Abstract
Background
Studies investigating cognitive impairments in psychosis and depression have typically compared the average performance of the clinical group against healthy controls (HC), and do not report on the actual prevalence of cognitive impairments or strengths within these clinical groups. This information is essential so that clinical services can provide adequate resources to supporting cognitive functioning. Thus, we investigated this prevalence in individuals in the early course of psychosis or depression.
Methods
A comprehensive cognitive test battery comprising 12 tests was completed by 1286 individuals aged 15–41 (mean age 25.07, s.d. 5.88) from the PRONIA study at baseline: HC (N = 454), clinical high risk for psychosis (CHR; N = 270), recent-onset depression (ROD; N = 267), and recent-onset psychosis (ROP; N = 295). Z-scores were calculated to estimate the prevalence of moderate or severe deficits or strengths (>2 s.d. or 1–2 s.d. below or above HC, respectively) for each cognitive test.
Results
Impairment in at least two cognitive tests was as follows: ROP (88.3% moderately, 45.1% severely impaired), CHR (71.2% moderately, 22.4% severely impaired), ROD (61.6% moderately, 16.2% severely impaired). Across clinical groups, impairments were most prevalent in tests of working memory, processing speed, and verbal learning. Above average performance (>1 s.d.) in at least two tests was present for 40.5% ROD, 36.1% CHR, 16.1% ROP, and was >2 SDs in 1.8% ROD, 1.4% CHR, and 0% ROP.
Conclusions
These findings suggest that interventions should be tailored to the individual, with working memory, processing speed, and verbal learning likely to be important transdiagnostic targets.
Keywords: Mental health, psychosis, working memory, processing speed, verbal learning
Introduction
Cognitive impairments are a prominent feature of early-stage mental illnesses, particularly in full-threshold psychotic disorder (Fioravanti, Bianchi, & Cinti, 2012; Mesholam-Gately, Giuliano, Goff, Faraone, & Seidman, 2009), clinical high-risk (CHR) for psychosis (Catalan et al., 2021; Fusar-Poli et al., 2012; Pukrop et al., 2006), and major depressive disorder (MDD; Ahern & Semkovska, 2017; Goodall et al., 2018). Such impairments can significantly impact an individual's everyday functioning and long-term outcomes. Cognition has been found to be predictive of progression through the psychosis spectrum (Koutsouleris et al., 2011). Further, in a transdiagnostic sample of young people with mental illness, baseline cognition was the strongest predictor of two-year functional outcomes (Lee et al., 2013). While the literature to date has clearly demonstrated a meaningful link between mental health difficulties and cognitive impairments, studies have largely taken a group-level approach to analysis. Such studies have shown that individuals with mental illnesses, on average, perform more poorly than healthy controls (HC) in multiple cognitive domains (East-Richard, R-Mercier, Nadeau, & Cellard, 2020). However, statistically significant differences at a group level cannot necessarily equal a clinically meaningful impairment (Abramovitch & Schweiger, 2015; Abramovitch, Short, & Schweiger, 2021; Michel, Ruhrmann, Schimmelmann, Klosterkötter, & Schultze-Lutter, 2014). At present there is no universally agreed definition of a clinically meaningful cognitive impairment (Abramovitch et al., 2021). However, by examining the amount to which individual performance deviates from the average (standard deviation difference), we can obtain a clearer picture of how common moderate or severe cognitive difficulties may be within early intervention services, and who may require further cognitive assessment or treatment.
Clinical guidelines for early psychosis (e.g. Early Psychosis Guidelines Working Group, 2016; NICE, 2014) and depression (e.g. Malhi et al., 2021; National Institute for Clinical Excellence, 2009) recommend an assessment of cognition and remediation therapies where cognition may be impairing functional recovery. Thus, it is important to estimate the prevalence of people presenting to services with impaired cognition who may need to access those additional services. This would allow for more adequate resourcing of clinical services and could inform relevant training for clinicians. Furthermore, because there may be a variation in the cognitive strengths or impairments in people presenting to services, it is important to screen cognition at service entry (Bryce & Allott, 2019; Bryce, Bowden, Wood, & Allott, 2021), and consider the cognitive profile of individuals who are in the early course of mental illness. Cognitive impairments are often present long before the onset of clinical symptoms (e.g. Mollon, David, Zammit, Lewis, & Reichenberg, 2018), and may also show further decline following the first-episode of psychosis (Fett et al., 2020; Flaaten et al., 2022), or with multiple depressive episodes (Allott, Fisher, Amminger, Goodall, & Hetrick, 2016; Semkovska et al., 2019). Thus, understanding the profile of cognitive performance in the early course of illness will allow clinicians to intervene early to preserve intact cognitive skills or prevent further decline (Pantelis, Wannan, Bartholomeusz, Allott, & McGorry, 2015), providing the best possible chance of functional recovery.
The idea that a subgroup of individuals with schizophrenia may be ‘neuropsychologically normal’ has been previously discussed (Keefe, 2008; Wilk et al., 2005), and some more recent studies have used data-driven approaches, which identified clusters of relatively spared v. impaired cognitive performance in early psychosis (Gould et al., 2014; Uren, Cotton, Killackey, Saling, & Allott, 2017; Wenzel et al., 2021). Few studies have examined the prevalence of cognitive impairments in this population. Two previous reports estimated that 70–80% of individuals with chronic schizophrenia demonstrate cognitive impairments (Allen, Goldstein, & Warnick, 2003; Palmer et al., 1997). Less is known about the prevalence of cognitive impairments in the early stages of the illness. In a sample of Ugandan inpatients with first-episode psychosis, 62% presented with a cognitive impairment, defined as mean scores of >2 SDs below HC in one domain, or of >1 SDs below HC in two or more domains (Mwesiga et al., 2022). Furthermore, to the best of our knowledge there are no previous studies which have investigated the prevalence of cognitive impairments in CHR.
The prevalence of cognitive impairments in MDD has been investigated in a few studies. Gualtieri and Morgan (2008) observed that up to 32% of their treated MDD sample had an impairment of 1–2 SDs relative to HC in at least one cognitive domain, which was >2 SDs in up to 18%. When cognitive impairment is defined as being >1 s.d. below HC in at least two cognitive tests, Douglas et al. (2018) observed a prevalence of 78.6% in their outpatient MDD sample, which was >2 SDs below HC in 14.3% of the sample. They also observed that their inpatient depression group had higher rates of impairment, at 91.4% (>1 s.d. in two tests) and 32.8% (>2 SDs in two tests). Tran, Milanovic, Holshausen, and Bowie (2021) also investigated the prevalence of impairment relative to the individuals' estimated premorbid performance in a community sample of individuals with MDD. They identified that 62.2% of the sample were performing at least 1 s.d. below their estimated premorbid performance. Finally, some studies have calculated the prevalence of a composite cognitive impairment (the average of cognitive test scores). The prevalence of composite impairment in outpatient MDD samples has ranged from 11.8% (Douglas et al., 2018) to 25.2% (Tran et al., 2021) at a level of >1 s.d. relative to HC, and has been observed in 1.5% of an outpatient MDD sample at >2 SDs relative to HC (Douglas et al., 2018). Thus, while rates of impairment appear high in adults with established MDD, less is known about the prevalence of cognitive impairment in younger recent-onset samples.
Beside understanding the prevalence of cognitive impairments during the early stages of serious mental illnesses, it is equally important to consider the prevalence of cognitive strengths. Current approaches to treatment of cognition often focus on the remediation of deficits. However, reinforcing and building on strengths could be useful as an adjunct to such remediation (Allott et al., 2020), to prevent further deterioration (Pantelis et al., 2015), and to leverage important psychological factors for the treatment process such as self-esteem and motivation (Allott et al., 2020). By understanding the prevalence of cognitive strengths, we can provide clinicians and services with a more comprehensive picture of cognition during the first-episode, which could be used to inform service delivery and training. To our knowledge, no study has yet examined the prevalence of cognitive strengths in mental illness.
In summary, the studies conducted to date have demonstrated that there is a significant association between serious mental disorders and cognitive impairment at the group level. Further, individuals with psychosis or depression may exhibit poorer performance on cognitive tasks than they would have if they had never developed the illness (Keefe, Eesley, & Poe, 2005; Tran et al., 2021). However, few studies have reported the prevalence of cognitive impairments in the earliest stages of mental illness, and no studies have reported the prevalence of cognitive strengths. We aimed to report the prevalence of clinically meaningful levels of cognitive strengths or impairments in a multi-diagnostic sample of individuals diagnosed with recent-onset psychosis (ROP) or depression, or identified at clinical high-risk for psychosis. We categorised cognitive test performance at various levels ranging from >2 SDs above average to >2 SDs below average. We defined impairments and strengths as both moderate (1–2 SDs) and extreme (>2 SDs) in two or more tests, as well as report a composite cognitive score across these levels. This definition allows for comparison with previous literature (e.g. Douglas et al., 2018; Tran et al., 2021), and, in the case of impairment, highlights that which might require clinical attention.
Methods
Participants
This sample comprised 1286 individuals aged 15–41 who were recruited into the multi-site ‘Personalised Prognostic Tools for Early Psychosis Management’ (PRONIA; https://www.pronia.eu) study and completed a cognitive test battery at baseline. Although the maximum age for inclusion was 40, one participant in the HC group was assessed on the date of their 41st birthday and retained in analysis. Full details on the methods and recruitment of the PRONIA study are provided in Koutsouleris et al. (2018). In summary, participants were recruited from ten international sites (Germany: Munich, Cologne, Münster, and Düsseldorf; UK: Birmingham; Italy: Udine, Bari, and Milan; Finland: Turku; Switzerland: Basel) if they met criteria for ROP, recent-onset depression (ROD), or clinical high risk for psychosis (CHR), or for HC with no personal or family history of mental illness. Inclusion and exclusion criteria for each study group are summarised in online Supplementary Table S1. All participants provided their written consent (or assent for participants aged <18) after having received all study information. All sites gained approval from their respective ethics committees.
Procedure
At baseline, participants were screened for inclusion and then completed the cognitive test battery and demographic measures including age, sex, education, and ethnicity. Cognitive tests were completed in a standardised order and in the native language of each site.
Measures
The cognitive test battery comprised the following tests and primary outcome scores (also see online Supplementary Material, ‘Description of Cognitive Tasks’ and online Supplementary Table S2): Trail Making Test (TMT) parts A & B, total reaction time (Army Individual Test Battery, 1944); phonetic (PVF) and semantic (SVF) verbal fluency, total number of correct words (Benton, Hamsher, & Sivan, 1994); Continuous Performance Task (CPT), d-Prime Sensitivity Index (Cornblatt, Risch, Faris, Friedman, & Erlenmeyer-Kimling, 1988); Rey Auditory Verbal Learning Test (RAVLT), sum of trials 1–5 (Rey, 1964); Rey-Osterrieth Complex Figure (ROCF), total score at immediate recall (Osterrieth, 1944; Rey, 1941); Self-Ordered Pointing Task (SOPT), total errors (Petrides & Milner, 1982); Auditory Digit Span task, number of correct trials forwards (FDS) and backwards (BDS) (Wechsler, 1939); Digit-Symbol Substitution Test (DSST), total score of number correct subtracted by number of errors (Copyright free version, component of the Wechsler Adult Intelligence Scale-Revised; Wechsler, 1981); Diagnostic Analysis of Non-Verbal Accuracy (DANVA), total number of correct faces identified (Nowicki & Duke, 1994); and the vocabulary and matrix reasoning subscales of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 2011). These tests were chosen to represent a comprehensive evaluation of the participant's cognitive functioning. Results were reported according to individual test scores to facilitate use of the findings for clinicians. Clinicians may use different cognitive test batteries in practice and thus, by reporting individual tests they will be able to directly check the expected prevalence of strength or impairment in a clinical population, relative to controls, on a particular test.
Statistical analysis
Statistical analysis was completed using IBM SPSS Statistics 25 software (IBM Corp, 2017). Group differences on demographic variables were examined using one-way ANOVA for continuous variables, or chi-squared for categorical variables. Post-hoc analysis then determined the direction of any significant group effects, using Tamhane or Bonferroni adjustment. The primary outcome scores for each cognitive test were chosen, with any ‘reaction time’ or ‘error’ scores reversed by subtracting the individual raw score from the highest raw score on that test, so that higher scores always indicated better performance. Following this, z-scores based on the mean and standard deviation (s.d.) of the HC study group were calculated using the formula: (raw score – HC mean) / HC s.d.. A ‘composite’ cognitive score was computed for each individual as the average of all twelve z-scores. Missing data values were small in this dataset, particularly in eleven of the twelve cognitive tasks (0.7–5.4%). However, more data was missing on the RAVLT (12.8%), as an alternative measure was used by the Finnish site, and those Finnish data were not used in the current study. The prevalence of various levels of cognitive impairment or strength was then investigated by allocating the proportion of z-scores for each cognitive test into the following categories: >2 SDs below HC (severely impaired); 1–2 SDs below HC (moderately impaired); ‘average performance’, meaning that performance was within 1 s.d. relative HC; 1–2 SDs above HC (above average), and >2 SDs above HC (extremely high). Chi-square analysis examined whether there was a significantly different proportion of the study groups meeting each level of cognitive performance. Post-hoc comparisons (with Bonferroni correction for multiple comparisons) were conducted to examine any group differences in each level of cognitive performance for each individual test. For each test and the composite score, we also calculated the odds ratio of each clinical group demonstrating a cognitive impairment (anything >1 s.d.) relative to HC and the 95% confidence intervals of the odds ratio, using the formula proposed by Altman (1990). Next, we examined the proportion of each study group showing moderate or severe cognitive impairment, or above average performance on a given number of tests. Cognitive impairment (or strength) was reported at the level of both 1–2 and >2 SDs relative to HC in at least two tests, or in the composite score. This definition was chosen to facilitate comparison with previous research (e.g. Douglas et al., 2018; Tran et al., 2021). For each participant group, the mean number of tests impaired or above average at both levels was also calculated.
Results
The characteristics of the sample are reported in Table 1. The four study groups significantly differed on age, sex, site, IQ estimate, years of education, and ethnicity. Post-hoc examinations of these group differences are shown in Table 1. Mean raw cognitive scores for each study group are presented in online Supplementary Table S3.
Table 1.
Characteristics of the sample
| Whole sample (N = 1286) | HC (N = 454) | ROD (N = 267) | CHR (N = 270) | ROP (N = 295) | Statistic | p value | Post-hoc | |
|---|---|---|---|---|---|---|---|---|
| Age | CHR<HC, p = 0.003* CHR<ROD, p = 0.001* CHR<ROP, p < 0.001* |
|||||||
| Mean (s.d.) | 25.07 (5.88) | 25.17 (5.99) | 26.64 (6.13) | 23.67 (5.26) | 25.67 (5.84) | F = 6.939 | <0.001* | |
| Range | 15–41a | 15–41a | 15–40 | 15–40 | 15–40 | |||
| Gender | ROP>HC (Male), p < 0.001* ROP>CHR (Male), p = 0.032* | |||||||
| Male % | 47.5 | 40.6 | 49.2 | 46.1 | 57.8 | χ2 = 21.771 | <0.001* | |
| Estimated Current IQ | HC>CHR, p < 0.001* HC>ROD, p < 0.001* HC>ROP, p < 0.001* CHR>ROP, p < 0.001* ROD>ROP, p < 0.001* |
|||||||
| Mean (s.d.) | 105.58 (12.09) | 110.00 (10.03) | 105.54 (11.83) | 105.46 (10.99) | 98.92 (13.10) | F = 56.655 | <0.001* | |
| Education Years | HC>CHR, p < 0.001* HC>ROD, p < 0.001* HC>ROP, p < 0.001* ROD>CHR, p = 0.014* |
|||||||
| Mean (s.d.) | 14.60 (3.12) | 15.71 (3.09) | 14.35 (3.06) | 13.60 (2.55) | 14.00 (3.20) | F = 34.339 | <0.001* | |
| Ethnicity | ||||||||
| N (%) | χ2 = 43.591 | <0.001* | ROD>ROP (White), p = 0.004* | |||||
| White | 1067 (83.0) | 381 (83.9) | 235 (88.0) | 226 (83.7) | 225 (76.3) | HC>ROD (Asian), p = 0.001* | ||
| Asian | 72 (5.6) | 39 (8.6) | 4 (1.5) | 11 (4.1) | 18 (6.1) | ROP>ROD (Asian), p = 0.026* | ||
| Black | 26 (2.0) | 6 (1.3) | 3 (1.1) | 4 (1.5) | 13 (4.4) | ROP>HC (Black), p = 0.041* | ||
| Mixed race | 28 (2.2) | 14 (3.1) | 3 (1.1) | 4 (1.5) | 7 (2.4) | CHR>HC (Other), p = 0.045* | ||
| Other | 62 (4.8) | 10 (2.2) | 16 (6.0) | 16 (5.9) | 20 (6.8) | ROD>HC (Other), p = 0.045* | ||
| Unknown | 31 (2.4) | 4 (0.9) | 6 (2.2) | 9 (3.3) | 12 (4.1) | ROP>HC(Other), p = 0.008* |
N, Number of Participants; s.d., Standard Deviation; F, one-way ANOVA; χ2, Chi-squared; *, Statistically significant at a level of p⩽0.05.
Note: One participant in the HC group was assessed on the date of their 41st birthday and retained in analysis.
Prevalence of cognitive strengths and impairment
The percentages of each study group demonstrating each level of cognitive performance are presented in Table 2. Figures 1a and b show the prevalence of any level of impairment (e.g. anything >1 s.d. below HC) and strengths (e.g. anything >1 s.d. above HC). Though the focus of this paper was only to report the prevalence of cognitive impairments and strengths, we also examined between-group differences. Chi-square analysis showed that there was a significant difference in the proportion of participants in each study group demonstrating each level of cognitive impairment in all cognitive tests. Post-hoc analysis of significant between group differences is presented in online Supplementary Table S6. Table 2 shows the odds ratio and 95% confidence interval for that odds ratio, showing how much higher the odds of a cognitive impairment (>1 s.d. below HC) are for the clinical group compared to HC.
Table 2.
Percentage of participants in each group meeting the criteria for each level of cognitive impairment or strengths per cognitive test
| Cognitive test (N) | HC | ROD | CHR | ROP | χ2 (df) | p value | Odds Ratio | 95% CI for Odds Ratio |
|---|---|---|---|---|---|---|---|---|
| TMT A | (433) | (248) | (256) | (281) | χ2 = 49.943 | <0.001* | ROD- 1.50 | ROD-1.01–2.23 |
| >2 SDs Above | 0 | 0.4 | 0 | 0 | (12) | CHR-1.91 | CHR-1.31–2.79 | |
| 1–2 SDs Above | 14.1 | 10.9 | 9 | 6.4 | ROP- 2.78 | ROP-1.95–3.97 | ||
| No impairment | 70 | 66.5 | 64.5 | 59.1 | ||||
| 1–2 SDs Below | 11.1 | 12.1 | 12.9 | 17.4 | ||||
| >2 SDs Below | 4.8 | 10.1 | 13.7 | 17.1 | ||||
| TMT B | (435) | (247) | (255) | (280) | χ2 = 101.057 | <0.001* | ROD- 1.67 | ROD-1.09–2.55 |
| >2 SDs Above | 0 | 0 | 0.4 | 0 | (12) | CHR- 2.22 | CHR-1.48–3.32 | |
| 1–2 SDs Above | 10.6 | 6.1 | 3.9 | 1.8 | ROP- 4.68 | ROP-3.23–6.77 | ||
| No impairment | 76.8 | 74.5 | 71.4 | 57.9 | ||||
| 1–2 SDs Below | 8.5 | 8.5 | 11.8 | 19.6 | ||||
| >2 SDs Below | 4.1 | 10.9 | 12.5 | 20.7 | ||||
| Phonetic verbal fluency | (450) | (264) | (266) | (288) | χ2 = 70.502 | <0.001* | ROD- 2 | ROD-1.35–2.96 |
| >2 SDs Above | 4.2 | 1.5 | 2.3 | 1 | (12) | CHR- 1.81 | CHR-1.22–2.70 | |
| 1–2 SDs Above | 12.9 | 8.3 | 10.5 | 4.9 | ROP- 3.35 | ROP-2.33–4.83 | ||
| No impairment | 69.6 | 66.7 | 65.4 | 60.1 | ||||
| 1–2 SDs Below | 12 | 21.6 | 21.1 | 27.8 | ||||
| >2 SDs Below | 1.3 | 1.9 | 0.8 | 6.3 | ||||
| Semantic verbal fluency | (451) | (264) | (266) | (287) | χ2 = 127.906 | <0.001* | ROD- 2.14 | ROD-1.47–3.13 |
| >2 SDs Above | 2.7 | 2.3 | 1.5 | 0.3 | (12) | CHR- 2.51 | CHR-1.73–3.64 | |
| 1–2 SDs Above | 12.2 | 9.8 | 5.6 | 3.1 | ROP- 5.66 | ROP-3.98–8.03 | ||
| No impairment | 70.7 | 61.4 | 63.2 | 47.7 | ||||
| 1–2 SDs Below | 12 | 20.1 | 24.8 | 33.1 | ||||
| >2 SDs Below | 2.4 | 6.4 | 4.9 | 15.7 | ||||
| CPT d’ | (451) | (263) | (266) | (288) | χ2 = 96.778 | <0.001* | ROD- 1.74 | ROD-1.17–2.59 |
| >2 SDs Above | 2.2 | 4.6 | 3.4 | 0 | CHR- 2.12 | CHR-1.44–3.10 | ||
| 1–2 SDs Above | 13.7 | 9.5 | 7.9 | 5.2 | (12) | ROP- 4.04 | ROP-2.83–5.76 | |
| No impairment | 70.1 | 63.9 | 63.2 | 55.2 | ||||
| 1–2 SDs Below | 12.4 | 17.9 | 24.4 | 30.9 | ||||
| >2 SDs Below | 1.6 | 4.2 | 1.1 | 8.7 | ||||
| RAVLT Trials 1–5 | (389) | (245) | (238) | (249) | χ2 = 123.299 | <0.001* | ROD- 1.69 | ROD-1.14–2.50 |
| >2 SDs Above | 0 | 0 | 0 | 0 | (9) | CHR- 2.21 | CHR-1.50–3.24 | |
| 1–2 SDs Above | 14.4 | 10.6 | 8 | 6 | ROP- 5.72 | ROP-3.97–8.23 | ||
| No impairment | 68.9 | 64.1 | 61.3 | 40.6 | ||||
| 1–2 SDs Below | 12.3 | 15.9 | 20.6 | 25.7 | ||||
| >2 SDs Below | 4.4 | 9.4 | 10.1 | 27.7 | ||||
| ROCF Immediate memory | (452) | (266) | (265) | (288) | χ2 = 88.896 | <0.001* | ROD- 1.55 | ROD-1.05–2.28 |
| >2 SDs Above | 0 | 0 | 0 | 0 | (9) | CHR- 1.80 | CHR-1.23–2.64 | |
| 1–2 SDs Above | 17 | 15.4 | 12.1 | 7.3 | ROP- 3.91 | ROP-2.76–5.53 | ||
| No impairment | 67.7 | 62.8 | 63.4 | 51.4 | ||||
| 1–2 SDs Below | 11.7 | 14.7 | 12.1 | 20.1 | ||||
| >2 SDs Below | 3.5 | 7.1 | 12.5 | 21.2 | ||||
| SOPT total errors | (449) | (263) | (267) | (286) | χ2 = 100.049 | <0.001* | ROD-1.75 | ROD-1.21–2.53 |
| >2 SDs Above | 0 | 0 | 0 | 0 | (9) | CHR- 1.58 | CHR-1.09–2.29 | |
| 1–2 SDs Above | 17.8 | 13.3 | 12 | 6.6 | ROP- 3.98 | ROP-2.83–5.58 | ||
| No impairment | 65.5 | 6.5 | 63.7 | 48.6 | ||||
| 1–2 SDs Below | 12.5 | 19.4 | 14.2 | 21.7 | ||||
| >2 SDs Below | 4.5 | 6.8 | 10.1 | 23.1 | ||||
| Forward digit span | (452) | (263) | (268) | (292) | χ2 = 62.584 | <0.001* | ROD- 2.02 | ROD-1.43–2.85 |
| >2 SDs Above | 3.3 | 1.9 | 3.7 | 1.4 | (12) | CHR- 1.41 | CHR-0.99–2.02 | |
| 1–2 SDs Above | 17.7 | 11.8 | 12.7 | 4.8 | ROP- 2.43 | ROP-1.74–3.38 | ||
| No impairment | 59.3 | 53.2 | 57.8 | 56.5 | ||||
| 1–2 SDs Below | 17.5 | 25.9 | 19 | 26 | ||||
| >2 SDs Below | 2.2 | 7.2 | 6.7 | 11.3 | ||||
| Backward digit span | (451) | (263) | (269) | (292) | χ2 = 126.958 | <0.001* | ROD- 1.85 | ROD-1.32–2.58 |
| >2 SDs Above | 0 | 0 | 0 | 0 | (9) | CHR- 2.03 | CHR-1.46–2.83 | |
| 1–2 SDs Above | 25.9 | 18.6 | 14.9 | 5.8 | ROP- 4.71 | ROP-3.42–6.48 | ||
| No impairment | 51.2 | 46 | 47.6 | 36 | ||||
| 1–2 SDs Below | 22.4 | 35 | 37.2 | 54.1 | ||||
| >2 SDs Below | 0.4 | 0.4 | 0.4 | 4.1 | ||||
| DSST Total score | (453) | (264) | (265) | (291) | χ2 = 271.929 | <0.001* | ROD- 2.32 | ROD-1.59–3.39 |
| >2 SDs Above | 2 | 1.9 | 1.5 | 0 | (12) | CHR- 3.29 | CHR-2.28–4.75 | |
| 1–2 SDs Above | 12.1 | 9.5 | 4.9 | 1 | ROP- 10.34 | ROP-7.24–14.77 | ||
| No impairment | 72 | 61.4 | 58.9 | 36.4 | ||||
| 1–2 SDs Below | 13 | 23.5 | 28.7 | 36.1 | ||||
| >2 SDs Below | 0.9 | 3.8 | 6 | 26.5 | ||||
| DANVA Total correct | (453) | (264) | (269) | (291) | χ2 = 64.349 | <0.001* | ROD- 0.94 | ROD-0.63–1.40 |
| >2 SDs Above | 0.9 | 1.9 | 0.4 | 0 | (12) | CHR- 1.36 | CHR-0.94–1.97 | |
| 1–2 SDs Above | 17 | 9.8 | 11.9 | 9.6 | ROP- 2.19 | ROP-1.56–3.09 | ||
| No impairment | 63.8 | 70.8 | 64.3 | 57.4 | ||||
| 1–2 SDs Below | 13.5 | 12.9 | 16 | 15.5 | ||||
| >2 SDs Below | 4.9 | 4.5 | 7.4 | 17.5 | ||||
| Composite scorea | (363) | (227) | (219) | (230) | χ2 = 138.974 | <0.001* | ROD- 2.98 | ROD-1.6–5.56 |
| >2 SDs Above | 0 | 0 | 0 | 0 | (9) | CHR- 3.74 | CHR-2.03–6.88 | |
| 1–2 SDs Above | 1.1 | 0.9 | 0.5 | 0 | ROP- 12.61 | ROP-7.24–21.97 | ||
| No impairment | 94.2 | 86.3 | 84 | 61.7 | ||||
| 1–2 SDs Below | 4.7 | 11 | 14.6 | 27.8 | ||||
| >2 SDs Below | 0 | 1.8 | 0.9 | 10.4 |
χ2, Chi-squared examining differences in the proportion of each study group at each level of performance; *, Statistically significant at a level of p ⩽ 0.05; Odds Ratio, The odds of the clinical group having any impairment >1 s.d., relative to HC; CI, Confidence Interval.
Calculated only for participants who completed all tests.
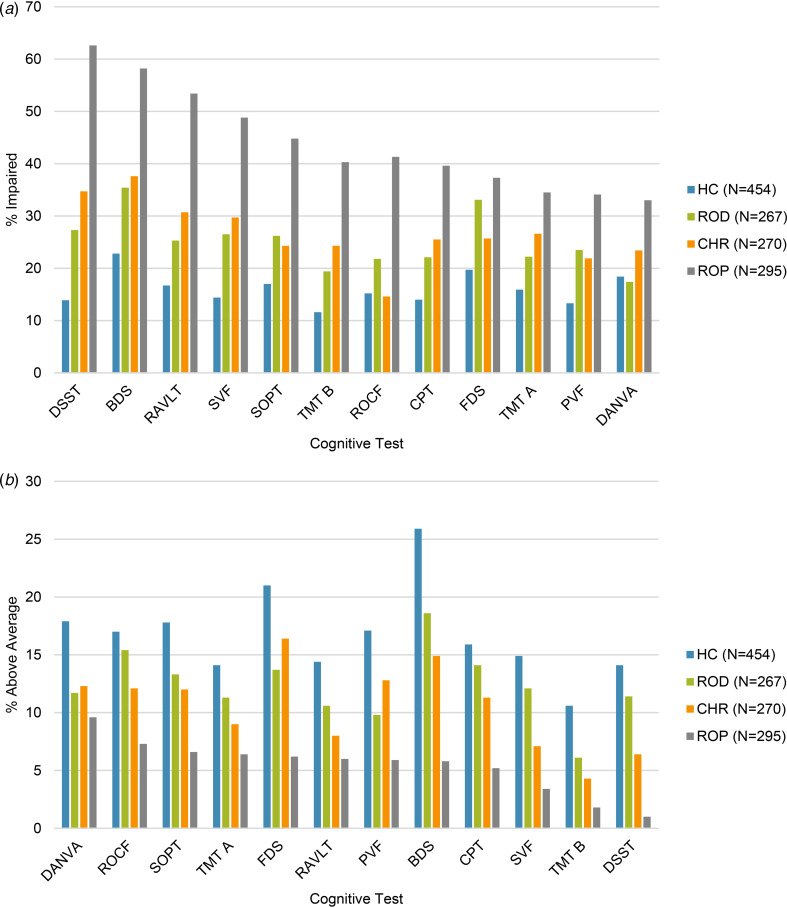
Figure 1.
Prevalence of impairments (a); >1 s.d. below average and strengths (b); >1 s.d. above average per group in the individual cognitive tests.
Note: Sample sizes provided are of the whole sample. Individual sample sizes for each test can be found in Table 2. Tests are presented in order from highest to lowest prevalence in the ROP group.
Next, we examined the proportion of each study group showing moderate or severe cognitive impairment on a given number of tests (Table 3). We observed that 88.3% of the ROP group were moderately impaired (1–2 SDs below HC) on at least two tests, and 45.1% showed severe impairment (>2 SDs below HC) on at least two tests i In the CHR group, 71.2% were moderately impaired on at least two tests, and 22.4% were severely impaired on at least two tests. In the ROD group, 61.6% were moderately impaired on at least two cognitive tests, and 16.2% were severely impaired on at least two tests.
Table 3.
The percentage of each group demonstrating moderate or severe impairment, above average, or extremely high performance on a given number of cognitive tests
| HC | ROD | CHR | ROP | χ2 (df) | p value | |
|---|---|---|---|---|---|---|
| Number of tests moderately impaired (1–2 SDs Below) | χ2 = 192.472 (15) | <0.001* | ||||
| 0 | 25.6 | 19.8 | 9.1 | 4.3 | ||
| 1 | 26.2 | 18.5 | 19.6 | 7.4 | ||
| 2–3 | 27.5 | 31.3 | 31.5 | 22.2 | ||
| 4–5 | 12.5 | 12.7 | 20.5 | 21.8 | ||
| 6–8 | 6.9 | 9.7 | 15.1 | 25.2 | ||
| 9–12 | 1.7 | 7.9 | 4.1 | 19.1 | ||
| % Moderately Impaired on Two or More Tests | 48.6 | 61.6 | 71.2 | 88.3 | ||
| Number of Tests Severely Impaired (>2 SDs Below) | χ2 = 175.494 (15) | <0.001* | ||||
| 0 | 75.8 | 66.1 | 55.7 | 34.3 | ||
| 1 | 17.6 | 17.6 | 21.9 | 20.4 | ||
| 2–3 | 5.8 | 11 | 16.4 | 23.5 | ||
| 4–5 | 0.9 | 3.5 | 5 | 11.3 | ||
| 6–8 | 0 | 1.7 | 0.5 | 9.1 | ||
| 9–12 | 0 | 0 | 0.5 | 1.2 | ||
| % Severely Impaired on Two or More Tests | 6.7 | 16.2 | 22.4 | 45.1 | ||
| Number of Tests Above Average (1–2 SDs Above) | (N = 363) | (N = 227) | (N = 219) | (N = 230) | χ2 = 108.104 (15) | <0.001* |
| 0 | 27.5 | 36.1 | 42.9 | 60.0 | ||
| 1 | 20.1 | 23.3 | 21 | 23.9 | ||
| 2–3 | 31.4 | 29.1 | 26.1 | 13.9 | ||
| 4–5 | 15.7 | 8.3 | 7.3 | 1.3 | ||
| 6–8 | 5.3 | 2.2 | 2.8 | 0.9 | ||
| 9–12 | 0 | 0.8 | 0 | 0 | ||
| % Above Average on Two or More Tests | 52.3 | 40.5 | 36.1 | 16.1 | ||
| Number of Tests Extremely High (>2 SDs Above) | (N = 363) | (N = 227) | (N = 219) | (N = 230) | χ2 = 19.083 (6) | 0.004* |
| 0 | 87.3 | 86.3 | 88.6 | 97.0 | ||
| 1 | 10.5 | 11.9 | 10 | 3 | ||
| 2–3 | 2.2 | 1.8 | 1.4 | 0 | ||
| 4–5 | 0 | 0 | 0 | 0 | ||
| 6–8 | 0 | 0 | 0 | 0 | ||
| 9–12 | 0 | 0 | 0 | 0 | ||
| % Extremely High on Two or More Tests | 2.2 | 1.8 | 1.4 | 0 |
χ2, Chi-squared; *, Statistically significant at a level of p ⩽ 0.05.
Regarding above average performance, 16.1% of the ROP group were above average (1–2 SDs above HC) on at least two tests, but no participant in the ROP group performed at the ‘extremely high’ level (>2 SDs above HC) on at least two tests. In the CHR group, 36.1% were above average on at least two tests, and 1.4% were extremely high on at least two tests. In the ROD group, 40.5% were above average on at least two cognitive tests, and 1.8% were extremely high on at least two cognitive tests.
Finally, the average number of impaired test performances at a moderate (1–2 SDs below) or severe (>2 SDs below) level was calculated per group (Table 4). One-way ANOVA demonstrated a significant difference between the groups in the number of tests moderately (F(3, 1035) = 72.810, p < 0.001), and severely (F(3, 1035) = 61.705, p < 0.001) impaired, as well as above average (F(3, 1035) = 31.708, p < 0.001) and extremely high (F(3, 1035) = 6.113, p < 0.001). Post-hoc examinations are presented in Table 4.
Table 4.
Average number of tests that each study group is impaired on
| HC | ROD | CHR | ROP | F | p | Tamhane Post Hoc | CI | |
|---|---|---|---|---|---|---|---|---|
| Number of tests moderately impaired | HC<ROD, p < 0.001* HC<CHR, p < 0.001* HC<ROP, p < 0.001* CHR<ROP, p < 0.001* ROD<ROP, p < 0.001* |
−1.50 to −0.34 −1.79 to −0.72 −3.90 to −2.63 −2.73 to −1.29 −3.09 to −1.59 |
||||||
| Mean (s.d.) | 2.04 (2.11) | 2.96 (2.84) | 3.29 (2.51) | 5.3 (3.22) | 72.810 | <0.001* | ||
| Min-Max | 0–10 | 0–12 | 0–12 | 0–12 | ||||
| Number of tests severely impaired | HC<ROD, p = 0.001* HC<CHR, p < 0.001* HC<ROP, p < 0.001* CHR<ROP, p < 0.001* ROD<ROP, p < 0.001* |
−0.65 to −0.11 −0.83 to −0.29 −2.11 to −1.27 −1.62 to −0.65 −1.79 to −0.84 |
||||||
| Mean (s.d.) | 0.35 (0.74) | 0.73 (1.4) | 0.91 (1.4) | 2.04 (2.34) | 61.705 | <0.001* | ||
| Min-Max | 0–5 | 0–8 | 0–9 | 0–11 | ||||
| Number of tests above average | HC>ROD, p = 0.022* HC>CHR, p < 0.001* HC>ROP, p < 0.001* CHR>ROP, p < 0.001* ROD>ROP, p < 0.001* |
0.04–0.85 0.29–1.06 1.01–1.65 0.32–0.99 0.53–1.24 |
||||||
| Mean (s.d.) | 2.00 (1.90) | 1.55 (1.75) | 1.32 (1.55) | 0.67 (1.06) | 31.708 | <0.001* | ||
| Min-Max | 0–8 | 0–10 | 0–7 | 0–6 | ||||
| Number of tests extremely high | HC>ROP, p < 0.001* CHR>ROP, p = 0.003* ROD>ROP, p < 0.001* |
0.05–0.18 0.02–0.17 0.05–0.20 |
||||||
| Mean (s.d.) | 0.15 (0.41) | 0.15 (0.41) | 0.13 (0.37) | 0.03 (0.17) | 6.113 | <0.001* | ||
| Min-Max | 0–2 | 0–2 | 0–2 | 0–1 |
*, Statistically significant at a level of p ⩽ 0.05; CI, 95% Confidence Intervals for Mean Difference.
Discussion
This study investigated the prevalence of cognitive impairments and strengths in a sample of individuals in the early stages of psychosis or depression. While previous studies have largely examined cognitive impairment in mental illness using group-level means, we examined the proportion of individuals in each group who were demonstrating various levels of performance from extremely high to severely impaired. Overall, the findings of this study are in line with the previous literature, in that a larger proportion of the clinical groups demonstrated both moderate and severe cognitive impairment than HC. However, even within the clinical groups, a subset of participants demonstrated unimpaired cognitive performance at the individual test level, and a small proportion demonstrated above average performance.
In this sample, cognitive impairment was most prevalent in the ROP group. Previous reports have estimated that 70–80% of individuals with chronic schizophrenia (Allen et al., 2003; Palmer et al., 1997), and 62% of individuals with first-episode psychosis (Mwesiga et al., 2022), will demonstrate cognitive impairment. We observed that 88.3% of the ROP group were moderately impaired (1–2 SDs below HC) in at least two tests, and 45.1% were severely impaired (>2 SDs below HC) in at least two tests, which is a slightly higher prevalence than those previous estimates. When an average composite cognitive score was calculated, 27.8% of the ROP group were demonstrating a global cognitive impairment of 1–2 SDs below HC, and severe impairment of >2 SDs below HC in 10.4% of the sample. We also saw a much higher proportion of the present ROP group showing significant, and widespread impairment on multiple tests when compared to the other study groups. Further, our ROP group demonstrated a significantly higher average number of tests impaired at both a moderate (5.3) and severely (2.04) impaired level than all other clinical groups.
We observed that 61.6% of the ROD group were moderately (1–2 SDs below HC) impaired in at least two cognitive tests, and 16.2% were severely (>2 SDs below HC) impaired in at least two cognitive tests. When an average composite cognitive score was calculated, 12.8% of the ROD group were demonstrating a moderate cognitive impairment (1–2 SDs below HC), and severe impairment (>2 SDs below HC) was seen in 1.8% of the sample. The prevalence of impairment in at least two tests in this sample is broadly comparable to the outpatient sample of Douglas et al. (2018). They observed that 78% were moderately impaired, and 14.3% were severely impaired, according to these criteria. Furthermore, the composite impairments of 11.8% <1 s.d. and 1.5% <2 SDs in that study are comparable to the present findings. Tran et al. (2021) observed a slightly higher prevalence of global impairment <1 s.d. in 25.2% of their sample of outpatients with MDD. Their sample was slightly older than the current sample (mean age 48.6 compared to 26.6 in this ROD group), and there was no restriction to first-episode MDD. Thus, it is possible that their sample may have experienced some further cognitive decline which can be associated with repeated or prolonged depressive episodes (Allott et al., 2016; Semkovska et al., 2019).
The current CHR group demonstrated a prevalence of cognitive impairment which was comparable, but less severe, than that seen in the ROP group; 71.2% were moderately impaired in at least two tests, and 22.4% were severely impaired in at least two tests. In the composite cognitive score, 14.6% of the CHR group were demonstrating an impairment of 1–2 SDs below HC, whereas 0.9% of the sample were severe (>2 SDs below HC). To the best of our knowledge, this is the first study to investigate the prevalence of cognitive impairment in individuals at CHR for psychosis. Nevertheless, these observations align with the previous literature demonstrating that cognitive impairments can begin well before the first-episode of psychosis (Catalan et al., 2021; Fusar-Poli et al., 2012). These findings also suggest that almost three quarters of CHR populations may be experiencing moderate deficits, and almost one quarter may already be experiencing severe deficits and require cognitive interventions by the time they seek help.
Current findings can also be compared with data-driven approaches, which have broadly identified either two (Amoretti et al., 2021; Wenzel et al., 2021) or three (Uren et al., 2017) distinct clusters of cognitive performance in individuals with ROP spanning from relatively spared to severely impaired. The relatively preserved profile of cognitive performance is often associated with milder clinical symptoms and better functioning (e.g. Crouse, Moustafa, Bogaty, Hickie, & Hermens, 2018; Oomen et al., 2021; Uren et al., 2017). Similar findings have emerged for samples with mood disorders (Cotrena, Branco, Ponsoni, Shansis, & Fonseca, 2017), but there are also suggestions that in depression samples there may be separable clusters based on the type of domains impaired, as opposed to the severity (Hermens et al., 2011; Tickell et al., 2019). Together, previous and current findings suggest that while cognitive impairments are a core feature of mental illnesses such as psychosis and depression, there will be a subsample of individuals who are not presenting with such impairments at illness onset. This paper further extends that notion by reporting the prevalence of each group with above average cognitive performance at baseline.
When we examined the individual tests showing the largest prevalence of impairment (Table 2, Fig. 1a), we saw largely the same six tests in all three clinical groups; Backwards Digit Span, Forward Digit Span, Digit-Symbol Substitution Test, Rey Auditory Verbal Learning Test Trials 1–5, Self-Ordered Pointing Test Total Errors, and Semantic Verbal Fluency. These tests assess working memory, processing speed, verbal learning and memory and verbal fluency. This observation aligns with previous systematic reviews and meta-analyses, in which deficits in these domains are often seen with the largest effect sizes in psychosis (Fioravanti et al., 2012; Schaefer, Giangrande, Weinberger, & Dickinson, 2013), CHR (Fusar-Poli et al., 2012; Pukrop & Klosterkötter, 2010; Zheng et al., 2018), and MDD (Ahern & Semkovska, 2017; Goodall et al., 2018). This would suggest that these are core cognitive functions that are impacted across diagnoses (East-Richard et al., 2020), and that these domains are particularly important when screening and providing treatment for cognitive impairment at the first-episode of mental illness.
To the best of our knowledge, no previous studies have reported the prevalence of cognitive strengths in the early course of psychosis or depression. Here, we observed that up to 40.5% of the ROD group, 36.1% of the CHR group, and 16.1% of the ROP group were performing at least one s.d. higher than average in at least two cognitive tests. This would indicate that, although cognitive impairments may be pervasive in the early course of mental illness, there may also be areas of relative strength or above average performance which clinicians could harness to enhance treatment, with a focus on bolstering motivation and functioning (Allott et al., 2020).
One limitation of this study is that the CHR sample was significantly younger than the other study groups, which may have had slight impacts on the standardised z-scores calculated based on the HC group. It is possible that the prevalence of cognitive strengths and impairments in the CHR group may need to be interpreted with more caution than the other clinical groups. The study groups did also significantly differ on the other demographic variables including estimated current IQ and years of education. However, differences on these factors reflect what is commonly seen in clinical practice. The purpose of this paper was not to determine the cause of cognitive impairment (e.g. potentially due to reduced education level in clinical groups), but rather to provide clinicians with a picture of cognitive performance which they might expect to see in individuals with recent-onset mental illness. Although the individual cognitive tests may each have their own norms which are matched on these demographic variables, these are often different normative samples for each test. By creating z-scores based on the present HC sample (who were recruited from the same areas as the clinical groups), we were able to provide a standardised picture of cognitive performance across all twelve tests. It must also be acknowledged that at present, there is no universally agreed definition of a clinically meaningful cognitive impairment. Here, we have provided the exact prevalence of three clinical groups performing at various levels of impairment to compare to previous literature and provide clinicians with a full picture of the patterns of performance that they are likely to observe in individuals with recent-onset mental illness. We have also provided the prevalence of impairments and strengths in a HC group recruited from the same locations for comparison. This is intended as a starting point for clinical services to estimate the need for cognitive treatments. However, cognitive impairment should always be interpreted within the individual's wider clinical picture, including their premorbid functioning, and their own treatment preferences.
Given the variability in the prevalence of impairment across individual tests, this suggests that while cognitive impairment may be common even at the early stages of serious mental illness, there is likely to be high variability in the profile of cognitive performance between individuals. These findings should help us to refine our approach to cognitive interventions for individuals with early psychosis or depression. By introducing, or promoting, routine cognitive screening at service entry (Bryce & Allott, 2019; Bryce et al., 2021), those individuals who are already demonstrating severe impairment could be efficiently referred to cognitive interventions as required. Such treatment could also be tailored towards the individual's specific profile of cognitive performance. In addition to interventions which take a deficit-reduction approach, patients may also benefit from an approach which aims to highlight their cognitive strengths (Allott et al., 2020) and to work on preserving cognitive domains which are intact at the first episode to prevent further decline (Pantelis et al., 2015). Here, we used the terms ‘strengths’ and ‘impairments’, referring to the degree to which cognitive performance is above or below average, taking a normative, as opposed to individual, approach (Lezak, Howieson, & Loring, 2004). We cannot report the extent to which an individual's performance may have declined from their own previous levels (Lezak et al., 2004; Tran et al., 2021), which can be associated with poorer outcomes even when performance is still within an ‘average’ range (Raucher-Chéné et al., 2022). Assessment of an individual's cognitive performance, including their relative strengths and weaknesses, would involve a full neuropsychological assessment in which the clinician brings together the full picture of their current performance, as well as estimated premorbid performance or intraindividual assessment, and demographic variables (Lezak et al., 2004). Further, the terms ‘strength’ and ‘impairment’ may not necessarily represent how the individual perceives their own current cognition relative to previous levels, and thus, targeted treatments for cognition should be planned in consultation with the individual to assist with both their objective and subjective areas of strength and impairment. In addition, this work reports on cognitive performance at baseline only. Cognition may take a heterogeneous trajectory following the first-episode, with some individuals potentially experiencing further declines in their cognitive performance (e.g. Fett et al., 2020; Flaaten et al., 2022). Further work is required to improve our understanding of the nature and trajectory of cognition both before and following the first episode of mental illness. Such work will allow clinicians to maximise the use of time and resources, ensuring that cognition is routinely assessed and treatment tailored to the individual's particular needs based on their presentation and stage of illness.
Conclusion
By understanding the prevalence of cognitive impairment and strength in early course mental illness, we can advocate for cognitive screening at intake, more accurately resource clinical services, and advise clinicians as to the proportion of young people who may need to access additional cognitive treatments or therapies.
Acknowledgements
The authors would also like to acknowledge the significant contributions of the PRONIA consortium members who performed the screening, recruitment, rating, examination, and follow-up of the study participants. They were involved in implementing the examination protocols of the study, setting up its IT infrastructure, and organising the flow and quality control of the data analysed in this manuscript between the local study sites and the central study database.
Contributor Information
PRONIA Consortium:
Nikolaos Koutsouleris, Dominic B. Dwyer, Lana Kambeitz-Ilankovic, Anne Ruef, Alkomiet Hasan, Claudius Hoff, Ifrah Khanyaree, Aylin Melo, Susanna Muckenhuber-Sternbauer, Yanis Köhler, Ömer Öztürk, Nora Penzel, David Popovic, Adrian Rangnick, Sebastian von Saldern, Rachele Sanfelici, Moritz Spangemacher, Ana Tupac, Maria Fernanda Urquijo, Johanna Weiske, Antonia Wosgien, Camilla Krämer, Shalaila S. Haas, Rebekka Lencer, Inga Meyhoefer, Marian Surmann, Udo Dannlowski, Olga Bienek, Georg Romer, Marlene Rosen, Theresa Lichtenstein, Stephan Ruhrmann, Joseph Kambeitz, Karsten Blume, Dominika Julkowski, Nathalie Kaden, Ruth Milz, Alexandra Nikolaides, Mauro Silke Vent, Martina Wassen, Christos Pantelis, Stefan Borgwardt, Christina Andreou, André Schmidt, Anita Riecher-Rössler, Laura Egloff, Fabienne Harrisberger, Ulrike Heitz, Claudia Lenz, Letizia Leanza, Amatya Mackintosh, Renata Smieskova, Erich Studerus, Anna Walter, Sonja Widmayer, Alexandra Korda, Rachel Upthegrove, Chris Day, Sian Lowri Griffiths, Mariam Iqbal, Mirabel Pelton, Pavan Mallikarjun, Alexandra Stainton, Ashleigh Lin, Paris Lalousis, Raimo K. R. Salokangas, Alexander Denissoff, Anu Ellilä, Tiina From, Markus Heinimaa, Tuula Ilonen, Päivi Jalo, Heikki Laurikainen, Antti Luutonen, Akseli Mäkela, Janina Paju, Henri Pesonen, Reetta-Liina Säilä, Anna Toivonen, Otto Turtonen, Frauke Schultze-Lutter, Eva Meisenzahl, Sonja Botterweck, Norman Kluthausen, Gerald Antoch, Julian Caspers, Hans-Jörg Wittsack, Ana Beatriz Solana, Manuela Abraham, Timo Schirmer, Alessandro Bertolino, Linda A. Antonucci, Giulio Pergola, Ileana Andriola, Barbara Gelao, Paolo Brambilla, Carlo Altamura, Marika Belleri, Francesca Bottinelli, Adele Ferro, Marta Re, Emiliano Monzani, Maurizio Sberna, Armando D'Agostino, Lorenzo Del Fabro, Giampaolo Perna, Maria Nobile, Alessandra Alciati, Matteo Balestrieri, Carolina Bonivento, Giuseppe Cabras, Franco Fabbro, Marco Garzitto, and Sara Piccin
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291723001770.
click here to view supplementary material
Financial support
PRONIA is a Collaborative Project funded by the European Union under the 7th Framework Programme under grant agreement n° 602152
Authors’ contributions
CP: Participated on Advisory Boards for Janssen-Cilag, AstraZeneca, Lundbeck, and Servier. Received honoraria for talks presented at educational meetings organised by AstraZeneca, Janssen-Cilag, Eli Lilly, Pfizer, Lundbeck, and Shire. NK and RS: Received honoraria for talks presented at education meetings organised by Otsuka/Lundbeck. RU: Received grants from the Medical Research Council, National Institute for Health Research: Health Technology Assessment, European Commission – Research: The Seventh Framework Programme, and personal speaker fees from Sunovion, outside the submitted work. TL: Supported by the Koeln Fortune Program/Faculty of Medicine, University of Cologne (Grant No. 370/2020). JW: Partly supported by the NARSAD Young Investigator Award of LK through the Brain & Behavior Research Foundation (grant number 28474). KA: Supported by a Dame Kate Campbell Fellowship from the University of Melbourne.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Abramovitch, A., & Schweiger, A. (2015). Misuse of cognitive neuropsychology in psychiatry research: The intoxicating appeal of neo-reductionism. Behavior Therapist, 38(7), 187–191. [Google Scholar]
- Abramovitch, A., Short, T., & Schweiger, A. (2021). The C factor: Cognitive dysfunction as a transdiagnostic dimension in psychopathology. Clinical Psychology Review, 86, 102007. doi: 10.1016/j.cpr.2021.102007 [DOI] [PubMed] [Google Scholar]
- Ahern, E., & Semkovska, M. (2017). Cognitive functioning in the first-episode of major depressive disorder: A systematic review and meta-analysis. Neuropsychology, 31(1), 52–72. doi: 10.1037/neu0000319 [DOI] [PubMed] [Google Scholar]
- Allen, D. N., Goldstein, G., & Warnick, E. (2003). A consideration of neuropsychologically normal schizophrenia. Journal of the International Neuropsychological Society, 9(1), 56–63. doi: 10.1017/S135561770391006X [DOI] [PubMed] [Google Scholar]
- Allott, K., Fisher, C. A., Amminger, G. P., Goodall, J., & Hetrick, S. (2016). Characterizing neurocognitive impairment in young people with major depression: State, trait, or scar? Brain and Behavior, 6(10), e00527. doi: 10.1002/brb3.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott, K., Steele, P., Boyer, F., de Winter, A., Bryce, S., Alvarez-Jimenez, M., & Phillips, L. (2020). Cognitive strengths-based assessment and intervention in first-episode psychosis: A complementary approach to addressing functional recovery? Clinical Psychology Review, 79, 101871. doi: 10.1016/j.cpr.2020.101871 [DOI] [PubMed] [Google Scholar]
- Altman, D. G. (1990). Practical statistics for medical research. London: CRC press. [Google Scholar]
- Amoretti, S., Rabelo-da-Ponte, F. D., Rosa, A. R., Mezquida, G., Sánchez-Torres, A. M., Fraguas, D., … Pina-Camacho, L. (2021). Cognitive clusters in first-episode psychosis. Schizophrenia Research, 237, 31–39. doi: 10.1016/j.schres.2021.08.021 [DOI] [PubMed] [Google Scholar]
- Army Individual Test Battery. (1944). Army Individual Test Battery: Manual of directions and scoring. In. Washington, DC: War Department, Adjutant General's Office.
- Benton, A. L., Hamsher, K. S., & Sivan, A. B. (1994). Multilingual aphasia examination: Token test. Iowa City: AJA associates. [Google Scholar]
- Bryce, S., & Allott, K. (2019). Cognitive screening: A significant unmet need in youth mental health. Australian & New Zealand Journal of Psychiatry, 53(8), 813–813. doi: 10.1177/0004867419834356 [DOI] [PubMed] [Google Scholar]
- Bryce, S., Bowden, S. C., Wood, S. J., & Allott, K. (2021). Brief, performance-based cognitive screening in youth aged 12–25: A systematic review. Journal of the International Neuropsychological Society, 27(8), 835–854. [DOI] [PubMed] [Google Scholar]
- Catalan, A., De Pablo, G. S., Aymerich, C., Damiani, S., Sordi, V., Radua, J., … Stone, W. S. (2021). Neurocognitive functioning in individuals at clinical high risk for psychosis: A systematic review and meta-analysis. JAMA Psychiatry, 78(8), 859–867. doi: 10.1001/jamapsychiatry.2021.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt, B. A., Risch, N. J., Faris, G., Friedman, D., & Erlenmeyer-Kimling, L. (1988). The continuous performance test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry research, 26(2), 223–238. doi: 10.1016/0165-1781(88)90076-5 [DOI] [PubMed] [Google Scholar]
- Cotrena, C., Branco, L. D., Ponsoni, A., Shansis, F. M., & Fonseca, R. P. (2017). Neuropsychological clustering in bipolar and major depressive disorder. Journal of the International Neuropsychological Society, 23(7), 584–593. doi: 10.1017/S1355617717000418 [DOI] [PubMed] [Google Scholar]
- Crouse, J. J., Moustafa, A. A., Bogaty, S. E., Hickie, I. B., & Hermens, D. F. (2018). Parcellating cognitive heterogeneity in early psychosis-spectrum illnesses: A cluster analysis. Schizophrenia Research, 202, 91–98. doi: 10.1016/j.schres.2018.06.060 [DOI] [PubMed] [Google Scholar]
- Douglas, K. M., Gallagher, P., Robinson, L. J., Carter, J. D., McIntosh, V. V., Frampton, C. M., … Porter, R. J. (2018). Prevalence of cognitive impairment in major depression and bipolar disorder. Bipolar Disorders, 20(3), 260–274. doi: 10.1111/bdi.12602 [DOI] [PubMed] [Google Scholar]
- Early Psychosis Guidelines Working Group. (2016). Australian clinical guidelines for early psychosis. Orygen The National Centre of Excellence in Youth Mental Health: Melbourne, Australia.
- East-Richard, C., R-Mercier, A., Nadeau, D., & Cellard, C. (2020). Transdiagnostic neurocognitive deficits in psychiatry: A review of meta-analyses. Canadian Psychology/Psychologie canadienne, 61(3), 190–214. doi: 10.1037/cap0000196 [DOI] [Google Scholar]
- Fett, A.-K. J., Velthorst, E., Reichenberg, A., Ruggero, C. J., Callahan, J. L., Fochtmann, L. J., … Kotov, R. (2020). Long-term changes in cognitive functioning in individuals with psychotic disorders: Findings from the Suffolk County mental health project. JAMA Psychiatry, 77(4), 387–396. doi: 10.1001/jamapsychiatry.2019.3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti, M., Bianchi, V., & Cinti, M. E. (2012). Cognitive deficits in schizophrenia: An updated metanalysis of the scientific evidence. BMC Psychiatry, 12(1), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaaten, C. B., Melle, I., Bjella, T., Engen, M. J., Asbo, G., Wold, K. F., … Ueland, T. (2022). Domain-specific cognitive course in schizophrenia: Group- and individual-level changes over 10 years. Schizophrenia Research. Cognition, 30, 100263. doi: 10.1016/j.scog.2022.100263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli, P., Deste, G., Smieskova, R., Barlati, S., Yung, A. R., Howes, O., … Borgwardt, S. (2012). Cognitive functioning in prodromal psychosis: A meta-analysis. Archives of General Psychiatry, 69(6), 10. doi: 10.1001/archgenpsychiatry.2011.1592 [DOI] [PubMed] [Google Scholar]
- Goodall, J., Fisher, C., Hetrick, S., Phillips, L. J., Parrish, E. M., & Allott, K. (2018). Neurocognitive functioning in depressed young people: A systematic review and meta-analysis. Neuropsychology Review, 28, 216–231. doi: 10.1007/s11065-018-9373-9 [DOI] [PubMed] [Google Scholar]
- Gould, I. C., Shepherd, A. M., Laurens, K. R., Cairns, M. J., Carr, V. J., & Green, M. J. (2014). Multivariate neuroanatomical classification of cognitive subtypes in schizophrenia: A support vector machine learning approach. NeuroImage: Clinical, 6, 229–236. doi: 10.1016/j.nicl.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri, C. T., & Morgan, D. W. (2008). The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: An unaccounted source of variance in clinical trials. Journal of Clinical Psychiatry, 69(7), 1122–1130. [DOI] [PubMed] [Google Scholar]
- Hermens, D. F., Redoblado Hodge, M. A., Naismith, S. L., Kaur, M., Scott, E., & Hickie, I. B. (2011). Neuropsychological clustering highlights cognitive differences in young people presenting with depressive symptoms. Journal of the International Neuropsychological Society, 17(2), 267–276. doi: 10.1017/S1355617710001566 [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2017). IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp,.
- Keefe, R. S. E. (2008). Should cognitive impairment be included in the diagnostic criteria for schizophrenia? World Psychiatry, 7(1), 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, R. S. E., Eesley, C. E., & Poe, M. P. (2005). Defining a cognitive function decrement in schizophrenia. Biological Psychiatry, 57(6), 688–691. doi: 10.1016/j.biopsych.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Koutsouleris, N., Davatzikos, C., Bottlender, R., Patschurek-Kliche, K., Scheuerecker, J., Decker, P., … Meisenzahl, E. M. (2011). Early recognition and disease prediction in the at-risk mental states for psychosis using neurocognitive pattern classification. Schizophrenia Bulletin, 38(6), 1200–1215. doi: 10.1093/schbul/sbr037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris, N., Kambeitz-Ilankovic, L., Ruhrmann, S., Rosen, M., Ruef, A., Dwyer, D. B., … the PRONIA Consortium. (2018). Prediction models of functional outcomes for individuals in the clinical high-risk state for psychosis or with recent-onset depression: A multimodal, multisite machine learning analysis. JAMA Psychiatry, 75(11), 1156–1172. doi: 10.1001/jamapsychiatry.2018.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, R. S. C., Hermens, D. F., Redoblado-Hodge, M. A., Naismith, S. L., Porter, M. A., Kaur, M., … Hickie, I. B. (2013). Neuropsychological and socio-occupational functioning in young psychiatric outpatients: A longitudinal investigation. PLoS ONE, 8(3), e58176. doi: 10.1371/journal.pone.0058176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak, M., Howieson, D., & Loring, D. (2004). The rationale of deficit measurement. Neuropsychological assessment, 4, 86–99. [Google Scholar]
- Malhi, G. S., Bell, E., Bassett, D., Boyce, P., Bryant, R., Hazell, P., … Porter, R. (2021). The 2020 royal Australian and New Zealand college of psychiatrists clinical practice guidelines for mood disorders. Australian & New Zealand Journal of Psychiatry, 55(1), 7–117. doi: 10.1177/0004867420979353 [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately, R. I., Giuliano, A. J., Goff, K. P., Faraone, S. V., & Seidman, L. J. (2009). Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology, 23(3), 315. doi: 10.1037/a0014708 [DOI] [PubMed] [Google Scholar]
- Michel, C., Ruhrmann, S., Schimmelmann, B. G., Klosterkötter, J., & Schultze-Lutter, F. (2014). A stratified model for psychosis prediction in clinical practice. Schizophrenia Bulletin, 40(6), 1533–1542. doi: 10.1093/schbul/sbu025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollon, J., David, A. S., Zammit, S., Lewis, G., & Reichenberg, A. (2018). Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry, 75(3), 270–279. doi: 10.1001/jamapsychiatry.2017.4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwesiga, E. K., Robbins, R., Akena, D., Koen, N., Nakku, J., Nakasujja, N., & Stein, D. J. (2022). Prevalence, profile and associations of cognitive impairment in Ugandan first-episode psychosis patients. Schizophrenia Research: Cognition, 28, 100234. doi: 10.1016/j.scog.2021.100234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence. (2009). Depression in adults: Recognition and management. Clinical guideline [CG90]. National Institute for Health and Clinical Excellence. Published October, 28.
- NICE. (2014). Psychosis and schizophrenia in adults: Prevention and management. NICE. https://www.nice.org.uk/guidance/cg178. [PubMed] [Google Scholar]
- Nowicki, S., & Duke, M. P. (1994). Individual differences in the nonverbal communication of affect: The diagnostic analysis of nonverbal accuracy scale. Journal of Nonverbal Behavior, 18(1), 27. doi: 10.1007/BF02169077 [DOI] [Google Scholar]
- Oomen, P. P., Begemann, M. J., Brand, B. A., de Haan, L., Veling, W., Koops, S., … van Beveren, N. (2021). Longitudinal clinical and functional outcome in distinct cognitive subgroups of first-episode psychosis: A cluster analysis. Psychological Medicine, 53(6), 2317–2327. doi: 10.1017/S0033291721004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth, P. A. (1944). Le test du copie d'une figure complexe. Archives de psychologie, 28, 206–356. [Google Scholar]
- Palmer, B. W., Heaton, R. K., Paulsen, J. S., Kuck, J., Braff, D., Harris, M. J., … Jeste, D. V. (1997). Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology, 11(3), 437. doi: 10.1037/0894-4105.11.3.437 [DOI] [PubMed] [Google Scholar]
- Pantelis, C., Wannan, C., Bartholomeusz, C. F., Allott, K., & McGorry, P. D. (2015). Cognitive intervention in early psychosis – preserving abilities versus remediating deficits. Current Opinion in Behavioral Sciences, 4, 63–72. doi: 10.1016/j.cobeha.2015.02.008 [DOI] [Google Scholar]
- Petrides, M., & Milner, B. (1982). Deficits on subject-ordered tasks after frontal-and temporal-lobe lesions in man. Neuropsychologia, 20(3), 249–262. doi: 10.1016/0028-3932(82)90100-2 [DOI] [PubMed] [Google Scholar]
- Pukrop, R., & Klosterkötter, J. (2010). Neurocognitive indicators of clinical high-risk states for psychosis: A critical review of the evidence. Neurotoxicity Research, 18(3-4), 272–286. doi: 10.1007/s12640-010-9191-1 [DOI] [PubMed] [Google Scholar]
- Pukrop, R., Schultze-Lutter, F., Ruhrmann, S., Brockhaus-Dumke, A., Tendolkar, I., Bechdolf, A., … Klosterkötter, J. (2006). Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first-and multiple-episode schizophrenia. Journal of Clinical and Experimental Neuropsychology, 28(8), 1388–1407. [DOI] [PubMed] [Google Scholar]
- Raucher-Chéné, D., Pochiet, G., Lavigne, K. M., Heinrichs, R. W., Malla, A., Joober, R., & Lepage, M. (2022). Normal-range verbal memory in the first-episode of psychosis: Clinical and functional outcomes across 24 months and impact of estimated verbal memory decrement. Schizophrenia Research, 246, 75–84. doi: 10.1016/j.schres.2022.06.007 [DOI] [PubMed] [Google Scholar]
- Rey, A. (1941). L'examen psychologique dans les cas d'encéphalopathie traumatique.(Les problems.). Archives de psychologie, 28, 215–285. [Google Scholar]
- Rey, A. (1964). L'examen clinique en psychologie [The clinical psychological examination]. Presses Universitaires de France: Paris. [Google Scholar]
- Schaefer, J. D., Giangrande, E., Weinberger, D. R., & Dickinson, D. (2013). The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophrenia Research, 150(1), 42–50. doi: 10.1016/j.schres.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semkovska, M., Quinlivan, L., O'Grady, T., Johnson, R., Collins, A., O'Connor, J., … Gload, T. (2019). Cognitive function following a major depressive episode: A systematic review and meta-analysis. The Lancet Psychiatry, 6(10), 851–861. doi: 10.1016/S2215-0366(19)30291-3 [DOI] [PubMed] [Google Scholar]
- Tickell, A. M., Scott, E. M., Davenport, T., Iorfino, F., Ospina-Pinillos, L., Harel, K., … Hermens, D. F. (2019). Neurocognitive clusters: A pilot study of young people with affective disorders in an inpatient facility. Journal of Affective Disorders, 242, 80–86. doi: 10.1016/j.jad.2018.08.045 [DOI] [PubMed] [Google Scholar]
- Tran, T., Milanovic, M., Holshausen, K., & Bowie, C. R. (2021). What is normal cognition in depression? Prevalence and functional correlates of normative versus idiographic cognitive impairment. Neuropsychology, 35(1), 33. doi: 10.1037/neu0000717 [DOI] [PubMed] [Google Scholar]
- Uren, J., Cotton, S. M., Killackey, E., Saling, M. M., & Allott, K. (2017). Cognitive clusters in first-episode psychosis: Overlap with healthy controls and relationship to concurrent and prospective symptoms and functioning. Neuropsychology, 31(7), 787. doi: 10.1037/neu0000367 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1939). The measurement and appraisal of adult intelligence (1st ed.). Baltimore: Williams and Wilkins Corporation. [Google Scholar]
- Wechsler, D. (1981). Wechsler Adult Intelligence Scale-Revised (WAIS-R) In. San Antonio, TX: Psychological Cooperation.
- Wechsler, D. (2011). Wechsler abbreviated scale of intelligence (2nd ed.) (WASI-II). NCS Pearson. [Google Scholar]
- Wenzel, J., Haas, S. S., Dwyer, D. B., Ruef, A., Oeztuerk, O. F., Antonucci, L. A., … the, P. c. (2021). Cognitive subtypes in recent onset psychosis: Distinct neurobiological fingerprints? Neuropsychopharmacology, 46(8), 1475–1483. doi: 10.1038/s41386-021-00963-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk, C. M., Gold, J. M., McMahon, R. P., Humber, K., Iannone, V. N., & Buchanan, R. W. (2005). No, it is not possible to be schizophrenic yet neuropsychologically normal. Neuropsychology, 19(6), 778. doi: 10.1037/0894-4105.19.6.778 [DOI] [PubMed] [Google Scholar]
- Zheng, W., Zhang, Q.-E., Cai, D.-B., Ng, C. H., Ungvari, G. S., Ning, Y.-P., & Xiang, Y.-T. (2018). Neurocognitive dysfunction in subjects at clinical high risk for psychosis: A meta-analysis. Journal of Psychiatric Research, 103, 38–45. doi: 10.1016/j.jpsychires.2018.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291723001770.
click here to view supplementary material