Abstract
Background:
Given ongoing donor shortages, appropriate patient selection for dual-organ transplantation is critical. We evaluated outcomes of heart retransplant with simultaneous kidney transplant (HRT-KT) versus isolated heart retransplant (HRT) across varying levels of renal dysfunction.
Methods:
The United Network for Organ Sharing database identified 1189 adult patients undergoing heart retransplantation between 2005 and 2020. Recipients undergoing HRT-KT (n=251) were compared to those undergoing HRT (n=938). The primary outcome was five-year survival; subgroup analyses and multivariable adjustment were performed utilizing the following three estimated glomerular filtration (eGFR) groups: < 30mL/min/1.73m2, 30–45mL/min/1.73m2, and > 45mL/min/1.73m2.
Results:
HRT-KT recipients were older and had longer waitlist times, longer inter-transplant periods, and lower eGFR levels. HRT-KT recipients were less likely to require pre-transplant ventilator (1.2% vs. 9.0%, p<0.001) or ECMO (2.0% vs. 8.3%, p<0.001) support but were more likely to have severe functional limitation (63.4% vs. 52.6%, p=0.001). After retransplantation, HRT-KT recipients had less treated acute rejection (5.2% vs 9.3%, p=0.02) and more dialysis requirement (29.1% vs. 20.2%, p < 0.001) before discharge. Survival at 5-years was 69.1% after HRT and 80.5% after HRT-KT (p<0.001). After adjustment, HRT-KT was associated with improved 5-year survival among recipients with eGFR < 30 ml/min/1.73m2 (HR:0.42, 95% CI: 0.26–0.67) and 30–45 ml/min/1.73m2 (HR:0.29, 95% CI 0.13–0.65), but not among those with eGFR>45 ml/min/1.73sm2 (HR 0.68, 95% CI 0.30–1.54).
Conclusion:
Simultaneous kidney transplantation is associated with improved survival following heart retransplantation in patients with eGFR < 45mL/min/1.73m2 and should be strongly considered to optimize organ allocation stewardship.
INTRODUCTION
Patients undergoing heart retransplantation are traditionally considered a higher-risk population due to prior sternotomy, higher sensitization status, potentially greater acuity at the time of transplantation, and increased comorbidities [1–4]. These patients are also at increased risk of developing significant renal dysfunctions due to the nephrotoxicity related to chronic immunosuppression in addition to the presence of end-stage heart failure [5]. Consequently, simultaneous kidney transplantation may be indicated at the time of heart retransplantation.
The utilization of simultaneous heart-kidney transplantation has experienced a 2.5-fold increase over the preceding decade, accounting for approximately 7% of all heart transplants in 2018 [6]. Appropriate patient selection remains a significant challenge, leading to substantial practice variation [7]. To address uncertainty surrounding organ allocation, a 2019 multi-disciplinary conference met to establish consensus guidelines, which favor consideration for simultaneous heart-kidney transplantation in patients with established intrinsic renal disease with estimated glomerular filtration rate (eGFR) < 45mL/min/1.73m2 [8]. A more recent proposal by the Organ Procurement and Transplant Network (OPTN) recommended a more stringent eGFR cutoff (< 30mL/min/1.73m2) for simultaneous heart-kidney transplantation, casting further uncertainty on future recipient eligibility.
A previous study comparing isolated heart retransplantation to simultaneous heart-kidney retransplantation from 1987 to 2011 demonstrated improved survival with simultaneous kidney transplant in recipients with eGFR < 30mL/min/1.73m2 or dialysis dependency [9]. However, there is a lack of contemporary data regarding the potential benefit of simultaneous kidney transplant in heart retransplant recipients, especially among those with less severe renal dysfunction (eGFR > 30mL/min/1.73m2). We therefore proposed to examine the effect of concomitant kidney transplant in heart retransplant recipients across the spectrum of renal dysfunction.
MATERIAL AND METHODS
Data Source
A retrospective analysis was performed utilizing the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research files, updated as of June 30, 2022. From the thoracic transplant recipient file, we identified all adult patients undergoing cardiac retransplantation between 1/1/2005 and 12/31/2020, after excluding patients undergoing simultaneous solid-organ transplantation other than kidney. Patients were stratified according to those who underwent isolated heart retransplantation (HRT) or simultaneous heart-kidney retransplantation (HRT-KT). The thoracic transplant follow-up file was utilized to identify chronic dialysis dependency following heart retransplantation. The kidney transplant file was utilized to analyze the kidney waitlist and transplantation events following heart retransplantation in these patients.
Recipient and donor baseline characteristics and patient outcomes were defined according to standard UNOS definitions. Estimated glomerular filtration rate (eGFR) was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation utilizing recipient creatinine level at the time of transplantation [10]. We categorized recipients into the following groups based on the Kidney Disease: Improving Global Outcomes (KDIGO) classification scheme: eGFR > 45mL/min/1.73m2 (Stages G1 – G3a), eGFR 30–45 mL/min/1.73m2 (Stage G3b), and eGFR < 30mL/min/1.73m2 (Stages G4 and G5) [11, 12]. Functional status was classified using the Karnofsky Performance Scale Index. Donor-recipient size mismatch was determined utilizing a previously validated formula for predicted heart mass which incorporates age, sex, height, and weight [13]. This study was approved by the Institutional Review Board at Cedars-Sinai Medical Center, with a waiver of informed consent (protocol ID: STUDY00001188, approval date 2/19/2021). The study is also in compliance with the International Society for Heart and Lung Transplantation (ISHLT) ethics statement.
Primary and Secondary Outcomes
The primary outcome was post-transplant survival truncated at 5 years, with patients censored at death, retransplant, or final follow-up date. Secondary outcomes included in-hospital complications (treated acute rejection episodes, stroke, new dialysis requirement, permanent pacemaker placement) and length of stay. Short-term (30-day and 90-day) mortality was also analyzed. Non-fatal 5-year secondary outcomes that were also examined included becoming chronic dialysis dependent, becoming waitlisted for a kidney transplant, and receiving a subsequent kidney transplant. The median follow-up time was 4.5 years (interquartile range (IQR) 1.8–8.4 years).
Statistical Analysis
Baseline recipient and donor characteristics were reported as mean +/− standard deviation or median (IQR) based on distribution for continuous variables and proportions for categorical variables. Between-groups comparisons were performed utilizing Student’s t test or Wilcoxon rank sum test for continuous variables depending on distribution. Pearson’s chi-squared test was utilized for categorical variables. Unadjusted 5-year survival was analyzed using the Kaplan-Meier method and compared between strata using the log-rank test. Competing risk analyses were performed for non-fatal secondary outcomes of chronic dialysis dependency, being waitlisted for a kidney, and kidney transplantation following heart transplantation with death as a competing risk event, and cumulative incidences of each event were compared between strata via Gray’s test.
To evaluate the independent effect of concomitant kidney transplant at the time of heart retransplantation, a multivariable Cox regression model for 5-year mortality was constructed for all patients undergoing heart retransplantation following initial univariate Cox regression analysis for each covariate included. Covariates included in the model were chosen a-priori based on clinical relevance. These included recipient characteristics (age, sex, body mass index (BMI), race, diabetes, cerebrovascular disease, malignancy, inotrope dependence, mechanical circulatory support, location prior to transplant, functional status, dialysis prior to transplant, waitlist duration, interval between prior and current heart transplant) and donor characteristics (age, diabetes, hypertension, sex-mismatch, size mismatch, inotropic support, ejection fraction < 50%, and ischemic time). Given the impact of pre-transplant renal function on the risk/benefit ratio of concomitant kidney transplant, an interaction term was created between type of retransplantation (simultaneous heart-kidney versus isolated heart) and eGFR (< 30mL/min/1.73m2, 30–45mL/min/1.73m2, > 45mL/min/1.73m2). The clustering of patients within each transplant center was accounted for using a robust variance estimator. Martingale residuals were used to check the proportional hazards assumption, and covariates that violated the assumption were addressed via stratification. All tests were two-tailed with an alpha level of 0.05. Statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, North Carolina).
Missing Data
Missing data are represented in Table 1 as total number (n) of patients with available data for each variable if not completely captured. For multivariable models, single imputation was used for variables with < 5% missing data; values were imputed to the most common category for categorical covariates and to the median or sub-group specific median for continuous covariates. For in-hospital outcomes, cases with missing data were treated as if the outcome of interest did not occur. Included variables and outcomes with missing data are reported in Table S1.
Table 1:
Baseline patient characteristics
| Heart-Kidney Retransplant (n=251) | Isolated Heart Retransplants (n=938) | p-value | |
|---|---|---|---|
|
| |||
| Recipient Characteristics | |||
|
| |||
| Age (years) | 47 (35–58) | 44 (28–57) | 0.02 |
|
| |||
| Male sex | 59.8% (150) | 64.1% (601) | 0.21 |
|
| |||
| Body mass index (kg/m2), n=1187 | 25.0 (21.3–28.4) | 26.3 (22.8–29.8) | 0.001 |
|
| |||
| Race | |||
| White | 58.6% (147) | 69.6% (653) | < 0.001 |
| Black | 26.3% (66) | 15.4% (144) | |
| Hispanic | 10.8% (27) | 10.8% (101) | |
| Others | 4.4% (11) | 4.3% (40) | |
|
| |||
| Indication for retransplant | 0.09 | ||
| Acute rejection | 1.6% (4) | 4.1% (38) | |
| Chronic rejection | 15.9% (40) | 12.5% (117) | |
| Cardiac allograft vasculopathy | 57.8% (145) | 54.5% (511) | |
| Primary graft failure | 6.0% (15) | 9.2% (86) | |
| Other | 18.7% (47) | 19.8% (186) | |
|
| |||
| Diabetes | 33.1% (83) | 23.4% (219) | 0.002 |
|
| |||
| Cerebrovascular disease | 7.6% (19) | 3.6% (34) | 0.02 |
|
| |||
| Prior malignancy | 11.2% (28) | 11.0% (103) | 0.60 |
|
| |||
| Creatinine (mg/dL), n=1183 | 2.7 (1.9–4.0) | 1.3 (1.0–1.8) | < 0.001 |
|
| |||
| Estimated GFR (mL/min/1.73m2), n=1183 | 24.7 (15.2–33.2) | 55.1 (39.3–74.8) | < 0.001 |
|
| |||
| Estimated GFR (mL/min/1.73m2) groups, n=1183 | |||
| eGFR < 30 mL/min/1.73m2 | 64.1% (161) | 11.3% (106) | < 0.001 |
| eGFR 30–45 mL/min/1.73m2 | 21.9% (55) | 22.1% (207) | |
| eGFR > 45 mL/min/1.73m2 | 13.9% (35) | 66.6% (625) | |
|
| |||
| Dialysis after listing | 41.4% (104) | 8.6% (81) | < 0.001 |
|
| |||
| Total bilirubin (mg/dL), n=1176 | 0.7 (0.4–1.0) | 0.8 (0.5–1.2) | 0.002 |
|
| |||
| Status 1A, 1, or 2 at transplant | 55.4% (139) | 51.1% (479) | 0.22 |
|
| |||
| Mechanical Support | |||
| IABP | 13.2% (33) | 11.2% (105) | 0.39 |
| ECMO | 2.0% (5) | 8.3% (78) | < 0.001 |
| VAD/TAH | 7.6% (19) | 8.9% (83) | 0.52 |
|
| |||
| Time since previous transplant (years) | 12.2 (6.9–18.3) | 9.7 (4.1–15.2) | < 0.001 |
|
| |||
| Retransplant within 1 year of prior transplant | 2.0% (5) | 10.5% (98) | < 0.001 |
|
| |||
| Heart waitlist duration (days) | 105 (39–265) | 66 (19–231) | < 0.001 |
|
| |||
| Chronic steroid use | 51.4% (129) | 53.6% (503) | 0.80 |
|
| |||
| Transfusions after listing | 21.5% (54) | 22.5% (211) | 0.81 |
|
| |||
| Mechanical ventilation | 1.2% (3) | 9.0% (84) | < 0.001 |
|
| |||
| Inotropic support | 44.6% (112) | 44.4% (416) | 0.94 |
|
| |||
| Location before transplant | 0.005 | ||
| Hospitalized, ICU | 43.8% (110) | 42.5% (399) | |
| Hospitalized, non-ICU | 22.3% (56) | 14.8% (139) | |
| Home | 33.9% (85) | 42.6% (400) | |
|
| |||
| Functional status | 0.001 | ||
| Mild limitation | 8.8% (22) | 16.4% (154) | |
| Moderate limitation | 21.9% (55) | 27.1% (254) | |
| Severe limitation | 63.4% (159) | 52.6% (493) | |
| Unknown | 6.0% (15) | 3.9% (37) | |
|
| |||
| Donor characteristics | |||
|
| |||
| Age (years) | 30 (22–39) | 29.5 (22–39) | 0.72 |
|
| |||
| Sex mismatch | 31.1% (78) | 29.1% (273) | 0.54 |
|
| |||
| Body mass index (kg/m2) | 25.8 (22.8–29.3) | 25.8 (22.5–29.6) | 0.94 |
|
| |||
| Race: White | 62.2% (156) | 59.8% (561) | 0.50 |
|
| |||
| Diabetes | 4.0% (10) | 2.6% (24) | 0.43 |
|
| |||
| Hypertension | 10.0% (25) | 11.4% (107) | 0.18 |
|
| |||
| Size mismatch (donor/recipient PHM ratio <0.86) | 11.6% (29) | 14.5% (136) | 0.23 |
|
| |||
| LVEF < 50% | 2.0% (5) | 2.4% (22) | 0.74 |
|
| |||
| Inotrope support at procurement, n=1186 | 43.4% (109) | 46.2% (433) | 0.48 |
|
| |||
| High-risk per PHS criteria | 21.5% (54) | 17.8% (167) | 0.18 |
|
| |||
| Total ischemic time (hours), n=1172 | 3.2 (2.5–3.9) | 3.3 (2.5–4.0) | 0.15 |
RESULTS
Patient Cohort and Characteristics
Of the 1189 patients undergoing heart retransplantation, 251 (21.1%) underwent simultaneous heart-kidney retransplant and 938 (78.9%) underwent isolated heart retransplant. Across the study period, there was increased utilization of simultaneous heart-kidney retransplant (Figure 1): in 2005, simultaneous heart-kidney retransplant accounted for 11.8% (8/68) of all cardiac retransplants compared to 37.0% (37/100) of all cardiac retransplants in 2020 (p < 0.001).
Figure 1:
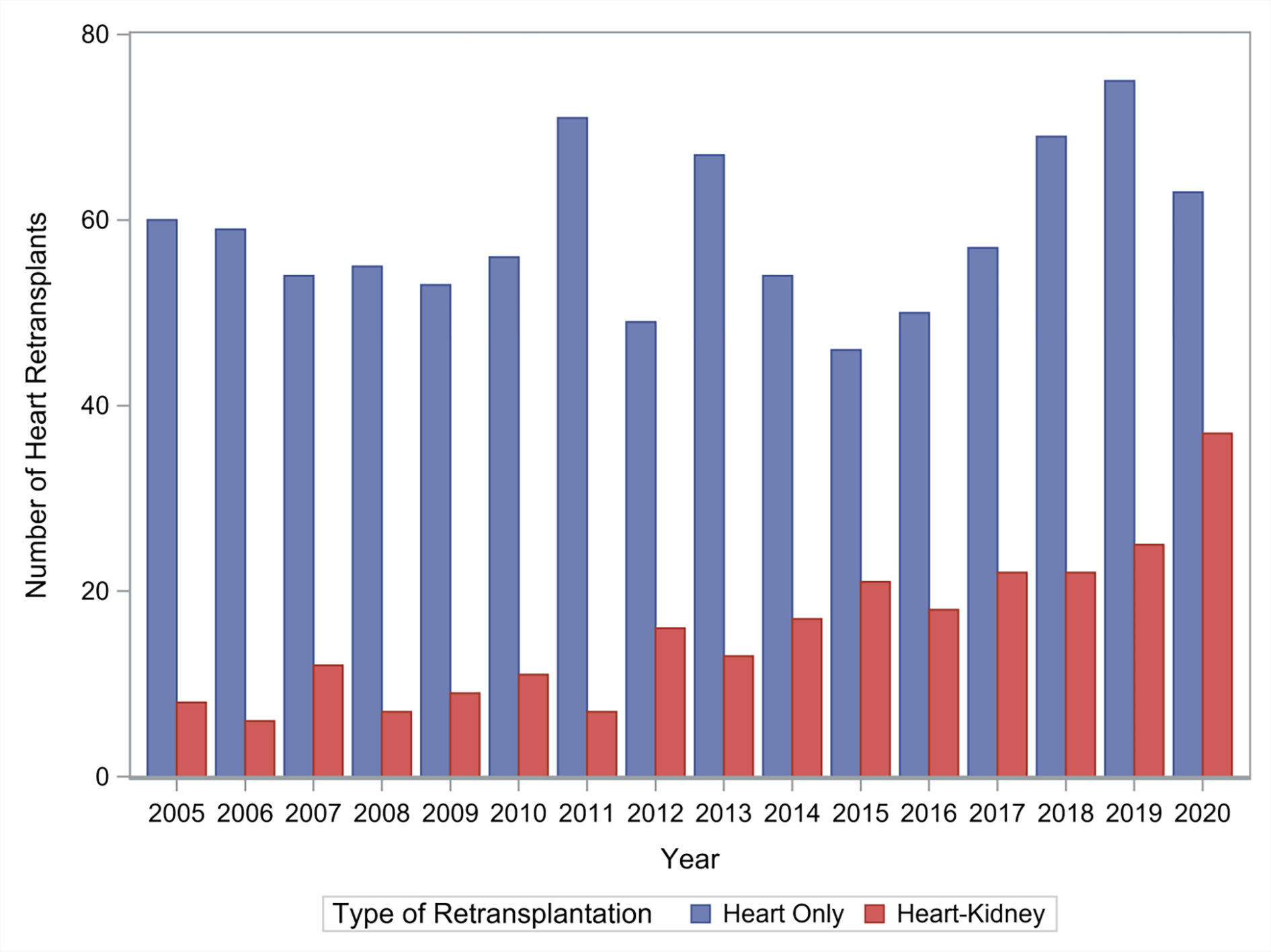
Trends in simultaneous heart-kidney utilization in heart retransplantation
Baseline recipient and donor characteristics are presented in Table 1. The most frequent primary indication for cardiac retransplantation was cardiac allograft vasculopathy. Notable differences existed between recipients of simultaneous heart-kidney versus isolated heart retransplant. Simultaneous heart-kidney recipients were older (median age 47 years, IQR:35–58 years vs. median age 44 years, IQR: 28–57 years, p=0.02), more frequently black (26.3% vs. 15.4%, p<0.001), had a longer inter-transplant period (median 12.2 years, IQR:6.9–18.3 years vs. median 9.7 years, IQR:4.1–15.2 years, p<0.001), and had longer waitlist durations (median 105 days, IQR:39–265 vs. median 66 days, IQR:19–231 days, p<0.001). Simultaneous heart-kidney recipients had a higher incidence of diabetes (33.1% vs. 23.4%, p=0.002) and cerebrovascular disease (7.6% vs. 3.6%, p=0.02); they required less preoperative ECMO (2.0% s. 8.3%, p<0.001) and mechanical ventilation (1.2% vs. 9.0%, p<0.001) but were more frequently severely functionally limited (63.4% vs. 52.6%, p=0.001). Donor characteristics between cohorts were similar.
Post-transplant Outcomes
In-hospital, short-term survival, and non-fatal secondary outcomes at 5-years are depicted in Table 2. Simultaneous heart-kidney recipients were less likely to experience treated acute rejection (5.2% vs. 9.4%, p=0.04) but were more likely to require new dialysis (29.1% vs. 20.2%, p=0.002). Length of stay was longer for simultaneous heart-kidney recipients (median 19 days vs. 14 days, p<0.001). Simultaneous heart-kidney recipients had improved survival at 30- and 90-days following transplantation.
Table 2:
In-hospital outcomes, short-term mortality, and nonfatal secondary outcomes after heart-retransplantation
| Heart-Kidney Retransplant (n=251) | Isolated Heart Retransplant (n=938) | p-value | |
|---|---|---|---|
| In-hospital outcomes | |||
| Treated acute rejection | 5.2% (13) | 9.3% (87) | 0.04 |
| Stroke | 3.6% (9) | 3.5% (33) | 0.96 |
| New dialysis requirement | 29.1% (73) | 20.2% (189) | 0.002 |
| Permanent pacemaker implant | 2.8% (7) | 2.6% (24) | 0.84 |
| Length of stay (days) | 19 (IQR:13–32) | 14 (IQR:10–26) | <0.001 |
| Short-term mortality | |||
| 30-day mortality | 3.2% (8) | 8.2% (77) | 0.006 |
| 90-day mortality | 5.2% (13) | 11.5% (108) | 0.003 |
| Non-fatal secondary outcomes at 5-year follow-up | |||
| Chronic dialysis dependency | 8.3% (4.3–13.9) | 14.6% (8.2–22.9) | 0.19 |
| Subsequent kidney waitlist | 2.4% (0.1–5.2) | 4.2% (2.9–5.8) | 0.31 |
| Subsequent kidney transplant | 0.5% (0.1–2.7) | 1.9% (1.1–3.1) | 0.18 |
All presented as % (n), median (IQR), or % (95% CI)
Unadjusted post-transplant survival is presented in Figure 2. Survival at 1, 3, and 5 years was 91.8% (95% CI: 87.6–94.6%), 85.0% (95% CI: 79.5–89.0%), and 80.5% (95% CI: 74.4–85.4%) in simultaneous heart-kidney recipients and 83.1% (95% CI: 80.5–85.3%), 74.1% (95% CI: 71.1–76.9%), and 68.9% (95% CI: 65.7–72.0%) in isolated heart retransplant recipients (p < 0.001). When stratified by pre-transplant eGFR, simultaneous heart-kidney recipients with eGFR < 30mL/min/1.73m2 and eGFR 30–45mL/min/1.73m2 had higher unadjusted 5-year survival, while no survival difference was observed when eGFR > 45mL/min/1.73m2. Unadjusted 5-year survival conditional on 30-day survival as well as 10-year post-transplant survival demonstrated a similar benefit of simultaneous heart-kidney transplantation (Figures S1 and S2). After multivariable Cox adjustment, simultaneous heart-kidney retransplant was associated with reduced risk of 5-year mortality when eGFR < 30mL/min/1.73m2 (HR:0.42, 95% CI: 0.26–0.67) and eGFR 30–45mL/min/1.73m2 (HR:0.29, 95% CI 0.13–0.65), but not when eGFR > 45mL/min/1.73m2 (HR:0.68, 95% CI 0.30–1.54) (Table 3 and Figure 3). Causes of death are demonstrated in Figure 4; simultaneous heart-kidney recipients had a higher incidence of death due to infection (22.0% vs. 9.0%, p=0.01) and malignancy (9.8% vs. 2.6%, p=0.02), while isolated heart recipients had a higher incidence of death due to multiorgan failure (13.4% vs. 2.4%, p=0.04) and a numerically higher incidence of death due to graft failure (14.2% vs. 7.3%, p=0.22) and cardiovascular causes (19.4% vs. 14.6%, p=0.47).
Figure 2:
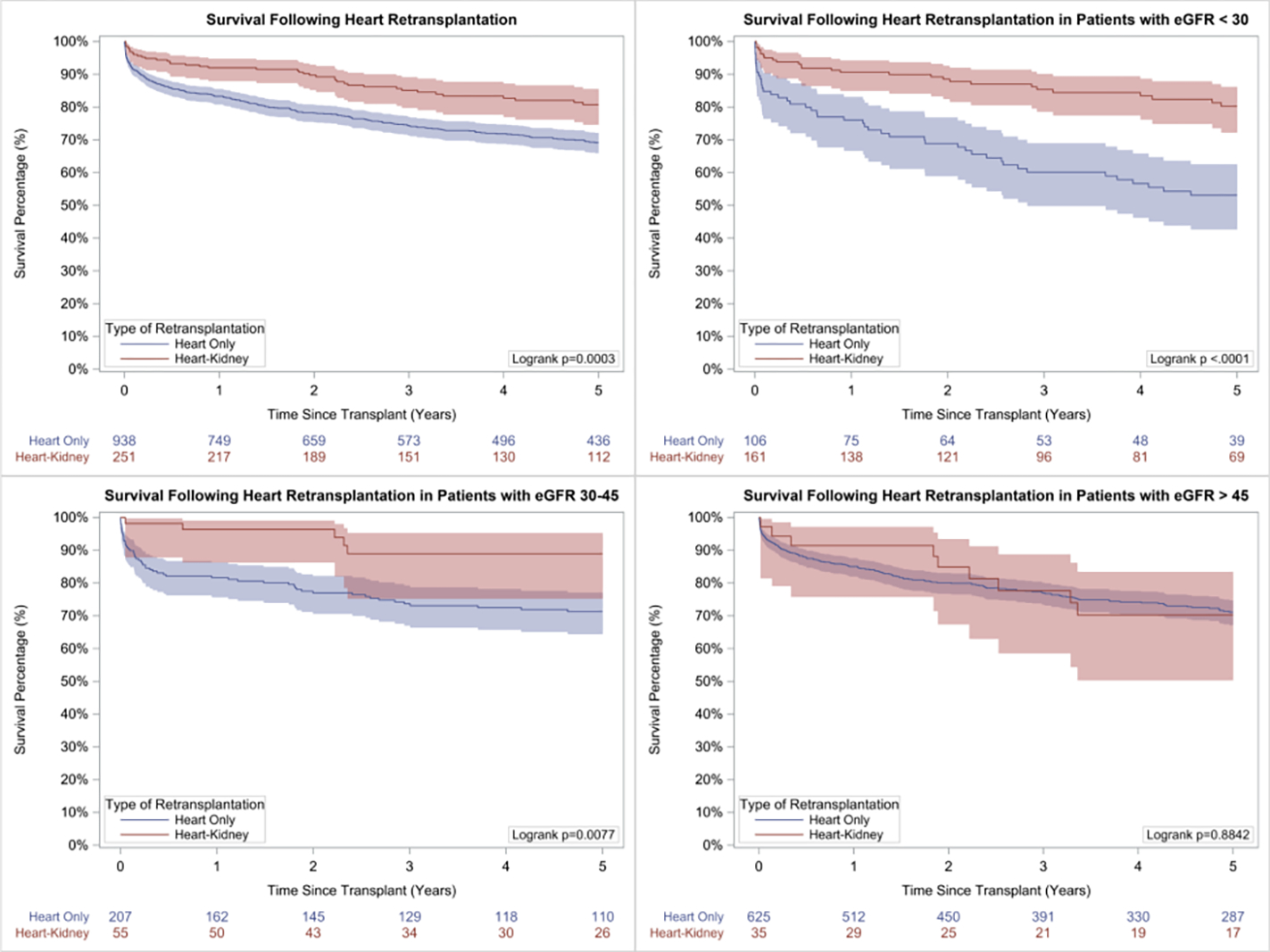
5-Year unadjusted survival following heart retransplantation in A. Entire cohort, B. Recipients with eGFR < 30 mL/min/1.73m2, C. Recipients with eGFR 30–45 mL/min/1.73m2, and D. Recipients with eGFR > 45 mL/min/1.73m2
Table 3:
Univariate and Multivariate Cox-Regression Analyses for 5-Year Mortality Following Heart Retransplantation
| Univariate Cox Analysis | Multivariate Cox Analysis | |||
|---|---|---|---|---|
| Covariate | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI |
| Heart-Kidney a at eGFR < 30mL/min/1.73m2 | 0.34 | 0.21 – 0.53 | 0.42 | 0.26 – 0.67 |
| Heart-Kidney a at eGFR 30–45mL/min/1.73m2 | 0.31 | 0.14 – 0.70 | 0.29 | 0.13 – 0.65 |
| Heart-Kidney a at eGFR > 45mL/min/1.73m2 | 0.95 | 0.44 – 2.08 | 0.68 | 0.30 – 1.54 |
| Ageb | 1.00 | 0.99 – 1.01 | `0.99 | 0.99 – 1.01 |
| BMIc | 1.04 | 1.02 – 1.07 | 1.04 | 1.01 – 1.06 |
| Waitlist durationd | 1.00 | 0.99 – 1.01 | 1.00 | 1.00 – 1.01 |
| Inter-transplant periodb | 0.95 | 0.94 – 0.97 | 0.98 | 0.96 – 0.99 |
| Female sex | 1.21 | 0.95 – 1.56 | 1.16 | 0.89 – 1.51 |
| Recipient race | ||||
| White | reference | reference | reference | reference |
| Black | 1.30 | 1.01 – 1.68 | 1.10 | 0.85 – 1.43 |
| Hispanic | 1.11 | 0.78 – 1.56 | 1.03 | 0.74 – 1.43 |
| Others | 0.96 | 0.59 – 1.57 | 0.92 | 0.55 – 1.53 |
| Status 1A or Adult Status 1, 2 | 1.32 | 1.01 – 1.72 | 0.61 | 0.39 – 0.96 |
| Dialysis prior to transplant | 1.65 | 1.23 – 2.21 | 1.80 | 1.34 – 2.43 |
| Recipient diabetes | 1.37 | 1.07 – 1.74 | 1.26 | 0.97 – 1.64 |
| Recipient cerebrovascular disease | 0.87 | 0.48 – 1.57 | 0.77 | 0.43 – 1.39 |
| Recipient history of malignancy | 0.84 | 0.56 – 1.25 | 0.86 | 0.56 – 1.31 |
| Inotrope dependency | 1.44 | 1.16 – 1.79 | 1.16 | 0.93 – 1.43 |
| Medical condition | ||||
| Home | reference | reference | reference | reference |
| Hospitalized, non-ICU | 1.08 | 0.77 – 1.51 | 1.27 | 0.82 – 1.97 |
| ICU | 1.69 | 1.30 – 2.21 | 1.55 | 0.92 – 2.60 |
| Ventricular assist device | 1.44 | 0.93 – 2.22 | 0.97 | 0.66 – 1.43 |
| Intra-aortic balloon pump | 1.53 | 1.07 – 2.20 | 1.31 | 0.94 – 1.81 |
| Functional Status | ||||
| Mild limitation | reference | reference | reference | reference |
| Moderate limitation | 1.88 | 1.23 – 2.86 | 1.83 | 1.19 – 2.81 |
| Severe Limitation | 2.10 | 1.42 – 3.12 | 1.40 | 0.89 – 2.21 |
| Donor ageb | 1.02 | 1.01 – 1.02 | 1.02 | 1.01 – 1.03 |
| Donor diabetes | 1.12 | 0.63 – 2.00 | 0.95 | 0.48 – 1.90 |
| Donor hypertension | 1.62 | 1.18 – 2.21 | 1.38 | 0.95 – 2.01 |
| Sex mismatch | 1.12 | 0.89 – 1.42 | 1.15 | 0.86 – 1.55 |
| Size mismatch (donor/recipient PHM ratio < 0.86) | 0.77 | 0.50 – 1.16 | 0.58 | 0.38 – 0.89 |
| Donor inotropic support | 0.92 | 0.75 – 1.12 | 0.97 | 0.77 – 1.21 |
| Donor LVEF < 50% | 1.11 | 0.56 – 2.21 | 0.96 | 0.44 – 2.11 |
| Ischemic timee | 1.12 | 1.01 – 1.23 | 1.14 | 1.02 – 1.27 |
Comparisons made to isolated heart retransplant
per 1-year increase
per 1-unit increase;
per 1-day increase
per 1-hour increase
eGFR=estimated glomerular filtration rate; BMI=body mass index; ICU=intensive care unit; PHM=predicted heart mass; LVEF= left ventricular ejection fraction
Figure 3:
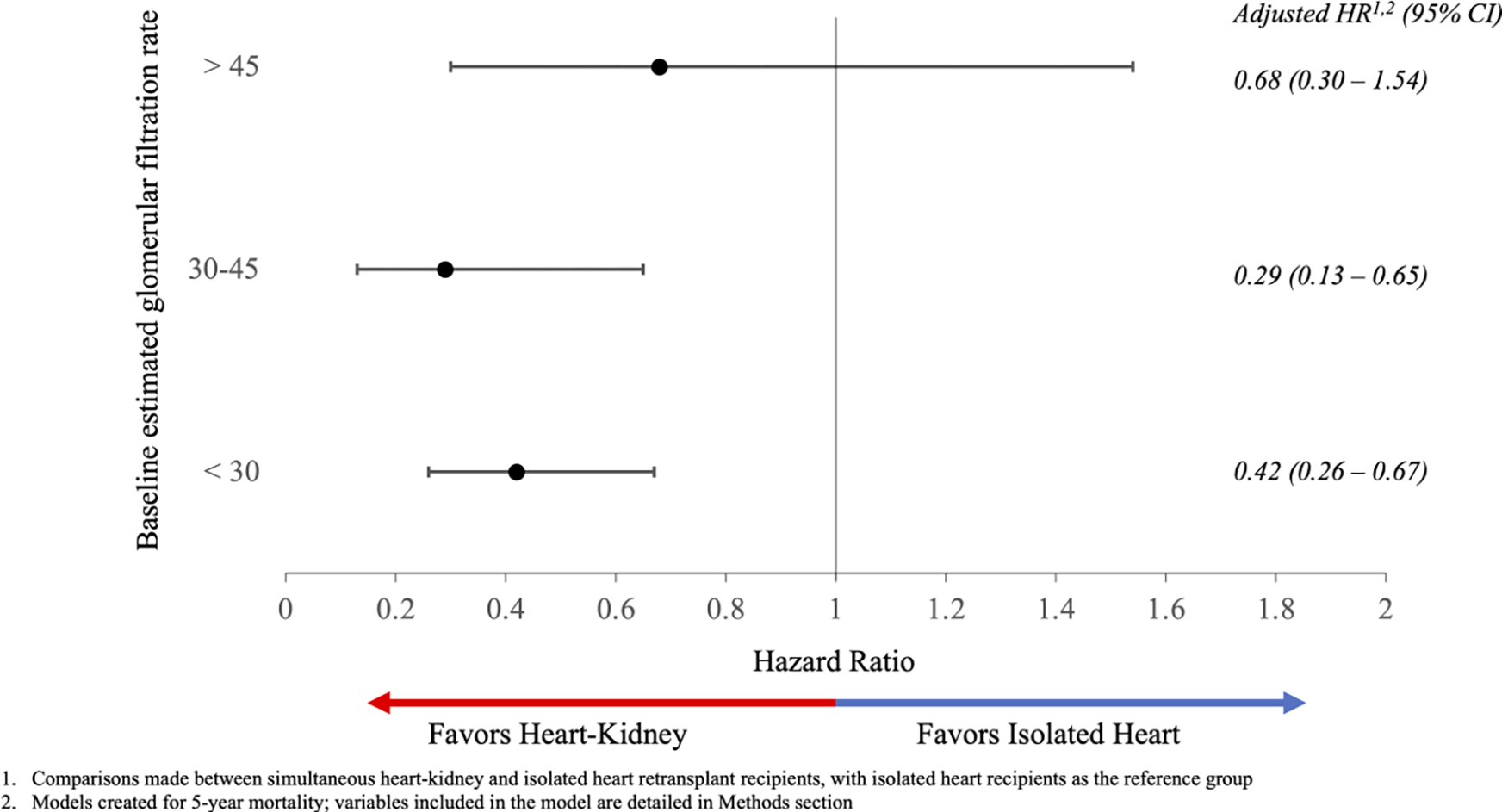
Multivariable Cox-adjusted 5-year mortality following heart retransplantation
Figure 4:
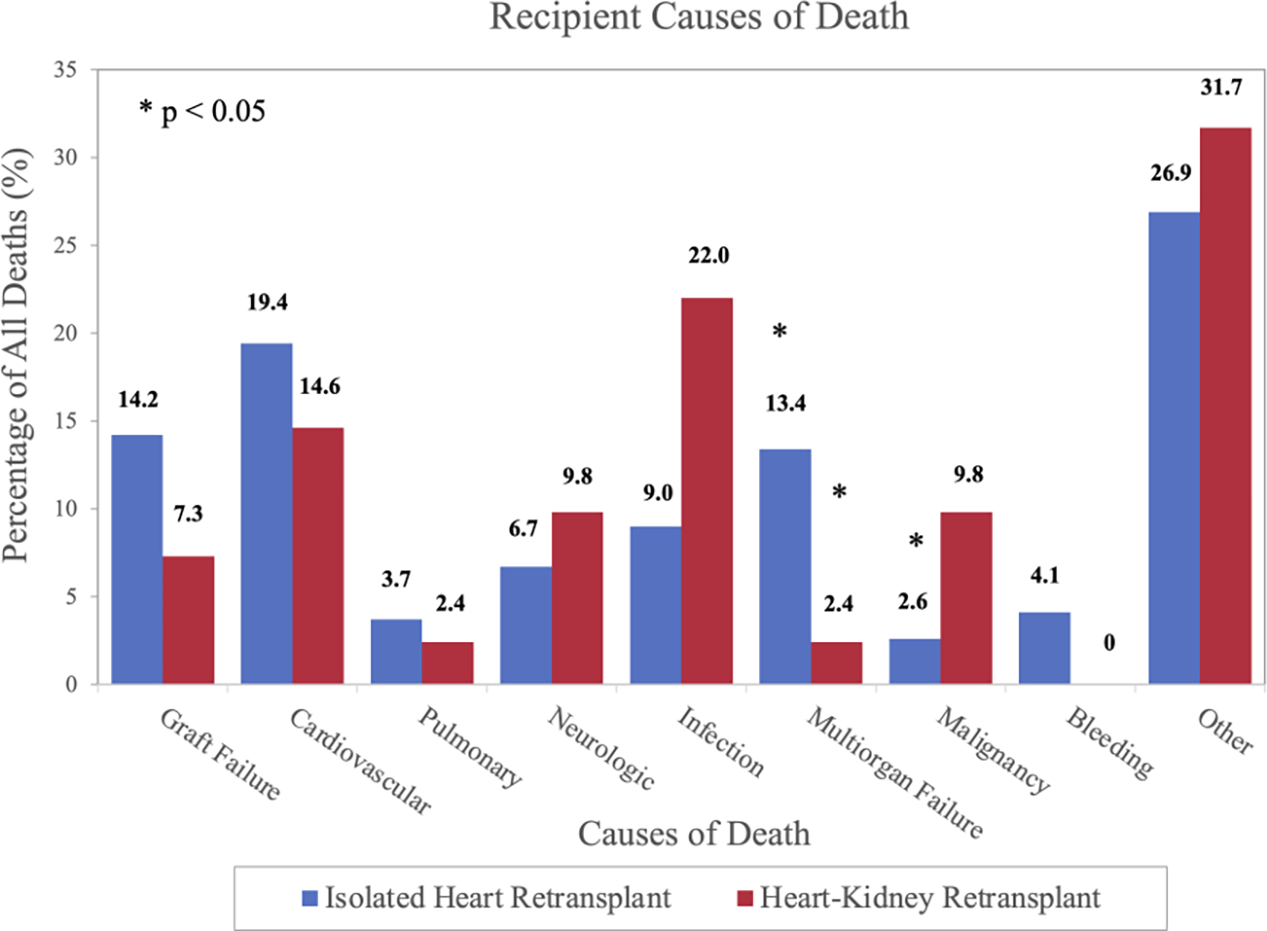
Causes of death after heart retransplantation at 5-years
Isolated heart retransplant recipients had numerically higher cumulative incidences of the nonfatal secondary outcomes of chronic dialysis dependency, subsequent kidney waitlisting, and subsequent kidney transplant (Table 2). Figures S3–S5 demonstrate cumulative incidences of non-fatal secondary outcomes stratified by eGFR group: in recipients with eGFR < 30mL/min/1.73m2, those who underwent isolated heart retransplant had higher incidences of subsequent kidney waitlisting (12.3%, 95% CI: 6.7 – 19.7% vs. 2.0%, 95% CI: 0.5 – 5.4%, p=0.001) and kidney transplant (5.3%, 95% CI: 2.0 – 11.3% vs. 0%, p=0.007); in recipients with eGFR > 45mL/min/1.73m2, those who received simultaneous heart-kidney retransplant had higher incidence of chronic dialysis dependency (10.7%, 95% CI: 2.6 – 25.7% vs. 2.9%, 95% CI: 1.7 – 4.7%, p=0.03).
DISCUSSION
Heart retransplant recipients remain a high-risk cohort of patients, and thus efforts to enhance outcomes are paramount to optimize organ stewardship. Furthermore, patient selection for simultaneous heart-kidney transplantation requires further study to identify groups deriving true benefit from dual organ transplantation to inform practice policies. As such, our analysis of the UNOS database highlights several important findings. First, simultaneous heart-kidney transplantation has been increasingly utilized in heart retransplant recipients, accounting for 37% of all cardiac retransplant cases in 2020. Second, post-transplant survival at 5-years for simultaneous heart-kidney retransplant recipients was 80.5%. This was comparable to the overall 5-year survival of all heart transplant recipients (80.9%) published in the most recent OPTN 2020 annual report [14], suggesting that significant renal disease in patients requiring heart retransplantation should not be a contraindication to retransplantation. Finally, after adjustment, simultaneous heart-kidney transplantation was associated with improved survival at 5-years in retransplant recipients with eGFR < 30mL/min/1.73m2 and eGFR 30–45mL/min/1.73m2, but not in patients with eGFR > 45mL/min/1.73m2.
Heart retransplant with simultaneous kidney transplant has increased dramatically across the study period. Concomitant renal disease is frequently encountered in patients with end-stage heart failure, which portends worse prognosis following isolated heart transplantation [15, 16]. Heart retransplantation candidates are at particularly increased risk for comorbid renal dysfunction due to the nephrotoxicity of chronic immunosuppression [3], and thus may benefit greater from consideration of dual organ transplantation. Indeed, the 2018 International Society for Heart and Lung Transplantation report demonstrated a much stronger preference towards multi-organ transplantation for retransplant recipients as compared to primary heart transplant recipients [17]. Our study further highlights this heightened enthusiasm for multiorgan transplantation and suggests a prominent role for simultaneous heart-kidney transplantation for cardiac retransplant recipients moving forward.
A key finding of this study was the survival benefit associated with simultaneous heart-kidney transplantation in retransplant recipients with eGFR 30–45mL/min/1.73m2, supporting an expanded role of concomitant kidney transplantation as compared to previous studies focused on only those with eGFR < 30mL/min/1.73m2 [9]. This is particularly pertinent in the context of the recent OPTN proposal to limit simultaneous heart-kidney transplantation to those with eGFR < 30mL/min/1.73m2. Under this proposal, those with eGFR 30–45mL/min/1.73m2 would fall under a safety net policy in which recipients would receive priority on the kidney waitlist between 60–365 days following heart transplantation under two conditions: eGFR < 20mL/min/1.73m2 or dialysis-dependency. While initial experience with a similar safety net policy in liver transplantation has demonstrated acceptable results [18], our results suggest that cardiac retransplant candidates with eGFR 30–45mL/min/1.73m2 may be better served from an upfront approach to concomitant renal transplantation.
Evidence-based patient selection is critical in optimizing outcomes and avoiding futile transplantations, especially in the setting of multiorgan transplants. Critics of heart retransplantation highlight the historically worse outcomes compared to primary transplantation, and understandably question the ethics of allocating multiple limited donor hearts to a single patient. Our findings suggest that selected cardiac retransplant recipients can achieve similar survival to primary heart recipients if underlying renal dysfunction is corrected via simultaneous kidney transplantation [14, 19]. However, this practice will reduce the donor pool of kidneys available to patients with isolated end-stage renal disease. Furthermore, simultaneous heart-kidney transplantation at borderline thresholds of renal dysfunction increases the likelihood of allocating kidneys to patients whose renal function would potentially improve following isolated heart transplantation. As such, the incremental survival benefit to heart retransplant recipients must be weighed against the detrimental reduction in available organs to those with isolated renal disease and the overall potential reduction in life-years gained by kidney transplantation [6, 20].
Limitations
This analysis of outcomes following simultaneous heart-kidney versus isolated heart retransplantation utilizing the UNOS database has several limitations. Most importantly, baseline renal function was calculated utilizing a single creatinine value prior to transplant, as this was the most reliable marker available within the registry. Therefore, we were unable to quantify the chronicity of renal dysfunction and identify potential markers used clinically to determine potential recoverability of kidney function (degree of proteinuria, echogenicity, and size of kidneys). Second, given significant national practice variation and lack of granular data in the UNOS database regarding clinician decision-making, we could not determine the exact indication or justification for performing simultaneous heart-kidney transplantation at any given eGFR level. Third, we were unable to compare outcomes of simultaneous heart-kidney transplantation versus sequential heart-kidney transplantation, a strategy that will likely gain traction following the recent OPTN safety net proposal. Fourth, while we performed a robust multivariable adjustment, it is possible that there are unmeasured confounders that impacted our results. Fifth, given the limitations of the UNOS database, we were unable to identify prior heart transplant recipients with advanced graft dysfunction and significant concomitant renal disease who were not considered for retransplantation. Lastly, given the small sample size of patients undergoing simultaneous heart-kidney transplantation with eGFR > 45mL/min/1.73m2, it is possible that our study is underpowered to detect subtle differences in 5-year survival in this subgroup.
CONCLUSION
The utilization of simultaneous heart-kidney transplantation in the setting of cardiac retransplantation has increased dramatically in the United States, accounting for over one-third of all cases in 2020. Significant renal disease in patients requiring heart retransplantation should not be a contraindication to retransplantation, and selected patients with chronic kidney disease and eGFR < 45mL/min/1.73m2 derive a survival benefit from simultaneous kidney transplantation. These findings are particularly important in the context of the recent OPTN policy to establish national guidelines and strict eGFR cutoffs for simultaneous heart-kidney transplant.
Supplementary Material
Acknowledgements
This work was supported in part by Health Resources and Services Administration contract HHSH250–2019-00001C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Financial Disclosure Statement
Jad Malas and Qiudong Chen are both supported by grants from the National Institutes of Health for advanced heart disease research (T32HL116273). The remaining authors have no relevant financial disclosures.
Abbreviations:
- HRT-KT
heart retransplant with simultaneous kidney transplant
- HRT
isolated heart retransplant
- eGFR
estimated glomerular filtration rate
- OPTN
Organ Procurement and Transplant Network
- UNOS
United Network for Organ Sharing
- ISHLT
International Society for Heart and Lung Transplantation
- ECMO
extracorporeal membrane oxygenation
- BMI
body mass index
- CI
confidence interval
- IQR
interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barghash MH, Pinney SP: Heart Retransplantation: Candidacy, Outcomes, and Management. Curr Transplant Rep 2020, 7(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess NR, Hickey GW, Sultan I et al. : Redo orthotopic heart transplantation in the current era. J Thorac Cardiovasc Surg 2021. [DOI] [PubMed] [Google Scholar]
- 3.Lund LH, Edwards LB, Kucheryavaya AY et al. : The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report-- 2014; focus theme: retransplantation. J Heart Lung Transplant 2014, 33(10):996–1008. [DOI] [PubMed] [Google Scholar]
- 4.Tsao L, Uriel N, Leitz K et al. : Higher rate of comorbidities after cardiac retransplantation contributes to decreased survival. J Heart Lung Transplant 2009, 28(10):1072–1074. [DOI] [PubMed] [Google Scholar]
- 5.Khush KK, Hsich E, Potena L et al. : The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult heart transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant 2021, 40(10):1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw BI, Sudan DL, Boulware LE et al. : Striking a Balance in Simultaneous Heart Kidney Transplant: Optimizing Outcomes for All Wait-Listed Patients. Journal of the American Society of Nephrology 2020, 31(8):1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw BI, Samoylova ML, Barbas AS et al. : Center variations in patient selection for simultaneous heart-kidney transplantation. Clin Transplant 2022, 36(5):e14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobashigawa J, Dadhania DM, Farr M et al. : Consensus conference on heart-kidney transplantation. Am J Transplant 2021, 21(7):2459–2467. [DOI] [PubMed] [Google Scholar]
- 9.Savla J, Lin KY, Pradhan M et al. : Heart Retransplant Recipients Have Better Survival With Concurrent Kidney Transplant Than With Heart Retransplant Alone. J Am Heart Assoc 2015, 4(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA : Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010, 55(4):622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Eckardt KU, Tsukamoto Y et al. : Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005, 67(6):2089–2100. [DOI] [PubMed] [Google Scholar]
- 12.Stevens PE, Levin A: Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013, 158(11):825–830. [DOI] [PubMed] [Google Scholar]
- 13.Kransdorf EP, Kittleson MM, Benck LR et al. : Predicted heart mass is the optimal metric for size match in heart transplantation. J Heart Lung Transplant 2019, 38(2):156–165. [DOI] [PubMed] [Google Scholar]
- 14.Colvin M, Smith JM, Ahn Y et al. : OPTN/SRTR 2020 Annual Data Report: Heart. American Journal of Transplantation 2022, 22(S2):350–437. [DOI] [PubMed] [Google Scholar]
- 15.Habib PJ, Patel PC, Hodge D et al. : Pre-orthotopic heart transplant estimated glomerular filtration rate predicts post-transplant mortality and renal outcomes: An analysis of the UNOS database. J Heart Lung Transplant 2016, 35(12):1471–1479. [DOI] [PubMed] [Google Scholar]
- 16.Hong KN, Iribarne A, Worku B et al. : Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg 2011, 92(2):520–527; discussion 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khush KK, Cherikh WS, Chambers DC et al. : The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant 2018, 37(10):1155–1168. [DOI] [PubMed] [Google Scholar]
- 18.Cannon RM, Goldberg DS, Eckhoff DE et al. : Early Outcomes With the Liver-kidney Safety Net. Transplantation 2021, 105(6):1261–1272. [DOI] [PubMed] [Google Scholar]
- 19.Miller RJH, Clarke BA, Howlett JG et al. : Outcomes in patients undergoing cardiac retransplantation: A propensity matched cohort analysis of the UNOS Registry. J Heart Lung Transplant 2019, 38(10):1067–1074. [DOI] [PubMed] [Google Scholar]
- 20.Cheng XS, Khush KK, Wiseman A et al. : To kidney or not to kidney: Applying lessons learned from the simultaneous liver-kidney transplant policy to simultaneous heart-kidney transplantation. Clin Transplant 2020, 34(6):e13878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


