Abstract
Posttraumatic stress disorder (PTSD) is a complex disorder that involves physiological, emotional, and cognitive dysregulation that may occur after exposure to a life-threatening event. In contrast with the condition of learned fear with resilience to extinction, abnormal fear with impaired fear extinction and exaggeration are considered crucial factors for the pathological development of PTSD. The prefrontal cortex (mPFC) is considered a critical region of top-down control in fear regulation, which involves the modulation of fear expression and extinction. The pathological course of PTSD is usually chronic and persistent; a number of studies have indicated temporal progression in gene expression and phenotypes may be involved in PTSD pathology. In the current study, we use a well-established modified single-prolonged stress (SPS&FS) rat model to feature PTSD-like phenotypes and compared it with a footshock fear conditioning model (FS model); we collected the frontal tissue after extreme stress exposure or fear conditioning and extracted RNA for transcriptome-level gene sequencing. We compared the genetic profiling of the mPFC at early (<2 h after solely FS or SPS&FS exposure) and late (7 days after solely FS or SPS&FS exposure) stages in these two models. First, we identified temporal differences in the expressional patterns between these two models and found pathways such as protein synthesis factor eukaryotic initiation factor 2 (EIF2), transcription factor NF-E2-related factor 2 (NRF2)-mediated oxidative stress response, and acute phase responding signaling enriched in the early stage in both models with significant p-values. Furthermore, in the late stage, the sirtuin signaling pathway was enriched in both models; other pathways such as STAT3, cAMP, lipid metabolism, Gα signaling, and increased fear were especially enriched in the late stage of the SPS&FS model. However, pathways such as VDR/RXR, GP6, and PPAR signaling were activated significantly in the FS model's late stage. Last, the network analysis revealed the temporal dynamics of psychological disorder, the endocrine system, and also genes related to increased fear in the two models. This study could help elucidate the genetic temporal alteration and stage-specific pathways in these two models, as well as a better understanding of the transcriptome-level differences between them.
Keywords: Posttraumatic stress disorder, Fear conditioning, Temporal gene profiling, Prefrontal cortex, Gene network
Highlights
-
•
Stage-specific pathways identified in rat models of conditioned fear and PTSD-like traits.
-
•
The findings elucidate the genetic temporal alteration in the prefrontal area linked to top-down fear control.
-
•
The study reveals genetic temporal changes at the transcriptome level and differences between the two models.
1. Introduction
Posttraumatic stress disorder (PTSD) is a stress-related disorder, that develops after exposure to an extremely traumatic event, experiencing as a severe injury or life-threatening situation. The pathological states of PTSD involve persistent affective, cognitive, somatic, and behavioral changes (van der Kolk et al., 1996). The persistence of fearful memories is a key component of PTSD pathology. Fear responses are essential for an individual's survival mechanism, but PTSD has been characterized as impaired fear extinction and exaggerated fear responses (Milad et al., 2006; Norrholm et al., 2011; Ressler and Mayberg, 2007). The abnormal fears experienced during PTSD could be a core factor in the maintenance of trauma re-experience and avoidance behavior in PTSD (Elzinga and Bremner, 2002; Pitman et al., 2012; VanElzakker et al., 2014; Yehuda and LeDoux, 2007).
The paradigm of Pavlovian fear conditioning has been widely employed for understanding the mechanism of pathological fears in animal models. The presence of neutral stimuli (conditioned stimulus, [CS]; e.g., neutral tones or light) paired with electrical shock (unconditioned stimulus, [US]) formed fear conditioning, [US] is used to evoke fear conditioning (Maren, 2001; Tovote et al., 2015; VanElzakker et al., 2014). While exposed to trauma, the presence of stimuli (e.g. smells, light, and sound) paired with an aversive experience (e.g. a traumatic event) could also lead to pathological conditions in PTSD (Careaga et al., 2016). Understanding the biological basis and mechanisms involved in fear expression is crucial for understanding the pathological changes involved in PTSD. Research has shown the prefrontal cortex (PFC) is a critical neural component for regulating fear expression in rodents (Almada et al., 2015). Studies in animal models have suggested the prelimbic (PrL) subregion participates in fear expression and sends excitatory projections to the basal nucleus of the amygdala (BLA). The activation of PrL increases freezing behavior, but the infralimbic (IL) cortex has an antagonistic modulation, the activation of IL reduced fear expression (Brinley-Reed et al., 1995; Vertes, 2004). Furthermore, it has been shown that fear regulation shares functional homology between rodent and human brain regions (VanElzakker et al., 2014). Specifically, the rodent PL region is homologous to the human dorsal anterior cingulate cortex (dACC), which projects to the amygdala. Similarly, the rodent IL region is likely a homologue of the human ventral mPFC, which projects to the basal nucleus and amygdala. Therefore, the importance of PFC in fear regulation appears to be conserved across species. The top-down control of the PFC also affects the function of two other critical brain regions in PTSD pathology: the hippocampus and the amygdala. These regions are involved in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis and the negative feedback loop of glucocorticoids for stress response (Jacobson and Sapolsky, 1991), contextual memory acquisition and expression (Phillips and LeDoux, 1992), defensive fear-related behavior (Kjelstrup et al., 2002), and abnormal contextualization and flashbacks of traumatic memories (Desmedt et al., 2015).
The development of PTSD has been suggested to be a longitudinal progress that could be divided into different stages. A proposed staging model (McFarlane et al., 2017) raised the concept that the regulation of glucocorticoid receptors, cytokine, immune, and metabolism responses possibly have differential expression levels during the early and later stages of PTSD's pathological course. Lines of evidence from the longitudinal transcriptomic research in mouse models featuring PTSD have supported this concept. The sequential regulation of inflammatory responses, cell cycle, and tissue remodeling processes revealed a temporal disease-perturbed molecular network associated with acute heart injury in a mouse model simulating PTSD (Cho et al., 2014). A two-site longitudinal study found that RNA expression in the immediate aftermath of trauma and a 6-month follow-up revealed critical gene candidates for PTSD development and also causal genes for PTSD and comorbid conditions, it was indicated that the longitudinal study could help promote the understanding of the causal mechanism in the development of PTSD (Wuchty et al., 2021).
In addition, genome studies have suggested a variety of candidate biomarkers could play important roles in PTSD pathology. Studies of the serotonin transporter (5-HTT) knockout rat models have shown these animals exhibited higher anxiety-like and depression-like phenotypes (Kalueff et al., 2010), and also impaired fear extinction (Nonkes et al., 2012). The dopaminergic system has also been associated with PTSD pathology. Research suggests it is involved in dopaminergic reward dysfunction and anhedonia in a rat model (Enman et al., 2015). HPA-axis dysregulation is one of the core features in PTSD, FK506 binding protein 5 (FKBP5), a cochaperone of hsp90, which mediates the sensitivities of glucocorticoid receptors (GRs) (Zannas et al., 2016), the protein complex of FKBP5 and GR which was found elevated in fear-conditioned mice, and blocking the formation of this protein complex resulted in impaired fear memory consolidation and recall in mice (Li et al., 2020). In addition, activation of the cyclic adenosine monophosphate (cAMP)-responsive element-binding protein (CREB) was shown to be necessary for fear retrieval in mice, which engaged in fear reconsolidation and extinction (Mamiya et al., 2009). In addition to cAMP and CREB, other molecules, such as brain-derived neurotrophic factor (BDNF) and mGluR5 are important mediators for fear conditioning (Liu et al., 2004; Riedel et al., 2000), protein kinases such as protein kinase A (PKA), protein kinase C (PKC) and cyclin-dependent kinase 5 (CDK5) are crucial for the consolidation of fear memory (Ahi et al., 2004; Fischer et al., 2002).
Our study involved the collection of PFC tissue samples from animal models of PTSD and fear, and we performed a two-site longitudinal genetic profile analysis. Understanding the gene expression at the early (<2 h after fear conditioning or extreme stress exposure) and late (7 days after fear conditioning or extreme stress exposure) stages could help illustrate temporal changes and distinguish genetic profiling differences in these two models.
2. Material and methods
2.1. Animals
Male Sprague-Dawley rats (300–400 g; purchased from BioLASCO Taiwan Co., Ltd, Taipei, Taiwan) were housed in ventilation cages with food and water access ad libitum. The temperature of the colony room was maintained at 20 ± 2 C with a 12 h light/dark cycle (light on from 7 a.m. to 7 p.m.). All of the experiments were performed during the light phase and within a specific time frame, from 12 p.m. to 5 p.m. Besides, we followed the standard ethical guidelines of the Academia Sinica Institutional Animal Care and Utilization Committee (Ethical Protocol ID: 19-12-1386). All efforts were made to minimize animals’ suffering and reduce the number of animals used. The animals were randomly divided into a control group and two experimental groups (N = three in each group).
2.2. Preparation of the animal models featuring PTSD and fear conditioning
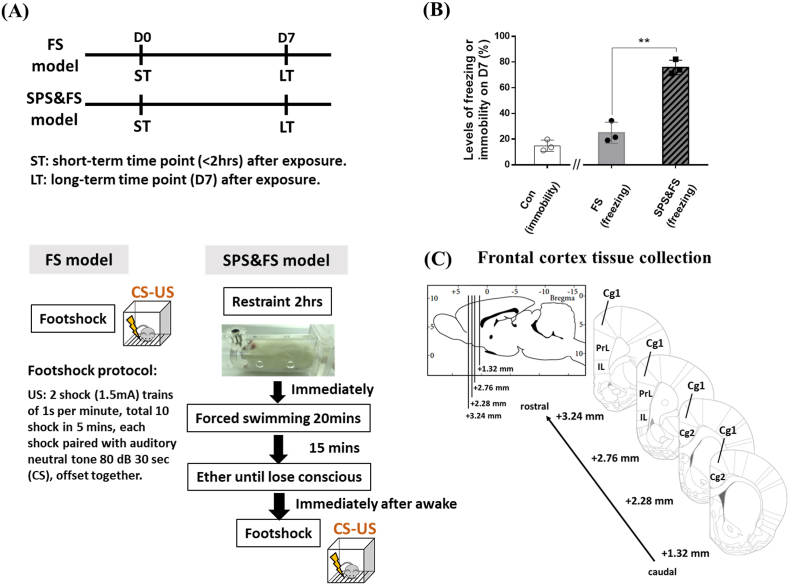
The single-prolonged stress (SPS) animal model (Liberzon et al., 1997) is a well-established animal model of the fear-related features of PTSD and one of the most frequently used models in rats. For model preparation in our study, we applied an enhanced SPS paradigm, a combination of SPS and footshock fear conditioning (FS) in rats (SPS&FS model) (Wang et al., 2008, 2015). The preparation procedure was conducted based on previous designs (Wang et al., 2008, 2015); rats were individually restrained for 2 h in a restraint tube, and then immediately introduced to forced swimming for 15 min. After 15 min of recovery, rats were exposed to ether until loss of consciousness (defined as rats showing no response to foot pinch). Before the animals awakened from ether exposure, they were introduced into a shock cage (50 × 50 × 50 cm), and immediately after the animals awoke, a modified footshock protocol (Mikics et al., 2008) was given: two shock trains of 1.5 mA for 1 s (US). Each US was paired with 30 s neutral auditory tone at 80 dB (CS) and offset together. A total of ten shocks were delivered in 5 min. However, in the FS model, animals only received the US-CS footshock protocol for fear conditioning without SPS exposure. For the SPS&FS model and FS model preparation, animals received SPS&FS or FS on Day 0 (Fig. 1A).
Fig. 1.
The study paradigm of the FS and SPS&FS models, freezing behavior measurement on Day 7 after FS or SPS&FS exposure, and tissue collection of the frontal cortex for RNA extraction. (A) The study paradigm of the FS and SPS&FS models, models were prepared on D0, and the steps of model preparation were illustrated, freezing behavior was measured on 7 days after FS or SPS&FS exposure, and tissue collection was divided into short-term (ST, < 2 h after FS or SPS&FS exposure) and long-term (LT, 7 days after FS or SPS&FS exposure) groups. (B) The levels of immobility in the control group and freezing levels in the FS or SPS&FS group (**p < 0.01). (C) Tissue collection from the frontal cortex for RNA extraction. The tissue samples were collected from two slice sections within the range of bregma +3.24 mm to +1.24 mm, which included the regions of Cg1, Cg2, IL, and PrL. The diagrams of sagittal and coronal sections modified from Paxinos and Watson (2004), 5th edition, Academic Press. Cg1 and 2, cingulate cortex, area 1 and 2; IL, infralimbic cortex; PrL, prelimbic cortex.
2.3. Context re-exposure after FS or SPS&FS
After the animals had received FS or SPS&FS on Day 0, they were returned to a footshock cage on Day 7 for context re-exposure and freezing level measurement (Fig. 1A and B). At each time of context re-exposure, freezing behavior was accessed with six neutral auditory tones at 80 dB 30 s (CS) and 30 s intervals between each CS. Freezing behavior was considered detected when animals did not move for 2 s (Karalis et al., 2016). Freezing levels were scored by the FreezeScan system (Clever Sys, Inc., Reston, VA).
2.4. Tissue collection and RNA extraction
For tissue collection, the PFC brain tissue from an early time point (<2 h after FS or SPS&FS) and a late time point (Day 7 after FS or SPS&FS exposure) was collected. The brain region and time points were selected based on our electrophysiology pilot study (Chang and Shyu, 2022). Our local field potentials (LFPs) results showed suppressed delta activities (0.5–4 Hz) correlating with freezing behavior before 2 h, characterized at 2 h, and persisting until later time points, such as D7 after SPS&FS exposure. The PFC exhibited the most significant delta activities compared to the amygdala and ventral hippocampus. Therefore, we considered the time point before delta activities increased (<2 h) as the early stage and the time point with significant delta activities (D7) as the late stage, which allowed us to identify significant underlying temporal genetic differences. Rats were euthanized with an overdose of sodium pentobarbital and later, the brain tissue was removed. Brain tissue was first sliced into thick coronal slices with a slicer that produced slices 1.0 mm thick. Tissue samples were collected from two slice sections within the range of bregma +3.24 mm to +1.24 mm, which included the regions of cingulate cortex area 1 and 2 (Cg1 and 2), IL, and PrL. Tissue slices were isolated and immediately frozen in dry ice (−30 °C). The tissue was later stored at −80 °C for further use. For total RNA extraction, total RNA was extracted following the RNeasy Mini Kit’s (Qiagen, Valencia, CA, United States) instructions. RNA was quantified using a NanoDrop ND-1000 (Thermo Fisher Scientific, Waltham, MA). RNA samples were subjected to next-generation sequencing, and all sequencing was performed at PhalanxBio Inc. (Taipei, Taiwan).
2.5. Next-generation sequencing data generation and analysis
2.5.1. Acquisition and analysis of expression data
Raw reads of RNA-seq from the sequencing instrument were first trimmed off the low-quality tranche and then checked. Besides, the RNA quality checks involve three main parts: (1) Sample quality control, the minimum required concentration is 500 ng high-quality total RNA, the RNA integrity number (RIN) is > 8, OD260/OD280 ≥ 1.8, OD260/OD230 ≥ 1.5. PolyA mRNA was purified and fragmented, and first- and second-strand cDNA was synthesized from the input of total RNA; (2) Library quality control includes ligating barcoded linkers to generate indexed libraries, which are then pooled and sequenced using an Illumina sequencer in paired-end 150-bp Rapid Run format to generate 20 million total reads per sample, the depth of sequencing is 50X; and (3) Sequencing data filtering and trimming is performed, where raw reads are trimmed based on two criteria: reads shorter than 35 bp are discarded, and reads with an average quality below 15 in a sliding window of four bases are removed. Spliced Transcripts Alignment to a Reference (STAR) software was used for mapping preprocessed read data to the reference genome (Ensembl Rnor_6.0). Phalanx Biotech performed Illumina TruSeq Stranded mRNA sequencing following the standard Illumina kit protocol (Illumina, San Diego, CA). Cufflinks (http://cufflinks.cbcb.umd.edu/) was used on the resulting alignment files to estimate the expression levels by calculating the number of RNA-seq fragments per kilobase of transcript per total million (FPKM) fragments mapped. Significance analysis of the RNA-seq data was performed using standard selection criteria to identify differentially expressed genes (DEGs; p < 0.05). The expression patterns of heat map and hierarchical clustering of Pearson correlation coefficients were used to demonstrate the expression patterns of these DEGs. The effect sizes of genes were calculated with Cohen’s d.
2.5.2. Differentiating expression patterns among groups
Principal component analysis (PCA) was performed using iDEP 0.93 (integrated differential expression and pathway analysis 0.93, http://bioinformatics.sdstate.edu/idep/) (Ge et al., 2018) to differentiate patterns among the control group and the early- and late-phase of FS and SPS&FS groups.
2.5.3. Gene function and regulatory network analysis
The fold changes of DEGs at time points of <2 h and 7days after FS or SPS&FS were uploaded to QIAGEN Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Inc. Redwood City, CA). The interactions among DEGs were revealed by the Ingenuity Pathway Knowledge Base, and IPA was applied to categorize DEGs in specific diseases and functions. To interpret the gene expression results in IPA, we compared all intervention groups with the control group. Two IPA scores were used to assess regulators and networks: the "enrichment” score, which measured the overlap of observed and predicted gene sets using Fisher’s exact test (FET) p-values, and the z-scores, which evaluated up/down-regulation pattern match and functioned as a significant measure and predictor for regulator activation state (Kramer et al., 2014). For group/subgroup comparisons, we specifically compared all intervention groups to the control group. Z-scores were computed by standardizing the dispersion of observed and predicted up/down regulation patterns within each intervention group compared to the control group. Analysis of “canonical pathways”, “upstream modulators”, “gene networks”, and “diseases and functions” was performed in IPA. The heat map of DEGs was illustrated using MATLAB packages (MathWorks, Inc., Natick, MA, USA) and Morpheus (https://software.broadinstitute.org/morpheus). For the network analysis, we first used the Build Connect tool in IPA to create connections between molecules, and colors of z-score levels were further illustrated by MATLAB packages.
2.6. Statistical analysis
Behavioral data from the freezing level measurement were expressed as mean ± SD. The comparison of freezing levels was only made between the FS and SPS&FS groups with Welch’s t-test, p < 0.05 was considered statistically significant. Bar graphs and associated statistical analysis were illustrated using GraphPad software (CA, USA).
3. Results
3.1. The study paradigm for understanding the temporal alterations after FS or SPS&FS exposure, freezing levels and tissue collection in the two models
To identify the temporal genetic alterations in the FS and SPS&FS models, animals were exposed to either solely fear conditioning (FS) or extreme stress (SPS&FS) on Day 0. Next, they were divided into two-time points: short-term (ST, <2 h after FS or SPS&FS exposure) and long-term time point (LT, Day 7 after FS or SPS&FS exposure), the model preparation process was outlined, following the preparation steps in Section 2.2 of the Materials and Methods (Fig. 1A). Freezing levels of the LT FS and SPS&FS groups were measured on Day 7 before animals were euthanized for tissue collection (Fig. 1B). The freezing levels of SPS&FS animals were significantly higher when compared with FS animals (t = 8.810, df = 3.599, p = 0.0015). In addition, we also measured the control group’s levels of immobilization. The average immobility level in the control group was 14.91%. The levels of immobile behavior in the control group and the freezing levels in the FS or SPS&FS group across the six auditory tones were shown in Supplemental Figure A1 mPFC tissues for RNA extraction were collected individually from the ST and LT groups in the FS and SPS&FS models, Fig. 1C illustrates the representative atlas of tissue collection sites, including the subregions of Cg1, Cg2, IL, and PrL of the mPFC, within the range of bregma +3.24 mm to +1.24 mm.
3.2. Analysis of the DEGs in the mPFC after FS or SPS&FS exposure
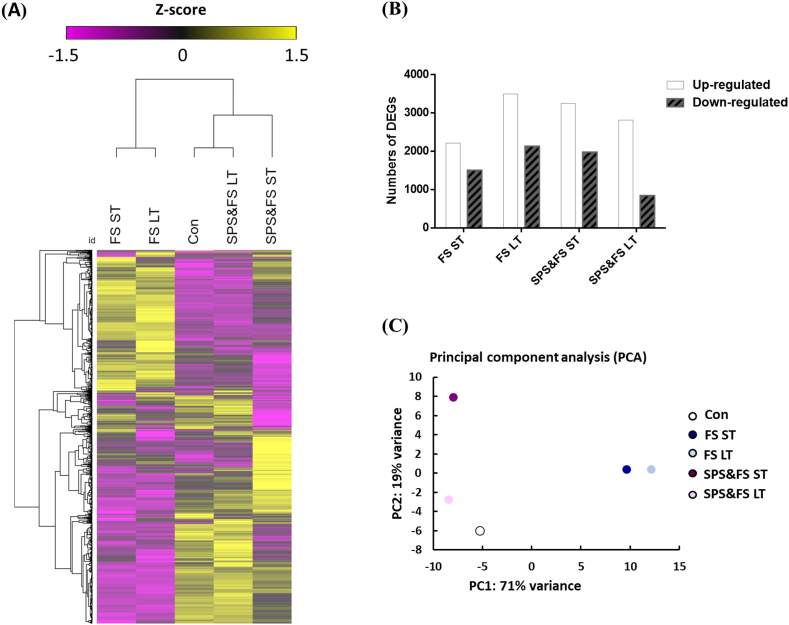
RNA-seq data analysis was performed to compare the temporal frontal transcriptomes of the control, FS, and SPS&FS groups. A number of DEGs were identified in the ST and LT groups. The gene expression patterns are shown in Fig. 2A. In addition, compared with the control group, many DEGs were identified in the ST and LT groups of the FS and SPS&FS models (Fig. 2B). We identified 1925 (ST cohort of the FS group) and 2228 (LT cohort of the FS group) upregulated genes, 1946 (ST cohort of the SPS&FS group), and 271 (LT cohort of the SPS&FS group) upregulated genes, and 2323 (ST cohort of the FS group) and 2572 (LT cohort of the FS group) downregulated genes, and 1185 (ST cohort of the SPS&FS group), and 126 (LT cohort of the SPS&FS group) downregulated genes in each group. The PCA plot (Fig. 2C) shows the separated clusters between the control, FS, and SPS&FS groups, presenting the temporal differences among the early and late phases and the genetic differences among the control, FS, and SPS&FS groups.
Fig. 2.
Expression of DEGs identified in early and late phases after FS or SPS&FS exposure. (A) Heat map of DEGs identified in the control, FS, and SPS&FS groups. The hierarchical clustering of Pearson correlation coefficients was used to demonstrate the expression patterns of these DEGs. (B) The numbers of DEGs at different time points in each group. (C) Principal component analysis (PCA) of DEGs in each group. Con: control group, ST: short-term (<2 h after FS or SPS&FS exposure); LT: long-term (7 days after FS or SPS&FS exposure), N = three in each group.
3.3. Analysis of the biological processes at the early and late phases after FS or SPS&FS exposure
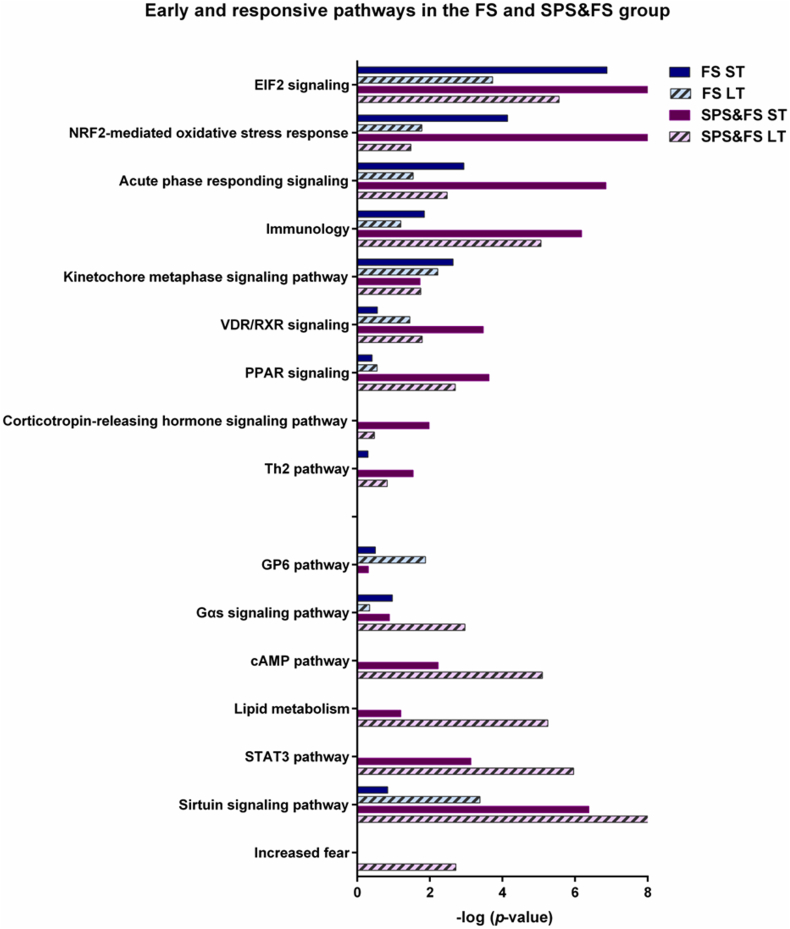
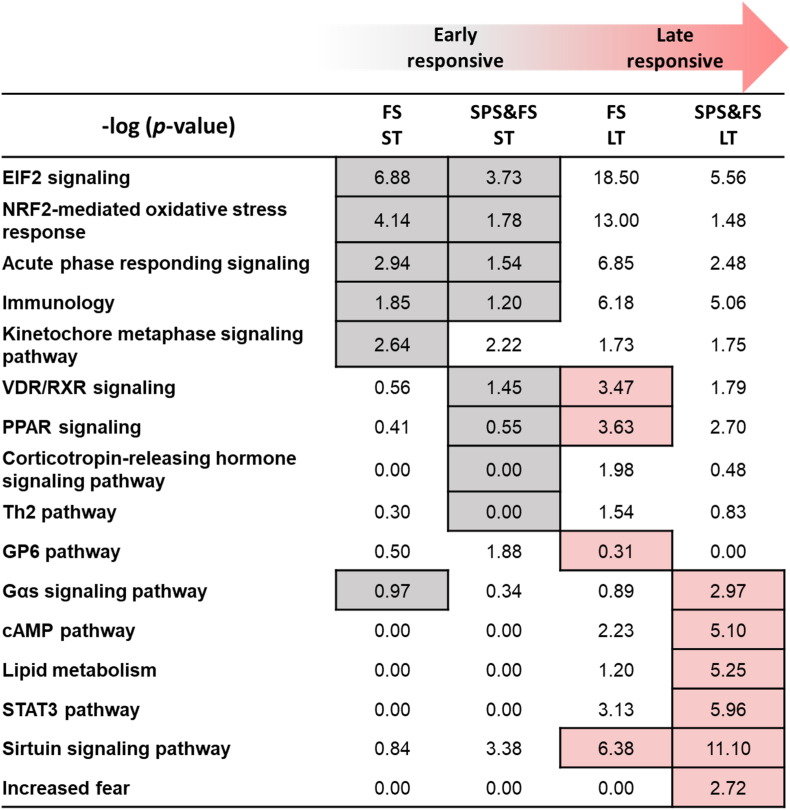
Functional enrichment analysis was performed to determine the enriched biological processes in association with DEGs. We identified many early and late responsive pathways in FS and SPS&FS models (Fig. 3). In the FS group, pathways enriched in the early stage included eukaryotic initiation factor 2 (EIF2) signaling, NF-E2-related factor 2 (NRF2)-mediated oxidative stress response, acute phase responding signaling, kinetochore metaphase signaling pathway, immunology, and Gαs signaling pathway. However, in the late phase of the FS group, pathways such as the sirtuin signaling pathway, VDR/RXR signaling, the glycoprotein 6 (GP6) pathway, and PPAR signaling were more enriched. In addition, several biological pathways were expressed with significant p-values (p < 0.05) and overlapped in both FS and SPS&FS groups. In the early phase of the SPS&FS model, we found biological pathways such as EIF2 signaling, acute phase responding signaling, immunology, peroxisome proliferator-activated receptor (PPAR) signaling, VDR/RXR signaling, corticotropin-releasing hormone (CRH) signaling pathway, and Th2 pathway activated in the early phase with significant p-values (p < 0.05). In addition, in the late stage of the SPS&FS model, we found biological processes such as the sirtuin signaling pathway, signal transducers and activators of the transcription 3 (STAT3) pathway, the cAMP pathway, lipid metabolism, the Gαs signaling pathway, and increased fear were more enriched in the late phase of the SPS&FS model. We found that a small number of pathways were more activated at a particular time; for example, the kinetochore metaphase signaling pathway was only significantly expressed in the early phase of the FS group and the GP6 pathway in the late phase of the FS group. Moreover, in the early stage of the SPS&FS group, the CRH signaling pathway was particularly more activated; other pathways such as STAT3, the cAMP pathway, lipid metabolism, and increased fear were particularly more enriched in the late stage of the SPS&FS group. We also identified pathways with reverse activation conditions in the SPS&FS and FS groups, including PPAR signaling and VDR/RXR signaling. These pathways were more involved in the early phase of the SPS&FS group. In comparison, these pathways were more enriched in the late phase of the FS group. The Gαs signaling pathway was more enriched in the late phase of the SPS&FS group, but in the FS group, it was found to have more involvement in the early phase. In addition, the pathway of increased fear was significantly enriched only in the late phase of the SPS&FS group, but not in the FS group, which may explain the key phenotype of abnormal fear maintenance in PTSD. Moreover, Table A.1 in the Appendices presents the p-values associated with each ingenuity canonical pathway, the analysis revealed an overall trend in the enriched pathways across the two stages. In both the SPS&FS and FS models, there was a notable increase in pathway enrichment from the early to the late stages. The trend suggests a dynamic temporal response, where early-stage pathways focus on immediate cellular and hormonal responses, while late-stage pathways involve more complex signaling processes and metabolic adaptations.
Fig. 3.
Early and late responsive pathways involved in the FS and SPS&FS groups. Pathways characterized in the early and late stages after FS and SPS&FS exposure. ST: short-term (<2 h after FS or SPS&FS exposure); LT: long-term (7 days after FS or SPS&FS exposure).
3.4. Analysis of temporal DEGs expression in the early and late phases after FS or SPS&FS exposure
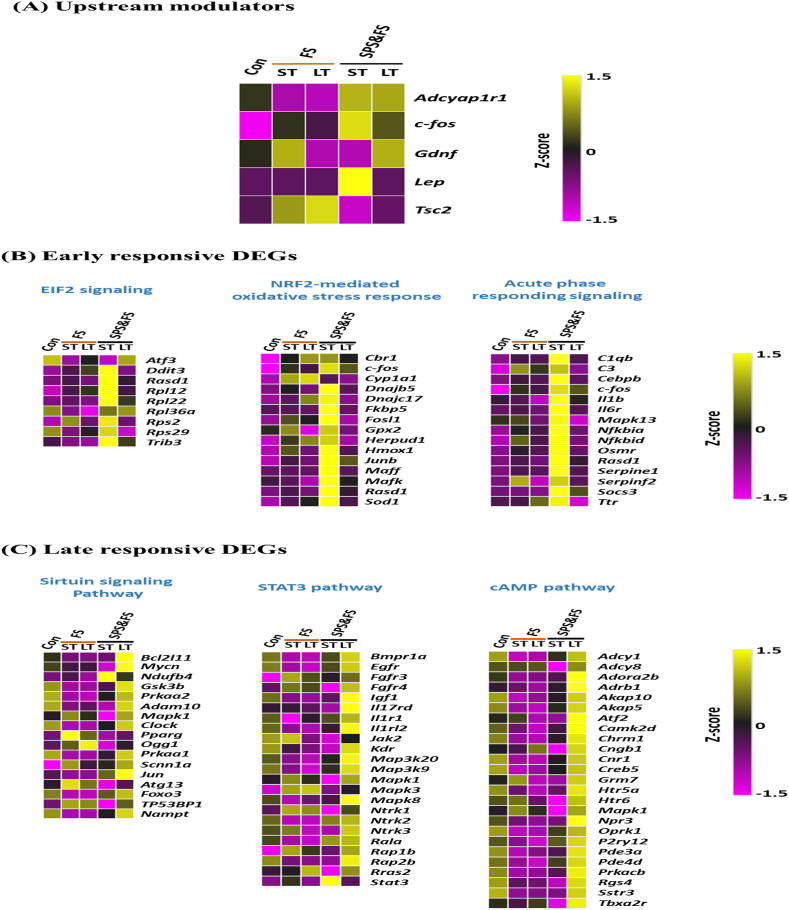
Next, from the analysis of upstream modulators by IPA (Fig. 4A), we found that genes such as Adcyap1r1, Lep, and c-fos were highly activated in the early phase of the SPS&FS group. Other genes such as Tsc2 and Gdnf were more downregulated. However, in the late phase of the SPS&FS group, upstream Gdnf and c-fos were upregulated compared with the control group. In addition, a reverse condition in the FS group, upstream Tsc2, was highly activated in both the early and late phases. Other modulator genes such as Adcyap1r1 were downregulated in both the early and late phases in the FS group.
Fig. 4.
Heat maps of upstream modulators and DEGs involved in the early and late biological processes after FS or SPS&FS exposure. (A) We identified genes of upstream modulators that may play critical roles in the early and late biological processes after FS or SPS&FS exposure. (B) Early responsive DEGs involved in EIF2 signaling, NRF2-mediated oxidative stress response, and acute phase responding signaling. (C) Late responsive DEGs involved in the sirtuin signaling pathway, STAT3 pathway, and cAMP pathway. Con: control group, ST: short-term (<2 h after FS or SPS&FS exposure); LT: long-term (7 days after FS or SPS&FS exposure).
In addition, we further analyzed the expression patterns of DEGs involved in the enriched biological process of early and late phases in both the FS and SPS&FS models. The representative heat maps of important genes are shown in Fig. 4B and C. In the early phase (Fig. 4B), we compared major pathways involved in both the FS and SPS&FS models, such as EIF2 signaling, NRF2-mediated oxidative stress response, and acute phase responding signaling. In the EIF2 signaling pathway, we identified a considerable number of DEGs activated during the early phase of SPS&FS, including a major pathway of ribosomal Rpl genes (Rpl12, Rpl22, and Rpl36a), Rps genes (Rps2 and Rps29), and other genes such as Trib3 encodes pseudokinase protein and Ddit3, which are involved in oxidative stress. In the NRF2-mediated oxidative stress response pathway, we also observed many inducible transcription factors activated during the early phase of SPS&FS, such as Maff, c-fos, JunB, Fosl1, and Mafk. In addition, we found genes such as antioxidant enzymes, Sod1, and Gpx2, and a gene encodes the enzyme heme oxygenase, Hmox1. Another gene, Fkbp5 encodes a significant regulator protein participated in the HPA axis, which was activated in the early phase of SPS&FS, but was less activated in the late phase of the FS and SPS&FS groups. In the acute phase responding signaling pathway, a number of genes were activated during the early phase of SPS&FS, including genes in signaling pathways implicated in anti-inflammatory responses (Socs3, Il1b, and IL6R); serpin family E member 1 (Serpine1), a gene associated with complement system gene C1qb and cytokine receptor gene Osmr. In the late phase (Fig. 4C), pathways such as the sirtuin signaling pathway, STAT3 pathway, cAMP pathway, lipid metabolism, and Gαs signaling pathway were more enriched. In the sirtuin signaling pathway, which is most notable for metabolic regulation, we identified genes that encode proteins important for apoptosis (Bcl2l11 and Foxo3), and cellular energy metabolism (Prkaa1 and Prkaa2). In addition, genes encode for other factors such as glycogen synthase kinase-3 (Gsk3b), metalloproteinases (Adam10), nicotinamide phosphoribosyltransferase (Nampt), and transcription factors (Mycn and Clock) showed convergent activation in the late phases of both the FS and SPS&FS models. In the STAT3 pathway, we found genes such as receptor proteins (Bmpr1a, Egfr, Fgfr3&4, and Kdr), interleukin receptor and related proteins (Il17rd and Il1rl2), kinases for cellular signaling (Mapk1 and Mapk8), and GTPase (Rap1b and Rras2) had higher activation in the late phase of the SPS&FS model. However, these genes in the FS group were less activated. In the cAMP pathway, a number of genes in the late phase of SPS&FS were upregulated. Among these genes, Adcy8 and Mapk1 were more activated in the early and late phases of the FS group and the late phase of SPS&FS. Furthermore, Htr6, Grm7, and Atf2 were more activated in the early phase of the FS group and the late phase of SPS&FS. Conversely, most of the genes in the FS group were downregulated. Additionally, the expression levels of upstream modulators and early- and late-responsive DEGs are provided in Table A.2 in the Appendices.
3.5. Canonical analysis of metabolic and signaling pathways involved in the early and late metabolic processes after FS or SPS&FS exposure
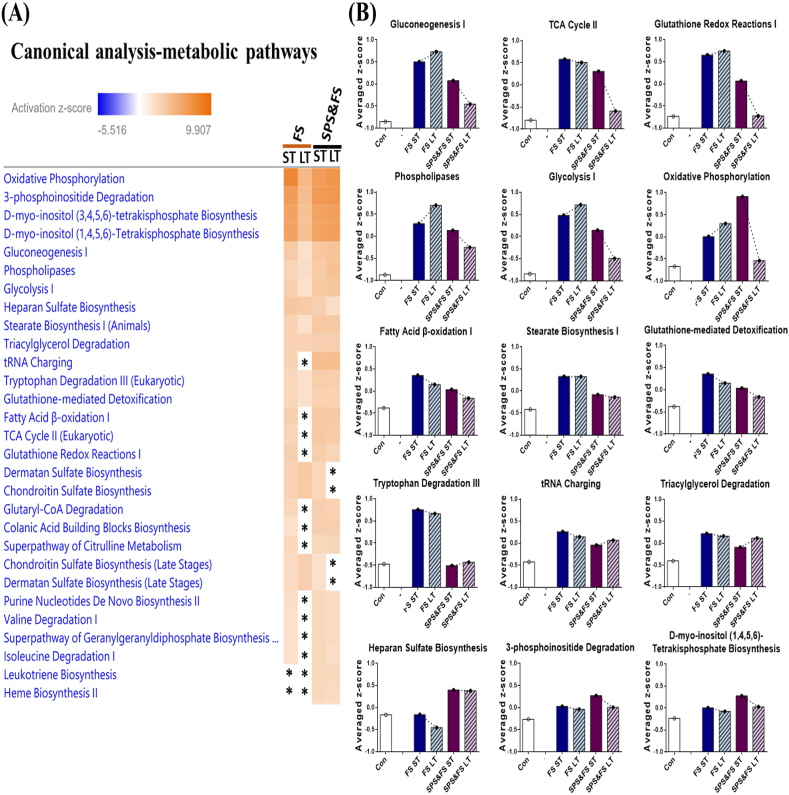
Canonical analyses were performed to determine the metabolic and signaling pathways in the early and late phases. Fig. 5 shows the metabolic pathways in the early and late phases after FS or SPS&FS exposure. After FS or SPS&FS exposure, we found pathways that showed a pattern similar to being downregulated during the LT SPS&FS phase and being upregulated during the ST FS, LT FS, and ST SPS&FS phases. These pathways include oxidative phosphorylation, 3-phosphoinositide degradation, tetrakisphosphate biosynthesis, gluconeogenesis I, phospholipases, glycolysis I, stearate biosynthesis I, tRNA charging, fatty acid β-oxidation I, TCA cycle II and glutathione redox reactions I. In addition, some pathways were maintained at similar levels during the early and late stages of the FS and SPS&FS models, including triacylglycerol degradation, tryptophan degradation III and glutathione-mediated detoxification.
Fig. 5.
The analysis of canonical pathways (metabolic pathways) involved in the early and late metabolic processes after FS or SPS&FS exposure. (A) The heat map represents the metabolic pathways involved in the early and late phases after FS or SPS&FS exposure. An asterisk (*) indicates a pathway that is statically insignificant (|activation z-score| ≤ 1.96). The color shows the z-scores of DEGs annotated in the specific metabolic biological process. ST: short-term (<2 h after FS or SPS&FS exposure); LT: long-term (7 days after FS or SPS&FS exposure). (B) The average expression profiles for DEGs involved in major metabolic processes. The y-axis shows the average z-score of genes in the specific metabolic process term. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
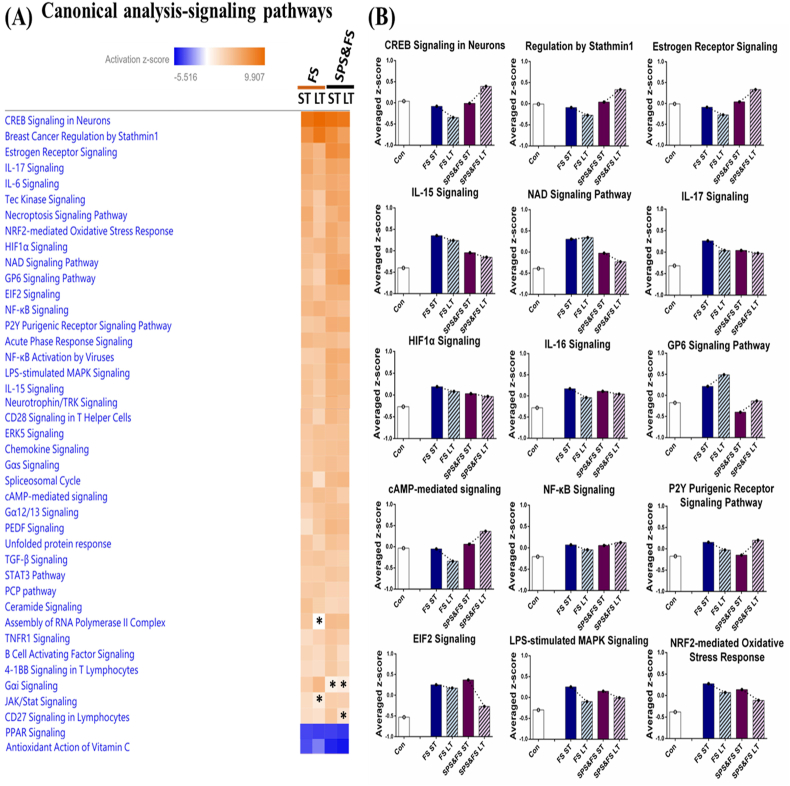
Next, Fig. 6 shows the pathways associated with signaling. Some of the results show a pattern of higher z-scores during the late phase of SPS&FS and lower z-scores during ST FS, LT FS, and ST SPS&FS, including pathways such as CREB signaling in neurons and regulation by stathmin1. In addition, some pathways exhibited a pattern of higher z-scores during the early and late phases of the FS group and lower z-scores during the early and late phases of the SPS&FS group. These pathways included estrogen receptor signaling, IL-15 signaling, NAD signaling, GP6 signaling, EIF2 signaling, P2Y purigenic receptor signaling, LPS-stimulated MAPK signaling, and NRF2-mediated oxidative stress response. Here, we observed that two pathways were significantly downregulated in the early and late phases of the FS and SPS&FS groups: they were PPAR signaling and antioxidant action of vitamin C.
Fig. 6.
The analysis of canonical pathways (signaling pathways) involved in the early and late signaling processes after FS or SPS&FS exposure. (A) The heat map represents the signaling pathways involved in the early and late phases after FS or SPS&FS exposure. An asterisk (*) indicates a pathway is statically insignificant (|activation z-score| ≤ 1.96). The color shows the z-scores of DEGs annotated in the specific signaling biological process. ST: short-term (<2 h after FS or SPS&FS exposure); LT: long-term (7 days after FS or SPS&FS exposure). (B) The average expression profiles for DEGs involved in major signaling processes. The y-axis shows the average z-score of genes in the specific signaling process term. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. The network analysis represents temporal alterations after FS or SPS&FS exposure
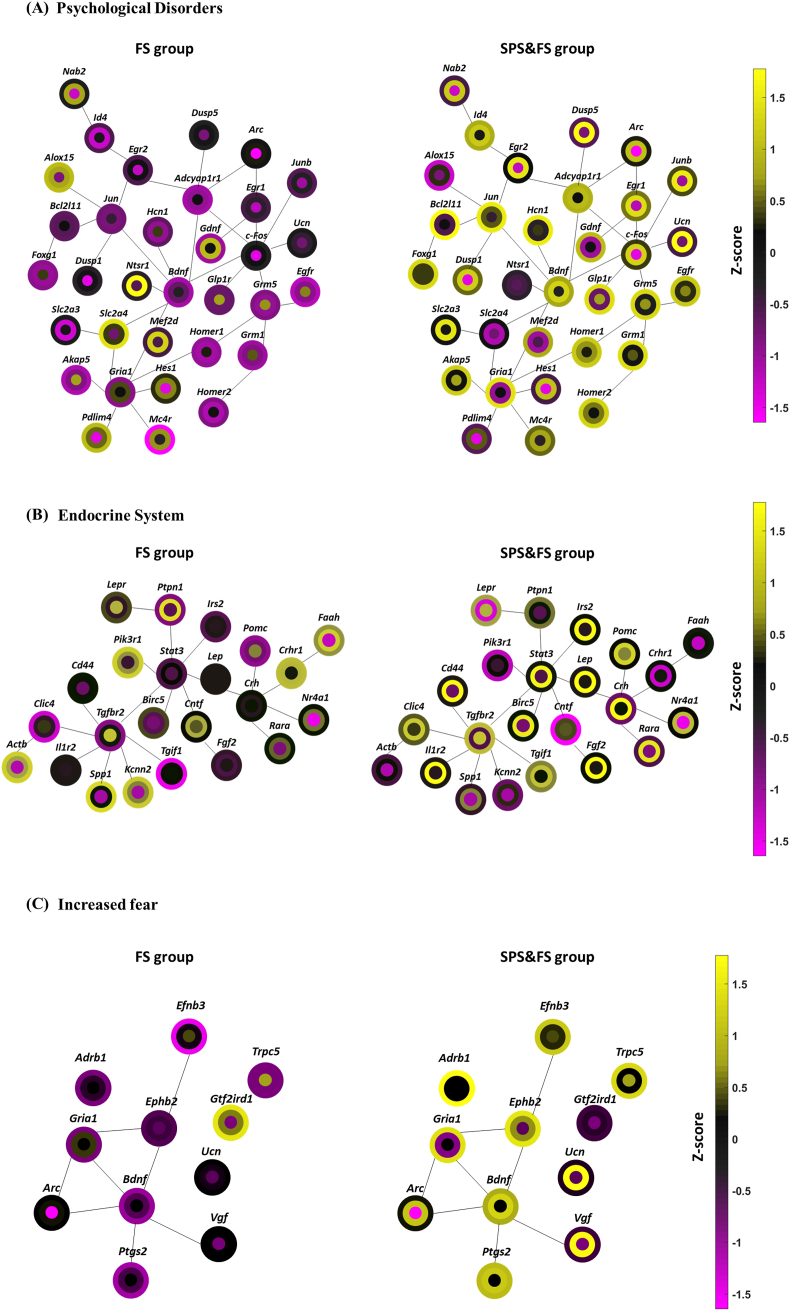
In this section, we compared the ST and LT network dynamic alterations in the FS and SPS&FS groups (Fig. 7). We characterized the temporal networks associated with psychological disorders, endocrine systems, and abnormal fears. Each gene’s expression level is presented as a circle with three rings. The control group’s expression level is in the center. The second-layer ring represents the expression level at the early stage, and the outer ring represents the expression level at the late stage. The linkage between each molecule presents the relation of protein-protein interaction derived from the IPA community network analysis.
Fig. 7.
The community networks represent the short-term (ST) and long-term (LT) alterations related to the psychological disorder, endocrine system and increased fear after FS or SPS&FS exposure. Network analysis of DEGs associated with (A) psychological disorders (B) endocrine system, and (C) increased fear in the FS or SPS&FS model. The expression level of each gene is presented as a circle with three rings; the expression level of the control group is in the center, the second-layer ring represents the expression level at the early stage, and the outer-layer ring represents the expression level at the late stage.
From the network analysis of psychological disorders (Fig. 7A), we found a number of genes associated with DNA-binding transcription factors or RNA-binding proteins: Arc, Junb, Egr1, c-fos, Dusp5, Dusp1, and Egr2 were enriched in the early phase after SPS&FS exposure. In addition, we found an increase in the gene expression of Bdnf (Bdnf engages in dendritic growth, synaptogenesis, and neurogenesis), Ucn (Ucn encodes for a stress response neuropeptide), and Slc2a3 (Slc2a3 encodes for a glucose transporter that mediated glucose uptake). In the late phase after SPS&FS exposure, the network analysis of psychological disorders shows the enrichment of genes such as Bcl2l11 (encodes for a Bcl-2-related protein, participating in apoptosis), Jun (transcription factor), Foxg1 (neural survival), Hcn1 (hyperpolarization-activated cyclic nucleotide-gated potassium channel 1), Glp1r (induces insulin secretion), Egfr (cellular growth, differentiation, and apoptosis), and Akap5 (a kinase anchor protein, participating in synaptic plasticity). The network analysis also showed other genes involved in the mGluR-eEF2-AMPAR pathway: Grm5, Grm1, Gria1, Homer1, and Homer2. However, in the FS group, we observed a different condition. A number of the genes in the psychological disorders network were downregulated or less activated. Here we identified only a few genes that were highly activated (z-score around 1–1.5) in the early phase after FS exposure, such as Nab2, Alox15 (lipoxygenase, and inflammation factor), Gdnf, Ntsr1, Mef2d, and Mc4r. We also identified a few genes activated in the late phase after FS exposure, including Alox15 (lipoxygenase), Slc2a4 (encodes for a glucose transporter), and Pdlim4 (encodes for an actin-binding protein). In addition, Bdnf was more activated in the SPS&FS model compared with the FS model in the late phase, and the higher Bdnf activation may imply more dendritic or synaptic formation after extreme stress exposure in the SPS&FS model.
In the network analysis of the endocrine system (Fig. 7B), we found a number of genes were highly activated in the early phase after SPS&FS exposure, including Irs2, Stat3, Lep, Birc5, Fgf2, Pomc, Crh, Rara, Cd44, Clic4, and Il1r2. We observed downregulated gene expression of Crhr1 in the late phase after SPS&FS exposure. Crhr1 encodes a G-protein coupled receptor that binds neuropeptides of the corticotropin-releasing family, and the downregulation of Crhr1 in the late phase of the SPS&FS group could imply the decreased sensitivity to negative feedback inhibition of HPA-axis function in the PTSD condition. In the FS group, genes associated with the endocrine system were less activated, but the pattern was different from the SPS&FS group. Only a few genes were more activated in the early phase after FS exposure, including Ptpn1, Cntf, and Faah. Moreover, other genes such as Actb and Spp1, which encode for extracellular matrix protein, Kcnn2, and Pik3r1 were more activated in the late phase after FS exposure. Crhr1 was activated in both the early and late phases after FS exposure, which is different from the downregulated condition in the SPS&FS model. In the network of increased fear (Fig. 7C), we found genes including Arc, Bdnf, Ucn, Ptgs2, and Vgf were highly activated at the early time point after SPS&FS exposure. In addition, Bdnf and Ptgs2 remained highly activated in the late phase of the SPS&FS group, as were other genes such as Gria1, Ephb2, Adrb1, Efnb3, and Trpc5. In the FS group, Arc, Ucn, and Vgf were activated in the early phase when compared with their levels in the control group, but the activation levels were low, and z-scores ranged between −0.5 and 0.5. We found most of the genes in the network of increased fear in the FS group were downregulated when compared with their levels in the control group; among them, only Gtf2ird1, which encodes for a transcription factor, was shown to have a higher activation level in the late phase after FS exposure. Furthermore, in Appendices Fig. A2, the expression level differences between FS and SPS&FS groups are presented in an alternative format, showing early and late-stage genetic network dynamics using circles with three rings to represent the expression levels of each gene. The center represents the control group, the second-layer ring represents the FS group, and the outer ring represents the SPS&FS group. This provides a clearer understanding of ST and LT network alterations in different groups.
3.7. A summary of temporal alteration in the FS model and the SPS&FS model
A summary of biological processes (Fig. 8) illustrates critical genetic pathways associated with the early and late responses involved in the FS model and the SPS&FS model, the p-values of each pathway was indicated. The biological processes are described in Section 3.3. Understanding this temporal alteration could help identify stage-specific differences between the two models.
Fig. 8.
A summary of biological processes involved in the early (marked with the grey color) and late (marked with the red color) responses after FS and SPS&FS exposure. The p-value of each pathway was indicated. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In this study, we investigated the genetic profiling of the frontal region in the early and late stages after fear conditioning in the FS model or extreme stress exposure in the SPS&FS model. From the genetic analysis, we aimed to elucidate further the genetic differences in the FS model or the model featuring PTSD. To reveal the genetic changes, we first performed the analysis of DEG patterns, and later we identified major early and late responsive pathways and upstream modulators. Further detailed analysis was performed on canonical pathways and networks to characterize the temporal changes in these two models. Here, many DEGs were identified at the early and late phases after FS or SPS&FS exposure. Based on the heat map of DEGs (Fig. 2A) and the PCA results (Fig. 2C), we found distinguishable patterns between the FS and SPS&FS groups and lines of evidence for temporal differences between early and late phases. From the functional enrichment analysis (Fig. 3), we found convergent pathways were enriched in the early stages of the FS and SPS&FS models, including EIF2 signaling, NRF2-mediated oxidative stress response, acute phase responding signaling, and immunology. The early activation of the EIF2 signaling pathway may explain the initiation of general translational control and effective induction of selected genes when adapted to various stress stimuli (Pakos-Zebrucka et al., 2016; Wek et al., 2006). In addition, the activation of the NRF2-mediated oxidative stress response could help balance redox homeostasis and prevent oxidative damage (Bergamini et al., 2004; Bouvier et al., 2017). Furthermore, previous research has indicated that the PERK/eIF2α pathway may mediate SPS-induced neural apoptosis (Wen et al., 2017), and inhibiting Nrf2 activation could lead to an increase in the inflammatory response and oxidative stress (Qiu et al., 2018) in a mouse model. Moreover, acute phase responding signaling is a core part of innate immunity and a first-line defense activated by stress, trauma, infection, and inflammation, and it can help mediate the acquired immune response (Cray et al., 2009; Huang et al., 2019). Furthermore, the activation of the immunology pathway in both the FS and SPS&FS models could be explained from an evolutionary perspective; an enhanced immune system could help repair wounds and prevent further infections, as part of an adaptive response to stressful conditions (Williams and Leaper, 1982). The convergent pathways observed in the FS and SPS&FS models may represent core biological responses to confronting fearful and stressful conditions. Other pathways such as PPAR signaling, VDR/RXR signaling, and corticotropin-releasing hormone signaling pathway were more significant in the early stage after SPS&FS exposure. PPAR signaling was indicated as actively regulating genes associated with lipid and carbohydrate metabolism (Thibaut, 2017), and two other mechanisms were observed to contribute to anxiety formation: (1) neuroinflammation and gene expression of cytokine and (2) the hydrolysis of neuropeptide CCK-4 (Domi et al., 2016; Rudko et al., 2020). Recent studies have suggested PPAR-α, which engages in neurosteroid biosynthesis, is especially critical for the regulation of emotion in PTSD and depression (Locci and Pinna, 2019; Nisbett and Pinna, 2018). The activation of VDR/RXR signaling, which regulates Ca2+ homeostasis and the synthesis of neurotrophins, and increased intracellular calcium levels were found in the mPFC after traumatic stress exposure (Ji et al., 2014; Wen et al., 2012). In the late phase after FS and SPS&FS exposure, pathways such as sirtuin, STAT3, cAMP, lipid metabolism, and Gα signaling pathways were enriched in the late stages of both the FS and SPS&FS models. Deficits in sirtuins were associated with impaired synaptic plasticity, and fear memory formation (Kim et al., 2018). Besides, deleting Sirt1 in the SPS mouse model led to reduced anxiety and freezing time (Li et al., 2019). STAT3 signaling is associated with corticotropin-releasing factor (CRF) via a PKA-CREB mechanism, which can potentially affect glucocorticoid receptor signaling pathways (Mynard et al., 2004). STAT3 signaling in the mid-brain was found to modulate anxiety-like behavior in female mice (Fernandes et al., 2021). In addition, activation of chronic social defeat stress in mice induced lipid dysregulation (Chuang et al., 2010). Chronic enhancement of Gαs signaling in the forebrain (Favilla et al., 2008) and cAMP signaling (Moss et al., 1992) were found to trigger increased anxiety-related behaviors. Noticeably, we found increased fear signaling in the late phase of the SPS&FS model but not in the FS model, the increased fear pathway represents a group of genes that are involved in the development and persistence of fear-related symptoms in PTSD, including adrb1, arc, bdnf, efnb3, ephb2, gria1, gtf2ird1, ptgs2, trpc5, ucn, and vgf, enhanced signaling through this pathway may imply abnormal fears are involved in PTSD maintenance (Fani et al., 2012). In this study, we also characterized some upstream modulators (Fig. 4A) that regulate early and late phases after SPS&FS and FS exposure. In the SPS&FS group, we found modulator genes such as Adcyap1r1 were highly activated in both early and late phases after extreme stress exposure. Adcyap1r1 encodes the pituitary adenylate cyclase-activating polypeptide 1 (PAC1) receptor, reported to participate in controlling the HPA axis and correlated with fear-, anxiety- and stress-related responses in PTSD (Oyola and Handa, 2017; Ressler et al., 2011). Another upstream modulatory gene, Lep encodes the leptin protein and participates in the downregulation of CRH (Arvaniti et al., 2001). Lep was more active in the early phase of the SPS&FS group. Previous studies have also indicated acutely increased leptin while responding to psychological stress (Epel et al., 2001). In addition, we found upregulated Gdnf, which is associated with neurogenesis and neuronal plasticity (Uchida et al., 2011). Increased Gdnf expression was found in the late phase after SPS&FS exposure. In the FS group, Tsc2 was enriched in both the early and late phases. An increase in Tsc2 expression could enhance the inhibitory regulation of the rapamycin (mTOR) pathway. The mTOR pathway has an important role in synaptic plasticity formation, cell growth, and proliferation (Bassetti et al., 2021; Tang et al., 2002). Lower activation of Tsc2 was characterized in the SPS&FS group, which could also imply less inhibition of the mTOR pathway in the SPS&FS group. To sum up, from the gene analysis of upstream modulators, we identified genes of modulators that contributed to the stress response and vulnerability to extreme stress; and we found that most of the genes of upstream modulators were less active in the FS group. In the analysis of canonical pathways, we identified a number of metabolic (Fig. 5) or signaling (Fig. 6) pathways activated differentially in the early and late phases. In the SPS&FS group, we identified pathways that trended toward activation in the early phase, followed by decreased activation in the late phase, including pathways of gluconeogenesis, glycolysis, the TCA cycle (de Guia et al., 2014), glutathione redox reactions, phospholipases, and oxidative phosphorylation (Richards et al., 2011; Spiers et al., 2014). These pathways are associated with changes in cortisol levels, hence the late-phase declined activation in these pathways may be due to the decreased sensitivity to negative feedback inhibition of HPA-axis regulation and low cortisol levels in the SPS&FS model. In comparison, regarding these pathways related to changes in cortisol levels, we found general activation without declines in the late phase in the FS model. In the analysis of signaling pathways (Fig. 6), we found enhanced activation in pathways such as CREB signaling in neurons, regulation by stathmin1, estrogen receptor signaling, and cAMP-mediated signaling in the late phase of the SPS&FS model. This result may be explained by the following reasons: (1) Activation of CREB signaling may represent enhanced gene expression control for long-term plasticity (Silva et al., 1998), and phosphorylation of CREB can also increase BDNF expression (Hetman and Kharebava, 2006). (2) Stathmin has been demonstrated as an important regulator of fear expression (Shumyatsky et al., 2002). (3) Reports have also suggested PTSD symptoms were affected by the cycle of estrogen levels: Low estrogen levels enhance phobic anxiety and depression (Glover et al., 2015; Ney et al., 2018; Nillni et al., 2015). (4) cAMP has been indicated as important in activating several intracellular signaling pathways, especially the cAMP-PKA pathway, which is important for synaptic plasticity and long-term memory formation (Waltereit and Weller, 2003). In addition, we noticed declined activation of EIF2 signaling in the late phase of the SPS&FS model, and EIF2 signaling activation has been suggested against anxiety/depressive-like behaviors (Lin and Sibille, 2015). Decreased EIF2 signaling may potentially contribute to enhanced anxiety and depression in the late phase of the SPS&FS model. From the network analysis related to psychological disorders (Fig. 7A), a number of genes associated with DNA-binding transcription factors or RNA-binding proteins were enriched in the early phase after SPS&FS exposure (Arc, Junb, Egr1, c-fos, Dusp5, Dusp1, and Egr2). In addition, we found an increase in Bdnf gene expression levels in both early and late phases after SPS&FS exposure. These outcomes reflect an early response to stress mediation and elevated Bdnf may imply an increase in synaptic plasticity and underlying LTPs (Minichiello, 2009). In comparison, a considerable number of genes from the FS group were downregulated or less activated in the psychological disorders network and the gene expression pattern was different from the SPS&FS group. In the network analysis of endocrine systems (Fig. 7B), one noticeable aspect is that Crhr1 was activated in both the early and late phases after FS exposure, but a reversed condition of downregulated Crhr1 gene expression was characterized in the late phase after SPS&FS exposure. This result may indicate the dysregulated negative feedback inhibition of HPA-axis function with less activation of molecular signaling for neuropeptides of the corticotropin-releasing family. In the network analysis of increased fear, Bdnf, Ptgs2, Gria1, Ephb2, Efnb3, Adrb1, and Trpc5 were activated in the late phase of the SPS&FS group. Bdnf as mentioned previously, is important for dendritic growth, synaptogenesis, and neurogenesis (Eadie et al., 2005; Henry et al., 2007; Horch, 2004). Ptgs2 encodes cyclo-oxygenase 2, which trended toward increasing when fear expression increased (Cho et al., 2016). Ephb2 and Ephb3 are important for synaptic efficacy and memory formation (Lai and Ip, 2009), and the adrenergic receptors Adrb1 and Trpc5 have been implied in fear memory and anxiety behavior (Riccio et al., 2009; Roozendaal et al., 2004; Rudoy and Van Bockstaele, 2007). For long-term fear regulation, we found a considerable number of genes associated with increased fears were activated in the SPS&FS group. In contrast, these genes were less activated in the FS group, which may explain the abnormal fear maintenance in PTSD. These network dynamics have revealed temporal genetic changes and a tendency toward pathological network alterations for PTSD. In conclusion, understanding the genetic profile of the frontal region revealed the temporal difference in the early and late stages after fear conditioning or extreme stress and further elucidated the biological progression involved in PTSD pathology, which may lead to better interventions for targeting particular temporal stages with specific pathways in PTSD treatment.
5. Conclusion
The frontal area is a crucial region involved in the top-down control of fear regulation. Our results demonstrate the temporal genetic alterations of the frontal area at the transcriptome level, and we compared them in the fear condition model and a model simulating the fear-related features of PTSD. Here, we identified differential expressional patterns in the two models, as well as stage-specific pathways involved in early or late responses and the temporal changes of genetic community networks (including psychological disorder, endocrine system, and increased fear). These results elucidate the crucial molecules involved in the early and late responses in the FS model and the model featuring PTSD. Further understanding of the temporal alterations could help better target stage-specific pathways and help elucidate the differences between the two models.
Credit author statement
Shao-Han Chang: Writing – original draft, data collection, Formal analysis, and Conceptualization, Yao-Ming Chang: Validation, analysis method instruction, Huan-Yuan Chen: Technical support, Fu-Zen Shaw: Conceptualization, Validation, Supervision, Bai-Chuang Shyu: Conceptualization, Validation, Supervision
Funding
This work was supported by the founding of the Ministry of Science and Technology of the Republic of China (Taiwan, MOST 109-2320-B-001-010).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
The authors gratefully acknowledge the support from the Institute of Biomedical Sciences (IBMS), Academia Sinica, Taiwan and also Academia Sinica Inflammation Core Facility, IBMS for technical support. The core facility is funded by the Academia Sinica Core Facility and Innovative Instrument Project (AS-CFII-111-213).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2023.100569.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Ahi J., Radulovic J., Spiess J. The role of hippocampal signaling cascades in consolidation of fear memory. Behav. Brain Res. 2004;149:17–31. doi: 10.1016/s0166-4328(03)00207-9. [DOI] [PubMed] [Google Scholar]
- Almada R.C., Coimbra N.C., Brandao M.L. Medial prefrontal cortex serotonergic and GABAergic mechanisms modulate the expression of contextual fear: intratelencephalic pathways and differential involvement of cortical subregions. Neuroscience. 2015;284:988–997. doi: 10.1016/j.neuroscience.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Arvaniti K., Huang Q., Richard D. Effects of leptin and corticosterone on the expression of corticotropin-releasing hormone, agouti-related protein, and proopiomelanocortin in the brain of ob/ob mouse. Neuroendocrinology. 2001;73:227–236. doi: 10.1159/000054639. [DOI] [PubMed] [Google Scholar]
- Bassetti D., Luhmann H.J., Kirischuk S. Presynaptic GABAB receptor-mediated network excitation in the medial prefrontal cortex of Tsc2(+/-) mice. Pflügers Archiv. 2021;473:1261–1271. doi: 10.1007/s00424-021-02576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini C.M., Gambetti S., Dondi A., Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharmaceut. Des. 2004;10:1611–1626. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- Bouvier E., Brouillard F., Molet J., Claverie D., Cabungcal J.H., Cresto N., Doligez N., Rivat C., Do K.Q., Bernard C., et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatr. 2017;22:1701–1713. doi: 10.1038/mp.2016.144. [DOI] [PubMed] [Google Scholar]
- Brinley-Reed M., Mascagni F., McDonald A.J. Synaptology of prefrontal cortical projections to the basolateral amygdala: an electron microscopic study in the rat. Neurosci. Lett. 1995;202:45–48. doi: 10.1016/0304-3940(95)12212-5. [DOI] [PubMed] [Google Scholar]
- Careaga M.B.L., Girardi C.E.N., Suchecki D. Understanding posttraumatic stress disorder through fear conditioning, extinction and reconsolidation. Neurosci. Biobehav. Rev. 2016;71:48–57. doi: 10.1016/j.neubiorev.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Cho J.H., Lee I., Hammamieh R., Wang K., Baxter D., Scherler K., Etheridge A., Kulchenko A., Gautam A., Muhie S., et al. Molecular evidence of stress-induced acute heart injury in a mouse model simulating posttraumatic stress disorder. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3188–3193. doi: 10.1073/pnas.1400113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.H., Shyu B.C. Horizons in Neuroscience Research. Nova Science Publishers; Hauppauge, NY: 2022. [Google Scholar]
- Cho J.H., Huang B.S., Gray J.M. RNA sequencing from neural ensembles activated during fear conditioning in the mouse temporal association cortex. Sci. Rep. 2016;6 doi: 10.1038/srep31753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J.C., Cui H., Mason B.L., Mahgoub M., Bookout A.L., Yu H.G., Perello M., Elmquist J.K., Repa J.J., Zigman J.M., Lutter M. Chronic social defeat stress disrupts regulation of lipid synthesis. J. Lipid Res. 2010;51:1344–1353. doi: 10.1194/jlr.M002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cray C., Zaias J., Altman N.H. Acute phase response in animals: a review. Comp. Med. 2009;59:517–526. [PMC free article] [PubMed] [Google Scholar]
- de Guia R.M., Rose A.J., Herzig S. Glucocorticoid hormones and energy homeostasis. Horm. Mol. Biol. Clin. Invest. 2014;19:117–128. doi: 10.1515/hmbci-2014-0021. [DOI] [PubMed] [Google Scholar]
- Desmedt A., Marighetto A., Piazza P.V. Abnormal fear memory as a model for posttraumatic stress disorder. Biol. Psychiatr. 2015;78:290–297. doi: 10.1016/j.biopsych.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Domi E., Uhrig S., Soverchia L., Spanagel R., Hansson A.C., Barbier E., Heilig M., Ciccocioppo R., Ubaldi M. Genetic deletion of neuronal PPARgamma enhances the emotional response to acute stress and exacerbates anxiety: an effect reversed by rescue of amygdala PPARgamma function. J. Neurosci. 2016;36:12611–12623. doi: 10.1523/JNEUROSCI.4127-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie B.D., Redila V.A., Christie B.R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Elzinga B.M., Bremner J.D. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect. Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enman N.M., Arthur K., Ward S.J., Perrine S.A., Unterwald E.M. Anhedonia, reduced cocaine reward, and dopamine dysfunction in a rat model of posttraumatic stress disorder. Biol. Psychiatr. 2015;78:871–879. doi: 10.1016/j.biopsych.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E., Lapidus R., McEwen B., Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Fani N., Tone E.B., Phifer J., Norrholm S.D., Bradley B., Ressler K.J., Kamkwalala A., Jovanovic T. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med. 2012;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favilla C., Abel T., Kelly M.P. Chronic Galphas signaling in the striatum increases anxiety-related behaviors independent of developmental effects. J. Neurosci. 2008;28:13952–13956. doi: 10.1523/JNEUROSCI.4986-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M.F., Lau D., Sharma S., Fulton S. Anxiety-like behavior in female mice is modulated by STAT3 signaling in midbrain dopamine neurons. Brain Behav. Immun. 2021;95:391–400. doi: 10.1016/j.bbi.2021.04.013. [DOI] [PubMed] [Google Scholar]
- Fischer A., Sananbenesi F., Schrick C., Spiess J., Radulovic J. Cyclin-dependent kinase 5 is required for associative learning. J. Neurosci. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. 20026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S.X., Son E.W., Yao R. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinf. 2018;19:534. doi: 10.1186/s12859-018-2486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover E.M., Jovanovic T., Norrholm S.D. Estrogen and extinction of fear memories: implications for posttraumatic stress disorder treatment. Biol. Psychiatr. 2015;78:178–185. doi: 10.1016/j.biopsych.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R.A., Hughes S.M., Connor B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur. J. Neurosci. 2007;25:3513–3525. doi: 10.1111/j.1460-9568.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- Hetman M., Kharebava G. Survival signaling pathways activated by NMDA receptors. Curr. Top. Med. Chem. 2006;6:787–799. doi: 10.2174/156802606777057553. [DOI] [PubMed] [Google Scholar]
- Horch H.W. Local effects of BDNF on dendritic growth. Rev. Neurosci. 2004;15:117–129. doi: 10.1515/revneuro.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- Huang C.F., Chiu S.Y., Huang H.W., Cheng B.H., Pan H.M., Huang W.L., Chang H.H., Liao C.C., Jiang S.T., Su Y.C. A reporter mouse for non-invasive detection of toll-like receptor ligands induced acute phase responses. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-55281-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Ji L.L., Tong L., Peng J.B., Jin X.H., Wei D., Xu B.K., Wang Z.Y. Changes in the expression of the vitamin D receptor and LVSCCA1C in the rat hippocampus submitted to single prolonged stress. Mol. Med. Rep. 2014;9:1165–1170. doi: 10.3892/mmr.2014.1934. [DOI] [PubMed] [Google Scholar]
- Kalueff A.V., Olivier J.D., Nonkes L.J., Homberg J.R. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci. Biobehav. Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Karalis N., Dejean C., Chaudun F., Khoder S., Rozeske R.R., Wurtz H., Bagur S., Benchenane K., Sirota A., Courtin J., Herry C. 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nat. Neurosci. 2016;19:605–612. doi: 10.1038/nn.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Kim H.S., Kaang B.K. Elevated contextual fear memory by SIRT6 depletion in excitatory neurons of mouse forebrain. Mol. Brain. 2018;11:49. doi: 10.1186/s13041-018-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup K.G., Tuvnes F.A., Steffenach H.A., Murison R., Moser E.I., Moser M.B. Reduced fear expression after lesions of the ventral hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A., Green J., Pollard J., Jr., Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K.O., Ip N.Y. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr. Opin. Neurobiol. 2009;19:275–283. doi: 10.1016/j.conb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Li W., Guo B., Tao K., Li F., Liu Z., Yao H., Feng D., Liu X. Inhibition of SIRT1 in hippocampal CA1 ameliorates PTSD-like behaviors in mice by protections of neuronal plasticity and serotonin homeostasis via NHLH2/MAO-A pathway. Biochem. Biophys. Res. Commun. 2019;518:344–350. doi: 10.1016/j.bbrc.2019.08.060. [DOI] [PubMed] [Google Scholar]
- Li H., Su P., Lai T.K., Jiang A., Liu J., Zhai D., Campbell C.T., Lee F.H., Yong W., Pasricha S., et al. The glucocorticoid receptor-FKBP51 complex contributes to fear conditioning and posttraumatic stress disorder. J. Clin. Invest. 2020;130:877–889. doi: 10.1172/JCI130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Krstov M., Young E.A. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- Lin L.C., Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol. Psychiatr. 2015;20:377–387. doi: 10.1038/mp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu I.Y., Lyons W.E., Mamounas L.A., Thompson R.F. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J. Neurosci. 2004;24:7958–7963. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci A., Pinna G. Stimulation of peroxisome proliferator-activated receptor-alpha by N-palmitoylethanolamine engages allopregnanolone biosynthesis to modulate emotional behavior. Biol. Psychiatr. 2019;85:1036–1045. doi: 10.1016/j.biopsych.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Mamiya N., Fukushima H., Suzuki A., Matsuyama Z., Homma S., Frankland P.W., Kida S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J. Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- McFarlane A.C., Lawrence-Wood E., Van Hooff M., Malhi G.S., Yehuda R. The need to take a staging approach to the biological mechanisms of PTSD and its treatment. Curr. Psychiatr. Rep. 2017;19:10. doi: 10.1007/s11920-017-0761-2. [DOI] [PubMed] [Google Scholar]
- Mikics E., Baranyi J., Haller J. Rats exposed to traumatic stress bury unfamiliar objects--a novel measure of hyper-vigilance in PTSD models? Physiol. Behav. 2008;94:341–348. doi: 10.1016/j.physbeh.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L., Pitman R.K., Quirk G.J. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol. Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Moss S.J., Smart T.G., Blackstone C.D., Huganir R.L. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science. 1992;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- Mynard V., Latchoumanin O., Guignat L., Devin-Leclerc J., Bertagna X., Barre B., Fagart J., Coqueret O., Catelli M.G. Synergistic signaling by corticotropin-releasing hormone and leukemia inhibitory factor bridged by phosphorylated 3',5'-cyclic adenosine monophosphate response element binding protein at the Nur response element (NurRE)-signal transducers and activators of transcription (STAT) element of the proopiomelanocortin promoter. Mol. Endocrinol. 2004;18:2997–3010. doi: 10.1210/me.2003-0417. [DOI] [PubMed] [Google Scholar]
- Ney L.J., Matthews A., Bruno R., Felmingham K.L. Modulation of the endocannabinoid system by sex hormones: implications for posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2018;94:302–320. doi: 10.1016/j.neubiorev.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Nillni Y.I., Pineles S.L., Patton S.C., Rouse M.H., Sawyer A.T., Rasmusson A.M. Menstrual cycle effects on psychological symptoms in women with PTSD. J. Trauma Stress. 2015;28:1–7. doi: 10.1002/jts.21984. [DOI] [PubMed] [Google Scholar]
- Nisbett K.E., Pinna G. Emerging therapeutic role of PPAR-alpha in cognition and emotions. Front. Pharmacol. 2018;9:998. doi: 10.3389/fphar.2018.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonkes L.J., de Pooter M., Homberg J.R. Behavioural therapy based on distraction alleviates impaired fear extinction in male serotonin transporter knockout rats. J. Psychiatry Neurosci. 2012;37:224–230. doi: 10.1503/jpn.110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm S.D., Jovanovic T., Olin I.W., Sands L.A., Karapanou I., Bradley B., Ressler K.J. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatr. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola M.G., Handa R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017;20:476–494. doi: 10.1080/10253890.2017.1369523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. fifth ed. Elsevier Academic Press; Burlington, MA: 2004. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y.L., Cheng X.N., Bai F., Fang L.Y., Hu H.Z., Sun D.Q. Aucubin protects against lipopolysaccharide-induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomed. Pharmacother. 2018;106:192–199. doi: 10.1016/j.biopha.2018.05.070. [DOI] [PubMed] [Google Scholar]
- Ressler K.J., Mayberg H.S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat. Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler K.J., Mercer K.B., Bradley B., Jovanovic T., Mahan A., Kerley K., Norrholm S.D., Kilaru V., Smith A.K., Myers A.J., et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A., Li Y., Moon J., Kim K.S., Smith K.S., Rudolph U., Gapon S., Yao G.L., Tsvetkov E., Rodig S.J., et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R.S., Nwose E.U., Bwititi P. Biochemical basis of circadian rhythms and diseases: with emphasis on post-traumatic stress disorder. Med. Hypotheses. 2011;77:605–609. doi: 10.1016/j.mehy.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Riedel G., Casabona G., Platt B., Macphail E.M., Nicoletti F. Fear conditioning-induced time- and subregion-specific increase in expression of mGlu5 receptor protein in rat hippocampus. Neuropharmacology. 2000;39:1943–1951. doi: 10.1016/s0028-3908(00)00037-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Hahn E.L., Nathan S.V., de Quervain D.J., McGaugh J.L. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J. Neurosci. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudko O.I., Tretiakov A.V., Naumova E.A., Klimov E.A. Role of PPARs in progression of anxiety: literature analysis and signaling pathways reconstruction. PPAR Res. 2020;2020 doi: 10.1155/2020/8859017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy C.A., Van Bockstaele E.J. Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2007;31:1119–1129. doi: 10.1016/j.pnpbp.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumyatsky G.P., Tsvetkov E., Malleret G., Vronskaya S., Hatton M., Hampton L., Battey J.F., Dulac C., Kandel E.R., Bolshakov V.Y. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- Silva A.J., Kogan J.H., Frankland P.W., Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Spiers J.G., Chen H.J., Sernia C., Lavidis N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2014;8:456. doi: 10.3389/fnins.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.J., Reis G., Kang H., Gingras A.C., Sonenberg N., Schuman E.M. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut F. Anxiety disorders: a review of current literature. Dialogues Clin. Neurosci. 2017;19:87–88. doi: 10.31887/DCNS.2017.19.2/fthibaut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P., Fadok J.P., Luthi A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Uchida S., Hara K., Kobayashi A., Otsuki K., Yamagata H., Hobara T., Suzuki T., Miyata N., Watanabe Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- van der Kolk B.A., Pelcovitz D., Roth S., Mandel F.S., McFarlane A., Herman J.L. Dissociation, somatization, and affect dysregulation: the complexity of adaptation of trauma. Am. J. Psychiatr. 1996;153:83–93. doi: 10.1176/ajp.153.7.83. [DOI] [PubMed] [Google Scholar]
- VanElzakker M.B., Dahlgren M.K., Davis F.C., Dubois S., Shin L.M. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol. Learn. Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes R.P. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Waltereit R., Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol. Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- Wang W., Liu Y., Zheng H., Wang H.N., Jin X., Chen Y.C., Zheng L.N., Luo X.X., Tan Q.R. A modified single-prolonged stress model for post-traumatic stress disorder. Neurosci. Lett. 2008;441:237–241. doi: 10.1016/j.neulet.2008.06.031. [DOI] [PubMed] [Google Scholar]
- Wang H.N., Bai Y.H., Chen Y.C., Zhang R.G., Wang H.H., Zhang Y.H., Gan J.L., Peng Z.W., Tan Q.R. Repetitive transcranial magnetic stimulation ameliorates anxiety-like behavior and impaired sensorimotor gating in a rat model of post-traumatic stress disorder. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R.C., Jiang H.Y., Anthony T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Wen Y., Li B., Han F., Wang E., Shi Y. Dysfunction of calcium/calmodulin/CaM kinase IIalpha cascades in the medial prefrontal cortex in post-traumatic stress disorder. Mol. Med. Rep. 2012;6:1140–1144. doi: 10.3892/mmr.2012.1022. [DOI] [PubMed] [Google Scholar]
- Wen L., Xiao B., Shi Y., Han F. PERK signalling pathway mediates single prolonged stress-induced dysfunction of medial prefrontal cortex neurons. Apoptosis. 2017;22:753–768. doi: 10.1007/s10495-017-1371-5. [DOI] [PubMed] [Google Scholar]
- Williams N.A., Leaper D.J. Oxford University Press; New York: 1982. Wounds: Biology and Management; pp. 71–87. [Google Scholar]
- Wuchty S., Myers A.J., Ramirez-Restrepo M., Huentelman M., Richolt R., Gould F., Harvey P.D., Michopolous V., Steven J.S., Wingo A.P., et al. Integration of peripheral transcriptomics, genomics, and interactomics following trauma identifies causal genes for symptoms of post-traumatic stress and major depression. Mol. Psychiatr. 2021 doi: 10.1038/s41380-021-01084-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R., LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zannas A.S., Wiechmann T., Gassen N.C., Binder E.B. Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. 2016;41:261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.