Abstract
The field of super-resolution ultrasound microvascular imaging has been rapidly growing over the past decade. By leveraging contrast microbubbles as point targets for localization and tracking, super-resolution ultrasound pinpoints the location of microvessels and measures their blood flow velocity. Super-resolution ultrasound is the first in vivo imaging modality that can image micron-scale vessels at a clinically relevant imaging depth without tissue destruction. These unique capabilities of super-resolution ultrasound provide structural (vessel morphology) and functional (vessel blood flow) assessments of tissue microvasculature on a global and local scale, which opens new doors for many enticing preclinical and clinical applications that benefit from microvascular biomarkers. The goal of this short review is to provide an update on recent advancements in super-resolution ultrasound imaging, with a focus on summarizing existing applications and discussing the prospects of translating super-resolution imaging to clinical practice and research. In this review, we also provide brief introductions of how super-resolution ultrasound works, how does it compare with other imaging modalities, and what are the tradeoffs and limitations for an audience who is not familiar with the technology.
Keywords: Super-resolution, Ultrasound, Microvascular imaging, Ultrasound localization microscopy
Introduction
Ultrasound is one of the most popular modalities for vascular imaging. Thanks to a fast imaging speed and high sensitivity to motion, ultrasound uniquely provides real-time imaging of blood flow with rich spatiotemporal information. To date, established techniques such as pulsed wave (PW) Doppler, color flow imaging (CFI), and power Doppler (PD) are ubiquitous on ultrasound scanners and widely used in clinic.
In recent years, a trending direction in ultrasound vascular imaging development is increasing the sensitivity to small vessels and the microvasculature. The key enabling technology behind these developments is ultrafast ultrasound imaging, which uses massive, parallel receive beamforming (e.g., plane wave imaging) to achieve an ultrafast imaging speed of tens of thousands of frames per second [1], [2]. Similar to the effect of long exposure photography, an ultrafast imaging speed allows rapid accumulation of blood flow signals within a short period of time, significantly boosting the sensitivity to small vessels that are otherwise invisible via conventional imaging methods [3], [4] (e.g., Fig. 1(a) vs. (b)). When combined with advanced tissue clutter filters such those based on singular value decomposition, these ultra-sensitive Doppler techniques become even more effective at extracting small vessel signals from the moving tissues [5], [6].
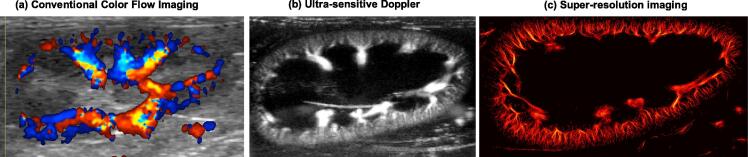
Figure 1.
Example ultrasound blood flow images taken from a rabbit kidney. (a) Conventional color Doppler flow imaging is limited to the larger vessels, predominantly in the medullary region. (b) Ultra-sensitive Doppler, enabled by ultra-fast ultrasound imaging, reveals small vessels in the kidney cortex (reprinted from [76]). (c) Super-resolution ultrasound imaging can resolve these cortical microvessels without sacrificing penetration depth (reprinted from [77]).
More recently, this pursuit of higher sensitivity to small vessels has entered a new era – super-resolution ultrasound microvascular imaging (Fig. 1(c). We will use the term “super-resolution ultrasound” or “super-resolution imaging” for the rest of the paper). Different from ultra-sensitive Doppler which is contrast-free, super-resolution ultrasound primarily relies on contrast microbubbles to gain sensitivity to microvessels, which is similar to the principles of contrast enhanced ultrasound (CEUS). In addition to the high sensitivity to small vessels, super-resolution ultrasound is also capable of resolving individual microvessels that are indiscernible with conventional ultrasound such as ultra-sensitive Doppler and CEUS. At present, super-resolution ultrasound remains the only imaging modality that offers micron-scale spatial resolution at a clinically relevant imaging depth (e.g., centimeters). The unique combination of high spatial resolution and deep imaging penetration has opened new doors for many enticing preclinical and clinical applications based on vascular and microvascular biomarkers. Here, we will provide a brief review of the principles of super-resolution ultrasound imaging and state-of-the-art techniques. We will then discuss trade-offs, challenges, and limitations of super-resolution imaging, followed by an overview of existing applications and future directions. For an extensive review of the super-resolution imaging technology, readers are referred to two seminal review papers by Couture et al. [7] and Christensen-Jeffries et al. [8].
Principles of super-resolution ultrasound microvascular imaging
Inspired by optical super-resolution imaging techniques such as PALM [9], [10] (Photoactivation Localization Microscopy), Couture et al. first introduced the concept of super-localizing disrupted contrast microbubbles to break the diffraction limit of ultrasound and achieve super-resolution [11]. Around the same time, Siepmann et al. used microbubble positions in tumor mouse models to improve microvascular imaging spatial resolution [12]. These early works were rapidly followed by various groups around the world [13], [14], [15], and the seminal works by Errico et al. [16] and Christensen-Jeffries et al. [17] in 2015 served as catalysts for the rapid growth of the super-resolution imaging field [18], [19], [20], [21], [22], [23]. The principle behind PALM and super-resolution ultrasound is straightforward (Fig. 2): by localizing the position of a point target (i.e., fluorophore in PALM, microbubble in ultrasound), which is much smaller than the size of the interrogating waves, one can effectively deblur the image and achieve super-resolution. For example, in optics, a fluorophore is a few nanometers in size while an emitted photon wavelength is typically on the scale of hundreds of nanometers; for ultrasound imaging, as illustrated in Fig. 3, a microbubble is a few microns in diameter and the wavelength of clinical ultrasound is several hundreds of microns. Without super-localization, a direct accumulation of microbubble signal (i.e., power Doppler) generates blurred vascular images (Fig. 2(d)). In contrast, by super-localizing and accumulating the location of microbubbles, one can generate super-resolved vascular images with much enhanced spatial resolution (Fig. 2(e)). The branch of super-resolution ultrasound imaging techniques that relies on microbubble localization to break the diffraction limit and achieve super-resolution is also commonly referred to as ultrasound localization microscopy (ULM).
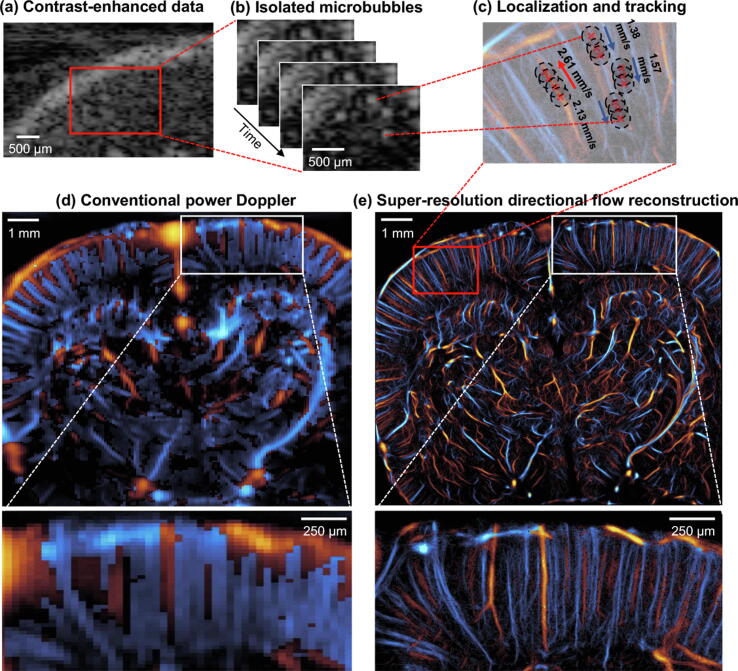
Figure 2.
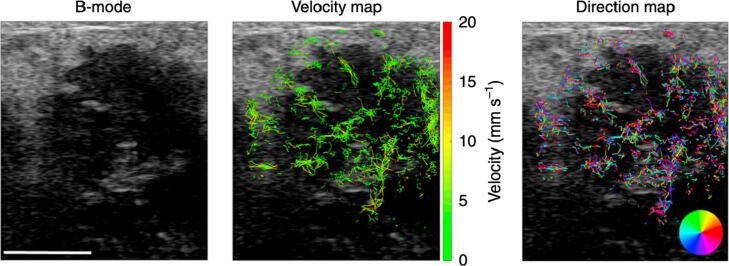
Super-resolution ultrasound imaging workflow. (a) Contrast-enhanced ultrasound imaging data undergoes clutter filtering to reveal (b) isolated microbubble signals. (c) Microbubble localization, frame-to-frame pairing, and tracking is performed to estimate super-resolved blood flow trajectories. (d) A conventional power Doppler processed coronel section of a rat brain is compared to a (e) super-resolved directional flow map of the same dataset.
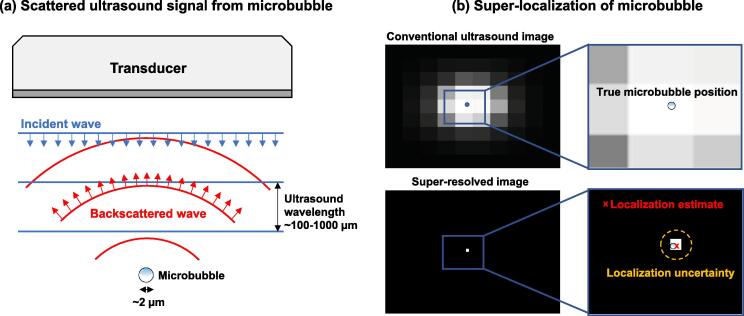
Figure 3.
Principles of microbubble super-localization. (a) The scattered ultrasound wave is several orders of magnitude larger that the microbubble diameter. (b) Super-localization strategies provide microbubble localization information that is much smaller than the ultrasound wavelength. The localization accuracy is inherently limited by the Cramér-Rao lower bound of the ultrasound imaging system and beamforming process which include factors such as ultrasound frequency, bandwidth, signal-to-noise-ratio (SNR), and correlation between the true microbubble signal and the estimated template [78].
The key condition for super-resolution imaging to work is to have spatially isolated point targets (e.g., Fig. 2(b)). In PALM, this condition is fulfilled by activating and deactivating photoactivatable molecules so that their signals become uncoupled in space. In super-resolution ultrasound, uncoupling is primarily achieved by the movement of microbubbles in the blood stream. For example: if the ultrasound signal from microbubble A overlaps with that from microbubble B in time t, they may become separated in time t+Δt if they move relative to one another. Although several studies have reported methods of active manipulation of microbubble signals for super-resolution [13], [24], [25], the majority of the existing techniques rely on the passive and stochastic process of blood flow-induced microbubble movement to achieve super-resolution.
Another unique feature of super-resolution ultrasound imaging is blood flow velocity quantification in small vessels. For example, as shown in Fig. 2(c), by tracking microbubble movement in the blood stream, one can infer blood flow speed and direction. This feature is distinct from conventional Doppler ultrasound which uses the Doppler frequency shift induced by the movement of red blood cells to measure blood velocity [26]. As such, super-resolution ultrasound is not subject to common sources of errors in Doppler ultrasound such as Doppler angle and spectral broadening [26]. However, super-resolution ultrasound may be less robust in measuring blood velocity in bigger vessels because of the higher microbubble concentration (i.e., less likely to localize isolated microbubbles) and faster blood flow (because it is difficult to track multiple microbubbles that are fast moving). In practice, as detailed below, blood flow measurements provided by super-resolution ultrasound is the time-averaged velocity during data acquisition. Instantaneous velocity measurements are available during the microbubble tracking process [27] but they may not be as robust as time-averaged estimates [16].
The big picture
How does super-resolution ultrasound compare with existing imaging modalities in terms of imaging resolution versus penetration? Fig. 4 shows the comparison. Compared to optical imaging and photoacoustic microscopy, super-resolution ultrasound offers better imaging penetration. Super-resolution ultrasound has comparable imaging depth as conventional ultrasound because the localization process does not compromise imaging penetration (i.e., it uses the same data as conventional ultrasound imaging). Compared to non-optical imaging methods such as magnetic resonance imaging (MRI), super-resolution ultrasound offers significantly higher spatial resolution. As indicated in Fig. 4, super-resolution ultrasound begins to occupy the penetration-resolution gap of existing biomedical imaging modalities.
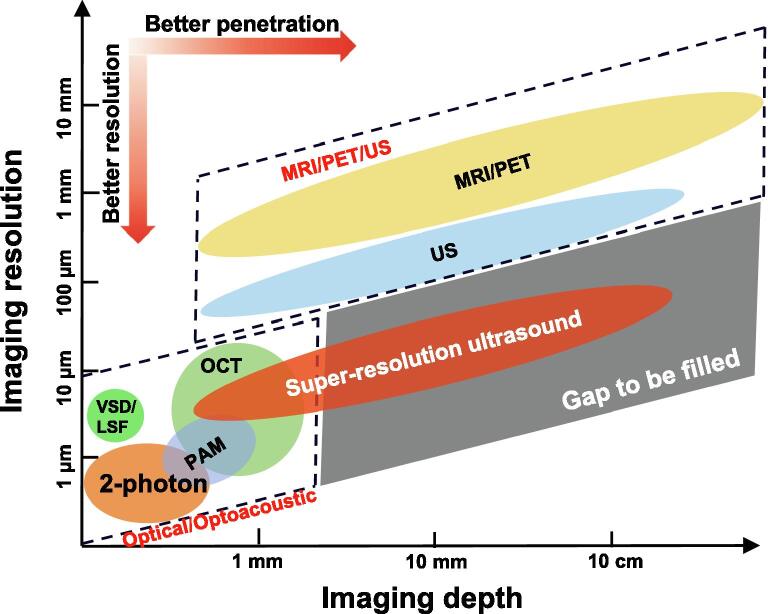
Figure 4.
Comparison of imaging resolution and imaging penetration depth for different modalities that are capable of vascular imaging. (2-photon: two-photon microscopy; LSF: laser-speckle flow imaging; MRI: magnetic resonance imaging; OCT: optical coherence tomography; PAM: photoacoustic microscopy (optical resolution); PET: positron emission tomography; US: ultrasound; VSD: voltage sensitive dye imaging).
What are the tradeoffs and limitations?
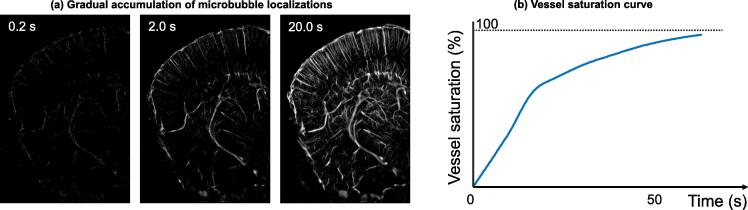
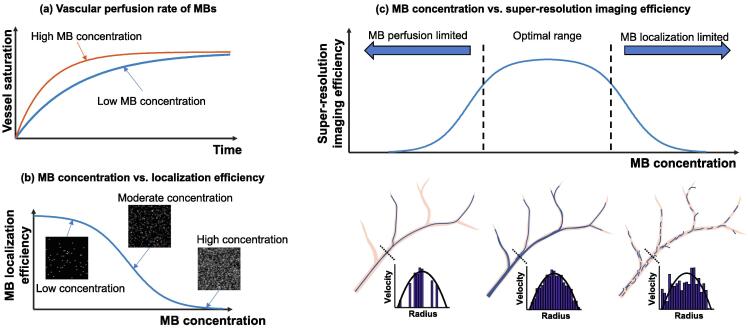
Invariably, there is a tradeoff. Fig. 5 helps to illustrate: since microbubble localization depends on the presence of spatially separated microbubble signals, the time it takes to generate a complete microvessel image is controlled by how quickly one can localize at least one microbubble at each spatial location where there is a vessel [28], [29], [30]. In practice, this process takes a long time because of the limited perfusion rate of microbubbles in the blood stream [31] (especially for smaller vessels where microbubble events can be rare) and the stochastic nature of microbubble uncoupling (e.g., being spatially isolated). In theory, increasing the microbubble concentration, which increases the perfusion rate (Fig. 6(a)), should decrease imaging time; however, more microbubbles decrease the likelihood of uncoupling and makes the localization process more challenging (Fig. 6(b)). As such, using the appropriate microbubble concentration is essential for successful super-resolution imaging. Unfortunately, the appropriate concentration is typically lower than the standard clinical dose of microbubble administration. Additionally, infusion of microbubbles is generally preferred over bolus injections for a stable microbubble concentration. Collectively, these factors contribute to a long data acquisition time for super-resolution imaging that ranges from several seconds to several minutes depending on the tissue or organ and the desired reconstruction fidelity.
Figure 5.
Illustration of super-resolution ultrasound accumulation process limited by microbubble perfusion rate. (a) Gradual accumulation of microbubble localizations in a coronel section of a mouse brain. (b) Example saturation curve for reconstructed cerebrovasculature from images taken in (a).
Figure 6.
Schematics for illustrating the relationships among microbubble (MB) concentration, vessel saturation rate, microbubble localization efficiency, and microvessel reconstruction fidelity. (a) A higher concentration of microbubbles will perfuse more vessels per unit time, leading to a faster vascular saturation rate. (b) Lower concentrations of microbubbles are more spatially sparse, making detection and localization easier. (c) Super-resolution ultrasound requires a “sweet spot” of microbubble concentration to enable accurate vessel reconstruction within a pragmatic amount of time.
Recognizing slow imaging speed as the key limitation of super-resolution ultrasound, the research community has proposed and developed many methods and techniques to address this challenge. These solutions span basic filtering techniques [32], advanced sparsity-based algorithms [22], [33] and recently, deep learning [22], [34], [35], [36], [37], [38]. Some of the solutions push the temporal resolution of super-resolution ultrasound to one second and even sub-seconds [22], [38], [39]. However, most of the existing fast super-resolution imaging techniques trade spatial resolution and completeness of the vessel structure for imaging speed. Also, some techniques do not offer the capability of blood flow velocity measurement provided by conventional super-resolution imaging (Fig. 2). Nevertheless, super-resolution imaging acceleration remains an active area of research and solutions for fast and robust imaging are anticipated in the near future.
Another limitation of super-resolution ultrasound imaging is the need for contrast injection, which diminishes the attraction of this otherwise excellent methodology. Areas already accustomed to contrast use may show faster transition to super-resolution imaging than those currently relying on intrinsic mechanisms such as scattering from moving red blood cells. Unfortunately, since the majority of clinical vascular imaging applications are non-contrast-based, the rate of adoption for super-resolution ultrasound may be limited. Compelling applications of super-resolution ultrasound with clear clinical evidence of effectiveness are necessary to facilitate the translational process. In the meantime, contrast-free techniques based on backscattering of native blood cells are also being actively developed [40], [41], [42]. Because of the absence of spatially isolated point targets such as microbubbles, contrast-free techniques do not provide spatial resolution as high as conventional super-resolution ultrasound. However, they may become more attractive alternatives to conventional super-resolution imaging for pragmatic reasons.
What are the existing applications?
To date, most super-resolution ultrasound applications are concentrated on preclinical animal models and pilot clinical studies. Table 1 summarizes representative existing applications of super-resolution ultrasound, which involve clinical areas including neurology, oncology, nephrology, and cardiology. Figure 7, Figure 8, Figure 9, Figure 10 presents representative examples of in vivo human super-resolution ultrasound imaging.
Table 1.
Summary of existing in vivo preclinical and clinical studies using super-resolution ultrasound imaging
| Organ | Condition | Imaging setting and model | Publication | Likelihood for transition to clinical research and practice |
|---|---|---|---|---|
| Brain | Stroke | Rat | Chavignon et al., 2022 [45] | Medium (non-neonates):
|
| Brain | Stroke | Human | Demené et al., 2021 [44] | |
| Brain | Alzheimer’s disease | Mouse | Lowerison et al., 2022 [47] | |
| Brain | Aging | Mouse | Lowerison et al., 2022 [48] | |
| Brain | Hydrocephalus | Pig | Zhang et al., 2022 [79] | |
| Brain | Normal animal for functional neural activity imaging; normal animal for dynamic pulsatility measurement | Rat | Renaudin et al., 2022 [39]; Bourquin et al., 2022 [67]; McCall et al., [80] | |
| Spinal cord | Normal | Rat | Claron et al., 2021 [81]; van Sloun et al., 2021 [34] | Low-medium:
|
| Breast | Cancer | Human | Opacic et al., 2018 [21]; Huang et al., 2021 [62] | High:
|
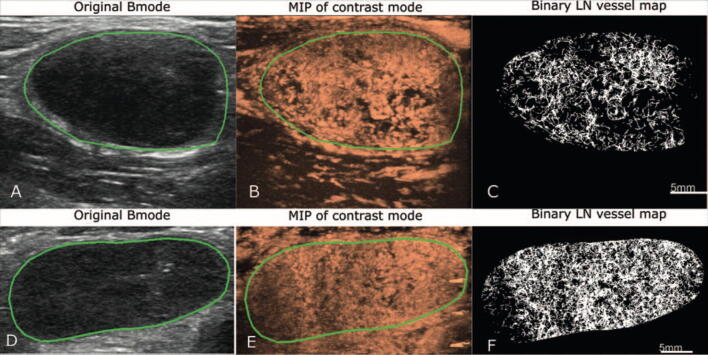
| Lymph Node | Normal | Rabbit | Zhu et al., 2019 [82] | High:
|
| Lymph Node | Metastatic cancer | Human | Zhu et al., 2022 [61] | |
| Tumor xenografts | Colorectal cancer; renal cell carcinoma; fibrosarcoma; lung carcinoma; epidermoid carcinoma; myxoid liposarcomas | Chicken embryo chorioallantoic membrane; rat; mouse | Lowerison et al., 2022 [50]; Lowerison et al., 2020 [49]; Lin et al., 2017 [18]; Opacic et al., 2018 [21] | N/A |
| Pancreas | Cancer | Human | Huang et al., 2021 [62] | Medium:
|
| Liver | Cancer | Rabbit | Zhang et al., 2021 [64] | Medium – High:
|
| Liver | Normal and acute-on-chronic liver failure | Human | Huang et al., 2021 [62] | |
| Kidney | Ischemia | Rat | Andersen et al., 2020 [23] | Medium – High:
|
| Kidney | Acute injury | Mouse | Chen et al., 2020 [83] | |
| Kidney | Normal | Human | Huang et al., 2021 [62] | |
| Kidney | Transplant allograft | Human | Bodard et al., 2023 [73] | High:
|
| Prostate | Unknown | Human | Solomon et al., 2019 [60] | High:
|
| Heart | Normal | Isolated rat heart; in vivo rat heart (transthoracic imaging) | Demeulenaere et al., 2022 [65]; Cormier et al., 2021 [66] | Low-Medium:
|
| Lower limb muscle | Normal | Human | Harput et al., 2018 [84] | High:
|
| Vasa vasorum | Atherosclerotic plaques; Takayasu arteritis | Rabbit and human | Chen et al., 2020 [85]; Goudot et al., 2023 [86] | Medium - High:
|
| Eye | Elevated intraocular pressure | Rabbit | Qian et al., 2022 [87] | Medium:
|
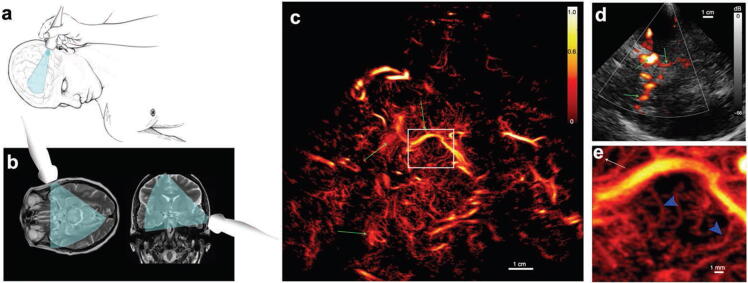
Figure 7.
In vivo human brain super-resolution imaging. (a) Schematic diagram indicating the handheld ultrasound imaging setup; (b) diagram indicating the ultrasound imaging field-of-view with respect to brain MRI images; (c) reconstructed super-resolution microvessel density map. The white box indicates the location of the magnified view in (e); (d) conventional power Doppler image of the same brain region as in (c). The green arrows serve as landmarks for comparison between (c) and (d). (Images adapted from Demené et al., 2021 [44]).
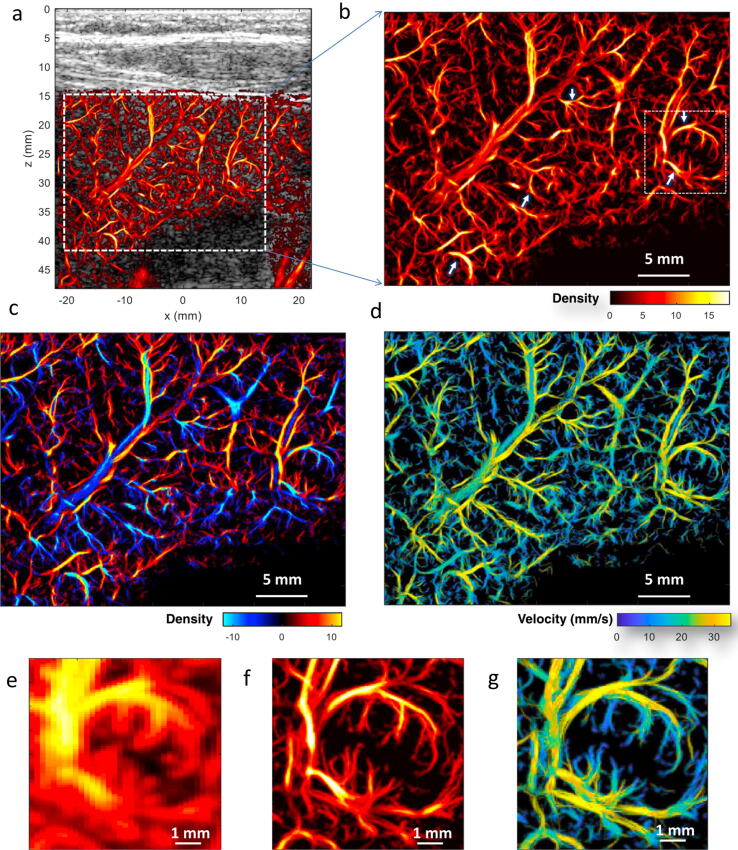
Figure 8.
In vivo human liver super-resolution imaging from a 38 year old patient with acute-on-chronic liver failure. (a) Super-resolved liver microvascular image overlaid on the B-mode image; (b) magnified view of the region indicated by the white dashed box in (a); (c) directional microvessel density map (red color indicating bottom-top flow direction and blue color indicating top-bottom flow direction); (d) super-resolved flow velocity map; (e) – (g) power Doppler, super-resolved vessel density, and flow velocity map of a local region marked by the white dashed box in (b). The white arrows denote distorted vessels with tortuosity and/or tapering in the main branches. (Images reprinted from Huang et al., 2021 [62]).
Figure 9.
Super-resolution ultrasound imaging of a patient with HER2 positive breast cancer. The vascular map reveals heterogeneously distributed microvessels throughout the tumor. (Images adapted from Opacic et al., 2018 [21]).
Figure 10.
In vivo human lymph node super-resolution imaging. (A-C) images from a reactive lymph node; (D-F) images from a metastatic lymph node. The first column presents the B-mode images, the second column presents the maximum intensity projection (MIP) images of the microbubble data, and the third column presents the reconstructed super-resolution microvessel density maps. (Images reprinted from Zhu et al., 2002 [61]).
For applications in the brain, super-resolution ultrasound found a niche in small animal disease models because it allows the probing of deep brain regions with high spatial resolution, which complements mainstream brain imaging modalities including optics and MRI. Several studies have also demonstrated intact skull imaging with super-resolution ultrasound (in animals [43] and humans [44]). For applications in stroke [45], [46], the high spatial resolution and quantitative hemodynamic measurements of microvessels (e.g., diffusion index, flow speed) allowed detection of deep seated aneurysm and separation between ischemic and hemorrhagic stroke. For applications in aging and Alzheimer’s disease [47], [48], super-resolution ultrasound indicated global reduction of vascularity and microvascular flow speed in mouse models. Super-resolution ultrasound also revealed early functional impairment of the cerebral microvasculature (e.g., reduced blood velocity) that preceded structural variations (e.g., reduced vascularity) in Alzheimer’s disease.
Cancer is the next major application of super-resolution ultrasound where noninvasive imaging of tumor microvasculature is significant for early detection, diagnosis, and prognosis. The ability of resolving detailed tumor microvasculature and providing quantitative metrics such as microvessel density, tortuosity, and flow velocity is essential for identifying microvessel phenotypes and characterizing the tumor vascular microenvironment, which is highly correlated with tumor invasiveness and metastatic potential [21], [49], [50]. Some of the assessments such as small vessel tortuosity and flow velocity are not readily available from other imaging modalities and unique to super-resolution ultrasound. These measurements reflect the structural and functional abnormalities of the tumor microvascular environment, which are useful for applications such as evaluating and predicting antiangiogenic therapy response [51], [52]. Nevertheless, the usefulness of super-resolution ultrasound for cancer and the advantages of super-resolution imaging over CEUS still need to be demonstrated by following existing CEUS cancer studies [51], [53], [54], [55]. In addition, as suggested by clinical evidence [56], [57], tumor perfusion measurements are sensitive biomarkers for evaluating cancer treatment response. In theory, super-resolution ultrasound is capable of evaluating tumor perfusion when combining the blood velocity measurements with vessel geometry. The emerging 3D super-resolution imaging techniques greatly facilitate the development of this new capability in the future [43], [45], [58].
Is super-resolution ultrasound ready for the clinic?
Although super-resolution ultrasound is likely ready for research investigations in clinic, a pressing issue for its application for large-scale clinical use is the lack of demonstrated clinical value of super-resolution imaging over conventional contrast-enhanced ultrasound (CEUS). This raises further questions: can super-resolution imaging provide better quantification (than CEUS) which translates to more accurate diagnoses? Is super-resolution imaging less susceptible to the sources of variabilities in CEUS [59]? Is super-resolution imaging repeatable? Since CEUS is a much simpler and faster technique than super-resolution imaging, the added benefit must be significant for super-resolution to gain traction in the clinic.
To answer these questions, one needs to have established super-resolution imaging solutions that are readily available in a clinical imaging setting. Unfortunately, super-resolution imaging largely remains as a research tool at present due to the aforementioned imaging speed limitation (and associated issues including expensive computational cost, tissue motion due to handheld scanning under elongated data acquisition time, etc.), which impedes the implementation of the technique on existing clinical scanners. However, studies have shown that super-resolution can be retrospectively performed using conventional CEUS data acquired from existing clinical scanners [60], [61]. Although the super-resolution imaging quality may be suboptimal because of the low imaging frame rate (e.g., 10-50 Hz) that is typical available on clinical imaging systems (low frame rate makes it more difficult to track and link the microbubbles to form microvessels), increasing numbers of newer generation ultrasound scanners have started to support high frame-rate imaging that is ideal for super-resolution [44], [62].
For clinical research investigations using super-resolution ultrasound, the widely available research ultrasound scanners (e.g., Verasonics) and open-source super-resolution data and processing codes (e.g., PALA [27] and ULTRA-SR [63]) provide a robust platform for the dissemination of the technology. Research investigations can follow existing clinical applications of CEUS to identify the unmet clinical needs and test whether super-resolution imaging can fill the penetration-resolution gap.
Finally, Table 2 summarizes the roadblocks for clinical translation of super-resolution ultrasound imaging.
Table 2.
Summary of roadblocks for clinical translation of super-resolution ultrasound imaging
| Name of the challenge | Solutions | |
|---|---|---|
|
Critical roadblocks for clinical translation |
Slow imaging speed/long data acquisition |
Technical development for fast super-resolution imaging |
| Tissue motion (especially for out-of-plane motion) |
|
|
| Use of contrast microbubbles |
Contrast-free solutions that provide enhanced spatial resolution (not as high as conventional super-resolution ultrasound that is localization-based) |
|
| “Soft” roadblocks for clinical translation | Slow imaging frame rate on conventional ultrasound scanners |
|
| Computational cost associated with beamforming and post-processing | Algorithm acceleration, optimization, and parallelization (e.g., GPU-based) |
What about basic research on animal models?
Preclinical applications of super-resolution ultrasound are associated with fewer pragmatic challenges than clinical imaging. For example, long data acquisition time is not a significant issue on animals because ultrasound probes can be mechanically fixed instead of handheld. Respiratory and cardiac motion can be mitigated by anesthesia, mechanical ventilation, and prospective or retrospective gating [64], [65], [66], [67]. Microbubble infusion is also convenient to implement for stable microbubble concentration in the blood stream. By providing on demand, noninvasive imaging of tissue microvasculature, super-resolution ultrasound offers an attractive alternative to histology in longitudinal study designs because repeated imaging on same animals can be conveniently achieved.
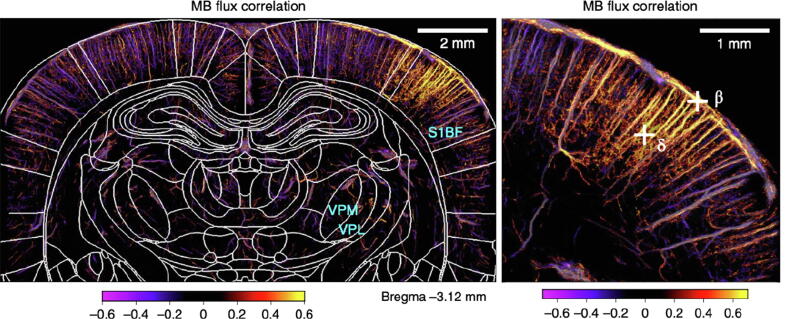
In neuroscience based on animal models, super-resolution ultrasound has rapidly gained traction because it allows whole-brain, micron-scale mapping of the cerebral vasculature [16], [43], which is unmatched by existing imaging modalities. This feature allows in vivo noninvasive imaging of deep brain regions with a spatial resolution that has never been achieved before, which is significant for many neurological disease applications including aging, Alzheimer’s disease, and ischemic and/or hemorrhagic stroke [45], [47], [48]. When combined with functional ultrasound [4], [39], [68] (fUS), super-resolution imaging provides an exciting new tool that allows neuroscientists to explore beyond the brain cortex and integrate the functional brain organizations across various brain regions at different scales (Fig. 11).
Figure 11.
Functional super-resolution imaging of the rat brain undergoing whisker stimulation. The microbubble (MB) flux correlation map demonstrates elevated microvascular blood flow in the S1 barrel field (S1BF) and ventro-posterio-median (VPM) thalamic nucleus for whisker stimulation. (Images adapted from Renaudin et al., 2022 [39]).
What’s next?
Because of the large discrepancies in imaging settings and safety regulations between preclinical and clinical imaging, the path of development of super-resolution ultrasound can be split into preclinical and clinical routes. Preclinical and clinical applications of super-resolution imaging present different technical challenges as well as different opportunities that lead to different possibilities. For example, as discussed above, for preclinical animal studies, longer data acquisition time may not be as significant of an issue as in clinical imaging. Therefore, reducing data acquisition time is not as critical in animals than in humans. However, improving the temporal resolution of super-resolution ultrasound is a common goal between preclinical and clinical settings. As another example, in animal studies, one can use non-FDA-approved contrast agents such as long-persistence microbubbles and nanodroplets to enhance the imaging performance of super-resolution ultrasound [24], [69]. The use of nanodroplets also provides opportunities to probe extravascular space by leveraging extravasation [70]. For clinical applications, the task of developing robust super-resolution imaging solutions that provide real/quasi-real-time imaging takes priority. If longer data acquisition is needed, the duration should not exceed 10-15 seconds (i.e., within a single breath hold). Tissue motion correction [71] is another priority for in vivo human imaging to account for handheld probe movement and other physiological motion. In addition, super-resolution ultrasound will also benefit from the development of newer and faster 3D ultrasound imaging techniques because 3D imaging is indispensable for localization and tracking microbubbles that travel in-and-out of the imaging plane, which results in biased flow velocity estimation using 2D imaging [43], [45], [58], [72].
For future clinical applications, one area that is potentially ripe for exploration with super-resolution ultrasound is to evaluate organ transplant rejections (e.g., kidney, pancreas, and liver). A new proof-of-concept study on human kidney allografts has been reported by Bodard et al [73]. Vascular rejection, manifesting as an arteritis which ranges of mild intimal inflammation and intimal thickening to necrosis of the intima with deposition of platelets, fibrin, and inflammatory cells requires biopsy for diagnosis [74]. The high spatial resolution of super-resolution ultrasound provides a powerful tool for detecting dysfunctional and morphologically damaged vessels in vivo without a biopsy. Such evaluations could be performed over time monitoring changes in vessel morphology with treatment. This would be a major advance in vascular rejection diagnosis and assessment. Further, transplant organs such as kidney and pancreas also provide a relatively benign environment for super-resolution ultrasound imaging: they do not present significant tissue motion and are easy to access, which is ideal for the prolonged data acquisition of super-resolution. Contrast microbubbles also have no organ toxicity, which is ideal for applications in transplanted organs.
Another similar target would be direct evaluation of microvessel damage as a means of diagnosis and assessing graft vs host disease (GVHD) after bone marrow transplantation. Without a tissue biopsy, anti-endothelial GVHD can be very hard to diagnose [75]. A method such as super-resolution that can actually visualize microvessel morphology in a benign way could have great utility in the management this condition. Bowel motion could be problematic, but the unique strengths of the method to visualize microvascular anatomy would make the method worth trying.
Funding
The study was partially supported by the National Institute of Biomedical Imaging and Bioengineering, National Cancer Institute, the National Institute on Aging, and National Institute of Child Health and Human Development of the National Institutes of Health under grant numbers R21EB030072, R21EB030072-01S1, R21AG077173, R00CA214523, and R21HD095501. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. MRL is partially supported by a Beckman Institute Postdoctoral Fellowship.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [P.S. and M.R.L. have patents in the field of super-resolution ultrasound imaging, some of which have been licensed.].
References
- 1.Montaldo G., Tanter M., Bercoff J., Benech N., Fink M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2009;56(3):489–506. doi: 10.1109/tuffc.2009.1067. in English. [DOI] [PubMed] [Google Scholar]
- 2.Tanter M., Fink M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrasonics Ferroelectr. Freq. Control. 2014;61(1):102–119. doi: 10.1109/tuffc.2014.6689779. [DOI] [PubMed] [Google Scholar]
- 3.Bercoff J., Montaldo G., Loupas T., Savery D., Meziere F., Fink M., et al. Ultrafast compound Doppler imaging: providing full blood flow characterization. IEEE Trans. Ultrasonics Ferroelectr. Freq. Control. 2011;58(1):134–147. doi: 10.1109/tuffc.2011.1780. [DOI] [PubMed] [Google Scholar]
- 4.Mace E., Montaldo G., Cohen I., Baulac M., Fink M., Tanter M. Functional ultrasound imaging of the brain. Nat Methods. 2011;8(8):662–U85. doi: 10.1038/nmeth.1641. [DOI] [PubMed] [Google Scholar]
- 5.Demene C., Deffieux T., Pernot M., Osmanski B.F., Biran V., Gennisson J.L., et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases doppler and fultrasound sensitivity. IEEE T Med Imaging. 2015;34(11):2271–2285. doi: 10.1109/TMI.2015.2428634. [DOI] [PubMed] [Google Scholar]
- 6.Baranger J., Arnal B., Perren F., Baud O., Tanter M., Demené C. Adaptive spatiotemporal SVD clutter filtering for ultrafast doppler imaging using similarity of spatial singular vectors. Ieee T Med Imaging. 2018;37(7):1574–1586. doi: 10.1109/TMI.2018.2789499. [DOI] [PubMed] [Google Scholar]
- 7.Couture O., Hingot V., Heiles B., Muleki-Seya P., Tanter M. Ultrasound localization microscopy and super-resolution: a state of the art. IEEE Trans Ultrason Ferroelectrics Frequency Control. 2018;65(8):1304–1320. doi: 10.1109/TUFFC.2018.2850811. [DOI] [PubMed] [Google Scholar]
- 8.Christensen-Jeffries K., Couture O., Dayton P.A., Eldar Y.C., Hynynen K., Kiessling F., et al. Super-resolution Ultrasound Imaging. Ultrasound Med Biol. 2020;46(4):865–891. doi: 10.1016/j.ultrasmedbio.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess S.T., Girirajan T.P., Mason M.D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys J. 2006;91(11):4258–4272. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betzig E., Patterson G.H., Sougrat R., Lindwasser O.W., Olenych S., Bonifacino J.S., et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 11.Couture O, Besson B, Montaldo G, Fink M, Tanter M. Microbubble ultrasound super-localization imaging (MUSLI). In: 2011 IEEE International Ultrasonics Symposium, 18-21 Oct. 2011 2011, p. 1285-7, doi: 10.1109/ULTSYM.2011.6293576.
- 12.Siepmann M, Schmitz G, Bzyl J, Palmowski M, Kiessling F. Imaging tumor vascularity by tracing single microbubbles. In: 2011 IEEE International Ultrasonics Symposium, 2011: IEEE, p. 1906-9.
- 13.Desailly Y., Couture O., Fink M., Tanter M. Sono-activated ultrasound localization microscopy. Appl Phys Lett. 2013;103(17) [Google Scholar]
- 14.O'Reilly M.A., Hynynen K. A super-resolution ultrasound method for brain vascular mapping. Med Phys. 2013;40(11) doi: 10.1118/1.4823762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viessmann O.M., Eckersley R.J., Christensen-Jeffries K., Tang M.X., Dunsby C. Acoustic super-resolution with ultrasound and microbubbles. Phys Med Biol. 2013;58(18):6447–6458. doi: 10.1088/0031-9155/58/18/6447. [DOI] [PubMed] [Google Scholar]
- 16.Errico C., Pierre J., Pezet S., Desailly Y., Lenkei Z., Couture O., et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nat Res Support, Non-U.S. Gov't. 2015;527(7579):499–502. doi: 10.1038/nature16066. in English. [DOI] [PubMed] [Google Scholar]
- 17.Christensen-Jeffries K., Browning R.J., Tang M.X., Dunsby C., Eckersley R.J. In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans Med Imaging. 2015;34(2):433–440. doi: 10.1109/TMI.2014.2359650. [DOI] [PubMed] [Google Scholar]
- 18.Lin F., Shelton S.E., Espindola D., Rojas J.D., Pinton G., Dayton P.A. 3-D Ultrasound localization microscopy for identifying microvascular morphology features of tumor angiogenesis at a resolution beyond the diffraction limit of conventional ultrasound. Theranostics. 2017;7(1):196–204. doi: 10.7150/thno.16899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song P., Trzasko J.D., Manduca A., Huang R., Kadirvel R., Kallmes D.F., et al. Improved super-resolution ultrasound microvessel imaging with spatiotemporal nonlocal means filtering and bipartite graph-based microbubble tracking. IEEE Trans Ultrason Ferroelectrics Frequency Control. 2018;65(2):149–167. doi: 10.1109/TUFFC.2017.2778941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackermann D., Schmitz G. Detection and tracking of multiple microbubbles in ultrasound B-Mode images. IEEE Trans Ultrason Ferroelectr Freq Control. 2016;63(1):72–82. doi: 10.1109/TUFFC.2015.2500266. [DOI] [PubMed] [Google Scholar]
- 21.Opacic T., Dencks S., Theek B., Piepenbrock M., Ackermann D., Rix A., et al. Motion model ultrasound localization microscopy for preclinical and clinical multiparametric tumor characterization. Nat Commun. 2018;9(1):1527. doi: 10.1038/s41467-018-03973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-Zion A., Solomon O., Tremblay-Darveau C., Adam D., Eldar Y.C. SUSHI: sparsity-based ultrasound super-resolution hemodynamic imaging. IEEE Trans Ultrason Ferroelectrics Freq Control. 2018;65(12):2365–2380. doi: 10.1109/TUFFC.2018.2873380. [DOI] [PubMed] [Google Scholar]
- 23.Andersen S.B., Taghavi I., Hoyos C.A.V., Søgaard S.B., Gran F., Lönn L., et al. Super-Resolution imaging with ultrasound for visualization of the renal microvasculature in rats before and after renal ischemia: a pilot study. Diagnostics. 2020;10(11):862. doi: 10.3390/diagnostics10110862. https://www.mdpi.com/2075-4418/10/11/862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang G., Harput S., Hu H., Christensen-Jeffries K., Zhu J., Brown J., et al. Fast Acoustic Wave Sparsely Activated Localization Microscopy (fast-AWSALM): ultrasound super-resolution using plane-wave activation of nanodroplets. IEEE Trans. Ultrason. Ferroelectr. Freq Control. 2019 doi: 10.1109/tuffc.2019.2906496. [DOI] [PubMed] [Google Scholar]
- 25.Kim J., Lowerison M.R., Sekaran N.V.C., Kou Z., Dong Z., Oelze M.L., et al. Improved ultrasound localization microscopy based on microbubble uncoupling via transmit excitation. IEEE Trans. Ultrason. Ferroelectr. Freq Control. 2022;69(3):1041–1052. doi: 10.1109/tuffc.2022.3143864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobbold RSC. Principles of Doppler Ultrasound. In: Foundations of Biomedical Ultrasound: Oxford University Press, 2007, p. 609-51 [chapter 9].
- 27.Heiles B., Chavignon A., Hingot V., Lopez P., Teston E., Couture O. Performance benchmarking of microbubble-localization algorithms for ultrasound localization microscopy. Nat Biomed Eng. 2022;6(5):605–616. doi: 10.1038/s41551-021-00824-8. [DOI] [PubMed] [Google Scholar]
- 28.Hingot V., Errico C., Heiles B., Rahal L., Tanter M., Couture O. Microvascular flow dictates the compromise between spatial resolution and acquisition time in Ultrasound Localization Microscopy. Sci Rep. 2019;9(1):2456. doi: 10.1038/s41598-018-38349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowerison M.R., Huang C., Kim Y., Lucien F., Chen S., Song P. In Vivo Confocal imaging of fluorescently labelled microbubbles: implications for ultrasound localization microscopy. IEEE Trans Ultrason Ferroelectrics Freq Control. 2020:1. doi: 10.1109/TUFFC.2020.2988159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen-Jeffries K., Brown J., Harput S., Zhang G., Zhu J., Tang M.X., et al. Poisson statistical model of ultrasound super-resolution imaging acquisition time. IEEE Transactions on Ultrason Ferroelectrics Freq Control. 2019;66(7):1246–1254. doi: 10.1109/TUFFC.2019.2916603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen-Jeffries K., Brown J., Harput S., Zhang G., Zhu J., Tang M.X., et al. Poisson statistical model of ultrasound super-resolution imaging acquisition time. IEEE Trans. Ultrason. Ferroelectr. Freq Control. 2019;66(7):1246–1254. doi: 10.1109/tuffc.2019.2916603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C., Lowerison M.R., Trzasko J.D., Manduca A., Bresler Y., Tang S., et al. Short acquisition time super-resolution ultrasound microvessel imaging via microbubble separation. Sci Rep. 2020;10(1):6007. doi: 10.1038/s41598-020-62898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You Q., Trzasko J.D., Lowerison M.R., Chen X., Dong Z., Sekaran N.V.C., et al. Curvelet transform-based sparsity promoting algorithm for fast ultrasound localization microscopy. IEEE Trans Med Imaging. 2022 doi: 10.1109/tmi.2022.3162839. in English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Sloun R.J.G., Solomon O., Bruce M., Khaing Z.Z., Wijkstra H., Eldar Y.C., et al. Super-Resolution ultrasound localization microscopy through deep learning. IEEE Trans Med Imaging. 2021;40(3):829–839. doi: 10.1109/tmi.2020.3037790. in English. [DOI] [PubMed] [Google Scholar]
- 35.Milecki L., Poree J., Belgharbi H., Bourquin C., Damseh R., Delafontaine-Martel P., et al. A deep learning framework for spatiotemporal ultrasound localization microscopy. IEEE Trans Med Imaging. 2021 doi: 10.1109/tmi.2021.3056951. in English. [DOI] [PubMed] [Google Scholar]
- 36.Bar-Zion A., Tremblay-Darveau C., Solomon O., Adam D., Eldar Y.C. Fast vascular ultrasound imaging with enhanced spatial resolution and background rejection. IEEE Trans Med Imaging. 2017;36(1):169–180. doi: 10.1109/tmi.2016.2600372. in English. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Zhou T., Lu M., Yang Y., He Q., Luo J. Deep learning for ultrasound localization microscopy. Ieee T Med Imaging. 2020;39(10):3064–3078. doi: 10.1109/TMI.2020.2986781. [DOI] [PubMed] [Google Scholar]
- 38.Chen X., Lowerison M.R., Dong Z., Sekaran N.V.C., Llano D.A., Song P. Localization free super-resolution microbubble velocimetry using a long short-term memory neural network. Ieee T Med Imaging. 2023:1. doi: 10.1109/tmi.2023.3251197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renaudin N., Demené C., Dizeux A., Ialy-Radio N., Pezet S., Tanter M. Functional ultrasound localization microscopy reveals brain-wide neurovascular activity on a microscopic scale. Nat Methods. 2022;19(8):1004–1012. doi: 10.1038/s41592-022-01549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You Q, Lowerison MR, Shin Y, Chen X, Chandra Sekaran NV, Dong Z et al., Contrast-free Super-resolution Doppler (CS Doppler) based on Deep Generative Neural Networks, bioRxiv 2022.09.29.510188, 2022, doi: 10.1101/2022.09.29.510188.
- 41.Jensen J.A., Schou M., Andersen S.B., Søgaard S.B., Sørensen C.M., Nielsen M.B., Gundlach C., Kjer H.M., Bjorholm Dahl A., Stuart M.B., Tomov B.G. Fast super resolution ultrasound imaging using the erythrocytes. (SPIE Medical Imaging) SPIE. 2022 [Google Scholar]
- 42.Park J.H., Choi W., Yoon G.Y., Lee S.J. Deep learning-based super-resolution ultrasound speckle tracking velocimetry. Ultrasound Med Biol. 2020;46(3):598–609. doi: 10.1016/j.ultrasmedbio.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Chavignon A., Heiles B., Hingot V., Orset C., Vivien D., Couture O. 3D Transcranial ultrasound localization microscopy in the rat brain with a multiplexed matrix probe. IEEE Trans Biomed Eng. 2022;69(7):2132–2142. doi: 10.1109/tbme.2021.3137265. [DOI] [PubMed] [Google Scholar]
- 44.Demené C., Robin J., Dizeux A., Heiles B., Pernot M., Tanter M., et al. Transcranial ultrafast ultrasound localization microscopy of brain vasculature in patients. Nature Biomed Eng. 2021;5(3):219–228. doi: 10.1038/s41551-021-00697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chavignon A., Hingot V., Orset C., Vivien D., Couture O. 3D transcranial ultrasound localization microscopy for discrimination between ischemic and hemorrhagic stroke in early phase. Sci Rep. 2022;12(1):14607. doi: 10.1038/s41598-022-18025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demené C., Robin J., Dizeux A., Heiles B., Pernot M., Tanter M., et al. Transcranial ultrafast ultrasound localization microscopy of brain vasculature in patients. Nat Biomed Eng. 2021;5(3):219–228. doi: 10.1038/s41551-021-00697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowerison MR, Chandra Sekaran NV, Dong Z, Chen X, You Q, Llano DA, et al. Super-resolution ultrasound imaging of cerebrovascular impairment in a mouse model of Alzheimer’s disease, bioRxiv, p. 2022.10.05.511008, 2022, doi: 10.1101/2022.10.05.511008.
- 48.Lowerison M.R., Sekaran N.V.C., Zhang W., Dong Z., Chen X., Llano D.A., et al. Aging-related cerebral microvascular changes visualized using ultrasound localization microscopy in the living mouse. Sci Rep. 2022;12(1):619. doi: 10.1038/s41598-021-04712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowerison M.R., Huang C., Lucien F., Chen S., Song P. Ultrasound localization microscopy of renal tumor xenografts in chicken embryo is correlated to hypoxia. Sci Rep. 2020;10(1):2478. doi: 10.1038/s41598-020-59338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowerison M.R., Zhang W., Chen X., Fan T.M., Song P. Characterization of anti-angiogenic chemo-sensitization via longitudinal ultrasound localization microscopy in colorectal carcinoma tumor xenografts. IEEE Trans. Biomed. Eng. 2022;69(4):1449–1460. doi: 10.1109/TBME.2021.3119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lassau N., Bonastre J., Kind M., Vilgrain V., Lacroix J., Cuinet M., et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol. 2014;49(12):794–800. doi: 10.1097/rli.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Connor J.P.B., Aboagye E.O., Adams J.E., Aerts H.J.W.L., Barrington S.F., Beer A.J., et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14(3):169–186. doi: 10.1038/nrclinonc.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dietrich C., Averkiou M., Correas J.-M., Lassau N., Leen E., Piscaglia F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall in der Medizin-Eur J Ultrasound. 2012;33(04):344–351. doi: 10.1055/s-0032-1313026. [DOI] [PubMed] [Google Scholar]
- 54.Lassau N., Koscielny S., Chami L., Chebil M., Benatsou B., Roche A., et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification–preliminary results. Radiology. Jan 2011;258(1):291–300. doi: 10.1148/radiol.10091870. [DOI] [PubMed] [Google Scholar]
- 55.Lassau N., Koscielny S., Albiges L., Chami L., Benatsou B., Chebil M., et al. Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res. 2010;16(4):1216–1225. doi: 10.1158/1078-0432.Ccr-09-2175. [DOI] [PubMed] [Google Scholar]
- 56.Jubb A.M., Harris A.L. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11(12):1172–1183. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 57.El Kaffas A., Hoogi A., Zhou J., Durot I., Wang H., Rosenberg J., et al. Spatial characterization of tumor perfusion properties from 3D DCE-US perfusion maps are early predictors of cancer treatment response. Sci Rep. 2020;10(1):6996. doi: 10.1038/s41598-020-63810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heiles B., Correia M., Hingot V., Pernot M., Provost J., Tanter M., et al. Ultrafast 3D ultrasound localization microscopy using a 32×32 matrix array. Ieee T Med Imaging. 2019:1. doi: 10.1109/TMI.2018.2890358. [DOI] [PubMed] [Google Scholar]
- 59.Tang M.X., Mulvana H., Gauthier T., Lim A.K., Cosgrove D.O., Eckersley R.J., et al. Quantitative contrast-enhanced ultrasound imaging: a review of sources of variability. Interface Focus. 2011;1(4):520–539. doi: 10.1098/rsfs.2011.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solomon O., van Sloun R.J.G., Wijkstra H., Mischi M., Eldar Y.C. Exploiting flow dynamics for superresolution in contrast-enhanced ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq Control. 2019;66(10):1573–1586. doi: 10.1109/tuffc.2019.2926062. [DOI] [PubMed] [Google Scholar]
- 61.Zhu J, Zhang C, Christensen-Jeffries K, Zhang G, Harput S, Dunsby C et al. Super-Resolution Ultrasound Localization Microscopy of Microvascular Structure and Flow for Distinguishing Metastatic Lymph Nodes - An Initial Human Study,“ (in eng), Ultraschall Med, Oct 7 2022, doi: 10.1055/a-1917-0016. Lokalisierungsmikroskopie mit Superresolution-Ultraschall der mikrovaskulären Struktur und des Flusses zur Unterscheidung metastatischer Lymphknoten – eine erste Studie am Menschen. [DOI] [PubMed]
- 62.Huang C., Zhang W., Gong P., Lok U.W., Tang S., Yin T., et al. Super-resolution ultrasound localization microscopy based on a high frame-rate clinical ultrasound scanner: an in-human feasibility study. Phys Med Biol. 2021;66(8):08NT01. doi: 10.1088/1361-6560/abef45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ULTRA-SR (Ultrasound Localisation and TRacking Algorithms for Super Resolution). (accessed) https://ultra-sr.com. [DOI] [PubMed]
- 64.Zhang W., Lowerison M.R., Dong Z., Miller R.J., Keller K.A., Song P. Super-Resolution ultrasound localization microscopy on a rabbit liver VX2 tumor model: an initial feasibility study. Ultrasound Med Biol. 2021;47(8):2416–2429. doi: 10.1016/j.ultrasmedbio.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demeulenaere O., Sandoval Z., Mateo P., Dizeux A., Villemain O., Gallet R., et al. Coronary flow assessment using 3-Dimensional ultrafast ultrasound localization microscopy. JACC: Cardiovasc Imaging. 2022;15(7):1193–1208. doi: 10.1016/j.jcmg.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Cormier P., Porée J., Bourquin C., Provost J. Dynamic myocardial ultrasound localization angiography. Ieee T Med Imaging. 2021;40(12):3379–3388. doi: 10.1109/TMI.2021.3086115. [DOI] [PubMed] [Google Scholar]
- 67.Bourquin C., Porée J., Lesage F., Provost J. In vivo pulsatility measurement of cerebral microcirculation in rodents using dynamic ultrasound localization microscopy. Ieee T Med Imaging. 2022;41(4):782–792. doi: 10.1109/TMI.2021.3123912. [DOI] [PubMed] [Google Scholar]
- 68.Deffieux T., Demené C., Tanter M. Functional ultrasound imaging: a new imaging modality for neuroscience. Neuroscience. 2021;474:110–121. doi: 10.1016/j.neuroscience.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Garg S., Thomas A.A., Borden M.A. The effect of lipid monolayer in-plane rigidity on in vivo microbubble circulation persistence. Biomaterials. 2013;34(28):6862–6870. doi: 10.1016/j.biomaterials.2013.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams R., Wright C., Cherin E., Reznik N., Lee M., Gorelikov I., et al. Characterization of submicron phase-change perfluorocarbon droplets for extravascular ultrasound imaging of cancer. Ultrasound Med Biol. 2013;39(3):475–489. doi: 10.1016/j.ultrasmedbio.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Hingot V., Errico C., Tanter M., Couture O. Subwavelength motion-correction for ultrafast ultrasound localization microscopy. Ultrasonics. 2017;77:17–21. doi: 10.1016/j.ultras.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Demeulenaere O., Bertolo A., Pezet S., Ialy-Radio N., Osmanski B., Papadacci C., et al. In vivo whole brain microvascular imaging in mice using transcranial 3D Ultrasound Localization Microscopy. eBioMedicine. 2022;79 doi: 10.1016/j.ebiom.2022.103995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bodard S., Denis L., Hingot V., Chavignon A., Hélénon O., Anglicheau D., et al. Ultrasound localization microscopy of the human kidney allograft on a clinical ultrasound scanner. Kidney Int. 2023 doi: 10.1016/j.kint.2023.01.027. [DOI] [PubMed] [Google Scholar]
- 74.Shimizu T., Tanabe T., Shirakawa H., Omoto K., Ishida H., Tanabe K. Acute vascular rejection after renal transplantation and isolated v-lesion. Clin Transplantation. 2012;26(s24):2–8. doi: 10.1111/j.1399-0012.2012.01673.x. [DOI] [PubMed] [Google Scholar]
- 75.Biedermann B.C. Vascular endothelium and graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21(2):129–138. doi: 10.1016/j.beha.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Song P., Trzasko J.D., Manduca A., Qiang B., Kadirvel R., Kallmes D.F., et al. Accelerated singular value-based ultrasound blood flow clutter filtering with randomized singular value decomposition and randomized spatial downsampling. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64(4):706–716. doi: 10.1109/TUFFC.2017.2665342. [DOI] [PubMed] [Google Scholar]
- 77.Tang S., Song P., Trzasko J.D., Lowerison M., Huang C., Gong P., et al. Kalman filter-based microbubble tracking for robust super-resolution ultrasound microvessel imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2020;67(9):1738–1751. doi: 10.1109/TUFFC.2020.2984384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desailly Y., Pierre J., Couture O., Tanter M. Resolution limits of ultrafast ultrasound localization microscopy. Phys Med Biol. 2015;60(22):8723–8740. doi: 10.1088/0031-9155/60/22/8723. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Z., Hwang M., Kilbaugh T.J., Sridharan A., Katz J. Cerebral microcirculation mapped by echo particle tracking velocimetry quantifies the intracranial pressure and detects ischemia. Nat Commun. 2022;13(1):666. doi: 10.1038/s41467-022-28298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCall J.R., Santibanez F., Belgharbi H., Pinton G.F., Dayton P.A. Non-invasive transcranial volumetric ultrasound localization microscopy of the rat brain with continuous, high volume-rate acquisition. Theranostics, Research Paper. 2023;13(4):1235–1246. doi: 10.7150/thno.79189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Claron J., Hingot V., Rivals I., Rahal L., Couture O., Deffieux T., et al. Large-scale functional ultrasound imaging of the spinal cord reveals in-depth spatiotemporal responses of spinal nociceptive circuits in both normal and inflammatory states. PAIN. 2021;162(4):1047–1059. doi: 10.1097/j.pain.0000000000002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu J., Rowland E.M., Harput S., Riemer K., Leow C.H., Clark B., et al. 3D super-resolution US imaging of rabbit lymph node vasculature in vivo by using microbubbles. Radiology. 2019 doi: 10.1148/radiol.2019182593. [DOI] [PubMed] [Google Scholar]
- 83.Chen Q., Yu J., Rush B.M., Stocker S.D., Tan R.J., Kim K. Ultrasound super-resolution imaging provides a noninvasive assessment of renal microvasculature changes during mouse acute kidney injury. Kidney Int. 2020;98(2):355–365. doi: 10.1016/j.kint.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harput S., Christensen-Jeffries K., Brown J., Li Y., Williams K.J., Davies A.H., et al. Two-Stage motion correction for super-resolution ultrasound imaging in human lower limb. IEEE Trans. Ultrason. Ferroelectr. Freq Control. 2018;65(5):803–814. doi: 10.1109/tuffc.2018.2824846. [DOI] [PubMed] [Google Scholar]
- 85.Chen Q., Yu J., Lukashova L., Latoche J.D., Zhu J., Lavery L., et al. Validation of ultrasound super-resolution imaging of vasa vasorum in rabbit atherosclerotic plaques. IEEE Trans Ultrason Ferroelectr Freq Control. 2020;67(8):1725–1729. doi: 10.1109/TUFFC.2020.2974747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goudot G., Jimenez A., Mohamedi N., Sitruk J., Khider L., Mortelette H., et al. Assessment of Takayasu's arteritis activity by ultrasound localization microscopy. eBioMedicine. 2023;90 doi: 10.1016/j.ebiom.2023.104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qian X., Huang C., Li R., Song B.J., Tchelepi H., Shung K.K., et al. Super-Resolution ultrasound localization microscopy for visualization of the ocular blood flow. IEEE Trans Biomed Eng. 2022;69(5):1585–1594. doi: 10.1109/tbme.2021.3120368. [DOI] [PMC free article] [PubMed] [Google Scholar]