Abstract
Parkinson disease (PD) is a chronic progressive neurodegenerative disease with increasing worldwide prevalence. Despite many trials of neuroprotective therapies in manifest PD, no disease-modifying therapy has been established. Over the past several decades, a series of breakthroughs have identified discrete populations at substantially increased risk of developing PD. Based on this knowledge, now is the time to design and implement PD prevention trials. This endeavor builds on experience gained from early prevention trials in Alzheimer disease and Huntington disease. This article first reviews prevention trial precedents in these other neurodegenerative diseases before focusing on the critical design elements for PD prevention trials, including whom to enroll for these trials, what therapeutics to test, and how to measure outcomes in prevention trials. Our perspective reflects progress and remaining challenges that motivated a 2021 conference, “Planning for Prevention of Parkinson: A Trial Design Symposium and Workshop.”
Now is the time to start comprehensively designing and implementing trials to prevent Parkinson disease (PD).1 Prevention, a once insurmountable challenge in PD is now a more realistic endeavor. A better understanding of PD, its molecular genetics, populations at risk for developing PD, improved enrollment strategies, and better defined multimodal prediagnostic progression metrics have paved the way for this logical next step. Previous studies have presciently laid the groundwork for individual elements of PD prevention trial planning.2-4 However, until now, little holistic planning has been pursued to integrate knowledge of targeted populations, specific interventions, and practical outcomes. Trials are more likely to ultimately succeed if they incorporate these basic design elements together with the perspectives of the affected community. Hence, prevention trial planning will benefit from early engagement of all key stakeholders, from advocates—both at-risk and manifesting—to treating physicians, to investigators, to investors, and from academia, government, industry, and philanthropy.
Enrolling at-risk participants just before PD phenoconversion (when diagnostic clinical criteria of PD are first met), or earlier, preceding the development of even subtle motor or imaging deficits, may eliminate a major reason for the consistent inefficacy of candidate disease-modifying therapy in de novo PD trials to date. Specifically, by the time of phenoconversion, it may be too late to impede the disease because therapies typically target mechanisms that mediate later steps in the PD pathophysiologic cascade. In other words, the proverbial parkinsonian horse may have already bolted when the (neuro)protective barn door is shut. This expansion of prevention research in the PD field follows on from studies in Alzheimer disease (AD) in individuals who are cognitively normal but at risk for AD (preclinical stage of disease) because of the presence of a biomarker of pathology (e.g., amyloid) or carrying pathogenic genetic variants associated with developing AD and from those with presymptomatic genetically confirmed Huntington disease (HD).
Throughout this article, we will emphasize the important, emerging role of the at-risk community in trial design and implementation. Expanding knowledge of prodromal features and high-risk determinants (e.g., pathogenic genetic variants and biomarkers of pathology) has increased the awareness of PD risk, which has understandably led to a sense of urgency to prevent PD in those at risk and their family members. These at-risk individuals broaden the traditional PD patient and advocacy community. Although their perspectives, motivations, and insights may vary based on distinctly different reasons for elevated risk, their engagement will be vital. Their contributions will improve trial design, implementation, interpretation, dissemination, and will help address the many ethical and practical challenges posed by prevention research.
In this article, we will first review prevention trial precedents in AD and HD, before focusing on the critical design elements for PD prevention trials (Figure 1), including whom to enroll for these trials, when, and how to recruit for them; what therapeutics to test; and how to measure outcomes in prevention trials (as detailed in the 9 other articles of this supplement issue). We conclude that the convergence of these advances and insights encourages the imminent initiation of PD prevention trials (as amplified in the incisive overview and outlook of Berg et al.5).
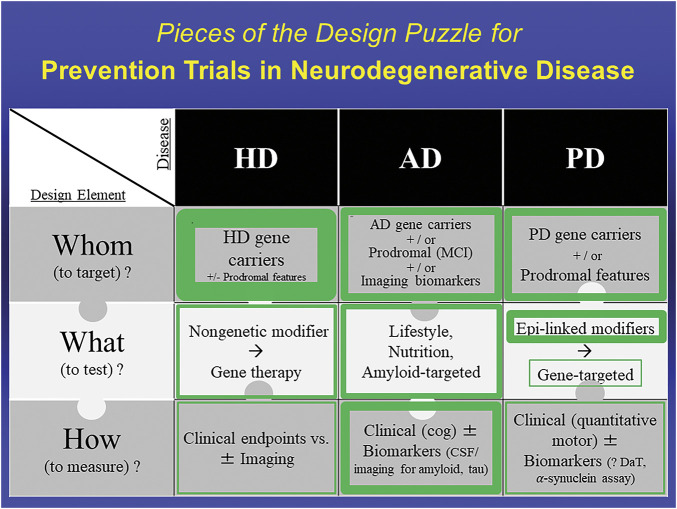
Figure 1. Pieces of the Design Puzzle for Prevention Trials in Neurodegenerative Disease: Comparing Early Progress in Selecting Design Elements for Prevention Trials Across HD, AD, and PD.
Thickness of green borders represents the relative extent to which the enclosed design element has been identified or established to enable initiation of prevention trials. For example, whom to target in HD is well established with the core feature of pathogenic expansion CAG repeats in the HD gene, possibly with prodromal features that increase likelihood of phenoconversion. In AD, all elements are sufficiently defined to have allowed numerous prevention trials. In PD, major advances in defining the genetics and prodromal state of PD have helped set the Whom piece of the trial design puzzle, and the relative safety and robustness of epidemiology (Epi)-linked risk modifiers compared to those in other neurodegenerative diseases have made them initially more attractive candidates for what to test, compared with emerging gene-targeted therapeutic strategies. How to measure prevention of PD before phenoconversion remains uncertain but is under intensive study. AD = Alzheimer disease; HD = Huntington disease; MCI = mild cognitive (cog) impairment; PD = Parkinson disease.
Prevention Trial Precedents in Other Neurodegenerative Conditions
AD is the most common neurodegenerative disorder worldwide, with affected individuals progressing from mild cognitive impairment (MCI) to dementia over several years. To date there have been several AD prevention trials, which have used different approaches for prevention, including lifestyle modification, nutritional supplementation, and drugs, typically targeting amyloid, a core pathologic feature, and potential mediator of AD. These studies have enrolled well-defined, enriched groups of individuals in preclinical at-risk populations, subdivided by genetic and biomarker risk. Trials based on genetic risk (presence of autosomal dominant variants or susceptibility genes) for AD include those conducted under the Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU), the Alzheimer Prevention Initiative (API), and the TOMMORROW study. The DIAN-TU studied solanezumab and gantenerumab, both antiamyloid monoclonal antibodies, in individuals with a variety of autosomal dominant pathogenic AD variants across cognitively normal, MCI, and mild dementia. Although the agents engaged with beta-amyloid, their target, they failed to show benefit in cognitive measures.6 The API program investigators ran 3 trials. They investigated amilomotide, an antiamyloid active vaccine, and umibecestat, a β-site amyloid precursor protein cleaving enzyme (BACE) inhibitor, in homozygous apolipoprotein E4 (APOE4) carriers who were cognitively normal older adults (Generation 1) and umibecestat in heterozygous APOE4 carriers who were cognitively normal older adults (Generation 2).7 Unfortunately, both umibecestat trials were discontinued early because of worsening cognition. As of October 2021, they have an ongoing study of crenezumab, an antiamyloid monoclonal antibody, in cognitively normal individuals who are carriers of a single variant of the autosomal dominant presenilin 1 gene (NCT01998841). The TOMMORROW trial investigated pioglitazone (a nuclear receptor peroxisome proliferator–activated receptor gamma [PPAR-γ] activator) in cognitively normal older adults who were carriers of both the APOE4 and TOMM40 susceptibility genes. This study was unfortunately stopped early because of lack of efficacy.8 There have also been AD biomarker–based trials targeting amyloid in individuals with elevated amyloid on PET imaging. These trials include the ongoing Anti-Amyloid Treatment in Asymptomatic AD (A4) study of solanezumab (NCT02008357), the EARLY trial of atabecestat (a BACE inhibitor),9 and the AHEAD A3-45 study of lecanemab (an antiamyloid monoclonal antibody) (NCT04468659).
Lifestyle modifications have also been explored in cohorts at risk for AD based on age or subjective cognitive decline and have ranged from reducing systolic blood pressure (Systolic Blood Pressure Intervention Trial - Memory and Cognition in Decreased Hypertension)10 to a multidomain approach of improved nutrition, exercise, cognitive training, and management of vascular risk factors (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability [FINGER study]).11 The results from these studies have suggested a lower hazard of progression to MCI or maintenance of cognitive function with these interventions. Given these successes, another lifestyle study, the US Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (US POINTER), is currently underway (NCT03688126). Nutritional supplements have failed to show any effect on cognition or prevention for AD. Ginkgo biloba was investigated in the Ginkgo Evaluation of Memory and the GuidAge studies; both studies failed to show benefit in reducing the incidence of dementia after 5 years or more of follow-up in older adults who primarily had normal cognition or subjective cognitive decline.12,13
The PD field can learn from both the successes and failures of these approaches for prevention studies in AD, including from their design and execution. The AD experience to date emphasizes the importance of studying a clearly defined, well-demarcated cohort; conscientious development of a trial infrastructure; and involvement of multiple stakeholders, given the high screen failure rates and poor retention in enriched preclinical AD trials.14 The establishment of registries or trial ready cohorts may reduce these challenges. As in the AD field, there is a need for better outcome measures in PD therapeutics development. Currently, there is only a single MCI outcome available, the Clinical Dementia Rating scale. The Food and Drug Administration (FDA) has agreed that for prevention trials in preclinical AD a single sensitive clinical outcome measure focused on cognition is sufficient to obtain conditional approval with the promise of demonstrating benefit in instrumental activities of daily living in postapproval trials or follow-up of participants.15 Until recently, there was also no FDA-approved primary outcome biomarker, although the testing of antiamyloid treatments as potential treatment or prevention measures has been greatly facilitated by availability of a quantitative brain imaging biomarker of this targeted pathologic protein in AD. In June 2021, in a controversial decision, the FDA granted accelerated approval for the use of aducanumab (an antiamyloid monoclonal antibody) in the treatment of AD based on evidence of effective removal of amyloid visualized on PET in 2 phase 3 trials in participants with MCI or very mild AD dementia. The trials unfortunately did not show a clear clinical benefit, and the FDA stipulated that a postapproval trial will need to provide that evidence. This has since paved the road for FDA submissions for accelerated approval based on biomarker evidence of efficacy and several companies with AD drugs in development have recently filed such applications. Of note, the testing of antiamyloid treatments as potential treatment or prevention measures has been greatly facilitated by availability of a quantitative brain imaging biomarker of this targeted pathologic protein in AD. By contrast, in PD there is as yet no validated imaging or other measure of alpha-synuclein or related pathologic markers that may serve as a biomarker of efficacy in prevention trials for PD (Figure 1). However, in the last decade, dopamine transporter imaging has demonstrated the ability to identify prodromal participants prior to the diagnosis of PD (see Whom to enroll below).
Another lesson from the early experience in prevention trials in the AD field is the value of bringing together various stakeholders to collaborate early in the process. The Collaboration for Alzheimer's Prevention (CAP) was assembled at the outset of prevention trials for AD16 and leveraged the motivation, resources, and skill of the patient community, academic, government, industry, philanthropic and regulatory partners to expedite rigorous harmonized trials for AD prevention. Beginning to convene their counterparts to build consensus on approaching prevention trials for PD was a key goal of our 2021 trial design conference and will be expanded on for future PD prevention initiatives to ensure their greatest impact.
HD, an autosomal dominantly inherited neurodegenerative disorder, should conceptually offer even more distinct advantages but some disadvantages for the pursuit of prevention trials (Figure 1). It causes motor, cognitive, and psychiatric symptoms in individuals who have inherited an abnormally long cytosine adenine guanine (CAG) trinucleotide DNA repeat expansion. Unique to HD is the effectively 100% penetrance of the variant, unlike the vast majority of those inherited in PD and AD patients. However, many individuals at risk for HD do not undergo genetic testing given concerns over genetic discrimination and psychosocial burden. The rare prevalence of this disorder of 1.2–13.1 persons per 100,000, based on insurance databases17 poses unique challenges for trials. To date, there have been few randomized clinical trials in premanifest, genetically confirmed carriers of the pathogenic HD variant. These trials include PREQUEL, a feasibility study of coenzyme Q1018; PRECREST, a phase 2 study of creatine19; and SIGNAL-HD, a phase 2 trial of pepinemab (NCT02481674). PREQUEL demonstrated that high-dose coenzyme Q10 was safe and well-tolerated, and PRECREST demonstrated the feasibility of using pharmacodynamic and neuroimaging biomarker measures of creatine treatment in premanifest individuals. However, neither compound was utilized in a phase 3 study following the futility in comparable studies in individuals already manifesting symptoms of HD, 2CARE and Creatine Safety, Tolerability, & Efficacy in Huntington's Disease, respectively. The SIGNAL-HD trial failed to meet its co-primary endpoints in either premanifest or early symptomatic HD participants. In spite of its autosomal dominant inheritance, HD is clinically heterogeneous, with a variable age at onset, even in individuals with the same CAG repeat length, and with respect to clinical manifestations. The HD field, like those of PD and AD, has limited FDA-approved surrogate endpoints, namely the HD Total Functional Capacity and Total Motor Scores, which are of limited use in the prodromal and presymptomatic stages given the floor effects of these clinical measures. The validation of surrogate biomarker endpoints for trials in HD is an active area of investigation and crucial for HD prevention trials.
Whom to Enroll for PD Prevention Trials?
Over the past 30 years, research on individuals with PD has identified several pathogenic genetic variants and nonmotor symptoms (NMSs) as strong predictors of subsequent PD development (See perspectives by Niotis et al.,20 Postuma,21 and Molsberry et al.22 in this supplement issue.) Individuals with these genetic variants or prodromal PD features, alone or in combination, are considered at risk for PD and related synucleinopathy disorders (multiple system atrophy and dementia with Lewy bodies). In 2015, the Movement Disorder Society (MDS) created MDS Research Criteria for Prodromal Parkinson disease, which included risk and prodromal markers for PD development.23 Using these criteria, individuals with a high likelihood ratio for prodromal PD could be identified. Over the past 10 years, several research studies and biobanks, like the Parkinson Progression Markers Initiative (PPMI), the Parkinson Associated Risk Study (PARS),24 the online Fox Insight study, the North American Prodromal Synucleinopathy (NAPS) Consortium for REM sleep behavior disorder (RBD), and many others, have enrolled cohorts of at-risk participants, who through their participation have provided cross-sectional or longitudinal clinical, biological and/or radiologic data on at-risk, preclinical, and prodromal PD states. In addition, genotyping initiatives, like PDGENEration (NCT04057794) and the Rostock International Parkinson Disease Study (NCT03866603) have increased awareness of genetic PD through the offering of genetic testing to individuals with PD. Collectively, these programs have begun to develop an infrastructure for PD prevention trials. They have identified potential participants for prevention trials, and fostered collaboration and unity amongst the many interested stakeholders in PD research. This critical and accelerating progress over the past 30 years presents a roadmap that will ultimately lead to PD prevention (Figure 2).
Figure 2. Roadmap to Designing PD Prevention Trials: This roadmap chronologically highlights many of the discoveries and initiatives in PD research that will lead the way to designing and implementing the first PD prevention trials.23,48-54.
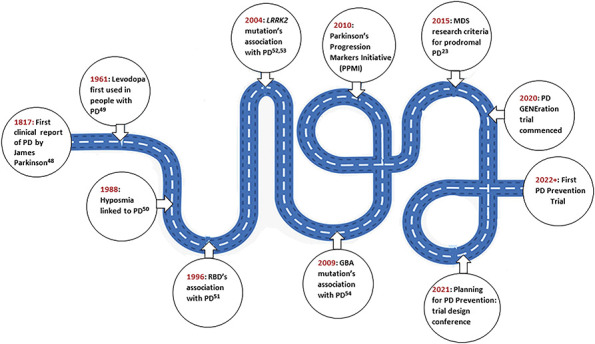
This roadmap chronologically highlights many of the discoveries and initiatives in PD research that will lead the way to designing and implementing the first PD prevention trials.23, 48-54 MDS = Movement Disorder Society; PD = Parkinson disease.
Genetically At-Risk Individuals
Although many genetic variants have been associated with PD, the 2 that are best positioned for prevention trials are the pathogenic LRRK2 and GBA variants.20 Prevention studies in genetically at-risk individuals are not unique to PD and are preceded by those in other neurodegenerative diseases, as above, along with other studies in medicine investigating BRCA1 and BRCA2 variants in breast and ovarian cancer, APC variants in familial adenomatous polyposis, and more recently, Phe508del allele carriers in cystic fibrosis. However, studying pathogenic variant carriers in PD does pose some distinct practical and ethical challenges. These PD-associated variants have incomplete penetrance and most individuals carrying them will never develop PD. Recent research reports penetrance of approximately 33% in LRRK2 G2019S variant carriers25 and 7%–15% pathogenic GBA variant carriers.26 It is also not known when individuals with these variants will develop PD (i.e., phenoconvert). Theoretically, given the presence of the variant at birth, phenoconversion could take place at any age. However, both the pathogenic LRRK2 and GBA variants demonstrate age-dependent penetrance with most individuals developing PD later in life, after 60 years in an age distribution similar to that of idiopathic PD. Nevertheless, there is still a ∼20–30 year time window for phenoconversion. Even if enrolled in the expected peak age range of onset, most carriers would not develop PD during a typical 18–24 month trial. Accordingly, further enrichment of genetically at-risk carrier cohorts with other predictors of imminent diagnosis (e.g., prodromal motor signs, dopamine transporter deficiency, nonmotor symptoms) may be necessary to realistically conduct phenoconversion-based prevention trials over a practical timeframe (see below). Conversely, relying on sufficiently sensitive clinical or molecular biomarker outcomes may be reasonable for proof-of-concept prevention studies that aim to demonstrate slowed progression prior to traditional PD diagnosis.
From an ethical standpoint, several questions arise regarding clinical trial participation of healthy individuals carrying pathogenic but incompletely penetrant variants. For example, are the benefits to an individual of learning they harbor a “PD gene” to participate in a prevention trial sufficient to offset any negative impact? This knowledge may result in psychological stress in some individuals27 and the chance, even if small, of added long-term care, disability, and life insurance costs or concerns of employment discrimination despite protections under the Genetic Information Nondiscrimination Act of 2008. Although these concerns may be modest for many who are motivated by self-knowledge and/or the opportunity to reduce disease risk for themselves or for others, the decision to get tested and to enroll should of course be made only after weighing the risks and benefits by each individual. Another question relatively unique to prevention trials is whether the safety risks of the intervention are sufficiently low given that the participant does not have manifest PD at the time of study treatment and may never get it with or without the intervention (see What below). Currently, routine genetic testing for pathogenic PD variants in unaffected individuals is not clinically recommended. However, testing in a research setting for those interested in discovering whether they have inherited a pathogenic variant is reasonable, especially if then presented with the option to take action by participating in a prevention trial. In addition, because of direct-to-consumer genetic testing, many people are self-discovering their increased risk for PD and many other genetic diseases and are presenting to neurologists and other specialists for education, counseling, or interest in clinical trial participation. As mentioned earlier, there is growing interest in these genetically at-risk populations with many individuals and families motivated to take part in prevention research.
Prodromally Defined “At-Risk” Populations
NMS have been identified throughout the various stages of PD from preclinical to advanced stages. Of the myriad of different NMS experienced, several have been associated with a future increased risk of PD. It is well-recognized that individuals with idiopathic RBD (iRBD) have the greatest increased risk for development of a synucleinopathy with approximately 6%–7% of participants phenoconverting per year to a synucleinopathy-related parkinsonism or dementia disorder.28 The extent of counseling regarding this increased risk varies.29 Those who are aware often present to neurologists or sleep medicine providers inquiring about prevention strategies and clinical trial opportunities. Through the International REM Sleep Behavior Study Group, NAPS Consortium, PPMI, and other initiatives, interested participants with iRBD are being enrolled into research cohorts. iRBD can be diagnosed with high sensitivity and specificity using a screening questionnaire.30 However, polysomnogram-diagnosed RBD remains the gold standard for diagnosis of this condition.
Other studied prodromal features include additional NMS such as constipation, hyposmia, body pain, excessive daytime sleepiness, mood disorders, and minimal parkinsonian symptoms,31 not yet meeting the clinical diagnostic criteria for PD. There are currently several studies using prospective longitudinal cohorts and administrative health care data to investigate prodromal features in the general population. Although many of these features are easily screened for using inexpensive questionnaires or through examination, individually they have relatively low sensitivity and specificity given their relatively high prevalence in the general and older population. A more useful approach may be considering them in combination with establishment of a composite prodromal cohort for prevention studies. In fact, a recent case-control study observed that the presence of constipation, RBD, and hyposmia conferred an age-adjusted odds ratio of 160 for having PD compared with their absence.32
Recent research has also identified a role for imaging markers, in particular DaT-SPECT imaging, which when integrated with genetic and clinical at-risk markers can further enrich the sample population, as seen in the Tübingen evaluation of Risk factors for Early detection of NeuroDegeneration study33 and the PARS,24 and potentially provide information on imminent phenoconversion. Reduced radiotracer uptake has been seen in iRBD and genetic cohorts. In a study of 32 participants, a DaT-SPECT scan could predict conversion in their nonmanifesting pathogenic LRRK2 variant carriers within 4 years. They found that a baseline scan with a ratio of bilateral striatal to occipital uptake below 1 predicted conversion to PD within the 4-year period with 100% sensitivity and specificity, occurring in 3 of their 32 participants.34 Iranzo et al.35 studied DaT-SPECT imaging in RBD participants and found that 20% of RBD individuals with an abnormal scan phenoconverted in 3 years (p = 0.006). Further studies are required to replicate these results but potentially using DaT-SPECT imaging in an already enriched population could identify those with the shortest lead time to developing PD, thus substantially reducing trial duration. The use of DaT-SPECT imaging alone in the general population is not feasible given its cost, radiation exposure, and limited availability.
What Therapeutics to Study in PD Prevention Trials?
Therapeutic options for prevention trials range from pharmacologic agents to genetic therapies to lifestyle measures. The intervention tested will likely vary depending on the at-risk study population enrolled (defined primarily by genetic vs prodromal risk factors) because the underlying driving disease pathogenesis and the target the investigators are seeking to engage would differ accordingly.36 For individuals at risk because of the presence of prodromal features, the focus may be on agents targeting α-synuclein given its core pathologic role in RBD and idiopathic PD. The focus may also be on less mechanistically defined agents of broad relevance to idiopathic PD such as its well-recognized inverse risk factors (e.g., caffeine, ibuprofen).37 For those at risk because of pathogenic genetic variants, attention logically turns to therapies precisely targeting the genetic mechanism implicated by the variant. However, those emerging mechanisms are uncertain and generally targeted at this point with new biological and chemical entities lacking established safety records. Accordingly, early attention may focus on relatively safe interventions plausibly hypothesized to confer protection against specific genetic forms of PD. Another key consideration in selecting what therapeutic candidates to first test for prevention is direct guidance from at-risk individuals and the newly assembling advocacy community they comprise. They of course are best able to understand the balance between the PD risk they face and the risk of adverse events they are willing to tolerate to potentially prevent the disease. Thus, such decisions should be made by researchers and regulators in partnership with representatives of those at risk.
Drug repurposing is a particularly appealing principle to invoke in selecting the first test agents because it can overcome several challenges associated with de novo drug discovery in offering established pharmacokinetics, tolerability, and safety profiles. Examples of potential repurposed agents and their study population include ambroxol, a mucolytic agent in pathogenic GBA variant carriers,38 and caffeine, an adenosine 2A antagonist, in pathogenic LRRK2 variant carriers.39 Another therapeutics approach for PD prevention trials would be retrialing an agent that was previously studied but failed to slow progression in a manifesting PD population (e.g., creatine, coenzyme Q10, inosine, or isradipine). Some were based on solid epidemiologic and basic science evidence for a role in PD development, are safe and tolerable, and if applied at an earlier stage in the neurodegenerative process may produce positive results. Although enthusiasm for revisiting former prospects would understandably be muted when phase 3 trial results were unequivocally negative, there may be greater motivation for candidates that showed some signal for disease modification even if insufficient for indication in manifesting PD (e.g., rasagiline).
For those individuals carrying a pathogenic LRRK2 or GBA variant, genetic therapy may be considered. Given the incomplete penetrance of these variants, at-risk carriers may not be interested in a precision medicine trial of an invasive or higher-risk treatment. Presently, there are several ongoing genetic therapy trials in individuals with LRRK2-mediated and GBA-mediated PD. If these studies were to demonstrate benefit or positive biomarker changes in individuals with PD along with good safety and tolerability, then reasonable next steps may include assessment in genetically at-risk individuals. In the meantime, foundational proof-of-concept trials for genetic PD prevention might assess putative protectants with well-known and acceptable side effect profiles that have been linked to resistance, for example, to LRRK2 PD as has been found for caffeine or ibuprofen.
Another potential avenue for the first PD prevention trial would be studying lifestyle factors commonly perceived as healthful (e.g., increased physical activity or bolstered nutrition) in at-risk populations. Exercise has been associated with both a reduced risk of PD development and a slower progression of disease.40 As reviewed by Janssen Daalen et al.41 in this supplement, current research is exploring different exercise prescriptions to identify the most beneficial exercise modality, intensity, and frequency for further study in a large clinical trial. Barriers and motivators to exercise in individuals with PD are also being identified.42 The increased availability of technology and motivational apps may improve participation in these clinical trials through gamification and social networking opportunities. Given the well-known general health benefits of exercise and its low-risk profile, this type of prevention trial may be more acceptable to the at-risk population and could be applied more generally and immediately to any or multiple at-risk populations.
How to Measure Outcomes of Prevention?
Clinical trials often have multiple primary and secondary outcomes of interest. In a PD prevention trial, the primary outcome would likely be an endpoint that demonstrates the ability of the intervention to prevent or reduce the likelihood of developing PD. Currently, the simplest and most obvious outcome that one might consider is phenoconversion. Although this clinically defined outcome is widely used in PD cohorts and case-control studies, it has significant limitations regarding its subjective nature, binary result (presence or absence), and poor sensitivity, especially in early disease.43 The Unified Parkinson Disease Rating Scale (UPDRS)44 or the Movement Disorders Society-revised UPDRS (MDS-UPDRS),45 which are routinely used to assess the core clinical characteristics of PD (bradykinesia, rigidity, rest tremor, and postural instability) are also unreliable in the setting of subtle prediagnostic motor deficits. These scales demonstrate high inter-rater variability, insensitivity to subtle motor impairment, confounding by other conditions (such as musculoskeletal issues as raters score what they see), and highly variable changes over time (with mean MDS-UPDRS total score changing by 2–7 points per year in individuals with newly diagnosed PD).46 Owing to these issues, the use of phenoconversion or a change in motor scale over time as the primary outcome of interest in a PD prevention trial would likely require enrollment of a large sample size, a long trial duration, and/or heavy enrichment for prediagnostic parkinsonian motor features—ultimately making the trial very costly or impractical at present. A sensitive and specific surrogate endpoint for PD remains to be identified and validated. As of January 2022, there are no FDA-approved PD surrogate markers. Discovery of such a biomarker of efficacy may permit a smaller sample size to be enrolled and a shorter trial duration, which collectively would make a PD prevention trial more realistic and appealing to investigators, participants, and funders.
Many PD biomarkers are being explored and range from early functional impairments of motor, cognitive, or autonomic domains to biological changes in the CSF, blood, skin, or on neuroimaging. Digital biomarkers, such as assessments made with a watch or smartphone app, are also being investigated. They are increasingly available and have demonstrated to be sensitive and reliable.47 These digital biomarkers can provide remote monitoring and the opportunity to capture passive data, with the potential to greatly improve on current trial data collection. They also reduce the need to come into the research clinic, thus making the trial more appealing to participants who are still working or leading active lives. Research initiatives such as PPMI are enrolling prodromal participants to establish and test biomarker outcomes. Involvement of these at-risk communities is important because endpoints chosen should be able to detect a clinically meaningful difference. The outcomes should also introduce minimal adverse effects or risk (e.g., radiation exposure or claustrophobia with nuclear medicine imaging) and not be too burdensome or bothersome (e.g., onerous daily log completion tasks or an itchy wristwatch strap) for participants. Finally, returning of individual or aggregated outcome measures to participants at the trial conclusion is important and could result in increased recruitment, retention, and compliance with the study protocols.
Conclusion
Research advances over the past 30 years now allow for the systematic design and implementation of PD prevention trials. Individuals at risk for PD are now more easily recognized, and the requisite trial infrastructure and methodology are rapidly developing. Key questions remain regarding which individuals will ultimately develop PD and how we can enrich these cohorts further. A variety of compelling therapeutic candidates are already available and include lifestyle measures, repurposed agents, and gene therapies. Identification and characterization of surrogate markers for PD progression before diagnosis is essential, and ongoing research in this area remains a high priority. Throughout the design and implementation of these trials, it is critical to have engagement and input from the at-risk community, which is just now organizing in part to improve emerging prospects for preventing PD.
Acknowledgment
Grant support from The Farmer Family Foundation Parkinson's Research Initiative, NIH R01NS110879, the Melvin Yahr Early Career Award in Movement Disorders Research, and Grant No. PF-MET-2011 from the Parkinson's Foundation. The authors thank Katherine Callahan for editorial assistance.
Glossary
- AD
Alzheimer disease
- API
Alzheimer Prevention Initiative
- APOE4
apolipoprotein E4
- BACE
precursor protein cleaving enzyme
- DIAN-TU
Dominantly Inherited Alzheimer Network Trials Unit
- HD
Huntington disease
- iRBD
idiopathic RBD
- MCI
mild cognitive impairment
- MDS
Movement Disorder Society
- NAPS
North American Prodromal Synucleinopathy
- NMS
nonmotor symptoms
- PARS
Parkinson Associated Risk Study
- PD
Parkinson disease
- PPMI
Parkinson Progression Markers Initiative
- RBD
REM sleep behavior disorder
- UPDRS
Unified Parkinson Disease Rating Scale
Appendix. Authors
Study Funding
Supported in part by Award ID PF-MET-2011 from the Parkinson's Foundation.
Disclosure
J.L. Keavney reports consultation fees from ESCAPE Bio. R.N. Alcalay reports consultation fees from Avrobio, Caraway, GSK, Merck, Ono Therapeutics, and Sanofi; and research support from NIH, DoD, the Parkinson's Foundation, and The Michael. J. Fox Foundation. K. Marek reports consultation fees from Michael J Fox Foundation, GE Healthcare, Roche, UCB, Bial, Denali, Takeda, Astellas, Biohaven, Neuron23, Aprinoia, Inhibikase, Alkahest, Genentech, Invicro. Ownership in Invicro, LLC. G.A. Marshall reports research salary support for serving as site principal investigator for clinical trials sponsored by Eisai Inc., Eli Lilly and Company, and Genentech. M.A. Schwarzschild reports consultation fees for steering committee, data monitoring committee or advisory services from Denali Therapeutics, Lilly, the Parkinson Study Group (for nQ Medical, Chase Therapeutics, Partner Therapeutics, and Bial Biotech), Parkinson’s Foundation (PF), Michael J. Fox Foundation (MJFF), Sutter Health, Northwestern University, Penn State University, and Xuanwu Hospital; and research grant support from the National Institute of Health, MJFF, PF, and Farmer Family Foundation; and licensing fee royalties from Massachusetts General Hospital for adenosine 2A receptor knockout mice. G.F. Crotty and H.D. Rosas report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Schwarzschild MA. Planning for prevention. It is time. In. WPC Blog2018.
- 2.Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology. 2015;84(11):1104-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennings D, Siderowf A, Stern M, et al. . Conversion to Parkinson disease in the PARS hyposmic and dopamine transporter-deficit prodromal cohort. JAMA Neurol. 2017;74(8):933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Videnovic A, Ju YS, Arnulf I, et al. . Clinical trials in REM sleep behavioural disorder: challenges and opportunities. J Neurol Neurosurg Psychiatry. 2020;91(7):740-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg D, Crotty GF, Keavney JL, Schwarzschild MA, Simuni T, Tanner C. Path to Parkinson disease prevention: conclusion and outlook. Neurology. 2022;99(7 Suppl):S76-S83. [DOI] [PubMed] [Google Scholar]
- 6.Salloway S, Farlow M, McDade E, et al. . A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer's disease. Nat Med. 2021;27(7):1187-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez Lopez C, Tariot PN, Caputo A, et al. . The Alzheimer's Prevention Initiative Generation Program: study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer's disease. Alzheimers Dement (NY). 2019;5:216-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns DK, Alexander RC, Welsh-Bohmer KA, et al. . Safety and efficacy of pioglitazone for the delay of cognitive impairment in people at risk of Alzheimer's disease (TOMMORROW): a prognostic biomarker study and a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021;20(7):537-547. [DOI] [PubMed] [Google Scholar]
- 9.Sperling R, Henley D, Aisen PS, et al. . Findings of efficacy, safety, and biomarker outcomes of atabecestat in preclinical Alzheimer disease: a truncated randomized phase 2b/3 clinical trial. JAMA Neurol. 2021;78(3):293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group SMIftSR, Williamson JD, Pajewski NM, et al. . Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kivipelto M, Solomon A, Ahtiluoto S, et al. . The Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER): study design and progress. Alzheimers Dement. 2013;9(6):657-665. [DOI] [PubMed] [Google Scholar]
- 12.Vellas B, Coley N, Ousset PJ, et al. . Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer's disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11(10):851-859. [DOI] [PubMed] [Google Scholar]
- 13.Snitz BE, O'Meara ES, Carlson MC, et al. . Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302(24):2663-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grill JD, Monsell SE. Choosing Alzheimer's disease prevention clinical trial populations. Neurobiol Aging. 2014;35(3):466-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Early Alzheimer's Disease: Developing Drugs for Treatment Guidance for Industry/Draft Guidance; 2018. Accessed January 26, 2022. Available at: fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM596728.pdf. [Google Scholar]
- 16.Reiman EM, Langbaum JB, Tariot PN, et al. . CAP—advancing the evaluation of preclinical Alzheimer disease treatments. Nat Rev Neurol. 2016;12(1):56-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Exuzides A, Crowell V, Reddy SR, Chang E, Yohrling G. Epidemiology of Huntington's disease (HD) in the US medicare population (670). Neurology. 2020;94(5 suppl):670.32156693 [Google Scholar]
- 18.Chandra A, Johri A, Beal MF. Prospects for neuroprotective therapies in prodromal Huntington's disease. Mov Disord. 2014;29(3):285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosas HD, Doros G, Gevorkian S, et al. . PRECREST: a phase II prevention and biomarker trial of creatine in at-risk Huntington disease. Neurology. 2014;82(10):850-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niotis K, West AB, Saunders-Pullman R. Who to enroll in Parkinson disease prevention trials? The case for genetically at-risk cohorts. Neurology. 2022;99(7 Suppl):S26-S33. [DOI] [PubMed] [Google Scholar]
- 21.Postuma RB. Neuroprotective trials in REM sleep behavior disorder: the way forward becomes clearer. Neurology. 2022;99(7 Suppl):S19-S25. [DOI] [PubMed] [Google Scholar]
- 22.Molsberry SA, Hughes KC, Schwarzschild MA, Ascherio A. Who to enroll in Parkinson disease prevention trials? The case for composite prodromal cohorts. Neurology. 2022;99(7 Suppl):S26-S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg D, Postuma RB, Adler CH, et al. . MDS research criteria for prodromal Parkinson disease. Mov Disord. 2015;30(12):1600-1611. [DOI] [PubMed] [Google Scholar]
- 24.Siderowf A, Jennings D, Eberly S, et al. . Impaired olfaction and other prodromal features in the Parkinson at-risk syndrome study. Mov Disord. 2012;27(3):406-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AJ, Wang Y, Alcalay RN, et al. . Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov Disord. 2017;32(10):1432-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcalay RN, Dinur T, Quinn T, et al. . Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol. 2014;71(6):752-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wade CH. What is the psychosocial impact of providing genetic and genomic health information to individuals? An overview of systematic reviews. Hastings Cent Rep. 2019;49(suppl 1):S88-S96. [DOI] [PubMed] [Google Scholar]
- 28.Postuma RB, Iranzo A, Hu M, et al. . Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malkani RG, Wenger NS. REM sleep behavior disorder as a pathway to dementia: if, when, how, what, and why should physicians disclose the diagnosis and risk for dementia. Curr Sleep Med Rep. 2021;7(3):57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postuma RB, Arnulf I, Hogl B, et al. . A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27(7):913-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maetzler W, Hausdorff JM. Motor signs in the prodromal phase of Parkinson disease. Mov Disord. 2012;27(5):627-633. [DOI] [PubMed] [Google Scholar]
- 32.Hughes KC, Gao X, Baker JM, et al. . Non-motor features of Parkinson disease in a nested case-control study of US men. J Neurol Neurosurg Psychiatry. 2018;89(12):1288-1295. [DOI] [PubMed] [Google Scholar]
- 33.Gaenslen A, Wurster I, Brockmann K, et al. . Prodromal features for Parkinson disease—baseline data from the TREND study. Eur J Neurol. 2014;21(5):766-772. [DOI] [PubMed] [Google Scholar]
- 34.Sierra M, Martinez-Rodriguez I, Sanchez-Juan P, et al. . Prospective clinical and DaT-SPECT imaging in premotor LRRK2 G2019S-associated Parkinson disease. Neurology. 2017;89(5):439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iranzo A, Santamaria J, Valldeoriola F, et al. . Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2017;82(3):419-428. [DOI] [PubMed] [Google Scholar]
- 36.Crotty GF, Schwarzschild MA. What to test in Parkinson disease prevention trials? Repurposed, low-risk, and gene-targeted drugs. Neurology. 2022;99(7 Suppl):S34-S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257-1272. [DOI] [PubMed] [Google Scholar]
- 38.Mullin S, Smith L, Lee K, et al. . Ambroxol for the treatment of patients with Parkinson disease with and without glucocerebrosidase gene mutations: a nonrandomized, noncontrolled trial. JAMA Neurol. 2020;77(4):427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crotty GF, Maciuca R, Macklin EA, et al. . Association of caffeine and related analytes with resistance to Parkinson disease among LRRK2 mutation carriers: a metabolomic study. Neurology. 2020;95(24):e3428-e3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crotty GF, Schwarzschild MA. Chasing protection in Parkinson disease: does exercise reduce risk and progression? Front Aging Neurosci. 2020;12:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen Daalen JM, Schootemeijer S, Richard E, Darweesh S.K.L., Bloem B.R. Lifestyle interventions for the prevention of Parkinson disease: a recipe for action. Neurology. 2022;99(7 Suppl):S42-S51. [DOI] [PubMed] [Google Scholar]
- 42.Schootemeijer S, van der Kolk NM, Ellis T, et al. . Barriers and motivators to engage in exercise for persons with Parkinson disease. J Parkinsons Dis. 2020;10(4):1293-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beach TG, Adler CH. Importance of low diagnostic Accuracy for early Parkinson disease. Mov Disord. 2018;33(10):1551-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fahn S, Elton R; Members of the UPDRS Development Committee. The unified Parkinson disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Development in Parkinson Disease. Vol 2. Macmillan Health Care Information; 1987:153-163. [Google Scholar]
- 45.Goetz CG, Tilley BC, Shaftman SR, et al. . Movement disorder society-sponsored revision of the unified Parkinson disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. [DOI] [PubMed] [Google Scholar]
- 46.Simuni T, Siderowf A, Lasch S, et al. . Longitudinal change of clinical and biological measures in early Parkinson disease: Parkinson progression markers initiative cohort. Mov Disord. 2018;33(5):771-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lipsmeier F, Taylor KI, Kilchenmann T, et al. . Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson disease clinical trial. Mov Disord. 2018;33(8):1287-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parkinson J. An Essay on the Shaking Palsy. Whittingham & Rowland, for Sherwood, Neely & Jones; 1817. [Google Scholar]
- 49.Fahn S. What is the most important and impactful paper related to movement disorder therapy published in the 20th century? Mov Disord Clin Pract. 2021;8(7):993-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38(8):1237-1244. [DOI] [PubMed] [Google Scholar]
- 51.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46(2):388-393. [DOI] [PubMed] [Google Scholar]
- 52.Paisan-Ruiz C, Jain S, Evans EW, et al. . Cloning of the gene containing mutations that cause PARK8-linked Parkinson disease. Neuron. 2004;44(4):595-600. [DOI] [PubMed] [Google Scholar]
- 53.Zimprich A, Biskup S, Leitner P, et al. . Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601-607. [DOI] [PubMed] [Google Scholar]
- 54.Sidransky E, Nalls MA, Aasly JO, et al. . Multicenter analysis of glucocerebrosidase mutations in Parkinson disease. N Engl J Med. 2009;361(17):1651-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]