Abstract
Background:
Individuals with bipolar disorder (BD) and schizophrenia (SCZ) show aberrant brain dynamics (i.e., altered recruitment or traversal through different brain states over time). Existing investigations of brain dynamics typically assume that one dominant brain state characterizes each time point. However, as multiple brain states likely are engaged at any given moment, this approach can obscure alterations in less prominent but critical brain states. Here, we examine brain dynamics in BD and SCZ by implementing a novel framework that simultaneously assesses the engagement of multiple brain states.
Methods:
Four recurring brain states were identified by applying nonlinear manifold learning and K-means clustering to the Human Connectome Project task-based fMRI data. We then assessed moment-to-moment state engagement in two independent samples of healthy controls (HCs) and patients with BD or SCZ using resting-state (N=336) or task-based fMRI (N=217). Relative state engagement and state engagement variability were extracted and compared across groups using MANCOVA, controlling for site, medication, age, and sex.
Results:
Our framework identified dynamic alterations in BD and SCZ while a state discretization approach revealed no significant group differences. Patient participants showed reduced state engagement variability, but not relative state engagement, across multiple brain states during resting-state and task-based fMRI. We found decreased state engagement variability in older participants and preliminary evidence suggesting an association with avolition.
Conclusions:
Findings suggest that assessing multiple brain states simultaneously reflects the complexity of aberrant brain dynamics in BD and SCZ, providing a more comprehensive understanding of the neural mechanisms underpinning these conditions.
Keywords: brain activation, psychotic disorders, mood disorders, cognitive control, clinical neuroscience, neuroimaging
Introduction
Bipolar disorder (BD) and schizophrenia (SCZ) are multifaceted, heterogeneous psychiatric disorders characterized by psychotic symptoms and impaired executive functioning (1, 2, 3). These illnesses and their cardinal symptoms putatively reflect disruptions in the interactions between functional brain networks, including the default mode and frontoparietal networks (4, 5, 6). Many previous studies have examined disruptions using “static” functional network structure, estimated over a minutes-long scan. Nevertheless, as communications within the brain are dynamic and fluctuate over small time windows (7, 8), our understanding of the neural correlates underpinning these disorders may be incomplete.
Indeed, a new wave of methods has emerged to capture this additional temporal information in the form of brain states (i.e., recurring brain activation and connectivity patterns) (8). Brain states can be characterized in terms of state engagement (i.e., the proportion of time points spent in a particular state, also called dwell time) and transition (i.e., the number of times individuals transitioned from one state to another). These methods have revealed aberrant brain dynamics in BD and SCZ that were undetectable using static approaches (9, 10, 11). Compared to healthy controls (HC), individuals with these disorders demonstrate aberrant transitions and dwell times (12, 13, 14, 15, 16, 17, 18, 19). Brain dynamic alterations are additionally associated with psychiatric symptoms in these groups, including elevated suicide risk, psychotic symptom severity, and hallucinations (14, 15, 18, 19).
However, these studies mainly assigned one state to each time unit despite the possibility that the activity or connectivity patterns at a given time window can reflect a combination of several patterns (15). As brain state recruitment can overlap temporally (20, 21), a state discretization approach might ignore meaningful but non-dominant constituent brain states that also contribute to the observed activation pattern at each time point. This approach can potentially misrepresent dynamic information. For instance, assigning one state to each time point makes it challenging to evaluate whether the elevated recruitment of one constituent brain state might accompany reduced engagement of another as an alternative or compensatory mechanism in clinical populations.
To address this limitation, we developed a novel method that estimates the extent to which each time point reflects the presence of multiple co-occurring states. We first applied nonlinear manifold learning and 2-step Diffusion Mapping (2sDM) to project task-based fMRI data from the Human Connectome Project (HCP; 22) onto a low-dimensional space. We subsequently identified reproducible brain activity patterns across multiple fMRI tasks (23). With non-negative least squares regression, we extended these recurring brain activity patterns to two ethnically diverse international datasets. We examined whether individuals with BD or SCZ differed from HC in either their engagement of or transitions through these brain states. Notably, this framework used 2sDM to learn task-relevant brain states for each time point in the HCP, followed by non-negative least squares regression to characterize brain state engagement for each time point in two other previously unseen datasets. We hypothesized that individuals with BD and SCZ would show aberrant brain dynamics (i.e., overall state engagement and engagement variability) compared to HC during rest. Since difficulties with executive functioning and cognitive flexibility have been consistently reported in individuals with BD and SCZ (2, 24, 25, 26), secondary analyses using fMRI data from a task-switching paradigm were performed to further investigate state transition in clinical populations and link altered brain state dynamics to clinical symptoms.
Methods and Materials
Participants
Data from three independent, publicly available datasets were analyzed in this study. First, we used the HCP S500 release (22) to identify recurring brain states. The moment-to-moment engagement of these brain states was explored in the HC, BD, and SCZ groups from the UCLA Consortium for Neuropsychiatric Phenomics (CNP) and the University of Tokyo Hospital site in the Japanese Strategic Research Program for the Promotion of Brain Sciences (SRPBS) datasets (27, 28). Demographic information for each dataset is shown in Table 1 (see Supplementary Table 1 for medication information).
Table 1.
Demographic information by dataset
| Characteristic | HC | BD | SCZ | |
|---|---|---|---|---|
| SRPBS | N | 86 | 40 | 33 |
| Self-reported sex (F/M) | 56/30 | 15/25 | 11/22 | |
| Age (years) (M±SD) | 47.07±15.18 | 34.48±9.11 | 31.64±10.77 | |
| CNP (Resting-state dataset) | N | 104 | 35 | 38* |
| Self-reported sex (F/M) | 49/54 | 15/20 | 9/29 | |
| Age (years) (M±SD) | 30.29±7.99 | 34.09±9.01 | 35.42±8.99 | |
| Ethnicity AI/Asian/Black/White/Other | 20/2/1/81/0 | 2/0/1/27/5 | 8/0/2/26/1 | |
| CNP (task-switching dataset) | N | 120 | 49 | 48** |
| Self-reported sex (F/M) | 56/64 | 21/28 | 11/37 | |
| Age (years) (M±SD) | 31.65±8.84 | 35.29±9.03 | 36.19±8.87 | |
| Race or ethnicity AI/Asian/Black/White/Other | 25/2/1/92/0 | 4/0/1/37/7 | 11/1/2/31/1 |
Note: This table reports the demographic information for all three datasets included in this study. Race or ethnicity information was not available for the SRPBS dataset. AI, American Indian or Alaskan Native, BD, bipolar disorder; HC, healthy control; Other, more than one race or ethnicity; SCZ, schizophrenia.
Race information missing from one participant.
Race information missing from two participants.
HCP fMRI data from six tasks (motor, working memory, social, emotional, relational, and gambling) were used to identify recurring brain states. As the HCP dataset contains tasks ranging from motor to cognitive and affective paradigms, it has the potential to reveal brain states underlying a rich set of cognitive processes. Brain states were identified in the HCP dataset to avoid circular analysis and overfitting when examining them in the CNP and SRPBS cohorts. We used the resting-state fMRI data from CNP and SRPBS dataset for primary analyses. Task-based fMRI data from the CNP task-switching paradigm were used for secondary analyses.
FMRI data preprocessing
Acquisition and imaging parameters for the HCP, CNP, and SRPBS datasets have been detailed elsewhere (22, 27, 28). The HCP minimal preprocessing pipeline was used for the HCP dataset (29). Standard preprocessing procedures described in previous work (23) were applied to the structural and functional data from the CNP and SRPBS datasets (see Supplementary Material for more details). While we have utilized the HCP and CNP datasets in previous works (23, 30), neither studies explored aberrant brain dynamics in individuals with BD or SCZ. We removed time points with over 0.45 framewise displacement to mitigate motion artifacts. Participants with over 20% of their time points censored due to motion were excluded from further analysis.
Additional quality control criteria for the HCP and CNP datasets were described in a previous study and are summarized in the Supplementary Material (23). After these exclusions, 390 HCP participants remained for brain state identification. For brain dynamics analyses, 336 participants from the CNP and SRPBS datasets were included in the resting-state analysis (N=177 participants from the CNP dataset, HC: n=104, BD: n=35, SCZ: n=38; N=159 participants from the SRPBS dataset: HC: n=86, BD: n=40, SCZ: n=33, Table 1), and 217 participants (CNP dataset, HC: n=120, BD: n=49, SCZ: n=48; Table 1) were included in the task-based analysis.
Brain state identification
As detailed information on 2sDM was described in earlier work (23), we provide a brief overview here. Nonlinear approaches like 2sDM can project complex neural data onto a manifold that is more representative of its temporal dynamics than linear methods to find robust brain states (23). In brief, diffusion maps was applied twice to fMRI timeseries data to reduce it in the participant’s and brain node’s dimensions as well as embedding it into a lower-dimensional space, where time points showing similar activity were located closer to each other (Figure 1). We applied K-means clustering for 100 iterations using the first three embedding dimensions and found the optimal number of clusters, or brain states, as four using the Calinski-Harabasz criterion (31; see discussion on the number of brain states in Supplementary Material). These labels can be applied to the original data to find time points associated with each state across participants. For each brain state, all associated fMRI data from all participants were averaged first across the individual level and next across time points to establish a brain state representative time point. These brain states are considered as recurring, as their activation patterns emerged during the performance of six different fMRI tasks across different time points. The same set of activation patterns can also be found when participants are at rest (23).
Figure 1: 2sDM pipeline.
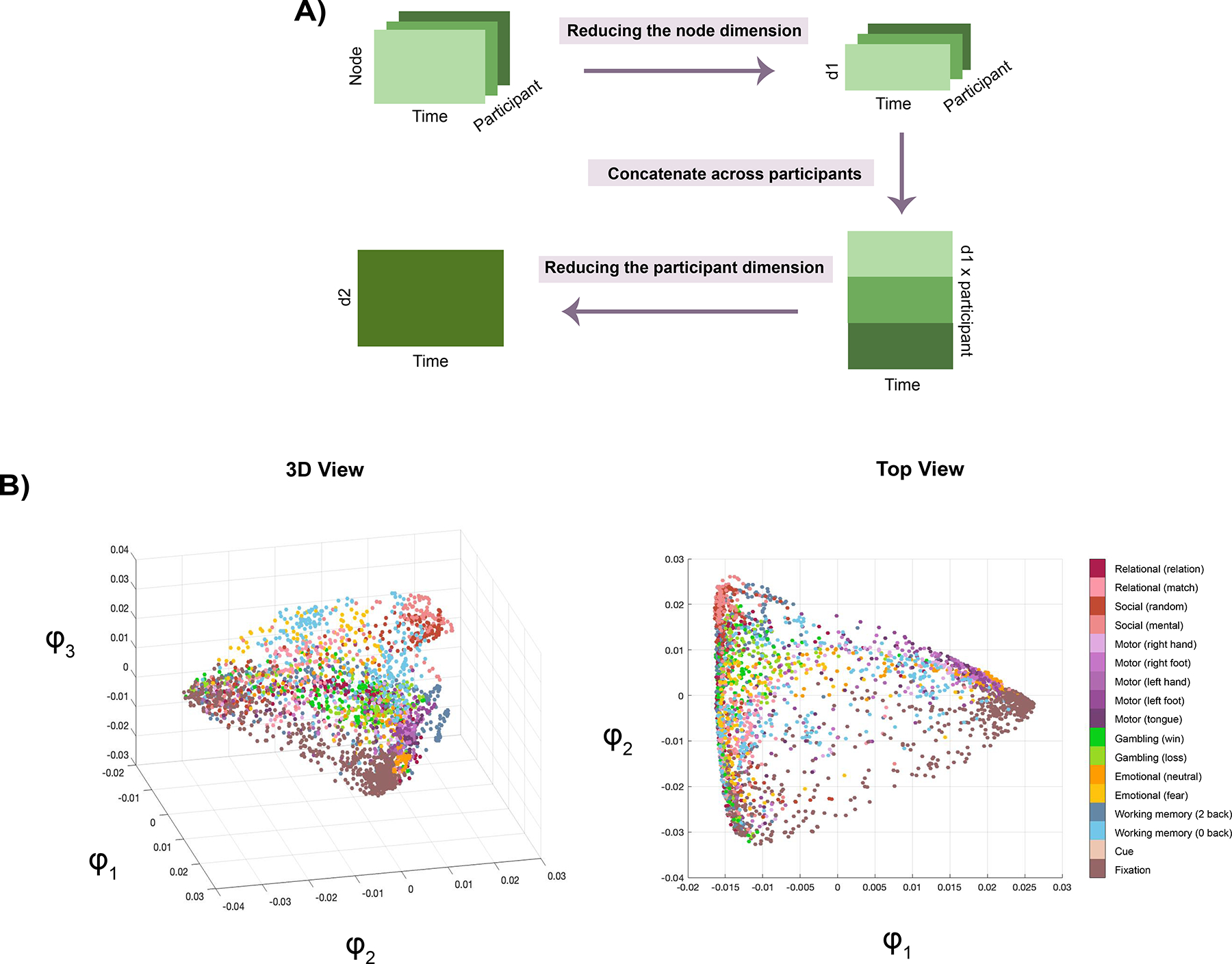
A) Flowchart showing how 2sDM was applied to the HCP dataset to first reduce the brain node’s (d1) and participant’s (d2) dimensions before projecting it to a low-dimensional space. B) Several views of the manifold. Each dot here represents a time point, color-coded by the task condition it is associated with. Further details about 2sDM can be found in Gao et al., 2021 (23).
Next, we investigated how canonical functional brain networks activated or deactivated for each brain state using the ten functional networks defined in the Shen-268 atlas (32,33). For each network, we first identified the activated and deactivated regions within each representative time point (defined as having activation above or below 0, respectively). An activation or deactivation percentage was computed by dividing the number of activated or deactivated regions by the total number of regions in a network.
Resting-state brain dynamics analyses
As brain state engagement can overlap spatially and temporally (20,21), we developed a framework to track the contributions of multiple brain states simultaneously in a continuous manner. Perhaps one helpful analogy to our approach is color. Red, yellow, and blue are the primary colors, which can be combined in various ways to form different secondary colors. Here, we first identified a set of recurring brain states, as described earlier, to serve as potential constituent brain states (similar to the primary colors). Next, to evaluate how they were engaged over time to form observed activation patterns (similar to secondary colors formed by the three primary ones), we regressed each representative time point from every CNP and SRPBS resting-state time point with non-negative least squares regression (MATLAB function lsqnonneg; Figure 2). Non-negative least squares was selected over standard regression to ensure brain state engagements were positive, as a negative state engagement, which is possible with standard regression, is difficult to interpret. Representative time points and all rest time points were first normalized by standard deviation before regression.
Figure 2: Assessing moment-to-moment brain state engagement with non-negative least squares regression.
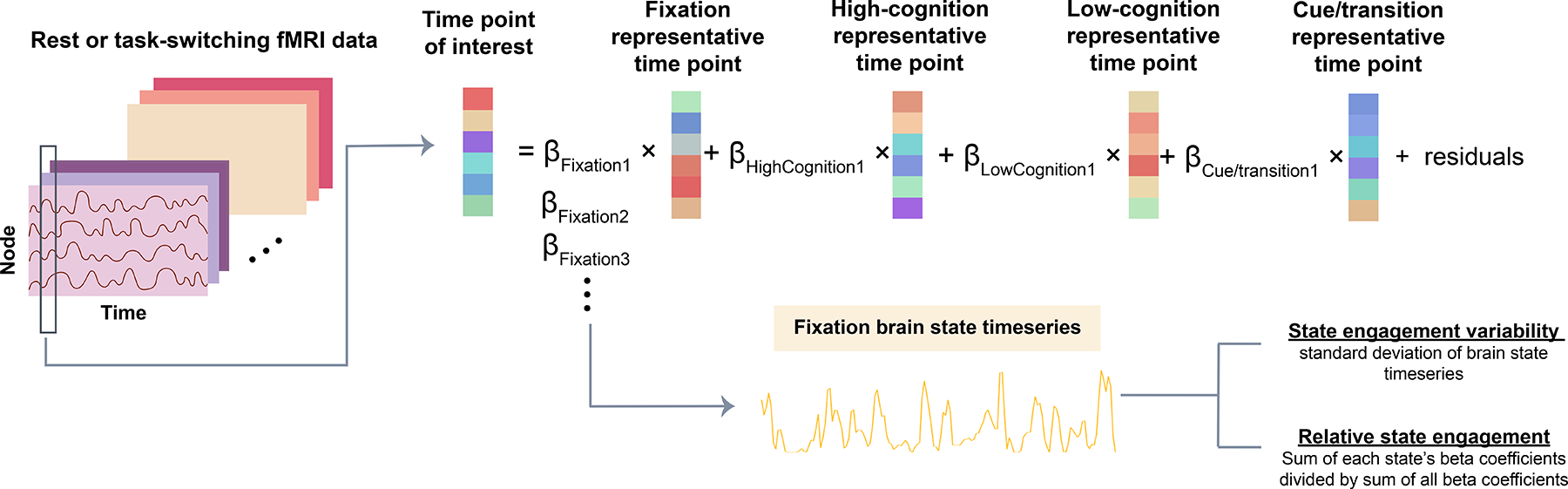
This flowchart illustrates how beta coefficients for each brain state are concatenated across time to create a brain state timeseries. Four recurring brain states were identified using the HCP dataset: fixation, high-cognition, low-cognition and cue/transition (see Figure 3). The representative time points for each HCP-derived state were regressed from each resting-state or task-switching time point of interest in the CNP and SRPBS datasets. The inputs to this regression are one time point of interest from an independent dataset (dependent variable; either resting-state or task-based data), as well as the representative time point from each of the four recurring brain states (independent variable). After the four representative brain states were regressed from the time point of interest. We received four beta coefficients. Each coefficient indicated the contribution of each brain state to the time point of interest. For each state, we can then create a brain state time series by concatenating beta coefficients across time. Two summary measures can be generated using the brain state time series: state engagement variability and relative state engagement.
By concatenating the output beta coefficients for each state across time, we can create a brain state timeseries indicating a particular state’s weighted contribution over time for each participant (Figure 2). Two summary measures can be extracted from each brain state timeseries: 1) relative state engagement —a continuous version of state engagement computed by dividing the sum of each state’s beta coefficients by the sum of all beta coefficients, and 2) state engagement variability —a continuous estimation of state transition defined as the standard deviation of brain state timeseries (see Supplementary Material for validations of these brain dynamic measures).
Secondary analysis with task-based fMRI data
We performed secondary analyses in a smaller sample with available fMRI data from a task-switching paradigm. This task required participants to flexibly switch between task rules (i.e., responding to the color vs. the shape of the stimulus) based on the cue presented over 96 trials (27). As this paradigm demands flexible responses to changing cognitive demands, it can supplement our resting-state analysis to provide insight into altered brain state engagement variability in clinical populations. Relative state engagement and engagement variability were extracted and examined in this dataset.
Statistical analysis
We combined the two datasets for resting-state group comparison to maximize statistical power for detecting brain dynamic alterations in BD and SCZ. Additionally, this allowed us to capitalize on similar study designs (e.g., collection of eyes-open, resting-state data from adult patient participants) and examine the extent to which these alterations generalize across cultures. As we have a relative state engagement and state engagement variability measure for each brain state, multivariate group differences were examined across all three groups using MANCOVA, with site (N=2), medication, age, and sex as covariates. The group comparison analysis was supplemented by assessing group differences between both patient groups and HC (i.e., BD vs HC and SCZ vs HC) with t-squared tests, controlling for site, medication, age and sex. Cohen’s d effect sizes in pairwise differences were computed to quantify how much each brain state contributed to multivariate group differences. For our primary and secondary analyses, p<0.0125 (i.e., p<0.05/4) was considered significant after Bonferroni correction for multiple comparisons. We did not correct for multiple comparisons for our post hoc or exploratory analyses. We additionally examined brain dynamics with a state discretization approach (see Supplementary Material for details).
Exploratory analysis with behavioral data
To further understand the implications of differences in state engagement variability, we examined the association between state engagement variability and symptom measures in patient participants. We focused on brain dynamic measures from task-based fMRI data, as the sample size was larger than the resting-state dataset. We first performed a principal component analysis (PCA) on the four state engagement variability measures to estimate a combined state engagement variability measure. This step allowed us to reduce dimensionality and decrease the number of statistical tests needed to investigate how state engagement variability across all four states was associated with clinical symptoms. Similar state engagement variability patterns were observed in both patient groups. Data from participants with BD and SCZ were additionally combined here to increase statistical power and examine transdiagnostic associations between altered brain dynamics and clinical symptoms. The first output component scores were then correlated with measures from the Scale for Assessment of Negative Symptoms (34) and Scale for Assessment of Positive Symptoms (35) using Spearman correlation. We specifically focused on avolition, anhedonia, and attention since the other factor measures were zero-inflated (Supplementary Figure 1). These symptom measures were only available in patient participants, not in HCs.
Results
Brain states and canonical functional brain networks
Replicating previous work (23), we identified four brain states with distinct activity patterns using 2sDM and K-means clustering. These brain states were characterized as high-cognition, low-cognition, fixation and cue/transition based on the task conditions involved in each cluster (Figure 3A; Supplementary Table 4).
Figure 3: Recurring brain states and their association with canonical functional brain networks.
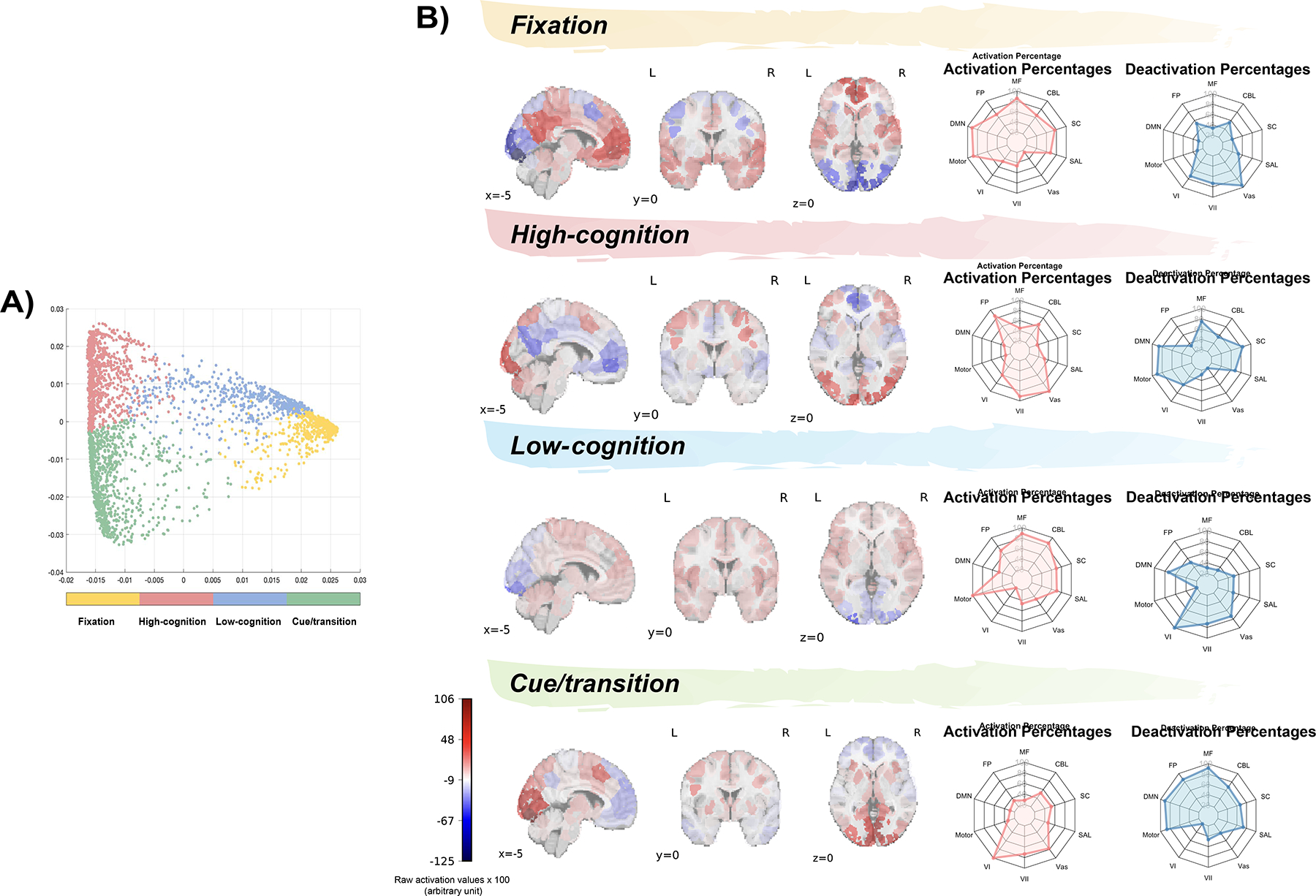
A) Four brain states identified from K-means clustering. These states were defined as fixation, high-cognition, low-cognition, and cue/transition based on the prominent task conditions associated with the time points in each cluster (each dot here represents a time point; see Figure 1 and Supplementary Table 4 for their task conditions). B) Regional brain activation corresponding to the four representative brain states. Spider plots show the relative extent to which functional brain networks were activated and deactivated in each representative timepoint. For illustrative purposes, the raw activation values were multiplied by 100. MF, medial frontal network; CBL, cerebellar network; SC, subcortical network; SAL, salience network; Vas, visual association network; VII, visual II network; VI, visual I network; motor, motor network; DMN, default mode network; FP, frontoparietal network.
In general, the networks associated with each brain state aligned with the presumed cognitive processes supported by each state (Figure 3B; Supplementary Table 5). For example, the entire motor network was activated for the low-cognition state, which contained time points from the motor task. Additionally, the high-cognition state, which included time points from working memory, emotional, social and relational tasks, was associated with frontoparietal network activation and default mode network deactivation.
Primary and secondary analysis: resting-state and task brain dynamics across groups
Before group comparisons, we examined the residual from non-negative least squares regression. No rest or task time point showed an outlier residual term (defined as 1.5 times the interquartile range), suggesting that the activity patterns of all rest and task time points can be considered as some weighted combinations of brain states obtained from the HCP (see discussion on residuals and model fitting in Supplementary Material).
During rest, we observed a significant effect of diagnostic group on state engagement variability (MANCOVA; F(8,648) =3.093, p=0.002; Figure 4B) but not for relative state engagement (MANCOVA; F(8,648)=1.327, p=0.227; Figure 4A). There were additionally site and age effects on state engagement variability (Supplementary Table 7; see Supplementary Material for site effect discussion). The effect of medication use was not significant (Supplementary Table 7).
Figure 4: Brain dynamics during rest.
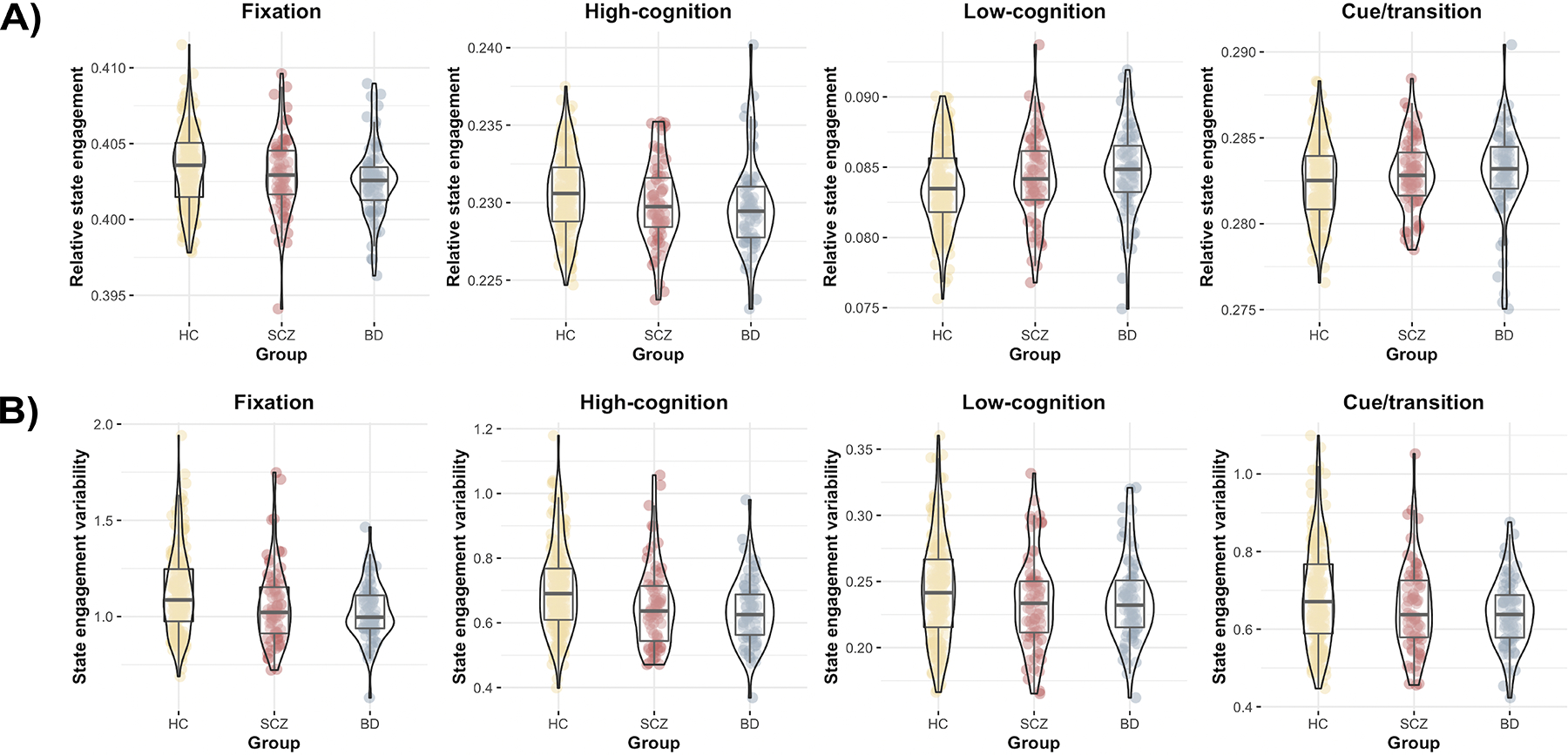
A) All three groups showed similar relative state engagement across all four states during rest. B) But we observed lower state engagement variability across multiple brain states in BD and SCZ compared to HC. Yellow, HC; red: SCZ; blue: BD. Violin plots created using the ggstatsplot R package (34).
Follow-up tests interrogated how clinical groups differed from HCs for state engagement variability. Participants with BD (t-squared; F(4,253)=4.850, p<0.001) and SCZ (t-squared; F(4,249)=2.883, p=0.023) showed significant differences in state engagement variability compared to HCs when including site, medication, age, and sex as covariates (Supplementary Table 8). Both clinical groups showed lower state engagement variability across multiple brain states, with the high-cognition brain state showing the largest effect size (Supplementary Tables 9 and 14).
During task-switching paradigm, we similarly observed a significant group effect on state engagement variability (MANCOVA; F(8,412)=2.536, p=0.011; Figure 5B) but not on relative state engagement (MANCOVA; F(8,412)=0.734, p=0.662; Figure 5A). While the effect of medication use was not significant, there were significant age, site, and sex effects (Supplementary Table 10; see Supplementary Material for site effect discussion). Both participants with SCZ (t-squared; F(4,157)=3.103, p=0.017) and BD (t-squared; F(4,158)=2.792, p=0.028) demonstrated lower state engagement variability in several brain states compared to HCs (Supplementary Table 11, 12 and 14). These findings indicate that aberrant brain dynamics in BD and SCZ affected multiple brain states during both paradigms. Our results further emphasize that implementing a multivariate approach when examining multiple brain states simultaneously can provide a more comprehensive understanding of brain dynamic alterations in BD and SCZ.
Figure 5: Brain dynamics during task-switching.
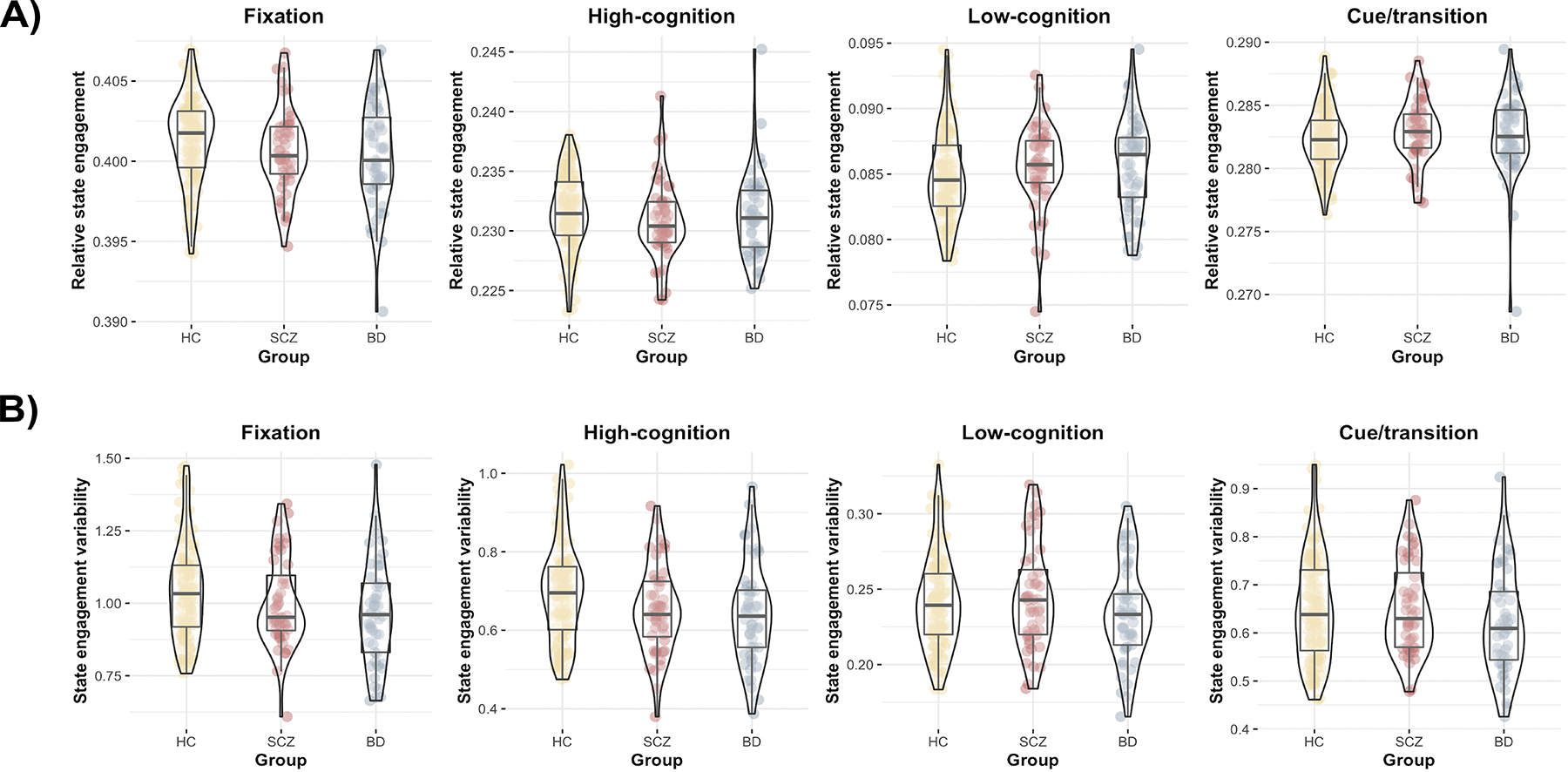
A) Similar to rest, we found that all three groups recruited the four brain states similarly during task-switching. B) The clinical populations also showed significantly lower state engagement variability in several brain states compared to HC during task-based fMRI. Yellow, HC; red: SCZ; blue: BD. Violin plots created using the ggstatsplot R package (36).
Brain dynamics with discrete states
We additionally compared brain dynamics that were extracted using a state discretization approach between groups. With discretized states, we did not observe significant group differences in state transition during resting-state (F(2,326)=1.404, p=0.247; Supplementary Table 15) or task-switching (F(2,208)=0.167, p=0.846; Supplementary Table 16). The group effect on dwell times was not significant for either resting-state or task-based data following Bonferroni correction (Supplementary Table 15 and 16).
Post hoc and exploratory analysis: age, symptom measures and state engagement variability
As we observed a strong age effect on state engagement variability in resting-state and task-switching (Supplementary Table 7 and 10), we conducted a post hoc analysis to investigate the direction of their association. Combined state engagement variability was computed to draw information from all four states while lowering the number of statistical tests run (Supplementary Table 17). At rest, combined state engagement variability decreased with age in the HC and SCZ groups (Pearson; HC: r=−0.414, p<0.001; SCZ: Pearson; r=−0.348, p=0.003; Figure 6A), but the correlation was not significant in the BD group (Pearson; r=−0.192, p=0.098; Figure 6A). The association between age and combined state engagement variability was significant for all groups during the task (Pearson; HC: r=−0.269, p=0.003; SCZ: r=−0.318, p=0.028; BD: r=−0.355, p=0.012; Figure 6B).
Figure 6: Associations between state engagement variability and age.
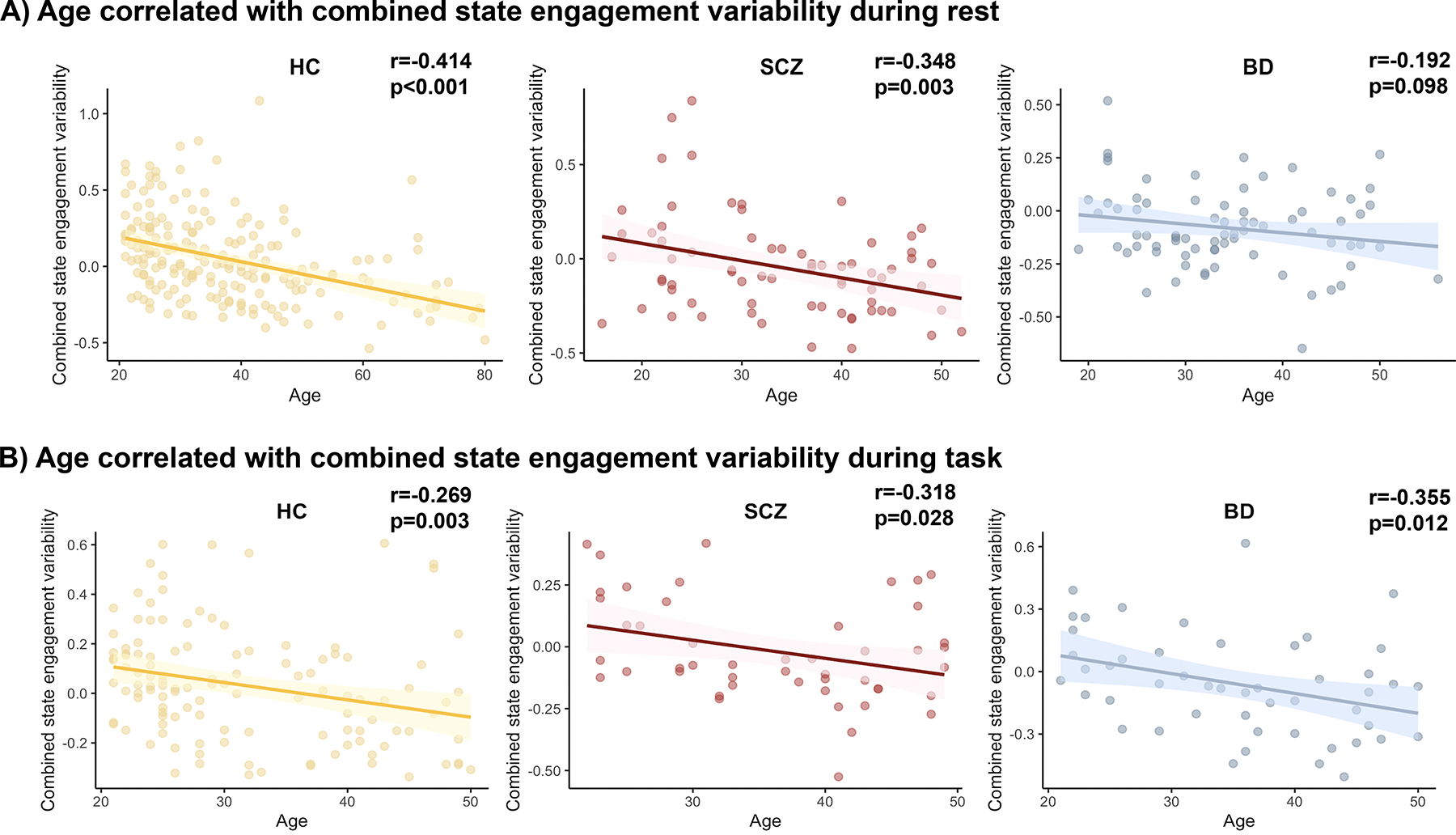
A) Overall state engagement variability was negatively related to age in HC and SCZ groups at rest. B) During task-switching, older participants showed decreased combined state engagement variability across all three groups.
Our exploratory analysis additionally revealed that decreased combined state engagement variability during task-switching was associated with elevated avolition in individuals with BD and SCZ (r=−0.222, p=0.029; Supplementary Figure 4; Supplementary Table 11), but not the other symptom measures (attention: r=0.154, p=0.132; anhedonia: r=−0.152, p=0.138; Supplementary Table 11).
Discussion
Here, we examined whether altered brain dynamics in individuals with BD or SCZ reflect differences in the simultaneous engagement of multiple brain states relative to unaffected controls. Our novel framework leveraged nonlinear manifold learning and non-negative least squares regression to assess the continuous engagement of brain states during resting-state and task-based fMRI. Both the BD and SCZ groups showed consistent reductions in state engagement variability across multiple brain states during rest and a cognitive control task. Decreased variability can indicate lower neural complexity, potentially reflecting the brain’s impaired ability to flexibly respond to ongoing cognitive demands (37,38). Detecting altered brain dynamics in a smaller sample using a task-switching paradigm supports prior arguments that more robust brain-behavior relationships can be identified when the construct of interest (i.e., cognitive flexibility) is engaged during the scan (39,40). A state discretization approach did not reveal aberrant brain dynamics in clinical populations. These results deepen our understanding of the neural basis of BD and SCZ, indicating that these illnesses reflect impaired fluid recruitment of different brain states, which may, in turn, contribute to difficulties with cognitive functioning or motivation.
Additional insight from assessing multiple brain states simultaneously
Prior studies of brain dynamics in BD and SCZ have reported mixed results, encompassing increased, similar, and decreased state transition and dwell time in clinical populations (12, 13, 15, 16, 17, 18). While these discrepancies could reflect variable analytic approaches, many studies often do not fully consider the temporal overlap in brain state engagement. Therefore, previous mixed findings might reflect potential bias contributed by the differences in the relative dominance of a given brain state. Assessment of state transition and engagement across multiple brain states simultaneously represents a powerful approach that is less biased by brain state dominance and might more closely reflect the complex cognitive alterations observed in patients with BD and SCZ.
We found similar relative state engagement across patient and control groups, which deviates from prior reports of altered dwell time in BD and SCZ (12, 13, 14, 18). The four brain states examined here shared spatial overlap with some of the brain states showing altered dynamics in patients in previous studies. For instance, studies have reported on states with DMN activation and frontoparietal network (FPN) deactivation or DMN deactivation and visual areas activation (18, 41). These states were analogous to our fixation and high-cognition states, respectively. But some spatial variations were also noted. Patients dwelled more in states showing opposite activation patterns in the FPN and the sensorimotor networks (18). Additionally, they spent less time in states where the DMN and FPN showed similar activation levels (i.e., activating or deactivating together; 41). None of our brain states showed these latter activation patterns. However, it is important to point out that our framework should still be able to capture these previously unseen patterns since it allows the simultaneous combinations of our four brain states. For example, a time point showing both DMN and FPN activation can be represented by the weighted sum of the high-cognition and fixation brain states.
One possible explanation underlying the differences in results is that our relative state engagement measure is not analogous to dwell time. The inconsistency in results likely reflects variations in the operationalization of engagement across studies. A state with less dwell time (i.e., considered dominant in fewer time points) can still show high relative state engagement if it is consistently engaged in the background. Our results also suggest that a state discretization approach might mask variability differences as dwell time differences. If a state demonstrated decreased engagement variability (as in most states examined here), it would likely show consistent engagement across time and be assigned to more or fewer time points, depending on its dominance. This variability difference might then be interpreted as increased or decreased dwell time, respectively. Our results indicated that altered brain dynamics in BD and SCZ stem from disrupted recruitment of various brain states across time, instead of the elevated or decreased engagement of a particular brain state relative to controls.
It is worth mentioning here that aberrant dwell times in patients have also been reported by papers using dynamic functional connectivity approaches. Specifically, patients spent more time in brain states that are characterized by weaker or sparser functional connections (12,13,42). These results echoed our main findings of decreased state engagement variability in BD and SCZ, indicating that patients might have decreased neural complexity to effectively engage different brain networks or brain states to support cognitive processes (37,38).
Speculating the contribution of the dopaminergic system
While we observed aberrant state engagement variability in BD and SCZ, the mechanisms underpinning these alterations remained unclear. The dopaminergic system is one potential candidate, as it is implicated in the pathogenesis of BD and SCZ (43, 44, 45, 46), cognitive control (47), aging (48, 49), and avolition (50). Emerging work has demonstrated that changes in the dopaminergic system (either induced by pharmacological manipulations or observed in psychiatric disorders such as SCZ) are associated with brain dynamic differences (51). Notably, a dopamine D2-receptor antagonist increased the effort required for state transition (51). These previous findings are important to consider since some patient participants were prescribed dopamine D2 antagonist medications at the time of scanning. While our analyses controlled for medication use, it remains possible that medication-induced alterations in dopamine functioning contributed to the observed group differences in engagement variability. Future research directly manipulating dopaminergic tone can further characterize how dopamine contributes to brain dynamic differences across individuals and in psychiatric disorders.
Limitations and future directions
While our framework provides a more comprehensive picture of brain dynamics by tracking multiple states concurrently, some limitations should be considered. As we used open-source data, medication information was unavailable for the SRPBS participants. However, when including medication as a covariate for our task-based analyses in the CNP dataset, we still observed aberrant state engagement variability in patient participants. These results suggest that group differences are potentially robust to medication effects. Brain states were identified using fMRI data from healthy HCP participants. While previous studies (18,21,23) have reported similar brain states in patients (including both BD and SCZ) and HC, model fit differences across groups could decrease or increase state engagement in patient groups. It is additionally possible that brain states outside of the ones studied here could contribute to impaired dynamics in BD and SCZ. One can easily modify our framework to study brain dynamics using brain states identified with other approaches (such as Neurosynth activation maps). Applying this framework to study brain dynamics in other clinical populations can shed light on whether the alteration patterns reported here are specific to BD and SCZ or can be generalized to other forms of psychopathology. Furthermore, the current study examined brain dynamics within each experimental condition. Future work can study if differences in brain dynamics between resting-state and task-based fMRI provide additional insights.
In summary, we introduced a novel approach to investigate multiple brain states that co-occur simultaneously. Findings from this study suggest that aberrant state engagement variability – instead of relative state engagement – drives brain dynamic differences in BD and SCZ. Our approach revealed brain dynamic alterations while a discrete state approach did not find significant group differences. By assessing multiple brain states simultaneously, our framework painted a more comprehensive picture of brain dynamics in a population-based cohort, as well as in patients with serious mental illness. This novel framework has the potential to provide a more comprehensive understanding of psychiatric disorders and uncover new findings not observable with previous methods.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Publicly available dataset | Homo sapiens; female/male | Human Connectome Project (HCP) | S500 release | |
| Publicly available dataset | Homo sapiens; female/male | UCLA Consortium for Neuropsychiatric Phenomics (CNP) | OpenfMRI ds000030 | |
| Publicly available dataset | Homo sapiens; female/male | Japanese Strategic Research Program for the Promotion of Brain Science (SRPBS) | DecNef Project Brain Data Repository | |
| Software | NA | MATLAB_R2021a | ||
| Software | NA | R |
Acknowledgements
Data was provided in part by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657 funded by the 16 NIH institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University) and the Consortium for Neuropsychiatric Phenomics (NIH Roadmap for Medical Research grants UL1-DE019580, RL1MH083268, RL1MH083269, RL1DA024853, RL1MH083270, RL1LM009833, PL1MH083271, and PL1NS062410). The CNP dataset was accessed through OpenNEURO (https://openneuro.org/datasets/ds000030/versions/00016). A portion of the data used in the preparation of this work were obtained from the DecNef Project Brain Data Repository (https://bicr-resource.atr.jp/srpbsopen/), collected as part of the Japanese Strategic Research Program for the Promotion of Brain Sciences (SRPBS) supported by the Japanese Advanced Research and Development Programs for Medical Innovation (AMED). JY was supported by the Gruber Science Fellowship from the Gruber Foundation via Yale University. MR was supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-2139841. RXR was supported by the National Research Service Award (award number: 5T32GM100884-09) from the National Institute of General Medicine. SN was supported by the National Institute of Mental Health under award number K00MH122372. MLW was supported by the National Institute on Drug Abuse (T32DA022975). Finally, DS was supported by R01 MH121095. A version of this work was published as a preprint on medRxiv (https://doi.org/10.1101/2022.10.07.22280835) and was previously presented at the 2022 Cognitive Neuroscience Society Annual Meeting and the 2022 Society for Neuroscience Annual Meeting. We thank Ignacio Ruiz-Sanchez, Huda Siddiqui and Iris Cheng for data preprocessing help.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, & McDonald C (2004). A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophrenia Research, 71(2), 405–416. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg DP, & Berman KF (2010). Executive Function, Neural Circuitry, and Genetic Mechanisms in Schizophrenia. Neuropsychopharmacology, 35(1), Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotrena C, Damiani Branco L, Ponsoni A, Samamé C, Milman Shansis F, & Paz Fonseca R (2020). Executive functions and memory in bipolar disorders I and II: New insights from meta-analytic results. Acta Psychiatrica Scandinavica, 141(2), 110–130. [DOI] [PubMed] [Google Scholar]
- 4.Chase HW, & Phillips ML (2016). Elucidating Neural Network Functional Connectivity Abnormalities in Bipolar Disorder: Toward a Harmonized Methodological Approach. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(3), 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheffield JM, & Barch DM (2016). Cognition and resting-state functional connectivity in schizophrenia. Neuroscience & Biobehavioral Reviews, 61, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong D, Wang Y, Chang X, Luo C, & Yao D (2018). Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophrenia Bulletin, 44(1), 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. (2013). Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage, 80, 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lurie DJ, Kessler D, Bassett DS, Betzel RF, Breakspear M, Kheilholz S, et al. (2020). Questions and controversies in the study of time-varying functional connectivity in resting fMRI. Network Neuroscience, 4(1), 30–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rashid B, Arbabshirani MR, Damaraju E, Cetin MS, Miller R, Pearlson GD, & Calhoun VD (2016). Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. NeuroImage, 134, 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y, Pearlson GD, Lin D, Sui J, Chen J, Salman M, et al. (2017). Identifying dynamic functional connectivity biomarkers using GIG-ICA: Application to schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Human Brain Mapping, 38(5), 2683–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TT, Kovacevic S, Dev SI, Lu K, Liu TT, & Eyler LT (2017). Dynamic functional connectivity in bipolar disorder is associated with executive function and processing speed: A preliminary study. Neuropsychology, 31, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, et al. (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage: Clinical, 5, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y, Pearlson GD, Yu Q, He H, Lin D, Sui J, et al. (2016). Interaction among subsystems within default mode network diminished in schizophrenia patients: A dynamic connectivity approach. Schizophrenia Research, 170(1), 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinen JM, Chén OY, Hutchison RM, Yeo BTT, Anderson KM, Sabuncu MR, et al. (2018). The human cortex possesses a reconfigurable dynamic network architecture that is disrupted in psychosis. Nature Communications, 9(1), Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RL, Yaesoubi M, Turner JA, Mathalon D, Preda A, Pearlson G, et al. (2016). Higher Dimensional Meta-State Analysis Reveals Reduced Resting fMRI Connectivity Dynamism in Schizophrenia Patients. PLOS ONE, 11(3), e0149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid B, Damaraju E, Pearlson GD, & Calhoun VD (2014). Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Frontiers in Human Neuroscience, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du M, Zhang L, Li L, Ji E, Han X, Huang G, et al. (2021). Abnormal transitions of dynamic functional connectivity states in bipolar disorder: A whole-brain resting-state fMRI study. Journal of Affective Disorders, 289, 7–15. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Peng X, Pelletier-Baldelli A, Orlov N, Farabaugh A, Nasr S, et al. (2021). Altered temporal, but intact spatial, features of transient network dynamics in psychosis. Molecular Psychiatry, 26(6), Article 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Zhu R, Tian S, Zhang S, Dai Z, Shao J, et al. (2022). Dynamic connectivity alterations in anterior cingulate cortex associated with suicide attempts in bipolar disorders with a current major depressive episode. Journal of Psychiatric Research, 149, 307–314. [DOI] [PubMed] [Google Scholar]
- 20.Karahanoğlu FI, & Van De Ville D (2015). Transient brain activity disentangles fMRI resting-state dynamics in terms of spatially and temporally overlapping networks. Nature Communications, 6, 7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piguet C, Karahanoğlu FI, Saccaro LF, Van De Ville D, & Vuilleumier P (2021). Mood disorders disrupt the functional dynamics, not spatial organization of brain resting state networks. NeuroImage: Clinical, 32, 102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, & Ugurbil K (2013). The WU-Minn Human Connectome Project: An overview. NeuroImage, 80, 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao S, Mishne G, & Scheinost D (2021). Nonlinear manifold learning in functional magnetic resonance imaging uncovers a low-dimensional space of brain dynamics. Human Brain Mapping, 42(14), 4510–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Donnell LA, Deldin PJ, Pester B, McInnis MG, Langenecker SA, & Ryan KA (2017). Cognitive flexibility: A trait of bipolar disorder that worsens with length of illness. Journal of Clinical and Experimental Neuropsychology, 39(10), 979–987. [DOI] [PubMed] [Google Scholar]
- 25.Cotrena C, Branco LD, Shansis FM, & Fonseca RP (2016). Executive function impairments in depression and bipolar disorder: Association with functional impairment and quality of life. Journal of Affective Disorders, 190, 744–753. [DOI] [PubMed] [Google Scholar]
- 26.Orellana G, & Slachevsky A (2013). Executive Functioning in Schizophrenia. Frontiers in Psychiatry, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poldrack RA, Congdon E, Triplett W, Gorgolewski KJ, Karlsgodt KH, Mumford JA, et al. (2016). A phenome-wide examination of neural and cognitive function. Scientific Data, 3(1), Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka SC, Yamashita A, Yahata N, Itahashi T, Lisi G, Yamada T, et al. (2021). A multi-site, multi-disorder resting-state magnetic resonance image database. Scientific Data, 8(1), Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. (2013). The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage, 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barron DS, Gao S, Dadashkarimi J, Greene AS, Spann MN, Noble S, et al. (2021). Transdiagnostic, Connectome-Based Prediction of Memory Constructs Across Psychiatric Disorders. Cerebral Cortex, 31(5), 2523–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caliński T, & Harabasz J (1974). A dendrite method for cluster analysis. Communications in Statistics, 3(1), 1–27. [Google Scholar]
- 32.Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, et al. (2015). Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nature Neuroscience, 18(11), Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble S, Spann MN, Tokoglu F, Shen X, Constable RT, & Scheinost D (2017). Influences on the Test-Retest Reliability of Functional Connectivity MRI and its Relationship with Behavioral Utility. Cerebral Cortex (New York, N.Y.: 1991), 27(11), 5415–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreasen NC (1983) The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, Iowa: The University of Iowa. [Google Scholar]
- 35.Andreasen NC (1984) The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, Iowa: The University of Iowa. [Google Scholar]
- 36.Patil I (2021). Visualization with statistical details: The ‘ggstatsplot’ approach. Journal of Open Source Software, 6(61), 3167, doi: 10.21105/joss.03167 [DOI] [Google Scholar]
- 37.Garrett DD, Samanez-Larkin GR, MacDonald SWS, Lindenberger U, McIntosh AR, & Grady CL (2013). Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neuroscience and Biobehavioral Reviews, 37(4), 610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin LQ (2020). Bring the Noise: Reconceptualizing Spontaneous Neural Activity. Trends in Cognitive Sciences, 24(9), 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene AS, Gao S, Scheinost D, & Constable RT (2018). Task-induced brain state manipulation improves prediction of individual traits. Nature Communications, 9(1), Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finn ES (2021). Is it time to put rest to rest? Trends in Cognitive Sciences, 25(12), 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H, Zhang H, Di X, Wang S, Meng C, Tian L, & Biswal B (2021). Reproducible coactivation patterns of functional brain networks reveal the aberrant dynamic state transition in schizophrenia. NeuroImage, 237, 118193. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Wang Y, Huang H, Jia Y, Zheng S, Zhong S, et al. (2020). Abnormal dynamic functional network connectivity in unmedicated bipolar and major depressive disorders based on the triple-network model. Psychological Medicine, 50(3), 465–474. [DOI] [PubMed] [Google Scholar]
- 43.Maia TV, & Frank MJ (2017). An Integrative Perspective on the Role of Dopamine in Schizophrenia. Biological Psychiatry, 81(1), 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howes OD, & Kapur S (2009). The Dopamine Hypothesis of Schizophrenia: Version III—The Final Common Pathway. Schizophrenia Bulletin, 35(3), 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tost H, Alam T, & Meyer-Lindenberg A (2010). Dopamine and psychosis: Theory, pathomechanisms and intermediate phenotypes. Neuroscience & Biobehavioral Reviews, 34(5), 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cousins DA, Butts K, & Young AH (2009). The role of dopamine in bipolar disorder. Bipolar Disorders, 11(8), 787–806. [DOI] [PubMed] [Google Scholar]
- 47.Cools R, & D’Esposito M (2011). Inverted-U–Shaped Dopamine Actions on Human Working Memory and Cognitive Control. Biological Psychiatry, 69(12), e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bäckman L, Ginovart N, Dixon RA, Wahlin T-BR, Wahlin Å, Halldin C, & Farde L (2000). Age-Related Cognitive Deficits Mediated by Changes in the Striatal Dopamine System. American Journal of Psychiatry, 157(4), 635–637. [DOI] [PubMed] [Google Scholar]
- 49.Li S-C, Lindenberger U, Nyberg L, Heekeren HR, & Bäckman L (2009). 5 Dopaminergic Modulation of Cognition in Human Aging. In Jagust W & D’Esposito M (Eds.), Imaging the Aging Brain (p. 0). Oxford University Press. [Google Scholar]
- 50.Heinz A, Knable MB, Coppola R, Gorey JG, Jones DW, Lee K-S, & Weinberger DR (1998). Psychomotor slowing, negative symptoms and dopamine receptor availability—An IBZM SPECT study in neuroleptic-treated and drug-free schizophrenic patients. Schizophrenia Research, 31(1), 19–26. [DOI] [PubMed] [Google Scholar]
- 51.Braun U, Harneit A, Pergola G, Menara T, Schäfer A, Betzel RF, et al. (2021). Brain network dynamics during working memory are modulated by dopamine and diminished in schizophrenia. Nature Communications, 12(1), Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


