Abstract
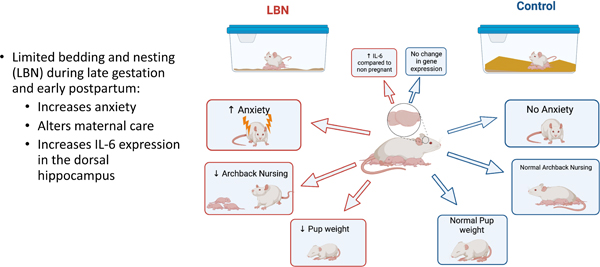
This study examined the effect of limited bedding and nesting (LBN) stress on postpartum anhedonia, maternal behaviors, anxiety-like behaviors, and neuroendocrine and neuroimmune function as a potential model of postpartum depression. Dams underwent sucrose preference tests prior to breeding, during gestation and again postpartum, to examine the potential onset of anhedonia. On embryonic day 19, dams were placed into either a limited bedding and nesting or control housing condition. Contrary to our predictions, LBN stress had no effect on postpartum sucrose preference. We also found no effect of LBN condition on fecal estradiol or corticosterone levels, both of which increased at birth and decreased postpartum. Regardless of housing conditions, approximately 40% of new mothers exhibited a decrease in sucrose preference, while others show no change, suggesting an individual susceptibility to postpartum anhedonia. In a separate cohort of LBN and Control dams, we measured pup retrieval, hoarding behavior, elevated plus maze (EPM), and marble burying. LBN dams exhibited increased anxiety, associated with decreased time spent in the open arms of the EPM. We also measured a significant increase in IL-6 expression in the dorsal hippocampus and medial prefrontal cortex of postpartum dams compared to non-pregnant dams. These findings suggest that while LBN stress has effects on anxiety and maternal care, it does not induce postpartum anhedonia. Rather, there are inherent differences in susceptibility to anhedonia in individual dams, and future studies should be conducted to better understand individual vulnerability and resilience to postpartum anhedonia.
Keywords: limited bedding and nesting, postpartum depression, anhedonia, anxiety, sucrose preference, IL-6, maternal behavior
Graphical Abstract
This study provides insight into the effects of short-term housing stress on postpartum mood-state and associated hormonal and immune changes.
Introduction
More than 85% of American women will be mothers in their lifetime (Gretchen Livingston et al., 2018). Approximately 85% of new mothers will experience the “baby blues” or feelings of sadness, anxiety, and anhedonia postpartum; yet these symptoms typically only last one to two weeks (Faisal-Cury et al., 2008; Pearlstein et al., 2009). When these symptoms last weeks, months, and sometimes years after birth, it is known as postpartum depression (PPD). PPD affects approximately 15% of new mothers and is associated with more severe symptoms including mood swings, increased anxiety, and social withdrawal (Miller, 2002; Wisner et al., 2013). PPD affects more than just the mental health of the mother but can also alter her ability to care for her baby, resulting in long-term consequences for her child (Brummelte & Galea, 2016; Patel et al., 2012; Schwarz, 2019). Certain risk factors are known to leave mothers more vulnerable to PPD including maternal isolation, negative life events, low socioeconomic status, and age (Gifford et al., 2021). These risk factors suggest that when mothers do not have the necessary resources and support available to them, they are at increased risk of developing PPD (Seyfried & Marcus, 2009). Although the risk factors and the effects of postpartum depression are well-documented, research in the etiology of postpartum depression is inadequate. For example, in an online search of the literature, only 0.01% of depression research mentions peripartum or postpartum; and when examining the preclinical literature, this number drops further. It is clear that research on the biological basis and underlying mechanisms of this devastating disorder need to be examined further.
One reason the postpartum period may not be the focus of preclinical research is because it is assumed that PPD and major depressive disorder (MDD) share a similar etiology. The current Diagnostic and Statistical Manual (DSM-V) classifies PPD as a subset of MDD characterized as “MDD with peripartum onset (American Psychiatric Association, 2013).” This simple lack of distinction between MDD and postpartum depression seems to suggest a shared etiology of the two disorders, which fails to recognize the extremely unique physiological differences in the pregnant/postpartum brain. Pregnancy and parturition are associated with dramatic changes in hormone levels, which have long been thought to underlie postpartum mood changes (Galea et al., 2001; Suda et al., 2008). More recently, alterations to the neuroimmune system and the effect on maternal health have begun to be examined but are still poorly understood (Sherer et al., 2018). This interaction between the neuroendocrine and neuroimmune changes during pregnancy and the postpartum period may reveal potential mechanisms leading to the onset of postpartum mood disorders and depression.
In support of this idea, a growing body of literature establishes a link between inflammation and depression. Pregnancy and maternal experience fundamentally alter the function of the immune system. While few clinical studies have examined associations between PPD and postpartum inflammation, patients with treatment-resistant MDD often show higher levels of inflammatory cytokines (Maes et al., 1997). Researchers have proposed that cytokines may serve as biomarkers for depression and predict treatment outcomes which could be used as a tool to predict the onset and treatment outcomes for PPD as well (Miller, 2002). Pregnancy and maternal experience fundamentally alters the function of the immune system and yet, few clinical studies have shown associations between PPD and postpartum inflammation, though the role of inflammation in the pathogenesis of major depressive disorder is increasingly acknowledged. Most notably, depressive symptoms have been linked to increased interleukin (IL)-6 in circulation in addition to increased levels of other inflammatory cytokines (Lee & Giuliani, 2019).
During pregnancy in particular, the immune system undergoes an adaptive change in function to support the semi-allogenic developing fetus while also trying to protect against pathogens (Robinson & Klein, 2012; Schwarz, 2019; Sherer et al., 2018). During a successful pregnancy, the immune system undergoes three phases: a proinflammatory state, anti-inflammatory state, and a return to the proinflammatory state at birth (Mor et al., 2017). This is followed by the postpartum period in which the body returns to the standard pre-pregnancy immune state, coinciding with significant decreases in hormone levels, as well as parturition-induced inflammation (Bränn et al., 2019). Alterations to the immune and endocrine system may underlie or predispose certain women to developing PPD yet the connections between stress, immune and neuroendocrine function have stayed a relatively under-examined area of research, particularly in preclinical studies.
In rats, we have previously observed significant postpartum anhedonia as measured by a decrease in sucrose preference immediately postpartum, on postnatal day (P) 1, which is accompanied by an increase in IL-4 in the hippocampus and IL-6 in the medial prefrontal cortex (Posillico & Schwarz, 2015). This change in postpartum sucrose preference was reversed by administration of an IL-6 antibody infused into the cerebrospinal fluid (Gomez et al., 2019). Further, increased peripheral IL-1β has been associated with repeated restraint stress during pregnancy and increased time immobile in the forced swim test during the last two days of gestation (O’Mahony et al., 2006). Combined, these studies suggest variations in the expression of inflammatory molecules during gestation, parturition and postpartum may play a role in maternal mood, corresponding with the clinical literature.
Hormones, including those of pregnancy, and the hypothalamic-pituitary-adrenal (HPA) axis are known to influence the function of the immune system (Robinson & Klein, 2012; Straub, 2007). Stress activates the immune system, suppresses neural plasticity and has been implicated in mood disorders (Bale, 2006). Interestingly, the highest predictors of PPD are personal and socio-environmental stressors (Gifford et al., 2021). Trauma, pregnancy complications, socioeconomic status, limited resources and unemployment are all associated with increased risk of PPD (Fok et al., 2012; Mukherjee et al., 2017; Pooler et al., 2013; Seyfried & Marcus, 2009). The Limited Bed and Nesting (LBN) model has been adopted and well-validated with rodents as a naturalistic approach to model a low resource, stressful environment similar to that of lower socioeconomic status conditions in humans (Walker et al., 2017). LBN reduces the amount of bedding in the environment creating an impoverished cage and a stressful environment due to the lack of nest-building material (Walker et al., 2017). To that end, LBN has been used as a method to study early-life stress adversity and its effects on the development of the offspring; and the use of LBN is cited to mimic depressed, severely stressed, abusive, and diminished quality of parental care (Sullivan et al., 2019; Walker et al., 2017). LBN increases depressive phenotypes and disrupts maternal behaviors through increased nest exits, licking, and grooming as well as decreased nest digging and time spent with pups (Pawluski et al., 2021). Using dams in the LBN stress condition and observing their behaviors may be a unique model of postpartum depression that would make it possible to study the physiological and neural mechanisms underlying PPD and enable improved treatment options for this unique disorder.
The present study sought to determine if there was a relationship between experiencing limited bedding and nesting (LBN) stress and the expression of negative maternal behaviors, anhedonia, anxiety, altered neuroimmune and endocrine function in female Sprague-Dawley rats. Our prediction was that inadequate bedding and nesting material would mimic the stressors experienced by some new mothers, including the lack of social support and economic resources (Gifford et al., 2021), thus making it difficult to care for her new pups while simultaneously precipitating postpartum anhedonia and anxiety.
Methods
Figure 1 provides an overview of the methods and experiments presented.
Figure 1. Experimental timeline of the present study.
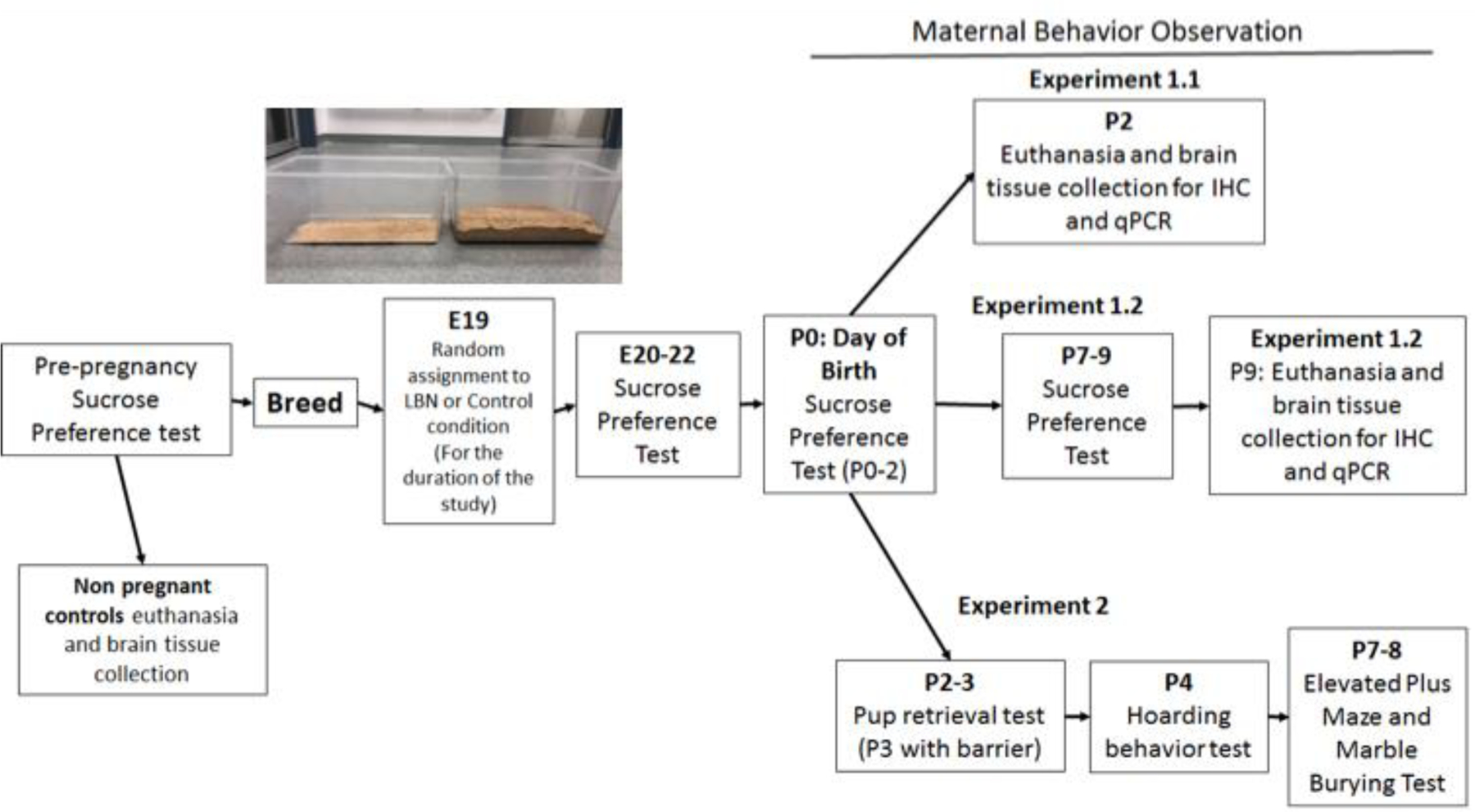
Inset image of LBN cage (left) compared to control cage (right).
Animals
All experiments used female Sprague-Dawley rats obtained from Charles River Laboratories (Wilmington, MA). All animals were housed in clear polypropylene cages (45 cm x 20.5 cm x 24 cm) with ad libitum access to food and water in rooms under a 12:12-hour light:dark cycle (7am on; 7 pm off) and maintained temperature and humidity. All experiments were performed during the light cycle and were in accordance with the Institutional Animal Care and Use Committee of the University of Delaware.
For breeding, each female rat was placed with a single male rat for a maximum of four days and checked daily for the presence of a sperm plug, which determined the day of conception and was assigned as Embryonic (E) Day 1. Day of birth (typically E23) was assigned as Postnatal (P) Day 0.
Limited Bedding and Nesting Procedure
On E19, animals were randomly assigned to one of two conditions: limited bedding and nesting (LBN) or control conditions and remained in these conditions for the duration of the study.. The LBN consisted of 250mL of wood chip bedding material while the control condition consisted of 4000mL of bedding in a standard polypropylene cage (45 cm × 20.5 cm x 24 cm) (see photo within Figure 1). The LBN volumes as based on literature suggesting 1–2cm of bedding (~250mL in our cages) creates an impoverished environment in which the bottom of the cage is just lightly covered (~1–2 cm deep) (Walker et al., 2017). Additional pilot work suggested the control condition volume of 4000mL (~2.5 inches deep) would create an environment which had more bedding than standard cages and provided dams with the ability to a nest within the bedding. Dams in both groups had one nesting square their cage, and all enrichment materials were removed to eliminate any confounding variables other than the amount of bedding present in the cages. The LBN condition cages were changed daily to maintain hygiene, while the control cages were changed once per week to support a stable pup-rearing environment. Animals were routinely handled in both groups for behavioral tests. The animals were maintained in these housing conditions throughout the duration of the study until the endpoints noted in each experiment. This paradigm has been approved by the University of Delaware Institutional Animal Care and Use Committee (IACUC). Both control and LBN litter sizes were comparable with control litters on average 13 pups/litter and LBN litters on average 14 pups/litter.
Groups
Experiment 1 consisted of four groups. Half of the cohort was finished at P2 resulting in a P2 control group and a P2 LBN group. The other half of the cohort was finished at P9 resulting in a P9 control group and P9 LBN group. For immunohistochemistry and qRT-PCR, an additional non pregnant control group was added for comparison to the postpartum animals.
Sucrose Preference Testing (for Experiment 1 and 2)
To measure sucrose preference, 2 bottles (one containing water and the other containing a 1% sucrose solution) were placed on the lid of the home cage for 48 hours. The parameters in this test were piloted in the lab for stable and consistent results. These bottles were weighed at the start of testing, after 24 hours, and again at the 48-hour endpoint. At the 24-hour mark, the 2 bottles were switched to the opposite of the cage to eliminate the possibility of a side preference. The weight of the bottles before and after testing were used to determine the amount of fluid each rat has consumed in grams. From this, the amount of sucrose consumed was divided by the total amount of liquid consumed to obtain a percent sucrose preference score. For example, 50% indicates no preference for the sucrose solution at all or an equal preference for water and sucrose solution, while 100% indicates that the rat exclusively prefers the sucrose solution. Prior to breeding, females were habituated to the conditions of the test and a baseline measure of sucrose preference was established. Sucrose preference was re-tested, over 48 hours, on E20–22, P0-P1 and P7-P8 (Baseline-P2: LBN N=24, Control N=21; P7–9: LBN N=11, Control N=9).
Experiment 1:
Maternal Behavior Observations
Automated recordings were collected at 7:00am during the light cycle, daily from P0 to P9. These videos were scored by manual coding of behaviors noted in minute by minute observations for the thirty-minute recording. Observed behaviors included: Pup-licking, hovering, arched back nursing, blanket nursing, passive nursing, self-grooming, eating, drinking, exploration, sleeping, sleeping away from the pups, pup retrieval, digging/nest building, tail chasing, and stepping on pups. Videos from the morning session (7 am) were scored for days P0, P1, P2, P4, P7, and P8. One cohort of animals was observed from P0-P2 (LBN N=24, Control N=24) and another cohort from P0–9 (LBN N=12, Control N=12).
Fecal Assays for Corticosterone and Estradiol
Fecal samples were collected from animals at a number of time points throughout the study (Baseline – prior to breeding, E18, E20, E22, P0, P2, P4, P6 and P9) (Baseline N=8 E18-P2: LBN N=16, Control N=16; P4: LBN N=8, Control N=8). Fecal samples were collected at 9 am to assess the glucocorticoid circadian peak, which occurs in circulation in the at the beginning of dark cycle and is excreted in feces approximately 12 hours later (Cavigelli et al., 2005; Thanos et al., 2009). Fecal samples were stored at −80℃, dried in a vacuum concentrator (SpeedVac), then steroid metabolites extracted using an ethanol extraction protocol (Cavigelli et al., 2005; Thanos et al., 2009). Fecal extracts were dried and reconstituted with methanol, then diluted in assay buffer to measure estradiol and corticosterone metabolite concentrations using an ELISA (kits from Arbor Assays, Ann Arbor, MI).
Euthanasia, Perfusion, Tissue collection
All euthanized rats were administered an overdose of the barbiturate Euthasol® (ANADA 200–071) via intraperitoneal injection. Sufficient anesthesia was assessed after the rat did not respond to a toe or tail pinch. Once anesthetized, rats were perfused with 0.9% saline solution to remove peripheral blood from the brain tissue, then the brain was split into the two hemispheres. One hemisphere was placed in 4% paraformaldehyde for post fixation and the other microdissected for the medial prefrontal cortex (mPFC) ~Bregma 3.72–3.24, nucleus accumbens (NAcc) ~Bregma 2.76–2.52, medial preoptic area (MPOA) ~Bregma −1.20 - −1.32, ventral hippocampus (vHP) ~Bregma −2.40 - −2.76, dorsal hippocampus (dHP) ~Bregma −5.04 - −5.28, and periaqueductal gray (PAG) ~Bregma −5.64 - −5.88. These regions were collected from brain tissue using the guide of a rat brain atlas along with liver, spleen, and plasma. Half brains were collected from half the cohort on postnatal day 2 with the remaining half being collected on postnatal day 9. Dissected brain regions were extracted using 800ul of Isol-RNA lysis reagent (Trizol) and homogenized. 160 ul chloroform was used separate samples into 3 phases for easy removal of the aqueous RNA phase. 360 ul molecular grade isopropanol was added to form a pellet of RNA which was subsequently washed repeatedly with 70% ethanol to purify. Once extracted, tissue was immediately frozen on dry ice and stored at −80°C until ready for processing.
Real-time quantitative PCR analysis
Dissected brain tissue was used for quantitative real-time PCR analysis of IL-1β (F: GAAGTCAAGACCAAAGTGG, R: TGAAGTCAACTATGTCCCG), IL-6, BDNF (IDT: F: ATCCCATGGGTTACACGAAGGAAG, R: AGTAAGGGCCCGAACATACGATTG) gene expression in the mPFC, vHP, dHP, and MPOA. ER-α (Qiagen, QT00369740) and ER-β (Qiagen, QT01292718), Oxytocin Receptor (OXTR; Qiagen QT00379036), and progesterone receptor (PGR; Qiagen QT00185430) gene expression was also observed in the MPOA. cDNA was synthesized from extracted RNA using the QuantiTect® Reverse Transcription Kit. Real-time PCR was then used to quantify relative gene expression using the RealMasterMix™ Fast SYBR Kit on a CFX96Touch real time PCR machine. GAPDH (F: GTTTGTGATGGGTGTGAACC, R: TCTTCTGAGTGGCAGTGATG) was used as the housekeeping gene as it has exhibited largely stable expression in our lab’s examinations of peripartum inflammation (Gomez et al., 2019; Posillico & Schwarz, 2015). All samples were run in duplicate or triplicate and for each reaction the average quantitative threshold amplification cycle number (Cq) value was determined and the 2- ΔΔCq method, where the ∆Ct is the difference in Ct values for your gene of interest and your housekeeping gene for a given sample, and the ∆∆Ct is the fold-change relative to a calibrator/reference sample(s) (sample(s) with the highest Ct value or the sample with the lowest gene expression). To ensure RNA quality and integrity, all samples were measured on the spectrophotometer and had absorbance ratios at 260/230 that were between 2.0 and 2.2, indicating these samples were pure. The samples were also measured on 260/280 absorbance ratios and only samples that had ratios between 1.9–2.1 were used in analyses. Additionally, no differences across conditions were observed in the expression levels of the housekeeping gene (GAPDH). Melt peak curves are provided in Supplementary Figure 1 to show specificity of the products measured.
ER- α immunohistochemistry
The half brains for immunohistochemistry were placed in 4% paraformaldehyde for 48 hours (replaced with fresh paraformaldehyde at 24 hours) and then transferred to fresh 30% sucrose with 0.1% sodium azide solution daily for 2 consecutive days to dehydrate until slicing. Brains were flash frozen in 2-methylbutane and sliced at 30 microns into 5 series on a Leica CM1950 cryostat. For consistency, brains with less than four sections from the MPOA, inconsistent staining, or tissue damage / folding that make microscopy impossible were removed from analysis. As a result, 18 of the 60 brains were removed. Despite that, we still had a sufficient number of dams in each condition [non-pregnant controls, n=5; P2 controls, n=8; P2 LBN, n=10; P9 controls, n=8; P9 LBN, n=6]. Each of these brains had 5–8 sections representing the MPOA. These sections were stained using anti-estrogen receptor α from Sigma (Cat No. 06–935) and Vector Laboratories goat anti-rabbit biotinylated secondary antibody to visual estrogen receptor (ER)-α. Sections were mounted onto a microscope slide and the MPOA was imaged using Stereo Investigator software. Densitometry staining in the MPOA was analyzed using ImageJ software and integrated area densities and percent area of staining from 6–8 sections were averaged within each animal to determine the density of estrogen receptor α in the MPOA for subsequent statistical analysis between conditions and time points. Figure 9A represents the process of image analysis in ImageJ -the initial 10x microscope image, the image after greyscale and the MPOA region of interest was outlined and finally the thresholded image with cells highlighted.
Figure 9. A. Sample images of MPOA and selection area for densitometry analysis in ImageJ.
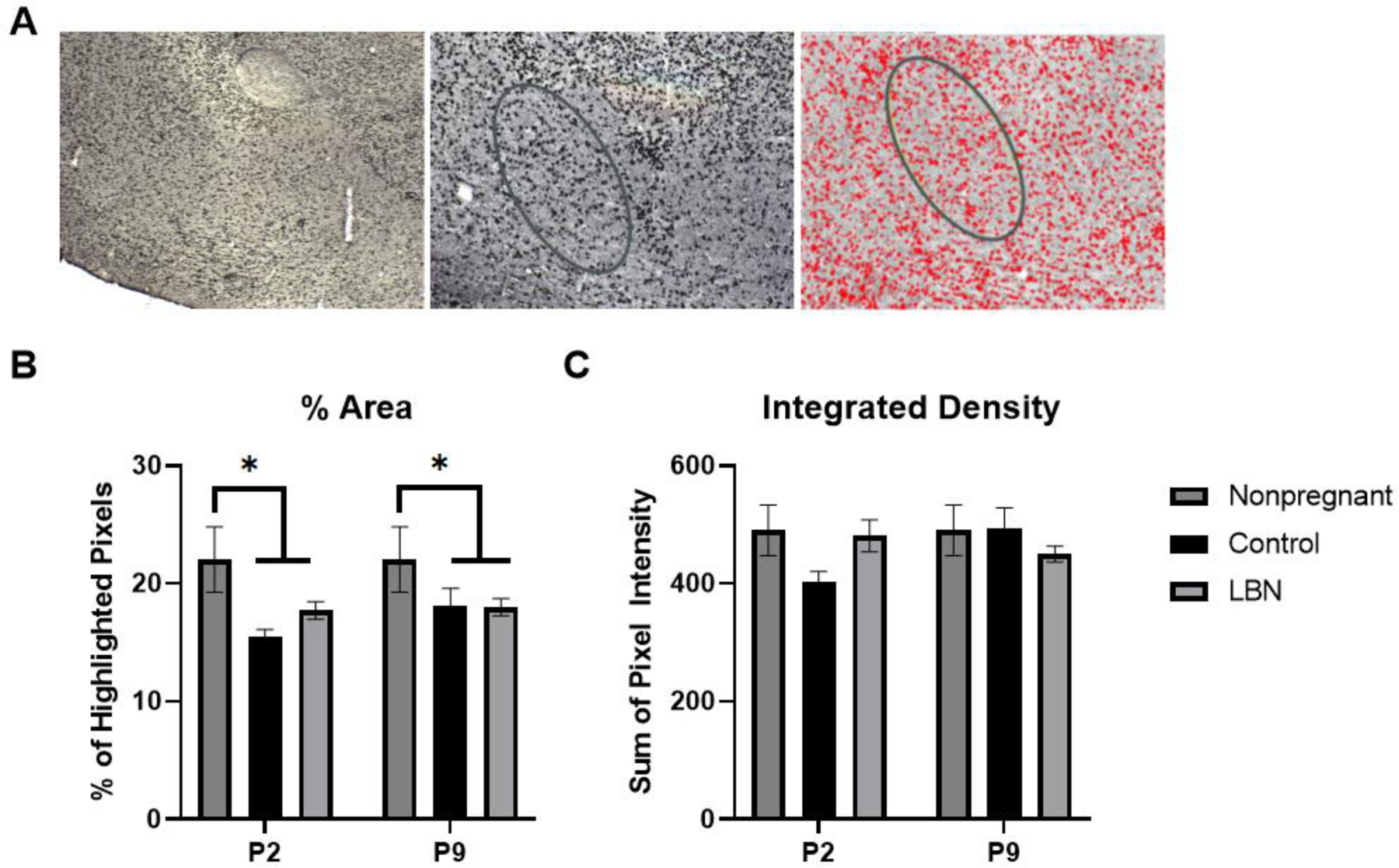
Image 1: 10x microscope image, Image 2: greyscale image created on ImageJ with MPOA region of interest outlined, image 3: image of highlighted cells after threshold and densitometry applied. B. % Area of highlighted ER-α cells within the selected region of interest inside the MPOA. A main effect of condition revealed that the animals of the postpartum conditions (LBN and Control) expressed a significant decrease in the fraction of highlighted pixels within the region of interest in the MPOA (F(2,36) = 6.188, p = 0.0049; *p < 0.05 relative to non-pregnant females at both time points examined). 5–8 sections were analyzed for non-pregnant control (n=5), P2 controls (n=8), P2 LBN (n=10), P9 controls (n=8), P9 LBN (n=6). C. Integrated density of the pixels within the selected region of interest. No main effects were found. * indicate p < 0.05. Error bars represent standard error of the mean (SEM).
Experiment 2:
Pup Retrieval Tests
A separate cohort of animals (LBN=16, Control=13) was used to examine alterations to other maternal behavior and motivation tasks, and anxiety-related behaviors. Another way to observe maternal behavior, other than that described above, is through pup retrieval testing. Two variations of pup-retrieval tests were employed to assess whether LBN stress impacts maternal motivation and behavior. In the first variant of the test, on postnatal day 2 the dam is temporarily removed from the home cage and placed in a small holding chamber. The litter was removed from the cage and 2 pups were placed in each corner of the cage, with the nest corner left empty (6 total pups were used) and any additional pups (on average 6–8 pups) were placed in a holding cage on a heating pad for the 10-minute test. The dam was returned to the home cage and observed for ten minutes, and the length of time for the dam to retrieve the first pup and to retrieve all 6 pups back to the “nest” is recorded, with a maximum of 10 minutes. The second variation of the test occurred on the following day (P3) in which a 4-inch-high cross-shaped Plexiglas barrier in placed into the maternal home cage to separate the cage into 4 quadrants and increase the difficulty of pup retrieval back to the “nest”. As in the first variant, the litter was divided into groups of 2 and placed in 3 corners of the cage and any additional pups (on average 6–8 pups) were placed in a holding cage on a heating pad for the 10-minute test. The dam was then placed in the 4th quadrant and observed for ten minutes. The length of time it took the dam to retrieve the first pup, the time to retrieve her entire litter, and total number of pups retrieved at the end of the test were recorded during the 10-minute session.
Hoarding behavior
Following pup retrieval testing on P2, animals (LBN=14, Control=13) were placed back into their home cage with their litter and a piece of candy (“Snack Size” 0.5oz Tootsie Roll™), which was similar in length to the pups, was placed in each cage. After 6 hours of acclimation, the candy was removed and weighed to determine amount consumed (baseline consumption test). Two days later, on P4, pups were removed from the cage for a 10-minute hoarding behavior test. In this procedure adapted from Numan et al (2005), two candies were placed in the cage in the quadrants diagonally opposite the nest. Time to first interaction with the candy and number of candies returned to the nest were recorded.
Elevated Plus Maze (EPM)
EPM is a common rodent test of anxiety with less time spent in the open arms indicative of anxiety-like behavior. The EPM consists of two runways that cross at a 4-way intersection positioned 50cm above the floors. Two arms, opposite one another, are enclosed by 40cm opaque black walls, while the other two runways are open. On P7 or P8 (counterbalanced with the marble burying test), rats (LBN=7, Control=12) were placed at the center of the intersection and recorded by an overhead camera for 5 minutes using AnyMaze tracking software following a protocol modified from Numan et al. (2004). The number of open-arm entries, time spent in the open arms, and distance traveled were recorded. EPM testing was counterbalanced across animals with the additional anxiety measure, marble burying, described below.
Marble Burying Test
The marble burying task is another test of anxiety with more marbles buried indicative of anxiety-like behavior. This test occurred on P7 or P8, counterbalanced with the EPM test. In this task, each rat (LBN=16, Control=13) was placed in a larger, novel cage (45 cm x 20.5 cm x 24 cm) with 5cm (1.5 L) of evenly flattened bedding. Twenty clean glass marbles were placed in the cage, evenly spaced in five rows of four marbles each prior to the test. Rats were transferred to the test cage with their pups, as pup separation is known to increase the number of marbles buried by dams. After 20 minutes in the test cage, the number of marbles that were buried was recorded. A marble was considered “buried” when it was at least two-thirds covered in bedding. This procedure was adapted from Angoa-Pérez et al. (2013). Marble burying was counterbalanced across animals with the EPM testing.
Statistical Analysis
Sucrose Preference Data.
Due to missing data in the sucrose preference dataset caused by leaky bottles, the multiple imputation method was used to estimate missing values (7 values imputed of 144 total values). Random number generation was set to a starting point of 200,000 using a Mersenne Twister. We created five multiple imputations and constrained the data within the confines of our data set (0–100%). The five imputations were averaged and a new dataset created. This dataset was used to run a repeated measures ANOVA for the P0–2 group as well as the P0–9 group.
Maternal Behavior Data.
Obstructed views of cage recordings occurred due to deep nests, non-visibility of the pups, or poor lighting which interrupted proper data collection. Thus, the multiple imputation method was used to estimate missing values in these cases (21 values imputed of 699 total values). Missing value patterns were assessed to confirm that the data was viable for use with multiple imputation. Random number generation was set to a starting point of 200,000 using a Mersenne Twister. We created five multiple imputations and constrained the data within the confines of our data set (0–30). The five imputations were averaged, and a new dataset created. This dataset was used to run a one-way ANOVA for the P0–2 group as well as a repeated measures ANOVA for the P0–9 group. All statistical tests underwent assumption testing including for homogeneity of variance using Levene’s test as well as for sphericity using Mauchly’s.
Other Data.
Pup retrieval, pup weight, elevated plus maze, and marble burying data were analyzed using a one-way ANOVA to determine differences between conditions. Fecal estradiol and corticosterone metabolite concentrations were analyzed using a two-way ANOVA for time and condition after using a natural log transformation of the data to achieve normality. All statistical tests underwent assumption testing including for homogeneity of variance using Levene’s test. All graphs represent the mean and error bars represent standard error of the mean for the data.
Results
Sucrose Preference Testing
P0–2
A repeated measures ANOVA revealed a main effect of day (F(1,19) = 23.79, p <0.001) but no effect of condition (p>0.05). In this case, both LBN and control displayed a significant decrease in sucrose preference on P0–2 compared to baseline testing (Figure 2).
Figure 2. Sucrose Preference in LBN and Control dams at baseline, E20–22, P0–2 and P7–9.
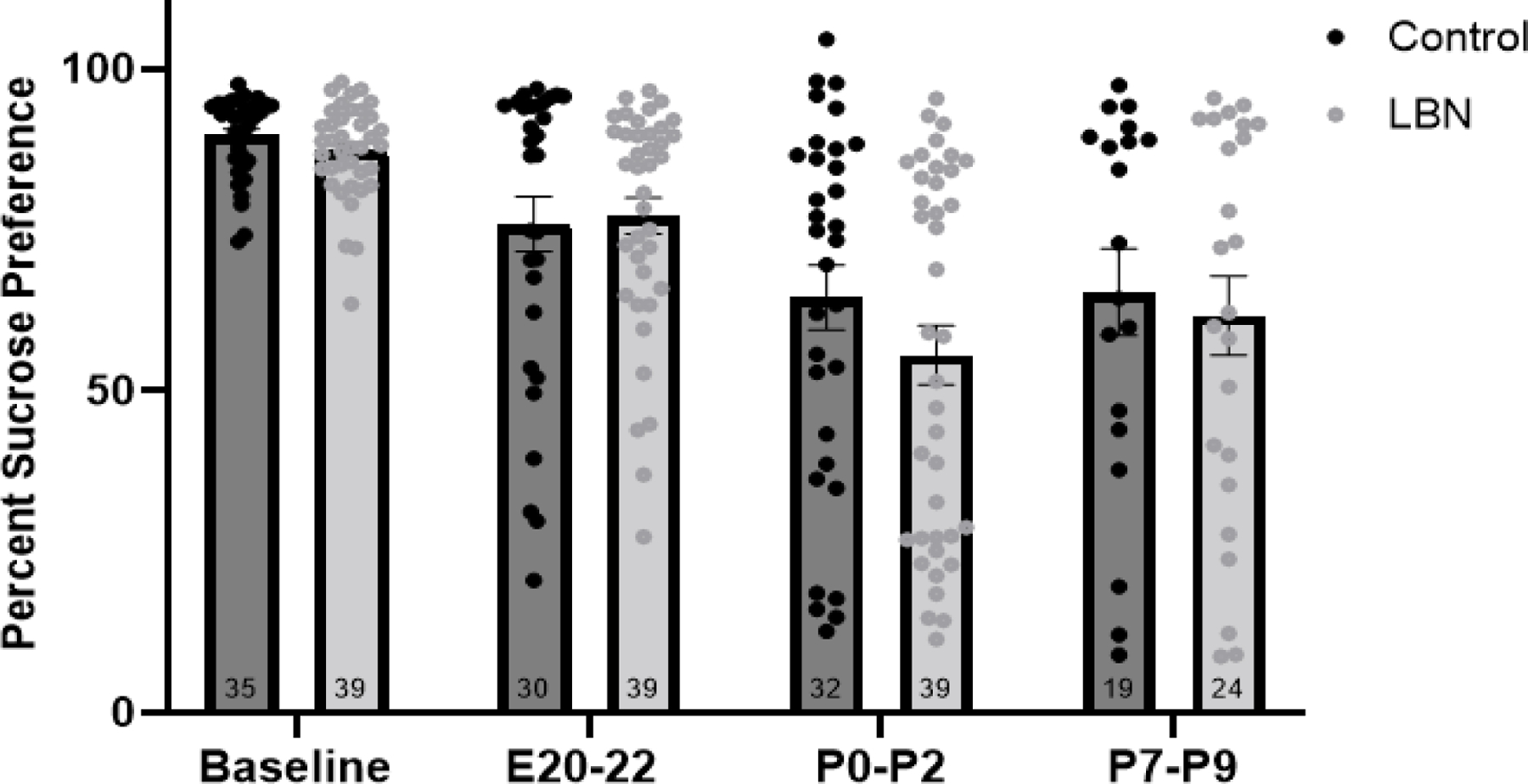
Analysis revealed a decrease in sucrose preference from baseline to P0–2 and P7–9. N/group is listed within the bar. (Baseline-P2: LBN N=24, Control N=21; P7–9: LBN N=11, Control N=9). Error bars represent standard error of the mean (SEM).
P0–9
Repeated measures ANOVA revealed a within subjects effect of day (F(2,34) = 14.868, p<0.001) but no main effect of condition (p>0.05). Both LBN and control animals showed decreased sucrose preference on P0–2 and on P7–9 compared to baseline testing (p<0.05) (Figure 2).
Interestingly, a closer examination of the sucrose preference data revealed that approximately 40% of dams within each condition exhibit postpartum anhedonia at P0–2 and P7–9, defined as a sucrose preference below 70%. Notably, no rats fell below 70% sucrose preference during their baseline testing suggesting that a subset of dams were more susceptible to postpartum anhedonia than their counterparts, within both the LBN and control conditions.
Maternal Behavior Observations
Analysis of maternal behaviors collected from P0-P2 revealed a significant between subjects effect of LBN stress condition for arched-back nursing (F(1,46) = 6.863, p = 0.012) (Figure 3A) and blanket nursing (F(1,46) = 9.576, p = 0.003) (Figure 3B), such that LBN significantly decreased the overall level of arched-back nursing during this time, while increasing simple blanket nursing relative to control dams. Analysis of maternal behaviors collected from P0-P9 revealed a significant between subjects effect of the stress condition for arched-back nursing (F(1,22) = 11.552, p = 0.003) (Figure 3C) and blanket nursing (F(1,22) = 6.416, p = 0.019) (Figure 3D), such that LBN significantly decreased the overall level of arched-back nursing during this time, while increasing simple blanket nursing relative to control dams. Because an interaction between day and condition was found for arched-back nursing in the P0-P9 analysis, a follow up one-way ANOVA was run and revealed a significant difference on P8 (F(1,22) = 10.156, p = 0.004). There were no significant main effects of stress condition on the pup-licking behaviors. As expected, there was a significant effect of day on all of these maternal behaviors, suggesting that the expression of maternal behaviors changes across the postpartum period.
Figure 3. Frequencies of observed maternal behaviors.
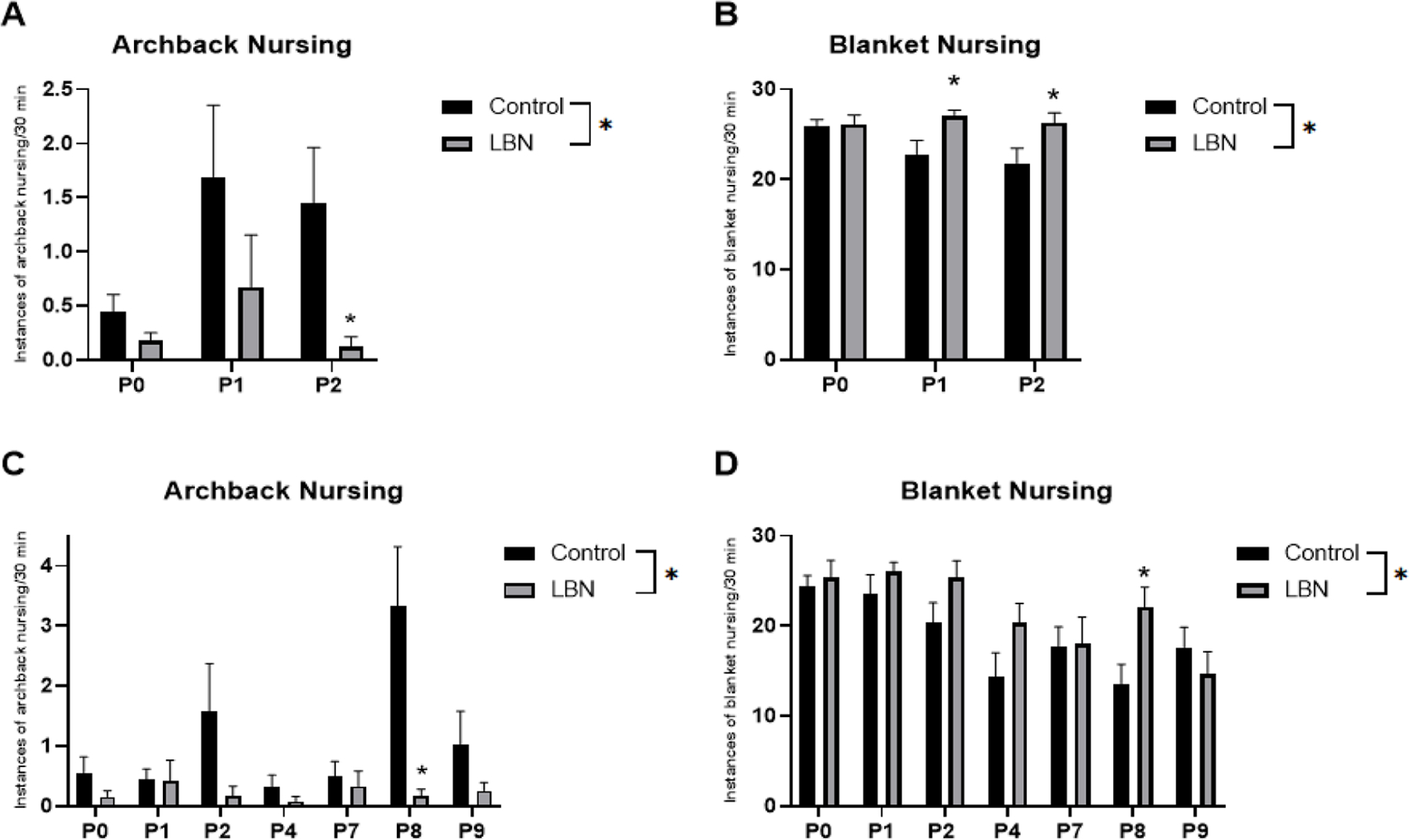
A. Number of instances observed over a 30 minute period of archback nursing from P0–2 (LBN N=24, Control N=24). B. Number of instances observed over a 30 minute period of blanket nursing from P0–2 (LBN N=24, Control N=24). C. Number of instances observed over a 30 minute period of archback nursing from P0–9 (LBN N=12, Control N=12). D. Number of instances observed over a 30 minute period of archback nursing from P0–9 (LBN N=12, Control N=12). * indicate p < 0.05. Error bars represent standard error of the mean (SEM).
Pup retrieval tests
Two variations of pup retrieval tasks were run as a measure of maternal behavior and maternal motivation. On P2, time to retrieve the first pup and the time to retrieve all pups during the no barrier variation of the test revealed no significant effect of condition (Time to retrieve first pup: F(1,28) = 0.99, p = 0.756 and time to retrieve all pups: F(1,28) = 0.828, p = 0.371) (Figure 4A). In the second variation of the pup retrieval task with a 4-inch barrier present in the cage, there was no significant difference in time to retrieve the first pup between conditions (F(1,28) = 1.823, p = 0.188) (Figure 4B). Only two dams were able to retrieve all pups within 10 minutes using this variation of the test.
Figure 4. Maternal care and anxiety measures between LBN and control dams.
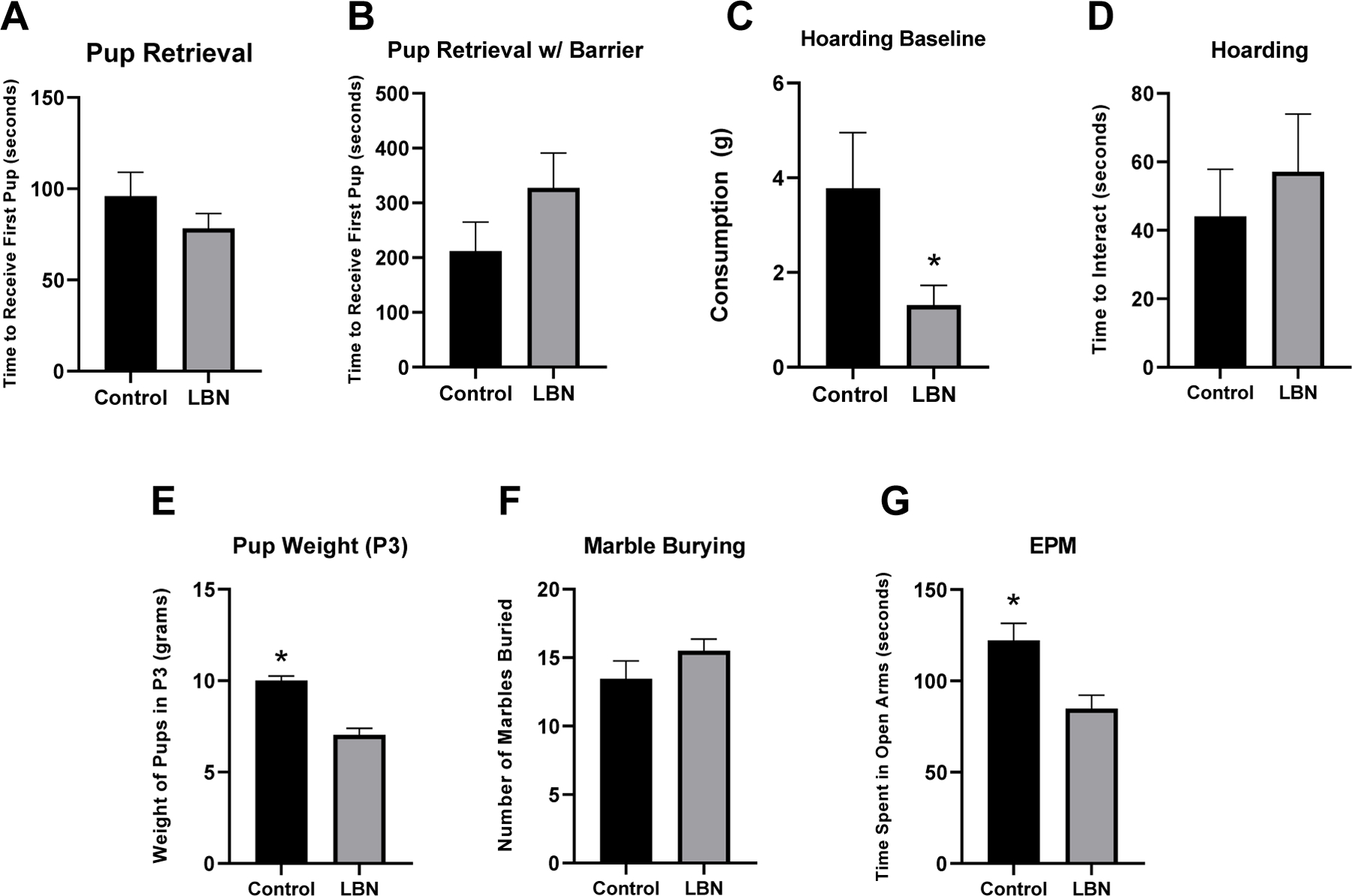
Analysis revealed decreased consumption of the sugary candy in the six hour hoarding baseline test, increased pup weight in control pups compared to LBN pups, and increased time spent in the open arm of the EPM. * indicate p < 0.05. Error bars represent standard error of the mean (SEM).
Hoarding behavior
In the baseline hoarding task in which animals had the candy in their home cage for 6 hours of acclimation, control animals consumed more of the candy than LBN animals (t(23)=2.397, p= 0.025; Control M=22.2% consumed; LBN M=7.7% consumed) (Figure 4C). No effects of hoarding behavior were observed when examining time to first interact with the candy (p>0.05) or the number of candies moved out of the initial quadrant (p>0.05) between LBN and control mothers during the 10-minute test (Figure 4D)
Pup weight
Weights were significantly different between LBN and control in P3 pups (F(1,26) = 39.396, p < 0.001) (Figure 4E). LBN pups weighed significantly less (M = 7.03 grams) compared to control pups (M = 10.0 grams) (LBN=15g, Control=12g).
Marble Burying
Marble burying was measured on P7 or P8 and the order of this test was counterbalanced with elevated plus maze testing. A one-way ANOVA revealed no difference in the number of marbles buried between LBN and control mothers (p>0.05) (Figure 4F).
Elevated Plus Maze
Elevated plus maze data was gathered from the AnyMaze tracking software (Stoelting, Wood Dale, IL, USA) and assessed various parameters during the test including distance traveled, number of freezing episodes, time spent freezing, open arm entries and time spent in the open arms. A series of one-way ANOVAs on each parameter revealed only an effect on time spent in the open arms (F(1,28) = 9.335, p = 0.005) with control mothers spending significantly more time in the open arm of the maze than LBN mothers (Figure 4G).
Fecal extracts
Estradiol
Fecal estradiol concentrations were measured at 4 timepoints throughout gestation (E18, P0, P2, P4) and these levels were compared with baseline estradiol levels in nonpregnant females (Figure 5A). A two-way ANOVA examined differences between control and LBN rats across gestation. Results indicated a significant effect of time (F(3,106) = 7.606, p < 0.001) but no effect of condition (LBN or control). Post hoc Tukey HSD revealed a significant difference between E18 and P2, as well as E18 and P4, P0 compared to P2 and P0 compared to P4 (p<0.05). Notably, levels measured during gestation and immediately postpartum (P0, P2) were significantly higher than levels measured in non-pregnant females.
Figure 5. Fecal 17β estradiol and corticosterone hormones across time in LBN and control dams.
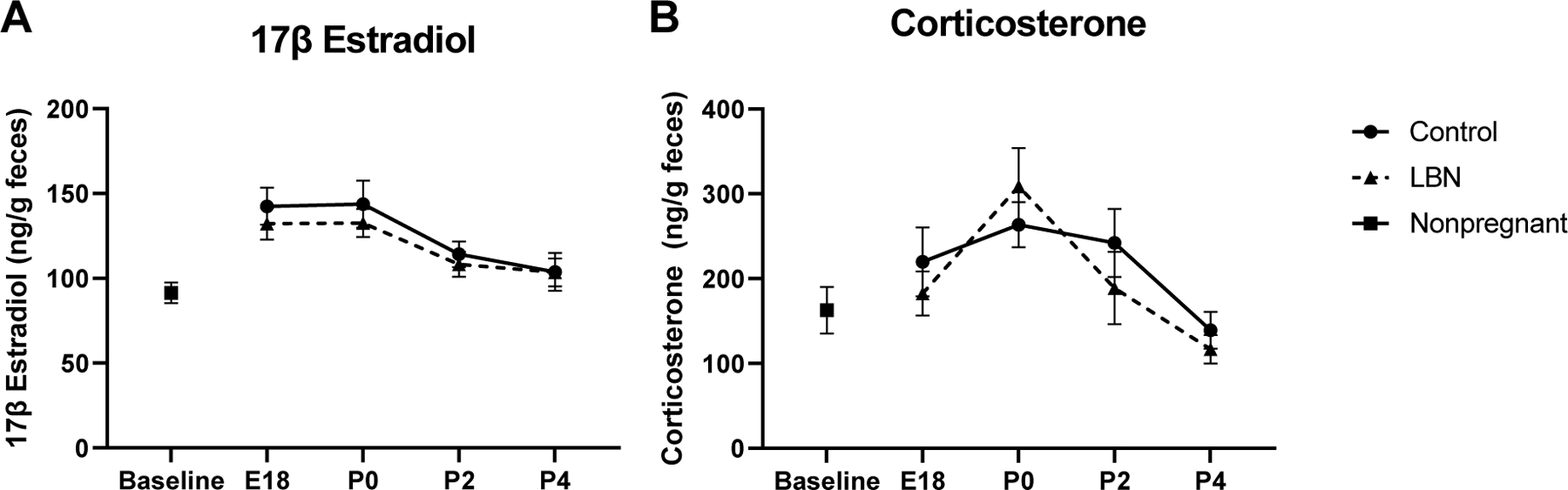
Analysis revealed no differences between LBN and control dams in either hormone. N/group is as follows: Baseline N=8, E18-P2: LBN N=16, Control N=16; P4: LBN N=8, Control N=8. Error bars represent standard error of the mean (SEM).
Corticosterone
Fecal corticosterone concentrations were measured at 4 timepoints throughout gestation (E18, P0, P2, P4) and these levels were compared with baseline corticosterone levels in nonpregnant females (Figure 5B). A two-way ANOVA examined differences between control and LBN rats across gestation. Results indicated a significant effect of time (F(3,110 = 2.120, p < 0.001) but no effect of condition (LBN or control). Post hoc Tukey HSD revealed a significant difference between P0 and all other time points (E18, P2 and P4) (p<0.05), such that corticosterone levels significantly dropped postpartum.
qPCR Results
P2
A series of one-way ANOVAs were run to examine differences in gene expression in IL-6, IL-1β, BDNF in the mPFC, dHP, and vHP (Figure 6) and ER-ɑ, ER-β, Oxytocin Receptor (OXTR), and progesterone receptor (PGR) in the MPOA between LBN, control and nonpregnant animals at P2 (Figure 8). Results indicated a trend towards significant differences in IL-6 expression in the dorsal hippocampus (F(2,27) = 2.7, p = 0.087). Tukey post hoc analyses showed that LBN had a trend towards significantly higher IL-6 expression compared to controls (p = 0.092). Similarly in the mPFC, there was a trend towards significant effect of condition (F(2,19) = 3.439, p = 0.05). Post hoc analyses again showed the LBN condition resulted in a trend of increased IL-6 expression in the mPFC compared to nonpregnant controls (p = 0.05). No effect of condition was observed in IL-6 expression in the vHP or MPOA at P2 (p>0.05). No effect of condition in the dHP, vHP, mPFC or MPOA were observed when examining IL-1β or BDNF expression (p > 0.05). No effect was observed of condition in the MPOA for ER-β, oxytocin receptor or ER-α (p>0.05). When collapsing LBN and control animals, an effect of pregnancy was revealed for ER-α in which significantly less ER-α was expressed identified in pregnant animals compared to nonpregnant controls (F(1,34) = 4.441, p = 0.043) (Figure 6). Further a trending increase of PGR was observed when collapsing LBN and control animals (p = 0.09).
Figure 6. PCR gene expression in nonpregnant, LBN and control dams at P2 in the mPFC, MPOA, dHP, and vHP.
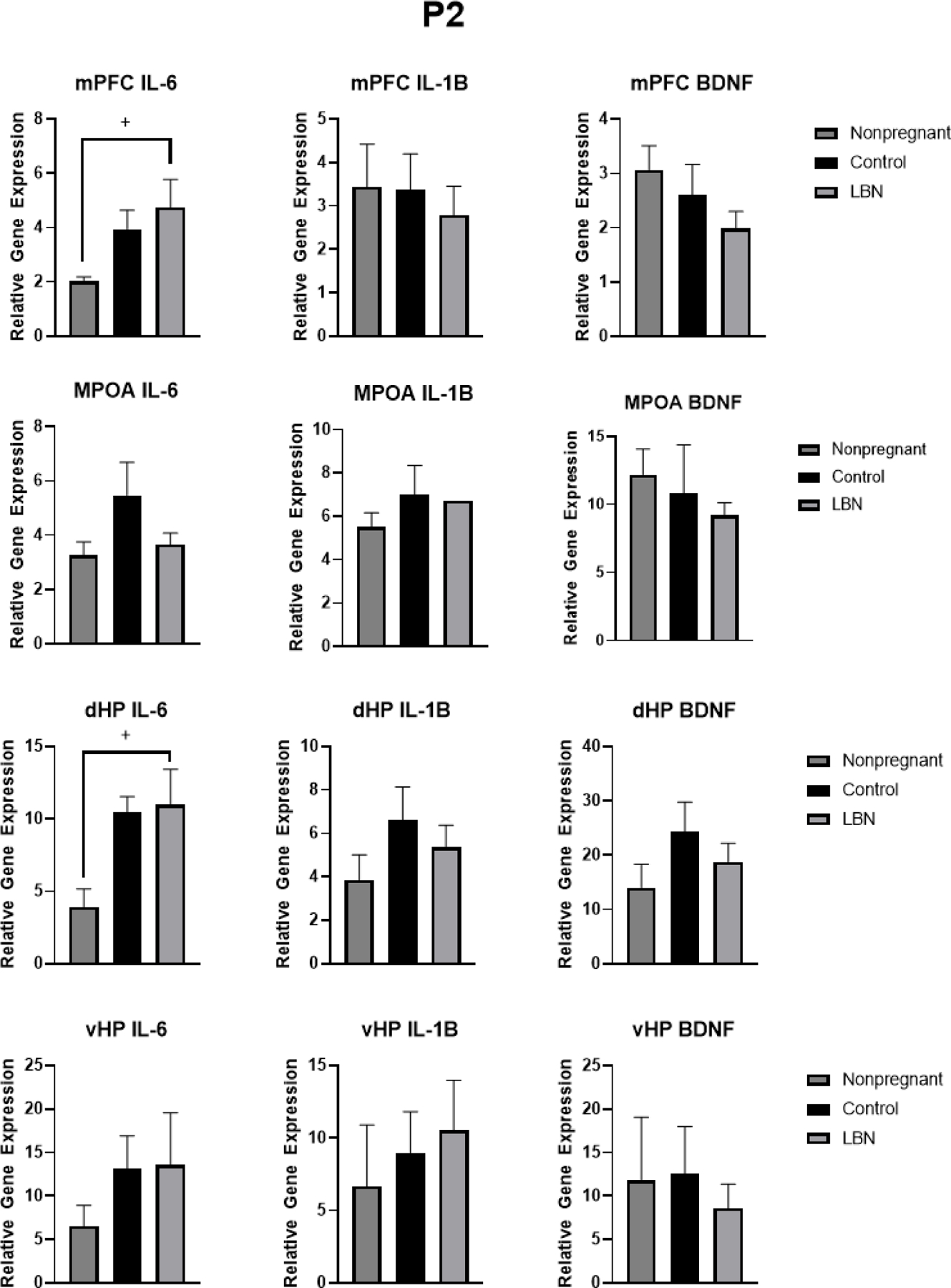
Analyses revealed an increase in IL-6 in the mPFC in LBN animals and a trend in an increase in IL-6 in the dHP of LBN animals compared to nonpregnant animals. + indicate p < 0.N per group is listed within each bar. Error bars represent standard error of the mean (SEM).
Figure 8. PCR gene expression in nonpregnant, LBN and control dams at P2 and P9 in the MPOA.
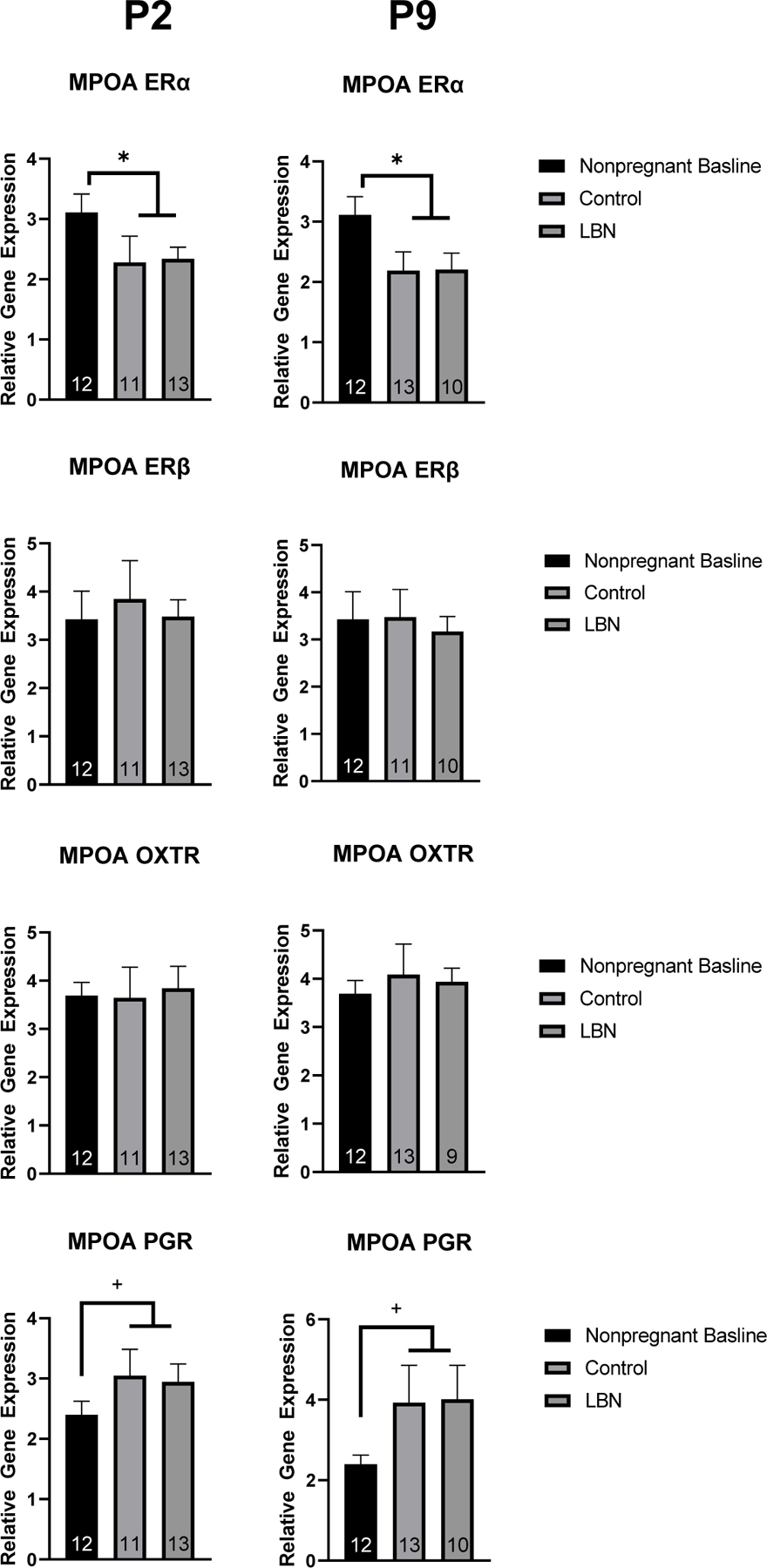
Analyses revealed a decrease in ERα in both control and LBN conditions and no changes in ERβ, OXTR, or PGR. N per group is listed within each bar. * indicate p < 0.05. Error bars represent standard error of the mean (SEM).
P9
A series of one-way ANOVAs were run to examine differences in gene expression in IL-6, Il-1β, and BDNF between LBN, control and nonpregnant animals at P9 (Figure 7). In the mPFC, a significant effect of condition was observed (F(2,18) = 10.616, p = 0.001). Further post hoc Tukey analysis revealed a difference between LBN and nonpregnant control animals (p<.001) and between LBN and control animals (p=.0.068). In the dHP, a trend towards an effect of condition was observed (F(2,32) = 2.954, p = 0.067). Further post hoc Tukey analysis revealed trend towards a difference between LBN and nonpregnant control animals (p = 0.087). No effect of condition was observed in IL-6 expression in the vHP or MPOA at P2. No effect of condition in the dHP, vHP, mPFC or MPOA were observed when examining IL-1β or BDNF expression. No effect was observed of condition in the MPOA for ER-β (F(2,59) = 0.833, p = 0.44), progesterone receptor (F(2,65) = 2.435, p = 0.096), oxytocin receptor (F(2,67) = 0.9791, p = 0.38) or ER-α (f(2,35)=2.164, p = 0.13) (Figure 8). When collapsing LBN and control animals, an effect of pregnancy was revealed for ER-α in which significantly less ER-α was expressed in pregnant animals compared to nonpregnant controls (F(1,34) = 6.443, p = 0.016). Further a trending increase of PGR was observed when collapsing LBN and control animals (p = 0.09).
Figure 7. PCR gene expression in nonpregnant, LBN and control dams at P9 in the mPFC, MPOA, dHP, and vHP.
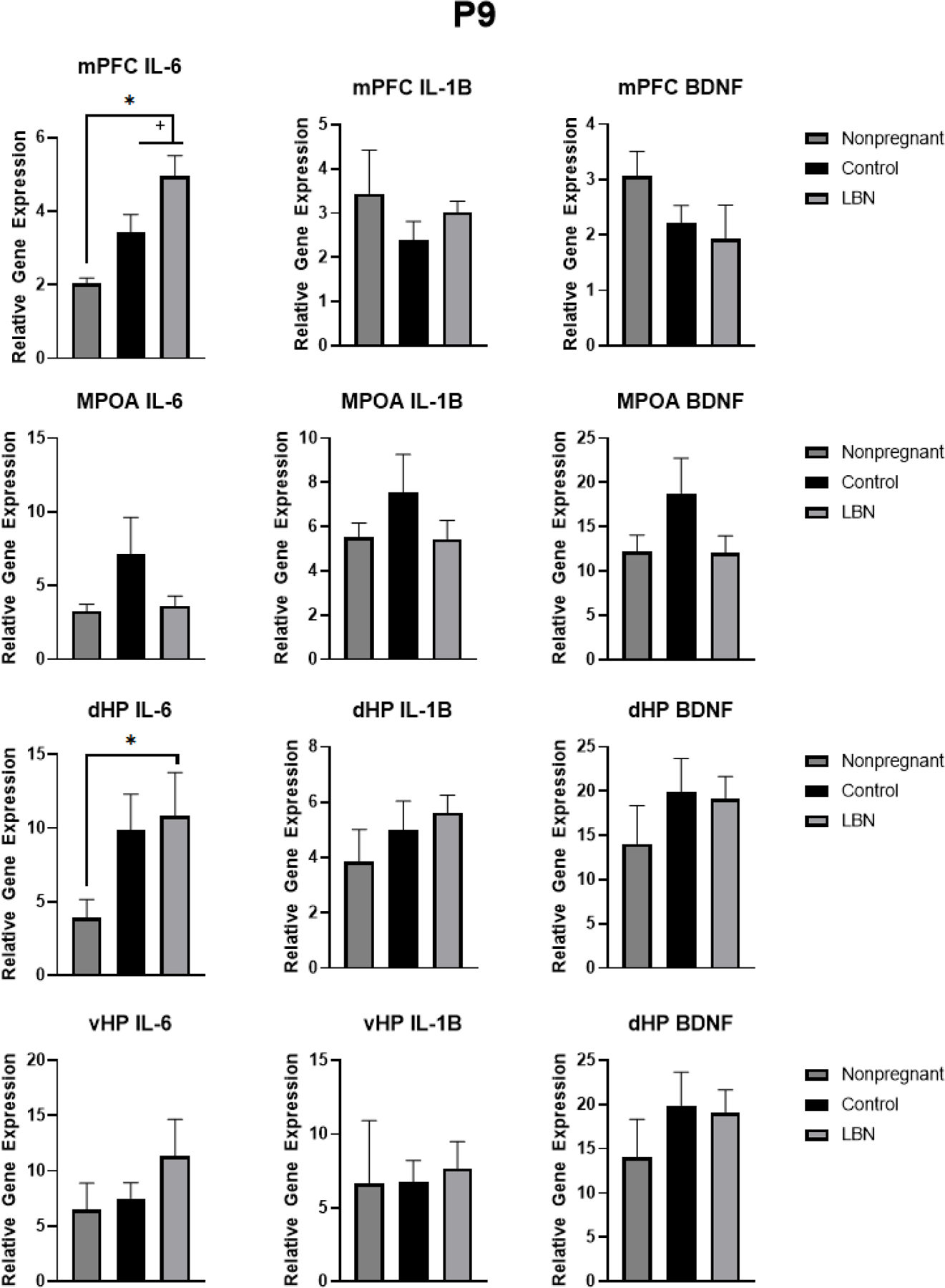
Analyses revealed an increase in IL-6 in the mPFC in LBN animals and a trend in an increase in IL-6 in the dHP of LBN animals compared to nonpregnant animals. . * indicate p < 0.05, + indicate p < 0.1. N per group is listed within each bar. Error bars represent standard error of the mean (SEM).
ER- α Densitometry
Analysis of ER-α percent area within the medial preoptic area revealed a main effect of condition: (F(2,36) = 6.188, p = 0.0049), such that the effect of the postpartum condition (LBN and Control) decreased the fraction of highlighted pixels within the region of interest compared to the nonpregnant females (Figure 9B). No significant differences were found for the integrated density of ER-α in the MPOA (Figure 9C). These observed results were validated with PCR ERα expression in the MPOA in which decreased ERα was observed in control and LBN animals.
Discussion
In humans, low socioeconomic status, lack of adequate resources and support can increase the risk of PPD up to ten times greater than that of mothers of higher socioeconomic status mothers (Gifford et al., 2021; Goyal et al., 2010). This study employed the LBN paradigm to model the low resource risk factor as a novel rodent model of PPD. Sucrose preference, maternal behavior, hoarding behavior and anxiety tasks were observed to determine the effect of LBN on postpartum mood state. Our results showed that LBN altered maternal nursing behaviors and resulted in smaller pup weight at P3 compared to control pups. Similarly, Kent et al. (2022) found that low bedding was associated with decreased pup weight at P9 and P21 suggesting LBN may result in direct weight gain effects in offspring. LBN had no effect on pup retrieval or hoarding behavior in the present study. The scoring of pup retrieval in the present study may have led to the lack of findings. Previous studies have examined pup retrieval as whether the behavior was engaged in or not as opposed to examining time to retrieval and number of pups retrieved in the present study. Additionally, we started timing when the female was placed in the cage with her pups as opposed to beginning the timing upon first interaction with pups (Li et al, 2003). Results suggest LBN conditions increased anxiety in the LBN animals compared to controls. Further, we investigated the effects of LBN on endocrine levels as well as growth and neuroimmune factors. Results indicated increased IL-6 in the mPFC of LBN animals and there was little effect of LBN experience on endocrine function. Overall, our results suggest while LBN negatively affected measures of maternal care and increased anxiety, it was unable to induce postpartum anhedonia in the same dams. Rather, it seems individual differences may underlie susceptibility to postpartum anhedonia. Our model employed LBN in the antenatal and postnatal period while many previous studies have used a classic postpartum LBN approach which may be why our results were only partially consistent with previous LBN studies (Molet et al., 2014, 2016). Additionally, the small amount of nesting materials in addition to the lack of bedding may explain some of the differences observed as previous studies have found that reduced nesting materials alone are sufficient to induce maternal stress and anxiety (Ivy et al., 2008.; Rice et al., 2008).
While no differences were observed between LBN and control conditions for anhedonia, in both groups anhedonia was observed in the form of decreased sucrose preference postpartum (P0–2 and P7–9) compared to baseline testing, replicating previous findings that pregnancy induces anhedonia postpartum (Posillico & Schwarz, 2015). In addition to sucrose preference testing the baseline hoarding task also served as a test of anhedonia in which animals were exposed to a novel piece of sugary candy for six hours of acclimation. Decreased consumption of the candy was observed in LBN animals in this six-hour baseline testing suggesting anhedonia in these animals. Future studies should aim to examine additional measures of anhedonia in postpartum dams exposed to LBN conditions. Interestingly, when examining the sucrose preference data closer, individual differences were evident in which some animals show decreased sucrose preference while others maintain high levels of sucrose preference postpartum (regardless of condition) which persisted from P2 to P9. Future studies should further examine the role of these individual differences.
Of interest to the present study, women with PPD report a lack of bond with their baby (Kerna, 2020). LBN stress has been well studied and understood to induce altered maternal behavior including decreased arched back nursing and rough pup handling and as such was hypothesized to be a relevant model of PPD (Walker et al., 2017). Within the LBN paradigm, few additional maternal behaviors have been observed. Both arched-back nursing and blanket nursing were impacted by the postpartum condition. Specifically, the LBN dams exhibited a significant decrease in the arched-back nursing behavior, while the LBN dams exhibited a significant increase in the blanket nursing behavior relative to control dams. Arched-back nursing requires more energy to maintain an erect spinal curve that achieves optimal nipple exposure to her pups while blanket nursing is a more relaxed posture which still achieves nursing, but to a lesser degree than arched-back nursing. Therefore, the data suggest that LBN condition altered the dam’s likelihood to engage in arched-back nursing over blanket nursing. A review by Orso and colleagues (2019) examined a number of studies which implemented early life stress (ELS) (in the form of limited bedding and nesting (LBN), in addition to maternal separation (MS)) and their effects on maternal behavior. Their findings support our results in which these forms of ELS resulted in an increase in arched-back nursing as well as a decrease in passive/blanket nursing (Bath et al., 2019). Yet, our results also differ from classic models of LBN in which limited bedding and nesting occurs only in the postpartum period. Previous studies have observed alterations to length of arched back nursing episodes, and overall nursing time was decreased in LBN animals (Ivy et al., 2008.; Molet et al., 2016). Ivy et al (2008) also observed a recovery of maternal care when increased bedding and nesting materials were made available. It would be of interest for future studies to examine whether returning animals to control bedding levels in the present model would reverse these altered maternal care behaviors.
The present study also indicated increased IL-6 gene expression in the mPFC and dHP o in the postpartum females compared to virgin controls. The observed increased IL-6 in mPFC replicates previous findings confirming parturition results in altered immune functioning in the mPFC (Posillico & Schwarz, 2015). Increased IL-6 in the dHP has not previously been reported in postpartum dams but this may be due to the fact that the hippocampus was separated into ventral and dorsal subregions. Similar to previous studies, no differences were observed in the ventral hippocampus in any of the cytokines or growth factors observed. This novel finding in the dHP may be unique to the dorsal hippocampus as compared to previous studies which examined the entire hippocampus.
While we had anticipated higher expression of ER-ɑ in control, non-stressed, dams relative to the LBN dams, our findings only revealed an effect of pregnancy alone, rather than a further, subsequent effect of the LBN paradigm. The relative gene expression of ER-β in the MPOA did not show any significant differences across the groups, however, we were able to note that the expression of ER-β in the nonpregnant females was decreased compared to the relative gene expression of ER-ɑ in nonpregnant females. ER-β is not as ubiquitously expressed as ER-ɑ in the MPOA, and as such, supporting previous research suggesting ER-ɑ and ER-β differ in their expression patterns across the brain, their binding efficacy and their resultant effects on reproductive and maternal behaviors (Champagne & Curley, 2008). Similar levels of oxytocin receptor gene expression were observed across all groups at both time points postpartum supporting previous findings that the expression of oxytocin postpartum returns to the levels expressed in nonpregnant rats and in the case of the present study, this likely occurs prior to postnatal day 2 (Meddle et al., 2007). The relative gene expression of progesterone (PGR) did not display significant differences among groups. This is supported by previous findings stating that the concentration of progesterone, much like estrogens, drop off relatively soon after birth and the time points observed (P2 and P9) may miss the window of increased progesterone (Trifu et al., 2019).
The results from the ER-ɑ staining revealed a significant difference in the postpartum condition (LBN and Control dams relative to non-pregnant controls), reflecting a decrease in the ER-ɑ expression in the MPOA when compared to the nonpregnant females. These findings are consistent with the qPCR results, which also showed a decrease in the expression of ER-ɑ in the MPOA following pregnancy and parturition. These results provide additional evidence to support the idea that circulating estrogen levels fall precipitously following parturition, which in turn may down-regulate the expression of the cognate receptor. Despite that, we found no significant differences in the expression of ER-α protein or mRNA between the LBN dams and the Control dams, an effect that was consistent throughout the experiments conducted in this study. This finding was contrary to our original expectations and thus proved that the specific parameters of the LBN paradigm used in this project may not have been successful at producing robust stress in the experimental group.
LBN had no effect on fecal estradiol or corticosterone metabolite levels but both show a peak around the time of birth (P0). Although this did not directly support our hypothesis that LBN would alter both hormones, the decrease in estradiol and corticosterone observed postpartum is consistent with the finding that these hormones remain high throughout pregnancy and fall off immediately after parturition (Lindsay & Nieman, 2005; Trifu et al., 2019). The similarity in circulating estradiol levels between the LBN and control dams could indicate that the LBN stress has no effect on the ability of the ovaries to produce estradiol, or again, that the stress itself was not robust enough to produce a change in this hormonal measure. Our goal was to noninvasively assess estradiol levels in fecal samples although fecal samples may not provide the same reliability or sensitivity of plasma blood samples. Further investigation will lead to a better understanding of the substrates involved and the mechanisms of postpartum depression, leading to more accurate diagnoses and increased patient education.
Peripartum anxiety is highly comorbid with PPD and shares many of the same risk factors and etiological theories (Brummelte & Galea, 2016). The results of the present study observed increased anxiety as measured by the elevated plus maze. In this task, LBN dams displayed decreased time in the open arms of the maze, a measure commonly associated with increased anxiety. No differences were observed in marble burying in the same mothers. In this task, the female was placed in a novel cage with marbles along with her pups. It is possible that while marble burying has been associated with anxiety-like behaviors, the presence of the pups in the task may have altered the anxiety response. Previous research has suggested pup separation increases the number of marbles buried by dams (Jury et al., 2015). Marble burying has been suggested to have several limitations including the differing assessment of what is considered a buried marble and how well this translates to a measure of anxiety (de Brouwer et al., 2018). Further studies should be conducted to examine the effects of LBN on additional anxiety tasks.
Short term antenatal LBN induced alterations to maternal care and anxiety but not to postpartum mood state. It is possible that the length of LBN exposure or form of LBN exposure in the present study was not long enough to induce the changes hypothesized. The present study examined these measures until P9 which may be overlooking alterations to maternal mood state and behavior that occur between P9 and weaning. Additionally, many classic models of LBN employ a wire rack to severely limit bedding while the present study only reduced the volume of bedding (Ivy et al., 2008.; Molet et al., 2016). Future studies should examine longer term LBN exposure, perhaps throughout gestation and further postpartum through weaning to understand the role of LBN on maternal mood state. While little effects of LBN were observed on maternal behavior and associated biomarkers, it is important to note that the pups of LBN animals weighed significantly less than the pups of control dams (Figure 4E). This suggests that while LBN does not manifest as expected in the maternal behavior and biomarkers, the pups are clearly affected. Another possible explanation for the results of the present study is that parturition may be such a stressful event to new dams that it overshadows the effects of living conditions, suggesting that both LBN and control dams may have baseline levels of stress during this transition to motherhood. Further research should examine the differential effects of LBN on the pup versus the dam. Additionally, the effects of early life stress might have a more significant effect in maternal outcomes for dams than gestational stressors which could support the individual sucrose preference differences observed in the present study. Overall, the present study provides insight into the effects of short-term housing stress on postpartum mood-state and associated hormonal and immune changes. Studies extending from this work may provide a clearer understanding of mechanisms underlying mood-state and associated changes in the early postpartum period with a focus on individual differences to provide more targeted therapeutics and treatments.
Supplementary Material
Funding:
This work was supported by the National Institutes of Health [R21MH122862].
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
Data availability:
Data is available upon request to the corresponding author.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders American Psychiatric Association. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, & Kuhn DM (2013). Marble Burying and Nestlet Shredding as Tests of Repetitive, Compulsive-like Behaviors in Mice. Journal of Visualized Experiments : JoVE, 82(82), 50978. 10.3791/50978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Opendak M, Brenhouse H, Grassi-Oliveira R, Orso R, Creutzberg KC, Wearick-Silva LE, Wendt Viola T, Gantes Tractenberg S, & Benetti F (2019). How Early Life Stress Impact Maternal Care: A Systematic Review of Rodent Studies. Frontiers in Behavioral Neuroscience | Www.Frontiersin.Org, 1. 10.3389/fnbeh.2019.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bränn E, Edvinsson Å, Rostedt Punga A, Sundström-Poromaa I, & Skalkidou A (2019). Inflammatory and anti-inflammatory markers in plasma: from late pregnancy to early postpartum. Scientific Reports, 9(1). 10.1038/S41598-018-38304-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, & Galea LAM (2016). Postpartum depression: Etiology, treatment and consequences for maternal care. Hormones and Behavior, 77, 153–166. 10.1016/J.YHBEH.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, & Mcclintock MK (2005). Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. Journal of Endocrinology, 184, 153–163. 10.1677/joe.1.05935 [DOI] [PubMed] [Google Scholar]
- Champagne FA, & Curley JP (2008). Maternal Regulation of Estrogen Receptor α Methylation 10.1016/j.coph.2008.06.018 [DOI] [PMC free article] [PubMed]
- de Brouwer G, Fick A, Harvey BH, & Wolmarans DW (2018). A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive–compulsive disorder: Mapping the way forward. Cognitive, Affective, & Behavioral Neuroscience 2018 19:1, 19(1), 1–39. 10.3758/S13415-018-00653-4 [DOI] [PubMed] [Google Scholar]
- Faisal-Cury A, Menezes PR, Tedesco JJA, Kahalle S, & Zugaib M (2008). Maternity “Blues”: Prevalence and Risk Factors. The Spanish Journal of Psychology Copyright, 11(2), 593. [PubMed] [Google Scholar]
- Fok CCT, Hayes DK, Curtis AB, Nihoa WK, & Shim MJ (2012). Hawai’i journal of health & social welfare (Vol. 79, Issue 5). https://www.scopus.com/inward/record.uri?eid=2-s2.0-85084961992&partnerID=40&md5=1feda1743b9ce121f1d21c61f3b0d36b [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, Wide JK, & Barr AM (2001). Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behavioural Brain Research, 122(1), 1–9. 10.1016/S0166-4328(01)00170-X [DOI] [PubMed] [Google Scholar]
- Gifford JJ, Pluchino JR, Della Valle R, & Schwarz JM (2021). Regional Differences in Various Risk Factors for Postpartum Depression: Applying Mixed Models to the PRAMS Dataset 2. 10.3389/fgwh.2021.726422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J, Haas NA, & Schwarz JM (2019). An IL-6 receptor antagonist attenuates postpartum anhedonia, but has no effect on anhedonia precipitated by subchronic stress in female rats. Psychopharmacology, 236(10), 2983–2995. 10.1007/s00213-019-05194-3 [DOI] [PubMed] [Google Scholar]
- Goyal D, Gay C, Lee KA, & Jose S (2010). How much does Low Socioeconomic Status Increase the Risk of Prenatal and Postpartum Depressive Symptoms in First Time Mothers? Womens Health Issues, 20(2), 96–104. 10.1016/j.whi.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretchen Livingston B, Livingston G, & Researcher Juliana Horowitz S (2018). FOR MEDIA OR OTHER INQUIRIES (Vol. 18). www.pewresearch.org. [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, & Baram TZ (n.d.). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress [DOI] [PMC free article] [PubMed]
- Jury NJ, Mccormick BA, Horseman ND, Benoit SC, & Gregerson KA (2015). Enhanced Responsiveness to Selective Serotonin Reuptake Inhibitors during Lactation. PLoS ONE, 10(2), 117339. 10.1371/journal.pone.0117339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent MH, Jacob JC, Bowen G, Bhalerao J, Desinor S, Vavra D, Leserve D, Ott KR, Angeles B, Martis M, Sciandra K, Gillenwater K, Glory C, Meisel E, Choe A, Olivares-Navarrete R, Puetzer JL, & Lambert K (2022). Disrupted development from head to tail: Pervasive effects of postnatal restricted resources on neurobiological, behavioral, and morphometric outcomes. Frontiers in Behavioral Neuroscience, 16, 313. 10.3389/FNBEH.2022.910056/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerna NNUDCOO and U. C. (2020). An Overview of the Management and Treatment of Postpartum Depression (PPD). EC Gynaecology, 28(9), 63–68. [Google Scholar]
- Lee C-H, & Giuliani F (2019). The Role of Inflammation in Depression and Fatigue. Frontiers in Immunology, 10. 10.3389/fimmu.2019.01696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay JR, & Nieman LK (2005). The Hypothalamic-Pituitary-Adrenal Axis in Pregnancy: Challenges in Disease Detection and Treatment. Endocrine Reviews, 26(6), 775–799. 10.1210/er.2004-0025 [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, & Neels H (1997). INCREASED SERUM IL-6 AND IL-1 RECEPTOR ANTAGONIST CONCENTRATIONS IN MAJOR DEPRESSION AND TREATMENT RESISTANT DEPRESSION. Cytokine, 9(11), 853–858. 10.1006/CYTO.1997.0238 [DOI] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, Van Leeuwen FW, & Douglas AJ (2007). Dynamic Changes in Oxytocin Receptor Expression and Activation at Parturition in the Rat Brain. Endocrinology, 148, 5095–5104. 10.1210/en.2007-0615 [DOI] [PubMed] [Google Scholar]
- Miller LJ (2002). Postpartum Depression. JAMA, 287(6), 762–765. 10.1001/JAMA.287.6.762 [DOI] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, & Stern H (2016). Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Translational Psychiatry 2016 6:1, 6(1), e702–e702. 10.1038/tp.2015.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Maras PM, Avishai-Eliner S, & Baram TZ (2014). Naturalistic rodent models of chronic early-life stress. Developmental Psychobiology, 56(8), 1675–1688. 10.1002/DEV.21230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Aldo P, & Alvero AB (2017). The unique immunological and microbial aspects of pregnancy REVIEWS E A R LY L I F E I M M U N O LO G Y. NATURE REVIEWS | IMMUNOLOGY, 17, 469. 10.1038/nri.2017.64 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Coxe S, Fennie K, Madhivanan P, & Trepka MJ (2017). Antenatal Stressful Life Events and Postpartum Depressive Symptoms in the United States: The Role of Women’s Socioeconomic Status Indices at the State Level. Journal of Women’s Health, 26(3), 276–285. 10.1089/jwh.2016.5872 [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, & Smith CD (2005). Medial preoptic area interactions with the nucleus accumbens–ventral pallidum circuit and maternal behavior in rats. Behavioural Brain Research, 158(1), 53–68. 10.1016/J.BBR.2004.08.008 [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Myint AM, Van Den Hove D, Desbonnet L, Steinbusch H, & Leonard BE (2006). Gestational Stress Leads to Depressive-Like Behavioural and Immunological Changes in the Rat. Neuroimmunomodulation, 13(2), 82–88. 10.1159/000096090 [DOI] [PubMed] [Google Scholar]
- Patel M, Bailey RK, Jabeen S, Ali S, Barker NC, & Osiezagha K (2012). Postpartum Depression: A Review. Journal of Health Care for the Poor and Underserved, 23(2), 534–542. 10.1353/HPU.2012.0037 [DOI] [PubMed] [Google Scholar]
- Pawluski J, Konkle ATM, Mateus V, Rincón-Cortés M, & Grace AA (2021). Early Pup Removal Leads to Social Dysfunction and Dopamine Deficit in Late Postpartum Rats: Prevention by Social Support 2, 694808. 10.3389/fgwh.2021.694808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein T, Howard M, Salisbury A, & Zlotnick C (2009). Postpartum depression 10.1016/j.ajog.2008.11.033 [DOI] [PMC free article] [PubMed]
- Pooler J, Perry DF, & Ghandour RM (2013). Prevalence and risk factors for postpartum depressive symptoms among women enrolled in WIC. Maternal and Child Health Journal, 17(10), 1969–1980. 10.1007/s10995-013-1224-y [DOI] [PubMed] [Google Scholar]
- Posillico CK, & Schwarz JM (2015). Implications of stress and infection on neuroimmune function and behavior as potential risk factors for developing postpartum depression. Brain, Behavior, and Immunity, 49, e6. 10.1016/J.BBI.2015.06.043 [DOI] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, & Baram TZ (2008). A Novel Mouse Model for Acute and Long-Lasting Consequences of Early Life Stress 10.1210/en.2008-0633 [DOI] [PMC free article] [PubMed]
- Robinson DP, & Klein SL (2012). Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Hormones and Behavior, 62(3), 263–271. 10.1016/J.YHBEH.2012.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM (2019). The future of mental health research: Examining the interactions of the immune, endocrine and nervous systems between mother and infant and how they affect mental health. Hormones and Behavior, 114. 10.1016/J.YHBEH.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried LS, & Marcus SM (2009). International Review of Psychiatry Postpartum mood disorders Postpartum mood disorders 10.1080/0954026031000136857 [DOI] [PubMed]
- Sherer ML, Posillico CK, & Schwarz JM (2018). An examination of changes in maternal neuroimmune function during pregnancy and the postpartum period. Frontiers in Neuroendocrinology, 51, 25–35. 10.1016/j.bbi.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH (2007). The Complex Role of Estrogens in Inflammation 10.1210/er.2007-0001 [DOI] [PubMed]
- Suda S, Segi-Nishida E, Newton SS, & Duman RS (2008). A Postpartum Model in Rat: Behavioral and Gene Expression Changes Induced by Ovarian Steroid Deprivation 10.1016/j.biopsych.2008.03.029 [DOI] [PMC free article] [PubMed]
- Sullivan R, Roth TL, Perry R, Bath KG, Gallo M, Shleifer DG, Godoy LD, Ofray D, Olaniyan A, & Campbell T (2019). Limited Bedding and Nesting Induces Maternal Behavior Resembling Both Hypervigilance and Abuse 10.3389/fnbeh.2019.00167 [DOI] [PMC free article] [PubMed]
- Thanos PK, Cavigelli SA, Michaelides M, Olvet DM, Patel U, Diep MN, & Volkow ND (2009). A Non-Invasive Method for Detecting the Metabolic Stress Response in Rodents: Characterization and Disruption of the Circadian Corticosterone Rhythm. Physiol. Res, 58, 219–228. www.biomed.cas.cz/physiolres [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifu S, Vladuti A, Popescu A, Trifu S, & Carol, “. (2019). The Neuroendocrinological aspects of pregnancy and postpartum depression. Acta Endocrinologica (Buc), XV(3), 410–415. 10.4183/aeb.2019.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, Morris MJ, Raineki C, Roth TL, Sullivan RM, Taché Y, & Baram TZ (2017a). Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential 10.1080/10253890.2017.1343296 [DOI] [PMC free article] [PubMed]
- Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, Morris MJ, Raineki C, Roth TL, Sullivan RM, Taché Y, & Baram TZ (2017b). Stress The International Journal on the Biology of Stress Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential 10.1080/10253890.2017.1343296 [DOI] [PMC free article] [PubMed]
- Wisner KL, Sit DKY, Mcshea MC, Rizzo DM, Zoretich RA, Hughes CL, Eng HF, Luther JF, Wisniewski SR, Michelle, ;, Moses-Kolko EL, Famy CS, & Hanusa BH (2013). Onset Timing, Thoughts of Self-harm, and Diagnoses in Postpartum Women With Screen-Positive Depression Findings. JAMA PSYCHIATRY, 70(5), 490–498. 10.1001/jamapsychiatry.2013.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request to the corresponding author.


