Abstract
FLASH radiotherapy, delivered with ultra-high dose rate (UHDR), may allow patients to be treated with less normal tissue toxicity for a given tumor dose compared to currently used conventional dose rate. Clinical trials are being carried out and are needed to test whether this improved therapeutic ratio can be achieved clinically. During the clinical trials, quality assurance and credentialing of equipment and participating sites, particularly pertaining to UHDR-specific aspects, will be crucial for the validity of the outcomes of such trials. This report represents an initial framework proposed by the NRG Oncology Center for Innovation in Radiation Oncology (CIRO) FLASH working group on quality assurance of potential UHDR clinical trials, and reviews current technology gaps to overcome. An important but separate consideration is the appropriate design of trials to answer clinical and scientific questions about FLASH most effectively.
This paper begins with an overview of UHDR radiotherapy delivery methods. UHDR beam delivery parameters are then covered, with a focus on electron and proton modalities. The definition and control of safe UHDR beam delivery and current and needed dosimetry technologies are reviewed and discussed. System and site credentialing for large, multi-institution trials are reviewed. Quality assurance is then discussed and new requirements are presented for treatment system standard analysis, patient positioning, and treatment planning.
The tables and figures in this paper are meant to serve as reference points as we move toward FLASH radiotherapy clinical trial performance. Some major questions regarding FLASH radiotherapy are discussed and next steps in this field are proposed. FLASH radiotherapy has potential but is associated with significant risks and complexities. We need to redefine optimization to focus not only on the dose, but also on the dose rate, in a manner that is robust and understandable and that can be prescribed, validated, and confirmed in real time. Robust patient safety systems and access to treatment data will be critical as FLASH radiotherapy moves into the clinical trials.
Keywords: FLASH, ultra-high dose rate, clinical trial, quality assurance, patient safety
Introduction
In 2014, Favaudon et al.(1) introduced “FLASH” radiotherapy (FLASH-RT), an ultra-high dose rate (UHDR) technique that spared normal tissues without compromising anti-tumor efficacy in mice compared to conventional dose rate irradiation. Their publication ignited strong interest in the radiotherapy community, and subsequent preclinical in-vivo studies similarly demonstrated improved therapeutic index using UHDR electron, proton, and photon beams(1–17). In recent years, with the increase in cancer survivors’ life expectancies, concern about potential radiation-induced toxicity has increased. FLASH-RT potentially enables the reduction of normal tissue toxicity with a standard tumor dose or the maintenance of comparable toxicity with an increased effective tumor dose(18–22). Furthermore, its ultra-rapid treatment delivery minimizes motion impacts. The performance of clinical trials of FLASH-RT examining normal tissue protection, tumor lethality, and UHDR treatment deliverability and definitions is the primary goal of the translation of preclinical research.
Clinical trials of FLASH-RT conducted with animals (e.g., cats and pigs) recently revealed late toxic effects(23). The FAST-01 trial (ClinicalTrials.gov no. NCT04592887) demonstrated the feasibility of proton FLASH-RT for human patients with multiple bone metastases(24, 25). The FAST-02 trial, designed to examine proton FLASH-RT for bone metastases in the chest, is now open for enrollment. A clinical trial examining electron FLASH-RT dose escalation for human patients with skin melanoma metastases (ClinicalTrials.gov no. NCT04986696) was initiated in 2021. Ongoing and planned clinical trials of FLASH-RT are designed to test the UHDR deliverability and safety, with the examination of the effectiveness of tumor control, normal tissue dose tolerance, the reproducibility of treatment effects across multiple institutions, and the safety and effectiveness of combined chemoradiation treatment paradigms. The success of planned FLASH clinical trials examining these and other hypotheses is contingent on the consistency and quality of UHDR technology implementation and reporting.
Presently, it is unclear what parameters of UHDR radiotherapy (including potential dose and/or dose rate thresholds) are required or optimal to produce FLASH effects. In early publications, 40 Gy/s was suggested as a dose rate threshold(1, 2). Subsequent studies have demonstrated that the dose, intra-pulse dose rate, and number of pulses play important roles in electron FLASH effects(26–28). Although in-vivo animal studies have been performed with single scattered and collimated beams, clinical particle-beam UHDR treatment delivery uses pencil-beam scanning(PBS) that uses lateral scanning of a series of locations at each depth to cover the tumor volume. Utilization of the plateau region of transmission beams(29, 30) or range modulation with the use of specific accessories(31, 32) were used to preserve the PBS specific ultra-high dose rate(29, 30, 33, 34).
Only when UHDR dose and dose-rate parameters are available can the impacts of clinical trial protocols on patients be evaluated systematically. The clinical trial treatment planning must consider beam delivery time structures to optimize UHDR dose distributions. Planning evaluation and reporting tools, such as voxel-based dose delivery time structure, and knowledge of the UHDR beam parameters are essential for the conduct of meaningful clinical trial studies, study reproducibility and translatability(35), and will facilitate the inclusion of these parameters for optimal UHDR implementation(36). Note that the UHDR parameters needed for optimal FLASH effects would most appropriately be determined through preclinical and clinical trials rather than specified as quality assurance metrics. However, the ability to document critical parameters accurately would fall under clinical trial credentialing.
Other critical requirements for FLASH-RT clinical trials are robust dosimetry methods, techniques, and equipment that enable the reliable and reproducible measurements of doses and dose rates. Traditional dosimetry tools need to be validated for the recording of doses under UHDR conditions. A new series of dosimetry systems needs to be developed and validated for the recording of dose delivery timing information at sufficient resolution. In addition, the safety and repeatability of UHDR dose delivery within and across institutions must be demonstrated, at least for the same modality.
The NRG Oncology Center for Innovation in Radiation Oncology (CIRO) formed a FLASH working group (NCFWG) with physicists and physicians from multiple institutions with the experience and intention to implement UHDR FLASH-RT. This report is based on the current team consensus regarding the framework on quality assurance and credentialing of potential FLASH clinical trials, and the technology status and challenges. It should not be referred in regular clinical practice. It addresses the following topics specific to electron and proton UHDR clinical trials: requirements for treatment delivery reports, the definition and control of safe UHDR delivery, dosimetry requirements, recommendations for treatment plan reporting, and requirements for FLASH-RT system credentialing and quality assurance. The recommended percentage uncertainties and thresholds are preliminary and should not be used as a basis for regulatory specifications. We conclude this report with a summary of the current state of technology and technological gaps relevant to future NRG FLASH-RT clinical trials.
FLASH-RT delivery reports
Various UHDR delivery technologies have been explored and invented, given the rapidly evolving nature of FLASH-RT research. Preclinical studies have been made possible with the development of dedicated experimental systems and modification of existing RT systems(37), including specialized electron linear accelerators (linacs)(1, 38), proton/particle beamlines(6, 39–42), synchrotron light sources producing kilovoltage x-rays(43), small animal irradiators with customized kilovoltage x-ray tubes(44, 45), and the conversion of clinical linacs(46–49). Newly designed systems with the main function of UHDR-RT(50), include the PHASER platform(51), electron FLASH system for intraoperative RT(52, 53), and external beam RT with very high-energy electrons(54, 55). Current translational studies and pilot human clinical trials have been conducted predominantly with UHDR electron and proton beams within conventional clinical therapeutic energy ranges(24, 38, 56). Here, to provide recommendations on reportable beam parameters for FLASH clinical trials that are ongoing and planned for the foreseeable future, the scope of the discussion is limited to UHDR-RT with electron and proton beams at energies used in current clinical practice; the considerations and recommendations may or may not apply to other modalities such as heavy-ion and photon UHDR-RT.
The NCFWG has reached the consensus that all reportable beam parameters for current clinical treatment with conventional dose rates for electron radiotherapy (CONV-eRT) and proton therapy (CONV-PT), preferably with definitions and measurement conditions specified in established professional guidelines, remain applicable to UHDR-RT. These parameters include 1) the treatment regimens, geometries, dose distributions, energies, percentage depth doses (PDDs), output factors, and lateral profiles for a range of field sizes for electron beams; and 2) the treatment regimens, geometries, dose distributions, energies, linear energy transfer (LET), spot profiles (for pencil beam) or lateral profiles (for scattered beam), integral depth doses (for pencil beam) or PDDs (for scattered beam), and output factors/halo effects for various field sizes and range modulations (e.g., spread-out Bragg peaks) for proton beams. Beam parameters of particular interest for electron and proton UHDR-RT are summarized here, with discussion and recommendations for delivery reports.
Electron beam therapy
Studies involving the use of UHDR electron beams, implemented primarily with specialized irradiators and modified medical linacs, have revealed significant variation in the pulse structure (instantaneous dose rate, dose per pulse, pulse width/duration, pulse repetition frequency, and mean dose rate)(1, 46, 48, 52, 53, 57–59). To facilitate the cross-platform interpretation of outcomes and reproducibility of irradiation when necessary, the recording and reporting of pulse structure details with specification of the aforementioned parameters, following the definitions provided in Figure 1a and Table 1(59, 60), are highly recommended. Although the standardization of reporting remains challenging due to significant variation across platforms, the pulse structure should be recorded and reported consistently at a minimum of one user-defined reference point. The temporal resolution of the recording at the reference point should be no coarser than the individual pulse duration (i.e., on the order of microseconds), with the measured dose rate(Table 1)(61). Finer-resolution (sub-microsecond) sub-pulse structure reporting is encouraged if achievable. With advancing UHDR-RT dosimetry technologies (Section Dosimeter requirements), recording at multiple points and dimensions with high spatial and temporal resolutions is desirable. When direct measurement is not possible, meaningful information on the spatiotemporal distributions of pertinent parameters can be obtained based on delivery information from a reference point for well-characterized beams, with the use of established calculation models such as analytical or Monte Carlo radiation transport models(55, 62).
Figure 1:
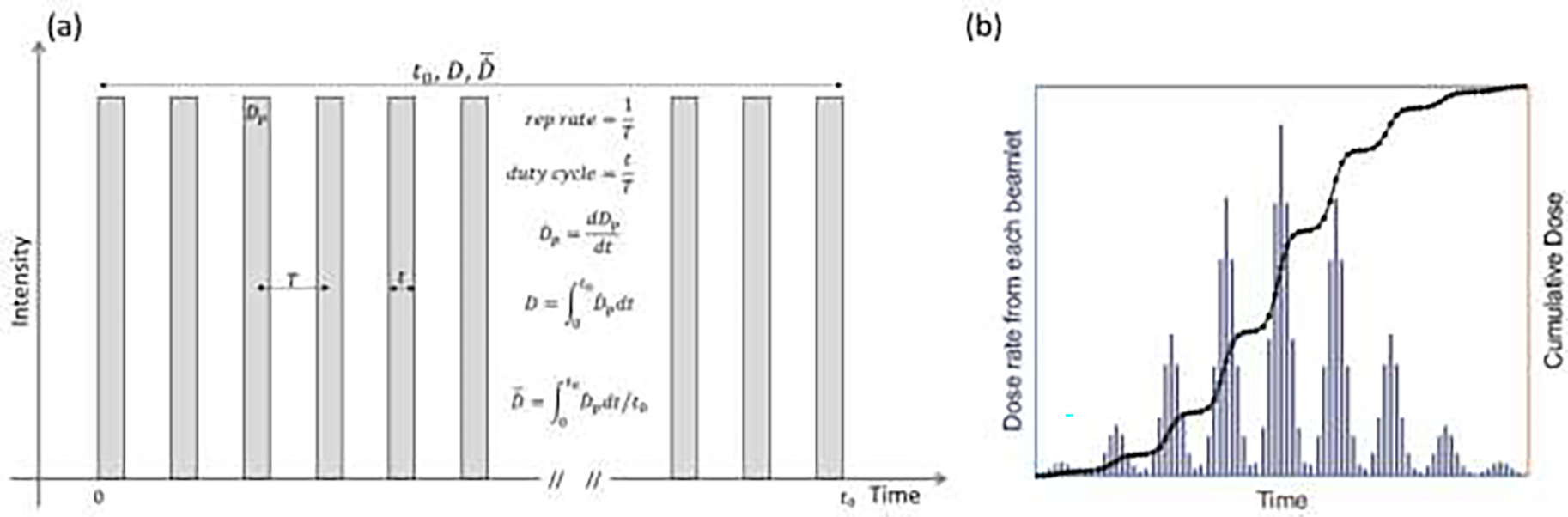
a) Schematic of temporal pulse structures of extracted beams with key parameters defined. b) Dose rates and cumulative doses from each spot on a square field of PBS delivery to the Bragg peak (central axis). The spots are of the same intensity and energy and are uniformly spaced. See Table 1 for variable definitions.
Table 1.
Reportable parameters for UHDR electron and proton beam deliveries
| Parameters | Definition | Electron | Cyclotron | Synchrocyclotron | Synchrotron |
|---|---|---|---|---|---|
| Beam Energy | Nominal energy | Delivered energies at nozzle | Delivered energies at nozzle | Delivered energies at nozzle | |
| Pulse repetition rate | Number of pulses per second | <400 Hz | near 100MHz | <1000 Hz | <1 Hz for spill |
| Duty cycle | Ratio of pulse ON time to OFF time | 1/2000 – 1/100 | quasicontinuous | <10% | 2- tens of ms per pill |
| Temporal pulse structure | Temporal sequence of pulses from the beginning to end of delivery, including the intervals between fields | To resolve individual pulses and pulse-shaped structures | To resolve per spot delivery time structure if PBS | To resolve beam pulses in addition to spot delivery time structure if PBS | To resolve spill duration and intervals, in addition to spot delivery time structure if PBS |
| Beam intensity | Number of particles irradiated | Per beam, can be replaced by the beam dose | Per beam and per spot if PBS | Per beam, per pulse and per spot if PBS | Per beam, per spill and per spot if PBS |
| Cumulative dose per delivery | in Fig. 1a at userdefined reference point(s) | ||||
| Dose per pulse | in Fig. 1a at userdefined reference point(s) | Per beam pulse | Per spot if PBS | Per beam pulse in addition to per spot if PBS | Per spot if PBS |
| Instantaneous dose rate | in Fig. 1a at userdefined reference point(s) | 10^4 – 10^6 Gy/sec | within a spot duration if PBS | within a spot duration if PBS | within a spot duration if PBS |
| Mean dose rate per beam | in Fig. 1a for each beam at userdefined reference point(s) | 40 – 3000 Gy/sec | Delivery specific spot pattern if PBS | Delivery specific spot pattern if PBS | Delivery specific spot pattern if PBS |
| Mean dose rate per fraction | in Fig. 1a for each fraction at userdefined reference point(s) | Delivery specific fields | Delivery specific fields | Delivery specific fields | Delivery specific fields |
| Volumetric dose rate distribution per beam and per fraction | Temporal dose distribution at each voxel in the treatment volume | Derived via beam modelling and monitoring data | Derived via beam modelling and monitoring data | Derived via beam modelling and monitoring data | Derived via beam modelling and monitoring data |
Proton beam therapy
Current UHDR irradiation with proton beams has been proposed and/or performed using several techniques, including 1) scattered transmission, 2) scattered transmission with range modulation, 3) PBS with transmission, and 4) PBS with range modulation(6, 29–32, 39, 63–65). With further technical development in beam structures and planning techniques, modification of the reported beam parameter requirements for spatiotemporal dose distribution reconstruction in patients is anticipated.
UHDR irradiation has been performed with clinical isochronous cyclotron, synchrotron, and synchrocyclotron-based proton machines that generate quasi-continuous or pulsed beams(6, 39–41). Under the assumption of a quasi-continuous beam from cyclotron accelerators or within one spill from synchrotron accelerators, the following are recommendations for the reporting of UHDR-PT beam parameters. For a passive scattered beam without dynamic range modulation, the minimal recommended recordings are of the dose and mean dose rate at a user-defined reference point per beam, providing the possession of prior knowledge of the aforementioned parameters of interest for CONV-PT. For scattering with dynamic range modulation and use of a modulation wheel, temporal modulations per range modulation step should be recorded as the dose is delivered by sweeping through the depth. For active scanning beams, the recording of dynamics is further complicated by the scanning pattern and speed, and any potential fluctuation in the beam intensity(Figure 1b) (63, 66, 67). Thus, the parameters to report ideally would be acquired from a cross-sectional reference plane at sufficiently high resolution to resolve the quantities of interest, including the spot position, profile, dose, and dose rate in spatial (millimeter-scale) and temporal (microsecond-scale) domains. For transmission beams, the reference plane can also be defined at the exit side. Although technologies enabling such measurement are emerging, with prototypes being tested (66), they are not yet ready for routine deployment (see Section Dosimeter requirements). Thus, the recording of the proton spot energy and intensity and spot delivery time structure at a resolution to resolve the dose per spot at a user-defined reference point (as in the example shown in Figure 1b) is recommended as a reporting minimum. It is ideal to record the delivery time structures at resolutions much finer than the spot duration (less than a few microseconds). Similarly, the reporting of spatiotemporal distributions of the parameters of interest, calculated for each voxel in the irradiation volume and based on the delivery information recorded via analytical or Monte Carlo calculations, is encouraged(29, 30, 68, 69). The results can be used to calculate protocol-defined dose rates in clinical trials.
Special consideration should be given to the beam temporal structure generated by synchrotron- and synchrocyclotron-based systems, for which the assumption of a quasi-continuous beam for single field delivery no longer holds. With synchrotron-based systems, proton spill from the accelerator typically supplies a number of spots, and the interval before the next spill generation is longer (i.e., a few seconds). Thus, the instantaneous spot dose rate within a spill and the interval between spills should be recorded. For synchrocyclotron-based systems, the time-resolved dose delivery at the temporal pulse structure of the extracted beam with a pulse repetition rate of about 1 kHz and duty cycle of a few percent at a reference point should be recorded and reported(40, 70) in addition to the parameters for quasi-continuous beams. Compared with those for cyclotron-based PBS beams, the reporting requirements for synchrotron- and synchrocyclotron-based UHDR PBS beams impose additional delivery timing information, for the resolution of per-pulse and spill delivery in addition to the capture of the cross-sectional spot scanning delivery.
When multiple fields are involved in electron and proton UHDR delivery fractions, the time intervals between the completion of one field and start of the other field must be recorded at millisecond-scale resolution. In addition to the aforementioned dosimetric recordings, the working group recommends the recording and reporting of any interruption to beam delivery, actions taken to resume delivery, unexpected discrepancies, deviations, and outliers during delivery. The group also encourages UHDR-RT system manufacturers to take these issues into consideration in the development of their intrinsic delivery monitoring and interlock devices and to make log files accessible to end users. Reportable parameters listed in Table 1 are also recommended to be included in the future DICOM-RT delivery report for UHDR deliveries.
Definition and control of safe UHDR beam delivery
Safe UHDR-RT delivery is defined as the delivery of radiation to a tissue volume in accordance with the protocol-defined dose, dose rate, and radiation modality and the approved spatial dose distribution from the prescribed treatment plan. Although the safety of modern radiotherapy systems has improved dramatically over time, UHDR beam delivery control and safety still pose unique challenges pertaining to the instantaneous nature of UHDRs. UHDR beam intensities are several hundreds to thousands of times greater than those of conventional clinical beams(37). For electron beams, each pulse (with a repetition period of milliseconds and duration of several microseconds) carries sufficient electrons to cause severe ion recombination in conventional transmission MU chambers, which are used for beam monitoring in CONV-RT. Such ion recombination effects need to be characterized and compensated for accurate recording of the delivered dose(71, 72). Although correlations of the UHDR-RT dose, dose rate, and dose distribution with biological effects are under investigation, the potential hazards of various UHDR-RT delivery failures are discussed in this section. Recommendations are made for the tolerance of delivery parameters based on the frequency and severity of potential failures in CONV-RT.
The protocol-defined dose is to be delivered correctly under UHDRs. Delivery machine performance must be stable and consistent with commissioning values. This includes characteristics that drive the physical dose distribution, such as depth dose/beam energy, dose linearity, spot position accuracy, output factors, and others. There is no current evidence to support deviation from current TG-40, TG-142, and TG-224 (72–74) guidelines, so at present these remain the most reasonable tolerances. Other characteristics for UHDR-RT are influenced by the dose rate. These require some additional consideration:
Output dependency on the dose rate in the monitoring MU chamber
The high instantaneous current in a pulsed beam causes severe ionization recombination in most transmission-type monitoring MU chambers. The two-voltage method and Boag’s method(73, 74) are inadequate for the correction of ion recombination loss at this level. We recommend that a MU chamber with a flat (or fully characterized) response to all possible dose rates and pulse structures be used for UHDR-RT to maintain <3% output variation.
Motion management
The active management of intra-fractional motion and residual setup error is critical to minimize delivery error in hypofractionated UHDR-RT. The rapid delivery of UHDR treatments to fields covering human patient tumor targets can increase the likelihood of partial misses in the presence of target motion. Passive and active motion control and/or gated beam delivery from CONV-RT protocols need careful consideration and implementation for accuracy in clinical trials(75). Motion management techniques should verifiably constrain residual motion to a magnitude much less than that of the target dimensions. The use of respiratory gating techniques is possible with the QA verification of reproducible target positioning, residual motion, and beam triggering in the gating window. These motion management tools should be credentialed prior to clinical trial participation via an end-to-end moving phantom test.
Image-guided treatment delivery
The verification of the patient setup and means of immobilization are of critical importance. Conventional kV, cone-beam computed tomography (CBCT) and/or four-dimensional CBCT imaging continue to be important for setup(56). Real-time image guidance provides clear benefit to ensure the patient is correctly positioned at the time the radiation is delivered; as a minimum, image guidance immediately before radiation delivery would be necessary to ensure that the setup is proper and the target remains inside the desired radiation portal while the beam is on. However, real-time imaging for motion detection requires a sufficiently fine temporal resolution of a duration much shorter than that of beam delivery, which is very challenging with current clinical imaging systems(76). Continuous surface imaging can serve as an alternative for the monitoring of intra-fractional target motion; the correlation between the surface position and the internal anatomy must be verified(77). Emerging in-vivo and functional imaging techniques(57, 66, 78) are promising for the monitoring of pulse-to-pulse beam delivery. Image guidance credentialing should be mandatory for any FLASH clinical trial.
Accessories
Clinic processes and workflows using treatment or patient specific matching accessories for UHDR-RT, such as the ridge filter and aperture, can be beneficial and developed. Such devices require robust positional quality control and any change in the latching status would need to result in the immediate termination of the beam.
Shielding
UHDR-RT increases the instantaneous dose rate by several orders of magnitude. In order to accrue patients to a UHDR-RT trial, it will be necessary that participating institutions have shielding in place that can meet regulatory requirements for UHDR beams (79, 80).
Dosimeter requirements
Dose measurement
The introduction of UHDR beams raises new dosimetry challenges(70, 81). Dosimeters for UHDR beams need to record the doses and dose rates accurately and reliably. The current clinical reference dosimetry tools for calibration, verification, and QA are air-filled ionization chambers (ICs) that are traceable to national standard laboratories(74, 82, 83). However, ICs are known to exhibit dose rate–dependent ion recombination effects(84), and great care and scrutiny are required when they are used for reference dosimetry under UHDR beams. An ideal UHDR dosimetry system must have well-defined dose-response curves and dose rate independence or a well-characterized dose rate dependence relationship. It should have little energy dependence or have dose and dose rate response curves characterized under specific beam energies. In addition, a dosimetry system used to measure the flatness and symmetry of UHDR lateral beam profiles needs to provide sufficient spatial resolution for this purpose. The use of many traditional dosimeters with UHDR beams has been explored(81) (Table 2). The upcoming AAPM report on FLASH dosimetry (TG 359) will provide further review and recommendations for dose measurement.
Table 2.
Dosimeters and their characteristics for electron and proton UHDR beams.
| Dose-rate Independent | Radiation Damage | 2D spatial resolution | LET independent | Readout immediately after delivery | Macropulse Dose information | |
|---|---|---|---|---|---|---|
| Radiochromic film | Y | No reuse | Y | N | N | N* |
| OSLD | Y | N | Y* | Y | N | N |
| TLD | Y | N | N | Y | N | N |
| Alanine | Y | N | N* | Y | N | N |
| Calorimeter | Y | N | N | Y | Y | Y* |
| Silicon diode detector | Y* | Y | Y* | Y | Y | Y* |
| Diamond detector | Y* | N | N* | Y | Y | Y* |
| Cylindrical ion chambers | N | N | N | N | Y | N* |
| Thin-gap parallel plate chamber | Y for P | N | Y | N | Y | Y* |
| Small volume cylindrical ion chamber | Y for P | N | Y* | N | Y | Y* |
| Faraday cup | Y | N | N | Y* | Y | Y* |
| Beam current transformer | Y | N | N | Y | Y | Y* |
| Plastic scintillator | N | Y | Y | N | Y | Y* |
| Inorganic scintillator | Y* | N* | Y* | N | Y | Y* |
Y - Yes, demonstrated and in use; Y* - Yes, but with caveats or to be demonstrated; N* - No, except for highly specialized settings or most likely not; N - No.
Dose rate measurement
Another important component of UHDR dosimetry is dose rate measurement. Given the lack of a well-established definition of the dose rate (see Section FLASH-RT delivery reports), a definition must be set at the initiation of a clinical trial and adhered to throughout the trial duration to facilitate the acquisition of consistent and reproducible observations. The dose rate dosimetry system must record the differential dose accumulation history and associated timing information. It must be verified and validated against an independent measurement to verify compliance with the dose rate defined in the trial protocol.
The selection of a dose rate verification technique depends on the radiation type and delivery modality. For instance, a broad UHDR electron field differs from a proton PBS field, temporally and in terms of the spatial structure. In addition, the dose rate definition may influence the optimization of UHDR treatment plans (see Section Treatment plan reporting). Thus, UHDR implementation should be verified with time-resolved dose measurements using a sampling rate that is suitable for the time structure of beam delivery. For pulsed beam delivery, if the micropulse time structure is too short to be feasibly captured at sub-microsecond time scale, then the dose reading rates should be sufficiently fast to reproducibly capture the macropulse structure with another system recording only the pulse timing.
Statically collimated electron and passive scattered proton fields can be considered to be spatiotemporally constant, which allows the extrapolation of a point measurement of the dose rate to the rest of the field. Various systems have been used to obtain UHDR delivery timing information; examples include a monitoring chamber(34), a diamond detector(85, 86), radioluminescence and Cherenkov emission(87, 88), and a beam current transformer(58). If the beam on and off signals are obtained from another system (e.g., beam control electronics), then the mean dose rate can also be inferred with a passive dosimeter.
Spatiotemporally varying beams, such as those employed in proton PBS, pose additional challenges for dose rate measurement. The dose from one PBS spot contributes varying amounts to the entire volume with a time structure characteristic of the PBS pattern (Figure 1b)(30, 63). Thus, at least 2D planar time-resolved dose measurement with sufficient spatial resolution is required to detect the dose modulation and gradients over time. Current log files that vendors build into systems can provide time-resolved 2D spot delivery information(58, 63), but the sampling frequency needs to be adapted to a time scale shorter than that of UHDR spot or pulse delivery (i.e., a few to tens of microseconds).
To extract the spatial dose and dose rate information, software (independent or integrated with the treatment planning system) should be available for the analysis of the delivered timing and dose information, display of a 2D or 3D dose rate map, and calculation of protocol-specific dose rate metrics analytically or via Monte Carlo simulation. The one-dimensional point measurement can then be extrapolated with a known 2D dose profile, such as percentage depth dose data, from the TPS or commissioning data(46, 55).
In summary, FLASH-RT clinical trials impose new and challenging requirements on dosimeters, which must record the integral dose, time-resolved point dose, or 2D dose delivery accurately, reproducibly, and at sufficient sampling frequency under UHDR beams (Table 2). Various efforts are ongoing, and further development is needed to test and validate suitable systems for UHDR beam reference dosimetry, characterization, and monitoring in FLASH-RT clinical trials.
FLASH-RT system credentialing and QA
Imaging and Radiation Oncology Core credentialing
Particularly in multi-institutional clinical trials, it is important to ensure that all institutions can and do deliver the intended treatment accurately and consistently(89). To be eligible for participation in NCTN clinical trials, institutions are required to undergo 1) site qualification (e.g., annual output checks, which all institutions perform and is a prerequisite for participation in any trial) and 2) credentialing (e.g., IMRT phantom irradiation under defined conditions, which is performed by potential participating institutions in response to the requirements of specific protocols). The Imaging and Radiation Oncology Core (IROC) manages both of these steps for NCTN trials(90). We anticipate that the same structure will be required for NCTN FLASH/UHDR RT clinical trials(56).
Site qualification should involve a high-level review of the institution’s capabilities and clinical practices. As it would be specific to the UHDR machine for a FLASH-RT trial, prior institutional qualification based on a standard linac or proton accelerator would not be sufficient. For FLASH-RT clinical trials, site qualification should include:
Ensuring that the UHDR machine is FDA approved or covered by an IDE.
Ensuring that the UHDR machine is capable of delivering the dose distribution and dose rate required by the protocol.
Ensuring that the appropriate dosimetry and ancillary equipment (e.g., ADCL-calibrated detectors, image guidance, motion management systems) is available for accurate UHDR delivery.
Ensuring that the programmatic practices (e.g., disease sites treated, planning techniques, typical margins, application of recording and verification systems) meet the guidelines established by the NCI and IROC.
Confirming basic dosimetry and beam timing characteristics.
Items 1–4 could be assessed using questionnaires and would rely on expert consensus developed through preclinical trials and with NCTN PIs. Broad information on FLASH-RT intent (i.e., treatment prescriptions) and delivery systems, for example, would facilitate IROC’s evaluation of the treatment planning, delivery, and verification processes at a given institution, and might provide insight into how community standards compare to clinical trial objectives. The information that should be collected includes 1) the general characteristics of the UHDR beam delivery system (e.g., beam pulsing structures and dose rate definition); 2) measurement results related to absolute dosimetry at different dose rates, including conventional clinical dose rates; and 3) descriptions of procedures for FLASH-RT prescription, pre-treatment QA, and handling of treatment interruptions.
The independent confirmation of basic dosimetry and beam timing characteristics is more involved, requiring remote or on-site auditing, and represents a major current gap in knowledge and capabilities. This task is performed annually for all machines at conventional dose rates; less-frequent monitoring for UHDR modalities is difficult to imagine, but annual on-site auditing would quickly become impractical. The independent auditing of delivered doses is well established and could likely be extended directly to UHDRs. The most challenging, and likely most important, component of dosimetry auditing would be the verification that the desired dose rate is achieved. This issue is complicated by the lack of a standard UHDR definition. At minimum, ensuring that one well-defined UHDR is achieved would be essential. However, such testing may be very challenging to implement remotely. On-site auditing is technically more straightforward but may be cost prohibitive. Ideally, and particularly as questions about the dose rate required remain unanswered, quantitative documentation of the pulse structure, dose per pulse, and other dose rate metrics is critical. Credentialing requirements will be developed and included in each specific protocol.
Protocol-specific credentialing involves deeper investigation of the institution’s capabilities and treatment process through the end-to-end testing of the treatment simulation, planning, and delivery. This process verifies that the intended physical dose and dose rate are delivered to the intended location, and it should be performed under clinically realistic conditions. At present, FLASH-RT trials are conducted with relatively simple clinical conditions and single unmodulated fields. This setting should guide current credentialing requirements (regardless of the treatment modality). For treatment planning algorithms that have been previously credentialed in non-UHDR settings, there may not be a need to re-test complex heterogeneities or structures when the dose rate is the only part of the delivery changing (although the optimization of the dose and dose rate is desirable). Credentialing requirements for a given protocol may extend beyond end-to-end testing (e.g., IGRT credentialing); such testing should be considered a minimum requirement.
One gap in the IROC’s current credentialing practice is related to the review of electron treatments. Although the electron output is checked regularly for reference conditions, no electron phantom is currently used for protocol-specific credentialing. Additionally, electron treatment planning algorithms have not been reviewed; in contrast, the use of only certain proton and photon algorithms is allowed in clinical trials. Thus, electron UHDR treatments may require more comprehensive credentialing than photon or proton UHDR protocols do.
As for non-UHDR clinical trials, all UHDR-RT site qualification and credentialing should be independent and consistent. The IROC should provide the entire measurement system (including the phantom and dosimeters) to facilitate the measurement of the dose and dose rate for a given beam delivery modality.
Other scientific and logistical questions also exist. Should credentialing be conducted more than once to verify the delivery system’s ongoing or long-term stability? If multiple machines or types of machine are used at a single institution, should unique credentialing of every machine be required or is complete credentialing of a single machine sufficient? What tolerance should be used in credentialing testing, particularly for dose rate evaluation? These issues require not only scientific evaluation, but also practical consideration, given the nature of clinical trials. Finally, credentialing should evolve to include more complex anatomical conditions as FLASH-RT trials evolve, for example to include moving targets, conformal or multi-field treatments.
IROC case review
Patient-specific case reviews for clinical trial enrollment is another major component needed to ensure trial consistency and quality. In addition to the procedures required for the evaluation of planned RT using conventional dose rates, UHDR treatment plan evaluation needs to be voxel based, including examination of the dose delivery time structure and the parameters associated directly with this treatment (e.g., the beam intensity, beam delivery sequence, and, when used, proton PBS scanning pattern and speed). The protocol and site evaluation should include appropriate UHDR definitions. Close coordination with clinical practice is essential.
Machine QA
The AAPM task group reports (91, 92) suggest various tests and test frequencies for the safety, dosimetric and spatial components of clinical RT systems. Imaging system QA for image-guided treatment delivery were also established clinically and should be followed(91–94). However, these established guidelines do not account for the unique consideration of UHDR therapy units. Moeckli et al.(53) reported the establishment of the IntraOp electron beam QA program, which entails output and energy constancy checks, based on the AAPM TG-72’s recommendations(95). A UHDR QA program in a clinical trial setting ensures that clinical trials are conducted accurately, while generating data that is reliable and sufficient to answer the questions that are the focus of the clinical trial. A UHDR QA program incorporates the elements of a conventional delivery QA program, while adding specific considerations for what makes UHDR different – namely, high dose rates. Important to note, electron and proton UHDR-RT systems are fundamentally different, potentially requiring different approaches, methods and tolerances in a modality-specific UHDR QA program. Given the infancy of the UHDR clinical trial environment, it is beyond the scope of this publication to recommend specific QC tests and tolerances. Clear and effective QA recommendations are a subject of research in the UHDR community to incorporate data-driven, FMEA principles, such as those recommended in AAPM TG-100. That said, since dose, temporal and spatial characteristics are the most critical parameters to any UHDR clinical trial, QC and tolerances for those parameters should be developed as part of a UHDR QA program that validates, tests, and reports the constancy of machine delivery parameters with appropriate dosimetry systems (see Section Dosimeter requirements).
In addition, treatment delivery records must be maintained for future study and evaluation, as the definition of the dose and dose rate for FLASH-RT effects is still evolving, especially for proton PBS delivery. Treatment records shall conform with, following the recommendations provided in Section FLASH-RT delivery reports and can be from the dosimetry systems discussed in Section Dosimeter requirements.
Patient QA
Patient-related, UHDR delivery pre-treatment QC performed according to current clinical standards(96, 97) for intensity-modulated x-ray, electron, and proton therapy is needed, while additional QC measurements that include delivery timing information are being developed. This ensures that not only the dose and spatial characteristics but also the protocol-defined dose rate meet the clinical trial requirements. Just as for machine QA, the UHDR modalities and beam delivery methods are heterogeneous, requiring each specific UHDR therapy machine to have a modality-specific patient-specific QA program. Point and planar dose rate QC, particularly compared to the treatment planning system, are important for satisfying the clinical trial design and ensuring reliable data. The clinical trial protocol should include clear patient-specific QA program. Appropriate, and currently limited, detector systems capable of resolving dose and dose rate should be used to ensure trial data quality and patient safety.
Treatment plan QA
General plan and chart review guidelines for initial, weekly, and end-of-treatment checks have been provided by Task Group 275(102). Aspects evaluated include the data transfer integrity, accuracy of calculations, image guidance requests, and plan quality. FLASH-RT should follow these recommendations, while also including UHDR treatment plan–specific parameters, such as the protocol-specific dose rate, dose per pulse, and pulse width. Any second and independent calculation check should also include these parameters. UHDR planning systems incorporating dose and dose rate distribution overlaying a patient image should be displayed and evaluated. A dose and dose rate volume histogram should also be displayed and evaluated. Furthermore, the tissue-specific relative biological effect of a given UHDR modality may be incorporated into the planning system. This effect should be noted in the clinical trial protocol.
Plan and chart review
The physicist should check the plan to ensure that the dose and dose rate distributions reflect the protocol specifications. The physicist should also perform standard plan and chart reviews to ensure that the requested imaging guidance and motion management would ensure safe UHDR delivery. The analysis of UHDR treatment plans’ robustness will need to be expanded from standard robustness analysis to include the reliability of defined dose rate achievement.
The physicist should also review the plan with the physician to ensure that if the UHDR is not achieved during RT, the plan will still be safe for target irradiation with adequate sparing of normal tissue as with conventional dose rates.
Special QA considerations
The end effect, partial treatment functionality, and log file QA are relevant topics for CONV-RT, but essential for UHDR treatments.
End effect dose
In 10CFR35, the NRC defines a medical event by, among other indications, misadministration exceeding 20% of the total prescribed dose or 50% of the prescribed fractional dose(103). These tolerances are often extended to non-isotopic treatments by various state regulatory agencies. Furthermore, IEC 60601–2-64 sets the standard that no accelerator shall deliver >10% of the dose after an interlock is triggered(104). The end effect or shutter dose is quantifiable, defined as the residual dose delivered from the time of interlock detection to that of dose termination(105, 106). Modern CONV-RT machines readily meet these requirements. However, for UHDR-capable machines used for FLASH-RT, the primary beam monitor and its interlock triggering software and hardware will need to have much faster responses than do CONV-RT setups and will need to be established for UHDR delivery to correctly record delivery dose and timing information. Due to the extremely short (millisecond-order) beam-on times, it is impractical, if not impossible, to test the door interlocks or manual emergency beam interruption under FLASH-RT delivery conditions. These tests may be performed with irradiation in CONV-RT mode if standard dose rate beams are available(53).
Partial treatment
Partial treatment is defined as the partial irradiation of a prescribed treatment field due to treatment interruption (e.g., interlock triggering). For UHDR delivery, partial treatment recovery breaks the prescribed field to be delivered into two or more distinct and interrupted fields. The UHDR machine must be able to reconstitute the prescribed field dose from the partial fields, verified with beam monitoring dosimeters. However, partial delivery likely means that the protocol-specific dose rate will not be achieved, and can affect the biological effect of FLASHRT in the patient. The interval between partial treatment occurrence and treatment resumption should be recorded. Extra care must be taken to ensure that any FLASH-RT clinical trial has a safety mitigation plan in place for partial treatment and irradiation. For example, the FAST-01 trial (Clinicaltrials.gov NCT04592887) (24, 25)was designed so that the physical dose of the prescribed field would be reconstructed in the event of an interlock, partial irradiation, or dose rate reduction, and then delivery to the rest of the field would be performed with CONV-RT, which is an acceptable standard of care.
Log file QA
QA using vendor-provided log files generated by treatment machine is an acceptable means of machine QA. With development and improvement, the beam monitors of UHDR machines may be the most comprehensive dosimeter systems capable of obtaining measurements such as time-resolved beam intensities and beam positions. Whereas the UHDR treatment commissioning process validates that the log files provide the timing resolution, beam intensity, and position monitoring with independent measurements suitable for specific UHDR modalities, the machine QA and daily QA procedures needs to involve the analysis of those log files before treatment. Furthermore, retrospective analysis of the log files, used solely or jointly with other QA metrology, can be performed to determine the beam dose-temporal distribution for recording and reporting purposes defined in Section FLASH-RT delivery reports that is a critical component to ensure safe FLASH-RT delivery in clinical trials.
Treatment plan reporting
Modern CONV-RT treatment planning can generate 3D dose distributions in voxel-based patient images. In typical RT clinical trials, the reporting and sharing of the structures and 3D dose files are needed, usually in standard DICOM format, are required(107, 108). These steps facilitate more detailed and flexible dose analysis for tumors and organs than can be performed with traditional reports on DVH indexes. The analysis and re-examination of the 3D dose distribution potentially allow for improved correlation of clinical outcomes with delivered doses, and the outcome findings and dose distribution can be overlaid on patient images. Thus, the provision/reporting of patient images, target and normal organ contours, and 3D dose distributions is required for FLASH-RT clinical trials.
For UHDR treatment planning, the protocol-specific dose rate should be defined, and is expected to be calculated and reported at the voxel level along with timing information for the expected differential dose delivery. Reporting of the 3D dose rate distribution will enable systematic analysis of the combined impact of the dose and dose rate for examination of the FLASH-RT sparing effect in patients in short- and long-term studies. The 3D dose rate and associated beam delivery information in the treatment plan are recommended to be included in DICOM plan information in standardized format. Plan reporting and evaluation should include voxel-based dose delivery time structure and parameters associated directly with UHDR delivery (e.g., the beam current, spot delivery sequence, scanning speed, and devices used to modify the beam), which are required to generate the dose rate defined in the treatment plan. The time structure of beam delivery is essential for the evolving definition of the FLASH-RT dose rate.
Although their reporting is not required, it is expected that the dose and dose rate distribution will be optimized in the treatment plan(69). Various proton FLASH treatment strategies have been investigated, with consideration of the beam properties with various dose rate definitions, including beam-specific device designs(29–32). In addition, it is expected that certain modeling of FLASH-RT biological effects will be considered in the dose and dose rate planning and optimization(109).
Personnel recommendations
The sheer speed with which UHDR-RT treatment delivery will likely necessitates higher staffing levels than traditional dose and dose rate therapies. UHDR-RT cases should likely require the presence of the physicist and physician for each treatment delivery, analogous to SBRT treatments. The physicist should verify all treatment plan parameters prior to each beam as well as the pre-treatment imaging and alignment in an online fashion. In addition, physicists should be responsible for performing and verifying that the appropriate machine QA and patient specific QA was performed prior to each UHDR-RT treatment. When possible, 3D imaging such as CBCT should be employed to appreciate the localization to the target and proximity of nearby organs at risk prior to each UHDR-RT fraction. It is critical that for each of the treatments, the attending physician reviews and approves the target localization, proximity of nearby organs at risk, prescription, dose and dose rate prior to beam delivery.
Investigator training recommendations
While several institutions are conducting extensive research in FLASH, there will likely be a large number of FLASH-capable machines available in the near-future. This means that many clinics will treat FLASH patients – including clinical trial patients - without prior FLASH training or clinical experience. As multi-institutional clinical trials for FLASH begin to accrue patients, it will be imperative that participating institutions have training specific to the complexities of the trial. For example, NCTN protocols for proton therapy often include more frequent PI meetings to discuss lessons learned and protocol deviations observed during the initial accrual to the trial. These collaborative training sessions help reduce deviation rates on the protocol and are highly recommended for NRG FLASH trials.
Summary and discussion
Preclinical research conducted with small animal models has shown that FLASH-RT has the potential to improve the therapeutic ratio between the tumor response and normal tissue toxicity. As interest in the incorporation of FLASH RT into clinical practice builds, we anticipate that many FLASH-RT clinical trials will open in the future. In this summary from the NCFWG on considerations for such trials, we review the current status of UHDR deliveries, identify technological gaps, and recommend standards that should be adhered to. The challenges and technical gaps that must be considered for FLASH-RT clinical trials and future outlooks are summarized in Table 3. The discussions in this article focused on electron and proton UHDR modalities. With the development of UHDR delivery with other modalities, such as heavy-ion and photon beams(16, 44, 51, 110), future work in the credentialing of FLASH-RT clinical trials based on these modalities is anticipated.
Table 3.
Summary of challenges and technical gaps for FLASH-RT clinical trials and future outlooks.
| Challenges and technical gaps | Future outlook | |
|---|---|---|
| Reporting parameters | ∎ Reporting the delivery time structure is not needed in current clinical practice; ∎ UHDR-RT systems have drastically different beam structures; Multiple definitions of dose rates exist; ∎ No clear understanding on if FLASH dose threshold exists and which definition of dose rate is related to the observation of FLASH effects. |
∎ Monitor the beam delivery current and sequence at a resolution that resolves every pulse and at finer resolution if possible. ∎ Build a recording and reporting system to derive and report the 3D voxel-based differential dose delivered with monitored beam current and time structure. |
| Dosimetry | ∎ Traditional ion chamber for reference dosimetry suffers from ion recombination effects under UHDR irradiation; ∎ Various dosimeters are being tested for their accuracy and reliability in dose measurements under UHDR; ∎ Systems that record UHDR delivery time structure are also under development. |
∎ Evolving dosimetry technologies are to be developed to reliably measure the integral dose, differential dose with associated timing structures; ∎ Uncertainties in the UHDR dose and dose rate measurements for various modalities are to be assessed; ∎ Establish dosimeter reference standard for UHDR beams. |
| Safety | ∎ Beam control system needs to have much quicker response to beam interruption and termination; ∎ Stability and repeatability in the deliveries of desired dose and dose needs to be checked; ∎ Safety on patient partial delivery; ∎ Facility shielding needs to be assessed for UHDRRT treatments. |
∎ UHDR system development groups and vendors work on solutions for faster beam controls, partial delivery monitor and treatment resume process. ∎ Use of appropriate dosimetry system to check the UHDR beam qualities; ∎ Shielding assessment under the work load of FLASH clinical trials. |
| IROC Quality Assurance | ∎ There is no established standards for UHDR related parameters; ∎ Appropriate dosimetry for UHDR dose and dose rate measurements is under development; ∎ Appropriate phantoms for credentialing are needed; ∎ Patient specific case review for clinical trial enrollment |
∎ Develop IROC/NIST traceable standards for dose and dose rate and validate in UHDR radiation systems; ∎ Appropriate phantoms are to be developed and used for end-to-end UHDR treatment credentialing; ∎ All site qualification and credentialing should be independent and consistently performed; ∎ Patient specific case review should include time structures for voxel level dose rate analysis and verification |
| Treatment plan | ∎ current TPS does not generate user-defined, voxel based dose rate in patient treatment volume by incorporating the beam delivery time structure; ∎ Similar to DVH, dose-rate-volume-histogram needs to be developed and displayed; ∎ Optimization on both dose and protocol defined dose rate. ∎ FLASH biological driven planning is likely to be necessary. |
∎ TPS vendors work with FLASH clinical community on UHDR treatment planning including calculation, optimization and reporting of the dose and dose rate; ∎ FLASH biological driven TPS using established relationship of FLASH sparing effects with treatment dose and dose rates. |
| Clinical QA | ∎ Machine QA on UHDR related parameters were not established; ∎ Patient specific QA on UHDR related parameters such as dose and dose rate in point, 2D and 3D needs to be established with appropriate dosimetry system; ∎ QA on the workflows under special situations such as partial treatment, needs to be established. |
∎ Stabilities of the beam parameters dictate the frequency of the QA on these parameters. ∎ Develop complete machine and patient QA programs including UHDR related parameters; ∎ Vendor provided log files can be an important component in establishing the QA programs. |
| IGRT and motion management | ∎ Patient setup and image guidance for UHDR delivery is challenging. ∎ Motion management for UHDR delivery needs development. |
∎ Develop motion control and mitigation strategies in treatment planning and delivery; ∎ Develop ultra fast and pulse-based in vivo dosimetry; ∎ Develop advanced functional and biological image guidance. |
Every new modality comes with benefits and challenges. FLASH treatment is delivered in a fraction of a second, much shorter than in current clinical practice. The understanding of the radiobiology, fractionation, treatment modalities, dosimetry, and QA for UHDRs is evolving. As described in Section FLASH-RT delivery reports, the use of multiple UHDR modalities and systems for FLASH-RT have been explored. FLASH clinical trials conducted by the NRG oncology cooperative group and other trial groups will require IROC credentialing of UHDR systems, and likely an investigational device exemption (IDE) from the FDA for these delivery systems. Although the current standard/basic clinical trial–specific requirements for conventional treatment reported in various NRG and other groups’ clinical trial protocols and publications (56) still hold, FLASH-RT clinical trial–specific requirements need to be developed and validated. The committee has recommended QA, monitoring, and reporting of critical UHDR treatment parameters, such as the beam intensity and delivery time structure, dose per pulse or PBS spot, and integral dose in 3D voxel-based treatment volumes. These requirements, in turn, add new challenges for current dosimetry systems and open up opportunities for the development of dosimetry technology for the measurement and recording of differential UHDR doses with time stamps. Measurement uncertainties in UHDR dosimetry systems need to be understood. Concerns about patient safety should be addressed during clinical trial design, with a protocol and validated mitigation strategy defined for the case of any potential discrepancy or deviation from the prescribed dose and dose rate. Image guidance and motion management for UHDR treatment need to be developed. Machine and patient-related QA programs are needed to address UHDR treatment–specific aspects with appropriate dosimetry systems. Further development of treatment planning systems and plan reports is needed for dose and dose rate optimization and calculation with the incorporation of machine- and beam-specific delivery parameters.
Currently, many questions about the effects of FLASH-RT remain. The existence of an UHDR dose threshold, the dose rate threshold for the observation of FLASH-RT effects, and acceptable and optimal treatment fractionation schemes remain unclear. Multiple definitions of the dose rate exist, especially for PBS treatment. Many preclinical studies of FLASH radiobiology mechanisms are ongoing. FLASH-RT effects may be tissue specific under certain UHDR delivery parameters. These questions may be answered in part by designing clinical trials by incorporating specific UHDR parameters prospectively in the prescription. The optimal design of such trials is an important question outside the scope of this report. The task of credentialing and quality assurance, which is the focus of this report, is to ensure treatments are reproducibly delivered according to the UHDR prescription across institutions. Correlations of the outcomes of FLASH-RT clinical trials can be performed by retrospectively analyzing reported parameters as specified in the protocol and measured during credentialing, QA, and delivery. The medical physics community needs to work together with physicians, radiobiologists, UHDR treatment system providers, and treatment planning system vendors to bridge the aforementioned technical gaps and to perform FLASH-RT clinical trials so that patients can benefit from the improved therapeutic ratio that FLASH-RT provides.
Acknowledgments:
Special thank you to Jennifer Piehl for proofreading the manuscript.
Funding:
NIH funding support 2U24CA180803–06, 2U10CA180868–06, P01CA257904–01A1.
Footnotes
Conflicts of Interest:
Drs Ayan, Buchsbaum, Kry, Li, Lu, Surucu, and Yu have nothing to declare.
Dr Bazalova-Carter declares in the past 36 months Grants or contracts from New Frontiers in Research Fund – Exploration – Canadian Tri-Council Canada Research Chairs Program.
Dr Bosch declares in the past 36 months Grants or contracts from PCS-1403–12804, PCS-2017C1–0422 (Mendenhall), Massachusetts General Hospital Consortium Agreement (Efstathiou) PARTIQoL, U24 CA 180803, U10 CA 180833, American Association of Physicists in Medicine-Service Contract.
Dr Diffenderfer declares in the past 36 months Grants or contracts from NIH/NCI 1P01CA210944–04 and NIH/NCI1P01CA257904–01A1.
Dr Dong declares in the past 36 months Grants or contracts from NIH 1P01CA257904–01A1, IBA Sponsored research on FLASH proton therapy, Speakers Bureaus from Varian Medical Systems.
Dr Gladstone declares in the past 36 months Grants or contracts from NCIP30CA023108, R01EB023909, U01 CA260446–01A1, R42CA224646–02, US Patent No. US10,201,718 B2, 2/12/2019, WO2016176265 A1, US20140114150 A1.
Dr Loo declares Since the initial planning of the work a grant from NCI P01CA244091, declares in the past 36 months Grants or contracts from Varian Medical Systems to Stanford University, cofounder, and board member of TibaRay.
Dr Maity declares in the past 36 months Grants or contracts from P01 CA257904 Translational Studies in FLASH Particle, Payment, or honoraria Sept. 2019 – given honorarium for giving lecture “Proton FLASH Radiotherapy at Penn” at IBA Proton Therapy Conference Radiotherapy.
Dr Mascia declares in the past 36 months Grants or contracts from Varian Medical Systems-Sponsored research projects; payment made to institution, Speakers’ Bureau; payment to institution from Varian Medical Systems.
Dr Maxim declares in the past 36 months Grants or contracts from NCI PO1CA244091, Founder (no payments) TibaRay, Inc.
Dr Moros declares in the past 36 months Grants or contracts from Submitted R01 grant in the Fall of 2021 to NCI on the application of radiation induced acoustics to FLASH monitoring.
Dr Petersson declares Since the initial planning of the work a grant from Medical Research Council (MRCMRC [MC_UU_00001/9] and MR/X006611/1), Cancer Research UK (RadNet Grant [C6078/A28736], declares in the past 36 months Member of the Scientific Advisory Board on FLASH for IBA. Unpaid position.
Dr Pollard-Larkin declares in the past 36 months Member of AAPM Board of Directors.
Dr Schueler declares in the past 36 months Grants or contracts from Supported in part by Cancer Center Support Grant P30 CA016672 from the National Cancer Institute of the National Institutes of Health, to The University of Texas MD Anderson Cancer Center, University Cancer Foundation via the Institutional Research Grant program at the University of Texas MD Anderson Cancer Center, The University of Texas MD Anderson Cancer Center, Division of Radiation Oncology, Support from Varian for travel, Support from IntraOp Medical for travel.
Dr Schuemann declares in the past 36 months Grants or contracts from National Institute of Health / National Cancer Inst. Damon Runyon Foundation, The Brain Tumour Charity, Damon Runyon Foundation, Leadership, or fiduciary role at Radiation Research Society.
Dr Sharma declares in the past 36 months Grants or contracts Sponsored Research Project from Sientra Inc.
Dr Simone declares in the past 36 months Honorarium and FlashForward Consortium Clinical Chair from Varian Medical Systems.
Dr Taylor declares in the past 36 months Grants or contracts from NCI grant 180803, Leadership or fiduciary role at Healthcare for the Homeless-Houston.
Dr Tsien declares in the past 36 months Payment or honoraria for Varian (2021), Support for attending meetings and/or travel from Zeiss (2022).
Dr Xiao declares in the past 36 months Grants or contracts from NCI U24CA180803–06(IROC) and 2U10CA180868–06(NRG).
Dr. Zhang declares in the past 36 months Grants or contracts from NCI U01 CA260446.
Dr Zhao declares in the past 36 months Grants or contracts from Varian, Mevion and NIH paid to their Institution, Data Safety Monitoring volunteer for Mevion.
Dr Zou declares in the past 36 months Grants or contracts from NCI P01CA257904, NIH R01HL-148272–01A1, R01HL152707, Varian Grant Support.
Author responsible for statistical analysis: N/A, no need for statistical analysis
Disclaimer- This article represents the opinions of the authors. It does not represent the opinion or policy of the National Institutes of Health of the US Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014;6:245ra93–245ra93. [DOI] [PubMed] [Google Scholar]
- 2.Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiotherapy and Oncology. 2017;124:365–369. [DOI] [PubMed] [Google Scholar]
- 3.Vozenin M-C, De Fornel P, Petersson K, et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res. 2019;25:35–42. [DOI] [PubMed] [Google Scholar]
- 4.Montay-Gruel P, Bouchet A, Jaccard M, et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiotherapy and Oncology. 2018;129:582–588. [DOI] [PubMed] [Google Scholar]
- 5.Vozenin M-C, Hendry JH, Limoli CL. Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin Oncol (R Coll Radiol). 2019;31:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diffenderfer ES, Verginadis II, Kim MM, et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int. J. Radiat. Oncol. Biol. Phys. 2020;106:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velalopoulou A, Karagounis IV, Cramer GM, et al. FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues While Preserving Sarcoma Response. Cancer Res. 2021;81:4808–4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggold JT, Chow S, Melemenidis S, et al. Abdominopelvic FLASH Irradiation Improves PD-1 Immune Checkpoint Inhibition in Preclinical Models of Ovarian Cancer. Mol Cancer Ther. 2022;21:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy K, Natarajan S, Wang J, et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci Rep. 2020;10:21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons DA, Lartey FM, Schüler E, et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiotherapy and Oncology. 2019;139:4–10. [DOI] [PubMed] [Google Scholar]
- 11.Soto LA, Casey KM, Wang J, et al. FLASH Irradiation Results in Reduced Severe Skin Toxicity Compared to Conventional-Dose-Rate Irradiation. Radiat Res. 2020;194:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karsch L, Pawelke J, Brand M, et al. Beam pulse structure and dose rate as determinants for the flash effect observed in zebrafish embryo. Radiother Oncol. 2022;173:49–54. [DOI] [PubMed] [Google Scholar]
- 13.Fouillade C, Curras-Alonso S, Giuranno L, et al. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-induced Senescence. Clin Cancer Res. 2020;26:1497–1506. [DOI] [PubMed] [Google Scholar]
- 14.Kim MM, Verginadis II, Goia D, et al. Comparison of FLASH Proton Entrance and the Spread-Out Bragg Peak Dose Regions in the Sparing of Mouse Intestinal Crypts and in a Pancreatic Tumor Model. Cancers (Basel). 2021;13:4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu H, Xie D, Yang Y, et al. Radioprotective effect of X-ray abdominal FLASH irradiation: Adaptation to oxidative damage and inflammatory response may be benefiting factors. Med Phys. 2022;49:4812–4822. [DOI] [PubMed] [Google Scholar]
- 16.Gao F, Yang Y, Zhu H, et al. First demonstration of the FLASH effect with ultrahigh dose rate high-energy X-rays. Radiother Oncol. 2022;166:44–50. [DOI] [PubMed] [Google Scholar]
- 17.Sørensen BS, Sitarz MK, Ankjærgaard C, et al. Pencil beam scanning proton FLASH maintains tumor control while normal tissue damage is reduced in a mouse model. Radiother Oncol. 2022:S0167-8140(22)00254–7. [DOI] [PubMed] [Google Scholar]
- 18.Pratx G, Kapp DS. Ultra-High-Dose-Rate FLASH Irradiation May Spare Hypoxic Stem Cell Niches in Normal Tissues. International Journal of Radiation Oncology*Biology*Physics. 2019;105:190–192. [DOI] [PubMed] [Google Scholar]
- 19.Durante M, Brauer-Krisch E, Hill M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. BJR. 2017:20170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington KJ. Ultrahigh Dose-rate Radiotherapy: Next Steps for FLASH-RT. Clin Cancer Res. 2019;25:3–5. [DOI] [PubMed] [Google Scholar]
- 21.Tubin S, Yan W, Mourad WF, et al. The future of radiation-induced abscopal response: beyond conventional radiotherapy approaches. Future Oncology. 2020;16:1137–1151. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Luo H, Zheng X, et al. FLASH radiotherapy: Research process from basic experimentation to clinical application. Precision Radiation Oncology. 2021;5:259–266. [Google Scholar]
- 23.Rohrer Bley C, Wolf F, Gonçalves Jorge P, et al. Dose- and Volume-Limiting Late Toxicity of FLASH Radiotherapy in Cats with Squamous Cell Carcinoma of the Nasal Planum and in Mini Pigs. Clinical Cancer Research. 2022:OF1–OF10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascia AE, Daugherty EC, Zhang Y, et al. Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases: The FAST-01 Nonrandomized Trial. JAMA Oncol. 2023;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daugherty EC, Mascia A, Zhang Y, et al. FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases (FAST-01): Protocol for the First Prospective Feasibility Study. JMIR Res Protoc. 2023;12:e41812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vozenin M-C, Montay-Gruel P, Limoli C, et al. All Irradiations that are Ultra-High Dose Rate may not be FLASH: The Critical Importance of Beam Parameter Characterization and In Vivo Validation of the FLASH Effect. Radiation Research. 2020;194. [DOI] [PubMed] [Google Scholar]
- 27.Montay-Gruel P, Acharya MM, Gonçalves Jorge P, et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clinical Cancer Research. 2021;27:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan J-L, Lee C, Wouters S, et al. Irradiation at Ultra-High (FLASH) Dose Rates Reduces Acute Normal Tissue Toxicity in the Mouse Gastrointestinal System. International Journal of Radiation Oncology*Biology*Physics. 2021;111:1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Water S, Safai S, Schippers JM, et al. Towards FLASH proton therapy: the impact of treatment planning and machine characteristics on achievable dose rates. Acta Oncologica. 2019;58:1463–1469. [DOI] [PubMed] [Google Scholar]
- 30.van Marlen P, Dahele M, Folkerts M, et al. Bringing FLASH to the Clinic: Treatment Planning Considerations for Ultrahigh Dose-Rate Proton Beams. International Journal of Radiation Oncology*Biology*Physics. 2020;106:621–629. [DOI] [PubMed] [Google Scholar]
- 31.Zhang G, Gao W, Peng H. Design of static and dynamic ridge filters for FLASH–IMPT: A simulation study. Medical Physics. 2022:mp.15717. [DOI] [PubMed] [Google Scholar]
- 32.Kang M, Wei S, Choi JI, et al. A Universal Range Shifter and Range Compensator Can Enable Proton Pencil Beam Scanning Single-Energy Bragg Peak FLASH-RT Treatment Using Current Commercially Available Proton Systems. International Journal of Radiation Oncology*Biology*Physics. 2022;113:203–213. [DOI] [PubMed] [Google Scholar]
- 33.Folkerts MM, Abel E, Busold S, et al. A framework for defining FLASH dose rate for pencil beam scanning. Med. Phys. 2020;47:6396–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou W, Diffenderfer ES, Ota K, et al. Characterization of a high-resolution 2D transmission ion chamber for independent validation of proton pencil beam scanning of conventional and FLASH dose delivery. Med Phys. 2021. [DOI] [PubMed] [Google Scholar]
- 35.Draeger E, Sawant A, Johnstone C, et al. A Dose of Reality: How 20 Years of Incomplete Physics and Dosimetry Reporting in Radiobiology Studies May Have Contributed to the Reproducibility Crisis. International Journal of Radiation Oncology*Biology*Physics. 2020;106:243–252. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz M, Traneus E, Safai S, et al. Treatment planning for Flash radiotherapy: General aspects and applications to proton beams. Medical Physics. 2022. [DOI] [PubMed] [Google Scholar]
- 37.Farr J, Grilj V, Malka V, et al. Ultra-high dose rate radiation production and delivery systems intended for FLASH. Medical Physics. 2022:mp.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourhis J, Sozzi WJ, Jorge PG, et al. Treatment of a first patient with FLASH-radiotherapy. Radiotherapy and Oncology. 2019;139:18–22. [DOI] [PubMed] [Google Scholar]
- 39.Patriarca A, Fouillade C, Auger M, et al. Experimental Set-up for FLASH Proton Irradiation of Small Animals Using a Clinical System. International journal of radiation oncology, biology, physics. 2018;102:619–626. [DOI] [PubMed] [Google Scholar]
- 40.Darafsheh A, Hao Y, Zwart T, et al. Feasibility of proton FLASH irradiation using a synchrocyclotron for preclinical studies. Med. Phys. 2020;47:4348–4355. [DOI] [PubMed] [Google Scholar]
- 41.Titt U, Yang M, Wang X, et al. Design and validation of a synchrotron proton beam line for FLASH radiotherapy preclinical research experiments. Medical Physics. 2022;49:497–509. [DOI] [PubMed] [Google Scholar]
- 42.Weber UA, Scifoni E, Durante M. FLASH radiotherapy with carbon ion beams. Medical Physics. 2022;49:1974–1992. [DOI] [PubMed] [Google Scholar]
- 43.Montay-Gruel P, Corde S, Laissue JA, et al. FLASH radiotherapy with photon beams. Medical Physics. 2022;49:2055–2067. [DOI] [PubMed] [Google Scholar]
- 44.Esplen N, Egoriti L, Paley B, et al. Design optimization of an electron-to-photon conversion target for ultra-high dose rate x-ray (FLASH) experiments at TRIUMF. Phys. Med. Biol. 2022;67:105003. [DOI] [PubMed] [Google Scholar]
- 45.Cecchi DD, Therriault-Proulx F, Lambert-Girard S, et al. Characterization of an x-ray tube-based ultrahigh dose-rate system for in vitro irradiations. Medical Physics. 2021;48:7399–7409. [DOI] [PubMed] [Google Scholar]
- 46.Rahman M, Ashraf MR, Zhang R, et al. Electron FLASH Delivery at Treatment Room Isocenter for Efficient Reversible Conversion of a Clinical LINAC. International Journal of Radiation Oncology*Biology*Physics. 2021;110:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szpala S, Huang V, Zhao Y, et al. Dosimetry with a clinical linac adapted to FLASH electron beams. J Appl Clin Med Phys. 2021;22:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schüler E, Trovati S, King G, et al. Experimental Platform for Ultra-high Dose Rate FLASH Irradiation of Small Animals Using a Clinical Linear Accelerator. International Journal of Radiation Oncology*Biology*Physics. 2017;97:195–203. [DOI] [PubMed] [Google Scholar]
- 49.Lempart M, Blad B, Adrian G, et al. Modifying a clinical linear accelerator for delivery of ultra-high dose rate irradiation. Radiother Oncol. 2019;139:40–45. [DOI] [PubMed] [Google Scholar]
- 50.Buchsbaum JC, Coleman CN, Espey MG, et al. FLASH Radiotherapy: New Technology Plus Biology Required. Int J Radiat Oncol Biol Phys. 2021. [DOI] [PubMed] [Google Scholar]
- 51.Maxim PG, Tantawi SG, Loo BW. PHASER: A platform for clinical translation of FLASH cancer radiotherapy. Radiother Oncol. 2019;139:28–33. [DOI] [PubMed] [Google Scholar]
- 52.Felici G, Barca P, Barone S, et al. Transforming an IORT Linac Into a FLASH Research Machine: Procedure and Dosimetric Characterization. Front. Phys. 2020;8:374. [Google Scholar]
- 53.Moeckli R, Gonçalves Jorge P, Grilj V, et al. Commissioning of an ultra-high dose rate pulsed electron beam medical LINAC for FLASH RT preclinical animal experiments and future clinical human protocols. Medical Physics. 2021;48:3134–3142. [DOI] [PubMed] [Google Scholar]
- 54.Whitmore L, Mackay RI, van Herk M, et al. Focused VHEE (very high energy electron) beams and dose delivery for radiotherapy applications. Sci Rep. 2021;11:14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Böhlen TT, Germond J, Traneus E, et al. Characteristics of very high-energy electron beams for the irradiation of deep-seated targets. Medical Physics. 2021;48:3958–3967. [DOI] [PubMed] [Google Scholar]
- 56.Taylor PA, Moran JM, Jaffray DA, et al. A roadmap to clinical trials for FLASH. Medical Physics. 2022;49:4099–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahman M, Ashraf MR, Zhang R, et al. Spatial and temporal dosimetry of individual electron FLASH beam pulses using radioluminescence imaging. Phys. Med. Biol. 2021;66:135009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonçalves Jorge P, Grilj V, Bourhis J, et al. Technical note: Validation of an ultrahigh dose rate pulsed electron beam monitoring system using a current transformer for FLASH preclinical studies. Medical Physics. 2022;49:1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schüler E, Acharya M, Montay-Gruel P, et al. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Medical Physics. 2022;49:2082–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashraf MR, Rahman M, Zhang R, et al. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Front. Phys. 2020;8:328. [Google Scholar]
- 61.Ashraf MR, Rahman M, Cao X, et al. Individual pulse monitoring and dose control system for pre-clinical implementation of FLASH-RT. Phys. Med. Biol. 2022;67:095003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahman M, Ashraf MR, Gladstone DJ, et al. Treatment Planning System for Electron FLASH Radiation Therapy: Open-Source for Clinical Implementation. International Journal of Radiation Oncology*Biology*Physics. 2022;112:1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou W, Diffenderfer ES, Cengel K, et al. Current Delivery Limitations of Proton PBS for FLASH. Radiotherapy and Oncology. 2020;155:212–218. [DOI] [PubMed] [Google Scholar]
- 64.Beyreuther E, Brand M, Hans S, et al. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiotherapy and Oncology. 2019;139:46–50. [DOI] [PubMed] [Google Scholar]
- 65.Grilj V, Buonanno M, Welch D, et al. Proton Irradiation Platforms for Preclinical Studies of High-Dose-Rate (FLASH) Effects at RARAF. Radiat Res. 2020;194:646–655. [DOI] [PubMed] [Google Scholar]
- 66.Rahman M, Brůža P, Langen KM, et al. Characterization of a new scintillation imaging system for proton pencil beam dose rate measurements. Physics in Medicine & Biology. 2020;65:165014. [DOI] [PubMed] [Google Scholar]
- 67.Jolly S, Owen H, Schippers M, et al. Technical challenges for FLASH proton therapy. Physica Medica. 2020;78:71–82. [DOI] [PubMed] [Google Scholar]
- 68.Nystrom H, Jensen MF, Nystrom PW. Treatment planning for proton therapy: what is needed in the next 10 years? BJR. 2020;93:20190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao H, Lin B, Lin Y, et al. Simultaneous dose and dose rate optimization (SDDRO) for FLASH proton therapy. Med Phys. 2020;47:6388–6395. [DOI] [PubMed] [Google Scholar]
- 70.Romano F, Bailat C, Jorge PG, et al. Ultra-high dose rate dosimetry: Challenges and opportunities for FLASH radiation therapy. Medical Physics. 2022:mp.15649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nesteruk KP, Togno M, Grossmann M, et al. Commissioning of a clinical pencil beam scanning proton therapy unit for ultrahigh dose rates (FLASH). arXiv:2101.01770 [physics]. 2021. [DOI] [PubMed] [Google Scholar]
- 72.Petersson K, Jaccard M, Germond JF, et al. High dose-per-pulse electron beam dosimetry - A model to correct for the ion recombination in the Advanced Markus ionization chamber. Medical physics. 2017;44:1157–1167. [DOI] [PubMed] [Google Scholar]
- 73.Boag JW, Hochhäuser E, Balk OA. The effect of free-electron collection on the recombination correction to ionization measurements of pulsed radiation. Phys. Med. Biol. 1996;41:885–897. [DOI] [PubMed] [Google Scholar]
- 74.IAEA, 2000. Absorbed Dose Determination in External Beam Radiotherapy An International Code of Practice for Dosimetry Based on Standards of Absorbed Dose to Water. Technical Reports Series, 398. 2000. [Google Scholar]
- 75.Czerska K, Emert F, Kopec R, et al. Clinical practice vs. state-of-the-art research and future visions: Report on the 4D treatment planning workshop for particle therapy – Edition 2018 and 2019. Physica Medica. 2021;82:54–63. [DOI] [PubMed] [Google Scholar]
- 76.El Naqa I, Pogue BW, Zhang R, et al. Image guidance for FLASH radiotherapy. Medical Physics. 2022;49:4109–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freislederer P, Kügele M, Öllers M, et al. Recent advances in Surface Guided Radiation Therapy. Radiat Oncol. 2020;15:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oraiqat I, Zhang W, Litzenberg D, et al. An ionizing radiation acoustic imaging (iRAI) technique for real-time dosimetric measurements for FLASH radiotherapy. Med Phys. 2020;47:5090–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anon. Recent Applications of the NCRP Public Dose Limit Recommendation for Ionizing Radiation NCRP Statement No. 10. 2004. [Google Scholar]
- 80.National Council on Radiation Protection and Measurements. Structural shielding design and evaluation for megavoltage X- and gamma-ray radiotherapy facilities: recommendations of the National Council on Radiation Protection and Measurements. Bethesda, MD: National Council on Radiation Protection and Measurements; 2005. [Google Scholar]
- 81.Esplen N, Mendonca MS, Bazalova-Carter M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: a topical review. Phys Med Biol. 2020;65:23TR03. [DOI] [PubMed] [Google Scholar]
- 82.Almond PR, Biggs PJ, Coursey BM, et al. AAPM’s TG-51 protocol for clinical reference dosimetry of high-energy photon and electron beams. Med. Phys. 1999;26:1847–1870. [DOI] [PubMed] [Google Scholar]
- 83.McEwen M, DeWerd L, Ibbott G, et al. Addendum to the AAPM’s TG-51 protocol for clinical reference dosimetry of high-energy photon beams. Med Phys. 2014;41:041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boag JW. Ionization measurements at very high intensities. Pulsed radiation beams. British Journal of Radiology. 1950;23. [DOI] [PubMed] [Google Scholar]
- 85.Bossini E, Minafra N. Diamond Detectors for Timing Measurements in High Energy Physics. Front. Phys. 2020;8:248. [Google Scholar]
- 86.Kranzer R, Schüller A, Bourgouin A, et al. Response of diamond detectors in ultra-high dose-per-pulse electron beams for dosimetry at FLASH radiotherapy. Phys. Med. Biol. 2022;67:075002. [DOI] [PubMed] [Google Scholar]
- 87.Kanouta E, Johansen JG, Kertzscher G, et al. Time structure of pencil beam scanning proton FLASH beams measured with scintillator detectors and compared with log files. Medical Physics. 2022:mp.15486. [DOI] [PubMed] [Google Scholar]
- 88.Favaudon V, Lentz J-M, Heinrich S, et al. Time-resolved dosimetry of pulsed electron beams in very high dose-rate, FLASH irradiation for radiotherapy preclinical studies. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2019;944:162537. [Google Scholar]
- 89.Anon. An Overview of NCI’s National Clinical Trials Network. [Google Scholar]
- 90.Zou W, Geng H, Teo B-KK, et al. NCTN clinical trial standardization for radiotherapy through IROC and CIRO. Med Phys. 2018;45:e850–e853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klein EE, Hanley J, Bayouth J, et al. Task Group 142 report: Quality assurance of medical acceleratorsa): Task Group 142 Report: QA of Medical Accelerators. Med. Phys. 2009;36:4197–4212. [DOI] [PubMed] [Google Scholar]
- 92.Arjomandy B, Taylor P, Ainsley C, et al. AAPM task group 224: Comprehensive proton therapy machine quality assurance. Med. Phys. 2019;46. [DOI] [PubMed] [Google Scholar]
- 93.Bissonnette J-P, Balter PA, Dong L, et al. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: A report of the AAPM TG-179: QA for image-guided radiation therapy utilizing CT-based technologies. Med. Phys. 2012;39:1946–1963. [DOI] [PubMed] [Google Scholar]
- 94.Fontenot JD, Alkhatib H, Garrett JA, et al. AAPM Medical Physics Practice Guideline 2.a: Commissioning and quality assurance of X-ray-based image-guided radiotherapy systems. Journal of Applied Clinical Medical Physics. 2014;15:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beddar AS, Biggs PJ, Chang S, et al. Intraoperative radiation therapy using mobile electron linear accelerators: Report of AAPM Radiation Therapy Committee Task Group No. 72: Task Group No. 72. Med. Phys. 2006;33:1476–1489. [DOI] [PubMed] [Google Scholar]
- 96.Miften M, Olch A, Mihailidis D, et al. Tolerance limits and methodologies for IMRT measurement-based verification QA: Recommendations of AAPM Task Group No. 218. Med. Phys. 2018;45:e53–e83. [DOI] [PubMed] [Google Scholar]
- 97.Mackin D, Zhu XR, Poenisch F, et al. Spot-Scanning Proton Therapy Patient-Specific Quality Assurance: Results from 309 Treatment Plans. International Journal of Particle Therapy. 2014;1:711–720. [Google Scholar]
- 98.Simeonov Y, Weber U, Penchev P, et al. 3D range-modulator for scanned particle therapy: Development, Monte Carlo simulations and experimental evaluation. Physics in Medicine and Biology. 2017;62:7075–7096. [DOI] [PubMed] [Google Scholar]
- 99.Zou W, Fisher T, Zhang M, et al. Potential of 3D printing technologies for fabrication of electron bolus and proton compensators. J Appl Clin Med Phys. 2015;16:4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vyas V, Palmer L, Mudge R, et al. On bolus for megavoltage photon and electron radiation therapy. Medical Dosimetry. 2013;38:268–273. [DOI] [PubMed] [Google Scholar]
- 101.Su S, Moran K, Robar JL. Design and production of 3D printed bolus for electron radiation therapy. Journal of Applied Clinical Medical Physics. 2014;15:194–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ford E, Conroy L, Dong L, et al. Strategies for effective physics plan and chart review in radiation therapy: Report of AAPM Task Group 275. Med. Phys. 2020;47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.10 CFR 35.3045. Medical use of a byproduct material; report and notification of a medical event. [Google Scholar]
- 104.IEC 60601–2-64. Medical electrical equipment, part 2–64: Particular requirements for the basic safety and essential performance of light ion beam medical electrical equipment. 2014. [Google Scholar]
- 105.Orton CG, Seibert JB. The measurement of teletherapy unit timer errors. Phys. Med. Biol. 1972;17:198–205. [DOI] [PubMed] [Google Scholar]
- 106.IAEA. Vienna. International Atomic Energy Association. Radiation Oncology Physics: A Handbook for Teachers and Students. In:; 2005. [Google Scholar]
- 107.Taichman DB, Sahni P, Pinborg A, et al. Data Sharing Statements for Clinical Trials — A Requirement of the International Committee of Medical Journal Editors. N Engl J Med. 2017;376:2277–2279. [DOI] [PubMed] [Google Scholar]
- 108.Moran J, Molineu A, Kruse J, et al. Guidance for the Physics Aspects of Clinical Trials. AAPM; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beddok A, Lahaye C, Calugaru V, et al. A comprehensive analysis of the relationship between dose-rate and biological effects in pre-clinical and clinical studies, from brachytherapy to flattening filter-free radiation therapy and FLASH irradiation. International Journal of Radiation Oncology*Biology*Physics. 2022:S0360301622001559. [DOI] [PubMed] [Google Scholar]
- 110.Tinganelli W, Sokol O, Quartieri M, et al. Ultra-High Dose Rate (FLASH) Carbon Ion Irradiation: Dosimetry and First Cell Experiments. International Journal of Radiation Oncology*Biology*Physics. 2022;112:1012–1022. [DOI] [PubMed] [Google Scholar]


