Abstract
Background:
Alzheimer disease (AD) is more prevalent in African American (AA) and Hispanic White (HIW) compared to Non-Hispanic White (NHW) individuals. Similarly, neuropsychiatric symptoms (NPS) vary by population in AD. This is likely the result of both sociocultural and genetic ancestral differences. However, the impact of these NPS on AD in different groups is not well understood.
Methods:
Self-declared AA, HIW, and NHW individuals were ascertained as part of ongoing AD genetics studies. Participants who scored higher than 0.5 on the Clinical Dementia Rating Scale (CDR) were included. Group similarities and differences on Neuropsychiatric Inventory Questionnaire (NPI-Q) outcomes (NPI-Q total score, NPI-Q items) were evaluated using univariate ANOVAs and post hoc comparisons after controlling for sex and CDR stage.
Results:
Our sample consisted of 498 participants (26% AA; 30% HIW; 44% NHW). Overall, NPI-Q total scores differed significantly between our groups, with HIW having the highest NPI-Q total scores, and by AD stage as measured by CDR. We found no significant difference in NPI-Q total score by sex. There were six NPI-Q items with comparable prevalence in all groups and six items that significantly differed between the groups (Anxiety, Apathy, Depression, Disinhibition, Elation, and Irritability). Further, within the HIW group, differences were found between Puerto Rican and Cuban American Hispanics across several NPI-Q items. Finally, Six NPI-Q items were more prevalent in the later stages of AD including Agitation, Appetite, Hallucinations, Irritability, Motor Disturbance, and Nighttime Behavior.
Conclusions:
We identified differences in NPS among HIW, AA, and NHW individuals. Most striking was the high burden of NPS in HIW, particularly for mood and anxiety symptoms. We suggest that NPS differences may represent the impact of sociocultural influences on symptom presentation as well as potential genetic factors rooted in ancestral background. Given the complex relationship between AD and NPS it is crucial to discern the presence of NPS to ensure appropriate interventions.
Keywords: Alzheimer disease; neuropsychiatric symptoms (NPS); NPI-Q, multi-ethnic
Introduction
Alzheimer disease (AD) is a major public health challenge, especially among underserved diverse populations. The prevalence of late-onset AD (onset after 65 years of age) varies significantly by self-declared racial/ethnic groups, where African American (AA) and Hispanic White (HIW) are 1.5 to 2 times more likely to develop AD compared to Non-Hispanic White (NHW) individuals1–5. In addition, AA and HIW also differ in clinical presentation, stage of disease at time of diagnosis, and dementia risk factors in relation to NHW6–9. While race-ethnicity are overly simplistic surrogates for cultural influences on behavior and clearly not biological classifications, they often serve as proxies, albeit simplistic, for non-biologic influences (e.g., sociocultural factors and social determinants of health) and genetic ancestry6,8–12. Non-biologic influences are especially pertinent to diverse groups and reflect a range of sociocultural factors that may impact the risk and course of AD13–16.
The core AD phenotype is characterized by progressive cognitive and functional decline, with the presence of at least one neuropsychiatric symptom (NPS) in 80% of AD cases17. NPS are non-cognitive multidimensional behavioral disturbances (e.g. depression, anxiety, and agitation) that are commonly prodromal to AD18–20. Further, while many of these symptoms are often overlooked or attributed to clinical manifestations of AD, they significantly affect daily living activities, often worsening prognosis and, have been shown tied to reduced lifespan21–26. Not surprisingly, NPS are major contributors to the social and economic burden of AD, with high costs for hospitalization, residential placement, and psychopharmacologic therapy27–29.
The assessment of NPS in AD is complicated as these symptoms are heterogenous and require clinical evaluation by trained specialists to optimize non-pharmacologic and pharmacologic approaches30. Multiple screening tools have been developed to identify NPS. The widely used Neuropsychiatric Inventory Questionnaire (NPI-Q) has demonstrated clinical reliability and validity to identify NPS31. Studies using the NPI-Q have shown that NPS are associated with faster progression from mild cognitive impairment (MCI) to dementia32, accelerated cognitive decline33, and increased functional decline34. However, most outcome studies using the NPI-Q in AD and related dementias have been restricted to NHW individuals and have not examined its utility in diverse populations.
Multiple studies suggest that there is good reason to examine the utility of the NPI-Q in diverse groups as it appears that AA and HIW individuals have different patterns of NPS compared to NHW individuals35–38. For instance, when examining NPS in AA individuals and NHW individuals with AD, psychosis and insomnia were more prominent among AA while NHW had higher levels of anxiety and depression39,40. Similarly, a study evaluating NPS differences between AD NHW and Hispanic individuals primarily from Central American countries showed that Hispanic individuals had a higher NPI-Q total score and a higher prevalence of specific NPS symptoms including Delusions, Hallucinations, Anxiety, Disinhibition, and Irritability compared to NHW individuals with AD41. Other studies have also demonstrated NPS differences in AD Hispanic individuals depending on the country of origin, for example the prevalence of Anxiety varies from 16% in Mexican Americans, 22% in Spaniards and 36% in Brazilians 17,37,42,43. Overall, these results lend support to both sociocultural and genetic ancestry influences on the expression of NPS.
Understanding variations of NPS in AD in different populations is critical to enhancing evaluation and management of NPS in all groups. The objective of this study is to identify similarities and differences in NPS as a function of self-declared race-ethnicity among individuals with AD and related dementias. We hypothesize there will be population specific NPI-Q patterns.
Methods
Participants
Our study sample consisted of self-declared AA, HIW, and NHW individuals who were ascertained for ongoing studies of AD genetics. Ascertainment sites included the University of Miami, Wake Forest University, and Case Western Reserve University. Our HIW group consisted mainly of Puerto Rican (PR) (residing in the Puerto Rico or the US) and Cuban American (CA) (residing in the US) individuals as well as a small set of individuals from various Latin American and Caribbean countries. Our AA and NHW groups were composed of individuals mainly from states in the Southeastern US (NC, SC, GA, TN, and FL). All participants underwent standard clinical evaluations, during which sociodemographic, cognitive, and behavioral data were collected through direct assessment and informant report. In addition, participants were assigned consensus diagnoses using standard criteria44–46. Individuals included in this study were selected from the parent studies based on the following criteria: (a) enrolled in a genetic study and completed study protocols; (b) age >60 years; (c) consensus diagnosis of MCI or AD; (d) Clinical Dementia Rating scale (CDR) Global score ≥ 0.5; (e) completed NPI-Q.
Neuropsychiatric Inventory Questionnaire (NPI-Q)
The NPI-Q is a 12-item questionnaire that is used to rate the presence of NPS (e.g., Hallucinations, Agitation, Depression, etc.) common to AD and dementia31. The NPI-Q was administered by trained clinical coordinators via an interview performed to the informant or caregiver of the individual for whom they care. Derived from the Neuropsychiatric Interview (NPI)47,48, the NPI-Q has demonstrated association with cognitive and functional decline33,34 as well as progression from MCI to dementia32. The NPI-Q has been validated in Spanish49, as well as in Spanish speaking countries including Brazil50, Mexico51, and Chile52. NPI-Q items are scored as absent (0) or present (1) and a total score is generated by summing positively endorsed items; higher NPI-Q total scores indicate greater NPS burden. For this study, the NPI-Q total score and individual item scores were used as dependent measures. The 12 NPI-Q items are shown in Supplementary table 1. NPI-Q items specifically reported will be italicized to differentiate them from general descriptions of NPS.
Clinical Dementia Rating scale (CDR)
The CDR is a semi-structured interview used to characterize cognitive and functional performance typical of AD and dementia53. The CDR consists of six domains: Memory, Orientation, Judgment & Problem Solving, Community Affairs, Home & Hobbies, and Personal Care, which are rated individually and then synthesized to assign a Global CDR score. The Global CDR score ranges from normal (Global CDR=0) to severe dementia (Global CDR = 3). For this study, the CDR was recoded into two levels: Early stage (i.e., mild dementia; a Global CDR=0.5 or 1) and Late stage (i.e., moderate-severe dementia; Global CDR > 1). Similar to the NPI-Q, the CDR information is often provided by the information or caregiver if the individual participating in the study is cognitively unable to answers these questions.
Analysis
A one-way ANOVA was used to compare self-declared race-ethnicity groups on sex, CDR severity (% Late stage), age at evaluation, age at onset, and years of education. A Factorial ANOVA was used to examine the main effects of race-ethnicity (AA, HIW and NHW), sex, CDR severity (Late Stage, Early Stage) on the NPI-Q total score and individual NPI-Q items (e.g., agitation). Both estimated mean differences (EMD) and estimated proportional differences (EPD) or the differences for each factor after adjusting for the other variables in the model are reported for the Factorial ANOVA post-hoc tests. As a secondary analysis, an independent-samples t-test was used to compare our PR (n = 44) and CA (n = 77) HIW individuals on NPI-Q total score and individual NPI-Q items. Bonferroni corrections were used for post hoc tests. All analyses were conducted using IBM SPSS Statistics for Windows, Version 27 (IBM SPSS Statistics for Windows, IBM Corporation, Armonk, NY).
Results
Our sample consisted of 498 individuals (AA=131; HIW=148; NHW=219). The one-way ANOVA (See Table 1) showed that our self-declared race-ethnicity groups differed significantly in the proportion of males-females (p < 0.001), years of education (p < 0.001), and proportion of CDR Category (p = 0.017), NPI-Q total scores at early stage (p = 0.044), and NPI-Q total scores at late stage (p = 0.010). There were no statistically significant differences between group on age at exam (p = 0.111) or age at onset (p = 0.563). Post-hoc tests revealed that NHW had a lower proportion of females than AA (p = 0.003) and HIW (p = 0.007) individuals, but there was no difference in the proportion of females between the AA and HIW (p = 1.000). NHW had significantly more years of education than both AA (p <0.001) and HIW (p < 0.001), and AA had more years of education than HIW (p = 0.004). HIW had a greater proportion of individuals in the CDR Late dementia stage than AA (p = 0.015) individuals. No proportional differences in CDR stages were seen between NHW and either AA (p = 0.154) or HIW (p = 0.737) individuals. Finally, HIW had a greater NPI-Q total score than NHW at both the early (p = 0.041) and late (p = 0.013) CDR stages, while no differences were found between AA and both HIW and NHW at either the early (HIW: p = 0.752; NHW p = 0.560) or late (HIW: p = 0.078; NHW: p = 1.000) CDR stages.
Table 1.
Race-ethnicity group comparisons (ANOVA)
| Variable | AA | NHW | HIW | Overall | p-value |
|---|---|---|---|---|---|
| % Female (n= 498) | 71.0% a | 53.4% a, c | 68.9% c | 62.7% | < 0.001 |
| % CDR >1 (n= 498) | 35.9% b | 46.6% | 52.7% b | 45.6% | 0.017 |
| NPIQ-Total CDR Early (sd) (n= 270) | 3.21(2.9) | 2.74(2.3)c | 3.69(2.5)c | 3.13(2.6) | 0.044 |
| NPIQ-Total CDR Late (sd) (n= 227) | 3.94(2.5) | 3.88(2.9)c | 5.05(2.5)c | 4.30(2.7) | 0.010 |
| AAE (sd) (n= 399) | 77.9 (9.4) | 78.9 (9.7) | 80.3 (9.3) | 79.2 (9.5) | 0.111 |
| AOO (sd) (n= 361) | 72.3 (9.9) | 73.5 (8.9) | 74.0 (9.5) | 73.4 (9.2) | 0.563 |
| Education (sd) (n= 389) | 12.0 (3.1) a, b | 13.6 (3.5) a, c | 10.2 (4.6) b, c | 12.4 (3.9) | < 0.001 |
= Difference between AA and NHW p < 0.05;
= Difference between AA and HIW p < 0.05;
= Difference between NHW and HIW p < 0.05;
AA: African American; AAE: age at exam; AOO: age of onset; CDR Early: Clinical Dementia Rating scale early stage (CDR global score <1); CDR Late: Clinical Dementia Rating scale late stage (CDR global score >= 1); HIW: Hispanic White; NHW: Non-Hispanic White;NPI-Q Total: Neuropsychiatric Inventory Questionnaire total score; sd = standard deviation; n: sample size.
NPI-Q Total Score Factorial ANOVA
We tested the main effects of self-declared race-ethnicity, CDR, and sex on NPI-Q total score using a factorial ANOVA. This analysis revealed main effects of race-ethnicity (p < 0.001) and CDR category (p < 0.001) on the NPI-Q total score. No main effects were found for sex (p = 0.48). Post-hoc comparisons showed that HIW had a greater NPI-Q total score compared to AA (EMD = 0.93, p = 0.009) and NHW (EMD = 1.14, p < 0.001), while there was no statistically significant difference between AA and NHW (EMD = 0.20, p = 1.000). Finally, those in the CDR Late stage (i.e., more severe dementia) had a significantly higher NPI-Q score than those in the CDR Early stage (EMD = 1.11, p < 0.001).
Neuropsychiatric Inventory Questionnaire (NPI-Q) Item Level Factorial ANOVAs
We tested the main effects of self-declared race-ethnicity, CDR, and sex on each of the NPI-Q items using factorial ANOVA. Significant results are presented in Table 2.
Table 2.
Factorial ANOVA main effects for NPI-Q total score and NPI-Q items
| Sexa (F, p) | Race/Ethnicity (F, p) | CDRb (F, p) | |
|---|---|---|---|
| NPI-Q Total Score | ns | 7.70, p < 0.001 | 22.17, p < 0.001 |
| Agitation | 4.48, p = 0.04 | ns | 11.92, p < 0.001 |
| Anxiety | ns | 22.347, p < 0.001 | ns |
| Apathy | ns | 7.03, p < 0.001 | ns |
| Appetite | ns | ns | 12.20, p < 0.001 |
| Delusions | ns | ns | ns |
| Depression | ns | 22.76, p < 0.001 | ns |
| Disinhibition | ns | 6.46, p < 0.01 | ns |
| Elation | ns | 3.06, p = 0.05 | ns |
| Hallucinations | 4.54, p = 0.03 | ns | 33.14, p < 0.001 |
| Irritability | 5.03, p = 0.03 | 14.00, p < 0.001 | 20.74, p < 0.001 |
| Motor Disturbance | ns | ns | 14.62, p < 0.001 |
| Nighttime Behavior | ns | ns | 15.69, p < 0.001 |
Reference=Female;
Reference=Early stage;
CDR = Clinical Dementia Rating scale; F = F-statistic; p= p-value; NPI-Q = Neuropsychiatric Inventory Questionnaire; ns= not significant.
Education was not significant for the NPI-Q total score and all NPI-Q items
Race-ethnicity
In total, six NPI-Q items showed similarities and six NPI-Q items showed differences among our groups (Figure 1). We found no statistical differences in the NPI-Q items Agitation, Appetite, Delusions, Hallucinations, Motor Disturbances and Nighttime Behaviors. In contrast, we found a significant main effect of self-declared race-ethnicity on NPI-Q items including Anxiety (p < 0.001), Apathy (p < 0.001), Depression (p < 0.001), Irritability (p < 0.001), Disinhibition (p = 0.002), and Elation (p = 0.046).
Figure 1.
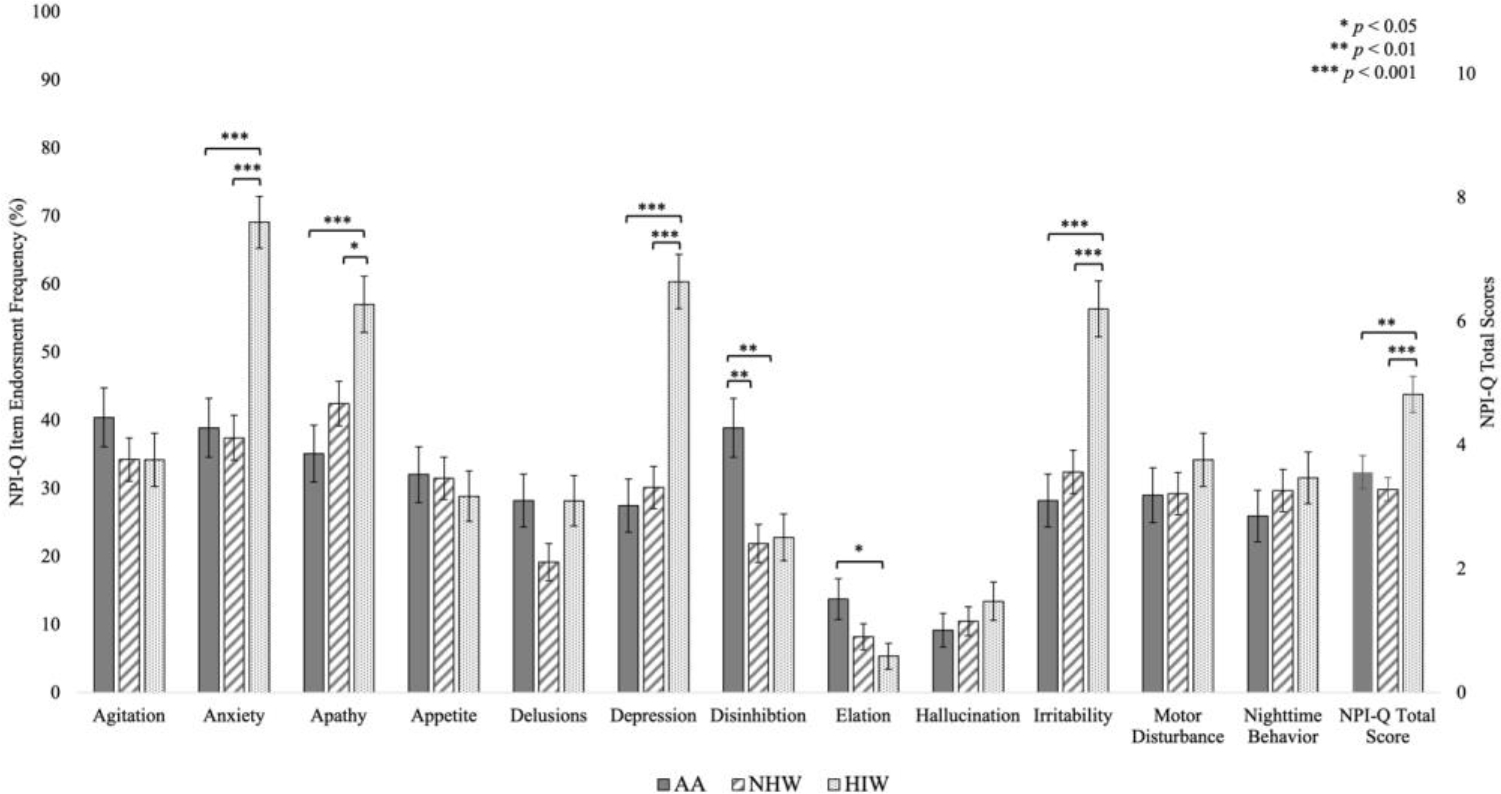
Frequencies of Positive Endorsement on NPI-Q Items and NPI-Q Total Scores by Race-Ethnicity.
AA: African American; HIW: Hispanic White; NHW: Non-Hispanic White; NPI-Q: Neuropsychiatric Inventory Questionnaire.
Post hoc comparisons revealed that compared to both AA and NHW individuals, HIW individuals had a higher prevalence of Anxiety (AA: EPD = 30.1%, p < 0.001; NHW: EPD = 32.2%, p < 0.001), Apathy (AA: EPD = 22.3%, p < 0.001; NHW: EPD = 15.0%, p =0.013), Depression (AA: EPD = 33.3%, p < 0.001; NHW: EPD = 30.7%, p < 0.001) and Irritability (AA: EPD = 27.8%, p < 0.001; NHW: EPD = 23.7%, p < 0.001), all symptoms common to mood disorders. AA and NHW individuals showed no statistically significant differences on the prevalence of Anxiety (p = 1.000), Apathy (p = 0.528), Depression (p = 1.000) or Irritability (p = 1.000).
AA individuals had a higher prevalence of Disinhibition, than both NHW (EPD = 17.0%, p = 0.003), and HIW individuals (EPD = 16.6%, p = 0.005). AA individuals also had a higher prevalence of Elation compared to HIW individuals (EPD = 8.3%, p = 0.04), but not NHW individuals (EPD = 5.5%, p = 0.23); HIW and NHW individuals did not differ on Elation (EPD = 2.8%, p = 1.000).
Clinical Dementia Rating (CDR) scale
There were significant main effects of CDR category on several NPI-Q items (Figure 2). Individuals in the CDR Late stage had significantly more Agitation (EPD = 14.9%, p < 0.001), Appetite (EPD = 14.5%, p < 0.001), Hallucinations (EPD = 15.9%, p < 0.001), Irritability (EPD = 19.1%, p < 0.001), Motor Disturbance (EPD = 15.8%, p < 0.001), and Nighttime Behavior (EPD = 16.1%, p < 0.001). NPI-Q items related to mood changes like Anxiety, Apathy, Depression, Disinhibition, and Elation did not differ by CDR stage.
Figure 2.
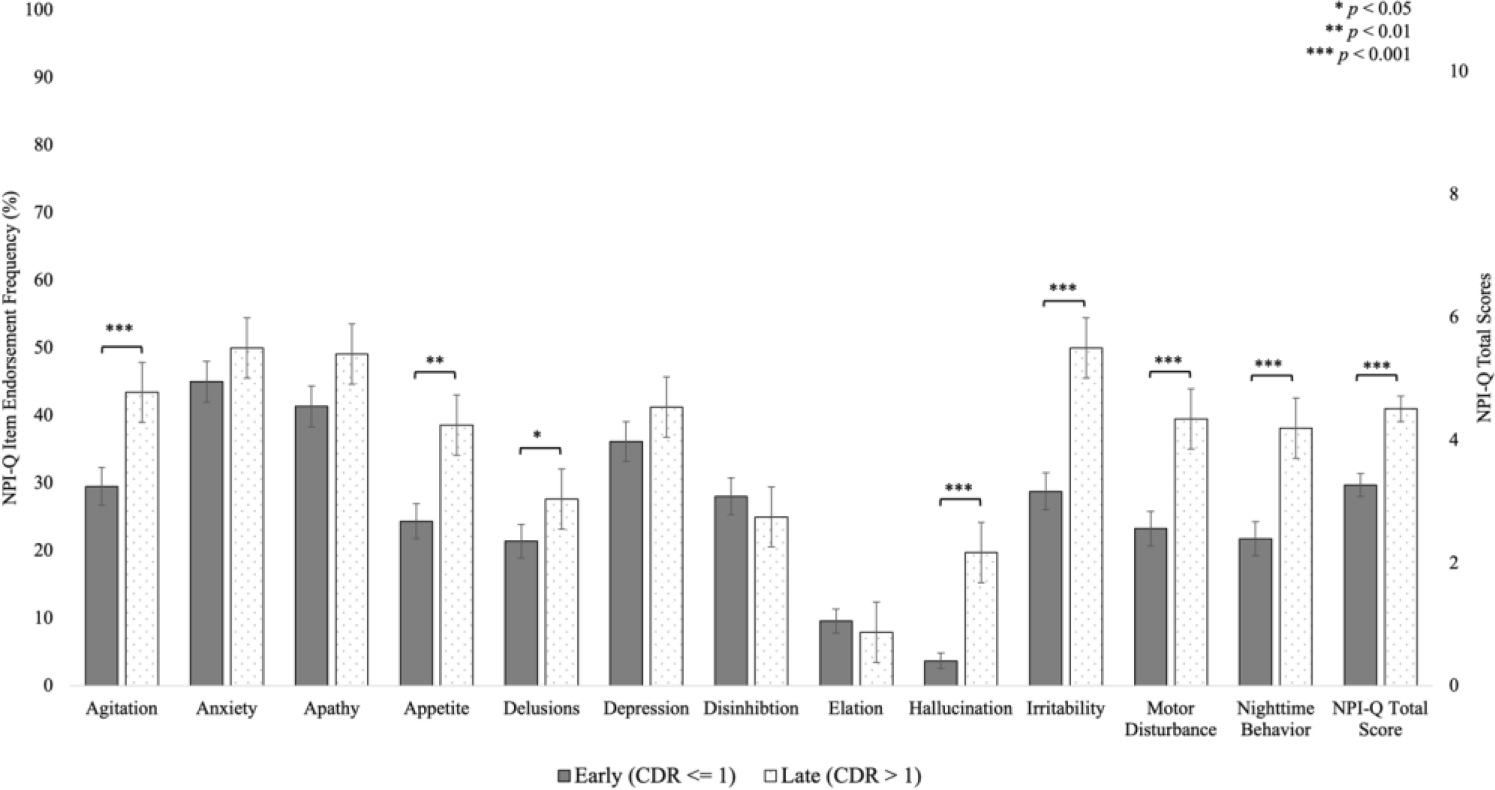
Frequencies of Positive Endorsement on NPI-Q Items and NPI-Q Total Scores by CDR Category.
CDR: Clinical Dementia Rating scale global score; NPI-Q Neuropsychiatric Inventory Questionnaire.
Sex
We found a significant main effect of sex on select NPI-Q items (Supplementary Figure 1). Males had a higher prevalence of Agitation (EPD = 9.5%, p = 0.04) and Irritability (EPD = 9.8%, p = 0.03), while females had a higher prevalence of Hallucinations (EPD = 6.1%, p =0.03).
HIW NPI-Q differences by country of origin
We evaluated NPS differences between our main HIW groups, PR and CA individuals (Figure 3). The results revealed that NPI-Q total scores were similar between PR and CA individuals (t = −1.32, p = 0.19), mean difference (MD) = 0.60). However, CA individuals had a greater prevalence of Anxiety (t = −3.62, p < 0.001, MD = 32.5%) and Irritability (t = −2.56, p = 0.01, MD = 22.1%), while PR individuals had a higher prevalence of Appetite (t = 3.38, p = 0.001, MD = 28.9%) and Motor Disturbance (t = 2.45, p = 0.02, MD = 22.1%). No differences between PR and CA individuals were found for the items Agitation (t = −0.76, p = 0.45, MD = 6.8%), Apathy (t = −1.47, p = 0.15, MD = 13.6%), Delusions (t = −1.33, p = 0.19, MD = 10.4%), Depression (t = −1.91, p =0.06, MD = 17.9%), Disinhibition (t = −0.81, p = 0.42, MD = 5.8%), Elation (t = 0.37, p = 0.72, MD = 1.6%), Hallucinations (t = 1.27, p = 0.21, MD = 8.1%), or Nighttime Behavior (t = −1.17, p = 0.25, MD = 9.7%).
Figure 3.
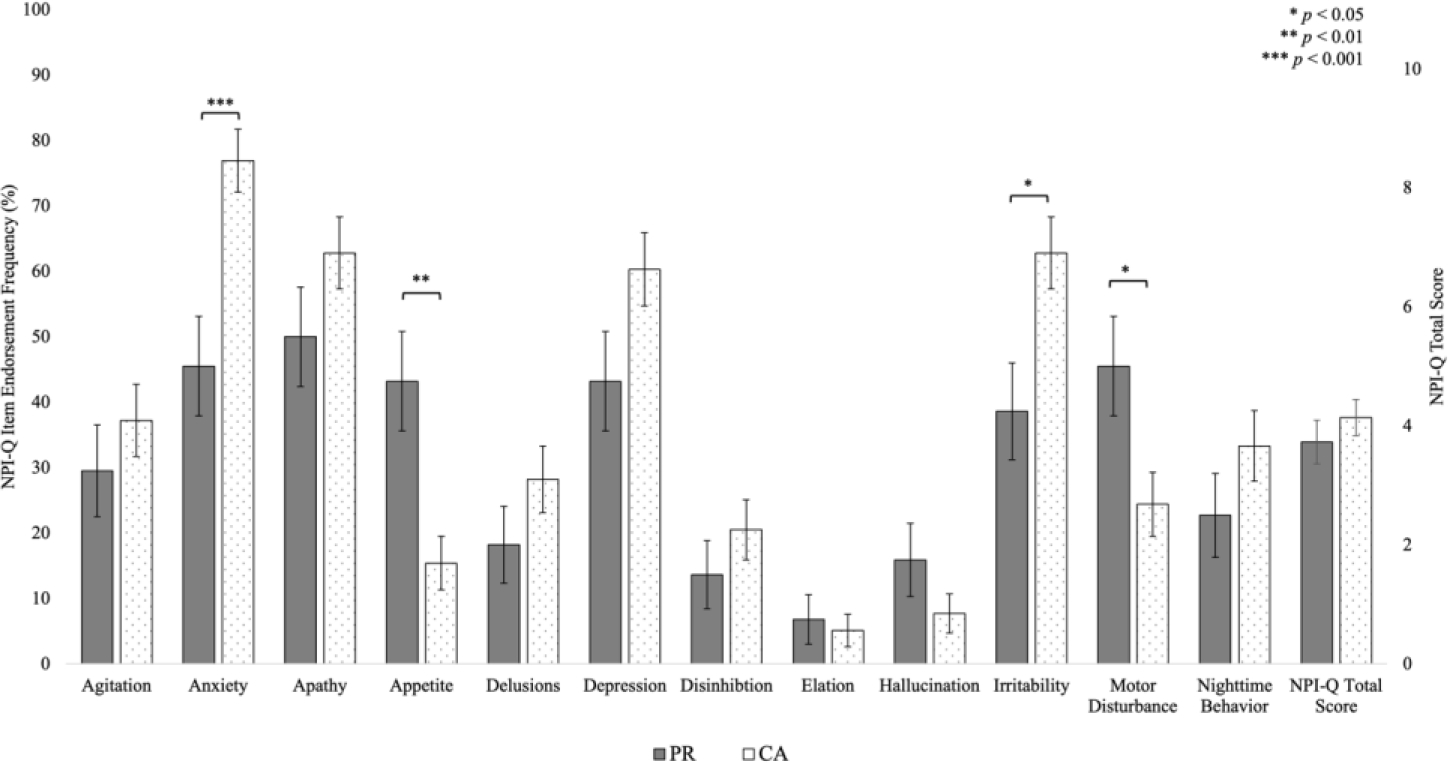
Frequencies of Positive Endorsement on NPI-Q Items and NPI-Q Total Scores for Puerto Rican and Cuban American individuals.
CA: Cuban; NPI-Q Neuropsychiatric Inventory Questionnaire; PR: Puerto Rican.
Discussion
The primary aim of this study was to examine similarities and differences in the prevalence of neuropsychiatric symptoms (NPS) among individuals with AD or MCI from different self-declared race-ethnicity groups using the NPI-Q. Our results demonstrated that while the prevalence of several NPS are comparable among race-ethnicity groups and AD stages, our HIW individuals had a significantly higher prevalence of select NPS including Anxiety, Apathy, Depression, and Irritability. The results also revealed that AA individuals had a higher prevalence of Disinhibition compared to both NHW and HIW individuals, and a higher prevalence of Elation compared to HIW individuals. As expected, those individuals in the later stages of dementia had more overall NPS, and a higher prevalence of Agitation, Appetite, Delusions, Hallucinations, Irritability, Motor Disturbance, and Nighttime Behavior. Finally, males had a higher prevalence of Agitation and Irritability, while females had a higher prevalence of Hallucinations.
Our results showing more Anxiety and mood symptoms among HIW individuals compared to AA and NHW individuals, are not surprising. Several studies have demonstrated that older HIW individuals with dementia (primarily Mexican Americans) have a higher prevalence of mood problems (as measured by the NPI) compared to older NHW individuals36,41,54. Further, anxiety and depression symptoms (as measured by the Spielberger State Trait Anxiety Inventory), appear to be greater among healthy older Hispanic adults as well suggesting that such symptoms may predate dementia55–57. Finally, our secondary analyses suggest that the role of country of origin may be an important factor in understanding NPS among HIW. Specifically, we found that the prevalence of Anxiety and Irritability were greater in CA vs PR individuals (with trends suggesting a higher prevalence of Apathy and Depression in CA as well). At a minimum, these results confirm that HIW are not uniform in the occurrence of NPS. However, they also point to the potential contributions of sociocultural differences58 between these groups as well as differences in genetic ancestry (i.e., different proportions of African, European, and Amerindian ancestry)59–61.
Our AA and NHW had a similar total number of neuropsychiatric symptoms, however there were differences in select symptoms. AA individuals had a higher prevalence of Disinhibition compared to both NHW and HIW individuals, and a higher prevalence of Elation compared to HIW individuals. However, unlike some other studies we did not find differences between AA and NHW individuals on Anxiety, Depression, or psychosis related symptoms39,40. We believe that the lack of differences may be related to measurement issues. This is especially pertinent when considering the lower prevalence of Anxiety and mood symptoms in AA individuals. An alternative explanation may be that there are reporting differences for select NPS (e.g., Depression) due to stigma or a focus on somatic symptoms that are common to depression62–63. All these results, highlight the importance of further study in this area as clinical decision making is guided by measures such as the NPI-Q and could lead to underutilization of well-established pharmacologic therapies.
Conceptualizing NPS in AD is a complex undertaking. Neuropathological studies have demonstrated that NPS might be the result of molecular and functional alteration in specific brain regions and not just a behavioral consequence of AD64. While neuropathological studies highlight potential biologic underpinnings it does not take into account non-biological differences between multi-ethnic groups. We suggest that sociocultural differences may exert a strong influence on the measurement of NPS and diagnostic endpoints65–66 Thus, awareness of sociocultural influences on measurement and uptake of treatment is critical to ensure that these services are delivered. At the same time, the role of genetic risk and its variation among groups with different ancestral backgrounds may also contribute to NPS endpoints. Ancestry informed genetic studies of NPS across different populations can identify potential ancestry specific targets for future interventions and also provide insight on ancestral influences in genes associated to individual NPS such as Anxiety and Depression. Finally, our results have implications for how NPS are measured and ultimately treated, with special consideration on the impact that caregiver or informant distress is present at the time NPS are being reported.
Limitations
First, while the NPI-Q is a well-validated measure of neuropsychiatric symptoms common to AD, it is brief and may overestimate the prevalence of these symptoms as opposed to clinical examination and formal diagnosis67. Second, NPI-Q results may be influenced by culturally related communication issues pertinent to NPS (e.g., understanding what constitutes the specific symptoms)66. This limitation can be addressed in future studies in which individuals from various groups are asked to describe their understanding of NPS and its expression. Third, while country of origin may be a proxy for socio-cultural practices, it does not consider acculturation among individuals who no longer live in their country of origin, future analyses will examine differences in those still living in their home country (e.g., PR, CA), vs those who have immigrated to a new country. Finally, language and socio-cultural language barriers may contribute to the differences observed. While all individuals were examined in their preferred language (native or acquired), the language of administration and socio-culture language interpretation of NPI-Q items could influence response patterns from informant and caregivers and is worth investigating.
Conclusions
Consistent with prior studies, our results point to self-declared race-ethnicity group differences in the prevalence of NPS among individuals with AD. The burden of these symptoms on top of the cognitive and functional impairments of AD or MCI is exacerbated by poor understanding of these phenomena and how to best treat them. Future efforts to optimize the treatment of neuropsychiatric symptoms in AD or MCI requires careful consideration of both biologic and non-biologic factors that disproportionately affect diverse populations. Even with improvements in dissecting the biology of these symptoms in diverse populations, translational benefit will hinge on addressing both biological and non-biological influences. Our task now is to further explore the way such information can be used to better inform culturally attuned interventions—particularly given evidence that effective and timely treatment of neuropsychiatric symptoms in AD or MCI can have broad effects on cognition and behavior as well as caregiver stress.
Supplementary Material
Acknowledgments:
We are grateful to the families and staff who participated in this study. This Investigation was supported by grant “Genetic Studies in Dementia” (AG052410, AG054074) from the National Institute of Health (NIH) and National Institute on Aging (NIA). We gratefully acknowledge the resources provided by the John P. Hussman Institute for Human Genomics.
Funding:
Financial support for the research, authorship and publication of this article was provided by the National Institutes of Health grant (AG052410; AG054074).
Footnotes
Conflict of interests: The authors declared no potential conflicts of interest.
Declaration of interest: None
Ethical Approval: This study received ethical approval from University of Miami Institutional Review Board (approved protocol #20070307)
Data Sharing: Data will be available upon request.
References
- 1.Lennon JC, Aita SL, Bene VAD, et al. : Black and White individuals differ in dementia prevalence, risk factors, and symptomatic presentation. Alzheimers Dement 2022; 18:1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green RC, Cupples LA, Go R, et al. : Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA 2002; 287:329–336 [DOI] [PubMed] [Google Scholar]
- 3.Tang MX, Maestre G, Tsai WY, et al. : Relative risk of Alzheimer disease and age-at-onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet 1996; 58:574–584 [PMC free article] [PubMed] [Google Scholar]
- 4.Tang MX, Cross P, Andrews H, et al. : Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001; 56:49–56 [DOI] [PubMed] [Google Scholar]
- 5.Perkins P, Annegers JF, Doody RS, et al. : Incidence and prevalence of dementia in a multiethnic cohort of municipal retirees. Neurology 1997; 49:44–50 [DOI] [PubMed] [Google Scholar]
- 6.Chin AL, Negash S, Hamilton R : Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord 2011; 25:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Bryant SE, Johnson L, Balldin V, et al. : Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 2013; 33:373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper C, Tandy AR, Balamurali TB, et al. : A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry 2010; 18:193–203 [DOI] [PubMed] [Google Scholar]
- 9.Babulal GM, Quiroz YT, Albensi BC, et al. : Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement 2019; 15:292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2022 Alzheimer’s disease facts and figures. Alzheimers Dement 2022; 18:700–789 [DOI] [PubMed] [Google Scholar]
- 11.Howell JC, Watts KD, Parker MW, et al. : Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimers Res Ther 2017; 9:88–017-0315–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Bryant SE, Xiao G, Edwards M, et al. : Biomarkers of Alzheimer’s disease among Mexican Americans. J Alzheimers Dis 2013; 34:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiscella K: Health care reform and equity: promise, pitfalls, and prescriptions. Ann Fam Med 2011; 9:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kontos EZ, Emmons KM, Puleo E, et al. : Determinants and beliefs of health information mavens among a lower-socioeconomic position and minority population. Soc Sci Med 2011; 73:22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson A: Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94:666–668 [PMC free article] [PubMed] [Google Scholar]
- 16.Williams DR, Mohammed SA, Leavell J, et al. : Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci 2010; 1186:69–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyketsos CG, Lopez O, Jones B, et al. : Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002; 288:1475–1483 [DOI] [PubMed] [Google Scholar]
- 18.van der Linde RM, Dening T, Stephan BC, et al. : Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry 2016; 209:366–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savva GM, Zaccai J, Matthews FE, et al. : Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. Br J Psychiatry 2009; 194:212–219 [DOI] [PubMed] [Google Scholar]
- 20.Edwards ER, Spira AP, Barnes DE, et al. : Neuropsychiatric symptoms in mild cognitive impairment: differences by subtype and progression to dementia. Int J Geriatr Psychiatry 2009; 24:716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eikelboom WS, van den Berg E, Singleton EH, et al. : Neuropsychiatric and Cognitive Symptoms Across the Alzheimer Disease Clinical Spectrum: Cross-sectional and Longitudinal Associations. Neurology 2021; 97:e1276–e1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li K, Zeng Q, Luo X, et al. : Neuropsychiatric symptoms associated multimodal brain networks in Alzheimer’s disease. Hum Brain Mapp 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters ME, Schwartz S, Han D, et al. : Neuropsychiatric symptoms as predictors of progression to severe Alzheimer’s dementia and death: the Cache County Dementia Progression Study. Am J Psychiatry 2015; 172:460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karttunen K, Karppi P, Hiltunen A, et al. : Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’s disease. Int J Geriatr Psychiatry 2011; 26:473–482 [DOI] [PubMed] [Google Scholar]
- 25.Scaricamazza E, Colonna I, Sancesario GM, et al. : Neuropsychiatric symptoms differently affect mild cognitive impairment and Alzheimer’s disease patients: a retrospective observational study. Neurol Sci 2019; 40:1377–1382 [DOI] [PubMed] [Google Scholar]
- 26.Garre-Olmo J, Lopez-Pousa S, Vilalta-Franch J, et al. : Grouping and trajectories of neuropsychiatric symptoms in patients with Alzheimer’s disease. Part II: two-year patient trajectories. J Alzheimers Dis 2010; 22:1169–1180 [DOI] [PubMed] [Google Scholar]
- 27.Beeri MS, Werner P, Davidson M, et al. : The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry 2002; 17:403–408 [DOI] [PubMed] [Google Scholar]
- 28.Mooijman D, Dey SS, Boisset JC, et al. : Single-cell 5hmC sequencing reveals chromosome-wide cell-to-cell variability and enables lineage reconstruction. Nat Biotechnol 2016; 34:852–856 [DOI] [PubMed] [Google Scholar]
- 29.Kales HC, Gitlin LN, Lyketsos CG: Assessment and management of behavioral and psychological symptoms of dementia. BMJ 2015; 350:h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kales HC, Gitlin LN, Lyketsos CG, et al. : Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc 2014; 62:762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufer DI, Cummings JL, Ketchel P, et al. : Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000; 12:233–239 [DOI] [PubMed] [Google Scholar]
- 32.Liew TM: Neuropsychiatric symptoms in early stage of Alzheimer’s and non-Alzheimer’s dementia, and the risk of progression to severe dementia. Age Ageing 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krell-Roesch J, Syrjanen JA, Machulda MM, et al. : Neuropsychiatric symptoms and the outcome of cognitive trajectories in older adults free of dementia: The Mayo Clinic Study of Aging. Int J Geriatr Psychiatry 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krell-Roesch J, Syrjanen JA, Mielke MM, et al. : Association Between Neuropsychiatric Symptoms and Functional Change in Older Non-Demented Adults: Mayo Clinic Study of Aging. J Alzheimers Dis 2020; 78:911–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sink KM, Covinsky KE, Newcomer R, et al. : Ethnic differences in the prevalence and pattern of dementia-related behaviors. J Am Geriatr Soc 2004; 52:1277–1283 [DOI] [PubMed] [Google Scholar]
- 36.Salazar R, Dwivedi AK, Royall DR: Cross-Ethnic Differences in the Severity of Neuropsychiatric Symptoms in Persons With Mild Cognitive Impairment and Alzheimer’s Disease. J Neuropsychiatry Clin Neurosci 2017; 29:13–21 [DOI] [PubMed] [Google Scholar]
- 37.Salazar Ricardo, Royall Donald R., Raymond F. Palmer: Neuropsychiatric symptoms in community-dwelling Mexican-Americans: results from the Hispanic Established Population for Epidemiological Study of the Elderly (HEPESE) study. Int J Geriatr Psychiatry 2015. Mar:300–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hargrave R, Stoeklin M, Haan M, et al. : Clinical aspects of dementia in African-American, Hispanic, and white patients. J Natl Med Assoc 2000; 92:15–21 [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen CI, Magai C: Racial differences in neuropsychiatric symptoms among dementia outpatients. Am J Geriatr Psychiatry 1999; 7:57–63 [PubMed] [Google Scholar]
- 40.Hargrave R, Stoeklin M, Haan M, et al. : Clinical aspects of Alzheimer’s disease in black and white patients. J Natl Med Assoc 1998; 90:78–84 [PMC free article] [PubMed] [Google Scholar]
- 41.Ortiz F, Fitten LJ, Cummings JL, et al. : Neuropsychiatric and behavioral symptoms in a community sample of Hispanics with Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2006; 21:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez Martinez M, Castro Flores J, Perez de las Heras S, et al. : Prevalence of neuropsychiatric symptoms in elderly patients with dementia in Mungialde County (Basque Country, Spain). Dement Geriatr Cogn Disord 2008; 25:103–108 [DOI] [PubMed] [Google Scholar]
- 43.Tatsch MF, Bottino CM, Azevedo D, et al. : Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: prevalence and relationship with dementia severity. Am J Geriatr Psychiatry 2006; 14:438–445 [DOI] [PubMed] [Google Scholar]
- 44.Albert MS, DeKosky ST, Dickson D, et al. : The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKhann G, Drachman D, Folstein M, et al. : Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944 [DOI] [PubMed] [Google Scholar]
- 46.McKhann GM, Knopman DS, Chertkow H, et al. : The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cummings JL: The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997; 48:S10–S16 [DOI] [PubMed] [Google Scholar]
- 48.Cummings JL, Mega M, Gray K, et al. : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314 [DOI] [PubMed] [Google Scholar]
- 49.Boada M, Cejudo JC, Tarraga L, Lopez OL, & Kaufer D (2002). Neuropsychiatric inventory questionnaire (NPI-Q): Spanish validation of an abridged form of the Neuropsychiatric Inventory (NPI). Neurologia (Barcelona, Spain), 17(6), 317–323. [PubMed] [Google Scholar]
- 50.Camozzato AL, Godinho C, Kochhann R, Massochini G, & Chaves ML (2015). Validity of the Brazilian version of the Neuropsychiatric Inventory Questionnaire (NPI-Q). Arquivos de Neuro-Psiquiatria, 73, 41–45. [DOI] [PubMed] [Google Scholar]
- 51.Zepeda MUP, Guerrero JAR, Carrasco OR, & Robledo LMG (2008). P3–038: Validation of the neuropsychiatric inventory questionnaire in a group of Mexican patients with dementia. Alzheimer’s & Dementia, 4, T527–T528. [Google Scholar]
- 52.Musa G, Henríquez F, Muñoz-Neira C, Delgado C, Lillo P, & Slachevsky A (2017). Utility of the Neuropsychiatric Inventory Questionnaire (NPI-Q) in the assessment of a sample of patients with Alzheimer’s disease in Chile. Dementia & neuropsychologia, 11, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris JC: The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 54.Hinton L, Haan M, Geller S, et al. : Neuropsychiatric symptoms in Latino elders with dementia or cognitive impairment without dementia and factors that modify their association with caregiver depression. Gerontologist 2003; 43:669–677 [DOI] [PubMed] [Google Scholar]
- 55.Camacho A, Gonzalez P, Buelna C, et al. : Anxious-depression among Hispanic/Latinos from different backgrounds: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Soc Psychiatry Psychiatr Epidemiol 2015; 50:1669–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camacho A, Tarraf W, Jimenez DE, et al. : Anxious Depression and Neurocognition among Middle-Aged and Older Hispanic/Latino Adults: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Results. Am J Geriatr Psychiatry 2018; 26:238–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wassertheil-Smoller S, Arredondo EM, Cai J, et al. : Depression, anxiety, antidepressant use, and cardiovascular disease among Hispanic men and women of different national backgrounds: results from the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2014; 24:822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harwood DG, Barker WW, Ownby RL, et al. : Depressive symptoms in Alzheimer’s disease. An examination among community-dwelling Cuban American patients. Am J Geriatr Psychiatry 2000; 8:84–91 [DOI] [PubMed] [Google Scholar]
- 59.Fortes-Lima C, Bybjerg-Grauholm J, Marin-Padron LC, et al. : Exploring Cuba’s population structure and demographic history using genome-wide data. Sci Rep 2018; 8:11422–018-29851–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreno-Estrada A, Gravel S, Zakharia F, et al. : Reconstructing the population genetic history of the Caribbean. PLoS Genet 2013; 9:e1003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Norris ET, Wang L, Conley AB, et al. : Genetic ancestry, admixture and health determinants in Latin America. BMC Genomics 2018; 19:861–018–5195–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riley M, Hill-Daniel J: What factors lead to underdiagnosis of depression in African Americans?. Evidence-Based Practice 2020:41–42 [Google Scholar]
- 63.Bailey RK, Mokonogho J, Kumar A: Racial and ethnic differences in depression: current perspectives. Neuropsychiatr Dis Treat 2019; 15:603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Dam D, Vermeiren Y, Dekker AD, et al. : Neuropsychiatric Disturbances in Alzheimer’s Disease: What Have We Learned from Neuropathological Studies?. Curr Alzheimer Res 2016; 13:1145–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braveman P, Gottlieb L: The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep 2014; 129 Suppl 2:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sayegh P, Knight BG: Functional assessment and neuropsychiatric inventory questionnaires: measurement invariance across hispanics and non-Hispanic whites. Gerontologist 2014; 54:375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tissot C, Therriault J, Pascoal TA, et al. : Association between regional tau pathology and neuropsychiatric symptoms in aging and dementia due to Alzheimer’s disease. Alzheimers Dement (N Y) 2021; 7:e12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


