Abstract
The human gut microbiota changes under the influence of environmental and genetic factors, affecting human health. Extensive studies have revealed that the gut microbiome is closely associated with many non-intestinal diseases. Among these, the influence of the gut microbiome on cancer biology and the efficacy of cancer therapy has attracted much attention. Prostate cancer cells are affected by direct contact with the microbiota of local tissues and urine, and a relationship between prostate cancer cells and the gut microbiota has been suggested. In the human gut microbiota, bacterial composition differs depending on prostate cancer characteristics, such as histological grade and castration resistance. Moreover, the involvement of several intestinal bacteria in testosterone metabolism has been demonstrated, suggesting that they may affect prostate cancer progression and treatment through this mechanism. Basic research indicates that the gut microbiome also plays an important role in the underlying biology of prostate cancer through multiple mechanisms owing to the activity of microbial-derived metabolites and components. In this review, we describe the evidence surrounding the emerging relationship between the gut microbiome and prostate cancer, termed the “gut-prostate axis.”
Keywords: Bacteria, Gut microbiome, Host microbial interactions, Prostate cancer, Testosterone
INTRODUCTION
Various bacterial species are present on every surface of the human body and form unique flora, depending on their location. In particular, there are approximately 1013–1014 total bacteria in the colon, making the gut microbiota the largest flora in the body [1]. It is now known that the bacteria that make up the gut microbiota interact with us through various factors. Lifestyle factors such as diet and exercise are the primary influencers of the composition of the gut microbiota [2]. In turn, intestinal bacterial components and their metabolites can influence human biological systems such as the immune system [3].
Some bacteria-induced diseases have long been known and studied; however, very few commensal bacteria have been targeted for the treatment and prevention of these diseases [4]. Recent advances in bacterial analysis have revealed that numerous diseases are affected by local or distant microbiomes. The study of the relationship between humans and their microbiota first focused on intestinal diseases, such as inflammatory bowel disease (IBD), because the local environment is rich in bacteria [5,6,7,8]. In addition, intervention with the gut microbiota by fecal microbiota transplantation and probiotics is becoming a therapeutic approach for these intestinal diseases [9]. More recently, the systemic effects of intestinal bacteria have been analyzed, and these symbiotic bacteria have been found to influence the state of diseases, including diabetes, dementia, hepatic steatosis, and non-intestinal cancers in distant organs [10,11,12]. Because the interactions between the intestinal tract and other distant organs are connected via their microbiota and believed to play an important role in human health, these major relationships have been termed, for example, the “gut-liver axis, gut-brain axis, and gut-kidney axis” [11,13,14].
Diseases involving the prostate have also been reported in several bacterial studies. Prostate cancer is strongly associated with lifestyle, factors, including diet, and and genetic factors, including race; in particular, animal fat intake and African-American race are respective risk factors for prostate cancer [15,16]. These factors modulate the risk of prostate cancer by altering the gut microbiome. In this review, we investigate the relationship between the gut microbiota and prostate cancer, using findings from previous studies.
CLINICAL RESEARCH ON GUT MICROBIOTA AND PROSTATE CANCER
The gut microbiota can affect the incidence and progression of various types of cancers, but fewer reports consider its relationship with prostate cancer than other cancers. Recent studies in the USA have reported that the composition of the gut microbiota differs depending on the status of prostate cancer. In 2018, Liss et al [17] analyzed the gut microbiota of 133 American men who underwent prostate biopsy and reported a relationship between human prostate cancer and the gut microbiome for the first time. Their cohort primarily included African-Americans and Caucasians. The composition of intestinal bacteria was significantly different between men with positive and those with negative biopsy results (p<0.01), and patients with prostate cancer had a higher ratio of Bacteroides and Streptococcus species in their gut microbiota (p<0.04). The researchers also performed a gene-based functional analysis of gut microbiota, revealing that the folate and arginine metabolism pathways were enriched in patients with prostate cancer. Because of the increased dependency on folate in prostate cancer cells, intensive research has been conducted on the relationship between them, and it has been shown that blood folate levels are correlated with prostate cancer risk and proliferation rate [18,19]. Furthermore, in a mouse model of prostate cancer, oral folate intake affected both the blood and prostate folate levels, and dietary folate deficiency improved cancer pathology [20]. Risk scoring based on 10 microbiome-derived metabolic pathways can detect prostate cancer with higher accuracy than the standard prostate-specific antigen (PSA) test. These results suggest that specific intestinal bacteria and their metabolic functions could play a role in the risk of prostate cancer, similar to that observed in several other diseases.
In another report, Bacteroides massiliensis was more common in the gut microbiota of Caucasian males with prostate cancer than in those with benign prostatic hyperplasia (BPH), whereas Faecalibacterium prausnitzii and Eubacterium rectalis were less common [21]. F. prausnitzii is a common anti-inflammatory bacterium, and its decrease is associated with a higher risk of Crohn’s disease recurrence [22]. In addition, culture supernatants of this bacterium exhibit anti-inflammatory effects by reducing NF-κB activity in intestinal epithelial cells, both in vitro and in vivo [22]. These results suggest that some metabolites secreted by F. prausnitzii may contribute to the prevention of prostate cancer and IBD; therefore, this species is potentially beneficial as a probiotic. The composition of the gut microbiota of non-Hispanic Caucasians did not differ significantly between patients with and without prostate cancer [23]. In Caucasian-centric communities, these results suggest that there may be no major specificity in the gut microbiota of patients with prostate cancer, but some Bacteroides species may predominate.
A few reports on the gut microbiome profile of patients with prostate cancer living outside the USA show different results. Our group has previously analyzed the gut microbiota composition of 152 Japanese patients who underwent prostate biopsy [24]. In this study, patients with high-grade prostate cancer (grade group >2) showed a significantly higher abundance of Alistipes and Lachnospira. Alistipes was enriched in the gut microbiota of patients with colorectal cancer, and Alistepes administration induced colitis in rodents by activating IL-6 signaling and promoting tumorigenesis [25,26]. The same mechanism may be responsible for the progression of prostate cancer. On the other hand, Lachnospira belongs to the family Lachnospiraceae, which is considered to contain beneficial bacteria that have anti-inflammatory effects and locally inhibit colon cancer [27,28]. However, as discussed later, short-chain fatty acids (SCFAs) produced by this family promote prostate cancer growth [29]. Moreover, a new index calculated from the abundance of 18 intestinal bacteria characteristic of patients with high-grade prostate cancer could discriminate the cancer status with greater accuracy than the PSA test (area under the curve [AUC]=0.81 vs. 0.67). Despite the different bacteria identified in studies in the USA, our functional analysis of the gut microbiome of patients with high-grade prostate cancer showed high starch and sucrose metabolism pathways, which are also common pathways seen in Americans. These results support previous reports that the profile of gut microbiota varies by region and race [30,31]. It also suggests that although different intestinal bacteria are involved in prostate cancer risk by region, their functions may be common across regions.
Since the microbiota, which consists of foreign elements, interacts with the immune system, it is often associated with inflammation. Urinary and prostatic tissue-residing microbiota can directly cause prostatitis and prostatic hyperplasia through inflammatory processes [32,33]. Gut microbiota are involved locally in the pathogenesis of these inflammatory diseases, but may also play a role in more distant organs via immune and inflammatory processes [34]. In a previous study, we found that men with enlarged prostates had significantly higher Firmicutes/Bacteroidetes ratios in the gut microbiota than those without enlarged prostates [35]. An imbalance between Firmicutes and Bacteroidetes in the human gut microbiota is considered an indicator of dysbiosis, and has also been observed in patients with obesity, IBD, nonalcoholic steatohepatitis, and myocarditis, suggesting a link to systemic inflammation [36,37,38,39,40]. Thus, our results indicate that inflammation caused by intestinal bacteria may also affect distant prostate cancers. Furthermore, gut microbiota may be associated not only with hypertrophy but also with inflammatory carcinogenesis in the prostate. In the future, there is a need to investigate the independent impact of these bacterial factors on prostate cancer risk, as both gut microbiota and prostate cancer development are affected by various lifestyle factors, such as diet and exercise.
THE REGULATION OF TESTOSTERONE BY INTESTINAL BACTERIA
Some bacteria reportedly affect testosterone levels in humans through deglucuronidation of testosterone in the gut, wherein the biologically active form is subsequently reabsorbed into the host [41]. Conversely, testosterone can alter the composition of gut microbiota [42,43]. We have previously shown that the fraction of the Firmicutes phylum in the gut microbiota of elderly Japanese men (median age, 71.0 y) was significantly correlated with serum testosterone levels (r=0.3323, p=0.01) [44]. A similar trend was observed in younger cohorts. Shin et al. also found that in 31 men with a median age of 37.45 years, serum testosterone levels were positively correlated with several bacteria belonging to the phylum Firmicutes, including Megamonas (r=0.4161, p=0.02) and Ruminococcus (r=0.4589, p=0.01) [45]. Similar to our study, in a group of adolescents (median age, 10.99 y), testosterone levels were positively correlated with Ruminococcus (r=0.371, p=0.040) and Dorea (r=0.471, p=0.007), both of which belong to Firmicutes [46].
Despite age-related changes in the gut microbiota composition, bacteria belonging to the phylum Firmicutes may be responsible for testosterone regulation in hosts. Fecal transplantation from adult male mice into immature female mice elevated their testosterone levels, indicating that the gut microbiome of males produces testosterone [47]. Oral administration of Lactobacillus, which belongs to Firmicutes, also increased testosterone levels in mice [48,49]. These results suggest that probiotics may improve testosterone-related health conditions and diseases in men.
The gut microbiota may also influence the development of breast cancer because of its ability to deconjugate conjugated estrogen excreted in the intestinal tract [50]. In addition, intestinal bacteria can metabolize estrogen-like compounds such as enterodiol [51]. Similarly, bacterial-derived testosterone analogs may be relevant in prostate cancer, and further studies are needed in this category.
The gut microbiota has recently been described as an androgen-producing “organ.” Accordingly, androgen deprivation therapy administered as a systemic treatment for prostate cancer can alter the gut microbiota. Sfanos et al [52] analyzed the gut microbiota of 30 American men with various stages of prostate cancer and healthy controls. The results showed that patients with prostate cancer had a significantly different microbial composition from healthy controls (p=0.02). In addition, treatment with anti-androgen therapy reduced the diversity of the gut microbiota in these patients and increased the proportion of Akkermansia muciniphila (0.002 vs. 0.055, p=0.002), which is also known to increase responses to immunotherapy [53]. In an in vivo study using a mouse model, Akkermansia muciniphila decreased in the gut microbiota after castration, and testosterone propionate supplementation increased this bacterium [54]. These conflicting results may be due to the underlying differences in the composition of the gut microbiota in humans and rodents. Further studies are needed to clarify the interaction between testosterone and Akkermansia muciniphila. Thus, during treatment selection, we should consider the possibility that androgen deprivation therapy influences the efficacy of other types of cancer treatments through gut microbiome-mediated mechanisms.
THE ROLE OF THE GUT MICROBIOME IN PROSTATE CANCER PATHOGENESIS
Although human microbiome studies suggest that the gut microbiota plays a role in prostate cancer progression, few basic studies have elucidated the mechanisms by which this can occur. In other diseases, including non-intestinal diseases, basic research has revealed that intestinal immunity or bacterial-derived metabolites influenced by the gut microbiota can have augmenting or suppressive effects. Thus, the two-sided mechanisms by which the intestinal microbiota affects human health are complex, but have become clearer in recent studies.
Previous findings have shown that animal fat intake promotes cancer growth in a prostate cancer mouse model, specifically in prostate-specific Pten knockout mice, through various mechanisms [55,56,57]. In our previous study, oral administration of antibiotics significantly reduced prostate cancer growth in mice fed a high-fat diet (HFD) (p=0.015) [29]. This study provides the first firm evidence that the gut microbiome is involved in prostate cancer progression. We found that this inhibitory effect involved a reduction in SCFAs produced by intestinal bacteria. SCFAs, major metabolites of the gut microbiota, stimulate systemic as well as local prostatic insulin-like growth factor 1 (IGF-1) production and activate the IGF-1 signaling pathway in prostate cancer cells, which in turn promotes cancer growth [29]. The results of our study suggest that influencing the gut microbiota by diet and medications may play a role in the pathogenesis of prostate cancer.
In contrast, butyrate, an SCFA, exerts anti-inflammatory effects through the activation of Gpr109a, inhibits carcinogenesis in the gut, and is a beneficial metabolite in humans [58]. Interestingly, this metabolite has opposing functions in the prostate and gut, indicating that the profile of healthy and beneficial gut microbiota may differ for each disease. For example, cohort studies have shown that some dairy products increase the risk of prostate cancer, whereas the opposite is observed in colorectal cancer [59,60,61]. The cause has not been identified, although the high butyrate content in milk suggests that this compound may be responsible for this discrepancy [62]. Since obese persons are at an increased risk of high-grade prostate cancer and have been found to have high levels of SCFAs in their stool, SCFAs are likely to be involved in prostate cancer risk [63,64].
Other mechanisms also suggest the involvement of gut microbiota in prostate cancer. We found that HFD-induced dysbiosis of the gut microbiota in mice elevated intestinal permeability and promoted lipopolysaccharide (LPS) leakage from this tract [65]. Serum LPS levels were significantly higher in prostate cancer model mice fed HFD than in those fed the control diet (p=0.005). Prostate local histamine signaling activated by LPS can promote inflammatory prostate cancer growth by stimulating the interleukin-6 (IL6)-signal transducer and activator of transcription 3 (STAT3) axis and increasing myeloid-derived suppressor cells (MDSCs); however, the LPS/toll-like receptor 4 (TLR4) signaling block inhibited HFD-induced prostate cancer growth [65]. In another report, it was found that gut dysbiosis and increased intratumoral LPS promoted prostate cancer progression and docetaxel resistance via the IL6-STAT3 axis in vivo and in vitro [66]. Intestinal bacterial components have also been reported to cause a variety of diseases in distant organs via inflammatory and immune mechanisms. The influx of gut microbiota-derived endotoxins in the portal circulation promotes TLR4 expression and is associated with hepatic inflammation and steatosis [67,68]. Obesity-induced hepatic translocation of lipoteichoic acid, one of the components of the cell wall of gram-positive bacteria, accelerates the senescence of hepatic stellate cells, promoting HCC progression via prostaglandin E2-mediated suppression of antitumor immunity [69]. Endotoxins and amyloids from gram-negative bacteria can penetrate the blood-brain barrier and induce amyloid β aggregation and neuroinflammation in the central nervous system, suggesting that bacterial molecules and metabolites may be involved in the onset and progression of Alzheimer’s disease [70]. Bacterial components other than LPS may also play key roles in the inflammatory and immune-related development and progression of prostate cancer.
Modulation of the immune system and other factors by intestinal bacteria is associated with the efficacy of various prostate cancer treatments. In an in vitro gut microbiota-simulated model, Daisley et al [71] demonstrated that abiraterone acetate, a hormone-based chemotherapeutic used to treat prostate cancer, increased Akkamansia muciniphila and suggested that the species enhanced the supply of bacteria-derived vitamin K2, which in turn increased the efficacy of this medication. Akkamansia in the gut microbiota can also improve the efficacy of immunotherapeutic checkpoint inhibitors in several cancer types [53]. In prostate cancer, extracellular vesicles from this species activate antitumor immunity by interacting with CD8-positive T cells and macrophages [72]. Gut microbiota can also affect immunity in the prostate, a distant organ, through bacterial components that circulate in the body.
Other mechanisms of prostate cancer regulation by androgens in the gut microbiome have also been demonstrated. Pernigoni et al [73] found that the gut microbiota of patients and murine models with castration-resistant prostate cancer (CRPC) was enriched with Ruminococcus and Bacteroides genera. These intestinal bacteria transform androgens into their active forms and are involved in prostate cancer progression by supplying the active androgens. Indeed, elimination of the microbiota by antibiotics delayed the acquisition of castration resistance in prostate cancer, but transplantation with stools of patients with CRPC in mice accelerated their prostate cancer progression. Conversely, transplantation of stools of patients with castration-sensitive prostate cancer or the particular species Prevotella stercorea inhibited cancer progression.
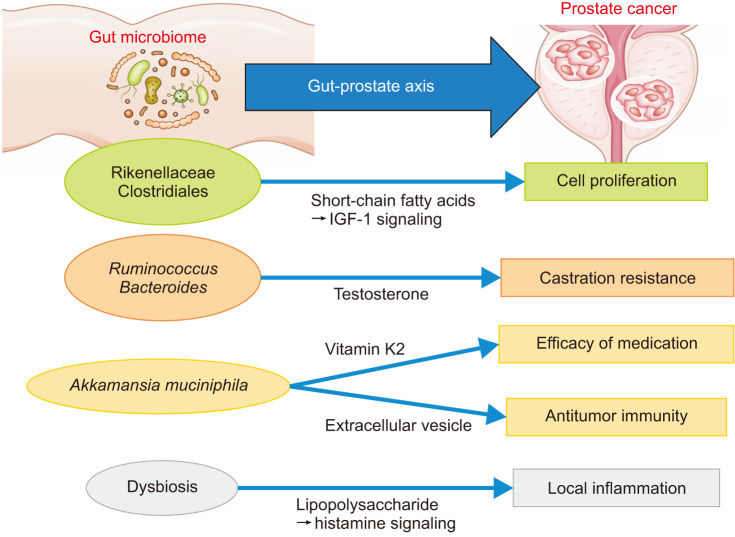
The reliable presence of multiple mechanisms, acting via metabolites, immunity, and androgens, suggests that a complex signaling network mediated by the microbiome is formed between the gut and prostate (Fig. 1). Future studies can help provide a more complete profile of this “gut-prostate axis.” Understanding this axis may lead to improved prevention and treatment of prostate cancer using specialized probiotics or prebiotics. In addition, fecal microbiota transplantation, as a more direct treatment method, has been in clinical trials for the treatment of some intestinal diseases [74], and its application in prostate cancer is expected soon.
Fig. 1. Overview of the “Gut-Prostate Axis”. Specific taxa and dysbiosis in the gut microbiome regulate crucial features of prostate cancer through multiple mechanisms.
PROSTATE CANCER AND THE LOCAL MICROBIOTA
In addition to the gut microbiome, the relationship between prostate cancer risk and the local microbiota has been studied. In the microbiota of human prostate tissue, Cutibacterium acnes, previously classified as Propionibacterium acnes, which commonly colonizes the skin, is more abundant in patients with prostate cancer. Chen and Wei [75] investigated the genomic sequence of C. acnes in three sets of RNA-seq data from prostatic tissues of Australian, Caucasian, and Chinese patients and detected C. acnes in both cancer and adjacent tissue samples, but not in non-cancerous samples. Cavarretta [76] also showed by bacterial genome sequencing of 16 prostatectomy specimens that Propionibacterium was more common in cancerous and peri-cancerous areas than in non-cancerous areas (60% vs. 49%).
In a study of 100 samples from patients with prostate cancer and 50 samples from men without cancer, C. acnes was found to be significantly more prevalent in patients with prostate cancer (60% vs. 26%, p=0.001) [77]. This is important, given that C. acnes can colonize prostate tissue and cause chronic inflammation in a rat model [78]. In addition, Escherichia has been detected in prostate cancer tissues in several studies, and is a genus of interest [79,80]. This genus mainly colonizes the gut, and the colibactin produced by some strains promotes carcinogenesis through DNA damage caused by double-strand breaks [81]. Escherichia-colonized prostate tissue may also have a similar oncogenic effect.
The association of prostate cancer with the microbiota present in urine and prostatic fluid that comes in close contact with the prostate gland has also been investigated, and several small-scale studies using metagenomic analysis have shown a common abundance of Streptococcus in patients with prostate cancer [23,82,83]. Although the mechanism underlying this remains unclear, these results suggest that the microbiome present in urine and prostatic fluid may be more directly involved in prostate cancer than in the gut. However, urine and prostate tissue contain far fewer numbers of bacteria than stool, and sequencing-based methods for detecting microbes in samples with low microbial concentrations are susceptible to contamination. Further verification, including contamination-free sample collection methods and appropriate sequencing controls, is required to obtain reliable results.
The gut microbiome may be indirectly involved in the carcinogenesis and progression of prostate cancer, but the local microbiome also appears to be directly involved. The composition of the microbiota in the prostate is different from that seen in stool, and it is likely that different bacterial associations and activities exist locally compared to indirect actions from the gut microbiota.
CONCLUSIONS
In addition to the importance of the local microbiome, the relationship between the gut microbiota and prostate cancer has been extensively investigated in both basic and clinical studies, and these suggest that the gut-prostate axis houses various bacterial associations through different mechanisms (Table 1). Furthermore, since the gut microbiome affects androgen production, it may be associated with the pathogenesis of several predominant male health conditions, such as the late onset of hypogonadism, erectile dysfunction, and BPH. However, the overall complexity of the axes remains unclear. Both genetic predisposition and environmental factors play a role in the pathogenesis of prostate cancer [84,85]. The gut microbiota is known to be susceptible to the same host factors [86,87]. Further studies should examine the gut-prostate axis as well as relevant genetic and environmental factors to determine the true impact of the gut microbiome on prostate cancer development and progression. These studies are expected to provide further evidence for the importance of the gut microbiome and support its modification for the prevention, diagnosis, and treatment of prostate cancer.
Table 1. List of bacteria-related factors with potential impacts on prostate cancer.
| Bacterial factor | Type | Influence | Study cohort | Location | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Alistipes | Bacterial name | Harmful | Humans (Japanese) | Gut | IL-6 signaling (prediction) | [25] |
| Lachnospira | Bacterial name | Harmful | Humans (Japanese) | Gut | SCFAs production (prediction) | [25] |
| Ruminococcus | Bacterial name | Harmful | Humans/mouse models | Gut | Active androgen supply | [74] |
| Bacteroides | Bacterial name | Harmful | Humans/mouse models | Gut | Active androgen supply | [74] |
| Cutibacterium acnes | Bacterial name | Harmful | Humans | Cancer tissue | Chronic inflammation (prediction) | [76,78] |
| Escherichia | Bacterial name | Harmful | Humans | Cancer tissue | DNA damage via colibactin (prediction) | [80,81] |
| Folate | Bacterial metabolite | Harmful | Humans (African-Americans/Caucasians) | Gut | Metabolism in prostate cancer cells (prediction) | [18] |
| SCFA | Bacterial metabolite | Harmful | Mouse models | Gut | IGF-1 signaling | [30] |
| LPS | Bacterial component | Harmful | Mouse models | Gut | Histamine signaling/IL-6 signaling | [66,67] |
| Dysbiosis | Micrbiome diversity | Harmful | Mouse models | Gut | Intestinal barrier dysfunction/LPS leakage | [66] |
| Faecalibacterium prausnitzii | Bacterial name | Beneficial | Humans (Caucasians) | Gut | Anti-inflammation (prediction) | [22] |
| Akkamansia muciniphila | Bacterial name | Beneficial | Humans/mouse models/cell lines | Gut | Vitamin K2 production | [72] |
| Antitumor immunity via extracellular vesicles | [73] | |||||
| Prevotella stercorea | Bacterial name | Beneficial | Humans/mouse models | Gut | Reduce androgen levels | [74] |
LPS: lipopolysaccharide, SCFA: short-chain fatty acid.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by Japan Society for the Promotion of Science KAKENHI (Grant No. JP21K09421).
- Conceptualization: MM, KF, KH, MADV, AT, HU, NN.
- Data curation: MM.
- Formal analysis: MM.
- Funding acquisition: MM.
- Investigation: MM.
- Methodology: MM.
- Project administration: MM, KF, KH, MADV, AT, HU, NN.
- Resources: MM.
- Software: MM.
- Supervision: AT, HU, NN.
- Validation: MM, KF.
- Visualization: MM.
- Writing – original draft: MM.
- Writing – review & editing: KF, KH, MADV, AT, HU, NN.
References
- 1.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strasser B, Wolters M, Weyh C, Krüger K, Ticinesi A. The effects of lifestyle and diet on gut microbiota composition, inflammation and muscle performance in our aging society. Nutrients. 2021;13:2045. doi: 10.3390/nu13062045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, et al. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25:5578–5589. doi: 10.3748/wjg.v25.i37.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilg H, Adolph TE, Gerner RR, Moschen AR. The intestinal microbiota in colorectal cancer. Cancer Cell. 2018;33:954–964. doi: 10.1016/j.ccell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 7.Ohkusa T, Sato N, Ogihara T, Morita K, Ogawa M, Okayasu I. Fusobacterium varium localized in the colonic mucosa of patients with ulcerative colitis stimulates species-specific antibody. J Gastroenterol Hepatol. 2002;17:849–853. doi: 10.1046/j.1440-1746.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- 8.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Imdad A, Nicholson MR, Tanner-Smith EE, Zackular JP, Gomez-Duarte OG, Beaulieu DB, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;11:CD012774. doi: 10.1002/14651858.CD012774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng NMY, Price CA, McKee AM, Hall LJ, Robinson SD. Exploring the impact of gut microbiota and diet on breast cancer risk and progression. Int J Cancer. 2021;149:494–504. doi: 10.1002/ijc.33496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 12.Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Stavropoulou E, Kantartzi K, Tsigalou C, Konstantinidis T, Romanidou G, Voidarou C, et al. Focus on the gut-kidney axis in health and disease. Front Med (Lausanne) 2021;7:620102. doi: 10.3389/fmed.2020.620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita M, Fujita K, Nonomura N. Influence of diet and nutrition on prostate cancer. Int J Mol Sci. 2020;21:1447. doi: 10.3390/ijms21041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gann PH. Risk factors for prostate cancer. Rev Urol. 2002;4(Suppl 5):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 17.Liss MA, White JR, Goros M, Gelfond J, Leach R, Johnson-Pais T, et al. Metabolic biosynthesis pathways identified from fecal microbiome associated with prostate cancer. Eur Urol. 2018;74:575–582. doi: 10.1016/j.eururo.2018.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price AJ, Travis RC, Appleby PN, Albanes D, Barricarte Gurrea A, Bjørge T, et al. Circulating folate and vitamin B12 and risk of prostate cancer: a collaborative analysis of individual participant data from six cohorts including 6875 cases and 8104 controls. Eur Urol. 2016;70:941–951. doi: 10.1016/j.eururo.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomaszewski JJ, Cummings JL, Parwani AV, Dhir R, Mason JB, Nelson JB, et al. Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate. Prostate. 2011;71:1287–1293. doi: 10.1002/pros.21346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bistulfi G, Foster BA, Karasik E, Gillard B, Miecznikowski J, Dhiman VK, et al. Dietary folate deficiency blocks prostate cancer progression in the TRAMP model. Cancer Prev Res (Phila) 2011;4:1825–1834. doi: 10.1158/1940-6207.CAPR-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golombos DM, Ayangbesan A, O’Malley P, Lewicki P, Barlow L, Barbieri CE, et al. The role of gut microbiome in the pathogenesis of prostate cancer: a prospective, pilot study. Urology. 2018;111:122–128. doi: 10.1016/j.urology.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alanee S, El-Zawahry A, Dynda D, Dabaja A, McVary K, Karr M, et al. A prospective study to examine the association of the urinary and fecal microbiota with prostate cancer diagnosis after transrectal biopsy of the prostate using 16sRNA gene analysis. Prostate. 2019;79:81–87. doi: 10.1002/pros.23713. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita M, Fujita K, Motooka D, Hatano K, Fukae S, Kawamura N, et al. The gut microbiota associated with high-Gleason prostate cancer. Cancer Sci. 2021;112:3125–3135. doi: 10.1111/cas.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao L, Chen Z, et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018;6:70. doi: 10.1186/s40168-018-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschen AR, Gerner RR, Wang J, Klepsch V, Adolph TE, Reider SJ, et al. Lipocalin 2 protects from inflammation and tumorigenesis associated with gut microbiota alterations. Cell Host Microbe. 2016;19:455–469. doi: 10.1016/j.chom.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Meehan CJ, Beiko RG. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol. 2014;6:703–713. doi: 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25:968–976. doi: 10.1038/s41591-019-0458-7. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita M, Fujita K, Hayashi T, Kayama H, Motooka D, Hase H, et al. Gut microbiota-derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res. 2021;81:4014–4026. doi: 10.1158/0008-5472.CAN-20-4090. [DOI] [PubMed] [Google Scholar]
- 30.Nishijima S, Suda W, Oshima K, Kim SW, Hirose Y, Morita H, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016;23:125–133. doi: 10.1093/dnares/dsw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Zhang YH, Huang T, Cai YD. Gene expression profiling gut microbiota in different races of humans. Sci Rep. 2016;6:23075. doi: 10.1038/srep23075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HY, Wang JW, Juan YS, Li CC, Liu CJ, Cho SY, et al. The impact of urine microbiota in patients with lower urinary tract symptoms. Ann Clin Microbiol Antimicrob. 2021;20:23. doi: 10.1186/s12941-021-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyake M, Tatsumi Y, Ohnishi K, Fujii T, Nakai Y, Tanaka N, et al. Prostate diseases and microbiome in the prostate, gut, and urine. Prostate Int. 2022;10:96–107. doi: 10.1016/j.prnil.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. 2020;17:7618. doi: 10.3390/ijerph17207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takezawa K, Fujita K, Matsushita M, Motooka D, Hatano K, Banno E, et al. The Firmicutes/Bacteroidetes ratio of the human gut microbiota is associated with prostate enlargement. Prostate. 2021;81:1287–1293. doi: 10.1002/pros.24223. [DOI] [PubMed] [Google Scholar]
- 36.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 37.Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallianou N, Christodoulatos GS, Karampela I, Tsilingiris D, Magkos F, Stratigou T, et al. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease: current evidence and perspectives. Biomolecules. 2021;12:56. doi: 10.3390/biom12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawicka-Śmiarowska E, Moniuszko-Malinowska A, Kamiński KA. Which microbes like my diet and what does it mean for my heart? Nutrients. 2021;13:4146. doi: 10.3390/nu13114146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, et al. High-fat, Western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. 2021;10:3164. doi: 10.3390/cells10113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross TL, Kasahara K, Rey FE. Sexual dimorphism of cardiometabolic dysfunction: gut microbiome in the play? Mol Metab. 2018;15:70–81. doi: 10.1016/j.molmet.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barroso A, Santos-Marcos JA, Perdices-Lopez C, Vega-Rojas A, Sanchez-Garrido MA, Krylova Y, et al. Neonatal exposure to androgens dynamically alters gut microbiota architecture. J Endocrinol. 2020;247:69–85. doi: 10.1530/JOE-20-0277. [DOI] [PubMed] [Google Scholar]
- 43.Moreno-Indias I, Sánchez-Alcoholado L, Sánchez-Garrido MA, Martín-Núñez GM, Pérez-Jiménez F, Tena-Sempere M, et al. Neonatal androgen exposure causes persistent gut microbiota dysbiosis related to metabolic disease in adult female rats. Endocrinology. 2016;157:4888–4898. doi: 10.1210/en.2016-1317. [DOI] [PubMed] [Google Scholar]
- 44.Matsushita M, Fujita K, Motooka D, Hatano K, Hata J, Nishimoto M, et al. Firmicutes in gut microbiota correlate with blood testosterone levels in elderly men. World J Mens Health. 2022;40:517–525. doi: 10.5534/wjmh.210190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170:192–201. doi: 10.1016/j.resmic.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Yuan X, Chen R, Zhang Y, Lin X, Yang X. Gut microbiota: effect of pubertal status. BMC Microbiol. 2020;20:334. doi: 10.1186/s12866-020-02021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 48.Lee J, Yang W, Hostetler A, Schultz N, Suckow MA, Stewart KL, et al. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69. doi: 10.1186/s12866-016-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dardmeh F, Alipour H, Gazerani P, van der Horst G, Brandsborg E, Nielsen HI. Lactobacillus rhamnosus PB01 (DSM 14870) supplementation affects markers of sperm kinematic parameters in a diet-induced obesity mice model. PLoS One. 2017;12:e0185964. doi: 10.1371/journal.pone.0185964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Tan Q, Fu Q, Zhou Y, Hu Y, Tang S, et al. Gastrointestinal microbiome and breast cancer: correlations, mechanisms and potential clinical implications. Breast Cancer. 2017;24:220–228. doi: 10.1007/s12282-016-0734-z. [DOI] [PubMed] [Google Scholar]
- 51.Parida S, Sharma D. The microbiome-estrogen connection and breast cancer risk. Cells. 2019;8:1642. doi: 10.3390/cells8121642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sfanos KS, Markowski MC, Peiffer LB, Ernst SE, White JR, Pienta KJ, et al. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018;21:539–548. doi: 10.1038/s41391-018-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. 2022;19:625–637. doi: 10.1038/s41575-022-00631-9. Erratum in: Rev Gastroenterol Hepatol 2022;19:682. [DOI] [PubMed] [Google Scholar]
- 54.Song CH, Kim N, Nam RH, Choi SI, Jang JY, Lee HN. Changes in gut microbiome upon orchiectomy and testosterone administration in AOM/DSS-induced colon cancer mouse model. Cancer Res Treat. 2022 doi: 10.4143/crt.2022.080. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi T, Fujita K, Matsushita M, Hayashi Y, Uemura M, Nonomura N. Metformin inhibits prostate cancer growth induced by a high-fat diet in Pten-deficient model mice. Int J Urol. 2019;26:307–309. doi: 10.1111/iju.13847. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi T, Fujita K, Nojima S, Hayashi Y, Nakano K, Ishizuya Y, et al. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 signaling. Clin Cancer Res. 2018;24:4309–4318. doi: 10.1158/1078-0432.CCR-18-0106. [DOI] [PubMed] [Google Scholar]
- 57.Narita S, Nara T, Sato H, Koizumi A, Huang M, Inoue T, et al. Research evidence on high-fat diet-induced prostate cancer development and progression. J Clin Med. 2019;8:597. doi: 10.3390/jcm8050597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orlich MJ, Mashchak AD, Jaceldo-Siegl K, Utt JT, Knutsen SF, Sveen LE, et al. Dairy foods, calcium intakes, and risk of incident prostate cancer in adventist health study-2. Am J Clin Nutr. 2022;116:314–324. doi: 10.1093/ajcn/nqac093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC, et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2015;101:87–117. doi: 10.3945/ajcn.113.067157. [DOI] [PubMed] [Google Scholar]
- 61.Collatuzzo G, Seyyedsalehi MS, Rezaeianzadeh A, Marzban M, Rashidian H, Hadji M, et al. Consumption of yoghurt and other dairy products and risk of colorectal cancer in Iran: the IROPICAN study. Nutrients. 2022;14:2506. doi: 10.3390/nu14122506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison S, Lennon R, Holly J, Higgins JPT, Gardner M, Perks C, et al. Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis. Cancer Causes Control. 2017;28:497–528. doi: 10.1007/s10552-017-0883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujita K, Hayashi T, Matsushita M, Uemura M, Nonomura N. Obesity, inflammation, and prostate cancer. J Clin Med. 2019;8:201. doi: 10.3390/jcm8020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 65.Matsushita M, Fujita K, Hatano K, Hayashi T, Kayama H, Motooka D, et al. High-fat diet promotes prostate cancer growth through histamine signaling. Int J Cancer. 2022;151:623–636. doi: 10.1002/ijc.34028. [DOI] [PubMed] [Google Scholar]
- 66.Zhong W, Wu K, Long Z, Zhou X, Zhong C, Wang S, et al. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome. 2022;10:94. doi: 10.1186/s40168-022-01289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, et al. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 70.Kim HS, Kim S, Shin SJ, Park YH, Nam Y, Kim CW, et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer’s disease: pathologic roles and therapeutic implications. Transl Neurodegener. 2021;10:49. doi: 10.1186/s40035-021-00273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Daisley BA, Chanyi RM, Abdur-Rashid K, Al KF, Gibbons S, Chmiel JA, et al. Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients. Nat Commun. 2020;11:4822. doi: 10.1038/s41467-020-18649-5. Erratum in: Nat Commun 2020;11:6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luo ZW, Xia K, Liu YW, Liu JH, Rao SS, Hu XK, et al. Extracellular vesicles from Akkermansia muciniphila elicit antitumor immunity against prostate cancer via modulation of CD8+ T cells and macrophages. Int J Nanomedicine. 2021;16:2949–2963. doi: 10.2147/IJN.S304515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pernigoni N, Zagato E, Calcinotto A, Troiani M, Mestre RP, Calì B, et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science. 2021;374:216–224. doi: 10.1126/science.abf8403. [DOI] [PubMed] [Google Scholar]
- 74.Wortelboer K, Nieuwdorp M, Herrema H. Fecal microbiota transplantation beyond Clostridioides difficile infections. EBioMedicine. 2019;44:716–729. doi: 10.1016/j.ebiom.2019.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y, Wei J. Identification of pathogen signatures in prostate cancer using RNA-seq. PLoS One. 2015;10:e0128955. doi: 10.1371/journal.pone.0128955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cavarretta I, Ferrarese R, Cazzaniga W, Saita D, Lucianò R, Ceresola ER, et al. The microbiome of the prostate tumor microenvironment. Eur Urol. 2017;72:625–631. doi: 10.1016/j.eururo.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 77.Davidsson S, Mölling P, Rider JR, Unemo M, Karlsson MG, Carlsson J, et al. Frequency and typing of Propionibacterium acnes in prostate tissue obtained from men with and without prostate cancer. Infect Agent Cancer. 2016;11:26. doi: 10.1186/s13027-016-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olsson J, Drott JB, Laurantzon L, Laurantzon O, Bergh A, Elgh F. Chronic prostatic infection and inflammation by Propionibacterium acnes in a rat prostate infection model. PLoS One. 2012;7:e51434. doi: 10.1371/journal.pone.0051434. Erratum in: PLoS One 2013;8:10.1371/annotation/2160e616-aa79-4097-96ab-e143d2a4d136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng Y, Jaratlerdsiri W, Patrick SM, Lyons RJ, Haynes AM, Collins CC, et al. Metagenomic analysis reveals a rich bacterial content in high-risk prostate tumors from African men. Prostate. 2019;79:1731–1738. doi: 10.1002/pros.23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng Y, Ramnarine VR, Bell R, Volik S, Davicioni E, Hayes VM, et al. Metagenomic and metatranscriptomic analysis of human prostate microbiota from patients with prostate cancer. BMC Genomics. 2019;20:146. doi: 10.1186/s12864-019-5457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 82.Ma X, Chi C, Fan L, Dong B, Shao X, Xie S, et al. The microbiome of prostate fluid is associated with prostate cancer. Front Microbiol. 2019;10:1664. doi: 10.3389/fmicb.2019.01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shrestha E, White JR, Yu SH, Kulac I, Ertunc O, De Marzo AM, et al. Profiling the urinary microbiome in men with positive versus negative biopsies for prostate cancer. J Urol. 2018;199:161–171. doi: 10.1016/j.juro.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demichelis F, Stanford JL. Genetic predisposition to prostate cancer: update and future perspectives. Urol Oncol. 2015;33:75–84. doi: 10.1016/j.urolonc.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 85.Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J, et al. Human prostate cancer risk factors. Cancer. 2004;101(10 Suppl):2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Tang M, Jiang H, Wu B, Cai W, Hu C, et al. The role of adrenergic activation on murine luteal cell viability and progesterone production. Theriogenology. 2016;86:1182–1188. doi: 10.1016/j.theriogenology.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]