Abstract
Background: Early-onset fetal growth restriction (FGR), an identifiable variant of FGR, exhibits divergences in its severity, management, and placental pathologies when juxtaposed with late-onset FGR. The objective of this cross-sectional investigation was to scrutinize placental pathologies in pregnancies afflicted by early-onset FGR, emphasizing a comparative analysis between cohorts with and without preeclampsia (PE). Patients, Materials and Methods: The study encompassed a cohort of 85 expectant mothers who received a diagnosis of early-onset FGR. Rigorous histopathological (HP) and immunohistochemical (IHC) assessments were conducted on the placentas. Comparative analyses were performed, distinguishing between individuals diagnosed with both PE and early-onset FGR, and those presenting normotensive early-onset FGR. Results: HP analysis unveiled a multitude of shared placental lesions, encompassing retroplacental hemorrhage, expedited villous maturation, infarctions, and calcification-associated fibrin deposits. IHC investigations displayed affirmative immunoreactivity for anti-hypoxia-inducible factor (HIF) and anti-vascular endothelial growth factor (VEGF) antibodies within the placental infarcted villitis. Moreover, noteworthy variances in placental measurements and distinctive lesions were discerned when comparing the PE and early-onset FGR cohort with the normotensive group. Conclusions: Maternal malperfusion emerged as a pivotal determinant linked to placental lesions in pregnancies affected by early-onset FGR. Remarkably, the occurrence of infarctions, specifically delayed infarctions, exhibited a noteworthy correlation with PE. These findings accentuate the significance of pursuing additional research endeavors aimed at unraveling the intricate mechanisms governing maternal malperfusion and its consequential influence on placental health in the context of early-onset FGR, with particular attention to the interplay with PE.
Keywords: placental pathology , fetal growth restriction , preeclampsia , maternal malperfusion
Introduction
Fetal growth restriction (FGR) is a pregnancy complication occurring in approximately 3–7% of all pregnancies [1]. This condition is dichotomized into two distinct entities, namely early-onset and late-onset FGR, contingent upon the temporal demarcation of diagnosis in relation to gestational age (GA) [2].
The classification of these two variants of FGR relies on several factors, including their association with hypertensive disorders, Doppler findings, severity, management, and placental characteristics [3, 4]. Early-onset FGR, transpiring prior to the 32nd week of gestation, exhibits a heightened susceptibility to perinatal morbidity and mortality, surpassing that observed in cases of late-onset FGR [2, 3].
Placental insufficiency, recognized as the prevailing etiological factor in FGR, results in fetal hypoxia and malnourishment. However, the intricate mechanisms underpinning placental dysfunction in FGR still elude comprehensive understanding [5, 6]. Moreover, it has been suggested that defective placentation primarily characterizes the pathogenesis of early-onset preeclampsia (PE) and FGR, arising from inadequate trophoblastic invasion of the maternal spiral arteries [7].
Aim
The aim of this study was the histopathological (HP) examination of placental pathology in pregnancies affected by early-onset FGR, focusing on a comparative analysis between cohorts with or without PE.
Materials and Methods
Settings and study design
A cross-sectional study was conducted on 85 consecutive women diagnosed with FGR, who gave birth at the Municipal Emergency Clinical Hospital, Timişoara, Romania, between 2020–2021. The study was conducted according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Guidelines.
The inclusion criteria for participants in the study were as follows: (i) diagnosis of early-onset FGR; (ii) singleton pregnancy; (iii) initiation of antenatal care during the first trimester of pregnancy, with precise determination of GA.
The exclusion criteria encompassed: (i) presence of fetal chromosomal anomalies or malformations; (ii) patients with incomplete or missing data.
Histological examination of the placentas
Following delivery, prior to undergoing standard paraffin embedding, the placentas were immersed in a 10% neutral buffered formalin solution for a duration of 24 hours. Subsequently, the placentas were sliced into longitudinal segments measuring 1–2 cm and immersed in a 10% neutral buffered formalin solution for several days. Upon completion of the fixation process, representative sections of placental tissue were excised and incorporated into paraffin. Paraffin-embedded placental fragments were then sectioned using a rotary microtome (Microm HM350) equipped with a section transfer mechanism (STS, microM). The sections were stained with Hematoxylin–Eosin (HE) for histological examination. All histological slides were blindly examined by a single pathologist experienced in placental pathology. The pathological variables assessed in this study were based on the Amsterdam Placental Workshop Group Consensus Statement and included the following: (1) avascular villi that may or may not have fetal vascular thrombi; (2) infarction (late or early); (3) non-infectious chronic villitis; (4) massive perivillous fibrin deposition (MPFD); (5) fetal vascular thrombi located within the proximal portion of the villous tree or chorionic plate; (6) retroplacental hemorrhage; (7) intervillous thrombi; (8) maternal side infarction; (9) meconium-laden macrophages in chorionic surface/placental membranes or free meconium; (10) increased syncytial knots; (11) deposit of intervillous fibrin; (12) immature intermediate trophoblasts and trophoblastic giant cells in the basal decidua; (13) agglutination of the villi; (14) hypoplasia of the distal villi; (15) hypertrophy of the capsular spiral artery; (16) basal and capsular decidual arteries with fibrinoid necrosis or atheroma; (17) muscularized arteries persist in the basal plate.
Note that while these pathological variables were included for assessment in our study, it is important to clarify that not all these variables were necessarily present in all study participants. The listed variables represent a comprehensive set of potential HP lesions that were considered based on the Amsterdam Placental Workshop Group Consensus Statement.
Immunohistochemical analysis
Sections have been collected on poly-L-lysine-coated slides and dried for 24 hours to 37ºC. The slides were then heated in a microwave oven for seven cycles of three minutes in a sodium citrate solution to a pH 6 to deparaffinize, hydrate, and unmask the antigen on the sections. After the heating process, histological sections were incubated in 3% hydrogen peroxide for 30 minutes at room temperature (RT), then for 10 minutes in distilled water and five minutes in 1% phosphate-buffered saline (PBS) to block endogenous peroxidase. The non-specific sites are then eliminated using 2% skim milk. Subsequently, the primary antibodies were then applied and incubated overnight at 4ºC. The secondary biotinylated antibody was applied the following day and left on for 30 minutes at RT before washing in 1% PBS. Next, the samples were incubated with Streptavidin–Horseradish peroxidase (HRP) for an additional 30 minutes at RT. After washing with 1% PBS, the chromogenic substrate was added to the samples and incubated for 10 minutes at RT. 3.3’-Diaminobenzidine (DAB, Dako) was used to detect the signal, and the reaction was stopped in 1% PBS. Following the contrast with Mayer’s Hematoxylin, alcohol dehydration, xylene clarification, and slide fixing in a DPX (Fluka) environment were performed.
The following IHC markers were used in this study: anti-cluster of differentiation (CD)34 (mouse monoclonal antibody), anti-CD20 (mouse monoclonal antibody), anti-CD3 (rabbit polyclonal antibody), anti-CD68 (mouse mono-clonal antibody), anti-tryptase (tryptase rabbit polyclonal antibody), anti-hypoxia-inducible factor-alpha [HIF-1α (H1a67) mouse monoclonal immunoglobulin G (IgG)], and anti-vascular endothelial growth factor (mouse monoclonal IgG).
Data sources and measurement
Two researchers extracted baseline characteristics of the participants, encompassing demographic and clinical data, from the electronic medical records of the patients. All antenatal care ultrasound (US) scans were conducted by a specialized researcher proficient in maternal–fetal medicine (A.C.R). The variables under investigation included maternal age, maternal weight, maternal height, body mass index (BMI), gestation, parity, GA at the time of diagnosis, GA at the time of delivery, as well as placental lesions, such as maternal vascular malperfusion and fetal vascular malperfusion.
GA was determined through the first trimester US examination. The classification of placental lesions adhered to the Guidelines presented in the “Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement” [8].
Results
The study comprised 85 pregnant women diagnosed with early-onset FGR. Table 1 presents the principal characteristics of the study population, encompassing the overall participants (n=85), individuals diagnosed with both PE and early-onset FGR (n=49), and those with normotensive early-onset FGR (n=36). On average, the participants had a mean age of 29.72 years, and the median GA at the diagnosis of FGR was 31 weeks (Table 1 ). In terms of fetal Doppler findings, the most prevalent abnormalities observed were reversed umbilical artery (UA) end-diastolic flow (45.9%) and absent UA end-diastolic flow (29.4%). Furthermore, 9.4% of the fetuses exhibited abnormal cardiotocography (CTG) results (Table 1 ). As for neonatal complications, the mortality rate stood at 3.5%, and 45% of the infants manifested varying degrees of intraventricular hemorrhage (Table 1 ).
Table 1.
Baseline characteristics of pregnant women with early-onset FGR
|
Maternal characteristics |
Overall (n=85) |
|
Maternal age [years] [mean ± SD] |
29.72±5.61 |
|
Maternal weight [kg] [median (IQR)] |
74 (14) |
|
Maternal height [cm] [median (IQR)] |
162 (8) |
|
Maternal BMI [kg/m2] [median (IQR)] |
28.19 (3.94) |
|
Gestation [median (IQR)] |
1 (1) |
|
Parity [median (IQR)] |
0 (1) |
|
GA on diagnosis [weeks] [median (IQR)] |
31 (2) |
|
GA on delivery [weeks] [median (IQR)] |
33 (3) |
|
Preeclampsia associated early-onset FGR [n/%] |
49/57.6% |
|
Normotensive early-onset FGR [n/%] |
36/42.4% |
|
Fetal Doppler results |
n/% |
|
UA AEDF |
25/29.4% |
|
UA REDF |
39/45.9% |
|
DVA absent |
14/16.5% |
|
Reversed DVA |
7/8.2% |
|
Abnormal CTG |
8/9.4% |
|
Abnormal BPP |
4/4.7% |
|
Neonatal complications |
n/% |
|
Neonatal death |
3/3.5% |
|
Bronchopulmonary dysplasia |
9/10.6% |
|
Intraventricular hemorrhage grades I/II |
24/28.2% |
|
Intraventricular hemorrhage grades III/IV |
17/20.0% |
|
Necrotizing enterocolitis |
2/2.4% |
AEDF: Absent end-diastolic flow; BMI: Body mass index; BPP: Biophysical profile; CTG: Cardiotocography; DVA: Ductus venosus agenesis; FGR: Fetal growth restriction; GA: Gestational age; IQR: Interquartile range; n: No. of cases; REDF: Reversed end-diastolic flow; SD: Standard deviation; UA: Umbilical artery.
The placental measurements revealed a median weight of 261 g, with a median maximum diameter of 15 cm, and a fetal-to-placenta weight ratio of 4.9 (Table 2 ). Significant distinctions were observed among the groups in various placental measurements. Specifically, PE and early-onset FGR exhibited diminished placental weight (242 g vs. 289 g; p<0.001), reduced maximum diameter (15 cm vs. 16 cm; p<0.001), and decreased minimum diameter (10 cm vs. 11 cm; p<0.001). Additionally, the volumetric analysis of the placenta indicated a notably lower placental volume in cases of PE and early-onset FGR (325 cm3 vs. 365 cm3; p<0.001). Notably, there were no statistically significant variations observed in the maximum and minimum placental plaque thickness, as well as the fetal/placental ratios, between the investigated groups (Table 2).
Table 2.
Placental characteristics among pregnancies complicated by early-onset FGR
|
Characteristics |
Overall (n=85) |
Preeclampsia and early-onset FGR (n=49) |
Normotensive early-onset FGR (n=36) |
p-value |
|
Placental weight [g] [median (IQI)] |
261 (94) |
242 (86) |
289 (110) |
<0.001 |
|
Maximum diameter [cm] [median (IQI)] |
15 (3) |
15 (2) |
16 (1.5) |
<0.001 |
|
Minimum diameter [cm] [median (IQI)] |
10.5 (3) |
10 (1) |
11 (1) |
<0.001 |
|
Maximum thickness [cm] [median (IQI)] |
3 (1) |
2.9 |
3 |
0.34 |
|
Minimum thickness [cm] [median (IQI)] |
1.5 (1) |
1.5 (1) |
1.5 (1) |
0.69 |
|
Placental volume [cm3] [median (IQI)] |
343 (162) |
325 (110) |
365 (135) |
<0.001 |
|
Fetal/placental weight ratio [median (IQI)] |
4.9 (2.1) |
4.85 (1.5) |
4.95 (1) |
0.49 |
FGR: Fetal growth restriction; IQI: Interquartile interval; n: No. of cases.
Among the discerned placental lesions in pregnancies complicated by early-onset FGR, as delineated by the Amsterdam Placental Workshop Group Consensus Statement, are as follows:
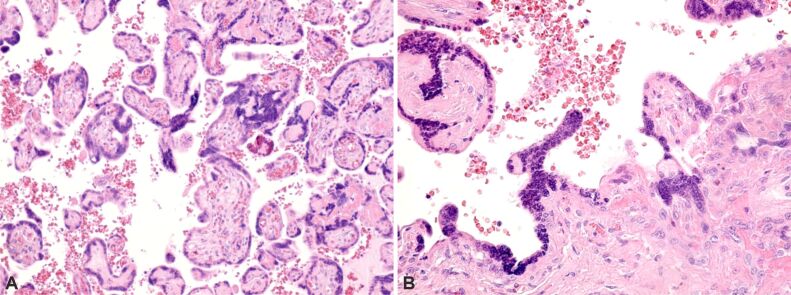
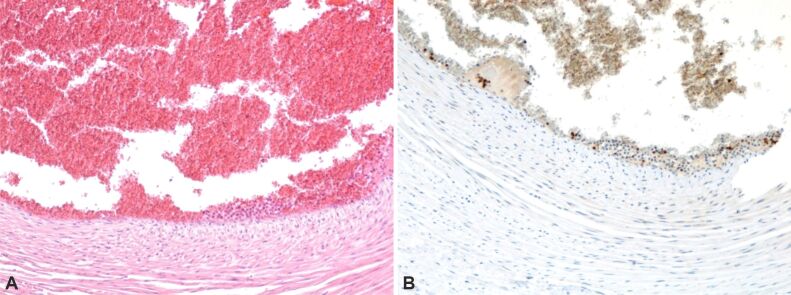
Retroplacental hemorrhage (Figure 1) – characterized by the accumulation of blood beneath the decidua, causing its dissection and compression of the overlying intervillous space. This leads to congestion, intravillous hemorrhage, and villous engorgement.
Figure 1.
Normal and pathological placental villi, characterized by the presence of small areas of extravillous calcification and intense extravillous hemorrhagic processes, as well as areas of intravillous vascular edema. Hematoxylin–Eosin (HE) staining, ×100.
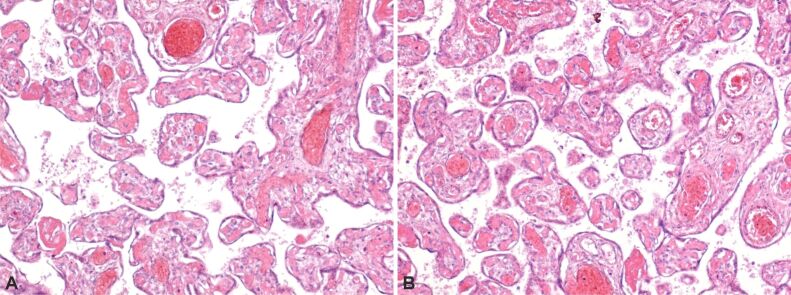
Accelerated villous maturation (Figure 2A and 2B) – characterized by the presence of shortened or short for GA hypermature villi, often accompanied by an increase in syncytial knots.
Figure 2.
(A and B) Normal and pathological placental villi with small areas of extravillous calcification and small syncytial knots. HE staining, ×100
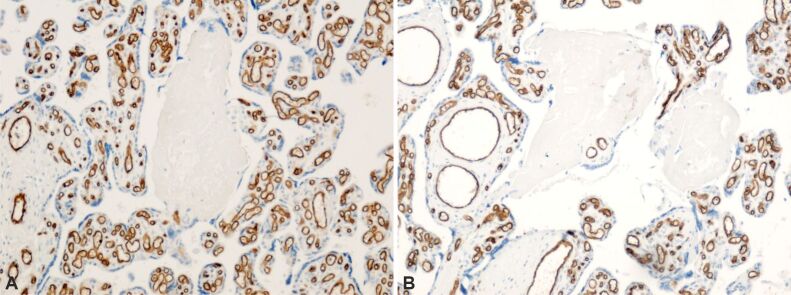
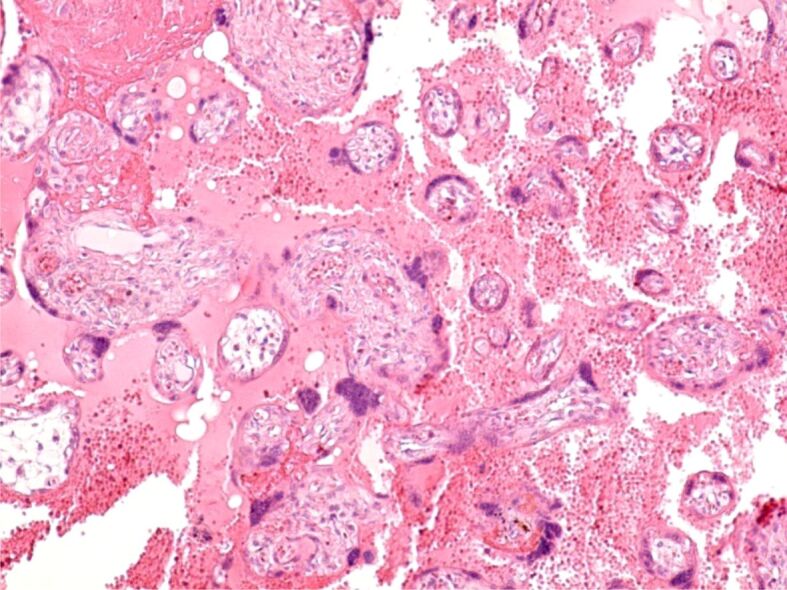
Placental infarctions (Figure 3A and 3B) – identified through immunohistochemical (IHC) analysis for CD34, which exhibits positive immunoreactivity in the basement membrane of capillary endothelium.
Figure 3.
A and B) Normal placental villi with presence of normal appearing intravillous capillaries and totally infarcted placental villi with absent vascular capillaries. The basement membrane of capillary endothelium is immunohistochemically labeled (brown). Anti-CD34 antibody immunomarking, ×100. CD34: Cluster of differentiation 34
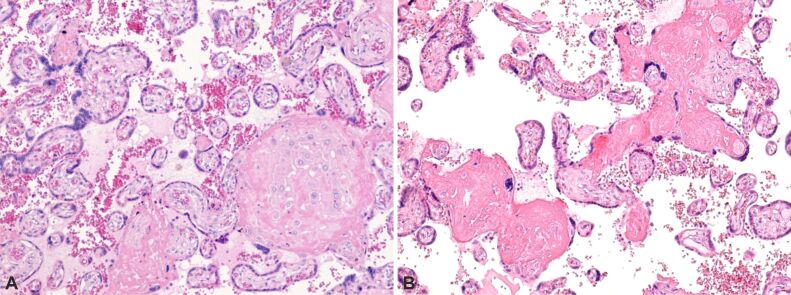
Fibrin deposits associated with calcification (Figure 4A and 4B; Figure 5) were also observed. Moreover, in placentas affected by early-onset FGR, regions of pronounced amyloid deposition (amyloid plaques) were detected (Figure 6).
Figure 4.
(A and B) Normal and pathological placental villi, with presence of intravillous and perivillous fibrinoid deposition (deep pink), small areas of extravillous and intravillous calcification. HE staining, ×100
Figure 5.
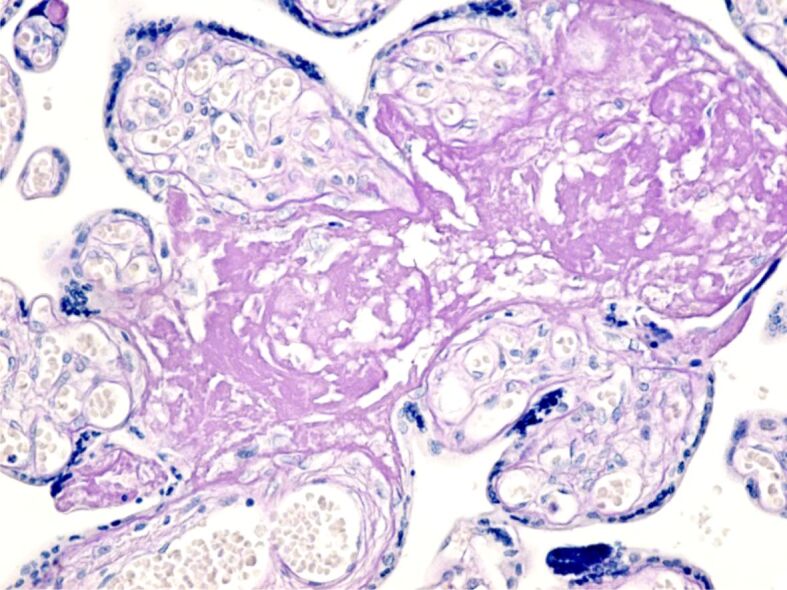
Placental villi showing intravillous and extravillous fibrinoid deposition with areas of perivillous calcification. PAS–Hematoxylin staining, ×100. PAS: Periodic Acid–Schiff
Figure 6.
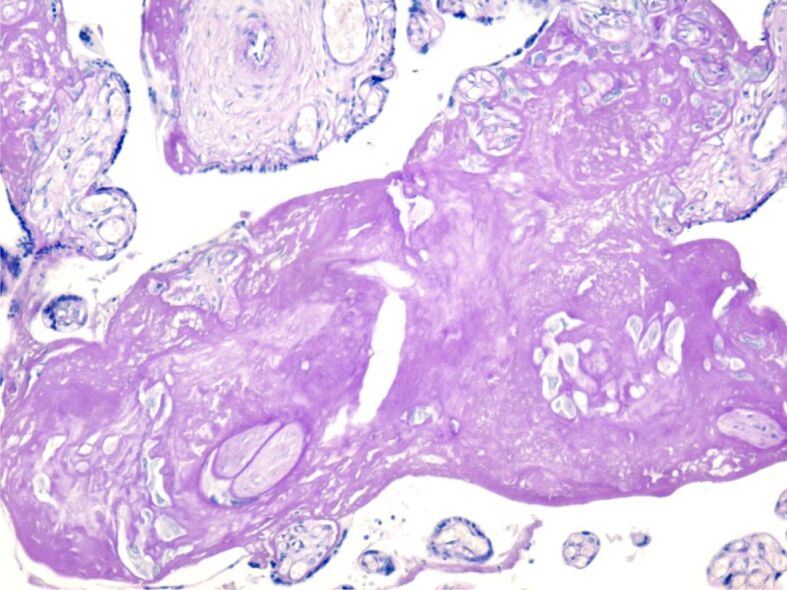
Areas of intense amyloid deposition (amyloid plaque). PAS–Hematoxylin staining, ×100
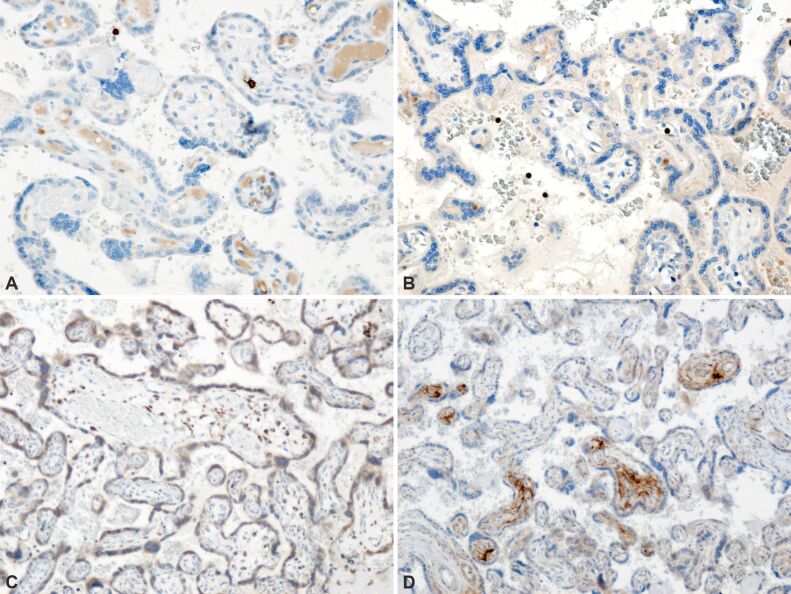
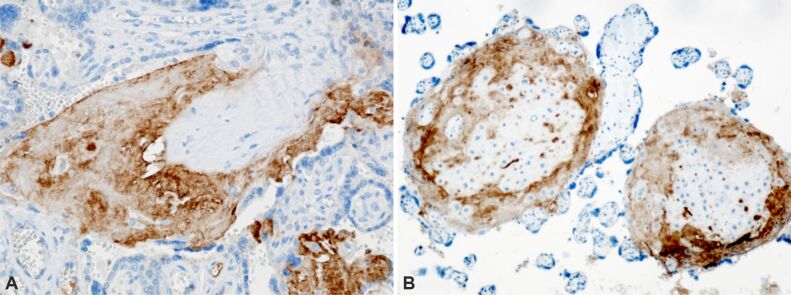
Furthermore, IHC analysis unveiled the presence of paravillous B-lymphocytes, identified through staining with an anti-CD20 antibody (Figure 7A), as well as T-lymphocytes, detected via staining with an anti-CD3 antibody (Figure 7B). Additionally, paravillous and intravillous macrophages (Figure 7C), along with intravillous mast cells, exhibited positive immunoreactivity upon staining with an anti-tryptase antibody (Figure 7D) in normal placental villi. Moreover, the presence of perilesional macrophages was observed in infarcted placental villi, as confirmed by staining with an anti-CD68 antibody (Figure 8). Furthermore, placental villi displaying positive immunoreactivity for anti-HIF and anti-VEGF antibodies were noted in placental infarcted villitis (Figure 9; Figure 10A and 10B). HP findings suggestive of fetal vascular malperfusion included umbilical or chorionic plate thrombosis (Figure 11A and 11B; Figure 12A and 12B).
Figure 7.
Normal placental villi with presence of normal appearing intravillous capillaries: (A) Perivilocytic B-lymphocytes are immunohistochemically stained (brown); (B) Perivilocytic T-lymphocytes are immunohistochemically stained (brown); (C) Perivillous and intravillous macrophages are immunohistochemically stained (brown); (D) Intravillous mast cells are immunohistochemically stained (brown). Anti-CD20 antibody immunomarking: (A) ×200. Anti-CD3 antibody immunomarking: (B) ×200. Anti-CD68 antibody immunomarking: (C) ×100. Anti-tryptase antibody immunomarking: (D) ×100. CD: Cluster of differentiation.
Figure 8.
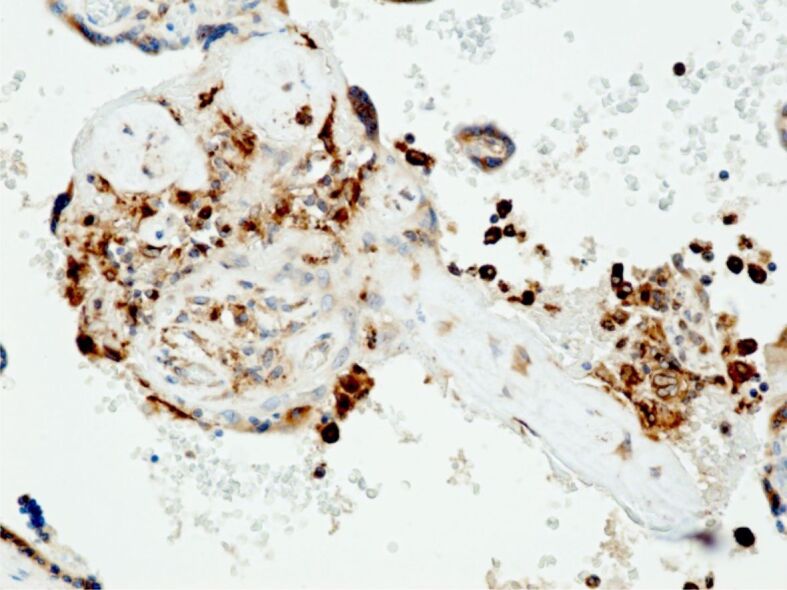
Placental microscopic aspects. Infarcted placental villi with presence of perivillous macrophages, immunohistochemically stained (brown). Anti-CD68 antibody immunomarking, ×200
Figure 9.
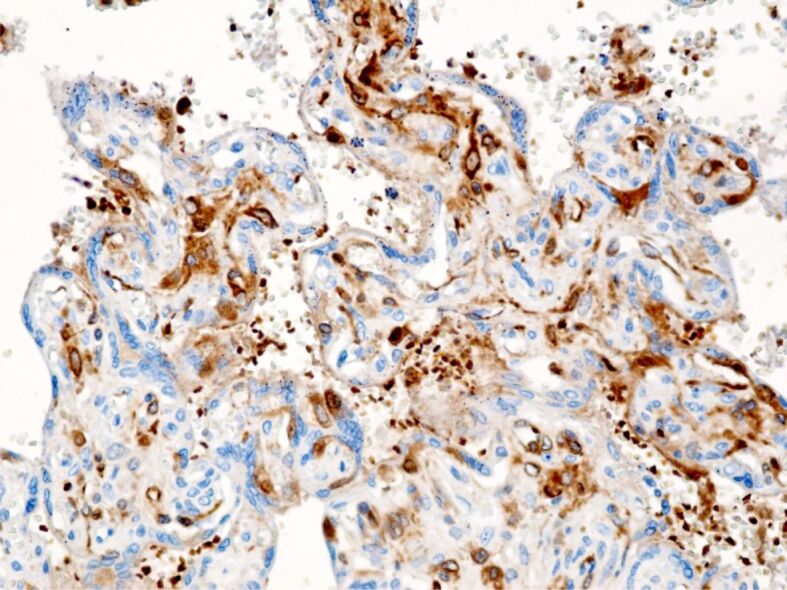
Placental villi with positive perivillous immunoreactivity (brown). Anti-HIF antibody immunomarking, ×100. HIF: Hypoxia-inducible factor
Figure 10.
Figure 10 – Positive immunoreaction (brown immunostaining): (A) Infarcted placental villi; (B) Infarcted placental villi and peridecidual areas. Anti-VEGF antibody immunomarking, ×200. VEGF: Vascular endothelial growth factor
Figure 11.
(A and B) Umbilical cord with a widely dilated blood vessel, with the presence of intravascular thrombosis partially blocking the blood vessel. HE staining: (A) ×100. Anti-CD3 antibody immunomarking: (B) ×100.
Figure 12.
(A and B) Placental cotyledons showing partially permeable blood vessels, but also thrombosed vessels blocking the vascular lumen. HE staining, ×100
Table 3 displays the placental findings in the study population, consisting of individuals diagnosed with PE, and early-onset FGR and those with normotensive early-onset FGR. The prevalence of various placental abnormalities was assessed and compared between the two groups. Maternal vascular malperfusion was observed in a significant proportion of cases, with overall rates of 67.05%. The most common were increased syncytial knots (81.2%), followed by infarctions (67.05%). Among the infarctions, late infarctions were more prevalent, accounting for 44.6% of overall cases (Table 3). Specifically, the incidence of infarctions was higher in the PE and early-onset FGR group compared to the normotensive group (83.7% vs. 44.4%; p=0.015). Furthermore, the PE and early-onset FGR group had a higher proportion of late infarctions compared to the normotensive group (58.1% vs. 27.8%; p<0.001) (Table 3 ). Regarding syncytial knots, both mildly increased (15–30%) and severely increased (≥30%) proportions were assessed. Mildly increased syncytial knots were observed in 40.0% of overall cases, with comparable rates between the PE and early-onset FGR group and the normotensive group (36.7% vs. 44.4%; p=0.50). However, severely increased syncytial knots were found in 41.2% of overall cases, with a higher prevalence in the PE and early-onset FGR group compared to the normotensive group (51.0% vs. 27.8%; p=0.04) (Table 3).
Table 3.
Individual placental lesions in women affected by early-onset FGR
|
Placental findings |
n/Total (%) |
Preeclampsia and early-onset FGR (n=49) |
Normotensive early-onset FGR (n=36) |
p-value |
|
Maternal vascular malperfusion |
||||
|
▪ Infarctions |
57/85 (67.05%) |
41/49 (83.7%) |
16/36 (44.4%) |
0.015 |
|
→ of which late infarctions |
38/85 (44.6%) |
28/49 (58.1%) |
10/36 (27.8%) |
<0.001 |
|
▪ Retroplacental hemorrhage |
11/85 (12.9%) |
6/49 (12.2%) |
5/36 (13.9%) |
0.53 |
|
▪ Accelerated villous maturation |
6/85 (7.05%) |
4/49 (8.2%) |
2/36 (5.6%) |
0.49 |
|
Syncytial knots |
||||
|
▪ Mildly increased (15–30%) |
34/85 (40.0%) |
18/49 (36.7%) |
16/36 (44.4%) |
0.50 |
|
▪ Severely increased (≥30%) |
35/85 (41.2%) |
25/49 (51.0%) |
10/36 (27.8%) |
0.04 |
|
▪ Massive perivillous fibrin deposition |
7/85 (8.2%) |
3/49 (6.1%) |
4/36 (11.1%) |
0.45 |
|
Fetal vascular malperfusion |
||||
|
▪ Thrombosis |
10/85 (11.7%) |
5/49 (8.2%) |
5/49 (16.7%) |
0.31 |
FGR: Fetal growth restriction; n: No. of cases
Retroplacental hemorrhage was observed in 12.9% of cases, with similar rates between the two study groups (p=0.53). Accelerated villous maturation, characterized by advanced maturation of placental villi, was present in 7.05% of cases, with no significant difference between the two groups (p=0.49). The presence of MPFD, a condition characterized by excessive fibrin accumulation, was identified in 8.2% of overall cases, with no significant difference between the two study groups (p=0.45).
In terms of lesions associated with fetal malperfusion, the only lesion identified was thrombosis, detected in 11.7% of cases, with no significant difference between the PE and early-onset FGR group (8.2%) and the normotensive group (16.7%) (p=0.31) (Table 3).
The logistic regression analysis revealed significant associations for certain placental findings. The presence of infarctions in the early-onset FGR placentas was significantly associated with PE, with an odds ratio (OR) of 6.40 [95% confidence interval (CI): 2.35–17.46, p<0.001] (Table 4 ). Late infarctions also show a significant association, with an OR of 3.467 (95% CI: 1.37–8.72, p=0.008). These findings suggest that the occurrence of infarctions, particularly late infarctions, increases the likelihood of PE.
Table 4.
Likelihood of individual placental lesions in women with early-onset FGR according to the evidence of PE
|
Placental findings |
B |
SE |
p-value |
OR |
95% CI |
|
|
Lower |
Upper |
|||||
|
Maternal vascular malperfusion |
||||||
|
▪ Infarctions |
1.857 |
0.512 |
<0.001 |
6.40 |
2.35 |
17.46 |
|
→ of which late infarctions |
1.243 |
0.471 |
0.008 |
3.467 |
1.37 |
8.72 |
|
▪ Retroplacental hemorrhage |
-0.145 |
0.650 |
0.824 |
0.865 |
0.24 |
3.09 |
|
▪ Accelerated villous maturation |
0.413 |
0.895 |
0.645 |
1.511 |
0.26 |
8.73 |
|
Syncytial knots |
||||||
|
▪ Mildly increased (15–30%) |
-0.320 |
0.448 |
0.474 |
0.726 |
0.30 |
1.74 |
|
▪ Severely increased (≥30%) |
0.996 |
0.469 |
0.034 |
2.708 |
1.08 |
6.79 |
|
▪ Massive perivillous fibrin deposition |
-0.651 |
0.798 |
0.415 |
0.522 |
0.10 |
2.49 |
|
Fetal vascular malperfusion |
||||||
|
▪ Thrombosis |
-0.811 |
0.687 |
0.238 |
0.444 |
0.11 |
1.70 |
CI: Confidence interval; FGR: Fetal growth restriction; OR: Odds ratio; PE: Preeclampsia; SE: Standard error. *The results indicate the probabilities in the PE group compared to the normotensive group
Discussions
Our study aimed to determine the histological placental lesions present in early-onset FGR. In the context of placental characteristics, the findings of this study reveal that PE and early-onset FGR exhibit notable distinctions when compared to normotensive early-onset FGR. Placental weight, diameter, and volume exhibit a significant decrease in the group of patients with early-onset FGR and PE compared to the group without PE. A cautious overall interpretation of the results would suggest that there are significant differences in placental characteristics between the group with PE and FGR and the group with normotensive early-onset FGR. Placental weight, maximum diameter, minimum diameter, and placental volume are all significantly affected by PE and FGR [9]. However, it is worth noting that there was no significant difference observed in placental thickness between the two groups. These findings align with previous studies that have also emphasized the role of placental characteristics in pregnancy complications [10, 11]. These findings support the hypothesis that placental alterations may play a significant role in the pathogenesis of PE and FGR [11, 12]. In comparison to previous studies, our observations reveal that the median values for placental weight, maximum diameter, minimum diameter, and placental volume align with the anticipated range based on the diagnoses of PE and FGR, as demonstrated in existing literature [13, 14]. Moreover, the absence of a statistically significant difference in placental thickness between the PE and normotensive groups corroborates the findings reported in prior investigations [13].
It was thus found that in early-onset FGR maternal malperfusion lesions are predominant, compared to those suggesting fetal malperfusion. These findings are consistent with other studies, which have also shown that lesions associated with maternal malperfusion can differentiate between early and late FGR with a sensitivity of 60% and specificity of 89% [13]. Therefore, it can be concluded that lesions indicating maternal malperfusion can be used as an effective tool to differentiate between early and late FGR. However, among the patients in this study, no lesions suggestive of severe maternal vascular malperfusion, such as atherosclerosis or persistent intravascular trophoblasts, were detected. Consequently, it can be considered that severe maternal vascular malperfusion may not be the main cause of adverse pregnancy outcomes in these patients.
Among patients with early-onset FGR in our study, various placental lesions indicative of maternal malperfusion were identified, such as retroplacental hemorrhage, accelerated villous maturation, infarcts, and extensive fibrin deposits. However, it is noteworthy that distal villous hypoplasia, which is typically observed in pregnancies of less than 32 weeks gestation, was not detected among the participants in this study.
The “placental theory” contends that abnormal placentation with altered trophoblast invasion may lead to the development of FGR [15]. FGR may result from the effects of defective trophoblast invasion on the villous tree development, the placenta’s final size, and the transfer of nutrients and oxygen from the mother to the fetus. Lesions consistent with maternal vascular malperfusion are the most frequent placental findings in these circumstances [7, 8]. Unlike early FGR, the late phenotype is not primarily determined by abnormal placentation in the first trimester, with HP examinations of the placenta from full-term FGR fetuses demonstrating a higher rate of vascular lesions (especially infarcts and thrombotic events) [16]. The histological findings in this study show that among pregnancies complicated with early FGR, lesions suggestive of fetal malperfusion are less common, with only thrombosis being identified.
The findings indicate significant differences in placental findings between the group with PE and early-onset FGR and the group with normotensive early-onset FGR. The proportions of maternal vascular malperfusion markers, such as infarctions and late infarctions, were higher in the PE and early-onset FGR group compared to the normotensive early-onset FGR group. However, there were no significant differences in retroplacental hemorrhage, accelerated villous maturation, MPFD, and fetal vascular malperfusion markers. Providing a cautious overall interpretation of the results, it can be concluded that maternal vascular malperfusion, specifically the presence of infarctions and late infarctions, may be associated with the development of PE and early-onset FGR [7]. The increased severity of syncytial knots in the PE and early-onset FGR group further supports the notion of altered placental development in these conditions [10].
The IHC study shows with positive anti-HIF and anti-VEGF antibodies immunoreaction are observed in placental infarcted villitis. Normal pregnancy corresponds with a weighed angiogenic state, whereas FGR-complicated pregnancies are frequently associated with an anti-angiogenic bias [17]. Angiogenesis is a key mechanism in the placentation process. VEGF family of growth factors and receptors is an important signaling pathway in angiogenesis [17]. Early onset of FGR is linked to increased VEGF gene activity in the placenta. Increased VEGF-A gene activity in the placenta, on the other hand, is regarded as a secondary response to the hypoxic state in utero, rather than being a critical factor in the etiology of FGR [18].
Strengths
The study has several strengths worth noting. Firstly, the accurate diagnosis of FGR and its specific phenotype was achieved through US scans performed by a single certified US specialist. This ensures consistency and reduces interobserver variability in the assessment of fetal growth. Additionally, the study utilized an accepted classification scheme for placental lesions, specifically the Amsterdam Placental Workshop Group Consensus Statement. By employing a standardized framework, the study enhances the reliability and comparability of its findings with other research studies.
Limitations
This study does have certain limitations that should be acknowledged. Firstly, the research was conducted in a single medical clinic, which may limit the generalizability of the findings to other populations or settings. It would be valuable to replicate the study in multiple centers to increase the diversity and representativeness of the sample.
Another limitation is the lack of consideration for other variables that could potentially influence the occurrence of placental lesions. Factors such as maternal medical history, lifestyle, and genetic predisposition could play a role in the development of placental lesions in early-onset FGR. The exclusion of these variables may limit the comprehensive understanding of the underlying mechanisms.
Additionally, the study focused exclusively on early-onset FGR and did not include patients with late-onset FGR. By not including the late-onset FGR group, it is not possible to differentiate lesions specific to early-onset FGR from those that may occur in both early- and late-onset cases. A comparative analysis with late-onset FGR could provide valuable insights into the distinct placental characteristics and lesions associated with each subtype.
Considering these limitations, it is important to interpret the findings within the context of the study’s scope and population, and further research involving larger and more diverse cohorts is warranted to validate and expand upon these findings.
Conclusions
Our study provides important insights into the placental lesions observed in patients with early-onset FGR, specifically highlighting the association with maternal malperfusion. The presence of maternal malperfusion-related lesions was consistently identified in these patients, indicating its significant role in the pathogenesis of early-onset FGR. Furthermore, our IHC analysis revealed a robust expression of VEGF in infarcted villi, suggesting its involvement in the vascular remodeling processes associated with placental dysfunction. The presence of infarctions, particularly late infarctions, was strongly associated with the occurrence of PE, indicating a potential link between these placental lesions and the development of PE in early-onset FGR cases. These findings contribute to our understanding of the underlying mechanisms and potential biomarkers involved in the pathogenesis of early-onset FGR. Further research is warranted to explore the clinical implications of these findings and develop targeted interventions for the management of early-onset FGR and its associated complications.
Conflict of interests
The authors declare that they have no conflict of interests
References
- 1.Chew LC, Verma RP. StatPearls . Treasure Island, FL, USA : StatPearls Publishing ; 2023 . Fetal growth restriction . [PubMed] [Google Scholar]
- 2.Melamed N, Baschat A, Yinon Y, Athanasiadis A, Mecacci F, Figueras F, Berghella V, Nazareth A, Tahlak M, McIntyre HD, Da Silva, Kihara AB, Hadar E, McAuliffe F, Hanson M, Ma RC, Gooden R, Sheiner E, Kapur A, Divakar H, Ayres-de-Campos D, Hiersch L, Poon LC, Kingdom J, Romero R, Hod M. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet. 2021;152(Suppl 1):3–57. doi: 10.1002/ijgo.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014;36(2):86–98. doi: 10.1159/000357592. [DOI] [PubMed] [Google Scholar]
- 4.Mifsud W, Sebire NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal Diagn Ther. 2014;36(2):117–128. doi: 10.1159/000359969. [DOI] [PubMed] [Google Scholar]
- 5.Krishna U, Bhalerao S. Placental insufficiency and fetal growth restriction. J Obstet Gynaecol India. 2011;61(5):505–511. doi: 10.1007/s13224-011-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardinger JE, Ambati S. StatPearls . Treasure Island, FL, USA : StatPearls Publishing ; 2022 . Placental insufficiency . [PubMed] [Google Scholar]
- 7.Mecacci F, Avagliano L, Lisi F, Clemenza S, Serena C, Vannuccini S, Rambaldi MP, Simeone S, Ottanelli S, Petraglia F. Fetal growth restriction: does an integrated maternal hemodynamic-placental model fit better. Reprod Sci. 2021;28(9):2422–2435. doi: 10.1007/s43032-020-00393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AEP, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PGJ, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, van der, van Lijnschoten, Gordijn SJ. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140(7):698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 9.Dahlstrøm B, Romundstad P, Øian P, Vatten LJ, Eskild A. Placenta weight in pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87(6):608–611. doi: 10.1080/00016340802056178. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens. 2012;2(2):72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi M, Makino S, Oguma K, Imai H, Takamizu A, Koizumi A, Yoshida K. Fetal growth restriction as the initial finding of preeclampsia is a clinical predictor of maternal and neonatal prognoses: a single-center retrospective study. BMC Pregnancy Childbirth. 2021;21(1):678–678. doi: 10.1186/s12884-021-04152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gathiram P, Moodley J. Pre-eclampsia: its pathogenesis and pathophysiology. Cardiovasc J Afr. 2016;27(2):71–78. doi: 10.5830/CVJA-2016-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spinillo A, Gardella B, Adamo L, Muscettola G, Fiandrino G, Cesari S. Pathologic placental lesions in early and late fetal growth restriction. Acta Obstet Gynecol Scand. 2019;98(12):1585–1594. doi: 10.1111/aogs.13699. [DOI] [PubMed] [Google Scholar]
- 14.Kovo M, Schreiber L, Elyashiv O, Ben-Haroush A, Abraham G, Bar J. Pregnancy outcome and placental findings in pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Reprod Sci. 2015;22(3):316–321. doi: 10.1177/1933719114542024. [DOI] [PubMed] [Google Scholar]
- 15.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2 Suppl):S745–S761. doi: 10.1016/j.ajog.2017.11.577. [DOI] [PubMed] [Google Scholar]
- 16.Figueras F, Caradeux J, Crispi F, Eixarch E, Peguero A, Gratacos E. Diagnosis and surveillance of late-onset fetal growth restriction. Am J Obstet Gynecol. 2018;218(2 Suppl):S790–S802. doi: 10.1016/j.ajog.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Tang L, He G, Liu X, Xu W. Progress in the understanding of the etiology and predictability of fetal growth restriction. Reproduction. 2017;153(6):R227–R240. doi: 10.1530/REP-16-0287. [DOI] [PubMed] [Google Scholar]
- 18.Szentpéteri I, Rab A, Kornya L, Kovács P, Joó JG. Gene expression patterns of vascular endothelial growth factor (VEGF-A) in human placenta from pregnancies with intrauterine growth restriction. J Matern Fetal Neonatal Med. 2013;26(10):984–989. doi: 10.3109/14767058.2013.766702. [DOI] [PubMed] [Google Scholar]