Abstract
Background
Suboptimal plasma retinol concentrations have been documented in US children with sickle cell disease (SCD) hemoglobin SS type (SCD-HbSS), but little is known about vitamin A kinetics and stores in SCD.
Objectives
The objectives were to quantify vitamin A total body stores (TBS) and whole-body retinol kinetics in young people with SCD-HbSS and use retinol isotope dilution (RID) to predict TBS in SCD-HbSS and healthy peers as well as after vitamin A supplementation in SCD-HbSS subjects.
Methods
Composite plasma [13C10]retinol response data collected from 22 subjects with SCD-HbSS for 28 d after isotope ingestion were analyzed using population-based compartmental modeling (“super-subject” approach); TBS and retinol kinetics were quantified for the group. TBS was also calculated for the same individuals using RID, as well as for healthy peers (n = 20) and for the subjects with SCD-HbSS after 8 wk of daily vitamin A supplements (3.15 or 6.29 μmol retinol/d [900 or 1800 μg retinol activity equivalents/d]).
Results
Model-predicted group mean TBS for subjects with SCD-HbSS was 428 μmol, equivalent to ∼11 mo of stored vitamin A; vitamin A disposal rate was 1.3 μmol/d. Model-predicted TBS was similar to that predicted by RID at 3 d postdosing (mean, 389 μmol; ∼0.3 μmol/g liver); TBS predictions at 3 compared with 28 d were not significantly different. Mean TBS in healthy peers was similar (406 μmol). RID-predicted TBS for subjects with SCD-HbSS was not significantly affected by vitamin A supplementation at either dose.
Conclusions
Despite differences in plasma retinol concentrations, TBS was the same in subjects with SCD-HbSS compared with healthy peers. Because 56 d of vitamin A supplementation at levels 1.2 to 2.6 times the Recommended Dietary Allowance did not increase TBS in these subjects with SCD-HbSS, further work will be needed to understand the effects of SCD on retinol metabolism. This trial was registered as NCT03632876 at clinicaltrials.gov.
Keywords: model-based compartmental analysis, population-based modeling, retinol isotope dilution, sickle cell disease, sickle cell disease hemoglobin SS type, vitamin A, vitamin A status
Introduction
Vitamin A is an essential nutrient that is required for growth, development, reproduction, tissue repair, immune function, and vision. Both vitamin A deficiency and suboptimal vitamin A status have been documented in populations worldwide, especially when there is inadequate availability of dietary vitamin A [1]. Normally, plasma retinol concentrations are homeostatically controlled, but they are low in vitamin A deficiency; in addition, plasma retinol is negatively impacted in other conditions, including inflammation, iron deficiency (for reviews, see [2,3]), and sickle cell disease (SCD) [[4], [5], [6]].
SCD affects millions of people worldwide [7], and it is the most common inherited blood disorder in the United States [8]. SCD is characterized by hemolytic anemia and vaso-occlusive complications related to the inherited hemoglobinopathies; the most severe disease presents in individuals with hemoglobin SS type (SCD-HbSS) [7,9]. Regarding plasma retinol, Schall et al. [4] found that, among US children with SCD-HbSS, those who had suboptimal plasma retinol concentrations, defined as <1.05 μmol/L [10], had poor growth, reduced hematologic status, and increased hospitalizations compared with those whose retinol concentrations were ≥1.05 μmol/L. Further, while vitamin A supplementation has been shown to improve vitamin A status in numerous populations (eg, see references in [2] as well as [11]), Dougherty et al. [5] reported that 12 mo of supplementation (either 1.05, 1.40, or 2.10 μmol/d) based on the RDA for children did not improve plasma retinol concentrations or hematologic status in children with SCD-HbSS. In a follow-up study, Brownell et al. [6] found that higher dose supplements (either 3.15 or 6.29 μmol/d) over a shorter duration (8 wk) were associated with improved growth and hematologic outcomes in subjects with SCD-HbSS but plasma retinol concentrations remained low.
Although plasma retinol is often used as an indicator of vitamin A status, its sensitivity is limited because concentrations are homeostatically controlled over a wide range of vitamin A stores [3,12]. At present, retinol isotope dilution (RID) is considered the best available method for estimating vitamin A status (ie, vitamin A total body stores [TBS]) in groups, and it is also used to estimate TBS in individuals; see, for example, [2,[13], [14], [15], [16]]. For an RID study, subjects ingest a dose of a vitamin A stable isotope, and a single blood sample is collected from each individual at a predetermined time; retinol specific activity in plasma (SAp) is determined and then used in an RID equation, along with coefficients that reflect assumptions related to dose absorption, catabolism, and mixing, to predict TBS; see, for example, [2,13,[17], [18], [19]] for more details. In addition to RID, TBS can also be determined using model-based compartmental analysis [2,20]. For modeling, subjects ingest a stable isotope-labeled dose of vitamin A, serial blood samples are collected, plasma retinol tracer response is analyzed in light of a proposed compartmental model, and TBS is calculated as one of the modeling results. Modeling also provides quantitative and descriptive information about whole-body retinol kinetics, including the movement of vitamin A between compartments and the rate of vitamin A utilization. The vitamin A system has been modeled in individual adults following frequent sampling [[21], [22], [23]] and in children using a population-based (“super-subject”) approach, which reduces sampling burden on individuals [[24], [25], [26], [27]]. For a super-subject study, several samples are collected from each subject, and modeling is done on a composite dataset to determine kinetic parameters and TBS predictions for the group.
Here, we used compartmental modeling and RID to study, for the first time, retinol kinetics and TBS in young people with SCD-HbSS, hypothesizing that, in addition to below-normal plasma retinol concentrations, they might have either lower or higher TBS than healthy peers, depending on how SCD-HbSS affects the vitamin A system. We also used RID to predict TBS before and after 8 wk of vitamin A supplementation in the subjects with SCD-HbSS.
Methods
Subjects
Young people included in the analyses described here were participants in a randomized, controlled, double-blind, dose-finding pilot study [6] that was designed to evaluate the efficacy and safety of vitamin A supplementation in subjects with SCD-HbSS and to assess vitamin A status in young people with SCD-HbSS compared to healthy subjects. This single-center study was conducted at Children’s Hospital of Philadelphia (CHOP); the trial was approved by the CHOP Institutional Review Board and is registered at clinicaltrials.gov (NCT03632876).
Details related to subject recruitment, demographics, anthropometrics, and data collected at baseline are presented in [6]. Briefly, young people (n = 22; 9–19 y old) who had a confirmed diagnosis of SCD-HbSS were recruited from the CHOP Comprehensive Sickle Cell Center between September 2015 and October 2016; subjects were randomly assigned to receive vitamin A supplements of either 3.15 or 6.29 μmol/d (900 or 1800 μg retinol activity equivalents [RAE]/d [i.e., 3000 or 6000 IU/d]) of retinol as retinyl palmitate (DSM Nutritional Products AG) for 8 wk after the initial kinetic studies. Healthy subjects of similar age (n = 20; 9–19 y old) who had no chronic diseases were recruited from CHOP Care Network primary care clinics in Philadelphia, PA, for baseline comparison to subjects with HbSS. For both groups, potential subjects were excluded if they had any recent hospitalizations, medication changes, or significant known laboratory abnormalities in the weeks preceding enrollment. Dietary intake was assessed at baseline for subjects in both groups using mean 3-d weighed food records; data were analyzed for micronutrient composition, including vitamin A (Nutrition Data System).
Stable isotope doses
8-,9-,10-,11-,12-,13-,14-,15-,19-,20-[13C10]Retinyl acetate (99.6% purity) was purchased from Buchem BV. Labeled vitamin A doses were prepared as follows at the CHOP Investigational Pharmacy. Aliquots of the isotope were solubilized in sunflower oil at a concentration of 0.5 mg/mL by sonication in amber vials for 30 min at 37°C. Doses were stored at −80°C for up to 7 d prior to isotope administration via oral syringes. Syringes were weighed before and after dose administration to determine the amount of isotope ingested by each subject (mean, 2.9 μmol [i.e., 829 μg RAE or 1 mg retinyl acetate]), calculated as 3.18 μmol [13C10]retinyl acetate/g oil × oil ingested (g).
Study design
The study protocol is shown in Supplemental Figure 1. In brief, following a standard dinner meal and an overnight fast of at least 10 h, baseline (day 0) blood samples were collected from all subjects (see next section); participants then ingested a dose of [13C10]retinyl acetate and consumed a standardized breakfast (a smoothie and a muffin that contained 40 g fat, as in [28]). For kinetic studies, participants with SCD-HbSS were assigned to be either frequently sampled (n = 7) or part of the super-subject group (n = 16). For the frequently sampled subjects, blood was obtained at 7, 9, and 13 h postdosing and, after a fast of at least 10 h, at 1, 3, 5, 7, 14, 21, and 28 d. For the super-subject group, fasting blood samples were collected from all individuals at 3 and 28 d postdosing (so that TBS could be estimated by RID at these times; see later section), and one additional fasting sample was obtained from each subject at one of the indicated remaining 8 times, providing data from 2 subjects at each of those times. Then, on day 28, subjects from both the frequently sampled and super-subject groups began to consume a daily vitamin A supplement (either 3.15 or 6.29 μmol/d) for 8 wk. After a 1-wk washout period, a second baseline fasting blood sample was collected (on day 91) and participants then ingested a second dose of [13C10]retinyl acetate. Blood was collected again on day 94 (3 d after the second isotope dose) to estimate TBS by RID after the vitamin A interventions. Finally, in the healthy subjects (n = 20), a baseline fasting blood sample was obtained on day 0 and the isotope dose was ingested; then, a second fasting blood sample was collected on day 3 for estimation of TBS by RID.
Sample procedures and retinol analyses
At each sampling time, venous blood was collected into Vacutainer tubes containing EDTA. Plasma was isolated by centrifugation and frozen at −80°C until shipment on dry ice to Newcastle University for LC-MS/MS analysis. For analysis, retinol was extracted into hexane as described by Oxley and Lietz [29]; chromatographic separation and MS/MS quantitation of analytes was done using the method of Oxley et al. [30]. Samples were protected from light during processing, storage, and shipment.
Composite dataset
Plasma [13C10]retinol concentrations at each time for each subject were converted to fraction of the ingested dose in plasma (FDp) as {plasma [13C10]retinol concentration (μmol/L) × estimated plasma volume (L)} / [13C10] dose (μmol), where plasma volume was estimated based on blood volume, which was calculated using a regression equation [31] and hematocrit calculated using erythrocyte count and mean cell volume. In order to obtain the most robust dataset for modeling, we combined data for participants in the super-subject and frequently sampled SCD-HbSS subgroups into one composite dataset. Our rationale was that, in the time since completion of this study, we have learned [32] that 2 children/time may not be optimal for super-subject modeling; in addition, there were problems quantifying the isotope in a number of samples, especially at later times, limiting the amount of data available. As a result, data from the frequently sampled subjects comprised a large portion of the composite dataset, and by chance, FDp data for all of these individuals were clustered at the higher end of the group, suggesting lower vitamin A stores. Therefore, to obtain a representative central tendency for the group FDp data for modeling, we adjusted each individual’s post-3 d samples based on their position relative to the geometric mean at 3 d; see Supplemental Methods. Geometric mean FDp at each time was calculated (using adjusted FDp at times after 3 d), generating a composite SCD-HbSS dataset for modeling.
Compartmental modeling
Geometric mean FDp data for SCD-HbSS subjects (pre-supplementation) were plotted using the Windows version of the Simulation, Analysis and Modeling software (WinSAAM v. 3.3.0 [[33], [34], [35]]) and weighted using a fractional standard deviation (FSD) of 0.05 at all times except day 3 and day 28, when all subjects were sampled; for those times, weight was increased to 0.025. Data were fit to a previously described [25] 6-component model for whole-body vitamin A metabolism (Figure 1); see figure legend for details. We assumed an absorption efficiency of 75% for preformed vitamin A, as in previous studies [22,36], and added an adjusted estimate for dietary vitamin A intake as weighted data (using an FSD of 0.05) to constrain the model output and improve confidence in model predictions as described in [36,37]. The value for adjusted vitamin A intake was calculated as preformed vitamin A + {provitamin A carotenoids [as RAE based on Institute of Medicine conversion factors [38]] / 0.75}, assuming 75% absorption efficiency for preformed vitamin A; this corrects ingested provitamin A carotenoids to an equivalent amount of preformed vitamin A.
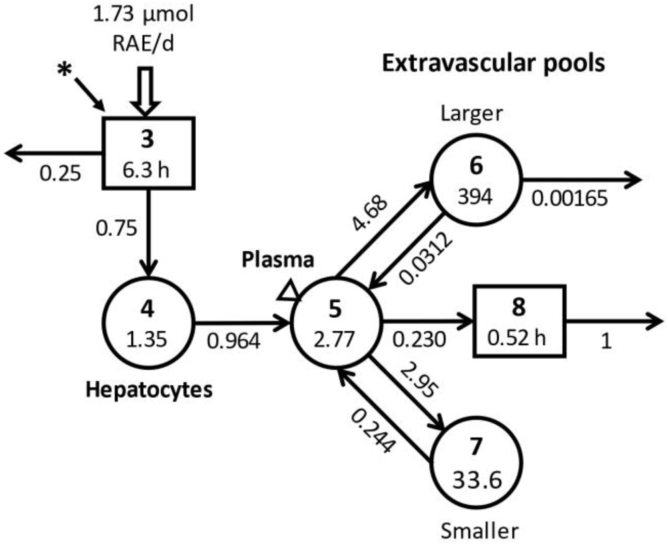
FIGURE 1.
Compartmental model for vitamin A metabolism in humans, with results obtained by modeling the composite SCD-HbSS dataset. The model is from [25]. Circles represent compartments, the rectangles are delay components, and arrows are fractional transfer coefficients [L(I,J)s, or the fraction of retinol in compartment J transferred to compartment I each day]; delay times [DT(I)s] correspond to the time (h) spent in delay component I. Delay component 3 is the site of input of tracer (∗) and dietary vitamin A, as well as the site of loss of unabsorbed vitamin A; it represents the processes of digestion and absorption of ingested vitamin A and then its packaging into chylomicrons and their subsequent metabolism until uptake of chylomicron remnants by hepatocytes (compartment 4). Retinol in hepatocytes is secreted into plasma compartment 5 bound to retinol-binding protein; plasma retinol is either irreversibly transferred to component 8 for rapid utilization by tissues or it exchanges with vitamin A in 2 extravascular pools (a larger compartment 6 and a smaller compartment 7, the sum of which is TBS). Irreversible loss from the system occurs from compartment 6 and component 8. During modeling, L(4,3) and L(0,3) were fixed to reflect 75% absorption efficiency and 25% of incoming vitamin A not absorbed; DT(8) was fixed at 75 min; and following WinSAAM convention, L(0,8)=1. Also, L(8,5) was calculated assuming that 50% of the disposal rate was from transfer of retinol from plasma to component 8; this was fixed in the model and the value for L(0,6) was constrained to reflect the remaining 50% of disposal rate coming from the larger extravascular pool. Final steady state model predictions for fractional transfer coefficients (d−1) are shown with each arrow and those for delay times are indicated in the rectangles; retinol pool sizes (i.e., compartment retinol masses; μmol) are shown for each compartment. RAE, retinol activity equivalents; SCD-HbSS, sickle cell disease hemoglobin SS type; TBS, vitamin A total body stores; WinSAAM, Windows version of Simulation, Analysis and Modeling software.
Once a satisfactory fit was obtained between the observed data and model predictions, weighted nonlinear least squares regression analysis was performed in WinSAAM to obtain final parameter values for fractional transfer coefficients [L(I,J)s, or the fraction of retinol in compartment J transferred to compartment I each day] and for the delay time in component 3 [DT(3), or the time (h) that retinol spends in delay component 3] as well as their statistical uncertainties. The geometric mean value for plasma retinol pool size [M(5); μmol of retinol in compartment 5; Figure 1] was calculated as plasma retinol concentration (μmol/L) × estimated plasma volume (L) for each sample from each individual; the geometric mean value for M(5) was fixed in the model and used in a steady state solution to calculate other compartment masses [M(I); μmol], including TBS, and transfer rates [R(I,J); μmol/d; calculated as L(I,J) × M(J)], including vitamin A disposal rate. Final model results were used to calculate time-related parameters including transit times, residence times, and plasma recycling time and number [20]. See Supplemental WinSAAM Deck.
Estimation of TBS by RID
To compare TBS in subjects with SCD-HbSS and healthy peers, as well as in the subjects with SCD-HbSS before and after supplementation, we used the RID equation presented by Green et al. [13], shown here as Equation 1:
| [1] |
where TBS is μmol of vitamin A in exchangeable extravascular pools (compartments 6 and 7; Figure 1); Fa is fraction of the oral isotope dose found in the exchangeable extravascular body pools at the time of sampling; S is the ratio of retinol specific activity in plasma (compartment 5) / retinol specific activity in the exchangeable extravascular pools at the time of sampling; and SAp is plasma retinol specific activity (FDp/μmol) at the time of sampling. Values for the composite coefficient FaS were calculated over time using the results from modeling the composite dataset as [F(6)t + F(7)t] × [F(5)t / M(5)] / {[F(6)t + F(7)t] / [M(6) + M(7)]}, where F(I)t is fraction of the tracer dose found in compartment I at time t. For the subjects with SCD-HbSS before intervention, the values for FaS at 3 and 28 d were used in Equation 1, along with each individual’s SAp at that time, to predict TBS. For the healthy subjects, TBS was estimated using the same 3 d value for FaS (because a more appropriate value was not available; see Discussion) and the subject’s SAp at that time. To compare TBS in the subjects with SCD-HbSS before and after the 8-wk supplementation period, we adopted the paired-RID approach [39], using TBS predicted 3 d after administration of the 2 isotope doses (Supplemental Figure 1); if residual 13C10 was quantifiable on day 91 after supplementation, it was subtracted from the measured value for a given subject. Note that, for several subjects, sampling was not done at 3 d after the second dose but at a different time (see Results); in those cases, we used the value for FaS corresponding to the actual sampling day to predict TBS.
Using the above-described values for TBS, we estimated liver vitamin A concentration for each subject as TBS (μmol) × fraction of TBS stored in the liver / estimated liver weight (g), assuming that 80% of TBS was in the liver [40] and using body surface area [41] to estimate liver weight.
Data management and statistics
Data were managed in Microsoft Excel; figures were generated in Microsoft PowerPoint and Adobe Photoshop or in GraphPad Prism. Subject demographics are reported as arithmetic mean (range); vitamin A intakes, RID predictions of TBS, and estimates of liver vitamin A concentration are presented as geometric mean (range). Model evaluation was performed using WinSAAM. Specifically, a parameter was considered identified if its FSD was <0.5 [42], and goodness of fit between model predictions and observed data was determined based on the ratio of calculated to observed data and improvement in the sum of weighted squared residuals. Predictions of TBS and liver vitamin A concentration for subjects with SCD-HbSS and healthy peers, as well as for subjects with SCD-HbSS before and after supplementation, were compared in GraphPad Prism using a paired t test, Wilcoxon test, or Mann-Whitney test, depending on whether the data were paired and normally distributed; P < 0.05 was considered significant.
Results
Subject characteristics
Selected demographics and plasma retinol concentrations for young people with SCD-HbSS and healthy peers are summarized in Table 1; see [6] for more details, including data on serum concentrations of retinol-binding protein (RBP; 18.7 ± 6.2 in individuals with SCD-HbSS compared with 20.2 ± 7.1 μg/mL in healthy peers). As shown in Table 1, healthy subjects and those with SCD-HbSS did not differ by age or sex; however, mean plasma retinol concentrations were significantly lower in the SCD-HbSS group, although as shown in Figure 1 in reference [6], there were individuals in both groups whose retinol concentrations fell below the cutoff of 1.05 μmol/L. Also, plasma retinol concentrations were not significantly different in the individuals with SCD-HbSS before and after supplementation (Table 1) or between the subgroups that received daily vitamin A supplements of either 3.15 or 6.29 μmol/d for 8 wk (data not shown). Vitamin A intake from foods ranged from 0.49 to 4.17 μmol RAE/d for 20 of the subjects with SCD-HbSS before supplementation.
TABLE 1.
Selected demographics and plasma retinol concentrations in healthy young people and in subjects with SCD-HbSS before and after vitamin A supplementation1
| Healthy (n = 20) | SCD-HbSS pre-supplementation (n = 22) | SCD-HbSS post-supplementation (n = 21) | |
|---|---|---|---|
| Sex, % males | 60 | 59 | 57 |
| Age, y | 14 (9–19) | 14 (9–19) | 14 (9–19) |
| Plasma retinol, μmol/L | 1.47 (0.87–2.27)2 | 1.12 (0.46–2.35) | 1.24 (0.74–2.37) |
SCD-HbSS, sickle cell disease hemoglobin SS type.
Subjects were participants in the studies reported by Brownell et al. [6]. Tabulated values are arithmetic mean (range), except for sex, which is reported as percent males. Plasma retinol concentration was calculated as [12C]retinol + [13C]retinol. Values for the healthy group include 19 subjects at 3 d after isotope ingestion and 1 at 5 d. For the SCD-HbSS group before supplementation, values include all 22 subjects at 3 d whereas after supplementation, values are for 20 of the same subjects (with n = 14 at 3 d, 4 at 4 d, 1 at 7 d, and 1 at 8 d). Retinol concentrations were compared between healthy subjects and subjects with SCD-HbSS pre-supplementation using Student’s unpaired t test. Subjects with SCD-HbSS were compared before and after vitamin A supplementation using a mixed effects linear regression model adjusting for hydroxyurea use and including a time × dose interaction term. The Benjamini and Hochberg false discovery rate method was applied with a maximum false discovery rate of 0.05 to derive corrected P values to address the issue of multiple testing.
Significant difference between group means using unpaired t test, P < 0.05.
Compartmental modeling
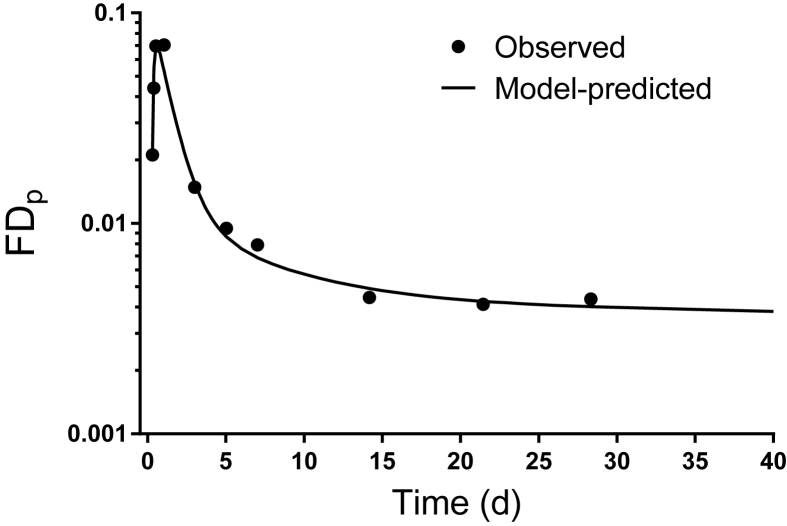
Geometric mean data for [13C10]retinol FDp (adjusted at times after 3 d as described in Supplemental Methods) for the group of subjects with SCD-HbSS before intervention are plotted over time in Figure 2, with the model-calculated fit to the data simulated to 40 d; an estimate of adjusted vitamin A intake (1.73 μmol RAE/d) was included as weighted data during modeling to allow us to better determine the terminal slope of the curve, as discussed previously [37]. As shown in Figure 2, the plasma [13C10]retinol tracer rose to a peak at ∼12 h, followed by a decline until 3 d as labeled retinol was distributed to tissues; subsequently (5–21 d), the curve bent as tracer recycled from tissues to plasma and then entered a terminal slope, reflecting the system fractional catabolic rate. Based on model simulations, the terminal slope of the isotope response curve was reached by ∼30 d.
FIGURE 2.
Composite [13C10]retinol FDp data vs. time for young people with SCD-HbSS before vitamin A supplementation and model-calculated fit. Symbols are geometric mean observed data and the line is the model-predicted curve simulated to 40 d. Geometric mean data include the following number of subjects at each time: 7 h (n = 9), 9 h (n = 9), 13 h (n = 8), 1 d (n = 9), 3 d (n = 22), 5 d (n = 7), 7 d (n = 8), 14 d (n = 7), 21 d (n = 5), and 28 d (n = 11). FDp, plasma retinol fraction of dose; SCD-HbSS, sickle cell disease hemoglobin SS type.
Model-predicted kinetic parameters, including fractional transfer coefficients and delay times for the SCD-HbSS pre-supplementation composite dataset, are presented in Figure 1. All 7 adjustable parameters were well-identified, with a mean parameter FSD of 0.14 (range, 0.012–0.28). Based on modeling results, DT(3), the mean time required for vitamin A digestion, absorption, and chylomicron assembly and catabolism until uptake of chylomicron remnants by hepatocytes (compartment 4), was 1.25 h; then 96%/d of retinol in hepatocytes was secreted into plasma bound to RBP. Of the total plasma retinol turned over each day, 59% was transferred to the larger extravascular vitamin A storage pool (compartment 6), 38% was transferred to the small extravascular pool (compartment 7), and the remaining 3% was rapidly utilized by tissues from which there was no recycling of retinol to plasma (component 8). Plasma retinol turnover was 4.7 and 3.0 pools/d to compartments 6 and 7, respectively; recycling from the smaller compartment 7 was 8 times faster (24%/d) compared to the larger compartment 6 (3.1%/d), and irreversible utilization from the larger compartment was 0.16%/d.
Model-predicted steady state compartment masses are also presented in Figure 1. Compared to the calculated mass of retinol in plasma compartment 5 (2.77 μmol), the model predicted that half that amount was present in hepatocytes (compartment 4). Model-predicted TBS was 428 μmol, with 92% of that found in the larger exchangeable pool (compartment 6) and the remainder in the smaller pool (compartment 7). Regarding transfer rates, the model predicted that 21 μmol of plasma retinol was transferred to the exchangeable pools each day, with 61% of that transferred to the larger storage pool; in addition, 12 μmol of retinol from the larger pool and 8.2 μmol from the smaller were recycled to plasma each day. The model-predicted adjusted vitamin A input for the group, and therefore the steady state disposal rate, was 1.3 μmol/d, assuming a vitamin A absorption efficiency of 75%. Based on the model-predicted TBS and the disposal rate, the system fractional catabolic rate was 0.30%/d and there was ∼11 mo of vitamin A in stores. An average retinol molecule spent 3.0 h in plasma compartment 5 during each transit and a total of 2.1 d in that compartment before leaving irreversibly; thus, retinol recycled to plasma from the exchangeable pools 16 times before being irreversibly lost from the system; retinol spent 21 d in the exchangeable extravascular pools before cycling back to plasma. In addition, retinol spent 30 d in compartment 6 compared to 4.1 d in compartment 7 during each transit and a total of 303 and 26 d, respectively, in these compartments. Overall, the system residence time was 331 d.
RID-predicted TBS and liver vitamin A concentrations
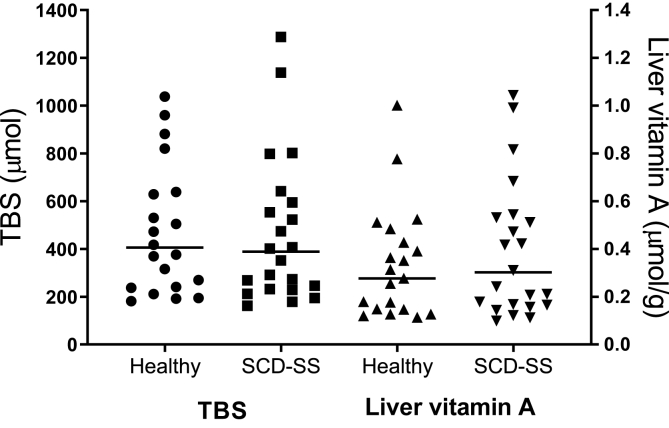
Predicted values for TBS, calculated using the model-derived values for FaS in RID Equation 1 along with each individual’s SAp at the same time, are summarized in Table 2. The geometric mean TBS at 3 d for the subjects with SCD-HbSS before supplementation was within 9% of the model-predicted value (389 compared with 428 μmol, respectively). When we compared TBS predictions at 3 d to values calculated at 28 d (or at 29 or 30 d for 2 of the subjects) for the 11 individuals whose data were above the limit of quantitation at the later times, mean predictions agreed well (252 compared with 248 μmol, respectively; NS; Supplemental Figure 2). In addition, as shown in Table 2, TBS predictions for the subjects with SCD-HbSS compared with healthy peers at 3 d (or at 5 d for one of the healthy individuals) were not significantly different (389 compared with 406 μmol, respectively); for a large majority of subjects (93%) in both groups, TBS was between 160 and 1000 μmol (Figure 3). Similar results were obtained for liver vitamin A concentrations (Table 2 and Figure 3).
TABLE 2.
RID-predicted TBS and estimated liver vitamin A concentrations in healthy young people and in subjects with SCD-HbSS before and after vitamin A supplementation1
| Group | TBS (μmol) | P value | Liver VA concentration (μmol/g) | P value |
|---|---|---|---|---|
| SCD-HbSS (n = 22) | 389 (162–1288) | 0.813 | 0.302 (0.0992–1.04) | 0.663 |
| Healthy (n = 20) | 406 (182–1038) | 0.277 (0.113–1.00) | ||
| SCD-HbSS paired comparisons | ||||
| 3.15 or 6.29 μmol retinol/d | ||||
| Before (n = 20) | 412 (162–1288) | 0.985 | 0.335 (0.112–1.04) | 0.841 |
| After (n = 20) | 415 (155–965) | 0.327 (0.104–0.826) | ||
| 3.15 μmol retinol/d | ||||
| Before (n = 9) | 540 (162–1288) | 0.0892 | 0.428 (0.112–1.04) | 0.838 |
| After (n = 9) | 435 (165–965) | 0.336 (0.113–0.826) | ||
| 6.29 μmol retinol/d | ||||
| Before (n = 11) | 323 (195–554) | 0.112 | 0.269 (0.144–0.544) | 0.178 |
| After (n = 11) | 381 (155–730) | 0.314 (0.104–0.648) | ||
RID, retinol isotope dilution; SAp, plasma retinol specific activity; SCD-HbSS, sickle cell disease hemoglobin SS type; TBS, vitamin A total body stores; VA, vitamin A.
Values are geometric mean (range) for TBS and liver vitamin A concentration for young people with SCD-HbSS before and after 8 wk of vitamin A supplementation (either 3.15 or 6.29 μmol/d) as well as for healthy subjects of similar age. For all subjects, TBS were calculated by RID Equation 1 (see Methods) using group values for the composite coefficient FaS derived from modeling the pre-supplementation super-subject dataset for the SCD-HbSS subjects along with each subject’s SAp at the corresponding time. For 19 of the 20 healthy individuals, TBS were calculated at 3 d after isotope dosing and for 1 subject at 5 d. For the subjects with SCD-HbSS before supplementation, TBS for all 22 participants were calculated at 3 d postdosing whereas after supplementation, TBS were calculated for 14 of the subjects with SCD-HbSS at 3 d, for 4 subjects at 4 d, 1 at 7 d, and 1 at 8 d. The following values for FaS were used for RID predictions: 2.40 at 3 d, 1.67 at 4 d, 1.34 at 5 d, 1.06 at 7 d, and 0.989 at 8 d. Liver VA concentration was estimated as TBS (μmol) × fraction of TBS stored in the liver / estimated liver weight (g), assuming that 80% of TBS are stored in the liver [40] and calculating liver weight using the regression equation presented in [41]. Predictions of TBS and liver VA concentration were compared by the specified groupings using a paired t test, Wilcoxon test, or Mann-Whitney test; results showed that there were no significant differences.
FIGURE 3.
Predictions of TBS and liver vitamin A concentration for healthy young people (n = 20) and subjects with SCD-HbSS before vitamin A supplementation (n = 22). Symbols are predictions for individual subjects at 3 d (except that 1 healthy subject was done at 5 d); lines are geometric means for each group. Values for TBS were calculated using Equation 1 (see Methods), with group values for the equation’s composite coefficient FaS calculated by modeling the SCD-HbSS pre-supplementation super-subject dataset along with each subject’s SAp at the corresponding time. Model-predicted values for FaS used in these calculations were 2.40 at 3 d and 1.34 at 5 d. Liver vitamin A concentration was estimated as TBS (μmol) × fraction of TBS in the liver / estimated liver weight (g), assuming that 80% of TBS was in the liver [40] and calculating liver weight using the regression equation in [41]. RID, retinol isotope dilution; SAp, plasma retinol specific activity; SCD-HbSS, sickle cell disease hemoglobin SS type; TBS, vitamin A total body stores.
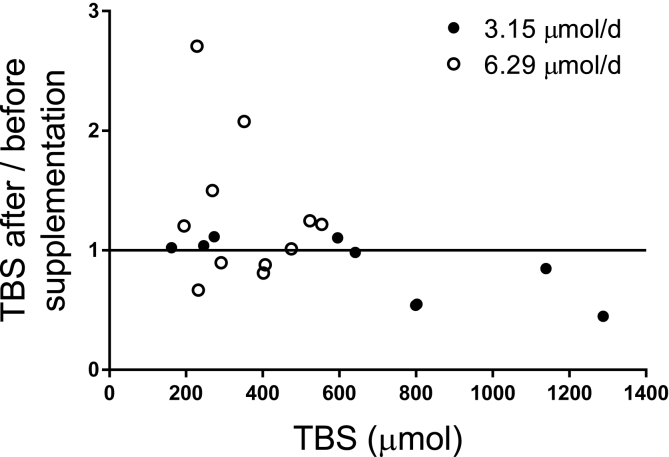
We then evaluated the effect of 8 wk of vitamin A supplementation (either 3.15 or 6.29 μmol/d) on TBS in the subjects with SCD-HbSS, using the paired-RID approach [2,39] to compare RID predictions of TBS based on samples collected 3 d after the first and second isotope doses. Because one subject with SCD-HbSS dropped out after intervention, data for another were below the limit of quantification, and actual sampling times varied from 3 d, TBS was calculated at 3 d after the second isotope dose for 14 subjects but at 4, 7, or 8 d postdosing for 6 others (using values for FaS derived for the same time in those cases). Overall, RID-predicted values of TBS before and after either level of intervention were not significantly different; the group mean TBS was ∼400 μmol after intervention (Table 2), and the mean ratio of TBS after / before supplementation was 1.0 (range, 0.45–2.7) (Figure 4). However, as illustrated in Figure 4, at lower values of TBS, vitamin A supplementation appeared to have more of a positive effect.
FIGURE 4.
Ratio of TBS after / before 8 wk of vitamin A supplementation (either 3.15 or 6.29 μmol/d) in young people with SCD-HbSS (n = 20) compared to TBS at baseline. Symbols represent TBS for each subject calculated using RID Equation 1 (see Methods) at 3 d after isotope administration; the line represents a ratio of 1. RID, retinol isotope dilution; SCD-HbSS, sickle cell disease hemoglobin SS type; TBS, vitamin A total body stores.
Discussion
SCD affects ∼100,000 individuals in the United States [43], and suboptimal vitamin A status, classified as plasma retinol concentration <1.05 μmol/L [10], has been reported in more than half of previously studied US children [4,5] and young people [6] with SCD-HbSS. Also, vitamin A supplementation, either at lower doses (1.05–2.10 μmol retinol/d) for a longer duration (12 mo) [5] or at higher doses (3.15 or 6.29 μmol retinol/d) for a shorter duration (8 wk) [6], did not result in increases in plasma retinol concentrations, although improvements were observed in some clinical and disease-related outcomes in the subjects who received the highest amount of supplemental vitamin A. In an effort to better understand the impacts of SCD on vitamin A status, we applied compartmental analysis to a composite (super-subject) retinol kinetics dataset in order to study whole-body vitamin A kinetics and to determine mean TBS, a more sensitive indicator of vitamin A status than plasma retinol concentrations [12], in the young people with SCD-HbSS studied by Brownell et al. [6] before vitamin A supplementation. To our knowledge, this is the first time the powerful tools of compartmental modeling have been used to study the vitamin A system in patients with SCD. We also used RID to predict TBS in individual subjects with SCD-HbSS before and after vitamin A supplementation, as well as in healthy peers.
There are several limitations to the current study, most importantly that kinetic studies were only done in the subjects with SCD-HbSS before intervention and that vitamin A interventions were not done in healthy subjects. If future experiments are planned to follow up on our initial observations, it would be ideal to include a super-subject kinetic study for each group and treatment to ensure that the most appropriate values of FaS (see a subsequent paragraph) are available for use in RID prediction equations and so that retinol kinetic parameters can be compared among groups. Although several vitamin A kinetic studies have been done in young children [[24], [25], [26],44] and in healthy young adults [22], such experiments have not yet been carried out in mid-childhood and adolescence. In addition, as pointed out in [6], sample sizes of both subjects with SCD-HbSS and healthy peers in this study were relatively small and, for the SCD-HbSS group, the duration of vitamin A supplementation was relatively short. Finally, it would have been optimal to use different stable isotopes of vitamin A to evaluate the impact of the vitamin A interventions to ensure that residual label from the first isotope dosing on day 0 did not confound SAp measurements after the second; it is also worth noting that increasing the dose of stable isotope would have improved detection at later sampling times. In spite of these limitations, our study provided some new and important results.
First, we noted that the tracer response profile for the composite group of SCD-HbSS subjects (Figure 2) is similar to patterns previously reported for healthy children [25,26]. The model-predicted TBS (428 μmol) indicate that these subjects with SCD-HbSS had adequate vitamin A stores; this finding is reasonable in light of their estimated adjusted dietary vitamin A intake (1.7 μmol RAE/d). Also, the model predicted that essentially all (92%) of total body exchangeable vitamin A was in compartment 6, the larger extravascular pool (presumed to be mainly but not exclusively the liver), with the balance in the small extravascular pool (compartment 7). The model-predicted proportion in the liver is higher than expected for individuals with adequate vitamin A status based on conventional wisdom [40,45], but it is not unreasonable.
In addition, our results show that model-predicted TBS for the young people with SCD-HbSS (428 μmol) was not significantly different from TBS predicted by RID at 3 d postdosing (389 μmol; Table 2); agreement between TBS predicted by population-based modeling and RID is not surprising [18], but it increases confidence in both approaches for estimating stores. Further, although mean plasma retinol concentration was lower in the subjects with SCD-HbSS than in healthy peers (Table 1), mean TBS predicted by RID at 3 d postdosing was not significantly different in the 2 groups (389 and 406 μmol, respectively; Table 2), and mean estimated liver vitamin A concentrations were adequate in both groups (0.302 μmol/g and 0.277 μmol/g, respectively) compared with the currently used cutoff for adequate (>0.1 μmol/g) [46] and assuming that 80% of TBS is in the liver [40]. Also of interest, among the 11 subjects with SCD-HbSS for whom data were available on both day 3 and day 28 postdosing, RID predictions of TBS were not significantly different. Although studies in theoretical subjects [16] indicate that later sampling times (>14 d in adults and >10 d in children) provide the most accurate RID predictions of TBS for the largest number of individuals, predictions for a group will be the same at all times if correct values for the composite coefficient FaS are used in the RID equation, along with the geometric mean SAp [18].
Regarding RID predictions, it is important to emphasize that TBS was estimated for healthy individuals and for the subjects with SCD-HbSS after vitamin A supplementation using values for FaS that were derived from modeling the (pre-supplementation) SCD-HbSS super-subject dataset. That is, since we did not obtain extensive retinol kinetic data after vitamin A supplementation in subjects with SCD-HbSS or in the healthy volunteers, we could not determine their group-specific values for FaS. Ideally, investigators should obtain group-specific values for the composite coefficient to ensure that the most accurate RID predictions of TBS for individuals will be calculated; when that is not possible, they should use values that were determined for a group that is as similar as possible to the one under study [16]. For example, if we had used 2.0, the d 3 value for FaS determined by Green and Green [47] for 20 theoretical healthy children who had a relatively low mean assigned value for TBS (284 μmol) rather than 2.4, the value for current subjects with SCD-HbSS, RID-predicted TBS for the healthy peer group would have been within 20% of the 406 μmol shown in Table 2.
In the current group of subjects with SCD-HbSS, mean TBS was not significantly increased after 8 wk of vitamin A supplementation (either 3.15 or 6.29 μmol/d). Assuming that these young people were in vitamin A balance before supplementation and using the steady state model-predicted vitamin A disposal rate (1.3 μmol/d), we estimated, based on the assumption that vitamin A absorption was 75%, that the maximum increase in TBS following the 2 interventions would have been 60 and 191 μmol, respectively. Compared with the group mean TBS before supplementation (428 μmol), these amounts correspond to 14% and 45% increases, respectively, and should have been detectable [48]. Because there is no reason to speculate that these levels of supplementation would alter vitamin A absorption, one must ask where the extra vitamin A is going. One previously discussed hypothesis is “adaptive preservation” [49], whereby during conditions of positive vitamin A balance, vitamin A disposal rate, specifically nonfunctional utilization, increases as a protective mechanism if stores are adequate, as in these subjects. That is, an increase in disposal rate mitigates part of the positive vitamin A balance. This would be reflected in our model as a time invariant fractional transfer coefficient for tracer irreversibly leaving the large vitamin A storage pool [L(0,6); Figure 1] and an increase in the corresponding transfer rate [the disposal rate, R(0,6)] as the mass of vitamin A in compartment 6 increases. The rate of transfer of retinol from compartment 6 to plasma would remain constant so that plasma retinol homeostasis would be maintained. Previous work in Stallings’ lab [5] showed that plasma retinol homeostasis is maintained in children with SCD-HbSS receiving long-term vitamin A supplements but at a concentration that is below normal.
Our findings—that TBS in young people with SCD-HbSS is not different from that in healthy peers and that vitamin A supplementation at the levels tested did not impact TBS or liver vitamin A concentrations in subjects with SCD-HbSS—do not resolve the question of why plasma retinol concentrations tend to be lower in US young people with SCD-HbSS. In addition to potential mechanisms discussed and rejected by Brownell et al. [6], it is known that chronic inflammation plays a central role in the pathophysiology of SCD [50]; inflammation is also associated with both decreased synthesis of the negative acute phase protein RBP (and thus hyporetinolemia) [2] and reductions in hepatic mobilization of vitamin A in rats with experimentally-induced inflammation [51]; although data are limited, inflammation may also interfere with vitamin A absorption [2,3]. It is likely that more extensive modeling studies in SCD compared with healthy subjects may be able to pinpoint the changes in whole-body vitamin A metabolism in SCD that account for suboptimal plasma retinol concentrations and the lack of impact of vitamin A supplementation on TBS.
Funding
Financial support for this work was provided by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; an Abbott Nutrition Advanced Fellowship in Pediatric Nutrition grant provided fellowship salary support to JNB, with no effect on study design, data collection, analysis, or interpretation, writing of the manuscript, or the decision to submit for publication. Additional support was provided by the Center for Human Phenomic Science (National Center for Advancing Translational Sciences, National Institutes of Health, UL1TR001878), Comprehensive Sickle Cell Center, Nutrition Center, and Cortner Endowed Chair at Children’s Hospital of Philadelphia (VAS). The [13C10]retinyl acetate used in these studies was donated by Newcastle University.
Author contributions
The authors responsibilities were as follows – VAS, JIS, MHG, GL: designed the experiment; VAS, JIS, JNB: conducted the clinical study; AO: performed retinol analyses; JLF, MHG: did the compartmental modeling and mathematical calculations; MHG, JLF, VAS, JIS, JNB, GL: interpreted the results; JLF, JIS, JNB: performed statistical analysis; JLF, MHG: drafted an initial version of the paper; JBG, MHG, VAS, GL: revised the manuscript, with input from other authors; JBG, MHG: finalized the article; MHG, VAS: have primary responsibility for the final content; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Data availability
Data described in the manuscript will be made available upon request pending publication and submission of an intended data use statement by a qualified investigator.
Acknowledgments
The authors thank DSM Nutritional Products, Basel, Switzerland, for providing the vitamin A supplements and Drs. Kim Smith-Whitley and Cynthia Norris (Division of Hematology, Children’s Hospital of Philadelphia, Philadelphia, PA). The authors would also like to express their appreciation to the patients with SCD and their families, as well as to the healthy subjects, for their participation in the study and their contributions to improving our understanding of this complex and debilitating disease.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.07.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M., et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 2.Lietz G., Furr H.C., Gannon B.M., Green M.H., Haskell M., Lopez-Teros V., et al. Current capabilities and limitations of stable isotope techniques and applied mathematical equations in determining whole-body vitamin A status. Food Nutr. Bull. 2016;37(2):S87–S103. doi: 10.1177/0379572116630642. suppl. [DOI] [PubMed] [Google Scholar]
- 3.Tanumihardjo S.A., Russell R.M., Stephensen C.B., Gannon B.M., Craft N.E., Haskell M.J., et al. Biomarkers of Nutrition for Development (BOND)-vitamin A review. J. Nutr. 2016;146(9):1816S–1848S. doi: 10.3945/jn.115.229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schall J.I., Zemel B.S., Kawchak D.A., Ohene-Frempong K., Stallings V.A. Vitamin A status, hospitalizations, and other outcomes in young children with sickle cell disease. J. Pediatr. 2004;145(1):99–106. doi: 10.1016/j.jpeds.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty K.A., Schall J.I., Kawchak D.A., Green M.H., Ohene-Frempong K., Zemel B.S., et al. No improvement in suboptimal vitamin A status with a randomized, double-blind, placebo-controlled trial of vitamin A supplementation in children with sickle cell disease. Am. J. Clin. Nutr. 2012;96(4):932–940. doi: 10.3945/ajcn.112.035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownell J.N., Schall J.I., Mcanlis C.R., Smith-Whitley K., Norris C.F., Stallings V.A. Effect of high-dose vitamin A supplementation in children with sickle cell disease: a randomized, double-blind, dose-finding pilot study. J. Pediatr. Hematol. Oncol. 2020;42(2):83–91. doi: 10.1097/MPH.0000000000001673. [DOI] [PubMed] [Google Scholar]
- 7.Wastnedge E., Waters D., Patel S., Morrison K., Goh M.Y., Adeloye D., et al. The global burden of sickle cell disease in children under five years of age: a systematic review and meta-analysis. J. Glob. Health. 2018;8(2) doi: 10.7189/jogh.08.021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health . NIH Publication No. 22-HL-3058; July 2022. Sickle cell disease. [Google Scholar]
- 9.Kawchak D.A., Schall J.I., Zemel B.S., Ohene-Frempong K., Stallings V.A. Adequacy of dietary intake declines with age in children with sickle cell disease. J. Am. Diet. Assoc. 2007;107(5):843–848. doi: 10.1016/j.jada.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Ballew C., Bowman B.A., Sowell A.L., Gillespie C. Serum retinol distributions in residents of the United States: Third National Health and Nutrition Examination Survey, 1988-1994. Am. J. Clin. Nutr. 2001;73(3):586–593. doi: 10.1093/ajcn/73.3.586. [DOI] [PubMed] [Google Scholar]
- 11.Imdad A., Mayo-Wilson E., Herzer K., Bhutta Z.A. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst. Rev. 2017;3(3):CD008524. doi: 10.1002/14651858.CD008524.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson J.A. Serum levels of vitamin A and carotenoids as reflectors of nutritional status. J. Natl. Cancer Inst. 1984;73(6):1439–1444. [PubMed] [Google Scholar]
- 13.Green M.H., Ford J.L., Green J.B., Berry P., Boddy A.V., Oxley A., et al. A retinol isotope dilution equation predicts both group and individual total body vitamin A stores in adults based on data from an early postdosing blood sample. J. Nutr. 2016;146(10):2137–2142. doi: 10.3945/jn.116.233676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Teros V., Green M.H., Haskell M.J., Green J.B. Influence of vitamin A status on the choice of sampling time for application of the retinol isotope dilution method in theoretical children. J. Nutr. 2021;151(12):3874–3881. doi: 10.1093/jn/nxab310. [DOI] [PubMed] [Google Scholar]
- 15.Pinkaew S., Udomkesmalee E., Davis C.R., Tanumihardjo S.A. Vitamin A–fortified rice increases total body vitamin A stores in lactating Thai women measured by retinol isotope dilution: a double-blind, randomized, controlled trial. Am. J. Clin. Nutr. 2021;113(5):1372–1380. doi: 10.1093/ajcn/nqaa418. [DOI] [PubMed] [Google Scholar]
- 16.Green M.H., Green J.B. The use of datasets for theoretical subjects to validate vitamin A–related methods and experimental designs. J. Nutr. 2022;152(3):707–713. doi: 10.1093/jn/nxab441. [DOI] [PubMed] [Google Scholar]
- 17.Furr H.C., Amedee-Manesme O., Clifford A.J., Bergen H.R., 3rd, Jones A.D., Anderson D.P., et al. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am. J. Clin. Nutr. 1989;49(4):713–716. doi: 10.1093/ajcn/49.4.713. [DOI] [PubMed] [Google Scholar]
- 18.Green M.H., Green J.B., Ford J.L. Better predictions of vitamin A total body stores by the retinol isotope dilution method are possible with deeper understanding of the mathematics and by applying compartmental modeling. J. Nutr. 2020;150(5):989–993. doi: 10.1093/jn/nxz321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gannon B.M., Tanumihardjo S.A. Comparisons among equations used for retinol isotope dilution in the assessment of total body stores and total liver reserves. J. Nutr. 2015;145(5):847–854. doi: 10.3945/jn.114.208132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cifelli C.J., Green J.B., Green M.H. Use of model-based compartmental analysis to study vitamin A kinetics and metabolism. Vitam. Horm. 2007;75:161–195. doi: 10.1016/S0083-6729(06)75007-5. [DOI] [PubMed] [Google Scholar]
- 21.Cifelli C.J., Green J.B., Wang Z., Yin S., Russell R.M., Tang G., et al. Kinetic analysis shows that vitamin A disposal rate in humans is positively correlated with vitamin A stores. J. Nutr. 2008;138(5):971–977. doi: 10.1093/jn/138.5.971. [DOI] [PubMed] [Google Scholar]
- 22.Green M.H., Ford J.L., Oxley A., Green J.B., Park H., Berry P., et al. Plasma retinol kinetics and β-carotene bioefficacy are quantified by model-based compartmental analysis in healthy young adults with low vitamin A stores. J. Nutr. 2016;146(10):2129–2136. doi: 10.3945/jn.116.233486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gannon B.M., Valentine A.R., Davis C.R., Howe J.A., Tanumihardjo S.A. Duration of retinol isotope dilution studies with compartmental modeling affects model complexity, kinetic parameters, and calculated vitamin A stores in US women. J. Nutr. 2018;148(8):1387–1396. doi: 10.1093/jn/nxy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Teros V., Ford J.L., Green M.H., Tang G., Grusak M.A., Quihui-Cota L., et al. Use of a “super-child” approach to assess the vitamin A equivalence of Moringa oleifera leaves, develop a compartmental model for vitamin A kinetics, and estimate vitamin A total body stores in young Mexican children. J. Nutr. 2017;147(12):2356–2363. doi: 10.3945/jn.117.256974. [DOI] [PubMed] [Google Scholar]
- 25.Ford J.L., Green J.B., Haskell M.J., Ahmad S.M., Mazariegos Cordero D.I., Oxley A., et al. Use of model-based compartmental analysis and a super-child design to study whole-body retinol kinetics and vitamin A total body stores in children from 3 lower-income countries. J. Nutr. 2020;150(2):411–418. doi: 10.1093/jn/nxz225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Teros V., Ford J.L., Green M.H., Monreal-Barraza B., García-Miranda L., Tanumihardjo S.A., et al. The “super-child” approach is applied to estimate retinol kinetics and vitamin A total body stores in Mexican preschoolers. J. Nutr. 2020;150(6):1644–1651. doi: 10.1093/jn/nxaa048. [DOI] [PubMed] [Google Scholar]
- 27.Engle-Stone R., Miller J.C., Reario M.F.D., Arnold C.D., Stormer A., Lafuente E., et al. Filipino children with high usual vitamin A intakes and exposure to multiple sources of vitamin A have elevated total body stores of vitamin A but do not show clear evidence of vitamin A toxicity. Curr. Dev. Nutr. 2022;6(8) doi: 10.1093/cdn/nzac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oxley A., Berry P., Taylor G.A., Cowell J., Hall M.J., Hesketh J., et al. An LC/MS/MS method for stable isotope dilution studies of β-carotene bioavailability, bioconversion, and vitamin A status in humans. J. Lipid Res. 2014;55(2):319–328. doi: 10.1194/jlr.D040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oxley A., Lietz G. Use of stable isotopes to study bioconversion and bioefficacy of provitamin A carotenoids. Methods Enzymol. 2022;670:399–422. doi: 10.1016/bs.mie.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Oxley A., Engle-Stone R., Miller J.C., Reario M.F.D., Stormer A., Capanzana M.V., et al. Determination of vitamin A total body stores in children from dried serum spots: application in a low- and middle-income country community setting. J. Nutr. 2021;151(5):1341–1346. doi: 10.1093/jn/nxaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadler S.B., Hidalgo J.H., Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232. [PubMed] [Google Scholar]
- 32.Ford J.L., Green J.B., Green M.H. A population-based (super-child) approach for predicting vitamin A total body stores and retinol kinetics in children is validated by the application of model-based compartmental analysis to theoretical data. Curr. Dev. Nutr. 2018;2(11):nzy071. doi: 10.1093/cdn/nzy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berman M., Weiss M.F. US Government Printing Office, US DHEW (NIH); Washington, DC: 1978. The SAAM Manual. [Google Scholar]
- 34.Wastney M.E., Patterson B.H., Linares O.A., Greif P.C., Boston R.C., WinSAAM . Academic Press; San Diego, CA: 1999. Investigating Biological Systems Using Modeling: Strategies and Software; pp. 95–138. [Google Scholar]
- 35.Stefanovski D., Moate P.J., Boston R.C. WinSAAM: a Windows-based compartmental modeling system. Metabolism. 2003;52(9):1153–1166. doi: 10.1016/s0026-0495(03)00144-6. [DOI] [PubMed] [Google Scholar]
- 36.Green M.H., Ford J.L., Green J.B. Inclusion of vitamin A intake data provides improved compartmental model-derived estimates of vitamin A total body stores and disposal rate in older adults. J. Nutr. 2019;149(7):1282–1287. doi: 10.1093/jn/nxz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford J.L., Green J.B., Green M.H. Addition of vitamin A intake data during compartmental modeling of retinol kinetics in theoretical humans leads to accurate prediction of vitamin A total body stores and kinetic parameters in studies of reasonable duration. J. Nutr. 2019;149(11):2065–2072. doi: 10.1093/jn/nxz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Institute of Medicine . National Academies Press; Washington, DC: 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. [PubMed] [Google Scholar]
- 39.Haskell M.J., Jamil K.M., Peerson J.M., Wahed M.A., Brown K.H. The paired deuterated retinol dilution technique can be used to estimate the daily vitamin A intake required to maintain a targeted whole body vitamin A pool size in men. J. Nutr. 2011;141(3):428–432. doi: 10.3945/jn.110.133124. [DOI] [PubMed] [Google Scholar]
- 40.Valentine A.R., Davis C.R., Tanumihardjo S.A. Vitamin A isotope dilution predicts liver stores in line with long-term vitamin A intake above the current Recommended Dietary Allowance for young adult women. Am. J. Clin. Nutr. 2013;98(5):1192–1199. doi: 10.3945/ajcn.113.063867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshizumi T., Gondolesi G.E., Bodian C.A., Jeon H., Schwartz M.E., Fishbein T.M., et al. A simple new formula to assess liver weight. Transplant. Proc. 2003;35(4):1415–1420. doi: 10.1016/s0041-1345(03)00482-2. [DOI] [PubMed] [Google Scholar]
- 42.Wastney M.E., Patterson B.H., Linares O.A., Greif P.C., Boston R.C. Investigating Biological Systems Using Modeling: Strategies and Software. Academic Press; San Diego, CA: 1999. Rejecting hypotheses and accepting a model; pp. 237–250. [Google Scholar]
- 43.Sickle Cell Disease (SCD) Centers for Disease Control and Prevention; 2023. Data & statistics.https://www.cdc.gov/ncbddd/sicklecell/data.html [Internet] Available from: [Google Scholar]
- 44.Lopez-Teros V., Quihui-Cota L., Méndez-Estrada R.O., Grijalva-Haro M.I., Esparza-Romero J., Valencia M.E., et al. Vitamin A-fortified milk increases total body vitamin A stores in Mexican preschoolers. J. Nutr. 2013;143(2):221–226. doi: 10.3945/jn.112.165506. [DOI] [PubMed] [Google Scholar]
- 45.Olson J.A. Recommended dietary intakes (RDI) of vitamin A in humans. Am. J. Clin. Nutr. 1987;45(4):704–716. doi: 10.1093/ajcn/45.4.704. [DOI] [PubMed] [Google Scholar]
- 46.Tanumihardjo S.A. Biological evidence to define a vitamin A deficiency cutoff using total liver vitamin A reserves. Exp. Biol. Med. (Maywood) 2021;246(9):1045–1053. doi: 10.1177/1535370221992731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green M.H., Green J.B. Use of model-based compartmental analysis and theoretical data to further explore choice of sampling time for assessing vitamin A status in groups and individual human subjects by the retinol isotope dilution method. J. Nutr. 2021;151(7):2068–2074. doi: 10.1093/jn/nxab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haskell M.J., Jamil K.M., Hassan F., Peerson J.M., Hossain M.I., Fuchs G.J., et al. Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. Am. J. Clin. Nutr. 2004;80(3):705–714. doi: 10.1093/ajcn/80.3.705. [DOI] [PubMed] [Google Scholar]
- 49.Green M.H., Green J.B. Vitamin A intake and status influence retinol balance, utilization and dynamics in rats. J. Nutr. 1994;124(12):2477–2485. doi: 10.1093/jn/124.12.477. [DOI] [PubMed] [Google Scholar]
- 50.Conran N., Belcher J.D. Inflammation in sickle cell disease. Clin. Hemorheol. Microcirc. 2018;68(2–3):263–299. doi: 10.3233/CH-189012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gieng S.H., Green M.H., Green J.B., Rosales F.J. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J. Lipid Res. 2007;48(4):904–913. doi: 10.1194/jlr.M600528-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request pending publication and submission of an intended data use statement by a qualified investigator.