these findings, multiple functional imaging studies have confirmed that cortisone administration significantly reduces blood flow and glucose metabolism in memory-related brain regions (such as the posterior-medial temporal lobe [251] and hippocampus [252, 253]) and exert detrimental effects on the excitability, structure, and functionality of the pre-frontal cortex, with a related impairment of working memory [254–258].
It might be expected that longer exposure to eGC treatment would result in more severe cognitive impairment. However, the actual impact of treatment duration on memory function is unclear. Early work [220] evaluated cognition in patients receiving chronic PRED treatment (16.4 mg/day for more than one year). In accordance with similar findings in later studies [259, 260], patients receiving chronic eGC treatment performed significantly worse than controls on hippocampal-dependent memory tasks. However, in these studies, memory impairment was not influenced by the duration of treatment. A recent double-blind, placebo-controlled, crossover study compared cognitive function and brain MRI morphology in patients with rheumatoid arthritis, either treated with chronic PRED therapy (7.5 mg/day for 5 years) or without eGCs. No difference was found between the two groups regarding memory performance or HV. Interestingly, acute PRED challenge before cognitive testing resulted in impaired delayed verbal memory recall in both groups, suggesting that acute, rather than chronic, exposure is responsible for memory deficits in these contexts [239]. Collectively, further evidence is needed to clarify the role of treatment duration in the pathophysiology of eGCs-induced cognitive impairment.
It is interesting to speculate that aging might influence the brain’s susceptibility to eGC-induced cognitive decline. Indeed, greater memory decline [220, 237] following exposure to eGCs has been described in older, when compared to younger patients. However, this was not confirmed in another study [261] in which younger subjects showed impaired short-term working memory tests, suggesting that older individuals might be less responsive to acute GC challenge due to the physiological atrophy of the frontal lobe found in these subjects [261]. There is, therefore, currently no conclusive evidence as to the predictive role of age in eGC-induced cognitive decline.
In contrast, there is a much clearer association between eGCs doses and the development of cognitive decline and neuropsychiatric symptoms [45, 216]. APSEs rarely occur at PRED-equivalent doses of < 20 mg/day [262], but doses above 40 mg PRED-equivalent per day exhibit the highest risk of acute events [263] up to a proper “steroid induced psychosis”. This is a quite difficult condition to manage, and a multidisciplinary approach (including dedicated support from psychiatrists) is strongly suggested. The most effective treatment consists of a combined strategy: both GC dose reduction or discontinuation (if possible) and administration of antipsychotic medications are required for the restitutio ad integrum of negative or delusional and hallucinatory symptoms. Among the antipsychotic drugs, haloperidol and risperidone have exhibited the best efficacy profile [264, 265] with symptoms often resolving in few weeks [262]. Liver and kidney diseases and function need to be carefully assessed while examining the safety profile of the antipsychotic drug. Quetiapine, aripiprazole or olanzapine, as well as mood stabilizers, selective serotonin re-uptake inhibitors, can be considered as second-line treatments [264, 265].
Regarding cognitive functioning, the relation between SIAGC dose and memory typically follows an inverted U-shaped function [241, 266], facilitating delayed memory retrieval at a threshold dose of 20 mg/day of hydrocortisone (which mirrors the physiological cortisol increases during mild stress), with higher doses resulting in impaired cognitive function [241].
Early reports documented a striking dose–response correlation between PRED dose and APSEs in hospitalized patients [267]. High eGC dosing (> 160 mg of hydrocortisone equivalent) induces a reversible but significant decrease in declarative and autobiographical memory [237, 268, 269]. The same effects were confirmed in healthy volunteers receiving high-dose intravenous hydrocortisone (0.45 mg/kg/day) compared to those under lower dose (0.15 mg/kg/day) [270]. Similarly, the administration of 40 mg/day of oral hydrocortisone worsens delayed recall performances compared to 20 mg/day [241].
Interestingly, higher eGC doses have also been associated with earlier symptom appearance, with memory deficits occurring within 3–5 days from administration [268, 271] and quicker recovery following medication withdrawal [272].eGCs also affect emotional memory retention in a dose-dependent fashion: low-to-moderate eGCs doses (< 20 mg/day) have been shown to increase inhibition of negative emotional stimuli [273, 274], whereas higher doses (40 mg/day) facilitate the experience of negative emotions [275, 276].
It is important to note that, aside of the mentioned direct detrimental effects on cognitive function caused by eGC administration, tapering down longstanding supraphysiological dose when the underlying disease has subsided or is well controlled with alternative non-GC medications, often results in an enigmatic phenomenon referred to as the glucocorticoid withdrawal syndrome (GWS) [277, 278]. This syndrome represents a unique challenge for the endocrinologist and manifests with symptoms resembling AI, often including irritability, mood swings, and psychiatric symptoms (depression, anxiety, panic attacks, up to psychotic state) [85, 278, 279]. The mechanisms behind GWS probably depend on the developed dependence on supraphysiological GC concentrations but are still not entirely understood. The pathogenesis seems to be multifactorial and, among the proposed mechanisms, the downregulation of CRH and proopiomelanocortin, as well as the upregulation of mediators such as vasopressin, central noradrenergic, and dopaminergic systems, seem to mediate cognitive disruption [280]. Indeed, an intact CRH system in the brain is necessary for adequate mesolimbic dopaminergic function; its alteration contributes to inadequate stimulation of dopaminergic neurons terminating in the nucleus accumbens, fuelling anxiety and depression [280]. In this context, there are no studies defining possible predictors for GWS development. Albeit there are data demonstrating possible predictor for recovery of the HPA axis in patients treated with chronic eGC [281, 282], studies investigating the effects of different titrating protocols on GWS development have so far been inconclusive [283] and, as such, an individualized approach is needed. Cognitive therapy in parallel with antidepressants (fluoxetine, sertraline, and trazodone) can be helpful to target specific patient symptoms and improve mood [284].
In conclusion, eGCs influence cognitive function, most notably impairing declarative, hippocampus-dependent memory. Chronic administration generally results in memory impairment; however, short-acting formulations can exert variable cognitive effects, depending on dosage and administration timing. The contribution of age, treatment dose, and duration have yet to be clearly established. The hippocampus, amygdala, and pre-frontal cortex are particularly affected by eGC excess and display structural alterations, that appear to be only partially reversible following treatment discontinuation.
A summary of the current evidence for cognitive function during eGC is shown in Table 1.
Conclusions
The HPA axis exerts important actions on the CNS in governing the physiological interactions between different brain areas involved in the cognition processes. GC fluctuation regulates a wide range of cognitive functions through a controlled interaction with GR and MR, guaranteed by substrate availability and receptor distribution. Any alterations in these complex processes can result in cognitive dysfunction. Due to the broad spectrum of unspecific symptoms complained by the patients, the recognition of cognitive deficit in patients with GC disorders is challenging, often delayed, or mistaken. Aside from neuropsychiatric symptoms, both hyper- and hypocortisolism, as well as exogenous steroid treatment, can all affect cognitive function (impacting mostly, but not only, memory), being the limbic system the more GC-sensitive brain area. A prompt recognition and treatment of underlying disease might be crucial to avoid permanent damage, albeit the resolution of hormonal imbalance does not guarantee complete recovery. Several authors are spending efforts to find possible pathogenetic factors and predictors for cognitive recovery after treatment. The contribution of absolute cortisol levels, the duration of exposure to altered cortisol concentrations or fluctuations, the balance between MR/GR activation, and the potential role of the androgen levels in CAH are all possible players involved in the damage. Sadly, to date, there are no accurate predictors for cognitive recovery following disease remission. Further studies are needed to find possible mechanisms involved to be targeted for future treatment strategies with the aim of a tailored precision-medicine approach.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
DDA and RP: conception and design of the study; DDA and DF: acquisition of data; DDA, DF, and RP: drafting the article; AMI, JWT, RP, and GM: revising the article critically for important intellectual content. All the authors: final approval of the version to be submitted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Human participants or animal rights
The study does not involve human participants and/or animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takahashi K, Totsune K, Sone M, Murakami O, Satoh F, Arihara Z, et al. Regional distribution of urocortin-like immunoreactivity and expression of urocortin mRNA in the human brain. Peptides. 1998;19(4):643–647. doi: 10.1016/S0196-9781(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 2.Claes SJ. Corticotropin-releasing hormone (CRH) in psychiatry: from stress to psychopathology. Ann Med. 2004;36(1):50–61. doi: 10.1080/07853890310017044. [DOI] [PubMed] [Google Scholar]
- 3.Keck ME, Holsboer F, Muller MB. Mouse mutants for the study of corticotropin-releasing hormone receptor function: development of novel treatment strategies for mood disorders. Ann N Y Acad Sci. 2004;1018:445–457. doi: 10.1196/annals.1296.055. [DOI] [PubMed] [Google Scholar]
- 4.Gravanis A, Margioris AN. The corticotropin-releasing factor (CRF) family of neuropeptides in inflammation: potential therapeutic applications. Curr Med Chem. 2005;12(13):1503–1512. doi: 10.2174/0929867054039008. [DOI] [PubMed] [Google Scholar]
- 5.Smagin GN, Heinrichs SC, Dunn AJ. The role of CRH in behavioral responses to stress. Peptides. 2001;22(5):713–724. doi: 10.1016/S0196-9781(01)00384-9. [DOI] [PubMed] [Google Scholar]
- 6.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 7.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 8.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 9.Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20(1):49–65. doi: 10.1038/s41583-018-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Kloet ER, Sutanto W, van den Berg DT, Carey MP, van Haarst AD, Hornsby CD, et al. Brain mineralocorticoid receptor diversity: functional implications. J Steroid Biochem Mol Biol. 1993;47(1–6):183–190. doi: 10.1016/0960-0760(93)90073-6. [DOI] [PubMed] [Google Scholar]
- 11.Arriza JL, Simerly RB, Swanson LW, Evans RM. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988;1(9):887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- 12.de Kloet ER, Van Acker SA, Sibug RM, Oitzl MS, Meijer OC, Rahmouni K, et al. Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int. 2000;57(4):1329–1336. doi: 10.1046/j.1523-1755.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- 13.de Kloet ER, Otte C, Kumsta R, Kok L, Hillegers MH, Hasselmann H, et al. Stress and depression: a crucial role of the mineralocorticoid receptor. J Neuroendocrinol. 2016 doi: 10.1111/jne.12379. [DOI] [PubMed] [Google Scholar]
- 14.Wingard JC, Packard MG. The amygdala and emotional modulation of competition between cognitive and habit memory. Behav Brain Res. 2008;193(1):126–131. doi: 10.1016/j.bbr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Vogel S, Klumpers F, Schroder TN, Oplaat KT, Krugers HJ, Oitzl MS, et al. Stress induces a shift towards striatum-dependent stimulus-response learning via the mineralocorticoid receptor. Neuropsychopharmacology. 2017;42(6):1262–1271. doi: 10.1038/npp.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes MC, Yau JL, Kotelevtsev Y, Mullins JJ, Seckl JR. 11 Beta-hydroxysteroid dehydrogenases in the brain: two enzymes two roles. Ann N Y Acad Sci. 2003;1007:357–366. doi: 10.1196/annals.1286.035. [DOI] [PubMed] [Google Scholar]
- 17.Konstantakou P, Mastorakos G, Vrachnis N, Tomlinson JW, Valsamakis G. Dysregulation of 11beta-hydroxysteroid dehydrogenases: implications during pregnancy and beyond. J Matern Fetal Neonatal Med. 2017;30(3):284–293. doi: 10.3109/14767058.2016.1171308. [DOI] [PubMed] [Google Scholar]
- 18.Gathercole LL, Lavery GG, Morgan SA, Cooper MS, Sinclair AJ, Tomlinson JW, et al. 11beta-hydroxysteroid dehydrogenase 1: translational and therapeutic aspects. Endocr Rev. 2013;34(4):525–555. doi: 10.1210/er.2012-1050. [DOI] [PubMed] [Google Scholar]
- 19.Stewart PM, Whorwood CB, Mason JI. Type 2 11 beta-hydroxysteroid dehydrogenase in foetal and adult life. J Steroid Biochem Mol Biol. 1995;55(5–6):465–471. doi: 10.1016/0960-0760(95)00195-6. [DOI] [PubMed] [Google Scholar]
- 20.Alfaidy N, Li W, MacIntosh T, Yang K, Challis J. Late gestation increase in 11beta-hydroxysteroid dehydrogenase 1 expression in human fetal membranes: a novel intrauterine source of cortisol. J Clin Endocrinol Metab. 2003;88(10):5033–5038. doi: 10.1210/jc.2002-021915. [DOI] [PubMed] [Google Scholar]
- 21.Pofi R, Tomlinson JW. Glucocorticoids in pregnancy. Obstet Med. 2020;13(2):62–69. doi: 10.1177/1753495X19847832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funder JW. Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242(4878):583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 23.Rajan V, Edwards CR, Seckl JR. 11 beta-hydroxysteroid dehydrogenase in cultured hippocampal cells reactivates inert 11-dehydrocorticosterone, potentiating neurotoxicity. J Neurosci. 1996;16(1):65–70. doi: 10.1523/JNEUROSCI.16-01-00065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Sanchez EP. Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis. Steroids. 2014;91:20–31. doi: 10.1016/j.steroids.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 26.Otte C, Moritz S, Yassouridis A, Koop M, Madrischewski AM, Wiedemann K, et al. Blockade of the mineralocorticoid receptor in healthy men: effects on experimentally induced panic symptoms, stress hormones, and cognition. Neuropsychopharmacology. 2007;32(1):232–238. doi: 10.1038/sj.npp.1301217. [DOI] [PubMed] [Google Scholar]
- 27.Piber D, Schultebraucks K, Mueller SC, Deuter CE, Wingenfeld K, Otte C. Mineralocorticoid receptor stimulation effects on spatial memory in healthy young adults: a study using the virtual Morris Water Maze task. Neurobiol Learn Mem. 2016;136:139–146. doi: 10.1016/j.nlm.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Lopez JF, Chalmers DT, Little KY, Watson SJ, A.E. Bennett Research Award Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43(8):547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 29.Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM. Genetic selection for coping style predicts stressor susceptibility. J Neuroendocrinol. 2003;15(3):256–267. doi: 10.1046/j.1365-2826.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- 30.Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4(3):965–994. doi: 10.1002/cphy.c130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma ST, Nieman LK, Feelders RA. Cushing's syndrome: epidemiology and developments in disease management. Clin Epidemiol. 2015;7:281–293. doi: 10.2147/CLEP.S44336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pivonello R, De Martino M, De Leo M, Tauchmanovà L, Faggiano A, Lombardi G, et al. Cushing’s syndrome: aftermath of the cure. Arq Bras Endocrinol Metabol. 2007;51(8):1381–1391. doi: 10.1590/s0004-27302007000800025. [DOI] [PubMed] [Google Scholar]
- 35.Scaroni C, Albiger NM, Palmieri S, Iacuaniello D, Graziadio C, Damiani L, et al. Approach to patients with pseudo-Cushing's states. Endocr Connect. 2020;9(1):R1–R13. doi: 10.1530/EC-19-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, et al. Consensus on diagnosis and management of Cushing's disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847–875. doi: 10.1016/S2213-8587(21)00235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BMK, Colao A. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611–629. doi: 10.1016/s2213-8587(16)00086-3. [DOI] [PubMed] [Google Scholar]
- 38.Pivonello R, Simeoli C, De Martino MC, Cozzolino A, De Leo M, Iacuaniello D, et al. Neuropsychiatric disorders in Cushing's syndrome. Front Neurosci. 2015;9:129. doi: 10.3389/fnins.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernini G, Tricò D. Cushing’s syndrome and steroid dementia. Recent Pat Endocr Metab Immune Drug Discovery. 2016;10(1):50–55. doi: 10.2174/1872214810666160809113021. [DOI] [PubMed] [Google Scholar]
- 40.Sonino N, Fava GA. Psychiatric disorders associated with Cushing’s syndrome. CNS Drugs. 2001;15(5):361–73. doi: 10.2165/00023210-200115050-00003. [DOI] [PubMed] [Google Scholar]
- 41.Pertichetti M, Serioli S, Belotti F, Mattavelli D, Schreiber A, Cappelli C, et al. Pituitary adenomas and neuropsychological status: a systematic literature review. Neurosurg Rev. 2020;43(4):1065–1078. doi: 10.1007/s10143-019-01134-z. [DOI] [PubMed] [Google Scholar]
- 42.Marsh L, Guinan E, Shah E, Powell M, Lowy C, Kopelman MD. A prospective study of the cognitive and psychiatric effects of pituitary tumours and their treatments. J Clin Neurosci. 2020;75:122–127. doi: 10.1016/j.jocn.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Malik O, Westphal B. A role for selective serotonin reuptake inhibitors in the management of residual cognitive dysfunction in pediatric Cushing's disease. J Child Adolesc Psychopharmacol. 2013;23(1):65–69. doi: 10.1089/cap.2012.0037. [DOI] [PubMed] [Google Scholar]
- 44.Starkman MN, Giordani B, Berent S, Schork MA, Schteingart DE. Elevated cortisol levels in Cushing’s disease are associated with cognitive decrements. Psychosom Med. 2001;63(6):985–993. doi: 10.1097/00006842-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Starkman MN. Neuropsychiatric findings in Cushing syndrome and exogenous glucocorticoid administration. Endocrinol Metab Clin North Am. 2013;42(3):477–488. doi: 10.1016/j.ecl.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Mauri M, Sinforiani E, Bono G, Vignati F, Berselli ME, Attanasio R, et al. Memory impairment in Cushing’s disease. Acta Neurol Scand. 1993;87(1):52–55. doi: 10.1111/j.1600-0404.1993.tb04075.x. [DOI] [PubMed] [Google Scholar]
- 47.Frimodt-Moller KE, Mollegaard Jepsen JR, Feldt-Rasmussen U, Krogh J. Hippocampal volume, cognitive functions, depression, anxiety, and quality of life in patients with Cushing syndrome. J Clin Endocrinol Metab. 2019;104(10):4563–4577. doi: 10.1210/jc.2019-00749. [DOI] [PubMed] [Google Scholar]
- 48.Resmini E, Santos A, Gomez-Anson B, Vives Y, Pires P, Crespo I, et al. Verbal and visual memory performance and hippocampal volumes, measured by 3-Tesla magnetic resonance imaging, in patients with Cushing's syndrome. J Clin Endocrinol Metab. 2012;97(2):663–671. doi: 10.1210/jc.2011-2231. [DOI] [PubMed] [Google Scholar]
- 49.Forget H, Lacroix A, Somma M, Cohen H. Cognitive decline in patients with Cushing’s syndrome. J Int Neuropsychol Soc. 2000;6(1):20–9. doi: 10.1017/s1355617700611037. [DOI] [PubMed] [Google Scholar]
- 50.Forget H, Lacroix A, Bourdeau I, Cohen H. Long-term cognitive effects of glucocorticoid excess in Cushing's syndrome. Psychoneuroendocrinology. 2016;65:26–33. doi: 10.1016/j.psyneuen.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 51.Starkman MN, Schteingart DE. Neuropsychiatric manifestations of patients with Cushing's syndrome. Arch Intern Med. 1981;141(2):215–219. doi: 10.1001/archinte.1981.00340020077021. [DOI] [PubMed] [Google Scholar]
- 52.Morelli V, Ghielmetti A, Caldiroli A, Grassi S, Siri FM, Caletti E, et al. Mental health in patients with adrenal incidentalomas: Is there a relation with different degrees of cortisol secretion? J Clin Endocrinol Metab. 2021;106(1):e130–e139. doi: 10.1210/clinem/dgaa695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu MS, Tian ZY, Zhang Z, Yang F, Lou Y, Wang YJ, et al. Impaired cognitive function in patients with autonomous cortisol secretion in adrenal incidentalomas. J Clin Endocrinol Metab. 2023;108(3):633–641. doi: 10.1210/clinem/dgac603. [DOI] [PubMed] [Google Scholar]
- 54.Michaud K, Forget H, Cohen H. Chronic glucocorticoid hypersecretion in Cushing's syndrome exacerbates cognitive aging. Brain Cogn. 2009;71(1):1–8. doi: 10.1016/j.bandc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56(8):1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- 56.Guldiken S, Guldiken B. Subclinical Cushing's syndrome is a potential cause of metabolic dementia and rapidly progressive Alzheimer-type dementia. Med Hypotheses. 2008;71(5):703–705. doi: 10.1016/j.mehy.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 57.de Quervain DJ, Poirier R, Wollmer MA, Grimaldi LM, Tsolaki M, Streffer JR, et al. Glucocorticoid-related genetic susceptibility for Alzheimer's disease. Hum Mol Genet. 2004;13(1):47–52. doi: 10.1093/hmg/ddg361. [DOI] [PubMed] [Google Scholar]
- 58.Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32(9):756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 59.Patil CG, Lad SP, Katznelson L, Laws ER. Brain atrophy and cognitive deficits in Cushing's disease. Neurosurg Focus. 2007;23(3):1–4. doi: 10.3171/foc-07/09/e11. [DOI] [PubMed] [Google Scholar]
- 60.Andela CD, van Haalen FM, Ragnarsson O, Papakokkinou E, Johannsson G, Santos A, et al. Mechanisms in endocrinology: Cushing's syndrome causes irreversible effects on the human brain: a systematic review of structural and functional magnetic resonance imaging studies. Eur J Endocrinol. 2015;173(1):R1–14. doi: 10.1530/EJE-14-1101. [DOI] [PubMed] [Google Scholar]
- 61.Bauduin S, van der Wee NJA, van der Werff SJA. Structural brain abnormalities in Cushing's syndrome. Curr Opin Endocrinol Diabetes Obes. 2018;25(4):285–289. doi: 10.1097/MED.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 62.Bourdeau I, Bard C, Bernard N, Leclerc I, Cordeau M, Be' Lair M, et al. Loss of brain volume in endogenous Cushing’s syndrome and its reversibility after correction of hypercortisolism. J Clin Endocrinol Metab. 2002;87(5):1949–1954. doi: 10.1210/jcem.87.5.8493. [DOI] [PubMed] [Google Scholar]
- 63.Bourdeau I, Bard C, Forget H, Boulanger Y, Cohen H, Lacroix A. Cognitive function and cerebral assessment in patients who have Cushing's syndrome. Endocrinol Metab Clin North Am. 2005;34(2):357–369. doi: 10.1016/j.ecl.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 64.Santos A, Resmini E, Crespo I, Pires P, Vives-Gilabert Y, Granell E, et al. Small cerebellar cortex volume in patients with active Cushing's syndrome. Eur J Endocrinol. 2014;171(4):461–469. doi: 10.1530/EJE-14-0371. [DOI] [PubMed] [Google Scholar]
- 65.Jiang H, Ren J, He N-Y, Liu C, Sun Y-H, Jian F-F, et al. Volumetric magnetic resonance imaging analysis in patients with short-term remission of Cushing's disease. Clin Endocrinol. 2017;87(4):367–374. doi: 10.1111/cen.13381. [DOI] [PubMed] [Google Scholar]
- 66.Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498(3):363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- 67.Tirosh A, RaviPrakash H, Papadakis GZ, Tatsi C, Belyavskaya E, Charalampos L, et al. Computerized analysis of brain MRI parameter dynamics in young patients with Cushing syndrome-a case-control study. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pires P, Santos A, Vives-Gilabert Y, Webb SM, Sainz-Ruiz A, Resmini E, et al. White matter alterations in the brains of patients with active, remitted, and cured Cushing syndrome: a DTI study. AJNR Am J Neuroradiol. 2015;36(6):1043–1048. doi: 10.3174/ajnr.A4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui M, Zhou T, Feng S, Liu X, Wang F, Zhang Y, et al. Altered microstructural pattern of white matter in Cushing's disease identified by automated fiber quantification. Neuroimage Clin. 2021;31:102770. doi: 10.1016/j.nicl.2021.102770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pires P, Santos A, Vives-Gilabert Y, Webb SM, Sainz-Ruiz A, Resmini E, et al. White matter involvement on DTI-MRI in Cushing's syndrome relates to mood disturbances and processing speed: a case-control study. Pituitary. 2017;20(3):340–348. doi: 10.1007/s11102-017-0793-y. [DOI] [PubMed] [Google Scholar]
- 71.Jiang H, He NY, Sun YH, Jian FF, Bian LG, Shen JK, et al. Altered gray and white matter microstructure in Cushing's disease: A diffusional kurtosis imaging study. Brain Res. 2017;1665:80–87. doi: 10.1016/j.brainres.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 72.Topiwala A, Ebmeier KP. Effects of drinking on late-life brain and cognition. Evid Based Ment Health. 2018;21(1):12–15. doi: 10.1136/eb-2017-102820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Res Health. 2003;27(2):146–152. [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson S, Bair JL, Thomas KM, Iacono WG. Problematic alcohol use and reduced hippocampal volume: a meta-analytic review. Psychol Med. 2017;47(13):2288–2301. doi: 10.1017/S0033291717000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34(4):468–483. [PMC free article] [PubMed] [Google Scholar]
- 76.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- 77.Sousa GMJ, Vargas HDQ, Barbosa FF, Galvao-Coelho NL. Stress, memory, and implications for major depression. Behav Brain Res. 2021;412:113410. doi: 10.1016/j.bbr.2021.113410. [DOI] [PubMed] [Google Scholar]
- 78.Hemmingsen SD, Wesselhoeft R, Lichtenstein MB, Sjogren JM, Stoving RK. Cognitive improvement following weight gain in patients with anorexia nervosa: a systematic review. Eur Eat Disord Rev. 2021;29(3):402–426. doi: 10.1002/erv.2796. [DOI] [PubMed] [Google Scholar]
- 79.Keeler J, Patsalos O, Thuret S, Ehrlich S, Tchanturia K, Himmerich H, et al. Hippocampal volume, function, and related molecular activity in anorexia nervosa: a scoping review. Expert Rev Clin Pharmacol. 2020;13(12):1367–1387. doi: 10.1080/17512433.2020.1850256. [DOI] [PubMed] [Google Scholar]
- 80.Gaudio S, Wiemerslage L, Brooks SJ, Schioth HB. A systematic review of resting-state functional-MRI studies in anorexia nervosa: evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neurosci Biobehav Rev. 2016;71:578–589. doi: 10.1016/j.neubiorev.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 81.Resmini E, Santos A, Aulinas A, Webb SM, Vives-Gilabert Y, Cox O, et al. Reduced DNA methylation of FKBP5 in Cushing's syndrome. Endocrine. 2016;54(3):768–777. doi: 10.1007/s12020-016-1083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kramlinger KG, Peterson GC, Watson PK, Leonard LL. Metyrapone for depression and delirium secondary to Cushing's syndrome. Psychosomatics. 1985;26(1):67–71. doi: 10.1016/s0033-3182(85)72906-4. [DOI] [PubMed] [Google Scholar]
- 83.Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C, et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing's syndrome. J Clin Endocrinol Metab. 2012;97(6):2039–2049. doi: 10.1210/jc.2011-3350. [DOI] [PubMed] [Google Scholar]
- 84.van der Lely AJ, Foeken K, van der Mast RC, Lamberts SWJ. Rapid reversal of acute psychosis in the Cushing syndrome with the cortisol-receptor antagonist mifepristone (RU 486) Ann Intern Med. 1991;114(2):143–144. doi: 10.7326/0003-4819-114-2-143. [DOI] [PubMed] [Google Scholar]
- 85.Dorn LD, Burgess ES, Firedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82(3):912–919. doi: 10.1210/jcem.82.3.3834. [DOI] [PubMed] [Google Scholar]
- 86.Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing's syndrome. Clin Endocrinol (Oxf) 1996;45(6):715–720. doi: 10.1046/j.1365-2265.1996.8690878.x. [DOI] [PubMed] [Google Scholar]
- 87.Starkman MN, Schteingart DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177–188. doi: 10.1016/0165-1781(86)90096-x. [DOI] [PubMed] [Google Scholar]
- 88.Hook JN, Giordani B, Schteingart DE, Guire K, Giles J, Kelley R, et al. Patterns of cognitive change over time and relationship to age following successful treatment of Cushing’s disease. J Int Neuropsychol Soc. 2007;13(1):21–29. doi: 10.1017/S1355617707070051. [DOI] [PubMed] [Google Scholar]
- 89.Levitsky LL. Cognitive dysfunction following treatment of Cushing's syndrome. Nat Clin Pract Endocrinol Metab. 2006;2(12):666–667. doi: 10.1038/ncpendmet0344. [DOI] [PubMed] [Google Scholar]
- 90.Pupier E, Santos A, Etchamendy N, Lavielle A, Ferriere A, Marighetto A, et al. Impaired quality of life, but not cognition, is linked to a history of chronic hypercortisolism in patients with Cushing's disease in remission. Front Endocrinol (Lausanne) 2022;13:934347. doi: 10.3389/fendo.2022.934347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forget H, Lacroix A, Cohen H. Persistent cognitive impairment following surgical treatment of Cushing’s syndrome. Psychoneuroendocrinology. 2002;27(3):367–83. doi: 10.1016/s0306-4530(01)00059-2. [DOI] [PubMed] [Google Scholar]
- 92.Ragnarsson O, Berglund P, Eder DN, Johannsson G. Long-term cognitive impairments and attentional deficits in patients with Cushing's disease and cortisol-producing adrenal adenoma in remission. J Clin Endocrinol Metab. 2012;97(9):E1640–E1648. doi: 10.1210/jc.2012-1945. [DOI] [PubMed] [Google Scholar]
- 93.Papakokkinou E, Johansson B, Berglund P, Ragnarsson O. Mental fatigue and executive dysfunction in patients with Cushing's syndrome in remission. Behav Neurol. 2015;2015:173653. doi: 10.1155/2015/173653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heald A, Parr C, Gibson C, O'Driscoll K, Fowler H. A cross-sectional study to investigate long-term cognitive function in people with treated pituitary Cushing's disease. Exp Clin Endocrinol Diabetes. 2006;114(9):490–497. doi: 10.1055/s-2006-924332. [DOI] [PubMed] [Google Scholar]
- 95.Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Soc Biol Psychiatry. 1999;46(12):1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 96.Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in learning associated with increase in hippocampal formation volume. Soc Biol Psychiatry. 2003 doi: 10.1016/S0002-3223(02)01750-X. [DOI] [PubMed] [Google Scholar]
- 97.Colwell MJ, Tagomori H, Chapman S, Gillespie AL, Cowen PJ, Harmer CJ, et al. Pharmacological targeting of cognitive impairment in depression: recent developments and challenges in human clinical research. Transl Psychiatry. 2022;12(1):484. doi: 10.1038/s41398-022-02249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crespo I, Esther GM, Santos A, Valassi E, Yolanda VG, De Juan-Delago M, et al. Impaired decision-making and selective cortical frontal thinning in Cushing's syndrome. Clin Endocrinol (Oxf) 2014;81(6):826–833. doi: 10.1111/cen.12564. [DOI] [PubMed] [Google Scholar]
- 99.Andela CD, van der Werff SJ, Pannekoek JN, van den Berg SM, Meijer OC, van Buchem MA, et al. Smaller grey matter volumes in the anterior cingulate cortex and greater cerebellar volumes in patients with long-term remission of Cushing's disease: a case-control study. Eur J Endocrinol. 2013;169(6):811–819. doi: 10.1530/EJE-13-0471. [DOI] [PubMed] [Google Scholar]
- 100.Bauduin S, van der Pal Z, Pereira AM, Meijer OC, Giltay EJ, van der Wee NJA, et al. Cortical thickness abnormalities in long-term remitted Cushing's disease. Transl Psychiatry. 2020;10(1):293. doi: 10.1038/s41398-020-00980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hou B, Gao L, Shi L, Luo Y, Guo X, Young GS, et al. Reversibility of impaired brain structures after transsphenoidal surgery in Cushing's disease: a longitudinal study based on an artificial intelligence-assisted tool. J Neurosurg. 2020 doi: 10.3171/2019.10.JNS191400. [DOI] [PubMed] [Google Scholar]
- 102.Resmini E, Santos A, Gomez-Anson B, Lopez-Mourelo O, Pires P, Vives-Gilabert Y, et al. Hippocampal dysfunction in cured Cushing's syndrome patients, detected by (1) H-MR-spectroscopy. Clin Endocrinol (Oxf) 2013;79(5):700–707. doi: 10.1111/cen.12224. [DOI] [PubMed] [Google Scholar]
- 103.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pofi R, Prete A, Thornton-Jones V, Bryce J, Ali SR, Faisal Ahmed S, et al. Plasma renin measurements are unrelated to mineralocorticoid replacement dose in patients with primary adrenal insufficiency. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgz055. [DOI] [PubMed] [Google Scholar]
- 105.Husebye ES, Pearce SH, Krone NP, Kampe O. Adrenal insufficiency. Lancet. 2021;397(10274):613–629. doi: 10.1016/S0140-6736(21)00136-7. [DOI] [PubMed] [Google Scholar]
- 106.Bleicken B, Hahner S, Loeffler M, Ventz M, Decker O, Allolio B, et al. Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2010;72(3):297–304. doi: 10.1111/j.1365-2265.2009.03596.x. [DOI] [PubMed] [Google Scholar]
- 107.Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, et al. Do corticosteroids damage the brain? J Neuroendocrinol. 2006;18(6):393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 108.Isidori AM, Venneri MA, Graziadio C, Simeoli C, Fiore D, Hasenmajer V, et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(3):173–185. doi: 10.1016/S2213-8587(17)30398-4. [DOI] [PubMed] [Google Scholar]
- 109.Bleicken B, Hahner S, Ventz M, Quinkler M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525–531. doi: 10.1097/MAJ.0b013e3181db6b7a. [DOI] [PubMed] [Google Scholar]
- 110.Johnstone PA, Rundell JR, Esposito M. Mental status changes of Addison's disease. Psychosomatics. 1990;31(1):103–107. doi: 10.1016/S0033-3182(90)72226-8. [DOI] [PubMed] [Google Scholar]
- 111.Henry M, Thomas KG, Ross IL. Episodic memory impairment in Addison's disease: results from a telephonic cognitive assessment. Metab Brain Dis. 2014;29(2):421–430. doi: 10.1007/s11011-014-9511-x. [DOI] [PubMed] [Google Scholar]
- 112.Tiemensma J, Andela CD, Kaptein AA, Romijn JA, van der Mast RC, Biermasz NR, et al. Psychological morbidity and impaired quality of life in patients with stable treatment for primary adrenal insufficiency: cross-sectional study and review of the literature. Eur J Endocrinol. 2014;171(2):171–182. doi: 10.1530/EJE-14-0023. [DOI] [PubMed] [Google Scholar]
- 113.Tytherleigh MY, Vedhara K, Lightman SL. Mineralocorticoid and glucocorticoid receptors and their differential effects on memory performance in people with Addison’s disease. Psychoneuroendocrinology. 2004;29(6):712–723. doi: 10.1016/s0306-4530(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 114.Schultebraucks K, Wingenfeld K, Heimes J, Quinkler M, Otte C. Cognitive function in patients with primary adrenal insufficiency (Addison's disease) Psychoneuroendocrinology. 2015;55:1–7. doi: 10.1016/j.psyneuen.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 115.Tiemensma J, Andela CD, Biermasz NR, Romijn JA, Pereira AM. Mild cognitive deficits in patients with primary adrenal insufficiency. Psychoneuroendocrinology. 2016;63:170–177. doi: 10.1016/j.psyneuen.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 116.Klement J, Hubold C, Hallschmid M, Loeck C, Oltmanns KM, Lehnert H, et al. Effects of glucose infusion on neuroendocrine and cognitive parameters in Addison disease. Metabolism. 2009;58(12):1825–1831. doi: 10.1016/j.metabol.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 117.Harbeck B, Kropp P, Monig H. Effects of short-term nocturnal cortisol replacement on cognitive function and quality of life in patients with primary or secondary adrenal insufficiency: a pilot study. Appl Psychophysiol Biofeedback. 2009;34(2):113–119. doi: 10.1007/s10484-009-9082-5. [DOI] [PubMed] [Google Scholar]
- 118.Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30(8):771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 119.Lupien SJ, Wilkinson CW, Briere S, Ng Ying Kin NM, Meaney MJ, Nair NP. Acute modulation of aged human memory by pharmacological manipulation of glucocorticoids. J Clin Endocrinol Metab. 2002;87(8):3798–3807. doi: 10.1210/jcem.87.8.8760. [DOI] [PubMed] [Google Scholar]
- 120.Minnetti M, Hasenmajer V, Pofi R, Venneri MA, Alexandraki KI, Isidori AM. Fixing the broken clock in adrenal disorders: focus on glucocorticoids and chronotherapy. J Endocrinol. 2020;246(2):R13–R31. doi: 10.1530/JOE-20-0066. [DOI] [PubMed] [Google Scholar]
- 121.de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 122.Henry M, Thomas KGF, Ross IL. Sleep, cognition and cortisol in addison's disease: a mechanistic relationship. Front Endocrinol (Lausanne) 2021;12:694046. doi: 10.3389/fendo.2021.694046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tytherleigh MY, Vedhara K, Lightman SL. Mineralocorticoid and glucocorticoid receptors and their differential effects on memory performance in people with Addison's disease. Psychoneuroendocrinology. 2004;29(6):712–723. doi: 10.1016/S0306-4530(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 124.Schultebraucks K, Wingenfeld K, Otte C, Quinkler M. The role of fludrocortisone in cognition and mood in patients with primary adrenal insufficiency (Addison's disease) Neuroendocrinology. 2016;103(3–4):315–320. doi: 10.1159/000438791. [DOI] [PubMed] [Google Scholar]
- 125.Otte C, Wingenfeld K, Kuehl LK, Kaczmarczyk M, Richter S, Quante A, et al. Mineralocorticoid receptor stimulation improves cognitive function and decreases cortisol secretion in depressed patients and healthy individuals. Neuropsychopharmacology. 2015;40(2):386–393. doi: 10.1038/npp.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hinkelmann K, Wingenfeld K, Kuehl LK, Fleischer J, Heuser I, Wiedemann K, et al. Stimulation of the mineralocorticoid receptor improves memory in young and elderly healthy individuals. Neurobiol Aging. 2015;36(2):919–924. doi: 10.1016/j.neurobiolaging.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 127.Wingenfeld K, Kuehl LK, Janke K, Hinkelmann K, Eckert FC, Roepke S, et al. Effects of mineralocorticoid receptor stimulation via fludrocortisone on memory in women with borderline personality disorder. Neurobiol Learn Mem. 2015;120:94–100. doi: 10.1016/j.nlm.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 128.Otte C, Wingenfeld K, Kuehl LK, Richter S, Regen F, Piber D, et al. Cognitive function in older adults with major depression: effects of mineralocorticoid receptor stimulation. J Psychiatr Res. 2015;69:120–125. doi: 10.1016/j.jpsychires.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 129.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 130.Shansky RM, Lipps J. Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front Hum Neurosci. 2013;7:123. doi: 10.3389/fnhum.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sapolsky RM. Glucocorticoid toxicity in the hippocampus: reversal by supplementation with brain fuels. J Neurosci. 1986;6(8):2240–2244. doi: 10.1523/JNEUROSCI.06-08-02240.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blacha AK, Rahvar AH, Flitsch J, van de Loo I, Kropp P, Harbeck B. Impaired attention in patients with adrenal insufficiency—Impact of unphysiological therapy. Steroids. 2021;167:108788. doi: 10.1016/j.steroids.2020.108788. [DOI] [PubMed] [Google Scholar]
- 134.Harbeck B, Danneberg S, Rahvar AH, Haas CS, Lehnert H, Kropp P, et al. Exploring the impact of short- and long-term hydrocortisone replacement on cognitive function, quality of life and catecholamine secretion: a pilot study. Appl Psychophysiol Biofeedback. 2016;41(3):341–347. doi: 10.1007/s10484-016-9338-9. [DOI] [PubMed] [Google Scholar]
- 135.Lovas K, Husebye ES, Holsten F, Bjorvatn B. Sleep disturbances in patients with Addison's disease. Eur J Endocrinol. 2003;148(4):449–456. doi: 10.1530/eje.0.1480449. [DOI] [PubMed] [Google Scholar]
- 136.Gillin JC, Jacobs LS, Snyder F, Henkin RI. Effects of ACTH on the sleep of normal subjects and patients with Addison's disease. Neuroendocrinology. 1974;15(1):21–31. doi: 10.1159/000122289. [DOI] [PubMed] [Google Scholar]
- 137.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60(12):1324–1330. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 138.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 139.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12(5):410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 140.Payne JD, Nadel L. Sleep, dreams, and memory consolidation: the role of the stress hormone cortisol. Learn Mem. 2004;11(6):671–678. doi: 10.1101/lm.77104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bennion KA, Mickley Steinmetz KR, Kensinger EA, Payne JD. Sleep and cortisol interact to support memory consolidation. Cereb Cortex. 2015;25(3):646–657. doi: 10.1093/cercor/bht255. [DOI] [PubMed] [Google Scholar]
- 142.Steiger A. Sleep and the hypothalamo-pituitary-adrenocortical system. Sleep Med Rev. 2002;6(2):125–138. doi: 10.1053/smrv.2001.0159. [DOI] [PubMed] [Google Scholar]
- 143.Groch S, Wilhelm I, Lange T, Born J. Differential contribution of mineralocorticoid and glucocorticoid receptors to memory formation during sleep. Psychoneuroendocrinology. 2013;38(12):2962–2972. doi: 10.1016/j.psyneuen.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 144.Henry M, Wolf PS, Ross IL, Thomas KG. Poor quality of life, depressed mood, and memory impairment may be mediated by sleep disruption in patients with Addison's disease. Physiol Behav. 2015;151:379–385. doi: 10.1016/j.physbeh.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hahner S, Loeffler M, Fassnacht M, Weismann D, Koschker AC, Quinkler M, et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab. 2007;92(10):3912–3922. doi: 10.1210/jc.2007-0685. [DOI] [PubMed] [Google Scholar]
- 146.Mallappa A, Debono M. Recent advances in hydrocortisone replacement treatment. Endocr Dev. 2016;30:42–53. doi: 10.1159/000439329. [DOI] [PubMed] [Google Scholar]
- 147.Garcia-Borreguero D, Wehr TA, Larrosa O, Granizo JJ, Hardwick D, Chrousos GP, et al. Glucocorticoid replacement is permissive for rapid eye movement sleep and sleep consolidation in patients with adrenal insufficiency. J Clin Endocrinol Metab. 2000;85(11):4201–4206. doi: 10.1210/jcem.85.11.6965. [DOI] [PubMed] [Google Scholar]
- 148.Staufenbiel SM, Andela CD, Manenschijn L, Pereira AM, van Rossum EF, Biermasz NR. Increased hair cortisol concentrations and BMI in patients with pituitary-adrenal disease on hydrocortisone replacement. J Clin Endocrinol Metab. 2015;100(6):2456–2462. doi: 10.1210/jc.2014-4328. [DOI] [PubMed] [Google Scholar]
- 149.Henry M, Ross IL, Wolf PSA, Thomas KGF. Impaired quality and efficiency of sleep impairs cognitive functioning in Addison's disease. Psychoneuroendocrinology. 2017;78:237–245. doi: 10.1016/j.psyneuen.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 150.El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194–2210. doi: 10.1016/S0140-6736(17)31431-9. [DOI] [PubMed] [Google Scholar]
- 151.Miller WL. Mechanisms in endocrinology: rare defects in adrenal steroidogenesis. Eur J Endocrinol. 2018;179(3):R125–R141. doi: 10.1530/EJE-18-0279. [DOI] [PubMed] [Google Scholar]
- 152.Bachelot A, Touraine P. Health status of adults with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Presse Med. 2014;43(4 Pt 1):428–437. doi: 10.1016/j.lpm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 153.Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, Arlt W, Auchus RJ, Falhammar H, et al. Congenital Adrenal Hyperplasia-Current Insights in Pathophysiology, Diagnostics, and Management. Endocr Rev. 2022;43(1):91–159. doi: 10.1210/endrev/bnab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Przybylik-Mazurek E, Kurzynska A, Skalniak A, Hubalewska-Dydejczyk A. Current approaches to the diagnosis of classical form of congenital adrenal hyperplasia. Recent Pat Endocr Metab Immune Drug Discov. 2015;9(2):103–110. doi: 10.2174/1872214809666151005123928. [DOI] [PubMed] [Google Scholar]
- 155.Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043–4088. doi: 10.1210/jc.2018-01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Browne WV, Hindmarsh PC, Pasterski V, Hughes IA, Acerini CL, Spencer D, et al. Working memory performance is reduced in children with congenital adrenal hyperplasia. Horm Behav. 2015;67:83–88. doi: 10.1016/j.yhbeh.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Helleday J, Bartfai A, Ritzen EM, Forsman M. General intelligence and cognitive profile in women with congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 1994;19(4):343–356. doi: 10.1016/0306-4530(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 158.Karlsson L, Gezelius A, Nordenstrom A, Hirvikoski T, Lajic S. Cognitive impairment in adolescents and adults with congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 2017;87(6):651–659. doi: 10.1111/cen.13441. [DOI] [PubMed] [Google Scholar]
- 159.Maheu FS, Merke DP, Schroth EA, Keil MF, Hardin J, Poeth K, et al. Steroid abnormalities and the developing brain: declarative memory for emotionally arousing and neutral material in children with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2008;33(2):238–245. doi: 10.1016/j.psyneuen.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Somajni F, Sovera V, Albizzati A, Russo G, Peroni P, Seragni G, et al. Neuropsychological assessment in prepubertal patients with congenital adrenal hyperplasia: preliminary study. Minerva Pediatr. 2011;63(1):1–9. [PubMed] [Google Scholar]
- 161.Amr NH, Baioumi AY, Serour MN, Khalifa A, Shaker NM. Cognitive functions in children with congenital adrenal hyperplasia. Arch Endocrinol Metab. 2019;63(2):113–120. doi: 10.20945/2359-3997000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Strandqvist A, Falhammar H, Lichtenstein P, Hirschberg AL, Wedell A, Norrby C, et al. Suboptimal psychosocial outcomes in patients with congenital adrenal hyperplasia: epidemiological studies in a nonbiased national cohort in Sweden. J Clin Endocrinol Metab. 2014;99(4):1425–1432. doi: 10.1210/jc.2013-3326. [DOI] [PubMed] [Google Scholar]
- 163.Hamed SA, Metwalley KA, Farghaly HS. Cognitive function in children with classic congenital adrenal hyperplasia. Eur J Pediatr. 2018;177(11):1633–1640. doi: 10.1007/s00431-018-3226-7. [DOI] [PubMed] [Google Scholar]
- 164.Johannsen TH, Ripa CP, Reinisch JM, Schwartz M, Mortensen EL, Main KM. Impaired cognitive function in women with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91(4):1376–1381. doi: 10.1210/jc.2005-1959. [DOI] [PubMed] [Google Scholar]
- 165.Nass R, Baker S. Learning disabilities in children with congenital adrenal hyperplasia. J Child Neurol. 1991;6(4):306–312. doi: 10.1177/088307389100600404. [DOI] [PubMed] [Google Scholar]
- 166.Pang S, Levine LS, Lorenzen F, Chow D, Pollack M, Dupont B, et al. Hormonal studies in obligate heterozygotes and siblings of patients with 11 beta-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1980;50(3):586–589. doi: 10.1210/jcem-50-3-586. [DOI] [PubMed] [Google Scholar]
- 167.Hines M. Gender development and the human brain. Annu Rev Neurosci. 2011;34:69–88. doi: 10.1146/annurev-neuro-061010-113654. [DOI] [PubMed] [Google Scholar]
- 168.Lombardo MV, Auyeung B, Pramparo T, Quartier A, Courraud J, Holt RJ, et al. Sex-specific impact of prenatal androgens on social brain default mode subsystems. Mol Psychiatry. 2018 doi: 10.1038/s41380-018-0198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Quartier A, Chatrousse L, Redin C, Keime C, Haumesser N, Maglott-Roth A, et al. Genes and pathways regulated by androgens in human neural cells, potential candidates for the male excess in autism spectrum disorder. Biol Psychiatry. 2018;84(4):239–252. doi: 10.1016/j.biopsych.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 170.Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14(5):636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- 171.McCreary JK, Truica LS, Friesen B, Yao Y, Olson DM, Kovalchuk I, et al. Altered brain morphology and functional connectivity reflect a vulnerable affective state after cumulative multigenerational stress in rats. Neuroscience. 2016;330:79–89. doi: 10.1016/j.neuroscience.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 172.Travers S, Bouvattier C, Fagart J, Martinerie L, Viengchareun S, Pussard E, et al. Interaction between accumulated 21-deoxysteroids and mineralocorticoid signaling in 21-hydroxylase deficiency. Am J Physiol Endocrinol Metab. 2020;318(2):E102–E110. doi: 10.1152/ajpendo.00368.2019. [DOI] [PubMed] [Google Scholar]
- 173.Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- 174.Berenbaum SA, Bryk KL, Beltz AM. Early androgen effects on spatial and mechanical abilities: evidence from congenital adrenal hyperplasia. Behav Neurosci. 2012;126(1):86–96. doi: 10.1037/a0026652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Collaer ML, Brook CG, Conway GS, Hindmarsh PC, Hines M. Motor development in individuals with congenital adrenal hyperplasia: strength, targeting, and fine motor skill. Psychoneuroendocrinology. 2009;34(2):249–258. doi: 10.1016/j.psyneuen.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33(7):973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Collaer ML, Hindmarsh PC, Pasterski V, Fane BA, Hines M. Reduced short term memory in congenital adrenal hyperplasia (CAH) and its relationship to spatial and quantitative performance. Psychoneuroendocrinology. 2016;64:164–173. doi: 10.1016/j.psyneuen.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Messina V, Karlsson L, Hirvikoski T, Nordenstrom A, Lajic S. Cognitive function of children and adolescents with congenital adrenal hyperplasia: importance of early diagnosis. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Debono M, Ross RJ, Newell-Price J. Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy. Eur J Endocrinol. 2009;160(5):719–729. doi: 10.1530/EJE-08-0874. [DOI] [PubMed] [Google Scholar]
- 180.Reisch N, Arlt W, Krone N. Health problems in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2011;76(2):73–85. doi: 10.1159/000327794. [DOI] [PubMed] [Google Scholar]
- 181.Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci. 2005;25(13):3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18(1):15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.O'Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat. 2005;207(3):271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88(4):1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- 186.Webb SM, Santos A, Resmini E, Martinez-Momblan MA, Martel L, Valassi E. Quality of life in Cushing's disease: a long term issue? Ann Endocrinol (Paris) 2018;79(3):132–137. doi: 10.1016/j.ando.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 187.Van't Westeinde A, Karlsson L, Thomsen Sandberg M, Nordenstrom A, Padilla N, Lajic S. Altered Gray matter structure and white matter microstructure in patients with congenital adrenal hyperplasia: relevance for working memory performance. Cereb Cortex. 2020;30(5):2777–2788. doi: 10.1093/cercor/bhz274. [DOI] [PubMed] [Google Scholar]
- 188.Bergamaschi R, Livieri C, Uggetti C, Candeloro E, Egitto MG, Pichiecchio A, et al. Brain white matter impairment in congenital adrenal hyperplasia. Arch Neurol. 2006;63(3):413–416. doi: 10.1001/archneur.63.3.413. [DOI] [PubMed] [Google Scholar]
- 189.Gaudiano C, Malandrini A, Pollazzon M, Murru S, Mari F, Renieri A, et al. Leukoencephalopathy in 21-beta hydroxylase deficiency: report of a family. Brain Dev. 2010;32(5):421–424. doi: 10.1016/j.braindev.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 190.Nass R, Heier L, Moshang T, Oberfield S, George A, New MI, et al. Magnetic resonance imaging in the congenital adrenal hyperplasia population: increased frequency of white-matter abnormalities and temporal lobe atrophy. J Child Neurol. 1997;12(3):181–186. doi: 10.1177/088307389701200306. [DOI] [PubMed] [Google Scholar]
- 191.Eriksson J, Vogel EK, Lansner A, Bergstrom F, Nyberg L. Neurocognitive architecture of working memory. Neuron. 2015;88(1):33–46. doi: 10.1016/j.neuron.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kurek A, Kucharczyk M, Detka J, Slusarczyk J, Trojan E, Glombik K, et al. Pro-apoptotic action of corticosterone in hippocampal organotypic cultures. Neurotox Res. 2016;30(2):225–238. doi: 10.1007/s12640-016-9630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mnif MF, Kamoun M, Mnif F, Charfi N, Kallel N, Rekik N, et al. Brain magnetic resonance imaging findings in adult patients with congenital adrenal hyperplasia: Increased frequency of white matter impairment and temporal lobe structures dysgenesis. Indian J Endocrinol Metab. 2013;17(1):121–127. doi: 10.4103/2230-8210.107833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Herting MM, Azad A, Kim R, Tyszka JM, Geffner ME, Kim MS. Brain differences in the prefrontal cortex, amygdala, and hippocampus in youth with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing's syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(2):739–769. doi: 10.1002/cphy.c130035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH, Joels M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol. 2018;49:124–145. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 197.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav Rev. 2003;27(3):233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 199.Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 200.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23(17):R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Miller WL, Witchel SF. Prenatal treatment of congenital adrenal hyperplasia: risks outweigh benefits. Am J Obstet Gynecol. 2013;208(5):354–359. doi: 10.1016/j.ajog.2012.10.885. [DOI] [PubMed] [Google Scholar]
- 202.Alexander N, Rosenlocher F, Stalder T, Linke J, Distler W, Morgner J, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544. doi: 10.1210/jc.2012-1970. [DOI] [PubMed] [Google Scholar]
- 203.Khalife N, Glover V, Taanila A, Ebeling H, Jarvelin MR, Rodriguez A. Prenatal glucocorticoid treatment and later mental health in children and adolescents. PLoS ONE. 2013;8(11):e81394. doi: 10.1371/journal.pone.0081394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hauser J, Knapman A, Zurcher NR, Pilloud S, Maier C, Diaz-Heijtz R, et al. Effects of prenatal dexamethasone treatment on physical growth, pituitary-adrenal hormones, and performance of motor, motivational, and cognitive tasks in juvenile and adolescent common marmoset monkeys. Endocrinology. 2008;149(12):6343–6355. doi: 10.1210/en.2008-0615. [DOI] [PubMed] [Google Scholar]
- 206.Trautman PD, Meyer-Bahlburg HF, Postelnek J, New MI. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinology. 1995;20(4):439–449. doi: 10.1016/0306-4530(94)00070-0. [DOI] [PubMed] [Google Scholar]
- 207.Hirvikoski T, Nordenstrom A, Lindholm T, Lindblad F, Ritzen EM, Wedell A, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab. 2007;92(2):542–548. doi: 10.1210/jc.2006-1340. [DOI] [PubMed] [Google Scholar]
- 208.Merce Fernandez-Balsells M, Muthusamy K, Smushkin G, Lampropulos JF, Elamin MB, Abu Elnour NO, et al. Prenatal dexamethasone use for the prevention of virilization in pregnancies at risk for classical congenital adrenal hyperplasia because of 21-hydroxylase (CYP21A2) deficiency: a systematic review and meta-analyses. Clin Endocrinol (Oxf) 2010;73(4):436–444. doi: 10.1111/j.1365-2265.2010.03826.x. [DOI] [PubMed] [Google Scholar]
- 209.Meyer-Bahlburg HF, Dolezal C, Haggerty R, Silverman M, New MI. Cognitive outcome of offspring from dexamethasone-treated pregnancies at risk for congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167(1):103–110. doi: 10.1530/EJE-11-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Adcock IM, Mumby S. Glucocorticoids. Handb Exp Pharmacol. 2017;237:171–196. doi: 10.1007/164_2016_98. [DOI] [PubMed] [Google Scholar]
- 211.Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford) 2011;50(11):1982–1990. doi: 10.1093/rheumatology/ker017. [DOI] [PubMed] [Google Scholar]
- 212.Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. doi: 10.1136/bmj.j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Minnetti M, Hasenmajer V, Sbardella E, Angelini F, Simeoli C, Di Paola N, et al. Susceptibility and characteristics of infections in patients with glucocorticoid excess or insufficiency: the ICARO tool. Eur J Endocrinol. 2022;187(5):719–731. doi: 10.1530/EJE-22-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Isidori AM, Arnaldi G, Boscaro M, Falorni A, Giordano C, Giordano R, et al. Towards the tailoring of glucocorticoid replacement in adrenal insufficiency: the Italian Society of Endocrinology Expert Opinion. J Endocrinol Invest. 2020;43(5):683–696. doi: 10.1007/s40618-019-01146-y. [DOI] [PubMed] [Google Scholar]
- 215.Othonos N, Pofi R, Arvaniti A, White S, Bonaventura I, Nikolaou N, et al. 11beta-HSD1 inhibition in men mitigates prednisolone-induced adverse effects in a proof-of-concept randomised double-blind placebo-controlled trial. Nat Commun. 2023;14(1):1025. doi: 10.1038/s41467-023-36541-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dubovsky AN, Arvikar S, Stern TA, Axelrod L. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53(2):103–115. doi: 10.1016/j.psym.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 217.Varney NR, Alexander B, MacIndoe JH. Reversible steroid dementia in patients without steroid psychosis. Am J Psychiatry. 1984;141:369–372. doi: 10.1176/ajp.141.3.369. [DOI] [PubMed] [Google Scholar]
- 218.Hall RC, Popkin MK, Stickney SK, Gardner ER. Presentation of the steroid psychoses. J Nerv Ment Dis. 1979;167(4):229–236. doi: 10.1097/00005053-197904000-00006. [DOI] [PubMed] [Google Scholar]
- 219.Brown ES, D JW, Frol A, Bobadilla L, Khan DA, Hanczyc M, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55(5):538–545. doi: 10.1016/j.biopsych.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 220.Keenan PA, Jacobson MW, Soleymani RM, Mayes MD, Stress ME, Yaldoo DT. The effect on memory of chronic prednisone treatment in patients with systemic disease. Am Acad Neurol. 1996;47(6):1396–1402. doi: 10.1212/wnl.47.6.1396. [DOI] [PubMed] [Google Scholar]
- 221.Brunner R, Schaefer D, Hess K, Parzer P, Resch F, Schwab S. Effect of corticosteroids on short-term and long-term memory. Neurology. 2005;64(2):335–337. doi: 10.1212/01.WNL.0000149523.35039.4C. [DOI] [PubMed] [Google Scholar]
- 222.Wolkowitz OM, Lupien SJ, Bigler ED. The “steroid dementia syndrome”: a possible model of human glucocorticoid neurotoxicity. Neurocase. 2007;13(3):189–200. doi: 10.1080/13554790701475468. [DOI] [PubMed] [Google Scholar]
- 223.Meijer OC, de Kloet ER. A refill for the brain mineralocorticoid receptor: the benefit of cortisol add-on to dexamethasone therapy. Endocrinology. 2017;158(3):448–454. doi: 10.1210/en.2016-1495. [DOI] [PubMed] [Google Scholar]
- 224.Warris LT, van den Heuvel-Eibrink MM, Aarsen FK, Pluijm SM, Bierings MB, van den Bos C, et al. Hydrocortisone as an intervention for dexamethasone-induced adverse effects in Pediatric patients with acute lymphoblastic Leukemia: results of a double-blind, randomized controlled trial. J Clin Oncol. 2016;34(19):2287–2293. doi: 10.1200/JCO.2015.66.0761. [DOI] [PubMed] [Google Scholar]
- 225.Warris LT, van den Heuvel-Eibrink MM, den Hoed MA, Aarsen FK, Pieters R, van den Akker EL. Does dexamethasone induce more neuropsychological side effects than prednisone in pediatric acute lymphoblastic leukemia? A systematic review. Pediatr Blood Cancer. 2014;61(7):1313–1318. doi: 10.1002/pbc.24988. [DOI] [PubMed] [Google Scholar]
- 226.Waber DP, McCabe M, Sebree M, Forbes PW, Adams H, Alyman C, et al. Neuropsychological outcomes of a randomized trial of prednisone versus dexamethasone in acute lymphoblastic leukemia: findings from Dana-Farber Cancer Institute All Consortium Protocol 00–01. Pediatr Blood Cancer. 2013;60(11):1785–1791. doi: 10.1002/pbc.24666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kadan-Lottick NS, Brouwers P, Breiger D, Kaleita T, Dziura J, Liu H, et al. A comparison of neurocognitive functioning in children previously randomized to dexamethasone or prednisone in the treatment of childhood acute lymphoblastic leukemia. Blood. 2009;114(9):1746–1752. doi: 10.1182/blood-2008-12-186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78(3):578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 229.Schwabe L, Wolf OT, Oitzl MS. Memory formation under stress: quantity and quality. Neurosci Biobehav Rev. 2010;34(4):584–591. doi: 10.1016/j.neubiorev.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 230.Wolf OT. The influence of stress hormones on emotional memory: relevance for psychopathology. Acta Psychol (Amst) 2008;127(3):513–531. doi: 10.1016/j.actpsy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 231.Keenan PA, Jacobson MW, Soleymani RM, Newcomer AJW. Commonly used therapeutic doses of glucocorticoids impair explicit memory. Ann NY Acad Sci. 1995;761:400–402. doi: 10.1111/j.1749-6632.1995.tb31402.x. [DOI] [PubMed] [Google Scholar]
- 232.Mrakotsky C, Forbes PW, Holmes Bernstein J, Grand RJ, Bousvaros A, Szigethy E, et al. Acute cognitive and behavioral effects of systemic corticosteroids in children treated for inflammatory bowel disease. J Int Neuropsychol Soc. 2012;19:96–109. doi: 10.1017/S1355617712001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.De Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3(4):313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 234.Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58(17):1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- 235.Buss C, Wolf OT, Witt J, Hellhammer DH. Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology. 2004;29(8):1093–1096. doi: 10.1016/j.psyneuen.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 236.Schlosser N, Wolf OT, Fernando SC, Riedesel K, Otte C, Muhtz C, et al. Effects of acute cortisol administration on autobiographical memory in patients with major depression and healthy controls. Psychoneuroendocrinology. 2010;35(2):316–320. doi: 10.1016/j.psyneuen.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 237.Newcomer AJW, Selke G, Melson AK, Hershey T, Craft S, Richards K, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- 238.Wingenfeld K, Wolf S, Krieg JC, Lautenbacher S. Working memory performance and cognitive flexibility after dexamethasone or hydrocortisone administration in healthy volunteers. Psychopharmacology. 2011;217(3):323–329. doi: 10.1007/s00213-011-2286-4. [DOI] [PubMed] [Google Scholar]
- 239.Coluccia D, Wolf OT, Kollias S, Roozendaal B, Forster A, de Quervain DJ. Glucocorticoid therapy-induced memory deficits: acute versus chronic effects. J Neurosci. 2008;28(13):3474–3478. doi: 10.1523/JNEUROSCI.4893-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lupien SJ, Wilkinson CW, Briere S, Menard C, Ng Ying Kin NM, Nair NP. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology. 2002;27(3):401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- 241.Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav Neurosci. 2003;117(3):505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- 242.Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann N Y Acad Sci. 2009;1179:41–55. doi: 10.1111/j.1749-6632.2009.04981.x. [DOI] [PubMed] [Google Scholar]
- 243.Oliveri RL, Sibilia G, Valentino P, Russo C, Romeo N, Quattrone A. Pulsed methvltxednisolone induces a reversible impairment of memory in patients with relapsing-remitting multiple sclerosis. Acta Neurol Scand. 1998;97:366–369. doi: 10.1111/j.1600-0404.1998.tb05967.x. [DOI] [PubMed] [Google Scholar]
- 244.Varney NR. A case of reversible steroid dementia. Arch Clin Neuropsychol. 1997;12(2):167–171. doi: 10.1093/arclin/12.2.167. [DOI] [PubMed] [Google Scholar]
- 245.Bentson J, Reza M, Winter J, Wilson G. Steroids and apparent cerebral atrophy on computed tomography scans. J Comput Assist Tomogr. 1978 doi: 10.1097/00004728-197801000-00003. [DOI] [PubMed] [Google Scholar]
- 246.Okuno T, Masatoshi I, Konishi K, Yoshioka M, Nakano Y. Cerebral atrophy following ACTH therapy. J Comput Assist Tomogr. 1980;4(1):20–23. doi: 10.1097/00004728-198002000-00004. [DOI] [PubMed] [Google Scholar]
- 247.Chapman C, Tubridy N, Cook MJ, Mitchell PJ, MacGregor LR, Lovelock C, et al. Short-term effects of methylprednisolone on cerebral volume in multiple sclerosis relapses. J Clin Neurosci. 2006;13(6):636–638. doi: 10.1016/j.jocn.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 248.Rao AB, Richert N, Howard T, Lewis BK, Bash CN, McFarland HF, et al. Methylprednisolone effect on brain volume and enhancing lesions in MS before and during IFNβ-1b. Neurology. 2002;59(5):688–694. doi: 10.1212/wnl.59.5.688. [DOI] [PubMed] [Google Scholar]
- 249.Brown ES, Beard L, Frol AB, Rush AJ. Effect of two prednisone exposures on mood and declarative memory. Neurobiol Learn Mem. 2006;86(1):28–34. doi: 10.1016/j.nlm.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 250.Spiegel W, McGeady SJ, Mansmann HC. Cerebral cortical atrophy and central nervous system (CNS) symptoms in a steroid-treated child with asthma. J Allergy Clin Immunol. 1992;89(4):918–919. doi: 10.1016/0091-6749(92)90449-C. [DOI] [PubMed] [Google Scholar]
- 251.de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, et al. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci. 2003;17(6):1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- 252.De Leon MJ, McRae T, Rusinek H, Convit A, De Santi S, Tarshish C, et al. Cortisol reduces hippocampal glucose metabolism in normal elderly, but not in Alzheimer’s disease. J Clin Endocrinol Metab. 1997;82(10):3251–3259. doi: 10.1210/jcem.82.10.4305. [DOI] [PubMed] [Google Scholar]
- 253.Ganguli R, Singh A, Brar J, Carter C, Mintun M. Hydrocortisone induced regional cerebral activity changes in schizophrenia: a PET scan stud. Schizophr Res. 2002;56:241–247. doi: 10.1016/S0920-9964(01)00219-5. [DOI] [PubMed] [Google Scholar]
- 254.Fleischer J, Metz S, Dusenberg M, Grimm S, Golde S, Roepke S, et al. Neural correlates of glucocorticoids effects on autobiographical memory retrieval in healthy women. Behav Brain Res. 2019;359:895–902. doi: 10.1016/j.bbr.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 255.Prouty EW, Chandler DJ, Gao WJ, Waterhouse BD. Selective vulnerability of dorsal raphe-medial prefrontal cortex projection neurons to corticosterone-induced hypofunction. Eur J Neurosci. 2019;50(1):1712–1726. doi: 10.1111/ejn.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav Neurosci. 1999;113(3):420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- 257.Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24(6):1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Young KD, Preskorn SH. Neuroscientific basis of corticosteroid-induced changes in human cognitive and emotional processing: implications for affective illness. J Psychiatr Pract. 2013;19(4):309–315. doi: 10.1097/01.pra.0000432601.09514.12. [DOI] [PubMed] [Google Scholar]
- 259.Wuppen K, Oesterle D, Lewicka S, Kopitz J, Plaschke K. A subchronic application period of glucocorticoids leads to rat cognitive dysfunction whereas physostigmine induces a mild neuroprotection. J Neural Transm (Vienna) 2010;117(9):1055–1065. doi: 10.1007/s00702-010-0441-4. [DOI] [PubMed] [Google Scholar]
- 260.Young AH, Sahakian BJ, Robbins TW, Cowen PJ. The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology. 1999;145:260–266. doi: 10.1007/s002130051057. [DOI] [PubMed] [Google Scholar]
- 261.Wolf OT, Convit A, McHugh PF, Kandil E, Thorn EL, De Santi S, et al. Cortisol differentially affects memory in young and elderly men. Behav Neurosci. 2001;115(5):1002–1011. doi: 10.1037/0735-7044.115.5.1002. [DOI] [PubMed] [Google Scholar]
- 262.Fietta P, Fietta P, Delsante G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin Neurosci. 2009;63(5):613–622. doi: 10.1111/j.1440-1819.2009.02005.x. [DOI] [PubMed] [Google Scholar]
- 263.Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491–497. doi: 10.1176/appi.ajp.2011.11071009. [DOI] [PubMed] [Google Scholar]
- 264.Huynh G, Reinert JP. Pharmacological management of steroid-induced psychosis: a review of patient cases. J Pharm Technol. 2021;37(2):120–126. doi: 10.1177/8755122520978534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hodgins GE, Saltz SB, Gibbs EP, Gonzalez R, Regan J, Nemeroff C. Steroid-induced psychosis in the pediatric population: a new case and review of the literature. J Child Adolesc Psychopharmacol. 2018;28(5):354–359. doi: 10.1089/cap.2018.0017. [DOI] [PubMed] [Google Scholar]
- 266.Filipini D, Gijsbers K, Birmingham MK, Dubrovsky B. Effects of adrenal steroids and their reduced metabolites on hippocampal long-term potentiation. J Steroid Biochem Mol Biol. 1991;40(1–3):87–92. doi: 10.1016/0960-0760(91)90171-z. [DOI] [PubMed] [Google Scholar]
- 267.Program TBCDS (1978) Acute adverse reactions to prednisone in relation to dosage [DOI] [PubMed]
- 268.Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days' corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25–31. doi: 10.1016/0306-4530(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 269.Prodan CI, Monnot M, Ross ED, Coleman AE. Reversible dementia with parkinsonian features associated with budesonide use. Neurology. 2006;67:723. doi: 10.1212/01.wnl.0000229924.76258.c1. [DOI] [PubMed] [Google Scholar]
- 270.Young K, Drevets WC, Schulkin J, Erickson K. Supplemental material for dose-dependent effects of hydrocortisone infusion on autobiographical memory recall. Behav Neurosci. 2011;125(5):735–741. doi: 10.1037/a0024764.supp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Brown ES, Suppes T, Khan DA, Carmody TJ., III Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55–61. doi: 10.1097/00004714-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 272.Wolkowitz OM, Rubinow D, Doran AR, Breier A, Berrettini WH, Kling MA, et al. Prednisone effects on neurochemistry and behavior. Arch Gen Psychiatry. 1990;47:963–968. doi: 10.1001/archpsyc.1990.01810220079010. [DOI] [PubMed] [Google Scholar]
- 273.Kuhlmann S, Kirschbaum C, Wolf OT. Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiol Learn Mem. 2005;83(2):158–162. doi: 10.1016/j.nlm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 274.van Marle HJ, Hermans EJ, Qin S, Overeem S, Fernandez G. The effect of exogenous cortisol during sleep on the behavioral and neural correlates of emotional memory consolidation in humans. Psychoneuroendocrinology. 2013;38(9):1639–1649. doi: 10.1016/j.psyneuen.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 275.Schmidt LA, Fox NA, Goldberg MC, Smith CG, Schulkin J. Effects of acute prednisone administration on memory, attention and emotion in healthy human adults. Psychoneuroendocrinology. 1999;24:461–483. doi: 10.1016/S0306-4530(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 276.Taylor VA, Ellenbogen MA, Washburn D, Joober R. The effects of glucocorticoids on the inhibition of emotional information: a dose-response study. Biol Psychol. 2011;86(1):17–25. doi: 10.1016/j.biopsycho.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 277.Bhattacharyya A, Kaushal K, Tymms DJ, Davis JR. Steroid withdrawal syndrome after successful treatment of Cushing's syndrome: a reminder. Eur J Endocrinol. 2005;153(2):207–210. doi: 10.1530/eje.1.01953. [DOI] [PubMed] [Google Scholar]
- 278.Theiler-Schwetz V, Prete A. Glucocorticoid withdrawal syndrome: what to expect and how to manage. Curr Opin Endocrinol Diabetes Obes. 2023 doi: 10.1097/MED.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 279.Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of Cushing's syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. doi: 10.1210/jc.2015-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev. 2003;24(4):523–538. doi: 10.1210/er.2001-0014. [DOI] [PubMed] [Google Scholar]
- 281.Pofi R, Feliciano C, Sbardella E, Argese N, Woods CP, Grossman AB, et al. The short synacthen (corticotropin) test can be used to predict recovery of hypothalamo-pituitary-adrenal axis function. J Clin Endocrinol Metab. 2018;103(8):3050–3059. doi: 10.1210/jc.2018-00529. [DOI] [PubMed] [Google Scholar]
- 282.Laugesen K, Broersen LHA, Hansen SB, Dekkers OM, Sorensen HT, Jorgensen JOL. Management of endocrine disease: glucocorticoid-induced adrenal insufficiency: replace while we wait for evidence? Eur J Endocrinol. 2021;184(4):R111–R122. doi: 10.1530/EJE-20-1199. [DOI] [PubMed] [Google Scholar]
- 283.Richter B, Neises G, Clar C. Glucocorticoid withdrawal schemes in chronic medical disorders. A systematic review. Endocrinol Metab Clin North Am. 2002;31(3):751–778. doi: 10.1016/s0889-8529(02)00008-7. [DOI] [PubMed] [Google Scholar]
- 284.He X, Findling JW, Auchus RJ. Glucocorticoid withdrawal syndrome following treatment of endogenous Cushing syndrome. Pituitary. 2022;25(3):393–403. doi: 10.1007/s11102-022-01218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.