Abstract
Background:
Platelet-rich plasma (PRP), a blood-based product containing platelets and growth factors, is being utilized to treat numerous non-hemostatic disorders. Studies have explored the use of PRP to provide rapid repair, healing, and recovery from various injuries; some studies mentioned the effectiveness of PRP as compared with other forms of treatment like the use of hyaluronic acid. Commercially available PRP systems are available now, and each varies from one another depending on how it is prepared, thus causing variations in platelet concentration and growth factor content. These variations also implicated different therapeutic applications.
Methods:
The paper reviews the various applications of PRP, including factors to consider before using PRP therapy, and provides an extensive list of PRP applications.
Results:
The administration of PRP as a standalone treatment or as a co-therapy results in observed positive outcomes. However, there is a lack of standardization for PRP preparation, increasing the risks for heterogeneity and bias amongst results.
Conclusion:
The use of PRP is indeed an option for regenerative therapy, but more research is needed before it can fully be recommended as a primary treatment modality.
Keywords: Platelet-rich plasma, Platelet, Biotherapy, Treatment, Regenerative medicine
Introduction
Platelet-rich plasma (PRP) is an autologous blood-based product prepared and administered to promote the repair and recovery of damaged tissue caused by a variety of conditions [1]. Applied locally to the site of injury, the regenerative action of PRP is mainly attributed to the release of stored growth factors in platelets, like platelet-derived growth factor [2], insulin-like growth factor [3, 4], and transforming growth factor-beta [5], and their respective actions on cells of the affected tissue. These growth factors promote the differentiation of precursor cells and the proliferation of mature cells, resulting in the formation of healthy tissue.
While the American Red Cross defines PRP to have a platelet concentration greater than or equal to 5.5 × 1010 platelets/50 mL, its composition varies widely, depending on the preparation. Most PRP preparation protocols promote a PRP with a platelet concentration ranging from 1.7 times to 6 times that of whole blood [6], and PRP may also contain leukocytes, and the most common classification for PRP describes whether the leukocyte content is increased (leukocyte-rich PRP/LR-PRP) or decreased (leukocyte-poor PRP/LP-PRP) [7].
While PRP is extensively studied and is currently applied as an alternative or supportive therapy for various injuries, there is more yet to be known about PRP components and their interactions with different cell and tissue types. This literature review will provide various conditions where PRP is being used as a potential therapy, as well as, present several factors to consider before using PRP as a therapy.
Current PRP preparation considerations
The term “platelet-rich plasma” was coined in the 1970s to refer to a plasma product with a platelet count higher than that of whole blood [8]. While the use of PRP for regenerative purposes has begun in the late 1980s [9] and its popularity has increased ever since, there is still contention amongst experts as to how to truly define “platelet-rich plasma”, as different established PRP preparation protocols result in PRP products that vary in composition and concentration, making standardization of PRP preparation difficult.
Two of the most important factors to consider in PRP preparation are the platelet concentration and the presence or absence of leukocytes. In 2001, Robert Marx proposed PRP to be a blood product that contains at least 1,000,000 platelets/uL (1000 × 109/L), a dosage of 2.5–5 times increased count compared to the normal peripheral platelet count of around 200,000–400,000 platelets/uL (200 × 109– 400 × 109/L) [10]. However, in vitro studies on cell cultures suggest that optimal platelet concentration in PRP may vary depending on the tissue type. Endothelial cell proliferation was observed to increase nearly sixfold when exposed to a platelet concentration of 1.25 platelets × 106/mL (1250 × 109/L), but the further increase may inhibit cell proliferation [11]. On the other hand, a 2021 paper presented different optimal platelet concentrations for the differentiation of mesenchymal stem cells (MSCs) into osteogenic (1500 × 109/L), adipogenic (> 1800 × 109/L), and chondrogenic cell populations (2000 × 109/L) [12].
There have been attempts to establish a standard for absolute platelet count in PRP, such as a 2021 clinical trial that observed long-term improvements in the WOMAC score of patients with osteoarthritis. administered with 8 mL of PRP containing a total of around 10 billion platelets [13]; however, more clinical trials should be performed to understand the absolute platelet count needed to elicit positive responses in various disease conditions where PRP is a treatment modality.
The presence of leukocytes in PRP may also influence tissue repair. The two main types of PRP are leukocyte-rich PRP or LR-PRP (minimum of 40–80% of leukocytes compared to baseline), and leukocyte-poor PRP or LP-PRP (less than 40% of leukocytes compared to baseline [7]. The presence of leukocytes, especially neutrophils, in PRP results in increased release of cytokines that may either inhibit or aggravate inflammation. In vitro studies on cultured chondrocytes from patients with osteoarthritis do not reduce gene expressions of OA-associated inflammatory markers [14]. In line with this, current evidence shows that LR-PRP demonstrated statistical improvement for tendinopathies and LP-PRP for cartilage pathology, while varying results were observed when testing the effectiveness of either LR-PRP or LP-PRP for acute muscle injuries [15, 16].
Several other factors also affect the final PRP product. Increased centrifugation speeds have been demonstrated to increase the number of platelets recovered by a factor of 2.16 at 500g and a factor of 3.48 at 1000g [17]. However, this may also increase platelet activation due to shear stress [18], as the expression of P-selectin was markedly increased in blood spun at 2000g [19]. Interestingly, the activation of PRP also influences its activity. A 2016 study on different platelet activators show that calcium chloride resulted in a gradual increase in measured mean platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), and vascular endothelial growth factor (VEGF) values for up to 24 h, while PRP added with thrombin and thrombin with calcium chloride saw a stable release of said growth factors over time [20]. Improvements in PRP preparation by adjusting temperature were explored, like a re-warming step at 37 °C may improve platelet activation [21] or a pre-incubation at 4 °C that resulted in an increase in VEGF and epidermal growth factor (EGF) release [22].
While the abovementioned papers have highlighted the different PRP preparation procedures that may influence its components and its medical applications, both the adaptability and adoptability of the PRP preparation and/or kit should also be heavily considered.
Most commercially available PRP kits are designed to require a centrifuge with rotor heads specific to their kit that could both accommodate the exact dimensions of the kit and deliver the recommended relative centrifugal force (RCF) needed for optimal regenerative ability. This, in addition to different centrifuges having different RCFs (as RCFs are dependent on both the revolutions per minute (RPM) and the dimensions of the rotor) [23], makes the kits not adaptable for most centrifuges readily available in most hospitals.
Some PRP kits have separation methods that depend entirely on the skill and handling of the person preparing the PRP. After centrifugation, the plasma is either pushed or pulled into the syringe. Any unexpected or unintended movement during PRP extraction may disrupt the layers and may result in an increase of red blood cell content and/or a decrease of platelets and white blood cells collected.
While most distributors would provide their centrifuge to the laboratory or clinic in a “tie-up” deal, the available number of centrifuge units may not be sufficient to meet demand should it increase. Aside from this, the user is required to purchase a specific number of PRP kits to avail of the centrifuge units, which could be too costly for most clinics or patients to meet. In the Philippines, the cost of PRP kits ranges between $175 and $300, which may not be financially viable for most patients, especially those who require multiple PRP injections.
Finally, most PRP protocols detail the preparation of only one type of PRP. As the PRP contents are dependent on several factors such as centrifugation forces and the number of spins, most published protocols prepare only either LR-PRP or LP-PRP. As mentioned previously, the therapeutic applications of PRP are influenced by numerous factors such as the leukocyte content and platelet activation. As a result, clinics should have at least two PRP preparation methods or kits that could produce the specific type of PRP needed, which could also increase the burden on the end user due to the cost of PRP.
Uses of PRP as a therapy
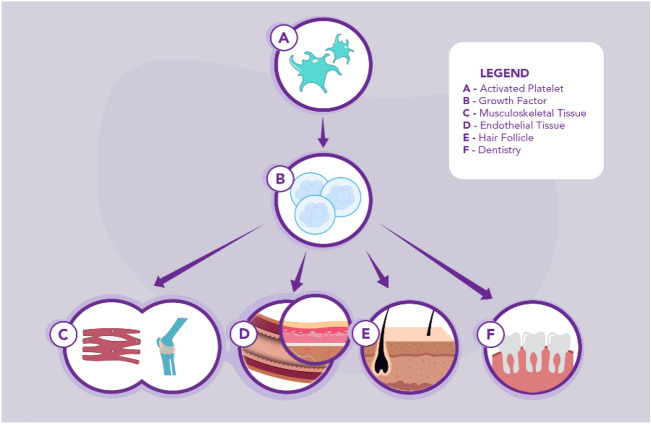
While there is a pronounced heterogeneity among the different PRP preparation protocols, because of PRP’s potential to promote mitogenesis, cell differentiation, and angiogenesis, PRP therapy is being used increasingly to treat a plethora of conditions. Despite the lack of standardization for PRP preparation, promising results have been observed and reported on the use of PRP therapies for chronic wounds, musculoskeletal disorders, dentistry, and hair loss (Fig. 1). Several meta-analyses (Table 1) and experimental studies (Table 2) discussing the use cases of PRP are highlighted in this paper. These are further discussed in the following sections.
Fig. 1.
The current uses of PRP as a treatment. When an injury has occurred, activated platelets (A) release growth factors (B) for connective tissue regeneration, cell growth, cell differentiation, and angiogenesis. In musculoskeletal injuries (C) and chronic wounds (D), growth factors played a significant role in regeneration and effectiveness in hemostasis, tissue repair, and reduction of healing time. These growth factors found in PRP are also impressive when used as a dental implant (F) in dental cell regeneration and as well in hair regrowth (E)
Table 1.
Summary Table of the discussed meta-analyses
| Study first author | Reference number | Year of publication | Type of study | Types of studies analyzed | Case definition | Findings |
|---|---|---|---|---|---|---|
| Qu et al. | [26] | 2021 | Meta-analysis | 20 randomized controlled trials and 5 observational studies | Lower-extremity diabetic ulcers, lower-extremity venous ulcers, and pressure ulcers | Significantly increased wound closure in lower-extremity diabetic ulcers (RR 1.20, 95% CI 1.09–1.32); insufficient strength of evidence for lower-extremity venous ulcers and pressure ulcers |
| Zheng et al. | [27] | 2022 | Meta-analysis | 2 double-blind randomized controlled trials, 1 randomized control trial, 5 prospective studies, and 5 retrospective studies | Burn wounds with or without grafts; 6 PRP treatments (3 topically applied on the wound; 3 injected into the wound), 5 autologous PRP gel applied on the wound. 1 platelet-rich fibrin sprayed on the wound, and 1 fibrin glue applied on the wound | Significant increase in healing rate (MD: 12.69%, 95% CI 9.08, 16.31; p < 0.00001; OR: 2.63, 95% CI 1.37, 5.08; p = 0.004) and decrease in healing time (MD: − 4.02 days, 95% CI − 5.23, − 2.81; p < 0.00001) |
| Moraes et al. | [36] | 2014 | Scoping review | 19 small single-center trials (17 randomized controlled trials and 2 quasi-randomized studies) | 6 trials—rotator cuff tear arthroscopic surgery; 1 trial—shoulder impingement syndrome surgery; 3 trials—elbow epicondylitis; 4 trials—ACL reconstruction; 2 trials—ACL donor graft site application; 1 trial—patellar tendinopathy; 1 trial—Achilles tendinopathy; 1 trial—Achilles rupture surgical repair | No significant differences in overall effect between both PRP and control for short-term (SMD 0.26; 95% CI − 0.19 to 0.71; p value 0.26; I2 = 51%; 162 participants; positive values favoring PRP), medium (SMD − 0.09, 95% CI − 0.56 to 0.39; p value 0.72; I2 = 50%; 151 participants), and long-term function (SMD 0.25, 95% CI − 0.07 to 0.57; p value 0.12; I2 = 66%; 484 participants) |
| Grassi et al. | [37] | 2018 | Meta-analysis | 6 randomized controlled trials | Acute muscle injury (3 studies exclusively hamstring injury; 3 studies that include hamstring, rectus femoris, quadriceps, gastrocnemius, thigh, foot and ankle, and shoulder) | The significant mean difference in return to sport favoring PRP (MD − 7.17, 95% CI − 12.26 to − 2.08, p < 0.00001; 6 trials), while no significant differences in risk of reinjury, complications, pain, strength, ROM/Flexibility, functional scores, and imaging were observed |
| Seow et al. | [38] | 2020 | Meta-analysis | 10 clinical studies | Hamstring injury (2 studies comparing PRP and control, 3 studies comparing PRP and PT, 2 studies comparing PRP + PT and PT alone, 1 study comparing PRP + PT and whole blood injection, 1 study comparing PRP + PT, PPP + PT, and PT alone, and 1 study comparing PRP + PT and placebo) | Nonsignificant decrease in mean time return to play, favoring PRP (MD − 5.67; 95% CI − 12.62 to 1.28; p = 0.11), and a nonsignificant reduction in reinjury rate in patients receiving PRP + PT versus PT alone (95% CI 0.45–1.71; I2 = 0%; p 0.70) |
| Chen et al. | [39] | 2017 | Meta-analysis | 21 randomized controlled trials | Tendon or ligament injury (8 studies on rotator cuff injury, 2 studies on tendinopathy, 3 studies on ACL injuries, and 8 studies on lateral epicondylitis) | Significantly improved reduction in short-term pain (MD − 0.72; 95% CI − 1.10 to − 0.34; p < 0.01; 17 trials), long-term pain (MD − 0.84; 95% CI − 1.23 to − 0.44; p < 0.01; 14 trials), and overall pain (MD, − 0.56; 95% CI − 0.76 to − 0.37; p < 0.01; 21 trials) in patients treated with PRP |
| Han et al. | [40] | 2019 | Meta-analysis | 13 randomized controlled trials | Arthroscopic rotator cuff repair (6 studies on single-row repair; 6 studies on double-row repair; 1 study on both single-row and double-row repair) | Significant reduction in retear rate (RR 1.18; 95% CI 1.03–1.18; overall effect p 0.004; 12 trials) and visual analog scale scores (MD − 0.35; 95% CI − 0.57 to − 0.13; overall effect p 0.002; 5 trials) for patients who underwent surgery augmented with PRP, as well as improved Constant shoulder score (MD 2.31; 95% CI 1.02–3.61; overall effect p 0.0005; 9 trials), UCLA shoulder scores (MD 0.98; 95% CI 0.27–1.69; overall effect p 0.007; 7 trials); and SST score for patients with PRP (MD 0.43; 95% CI 0.11–0.75; overall effect p 0.008; 4 trials) |
| Kim et al. | [41] | 2021 | Meta-analysis | 4 studies (2 retrospective cohort studies, and 2 randomized controlled trials) | Lateral elbow tendinosis | No significant difference in VAS scores after 2 months (p 0.55; 2 studies), 6 months, (p 0.67; 2 studies), and 1 year (p 0.36; 3 trials), as well as no significant difference in PRTEE scores after 12 weeks (p 0.86; 2 studies), 24 weeks, (p 0.72; 2 studies), and 52 weeks (p 0.66; 2 trials) |
| Li et al. | [42] | 2019 | Meta-analysis | 5 studies | Lateral epicondylitis (2 studies compared PRP and triamcinolone, 1 study comparing PRP and methylprednisolone alone, 1 study comparing PRP and methylprednisolone + lignocaine, and 1 study comparing PRP + lignocaine and methylprednisolone + lignocaine) | Significantly improved mean VAS scores at 24 weeks (MD − 2.61; 95% CI − 5.18 to − 0.04; overall effect p 0.05; 2 trials) and mean DASH scores at 24 weeks, (MD − 7.73; 95% CI − 9.99 to − 5.46; overall effect p < 0.001; 2 trials) for patients receiving PRP treatment |
| Franchini et al. | [50] | 2016 | Meta-analysis | 11 randomized controlled trials | Periodontal intrabony defects | Significantly improved PD (MD − 0.39; 95% CI − 0.80 to 0.02; p < 0.01; 11 trials), CAL gain (MD − 0.57; 95% CI − 0.93 to − 0.20; effect p 0.002; 11 trials). GR gain (MD − 0.46; 95% CI − 0.77 to − 0.15; effect p 0.0035; 9 trials), and BD gain (MD − 0.46; 95% CI − 0.77 to − 0.15; effect p 0.0035; 6 trials) in groups treated with PRP |
RR risk ratio, CI confidence interval, MD mean difference, OR odds ratio, ACL anterior cruciate ligament, SMD standardized mean difference, ROM range of motion, PT physical therapy, UCLA University of California, Los Angeles, VAS visual analog score, PRTEE patient-rated tennis elbow evaluation, DASH disabilities of arm, shoulder and hand score, PD probing depth, CAL clinical attachment level, GR gingival recession, BD bone defect
Table 2.
Summary table of the discussed experimental studies
| Study first author | Reference number | Year of publication | Type of study | Patient characteristics | Patient case definition | Results |
|---|---|---|---|---|---|---|
| Cieslik-Bielecka et al. | [28] | 2018 | Experimental study | 5 patients with AIDS ulcers | Venous or arteriovenous insufficiency-related crural ulcers in AIDS patients | Statistically significant IHC expression of VEGF (p 0.001523), FLK-1 (p 0.008241), and CD34 (p 0.006982) |
| Eichler et al. | [30] | 2020 | Consecutive, retrospective, experimental study | 163 patients (81 control patients and 82 receiving PRP injection) | Patients who underwent a single-incision sentinel lymph node biopsy under the arm | No significant increase in major and minor complication rates, with a 100% overall survival after 30 months |
| Berna-Serna et al. | [31] | 2020 | Prospective observational study | 23 patients (15 patients without cancer and 8 patients with a history of breast cancer) | Patients with chronic wounds of the breast | Complete wound closure was observed in 19 out of 23 patients (82.6%) within 4 weeks, and no malignant breast pathologies were observed in the cancer patients after > 4 years |
| Singhal et al. | [63] | 2015 | Experimental observational study | 16 males and 4 females (10 patients treated with PRP (8 males, 2 females; 10 patients without PRP treatment (8 males, 2 females) | Patients with mild to moderate AGA, having either a Hamilton-Norwood score I to IV for males or a Ludwig alopecia score I or II for females | An 89.4% reduction in mean hair pulled was observed in patients treated with PRP after 12 weeks (12 weeks 0.6 hair/pull vs. baseline 4.4 hair/pull), as the growth of new hair was observed after an average of 1.4 weeks |
| Bayat et al. | [64] | 2019 | Experimental observational study | 19 patients were given PRP injections at week 0, week 4, and week 8 | Patients with AGA, having a Hamilton-Norwood score of III–V, and excluding patients using other topical and systemic medications | Significant increase in hair count (3 months 38.58 ± 7.574 vs. baseline 30.11 ± 7.055; p < 0.001) and hair thickness (3 months 0.054 ± 0.0124 vs. baseline 0.041 ± 0.0076; p < 0.001) were observed 3 months after last PRP injection |
| Butt et al. | [65] | 2019 | Experimental observational study | 30 patients with AGA (20 males and 10 females) | Patients with AGA, having either a Hamilton-Norwood score of III–VI for males or a Ludwig alopecia score of I–III for females | Significant decrease in the number of hairs pulled (6 months 7.7 ± 3.8 vs. baseline 11.0 ± 4.05; p < 0.05) and increase in mean hair density (6 months 50.20 ± 15.91/cm2 vs. baseline 34.18 ± 14.36/cm2; p < 0.05) were observed 6 months after last PRP injection, with 18 out of 30 patients (60%) reported an increase in terminal to vellus hair ratio |
| Dubin et al | [66] | 2019 | Experimental observational study | 30 female patients with AGA (15 patients treated with PRP and 15 patients treated with placebo) | Patients with AGA, having a Ludwig alopecia score of I–III, and excluding patients using topical medications | Significant increases in mean hair density (PRP + 71.1 hairs/cm2 vs. placebo − 26.7 hairs/cm2; p < 0.01), and mean hair caliber (PRP + 0.0043 mm vs. placebo − 0.0034 mm; p < 0.01) were observed in patients 8 weeks after PRP treatment, and significant increases in mean hair density (PRP + 105.9 hairs/cm2 vs. placebo − 52.4 hairs/cm2; p < 0.01) and mean hair caliber (PRP + 0.0053 mm vs. placebo − 0.0060 mm; p < 0.01) were observed in patients 24 weeks after PRP treatment |
IHC immunohistochemistry, VEGF vascular endothelial growth factor, FLK-1 fetal liver kinase 1, CD34 cluster of differentiation 34, AGA androgenetic alopecia
Chronic wound healing
Chronic wounds, when untreated, can be debilitating for those afflicted. These wounds are either recurring or do not heal fully due to various disease conditions which include but are not limited to inadequate blood supply (i.e., cardiovascular disease), metabolic disorders (i.e., diabetes), nutrient deficiencies (i.e., vitamin C or K deficiencies), impaired immune function (i.e., immunodeficiency), or extensive tissue damage (i.e., burns or radiation therapy) [24]. Although wound care methods for chronic wounds are dependent on the type of ulcer present, advanced wound care technologies like growth factors can be administered to improve chronic wound healing [25] Because platelets and the growth factors within play a pivotal role in hemostasis and tissue repair, PRP is being explored for use in chronic wounds.
A report prepared by clinicians of the Mayo Clinic for the US Department of Health and Human Services presented the results of a systematic review on the effectiveness of PRP in the treatment of lower-extremity diabetic ulcers, lower-extremity venous ulcers, and pressure ulcers [26]. Results of their meta-analysis show that lower extremity diabetic ulcers managed with PRP resulted in more significant wound closure, reduction in the wound area, reduced time for wound closure, and reduced wound depth. Although the authors reported no significant difference in evaluated subgroups for lower-extremity diabetic ulcers and pressure ulcers due to insufficient evidence, they recognize that further studies should be more rigorous, with the characterization of PRP preparation being one of the main factors that need to be reported. In 2022, another meta-analysis of 13 studies shows a significant increase in wound healing rate and reduction in healing time for burn wounds treated with PRP, but little change in the percentage of burn skin graft take [27].
Nevertheless, the use of PRP therapy to enhance wound healing for various other conditions has resulted in positive outcomes. A 2018 study on the potential uses of LR-PRP on wounds of AIDS patients showed increased healing, with a nonsignificant increase in epidermal processes between baseline and 10 days after PRP administration. Additionally, IHC of VEGF immunoexpression revealed a significant increase in mean VEGF 10 days after PRP treatment (118.9 vessels/mm2 vs. baseline = 114.3 vessels/mm2), as well as increased immunoexpression of CD34, suggesting improved wound vascularity [28].
There is increasing discussion on the use of PRP to facilitate the healing of surgical wounds related to cancers. Although there were initial reservations against the use of PRP due to its angiogenic and mitogenic effects conferring a potential for tumor regrowth [29], recent studies have indicated that PRP may be oncological inert. No long-term adverse effects were seen when PRP was administered to patients with breast cancers, with no cancer reoccurrence being observed when PRP was injected into sentinel lymph node biopsy sites of low-risk oncological cohorts [30]. These results, combined with a successful decrease in the time of closure for chronic wounds of the breast after PRP administration with little to no complications [31], could further reinforce the notion that PRP may be a beneficial therapy for other cancer complications.
Musculoskeletal injuries
Nearly half of the more common musculoskeletal injuries reported involved tendon and ligament injuries [32], while about 30% of sports injuries are on muscles [33], with hamstring injuries being common with acute hamstring injuries composing an average of 17% of all injuries in association football players [34] and 25.6% of all injuries among American football athletes [35].
Several case studies on PRP use for musculoskeletal diseases have reported positive outcomes. However, reviews of several case series and clinical trials are inconclusive, especially since different musculoskeletal tissue may react differently to PRP treatment. A 2014 systematic review of the use of PRP on musculoskeletal soft tissue injuries reported a marginal reduction in short-term pain reported in four trials, while no significant differences were observed in four trials that compared the incidence of adverse effects between treatment groups [36].
In 2018, a review of 6 studies involving 374 patients with acute muscle injury reported a significantly shorter time to return to sport from various types of acute muscle injury but a non-significant difference in re-injury rate, while outcomes of complications, pain, strength, range of motion/flexibility, functional scores, and imaging were too heterogenous [37]. Two years later, a systematic review of 10 studies involving patients with acute hamstring muscle injury presents a non-significant decrease in the mean time to return to play and reinjury rates, favoring PRP in combination with physical therapy (PT) compared to either PRP or PT alone [38].
The use of PRP and its effectiveness on both tendon and ligament injuries were explored by a 2018 systematic review of 37 randomized controlled trials, with rotator cuff injuries and lateral epicondylitis being the most studied, each representing 38.1% of the studies included. Patients who received PRP reported improved VAS scores upon short-term (2–6.5 months) and long-term follow-ups [39]. A significant decrease in retear rate of arthroscopic rotator cuff repair was also reported by a 2019 review, along with improved VAS scores [40].
Several studies have also compared PRP with commonly provided treatments for lateral elbow tendinosis and elbow epicondylitis. When comparing cases treated with PRP to those who underwent surgery for lateral elbow tendinosis, no significant differences were observed in both their pain scores and functional outcomes, suggesting that the use of PRP may be an alternative if surgery is not viable [41]. As for lateral epicondylitis, no statistically significant differences were observed in the short-term and long-term VAS scores and MAYO index between groups injected with either PRP or corticosteroids. However, while the DASH score of the corticosteroid group was significantly lower than the PRP group in the short-term, the DASH score of PRP becomes lower than that of the corticosteroid group at the 24-week follow-up [42].
It is important to note that heterogeneity was observed in most models in the meta-analyses highlighted. This can be attributed to the differences in reporting of outcomes, differences in PRP preparation, and variations in PRP administration and follow-up.
Orthodontics and dentistry
Current medical procedures for most oral diseases, like periodontitis and dental cavities, do not fully regenerate tissue; as a result, most of these procedures instead focus on preventing further damage or by replacing damaged tissue with artificially synthesized materials i.e. fillings and metal implants [43]. Due to the abundance of growth factors and cytokines in PRP and platelet-rich fibrin (PRF) which could promote cell growth and proliferation of the stem cell populations present in dental tissue, its applications in regenerative dentistry are also being studied extensively, in the hopes that it could promote significant, if not complete, regeneration of dental tissue [44]. Its use in dental and oral surgery has been extensively reviewed, with previous studies reporting varying, but promising improvements in bone and soft tissue healing post-operation [45].
An estimated 90% of the entire population is affected by some sort of periodontal disease [46]. The periodontium is a structure serving to anchor the teeth in place and provide protection against stress. A combination of epithelial tissue, mineralized tissue, and connective tissue comprises the periodontium, with the alveolar bone, cementum, and interposed periodontal ligament forming the functional structure [47]. Due to the prevalence of fibroblasts in gingival tissue on which growth factors can act, both PRP and PRF have been adopted as a therapy for soft tissue or hard tissue damage [48, 49], resulting in favorable prognosis for patients with intrabony defects.
However, meta-analyses of trials studying the potential applications of PRP for orthodontics and oral surgery show mixed results. In 2019, a meta-analysis of 11 randomized controlled trials that evaluated the use of PRP in oral surgery reported no difference in probe depth and a slight decrease in clinical attachment level. In the same review, a slight decrease in gingival recession was observed across 9 trials, and data from 6 trials also show a slight decrease in bony defects [50].
Hair loss
Alopecia is a term referring to several dermatological disorders that result in hair thinning or hair loss. One of the most common types of alopecia is androgenic alopecia (AGA), also known as pattern baldness, which has a worldwide prevalence of about 60–70% [51]. AGA is characterized by hair thinning due to the effects of dihydrotestosterone on dermal papillae cells, resulting in a shortened anagen phase [52]. Another type of alopecia is alopecia areata (AA), also known as spot baldness, and is characterized as an autoimmune disorder caused by localized inflammation due to T cells infiltrating hair follicle bulbs, resulting in nonscarring alopecia [53]. AA is seen in an estimated 0.1–0.2% of people, with a 2% risk of developing AA throughout life [54].
Growth factors found in platelets are also known to promote hair growth through stimulation of the anagen phase in hair follicles by fibroblast growth factor (FGF) [55] and insulin-like growth factor (IGF) [56], enhanced proliferation of hair follicle stem cells by platelet-derived growth factor (PDGF) [57], and the synergistic regeneration of hair follicles by epidermal growth factor (EGF) and Jagged-1, a Notch ligand [58]. The increased VEGF levels found in PRP also promote angiogenesis, which in turn increases nutrient delivery into the hair follicle [59] This is supported by the results of a 2015 half-head placebo study, where a slight but significant increase in blood cell vascularization and hair growth was observed on the side treated with PRP [60].
PRP therapy as an additional treatment for hair loss has resulted in an improvement in overall hair growth and thickness between 50% [61] and 62% [62]. In 2015, a study on 10 patients with AGA showed a 65% reduction in hair pulled out 12 weeks after having PRP therapy, and hair growth was observable after an average of 1.4 weeks [63]. Significant increases in hair count and thickness were observed in 19 patients 12 weeks after three monthly injections of PRP [64]. Another study that includes 30 patients with AGA who had two PRP injections 1 month apart showed that after 6 months, 73.3% of them had a significant decrease in vellus hair, and 60% of them had a 1.46 times increase in mean hair density [65]. A 2020 comparative study observed 30 women with AGA that were given three monthly injections of either PRP or placebo. After 24 weeks, the PRP group observed significant improvements in mean hair density and mean hair caliber [66].
PRP as a co-treatment
The regenerative capabilities of platelets have been studied extensively, and PRP as a standalone therapy has been used to promote the healing of chronic wounds, treat and manage musculoskeletal disorders, stimulate regeneration of oral and dental tissue, and increase hair growth, as discussed in the previous sections. Research on how PRP can augment other regenerative therapies can lead to the implementation of more effective co-treatments. The use of PRP with hyaluronic acid (HA), minoxidil, and mesenchymal stem cells are some of the co-treatment approaches being explored by clinicians.
Hyaluronic acid
Joint disorders, like osteoarthritis, are often degenerative due to the lack of capacity for affected tissues to self-repair [67]. Cartilage, the type of tissue found in joints, acts both to reduce friction and absorb compressing forces. However, cartilage does not repair fully as the tissue is avascular, lacks nerves, and is composed of cells that have a low metabolic rate [68]. As a result, current treatments for joint disorders commonly support a palliative approach in combination with rehabilitation exercises and medication. Although the use of non-steroidal anti-inflammatory drugs (NSAIDs) has the highest level of evidence for pain relief, they are not recommended for long-term use due to the adverse side effects associated with their use such as cardiovascular disease, renal damage, and increased GI bleeding [58, 59]. Also, several NSAIDs are contraindicated against bleeding conditions [69], and may increase risk of developing cardiovascular and gastrointestinal problems [70] due to their mechanism of action being cyclooxygenase inhibitors [71].
Because of this, other treatment methods for osteoarthritis are being explored. The use of exogenous hyaluronic acid injections as a palliative treatment is being considered because it adds lubrication and increases shock absorption while increasing endogenous HA production [72]. PRP has also been studied extensively as a potential treatment for cartilage disorders due to its aforementioned regenerative capabilities [73]. In terms of comparison, numerous meta-analyses have described that PRP provided more long-term relief than HA with fewer side effects [63, 64]. However, what is interesting is that the growth factor release of PRP is potentiated by the presence of HA. A 2016 in vitro study of PRP incubated with HA showed that compared to PRP alone, the presence of HA led to significant increases in TGF-β (PRP with HA 33.7 ± 8.3 mg/mL vs. PRP only 12.7 ± 10.5 mg/mL; p = 0.034) and PDGF-AA levels (PRP with HA 2.00 ± 0.52 ug/mL vs. PRP only 1.51 ± 0.40 ug/mL; p = 0.003) at day 5 [74]. The study performed by Yu et al. demonstrates the positive anti-inflammatory effect of a co-treatment of PRP and HA after it was revealed that expression of platelet derived-endothelial cell growth factor, VEGF, IL-6, and IL-10 were further upregulated compared to standalone PRP or HA therapies, as well as significant improvements in WOMAC scores, showing a 62% decrease of total mean WOMAC score 52 weeks after administration of combination therapy (52 weeks 14.40 ± 11.73 vs. baseline 38.21 ± 17.25; p ≤ 0.0001) [75].
The synergistic effect of both PRP and HA has prompted studies on the effectiveness of the cotreatment, as a 2020 meta-analysis reported that PRP combined with HA resulted in overall improved outcome scores with no increase in manifestations of side effects [76]. This is supported by the results of a 2021 retrospective study comparing the cotreatment with LR-PRP alone, which reported that not only did the combination of PRP and HA result in the recipients reporting improved outcome scores over time, but knee mobility and function were also reported to be improved [77].
Despite the favorable potentiality of PRP in combination with HA as a therapeutic agent for degenerative diseases, certain limitations of the co-treatment were identified. PRP preparation protocols should be standardized, as leukocytes that may be present in the prepared PRP may lead to inflammation in the injury site. This was reported by a 2021 paper when an increase in post-injection pain and swelling was seen in the PRP group [78]. Fortunately, no further treatment was needed, as the pain and swelling subsided over time. Other adverse side effects, including hypertension and proteinuria, were also reported in patients treated with PRP and HA [75]. The aforementioned 2018 study also suggested a maximum dose of 8 mL of PRP with 0.20 mg of HA, with more side effects like diarrhea, constipation, and hyperlipidemia being reported as doses increase. Further research should be performed to understand the connection between the cotreatment and the side effects reported in the study.
Minoxidil
The current treatment approach to AGA involves the topical application of a 5% minoxidil solution [79]. As a standalone treatment, PRP therapy has resulted in mixed outcomes. Interestingly, PRP as an adjuvant in combination with other therapies shows more promise and can result in better outcomes [80]. As topical minoxidil is known to have side effects like localized irritation [81] and varying degrees of hair shedding during the early stages of treatment [82], it is believed that supplementation with PRP may help reduce these side effects while significantly improving outcomes.
One study in 2018 showed that the group treated with PRP combined with 5% minoxidil showed significantly increased improvements in hair count (9.8 ± 26.9/0.65 cm2 vs. placebo 3.7 ± 14.5/0.65 cm2), density (12.3 ± 34.2/cm2 vs. placebo 5.1 ± 23.9/cm2), and anagen/telogen ratio (69.6 ± 234% vs. placebo 1.7 ± 192.5%) compared to groups treated with placebo [83]. Significant improvements in hair density [84] and concentration of positive immunohistochemical indicators of hair growth [85] seen in treated areas further support the use of PRP with minoxidil, while reporting minimal side effects.
Mesenchymal stem cells
Interest in the use of stem cells as a regenerative therapy is not new. Because stem cells are unspecialized, these can be directed to differentiate into more specialized cells in the presence of the appropriate cell signals [86]. Their use in medicine is predicated on their potential to transform into cells needed to constitute the specific tissue needed. As a result, applications of stem cells to manage different diseases, including hematologic disorders [87–89], neurodegenerative diseases [90–92], and musculoskeletal disorders [93–95] are continuously being explored.
Mesenchymal stem cells (MSCs) are non-hematopoietic (do not become blood cells) and are found in most parts of the body, including bone marrow and adipose tissue. Due to their pluripotency, these are the type of stem cells used for most therapeutic applications [86]. While stem cell therapies and transplantations have been proven effective, there is still growing interest in supplementing this treatment to further increase its effectiveness. Because platelets and their growth factors have mitogenic and proliferative properties, pairing PRP with mesenchymal stem cells may be the ultimate cell-based regenerative biotherapy that we may know of.
As platelets are activated by multiple signaling pathways, it is thought that the activation of platelets in PRP may also promote stem cell growth when both are combined. Adipose-derived stem cells (ADSCs) treated with inhibitors for the ERK1/2 pathway, PI3k/Akt pathway, and JNK pathway demonstrated cell growth when PRP or PDGF-BB was added. In the presence of PRP, phosphorylation of ERK1/2 and Akt was observed, and JNK activity was markedly increased [96]. PRP may also influence cell migration, as the expression of Cdc 42, Rac 1, and Rho A in ADSCs gradually increased when PRP is present, and Transwell invasion results show a significantly increased cell migration of ADSCs when treated with PRP [97].
A 2013 study demonstrated ADSCs that were cultured with PRP in vitro proliferated more efficiently and differentiated into chondrogenic cells as genes essential to chondrogenesis, namely genes for type II collagen, Sox9, and aggrecan, showed increased expression. In vivo, ADSCs prepared with 15% PRP resulted in a reduction of time until recovery by 15 days and an increase of regenerated cartilage to 45%, seen in routine H&E staining [98]. ADSCs with PRP were also shown to promote faster wound closure in mice. Trichrome-stained tissue of samples treated with ADSCs and PRP exhibited increased granulation tissue and collagen fiber formation as early as 3 days after treatment [97].
Muscle-derived mesenchymal stem cells (M-MSCs) were also paired with PRP gel to stimulate the formation of bone tissue in rabbits with an induced humeral bone defect. In vitro, M-MSC exposure to PRP demonstrated an increase in Cbfa-1, an osteogenic transcription factor, and a subsequent decrease in MyoD1, a myogenic transcription factor. This was accompanied by elevated type I collagen expression and increased ALP activity, supporting the differentiation of M-MSCs into osteoprogenitor cells [99].
Although limited, there is evidence that supports the supplementation of stem cell treatment with PRP for wounds affected by or resulting from radiotherapy. When irradiated, co-cultures of human-derived microvascular endothelial cells (HDMECs) and ADSCs demonstrated a significant increase in basic fibroblast growth factor amounts and expression of IL-6 upon the addition of PRP [100]. Atrophy of oral mucosal tissue due to radiotherapy was also reduced, as observed in irradiated rat tongue models treated with bone marrow MSCs and PRP [101]. A combined MSC-PRP therapy increased the closure rate of irradiated wounds in rat models. Simultaneously, histological analysis show increased VEGF and CD31 expression [102]. Still, further research into the effects of PRP and its other components on other cancers should be performed.
Rationale for PRP use
Due to it being considered a “substance intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease”, PRP can be defined as a drug [103]. However, PRP is not considered a drug. Instead, PRP is classified by the US Food and Drug Administration as a “human blood and blood products” instead of a drug [104]. While numerous PRP preparation techniques and kits have been granted US FDA clearance, there is no FDA approval on the use of PRP as a specific treatment for the various conditions, due to a lack of standardized PRP preparation protocols and inconclusive, albeit positive, results from published studies and clinical trials [105].
Interestingly, because PRP is a blood product, it is not considered to be a “human cells, tissues, or cellular and tissue-based product” (HCT/P). Therefore, PRP, like all other blood products, requires further regulatory compliance for clinical trials and marketing [106]. Regardless of the designation, PRP is still being offered as a treatment modality for various conditions. However, due to the lack of standardization of PRP preparation methods, there is little shared consensus among physicians on the proper preparation and use of PRP, which may also influence both the healing process and the patient self-assessment of the healing process.
Preference for PRP use
In 2020, 148 patients of the Department of Orthopaedic Surgery of the University of Missouri, who have received at least one PRP injection for various musculoskeletal conditions between 2011 and 2016, were contacted, with 40.5% responding to the survey invite (n = 60 respondents) [107]. Among the 60 respondents, 35 of them received LP-PRP (58.0%). 43 of the respondents returned to full work duty after at most 60 days, with an overall level of satisfaction of 67.9%. Among the remaining 17 respondents, 6 had a planned retirement, 1 had an unexpected retirement, 4 reported disability, 3 reported pain, and 3 required surgeries. While it is interesting that the use of LR-PRP resulted in a 2.7× increase in lack of return to full-time work, 33 of them had PRP injected in a joint, and 19 of those had osteoarthritis.
A 2021 survey of 599 members of the American Orthopedic Society for Sports Medicine assessed their use of orthobiologics [108]. Only 165 members completed the survey (27.5% of total respondents). However, the two most commonly used orthobiologic therapy among those interviewed were LP-PRP (n = 83; 76.1%) and LR-PRP (n = 77; 70.6%). For osteoarthritis, LP-PRP was the most commonly used therapy for surgical (n = 36; 33.0% of cases) and non-surgical (n = 52; 47.7% of cases) cases, with the knee joint being the most commonly treated with PRP (94.2% of nonsurgical cases treated with PRP; 97.2% of surgical cases). Conversely, LR-PRP is the most commonly used therapy for muscle injuries (8.3% of surgical, cases n = 9; 23.9% of non-surgical cases, n = 26), with the most common muscle group treated with PRP being the hamstring (88.5% of nonsurgical cases treated with PRP; 88.9% of surgical cases). LR-PRP is also the most commonly used therapy for injuries involving the tendon (34.0% of surgical cases, n = 9; 23.9% of non-surgical cases, n = 26) and ligament (14.7% of surgical cases, n = 16; 75.0% of non-surgical cases, n = 12).
Staying on musculoskeletal injuries, 149 team physicians from various American sports leagues were contacted in a 2021 survey, with only 46 of them completing the survey (30.9% completion rate) [109]. Among the 46 physicians who responded, 93% of them stated that they have administered PRP therapy. The same study also presented differences in the usage and administration of PRP. 98% of those who administer PRP therapy say that they administer it in the office, but administration of PRP in the operating room was endorsed by 41%. 26 out of the 46 physicians (56%) have used PRP to treat athletes from the recreational level through the professional level.
33 of the 46 physicians (72%) administer PRP through the guidance of ultrasound. When asked about when they administer PRP, 32.6% of the respondents use PRP initially as a first-line treatment, while 32.6% use PRP as a last-line treatment, when options are low. Of the remaining respondents, 19% administer PRP following physical therapy and rehabilitation; 13% administer PRP after the RICE method, and around 2% have given PRP during or after surgery.
On average, the most athletes treated with PRP were under the supervision of physicians of collegiate teams (69.4 ± 100 athletes) and the National Football League (60.4 ± 87.8 athletes), while physicians of the National Hockey League treated the least number of athletes (18.0 ± 12.7 athletes). When asked about the complications that their patients experienced, 32 physicians (70%) reported no complications, and for the 14 that have reported complications, 12 (26) reported that their patient/s experienced some form of pain.
While the three surveys give extensive insight into the opinions and preferences on PRP therapy, their main limitation is that the three studies have a low completion rate. These studies also do not mention in detail how PRP was prepared, which increases the likelihood of observing heterogeneous events when replicated. Finally, either because or despite the lack of standardization of PRP contents and preparation, there is no consensus as to the proper timing and frequency of PRP administration for each injury. These were acknowledged and are thought to be attributed to the broad profile of usage, as the respondents or their patients experienced PRP treatment for a broad range of musculoskeletal disorders.
Advantages of using PRP
The interest in PRP use has been due to the advantages it offers, namely its abundance of regenerative growth factors, ease of preparation, and patient safety. Growth factors found in platelets are known to be potent mitogens. Because PRP has increased concentrations of growth factors, localized application of PRP to the injured site increases the mobilization of endothelial progenitor cells, which subsequently promotes the migration of resident progenitor and endothelial cells [110]. The individual effects of several growth factors were already discussed in previous sections. Rather, this section will elaborate on why PRP is appealing to most clinicians based on its ease of preparation and its safety to the patients relative to other similar therapies.
Although the specifics of PRP preparation have yet to be standardized, commercial PRP products have simplified their preparation through the patented kits that manufacturers have developed. Most PRP kits provide both the collection methods and the anticoagulant used. Although most PRP preparations in literature use low centrifugation speeds, PRP preparation kit protocols usually require around 1500 rpm (1776g) centrifugation speeds, which can be provided by most tabletop centrifuges, although some kits require specific rotor heads that could fit their specialized preparation vessels. Also, most PRP kits do not require specialized temperatures, and PRP can be prepared at room temperatures (20–25 °C).
PRP is arguably safer than other similar therapies due to it being autologous, designed to be compatible with homeostatic conditions, and easier to collect and prepare. Since PRP is made from the patient’s blood, it does not have the disadvantages that most allogeneic blood products have. It eliminates the need for testing to check for transfusion-transmissible diseases and compatibility, as there is no exposure to donor blood when autologous units are used [111]. Compatibility is also not an issue, as crossmatching is also not required for autologous blood units [112]. Most PRP kits also do not require enormous amounts of blood to be collected. While several PRP preparation protocols typically require around 20–60 mL of whole blood to be collected [113], more modern PRP preparation kits developed by various pharmaceutical companies have reduced the amount of blood required to achieve a therapeutic effect, down to around 8–15 mL of whole blood. Because modern PRP preparations consider overall cellular composition, clinicians may choose between using LR-PRP or LP-PRP depending on the type of injury treated. LR-PRP contains an increase of proinflammatory cytokines and growth factors which may benefit tendon healing; meanwhile, LP-PRP reduces the severity of the inflammatory response, and may confer therapeutic benefit for osteoarthritis [16].
The amount of anticoagulant is specific, to maintain the proper ratio of 3.2% sodium citrate (SC) to blood. Most PRP preparations use an anticoagulant-to-blood ratio of 1:9, as this ratio is used for platelet function and coagulation tests [114]. However, improvements in the effectiveness of PRP were observed when the amount of anticoagulant used was reduced in half. When treating excisional wounds in mice models, both proliferation and migration of fibroblasts treated with PRP containing 1/2 SC were observed in tissue sections, and almost complete closure was observed after 6 days [115].
Disadvantages of using PRP
PRP shares the potential issues that other similar blood products may have. For instance, PRP should be kept at room temperature (22 °C) as cold temperatures may alter platelet morphology, affect glycoprotein Ib/IX/V/alpha (GPIbα), and promote platelet clearance [116]. As a result, it is much more prone to bacterial growth compared to other blood units when stored for more than 24 h [117]. Additionally, several growth factors in platelets are observed to have shortened clearances, which may limit the overall effectiveness of PRP. PDGF has a biological half-life of 2.4 h, while VEGF has an even shorter half-life of less than 30 min [118]. Because of these, PRP should be used almost immediately after preparation, and most PRP kits recommend the use of PRP within 30 min after preparation.
Due to the lack of standardization of PRP preparation, the presence of other formed elements may lead to further tissue damage. While the presence of erythrocytes is kept to a minimum and is more easily separated from the platelets during PRP preparation, it is more difficult for platelets and leukocytes to be separated, as their specific gravities are close to each other. Although the presence of leukocytes in PRP could promote cell migration and enhance the proliferation of fibroblasts [119], it could also lead to increased inflammation due to increased gene expression and production of interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) [120]. Expression of genes related to autophagy and production of reactive oxygen species (ROS) were observed to be increased in tendon fibroblasts treated with PRP [121].
It is important to note that most adverse effects attributed to the presence of leukocytes in PRP result from the presence of neutrophils and their contents. Neutrophil elastase (NE) and cathepsin G (CG) is released from neutrophils during inflammation, and both cleave elastin, reducing the integrity of the extracellular matrix of connective tissue and its elasticity [122]. Both NE and CG indirectly promote further tissue destruction by playing a role in the activation of matrix metalloproteinases and the inactivation of protease inhibitors [123, 124]. Overproduction of nitric oxide (NO) as a response to cytokine signaling at the site of injury could activate neutrophils present [125] and promote further proinflammatory effects [126].
Because PRP lacks a standardized method of preparation, most manufacturers have patented their PRP preparation protocols and are selling them as PRP kits. Additionally, most PRP preparation procedures require specific centrifuges. Professional fees may further increase the price of treatment, as administering PRP requires specialized training. As a result, the cost of these kits varies widely, from $175 to $1150 per kit [17]. The procedure may cost between $500 and $2000, and because the treatment may not be covered by insurance, regular additional PRP treatment may be costly for the patient [105].
Conclusion
Due to the presence of growth factors in platelets, the use of PRP for regenerative therapy is becoming prevalent. While PRP is shown to have great therapeutic potential as supported by improvements in the recovery of individual cases, its efficacy is still questioned, as clinical trials have returned mixed results. Most systematic reviews regarding PRP use in clinical trials report an increase in heterogeneity and risk of bias, while also having low-quality of evidence.
Although differences in patient recruitment, the extent of injury, and observation time may have caused disagreement on the effectiveness of PRP, PRP preparation is also reported to be a major contributor. The numerous PRP classification systems based on the quantity and quality of its components resulted in varying PRP preparation protocols intended to produce different types of PRP. These variations in preparation protocols make standardization of the preparation procedure difficult. Aside from improvements in PRP standardization and clinical trial design, further research on the interactions between PRP components and different tissue microenvironments may help us understand the extent of the regenerative capacity of PRP.
Considering everything discussed previously, the use of PRP is an option of regenerative therapy for most disorders. Aside from the presence of growth factors, cytokines, and bioactive formed elements in PRP, its autologous preparation removes the potential for any unintended elements to be introduced to the tissue site, and it is more affordable or less invasive compared to other possible treatments available, like stem cell treatment, reparative surgery, or tissue/organ replacement. Ultimately, the prescription of PRP therapy as an alternative or supportive intervention remains at the discretion of the attending physician, after careful consideration of the individual case presentation.
Acknowledgements
The authors would like to thank the DOST-PCHRD for the support given to the research program, without which the authorship of this literature review would be difficult. This literature review is part of a research program titled “Research Center for the Rehabilitation/Sports Medicine (Oplan Atletang Pinoy)”, funded by the Department of Science and Technology—Philippine Council for Health Research and Development (Ref. No. 001911).
Author contributions
MRPC and AV wrote the main manuscript text. CS, JTC and MMBB contributed to the writing of Sections “Use of PRP as a Therapy” and “PRP as a co-treatment”. MD contributed to the writing of Section “Rationale for PRP Use”. RCV edited the flow of the manuscript and prepared the figures in the text. All authors reviewed the manuscript prior to publication.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Statement
The review article submitted to this journal is the work of the authors only and has not been published elsewhere. All authors have meaningfully and substantially contributed to the research, synthesis, and writing of this article. All resources used are properly cited and referenced. No human or animal experiments were conducted for this review.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.dos Santos RG, Santos GS, Alkass N, et al. The regenerative mechanisms of platelet-rich plasma: a review. Cytokine. 2021;144:155560. doi: 10.1016/j.cyto.2021.155560. [DOI] [PubMed] [Google Scholar]
- 2.Chen PH, Chen X, He X. Platelet-derived growth factors and their receptors: structural and functional perspectives. Biochim Biophys Acta. 2013;1834:2176–2186. doi: 10.1016/j.bbapap.2012.10.015.Platelet-derived. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren M, Zhong X, Ma CY, et al. Insulin-like growth factor-1 promotes cell cycle progression via upregulation of cyclin D1 expression through the phosphatidylinositol 3-kinase/nuclear factor-kB signaling pathway in FRTL thyroid cells. Acta Pharmacol Sin. 2009;30:113–119. doi: 10.1038/aps.2008.8. [DOI] [PubMed] [Google Scholar]
- 4.Gan QF, Choy KW, Foo CN, Leong PP, Cheong SK. Incorporating insulin growth Factor-1 into regenerative and personalised medicine for musculoskeletal disorders: a systematic review. J Tissue Eng Regen Med. 2021;15:419–441. doi: 10.1002/term.3192. [DOI] [Google Scholar]
- 5.Poniatowski LA, Wojdasiewicz P, Gasik R, Szukiewicz D. Transforming growth factor beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediat Inflamm. 2015 doi: 10.1155/2015/137823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pachito DV, Bagattini AM, de Almeida AM, Mendrone-Júnior A, Riera R. Technical procedures for preparation and administration of platelet-rich plasma and related products: a scoping review. Front Cell Dev Biol. 2020;8:1–11. doi: 10.3389/fcell.2020.598816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Ski Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mościcka P, Przylipiak A. History of autologous platelet-rich plasma: a short review. J Cosmet Dermatol. 2021;20:2712–2714. doi: 10.1111/jocd.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marx RE. Platelet-rich plasma (PRP): What is PRP and what is not PRP? What is PRP? What is PRP in relation to recombinant growth factors? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rughetti A, Giusti I, D’Ascenzo S, et al. Platelet gel-released supernatant modulates the angiogenic capability of human endothelial cells. Blood Transfus. 2008;6:12–17. doi: 10.2450/2008.0026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Li Z, Li J, et al. Optimization of the platelet-rich plasma concentration for mesenchymal stem cell applications. Tissue Eng Part A. 2019;25:333–351. doi: 10.1089/ten.tea.2018.0091. [DOI] [PubMed] [Google Scholar]
- 13.Bansal H, Leon J, Pont JL, et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci Rep. 2021;11:1–10. doi: 10.1038/s41598-021-83025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rikkers M, Dijkstra K, Terhaard BF, et al. Platelet-rich plasma does not inhibit inflammation or promote regeneration in human osteoarthritic chondrocytes in vitro despite increased proliferation. Cartilage. 2020;13:991S–1003S. doi: 10.1177/1947603520961162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins T, Alexander D, Barkatali B. Platelet-rich plasma: a narrative review. EFORT Open Rev. 2021;64:225–235. doi: 10.1302/2058-5241.6.200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624–634. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kececi Y, Ozsu S, Bilgir O. A cost-effective method for obtaining standard platelet-rich plasma. Wounds. 2014;26:232–238. [PubMed] [Google Scholar]
- 18.Merolla M, Nardi MA, Berger JS. Centrifugation speed affects light transmission aggregometry. Int J Lab Hematol. 2012;34:81–85. doi: 10.1111/j.1751-553X.2011.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Söderström AC, Nybo M, Nielsen C, Vinholt PJ. The effect of centrifugation speed and time on pre-analytical platelet activation. Clin Chem Lab Med. 2016;54:1913–1920. doi: 10.1515/cclm-2016-0079. [DOI] [PubMed] [Google Scholar]
- 20.Cavallo C, Roffi A, Grigolo B, et al. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int. 2016 doi: 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du L, Miao Y, Li X, Shi P, Hu Z. A novel and convenient method for the preparation and activation of PRP without any additives: temperature controlled PRP. Biomed Res Int. 2018;2018:1761865. doi: 10.1155/2018/1761865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etulain J, Mena HA, Meiss RP, et al. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci Rep. 2018;8:1–15. doi: 10.1038/s41598-018-19419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonmez C, Gümüs A, Senes M, Aykal G, Taneli F, Aksungar F, et al. An important source of preanalytical error in medical laboratories: centrifugation. Turk J Biochem. 2021;46:399. doi: 10.1515/TJB-2020-0262. [DOI] [Google Scholar]
- 24.Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34:599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4:560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu W, Wang Z, Hunt C, et al. The effectiveness and safety of platelet-rich plasma for chronic wounds: a systematic review and meta-analysis. Mayo Clin Proc. 2021;96:2407–2417. doi: 10.1016/j.mayocp.2021.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Zheng W, Zhao D, Zhao Y, Li Z. Effectiveness of platelet rich plasma in burn wound healing: a systematic review and meta-analysis. J Dermatol Treat. 2022;33:131–137. doi: 10.1080/09546634.2020.1729949. [DOI] [PubMed] [Google Scholar]
- 28.Cieslik-Bielecka A, Skowroński R, Jędrusik-Pawłowska M, Pierchała M. The application of L-PRP in AIDS patients with crural chronic ulcers: a pilot study. Adv Med Sci. 2018;63:140–146. doi: 10.1016/j.advms.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Spartalis ED, Tomos P, Dimitroulis D, Kouraklis G. Platelet-rich plasma in surgical oncology. Surg Innov. 2014;21:441. doi: 10.1177/1553350613520516. [DOI] [PubMed] [Google Scholar]
- 30.Eichler C, Baucks C, Üner J, et al. Platelet-rich plasma (PRP) in breast cancer patients: an application analysis of 163 sentinel lymph node biopsies. Biomed Res Int. 2020;2020:3432987. doi: 10.1155/2020/3432987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berná-Serna JD, Guzmán-Aroca F, García-Vidal JA, et al. A new therapeutic application of platelet-rich plasma to chronic breast wounds: a prospective observational study. J Clin Med. 2020;9:1–10. doi: 10.3390/jcm9103063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Valle X. Clinical practice guide for muscular injuries: epidemiology, diagnosis, treatment and prevention. Br J Sports Med. 2011;45:e2. doi: 10.1136/bjsm.2010.081570.20. [DOI] [Google Scholar]
- 34.Bisciotti GN, Chamari K, Cena E, et al. Hamstring injuries prevention in soccer: a narrative review of current literature. Joints. 2019;07:115–126. doi: 10.1055/s-0040-1712113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack CD, Kent RW, Coughlin MJ, et al. Incidence of lower extremity injury in the national football league: 2015 to 2018. Am J Sports Med. 2020;48:2287–2294. doi: 10.1177/0363546520922547. [DOI] [PubMed] [Google Scholar]
- 36.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD010071.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Grassi A, Napoli F, Romandini I, et al. Is platelet-rich plasma (PRP) effective in the treatment of acute muscle injuries? A systematic review and meta-analysis. Sport Med. 2018;48:971–989. doi: 10.1007/s40279-018-0860-1. [DOI] [PubMed] [Google Scholar]
- 38.Seow D, Shimozono Y, TengkuYusof TNB, Yasui Y, Massey A, Kennedy JG. Platelet-rich plasma injection for the treatment of hamstring injuries: a systematic review and meta-analysis with best-worst case analysis. Am J Sports Med. 2021;49:529–537. doi: 10.1177/0363546520916729. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Jones IA, Park C, Vangsness CTJ. The efficacy of platelet-rich plasma on tendon and ligament healing: a systematic review and meta-analysis with bias assessment. Am J Sports Med. 2018;46:2020–2032. doi: 10.1177/0363546517743746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han C, Na Y, Zhu Y, et al. Is platelet-rich plasma an ideal biomaterial for arthroscopic rotator cuff repair? A systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2019;14:183. doi: 10.1186/s13018-019-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim CH, Park YB, Lee JS, Jung HS. Platelet-rich plasma injection vs. operative treatment for lateral elbow tendinosis: a systematic review and meta-analysis. J Shoulder Elb Surg. 2022;31:428–436. doi: 10.1016/j.jse.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Li A, Wang H, Yu Z, et al. Platelet-rich plasma vs corticosteroids for elbow epicondylitis: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e18358. doi: 10.1097/MD.0000000000018358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birjandi AA, Neves VCM, Sharpe P. Advances in regenerative dentistry; building with biology. Regen Med. 2021;16:343–345. doi: 10.2217/rme-2021-0003. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Gou L, Zhang P, Li H, Qiu S. Platelet-rich plasma and regenerative dentistry. Aust Dent J. 2020;65:131–142. doi: 10.1111/adj.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;10:23. doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 47.Fraser D, Caton J, Benoit DSW. Periodontal wound healing and regeneration: insights for engineering new therapeutic approaches. Front Dent Med. 2022;3:1–24. doi: 10.3389/fdmed.2022.815810. [DOI] [Google Scholar]
- 48.Cho YD, Kim KH, Lee YM, Ku Y, Seol YJ. Periodontal wound healing and tissue regeneration: a narrative review. Pharmaceuticals. 2021;14:1–17. doi: 10.3390/ph14050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paradowska-Stolarz A, Wieckiewicz M, Owczarek A, Wezgowiec J. Natural polymers for the maintenance of oral health: review of recent advances and perspectives. Int J Mol Sci. 2021;22:10337. doi: 10.3390/ijms221910337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franchini M, Cruciani M, Mengoli C, et al. The use of platelet-rich plasma in oral surgery: a systematic review and meta-analysis. Blood Transfus. 2019;17:357–367. doi: 10.2450/2019.0177-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain R, De-Eknamkul W. Potential targets in the discovery of new hair growth promoters for androgenic alopecia. Expert Opin Ther Targets. 2014;18:787–806. doi: 10.1517/14728222.2014.922956. [DOI] [PubMed] [Google Scholar]
- 52.Ustuner ET. Cause of androgenic alopecia: crux of the matter. Plast Reconstr Surg. 2013;1:1–5. doi: 10.1097/GOX.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1–12. doi: 10.1016/j.jaad.2017.04.1141. [DOI] [PubMed] [Google Scholar]
- 55.Lin W, Xiang LJ, Shi HX, et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. Biomed Res Int. 2015;2015:730139. doi: 10.1155/2015/730139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn SY, Pi LQ, Hwang ST, Lee WS. Effect of IGF-I on hair growth is related to the anti-apoptotic effect of IGF-I and up-regulation of PDGF-A and PDGF-B. Ann Dermatol. 2012;24:26–31. doi: 10.5021/ad.2012.24.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.González R, Moffatt G, Hagner A, et al. Platelet-derived growth factor signaling modulates adult hair follicle dermal stem cell maintenance and self-renewal. NPJ Regen Med. 2017;2:11. doi: 10.1038/s41536-017-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Y, Liu C, Zhan X, Wang B, Li K, Li J. Jagged1 and epidermal growth factor promoted androgen-suppressed mouse hair growth in vitro and in vivo. Front Pharmacol. 2020;10:1–12. doi: 10.3389/fphar.2019.01634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gnann LA, Castro RF, Azzalis LA, et al. Hematological and hepatic effects of vascular epidermal growth factor (VEGF) used to stimulate hair growth in an animal model. BMC Dermatol. 2013;13:2–6. doi: 10.1186/1471-5945-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V. The effect of platelet-rich plasma in hair regrowth: a randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4:1317–1323. doi: 10.5966/sctm.2015-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gentile P, Garcovich S. Systematic review of platelet-rich plasma use in androgenetic alopecia compared with minoxidil®, finasteride®, and adult stem cell-based therapy. Int J Mol Sci. 2020;21:1–26. doi: 10.3390/ijms21082702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roohaninasab M, Goodarzi A, Ghassemi M, Sadeghzadeh-Bazargan A, Behrangi E, NajarNobari N. Systematic review of platelet-rich plasma in treating alopecia: focusing on efficacy, safety, and therapeutic durability. Dermatol Ther. 2021;34:e14768. doi: 10.1111/dth.14768. [DOI] [PubMed] [Google Scholar]
- 63.Singhal P, Agarwal S, Dhot PS, Sayal SK. Efficacy of platelet-rich plasma in treatment of androgenic alopecia. Asian J Transfus Sci. 2015;9:159–162. doi: 10.4103/0973-6247.162713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bayat M, Yazdanpanah MJ, HamidiAlamdari D, Banihashemi M, Salehi M. The effect of platelet-rich plasma injection in the treatment of androgenetic alopecia. J Cosmet Dermatol. 2019;18:1624–1628. doi: 10.1111/jocd.12907. [DOI] [PubMed] [Google Scholar]
- 65.Butt G, Hussain I, Ahmed FJ, Choudhery MS. Efficacy of platelet-rich plasma in androgenetic alopecia patients. J Cosmet Dermatol. 2019;18:996–1001. doi: 10.1111/jocd.12810. [DOI] [PubMed] [Google Scholar]
- 66.Dubin DP, Lin MJ, Leight HM, et al. The effect of platelet-rich plasma on female androgenetic alopecia: a randomized controlled trial. J Am Acad Dermatol. 2020;83:1294–1297. doi: 10.1016/j.jaad.2020.06.1021. [DOI] [PubMed] [Google Scholar]
- 67.Onishi K, Utturkar A, Chang E, Panush R, Hata J, Perret-Karimi D. Osteoarthritis: a critical review. Crit Rev Phys Rehabil Med. 2012;24:251–264. doi: 10.1615/critrevphysrehabilmed.2013007630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mescher AL. Junqueira’s basic histology text and atlas. Fourteenth: McGraw-Hill; 2016. [Google Scholar]
- 69.Davis A, Robson J. The dangers of NSAIDs: look both ways. Br J Gen Pract. 2016;66:172–173. doi: 10.3399/bjgp16X684433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Curtis E, Fuggle N, Shaw S, et al. Safety of cyclooxygenase-2 inhibitors in osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging. 2019;36:25–44. doi: 10.1007/s40266-019-00664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patrono C. Cardiovascular effects of cyclooxygenase-2 inhibitors: a mechanistic and clinical perspective. Br J Clin Pharmacol. 2016;82:957–964. doi: 10.1111/bcp.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45:S28–33. doi: 10.1016/j.semarthrit.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Laver L, Marom N, Dnyanesh L, Mei-Dan O, Espregueira-Mendes J, Gobbi A. PRP for degenerative cartilage disease: a systematic review of clinical studies. Cartilage. 2017;8:341–364. doi: 10.1177/1947603516670709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iio K, Furukawa KI, Tsuda E, et al. Hyaluronic acid induces the release of growth factors from platelet-rich plasma. Asia-Pac J Sport Med Arthrosc Rehabil Technol. 2016;4:27–32. doi: 10.1016/j.asmart.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu W, Xu P, Huang G, Liu L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Ther Med. 2018;16:2119–2125. doi: 10.3892/etm.2018.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao J, Huang H, Liang G, Zeng LF, Yang W, Liu J. Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2020;21:1–12. doi: 10.1186/s12891-020-03262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palco M, Fenga D, Basile GC, et al. Platelet-rich plasma combined with hyaluronic acid versus leucocyte and platelet-rich plasma in the conservative treatment of knee osteoarthritis. A retrospective study. Medicina (Kaunas) 2021;57:232. doi: 10.3390/medicina57030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li M, Huang Z, Wang S, Di Z, Zhang J, Liu H. Intra-articular injections of platelet-rich plasma vs. hyaluronic acid in patients with knee osteoarthritis: preliminary follow-up results at 6-months. Exp Ther Med. 2021;21:1–7. doi: 10.3892/etm.2021.10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta AK, Talukder M, Venkataraman M, Bamimore MA. Minoxidil: a comprehensive review. J Dermatol Treat. 2021;33:1–11. doi: 10.1080/09546634.2021.1945527. [DOI] [PubMed] [Google Scholar]
- 80.Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491–497. doi: 10.1097/DSS.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 81.Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737–746. doi: 10.1016/j.jaad.2020.06.1009. [DOI] [PubMed] [Google Scholar]
- 82.Pachar S, Chouhan C, Rao P, Kachhawa D, Singh H, Yadav C. A Comparative study of efficacy of 5% minoxidil and 5% minoxidil plus platelet-rich plasma in same patient for treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2022;15:71–76. doi: 10.4103/JCAS.JCAS_232_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alves R, Grimalt R. Platelet-rich plasma in combination with 5% minoxidil topical solution and 1 mg oral finasteride for the treatment of androgenetic alopecia: a randomized placebo-controlled, double-blind, half-head study. Dermatol Surg. 2018;44:126–130. doi: 10.1097/DSS.0000000000001198. [DOI] [PubMed] [Google Scholar]
- 84.Singh SK, Kumar V, Rai T. Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: a randomized, double-blind placebo control trial. Indian J Dermatol Venereol Leprol. 2020;86:150–157. doi: 10.4103/ijdvl.IJDVL_589_18. [DOI] [PubMed] [Google Scholar]
- 85.Pakhomova EE, Smirnova IO. Comparative evaluation of the clinical efficacy of prp-therapy, minoxidil, and their combination with immunohistochemical study of the dynamics of cell proliferation in the treatment of men with androgenetic alopecia. Int J Mol Sci. 2020;21:1–16. doi: 10.3390/ijms21186516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hatzimichael E, Tuthill M. Hematopoietic stem cell transplantation. Stem Cells Cloning Adv Appl. 2010;3:105–117. doi: 10.2147/SCCAA.S6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferrari G, Cavazzana M, Mavilio F. Gene therapy approaches to hemoglobinopathies. Hematol Oncol Clin N Am. 2017;31:835–852. doi: 10.1016/j.hoc.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 89.Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TML. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:2851–2857. doi: 10.1200/JCO.2015.66.2866. [DOI] [PubMed] [Google Scholar]
- 90.Hiller BM, Marmion DJ, Thompson CA, et al. Optimizing maturity and dose of iPSC-derived dopamine progenitor cell therapy for Parkinson’s disease. NPJ Regen Med. 2022;7:24. doi: 10.1038/s41536-022-00221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan Y, Ma T, Gong K, Ao Q, Zhang X, Gong Y. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen Res. 2014;9:798–805. doi: 10.4103/1673-5374.131596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goutman SA, Savelieff MG, Sakowski SA, Feldman EL. Stem cell treatments for amyotrophic lateral sclerosis: a critical overview of early phase trials. Expert Opin Investig Drugs. 2019;28:525–543. doi: 10.1080/13543784.2019.1627324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Biressi S, Filareto A, Rando TA. Stem cell therapy for muscular dystrophies. J Clin Investig. 2020;130:5652–5664. doi: 10.1172/JCI142031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hevesi M, LaPrade M, Saris DBF, Krych AJ. Stem cell treatment for ligament repair and reconstruction. Curr Rev Musculoskelet Med. 2019;12:446–450. doi: 10.1007/s12178-019-09580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iaquinta MR, Mazzoni E, Bononi I, et al. Adult stem cells for bone regeneration and repair. Front Cell Dev Biol. 2019;7:1–15. doi: 10.3389/fcell.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lai F, Kakudo N, Morimoto N, et al. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res Ther. 2018;9:1–10. doi: 10.1186/s13287-018-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang L, Zhang B, Liao B, et al. Platelet-rich plasma in combination with adipose-derived stem cells promotes skin wound healing through activating rho gtpase-mediated signaling pathway. Am J Transl Res. 2019;11:4100–4112. [PMC free article] [PubMed] [Google Scholar]
- 98.Van Pham P, Bui KHT, Ngo DQ, et al. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther. 2013;4:1. doi: 10.1186/scrt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yin N, Wang Y, Ding L, et al. Platelet-rich plasma enhances the repair capacity of muscle-derived mesenchymal stem cells to large humeral bone defect in rabbits. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-63496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haubner F, Muschter D, Schuster N, et al. Platelet-rich plasma stimulates dermal microvascular endothelial cells and adipose derived stem cells after external radiation. Clin Hemorheol Microcirc. 2015;61:279–290. doi: 10.3233/CH-151982. [DOI] [PubMed] [Google Scholar]
- 101.Elsaadany B, El Kholy S, El Rouby D, Rashed L, Shouman T. Effect of transplantation of bone marrow derived mesenchymal stem cells and platelets rich plasma on experimental model of radiation induced oral mucosal injury in albino rats. Int J Dent. 2017 doi: 10.1155/2017/8634540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myung H, Jang H, Myung JK, et al. Platelet-rich plasma improves the therapeutic efficacy of mesenchymal stem cells by enhancing their secretion of angiogenic factors in a combined radiation and wound injury model. Exp Dermatol. 2020;29:158–167. doi: 10.1111/exd.14042. [DOI] [PubMed] [Google Scholar]
- 103.US Food and Drug Administration. Drugs@FDA glossary of terms. Published 2017. https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-glossary-terms#D. Accessed 7 Nov 2022.
- 104.US Food and Drug Administration. CFR—code of federal regulations title 21 part 640—additional standards for human blood and blood products. 2022. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-640.
- 105.Jones IA, Togashi RC, Thomas Vangsness C. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558–565. doi: 10.1007/s12178-018-9514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marks P, Gottlieb S. Balancing safety and innovation for cell-based regenerative medicine. N Engl J Med. 2018;378:954–959. doi: 10.1056/NEJMsr1715626. [DOI] [PubMed] [Google Scholar]
- 107.Melbourne CS, Smith CA, Patel J, Platt B, Cook JL. Indications and associated outcomes for platelet-rich plasma injections performed in an academic orthopaedic-specific hospital: a patient satisfaction survey. Curr Orthop Pract. 2020;31:263–266. doi: 10.1097/BCO.0000000000000865. [DOI] [Google Scholar]
- 108.Noback PC, Donnelley CA, Yeatts NC, et al. Utilization of orthobiologics by sports medicine physicians: a survey-based study. J Am Acad Orthop Surg Glob Res Rev. 2021;5:e20.00185. doi: 10.5435/JAAOSGlobal-D-20-00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kantrowitz DE, Padaki AS, Ahmad CS, Lynch TS. Defining platelet-rich plasma usage by team physicians in elite athletes. Orthop J Sport Med. 2018;6:1–10. doi: 10.1177/2325967118767077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Lucas B, Pérez LM, Gálvez BG. Importance and regulation of adult stem cell migration. J Cell Mol Med. 2018;22:746–754. doi: 10.1111/jcmm.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. 6.1: Autologous blood transfusion (collection and reinfusion of the patient’s own red blood cells). Transfusion Handbook. Published 2014. https://www.transfusionguidelines.org/transfusion-handbook/6-alternatives-and-adjuncts-to-blood-transfusion/6-1-autologous-blood-transfusion-collection-and-reinfusion-of-the-patient-s-own-red-blood-cells. Accessed 16 June 2022.
- 112.Adias TC, Jeremiah Z, Uko E, Osaro E. Autologous blood transfusion—a review. S Afr J Surg. 2006;44:114–118. [PubMed] [Google Scholar]
- 113.Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg. 2014;7:189–197. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Germanovich K, Femia EA, Cheng CY, Dovlatova N, Cattaneo M. Effects of pH and concentration of sodium citrate anticoagulant on platelet aggregation measured by light transmission aggregometry induced by adenosine diphosphate. Platelets. 2018;29:21–26. doi: 10.1080/09537104.2017.1327655. [DOI] [PubMed] [Google Scholar]
- 115.Oneto P, Zubiry PR, Schattner M, Etulain J. Anticoagulants interfere with the angiogenic and regenerative responses mediated by platelets. Front Bioeng Biotechnol. 2020;8:1–12. doi: 10.3389/fbioe.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Handigund M, Cho YG. Review: insights into platelet storage and the need for multiple approaches. Ann Clin Lab Sci. 2015;45:713–719. [PubMed] [Google Scholar]
- 117.Védy D, Robert D, Gasparini D, Canellini G, Waldvogel S, Tissot JD. Bacterial contamination of platelet concentrates: pathogen detection and inactivation methods. Hematol Rev. 2009;1:e5. doi: 10.4081/hr.2009.e5. [DOI] [Google Scholar]
- 118.Roh YH, Kim W, Park KU, Oh JH. Cytokine-release kinetics of platelet-rich plasma according to various activation protocols. Bone Jt Res. 2016;5:37–45. doi: 10.1302/2046-3758.52.2000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Devereaux J, Dargahi N, Fraser S, Nurgali K, Kiatos D, Apostolopoulos V. Leucocyte-rich platelet-rich plasma enhances fibroblast and extracellular matrix activity: implications in wound healing. Int J Mol Sci. 2020;21:1–17. doi: 10.3390/ijms21186519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang SZ, Fan WM, Jia J, Ma LY, Bin YuJ, Wang C. Is exclusion of leukocytes from platelet-rich plasma (PRP) a better choice for early intervertebral disc regeneration? Stem Cell Res Ther. 2018;9:1–11. doi: 10.1186/s13287-018-0937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hudgens JL, Sugg KB, Grekin JA, Gumucio JP, Bedi A, Mendias CL. Platelet-rich plasma activates proinflammatory signaling pathways and induces oxidative stress in tendon fibroblasts. Am J Sports Med. 2016;44:1931–1940. doi: 10.1177/0363546516637176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heinz A. Elastases and elastokines: elastin degradation and its significance in health and disease. Crit Rev Biochem Mol Biol. 2020;55:252–273. doi: 10.1080/10409238.2020.1768208. [DOI] [PubMed] [Google Scholar]
- 123.Garratt LW, Sutanto EN, Ling KM, et al. Matrix metalloproteinase activation by free neutrophil elastase contributes to bronchiectasis progression in early cystic fibrosis. Eur Respir J. 2015;46:384–394. doi: 10.1183/09031936.00212114. [DOI] [PubMed] [Google Scholar]
- 124.Son ED, Kim H, Choi H, et al. Cathepsin G increases MMP expression in normal human fibroblasts through fibronectin fragmentation, and induces the conversion of proMMP-1 to active MMP-1. J Dermatol Sci. 2009;53:150–152. doi: 10.1016/j.jdermsci.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 125.Galkina SI, Golenkina EA, Viryasova GM, Romanova YM, Sud’ina GF. Nitric oxide in life and death of neutrophils. Curr Med Chem. 2019;26:5764–5780. doi: 10.2174/0929867326666181213093152. [DOI] [PubMed] [Google Scholar]
- 126.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]