Abstract
Objective:
To assess whether prenatal risk phenotypes are associated with neurobehavioral impairment children born < 30 weeks’ gestational age (GA) at NICU discharge and 24-month follow-up.
Study Design:
We studied infants from the Neonatal Neurobehavior and Outcomes in Very Preterm Infants (NOVI) study, a multi-site investigation of infants born < 30 weeks’ GA. There were 704 newborns enrolled in NOVI; of these 679 (96%) had neonatal neurobehavioral data and 556 (79%) had 24-month follow-up data. Maternal prenatal phenotypes (physical and psychological risk groups) were characterized from 24 physical and psychological health risk factors. Neurobehavior was assessed at NICU discharge using the NICU Network Neurobehavioral Scales and at two-year follow up using the Bayley Scales of Infant and Toddler Development and the Child Behavior Checklist.
Results:
Children born to mothers in the psychological risk group were at increased risk for dysregulated neonatal neurobehavior (OR = 2.04; 95% CI = 1.08 to 3.87), severe motor delay (OR = 3.80, 95% CI = 1.48 to 9.75), and clinically significant externalizing problems (OR = 2.84, 95% CI = 1.28 to 6.31) at age 24 months, compared with children born to mothers in the low-risk group. Children born to mothers in the physical risk group were more likely to have severe motor delay (OR = 2.70, 95% CI = 1.07 to 6.85) compared with the low-risk group.
Conclusion:
High-risk maternal prenatal phenotypes were associated with neurobehavioral impairment for children born very preterm. This information could identify newborns at risk for adverse neurodevelopmental outcomes.
Keywords: prenatal, risk factor, phenotype, neurobehavior, preterm birth
Infants born < 30 weeks’ gestational age (GA) are at increased risk for neurodevelopmental impairment,1–3 but there is great variability in outcomes among this group. Longitudinal follow-up studies have identified distinct subgroups of children born very preterm, ranging from above average to severely impaired on standardized measures of cognition and behavior.4–6 Being able to predict which preterm infants are at greatest risk for long-term impairment could have enormous benefits in terms of provision of targeted services and follow-up care.
While morbidities related to prematurity (e.g., brain injury, sepsis) have been shown to predict outcomes in this population7,8, more recent research has shown that risk factors prior to birth, including mothers’ prenatal physical (e.g., gestational diabetes) and psychological (e.g., depression) health problems, are associated with adverse health and behavioral outcomes in infants born preterm.9,10 These findings are very much in line with a Developmental Origins of Health and Disease (DOHAD) perspective that emphasizes impacts of the fetal environment on long-term outcome. In the current sample, we previously reported that maternal prenatal depression and anxiety were associated with poorer neonatal neurobehavior, specifically poorer attention and increased lethargy.9 One limitation of earlier studies is that they have tended to investigate single risk factors in isolation, though prenatal risk factors tend to co-occur and likely work together to influence child outcomes. A different approach is to holistically assess multiple risk factors as they co-occur in pregnancy, to evaluate associations between distinct prenatal phenotypes and child outcomes.11,12 Associations between prenatal risk phenotypes and neurobehavioral profiles in children born preterm are unknown.
To address these gaps, the objective of this study is to examine whether prenatal risk phenotypes are associated with neurobehavioral impairment at NICU discharge and 24-month follow up for children born < 30 weeks’ GA. In line with prior work investigating single risk factors, our hypothesis is that infants born to mothers with elevated physical or psychological risk phenotypes will have worse neurobehavioral outcomes compared with infants born to mothers with low-risk phenotypes.
Methods
Study Sample and Procedures
As previously described, the Neonatal Neurobehavior and Outcomes in Very Preterm Infants (NOVI) study enrolled infants born < 30 weeks’ postmenstrual age (PMA) from nine NICUs affiliated with six universities from April 2014 to June 2016.13,14 Inclusion criteria included: (a) birth < 30 weeks PMA; (b) parental ability to read and speak English or Spanish; and (c) residence within 3 hours of the NICU and follow-up clinic. Infants were excluded for major congenital anomalies15, NICU death, maternal age < 18 years, maternal cognitive impairment, or maternal death. Study procedures were explained and informed consent was obtained in accordance with each institution’s review board.
Information about maternal and infant demographics and prenatal variables were obtained via maternal interview and medical record review. Certified examiners conducted neurobehavioral assessments at NICU discharge and at a 24-month follow-up visit (mean corrected age = 25.3 months).
There were 704 infants and 617 caregivers enrolled in NOVI. Of these, 601 caregivers (97%) had interview data and were included in the derivation of prenatal risk phenotypes. Of the 704 infants enrolled, 679 (96%) had neonatal outcome data and 556 (79%) had 24-month follow-up data (Figure 1). Though we previously reported associations between individual prenatal risk factors and neonatal outcomes9, the current study represents pre-planned analysis of prenatal phenotypes in relation to NICU Network Neurobehavioral Scales (NNNS) neurobehavioral profiles and 2 year neurodevelopmental outcomes.
Figure 1.
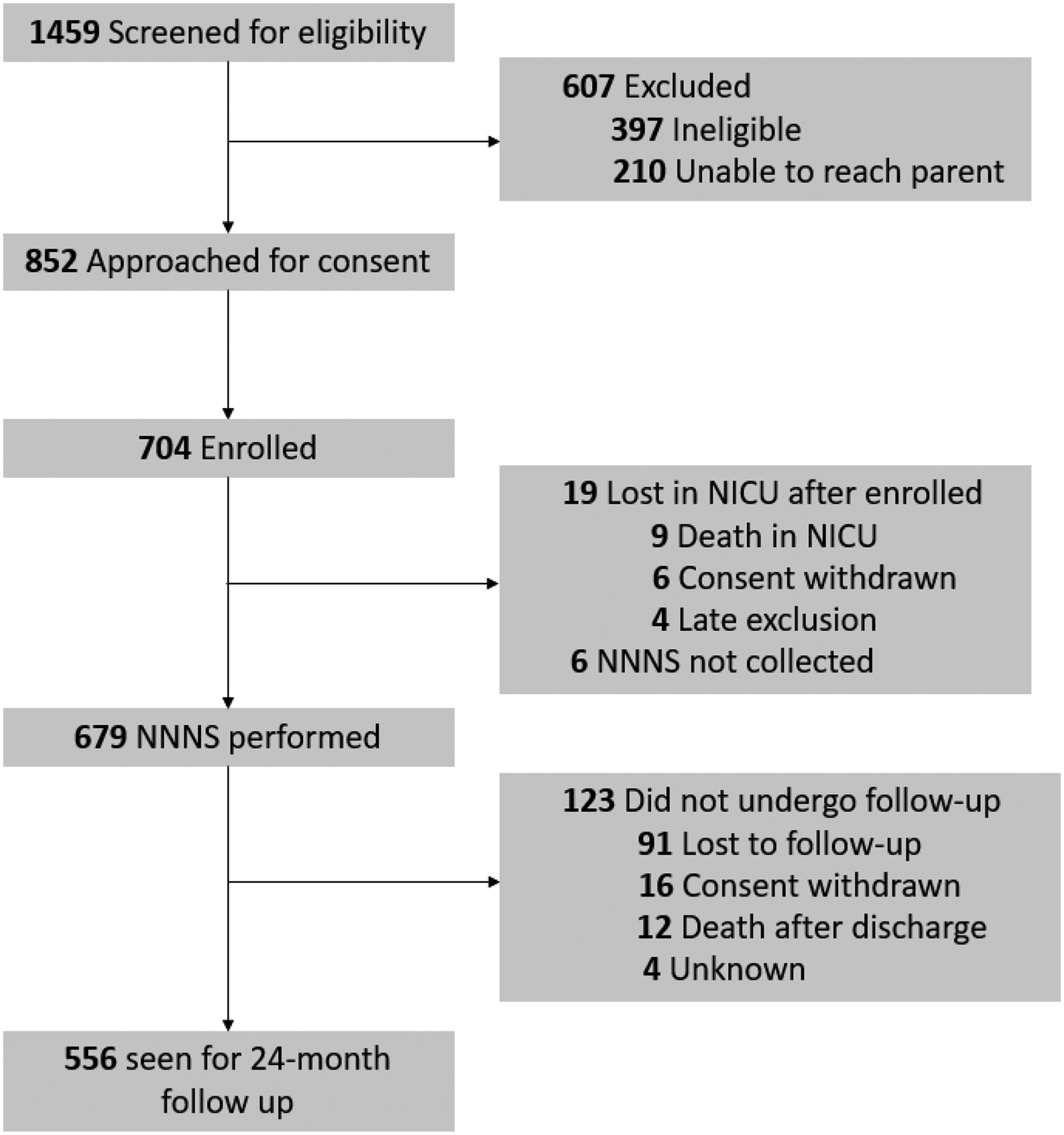
Study flowchart
Measures
Prenatal risk phenotypes.
We previously described three prenatal risk phenotypes: a typical/low-risk group (61%) with few prenatal risk factors, a physical risk group (26%) with elevated physical health problems, and a psychological risk group (13%) with elevated substance use and psychological problems.11 Briefly, we determined these phenotypes by applying latent class analysis to 24 prenatal risk factors, spanning demographic (e.g., maternal age, SES, education, minority race/ethnicity), medical (e.g., maternal underweight/obesity, gestational weight gain, hypertension, pre-eclampsia), substance use (e.g., alcohol, tobacco, marijuana, illegal drugs), and psychological (e.g., depression, anxiety) variables. A 3-class solution was found to provide the best fit to the data and mothers were grouped according to their mostly likely latent class. A visualization of the prenatal risk phenotypes can be viewed elsewhere.11
NICU Network Neurobehavioral Scales (NNNS).
The NNNS is a standardized neurobehavioral assessment that examines newborn muscle tone, reflexes, movement, attention, regulation, and signs of stress and abstinence and results in 12 summary scores.16–19 Examiners were blinded to neonatal clinical variables. We previously applied latent profile analysis to NNNS summary scores in the NOVI study, in order to group children with similar patterns of scores into 6 distinct and mutually-exclusive groups (Table 1; online)13,20 Infants in profiles 5 and 6 showed the most dysregulated neurobehavior. Infants in profile 5 (23%) were hypo-aroused, with poor attention, low arousal, high lethargy and hypotonia, and the most nonoptimal reflexes compared with infants in all other profiles. Infants in profile 6 (7%) were hyper-aroused, demonstrating poor attention and self-regulation and low quality of movement alongside high arousal, excitability, hypertonia, and many signs of stress and abstinence. In this study, membership in either dysregulated NNNS profile (5 or 6) was our primary neonatal neurobehavioral outcome.
Table 1.
Standardized summary scores by NICU Network Neurobehavioral Scales (NNNS) Profile
| NNNS Summary Score | Profile 1 (n=79; 11.6%) | Profile 2 (n=209; 30.7%) | Profile 3 (n=78; 11.5%) | Profile 4 (n=108, 15.9%) | Profile 5 (n=158; 23.3%) | Profile 6 (n=47; 6.9%) |
|---|---|---|---|---|---|---|
| Attention | 1.33 | −0.17 | −0.26 | 0.49 | −0.49 | −0.48 |
| Handling | −0.17 | −0.51 | 0.79 | 0.54 | −0.22 | 0.72 |
| Regulation | 1.32 | 0.23 | −0.79 | 0.58 | −0.50 | −1.55 |
| Arousal | −0.88 | −0.30 | 1.34 | 0.27 | −0.40 | 1.29 |
| Excitability | −0.53 | −0.64 | 1.13 | 0.01 | −0.14 | 2.32 |
| Lethargy | −0.32 | 0.14 | −0.47 | −0.64 | 0.74 | −0.35 |
| Hypertonicity | −0.08 | −0.14 | 0.43 | −0.16 | −0.14 | 0.85 |
| Hypotonicity | −0.34 | −0.16 | −0.14 | −0.32 | 0.67 | −0.02 |
| Nonoptimal reflexes | −0.55 | −0.24 | −0.26 | −0.63 | 1.05 | 0.35 |
| Asymmetric reflexes | 0.57 | −0.43 | −0.53 | 0.82 | −0.04 | 0.07 |
| Quality of movement | 0.75 | 0.43 | 0.38 | −0.08 | −0.57 | −1.69 |
| Stress abstinence | −0.30 | −0.66 | 0.16 | 0.51 | 0.25 | 1.16 |
Bayley Scales of Infant and Toddler Development, 3rd edition (Bayley-III).
The Bayley-III is a widely used developmental assessment that captures cognitive, motor, and language domains.21 The Bayley-III was administered by certified examiners at the 24-month follow-up visit. Cognitive, motor, and language composite scores were calculated, which are normed scores with a mean of 100 and a standard deviation (SD) of 15. Our primary Bayley outcomes were moderate (<85; 1 SD below mean) and severe (<70; 2 SD below mean) cognitive, motor, and language delay.21–23
Child Behavior Checklist - Preschool(1 ½ - 5 years; CBCL).24
Parents completed the CBCL at the 24-month follow-up visit. The CBCL is a parent-report measure that contains 100 statements about the child’s behavior. Responses are recorded as 0 (not true), 1 (somewhat or sometimes true), or 2 (very true or often true). Individual items are grouped into subscales that are categorized into three broad composite scores: internalizing (emotionally reactive, anxious and/or depressed, somatic complaints, and withdrawn behavior), externalizing (attention problems, aggressive problems), and total problems (sum of all). Raw scores were assigned to normalized T-scores according to test creators; internalizing, externalizing, and total problems T-scores above 63 were considered clinically significant24 and were our primary CBCL outcomes.
Covariates
Neonatal medical morbidities were collected according to Vermont Oxford Network criteria and included a count of 4 possible morbidities: brain injury (periventricular leukomalacia, moderate to severe ventriculomegaly, or parenchymal echodensity with or without intraventricular hemorrhage), bronchopulmonary dysplasia (BPD; requiring supplemental oxygen at 36 weeks’ PMA), severe retinopathy of prematurity (ROP; stage 4–5 or surgery), and necrotizing enterocolitis (≥Bell’s stage 2)/culture positive sepsis (includes early- or late-onset sepsis from blood or cerebrospinal fluid culture).25,26 Maternal postnatal psychological distress was accounted for using the Brief Symptom Inventory (BSI),27 a self-report measure of psychological symptoms across nine domains (somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism). The BSI was collected as part of a maternal interview at NICU discharge and 2-year follow-up. Mothers’ global severity index (GSI), measuring overall symptom severity, was averaged across the entire postnatal period (discharge and 2 years) and used as a measure of postnatal psychological distress.27
Statistical Analysis
First, we summarized neurobehavioral outcomes by prenatal risk phenotype, using frequencies for categorical outcomes. The referent group was infants born to mothers in the typical/low-risk prenatal phenotype. Associations between prenatal risk phenotypes and infant neurobehavioral outcomes were determined using generalized estimating equations (GEE) that accounted for a binomial outcome distribution and nesting of multiple births within families. Covariates were selected a priori and included study site, maternal primary language, infant GA at birth, infant sex, neonatal medical risk, and maternal postnatal psychological distress. Note that other demographic factors (e.g., SES) were not included as covariates since these variables were included as part of the original prenatal risk LCA.
Results
Maternal and infant characteristics are shown in Table 2. The sample included 58% of mothers identifying as a minoritized race or ethnicity (24% Black, 13% Asian, 3% Native American, 9.5% Hawaiian, 3.3% Pacific Islander, 20% Hispanic/Latino), 13% having less than a high school degree, and 25% having no relationship partner. Approximately half of the infants (56%) were male and the average PMA at birth was 27 weeks (SD = 1.9 weeks). The most common neonatal medical morbidity was BPD, impacting 51% of infants in the sample.
Table 2.
Maternal and infant characteristics
| Full Sample | |
|---|---|
| M (SD) or % (n) | |
| Maternal characteristics (N = 617) | |
| Minoritized race or ethnicity | 58% (347/601) |
| White race | 57% (340/601) |
| Black race | 24% (146/601) |
| Asian race | 13% (80/601) |
| Native American race | 3.0% (18/601) |
| Hawaiian race | 9.5% (57/601) |
| Pacific Islander race | 3.3% (20/601) |
| Other race | 10% (63/601) |
| Hispanic/Latino ethnicity | 20% (121/601) |
| Low SES: Hollingshead level 5 | 9.9% (59/599) |
| Maternal education: < HS/GED | 13% (79/598) |
| No partner | 25% (152/600) |
| Infant characteristics (N = 704) | |
| Sex = Male | 56% (388/697) |
| Multiple gestation | 26% (184/697) |
| Cesarean delivery | 71% (495/696) |
| PMA at birth (weeks) | 27.0 (1.9) |
| Birth weight (g) | 948.3 (280.6) |
| Head circumference (cm) | 24.5 (2.43) |
| PMA at NICU discharge (weeks) | 40.5 (5.43) |
| Length of NICU stay | 93.5 (41.9) |
| Weight at discharge (g) | 3013 (905) |
| Severe retinopathy of prematurity (ROP) | 5.9% (41/697) |
| Necrotizing enterocolitis/sepsis | 18% (128/697) |
| Bronchopulmonary dysplasia (BPD) | 51% (357/697) |
| Serious brain injury* | 13% (92/694) |
Note. Minoritized race or ethnicity was defined as any non-White race (e.g., Black, Asian) or ethnicity (e.g., Hispanic and/or Latino/a). Percentages of race and ethnicity do not sum to 100 because participants could select multiple races.
Serious brain injury was defined as periventricular leukomalacia, moderate to severe ventriculomegaly, or parenchymal echodensity with or without intraventricular hemorrhage.
SES = socioeconomic status; HS = high school; GED = General Equivalency Diploma; PMA = postmenstrual age; NICU = neonatal intensive care unit; g = grams; cm = centimeters.
Infants born to mothers in the physical risk group had older GA (M = 27.7 weeks; SD = 1.51 weeks) compared with infants born to mothers in both the low-risk group (M = 26.9 weeks; SD = 1.99) and the psychological risk group (M = 26.6 weeks, SD = 1.92 weeks), all p < .05. There were no differences in GA between infants born to mothers in the psychological risk group compared with the low-risk group (p > .05). There were no differences between the groups in terms of sex or neonatal medical morbidities (all p > .05). There were some demographic differences between the groups. Compared with women in the low-risk group, women in the psychological risk group were more likely to have low SES (18% vs. 6.5%), to have less than a high school education (26% vs 12%), and to have no relationship partner (62% vs. 21%), all p < .002.
Neurobehavioral outcomes for the entire sample and by prenatal risk phenotype are shown in Table 3. About one third (30%) of infants had a dysregulated NNNS profile at NICU discharge. Between 20–38% of the sample had a moderate delay on the Bayley, with language delay being the most common. Between 6–12% of the sample had a severe delay on the Bayley, with language delay again being the most common. Fewer than 10% of the sample had clinically significant CBCL internalizing (7.7%), externalizing (9.7%), and total problem scores (9.5%).
Table 3.
Neonatal and two-year outcomes by maternal prenatal phenotype
| Child outcomes | Full Sample | Low Risk | Physical Risk | Psych Risk | Phys vs. Low Risk aOR (95% CI) | Psych vs. Low Risk aOR (95% CI) |
|---|---|---|---|---|---|---|
| Dysregulated NNNS Profile (5 or 6) | 30% (205/679) | 29% (120/419) | 31% (50/161) | 36% (32/88) | 1.03 (0.61–1.74) | 2.04* (1.08–3.87) |
| Bayley cognitive composite < 85 | 25% (133/541) | 24% (78/329) | 24% (33/138) | 27% (18/66) | 1.14 (0.66–1.95) | 0.97 (0.49–1.90) |
| Bayley cognitive composite < 70 | 6.3% (34/541) | 5.5% (18/329) | 6.5% (9/138) | 11% (7/66) | 1.36 (0.52–3.56) | 2.08 (0.66–6.53) |
| Bayley motor composite < 85 | 20% 106/541 | 18% (58/329) | 23% (31/138) | 23% (15/66) | 1.64 (0.93–2.92) | 1.35 (0.66–2.73) |
| Bayley motor composite < 70 | 8.5% (46/541) | 6.4% (21/329) | 10% (14/138) | 15% (10/66) | 2.70* (1.07–6.85) | 3.80** (1.48–9.75) |
| Bayley language composite < 85 | 38% (208/541) | 39% (129/329) | 34% (47/138) | 44% (29/66) | 0.90 (0.57–1.41) | 1.09 (0.59–2.01) |
| Bayley language composite < 70 | 12% (63/541) | 11% (37/329) | 12% (16/138) | 15% (10/66) | 1.17 (0.58–2.35) | 1.03 (0.44–2.43) |
| CBCL Internalizing T-score > 63 | 7.7% (43/556) | 5.9% (20/341) | 7.9% (11/139) | 18% (12/68) | 1.66 (0.73–3.77) | 2.17 (0.95–4.97) |
| CBCL Externalizing T-score > 63 | 9.7% (54/556) | 7.6% (26/341) | 10% (14/139) | 21% (14/68) | 1.65 (0.78–3.46) | 2.54* (1.15–5.56) |
| CBCL Total Problems T-score > 63 | 9.5% (53/556) | 7.0% (24/341) | 9.4% (13/139) | 24% (16/68) | 1.64 (0.77–3.48) | 2.84* (1.28–6.31) |
Note. Adjusted models accounted for nesting of multiple births within families and a priori covariates (study site, primary language, infant GA at birth, infant sex, neonatal medical morbidities, maternal postnatal psychological distress). Dysregulated NNNS profile refers to infants with either a hypo- or hyper-aroused neurobehavioral profile. aOR = adjusted odds ratio; Phys = Physical Risk; Psych = Psychological Risk; NNNS = NICU Network Neurobehavioral Scales; CBCL = Child Behavior Checklist.
p < .05,
p < .01.
Results from adjusted GEE models (Table 3) show that infants born to mothers in the psychological risk group were at increased risk for dysregulated neonatal neurobehavior compared with infants in the low-risk group (OR = 2.04; 95% CI = 1.08 to 3.87). At 24-month follow-up, infants born to mothers in the psychological risk group were at increased risk for severe motor delay (OR = 3.80, 95% CI = 1.48 to 9.75), clinically significant externalizing problems (OR = 2.54, 95% CI = 1.16 to 5.56), and clinically significant total problems (OR = 2.84, 95% CI = 1.28 to 6.31) compared with infants born to mothers in the low-risk group. Infants born to mothers in the physical risk group were also more likely to have severe motor delay (OR = 2.70, 95% CI = 1.07 to 6.85) compared with infants born to mothers in the low-risk group.
Discussion
The aim of the current study was to investigate links between maternal prenatal phenotypes and neonatal and 2-year neurobehavioral outcomes in a sample of children born < 30 weeks’ GA. We found differential associations between two high risk prenatal phenotypes and child outcomes. Infants born to mothers with high psychological risk factors during pregnancy were at increased risk for dysregulated neonatal neurobehavior as well as severe motor delay and clinically significant behavior problems at age 2, compared with infants born to mothers with few risk factors. Increased risk for severe motor delay was also evident among infants born to mothers with high physical risk factors during pregnancy. Together these results show that maternal prenatal phenotypes are important antecedents of outcomes for children born < 30 weeks’ GA.
A prior study examining prenatal phenotypes also found evidence for distinct profiles of women experiencing elevated psychological or physical health conditions during pregnancy, alongside a low-risk, “healthy” group.12 Children of mothers in the physical risk group had poorer fetal central nervous system development whereas fetuses of mothers in the psychological risk group had greater heart rate reactivity during a laboratory stressor.12 This prior study included children born preterm and at term, but did not evaluate neurobehavioral outcomes of the children at birth or beyond. Nonetheless, there is growing attention to the importance of studying prenatal risk phenotypes and their implications for fetal and child development.
In our previous paper using the current sample, we found that children born to women in the psychological and physical risk profiles also had differential DNA methylation signatures compared with one another and to children born to women in the low risk phenotype.11 Therefore, there appear to be both biological and behavioral consequences of exposure to these different types of perinatal risk. One future direction important to examine is whether exposure to different types of perinatal risk has an impact on child outcomes via epigenetic changes detectable at birth. This type of research could serve as a clue to the etiology of developmental delay and mental health disorders of childhood, and possibly suggest means by which concerning neurodevelopmental outcomes could be mitigated.
Our results are broadly in line with prior research showing that prenatal risk factors predict developmental outcomes for infants born preterm. In our current sample, we have shown that maternal prenatal depression and anxiety and medical complications (e.g., diabetes, hypertension, obesity, infection) are associated with worse NNNS summary scores in multiple domains (e.g., attention, lethargy, self-regulation, reflexes).13,28 The current findings extend our prior work by demonstrating that prenatal antecedents were associated with NNNS risk profiles that reflect extreme dysregulation of neonatal tone, movement, attention and arousal, as well as with motor delays and behavior problems at two-years adjusted age. Prior evidence links prenatal psychological and medical conditions to child motor development, although these studies have been conducted primarily in children born at term.29–31 Our finding that maternal psychological risk was associated with child behavior problems is also consistent with a large body of work showing that maternal depression and anxiety during pregnancy are robust predictors of child mental health outcomes32–34, though an ongoing challenge in this area is understanding whether these associations are causal or due to genetic or environmental confounding.35 If these associations are found to be causal, then it suggests a need to intervene on the modifiable risk factors that define the high-risk prenatal phenotypes (e.g., provision of treatment for depression, anxiety, and/or substance use disorders, monitoring and preventative care measures for hypertension and diabetes).
Notably, women in the psychological risk group were also the most socioeconomically disadvantaged in the sample: they were almost three times as likely to be classified as having low SES (18% vs 6.5%) and to have no partner (62% vs 21%) compared with women in the low-risk group and were twice as likely to have less than a high school education (26% vs 12%). A similar trend was observed in the prior study of prenatal risk phenotypes, with women in the high psychological stress group being more likely to be in a minoritized group, to have fewer years of education, and to have lower household income.12 Socioeconomic inequities are linked to poor mental health, and this may be even more true during pregnancy.36 Moreover, maternal mental health and SES both contribute to the quality of the postnatal environment children are raised in.37,38 Therefore, the combination of low SES, mental health difficulties, and substance use observed in our high psychological risk group may have collectively contributed to adverse prenatal and postnatal conditions for children in this sample, with concomitant risks for child outcomes. Our use of a LCA approach allowed us to better understand how these different prenatal risk factors co-occur within groups of women and how they may have compounding negative associations with child neurobehavioral development. Our novel person-centered method may also have led us to find stronger associations with child outcomes as opposed to traditional variable-centered analyses that describe relationships among single pairs of variables (i.e., one prenatal risk factor and one child outcome) while controlling for other (potentially correlated) risk factors.
There are several limitations of the current study. First, although we assessed 24 risk factors across multiple domains, we were limited by the data that were previously collected and could not assess all possible risk factors, nor were we able to assess potential protective factors (e.g., maternal mental healthcare utilization). Some data (e.g., pregnancy moods and feelings) were also assessed retrospectively. Additionally, although we had some data on postnatal maternal mental health, we were unable to account for other aspects of the postnatal environment that may be associated with prenatal phenotypes and child outcomes (e.g., caregiver sensitivity). We also could not account for genetic confounding that might explain links between maternal and child psychopathology. While we have not found differences in the medical or demographic characteristics of children lost to follow-up14, there is the possibility that those lost to follow-up differed based on variables we did not collect, which may have influenced our results. Finally, we report these associations in a specific sample of infants born < 30 weeks’ GA. It is unknown whether similar findings would be observed for preterm children born > 30 weeks’ GA, or for children born at term. It is also unknown whether the prenatal risk factors and phenotypes studied here potentially contributed to the underlying cause of prematurity for infants in this study. Further studies are needed to understand whether prenatal risk phenotypes are linked to neurobehavioral outcomes in children born across the GA spectrum (i.e., from 22 to 42 weeks).
Future research should also examine whether the prenatal risk phenotypes identified in the current and prior studies generalize to different populations of women (e.g., high vs low risk pregnancies, women in different geographical areas) and how the prevalence of the two high risk phenotypes differ across these contexts. It would also be interesting to examine how maternal biomarkers associated with stress and health (e.g., cortisol, inflammatory cytokines) differ across the groups at different points in time.
In conclusion, we found that high-risk prenatal phenotypes were associated with neurobehavioral impairment at NICU discharge and 24-month follow up for infants born < 30 weeks’ GA. This information could help improve identification of very preterm infants at highest risk for poor developmental outcomes. These results also support the importance of interventions aimed at reducing maternal psychological stress in pregnancy.
Supplementary Material
Financial Support:
This work was funded by the National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant R01HD072267 (to B.M.L. and T.M.O.S.). MC was additionally supported by career development award from the National Institutes of Mental Health (NIMH), grant K01MH129510.
List of Abbreviations:
- BPD
bronchopulmonary dysplasia
- BSI
Brief Symptom Inventory
- CBCL
Child Behavior Checklist
- GA
gestational age
- GEE
generalized estimating equations
- GSI
global severity index
- NNNS
NICU Network Neurobehavioral Scales
- PMA
postmenstrual age
- ROP
retinopathy of prematurity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no conflicts of interest.
Declaration of Interest Statement: The authors declare no conflicts of interest.
References
- 1.Stephens BE, Vohr BR. Neurodevelopmental Outcome of the Premature Infant. Pediatr Clin North Am 2009; 56:631–646. [DOI] [PubMed] [Google Scholar]
- 2.Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol 2008;21:123–128. [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR. Neurodevelopmental Outcomes of Extremely Low Birth Weight Infants <32 Weeks’ Gestation Between 1993 and 1998. Pediatrics 2005; 116:635–643. [DOI] [PubMed] [Google Scholar]
- 4.Heeren T, Joseph RM, Allred EN, O’Shea TM, Leviton A, Kuban KCK. Cognitive functioning at the age of 10 years among children born extremely preterm: A latent profile approach. Pediatr Res 2017; 82:614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett AC, Youssef G, Anderson PJ, Duff J, Doyle LW, Cheong JLY. Exploring the “preterm Behavioral Phenotype” in Children Born Extremely Preterm. J Dev Behav Pediatr 2019; 40:200–207. [DOI] [PubMed] [Google Scholar]
- 6.Camerota M, McGowan EC, Hofheimer JA, O’Shea TM, Carter BS, Helderman JB, et al. Neurodevelopmental profiles of infants born <30 weeks gestation at 2 years of age. Pediatr Res 2022; 91:1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai S, Thompson DK, Anderson PJ, Yang JYM. Short- and Long-Term Neurodevelopmental Outcomes of Very Preterm Infants with Neonatal Sepsis: A Systematic Review and Meta-Analysis. Children 2019; 6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Shea TM, Allred EN, Kuban KCK, Hirtz D, Specter B, Durfee S, et al. Intraventricular Hemorrhage and Developmental Outcomes at 24 Months of Age in Extremely Preterm Infants. J Child Neurol 2012; 27:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofheimer JA, Smith LM, McGowan EC, O’Shea TM, Carter BS, Neal CR, et al. Psychosocial and medical adversity associated with neonatal neurobehavior in infants born before 30 weeks gestation. Pediatr Res 2020; 87:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung GPG, Chan LM, Ho YC, To WK, Chan HB, Lao TT. Does gestational diabetes mellitus affect respiratory outcome in late-preterm infants? Early Hum Dev 2014; 90:527–530. [DOI] [PubMed] [Google Scholar]
- 11.Camerota M, Graw S, Everson TM, McGowan EC, Hofheimer JA, O’Shea TM, et al. Prenatal risk factors and neonatal DNA methylation in very preterm infants. Clin Epigenetics 2021; 13:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh K, McCormack CA, Webster R, Pinto A, Lee S, Feng T, et al. Maternal prenatal stress phenotypes associate with fetal neurodevelopment and birth outcomes. Proc Natl Acad Sci 2019; 116:23996–24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGowan EC, Hofheimer JA, O’Shea TM, Carter BS, Helderman J, Neal CR, et al. Sociodemographic and medical influences on neurobehavioral patterns in preterm infants: A multi-center study. Early Hum Dev 2020; 142:104954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowan EC, Hofheimer JA, O’Shea TM, Kilbride H, Carter BS, Check J, et al. Analysis of Neonatal Neurobehavior and Developmental Outcomes Among Preterm Infants. JAMA Netw Open 2022; 5:e2222249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walden RV, Taylor SC, Hansen NI, Poole WK, Stoll BJ, Abuelo D, et al. Major congenital anomalies place extremely low birth weight infants at higher risk for poor growth and developmental outcomes. Pediatrics 2007; 120:e1512–9. [DOI] [PubMed] [Google Scholar]
- 16.Lester BM, Tronick EZ, in collaboration with T. Berry Brazelton, MD. The Neonatal Intensive Care Unit Network Neurobehavioral Scale Procedures. Pediatrics 2004; 113:641–667. [PubMed] [Google Scholar]
- 17.Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 2004; 113:676–678. [PubMed] [Google Scholar]
- 18.Fink NS, Tronick E, Olson K, Lester B. Healthy newborns’ neurobehavior: Norms and relations to medical and demographic factors. J Pediatr 2012; 161:1073–1079.e3. [DOI] [PubMed] [Google Scholar]
- 19.Tronick E, Lester BM. Grandchild of the NBAS: The NICU Network Neurobehavioral Scale (NNNS): A review of the research using the NNNS. J Child Adolesc Psychiatr Nurs 2013; 26:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everson TM, Marsit CJ, O’Shea TM, Burt A, Hermetz K, Carter BS, et al. Epigenome-wide Analysis Identifies Genes and Pathways Linked to Neurobehavioral Variation in Preterm Infants. Sci Rep 2019;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayley N. Bayley Scales of Infant and Toddler Development - Third Edition. Harcourt Assessment; 2006. [Google Scholar]
- 22.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res 2014; 75:670–674. [DOI] [PubMed] [Google Scholar]
- 23.Anderson PJ, Burnett A. Assessing developmental delay in early childhood - concerns with the Bayley-III scales. Clin Neuropsychol 2017; 31:371–381. [DOI] [PubMed] [Google Scholar]
- 24.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles. University of Vermont Department of Psychiatry; 2000. [Google Scholar]
- 25.Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CMT, et al. Using a Count of Neonatal Morbidities to Predict Poor Outcome in Extremely Low Birth Weight Infants: Added Role of Neonatal Infection. Pediatrics 2009; 123:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermont Oxford Network. Manual of Operations: Part 2. Data Definitions and Infant Data Forms. Vermont Oxford Network; 2018. [Google Scholar]
- 27.Deragotis LR. The Brief Symptom Inventory (BSI): Administration, Scoring and Procedures Manual. 3rd ed. National Computer Systems; 1993. [Google Scholar]
- 28.Nosavan NP, Smith LM, Dansereau LM, Roberts MB, Hofheimer JA, Carter BS, et al. Associations between maternal pre-pregnancy body mass index and neonatal neurobehavior in infants born before 30 weeks gestation. J Perinatol 2022; 42:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huizink AC, Robles De Medina PG, Mulder EJH, Visser GHA, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry 2003; 44:810–818. [DOI] [PubMed] [Google Scholar]
- 30.Arabiat D, AL Jabery M, Kemp V, Jenkins M, Whitehead LC, Adams G. Motor Developmental Outcomes in Children Exposed to Maternal Diabetes during Pregnancy: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2021; 18:1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grace T, Bulsara M, Robinson M, Hands B. The Impact of Maternal Gestational Stress on Motor Development in Late Childhood and Adolescence: A Longitudinal Study. Child Dev 2016; 87:211–220. [DOI] [PubMed] [Google Scholar]
- 32.Huizink AC, Mulder EJH, Buitelaar JK. Prenatal Stress and Risk for Psychopathology: Specific Effects or Induction of General Susceptibility? Psychol Bull. 2004; 130:115–142. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor TG, Heron J, Golding J, Glover V, the ALSPAC Study Team. Maternal antenatal anxiety and behavioural / emotional problems in children: A test of a programming hypothesis. J Child Psychol Psychiatry 2003; 44:1025–1036. [DOI] [PubMed] [Google Scholar]
- 34.Doyle C, Cicchetti D. Future directions in prenatal stress research: Challenges and opportunities related to advancing our understanding of prenatal developmental origins of risk for psychopathology. Dev Psychopathol 2018; 30:721–724. [DOI] [PubMed] [Google Scholar]
- 35.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr Perinat Epidemiol 2008; 22:438–466. [DOI] [PubMed] [Google Scholar]
- 36.Björkstedt SM, Koponen H, Kautiainen H, Gissler M, Pennanen P, Eriksson JG, et al. Preconception Mental Health, Socioeconomic Status, and Pregnancy Outcomes in Primiparous Women. Front Public Health 2022; 10:880339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NICHD Early Child Care Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Dev Psychol 1999; 35:1297–1310. [DOI] [PubMed] [Google Scholar]
- 38.Conger RD, Conger KJ, Martin MJ. Socioeconomic Status, Family Processes,and Individual Development. J Marriage Fam 2010; 72:685–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


