Abstract
The gastrointestinal system is now considered the largest endocrine organ, highlighting the importance of gut-derived peptides and metabolites in metabolic homeostasis. Gut peptides are secreted from intestinal enteroendocrine cells in response to nutrients, microbial metabolites, and neural and hormonal factors, and regulate systemic metabolism via multiple mechanisms. While extensive research is focused on the neuroendocrine effects of gut peptides, evidence suggests that several of these hormones act as endocrine signaling molecules with direct effects at the target organ, especially in a therapeutic setting. Additionally, the gut microbiota metabolizes ingested nutrients and fiber to produce compounds that impact host metabolism indirectly, through gut peptide secretion, and directly, acting as endocrine factors. This review will provide an overview of the role of endogenous gut peptides in metabolic homeostasis and disease, as well as the potential endocrine impact of microbial metabolites on host metabolic tissue function.
Introduction
Energy and glucose homeostasis are tightly controlled by coordinated neural and endocrine signals that facilitate tissue crosstalk and central nervous system (CNS) integration to regulate food intake, energy expenditure, and glycemia. The liver, pancreas, and adipose tissue are traditionally considered organs of the endocrine system involved in regulating metabolic homeostasis. The endocrine pancreas secretes insulin in response to the postprandial rise in blood glucose, repressing hepatic glucose production and facilitating glucose uptake in adipose tissue and skeletal muscle while glucagon secretion generally opposes these actions (Campbell and Newgard, 2021). Further, adipocytes secrete adipokines, including leptin and adiponectin, to regulate food intake and maintain fat stores (Scheja and Heeren, 2019). The textbook functions of the gastrointestinal (GI) system are digestion and nutrient absorption; however, the gut is now considered the largest endocrine organ, maintaining energy and glucose homeostasis both directly and indirectly via gut peptides produced by enteroendocrine cells (EECs) and endocrine metabolites produced or altered by the gut microbiota (Ahlman and Nilsson, 2001).
EECs are dispersed throughout the GI tract, comprising only 1% of the total intestinal epithelial cell population (Worthington et al., 2018). Despite the low abundance of EECs, they have a major role in the maintenance of energy and glucose homeostasis, evidenced by glucose intolerance in mice lacking normal EEC development (Terry et al., 2014). Gut peptides are secreted in response to the sensing of luminal contents and function to coordinate digestion, nutrient absorption, appetite, energy expenditure, and insulin secretion (Table 1) (Gribble and Reimann, 2019). Recent advances have demonstrated the complexity and redundancy of these signaling molecules, as peptides impacting metabolic homeostasis are still being identified while the mechanisms of action and metabolic effects of previous peptides are continually redefined or discovered. While a large proportion of gut peptides act in a paracrine fashion on nearby intestinal epithelial cells or peripheral nerves, like vagal afferent neurons or spinal afferents that can signal to the brain (Wachsmuth et al., 2022), studies suggest many intestinally-derived peptides can enter the bloodstream and act in an endocrine fashion. This review focuses on the endocrine signaling capabilities of gut peptides, as other recent reviews have highlighted the role of neural signaling in regulating the metabolic effects (see Wachsmuth et al. (2022), Duca et al. (2021) for more).
Table 1.
Summary of intestinal gut peptides.
| Peptide | EEC Type | Tissue Location of Secretion | Function | References |
|---|---|---|---|---|
| Serotonin | Enterochromaffin Cell | Throughout the GI tract | Regulation of intestinal motility and inflammation, gluconeogenesis and glucose uptake, adipose tissue lipolysis, brown adipose tissue thermogenesis | (Sumara et al., 2012, Heredia et al., 2013, Margolis et al., 2014, Crane et al., 2015) |
| CCK | I cell | Small intestine | Regulation of gallbladder contraction, gastric emptying, pancreatic exocrine secretion, brown adipose tissue thermogenesis and hepatic glucose production, decreases food intake | (Blouet and Schwartz, 2012, Cheung et al., 2009, Li and Owyang, 1993, Lorenz and Goldman, 1982, Schwartz et al., 1993, Sonobe et al., 1995) |
| GIP | K cell (also found in some GLP-1 secreting cells) | Small intestine | Amplifies glucose-stimulated insulin secretion, promotes β-cell survival and proliferation | (Gasbjerg et al., 2019, Kim et al., 2005) |
| Neurotensin | N cell | Small intestine | Increases bile acid reabsorption and gallbladder motility, regulates insulin, somatostatin and glucagon secretion | (Dolais-Kitabgi et al., 1979, Yamasato and Nakayama, 1988, Béraud-Dufour et al., 2010, Li et al., 2021b) |
| GLP-1 | L cell | Small intestine through rectum | Amplifies glucose-stimulated insulin secretion, promotes β-cell survival and proliferation, decreases food intake, delays gastric emptying | (Li et al., 2005, Hare et al., 2010, Lamont et al., 2012, Turton et al., 1996, Davis et al., 1998, Zhang et al., 2022) |
| GLP-2 | L cell | Small intestine through rectum | Increases epithelial cell proliferation, intestinal barrier function and intestinal hexose transport, inhibits gastric acid secretion | (Drucker et al., 1996, Benjamin et al., 2000, Wøjdemann et al., 1999, Cheeseman and Tsang, 1996) |
| PYY | L cell | Small intestine through rectum | Inhibits gastric acid secretion, gastric emptying and pancreatic exocrine secretion, decreases food intake | (Adrian et al., 1985, Grandt et al., 1995, Moran et al., 2005, Challis et al., 2003, Degen et al., 2005) |
| Oxyntomodulin | L cell | Colon | Decreases food intake, amplifies glucose-stimulated insulin secretion | (Dakin et al., 2004, Maida et al., 2008) |
| INSL5* | L cell | Colon | Increases food intake and hepatic glucose production, regulates islet development and insulin secretion | (Grosse et al., 2014, Lee et al., 2016, Zaykov et al., 2019, Burnicka-Turek et al., 2012) |
(GI, gastrointestinal; CCK, cholecystokinin; GIP, glucose-dependent insulinotropic peptide; GLP-1, glucagon-like peptide 1; GLP-2, glucagon-like peptide 2; PYY, peptide YY; INSL5, insulin-like peptide 5).
Several actions of INSL5 are debated; see text for more details.
While the role of the intestine in regulating food intake and glucose homeostasis is well documented, the gut microbiota is also now considered a critical component of the intestinal endocrine system (Clarke et al., 2014). The gut microbiota, composed of all bacteria, archaea, and fungi residing in the GI tract, is both directly and indirectly implicated in host metabolic homeostasis (Howard et al., 2022). Many of the effects of the gut microbiota on energy and glucose homeostasis are linked to compounds produced or altered by gut bacteria that act directly on EECs, or alternatively enter circulation and target metabolic tissue function (Agus et al., 2021). For example, short chain fatty acids (SCFAs) produced by gut bacterial fermentation of ingested fiber induce secretion of glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) from EECs, thereby indirectly impacting the gut endocrine system, but also enter circulation to impact hepatic glucose metabolism (Shimizu et al., 2019), lipid metabolism (Yu et al., 2019), and regulate brown adipose thermogenesis (Christiansen et al., 2018, Cani et al., 2006). In addition, molecular components of microbes, like LPS, can activate EECs via innate immune recognition (Nguyen et al., 2014, Anhê et al., 2021). As the gut microbiota-metabolome axis impacts host metabolism, this review will discuss several metabolites and bacterial components with endocrine action that participate in host maintenance of energy and glucose homeostasis. Given the complex interaction of diet, gut microbiota, and the GI tract, it is crucial to better understand how these pathways work in unison to impact host metabolic health.
Gut Peptides/Hormones
EECs are specialized secretory cells located throughout the GI tract. While EEC subtypes are classically characterized based on the gut peptide they produce (e.g. K-cells secrete glucose-dependent insulinotropic peptide (GIP), L-cells secrete PYY and GLP-1, and I-cells secrete cholecystokinin (CCK); see Fig. 1), it is now accepted that EEC location may more accurately dictate peptide expression based on migration from crypt to villus (Beumer et al., 2018) and anatomical location (e.g. small intestine vs. colon) (Habib et al., 2012). Here we review the endocrine effect of gut peptides, while there is substantial evidence that many gut peptides act in a paracrine fashion on vagal and spinal afferent neurons innervating the gut to regulate energy and glucose homeostasis (see (Wachsmuth et al., 2022, Duca et al., 2021) for more). For example, CCK is a gut hormone secreted by I-cells of the upper small intestine in response to luminal fat and protein, and the CCK receptor is expressed in the GI tract and vagal afferent neurons (Fakhry et al., 2017, Wang et al., 2019). A gut-brain vagal signaling axis is implicated in the effects of CCK on gallbladder contraction (Sonobe et al., 1995), gastric emptying (Schwartz et al., 1993), pancreatic exocrine secretion (Li and Owyang, 1993), brown adipose tissue thermogenesis (Blouet and Schwartz, 2012), hepatic glucose production (Cheung et al., 2009) and control of feeding behavior (Lorenz and Goldman, 1982). However, non-neural signaling pathways for gut peptides are also critical for metabolic homeostasis, especially in the effect of incretin hormones.
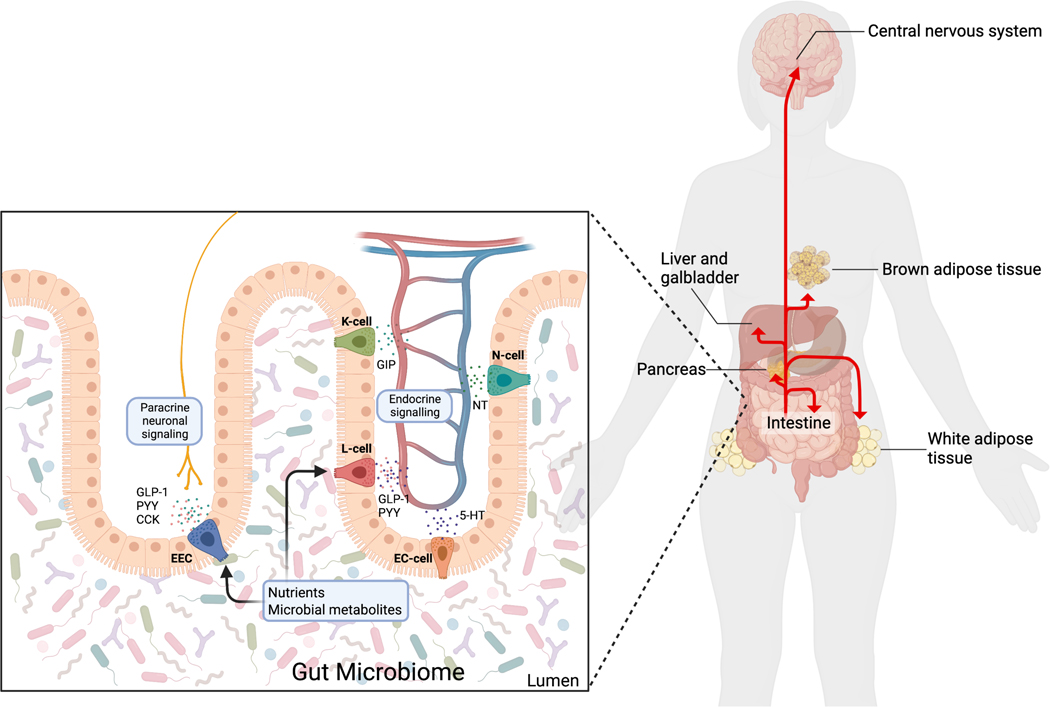
Figure 1. Gut peptide secretion and endocrine effects.
Enteroendocrine cells (EECs), dispersed throughout the intestine, sense luminal nutrients and microbial metabolites and secrete gut peptides that impact metabolism. K-cells secrete glucose-dependent insulinotropic peptide (GIP); L-cells secrete glucagon-like peptide 1 (GLP-1) and peptide YY (PYY); enterochromaffin cells (EC cells) secrete 5-hydroxytryptamine (5-HT, also known as serotonin); and N-cells secrete neurotensin (NT). Some of these gut peptides, especially GLP-1, PYY, and CCK, impact metabolism via paracrine neuronal signaling. Further, gut peptides enter circulation and act as endocrine factors at the intestine, pancreas, liver, gallbladder, central nervous system, and brown and white adipose tissue. Figure created with BioRender.com.
Incretin hormones
There are over 20 known gut peptides secreted by EECs that have both independent and overlapping effects on metabolism. A subset of gut peptides, termed incretins, are released in response to ingested nutrients and perpetuate glucose-stimulated insulin secretion from pancreatic β-cells, accounting for 50–70% of total insulin secretion following meal consumption (Nauck et al., 1986). The incretin hormones, GIP and GLP-1 are secreted in response to meal consumption, with the magnitude of secretion proportional to both rate of nutrient appearance (or rate of gastric emptying) and energy content (Vilsbøll et al., 2003, Ahrén, 2022). Traditionally, K-cells located in the duodenum and upper jejunum were thought to exclusively secrete GIP, and L-cells located in the ileum and colon were thought to exclusively secrete GLP-1. However, GIP and GLP-1 have been shown to colocalize in a subset of human, rat, and porcine small intestinal EECs, indicating simultaneous postprandial secretion of these peptides (Mortensen et al., 2003, Habib et al., 2012). GIP, the first identified incretin hormone, is secreted in response to luminal glucose and lipids (Wu et al., 2017, Wu et al., 2012). Interestingly, in humans, GIP secretion is greater in response to fat than carbohydrates, despite the glucose-dependent insulinotropic effect of this peptide (Wu et al., 2017). GLP-1 is secreted in response to ingested macronutrients and fiber, as well as neural and hormonal factors (Wang et al., 2015). Further, GLP-1 secretion in response to nutrients and other secretagogues appears to be specific to L-cell localization in the intestine. For example, L-cells of the small intestine are indispensable for the secretion of ingested nutrient-induced GLP-1 (Sun et al., 2017), whereas colonic L cells mediate GLP-1 secretion in response to activation of the G-protein coupled receptors, GPR119 and melanocortin 4 receptor (MC4R), metformin, bile acids, as well as maximal LPS-induced GLP-1 secretion (Panaro et al., 2020, Christiansen et al., 2019). In addition, microbial metabolites, such as SCFAs, can induce incretin hormone secretion (see section below). Both GIP and GLP-1 are degraded by dipeptidyl-peptidase 4 (DPP-4) within minutes of secretion (Kieffer et al., 1995), such that only a small percentage of these hormones reach systemic circulation, calling into question the endocrine ability of these peptides.
Despite the short half-life, GIP and GLP-1 function to amplify glucose-stimulated insulin secretion via direct activation of the GIP receptor (GIPR) and GLP-1 receptor (GLP-1R) expressed on pancreatic β-cells. Both are members of the B family of G-protein coupled receptors and have overlapping signaling mechanisms to potentiate glucose-stimulated insulin secretion (Mayo et al., 2003). Binding of GIP or GLP-1 to their associated receptors induces recruitment and activation of the Gas protein, adenylate cyclase activation and elevated intracellular cyclic AMP (cAMP), resulting in protein kinase A (PKA) and exchange protein directly activated by cAMP (EPAC)-mediated potentiation of insulin granular exocytosis (Kashima et al., 2001, Kaihara et al., 2013, Dyachok et al., 2006). Further, GLP-1 and GIP activate divergent, PKA-independent signaling mechanisms to promote β-cell survival and proliferation (Li et al., 2005, Kim et al., 2005). In addition to the insulin stimulating effects of incretins, GLP-1, but not GIP, inhibits glucagon secretion from α-cells, with equal contributions from glucagon inhibition and insulin secretion on glucose homeostasis (Hare et al., 2010). Although there is evidence for a neural GLP-1-mediated regulation of glucose homeostasis, potentially mediated by hepatic portal vein or gut-innervating GLP-1R expressing neurons (Balkan and Li, 2000, Vahl et al., 2007, Burcelin et al., 2001, Borgmann et al., 2021), recent work involving transgenic mice highlights the importance of pancreatic GLP-1R in glucose homeostasis (Lamont et al., 2012). Indeed, knockdown of the GLP-1R in β-cells abolishes the effects of GLP-1 on insulin secretion (Smith et al., 2014). Further, whole-body GLP-1R-deficient mice have impaired glucose-stimulated insulin secretion and glucose tolerance, whereas reintroduction of the GLP-1R only in pancreatic islets normalized glucose homeostasis and glucose-stimulated insulin secretion (Lamont et al., 2012) while deletion of GLP-1R in neurons does not impair oral glucose-stimulated insulin secretion (Varin et al., 2019, Sisley et al., 2014), all indicating that GLP-1 likely augments glucose-stimulated insulin secretion in an endocrine fashion via pancreatic rather than neural GLP-1Rs.
In addition to direct receptor binding on pancreatic islet cells, GLP-1 is proposed to act on the peripheral and CNS to induce satiation and decrease food intake postprandially. The GLP-1R is expressed on neurons in the hindbrain and hypothalamus (Turton et al., 1996, Adams et al., 2018), key regions regulating feeding behavior, as well as a subset of vagal afferent neurons in the nodose ganglion (Nakagawa et al., 2004). Both central and peripheral GLP-1 administration decreases food intake and GLP-1 secretion activates vagal afferent neurons (Davis et al., 1998, Turton et al., 1996, Nakabayashi et al., 1996, Buckley et al., 2020), sparking debate regarding the neural circuit involved in the effect of GLP-1 on food intake. However, studies utilizing vagal lesioning and deafferentation prompted in part by the rate of GLP-1 degradation by DDP-4 suggest that endogenous GLP-1 acts as a paracrine peptide through a gut-brain vagal circuit to regulate feeding behavior (Diepenbroek et al., 2017, Abbott et al., 2005a, Plamboeck et al., 2013, Brierley and de Lartigue, 2022, Borgmann et al., 2021). Conversely, more recently, the impact of vagal GLP-1R on energy homeostasis has been debated, as viral and transgenic knockout studies have shown a limited role in vagal afferent GLP-1 signaling on energy homeostasis (Varin et al., 2019, Sisley et al., 2014, Brierley et al., 2021). Interestingly, in an elegant study, it was demonstrated that the effects of GLP-1 on food intake and gastric emptying are mediated by GLP-1R expressing ileal enteric neurons (Zhang et al., 2022). Thus, while the endocrine action of GLP-1 is likely limited to the pancreas, the overall impact of neural endogenous GLP-1 signaling is contentious (see McLean et al. (2021) for more detailed endocrine and paracrine signaling of GLP-1).
Oxyntomodulin, like GLP-1, is derived from posttranslational modifications of proglucagon, is secreted postprandially by colonic EECs, and binds both the GLP-1 and glucagon (GCG) receptor (Baggio et al., 2004, Baldissera et al., 1988). Similar to GLP-1, oxyntomodulin acutely decreases food intake in rodents when administered directly to the brain (Dakin et al., 2001) and peripherally (Dakin et al., 2004), likely dependent on hypothalamic GLP-1R activation (Baggio et al., 2004). Further, oxyntomodulin production is blunted individuals with type 2 diabetes (T2D) (Wewer Albrechtsen et al., 2016), and treatment with oxyntomodulin is beneficial for glucose homeostasis via amplification of glucose-stimulated insulin secretion and body weight in individuals with T2D and obesity (Shankar et al., 2018, Wynne et al., 2005, Maida et al., 2008). However, more research is needed into determining the mechanism of action of oxyntomodulin, given it has a longer half-life than GLP-1 (~12 min) (Schjoldager et al., 1988).
GLP-2
Glucagon-like peptide 2 (GLP-2), co-secreted with GLP-1 from intestinal L cells in response to nutrients (Hartmann et al., 2000), has intestinotrophic as well as metabolic effects. At the intestine, GLP-2 plays a protective role in gut barrier function (Benjamin et al., 2000, Chen et al., 2012, Chang et al., 2021) and enhances nutrient absorption (Meier et al., 2006). Additionally, GLP-2 induces glucagon secretion, but has no effect on glycemia, in healthy individuals, suggesting minimal contribution of this peptide in normal glucose homeostasis (Meier et al., 2006, Sørensen et al., 2003). On the contrary, GLP-2 signaling is an attractive target for obesity-associated hyperglycemia, given that, in rodents with obesity, blocking endogenous GLP-2 action worsens glucose tolerance (Baldassano et al., 2015), and peripheral GLP-2 analog treatment improves glucose tolerance independent of body weight (Ejarque et al., 2021). Further, GLP-2 signaling is necessary and sufficient for the metabolic improvements associated with prebiotic supplementation in high fat feeding (Cani et al., 2009). The effect of GLP-2 on glucose regulation is hypothesized to occur due to decreased adipose tissue inflammation (Ejarque et al., 2021), improved gut barrier that attenuates metabolic endotoxemia (Cani et al., 2009), and/or neuroendocrine action via activation of Pro-opiomelanocortin (POMC)-expressing neurons of the hypothalamus (Shi et al., 2013); however, the exact mechanism remains to be fully elucidated.
PYY
PYY is a gut peptide expressed in L-cells of the distal intestine, where it is co-secreted with GLP-1 (Habib et al., 2013). PYY exists in two isoforms: PYY1–36 and PYY3–36, formed by DPP-4 mediated N-terminal cleavage following secretion (Mentlein et al., 1993). PYY3–36, the dominant form in circulation postprandially (Grandt et al., 1994), principally binds the Y2 receptor found in the CNS, including the hypothalamus and brain stem, as well as peripheral tissues, including the colon and kidney (Yi et al., 2018). PYY is secreted in response to luminal lipids and protein (Mangan et al., 2019, Batterham et al., 2006), as well as neural and gut microbial factors, including SCFAs (Zhang et al., 1993, Larraufie et al., 2018) (see below). PYY functions to inhibit gastric acid secretion, gastric emptying, and pancreatic exocrine secretion (Adrian et al., 1985, Grandt et al., 1995, Moran et al., 2005). Exogenous PYY administration also decreases food intake in rodents and humans (Challis et al., 2003, Degen et al., 2005), suggesting a role for this peptide in suppression of food intake following meal consumption. Indeed, mice lacking PYY develop obesity, and replacing PYY via once daily injection or continuous delivery via osmotic minipump induces weight loss these mice (Batterham et al., 2006), indicating that PYY is an endogenous regulator of food intake. Mechanistically, PYY is proposed to activate Y2 receptors in the nucleus tractus solitarius and/or the arcuate nucleus of the hypothalamus, activating anorexigenic neurons and inhibiting orexigenic neurons (Batterham et al., 2002, Blevins et al., 2008, Gustafson et al., 1997, Abbott et al., 2005b), indicating a clear endocrine action. However, the hypophagic effect of PYY is likely at least in part mediated by a gut-brain vagal circuit, as vagotomy and midbrain transection abolish the effect of PYY on food intake in rats (Koda et al., 2005).
In addition to energy homeostasis, PYY is also implicated in control of glucose homeostasis. In the pancreas, PYY is co-expressed with glucagon in α-cells and somatostatin in δ-cells (Böttcher et al., 1989, Khan et al., 2016), suggesting an endocrine effect of PYY on insulin secretion. In accordance with the inhibitory actions of this peptide, PYY inhibits glucose-stimulated insulin secretion in vivo (Böttcher et al., 1989). However, as PYY3–36 has no effect on insulin secretion in isolated islets and the Y2 receptor is not expressed in pancreatic islets (Chandarana et al., 2013), locally secreted PYY1–36 can act directly at the islet, whereas gut-derived PYY3–36 has no direct effect on β-cell insulin secretion. In contrast, peripheral PYY3–36 administration improves glucose tolerance likely via EEC Y2 receptor activation and increased GLP-1 secretion that subsequently increases insulin release (Chandarana et al., 2013). Thus, PYY1–36 secreted within pancreatic islets may represent a negative feedback mechanism for glucose-stimulated insulin secretion.
5-hydroxytryptamine (Serotonin)
While 5-hydroxytryptamine (5-HT, also known as serotonin) is canonically considered a neurotransmitter, serotonin is also synthesized by enterochromaffin (EC) cells of the intestine from tryptophan, a process critically regulated by tryptophan availability, tryptophan hydroxylase (the rate-limiting enzyme in serotonin synthesis), and gut microbial metabolism of tryptophan (Yano et al., 2015, Yabut et al., 2019). Serotonin is secreted from EC cells in response to changes in luminal nutrients, microbial metabolites, or stretch following meal consumption (Wang et al., 2017, Martin et al., 2017, Reigstad et al., 2015). Following secretion, the majority of serotonin is taken up and stored or degraded in platelets (Mercado and Kilic, 2010), with a small proportion remaining in plasma to act as a signaling factor in peripheral tissues. Because serotonin typically cannot cross the blood-brain barrier, the actions of peripheral serotonin are distinct from central serotonin; as such, gut-derived serotonin is an independent regulator of metabolic tissue function.
Serotonin primarily acts on peripheral tissues via activation of one of fourteen 5-HT receptors (HTRs), all except one of which are classified as G-protein coupled receptors (Sahu et al., 2018). Peripheral serotonin participates in intestinal homeostasis, including regulation of gut motility via enteric neuron signaling and intestinal inflammation (Heredia et al., 2013, Margolis et al., 2014). In addition, serotonin is implicated in adipose tissue lipid metabolism, as it promotes adipocyte glucose uptake and decreases lipolysis via HTR2A receptor activation (Hansson et al., 2016), and may also play a role in inhibition of brown adipose tissue thermogenesis, especially during diet-induced obesity (Crane et al., 2015). However, adipocytes synthesize and reuptake serotonin directly, so many of the actions of serotonin on adipose tissue are attributed to local, adipocyte-derived serotonin (Kinoshita et al., 2010, Oh et al., 2015). Interestingly, serotonin is increased during fasting, and promotes hepatic gluconeogenesis and inhibits hepatic glucose uptake, and promotes adipose tissue lipolysis via HTR2B activation in the fasted state (Sumara et al., 2012). On the contrary, during states of nutrient availability, serotonin may increase hepatic triglyceride accumulation (Osawa et al., 2011), providing evidence for the potential of HTR3 antagonists for treatment of non-alcoholic fatty liver disease (Haub et al., 2011).
INSL5
As previously mentioned, there are over 20 identified gut peptides, and recent research has identified novel gut peptides as well as functions of known peptides in metabolism. Among these, insulin-like peptide 5 (INSL5), produced by colonic L-cells (Billing et al., 2018), is secreted during fasting and has orexigenic properties (Lewis et al., 2020, Grosse et al., 2014); however, this effect is inconsistent (Zaykov et al., 2019). Interestingly, INSL5 receptor (relaxin/insulin-like family peptide receptor 4) expressing neurons in the hypothalamus were recently found to play a role in the regulation of feeding behavior associated with INSL5 (Lewis et al., 2022). However, as evidence for INSL5 production or presence in the brain is lacking, the physiological role of these neurons in INSL5-mediated feeding behavior is unclear, and it is unknown if these neurons are targeted by gut-derived INSL5. While the biological role of INSL5 is not fully elucidated, INSL5 signaling may participate in islet development and insulin secretion, as mice lacking INSL5 have decreased basal and glucose-stimulated insulin secretion and smaller pancreatic islets compared to wild-type controls, likely due to decreased INSL5-mediated activation of the relaxin family peptide receptor 4 (Burnicka-Turek et al., 2012). Further, INSL5 expression is regulated by the gut microbiota and may act to increase hepatic glucose production, in accordance with its secretion profile during low nutrient availability (Lee et al., 2016).
Neurotensin
Neurotensin, secreted by enteroendocrine N-cells and hypothalamic neurons (Polak et al., 1977), has major implications in the physiology of the CNS, but also plays a role in intestinal and metabolic homeostasis. Neurotensin is secreted primarily in response to luminal lipids (Draviam et al., 1990), acting locally to increase lipid absorption via increasing bile acid reabsorption and gallbladder motility (Gui and Carraway, 2001, Yamasato and Nakayama, 1988, Li et al., 2021b). Further, neurotensin may regulate glucose homeostasis, as systemic neurotensin administration results in hepatic glucose production from glycogenolysis and hyperglycemia (Carraway et al., 1976); this effect is likely due to the regulatory effect of neurotensin on insulin, glucagon, and somatostatin secretion from pancreatic islets (Dolais-Kitabgi et al., 1979, Béraud-Dufour et al., 2010). While peripheral neurotensin certainly plays a role in metabolic homeostasis, much of the research is focused on the effects of intracerebroventricular neurotensin on metabolic and energy homeostasis. Further, as the half-life of this peptide is ~30 seconds in rodents (Aronin et al., 1982), the endocrine effects of peripheral neurotensin have yet to be fully elucidated but are likely extremely limited.
Gut Microbiota
The complex gut microbiota-host relationship integrates intestinal and systemic metabolism, impacting gut peptide secretion and overall metabolic tissue function (Agus et al., 2021). As mentioned earlier, the gut microbiota encompasses all microbes residing in the GI tract. However, the majority of research thus far has focused on the impact of the gut bacteria, while only recently have other microbes, like fungi or bacteriophages, been implicated in regulating host metabolic health (Sun et al., 2021a, Heisel et al., 2017, de Jonge et al., 2022).
Interaction of the gut microbiota and gut peptide signaling
The gut microbiota is a key factor for coordinated gut peptide secretion, as germ-free and antibiotic-treated mice have alterations in nutrient-sensing and chemosensory machinery, EEC number, and gut peptide release (Table 2) (Duca et al., 2012, Lee et al., 2016, Modasia et al., 2020). For example, germ-free mice exhibit dysregulated diurnal GLP-1 secretion and consistently increased circulating basal and fed GLP-1 (Martchenko et al., 2020, Bäckhed et al., 2004, Heiss et al., 2021, Zarrinpar et al., 2018), despite discrepancies in intestinal expression in the literature (Duca et al., 2012, Wichmann et al., 2013). This increase in GLP-1 secretion likely mediates the increase in gut transit time observed in germ-free mice compared to conventional mice (Wichmann et al., 2013); however, this has also been attributed to modulation of bile acids by intestinal bacteria (Li et al., 2021c). Similarly, INSL5 expression is increased in antibiotic-treated and germ-free mice (Lee et al., 2016), whereas circulating PYY is decreased in germ-free mice during fasting and in response to ingested lipids (Samuel et al., 2008, Duca et al., 2012), suggesting that the gut microbiota regulate L-cell secretion profiles. Germ-free mice also have decreased colonic tryptophan hydroxylase expression and circulating serotonin (Sjögren et al., 2012, Wikoff et al., 2009, Yano et al., 2015), likely due to the key role of the gut microbiota in tryptophan metabolism and serotonin biosynthesis.
Table 2.
Summary of gut peptide and expression in germ-free mice compared to conventional mice.
| Peptide | GF vs. conventional mice | References |
|---|---|---|
| Serotonin | Decreased in circulation | (Sjögren et al., 2012, Yano et al., 2015, Wikoff et al., 2009) |
| CCK | Increased in circulation | (Martinez-Guryn et al., 2018) |
| Decreased expression in the proximal intestine | (Duca et al., 2012) | |
| GIP | Increased GIP+ cells in jejunum and colon | (Modasia et al., 2020) |
| Neurotensin | No data | |
| GLP-1 | Increased in circulation | (Heiss et al., 2021, Wichmann et al., 2013, Zarrinpar et al., 2018) |
| Increased cecal and colon Gcg expression | (Wichmann et al., 2013) | |
| Decreased expression in the proximal intestine | (Duca et al., 2012) | |
| GLP-2 | No data | |
| PYY | Decreased in circulation and decreased expression in the proximal intestine | (Duca et al., 2012) |
| Decreased in circulation compared to mice colonized with B. thetaiotaomicron and M. smithii | (Samuel et al., 2008) | |
| Oxyntomodulin | No data | |
| INSL5 | Increased expression in colon | (Lee et al., 2016) |
(GF, germ-free; GI, gastrointestinal; CCK, cholecystokinin; GIP, glucose-dependent insulinotropic peptide; GLP-1, glucagon-like peptide 1; GLP-2, glucagon-like peptide 2; PYY, peptide YY; INSL5, insulin-like peptide 5).
Further, germ-free mice have lower fasting insulin and body weight, and it is therefore proposed that the gut microbiota coordinates nutrient harvest and lipid metabolism and storage as a beneficial survival mechanism (Bäckhed et al., 2004). In addition, modulation, rather than ablation, of the gut microbiome with fermentable fiber supplementation induces GLP-1 secretion and glucose tolerance in healthy animals (Massimino et al., 1998a), providing a more physiologically relevant model implicating the importance of the gut microbiota in metabolic homeostasis. Thus, modifying the gut microbiota is a promising therapy for obesity and glucose intolerance. Indeed, fermentable fiber supplementation reduces body weight gain in models of diet-induced obesity (Meyer et al., 2022b) and improves glucose tolerance and insulin sensitivity in diabetic rodents dependent on GLP-1R signaling (Cani et al., 2005, Cani et al., 2006). However, this effect remains controversial in human studies, as fermentable fiber supplementation in individuals with T2D has shown both no effect on postprandial GLP-1 secretion (Birkeland et al., 2021) and increased postprandial GLP-1 and improved glucose tolerance (Zhao et al., 2018). The impact of fiber on GLP-1 signaling could be due to increased number of L-cells or expression of the preproglucagon gene (Massimino et al., 1998b, Kaji et al., 2011, Everard et al., 2011), although alterations in the gut microbiota via fermentable fibers induce myriad of other effects that could impact host metabolism, such as production and alterations in gut-derived metabolites (Meyer et al., 2022a). For example, both SCFAs and bile acids have been linked with the effect of dietary fiber on gut peptide secretion and subsequent effects on host metabolic homeostasis (Makki et al., 2022, Cani et al., 2006). The specific signaling pathways for which SCFAs, bile acids, and other gut derived metabolites is discussed in detail in the following section. Altogether, the gut microbiota has a significant impact on secretion of gut peptides that can impact metabolic homeostasis.
Gut-derived Metabolites
Perhaps the most investigated mechanism by which the gut microbiota impacts the energy and glucose homeostasis is via the host metabolome, as gut microbes metabolize dietary components and endogenous substances to produce novel bioactive chemicals (Agus et al., 2021). A notable class of compounds produced by intestinal bacteria is SCFAs generated by gut bacterial fiber fermentation. Specifically, fermentable soluble fibers, including resistant starch, β-glucan, inulin/inulin-type fructans, pectin, and soluble corn fiber, are well-established substrates for SCFA production by intestinal bacteria (Martinez et al., 2021). SCFAs can induce gut peptide secretion locally or can enter systemic circulation to act on peripheral metabolic tissues like the liver and adipose tissue (Li et al., 2018b, den Besten et al., 2015). Further, amino acids from the diet are modified by gut microbial metabolism, resulting in altered circulating metabolites that act as endocrine factors to regulate energy and glucose homeostasis (Jo et al., 2021, Hubbard et al., 2015). For example, branched chain amino acids (BCAAs) are produced by bacterial metabolism of the amino acids, glycine, serine, or threonine (Amorim Franco and Blanchard, 2017, Gojda and Cahova, 2021). Similarly, bacterial metabolism of histidine produces imidazole propionate, which regulates hepatic metabolism (Koh et al., 2018), and bacterial metabolism of tryptophan produces tryptamine, indoleacetic acid, indole aldehyde, and others, that regulate inflammation and metabolism via cellular signaling mechanisms (Roager and Licht, 2018). Endogenous compounds can also be modified by intestinal bacteria. Bile acids, produced in the liver, are modified by gut bacteria via deconjugation by bile salt hydrolase and production of exogenous bile acid species (termed secondary and tertiary bile acids) by coordinated bacterial dihydroxylation, oxidation, and epimerization enzymes and resulting in a diverse bile acid pool (Guzior and Quinn, 2021); these modifications impact host receptor signaling to alter gut peptide secretion and tissue metabolism (Ridlon et al., 2014). As these compounds both impact intestinal endocrine function and act as endocrine factors themselves, this review will discuss in detail the effects of metabolites produced or altered by the gut microbiota on host energy and glucose homeostasis.
Short chain fatty acids
As previously mentioned, fiber consumption induces gut peptide secretion at least in part by increasing SCFA production by gut bacteria. It is thought that SCFAs impact gut peptide secretion via the G-protein coupled receptors GPR41 (FFAR3) and GPR43 (FFAR2) expressed on EECs (Fig. 2, Table 3) (Brooks et al., 2017, Christiansen et al., 2018). Knockout of either FFAR2 or FFAR3 reduces GLP-1 secretion in response to either propionate or acetate (Tolhurst et al., 2012), and activation of a mutant FFAR2-DREADD unresponsive to SCFAs induces GLP-1 secretion similar to propionate administration in wild-type mice (Bolognini et al., 2019). Because the concentration of certain SCFAs, like acetate, in the intestinal lumen is consistently maintained to achieve FFAR2 activation (Cummings et al., 1987), it is proposed that SCFAs may impact gut peptide secretion via basolateral receptor activation. In line with this, FFAR2 has been shown to be expressed on the basolateral EEC membrane, and dietary and vascular SCFAs have differential effects on GLP-1 and PYY secretion (Christiansen et al., 2018, Karaki et al., 2006), indicating that absorption may be necessary for SCFA sensing. However, FFAR3 has a higher affinity for butyrate than acetate and propionate (Brown et al., 2003, Le Poul et al., 2003); therefore, luminal butyrate may be sensed by FFAR3 to induce gut peptide secretion. Nonetheless, it is possible that despite the open-faced nature of EECs to the luminal environment, SCFAs may induce gut peptide secretion via an endocrine mechanism that targets the basolateral side of the EECs; the reasoning and exact pathway for this unique mechanism warrants further investigation.
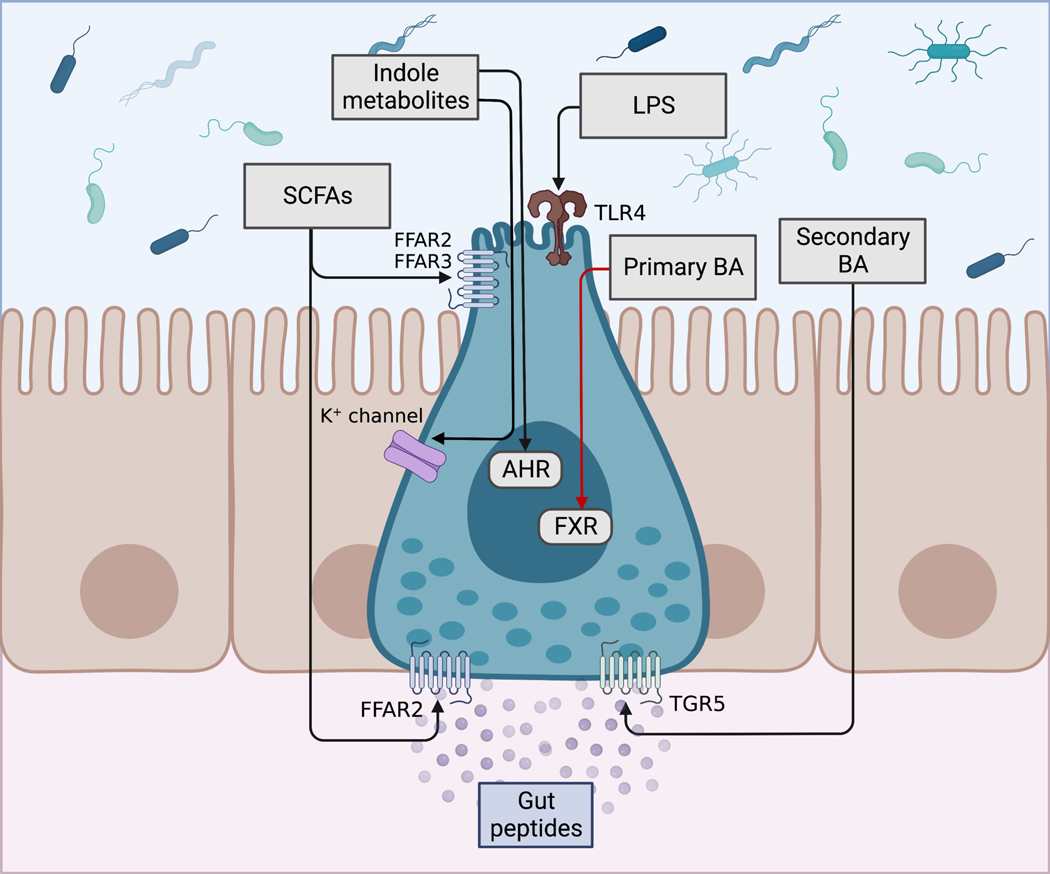
Figure 2. Signaling mechanisms of gut peptide secretion by microbially produced metabolites.
Metabolites produced or altered by the gut microbiota that impact gut peptide secretion include short chain fatty acids (SCFAs), indole metabolites produced from bacterial metabolism of tryptophan, primary bile acids (BAs) that can be deconjugated by bacterial bile salt hydrolase, and secondary BAs produced by bacterial metabolism of primary Bas, among others. SCFAs are proposed to induce secretion of glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) via FFAR2 and/or FFAR3; however, some studies suggest that SCFA absorption and basolateral FFAR2 is responsible for SCFA-induced gut peptide secretion. Indole metabolites inhibit voltage-gated K+ channels to increase EEC action potential and intracellular Ca2+, and induce GLP-1 secretion; alternatively, indole metabolites may activate the aryl hydrocarbon receptor (AHR) to induce GLP-1 secretion. Bacterial lipopolysaccharide (LPS) induces GLP-1 secretion via toll-like receptor 4 (TLR4). Primary BAs primarily activate the Farnesoid X receptor (FXR) to inhibit GLP-1 secretion, whereas secondary BAs primarily activate the basolateral G-protein bile acid receptor 1 (Gpbar1, also known as TGR5) to induce gut peptide secretion. Black arrows indicate signaling pathways resulting in induction of gut peptide secretion; red arrow indicates signaling pathways resulting in inhibition of gut peptide secretion. Figure created with BioRender.com.
Table 3.
Effects of microbial metabolites or components on gut peptide secretion.
| Compound | Effect on gut peptide secretion | Proposed Mechanism | References |
|---|---|---|---|
| SCFA | Increased GLP-1 and PYY | Activation of FFAR2/FFAR3 | (Brooks et al., 2017, Christiansen et al., 2018, Tolhurst et al., 2012) |
| Primary and some secondary bile acids | Decreased GLP-1 | Activation of FXR | (Li et al., 2019b, Li et al., 2019c, Trabelsi et al., 2015) |
| Secondary bile acids | Increased GLP-1 and PYY | Activation of TGR5 (Gpbar1) | (Brighton et al., 2015, Christiansen et al., 2019, Kuhre et al., 2018) |
| Tryptophan metabolites | Increased GLP-1 | Activation of AHR | (Natividad et al., 2018) |
| Increased GLP-1 (acute) | Inhibition of voltage-gated K+ channels (acute) | (Chimerel et al., 2014) | |
| Decreased GLP-1 (prolonged) | Decreased ATP production via inhibition of NADH dehydrogenase | ||
| LPS (E. coli) | Increased GLP-1 | Activation of TLR4 | (Lebrun et al., 2017, Anhê et al., 2021) |
| LPS (R. sphaeroides) | No effect on GLP-1 secretion | No (or possibly antagonistic) effect on TLR4 activation | (Anhê et al., 2021) |
(SCFA, short chain fatty acids; FFAR2, free fatty acid receptor 2; FFAR3, free fatty acid receptor 3; GLP-1, glucagon-like peptide 1; PYY, peptide YY; FXR, Farnesoid X Receptor; TGR5, G-protein-coupled bile acid receptor (Gpbar1); AHR, arylhydrocarbon receptor; LPS, lipopolysaccharide; TLR4, toll-like receptor 4).
In addition to their role in the stimulation of gut peptide secretion, SCFAs are also absorbed into general circulation and can act as endocrine factors in metabolically active tissues (Fig. 3). The majority of SCFAs are removed via first pass by the liver, where they impact hepatic metabolism. For example, butyrate decreases lipogenesis and increases hepatic oxidative respiration and beta-oxidation via activation of AMP-activated protein kinase (AMPK) (Mollica et al., 2017), dependent on peroxisome proliferator- activated receptor gamma (PPAR-γ) (den Besten et al., 2015). Acetate and propionate can also be used by hepatocytes for ATP production and gluconeogenesis, respectively(Fujino et al., 2001, Anderson and Bridges, 1984). A small amount of SCFAs escape hepatocyte uptake and enter general circulation to regulate adipocyte thermogenesis and browning. Specifically, butyrate, and, to a lesser extent, acetate, are consistently shown to induce adipocyte browning and increase thermogenesis in mice (Gao et al., 2009, Wang et al., 2020, Li et al., 2019a, Sahuri-Arisoylu et al., 2016). However, there are differential effects of circulating acetate compared to acetate derived from adipocytes acting as a paracrine signal (Sun et al., 2021b), indicating the effect acetate on adipocyte browning may be dependent on source and concentration. Circulating SCFAs alter tissue metabolism via two primary mechanisms: activation of GPCR signaling and epigenetic regulation. As GPR41 and GPR43 are widely expressed (Brown et al., 2003), these receptors may mediate the endocrine actions of SCFAs in the liver (Aoki et al., 2021) and adipose tissue (Kimura et al., 2013). SCFAs also directly impact gene expression via epigenetic modulation to regulate metabolic function. Indeed, SCFA administration in diet-induced obese mice induces expression of adiponectin and resistin via decreasing CpG methylation at adiponectin and resistin promotor regions (Lu et al., 2018). In addition, butyrate acts as a histone deacetylase (HDAC) inhibitor to alter gene expression (Vidali et al., 1978), including hepatic fibroblast growth factor 21 (FGF21) through HDAC3 inhibition (Li et al., 2012) and skeletal muscle insulin receptor substrate 1 (IRS1), peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC1a), and sirtuins to regulate insulin receptor signaling (Chriett et al., 2019). The effects of butyrate on HDAC inhibition have also been extensively investigated in the context of inflammatory bowel disease (Li et al., 2021a) , gut immunity (Yang et al., 2020) and asthma (Islam et al., 2022), implicating butyrate as a key epigenetic regulator of both metabolic and immune cell function. However, the impact of endogenous SCFA on host metabolism is less resolved, as a majority of the studies outlined above utilize orally or intraperitoneally administrated SCFAs, which is not physiologically relevant. Additionally, several studies suggest SCFAs act in a neural fashion to regulate host metabolism (Goswami et al., 2018, Muller et al., 2020, Li et al., 2018a), and at least one study demonstrated that intravenous administration had no impact on improving energy homeostasis (Li et al., 2018a). Future studies examining the metabolic impact of SCFAs should aim to deliver SCFAs directly to the large intestine to more closely mimic endogenous production, or at the very least, should try to replicate post prandial levels in portal and general circulation (Meyer et al., 2022b). At least one study though has elegantly demonstrated that endogenous SCFAs derived from dietary fiber fermentation can enter circulation and reach the CNS to impact energy homeostasis (Frost et al., 2014), thus underscoring the need for further investigation.
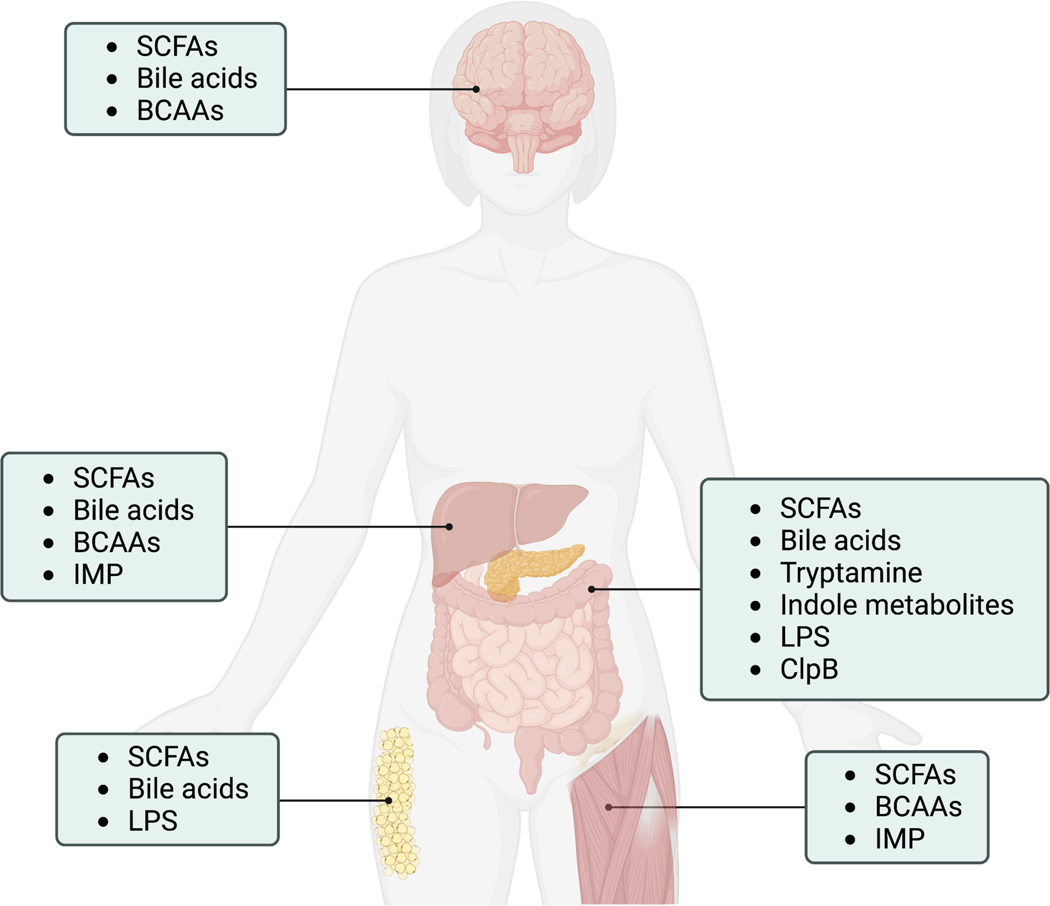
Figure 3. Microbial metabolites enter circulation and impact metabolic organ function.
Microbial metabolites discussed in the text are listed with their metabolic organ targets. Short chain fatty acids (SCFAs); branched chain amino acids (BCAAs), imidazole propionate (IMP), lipopolysaccharide (LPS); caseinolytic peptidase B protein homolog (ClpB). Figure created with BioRender.com.
Bile acids
Bile acid concentrations increase in the intestinal lumen postprandially, playing a critical role in lipid absorption in the proximal small intestine as emulsifying agents. However, it is now known that bile acid signaling is a critical regulator of metabolic homeostasis, via paracrine and endocrine actions that are mediated in part by interactions with the gut bacteria. For example, bile acids regulate food intake through distinct signaling pathways via induction of gut peptide secretion by acting as ligands for both the G-protein coupled bile acid receptor-1 (Gpbar1, also known as TGR5) and Farnesoid X Receptor (FXR) (Fig. 2, Table 3) (Chiang, 2013). Indeed, the effects of intraluminal bile acids on gut peptide secretion is well-documented in rodents (Kuhre et al., 2018, Christiansen et al., 2019) as well as humans (Adrian et al., 1993, Adrian et al., 2012, Hansen et al., 2016), and is induced via TGR5 (Christiansen et al., 2019). TGR5 is highly expressed in the colon, where secondary bile acids are produced; as such, the endogenous ligands of TGR5 are conjugated secondary bile acids produced by gut bacteria from host-derived primary bile acids, with taurine-conjugated lithocholic acid being the most potent agonist (Duboc et al., 2014). While luminal bile acids were thought to induce GLP-1 and PYY secretion dependent on apical TGR5, more recent research suggests that bile acid absorption and basolateral TGR5 are required for the effect of bile acids on gut peptide secretion (Kuhre et al., 2018, Brighton et al., 2015), as intraluminal TGR5 agonism has no effect on gut peptide secretion, but intravascular administration of a TGR5 agonist induces robust GLP-1 responses (Christiansen et al., 2019). Additionally, based on the gut peptide-stimulating effect of TGR5 agonism, this signaling pathway has recently been implicated in treatments for obesity and T2D (Zheng et al., 2021), including fiber supplementation and gastric bypass surgeries (Ding et al., 2016, McGavigan et al., 2017), both of which are associated with increased plasma GLP-1 and attenuated food intake.
FXR is expressed in the ileum where primary bile acid concentrations are the greatest, thus FXR activity is largely regulated by primary bile acid species, with chenodeoxycholic acid (CDCA) being the most potent agonist, and rodent taurine-conjugated beta-muricholic acid a potent FXR antagonist (Sayin et al., 2013, Makishima et al., 1999). Whereas TGR5 induces gut peptide secretion, FXR inhibits proglucagon expression and GLP-1 secretion via interaction with cAMP response element binding protein (CREB) in EECs (Li et al., 2019b, Li et al., 2019c, Trabelsi et al., 2015). Further, FXR activation impairs SCFA-induced gut peptide secretion via inhibition of FFAR2 signaling (Ducastel et al., 2020), demonstrating complex interactions and converging signaling pathways between different classes of microbial metabolites. Interestingly, despite the antagonistic role of FXR signaling in bile acid- and SCFA-mediated GLP-1 secretion in metabolically healthy individuals, FXR activation promotes weight loss and improvements in glucose regulation following gastric bypass surgery (Ryan et al., 2014) and increases intestinal EEC number ex vivo (Kim et al., 2022), suggesting dynamic FXR signaling dependent on physiological state. Additionally, FXR is localized in peripheral metabolic tissues (Zhang et al., 2014, Cariou et al., 2006), and it is plausible that the differing metabolic outcomes observed during studies involving FXR are due to action in the intestine versus other tissues like the liver.
Aside from their role in the induction of gut peptides, bile acids can also impact host metabolism in peripheral tissues and within the CNS (Fig. 3). As bile acids undergo enterohepatic circulation, they can both directly and indirectly alter systemic physiology through hepatic and intestinal FXR, respectively; however, the role of FXR remains contentious in individuals with normal metabolic function and metabolic syndrome. For example, while global FXR deficient mice on a normal chow diet display peripheral insulin resistance and elevated serum free fatty acids (Cariou et al., 2006), mice with global, but not liver-specific, FXR deficiency are protected from diet-induced obesity and insulin resistance (Prawitt et al., 2011). On the contrary, intestinal FXR agonism prevents diet-induced obesity and insulin resistance (Fang et al., 2015), further complicating the role of FXR in obesity and metabolic disease. Intestinal FXR may exert beneficial effects via secretion of FGF19 (rodent FGF15) that acts on the fibroblast growth factor receptor 4 (FGFR4) to control bile acid, glucose, and lipid metabolism (Stroeve et al., 2010), as FGF15/19 represses gluconeogenic enzyme expression and postprandial lipogenesis and induces glycogen synthesis (Kim et al., 2020, Potthoff et al., 2011, Kir et al., 2011). However, bile acids also exert an FGF19-independent effect on hepatic lipid metabolism through FXR, as hepatic FXR-deficiency induces hepatic triglyceride accumulation and elevated serum cholesterol, whereas intestinal FXR-deficiency has no effect on hepatic or circulating lipids (Schmitt et al., 2015). FGF15/19 is also involved in the adipose tissue thermogenic response to cold (Fang et al., 2015, Morón-Ros et al., 2021). Finally, levels of the FXR agonist taurochenodeoxycholic acid (TCDCA) increase with high fat-feeding due to small intestinal gut microbiota modulation and impair insulin action in the dorsal vagal cortex dependent on FXR (Zhang et al., 2021, Meyer et al., 2022a), implicating central FXR in control of glucose homeostasis.
TGR5, on the other hand, is consistently reported to be metabolically beneficial. Following a meal, bile acids increase temporally in the hypothalamus, where TGR5 activation participates in satiety and decreases food intake (Perino et al., 2021). Therefore, TGR5 is a prime target for obesity, as central TGR5 agonism in obesity reduces body weight and food intake and increases energy expenditure via the sympathetic nervous system (Castellanos-Jankiewicz et al., 2021). Peripheral TGR5 also increases energy expenditure in humans (Broeders et al., 2015) and mice via TGR5-mediated intracellular thyroid hormone activation and adipose tissue beiging (Velazquez-Villegas et al., 2018, Watanabe et al., 2006).
Amino acids and derivatives
Large scale metabolomic studies have identified gut microbiota-related amino acid metabolites that regulate metabolic homeostasis via endocrine action. Among these, BCAAs are essential amino acids derived from the diet or gut bacterial biosynthesis. Following absorption, BCAA catabolism occurs primarily in skeletal muscle, where activity of the first enzyme in the BCAA catabolic pathway, branched-chain-amino-acid aminotransferase, is high. In healthy individuals, BCAAs, especially leucine, promote protein synthesis and inhibit proteolysis through mammalian target of rapamycin (mTOR) signaling (Suryawan et al., 2008). In the brain, BCAAs compete for transport with other aromatic amino acids (tryptophan, tyrosine, and phenylalanine) and can thus decrease production of certain neurotransmitters, including serotonin (Gijsman et al., 2002, Choi et al., 2013). In addition, BCAA catabolism results in production of alanine, a key gluconeogenic amino acid, and can therefore promote hepatic glucose production during starvation when BCAA levels increase (Fig. 3) (Holecek et al., 2016). These metabolic effects provide the basis for BCAA supplementation for athletes; however, human studies suggest that the benefits of BCAAs are limited (Plotkin et al., 2021).
Interestingly, plasma BCAAs are elevated in obesity and correlate with insulin resistance and are a predictor of T2D (Newgard et al., 2009, Vanweert et al., 2021, Felig et al., 1969, Wang et al., 2011b). Evidence suggests that both peripheral and hepatic insulin resistance occurs with elevated BCAAS in obesity and T2D, independent of body weight. BCAA supplementation in diet-induced obesity induces skeletal muscle insulin resistance via phosphorylation of mTOR and IRS1, in accordance with the known functions of BCAAs in skeletal muscle (Newgard et al., 2009). Mechanistically, elevated BCAAs in T2D occurs at least in part due to altered expression of enzymes involved in BCAA metabolism in liver, skeletal muscle, and adipose tissue (She et al., 2007, Lian et al., 2015), as well as due to increased abundance of BCAA-producing bacteria and decreased abundance of bacteria that uptake BCAAs in the gut (Pedersen et al., 2016). In the liver, enzymes that regulate BCAA catabolism also control hepatic lipogenesis; therefore, dysregulated expression of these enzymes could contribute to hepatic insulin resistance (White et al., 2018). On the other hand, strategies that reduce circulating BCAAs, like gastric bypass surgery, improve peripheral insulin sensitivity independent of body weight (Lips et al., 2014, Magkos et al., 2013) at least in part by decreasing muscle fatty acyl CoA and glycine accumulation (White et al., 2016).
In addition to BCAAs, imidazole propionate (IMP), a metabolite produced by gut bacterial histidine metabolism, has recently gained attention in the context of T2D. Individuals with T2D have increased portal vein and circulating IMP levels (Koh et al., 2018), increased pro-inflammatory gut bacteria (Molinaro et al., 2020), and low gut microbial diversity (Menni et al., 2020). Despite no differences in dietary histidine intake, IMP is positively correlated with saturated fat and negatively correlated with fiber and unsaturated fat consumption in individuals with T2D, indicating that diet-mediated gut microbiota modulation is critical for IMP production (Molinaro et al., 2020). Following absorption, IMP impairs glucose tolerance and insulin signaling in mice through a p38γ mitogen activated protein kinase (MAPK)-p62-mTOR complex 1 (mTORC1) signaling axis (Fig. 3) (Koh et al., 2018). Interestingly, individuals with T2D and high blood glucose taking metformin have increased IMP levels, and the blood glucose lowering effect of metformin is blunted with IMP pretreatment in mice, dependent on p38γ MAPK-Akt mediated inhibitory AMPK phosphorylation (Koh et al., 2020). Taken together, these data provide promising framework for therapeutics targeting IMP-producing bacteria for treatment of T2D.
The amino acid tryptophan is also metabolized by gut bacteria, producing metabolites that impact host receptor activity. While over 95% of dietary tryptophan is metabolized directly by the host via indoleamine 2,3-dioxygenase 1 (IDO1), gut bacteria can metabolize tryptophan into tryptamine and indole metabolites. Production of the metabolite tryptamine in the gut is impacted by bacterial metabolism of tryptophan, as germ-free mice have lower fecal tryptamine than humanized mice (Marcobal et al., 2013), and it is estimated that >10% of individuals harbor gut microbes that express at least one of the enzymes for decarboxylation of tryptophan to produce tryptamine (Williams et al., 2014). Further, metabolic syndrome is associated with blunted production of tryptamine and indole metabolites from dietary tryptophan due to gut microbiome dysbiosis (Natividad et al., 2018). Although a relatively low-abundance metabolite, tryptamine induces serotonin secretion from gut EC cells (Takaki et al., 1985), potentially indirectly impacting peripheral tissue metabolism. Tryptamine is also a proposed therapeutic for gut inflammatory disorders, as it induces mucus secretion from goblet cells via the G-protein coupled serotonin receptor 5-HTR4 (Bhattarai et al., 2020). The effects of tryptophan metabolites on metabolic homeostasis are at least partially dependent on the aryl hydrocarbon receptor (AHR). Indeed, high fat-fed mice treated with either an AHR agonist or Lactobacillus reuteri, a bacteria with high AHR ligand production, have improved gut barrier function and metabolic homeostasis possibly mediated by AHR-induced GLP-1 secretion from EECs (Natividad et al., 2018). However, a previous study found that indole induces acute GLP-1 secretion from EECs via voltage-gated K+ channel inhibition (Chimerel et al., 2014); therefore, multiple potential intersecting pathways may be responsible for indole-mediated GLP-1 secretion (Fig. 2, Table 3). On the other hand, exposure to the AHR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, also known as dioxin) is correlated with hyperglycemia and insulin resistance in humans (Henriksen et al., 1997, Cranmer et al., 2000), and mice expressing a low-binding affinity AHR variant and global AHR deficient mice are resistant to diet induced obesity and associated metabolic perturbances (Xu et al., 2015, Kerley-Hamilton et al., 2012, Wang et al., 2011a). Taken together, while these data indicate a potential impact of indole metabolites impacting metabolic homeostasis via AHR, there is much to be determined in regards to the site of action and mechanism. Indeed, AHR is expressed in a variety of cell types, and is a critical regulator of NF-KB inflammatory signaling (Ishihara et al., 2021). Thus, it is possible that there exists a balance of pro- and anti-inflammatory signaling required to maintain homeostasis, that is further dependent on the specific tissue affected. For example, intestinal AHR activation improves intestinal inflammation associated with obesity (Postal et al., 2020), and promotes secretion of anti-inflammatory cytokines in the intestine to improve gut barrier and metabolic homeostasis in mice challenged with high fat feeding (Lin et al., 2019). Nonetheless, bacterially-derived tryptophan metabolites represent an exciting new area of research in metabolic disease, and warrant further research.
Bacterial Components
Chronic low-grade inflammation often occurs in obesity and obesity-associated metabolic disorders, at least in part due to LPS exposure. Western-style high fat diet-feeding and loss of gut barrier integrity in obesity promote LPS absorption, resulting in host low-grade inflammation and impaired glucose homeostasis, termed metabolic endotoxemia (Fig. 3) (Pendyala et al., 2012, Cani et al., 2007a). High fat diet-fed mice have increased circulating LPS, and chronic LPS exposure increases body weight, worsens glucose tolerance and insulin sensitivity, and increases inflammatory cytokine expression dependent on the cell surface receptor cluster of differentiation 14 (CD14) (Cani et al., 2007a). Both rodent and human obesity is associated with increased adipose tissue expression of proinflammatory cytokines, including tumor necrosis factor (TNF)-α that is correlated with hyperinsulinemia and inhibits insulin receptor tyrosine kinase activity through IRS-1 (Hotamisligil et al., 1993, Hotamisligil et al., 1995, Hotamisligil et al., 1996). Further, OFS supplementation in high fat-feeding improves glucose homeostasis, reduces adipose and circulating inflammatory cytokines, and increases gut Bifidobacterium sp. that are negatively associated with endotoxemia, further implicating the gut microbiota composition and in the detrimental inflammatory and metabolic effects of high fat-feeding (Cani et al., 2007b). Circulating LPS forms a complex with LPS-binding protein, which can interact with cell surface receptors CD14, toll-like receptor 4 (TLR4), and toll-like receptor 2 (TLR2), inducing proinflammatory cytokine release (Mohammad and Thiemermann, 2020). As such, some reports suggest that CD14 and TLR4 deficient mice are protected from diet-induced obesity and insulin resistance (Kim et al., 2007, Poggi et al., 2007, Jia et al., 2014, Roncon-Albuquerque et al., 2008), whereas others suggest that neither TLR4 or CD14 mediate diet-induced obesity (Dalby et al., 2018, Young et al., 2012). These discrepancies in the literature may be due to differences in knockout tissue specificity, genetic background, or diet, and indicate a need to further elucidate the significance of TLR4 in the pathophysiology of metabolic disorders. Interestingly, hexa-acylated LPS derived from Escherichia coli induces GLP-1 secretion from enteroendocrine L-cells in response to intestinal injury to reduce inflammation via TLR4 activation (Fig. 2, Table 3) (Lebrun et al., 2017), whereas penta-acylated LPS from R. sphaeroides has no effect on GLP-1 secretion (Table 3) (Anhê et al., 2021), indicating that the effects of LPS on metabolism are dependent on diet, physiological state, and species-specific LPS type. In addition to TLR4, bacterial LPS agonizes TLR2, altering cellular metabolism and immune cell activation (Kirschning et al., 1998). Further, mice lacking TLR2 are resistant to diet-induced obesity and glucose intolerance (Guo et al., 2021, Ehses et al., 2010), and inhibition of TLR2 signaling improves insulin sensitivity (Caricilli et al., 2008). On the contrary, flaggelin, the primarily protein found in bacterial flagella, may reduce metabolic endotoxemia via TLR5-mediated gut microbiota remodeling. Upon activation by flaggelin, TLR5, expressed in the intestinal epithelium, modulates the presence of pathogenic gut bacteria (Carvalho et al., 2012). Interestingly, mice lacking whole body and intestinal TLR5 develop metabolic endotoxemia, with increased body weight and adiposity and abnormal glucose regulation, as well as susceptibility to colonization with pathogenic bacteria (Chassaing et al., 2014, Vijay-Kumar et al., 2010), implicating an intestinal feedback loop in which pathogenic bacteria stimulate TLR5 that, in turn, impairs pathogenic bacterial growth to regulate intestinal inflammation and prevent metabolic endotoxemia. Together, these studies suggest a role for multiple TLRs in inflammation-associated metabolic perturbances.
The nucleotide-binding oligomerization domain-containing proteins, NOD1 and NOD2, are ubiquitously expressed pattern recognition receptors that recognize bacterial cell wall components, including peptidoglycans from gram-negative and some gram-positive bacteria (Rivers et al., 2019). In particular, NOD1 and NOD2 have been studied in bacterial induction of inflammatory signaling that results in insulin resistance. Expression of NOD1 and NOD2 is elevated in individuals with metabolic syndrome (Lappas, 2014, Zhou et al., 2015, Shiny et al., 2013) and diet-induced obese rodents (Sharma et al., 2022), and mice lacking NOD1, but not NOD2, are resistant to diet-induced body weight gain and glucose intolerance (Amar et al., 2011). Further, activation of NOD1 is consistently linked to adipose tissue inflammation and peripheral insulin resistance (Zhao et al., 2011, Schertzer et al., 2011, Zhou et al., 2012). Taken together, inflammatory signaling induced by bacterial activation of TLRs and/or NOD-like receptors may be a promising target for treatment of metabolic disease.
The bacterial protein, caseinolytic peptidase B protein homolog (ClpB), expressed by E. coli has been identified as an antigen mimetic protein of α-melanocyte stimulating hormone (α-MSH) (Tennoune et al., 2014), a key neuropeptide involved in regulation of food intake. While little is known about the physiological effects of ClpB, this protein has been implicated in the development of eating disorders, and, more recently, obesity, as gut bacterial ClpB-like gene function is negatively correlated with obesity in humans (Arnoriaga-Rodríguez et al., 2020). Further, chronic intragastric E. coli treatment decreases food intake, while treatment with Clpb-deficient E. coli has no effect on food intake (Tennoune et al., 2014); this effect is proposed to be mediated by increased PYY secretion with ClpB exposure (Dominique et al., 2019). Additionally, treatment with a strain Hafnia alvei expressing ClpB with an α-MSH-like motif reduces food intake and body weight in diet-induced obese mice, reduces food intake in genetically obese ob/ob mice (Legrand et al., 2020) and improves body weight loss in humans (Déchelotte et al., 2021), providing the foundation for research into novel probiotic ClpB-expressing bacterial strains for obesity.
Conclusions and Future perspectives
Given the expanding viewpoint for the GI tract as an important endocrine organ in the regulation of metabolic homeostasis, it is no surprise that several of the most successful treatment options for obesity and diabetes are gut-derived in nature. For example, two classes of drugs, GLP-1R agonists (GLP-1RA), like liraglutide, and DPP-4 inhibitors, like sitagliptin, improve T2D via activating GLP-1R signaling mechanisms. Interestingly, GLP-1RAs possess a long half-life, while DPP-4 inhibitors increase the half-life of endogenous GLP-1 (Nauck et al., 2021, Omar and Ahrén, 2014); therefore, these drugs can target endocrine actions of GLP-1R signaling. For example, it is likely that GLP-1RAs improve glucose homeostasis via amplification of glucose-stimulated insulin secretion at the β-cell and induce significant weight loss via CNS action (Varin et al., 2019, Lamont et al., 2012). More recently, clinical trials investigating both dual GLP-1R/GIPR agonists and GLP-1/glucagon receptor (GCGR) agonists as well as GLP-1R/GIPR/GCGR triagonists indicate positive effects on weight loss and glycemia, with GCGR and GLP-1R agonism promoting weight loss and GIPR agonism negating the effects of glucagon signaling on hepatic glucose production (Capozzi et al., 2018, Frias et al., 2018, Coskun et al., 2018, Ji et al., 2021). For example, the “twincretin” tirzepatide is generally more effective at reducing glycemia and body weight compared to the GLP-1 analog semaglutide with the same safety profile (Vadher et al., 2022), whereas GLP-1R/GIPR/GCGR triagonists show early synergistic effects on metabolism, improving glycemic control and body weight to a greater extent than dual incretin receptor agonists in rodents (Finan et al., 2015). However, with all these current treatments, there are moderate side effects, including nausea, diarrhea, and, to a lesser extent, vomiting, constipation, abdominal pain, and dyspepsia (Filippatos et al., 2014). Therefore, future studies must continue to understand the endocrine action of gut peptide signals, as a better understanding of potential sites of action could lead to more personalized and targeted therapies that limit side effects.
In contrast to the establishment and success of GLP-1-mediated therapies, therapies targeting the vast potential of the gut microbiota are still in infancy. As such, while many studies have highlighted the potential of various probiotics in metabolic homeostasis (Bauer et al., 2018, Stenman et al., 2014), only a few have been successful in clinical trials (Bernini et al., 2016, Minami et al., 2015, Kadooka et al., 2010, Depommier et al., 2019). However, as sequencing efforts become more advanced, there is a greater likelihood that gut bacteria will be discovered that have novel roles in mediating energy and glucose homeostasis. For example, one group has discovered a gut bacteria that is capable of producing ClpB, which could have major implications in metabolism (Tennoune et al., 2014). Additionally, there is the emerging field of bioengineered bacteria, with several groups generating bacteria capable of producing specific metabolites, like leptin and GLP-1, that target metabolic organs to prevent or treat metabolic disease (Bermúdez-Humarán et al., 2007, Arora et al., 2016). Nonetheless, despite these efforts, it is possible that probiotic treatment may be highly personalized, as some individuals are permissive to colonization of probiotics while others are resistant, depending on their pre-existing gut microbiota (Zmora et al., 2018). Indeed, the gut microbiome is highly complex and individualized, thus baseline gut microbiome and metabolome conditions could influence whether treatments targeting the gut microbiome are successful. For example, an individual’s baseline gut microbiome and metabolome can dictate the successful glucoregulatory effect of exercise, while machine-learning algorithm can this information to predict if an individual will “respond” to exercise based on microbial characteristics (Liu et al., 2020). A similar program uses a machine learning algorithm to personalize dietary interventions for glucose tolerance using baseline gut microbial signatures in combination with diet and health information (Berry et al., 2020). Altogether, this highlights the importance of comprehensive clinical studies that incorporate not only phenotypic characteristics, but also baseline gut metagenomic and metabolomic analyses to determine if drug-gut interactions dictate the successful or failure of treatments toward obesity and diabetes. While the exact mechanisms are not completely elucidated, it is evident that both gut peptides and gut microbiota-derived compounds act as endocrine factors to impact host signaling and metabolic homeostasis, representing a relatively novel and exciting collection of compounds and receptors that can be targeted for treatment of metabolic disease.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH (R01DK121804).
Footnotes
Declaration of Interest
The authors declare no conflicts of interest.
References
- ABBOTT CR, MONTEIRO M, SMALL CJ, SAJEDI A, SMITH KL, PARKINSON JR, GHATEI MA & BLOOM SR 2005a. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res, 1044, 127–31. [DOI] [PubMed] [Google Scholar]
- ABBOTT CR, SMALL CJ, KENNEDY AR, NEARY NM, SAJEDI A, GHATEI MA & BLOOM SR 2005b. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res, 1043, 139–44. [DOI] [PubMed] [Google Scholar]
- ADAMS JM, PEI H, SANDOVAL DA, SEELEY RJ, CHANG RB, LIBERLES SD & OLSON DP 2018. Liraglutide Modulates Appetite and Body Weight Through Glucagon-Like Peptide 1 Receptor-Expressing Glutamatergic Neurons. Diabetes, 67, 1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADRIAN TE, BALLANTYNE GH, LONGO WE, BILCHIK AJ, GRAHAM S, BASSON MD, TIERNEY RP & MODLIN IM 1993. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut, 34, 1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADRIAN TE, GARIBALLA S, PAREKH KA, THOMAS SA, SAADI H, AL KAABI J, NAGELKERKE N, GEDULIN B. & YOUNG AA 2012. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia, 55, 2343–7. [DOI] [PubMed] [Google Scholar]
- ADRIAN TE, SAVAGE AP, SAGOR GR, ALLEN JM, BACARESE-HAMILTON AJ, TATEMOTO K, POLAK JM & BLOOM SR 1985. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology, 89, 494–9. [DOI] [PubMed] [Google Scholar]
- AGUS A, CLÉMENT K. & SOKOL H. 2021. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut, 70, 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHLMAN H. & NILSSON 2001. The gut as the largest endocrine organ in the body. Ann Oncol, 12 Suppl 2, S63–8. [DOI] [PubMed] [Google Scholar]
- AHRÉN B. 2022. Glucose-dependent insulinotropic polypeptide secretion after oral macronutrient ingestion: The human literature revisited and a systematic study in model experiments in mice. J Diabetes Investig, 13, 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMAR J, CHABO C, WAGET A, KLOPP P, VACHOUX C, BERMÚDEZ-HUMARÁN LG, SMIRNOVA N, BERGÉ M, SULPICE T, LAHTINEN S, OUWEHAND A, LANGELLA P, RAUTONEN N, SANSONETTI PJ & BURCELIN R. 2011. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med, 3, 559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMORIM FRANCO TM & BLANCHARD JS 2017. Bacterial Branched-Chain Amino Acid Biosynthesis: Structures, Mechanisms, and Drugability. Biochemistry, 56, 5849–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON JW & BRIDGES SR 1984. Short-chain fatty acid fermentation products of plant fiber affect glucose metabolism of isolated rat hepatocytes. Proc Soc Exp Biol Med, 177, 372–6. [DOI] [PubMed] [Google Scholar]
- ANHÊ FF, BARRA NG, CAVALLARI JF, HENRIKSBO BD & SCHERTZER JD 2021. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep, 36, 109691. [DOI] [PubMed] [Google Scholar]
- AOKI R, ONUKI M, HATTORI K, ITO M, YAMADA T, KAMIKADO K, KIM YG, NAKAMOTO N, KIMURA I, CLARKE JM, KANAI T. & HASE K. 2021. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome, 9, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNORIAGA-RODRÍGUEZ M, MAYNERIS-PERXACHS J, BUROKAS A, PÉREZ-BROCAL V, MOYA A, PORTERO-OTIN M, RICART W, MALDONADO R. & FERNÁNDEZ-REAL JM 2020. Gut bacterial ClpB-like gene function is associated with decreased body weight and a characteristic microbiota profile. Microbiome, 8, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARONIN N, CARRAWAY RE, FERRIS CF, HAMMER RA & LEEMAN SE 1982. The stability and metabolism of intravenously administered neurotensin in the rat. Peptides, 3, 637–42. [DOI] [PubMed] [Google Scholar]
- ARORA T, WEGMANN U, BOBHATE A, LEE YS, GREINER TU, DRUCKER DJ, NARBAD A. & BÄCKHED F. 2016. Microbially produced glucagon-like peptide 1 improves glucose tolerance in mice. Mol Metab, 5, 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÄCKHED F, DING H, WANG T, HOOPER LV, KOH GY, NAGY A, SEMENKOVICH CF & GORDON JI 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A, 101, 15718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAGGIO LL, HUANG Q, BROWN TJ & DRUCKER DJ 2004. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology, 127, 546–58. [DOI] [PubMed] [Google Scholar]
- BALDASSANO S, RAPPA F, AMATO A, CAPPELLO F. & MULÈ F. 2015. GLP-2 as Beneficial Factor in the Glucose Homeostasis in Mice Fed a High Fat Diet. J Cell Physiol, 230, 3029–36. [DOI] [PubMed] [Google Scholar]
- BALDISSERA FG, HOLST JJ, KNUHTSEN S, HILSTED L. & NIELSEN OV 1988. Oxyntomodulin (glicentin-(33–69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul Pept, 21, 151–66. [DOI] [PubMed] [Google Scholar]
- BALKAN B. & LI X. 2000. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol, 279, R1449–54. [DOI] [PubMed] [Google Scholar]
- BATTERHAM RL, COWLEY MA, SMALL CJ, HERZOG H, COHEN MA, DAKIN CL, WREN AM, BRYNES AE, LOW MJ, GHATEI MA, CONE RD & BLOOM SR 2002. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature, 418, 650–4. [DOI] [PubMed] [Google Scholar]
- BATTERHAM RL, HEFFRON H, KAPOOR S, CHIVERS JE, CHANDARANA K, HERZOG H, LE ROUX CW, THOMAS EL, BELL JD & WITHERS DJ 2006. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab, 4, 223–33. [DOI] [PubMed] [Google Scholar]
- BAUER PV, DUCA FA, WAISE TMZ, DRANSE HJ, RASMUSSEN BA, PURI A, RASTI M, O’BRIEN CA & LAM TKT 2018. Lactobacillus gasseri in the Upper Small Intestine Impacts an ACSL3-Dependent Fatty Acid-Sensing Pathway Regulating Whole-Body Glucose Homeostasis. Cell Metab, 27, 572–587 e6. [DOI] [PubMed] [Google Scholar]
- BENJAMIN MA, MCKAY DM, YANG PC, CAMERON H. & PERDUE MH 2000. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut, 47, 112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÉRAUD-DUFOUR S, ABDERRAHMANI A, NOEL J, BRAU F, WAEBER G, MAZELLA J. & COPPOLA T. 2010. Neurotensin is a regulator of insulin secretion in pancreatic beta-cells. Int J Biochem Cell Biol, 42, 1681–8. [DOI] [PubMed] [Google Scholar]
- BERMÚDEZ-HUMARÁN LG, NOUAILLE S, ZILBERFARB V, CORTHIER G, GRUSS A, LANGELLA P. & ISSAD T. 2007. Effects of intranasal administration of a leptin-secreting Lactococcus lactis recombinant on food intake, body weight, and immune response of mice. Appl Environ Microbiol, 73, 5300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNINI LJ, SIMAO AN, ALFIERI DF, LOZOVOY MA, MARI NL, DE SOUZA CH, DICHI I. & COSTA GN 2016. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: A randomized trial. Effects of probiotics on metabolic syndrome. Nutrition, 32, 716–9. [DOI] [PubMed] [Google Scholar]
- BERRY SE, VALDES AM, DREW DA, ASNICAR F, MAZIDI M, WOLF J, CAPDEVILA J, HADJIGEORGIOU G, DAVIES R, AL KHATIB H, BONNETT C, GANESH S, BAKKER E, HART D, MANGINO M, MERINO J, LINENBERG I, WYATT P, ORDOVAS JM, GARDNER CD, DELAHANTY LM, CHAN AT, SEGATA N, FRANKS PW & SPECTOR TD 2020. Human postprandial responses to food and potential for precision nutrition. Nat Med, 26, 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEUMER J, ARTEGIANI B, POST Y, REIMANN F, GRIBBLE F, NGUYEN TN, ZENG H, VAN DEN BORN M, VAN ES JH & CLEVERS H. 2018. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat Cell Biol, 20, 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATTARAI Y, JIE S, LINDEN DR, GHATAK S, MARS RAT, WILLIAMS BB, PU M, SONNENBURG JL, FISCHBACH MA, FARRUGIA G, SHA L. & KASHYAP PC 2020. Bacterially Derived Tryptamine Increases Mucus Release by Activating a Host Receptor in a Mouse Model of Inflammatory Bowel Disease. iScience, 23, 101798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILLING LJ, SMITH CA, LARRAUFIE P, GOLDSPINK DA, GALVIN S, KAY RG, HOWE JD, WALKER R, PRUNA M, GLASS L, PAIS R, GRIBBLE FM & REIMANN F. 2018. Co-storage and release of insulin-like peptide-5, glucagon-like peptide-1 and peptideYY from murine and human colonic enteroendocrine cells. Mol Metab, 16, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKELAND E, GHARAGOZLIAN S, GULSETH HL, BIRKELAND KI, HARTMANN B, HOLST JJ, HOLST R. & AAS AM 2021. Effects of prebiotics on postprandial GLP-1, GLP-2 and glucose regulation in patients with type 2 diabetes: A randomised, double-blind, placebo-controlled crossover trial. Diabet Med, 38, e14657. [DOI] [PubMed] [Google Scholar]
- BLEVINS JE, CHELIKANI PK, HAVER AC & REIDELBERGER RD 2008. PYY(3–36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides, 29, 112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOUET C. & SCHWARTZ GJ 2012. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS One, 7, e51898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLOGNINI D, BARKI N, BUTCHER AJ, HUDSON BD, SERGEEV E, MOLLOY C, MOSS CE, BRADLEY SJ, LE GOUILL C, BOUVIER M, TOBIN AB & MILLIGAN G. 2019. Chemogenetics defines receptor-mediated functions of short chain free fatty acids. Nat Chem Biol, 15, 489–498. [DOI] [PubMed] [Google Scholar]
- BORGMANN D, CIGLIERI E, BIGLARI N, BRANDT C, CREMER AL, BACKES H, TITTGEMEYER M, WUNDERLICH FT, BRÜNING JC & FENSELAU H. 2021. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metab, 33, 1466–1482.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÖTTCHER G, AHRÉN B, LUNDQUIST I. & SUNDLER F. 1989. Peptide YY: intrapancreatic localization and effects on insulin and glucagon secretion in the mouse. Pancreas, 4, 282–8. [PubMed] [Google Scholar]
- BRIERLEY DI & DE LARTIGUE G. 2022. Reappraising the role of the vagus nerve in GLP-1-mediated regulation of eating. Br J Pharmacol, 179, 584–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIERLEY DI, HOLT MK, SINGH A, DE ARAUJO A, MCDOUGLE M, VERGARA M, AFAGHANI MH, LEE SJ, SCOTT K, MASKE C, LANGHANS W, KRAUSE E, DE KLOET A, GRIBBLE FM, REIMANN F, RINAMAN L, DE LARTIGUE G. & TRAPP S. 2021. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab, 3, 258–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIGHTON CA, RIEVAJ J, KUHRE RE, GLASS LL, SCHOONJANS K, HOLST JJ, GRIBBLE FM & REIMANN F. 2015. Bile Acids Trigger GLP-1 Release Predominantly by Accessing Basolaterally Located G Protein-Coupled Bile Acid Receptors. Endocrinology, 156, 3961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROEDERS EP, NASCIMENTO EB, HAVEKES B, BRANS B, ROUMANS KH, TAILLEUX A, SCHAART G, KOUACH M, CHARTON J, DEPREZ B, BOUVY ND, MOTTAGHY F, STAELS B, VAN MARKEN LICHTENBELT WD & SCHRAUWEN P. 2015. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab, 22, 418–26. [DOI] [PubMed] [Google Scholar]
- BROOKS L, VIARDOT A, TSAKMAKI A, STOLARCZYK E, HOWARD JK, CANI PD, EVERARD A, SLEETH ML, PSICHAS A, ANASTASOVSKAJ J, BELL JD, BELL-ANDERSON K, MACKAY CR, GHATEI MA, BLOOM SR, FROST G. & BEWICK GA 2017. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol Metab, 6, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN AJ, GOLDSWORTHY SM, BARNES AA, EILERT MM, TCHEANG L, DANIELS D, MUIR AI, WIGGLESWORTH MJ, KINGHORN I, FRASER NJ, PIKE NB, STRUM JC, STEPLEWSKI KM, MURDOCK PR, HOLDER JC, MARSHALL FH, SZEKERES PG, WILSON S, IGNAR DM, FOORD SM, WISE A. & DOWELL SJ 2003. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem, 278, 11312–9. [DOI] [PubMed] [Google Scholar]
- BUCKLEY MM, O’BRIEN R, BROSNAN E, ROSS RP, STANTON C, BUCKLEY JM & O’MALLEY D. 2020. Glucagon-Like Peptide-1 Secreting L-Cells Coupled to Sensory Nerves Translate Microbial Signals to the Host Rat Nervous System. Front Cell Neurosci, 14, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURCELIN R, DA COSTA A, DRUCKER D. & THORENS B. 2001. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes, 50, 1720–8. [DOI] [PubMed] [Google Scholar]
- BURNICKA-TUREK O, MOHAMED BA, SHIRNESHAN K, THANASUPAWAT T, HOMBACH-KLONISCH S, KLONISCH T. & ADHAM IM 2012. INSL5-deficient mice display an alteration in glucose homeostasis and an impaired fertility. Endocrinology, 153, 4655–65. [DOI] [PubMed] [Google Scholar]
- CAMPBELL JE & NEWGARD CB 2021. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol, 22, 142–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANI PD, AMAR J, IGLESIAS MA, POGGI M, KNAUF C, BASTELICA D, NEYRINCK AM, FAVA F, TUOHY KM, CHABO C, WAGET A, DELMÉE E, COUSIN B, SULPICE T, CHAMONTIN B, FERRIÈRES J, TANTI JF, GIBSON GR, CASTEILLA L, DELZENNE NM, ALESSI MC & BURCELIN R. 2007a. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes, 56, 1761–72. [DOI] [PubMed] [Google Scholar]
- CANI PD, DAUBIOUL CA, REUSENS B, REMACLE C, CATILLON G. & DELZENNE NM 2005. Involvement of endogenous glucagon-like peptide-1(7–36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol, 185, 457–65. [DOI] [PubMed] [Google Scholar]
- CANI PD, KNAUF C, IGLESIAS MA, DRUCKER DJ, DELZENNE NM & BURCELIN R. 2006. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes, 55, 1484–90. [DOI] [PubMed] [Google Scholar]
- CANI PD, NEYRINCK AM, FAVA F, KNAUF C, BURCELIN RG, TUOHY KM, GIBSON GR & DELZENNE NM 2007b. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia, 50, 2374–83. [DOI] [PubMed] [Google Scholar]
- CANI PD, POSSEMIERS S, VAN DE WIELE T, GUIOT Y, EVERARD A, ROTTIER O, GEURTS L, NASLAIN D, NEYRINCK A, LAMBERT DM, MUCCIOLI GG & DELZENNE NM 2009. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut, 58, 1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPOZZI ME, DIMARCHI RD, TSCHÖP MH, FINAN B. & CAMPBELL JE 2018. Targeting the Incretin/Glucagon System With Triagonists to Treat Diabetes. Endocr Rev, 39, 719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARICILLI AM, NASCIMENTO PH, PAULI JR, TSUKUMO DM, VELLOSO LA, CARVALHEIRA JB & SAAD MJ 2008. Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol, 199, 399–406. [DOI] [PubMed] [Google Scholar]
- CARIOU B, VAN HARMELEN K, DURAN-SANDOVAL D, VAN DIJK TH, GREFHORST A, ABDELKARIM M, CARON S, TORPIER G, FRUCHART JC, GONZALEZ FJ, KUIPERS F. & STAELS B. 2006. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem, 281, 11039–49. [DOI] [PubMed] [Google Scholar]
- CARRAWAY RE, DEMERS LM & LEEMAN SE 1976. Hyperglycemic effect of neurotensin, a hypothalamic peptide. Endocrinology, 99, 1452–62. [DOI] [PubMed] [Google Scholar]
- CARVALHO FA, KOREN O, GOODRICH JK, JOHANSSON ME, NALBANTOGLU I, AITKEN JD, SU Y, CHASSAING B, WALTERS WA, GONZÁLEZ A, CLEMENTE JC, CULLENDER TC, BARNICH N, DARFEUILLE-MICHAUD A, VIJAY-KUMAR M, KNIGHT R, LEY RE & GEWIRTZ AT 2012. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe, 12, 139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTELLANOS-JANKIEWICZ A, GUZMÁN-QUEVEDO O, FÉNELON VS, ZIZZARI P, QUARTA C, BELLOCCHIO L, TAILLEUX A, CHARTON J, FERNANDOIS D, HENRICSSON M, PIVETEAU C, SIMON V, ALLARD C, QUEMENER S, GUINOT V, HENNUYER N, PERINO A, DUVEAU A, MAITRE M, LESTE-LASSERRE T, CLARK S, DUPUY N, CANNICH A, GONZALES D, DEPREZ B, MITHIEUX G, DOMBROWICZ D, BÄCKHED F, PREVOT V, MARSICANO G, STAELS B, SCHOONJANS K. & COTA D. 2021. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab, 33, 1483–1492.e10. [DOI] [PubMed] [Google Scholar]
- CHALLIS BG, PINNOCK SB, COLL AP, CARTER RN, DICKSON SL & O’RAHILLY S. 2003. Acute effects of PYY3–36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem Biophys Res Commun, 311, 915–9. [DOI] [PubMed] [Google Scholar]
- CHANDARANA K, GELEGEN C, IRVINE EE, CHOUDHURY AI, AMOUYAL C, ANDREELLI F, WITHERS DJ & BATTERHAM RL 2013. Peripheral activation of the Y2-receptor promotes secretion of GLP-1 and improves glucose tolerance. Mol Metab, 2, 142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG Y, DENG Q, ZHANG Z, ZHAO H, TANG J, CHEN X, LIU G, TIAN G, CAI J. & JIA G. 2021. Glucagon-like peptide 2 attenuates intestinal mucosal barrier injury through the MLCK/pMLC signaling pathway in a piglet model. J Cell Physiol, 236, 3015–3032. [DOI] [PubMed] [Google Scholar]
- CHASSAING B, LEY RE & GEWIRTZ AT 2014. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology, 147, 1363–77.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEESEMAN CI & TSANG R. 1996. The effect of GIP and glucagon-like peptides on intestinal basolateral membrane hexose transport. Am J Physiol, 271, G477–82. [DOI] [PubMed] [Google Scholar]
- CHEN X, ZHAO HX, FU XS, LI CPXL 2012. Glucagonlike peptide 2 protects intestinal barrier in severe acute pancreatitis through regulating intestinal epithelial cell proliferation and apoptosis. Pancreas, 41, 1080–5. [DOI] [PubMed] [Google Scholar]
- CHEUNG GW, KOKOROVIC A, LAM CK, CHARI M. & LAM TK 2009. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab, 10, 99–109. [DOI] [PubMed] [Google Scholar]
- CHIANG JY 2013. Bile acid metabolism and signaling. Compr Physiol, 3, 1191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIMEREL C, EMERY E, SUMMERS DK, KEYSER U, GRIBBLE FM & REIMANN F. 2014. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep, 9, 1202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI S, DISILVIO B, FERNSTROM MH & FERNSTROM JD 2013. Oral branched-chain amino acid supplements that reduce brain serotonin during exercise in rats also lower brain catecholamines. Amino Acids, 45, 1133–42. [DOI] [PubMed] [Google Scholar]
- CHRIETT S, DĄBEK A, WOJTALA M, VIDAL H, BALCERCZYK A. & PIROLA L. 2019. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci Rep, 9, 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIANSEN CB, GABE MBN, SVENDSEN B, DRAGSTED LO, ROSENKILDE MM & HOLST JJ 2018. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol, 315, G53–G65. [DOI] [PubMed] [Google Scholar]
- CHRISTIANSEN CB, TRAMMELL SAJ, WEWER ALBRECHTSEN NJ, SCHOONJANS K, ALBRECHTSEN R, GILLUM MP, KUHRE RE & HOLST JJ 2019. Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Am J Physiol Gastrointest Liver Physiol, 316, G574–g584. [DOI] [PubMed] [Google Scholar]
- CLARKE G, STILLING RM, KENNEDY PJ, STANTON C, CRYAN JF & DINAN TG 2014. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol, 28, 1221–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSKUN T, SLOOP KW, LOGHIN C, ALSINA-FERNANDEZ J, URVA S, BOKVIST KB, CUI X, BRIERE DA, CABRERA O, ROELL WC, KUCHIBHOTLA U, MOYERS JS, BENSON CT, GIMENO RE, D’ALESSIO DA & HAUPT A. 2018. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab, 18, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE JD, PALANIVEL R, MOTTILLO EP, BUJAK AL, WANG H, FORD RJ, COLLINS A, BLÜMER RM, FULLERTON MD, YABUT JM, KIM JJ, GHIA JE, HAMZA SM, MORRISON KM, SCHERTZER JD, DYCK JR, KHAN WI & STEINBERG GR 2015. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med, 21, 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANMER M, LOUIE S, KENNEDY RH, KERN PA & FONSECA VA 2000. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is associated with hyperinsulinemia and insulin resistance. Toxicol Sci, 56, 431–6. [DOI] [PubMed] [Google Scholar]
- CUMMINGS JH, POMARE EW, BRANCH WJ, NAYLOR CP & MACFARLANE GT 1987. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut, 28, 1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAKIN CL, GUNN I, SMALL CJ, EDWARDS CMB, HAY DL, SMITH DM, GHATEI MA & BLOOM SR 2001. Oxyntomodulin Inhibits Food Intake in the Rat. Endocrinology, 142, 4244–4250. [DOI] [PubMed] [Google Scholar]
- DAKIN CL, SMALL CJ, BATTERHAM RL, NEARY NM, COHEN MA, PATTERSON M, GHATEI MA & BLOOM SR 2004. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology, 145, 2687–95. [DOI] [PubMed] [Google Scholar]
- DALBY MJ, AVIELLO G, ROSS AW, WALKER AW, BARRETT P. & MORGAN PJ 2018. Diet induced obesity is independent of metabolic endotoxemia and TLR4 signalling, but markedly increases hypothalamic expression of the acute phase protein, SerpinA3N. Sci Rep, 8, 15648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS HR JR., MULLINS DE, PINES JM, HOOS LM, FRANCE CF, COMPTON DS, GRAZIANO MP, SYBERTZ EJ, STRADER CD & VAN HEEK M. 1998. Effect of chronic central administration of glucagon-like peptide-1 (7–36) amide on food consumption and body weight in normal and obese rats. Obes Res, 6, 147–56. [DOI] [PubMed] [Google Scholar]
- DE JONGE PA, WORTELBOER K, SCHEITHAUER TPM, VAN DEN BORN B-JH, ZWINDERMAN AH, NOBREGA FL, DUTILH BE, NIEUWDORP M. & HERREMA H. 2022. Gut virome profiling identifies a widespread bacteriophage family associated with metabolic syndrome. Nature Communications, 13, 3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DÉCHELOTTE P, BRETON J, TROTIN-PICOLO C, GRUBE B, ERLENBECK C, BOTHE G, FETISSOV SO & LAMBERT G. 2021. The Probiotic Strain H. alvei HA4597(®) Improves Weight Loss in Overweight Subjects under Moderate Hypocaloric Diet: A Proof-of-Concept, Multicenter Randomized, Double-Blind Placebo-Controlled Study. Nutrients, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEGEN L, OESCH S, CASANOVA M, GRAF S, KETTERER S, DREWE J. & BEGLINGER C. 2005. Effect of peptide YY3–36 on food intake in humans. Gastroenterology, 129, 1430–6. [DOI] [PubMed] [Google Scholar]
- DEN BESTEN G, BLEEKER A, GERDING A, VAN EUNEN K, HAVINGA R, VAN DIJK TH, OOSTERVEER MH, JONKER JW, GROEN AK, REIJNGOUD DJ & BAKKER BM 2015. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARgamma-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes, 64, 2398–408. [DOI] [PubMed] [Google Scholar]
- DEPOMMIER C, EVERARD A, DRUART C, PLOVIER H, VAN HUL M, VIEIRA-SILVA S, FALONY G, RAES J, MAITER D, DELZENNE NM, DE BARSY M, LOUMAYE A, HERMANS MP, THISSEN J-P, DE VOS WM & CANI PD 2019. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nature Medicine, 25, 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIEPENBROEK C, QUINN D, STEPHENS R, ZOLLINGER B, ANDERSON S, PAN A. & DE LARTIGUE G. 2017. Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am J Physiol Gastrointest Liver Physiol, 313, G342–g352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DING L, SOUSA KM, JIN L, DONG B, KIM BW, RAMIREZ R, XIAO Z, GU Y, YANG Q, WANG J, YU D, PIGAZZI A, SCHONES D, YANG L, MOORE D, WANG Z. & HUANG W. 2016. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology, 64, 760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLAIS-KITABGI J, KITABGI P, BRAZEAU P. & FREYCHET P. 1979. Effect of neurotensin on insulin, glucagon, and somatostatin release from isolated pancreatic islets. Endocrinology, 105, 256–60. [DOI] [PubMed] [Google Scholar]
- DOMINIQUE M, BRETON J, GUÉRIN C, BOLE-FEYSOT C, LAMBERT G, DÉCHELOTTE P. & FETISSOV S. 2019. Effects of Macronutrients on the In Vitro Production of ClpB, a Bacterial Mimetic Protein of α-MSH and Its Possible Role in Satiety Signaling. Nutrients, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAVIAM EJ, UPP JR JR., GREELEY GH JR., TOWNSEND CM JR. & THOMPSON JC 1990. Effect of oral fat on plasma levels of neurotensin and neurotensin fragments in humans. Characterization by high-pressure liquid chromatography. Dig Dis Sci, 35, 200–4. [DOI] [PubMed] [Google Scholar]
- DRUCKER DJ, ERLICH P, ASA SL & BRUBAKER PL 1996. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A, 93, 7911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOC H, TACHÉ Y. & HOFMANN AF 2014. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis, 46, 302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCA FA, SWARTZ TD, SAKAR Y. & COVASA M. 2012. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS One, 7, e39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCA FA, WAISE TMZ, PEPPLER WT & LAM TKT 2021. The metabolic impact of small intestinal nutrient sensing. Nat Commun, 12, 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCASTEL S, TOUCHE V, TRABELSI MS, BOULINGUIEZ A, BUTRUILLE L, NAWROT M, PESCHARD S, CHÁVEZ-TALAVERA O, DORCHIES E, VALLEZ E, ANNICOTTE JS, LANCEL S, BRIAND O, BANTUBUNGI K, CARON S, BINDELS LB, DELZENNE NM, TAILLEUX A, STAELS B. & LESTAVEL S. 2020. The nuclear receptor FXR inhibits Glucagon-Like Peptide-1 secretion in response to microbiota-derived Short-Chain Fatty Acids. Sci Rep, 10, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYACHOK O, ISAKOV Y, SÅGETORP J. & TENGHOLM A. 2006. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting β-cells. Nature, 439, 349–352. [DOI] [PubMed] [Google Scholar]
- EHSES JA, MEIER DT, WUEEST S, RYTKA J, BOLLER S, WIELINGA PY, SCHRAENEN A, LEMAIRE K, DEBRAY S, VAN LOMMEL L, POSPISILIK JA, TSCHOPP O, SCHULTZE SM, MALIPIERO U, ESTERBAUER H, ELLINGSGAARD H, RÜTTI S, SCHUIT FC, LUTZ TA, BÖNI-SCHNETZLER M, KONRAD D. & DONATH MY 2010. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia, 53, 1795–1806. [DOI] [PubMed] [Google Scholar]
- EJARQUE M, SABADELL-BASALLOTE J, BEIROA D, CALVO E, KEIRAN N, NUÑEZ-ROA C, RODRÍGUEZ MDM, SABENCH F, DEL CASTILLO D, JIMENEZ V, BOSCH F, NOGUEIRAS R, VENDRELL J. & FERNÁNDEZ-VELEDO S. 2021. Adipose tissue is a key organ for the beneficial effects of GLP-2 metabolic function. Br J Pharmacol, 178, 2131–2145. [DOI] [PubMed] [Google Scholar]
- EVERARD A, LAZAREVIC V, DERRIEN M, GIRARD M, MUCCIOLI GG, NEYRINCK AM, POSSEMIERS S, VAN HOLLE A, FRANCOIS P, DE VOS WM, DELZENNE NM, SCHRENZEL J. & CANI PD 2011. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes, 60, 2775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAKHRY J, WANG J, MARTINS P, FOTHERGILL LJ, HUNNE B, PRIEUR P, SHULKES A, REHFELD JF, CALLAGHAN B. & FURNESS JB 2017. Distribution and characterisation of CCK containing enteroendocrine cells of the mouse small and large intestine. Cell and Tissue Research, 369, 245–253. [DOI] [PubMed] [Google Scholar]
- FANG S, SUH JM, REILLY SM, YU E, OSBORN O, LACKEY D, YOSHIHARA E, PERINO A, JACINTO S, LUKASHEVA Y, ATKINS AR, KHVAT A, SCHNABL B, YU RT, BRENNER DA, COULTER S, LIDDLE C, SCHOONJANS K, OLEFSKY JM, SALTIEL AR, DOWNES M. & EVANS RM 2015. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med, 21, 159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELIG P, MARLISS E. & CAHILL GF JR. 1969. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med, 281, 811–6. [DOI] [PubMed] [Google Scholar]
- FILIPPATOS TD, PANAGIOTOPOULOU TV & ELISAF MS 2014. Adverse Effects of GLP-1 Receptor Agonists. Rev Diabet Stud, 11, 202–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINAN B, YANG B, OTTAWAY N, SMILEY DL, MA T, CLEMMENSEN C, CHABENNE J, ZHANG L, HABEGGER KM, FISCHER K, CAMPBELL JE, SANDOVAL D, SEELEY RJ, BLEICHER K, UHLES S, RIBOULET W, FUNK J, HERTEL C, BELLI S, SEBOKOVA E, CONDE-KNAPE K, KONKAR A, DRUCKER DJ, GELFANOV V, PFLUGER PT, MÜLLER TD, PEREZ-TILVE D, DIMARCHI RD & TSCHÖP MH 2015. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med, 21, 27–36. [DOI] [PubMed] [Google Scholar]
- FRIAS JP, NAUCK MA, VAN J, KUTNER ME, CUI X, BENSON C, URVA S, GIMENO RE, MILICEVIC Z, ROBINS D. & HAUPT A. 2018. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet, 392, 2180–2193. [DOI] [PubMed] [Google Scholar]
- FROST G, SLEETH ML, SAHURI-ARISOYLU M, LIZARBE B, CERDAN S, BRODY L, ANASTASOVSKA J, GHOURAB S, HANKIR M, ZHANG S, CARLING D, SWANN JR, GIBSON G, VIARDOT A, MORRISON D, LOUISE THOMAS E. & BELL JD 2014. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun, 5, 3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJINO T, KONDO J, ISHIKAWA M, MORIKAWA K. & YAMAMOTO TT 2001. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J Biol Chem, 276, 11420–6. [DOI] [PubMed] [Google Scholar]
- GAO Z, YIN J, ZHANG J, WARD RE, MARTIN RJ, LEFEVRE M, CEFALU WT & YE J. 2009. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes, 58, 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASBJERG LS, HELSTED MM, HARTMANN B, JENSEN MH, GABE MBN, SPARRE-ULRICH AH, VEEDFALD S, STENSEN S, LANNG AR, BERGMANN NC, CHRISTENSEN MB, VILSBØLL T, HOLST JJ, ROSENKILDE MM & KNOP FK 2019. Separate and Combined Glucometabolic Effects of Endogenous Glucose-Dependent Insulinotropic Polypeptide and Glucagon-like Peptide 1 in Healthy Individuals. Diabetes, 68, 906–917. [DOI] [PubMed] [Google Scholar]
- GIJSMAN HJ, SCARNÀ A, HARMER CJ, MCTAVISH SB, ODONTIADIS J, COWEN PJ & GOODWIN GM 2002. A dose-finding study on the effects of branch chain amino acids on surrogate markers of brain dopamine function. Psychopharmacology (Berl), 160, 192–7. [DOI] [PubMed] [Google Scholar]
- GOJDA J. & CAHOVA M. 2021. Gut Microbiota as the Link between Elevated BCAA Serum Levels and Insulin Resistance. Biomolecules, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSWAMI C, IWASAKI Y. & YADA T. 2018. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J Nutr Biochem, 57, 130–135. [DOI] [PubMed] [Google Scholar]
- GRANDT D, SCHIMICZEK M, BEGLINGER C, LAYER P, GOEBELL H, EYSSELEIN VE & REEVE JR 1994. Two molecular forms of Peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regulatory Peptides, 51, 151–159. [DOI] [PubMed] [Google Scholar]
- GRANDT D, SIEWERT J, SIEBURG B, AL TAI O, SCHIMICZEK M, GOEBELL H, LAYER P, EYSSELEIN VE, REEVE JR JR. & MÜLLER MK 1995. Peptide YY inhibits exocrine pancreatic secretion in isolated perfused rat pancreas by Y1 receptors. Pancreas, 10, 180–6. [DOI] [PubMed] [Google Scholar]
- GRIBBLE FM & REIMANN F. 2019. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nature Reviews Endocrinology, 15, 226–237. [DOI] [PubMed] [Google Scholar]
- GROSSE J, HEFFRON H, BURLING K, AKHTER HOSSAIN M, HABIB AM, ROGERS GJ, RICHARDS P, LARDER R, RIMMINGTON D, ADRIAENSSENS AA, PARTON L, POWELL J, BINDA M, COLLEDGE WH, DORAN J, TOYODA Y, WADE JD, APARICIO S, CARLTON MB, COLL AP, REIMANN F, O’RAHILLY S. & GRIBBLE FM 2014. Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proc Natl Acad Sci U S A, 111, 11133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUI X. & CARRAWAY RE 2001. Enhancement of jejunal absorption of conjugated bile acid by neurotensin in rats. Gastroenterology, 120, 151–60. [DOI] [PubMed] [Google Scholar]
- GUO Z, ZHANG Y, LIU C, YOUN JY & CAI H. 2021. Toll-Like Receptor 2 (TLR2) Knockout Abrogates Diabetic and Obese Phenotypes While Restoring Endothelial Function via Inhibition of NOX1. Diabetes, 70, 2107–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSON EL, SMITH KE, DURKIN MM, WALKER MW, GERALD C, WEINSHANK R. & BRANCHEK TA 1997. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Brain Res Mol Brain Res, 46, 223–35. [DOI] [PubMed] [Google Scholar]
- GUZIOR DV & QUINN RA 2021. Review: microbial transformations of human bile acids. Microbiome, 9, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABIB AM, RICHARDS P, CAIRNS LS, ROGERS GJ, BANNON CA, PARKER HE, MORLEY TC, YEO GS, REIMANN F. & GRIBBLE FM 2012. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology, 153, 3054–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABIB AM, RICHARDS P, ROGERS GJ, REIMANN F. & GRIBBLE FM 2013. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia, 56, 1413–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSEN M, SCHELTEMA MJ, SONNE DP, HANSEN JS, SPERLING M, REHFELD JF, HOLST JJ, VILSBØLL T. & KNOP FK 2016. Effect of chenodeoxycholic acid and the bile acid sequestrant colesevelam on glucagon-like peptide-1 secretion. Diabetes Obes Metab, 18, 571–80. [DOI] [PubMed] [Google Scholar]
- HANSSON B, MEDINA A, FRYKLUND C, FEX M. & STENKULA KG 2016. Serotonin (5-HT) and 5-HT2A receptor agonists suppress lipolysis in primary rat adipose cells. Biochem Biophys Res Commun, 474, 357–363. [DOI] [PubMed] [Google Scholar]
- HARE KJ, VILSBØLL T, ASMAR M, DEACON CF, KNOP FK & HOLST JJ 2010. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes, 59, 1765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMANN B, JOHNSEN AH, ORSKOV C, ADELHORST K, THIM L. & HOLST JJ 2000. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides, 21, 73–80. [DOI] [PubMed] [Google Scholar]
- HAUB S, RITZE Y, LADEL I, SAUM K, HUBERT A, SPRUSS A, TRAUTWEIN C. & BISCHOFF SC 2011. Serotonin receptor type 3 antagonists improve obesity-associated fatty liver disease in mice. J Pharmacol Exp Ther, 339, 790–8. [DOI] [PubMed] [Google Scholar]
- HEISEL T, MONTASSIER E, JOHNSON A, AL-GHALITH G, LIN YW, WEI LN, KNIGHTS D. & GALE CA 2017. High-Fat Diet Changes Fungal Microbiomes and Interkingdom Relationships in the Murine Gut. mSphere, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEISS CN, MANNERÅS-HOLM L, LEE YS, SERRANO-LOBO J, HÅKANSSON GLADH A, SEELEY RJ, DRUCKER DJ, BÄCKHED F. & OLOFSSON LE 2021. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep, 35, 109163. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN GL, KETCHUM NS, MICHALEK JE & SWABY JA 1997. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology, 8, 252–8. [DOI] [PubMed] [Google Scholar]
- HEREDIA DJ, GERSHON MD, KOH SD, CORRIGAN RD, OKAMOTO T. & SMITH TK 2013. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol, 591, 5939–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLECEK M, SIMAN P, VODENICAROVOVA M. & KANDAR R. 2016. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr Metab (Lond), 13, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTAMISLIGIL GS, ARNER P, CARO JF, ATKINSON RL & SPIEGELMAN BM 1995. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest, 95, 2409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTAMISLIGIL GS, PERALDI P, BUDAVARI A, ELLIS R, WHITE MF & SPIEGELMAN BM 1996. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science, 271, 665–8. [DOI] [PubMed] [Google Scholar]
- HOTAMISLIGIL GS, SHARGILL NS & SPIEGELMAN BM 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science, 259, 87–91. [DOI] [PubMed] [Google Scholar]
- HOWARD EJ, LAM TKT & DUCA FA 2022. The Gut Microbiome: Connecting Diet, Glucose Homeostasis, and Disease. Annu Rev Med, 73, 469–481. [DOI] [PubMed] [Google Scholar]
- HUBBARD TD, MURRAY IA & PERDEW GH 2015. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos, 43, 1522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIHARA Y, KADO SY, BEIN KJ, HE Y, POURARYAN AA, URBAN A, HAARMANN-STEMMANN T, SWEENEY C. & VOGEL CFA 2021. Aryl Hydrocarbon Receptor Signaling Synergizes with TLR/NF-κB-Signaling for Induction of IL-22 Through Canonical and Non-Canonical AhR Pathways. Front Toxicol, 3, 787360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISLAM R, DASH D. & SINGH R. 2022. Intranasal curcumin and sodium butyrate modulates airway inflammation and fibrosis via HDAC inhibition in allergic asthma. Cytokine, 149, 155720. [DOI] [PubMed] [Google Scholar]
- JI L, JIANG H, AN P, DENG H, LIU M, LI L, FENG L, SONG B, HAN-ZHANG H, MA Q. & QIAN L. 2021. IBI362 (LY3305677), a weekly-dose GLP-1 and glucagon receptor dual agonist, in Chinese adults with overweight or obesity: A randomised, placebo-controlled, multiple ascending dose phase 1b study. EClinicalMedicine, 39, 101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIA L, VIANNA CR, FUKUDA M, BERGLUND ED, LIU C, TAO C, SUN K, LIU T, HARPER MJ, LEE CE, LEE S, SCHERER PE & ELMQUIST JK 2014. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun, 5, 3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JO JK, SEO SH, PARK SE, KIM HW, KIM EJ, KIM JS, PYO JY, CHO KM, KWON SJ, PARK DH & SON HS 2021. Gut Microbiome and Metabolome Profiles Associated with High-Fat Diet in Mice. Metabolites, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KADOOKA Y, SATO M, IMAIZUMI K, OGAWA A, IKUYAMA K, AKAI Y, OKANO M, KAGOSHIMA M. & TSUCHIDA T. 2010. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr, 64, 636–43. [DOI] [PubMed] [Google Scholar]
- KAIHARA KA, DICKSON LM, JACOBSON DA, TAMARINA N, ROE MW, PHILIPSON LH & WICKSTEED B. 2013. β-Cell-specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes, 62, 1527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAJI I, KARAKI S, TANAKA R. & KUWAHARA A. 2011. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J Mol Histol, 42, 27–38. [DOI] [PubMed] [Google Scholar]
- KARAKI S, MITSUI R, HAYASHI H, KATO I, SUGIYA H, IWANAGA T, FURNESS JB & KUWAHARA A. 2006. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res, 324, 353–60. [DOI] [PubMed] [Google Scholar]
- KASHIMA Y, MIKI T, SHIBASAKI T, OZAKI N, MIYAZAKI M, YANO H. & SEINO S. 2001. Critical Role of cAMP-GEFII·Rim2 Complex in Incretin-potentiated Insulin Secretion*. Journal of Biological Chemistry, 276, 46046–46053. [DOI] [PubMed] [Google Scholar]
- KERLEY-HAMILTON JS, TRASK HW, RIDLEY CJ, DUFOUR E, RINGELBERG CS, NURINOVA N, WONG D, MOODIE KL, SHIPMAN SL, MOORE JH, KORC M, SHWORAK NW & TOMLINSON CR 2012. Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a Western diet. Environ Health Perspect, 120, 1252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN D, VASU S, MOFFETT RC, IRWIN N. & FLATT PR 2016. Islet distribution of Peptide YY and its regulatory role in primary mouse islets and immortalised rodent and human beta-cell function and survival. Molecular and Cellular Endocrinology, 436, 102–113. [DOI] [PubMed] [Google Scholar]
- KIEFFER TJ, MCINTOSH CH & PEDERSON RA 1995. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology, 136, 3585–96. [DOI] [PubMed] [Google Scholar]
- KIM F, PHAM M, LUTTRELL I, BANNERMAN DD, TUPPER J, THALER J, HAWN TR, RAINES EW & SCHWARTZ MW 2007. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res, 100, 1589–96. [DOI] [PubMed] [Google Scholar]
- KIM KS, PECK BC, HUNG YH, KOCH-LASKOWSKI K, WOOD L, DEDHIA PH, SPENCE JR, SEELEY RJ, SETHUPATHY P. & SANDOVAL DA 2022. Vertical sleeve gastrectomy induces enteroendocrine cell differentiation of intestinal stem cells through bile acid signaling. JCI Insight, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM SJ, WINTER K, NIAN C, TSUNEOKA M, KODA Y. & MCINTOSH CH 2005. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem, 280, 22297–307. [DOI] [PubMed] [Google Scholar]
- KIM YC, SEOK S, ZHANG Y, MA J, KONG B, GUO G, KEMPER B. & KEMPER JK 2020. Intestinal FGF15/19 physiologically repress hepatic lipogenesis in the late fed-state by activating SHP and DNMT3A. Nat Commun, 11, 5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA I, OZAWA K, INOUE D, IMAMURA T, KIMURA K, MAEDA T, TERASAWA K, KASHIHARA D, HIRANO K, TANI T, TAKAHASHI T, MIYAUCHI S, SHIOI G, INOUE H. & TSUJIMOTO G. 2013. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun, 4, 1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINOSHITA M, ONO K, HORIE T, NAGAO K, NISHI H, KUWABARA Y, TAKANABE-MORI R, HASEGAWA K, KITA T. & KIMURA T. 2010. Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5. Mol Endocrinol, 24, 1978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIR S, BEDDOW SA, SAMUEL VT, MILLER P, PREVIS SF, SUINO-POWELL K, XU HE, SHULMAN GI, KLIEWER SA & MANGELSDORF DJ 2011. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science, 331, 1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSCHNING CJ, WESCHE H, MERRILL AYRES T. & ROTHE M. 1998. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med, 188, 2091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KODA S, DATE Y, MURAKAMI N, SHIMBARA T, HANADA T, TOSHINAI K, NIIJIMA A, FURUYA M, INOMATA N, OSUYE K. & NAKAZATO M. 2005. The Role of the Vagal Nerve in Peripheral PYY3–36-Induced Feeding Reduction in Rats. Endocrinology, 146, 2369–2375. [DOI] [PubMed] [Google Scholar]
- KOH A, MANNERÅS-HOLM L, YUNN NO, NILSSON PM, RYU SH, MOLINARO A, PERKINS R, SMITH JG & BÄCKHED F. 2020. Microbial Imidazole Propionate Affects Responses to Metformin through p38γ-Dependent Inhibitory AMPK Phosphorylation. Cell Metab, 32, 643–653.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH A, MOLINARO A, STÅHLMAN M, KHAN MT, SCHMIDT C, MANNERÅS-HOLM L, WU H, CARRERAS A, JEONG H, OLOFSSON LE, BERGH PO, GERDES V, HARTSTRA A, DE BRAUW M, PERKINS R, NIEUWDORP M, BERGSTRÖM G. & BÄCKHED F. 2018. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell, 175, 947–961.e17. [DOI] [PubMed] [Google Scholar]
- KUHRE RE, WEWER ALBRECHTSEN NJ, LARSEN O, JEPSEN SL, BALK-MØLLER E, ANDERSEN DB, DEACON CF, SCHOONJANS K, REIMANN F, GRIBBLE FM, ALBRECHTSEN R, HARTMANN B, ROSENKILDE MM & HOLST JJ 2018. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab, 11, 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMONT BJ, LI Y, KWAN E, BROWN TJ, GAISANO H. & DRUCKER DJ 2012. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest, 122, 388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPPAS M. 2014. NOD1 expression is increased in the adipose tissue of women with gestational diabetes. J Endocrinol, 222, 99–112. [DOI] [PubMed] [Google Scholar]
- LARRAUFIE P, MARTIN-GALLAUSIAUX C, LAPAQUE N, DORE J, GRIBBLE FM, REIMANN F. & BLOTTIERE HM 2018. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Scientific Reports, 8, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE POUL E, LOISON C, STRUYF S, SPRINGAEL JY, LANNOY V, DECOBECQ ME, BREZILLON S, DUPRIEZ V, VASSART G, VAN DAMME J, PARMENTIER M. & DETHEUX M. 2003. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem, 278, 25481–9. [DOI] [PubMed] [Google Scholar]
- LEBRUN LJ, LENAERTS K, KIERS D, PAIS DE BARROS J-P, LE GUERN N, PLESNIK J, THOMAS C, BOURGEOIS T, DEJONG CHC, KOX M, HUNDSCHEID IHR, KHAN NA, MANDARD S, DECKERT V, PICKKERS P, DRUCKER DJ, LAGROST L. & GROBER J. 2017. Enteroendocrine L Cells Sense LPS after Gut Barrier Injury to Enhance GLP-1 Secretion. Cell Reports, 21, 1160–1168. [DOI] [PubMed] [Google Scholar]
- LEE YS, DE VADDER F, TREMAROLI V, WICHMANN A, MITHIEUX G. & BÄCKHED F. 2016. Insulin-like peptide 5 is a microbially regulated peptide that promotes hepatic glucose production. Mol Metab, 5, 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGRAND R, LUCAS N, DOMINIQUE M, AZHAR S, DEROISSART C, LE SOLLIEC MA, RONDEAUX J, NOBIS S, GUÉRIN C, LÉON F, DO REGO JC, PONS N, LE CHATELIER E, EHRLICH SD, LAMBERT G, DÉCHELOTTE P. & FETISSOV SO 2020. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice-a new potential probiotic for appetite and body weight management. Int J Obes (Lond), 44, 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS JE, MIEDZYBRODZKA EL, FOREMAN RE, WOODWARD ORM, KAY RG, GOLDSPINK DA, GRIBBLE FM & REIMANN F. 2020. Selective stimulation of colonic L cells improves metabolic outcomes in mice. Diabetologia, 63, 1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS JE, WOODWARD OR, NUZZACI D, SMITH CA, ADRIAENSSENS AE, BILLING L, BRIGHTON C, PHILLIPS BU, TADROSS JA, KINSTON SJ, CIABATTI E, GÖTTGENS B, TRIPODI M, HORNIGOLD D, BAKER D, GRIBBLE FM & REIMANN F. 2022. Relaxin/insulin-like family peptide receptor 4 (Rxfp4) expressing hypothalamic neurons modulate food intake and preference in mice. Mol Metab, 66, 101604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI B, LI L, LI M, LAM SM, WANG G, WU Y, ZHANG H, NIU C, ZHANG X, LIU X, HAMBLY C, JIN W, SHUI G. & SPEAKMAN JR 2019a. Microbiota Depletion Impairs Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. Cell Rep, 26, 2720–2737.e5. [DOI] [PubMed] [Google Scholar]
- LI G, LIN J, ZHANG C, GAO H, LU H, GAO X, ZHU R, LI Z, LI M. & LIU Z. 2021a. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes, 13, 1968257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI H, GAO Z, ZHANG J, YE X, XU A, YE J. & JIA W. 2012. Sodium Butyrate Stimulates Expression of Fibroblast Growth Factor 21 in Liver by Inhibition of Histone Deacetylase 3. Diabetes, 61, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J, SONG J, YAN B, WEISS HL, WEISS LT, GAO T. & EVERS BM 2021b. Neurotensin differentially regulates bile acid metabolism and intestinal FXR-bile acid transporter axis in response to nutrient abundance. Faseb j, 35, e21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI N, KOESTER ST, LACHANCE DM, DUTTA M, CUI JY & DEY N. 2021c. Microbiome-encoded bile acid metabolism modulates colonic transit times. iScience, 24, 102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI P, GAO X, SUN X, LI W, YI B. & ZHU L. 2019b. A novel epigenetic mechanism of FXR inhibiting GLP-1 secretion via miR-33 and its downstream targets. Biochem Biophys Res Commun, 517, 629–635. [DOI] [PubMed] [Google Scholar]
- LI P, ZHU L, YANG X, LI W, SUN X, YI B. & ZHU S. 2019c. Farnesoid X receptor interacts with cAMP response element binding protein to modulate glucagon-like peptide-1 (7–36) amide secretion by intestinal L cell. J Cell Physiol, 234, 12839–12846. [DOI] [PubMed] [Google Scholar]
- LI Y, CAO X, LI L-X, BRUBAKER PL, EDLUND H. & DRUCKER DJ 2005. β-Cell Pdx1 Expression Is Essential for the Glucoregulatory, Proliferative, and Cytoprotective Actions of Glucagon-Like Peptide-1. Diabetes, 54, 482–491. [DOI] [PubMed] [Google Scholar]
- LI Y. & OWYANG C. 1993. Vagal afferent pathway mediates physiological action of cholecystokinin on pancreatic enzyme secretion. J Clin Invest, 92, 418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Z, YI CX, KATIRAEI S, KOOIJMAN S, ZHOU E, CHUNG CK, GAO Y, VAN DEN HEUVEL JK, MEIJER OC, BERBEE JFP, HEIJINK M, GIERA M, WILLEMS VAN DIJK K, GROEN AK, RENSEN PCN & WANG Y. 2018a. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut, 67, 1269–1279. [DOI] [PubMed] [Google Scholar]
- LI Z, YI CX, KATIRAEI S, KOOIJMAN S, ZHOU E, CHUNG CK, GAO Y, VAN DEN HEUVEL JK, MEIJER OC, BERBÉE JFP, HEIJINK M, GIERA M, WILLEMS VAN DIJK K, GROEN AK, RENSEN PCN & WANG Y. 2018b. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut, 67, 1269–1279. [DOI] [PubMed] [Google Scholar]
- LIAN K, DU C, LIU Y, ZHU D, YAN W, ZHANG H, HONG Z, LIU P, ZHANG L, PEI H, ZHANG J, GAO C, XIN C, CHENG H, XIONG L. & TAO L. 2015. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes, 64, 49–59. [DOI] [PubMed] [Google Scholar]
- LIN Y-H, LUCK H, KHAN S, SCHNEEBERGER PHH, TSAI S, CLEMENTE-CASARES X, LEI H, LEU Y-L, CHAN YT, CHEN H-Y, YANG S-H, COBURN B, WINER S. & WINER DA 2019. Aryl hydrocarbon receptor agonist indigo protects against obesity-related insulin resistance through modulation of intestinal and metabolic tissue immunity. International Journal of Obesity, 43, 2407–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPS MA, VAN KLINKEN JB, VAN HARMELEN V, DHARURI HK, T HOEN PA, LAROS JF, VAN OMMEN GJ, JANSSEN IM, VAN RAMSHORST B, VAN WAGENSVELD BA, SWANK DJ, VAN DIELEN F, DANE A, HARMS A, VREEKEN R, HANKEMEIER T, SMIT JW, PIJL H. & WILLEMS VAN DIJK K. 2014. Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care, 37, 3150–6. [DOI] [PubMed] [Google Scholar]
- LIU Y, WANG Y, NI Y, CHEUNG CKY, LAM KSL, WANG Y, XIA Z, YE D, GUO J, TSE MA, PANAGIOTOU G. & XU A. 2020. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab, 31, 77–91 e5. [DOI] [PubMed] [Google Scholar]
- LORENZ DN & GOLDMAN SA 1982. Vagal mediation of the cholecystokinin satiety effect in rats. Physiol Behav, 29, 599–604. [DOI] [PubMed] [Google Scholar]
- LU Y, FAN C, LIANG A, FAN X, WANG R, LI P. & QI K. 2018. Effects of SCFA on the DNA methylation pattern of adiponectin and resistin in high-fat-diet-induced obese male mice. British Journal of Nutrition, 120, 385–392. [DOI] [PubMed] [Google Scholar]
- MAGKOS F, BRADLEY D, SCHWEITZER GG, FINCK BN, EAGON JC, ILKAYEVA O, NEWGARD CB & KLEIN S. 2013. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes, 62, 2757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIDA A, LOVSHIN JA, BAGGIO LL & DRUCKER DJ 2008. The Glucagon-Like Peptide-1 Receptor Agonist Oxyntomodulin Enhances β-Cell Function but Does Not Inhibit Gastric Emptying in Mice. Endocrinology, 149, 5670–5678. [DOI] [PubMed] [Google Scholar]
- MAKISHIMA M, OKAMOTO AY, REPA JJ, TU H, LEARNED RM, LUK A, HULL MV, LUSTIG KD, MANGELSDORF DJ & SHAN B. 1999. Identification of a nuclear receptor for bile acids. Science, 284, 1362–5. [DOI] [PubMed] [Google Scholar]
- MAKKI K, BROLIN H, PETERSEN N, HENRICSSON M, CHRISTENSEN DP, KHAN MT, WAHLSTRÖM A, BERGH PO, TREMAROLI V, SCHOONJANS K, MARSCHALL HU & BÄCKHED F. 2022. 6α-hydroxylated bile acids mediate TGR5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANGAN AM, AL NAJIM W, MCNAMARA N, MARTIN WP, ANTANAITIS A, BLEIEL SB, KENT RM, LE ROUX CW & DOCHERTY NG 2019. Effect of Macronutrient Type and Gastrointestinal Release Site on PYY Response in Normal Healthy Subjects. J Clin Endocrinol Metab, 104, 3661–3669. [DOI] [PubMed] [Google Scholar]
- MARCOBAL A, KASHYAP PC, NELSON TA, ARONOV PA, DONIA MS, SPORMANN A, FISCHBACH MA & SONNENBURG JL 2013. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. Isme j, 7, 1933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIS KG, STEVANOVIC K, LI Z, YANG QM, ORAVECZ T, ZAMBROWICZ B, JHAVER KG, DIACOU A. & GERSHON MD 2014. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut, 63, 928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTCHENKO SE, MARTCHENKO A, COX BJ, NAISMITH K, WALLER A, GURGES P, SWEENEY ME, PHILPOTT DJ & BRUBAKER PL 2020. Circadian GLP-1 Secretion in Mice Is Dependent on the Intestinal Microbiome for Maintenance of Diurnal Metabolic Homeostasis. Diabetes, 69, 2589–2602. [DOI] [PubMed] [Google Scholar]
- MARTIN AM, LUMSDEN AL, YOUNG RL, JESSUP CF, SPENCER NJ & KEATING DJ 2017. Regional differences in nutrient-induced secretion of gut serotonin. Physiol Rep, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINEZ TM, MEYER RK & DUCA FA 2021. Therapeutic Potential of Various Plant-Based Fibers to Improve Energy Homeostasis via the Gut Microbiota. Nutrients, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINEZ-GURYN K, HUBERT N, FRAZIER K, URLASS S, MUSCH MW, OJEDA P, PIERRE JF, MIYOSHI J, SONTAG TJ, CHAM CM, REARDON CA, LEONE V. & CHANG EB 2018. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe, 23, 458–469.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSIMINO SP, MCBURNEY MI, FIELD CJ, THOMSON AB, KEELAN M, HAYEK MG & SUNVOLD GD 1998a. Fermentable dietary fiber increases GLP-1 secretion and improves glucose homeostasis despite increased intestinal glucose transport capacity in healthy dogs. J Nutr, 128, 1786–93. [DOI] [PubMed] [Google Scholar]
- MASSIMINO SP, MCBURNEY MI, FIELD CJ, THOMSON ABR, KEELAN M, HAYEK MG & SUNVOLD GD 1998b. Fermentable Dietary Fiber Increases GLP-1 Secretion and Improves Glucose Homeostasis Despite Increased Intestinal Glucose Transport Capacity in Healthy Dogs. The Journal of Nutrition, 128, 1786–1793. [DOI] [PubMed] [Google Scholar]
- MAYO KE, MILLER LJ, BATAILLE D, DALLE S, GÖKE B, THORENS B. & DRUCKER DJ 2003. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev, 55, 167–94. [DOI] [PubMed] [Google Scholar]
- MCGAVIGAN AK, GARIBAY D, HENSELER ZM, CHEN J, BETTAIEB A, HAJ FG, LEY RE, CHOUINARD ML & CUMMINGS BP 2017. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut, 66, 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN BA, WONG CK, CAMPBELL JE, HODSON DJ, TRAPP S. & DRUCKER DJ 2021. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocr Rev, 42, 101–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIER JJ, NAUCK MA, POTT A, HEINZE K, GOETZE O, BULUT K, SCHMIDT WE, GALLWITZ B. & HOLST JJ 2006. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology, 130, 44–54. [DOI] [PubMed] [Google Scholar]
- MENNI C, ZHU J, LE ROY CI, MOMPEO O, YOUNG K, REBHOLZ CM, SELVIN E, NORTH KE, MOHNEY RP, BELL JT, BOERWINKLE E, SPECTOR TD, MANGINO M, YU B. & VALDES AM 2020. Serum metabolites reflecting gut microbiome alpha diversity predict type 2 diabetes. Gut Microbes, 11, 1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENTLEIN R, DAHMS P, GRANDT D. & KRÜGER R. 1993. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept, 49, 133–44. [DOI] [PubMed] [Google Scholar]
- MERCADO CP & KILIC F. 2010. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv, 10, 231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER RK, BIME MA & DUCA FA 2022a. Small intestinal metabolomics analysis reveals differentially regulated metabolite profiles in obese rats and with prebiotic supplementation. Metabolomics, 18, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER RK, LANE AI, WENINGER SN, MARTINEZ TM, KANGATH A, LAUBITZ D. & DUCA FA 2022b. Oligofructose restores postprandial short-chain fatty acid levels during high-fat feeding. Obesity (Silver Spring), 30, 1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINAMI J, KONDO S, YANAGISAWA N, ODAMAKI T, XIAO JZ, ABE F, NAKAJIMA S, HAMAMOTO Y, SAITOH S. & SHIMODA T. 2015. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J Nutr Sci, 4, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MODASIA A, PARKER A, JONES E, STENTZ R, BRION A, GOLDSON A, DEFERNEZ M, WILEMAN T, ASHLEY BLACKSHAW L. & CARDING SR 2020. Regulation of Enteroendocrine Cell Networks by the Major Human Gut Symbiont Bacteroides thetaiotaomicron. Front Microbiol, 11, 575595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHAMMAD S. & THIEMERMANN C. 2020. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front Immunol, 11, 594150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINARO A, BEL LASSEN P, HENRICSSON M, WU H, ADRIOUCH S, BELDA E, CHAKAROUN R, NIELSEN T, BERGH PO, ROUAULT C, ANDRÉ S, MARQUET F, ANDREELLI F, SALEM JE, ASSMANN K, BASTARD JP, FORSLUND S, LE CHATELIER E, FALONY G, PONS N, PRIFTI E, QUINQUIS B, ROUME H, VIEIRA-SILVA S, HANSEN TH, PEDERSEN HK, LEWINTER C, SØNDERSKOV NB, KØBER L, VESTERGAARD H, HANSEN T, ZUCKER JD, GALAN P, DUMAS ME, RAES J, OPPERT JM, LETUNIC I, NIELSEN J, BORK P, EHRLICH SD, STUMVOLL M, PEDERSEN O, ARON-WISNEWSKY J, CLÉMENT K. & BÄCKHED F. 2020. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun, 11, 5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLICA MP, MATTACE RASO G, CAVALIERE G, TRINCHESE G, DE FILIPPO C, ACETO S, PRISCO M, PIROZZI C, DI GUIDA F, LAMA A, CRISPINO M, TRONINO D, DI VAIO P, BERNI CANANI R, CALIGNANO A. & MELI R. 2017. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes, 66, 1405–1418. [DOI] [PubMed] [Google Scholar]
- MORAN TH, SMEDH U, KINZIG KP, SCOTT KA, KNIPP S. & LADENHEIM EE 2005. Peptide YY(3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 288, R384–R388. [DOI] [PubMed] [Google Scholar]
- MORÓN-ROS S, URIARTE I, BERASAIN C, AVILA MA, SABATER-MASDEU M, MORENO-NAVARRETE JM, FERNÁNDEZ-REAL JM, GIRALT M, VILLARROYA F. & GAVALDÀ-NAVARRO A. 2021. FGF15/19 is required for adipose tissue plasticity in response to thermogenic adaptations. Mol Metab, 43, 101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTENSEN K, CHRISTENSEN LL, HOLST JJ & ORSKOV C. 2003. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regulatory Peptides, 114, 189–196. [DOI] [PubMed] [Google Scholar]
- MULLER PA, SCHNEEBERGER M, MATHEIS F, WANG P, KERNER Z, ILANGES A, PELLEGRINO K, DEL MÁRMOL J, CASTRO TBR, FURUICHI M, PERKINS M, HAN W, RAO A, PICKARD AJ, CROSS JR, HONDA K, DE ARAUJO I. & MUCIDA D. 2020. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature, 583, 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKABAYASHI H, NISHIZAWA M, NAKAGAWA A, TAKEDA R. & NIIJIMA A. 1996. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol, 271, E808–13. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA A, SATAKE H, NAKABAYASHI H, NISHIZAWA M, FURUYA K, NAKANO S, KIGOSHI T, NAKAYAMA K. & UCHIDA K. 2004. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci, 110, 36–43. [DOI] [PubMed] [Google Scholar]
- NATIVIDAD JM, AGUS A, PLANCHAIS J, LAMAS B, JARRY AC, MARTIN R, MICHEL ML, CHONG-NGUYEN C, ROUSSEL R, STRAUBE M, JEGOU S, MCQUITTY C, LE GALL M, DA COSTA G, LECORNET E, MICHAUDEL C, MODOUX M, GLODT J, BRIDONNEAU C, SOVRAN B, DUPRAZ L, BADO A, RICHARD ML, LANGELLA P, HANSEL B, LAUNAY JM, XAVIER RJ, DUBOC H. & SOKOL H. 2018. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab, 28, 737–749.e4. [DOI] [PubMed] [Google Scholar]
- NAUCK MA, HOMBERGER E, SIEGEL EG, ALLEN RC, EATON RP, EBERT R. & CREUTZFELDT W. 1986. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab, 63, 492–8. [DOI] [PubMed] [Google Scholar]
- NAUCK MA, QUAST DR, WEFERS J. & MEIER JJ 2021. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab, 46, 101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWGARD CB, AN J, BAIN JR, MUEHLBAUER MJ, STEVENS RD, LIEN LF, HAQQ AM, SHAH SH, ARLOTTO M, SLENTZ CA, ROCHON J, GALLUP D, ILKAYEVA O, WENNER BR, YANCY WS JR., EISENSON H, MUSANTE G, SURWIT RS, MILLINGTON DS, BUTLER MD & SVETKEY LP 2009. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab, 9, 311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN AT, MANDARD S, DRAY C, DECKERT V, VALET P, BESNARD P, DRUCKER DJ, LAGROST L. & GROBER J. 2014. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: involvement of the GLP-1 pathway. Diabetes, 63, 471–82. [DOI] [PubMed] [Google Scholar]
- OH CM, NAMKUNG J, GO Y, SHONG KE, KIM K, KIM H, PARK BY, LEE HW, JEON YH, SONG J, SHONG M, YADAV VK, KARSENTY G, KAJIMURA S, LEE IK, PARK S. & KIM H. 2015. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun, 6, 6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMAR B. & AHRÉN B. 2014. Pleiotropic Mechanisms for the Glucose-Lowering Action of DPP-4 Inhibitors. Diabetes, 63, 2196–2202. [DOI] [PubMed] [Google Scholar]
- OSAWA Y, KANAMORI H, SEKI E, HOSHI M, OHTAKI H, YASUDA Y, ITO H, SUETSUGU A, NAGAKI M, MORIWAKI H, SAITO K. & SEISHIMA M. 2011. L-tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J Biol Chem, 286, 34800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANARO BL, YUSTA B, MATTHEWS D, KOEHLER JA, SONG Y, SANDOVAL DA & DRUCKER DJ 2020. Intestine-selective reduction of Gcg expression reveals the importance of the distal gut for GLP-1 secretion. Mol Metab, 37, 100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEDERSEN HK, GUDMUNDSDOTTIR V, NIELSEN HB, HYOTYLAINEN T, NIELSEN T, JENSEN BAH, FORSLUND K, HILDEBRAND F, PRIFTI E, FALONY G, LE CHATELIER E, LEVENEZ F, DORÉ J, MATTILA I, PLICHTA DR, PÖHÖ P, HELLGREN LI, ARUMUGAM M, SUNAGAWA S, VIEIRA-SILVA S, JØRGENSEN T, HOLM JB, TROŠT K, CONSORTIUM M, KRISTIANSEN K, BRIX S, RAES J, WANG J, HANSEN T, BORK P, BRUNAK S, ORESIC M, EHRLICH SD & PEDERSEN O. 2016. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature, 535, 376–381. [DOI] [PubMed] [Google Scholar]
- PENDYALA S, WALKER JM & HOLT PR 2012. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology, 142, 1100–1101.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERINO A, VELÁZQUEZ-VILLEGAS LA, BRESCIANI N, SUN Y, HUANG Q, FÉNELON VS, CASTELLANOS-JANKIEWICZ A, ZIZZARI P, BRUSCHETTA G, JIN S, BALEISYTE A, GIOIELLO A, PELLICCIARI R, IVANISEVIC J, SCHNEIDER BL, DIANO S, COTA D. & SCHOONJANS K. 2021. Central anorexigenic actions of bile acids are mediated by TGR5. Nat Metab, 3, 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLAMBOECK A, VEEDFALD S, DEACON CF, HARTMANN B, WETTERGREN A, SVENDSEN LB, MEISNER S, HOVENDAL C, VILSBØLL T, KNOP FK & HOLST JJ 2013. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol, 304, G1117–27. [DOI] [PubMed] [Google Scholar]
- PLOTKIN DL, DELCASTILLO K, VAN EVERY DW, TIPTON KD, ARAGON AA & SCHOENFELD BJ 2021. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. Int J Sport Nutr Exerc Metab, 31, 292–301. [DOI] [PubMed] [Google Scholar]
- POGGI M, BASTELICA D, GUAL P, IGLESIAS MA, GREMEAUX T, KNAUF C, PEIRETTI F, VERDIER M, JUHAN-VAGUE I, TANTI JF, BURCELIN R. & ALESSI MC 2007. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia, 50, 1267–76. [DOI] [PubMed] [Google Scholar]
- POLAK JM, SULLIVAN SN, BLOOM SR, BUCHAN AM, FACER P, BROWN MR & PEARSE AG 1977. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature, 270, 183–4. [DOI] [PubMed] [Google Scholar]
- POSTAL BG, GHEZZAL S, AGUANNO D, ANDRÉ S, GARBIN K, GENSER L, BROT-LAROCHE E, POITOU C, SOULA H, LETURQUE A, CLÉMENT K. & CARRIÈRE V. 2020. AhR activation defends gut barrier integrity against damage occurring in obesity. Mol Metab, 39, 101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTHOFF MJ, BONEY-MONTOYA J, CHOI M, HE T, SUNNY NE, SATAPATI S, SUINO-POWELL K, XU HE, GERARD RD, FINCK BN, BURGESS SC, MANGELSDORF DJ & KLIEWER SA 2011. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab, 13, 729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRAWITT J, ABDELKARIM M, STROEVE JH, POPESCU I, DUEZ H, VELAGAPUDI VR, DUMONT J, BOUCHAERT E, VAN DIJK TH, LUCAS A, DORCHIES E, DAOUDI M, LESTAVEL S, GONZALEZ FJ, ORESIC M, CARIOU B, KUIPERS F, CARON S. & STAELS B. 2011. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes, 60, 1861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIGSTAD CS, SALMONSON CE, RAINEY JF 3RD, SZURSZEWSKI JH, LINDEN DR, SONNENBURG JL, FARRUGIA G. & KASHYAP PC 2015. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. Faseb j, 29, 1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDLON JM, KANG DJ, HYLEMON PB & BAJAJ JS 2014. Bile acids and the gut microbiome. Curr Opin Gastroenterol, 30, 332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVERS SL, KLIP A. & GIACCA A. 2019. NOD1: An Interface Between Innate Immunity and Insulin Resistance. Endocrinology, 160, 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROAGER HM & LICHT TR 2018. Microbial tryptophan catabolites in health and disease. Nat Commun, 9, 3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONCON-ALBUQUERQUE R JR., MOREIRA-RODRIGUES M, FARIA B, FERREIRA AP, CERQUEIRA C, LOURENÇO AP, PESTANA M, VON HAFE P. & LEITE-MOREIRA AF 2008. Attenuation of the cardiovascular and metabolic complications of obesity in CD14 knockout mice. Life Sci, 83, 502–10. [DOI] [PubMed] [Google Scholar]
- RYAN KK, TREMAROLI V, CLEMMENSEN C, KOVATCHEVA-DATCHARY P, MYRONOVYCH A, KARNS R, WILSON-PÉREZ HE, SANDOVAL DA, KOHLI R, BÄCKHED F. & SEELEY RJ 2014. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature, 509, 183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHU A, GOPALAKRISHNAN L, GAUR N, CHATTERJEE O, MOL P, MODI PK, DAGAMAJALU S, ADVANI J, JAIN S. & KESHAVA PRASAD TS 2018. The 5-Hydroxytryptamine signaling map: an overview of serotonin-serotonin receptor mediated signaling network. J Cell Commun Signal, 12, 731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHURI-ARISOYLU M, BRODY LP, PARKINSON JR, PARKES H, NAVARATNAM N, MILLER AD, THOMAS EL, FROST G. & BELL JD 2016. Reprogramming of hepatic fat accumulation and ‘browning’ of adipose tissue by the short-chain fatty acid acetate. Int J Obes (Lond), 40, 955–63. [DOI] [PubMed] [Google Scholar]
- SAMUEL BS, SHAITO A, MOTOIKE T, REY FE, BACKHED F, MANCHESTER JK, HAMMER RE, WILLIAMS SC, CROWLEY J, YANAGISAWA M. & GORDON JI 2008. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A, 105, 16767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAYIN SI, WAHLSTRÖM A, FELIN J, JÄNTTI S, MARSCHALL HU, BAMBERG K, ANGELIN B, HYÖTYLÄINEN T, OREŠIČ M. & BÄCKHED F. 2013. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab, 17, 225–35. [DOI] [PubMed] [Google Scholar]
- SCHEJA L. & HEEREN J. 2019. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol, 15, 507–524. [DOI] [PubMed] [Google Scholar]
- SCHERTZER JD, TAMRAKAR AK, MAGALHÃES JG, PEREIRA S, BILAN PJ, FULLERTON MD, LIU Z, STEINBERG GR, GIACCA A, PHILPOTT DJ & KLIP A. 2011. NOD1 activators link innate immunity to insulin resistance. Diabetes, 60, 2206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHJOLDAGER BT, BALDISSERA FG, MORTENSEN PE, HOLST JJ & CHRISTIANSEN J. 1988. Oxyntomodulin: a potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur J Clin Invest, 18, 499–503. [DOI] [PubMed] [Google Scholar]
- SCHMITT J, KONG B, STIEGER B, TSCHOPP O, SCHULTZE SM, RAU M, WEBER A, MÜLLHAUPT B, GUO GL & GEIER A. 2015. Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal. Liver Int, 35, 1133–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ GJ, BERKOW G, MCHUGH PR & MORAN TH 1993. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol, 264, R630–7. [DOI] [PubMed] [Google Scholar]
- SHANKAR SS, SHANKAR RR, MIXSON LA, MILLER DL, PRAMANIK B, O’DOWD AK, WILLIAMS DM, FREDERICK CB, BEALS CR, STOCH SA, STEINBERG HO & KELLEY DE 2018. Native Oxyntomodulin Has Significant Glucoregulatory Effects Independent of Weight Loss in Obese Humans With and Without Type 2 Diabetes. Diabetes, 67, 1105–1112. [DOI] [PubMed] [Google Scholar]
- SHARMA A, SINGH S, MISHRA A, RAI AK, AHMAD I, AHMAD S, GULZAR F, SCHERTZER JD, SHRIVASTAVA A. & TAMRAKAR AK 2022. Insulin resistance corresponds with a progressive increase in NOD1 in high fat diet-fed mice. Endocrine, 76, 282–293. [DOI] [PubMed] [Google Scholar]
- SHE P, VAN HORN C, REID T, HUTSON SM, COONEY RN & LYNCH CJ 2007. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab, 293, E1552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHI X, ZHOU F, LI X, CHANG B, LI D, WANG Y, TONG Q, XU Y, FUKUDA M, ZHAO JJ, LI D, BURRIN DG, CHAN L. & GUAN X. 2013. Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab, 18, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMIZU H, MASUJIMA Y, USHIRODA C, MIZUSHIMA R, TAIRA S, OHUE-KITANO R. & KIMURA I. 2019. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Scientific Reports, 9, 16574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINY A, REGIN B, BALACHANDAR V, GOKULAKRISHNAN K, MOHAN V, BABU S. & BALASUBRAMANYAM M. 2013. Convergence of innate immunity and insulin resistance as evidenced by increased nucleotide oligomerization domain (NOD) expression and signaling in monocytes from patients with type 2 diabetes. Cytokine, 64, 564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISLEY S, GUTIERREZ-AGUILAR R, SCOTT M, D’ALESSIO DA, SANDOVAL DA & SEELEY RJ 2014. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest, 124, 2456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJÖGREN K, ENGDAHL C, HENNING P, LERNER UH, TREMAROLI V, LAGERQUIST MK, BÄCKHED F. & OHLSSON C. 2012. The gut microbiota regulates bone mass in mice. J Bone Miner Res, 27, 1357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH EP, AN Z, WAGNER C, LEWIS AG, COHEN EB, LI B, MAHBOD P, SANDOVAL D, PEREZ-TILVE D, TAMARINA N, PHILIPSON LH, STOFFERS DA, SEELEY RJ & D’ALESSIO DA 2014. The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab, 19, 1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONOBE K, SAKAI T, SATOH M, HAGA N. & ITOH Z. 1995. Control of gallbladder contractions by cholecystokinin through cholecystokinin-A receptors in the vagal pathway and gallbladder in the dog. Regul Pept, 60, 33–46. [DOI] [PubMed] [Google Scholar]
- SØRENSEN LB, FLINT A, RABEN A, HARTMANN B, HOLST JJ & ASTRUP A. 2003. No effect of physiological concentrations of glucagon-like peptide-2 on appetite and energy intake in normal weight subjects. Int J Obes Relat Metab Disord, 27, 450–6. [DOI] [PubMed] [Google Scholar]
- STENMAN LK, WAGET A, GARRET C, KLOPP P, BURCELIN R. & LAHTINEN S. 2014. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef Microbes, 5, 437–45. [DOI] [PubMed] [Google Scholar]
- STROEVE JH, BRUFAU G, STELLAARD F, GONZALEZ FJ, STAELS B. & KUIPERS F. 2010. Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice. Lab Invest, 90, 1457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMARA G, SUMARA O, KIM JK & KARSENTY G. 2012. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab, 16, 588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN EW, DE FONTGALLAND D, RABBITT P, HOLLINGTON P, SPOSATO L, DUE SL, WATTCHOW DA, RAYNER CK, DEANE AM, YOUNG RL & KEATING DJ 2017. Mechanisms Controlling Glucose-Induced GLP-1 Secretion in Human Small Intestine. Diabetes, 66, 2144–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN S, SUN L, WANG K, QIAO S, ZHAO X, HU X, CHEN W, ZHANG S, LI H, DAI H. & LIU H. 2021a. The gut commensal fungus, Candida parapsilosis, promotes high fat-diet induced obesity in mice. Communications Biology, 4, 1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN W, DONG H. & WOLFRUM C. 2021b. Local acetate inhibits brown adipose tissue function. Proc Natl Acad Sci U S A, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SURYAWAN A, JEYAPALAN AS, ORELLANA RA, WILSON FA, NGUYEN HV & DAVIS TA 2008. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab, 295, E868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAKI M, MAWE GM, BARASCH JM, GERSHON MD & GERSHON MD 1985. Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience, 16, 223–40. [DOI] [PubMed] [Google Scholar]
- TENNOUNE N, CHAN P, BRETON J, LEGRAND R, CHABANE YN, AKKERMANN K, JÄRV A, OUELAA W, TAKAGI K, GHOUZALI I, FRANCOIS M, LUCAS N, BOLE-FEYSOT C, PESTEL-CARON M, DO REGO JC, VAUDRY D, HARRO J, DÉ E, DÉCHELOTTE P. & FETISSOV SO 2014. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl Psychiatry, 4, e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRY NA, WALP ER, LEE RA, KAESTNER KH & MAY CL 2014. Impaired enteroendocrine development in intestinal-specific Islet1 mouse mutants causes impaired glucose homeostasis. Am J Physiol Gastrointest Liver Physiol, 307, G979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLHURST G, HEFFRON H, LAM YS, PARKER HE, HABIB AM, DIAKOGIANNAKI E, CAMERON J, GROSSE J, REIMANN F. & GRIBBLE FM 2012. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes, 61, 364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRABELSI MS, DAOUDI M, PRAWITT J, DUCASTEL S, TOUCHE V, SAYIN SI, PERINO A, BRIGHTON CA, SEBTI Y, KLUZA J, BRIAND O, DEHONDT H, VALLEZ E, DORCHIES E, BAUD G, SPINELLI V, HENNUYER N, CARON S, BANTUBUNGI K, CAIAZZO R, REIMANN F, MARCHETTI P, LEFEBVRE P, BÄCKHED F, GRIBBLE FM, SCHOONJANS K, PATTOU F, TAILLEUX A, STAELS B. & LESTAVEL S. 2015. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun, 6, 7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURTON MD, O’SHEA D, GUNN I, BEAK SA, EDWARDS CM, MEERAN K, CHOI SJ, TAYLOR GM, HEATH MM, LAMBERT PD, WILDING JP, SMITH DM, GHATEI MA, HERBERT J. & BLOOM SR 1996. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature, 379, 69–72. [DOI] [PubMed] [Google Scholar]
- VADHER K, PATEL H, MODY R, LEVINE JA, HOOG M, CHENG AY, PANTALONE KM & SAPIN H. 2022. Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: An adjusted indirect treatment comparison. Diabetes, Obesity and Metabolism, 24, 1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAHL TP, TAUCHI M, DURLER TS, ELFERS EE, FERNANDES TM, BITNER RD, ELLIS KS, WOODS SC, SEELEY RJ, HERMAN JP & D’ALESSIO DA 2007. Glucagon-Like Peptide-1 (GLP-1) Receptors Expressed on Nerve Terminals in the Portal Vein Mediate the Effects of Endogenous GLP-1 on Glucose Tolerance in Rats. Endocrinology, 148, 4965–4973. [DOI] [PubMed] [Google Scholar]
- VANWEERT F, DE LIGT M, HOEKS J, HESSELINK MKC, SCHRAUWEN P. & PHIELIX E. 2021. Elevated Plasma Branched-Chain Amino Acid Levels Correlate With Type 2 Diabetes-Related Metabolic Disturbances. J Clin Endocrinol Metab, 106, e1827–e1836. [DOI] [PubMed] [Google Scholar]
- VARIN EM, MULVIHILL EE, BAGGIO LL, KOEHLER JA, CAO X, SEELEY RJ & DRUCKER DJ 2019. Distinct Neural Sites of GLP-1R Expression Mediate Physiological versus Pharmacological Control of Incretin Action. Cell Reports, 27, 3371–3384.e3. [DOI] [PubMed] [Google Scholar]
- VELAZQUEZ-VILLEGAS LA, PERINO A, LEMOS V, ZIETAK M, NOMURA M, POLS TWH & SCHOONJANS K. 2018. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat Commun, 9, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIDALI G, BOFFA LC, BRADBURY EM & ALLFREY VG 1978. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A, 75, 2239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIJAY-KUMAR M, AITKEN JD, CARVALHO FA, CULLENDER TC, MWANGI S, SRINIVASAN S, SITARAMAN SV, KNIGHT R, LEY RE & GEWIRTZ AT 2010. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science, 328, 228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILSBØLL T, KRARUP T, SONNE J, MADSBAD S, VØLUND A, JUUL AG & HOLST JJ 2003. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab, 88, 2706–13. [DOI] [PubMed] [Google Scholar]
- WACHSMUTH HR, WENINGER SN & DUCA FA 2022. Role of the gut-brain axis in energy and glucose metabolism. Exp Mol Med, 54, 377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C, XU CX, KRAGER SL, BOTTUM KM, LIAO DF & TISCHKAU SA 2011a. Aryl hydrocarbon receptor deficiency enhances insulin sensitivity and reduces PPAR-α pathway activity in mice. Environ Health Perspect, 119, 1739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG D, LIU CD, LI HF, TIAN ML, PAN JQ, SHU G, JIANG QY, YIN YL & ZHANG L. 2020. LSD1 mediates microbial metabolite butyrate-induced thermogenesis in brown and white adipose tissue. Metabolism, 102, 154011. [DOI] [PubMed] [Google Scholar]
- WANG F, KNUTSON K, ALCAINO C, LINDEN DR, GIBBONS SJ, KASHYAP P, GROVER M, OECKLER R, GOTTLIEB PA, LI HJ, LEITER AB, FARRUGIA G. & BEYDER A. 2017. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol, 595, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG R, LU Y, CICHA MZ, SINGH MV, BENSON CJ, MADDEN CJ, CHAPLEAU MW & ABBOUD FM 2019. TMEM16B determines cholecystokinin sensitivity of intestinal vagal afferents of nodose neurons. JCI Insight, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG TJ, LARSON MG, VASAN RS, CHENG S, RHEE EP, MCCABE E, LEWIS GD, FOX CS, JACQUES PF, FERNANDEZ C, O’DONNELL CJ, CARR SA, MOOTHA VK, FLOREZ JC, SOUZA A, MELANDER O, CLISH CB & GERSZTEN RE 2011b. Metabolite profiles and the risk of developing diabetes. Nat Med, 17, 448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG X, LIU H, CHEN J, LI Y. & QU S. 2015. Multiple Factors Related to the Secretion of Glucagon-Like Peptide-1. Int J Endocrinol, 2015, 651757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE M, HOUTEN SM, MATAKI C, CHRISTOFFOLETE MA, KIM BW, SATO H, MESSADDEQ N, HARNEY JW, EZAKI O, KODAMA T, SCHOONJANS K, BIANCO AC & AUWERX J. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature, 439, 484–9. [DOI] [PubMed] [Google Scholar]
- WEWER ALBRECHTSEN NJ, HORNBURG D, ALBRECHTSEN R, SVENDSEN B, TORÄNG S, JEPSEN SL, KUHRE RE, HANSEN M, JANUS C, FLOYD A, LUND A, VILSBØLL T, KNOP FK, VESTERGAARD H, DEACON CF, MEISSNER F, MANN M, HOLST JJ & HARTMANN B. 2016. Oxyntomodulin Identified as a Marker of Type 2 Diabetes and Gastric Bypass Surgery by Mass-spectrometry Based Profiling of Human Plasma. EBioMedicine, 7, 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE PJ, LAPWORTH AL, AN J, WANG L, MCGARRAH RW, STEVENS RD, ILKAYEVA O, GEORGE T, MUEHLBAUER MJ, BAIN JR, TRIMMER JK, BROSNAN MJ, ROLPH TP & NEWGARD CB 2016. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab, 5, 538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE PJ, MCGARRAH RW, GRIMSRUD PA, TSO SC, YANG WH, HALDEMAN JM, GRENIER-LAROUCHE T, AN J, LAPWORTH AL, ASTAPOVA I, HANNOU SA, GEORGE T, ARLOTTO M, OLSON LB, LAI M, ZHANG GF, ILKAYEVA O, HERMAN MA, WYNN RM, CHUANG DT & NEWGARD CB 2018. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metab, 27, 1281–1293.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICHMANN A, ALLAHYAR A, GREINER TU, PLOVIER H, LUNDÉN G, LARSSON T, DRUCKER DJ, DELZENNE NM, CANI PD & BÄCKHED F. 2013. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe, 14, 582–90. [DOI] [PubMed] [Google Scholar]
- WIKOFF WR, ANFORA AT, LIU J, SCHULTZ PG, LESLEY SA, PETERS EC & SIUZDAK G. 2009. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A, 106, 3698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS BB, VAN BENSCHOTEN AH, CIMERMANCIC P, DONIA MS, ZIMMERMANN M, TAKETANI M, ISHIHARA A, KASHYAP PC, FRASER JS & FISCHBACH MA 2014. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe, 16, 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WØJDEMANN M, WETTERGREN A, HARTMANN B, HILSTED L. & HOLST JJ 1999. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J Clin Endocrinol Metab, 84, 2513–7. [DOI] [PubMed] [Google Scholar]
- WORTHINGTON JJ, REIMANN F. & GRIBBLE FM 2018. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol, 11, 3–20. [DOI] [PubMed] [Google Scholar]
- WU T, RAYNER CK, WATSON LE, JONES KL, HOROWITZ M. & LITTLE TJ 2017. Comparative effects of intraduodenal fat and glucose on the gut-incretin axis in healthy males. Peptides, 95, 124–127. [DOI] [PubMed] [Google Scholar]
- WU T, ZHAO BR, BOUND MJ, CHECKLIN HL, BELLON M, LITTLE TJ, YOUNG RL, JONES KL, HOROWITZ M. & RAYNER CK 2012. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am J Clin Nutr, 95, 78–83. [DOI] [PubMed] [Google Scholar]
- WYNNE K, PARK AJ, SMALL CJ, PATTERSON M, ELLIS SM, MURPHY KG, WREN AM, FROST GS, MEERAN K, GHATEI MA & BLOOM SR 2005. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes, 54, 2390–5. [DOI] [PubMed] [Google Scholar]
- XU CX, WANG C, ZHANG ZM, JAEGER CD, KRAGER SL, BOTTUM KM, LIU J, LIAO DF & TISCHKAU SA 2015. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int J Obes (Lond), 39, 1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YABUT JM, CRANE JD, GREEN AE, KEATING DJ, KHAN WI & STEINBERG GR 2019. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr Rev, 40, 1092–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMASATO T. & NAKAYAMA S. 1988. Effects of neurotensin on the motility of the isolated gallbladder, bile duct and ampulla in guinea-pigs. Eur J Pharmacol, 148, 101–6. [DOI] [PubMed] [Google Scholar]
- YANG W, YU T, HUANG X, BILOTTA AJ, XU L, LU Y, SUN J, PAN F, ZHOU J, ZHANG W, YAO S, MAYNARD CL, SINGH N, DANN SM, LIU Z. & CONG Y. 2020. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun, 11, 4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANO JM, YU K, DONALDSON GP, SHASTRI GG, ANN P, MA L, NAGLER CR, ISMAGILOV RF, MAZMANIAN SK & HSIAO EY 2015. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell, 161, 264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YI M, LI H, WU Z, YAN J, LIU Q, OU C. & CHEN M. 2018. A Promising Therapeutic Target for Metabolic Diseases: Neuropeptide Y Receptors in Humans. Cell Physiol Biochem, 45, 88–107. [DOI] [PubMed] [Google Scholar]
- YOUNG JL, MORA A, CERNY A, CZECH MP, WODA B, KURT-JONES EA, FINBERG RW & CORVERA S. 2012. CD14 deficiency impacts glucose homeostasis in mice through altered adrenal tone. PLoS One, 7, e29688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU C, LIU S, CHEN L, SHEN J, NIU Y, WANG T, ZHANG W. & FU L. 2019. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J Endocrinol, 243, 125–135. [DOI] [PubMed] [Google Scholar]
- ZARRINPAR A, CHAIX A, XU ZZ, CHANG MW, MAROTZ CA, SAGHATELIAN A, KNIGHT R. & PANDA S. 2018. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun, 9, 2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAYKOV AN, GELFANOV VM, PEREZ-TILVE D, FINAN B. & DIMARCHI RD 2019. Insulin-like peptide 5 fails to improve metabolism or body weight in obese mice. Peptides, 120, 170116. [DOI] [PubMed] [Google Scholar]
- ZHANG SY, LI RJW, LIM YM, BATCHULUUN B, LIU H, WAISE TMZ & LAM TKT 2021. FXR in the dorsal vagal complex is sufficient and necessary for upper small intestinal microbiome-mediated changes of TCDCA to alter insulin action in rats. Gut, 70, 1675–1683. [DOI] [PubMed] [Google Scholar]
- ZHANG T, PERKINS MH, CHANG H, HAN W. & DE ARAUJO IE 2022. An inter-organ neural circuit for appetite suppression. Cell, 185, 2478–2494.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG T, UCHIDA T, GOMEZ G, LLUIS F, THOMPSON JC & GREELEY GH JR. 1993. Neural regulation of peptide YY secretion. Regul Pept, 48, 321–8. [DOI] [PubMed] [Google Scholar]
- ZHANG X, HUANG S, GAO M, LIU J, JIA X, HAN Q, ZHENG S, MIAO Y, LI S, WENG H, XIA X, DU S, WU W, GUSTAFSSON J. & GUAN Y. 2014. Farnesoid X receptor (FXR) gene deficiency impairs urine concentration in mice. Proc Natl Acad Sci U S A, 111, 2277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO L, HU P, ZHOU Y, PUROHIT J. & HWANG D. 2011. NOD1 activation induces proinflammatory gene expression and insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab, 301, E587–98. [DOI] [PubMed] [Google Scholar]
- ZHAO L, ZHANG F, DING X, WU G, LAM YY, WANG X, FU H, XUE X, LU C, MA J, YU L, XU C, REN Z, XU Y, XU S, SHEN H, ZHU X, SHI Y, SHEN Q, DONG W, LIU R, LING Y, ZENG Y, WANG X, ZHANG Q, WANG J, WANG L, WU Y, ZENG B, WEI H, ZHANG M, PENG Y. & ZHANG C. 2018. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science, 359, 1151–1156. [DOI] [PubMed] [Google Scholar]
- ZHENG X, CHEN T, JIANG R, ZHAO A, WU Q, KUANG J, SUN D, REN Z, LI M, ZHAO M, WANG S, BAO Y, LI H, HU C, DONG B, LI D, WU J, XIA J, WANG X, LAN K, RAJANI C, XIE G, LU A, JIA W, JIANG C. & JIA W. 2021. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab, 33, 791–803.e7. [DOI] [PubMed] [Google Scholar]
- ZHOU YJ, LIU C, LI CL, SONG YL, TANG YS, ZHOU H, LI A, LI Y, WENG Y. & ZHENG FP 2015. Increased NOD1, but not NOD2, activity in subcutaneous adipose tissue from patients with metabolic syndrome. Obesity (Silver Spring), 23, 1394–400. [DOI] [PubMed] [Google Scholar]
- ZHOU YJ, ZHOU H, LI Y. & SONG YL 2012. NOD1 activation induces innate immune responses and insulin resistance in human adipocytes. Diabetes Metab, 38, 538–43. [DOI] [PubMed] [Google Scholar]
- ZMORA N, ZILBERMAN-SCHAPIRA G, SUEZ J, MOR U, DORI-BACHASH M, BASHIARDES S, KOTLER E, ZUR M, REGEV-LEHAVI D, BRIK RB, FEDERICI S, COHEN Y, LINEVSKY R, ROTHSCHILD D, MOOR AE, BEN-MOSHE S, HARMELIN A, ITZKOVITZ S, MAHARSHAK N, SHIBOLET O, SHAPIRO H, PEVSNER-FISCHER M, SHARON I, HALPERN Z, SEGAL E. & ELINAV E. 2018. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell, 174, 1388–1405 e21. [DOI] [PubMed] [Google Scholar]