Abstract
Objective:
Xerostomia, (or oral dryness), is most commonly caused by medications that affect saliva secretion and is often accompanied by symptoms of orofacial pain. Medication-induced xerostomia may or may not be associated with objectively demonstrable hyposalivation. In this study, we attempt to systematically identify an association between medication-induced xerostomia and orofacial pain.
Methods:
A systematic search was conducted using the following databases: WoS, PubMed, SCOPUS, and MEDLINE. The search terms used were: xerostomia OR “dry mouth” AND medication AND (“oral pain” OR “orofacial pain” OR “craniofacial pain” OR “burning mouth” OR “glossodynia”) NOT Sjögren’s NOT cancer. Inclusion criteria were medication-induced xerostomia and reported symptoms of orofacial pain. Four researchers performed the selection process and quality assessment and two researchers conducted data extraction.
Results:
Seven studies with a total of 1,029 patients were included. These studies were conducted between 2009 – 2022 and consisted of cross-sectional studies, case-control studies, and one randomized crossover trial. The studies consisted of a total of 1029 participants. All studies included male and female participants whose mean ages ranged from 43 to 100 years.
Conclusions:
A positive association was found between medication-induced xerostomia and orofacial pain. We found no associations between salivary flow measurements (hyposalivation) and medication use. Future research should focus on saliva flow measurements, standardized assessment of medication-induced xerostomia as well as the inclusion of accompanying orofacial pain diagnosis in the medical history to allow for higher level of evidence in establishing reliable predictors of medication-induced oral health damage to facilitate clinical prevention and management.
Keywords: xerostomia, dry mouth, medications, orofacial pain, oral pain
INTRODUCTION/BACKGROUND
Xerostomia is the subjective feeling of dryness in the oral cavity. Hyposalivation is the objective finding of decreased salivary production and is defined as an unstimulated salivation rate of <0.3 ml/min.1,2 Although there is often an association between xerostomia and hyposalivation, these terms are not interchangeable. Some patients presenting with xerostomia may still maintain normal salivary production and conversely, patients with severe hyposalivation may not complain of xerostomia.3,4 The etiology of xerostomia is multi-factorial and possible causes include medications, systemic diseases such as Sjögren’s syndrome, diabetes, HIV and other infections, sleep apnea, radiation of the head and neck, chemotherapy, radioiodine treatment, and nerve damage.5–7 The most frequent cause is medication-triggered side effect.8 Several reports describe commonly prescribed medications, for chronic conditions, that have inadvertent anticholinergic effects, resulting in medication-induced xerostomia.9 The intake of medication among the US population has substantially increased in recent years and is currently at 37% in adults aged 26 to 87 years.10 Over 600 medications have anticholinergic effects and these medications have the potential to bind cholinergic receptors and inhibit the action of acetylcholine which, among other effects, ultimately causes a reduction in saliva production.11
The prevalence of xerostomia in the adult population ranges widely between 14–46% and is more prevalent in women. The frequency of xerostomia is also higher among older adults.3 Two studies reported prevalence of medication-related xerostomia as 27 – 30 % while the same studies reported the prevalence in non-medicated patients as 14–16 %.12,13 The consequences of xerostomia include high prevalence of caries, oral discomfort, taste disturbances, increased risk of oral infections, difficulty in mastication, swallowing, and speech.1,14,15 Xerostomia has a prominent, negative impact on oral health and quality of life.16
The International Association for the Study of Pain defines orofacial pain as “a frequent form of pain perceived in the face and/or oral cavity. It may be caused by diseases or disorders of regional structures, dysfunction of the nervous system, or through a referral from distant sources”.17 Terms such as oral pain, craniofacial pain, and facial pain have also been used in the literature to describe similar conditions. Orofacial pain is one of the most common causes of chronic pain affecting 10–15% of the adult population and has been shown to significantly impact the quality of a patient’s life.18,19 20
There is limited research investigating the association between medication-induced xerostomia and pain in the orofacial region. Evidence based on scientific reports is lacking regarding the impact of medication-triggered xerostomia and orofacial pain on oral and general health. Therefore, the objective of the current study was to systematically identify whether an association exists between medication-induced xerostomia and orofacial pain.
METHODS
Design
This systematic review was reported according to the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA).21 All studies published in the English language that investigated the association of medication-induced xerostomia with orofacial pain were included.
Focused Question
Is there an association between xerostomia and orofacial pain in patients taking medications?
Patient, Intervention, Comparison, Outcome (PICO) Question
P (Population): Patients taking medications that cause xerostomia
I (Intervention): Medications causing xerostomia
C (Comparison): Patients without xerostomia (xerostomia vs no xerostomia)
O (Outcome): Orofacial pain
SEARCH METHODS FOR IDENTIFICATION OF STUDIES - STUDY SELECTION
Databases.
A systematic search was conducted using the following electronic bibliographic databases to identify potential studies for inclusion in the systematic review:
Web of Science (WoS) via Nova Southeastern University Library
PubMed via National Library of Medicine
SCOPUS via University of Iowa Libraries
MEDLINE via Ovid
The reference lists of included studies were searched for additional eligible studies not retrieved by our search. The searches were re-run immediately prior to final analyses to retrieve the most recent studies eligible for inclusion.
Search terms.
The terms for medication-induced xerostomia and orofacial pain used in the search included the following: “medication” AND “xerostomia OR dry mouth” AND “oral pain OR orofacial pain OR craniofacial pain” NOT “Sjogren’s” NOT “cancer”. The search strategies and databases used are listed in Table 1. The references from articles that met the inclusion criteria were also searched.
Table 1.
Finalized search strategy table
| Database | Search Strategy |
|---|---|
| PubMed | medication AND (xerostomia OR “dry mouth”) AND (“oral pain” OR “orofacial pain” OR “craniofacial pain”) NOT sjogren’s NOT cancer |
| WoS | (ALL=(xerostomia) OR ALL=(“dry mouth”)) AND ALL=(medication) AND (ALL=(“oral pain”) OR ALL=(“orofacial pain”) OR ALL=(“craniofacial pain”)) NOT ALL=(Sjogren’s) NOT All=(cancer) |
| SCOPUS | ALL ((xerostomia OR “dry mouth”) AND medication AND (“oral pain” OR “orofacial pain” OR “craniofacial pain”) NOT sjogren’s NOT cancer) |
| MEDLINE | (xerostomia OR “dry mouth”) AND medication AND (“oral pain” OR “orofacial pain” OR “craniofacial pain”) NOT sjogren’s NOT cancer |
Search Strategy
Database
Inclusion/Exclusion Criteria
Studies were considered eligible for inclusion if the PICO criteria were met. This review considered original research articles written in English that reported on/tested medication-induced xerostomia and orofacial pain. Studies were eligible for inclusion if they analyzed patients with and without xerostomia. Studies that did not report outcomes for orofacial, oral, craniofacial pain, or xerostomia were excluded, as well as studies that reported exclusively on burning mouth syndrome. Conference, abstracts or review articles, and books that did not contain new research data were excluded. There was no restriction on year of publication.
Outcome measures
Assessments of xerostomia and assessments of pain were considered as primary outcomes. Assessments of xerostomia included a Visual Analog Scale (VAS) and different kinds of xerostomia questionnaires. Assessments of pain included scales such as Visual Analog Scale and Numeric Rating Scale (NRS), as well as questionnaires assessing pain complaints and characteristics.
Additional outcome measures observed were characteristics of medications taken by the study population and assessments of hyposalivation such as whole saliva flow rate.
Data collection and analysis
Selection of studies and data extraction.
All references identified were exported to reference manager software (Endnote 20) for tracking and removal of duplicates. The screening was performed in two phases, initial screening based on the title and abstract (phase 1), followed by a full-text screening of the eligible articles for final inclusion (phase 2). Phase 1: Two independent reviewers (DK and NM) assessed the titles and abstracts of the articles obtained from the electronic database searches to determine if the articles met the inclusion criteria. Articles that did not fulfill the inclusion criteria were removed sequentially. Disagreements were resolved through a discussion or by consulting a third reviewer (OAK) and a fourth reviewer (SA). Phase 2: Subsequent to the title and abstract review, full text was obtained for the articles that appeared to meet the inclusion criteria based on the title and/or abstract or for which there was insufficient information in the title or abstract. Two primary reviewers independently assessed the full text of each paper that appeared to meet the inclusion criteria. Any disagreements between the two primary reviewers over the eligibility of a particular study were resolved through a discussion and if a consensus could not be reached, the third reviewer (OAK) was consulted to determine the eligibility of the article.
Prioritization of the exclusion criteria.
Phase 1: Title-abstract screening
Not an original full research paper
No reports of xerostomia
No reports of orofacial pain
Xerostomia secondary to BMS (burning mouth syndrome)
No appropriate control group was used
Phase 2: Full-text screening
As above, with the addition of:
No relevant outcome measures were reported for the assessment of pain
No relevant outcome measures were reported for the assessment of xerostomia
Xerostomia reported, however, no medications/systemic illness associated
Entire study population exclusively diagnosed with BMS
Data extraction and management.
Data were extracted from each paper that met the inclusion criteria as determined by the two primary reviewers. Microsoft Excel (Microsoft Office Professional Plus 2016) was used to create the data extraction form. Two reviewers independently extracted the data from each article. Numerical data from tables, text, or figures were extracted first; when these were not reported, this data was estimated from graphs and appropriately specified in the data extraction form. The following data were recorded: author and year of publication, number of subjects, gender, age, study groups, assessment of xerostomia, assessment of pain, reported medications/systemic illnesses, and assessment of salivary flow.
Additional data related to the assessment of xerostomia, assessment of pain, assessment of salivary flow, and medications were also recorded.
A narrative report of the results is provided to summarize the association between medication-induced xerostomia and orofacial pain based on the systematic review of the literature.
Assessment of Risk of Bias of Individual Studies
The Newcastle – Ottawa Scale was used to assess the methodological quality of the studies included in this review. Criteria of Selection (S), Comparability (C), and Exposure (E), were systematically assessed and graded with star allocation when satisfying the sub-criteria (detailed in Table 2). The overall score was calculated as the sum of the subcriteria scores.
Table 2.
Quality of reporting of including studies using the Newcastle-Ottawa Assessment criteria
| Newcastle-Ottawa Assessment criteria | Barbe et al. (2018) | Beker et al. (2019) | da Silva et al. (2011) | de Siqueria et al. (2021) | Michalak et al. (2022) | Van de Rijt et al. (2020) | Yuen et al. (2009) |
|---|---|---|---|---|---|---|---|
| SELECTION | |||||||
| • Is the case definition adequate? | * | * | * | * | - | * | - |
| • Representativeness of the cases | * | - | * | * | - | * | - |
| • Selection of controls | - | * | * | - | * | * | * |
| • Definition of controls | - | - | * | * | * | * | - |
| COMPARABILITY | |||||||
| • Comparability of cohorts | - | - | - | - | - | * | ** |
| EXPOSURE | |||||||
| • Ascertainment of exposure | * | * | - | - | * | * | * |
| • Same method of ascertainment for cases and controls | - | * | - | * | - | * | * |
| • Non-response rate | * | * | * | * | - | * | - |
| TOTAL | 4 | 5 | 5 | 5 | 3 | 8 | 5 |
A study can be awarded a maximum of one star (*) for each numbered item within the Selection and Exposure categories. A maximum of two stars can be given for Comparability.Good studies: 7–8 points Satisfactory studies: 5–6 points Unsatisfactory studies: 0 to 4 points
RESULTS
Study selection
A total of seven studies were included in the present review. These studies were conducted between 2009 – 2022 and consisted of cross-sectional studies, case-control studies, and one randomized crossover trial. Results of the electronic bibliographic database searches and the application of the selection criteria are shown in Figure 1.
Figure 1.
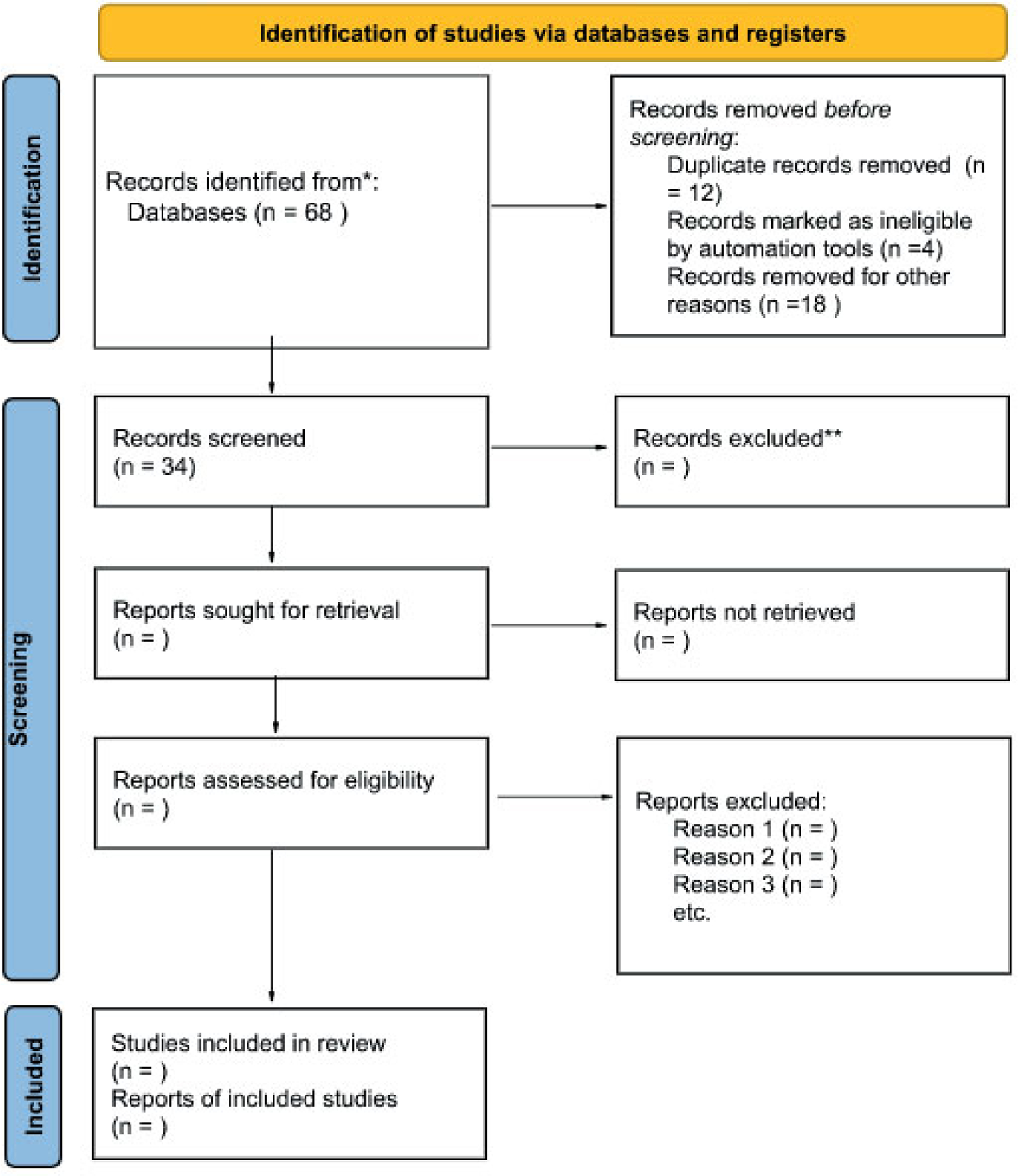
PRISMA flow diagram
Study characteristics
The studies consisted of a total of 1029 participants. All studies included male and female participants whose mean ages ranged from 43 to 100 years. All studies were published in English. The study characteristics including author and year of publication, number of subjects, gender, age, study groups, assessment of xerostomia, assessment of pain, reported medications/systemic illnesses, and assessment of salivary flow are summarized in Table 3.
Table 3.
Summary of characteristics of studies reporting on medication-induced xerostomia and orofacial pain
| Index | Author (Year) | Number of subjects | Gender | Age (Mean) | Groups (N) | Assesment of xerostomia | Assessment of pain | Reported Medications/Systemic Illnesses | Assessment of Salivary Flow |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Barbe, AG., et al. (2018) | 40 | 14M, 26F | 61 | Xerostomia and GUM Hydral Xerostomia and Biotene | VAS, Xerostomia Questionnaire | OHIPG-14, XQPart1 | Not specified | Whole stimulated salivation rate |
| 2 | Beker, N., et al. (2019) | 162 | 38 M, 120 F | 100.9 | NA | Xerostomia Questionnaire | Questionnaire | Reported medications | NA |
| 3 | da Silva, L.A., et al. (2011) | 138 | 41 M, 97 F | N/A | Patients with orofacial pain (82) | Xerostomia Inventory | VAS, Pain characterstics | Reported medications | Salivary Flow |
| Control (56) | |||||||||
| 4 | de Siqueira, SRDT., et al. (20211) | 306 | 89M, 217F | 52.32 | Patients with facial pain (174) | Xerostomia Inventory | Pain complaints, Pain characterstics | Reported systemic illnesses | N/A |
| Control (132) | |||||||||
| 5 | Michalak, P., et al. (2022) | 80 | 28 M, 52 F | 72.05 | Institutionalised center MHCOD (50) | Oral and mucosal complaints, Fox’s questionnaire, Challacombe Scale, CODS and Mirror sliding test | VAS, Oral Cavity Complaints.Oral Mucosal Complaints | Reported medications | N/A |
| Institutionalised center DMCH (30) | |||||||||
| 6 | Van de Rijt, L. L., et al (2020) | 111 | 42 M, 69 F | 83.9 | Group 1: Patients with dementia (84) | Summated Xerostomia Inventory | NRS, OPS-NVI | Reported medications | N/A |
| Group 2 : Patients without dem | |||||||||
| 7 | Yuen, H.K., et al (2009) | 192 | 115 M, 77 F | 43.9 | N/A | Questionnaire | Questionnaire | Not specified | N/A |
Abbreviations
M Male
F Female
VAS Visual Analogue Scale
OHIPG-14 Oral Health Impact Profile
OPS-NVI Orofacial-Pain Scale for Non-Verbal Individuals
NRS Numeric Rating Scale
CODS Clinical Oral Dryness Score
Results of individual studies
We considered two main outcome measures: assessment of xerostomia and pain. The assessment of salivary flow and details of characteristics of medications was also reported. The results of individual studies are briefly summarized in terms of the association of medication-induced xerostomia and orofacial pain. A detailed summary of these results is provided in Table 3. Michalak, P., et al. (2022)22 found that the population of Institutionalized Care Seniors reports oral and mucosal complaints like dryness, swallowing problems, lack of saliva, etc. The authors assessed xerostomia by using Fox questionnaire, Challacombe Scale, Clinical Oral Dryness Score (CODS) (over 65 % CODS with a 4–10 score), and mirror sliding to assess dry mouth. For assessment of pain, the authors reported VAS, oral and mucosal complaints like pain, burning, and mucosal burning. The most reported oral complaints were dryness 36%, lack of saliva 30%, and pain 13.8%, while the mucosal complaints were decreased saliva 45%, and difficulty in taking food 23.8%. Patients with significant resistance to the dental mirror sliding test, which was indicative of dryness, reported higher VAS scores for oral pain (p=0.018). Van de Rijt, L.L., et al (2020)23 found that orofacial pain was more prevalent in patients with dementia than residents without dementia. 48.8 % of dementia patients reported orofacial pain with an Orofacial-Pain Scale for Non-Verbal Individuals (OPS-NVI) while self-reported orofacial pain was present in 37.8%. The summated xerostomia inventory score for patients with dementia is reported as median 6 (IQR 5–10). The authors report having xerostomia (apart from other factors) as one of the significant predictors of orofacial pain in residents with dementia (OR 1.04, p <0.05). Yuen, H.K., et al (2009)24 found that adults with SCI reported a high prevalence of oral problems including experiencing oral pain in the past 12 months (42%) and around 60% patients reported xerostomia. The authors report that oral pain was positively associated with dry mouth. The results of each method of assessment of xerostomia and pain in each study are summarized in Table 4 and 5 respectively.
Table 4.
Assessment of xerostomia
| Index | Author (Year) | Assesment of dry mouth | Outcome | Scale | Group(s) | Mean ± SD | N (%) |
|---|---|---|---|---|---|---|---|
| 1 | Barbe, AG., et al. (2018) | Visual Analogue Scale (VAS) | How dry is your mouth? | 0–100 | Gum Hydral | 80 | |
| Biotene | 70 | ||||||
| Xerostomia Questionnaire Part 1 | Mean oral dryness score | Gum Hydral | 2.5 ± 0.6 | ||||
| Biotene | 2.3 ± 0.6 | ||||||
| 2 | Beker, N., et al (2019) | Xerostomia Questionnaire | My mouth feels dry when eating | 5–15 | Yes (scores ≥8) | 30 (18) | |
| My mouth feels dry | |||||||
| I have difficulty eating dry foods | |||||||
| I have difficulties swallowing certain foods | |||||||
| My lips feel dry | |||||||
| 3 | da Silva, L.A., et al. (2011) | Xerostomia Inventory | Difficulty of chewing due to xerostomia | Yes/No | Patients with orofacial pain | 11 (13.4) | |
| Control | 2 (3.6) | ||||||
| Dry mouth senstation | Patients with orofacial pain | 45 (54.9) | |||||
| Control | 22 (39.3) | ||||||
| Needs of liquids to swallow | Patients with orofacial pain | 17 (20.7) | |||||
| Control | 5 (8.9) | ||||||
| Avoiding food due to xerostomia | Patients with orofacial pain | 8 (9.8) | |||||
| Control | 0 (0) | ||||||
| Abnormal saliva | Patients with orofacial pain | 30 (36.6) | |||||
| Control | 11 (19.6) | ||||||
| Discomfort at the oral cavity | Patients with orofacial pain | 60 (73.2) | |||||
| Control | 8 (14.3) | ||||||
| Lost in taste due to xerostomia | Patients with orofacial pain | 24 (29.3) | |||||
| Control | 6 (10.7) | ||||||
| Difficulty to use dentres due to xerostomia | Patients with orofacial pain | 4 (4.9) | |||||
| Control | 1 (1.8) | ||||||
| 4 | de Siqueira SRDT., et al. (2021) | Xerostomia Inventory | Dry mucosa score | Patients with facial pain | 9.76 ± 4.488 | ||
| Control | 6.69 ± 3.535 | ||||||
| Xerostomia score | Patients with facial pain | 7.14 ± 3.482 | |||||
| Control | 5.04 ± 2.698 | ||||||
| Reported xerostomia | Yes/No | Patients with facial pain, yes | 109 (62.6) | ||||
| Control, yes | 45 (34.1) | ||||||
| 5 | Michalak, P., et al. (2022) | Oral cavity complaints | Dryness | Yes/No | Both groups, yes | 29 (36.3) | |
| Swallowing problems | Yes/No | Both groups, yes | 11 (13.8) | ||||
| Lack of saliva | Yes/No | Both groups, yes | 24 (30) | ||||
| Oral muscosa complaints | Decrease in saliva | Yes/No | Both groups, yes | 36 (45) | |||
| Difficulty in taking food | Yes/No | Both groups, yes | 19 (15.2) | ||||
| Fox’s questionaire | Do you experience mouth dryness during the night or upon wakin up? | Yes/No | Both groups, yes | 34 (42.5) | |||
| Do you experience mouth dryness during the day? | Yes/No | Both groups, yes | 33 (41.3) | ||||
| Do you keep a glass of water next to your bed? | Yes/No | Both groups, yes | 30 (37.5) | ||||
| Do you drink fluids while swallowing dry foods? | Yes/No | Both groups, yes | 37 (46.3) | ||||
| Do you experience mouth dryness during meals? | Yes/No | Both groups, yes | 30 (37.5) | ||||
| Do you experience problems with swallowing foods? | Yes/No | Both groups, yes | 17 (21.3) | ||||
| Do you use chewing gum on a daily basis to eliminate a feeling of mouth dryness? | Yes/No | Both groups, yes | 7 (8.8) | ||||
| Do you use hard fruit or mint candies on a daily basis to eliminate a feeling of mouth dryness? | Yes/No | Both groups, yes | 18 (22.5) | ||||
| Do you perceive the volume of saliva in your mouth as too small/excessive, or do you just not notice it? | Yes/No | Both groups, yes | 42 (52.5) | ||||
| Do you need to moisten your mouth frequently? | Yes/No | Both groups, yes | 29 (36.3) | ||||
| Challacombe Scale | Mirror sticks to buccal mucosa | Yes/No | Both groups, yes | 41 (51.3) | |||
| Mirror sticks to tongue | Yes/No | Both groups, yes | 49 (61.3) | ||||
| Saliva frothy | Yes/No | Both groups, yes | 17 (21.3) | ||||
| No saliva pooling in floor of mouth | Yes/No | Both groups, yes | 49 (61.3) | ||||
| Tongue shows generalised shortened papillae (mild depapillation) | Yes/No | Both groups, yes | 53 (66.3) | ||||
| Altered gingival structure (i.e., smooth) | Yes/No | Both groups, yes | 43 (53.8) | ||||
| Glassy appearance of oral mucosa, especially palate | Yes/No | Both groups, yes | 17 (21.3) | ||||
| Tongue lobulated/fissured | Yes/No | Both groups, yes | 53 (66.3) | ||||
| Cervical caries (more than 2 teeth) | Yes/No | Both groups, yes | 11 (13.8) | ||||
| Debris on palate or sticking to teeth | Yes/No | Both groups, yes | 24 (30) | ||||
| Clinical Oral Dryness Score (CODS) | 1–10 | 1–3 | 28 (35) | ||||
| 4–6 | 42 (52.5) | ||||||
| 7–10 | 10 (12.5) | ||||||
| Mirror sliding test | 1–3 | 1 - No Resistance | 29 (36.2) | ||||
| 2 - Slightly Resistance | 31 (38.8) | ||||||
| 3 - Significant Resistance | 20 (25) | ||||||
| 6 | Van de Rijt, L. L., et al (2020) | Summated Xerostomia Inventory | Group 1 | 6 (5–10)* | |||
| Group 2 | 7 (5.5–13)* | ||||||
| 7 | Yuen, H.K., et al (2009) | Questionnaire | Do you sip liquids to aid in swallowing dry foods? | Yes/No | Yes | 59.90% | |
| Does your mouth feel dry when eating a meal? | |||||||
| Do you have difficulties swallowing any foods? | |||||||
| Does the amount of saliva in your mouth seem to be too little? |
Median (IQR)
Table 5.
Assessment of orofacial pain
| Index | Author (Year) | Assessment of pain | Outcomes | Scale | Group(s) | Mean (±SD) | N (%) | |
|---|---|---|---|---|---|---|---|---|
| 1 | Barbe, AG., et al. (2018) | OHIPG-14 | Pain in oral area | 0–4 | Gum Hydral | No score available | ||
| Biotene | No score available | |||||||
| Xerostomia Questionnaire Part 1 | Mean Oral Pain score | 0–3 | Gum Hydral | 0.8 ± 0.9 | ||||
| Biotene | 0.8 ± 1.0 | |||||||
| 2 | Beker, N., et al. (2019) | Questionnaire | Do you experience pain or discomfort in your mouth | Yes/No/Sometimes | Patients with orofacial pain, Yes | 7 (4) | ||
| Patients with orofacial pain, Sometimes | 13 (8) | |||||||
| 3 | da Silva, L.A., et al. (2011) | VAS | 0–10 | Pt with Orofacial pain | 8.01 ± 2.72 | |||
| Control | NA | |||||||
| Pain Characterstics | Pain in mandibular movements | Pt with Orofacial pain, Present | 15 (18.3) | |||||
| Control, Present | 1 (1.8) | |||||||
| TMD | Pt with Orofacial pain, Present | 36 (43.9) | ||||||
| Control, Present | 2 (3.6) | |||||||
| Pain at facial palpation | Pt with Orofacial pain, Present | 26 (31.7) | ||||||
| Control, Present | 0 (0) | |||||||
| Neckache | Pt with Orofacial pain, Present | 32 (39.0) | ||||||
| Control, Present | 7 (12.5) | |||||||
| Masticatory pain | Pt with Orofacial pain, Present | 21 (25.6) | ||||||
| Control, Present | 1 (1.8) | |||||||
| 4 | de Siqueira SRDT., et al (2021) | Pain complaints | Pain (except at the craniofacial region) | Yes/No | Pt with facial pain, Yes | 152 (87.4) | ||
| Control, Yes | 80 (60.6) | |||||||
| Pain when waking up | Yes/No | Pt with facial pain, Yes | 101 (58.0) | |||||
| Control, Yes | 41 (31.1) | |||||||
| Sensation of tired face | Yes/No | Pt with facial pain, Yes | 81 (46.6) | |||||
| Control, Yes | 20 (15.2) | |||||||
| Pain in mandibular movements | Yes/No | Pt with facial pain, Yes | 64 (36.8) | |||||
| Control, Yes | 5 (3.8) | |||||||
| Pain characteristics | Number of cranial areas with headache | Pt with facial pain | 0.90 ± 0.761 | |||||
| Control | 0.50 ± 0.704 | |||||||
| Number of pain areas (except at the craniofacial region) | Pt with facial pain | 1.61 ± 0.995 | ||||||
| Control | 0.80 ± 0.766 | |||||||
| Number of pain areas when waking up | Pt with facial pain | 1.83 ± 2.000 | ||||||
| Control | 0.54 ± 1.087 | |||||||
| Number of areas with “tired” sensation | Pt with facial pain | 0.70 ± 0.954 | ||||||
| Control | 0.16 ± 0.369 | |||||||
| Number of painful mandibular movements | Pt with facial pain | 0.85 ± 1.404 | ||||||
| Control | 0.04 ± 0.193 | |||||||
| Number trigger points | Pt with facial pain | 2.69 ± 2.379 | ||||||
| Control | 0.25 ± 0.651 | |||||||
| 5 | Michalak, P., et al. (2022) | VAS | Not specified | No resistance | 1.1 | |||
| Slight resistance | 1.42 | |||||||
| Significant resistance | 3.05 | |||||||
| Oral complaints | Pain | Yes/No | Both groups, Yes | 11 (13.75) | ||||
| Burning | Yes/No | Both groups, Yes | 2 (2.5) | |||||
| Pain, crackling, skipping in temporomandibular joint | Yes/No | Both groups, Yes | 3 (3.75) | |||||
| Oral mucosal complaints | Mucosal burning | Yes/No | Both groups, Yes | 10 (12.5) | ||||
| 6 | Van de Rijt, L.L., etal (2020) | NRS | Verbal participants of Group 1 (37) | 14 (37.8) | ||||
| Group 2 | 4 (14.8) | |||||||
| OPS-NVI | Group 1 | 41 (48.8) | ||||||
| Group 2 | 4 (14.8) | |||||||
| 7 | Yuen, H.K., et al (2009) | Questionnaire | In the past 12 months, have you had any of the following incidents as a result of your teeth orgums hurting? | Pain medication used | Yes/No | 43 (22.4) | ||
| Eating disrupted | Yes/No | 38 (19.8) | ||||||
| Dentist visited | Yes/No | 35 (18.2) | ||||||
| Sleep disrupted | Yes/No | 17 (8.8) | ||||||
| Work or school missed | Yes/No | 3 (1.6) | ||||||
| Emergency room visited | Yes/No | 1 (0.5) | ||||||
Abbreviations
VAS Visual Analogue Scale
OHIPG-14 Oral Health Impact Profile
OPS-NVI Orofacial-Pain Scale for Non-Verbal Individuals
NRS Numeric Rating Scale
Barbe, AG., et al. (2018)25 and da Silva, L.A., et al. (2011)26 were the only two studies that performed the assessment of salivary flow. Both studies used different ways to measure salivary flow. The whole stimulated salivation rate was measured in ml/min by Barbe, AG., et al. (2018)25 at a mean of 0.3–0.4 ml/min, indicating the presence of hyposalivation associated with xerostomia.
da Silva, L.A., et al. (2011)26 quantified salivary flow by measuring the weight of cotton balls placed intraorally and soaked in saliva for 5 minutes. They reported a mean of 0.125±0.05 g/min in patients with orofacial pain and 0.175±0.05 g/min in patients without. It is unclear whether there was a cut-off value indicative of hyposalivation with this method of assessment, but the authors stated salivary flow was lesser in patients than in the controls. Table 6 summarizes the assessment of salivary flow in two aforementioned studies.
Table 6.
Assessment of salivary flow
| Index | Author (Year) | Salivary flow measure | Scale | Group(s) | Mean (±SD) |
|---|---|---|---|---|---|
| 1 | Barbe, AG., etal. (2018) | Whole stimulated salivation rate | ml/min | Gum Hydral | 0.3 ± 0.2 |
| Biotene | 0.4 ± 0.2 | ||||
| 2 | Beker, N., etal.(2019) | NA | NA | NA | NA |
| 3 | da Silva, L.A., etal. (2011) | Salivary flow | g/min | Patients with orofacial pain | 0.125 ± 0.05* |
| Control | 0.175 ± 0.05* | ||||
| 4 | de Siqueira, SRDT., et al. (2021) | NA | NA | NA | NA |
| 5 | Michalak, P., et al. (2022) | NA | NA | NA | NA |
| 6 | Van de Rijt, L.L., et al (2020) | NA | NA | NA | NA |
| 7 | Yuen, H.K., et al (2009) | NA | NA | NA | NA |
Estimated
Four out of the seven studies included in this review reported the medications being used by their study population which was used to establish the possibility of medication-induced xerostomia.22,23,26,27 These medications ranged from antidepressants, antihypertensives, and antihistamines, which are known to have anticholinergic effects that limit the production of saliva. Unsurprisingly, Beker, N., et al.(2019)27 observed xerostomia was positively associated with the use of medications (p=0.001) taken by the population, namely antidepressants, and diuretics. According to da Silva, L.A., et al. (2011), 78% of patients were on medications.26 Medications taken by patients with orofacial pain included amitriptyline (35%) and antihypertensives (16%) that are likewise known to have anticholinergic side effects and appeared to be associated with more frequent dry mouth complaints (p=0.007). Michalak, P., et al. (2022)22 observed patients with higher resistance to the dental mirror sliding test also took more medications (p=0.014).
de Siqueira, SRDT., et al. (2021)28 reported the types of systemic illness the study population had, suggesting that these were being controlled by medication. These systemic illnesses included cardiovascular and psychiatric conditions. A weak, but positive correlation between the number of medications and dry mucosa score (0.249 < 0.001) was observed. Although the types of medications were only grouped as analgesic and non-analgesic, patients with craniofacial pain were taking more medication (p < 0.001) than the control group. The control group reportedly only took pain analgesics, while 46% of patients with craniofacial pain took medications that were not pain analgesics.
Two studies did not report medications being taken by the population. However, Barbe, AG., et al. (2018)25 reported that their entire population had an established diagnosis of medication-induced xerostomia. Yuen, H.K., et al (2009)24 likewise stated all patients included in the study had spinal cord injuries. The authors in their introduction established that such patients (with SCI) are usually on medications to reduce muscle spasms and to regulate neurogenic bladder problems. Details of the available data related to medication in each study can be found in Table 7.
Table 7.
Characteristics of medications and illnesses reported
| Index | Author (Year) | Group | Types of medications | Known side effect of xerostomia | Percentage of patients taking medication (%) | Number of medication (Mean ± SD) | Type of systemic illness (N%) |
|---|---|---|---|---|---|---|---|
| 1 | Barbe, AG., et al. (2018) | Not specified | NA | ||||
| 2 | Beker, N., et al.(2019) | NA | Cardiac medication | 53.00 | |||
| Diuretics | Y | 47.00 | |||||
| Anti-coagulants | 45.00 | ||||||
| Antacids | 41.00 | ||||||
| Analgesics | 25.00 | ||||||
| Sedatives | 16.00 | ||||||
| Laxatives | 18.00 | ||||||
| Antidiabetics | 5.00 | ||||||
| Antidepressants | Y | 3.00 | |||||
| Iron supplements | 7.00 | ||||||
| Other | 49.00 | ||||||
| 3 | da Silva, L.A., (2011) | Patients with orofacial pain | Amitriptyline | Y | 35.30 | ||
| Carbamazepine | 26.80 | ||||||
| Antihypertensive | Y | 15.90 | |||||
| Common analgesics | 4.90 | ||||||
| Others | 14.60 | ||||||
| Control | Anti-hypertensive | Y | 53.60 | ||||
| 4 | de Siqueira, SRDT., et al. (2021) | Patients with facial pain | Including pain analgesics | 82.80 | 1.91 ± 1.491 | Cardiovascular (39.2) | |
| Otorhinolaryngologic (20.9) | |||||||
| Excluding pain analgesics | 46.60 | 0.76 ± 1.068 | Gastric (19.0) | ||||
| Psychiatric (14.7) | |||||||
| Control | Including pain analgesics | 43.20 | 0.64 ± 1.006 | Endocrine (12.1) | |||
| Rheumatologic (13.1) | |||||||
| Excluding pain analgesics | 43.20 | 0.64 ± 1.006 | Nephrologic (3.3) | ||||
| Neurologic (3.3) | |||||||
| 5 | Michalak, P., et al. (2022) | Both groups | Anticholinergics | Y | 5.65 | MHCOD: 6.32 ± 2.34 DMCH : 6.23 ± 3.13 | |
| Antihistamines | Y | 9.65 | |||||
| Antihypertensive | Y | 71.00 | |||||
| For Parkinson’s disease | 10.65 | ||||||
| Cytotoxic | 4.65 | ||||||
| Sedative | 55.00 | ||||||
| Relaxants | 9.35 | ||||||
| Antidepressants | Y | 34.65 | |||||
| Anticoagulants | 28.00 | ||||||
| Bone resorption inhibitors | 3.65 | ||||||
| Other | 78.00 | ||||||
| 6 | Van de Rijt, L. L., et al (2020) | Both groups | Analgesics | 82.90 | |||
| Regular | 58.10 | ||||||
| Anti-depressants | Y | 26.10 | |||||
| Anti-epileptics | 17.10 | ||||||
| Anti-psychotics | 16.20 | ||||||
| 7 | Yuen, H.K., et al (2009) | NA | Reduce muscle spasms (eg, phenytoin and baclofen) | ||||
| Regulate neurogenic bladder problems (eg, bethanechol and oxybutynin) |
DISCUSSION
In this systematic review, we aimed to clarify the relationship between xerostomia and orofacial pain in patients taking medications. The results of our systematic review suggest presence of a positive association between medication-induced xerostomia and orofacial pain.
Medication-induced xerostomia is the subjective feeling of dry mouth secondary to medication. Drug-induced hyposalivation is when there is a measurable, objectively demonstrable reduction of salivation associated with the use of medications. The former may be associated with a change in the property of saliva or change in the patient’s perception mechanism. Xerostomia with hyposalivation is caused by both salivary gland dysfunction and salivary innervation disorder. It has been postulated that medication-induced hyposalivation may be a result of a disorder in salivary innervation.29 In the current article, the authors focused on medication induced xerostomia as a criterion for inclusion. If the included articles also reported medication induced hyposalivation, this was reported as an additional finding.
Salivary gland innervation is via the parasympathetic and sympathetic innervations. Bidirectional interactions have been shown to be present between the autonomic nervous system and pain. There is a considerable overlap in the brain regions involved in pain processing and modulation as well as central autonomic networks.30 Xerogenic drugs have been shown to affect CNS or neuroglandular junction. Acini cells in salivary glands are regulated by muscarinic and adrenergic receptors that are stimulated during saliva secretion. Some xerogenic medications have anticholinergic effects and can centrally suppress the production of acetylcholine or block these muscarinic and adrenergic receptors in salivary glands.31
Most of the studies included in the review exclusively used self-administered questionnaires to determine the presence of xerostomia.23,24,26–28 All studies used different methods of pain assessment, the most common being VAS. Barbe, AG., et al. (2018)25 found that patients with medication-induced xerostomia reported a mean oral pain score of 0.8 and a mean dryness score of 2.3–2.5 in the xerostomia questionnaire administered. Patients also reported a VAS score for dryness between 70 to 80 on average, out of 100. Beker, N., et al.(2019)27 conducted a five-point xerostomia questionnaire on a group of centenarians and reported 18% of the population had symptoms of xerostomia. Results of another questionnaire conducted, reported 12% of the population complained of oral pain or discomfort. da Silva, L.A., et al. (2011)26 found that patients with orofacial pain reported more complaints of xerostomia. Results from the Xerostomia Inventory questionnaire indicated 45% and 60% of patients with orofacial pain answered “yes” to statements like experiencing dry mouth and oral discomfort, respectively, compared with controls without orofacial pain (39% and 14%). Patients with orofacial pain reported a mean score of 8.01 on a 1–10 VAS and reported pain characteristics such as neckache (39%) and masticatory pain (25%). de Siqueira, SRDT., et al. (2021)28 similarly conducted a Xerostomia Inventory questionnaire on patients with and without chronic craniofacial pain and found higher xerostomia scores in patients with facial pain compared with controls (7.14 vs. 5.04, p<0.001). 62.6% patients with craniofacial pain reported xerostomia.
None of the studies included both dry mouth and pain as their primary outcomes. Only one of the seven studies was a randomized control trial, limiting the ranking of scientific evidence gathered. In most studies, data collection of xerostomia and orofacial pain relied heavily on patient’s recall ability. Additionally, medication use was reported in all studies as a covariant and not an explanatory variable. Important pharmacological factors like dosage and duration of use were not provided and therefore not considered. This again, further limits the strength of the association between orofacial pain and medication-induced xerostomia.
Assessment of xerostomia
Yes/No format questionnaires were used to assess the presence of xerostomia by da Silva, L.A., et al. (2011)26 using Xerostomia inventory, by de Siqueira, SRDT., et al. (2021)28 using Reported xerostomia, by Michalak, P., et al. (2022)22 using Oral cavity complaints, Oral mucosa complaints, Fox’s questionnaire, and Challacombe Scale and by Yuen, H.K., et al (2009)24. Some of the questions asked were common to different questionnaires, however, a lack of standardization limits their comparability.
Barbe, AG., et al. (2018)25, Beker, N., et al.(2019)27, de Siqueira, SRDT., et al. (2021)28, Van de Rijt, L.L., et al (2020)23 used multiple-point summated questionnaires to determine and compare the presence of xerostomia within their study populations. Because these studies chose different points or did not mention the scale in their questionnaires, their summated scores could not be compared.
Michalak, P., et al. (2022)22 was the only included study to also use the mirror sliding test and Clinical Oral Dryness Score (CODS) to assess the severity of xerostomia. Likewise, Barbe, AG., et al. (2018)25 also used a 0–100 VAS for the question, “How dry is your mouth?” to subjectively quantify the presence of xerostomia.
Assessment of pain
Beker, N., et al.(2019)27, Michalak, P., et al. (2022)22 and Yuen, H.K., et al (2009)24 used only Yes/No format questionnaires to determine the presence of orofacial pain in study participants. Yuen, H.K., et al (2009)24 established the presence of pain by asking if certain adverse events had occurred in the last 12 months. da Silva, L.A., et al. (2011)26 and de Siqueira, SRDT., et al. (2021)28 however, used a combination of Yes/No and open questionnaires to collect information about pain complaints and characteristics. Barbe, AG., et al. (2018)25 used mean oral pain score derived from the xerostomia questionnaire and the OHIPG-14 summated questionnaire to determine the presence of pain.
da Silva, L.A., et al. (2011)26, Michalak, P., et al. (2022)22 and Van de Rijt, L.L., et al (2020)23 used scales such as VAS, NRS, and OPS-NVI to quantify orofacial pain, although each study’s scale was calibrated differently and therefore not directly comparable.
Only two studies26,28 specified the diagnoses of orofacial pain patients recruited that included neuralgias, temporomandibular disorders, atypical facial pain, and neuropathies. The remaining studies did not distinguish the types of orofacial pain. The pain outcomes for each study are elaborated further in Table 5.
Characteristics of Medications
Barbe, AG., et al. (2018)25 and Yuen, H.K., et al (2009)24 were the only two studies in this review that did not specify the medications taken by the population, nor any systemic illnesses in the population. Yuen, H.K., et al (2009)24 however, did mention that their study population of SCI patients typically take medications that reduce muscle spasms and regulate neurogenic bladder problems, some of which have anticholinergic side effects. Moreover, the authors also did not mention what percentage of the current study population was on these medications.
de Siqueira, SRDT., et al. (2021)28 was the only study that reported types of systemic illnesses. They also reported medications in two categories: medications that were pain analgesics, and medications that were not. Both outcomes included respective percentages of the study population.
Beker, N., et al.(2019)27, da Silva, L.A., et al. (2011)26, Michalak, P., et al. (2022)22 and Van de Rijt, L.L., et al (2020)23 specified the types of medications taken by their populations and reported their respective percentages. The percentage of participants taking antidepressants was mentioned in all four studies.
Assessment of salivary flow
Two studies utilized another clinical measure of hyposalivation: salivary flow. Barbe, AG., et al. (2018)25 measured the whole stimulated salivation rate at 0.3 ± 0.2 ml/min (mean ± SD) participants in the Gum Hydral group and 0.4 ± 0.2 ml/min (mean ± SD) in the Biotene group. da Silva, L.A., et al. (2011)26 measured quantitative salivary flow which was significantly lower in patients with orofacial pain than controls (0.125 g/min vs. 0.175 g/min, p=0.008).
LIMITATIONS
There are several limitations associated with this review. First, none of the studies included a control group without xerostomia. Barbe, AG., et al. (2018)25 is the only study that exclusively enrolled patients with medication-induced xerostomia. Pain assessments were performed indiscriminately on the entire study population, thus the determination of whether the same subject with xerostomia also reported pain was not possible unless a direct correlation was reported.
With regard to outcome measures, most studies assessed only the presence or absence of xerostomia. Using validated xerostomia questionnaires could allow quantification of the correlation with the severity of pain. Only two studies reported objective measures of hyposalivation. Measuring hyposalivation would allow association with medication-induced dry mouth.
Furthermore, few studies specified the category of medications or the drugs taken by the study groups. It is well known that side effects from medications are dose-dependent, but no dosages were reported. Duration of drug intake was not included, which could also have an effect on xerostomia. Most studies included the combination of xerogenic and non-xerogenic medications. Therefore, a low correlation was found in those studies between xerostomia and medications, as non-xerogenic medications were combined with xerogenic medications. The risk of bias within the studies cannot be assessed as there was only one randomized controlled trial included, limiting the weight of the conclusions derived.
CONCLUSION
This is the first review to summarize current knowledge on the association between xerostomia and orofacial pain in patients taking medications. This review highlights the lack of high level of evidence studies investigating orofacial pain and medication-induced dry mouth. Similarly, there is a lack of sufficient evidence regarding the characteristics of medications responsible for xerostomia and orofacial pain. The inability to perform a metanalysis due to the limited number and quality of studies addressing this topic requires that the reader rely on the conclusions of this study with prudence. Both chronic orofacial pain and xerostomia have been shown to negatively impact quality of life. Healthcare professionals including medical providers and the dental community are encouraged to investigate symptoms of orofacial pain and dry mouth within the scope of their practice. Future research should focus on saliva flow measurements, standardized assessment of medication-induced xerostomia as well as the inclusion of accompanying orofacial pain diagnosis in the medical history to allow for higher level of evidence in establishing reliable predictors of medication-induced oral health damage to facilitate clinical prevention and management.
FUNDING
Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Number K23DE031021. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
LIST OF ABBREVIATIONS
- BMS
Burning mouth syndrome
- SCI
Spinal Cord Injury
- VAS
Visual analogue scale
- OHIP-14
Oral Health Impact Profile-14
- OPS-NVI
Orofacial-Pain Scale for Non-Verbal Individuals
- NRS
Numeric Rating Scale
- CODS
Clinical Oral Dryness Score
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to difference between this version and the Version of Record.
Disclosure Statement: Author Divya Kohli declares that she has nothing to disclose. Author Nikkita Madhu declares that she has nothing to disclose. Author Olga A. Korczeniewska declares that she has nothing to disclose. Author Szilvia Arany declares that she has nothing to disclose. Author Tal Eliav declares that she has nothing to disclose.
Contributor Information
Divya Kohli, Department of Oral and Maxillofacial Surgery, Nova Southeastern University College of Dental Medicine, Fort Lauderdale, Florida.
Nikkita Madhu, Department of Prosthodontics, University of Iowa College of Dentistry, Iowa City, Iowa.
Olga A. Korczeniewska, Center for Orofacial Pain and Temporomandibular Disorders, Department of Diagnostic Sciences, Rutgers School of Dental Medicine, Rutgers, The State University of New Jersey, Newark, New Jersey.
Tal Eliav, Medical School for International Health, Faculty of Health Sciences, Ben Gurion University of the Negev, Beer Sheva, Israel.
Szilvia Arany, Specialty Care, Eastman Institute for Oral Health, University of Rochester, Rochester, NY.
References
- 1.Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis 2002;8:117–129. [DOI] [PubMed] [Google Scholar]
- 2.Heintze U, Birkhed D, Bjorn H. Secretion rate and buffer effect of resting and stimulated whole saliva as a function of age and sex. Swed Dent J 1983;7:227–238. [PubMed] [Google Scholar]
- 3.Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J 2000;50:140–161. [DOI] [PubMed] [Google Scholar]
- 4.Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc 2003;134:61–69; quiz 118–119. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson JC, Wu AJ. Salivary gland dysfunction: causes, symptoms, treatment. J Am Dent Assoc 1994;125:409–416. [DOI] [PubMed] [Google Scholar]
- 6.Tanasiewicz M, Hildebrandt T, Obersztyn I. Xerostomia of Various Etiologies: A Review of the Literature. Adv Clin Exp Med 2016;25:199–206. [DOI] [PubMed] [Google Scholar]
- 7.Mortazavi H, Baharvand M, Movahhedian A, Mohammadi M, Khodadoustan A. Xerostomia due to systemic disease: a review of 20 conditions and mechanisms. Ann Med Health Sci Res 2014;4:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen-Brown G, Ship JA. Diagnosis and treatment of salivary gland disorders. Quintessence Int 2004;35:108–123. [PubMed] [Google Scholar]
- 9.Smidt D, Torpet LA, Nauntofte B, Heegaard KM, Pedersen AM. Associations between labial and whole salivary flow rates, systemic diseases and medications in a sample of older people. Community Dent Oral Epidemiol 2010;38:422–435. [DOI] [PubMed] [Google Scholar]
- 10.Delara M, Murray L, Jafari B, et al. Prevalence and factors associated with polypharmacy: a systematic review and Meta-analysis. BMC Geriatr 2022;22:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arany S, Kopycka-Kedzierawski DT, Caprio TV, Watson GE. Anticholinergic medication: Related dry mouth and effects on the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;132:662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villa A, Polimeni A, Strohmenger L, Cicciu D, Gherlone E, Abati S. Dental patients’ self-reports of xerostomia and associated risk factors. J Am Dent Assoc 2011;142:811–816. [DOI] [PubMed] [Google Scholar]
- 13.Nederfors T, Isaksson R, Mornstad H, Dahlof C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population--relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol 1997;25:211–216. [DOI] [PubMed] [Google Scholar]
- 14.Villa A, Abati S. Risk factors and symptoms associated with xerostomia: a cross-sectional study. Aust Dent J 2011;56:290–295. [DOI] [PubMed] [Google Scholar]
- 15.Villa A, Wolff A, Aframian D, et al. World Workshop on Oral Medicine VI: a systematic review of medication-induced salivary gland dysfunction: prevalence, diagnosis, and treatment. Clin Oral Investig 2015;19:1563–1580. [DOI] [PubMed] [Google Scholar]
- 16.Barbe AG. Medication-Induced Xerostomia and Hyposalivation in the Elderly: Culprits, Complications, and Management. Drugs Aging 2018;35:877–885. [DOI] [PubMed] [Google Scholar]
- 17.Pain IAftSo. Orofacial Pain. Volume 2023, 2021. [Google Scholar]
- 18.Haggman-Henrikson B, Liv P, Ilgunas A, et al. Increasing gender differences in the prevalence and chronification of orofacial pain in the population. Pain 2020;161:1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovgren A, Haggman-Henrikson B, Visscher CM, Lobbezoo F, Marklund S, Wanman A. Temporomandibular pain and jaw dysfunction at different ages covering the lifespan--A population based study. Eur J Pain 2016;20:532–540. [DOI] [PubMed] [Google Scholar]
- 20.Shueb SS, Nixdorf DR, John MT, Alonso BF, Durham J. What is the impact of acute and chronic orofacial pain on quality of life? J Dent 2015;43:1203–1210. [DOI] [PubMed] [Google Scholar]
- 21.Welch V, Petticrew M, Tugwell P, et al. PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med 2012;9:e1001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalak P, Polak-Szlosarczyk P, Dyduch-Dudek W, et al. Oral and Mucosal Complaints among Institutionalized Care Seniors in Malopolska Voivodeship-The Utility of the Mirror Sliding Test in an Assessment of Dry Mouth. Int J Environ Res Public Health 2022;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Rijt LJ, Feast AR, Vickerstaff V, Lobbezoo F, Sampson EL. Prevalence and associations of orofacial pain and oral health factors in nursing home residents with and without dementia. Age and Ageing 2020;49:418–424. [DOI] [PubMed] [Google Scholar]
- 24.Yuen HK, Shotwell MS, Magruder KM, Slate EH, Salinas CF. Factors Associated With Oral Problems Among Adults With Spinal Cord Injury. Journal of Spinal Cord Medicine 2009;32:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbe AG, Schmidt-Park Y, Hamacher S, Derman SHM, Noack MJ. Efficacy of GUM® Hydral versus Biotène® Oralbalance mouthwashes plus gels on symptoms of medication-induced xerostomia: a randomized, double-blind, crossover study. Clin Oral Investig 2018;22:169–180. [DOI] [PubMed] [Google Scholar]
- 26.da Silva LA, Teixeira MJ, de Siqueira JT, de Siqueira SR. Xerostomia and salivary flow in patients with orofacial pain compared with controls. Arch Oral Biol 2011;56:1142–1147. [DOI] [PubMed] [Google Scholar]
- 27.Beker N, van der Maarel-Wierink CD, de Baat C, Holstege H. Self-reported oral health in the Dutch 100-plus Study of cognitively healthy centenarians: an observational cohort study. Bmc Geriatrics 2019;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Siqueira SRDT, de Siqueira JTT, Teixeira MJ. Association between craniofacial pain complaints, somatoform symptoms and chronic diseases. Archives of oral biology 2021;122:104892. [DOI] [PubMed] [Google Scholar]
- 29.Xerostomia Sugiya H.. Reference Module in Biomedical Sciences: Elsevier, 2014.
- 30.Hohenschurz-Schmidt DJ, Calcagnini G, Dipasquale O, et al. Linking Pain Sensation to the Autonomic Nervous System: The Role of the Anterior Cingulate and Periaqueductal Gray Resting-State Networks. Front Neurosci 2020;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolff A, Joshi RK, Ekström J, et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs in R&D 2017;17:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


