Abstract
Background:
Rescue of mitochondrial function is a promising neuroprotective strategy for Parkinson’s disease (PD). Ursodeoxycholic acid (UDCA) has shown considerable promise as a mitochondrial rescue agent across a range of pre-clinical in vitro and in vivo models of PD.
Objectives:
To investigate the safety and tolerability of high dose UDCA in PD and determine midbrain target engagement.
Methods:
The UP (UDCA in PD) study was a phase II, randomised, double-blind, placebo-controlled trial of UDCA (30mg/kg daily, 2:1 randomisation UDCA vs placebo) in 30 participants with PD for 48 weeks. Primary outcome was safety and tolerability. Secondary outcomes included 31Phosphorus Magnetic Resonance Spectroscopy (31P-MRS) to explore target engagement of UDCA in PD midbrain and assessment of motor progression, applying both the Movement Disorders Society Unified Parkinson’s Disease Rating Scale, part III (MDS-UPDRS-III) and objective, motion sensor-based quantification of gait impairment.
Results:
UDCA was safe and well tolerated, only mild transient gastrointestinal adverse events were more frequent in the UDCA treatment group. Midbrain 31P-MRS demonstrated an increase in both Gibb’s free energy and inorganic phosphate levels in the UDCA treatment group compared to placebo, reflecting improved ATP hydrolysis. Sensor-based gait analysis indicated a possible improvement of cadence (steps per minute) and other gait parameters in the UDCA group compared to placebo. In contrast, subjective assessment applying the MDS-UPDRS-III failed to detect a difference between treatment groups.
Conclusions:
High dose UDCA is safe and well tolerated in early PD. Larger trials are needed to further evaluate the disease-modifying effect of UDCA in PD.
Introduction
Mitochondrial dysfunction was first identified in sporadic Parkinson’s disease (PD) and has since been implicated in all forms of familial PD.1,2 Rescue of mitochondrial function has therefore been proposed as a promising neuroprotective strategy.3,4
Our group undertook the first screen of an entire compound library in genetically stratified PD patient tissue which led to the identification of the naturally occurring bile acid ursodeoxycholic acid (UDCA) as a promising mitochondrial rescue compound for PD.5,6 We subsequently confirmed the mitochondrial rescue effect of UDCA in mechanistically stratified sporadic PD patient tissue.7 Other groups have independently reported a beneficial effect of both UDCA and its taurine conjugate tauroursodeoxycholic acid (TUDCA) in toxin-induced cell culture models as well as in the classical MPTP- and rotenone-induced rodent models of PD.8–14 UDCA appears to exert its neuroprotective effect via improved mitochondrial function and transport as well as ameliorated autophagic flux, involving the AMPK/mTOR and PINK1/Parkin pathways.14
UDCA has been licensed to treat primary biliary cholangitis at the dose of 15mg/kg for > 30 years. Its excellent safety and tolerability profile makes it ideally suited for the drug repurposing strategy.15,16
Pharmacokinetic studies in patients with amyotrophic lateral sclerosis (ALS) confirmed blood-brain-barrier penetrance of UDCA, especially at higher doses.17 In 2015, the international Linked Clinical Trials Initiative (iLCT) named UDCA as its most highly prioritized neuroprotective compound for investigation in clinical trials to further validate its neuroprotective potential in PD.
Here we present the results of a phase II, double-blind, randomised, placebo-controlled trial of 30 mg/kg of UDCA in early PD, the UP study. The primary outcome of our study was safety and tolerability of UDCA in PD. We also applied 31Phosphorus magnetic resonance spectroscopy (31P-MRS) to provide evidence of mechanistic target engagement for UDCA in PD midbrain tissue, including the substantia nigra as the key site of PD pathology. 18,19 In addition, we combined “gold standard” clinical rating scales with sensor-based objective gait analysis to explore the effect of UDCA vs placebo on PD motor progression. We also assessed the effect of high dose UDCA medication on bile acid composition throughout the trial and undertook genetic screening for pathogenic mutations in Mendelian inherited PD genes and risk variants in glucocerebrosidase (GBA).
Methods
Study design and participants
A comprehensive protocol for this trial has previously been published.20 The full trial protocol is also provided in Supplement 1. In brief, The UP Study (trial registration: EudraCT no. 2018–001887-46) was a phase II, two-centre, double-blind, randomised, placebo-controlled trial of 30 mg/kg of UDCA in recent-onset PD (≤3 years since diagnosis according to Queen Square Brain Bank Criteria) with sustained (>3 months) motor response to dopaminergic medication. UDCA was administered orally for 48 weeks with a subsequent 8-week washout phase to 31 participants with a 2:1 randomisation of drug vs placebo. Changes in the dose of the symptomatic dopaminergic medication by the treating physician were permissible. The trial was conducted at two sites, Sheffield Teaching Hospitals (STH) and University College London Hospitals (UCLH) and approved by the East of England Ethics committee (Protocol ID: 18/EE0280). STH acted as sponsor of the study (STH18493). All participants provided written informed consent prior to any study related activities in accordance with the Declaration of Helsinki.
Following a screening visit to confirm eligibility, participants attended six further visits: baseline (start of treatment period), week 12, week 24, week 36, week 48 (end of treatment period) and week 56 (end of washout period). Treatment with either UDCA or placebo was commenced at a dose of 250 mg per day and increased by 250 mg every three days until the target weight-dependant dose of 30mg/kg was achieved. Placebo and UDCA were provided by PRO.MED.CS Praha a.s. and matched with no identifiable differences in taste, appearance, or smell. PRO.MED.CS Praha a.s. did not have any input upon trial design, protocol development or delivery.
Outcomes
The primary outcome was to compare the safety and tolerability of UDCA at 30 mg/kg to placebo in PD as indicated by the following: number of serious adverse events (SAE’s), number of adverse treatment-reactions and number of participants who completed the study. At each visit, safety monitoring was performed, and adverse events (AE’s) were reviewed and assessed for severity and likely relationship to UDCA. Compliance was assessed by counting the number of investigational medicinal product (IMP) capsules returned and expressed as (IMP dispensed - IMP returned)/IMP prescribed.
The predefined secondary outcomes included: 1. Changes of bioenergetic metabolite estimates derived from 31P-MRS in the midbrain; 2. Changes in the supervised gait analysis; 3. Changes in the Movement Disorders Society Unified Parkinson’s Disease Rating Scale Part III (MDS-UPDRS-III). Changes for all secondary outcomes were assessed from baseline to week 48 in the UDCA vs placebo group, both the supervised gait analysis and the MDS-UPDRS were undertaken in the practically defined “OFF” medication state.
Exploratory outcomes included the change of MDS-UPDRS-III in the practically defined “OFF” medication state at other time points (week 48 to week 56 and baseline to week 56). We also recorded the levodopa equivalent dosage (LED), MDS-UPDRS I-IV in the ‘ON’ state, Montreal Cognitive Assessment (MoCA), Montgomery-Asberg depression rating scale (MADRS), Non-motor Symptom Questionnaire (NMSQ) and the Parkinson’s disease 39 item quality of life questionnaire.21–26 Whenever COVID-19 restrictions prevented face-to-face review of participants, clinical assessments were conducted remotely over video. A summary of the safety monitoring and clinical assessment study procedures are provided in Supplementary Figure 1 and Supplement 2.1.
Sample collection, gait analysis and 31P-MRS imaging
Genetic and bile acid analyses were undertaken in all participants. Further detail is provided in Supplement 2.1 and 2.2. Sensor-based gait analysis was undertaken at the Clinical Research Facility of the STH study site only for STH recruited participants due to COVID-19.
Gait outcomes included spatiotemporal metrics and gait quality measures related to intensity, regularity and variability.27–29 The experimental design of the gait analysis is depicted in Supplementary Figure 2 with further detail in Supplement 2.3.
All participants in the trial were invited for 31P-MRS scans both at baseline and week 48 at the STH site, further details of 31P-MRS acquisition and analysis are provided in Figure 1 and Supplement 2.4.
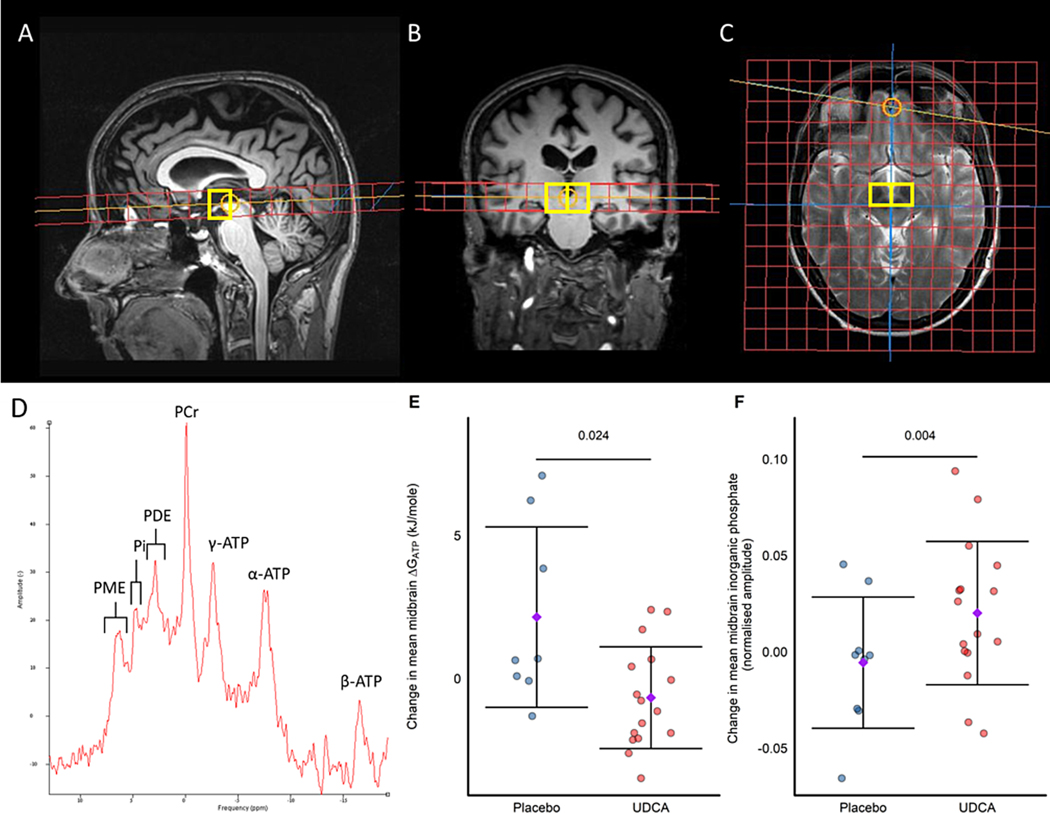
Figure 1: 31Phosphorus Magnetic Resonance voxel localisation, example spectra and results.
Sagittal (A), coronal (B) and axial (C) images demonstrating spectroscopic grid positioning for the midbrain voxels. (D) Example spectrum obtained from the midbrain of an UP study participant. Change from baseline to week 48 in key 31P-MRS parameters from the midbrain for; (E) ΔGATP and (F) inorganic phosphate; purple diamond and error bars signify mean ± standard deviation; P values depicted on each panel above the bar are for the significance of the estimated treatment coefficient with UDCA as assessed by linear regression. UDCA n=16, placebo n=9 except panel (E) where placebo n=8 due to an excluded magnesium value prior to unblinding required for calculation of ΔGATP. PME=phosphomonoesters, PDE= phosphodiesters, Pi= inorganic phosphate, PCr= phosphocreatine, γ-ATP= gamma adenosine triphosphate, α-ATP= gamma adenosine triphosphate, β-ATP= gamma adenosine triphosphate, ΔGATP = Gibbs free energy of ATP hydrolysis.
Statistical Analysis
Statistical analyses were by intention-to-treat (ITT). Some assessments were delayed due to the COVID pandemic; sensitivity analyses were performed for each analysis excluding data collected outside of the planned assessment window. All results presented are using the full analysis dataset unless stated otherwise. SAE’s and adverse treatment reactions are presented descriptively, in summaries individual AE’s (by preferred term) are counted once per participant at the worst severity. We considered the rate of SAE’s reported in the exenatide trial in PD to be tolerable and acceptable (i.e., 20%). If no SAE’s were found in the group receiving UDCA (n=20) then the likelihood that the true SAE rate is less than 20% is 0.990778 (i.e., there is a less than 1% chance that the true SAE rate is >=20%).30 The study was not powered to detect differences in the secondary or exploratory endpoints and therefore, the interpretation of observed differences and confidence intervals (CI’s) will take priority over statistical significance conferred by p-values and no adjustment is made for multiple testing.
Demographic and clinical assessment data were summarised using relevant summary statistics. Multi-variate statistical analysis of serum bile acid profiling data was performed using SIMCA 17.0 (MKS Umetrics AB) and Pareto-scaled, log-transformed data. Between-group differences for changes in clinical parameters were assessed using t-tests for continuous data. Between-group differences in gait analysis parameters from baseline to week 48 were compared using Mann-Whitney U tests as data did not appear to be normally distributed. The change in 31P-MRS parameters from baseline to week 48 was compared between groups using t-tests for each metabolite in turn with further exploratory analysis using linear regression. Additional information on the statistical analysis is also included in Supplements 2.2–2.4. We also refer to the full Statistical Analysis Plan (SAP, Supplement 3), pre-planned analysis was carried out using SAS v9.4.
Data Sharing
Raw data are available from the corresponding author on reasonable request.
Results
Patient characteristics
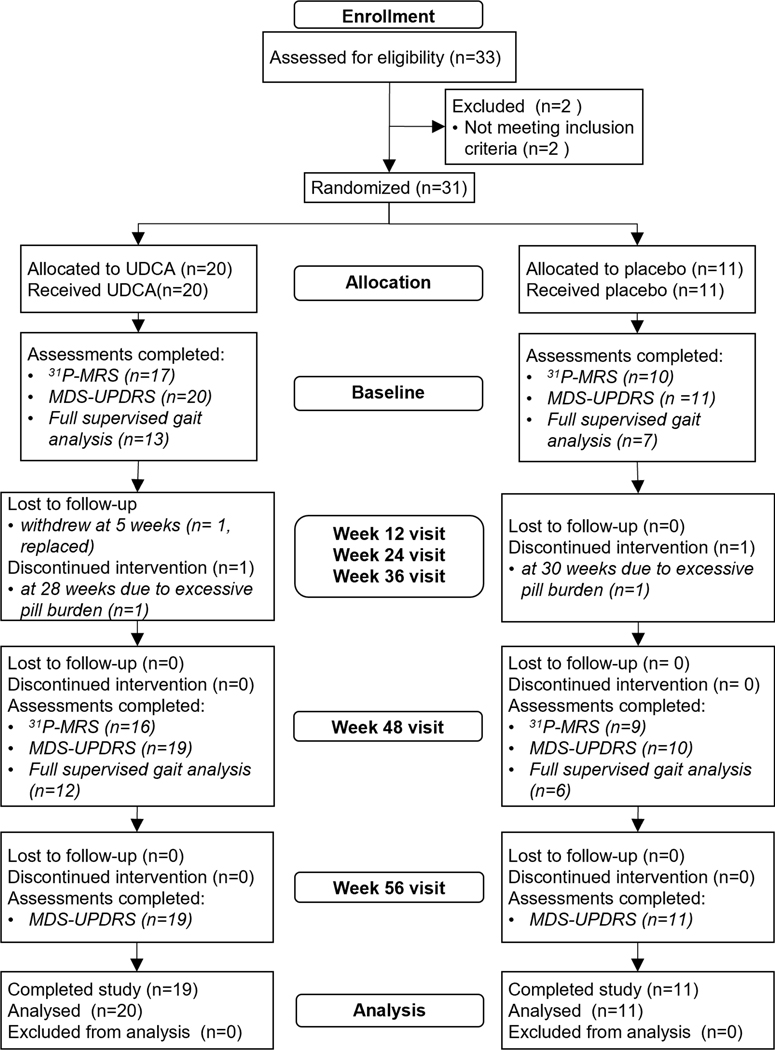
33 participants were assessed for eligibility from January 2019 to October 2019, with 22 participants assessed at STH and 11 at UCLH.20 Two participants were excluded due to a MoCA score < 25. In total 31 participants were randomised, 11 to placebo and 20 to 30mg/kg of UDCA daily, titrated to target dose over approximately 8 weeks. Full details of cohort enrollment are shown in Figure 2. Demographic and clinical characteristics are summarized in Table 1.
Figure 2: Consort flowchart of enrollment, allocation and follow-up assessments performed.
Analysis used an intention-to-treat population therefore all participants randomised were included in the analysis dataset. Details of key secondary outcome assessments are included to demonstrate data completeness.
Table 1.
Demographic and clinical features
| UDCA (n=20) | Placebo (n=11) | ||
|---|---|---|---|
| Age (years) | Mean ± SD | 56.3 ± 7.6 | 61.9 ± 8.28 |
| Range | 40–74 | 53–73 | |
| Sex (n, %) | Male (%) | 14 (70) | 5 (45.5) |
| Female (%) | 6 (30) | 6 (54.5) | |
| BMI (kg/m2) | Mean ± SD | 26.4 ± 3.23 | 24.9 ± 3.54 |
| Range | 20.9 – 31.8 | 18.1 – 32.4 | |
| Disease Duration (months) | Mean ±SD | 16.3 ± 11.7 | 22.1 ± 7.2 |
| Range | 2.3 – 41.5 | 10.7 – 32.7 | |
| Family History of PD in a first degree relative (n, %) | Present | 1 (5) | 2 (18.2) |
| Absent | 19 (95) | 9 (81.8) | |
| Modified Hoehn &Yahr
(n, %) |
Stage 1 | 5 (25) | 2 (18.2) |
| Stage 1.5 | 2 (10) | 2 (18.2) | |
| Stage 2 | 13 (65) | 7 (63.6) | |
| Predicted Risk of Rapid Disease Progression | Mean ±SD | 0.31 ± 0.16 | 0.28 ± 0.21 |
| Range | 0.09 – 0.77 | 0.10 – 0.69 |
Summary of demographic and clinical features for trial participants in the UDCA vs placebo arm.
The mean age of the UDCA treatment group was approx. 5 years younger than the mean age of the placebo group (56.3 years vs 61.9 years), prompting us to undertake additional ANCOVA analysis to allow for a possible effect of the lower age in the UDCA treatment group (see below).
Genetic analysis and Serum bile acid analysis
None of the trial participants carried pathogenic mutations in monogenic PD genes or pathogenic risk variants of GBA. Following commencement of treatment with UDCA, marked enrichment for UDCA and its conjugates glycoursodeoxycholic acid (GUDCA) and TUDCA compared to baseline were found at all visits during the treatment period. Bile acid profiles returned to levels comparable to baseline values at week 56 following the 8-week washout. No changes compared to baseline were seen at any time point in placebo-treated patients. Detailed results of the bile acid analysis are provided in Supplement 2.2 and Supplementary Figure 3.
Primary outcome
One participant withdrew after 5 weeks of treatment due to difficulties swallowing the number of IMP capsules in addition to their regular medication, but not pharmacological side effects of the IMP as such. This participant was subsequently replaced. The remaining 30 participants all completed the trial, resulting in a total ITT analysis cohort of 31 trial participants. Two participants stopped taking the IMP early at 28 weeks (UDCA group) and 30 weeks (placebo group) respectively (Fig. 2). Both cited the burden of taking an additional 9–10 tablets in addition to their usual medications. All other trial participants (19/20 in the UDCA group and 9/11 in the placebo group) completed the full treatment period. Compliance was excellent in participants completing the 48-week treatment period (mean ± SD; 97.6±5.4% in UDCA vs 95.2±8.4% in placebo).
Two SAE’s occurred, both in the same participant on placebo, namely retroperitoneal haemorrhage leading to hospital admission and subsequent hospital-acquired pneumonia. Since we found no SAEs in the UDCA group in the full ITT population (n = 20), the likelihood that the true SAE rate for UDCA was less than 20% is 0.990778.30 Twenty-four adverse reactions (AR’s) were observed in 14/31 participants (10 UDCA and 4 placebo, Table 2). The most frequent AR’s were gastrointestinal symptoms: 5/20 (25.0%) participants on UDCA developed mild diarrhoea (i.e., not requiring any treatment) with three episodes resolving within 48 hours or less; a further two participants had episodes that resolved within 72 hours. In the placebo group, 1/11 (9.1%) developed diarrhoea that resolved within 24 hours. Mild nausea (i.e., not requiring any treatment) occurred in 2/20 (10%) of participants taking UDCA, in one participant this episode resolved within 24 hours, in the second participant the nausea was of unspecified duration due to missing data. No other AR’s occurred in the UDCA treatment group at a frequency of more than 1 of the 20 participants. Blood monitoring performed at all face-to-face visits revealed no clinically significant changes in any blood tests performed other than one incidental finding of asymptomatic hyperkalaemia (5.6mmol/L), present at baseline prior to commencement of treatment in the UDCA group and one isolated increase in alkaline phosphatase (194 IU/L) after retroperitoneal haemorrhage (week 36, placebo group) with subsequent normalization.
Table 2.
Adverse Treatment Reactions
| System Organ Class | Adverse Treatment Reactiona | UDCA (n=20) | Placebo (n=11) |
|---|---|---|---|
| Gastrointestinal disorders | Abdominal distension | 1 (5.0%) | 1 (9.1%) |
| Abdominal pain | 1 (5.0%) | 1 (9.1%) | |
| Constipation | 1 (5.0%) | 1 (9.1%) | |
| Diarrhoea | 5 (25.0%) | 1 (9.1%) | |
| Dry mouth | 0 (0.0%) | 1 (9.1%) | |
| Gastroesophageal reflux disease | 0 (0.0%) | 1 (9.1%) | |
| Nausea | 2 (10.0%) | 0 (0.0%) | |
| Salivary hypersecretion | 1 (5.0%) | 0 (0.0%) | |
| Metabolism and nutrition disorders | Abnormal loss of weight | 1 (5.0%) | 0 (0.0%) |
| Musculoskeletal disorders | Arthralgia | 1 (5.0%) | 0 (0.0%) |
| Nervous system disorders | Parkinson’s Disease progression | 0 (0.0%) | 1 (9.1%) |
| Restless legs syndrome | 1 (5.0%) | 0 (0.0%) | |
| Skin and subcutaneous tissue disorders | Pruritus | 1 (5.0%) | 0 (0.0%) |
| Rash | 1 (5.0%) | 0 (0.0%) |
All participants with at least 28 days exposure to study treatment are listed. Only adverse reactions recorded as having a definite, probable or possible relationship to trial medication which started on or after first dose are included. Participants are counted once per row but may appear in more than one row. Adverse treatment reactions observed in more than one trial participant are highlighted in italics and bold.
Secondary outcomes
Evidence of target engagement was assessed using 31P-MRS to determine the effect of the IMP on the bioenergetic profile in the midbrain (including the substantia nigra Fig. 1A–C). A typical 31P-MRS spectrum is depicted in Fig 1D. In total, 25 participants underwent 31P-MRS before and after treatment (UDCA n=16, placebo n=9). Mean midbrain Gibbs free energy of ATP hydrolysis (ΔGATP) reduced by −0.672 kJ/mole (95% CI, −1.62, 0.277) in the UDCA group, but increased by +2.145 kJ/mole (95% CI −0.491, 4.781) in the placebo group from baseline to week 48 (treatment estimate −1.929, 95% CI −3.472, −0.385, P=0.024; Fig. 1E). ΔGATP reflects the amount of energy released from the hydrolysis of ATP to ADP and Pi. As this reaction is exergonic, the value is negative, with more negative values representing greater amounts of energy released to the tissue examined. The observation of an increase in mean midbrain Pi by +0.02 (95% CI 0.00, 0.04) in the UDCA group and reduction by −0.006 (95% CI −0.032, 0.02) in the placebo group (treatment estimate 0.032, 95% CI 0.013, 0.051, P=0.004, Fig. 1F) is in keeping with increased hydrolysis of ATP in the UDCA treatment group. None of the other 31P-MRS-derived parameters changed significantly (Supplementary Table 1).
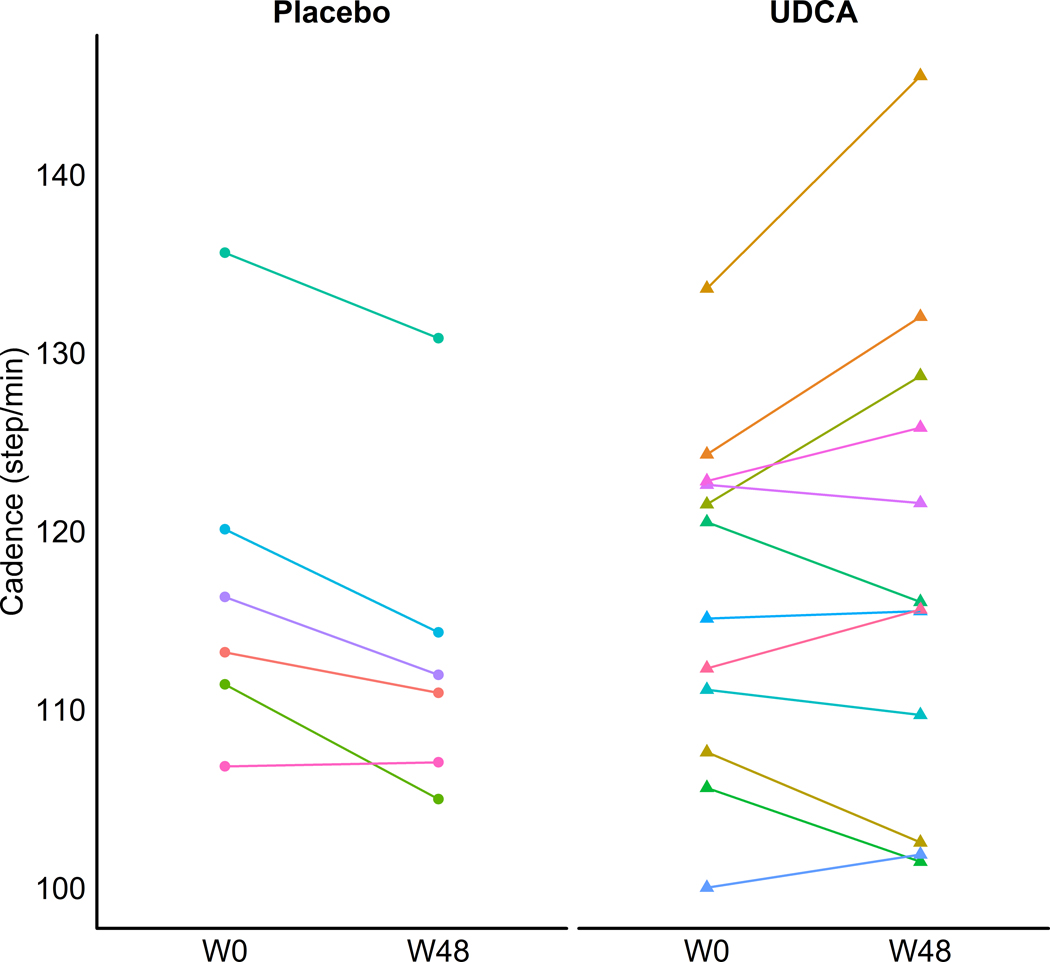
Objectively measured changes in motor impairment before and after treatment, using a quantitative supervised, sensor-based gait analysis approach was only available for STH participants due to COVID-19 (12/19 in the UDCA group and 6/11 in the placebo group). Cadence (steps per minute) increased in the UDCA group (median change +1.5 step/min) but decreased in the placebo group between baseline and 48 weeks, (median change −4.5 step/min, group-difference P=0.0253, Fig. 3). Of note, there was no correlation between changes in cadence and changes in LED (see Supplementary Figure 4). Additionally, consistent with the improvement in cadence we observed small but significant reductions in absolute stride time and stance time as well as stride time variability and stance time variability in the UDCA group compared to placebo (see Supplementary Figure 2). The observed changes in gait parameters, whilst of interest, need to be interpreted with caution due to the small magnitude of changes and overall small cohort size (see discussion). Further details of the gait analysis results are shown in Supplement 2.3 and Supplementary Table 2.
Figure 3: Supervised gait analysis.
Comparison of changes in cadence from baseline to week 48 (placebo n= 6, UDCA n = 12). Data is shown at the individual level with each line representing a different participant. Cadence improved in the UDCA group (median change +1.5 step/min) but deteriorated in the placebo group (median change −4.5 step/min). This was significant at the group level (P=0.0253) as assessed with the Mann-Whitney U test.
MDS-UPDRS-III scores in the practically defined ‘OFF’ state improved from baseline to week 48 by a mean of 1.68 points (95% CI −4.90, 1.53) in the UDCA group and by 5.2 points in the placebo group (95% CI −9.82, −0.58) with a mean difference between UDCA and placebo of 3.52 (95% CI −1.83, 8.86, P=0.1844). To investigate any effect of age on these results an ANCOVA model including an age covariate was fitted, with results consistent with the pre-planned analysis detailed above (mean difference 3.45, 95% CI −2.15, 9.04, P=0.2165).
Exploratory outcomes
Both the comparison of MDS-UPDRS-III “OFF” scores between week 48 vs week 56 and between baseline vs week 56 were non-significant. The mean MDS-UPDRS-III scores in the ‘ON’ state showed similar patterns from baseline to week 48, week 48 to week 56 and baseline to week 56 to those seen in the ‘OFF’ state, again with no significant treatment effect between groups seen at any time point. Changes in LED were not normally distributed, the median change in LED from baseline to week 48 was 0 in both the UDCA group and the placebo group. 8 (40%) patients in the UDCA group had no change in their LED and 7 (64%) in the placebo group also had no change. Of the remaining patients in the UDCA group, 2 (10%) had a reduction in LED, 7 (35%) had an increase and 1 (5%) had missing data; in the placebo group all 4 (36%) remaining patients had an increase in LED.
We observed a small but significant increase in the MADRS scores in the UDCA group compared to placebo from baseline to both week 48 (treatment estimate 2.05 points, 95% CI 0.15, 3.94, P = 0.0353) and week 56 (treatment estimate 3.94 points, 95% CI 1.14, 6.74, P = 0.0075), however overall scores remained low in the UDCA group (week 48 mean ± SD 4.1 ± 4.7 and week 56 mean ± SD 4.6 ± 5.3) and well below the cut off of 14/15 points likely to indicate a depressive disorder.31 We also acknowledge the minor worsening in the NMSQ score in the UDCA treatment group from baseline to week 56 (treatment estimate 2.11 points, 95% CI 0.37, 3.84, P=0.0193), but not from baseline to week 48. However, scores at week 56 (mean ± SD 6.5 ± 4.3) remained consistent with previously reported scores in mild PD (mean ± SD, 8.0 ± 5.3).25 This data and all other exploratory clinical outcomes are summarized in Supplement 2.5 and Supplementary Table 3.
Discussion
The UP study has confirmed that UDCA at a dose of 30mg/kg is safe and extremely well tolerated in PD with no SAE’s and only mild, transient side effects reported in the UDCA treatment group (primary outcome). This is reflected by the high compliance rate (mean of 97.6%) of those participants in the UDCA treated group who completed the full treatment duration. The lack of clinically significant changes in blood monitoring throughout the trial related to UDCA is also extremely reassuring. This safety profile contrasts with the side effect profile of other recently explored putative neuroprotective compounds.32,33
We acknowledge that the excellent safety profile of UDCA in this study needs to be confirmed in larger trials. Although rare SAEs cannot be ruled out, due to the sample size, given the lack of SAEs observed in a sample of 20 UDCA patients the estimated probability of an SAE rate of 14% or more (approximately one in 7) in larger trials is less than 5%. Further, high dose UDCA (≥30mg/kg daily) has been trialed in other conditions such as ALS and primary sclerosing cholangitis with reassuring safety data in similar sized cohorts.17,34,35
Non-significant improvements of MDS-UPDRS-III “OFF” scores over the treatment period were observed in both the UDCA and the placebo group, but were more marked in the placebo group. This is unlikely to be due to changes in dopaminergic medication as the increases in LED over the course of the trial were generally small and similar in both treatment arms. The marked placebo effect may have reduced our ability to detect a clear treatment effect, as observed in other PD neuroprotection trials.36
To address the inherent shortcomings of clinical rating scales to quantify motor progression in PD, we included supervised, sensor-based objective quantification of motor impairment as a secondary trial outcome. This demonstrated a reduction in cadence and an associated increase in both stride time, stance time and their variabilities in the placebo treatment group. The longitudinal deterioration of these measures in the placebo group is comparable with previous studies of PD.37–40 In contrast, we observed either an improvement in the UDCA treatment group or comparatively less worsening in gait over the treatment period. Importantly, this improvement was not correlated with LED. Whilst encouraging, these changes in gait parameters were mostly small, should therefore be interpreted with caution and need to be validated in larger trials. Changes in MDS-UPDRS-III scores did not correlate with any gait parameters (data not shown) which is not unexpected as only a small proportion of MDS-UPDRS-III is comprised of comparatively crude gait-related assessments.
Conceptually, the proof of target engagement is a key aspect of early, proof of concept studies for any compound, but has been lacking for many PD neuroprotection studies. Elevated (i.e. less negative) 31P-MRS measured ΔGATP has previously been observed in mitochondrial cytopathies and is therefore consistent with mitochondrial dysfunction.41 Administration of coenzyme Q10 in mitochondrial cytopathies resulted in an improvement (lowering) of ΔGATP, providing evidence of target engagement in muscle tissue.42 Similarly, the observed lowering of ΔGATP in the UDCA treatment arm of our study is in keeping with the assumption of mechanistic target engagement for UDCA in the midbrain, resulting in improved mitochondrial function. Sathe and co-workers also reported 31P-MRS based evidence of target engagement for UDCA in PD in a small open-label pilot-study.43 However, a different imaging protocol focussing on the occipital cortex was applied, ΔGATP was not calculated and only three PD patients had 31P-MRS imaging before and after a 6-week course of UDCA at a dose of 50 mg/kg. Notably, 31P-MRS is also being applied in other completed or on-going proof-of-concept studies for mitochondrial rescue compounds in PD.44–46
There are limitations to our trial. The latter part of our UP study was compromised by the COVID-19 pandemic. However, our sensitivity analysis of data only collected at the correct time points suggests that this did not have a significant effect on the overall outcome of the trial (data not shown). We excluded PD patients with a more advanced age (˃75) to limit the impact of additional co-morbidities on secondary outcome measures such as the gait analysis. Further, the UDCA treatment group consisted of more males and had a shorter disease duration than the placebo. Although unlikely, we cannot exclude that these limitations may have impacted secondary outcome results. Additionally, the gait analysis was only conducted in a subset of trial participants due to Covid-related issues, most changes observed were small and await confirmation in larger trials and should therefore be interpreted with caution.
The action of UDCA might be pleiotropic and is yet to be fully elucidated. Changes in the PD gut microbiome are associated with alterations in the bile acid pool.47 Intriguingly, reductions of (endogenous) UDCA and TUDCA have been reported in an experimental model of prodromal PD; in addition, UDCA treatment partially restores the gut microbial profile in other conditions.48–50 A beneficial effect of UDCA in PD may therefore not be limited to mitochondrial rescue but also an additional, as yet speculative effect on the microbiome and the gut-brain axis.
As stated above, the UP study was not formally powered to confirm or refute a neuroprotective effect of UDCA. Subsequent, considerably larger and more expensive phase IIb/III studies will be required to confirm or refute such a neuroprotective effect for UDCA. However, the excellent safety profile of UDCA at 30 mg/kg, combined with the 31P-MRS-based evidence of target engagement and the promising results from the gait analysis provide strong rationale for future trials of UDCA in PD and significantly de-risked the required major investment for a definitive study.
Supplementary Material
Acknowledgements
We would like to thank all research participants. We would also like to thank the members of the independent trial steering committee (TSC) Prof Donald Grosset (chair) and Helen Matthews as well as the members of the independent data monitoring committee (IDMC) Prof Camille Carroll (Chair) and Prof John Newell-Price.
Funding:
This research was supported and co-funded by the National Institute of Health Research (NIHR) Sheffield Biomedical Research Centre (BRC) / NIHR Sheffield Clinical Research Facility (CRF). It was also supported by the NIHR UCLH Biomedical Research Centre, and the NIHR UCLH Clinical Research Facility - Leonard Wolfson Experimental Neurology Centre. TP, MS, SM, RT, SL, TJ, OB: JP Moulton Charitable Foundation; The Cure Parkinson’s Trust. SWS was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke (program: 1ZIANS003154). LS is funded by Alzheimer’s Research UK (ARUK-SRF2017B-1). Metabolomics studies were performed at the MRC-NIHR National Phenome Centre at Imperial College London; this centre receives financial support from the Medical Research Council (MRC) and NIHR (grant number MC_PC_12025). BHM is the recipient of an NIHR Academic Clinical Lectureship (CL-2019-21-002). The Division of Digestive Diseases and MRC-NIHR National Phenome Centre at Imperial College London receive financial and infrastructure support from the NIHR Imperial Biomedical Research Centre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care (DHSC).
Financial Disclosures of all authors
MS is the recipient of an NIHR Academic clinical Fellowship. AM has received funding from an MND association project grant, Bailey Thomas foundation project grant, EJHG editorial office funding from Springer Nature and PhD studentships from Saudi Arabian cultural ministry. BHM has received consultancy fees from Finch Therapeutics Group, Akebia Therapeutics, and Summit Therapeutics and contributor fees from the European Society of Neurogastroenterology and Motility. SWS receives research support from Cerevel Therapeutics. SWS is a member of the Scientific Advisory Council of the Lewy Body Dementia Association and the Multiple System Atrophy Coalition. SWS is an editorial board member of JAMA Neurology and the Journal of Parkinson’s Disease. JRM has received consultancy fees from Cultech Ltd and Enterobiotix Ltd. TF has served on Advisory Boards and received honoraria from Peptron, Bayer, Bluerock, Abbvie, Neuroderm, Treefrog, Trialspark, Gain therapeutics & Novonordisk. He has received grant funding from NIHR, Edmond J Safra Foundation, John Black Charitable Foundation, Cure Parkinson’s, Van Andel Institute, Defeat MSA, Innovate UK, Michael J Fox Foundation. OB has served on Advisory Boards and received honoraria from Bluerock and Abbvie. He has received grant funding from Cure Parkinson’s, Jon Moulton Charity Trust and the Medical Research Council (MRC) Centre of Excellence (CoEN) initiative. All other authors have no financial disclosures.
Footnotes
Supplementary material
Supplementary material is available at Movement Disorders online.
Financial Disclosure/Conflict of Interest: The authors report no competing interests.
References
- 1. Borsche M, Pereira SL, Klein C, Grunewald A. Mitochondria and Parkinson’s Disease: Clinical, Molecular, and Translational Aspects. J Parkinsons Dis. 2021;11(1):45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54(3):823–7. [DOI] [PubMed] [Google Scholar]
- 3. Zambrano K, Barba D, Castillo K, et al. Fighting Parkinson’s disease: The return of the mitochondria. Mitochondrion. 2022;64:34–44. [DOI] [PubMed] [Google Scholar]
- 4. Schapira AH, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: future therapeutic perspectives. Lancet. 2014;384(9942):545–55. [DOI] [PubMed] [Google Scholar]
- 5. Mortiboys H, Aasly J, Bandmann O. Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease. Brain. 2013;136(Pt 10):3038–50. [DOI] [PubMed] [Google Scholar]
- 6. Mortiboys H, Furmston R, Bronstad G, Aasly J, Elliott C, Bandmann O. UDCA exerts beneficial effect on mitochondrial dysfunction in LRRK2(G2019S) carriers and in vivo. Neurology. 2015;85(10):846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carling PJ, Mortiboys H, Green C, et al. Deep phenotyping of peripheral tissue facilitates mechanistic disease stratification in sporadic Parkinson’s disease. Prog Neurobiol. 2020;187:101772. [DOI] [PubMed] [Google Scholar]
- 8. Castro-Caldas M, Carvalho AN, Rodrigues E, et al. Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Mol Neurobiol. 2012;46(2):475–86. [DOI] [PubMed] [Google Scholar]
- 9. Abdelkader NF, Safar MM, Salem HA. Ursodeoxycholic Acid Ameliorates Apoptotic Cascade in the Rotenone Model of Parkinson’s Disease: Modulation of Mitochondrial Perturbations. Mol Neurobiol. 2016;53(2):810–817. [DOI] [PubMed] [Google Scholar]
- 10. Cuevas E, Burks S, Raymick J, et al. Tauroursodeoxycholic acid (TUDCA) is neuroprotective in a chronic mouse model of Parkinson’s disease. Nutr Neurosci. 2020;(1476–8305):1–18. [DOI] [PubMed] [Google Scholar]
- 11. Rosa AI, Duarte-Silva S, Silva-Fernandes A, et al. Tauroursodeoxycholic Acid Improves Motor Symptoms in a Mouse Model of Parkinson’s Disease. Mol Neurobiol. 2018;55(12):9139–9155. [DOI] [PubMed] [Google Scholar]
- 12. Moreira S, Fonseca I, Nunes MJ, et al. Nrf2 activation by tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Exp Neurol. 2017;295:77–87. [DOI] [PubMed] [Google Scholar]
- 13. Chun HS, Low WC. Ursodeoxycholic acid suppresses mitochondria-dependent programmed cell death induced by sodium nitroprusside in SH-SY5Y cells. Toxicology. 2012;292(2–3):105–12. [DOI] [PubMed] [Google Scholar]
- 14. Qi H, Shen D, Jiang C, Wang H, Chang M. Ursodeoxycholic acid protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis in MPTP/MPP(+)-induced Parkinson’s disease. Neurosci Lett. 2021;741:135493. [DOI] [PubMed] [Google Scholar]
- 15. Goulis J, Leandro G, Burroughs AK. Randomised controlled trials of ursodeoxycholic-acid therapy for primary biliary cirrhosis: a meta-analysis. Lancet. 1999;354(9184):1053–60. [DOI] [PubMed] [Google Scholar]
- 16. Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J Hepatol. 2001;35(1):134–146. [DOI] [PubMed] [Google Scholar]
- 17. Parry GJ, Rodrigues CM, Aranha MM, et al. Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis. Clin Neuropharmacol. 2010;33(1):17–21. [DOI] [PubMed] [Google Scholar]
- 18. Henchcliffe C, Shungu DC, Mao X, et al. Multinuclear magnetic resonance spectroscopy for in vivo assessment of mitochondrial dysfunction in Parkinson’s disease. Ann N Y Acad Sci. 2008;1147:206–20. [DOI] [PubMed] [Google Scholar]
- 19. Buckley C, Alcock L, McArdle R, et al. The Role of Movement Analysis in Diagnosing and Monitoring Neurodegenerative Conditions: Insights from Gait and Postural Control. Brain Sci. 2019;9(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Payne T, Sassani M, Buckley E, et al. Ursodeoxycholic acid as a novel disease-modifying treatment for Parkinson’s disease: protocol for a two-centre, randomised, double-blind, placebo-controlled trial, The ‘UP’ study. BMJ Open. 2020;10(8):e038911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. [DOI] [PubMed] [Google Scholar]
- 22. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–53. [DOI] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 24. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- 25. Chaudhuri KR, Martinez-Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord. 2006;21(7):916–23. [DOI] [PubMed] [Google Scholar]
- 26. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997;26(5):353–7. [DOI] [PubMed] [Google Scholar]
- 27. Trojaniello D, Ravaschio A, Hausdorff JM, Cereatti A. Comparative assessment of different methods for the estimation of gait temporal parameters using a single inertial sensor: application to elderly, post-stroke, Parkinson’s disease and Huntington’s disease subjects. Gait Posture. 2015;42(3):310–6. [DOI] [PubMed] [Google Scholar]
- 28. Spain RI, St George RJ, Salarian A, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture. 2012;35(4):573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moe-Nilssen R, Helbostad JL. Estimation of gait cycle characteristics by trunk accelerometry. J Biomech. 2004;37(1):121–6. [DOI] [PubMed] [Google Scholar]
- 30. Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leentjens AFG, Verhey FRJ, Lousberg R, Spitsbergen H, Wilmink FW. The validity of the Hamilton and Montgomery-Åsberg depression rating scales as screening and diagnostic tools for depression in Parkinson’s disease. Int J Geriatr Psychiatry. 2000;15(7):644–649. [DOI] [PubMed] [Google Scholar]
- 32. Simuni T, Fiske B, Merchant K, et al. Efficacy of Nilotinib in Patients With Moderately Advanced Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2021;78(3):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pagan FL, Hebron ML, Wilmarth B, et al. Nilotinib Effects on Safety, Tolerability, and Potential Biomarkers in Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2020;77(3):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cullen SN, Rust C, Fleming K, Edwards C, Beuers U, Chapman RW. High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol. 2008;48(5):792–800. [DOI] [PubMed] [Google Scholar]
- 35. Harnois DM, Angulo P, Jorgensen RA, LaRusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96(5):1558–1562. [DOI] [PubMed] [Google Scholar]
- 36. Whone A, Luz M, Boca M, et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain. 2019;142(3):512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mico-Amigo ME, Kingma I, Heinzel S, et al. Potential Markers of Progression in Idiopathic Parkinson’s Disease Derived From Assessment of Circular Gait With a Single Body-Fixed-Sensor: A 5 Year Longitudinal Study. Front Hum Neurosci. 2019;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Del Din S, Elshehabi M, Galna B, et al. Gait analysis with wearables predicts conversion to parkinson disease. Ann Neurol. 2019;86(3):357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hobert MA, Nussbaum S, Heger T, Berg D, Maetzler W, Heinzel S. Progressive Gait Deficits in Parkinson’s Disease: A Wearable-Based Biannual 5-Year Prospective Study. Front Aging Neurosci. 2019;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amboni M, Iuppariello L, Iavarone A, et al. Step length predicts executive dysfunction in Parkinson’s disease: a 3-year prospective study. J Neurol. 2018;265(10):2211–2220. [DOI] [PubMed] [Google Scholar]
- 41. Barbiroli B, Montagna P, Martinelli P, et al. Defective brain energy metabolism shown by in vivo 31P MR spectroscopy in 28 patients with mitochondrial cytopathies. J Cereb Blood Flow Metab. 1993;13(3):469–74. [DOI] [PubMed] [Google Scholar]
- 42. Barbiroli B, Iotti S, Lodi R. Improved brain and muscle mitochondrial respiration with CoQ. An in vivo study by 31P-MR spectroscopy in patients with mitochondrial cytopathies. Biofactors. 1999;9(2–4):253–60. [DOI] [PubMed] [Google Scholar]
- 43. Sathe AG, Tuite P, Chen C, et al. Pharmacokinetics, Safety, and Tolerability of Orally Administered Ursodeoxycholic Acid in Patients With Parkinson’s Disease-A Pilot Study. J Clin Pharmacol. 2020;60(6):744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prasuhn J, Bruggemann N, Hessler N, et al. An omics-based strategy using coenzyme Q10 in patients with Parkinson’s disease: concept evaluation in a double-blind randomized placebo-controlled parallel group trial. Neurol Res Pract. 2019;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schultz JL, Brinker AN, Xu J, et al. A pilot to assess target engagement of terazosin in Parkinson’s disease. Parkinsonism Relat Disord. 2022;94:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brakedal B, Dölle C, Riemer F, et al. The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab. 2022;34(3):396–407.e6. [DOI] [PubMed] [Google Scholar]
- 47. Li P, Killinger BA, Ensink E, et al. Gut Microbiota Dysbiosis Is Associated with Elevated Bile Acids in Parkinson’s Disease. Metabolites. 2021;11(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Graham SF, Rey NL, Ugur Z, et al. Metabolomic Profiling of Bile Acids in an Experimental Model of Prodromal Parkinson’s Disease. Metabolites. 2018;8(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li H, Wang Q, Chen P, Zhou C, Zhang X, Chen L. Ursodeoxycholic Acid Treatment Restores Gut Microbiota and Alleviates Liver Inflammation in Non-Alcoholic Steatohepatitic Mouse Model. Front Pharmacol. 2021;12(1663–9812):788558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang R, Wei Y, Li Y, et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67(3):534–541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.