Abstract
Disadvantaged populations, including inhabitants of developing countries as well as racial/ethnic and sexual minorities in the U.S., are disproportionally burdened by Human Immunodeficiency Virus (HIV) infection, delayed HIV diagnosis, and unfavorable HIV-treatment outcomes. HIV interventions targeting single behaviors (e.g., testing) in these populations have shown to be efficacious at producing behavioral and clinical change but have been unable to eliminate the social health disparities associated with syndemics (i.e., a set of connected risks, interacting synergistically, and contributing to excess burden of disease in a population). This meta-analysis of 331 reports (clusters; Number of Effect Sizes [k] = 1364) assessed whether multiple-behavior interventions that target clusters of syndemic risks are more efficacious for those in disadvantaged regions and social groups. Across the board, multiple-behavior interventions were more efficacious than single-behavior ones as well as passive control groups among samples from countries with lower log Gross Domestic Product (GDP), lower Human Development Index (HDI) and lower Healthcare Access and Quality (HAQ) Index. Within the United States, the efficacy of multiple-behavior interventions was similar across different levels of representation of racial/ethnic and sexual minorities. The analyses used robust variance estimation (RVE) with small-sample corrections to assess the differential effects of multiple-behavior interventions and Egger Sandwich test with the MLMA (multilevel meta-analysis) approach to detect selection biases.
Keywords: HIV intervention, disadvantaged groups, country development, syndemics, meta-analysis
From its beginning, the Human Immunodeficiency Virus (HIV) epidemic has disproportionately burdened disadvantaged populations with differential access to prevention resources, decision-making ability, and treatment (Bonvicini, 2017; Operario et al., 2022; Zea et al., 2022). Globally, HIV is concentrated in low-income regions (GBD 2019 HIV Collaborators, 2021; WHO, 2022a), and this disparity has remained stable even after decades of HIV prevention and treatment efforts (CDC, 2019; WHO, 2022). Within the United States, HIV prevalence and incidence have remained higher among under-resourced and stigmatized groups such as racial/ethnic and sexual minorities (CDC, 2019), and treatment adherence is lower in under-resourced and stigmatized groups in the U.S. and other countries (Galea et al., 2021). Furthermore, adherence to treatment with antiretroviral medication ranges from 20 – 95 percent in low-income countries or territories (Hudelson & Cluver, 2015) but about 67.7 – 83.5 percent in the U.S. (McComsey et al., 2021).1 These social health disparities have continued despite efforts to reverse them and are the focus of this paper.
Syndemic theory offers an explanation for disparities in both health and health intervention outcomes: Populations from racial/ethnic minorities, sexual minorities, economically disadvantaged areas, and low- and middle-income countries are burdened by adversity across the lifespan and develop psychosocial health problems with multiplicative negative effects on their health (Garcia & Kushel, 2022; Kerrigan & Barrington, 2022; Operario et al., 2022; P. A. Wilson et al., 2014). For example, systematic marginalization impairs psychological wellbeing via heightened stress, which leads to alcohol and substance use, which are themselves highly correlated as well (Collins, 2016). Alcohol and other substance use can lead to more sexual violence and condomless sex, thus increasing the risk of HIV infection (Decker et al., 2016; Meyer et al., 2011; Parks et al., 2012). Indeed, heavy drinking and nonmedical drug use both correlate with higher HIV seropositivity (des Jarlais et al., 2020; Guerras et al., 2021), higher levels of unsafe sex (Hutton et al., 2019; Ip et al., 2019), decreased psychological wellbeing (Skalski et al., 2015), higher viral load (Ladak et al., 2019), more sexual violence (Wechsberg et al., 2006), and greater HIV-treatment non-adherence (Bauman et al., 2013; Glynn et al., 2019; Kalichman, 2008). Likewise, harmful social conditions such as poverty are often associated with food insecurity, homelessness, and comorbidities that make HIV treatment challenging to maintain (Garcia & Kushel, 2022; Tang et al., 2011). In a nutshell, rather than individual risk factors, these findings suggest the existence of patterns of correlated risk factors like the ones proposed in syndemic theory and the literature on multimorbidity (Freedland et al., 2021; Jain et al., 2022; Strathdee et al., 2013). We define syndemics in HIV as the synergistic epidemics of substance use, violence, diminished psychological wellbeing, and HIV among impacted populations, particularly those burdened by socioeconomic disadvantages (Sheehan et al., 2020; Tsai, 2018; Tsai & Burns, 2015).
Intervention researchers and policymakers have been acutely aware of syndemics and the needs of disadvantaged groups (Bonevski et al., 2014; Glynn et al., 2019) and have identified ways to maximize intervention efficacy for these groups. In particular, multiple-behavior interventions concurrently target multiple risks and are likely to be most efficacious for populations susceptible to syndemics (Rotheram-Borus et al., 2009). For example, nonmedical substance use is associated with lower rates of condom use (Ritchwood et al., 2015). In that case, interventions recommending both condom use and alcohol use should produce more change than interventions recommending condom use solely. This difference between multiple- and single-behavior interventions may hence be larger for groups with syndemic risks, including populations from low-resource regions or racial/ethnic and sexual minorities within the U.S. Nonetheless, prior intervention research has studied program efficacy among disadvantaged populations without comparing these effects with those among more advantaged ones. In this paper, we conducted a meta-analysis to examine whether multiple- versus single-behavior interventions are differentially successful across samples with different levels of disadvantage. First, we gauged differences in efficacy across higher versus lower income/development countries. Second, we examined possible differences in efficacy across racial/ethnic and sexual minorities and majority samples in the U.S. We asked the question of whether, relative to single-behavior interventions, multiple-behavior interventions, which address syndemics, would result in higher intervention efficacy in disadvantaged samples.
Health Disparities and Multiple Risks
Social health disparities in the area of HIV are associated with syndemic differences across countries. In particular, Gross Domestic Product (GDP), Human Development Index (HDI) and Healthcare Access and Quality (HAQ) Index are useful to differentiate the level of resources across countries. Overall, lower scores indicate lower financial resources, lower human development, and poorer healthcare access and quality. Most countries scoring poorly on these indices are in Africa and Central Asia, and their gaps relative to the U.S. and European countries are large. Lower (versus higher) GDP, HDI, and HAQ countries often have higher HIV incidence (Essig et al., 2015; Lou et al., 2014), as well as lower healthcare access, lower HIV testing rates, lower antiretroviral therapy (ART) coverage, and lower uptake of preventive measures (e.g., pre-exposure prophylaxis [PrEP] and protected sex; Levi et al., 2018). Thus, we were interested in assessing whether multiple-behavior interventions are more efficacious in countries with lower GDP, HDI, and HAQ than in countries with higher GDP, HDI, and HAQ.
Both in the U.S. and abroad, MSM are at substantially higher HIV risk than other groups. First, MSM are at greater risk because of the higher level of HIV prevalence in the MSM community (CDC, 2019). Compared to other men, MSM are also more likely to engage in unprotected anal intercourse (Koblin et al., 2003), have multiple sexual partners (Rosenberg et al., 2011), and use substances nonmedically (Stall & Purcell, 2000). These behaviors, which often co-occur (Outlaw et al., 2011), are all associated with a greater risk of contracting HIV (Koblin et al., 2006), and substance use also decreases adherence to ART (Hendershot et al., 2009) and PrEP (Van der Elst et al., 2013).
Within the U.S., racial/ethnic minorities are also at greater risk for HIV and other health problems than other groups. Compared to White individuals, Latinx individuals have a higher prevalence and incidence of HIV (CDC, 2019), higher rates of substance abuse (SAMHSA, 2013), higher rates of binge drinking (Chartier & Caetano, 2010), worse educational attainment (Woolf & Braveman, 2011), lower rates of condom use among females (Reece et al., 2010), greater stress (Mulia et al., 2008), and worse ART adherence (CDC, 2019). Compared to White people, Black individuals have higher levels of condom use (Reece et al., 2010) but a greater risk of HIV infection because of the higher HIV prevalence in their social networks (CDC, 2019). Black populations are also more likely to be undiagnosed for HIV (Singh et al., 2018), to have low ART adherence rates (Beer & Skarbinski, 2014), to have lower educational attainment and income (Woolf & Braveman, 2011), to experience greater stress (Beckie, 2012), to use substance nonmedically (e.g., crack; SAMHSA, 2019), and to have higher rates of sexually transmitted infections (STIs; De Francesco et al., 2018). Finally, younger adults also have a unique pattern of risk. Specifically, adolescents and young adults have higher rates of unprotected sex (SAMHSA, 2013), alcohol use (Hingson & White, 2014), nonmedical substance use (Baumann et al., 2007), anxiety and suicide (Organization, 2017), as well as greater medication non-adherence (Reisner et al., 2009). The picture is further complicated by varying disparities among younger people in different racial/ethnic minority groups (Valleroy et al., 2000), suggesting that disparities may compound and exacerbate each other.
Multiple-Behavior Interventions
Whether multiple-behavior interventions work better than single ones in HIV is an empirical question. A prior meta-analytic review of 15 randomized controlled trials found that interventions recommending at least two behaviors that related to transmission risk, care management, or medication adherence had lower rates of unprotected sexual intercourse than did other interventions (Crepaz et al., 2015). Although this meta-analysis was small and only included studies targeting people living with HIV, it did suggest that multiple-behavior interventions may be more efficacious than single-behavior ones across the board. A more recent meta-analysis focusing on trials with sexual minority men also led to the conclusion that multiple-behavior interventions were associated with improvements in mental health and substance use (Pantalone et al., 2020).
Multiple-behavior interventions may lead to greater engagement with the intervention and, therefore, more change because the intervention offers several pathways and increases the probability that at least one would be more interesting to a participant. In addition, multiple-behavior interventions may require greater effort to meet multiple goals and promote more change due to higher expectations of success (Förster et al., 2005). Multiple-behavior interventions are also likely to leverage “motivational spill-over,” such that success in changing one behavior increases motivation and self-regulation for other behaviors (Mata et al., 2009; K. Wilson et al., 2015). An example of HIV would be that successfully reducing alcohol and nonmedical substance use may promote positive expectations and, in turn, increase the regulation of sexual risk (Parsons et al., 2014).
Critical to our research question, multiple-behavior interventions are one response to syndemics because they target change in two or more risky behaviors either simultaneously or sequentially (Prochaska et al., 2008). By doing so, these interventions can take advantage of the ability of change in one behavior to promote changes in another (Albarracín et al., 2017; Albarracín, Sunderrajan, et al., 2018; Albarracín, Wang, et al., 2018). For example, reducing substance use may lead to reductions in alcohol use, sexual violence, having sex under the influence, and increases in condom use (Pitpitan et al., 2018). Addressing multiple behaviors that correlate with a target behavior in the syndemics cluster may also increase success in implementing the target behavior. For example, one intervention targeted alcohol use and medication adherence together as a way of improving intervention efficacy (Bachanas et al., 2016). Additionally, an intervention combining ART adherence with the introduction of mindfulness meditation practice has been shown to reduce self-management barriers (e.g., panic, lack of focus, and timing problems) to adherence (Creswell et al., 2009).
To summarize, multiple-behavior interventions may address syndemics better than single-behavior interventions. Individual multiple-behavior interventions for HIV prevention, testing, and treatment have shown promising results by addressing correlated risk behaviors (e.g., injection and sexual risk behaviors, Garfein et al., 2007). For example, one study of young MSM utilized motivational interviewing to reduce two high-risk behaviors – the use of recreational drugs and unprotected anal sex (Parsons et al., 2014). This study found that the multiple-behavior approach to change was more successful than a typical single-behavior intervention for MSM. In this meta-analysis, we examined whether multiple-behavior intervention addresses syndemics with a comprehensive dataset of 331 reports (see Supplemental Materials), considering the income and development of countries, and within the US samples, representation by racial/ethnic and sexual minorities and age.
The Present Meta-Analysis
We assessed the associations between the efficacy of multiple-behavior interventions and the disadvantaged population status in two ways. First, we used data from all the included countries to assess whether multiple-behavior interventions are more efficacious in countries with different levels of log GDP, GDP per capita, HDI, and HAQ index, in addition to the proportion of gay/bisexual or MSM in the sample and being young adults. Second, we used US data to assess whether, relative to single-behavior interventions, multiple-behavior interventions are more efficacious for racial/ethnic minorities (i.e., Black and Latinx participants), MSM (Grey et al., 2016), and young adults. The analyses involved meta-regression models using Robust Variance Estimation (RVE) clustered by report with small-sample corrections (Pustejovsky & Tipton, 2022; Tipton & Pustejovsky, 2015) to account for the statistical non-independence between effect sizes from the same study samples (Hedges et al., 2010). These models controlled for methodological and sample characteristics. We also specified the single-behavior intervention as the reference group. The effect sizes represent changes in outcomes from the pretest to the posttest, which is the optimal approach when clinical trials use a variety of control groups (Dai et al., 2020; Sunderrajan et al., 2021a). A review of the literature showed only one meta-analysis about multiple-behavior interventions (Sunderrajan et al., 2021b). However, this work focused on examining whether the number of recommendations included in HIV interventions improved intervention efficacy. It did not answer the question of the differential effects of three conditions (i.e., multiple-behavior, single-behavior, and passive controls) across more and less advantaged groups. We pre-registered this project as RQ3 (i.e., research question 3) in https://osf.io/mq5kr. The full dataset and analysis codes are available at https://osf.io/cg7ea/?view_only=a5a9e84d73e3403ea56d29ecd5b3131a.
Method
Search Strategy
We used the same methods as other recent publications that have used other components of this project (Dai et al., 2020; Sunderrajan et al., 2021a), which spanned various domains. We searched MEDLINE, PsycINFO, and EBSCO through July 27, 2022. Our search terms included: intervention, health education, persuasion, recommendation, treatment, educational program, rehabilitation, counseling outcomes, treatment outcomes, treatment effectiveness evaluation, treatment compliance, health promotion, behavior change, and randomized trial. To identify HIV-related recommendations, these keywords were entered in combination with keywords for interventions promoting change in HIV and related behaviors, including HIV, AIDS, STI/STD, condom use, circumcision, alcohol use, drug use, and adherence. In addition, we searched conference titles (i.e., conferences of American Public Health Association), emailed the most published authors in our database to request published and unpublished works, sent requests to relevant organizations and listservs, and examined the reference lists of prior reviews to identify other possible reports for inclusion. This search yielded 17,387 total possible articles.
Inclusion Criteria
Once our search was complete, we used the following criteria to determine the inclusion and exclusion of reports from our analysis (see Figure 1):
Figure 1.
PRISMA Diagram
Presence of at least two groups. Reports had to include both a comparison group and a multiple-behavior intervention group. Comparison groups were separated into three categories: (a) passive controls (no recommendations), (b) active control or single-behavior interventions (i.e., interventions making one recommendation), and (c) multiple-behavior interventions (i.e., interventions making two or more recommendations).
Presence of at least one behavioral or clinical outcome. Reports had to include at least one behavioral or one clinical outcome. We excluded reports that only included information on behavioral intentions, attitudes, perceived social norms, or other non-behavioral or non-clinical outcomes.
Presence of appropriate statistics to estimate change over time. Reports had to include enough statistical information to calculate effect sizes representing change over time. Thus, reports had to include outcome values at both the baseline and at least one follow-up. When information was only reported for the follow-up, reports were excluded. Studies that only included reported analyses with statistical controls or other statistical corrections were excluded as well.
After screening abstracts, we excluded 15599 articles and assessed 1788 full-text reports for eligibility. After applying our inclusion criteria, 331 reports from 153 studies were included in our analyses (see Supplemental Material).
Coding and Data Extraction
The coding and data extraction was comprehensive and included determining the type of condition to which a group was assigned, moderators related to social disadvantages, and other exploratory moderators related to the report, the intervention, and the methods. All coding and data extraction were conducted via an in-house online data entry system using a coding and data entry manual developed by the research team. One researcher extracted the means and standard deviations or proportions for each available study outcome. A second researcher later rechecked the entries of these data entry for each paper.
Coding of Reports about Syndemic Conditions
We combined a text analysis of the study titles and gave a score of 1 (versus 0) to any study that had any of the following keywords: “acute hiv infection”, “aids/hiv patient”, “antiretroviral”, “concordant”, “hiv infection”, “hiv-infectat”, “hiv-infected”, “hiv-positive”, “hiv/aids-infected”, “hiv+”, “living with hiv”, “living with hiv/aid”, “men with aids”, “medication adherence”, “nonadherence”, “seropositive”, “serostatus”, “women with aids”, “addiction”, “binge substance use”, “cocaine”, “crack”, “depress”, “drug use”, “hazardous alcohol”, “hazardous drinker”, “heavy drinker”, “history of drug use”, “homeless”, “incarcerated”, “inject drug”, “injection”, “intravenous”, “jail”, “justice-involved”, “marginalized”, “marijuana”, “methadone”, “methamphetamine”, “migrants”, “narcology”, “offender”, “opioid”, “probationer”, “relapse”, “residential drug abuse”, “sex worker”, “substance abuser”, “substance misuse”, “substance user”, “substance-dependent”, “substance-using”, “trauma”, “unhealthy alcohol use”, “use stimulant”, and/or “violence.” Studies without those keywords were coded 0. In addition, we coded whether studies included participants from a clinical population or other vulnerable populations (a report scored as 1 if participants were at-risk, such as drug dependent or female sex workers and 0 otherwise; see Table 1). We next calculated the sum of these three indicators. Scores equal to one or above indicate the reports were with participants who were at-risk or experienced syndemic conditions.
Table 1.
Descriptive Statistics of Three Hundred and Thirty-One Included Reports (k = 1,364)
| Variable | Inter-rater reliability | Count | M (SD) or percentages |
|---|---|---|---|
| Report characteristics: | |||
| Publication year^ | 1.00 | 311 | 2011 (6.7) |
| Source type | 1.00 | ||
| Journal article | 325 | 98.19% | |
| Other | 2 | 0.60% | |
| Institutional area | 1.00 | ||
| Psychology | 31 | 9.37% | |
| Community/public health | 97 | 29.31% | |
| Medicine | 110 | 33.23% | |
| Epidemiology | 18 | 5.44% | |
| Social work | 9 | 2.72% | |
| Other | 54 | 16.31% | |
| Language of the report | 1.00 | ||
| English | 329 | 99.4% | |
| Six-month follow up | 1.00 | ||
| Yes | 250 | 75.53% | |
| No | 79 | 23.87% | |
| Number of participants^ | 1.00 | 327 | 254.80 (691.18) |
| Age in years^ | 1.00 | 329 | 29.49 (9.98) |
| Gender | |||
| % men^ | 1.00 | 313 | 54.53 (36.01) |
| % women^ | 1.00 | 314 | 45.97 (35.91) |
| Racial/ethnic descenta | |||
| % White American^ | 1.00 | 254 | 21.94 (28.60) |
| % African American^ | 1.00 | 264 | 33.44 (35.75) |
| % Latin American^ | 1.00 | 245 | 15.32 (25.51) |
| % Asian American^ | 1.00 | 209 | 5.61 (21.19) |
| % Native American Indian^ | 1.00 | 210 | 2.3 (13.72) |
| % High school graduates^ | 1.00 | 180 | 44.24 (33.98) |
| Self-identified sexual orientation | |||
| % heterosexual^ | 1.00 | 102 | 47.95 (42.7) |
| % Gay/bisexual or MSM^ | 1.00 | 94 | 53.68 (42.98) |
| Intention to treat | 0.42 | ||
| Yes | 91 | 27.49% | |
| No | 179 | 54.08% | |
| Intervention characteristics: | |||
| Sample targeted by race/ethnicity | 1.00 | ||
| Yes | 67 | 20.24% | |
| No | 249 | 75.23% | |
| Sample targeted by gender | 1.00 | ||
| Yes | 127 | 38.37% | |
| No | 189 | 57.10% | |
| Targeted sample | 1.00 | ||
| HIV positive* | 55 | 16.52% | |
| Intravenous drug user* | 18 | 5.44% | |
| Drug dependent* | 21 | 6.34% | |
| Men who have sex with men* | 25 | 7.55% | |
| Female sex workers* | 2 | 0.60% | |
| Prison inmates* | 5 | 1.51% | |
| Minority women* | 3 | 0.91% | |
| African American women* | 8 | 2.42% | |
| College students | 8 | 2.42% | |
| Middle school students | 5 | 1.51% | |
| Combination* | 42 | 12.69% | |
| Self-selected sample | 1.00 | ||
| Yes | 278 | 83.99% | |
| Nob | 42 | 12.69% | |
| Both | 2 | 0.60% | |
| Recruitment context | 1.00 | ||
| Hospital/health clinic | 141 | 42.60% | |
| Drug treatment | 26 | 7.85% | |
| Social service | 10 | 3.02% | |
| Bar | 8 | 2.42% | |
| Street | 13 | 3.93% | |
| Classroom | 23 | 6.95% | |
| Multiple | 40 | 12.08% | |
| Exposure setting | 1.00 | ||
| Health clinic | 132 | 39.88% | |
| Community | 35 | 10.57% | |
| Schools | 19 | 5.74% | |
| Mass media | 2 | 0.60% | |
| Multiple contexts | 13 | 3.93% | |
| Delivery medium | 0.97 | ||
| Face-to-face | 239 | 72.21% | |
| Software | 10 | 3.02% | |
| Internet | 9 | 2.72% | |
| Television | 9 | 2.72% | |
| Radio | 3 | 0.91% | |
| Multiple context | 11 | 3.32% | |
| Delivery format | 1.00 | ||
| Groups | 107 | 32.33% | |
| Individuals | 136 | 41.09% | |
| Both | 42 | 12.69% | |
| Facilitator | 0.93 | ||
| Doctor/nurse | 43 | 12.99% | |
| Public health educator | 47 | 14.20% | |
| Clinical psychologist | 17 | 5.14% | |
| Community leader | 1 | 0.30% | |
| Multiple | 19 | 5.71% | |
| Culturally appropriate | 0.89 | ||
| Yes | 77 | 23.26% | |
| No | 241 | 72.81% | |
| Days between intervention and post-test^ | 0.88 | 317 | 164.79 (185.04) |
| Duration of the intervention (in hours) | 0.88 | 217 | 9.07 (15.79) |
| Ensuring high intervention fidelity | 0.97 | ||
| Yes | 65 | 19.64% | |
| No | 266 | 80.36% | |
| Attitudinal elements: | |||
| Attitude arguments | 0.63 | ||
| Yes | 118 | 35.65% | |
| No | 114 | 34.44% | |
| Threat arguments | 0.45 | ||
| Yes | 40 | 12.08% | |
| No | 145 | 43.81% | |
| Norm arguments | 0.64 | ||
| Yes | 60 | 18.13% | |
| No | 139 | 41.99% | |
| Informational arguments | 0.55 | ||
| Yes | 243 | 73.41% | |
| No | 32 | 9.67% | |
| Motivational elements: | |||
| Feedback | 0.51 | ||
| Yes | 64 | 19.34% | |
| No | 153 | 46.22% | |
| Skill training elements: | |||
| Skill training | 0.69 | ||
| Yes | 146 | 44.11% | |
| No | 89 | 26.89% | |
| Goal setting | 0.64 | ||
| Yes | 66 | 19.94% | |
| No | 145 | 43.81% | |
| Role playing | 0.54 | ||
| Yes | 57 | 17.22% | |
| No | 160 | 48.34% |
Note. M = mean, SD = standard deviation. A caret indicates variables used r = intercoder reliability for continuous variables; other variables used κ = intercoder reliability for categorical variables. An asterisk indicates the at-risk populations.
reported for interventions conducted in North America, as these categories are not applicable to most other continents.
includes captive audiences (e.g., participants recruited from a prison setting).
Determination of Multiple-Behavior Intervention, Single-Behavior Intervention, and Passive Control
Each report condition was coded into one of three categories: (a) multiple-behavior intervention, (b) single-behavior intervention, or (c) passive control group. Multiple-behavior interventions had two or more behavioral recommendations (e.g., use condoms more frequently and reduce alcohol consumption). As an example, (Amaro et al., 2007) recommended that participants both reduce their substance use and reduce their sexual risk through abstinence. This intervention was therefore coded as a multiple-behavior intervention. Similarly, Go et al. (2015) included one intervention group that recommended clients (a) reduce their sexual risk behaviors and (b) reduce their injection drug use, (c) seek social support, (d) disclose their HIV status, and (e) ask about their partners to get tested. As this group involved changing more than two behaviors, it was coded as a multiple-behavior intervention.
Single-behavior interventions had only one behavioral recommendation. For instance, one study of HIV adherence had a group whose only recommendation was to take their pills as prescribed (Pop-Eleches et al., 2011). Other general examples of single-behavior interventions include intervention arms that only recommended HIV adherence or only HIV testing (e.g., (Barnett et al., 2009). Finally, if a group had no direct behavioral recommendations, it was coded as a passive control condition. An example of a no-recommendation condition would be either a waitlist control or a condition providing general information without a recommendation to change a behavior (Santos et al., 2014).
Coding of Moderators Related to Population Disadvantages
Country Code and Country Development Indices.
Each report was coded for the country where the study was conducted. Country indices of development were included by obtaining GDP and GDP per capita from the World Bank, HDI from the United Nations Human Development Report, and the HAQ index from the Institute for Health Metrics and Evaluation (University of Washington School of Medicine, 2023).
For a given report, we estimated the developmental indices using the closest year available. Therefore, studies from the same countries (e.g., the U.S.) but conducted at different time periods were linked to different developmental indices. Four studies included in our dataset provided data with a sample representing multiple countries (Amirkhanian et al., 2005; Bachanas et al., 2016b; Latkin et al., 2009; Nöstlinger et al., 2016). All of these studies, except for Latkin et al. (2009), reported only overall sample sizes (e.g., n = 488). We, therefore, assumed even representation from each country and calculated a simple average of the country indices corresponding to the represented countries. For example, the sample included 244 US participants and 244 Thai participants, the calculation of log GDP was [244*30 + 244*25.6]/(244+244) = 27.8.
MSM and Race/Ethnicity.
We coded the following sample characteristics: (a) percentage of females in each group, (b) percentages of gay/bisexual or MSM, (c) percentages of participants of European, African, Latin, Asian, and Native American descent, and (d) mean age. Most of the included reports did not include all sample characteristics. In some cases, specific races/ethnicities were missing despite others being reported (e.g., only White and Black representation was reported). In these cases, the unknown portions of the sample were coded as percentages of “Other” racial/ethnic representation.
Coding of Exploratory Moderators
Relevant characteristics of the reports, as well as the methods used in the reports, were coded. Intercoder reliability coefficients (Fleiss′ kappa for categorical variables and Pearson correlations for continuous variables) are summarized in Table 1. Disagreements between coders were resolved by discussion, further examination of the reports, and consultation with an additional coder if needed.
Report Characteristics.
We coded (a) publication year, (b) first authors’ institution (e.g., college, hospital, research center), (c) first authors’ institutional area (e.g., psychology, public health, and medicine), (d) source type (e.g., journal article and dissertation), (e) language of the report, and (f) research quality by calculating a total score of three binary indicators (i.e., 1 = yes versus 0 = no), including whether the study had random assignment of participants into groups, included a 6-month follow-up, and had analyses using intention-to-treat principles.
Methodological and Other Sample Characteristics.
We coded for factors related to the design of the intervention, including (a) whether the intervention involved samples with limited refusal freedom (e.g., prisoners) or samples without such limitations, (b) what the exposure setting (e.g., school, community) was, which was recoded to represent a clinical versus non-clinical setting, (c) what the exposure format (e.g., radio, brochure) was, which was recoded to indicate face-to-face versus other formats, (d) whether the intervention was delivered to a group, to individuals, or to a combination of the two, (e) whether the intervention was described as culturally appropriate, (f) the number of days between the intervention and posttest, (g) the duration of intervention (in hours), (h) whether the intervention facilitator was a professional (e.g., physician, nurse, social worker, and professional counselor) or lay community member (e.g., community leaders and peers), (i) whether the measured outcome was targeted in the intervention (e.g., HIV testing for a testing intervention versus depression for an HIV treatment intervention that did not target depression), (j) whether the intervention targeted specific populations (e.g., race and gender), (k) where participants were recruited (e.g., drug treatment facility, social service agency), which was recoded to describe a medical versus non-medical setting, (l) whether the intervention was ensured to have high fidelity, for example, the intervention implemented any best practices and recommendations from the NIH Behavior Change Consortium (Bellg et al., 2004), and whether the intervention relied on (m) attitudinal elements (e.g., attitudinal arguments, threat arguments), (n) passive strategies, e.g., informational arguments (Albarracín et al., 2005), (o) motivational elements (e.g., feedback, encouragement), or (p) skills training elements (e.g., role playing, goal setting; Michie et al., 2014).
Effect Sizes
Our effect size of interest was within-group change over time (d) for all intervention-relevant behaviors and clinical outcomes and for all available follow-ups. The decision to use change as our effect size was based on careful consideration of the characteristics of our data. First, a substantive problem with between-group effect sizes is that a control group may be a passive or active control (Karlsson & Bergmark, 2015). Comparing a treatment group to a passive control group is an absolute comparison (i.e., the maximum amount of change relative to no treatment) that tends to produce larger effect sizes, whereas comparing a treatment group to an active control group is a relative comparison (i.e., the amount of change relative to another treatment) that produces smaller effects (Brookmeyer et al., 2016). In addition, many control groups in this literature are themselves multiple-behavior interventions. Therefore, the impact of these conditions is best verified over time. Another advantage of analyzing changes over time is that this approach allows for individual slope models to assess if moderators have differential effects on multiple-and single-behavior intervention groups, allowing for more informative conclusions. Ascertaining intervention efficacy over time, with everyone serving as their own control, was therefore optimal.
To calculate d for continuous outcomes, the mean of the pre-test measure was subtracted from the mean of the post-test measure, and this difference was then divided by the pooled standard deviation of the means (Borenstein et al., 2009). To calculate d for proportional outcomes, an odds ratio was calculated by dividing the odds of the behavior at the post-test by the odds of the behavior at the pre-test and then converting it into a d by taking the natural log and dividing it by 1.81 (Borenstein et al., 2009). In cases where the reported proportions were 0 or 1, a correction of 0.005 was added to 0’s and subtracted from 1’s to reduce the skew produced by extreme odds ratio calculations (Sweeting et al., 2004). All effect sizes were corrected for small sample sizes (Freeman et al., 1986) and coded such that positive effect sizes reflect health improvement (e.g., increased testing rates and decreases in unprotected sex). As the correlation between pre-test and post-test observations was unknown, we assumed that r = .5 in our analyses (Morris et al., 2000). We used corrected estimates and standard errors for studies reporting results from three reports of cluster-randomized trials (Hedges, 2007). Previous analyses from other domains of the larger dataset have found that models are robust to changes in the assumed correlation (K. Wilson et al., 2015). Due to potential limitations of this approach, we also repeated all analyses with difference-in-differences scores (see Supplemental Material).
Our dataset included 39 different outcome types, which are shown in Table 2. These measures are related to HIV/sexual risk, substance use, psychological wellbeing, and other syndemic outcomes. Examples of HIV/sexual risk outcomes are measures like the numbers of unprotected sexual encounters in a specific time period, proportions of the sample that received an HIV test since the last measurement, HIV viral load measures, or proportions of the sample meeting treatment adherence standards. Examples of substance use outcomes include self-reports of the amount of alcohol or substance use in a given period, as well as clinical measures such as chemical representation in urine samples. Psychological wellbeing measures include reports of depression and quality of life. Other syndemic outcomes includes quality of life, body mass index, and unwanted pregnancy.
Table 2.
Outcome Type and Syndemic Categorization
| Outcome | Syndemic category |
|---|---|
| Abstinence | HIV/Sexual risk |
| Adolescent marriage | Other syndemic |
| Alcohol use | Substance use |
| Body mass index | Other syndemic |
| CD4 | HIV/Sexual risk |
| Condom use/unprotected sex | HIV/Sexual risk |
| Condom-protected intercourse | HIV/Sexual risk |
| Contraception (other than condoms) | HIV/Sexual risk |
| Lower depression | Psychological wellbeing |
| Drug use | Substance use |
| HIV adherence | HIV/Sexual risk |
| HIV disclosure | HIV/Sexual risk |
| HIV prevalence | HIV/Sexual risk |
| HIV testing | HIV/Sexual risk |
| HIV treatment | HIV/Sexual risk |
| Intervention attendance | Other syndemic |
| Medication adherence | Substance use |
| Mental health | Psychological wellbeing |
| Past year physical and/or sexual IPV | HIV/Sexual risk |
| Physical health | Other syndemic |
| Quality of life | Other syndemic |
| Quality of life environmental | Other syndemic |
| Quality of life physical | Other syndemic |
| Quality of life psychological | Psychological wellbeing |
| Quality of life social | Psychological wellbeing |
| Risk sexual behavior | HIV/Sexual risk |
| Safe sex strategies | HIV/Sexual risk |
| School dropout | Other syndemic |
| Sex | HIV/Sexual risk |
| Sex and drinking | HIV/Sexual risk |
| Sex for money | HIV/Sexual risk |
| Sex partner selection | HIV/Sexual risk |
| STI rates | HIV/Sexual risk |
| STI testing | HIV/Sexual risk |
| Substance use | Substance use |
| Talk about HIV | HIV/Sexual risk |
| Tobacco treatment | Substance use |
| Unwanted pregnancy | Other syndemic |
| Viral load | HIV/Sexual risk |
Main Analyses
We first examined selection bias by conducting the Egger Sandwich test (Sterne et al., 2006) with the use of a multilevel meta-analysis (MLMA) approach and a modified covariate (Pustejovsky & Rodgers, 2019). We did not use PET-PEESE (precision-effect test and precision-effect estimate with standard errors), trim-and-fill, or selection models because these detection methods are biased when the data contain statistically dependent effect sizes (Rodgers & Pustejovsky, 2021). Second, we estimated the average effect in each of the three types of conditions and estimated the impact of exploratory moderators to be included in our main analyses using robust variance estimation (RVE) models with small-sample corrections (Tipton & Pustejovsky, 2015). Third, we conducted a confirmatory factor analysis among changes in HIV/sexual risk, substance use, psychological wellbeing, and other syndemic measures. The presence of a satisfactory model fit would support the notion that these outcomes are, in fact, intercorrelated as proposed by syndemics.
The key analyses were to test the differential effects of multiple-behavior intervention as a function of country development indices, proportion of gay/bisexual or MSM, different races/ethnicities, and different ages. Using log GDP as an example, we fit a meta-regression model clustered by report with small-sample corrections that include the main effects for intervention groups (with single-behavior intervention as a reference group) and log GDP, the interaction between these two moderators, also controlling for covariates suggested by our exploratory moderators. A significant interaction between multiple-behavior intervention and log GDP implies differential effects of multiple-behavior intervention programs related to log GDP, relative to the effects of single-behavior intervention programs. The analyses for country development indices, gay/bisexual or MSM, and different ages were conducted with the entire dataset from all countries.
We next analyzed the US data to examine the differential effects of multiple-behavior intervention for racial/ethnic minority (e.g., non-White Americans) versus majority representation, specific racial/ethnic minorities (e.g., African Americans, Latinx people, and Other2), gay/bisexual or MSM, different ages, and the intersection of racial/ethnic minority (see CDC, 2019). These meta-regression models were specified in a similar way to the international ones, except that the country development indices were replaced with the gay/bisexual or MSM, race/ethnicity, and age variables. For the meta-regression model testing the interaction between racial/ethnic minorities and gay/bisexual or MSM, we only analyzed multiple-behavior intervention groups due to insufficient data in other conditions.
In addition to coefficients from the random-effects models, we calculated I2, which indicates the proportion of total variance due to random-effects variance (Higgins et al., 2003; Huedo-Medina et al., 2006). We used R packages metafor (Viechtbauer, 2019) to perform the bias test and combined the metafor object with the package clubSandwich (Pustejovsky, 2022) to conduct the main analyses, including the mean effect size estimation and meta-regression analyses clustered by report with small-sample corrections. Following the significant interactions between multiple behavior intervention and country development indices identified in the meta-regression models, we computed the predicted ds and included them in the analyses of pair-wise comparisons to interpret the interaction. The interpretation of effect sizes was based on Cohen (1988).
Results
The meta-analysis included 331 reports, which provided 205 intervention groups recommending multiple behaviors (MBI), 76 intervention groups recommending a single behavior (SBI), and 50 passive control (PC) groups. About 96 percent of the included reports (i.e., 317; 98% of the records) were with samples that were at-risk or experienced syndemic conditions (e.g., HIV infection and substance use disorder). The average study group had 4.11 effect sizes, and a total of 32 countries were represented in the dataset. About fifty-nine percent of the reports were conducted in the U.S. (n = 810), and of the non-US studies, the more represented countries outside the U.S. were South Africa (n = 105), Thailand (n = 74), China (n = 66), Kenya (n = 50), and. Overall, the data had adequate variability to assess differences across countries.
Within the U.S., 59 percent of the samples reported being gay/bisexual or MSM, 27 percent of participants were of European descent, 49 percent of participants were of African descent, 19 percent of Latinx descent, and 1 percent of participants were of Asian descent. The average age was 29.60 years (SD = 11.34), and the samples were 48 percent female. As with countries, there was considerable demographic variability in assessing demographic differences within the U.S.
Selection Bias
Figure 2 shows a contoured-enhanced funnel plot of effect sizes, which plots ds on the x-axis and the standard error of each effect on the y-axis. Although the funnel plot must be viewed with caution due to the inclusion of non-independent effect sizes, asymmetry is often considered as possibly associated with bias. We followed Rodgers and Pustejovsky (2020)’s proposed analytical procedures to conduct Egger Sandwich tests with the MLMA approach for all effect sizes and then separately for the effects from multiple-behavior interventions, single-behavior interventions, and passive controls. The Egger Sandwich test is essentially a modified regression test using a modified covariate proposed in Pustejovsky and Rodgers (2019) to test the significance of a regression slope as an indicator of asymmetry in the funnel plot. The overall analysis showed a significant slope, p = .026, suggesting potential selection bias in the overall dataset. When dividing the dataset according to intervention types, we also detected selection bias in the effect sizes from multiple-behavior interventions, p = .044 and marginally significant for passive controls, p = .069, but not from single-behavior interventions, p = .182. Given the possible presence of selection bias from results of the Egger Sandwich test, we included the modified covariate in the following analyses to mitigate bias in our results.
Figure 2.
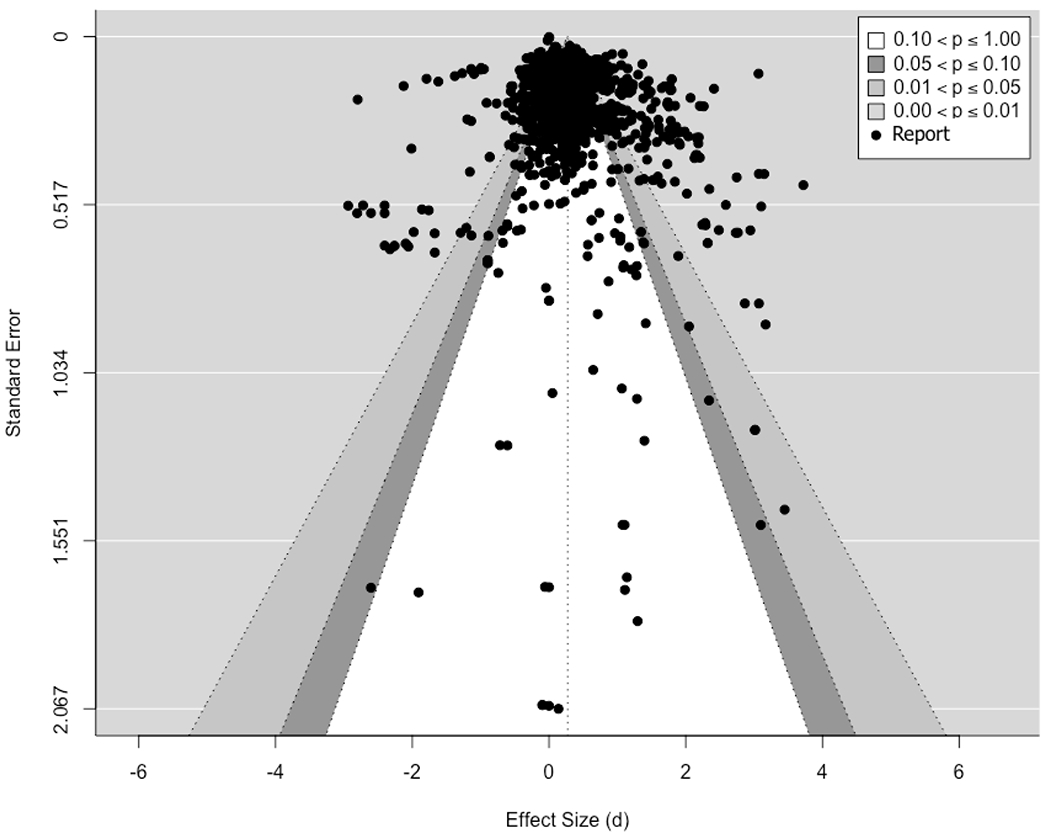
Contour-Enhanced Funnel Plot
Note. The dotted vertical line corresponds to the random-effects model estimate of average effect (0.273), and the funnel shape corresponds to the 90%, 95%, and 99% pseudo confidence intervals.
Average Intervention Efficacy
In the preliminary analyses, we first assessed possible differences in efficacy across interventions by testing whether the effect sizes were associated with three dummy indicators for multiple-behavior intervention, single-behavior intervention, and passive control group. Results showed no difference in intervention efficacy between multiple-behavior intervention, d = 0.29, 95% CI [0.12, 0.45], and single-behavior intervention, d = 0.25, 95% CI [0.02, 0.48], b = 0.03, SE = 0.09, p = .697. In contrast, passive controls had lower efficacy than both multiple-behavior and single-behavior interventions (see Table 3). The estimated mean effect size of passive controls was nonsignificant, d = 0.04, 95% CI [−0.14, 0.23]. There was also substantial heterogeneity among effect sizes, I2 = 94.63%.
Table 3.
Mean Estimated Effect Sizes of Different Intervention Types (Cluster = 324, k = 1348)
| Variable | b (SE) | df |
|---|---|---|
| Single-behavior intervention | 0.25* (0.12) | 76.77 |
| Multiple-behavior intervention | 0.29** (0.08) | 90.98 |
| Passive control | 0.04 (0.09) | 56.85 |
| I 2 | 94.63 |
Note. Report of the robust variance estimation meta-regression using single-behavior intervention as the reference (i.e., intercept). b = unstandardized coefficient; SE = standard error; df = degrees of freedom; p = p value; k = number of effect sizes; I2 = proportion of variance due to between-group heterogeneity.
p < .10,
p < .05,
p < .01,
p < .001
Identifying Covariates among Exploratory Moderators
We next assessed whether the report, methodological, and sample characteristics moderated effect sizes beyond intervention type and needed to be controlled for in subsequent analyses. We evaluated each covariate by adding it as a predictor to the model reported in Table 3 if the moderators were had fewer than 10 percent missing data (i.e., NA) in the dataset. For report characteristics, we tested (a) whether the report’s institutional area explained the intervention efficacy, ps = .106 - .769, as well as (b) whether the research quality explained the intervention efficacy, p = .572. For methodological characteristics, we assessed (c) whether the report indicated that the intervention was culturally appropriate, b = −0.11, SE = 0.06, p = .070, (d) whether the intervention involved samples with limited refusal freedom (e.g., prisoners) or samples without such limitations, ps = .104 - .920, (e) whether exposure to the interventions took place in clinical or non-clinical (e.g., community) settings, p = .129, (f) whether exposure was face-to-face or not, p = .795, (g) whether the intervention was delivered as solely individual or non-individual sessions (e.g., group or both individual and group sessions), p = .401, (h) the number of days between the intervention and the posttest, p = .951, (i) whether the intervention involved expert facilitators or lay community members, b = 0.22, SE = 0.07, p = .001, (j) whether the measured outcome was targeted in the intervention, p = .141, (k) whether the intervention targeted specific race, p = .321, (l) whether the intervention targeted specific gender, p = .696, (m) whether participants were recruited from medical settings, b = 0.15, SE = 0.06, p = .011, and (n) whether the intervention was ensured to have high fidelity, p = .575. For sample characteristics, we examined (o) participants’ average age, b = 0.01, SE = 0.00, p < .001, and (p) the percentage of females in the samples, b = −0.00, SE = 0.00, p = .081. In summary, results showed larger intervention effects for (a) older participants, (b) female participants, (c) interventions delivered by expert facilitators (e.g., physicians or professionally trained counselors), (d) participants recruited from medical settings, and (e) interventions not described as culturally appropriate. Therefore, we included these five covariates in the main analyses.
Confirmatory Factor Analysis (CFA) among Different Syndemic Outcomes
We next conducted a CFA to examine if HIV/sexual risk, substance use, psychological wellbeing, other syndemic outcomes are interrelated as proposed by the notion of syndemics. We first calculated two latent factors of HIV/sexual risk and substance use, each with many outcomes. These two latent factors were subsumed under a single factor representing syndemic outcomes, which also included psychological well-being and other syndemic outcomes.
Table 4 shows the significant factor loadings of all first order factors on the overall syndemic factor, p < .001. As for the goodness of fit, Comparative Fit Index (CFI), Relative Fit Index (RFI), and Incremental Fit Index (IFI), which adjust for and thus are less sensitive to small sample size, showed satisfactory goodness of fit. However, Chi-squared and Root Mean Square Error of Approximation (RMSEA), which are more sensitive to sample size, showed poorer fit. Taken together, the CFA results showed substantial support for HIV/sexual risk, substance use, psychological wellbeing, and other syndemic outcomes as interrelated. Therefore, improvements in one syndemic outcome may be tied to improvements in other syndemic outcomes.
Table 4.
Confirmatory Factor Analyses of HIV/Sexual Risk, Substance Use, Psychological Wellbeing and Other Syndemic Outcomes
| Variable | b* |
|---|---|
|
| |
| Factor loading | |
| HIV/sexual risk | |
| HIV/sexual risk latent variable 1 | 0.31*** |
| HIV/sexual risk latent variable 2 | 1.49*** |
| Substance use | |
| Substance use latent variable 1 | 1.00*** |
| Substance use latent variable 2 | 1.00*** |
| Overall syndemic | |
| HIV/sexual risk | 0.56** |
| Substance use | 0.76*** |
| Psychological wellbeing | 0.89*** |
| Other syndemic | 1.06*** |
|
| |
| Fit Indices | |
| χ2 (df) | 50.90*** (7) |
| RMSEA | 0.1 |
| CFI | 0.94 |
| TLI | 0.87 |
| SRMR | 0.15 |
| RFI | 0.85 |
| IFI | 0.94 |
Note. b* = Parameter estimates when all variables are standardized; RMSEA = Root Mean Square Error of Approximation; CFI = Comparative Fit Index; TLI = Tucker Lewis index; SRMR = (Standardized) Root Mean Square Residual; RFI = Relative Fit Index; IFI = Incremental Fit Index. Bold style indicates a good fit (i.e., CFI and IFI > .90 and RFI ~ 1).
p < .001
Differential Effects of Multiple-Behavior Interventions Related to Country Development, Gay/bisexual or MSM Representation, and Being Young Adults
Table 5 shows the results of the meta-regression models clustered by report and with small-sample corrections. Log GDP, GDP per capita, HDI, and HAQ all revealed the same pattern of interactions with multiple-behavior intervention across the multi-level meta-regression models, bs = −1.90 – −0.00, ps < .003 – .072. Specifically, participants from less developed countries, where syndemics are presumably more pronounced, improved more in response to multiple-behavior interventions than in response to single-behavior interventions. Figure 3 illustrates the interactions involving the log GDP, GDP per capita, HDI, and HAQ in the multi-level meta-regression models. As shown, intervention efficacy increases in multiple-behavior interventions as log GDP, GDP per capita, HDI, and HAQ decreases. Next, we included the syndemic categories (i.e., HIV/sexual risk, substance use, psychological wellbeing, and other syndemic) as additional moderators to assess whether the interaction effects were robust to these controls. The direction, strength, and significance of the interaction effects remained the same after controlling for the syndemic categories.3 Overall, the results indicated that multiple-behavior interventions, rather than single-behavior interventions, were associated with larger improvements in samples from less developed countries.
Table 5.
Differential Effects of Multiple-Behavior Interventions Related to Country Log GDP, GDP Per Capita, HDI, and HAQ
| Development indices | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Log GDP | GDP per capita | HDI | HAQ | ||||
|
| ||||||||
| b (SE) | df | b (SE) | df | b (SE) | df | b (SE) | df | |
| (Cluster = 278, k = 1180) | (Cluster = 278, k = 1180) | (Cluster = 276, k = 1172) | (Custer = 282, k = 1192) | |||||
| Intercept | −0.96 (0.72) | 29.00 | −0.16 (0.18) | 64.95 | −0.68† (0.36) | 19.94 | −0.41 (0.26) | 28.28 |
| Intervention type: MBI | 2.63* (1.00) | 50.99 | 0.22 (0.14) | 55.28 | 1.57** (0.49) | 32.25 | 0.9** 0.31) | 39.26 |
| Intervention type: PC | 0.67 (0.90) | 33.96 | −0.04 (0.16) | 39.67 | 0.5 0.44) | 20.55 | 0.2 (0.31) | 22.18 |
| Development index | 0.03 (0.03) | 32.07 | 0.00 (0.00) | 36.78 | 0.68 (0.46) | 20.40 | 0.00 (0.00) | 28.19 |
| Development index × Intervention type: MBI | −0.09* (0.04) | 55.89 | −0.00† (0.00) | 67.07 | −1.9** (0.6) | 38.75 | −0.01** (0.00) | 52.84 |
| Development index × Intervention type: PC | −0.03 (0.03) | 38.82 | −0.00 (0.00) | 57.83 | −0.77 (0.56) | 26.73 | −0.00 (0.00) | 31.82 |
| Covariates: | ||||||||
| Average age | 0.01** (0.00) | 94.75 | 0.01** (0.00) | 99.36 | 0.01** (0.00) | 94.71 | 0.01** (0.00) | 100.51 |
| Percentages of females | 0.00 (0.00) | 117.30 | 0.00 (0.00) | 117.90 | 0.00 (0.00) | 116.52 | 0.00 (0.00) | 119.32 |
| Expert facilitator | 0.13* (0.07) | 145.25 | 0.15* (0.07) | 151.71 | 0.13† (0.07) | 145.14 | 0.12† (0.07) | 147.31 |
| Medical recruitment setting | 0.02 (0.06) | 154.86 | 0.01 (0.06) | 152.54 | 0.01 (0.06) | 152.80 | 0.04 (0.06) | 157.98 |
| Culturally appropriate | −0.05 (0.06) | 100.37 | −0.07 (0.07) | 100.52 | −0.06 (0.07) | 99.58 | −0.07 (0.07) | 98.70 |
| Modified covariate for potential biases | 0.44 (0.38) | 26.41 | 0.48 (0.4) | 26.33 | 0.48 (0.39) | 25.98 | 0.38 (0.37) | 27.21 |
| I2 | 94.30 | 94.36 | 94.42 | 94.57 | ||||
Note. Log GDP = the log form of a country’s overall gross domestic product; GDP per capita = overall gross domestic product of a country divided by its total population; HDI = human development index; HAQ = healthcare access and quality index; b = unstandardized coefficient; SE = standard error; df = degrees of freedom; p = p value; k = total number of effect sizes; average age = average age of the sample; percentages of females = percentages of female participants; expert facilitator = the intervention program was facilitated by experts (e.g., clinical psychologists); medical recruitment setting = participant recruited from medical settings; culturally appropriate = the intervention was culturally appropriate; clinical delivery setting = the intervention was delivered in clinical settings. I2 = proportion of variance due to between-group heterogeneity.
p < .10,
p < .05,
p < .01,
p < .001
Figure 3.
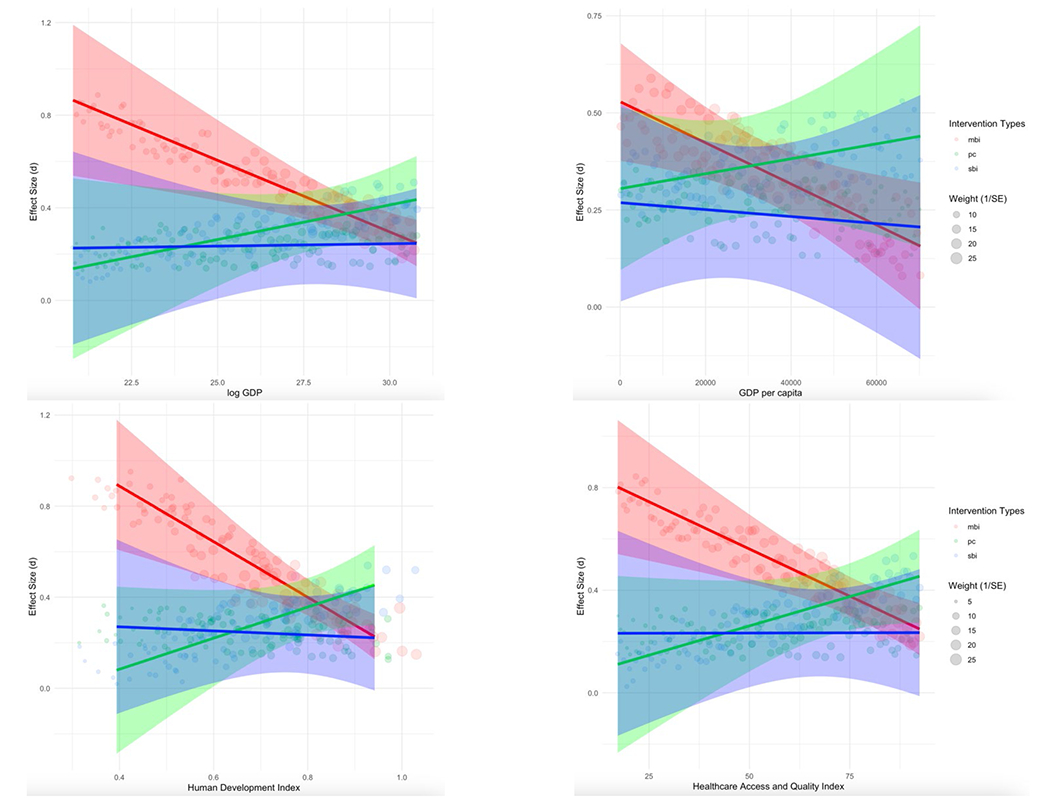
Modeled Condition Impact
Note. Lines depict the interaction effects between intervention types and development indices, including log GDP (top left), GDP per capita (top right), HDI (bottom left), and HAQ (bottom right). They were computed from the meta-regression model clustered by report with small-sample corrections as reported in Table 4 using a sequence of 100 development indices (i.e., from min to max at an increment interval of max minus min divided by 100). The shading corresponds to 95% CI, while keeping the covariates of average age (28.79), percentages of females (43.65), expert facilitator (0.33), medical recruitment setting (0.49), culturally appropriate (0.23), and modified covariate for potential biases (0.23) at their grand means.
Next, we conducted planned contrasts to decompose the significant interactions between multiple-behavior interventions and development indices at three levels using the current data set (i.e., higher = M + 1 SD, medium = M, and lower = M – 1 SD). The bottom panel of Table 6 shows the planned contrasts for countries with lower development indices. As hypothesized, in countries with lower development indices, multiple-behavior interventions had greater efficacy than single-behavior interventions in all four development indices, ts = 3330.36 - 5624.39, ps < .001. The same pattern of results also revealed in the contrasts between multiple-behavior interventions and passive controls, ts = 3736.16 - 5427.65, ps < .001.
Table 6.
Results of Planned Contrasts of Intervention Type across Levels of Development Index
| Intervention type contrast | Log GDP | GDP per capita | HDI | HAQ | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mdiff (SE) | df | Mdiff (SE) | df | Mdiff (SE) | df | Mdiff (SE) | df | |
| Higher = 31.1 | Higher = 53680 | Higher = 0.956 | Higher = 93.4 | |||||
| SBI vs. MBI | 0.21*** (0.00) | 55.89 | 0.16*** (0.00) | 67.07 | 0.25*** (0.00) | 38.75 | 0.22*** (0.00) | 52.84 |
| SBI vs. PC | 0.2 (0.24) | 38.82 | 0.19 (0.20) | 57.83 | 0.24 (0.28) | 26.73 | 0.22 (0.26) | 31.82 |
| MBI vs . PC | −0.02*** (0.00) | 30.01 | 0.02*** (0.00) | 43.16 | −0.01*** (0.00) | 21.15 | 0.01*** (0.00) | 24.32 |
| Medium = 28.7 | Medium = 31598 | Medium = 0.816 | Medium = 73.6 | |||||
| SBI vs. MBI | 0.00*** (0.00) | 55.89 | 0.00*** (0.00) | 67.07 | −0.01*** (0.00) | 38.75 | −0.02*** (0.00) | 52.84 |
| SBI vs. PC | 0.13 (0.24) | 38.82 | 0.12 (0.20) | 57.83 | 0.13 (0.28) | 26.73 | 0.13 (0.26) | 31.82 |
| MBI vs . PC | 0.14*** (0.00) | 30.01 | 0.12*** (0.00) | 43.16 | 0.15*** (0.00) | 21.15 | 0.15*** (0.00) | 24.32 |
| Lower = 26.3 | Lower = 9516 | Lower = 0.677 | Lower = 54.0 | |||||
| SBI vs. MBI | −0.22*** (0.00) | 55.89 | −0.16*** (0.00) | 67.07 | −0.28*** (0.00) | 38.75 | −0.25*** (0.00) | 52.84 |
| SBI vs. PC | 0.06 (0.24) | 38.82 | 0.06 (0.20) | 57.83 | 0.03 (.28) | 26.73 | 0.05 (0.26) | 31.82 |
| MBI vs . PC | 0.29*** (0.00) | 30.01 | 0.22*** (0.00) | 43.16 | 0.3*** (0.00) | 21.15 | 0.3*** (0.00) | 24.32 |
Note. MBI = multiple-behavior intervention; SBI = single-behavior intervention; PC = passive control. SBI vs. MBI = comparing difference from SBI to MBI; SBI vs. PC = comparing difference from SBI to PC; MBI vs. PC = comparing difference from MBI to PC. Mean differences between contrast groups and standard errors in paratheses were derived using all the data (i.e., regressing predicted ds on intervention types and development indices clustered by report). Degrees-of-freedom (df) method: Derived from the RVE meta-regression models with small-sample corrections.
p < .10,
p < .05,
p < .01,
p < .001
The top panel in Table 6 reports the results for countries with higher development indices. Single-behavior interventions appeared to have greater intervention efficacy than multiple-behavior interventions and passive controls in the models that included each development index, ts = 3771.92 - 4556.62, ps < .001. However, this pattern of results should not be understood alone. As shown in Figure 3, there is substantial overlap of effect sizes among multiple- and single-behavior interventions and passive controls as development indices increase (i.e., the right side of the x-axis), suggesting no differences in intervention efficacy.
We next assessed whether the efficacy of multiple-behavior interventions varied with gay/bisexual or MSM representation and age, again using the total sample of all countries. These results, which appear in Table 7, showed statistically significant interactions between the proportion of gay/bisexual or MSM representation in the samples and multiple-behavior interventions, b = −0.01, SE = 0.00, p = .010, and the proportion of gay/bisexual or MSM representation and passive controls, b = −0.01, SE = 0.00, p = .023. As the interaction effect between the proportion of gay/bisexual or MSM and multiple-behavior interventions was weak and unexpected, we were skeptical to interpret the result (see Table S10 for an additional analysis with other methodological characteristics). Lastly, no significant interaction was found between multiple-behavior interventions and age, p = .484.
Table 7.
Differential Effects of Multiple-Behavior Interventions Related to Gay/bisexual or MSM Representation and Being Young Adults Across Countries
| Variable | Disadvantaged group status | |||
|---|---|---|---|---|
| Gay/bisexual or MSM representation | Youne adult | |||
| b (SE) | df | b (SE) | df | |
| (Cluster = 89, k = 415) | (Cluster = 300, k = 1261) | |||
| Intercept | −0.24 (0.25) | 9.48 | −0.16 (0.33) | 30.86 |
| Intervention type: MBI | 0.63** (0.19) | 8.73 | 0.25 (0.31) | 41.74 |
| Intervention type: PC | 0.5 (0.35) | 9.51 | 0.07 (0.34) | 36.40 |
| Disadvantaged group status | −0.00 (0.00) | 11.63 | −0.01 (0.01) | 31.47 |
| Disadvantaged group status × Intervention type: MBI | −0.01* (0.00) | 16.77 | −0.01 (0.01) | 51.51 |
| Disadvantaged group status × Intervention type: PC | −0.01* (0.00) | 13.44 | −0.01 (0.01) | 29.53 |
| Covariates: | ||||
| Average age | 0.00 (0.00) | 29.11 | - | - |
| Percentages of females | −0.01* (0.00) | 25.71 | 0.00 (0.00) | 127.03 |
| Expert facilitator | 0.05 (0.12) | 40.71 | 0.15* (0.07) | 161.79 |
| Medical recruitment setting | 0.12 (0.15) | 32.50 | 0.08 (0.06) | 168.61 |
| Culturally appropriate | 0.06 (0.09) | 17.83 | −0.06 (0.06) | 105.75 |
| Modified covariate for potential biases | 1.13* (00.4) | 22.11 | 0.25 (0.33) | 29.78 |
| I2 | 87.12 | 94.56 | ||
Note. Report of the robust variance estimation meta-regression using single-behavior intervention as the reference (i.e., intercept). b = unstandardized coefficient; SE = standard error; p = p value; k = the number of effect sizes; gay/bisexual or MSM representation = percentage of gay/bisexual or MSM in the sample; average age = average age of the sample; percentages of females = percentages of female participants; expert facilitator = the intervention program was facilitated by experts (e.g., clinical psychologists); medical recruitment setting = participant recruited from medical settings; culturally appropriate = the intervention was culturally appropriate; clinical delivery setting = the intervention was delivered in clinical settings; I2 = proportion of variance due to between-group heterogeneity.
p < .10,
p < .05,
p < .01,
p < .001
Differential Effects of Multiple-Behavior Interventions Related to Racial/Ethnic Minority Representation in the U.S.
We last considered disadvantaged status associated with being a racial/ethnic minority or a sexual minority in the U.S. (62% of all the included reports). Specifically, we analyzed the proportion of the sample that was non-White, Black/African-American, Latinx, or “Other”, and the proportion of gay/bisexual or MSM in the sample. We also analyzed age, as young age is a risk factor for HIV infection. These results, which appear in Table 8, showed largely no evidence of differential effects of multiple-behavior interventions across groups in the US sample. Only a weak marginally significant interaction between multiple-behavior intervention and gay/bisexual or MSM representation was found, b = −0.01, SE = 0.00, p = .097. That is, multiple-behavior interventions appeared to benefit all the groups in a similar way.
Table 8.
Differential Effects of Multiple-Behavior Interventions Related to Minority Group Status and Being Young Adults in the US
| Variable | Minority group status | |||||
|---|---|---|---|---|---|---|
| Non-White representation | Black representation | Latinx representation | ||||
|
| ||||||
| b (SE) | df | b (SE) | df | b (SE) | df | |
| (Cluster = 174, k = 737) | (Cluster = 151, k = 604) | (Cluster = 139, k = 584) | ||||
| Intercept | 0.16 (0.19) | 24.03 | −0.11 (0.27) | 30.57 | 0.03 (0.33) | 36.29 |
| Intervention type: MBI | −0.36† (0.18) | 19.33 | −0.21 (0.22) | 27.49 | −0.37† (0.21) | 31.63 |
| Intervention type: PC | −0.3 (0.25) | 10.22 | −0.51 (0. 29) | 14.79 | −0.26 (0.27) | 18.91 |
| Disadvantaged group status | 0.00 (0.00) | 17.29 | 0.00 (0.00) | 15.00 | −0.01 (0.01) | 3.51 |
| Disadvantaged group status × Intervention type: MBI | 0.00 (0.00) | 25.31 | 0.00 (0.00) | 23.19 | 0.01 (0.01) | 6.00 |
| Disadvantaged group status × Intervention type: PC | 0.00 (0.00) | 17.12 | 0.01 (0.01) | 22.64 | 0.00 (0.01) | 2.78 |
| Covariates: | ||||||
| Average age | 0.01** (0.00) | 58.94 | 0.01** (0.00) | 70.94 | 0.01* (0.00) | 61.70 |
| Percentages of females | 0.00 (0.00) | 59.56 | 0.00 (0.00) | 51.83 | 0.00 (0.00) | 51.80 |
| Expert facilitator | 0.11 (0.07) | 88.47 | 0.08 (0.09) | 74.72 | 0.16† (0.09) | 68.62 |
| Medical recruitment setting | 0.03 (0.07) | 81.90 | 0.01 (0.08) | 73.10 | −0.01 (0.08) | 66.56 |
| Culturally appropriate | 0.00 (0.00) | 47.87 | 0.01 (0.08) | 43.14 | −0.01 (0.09) | 42.03 |
| Modified covariate for potential biases | 0.4 (0.45) | 18.49 | 0.6 (0.64) | 14.36 | 0.66 (0.66) | 14.14 |
| I2 | 93.13 | 94.16 | 94.22 | |||
| Other representation | Gay/bisexual MSM representation | Young adult | ||||
| b (SE) | df | b (SE) | df | b (SE) | df | |
|
| ||||||
| (Cluster = 138, k = 573) | (Cluster = 59, k = 252) | (Cluster = 174, k = 737) | ||||
| Intercept | −0.09 (0.33) | 42.67 | −0.02 (0.21) | 4.43 | −0.46 (0.51) | 16.99 |
| Intervention type: MBI | −0.10 (0.20) | 28.82 | 0.28† (0.14) | 7.37 | 0.36 (0.45) | 20.17 |
| Intervention type: PC | −0.04 (0.26) | 17.72 | 0.05 (0.56) | 4.09 | 0.39 (0.47) | 21.45 |
| Disadvantaged group status | 0.00 (0.00) | 2.43 | −0.00† (0.00) | 5.71 | 0.02 (0.01) | 18.59 |
| Disadvantaged group status × Intervention type: MBI | −0.00 (0.01) | 4.95 | −0.00† (0.00) | 11.92 | −0.01 (0.01) | 29.20 |
| Disadvantaged group status × Intervention type: PC | −0.01 (0.01) | 3.75 | −0.00 (0.00) | 5.60 | −0.02 (0.01) | 21.41 |
| Covariates: | ||||||
| Average age | 0.01* (0.00) | 52.90 | −0.01 (0.00) | 16.29 | - | - |
| Percentages of females | 0.00 (0.00) | 53.41 | 0.00 (0.00) | 11.39 | 0.00 (0.00) | 60.13 |
| Expert facilitator | 0.13 (0.10) | 74.80 | 0.15† (0.08) | 20.72 | 0.1 (0.07) | 89.51 |
| Medical recruitment setting | 0.04 (0.09) | 68.14 | −0.05 (0.07) | 15.65 | 0.04 (0.07) | 73.95 |
| Culturally appropriate | −0.04 (0.10) | 44.13 | −0.01 (0.11) | 6.04 | 0.00 (0.00) | 43.52 |
| Modified covariate for potential biases | 0.51 (0.70) | 12.78 | 0.8* (036) | 17.88 | 0.35 (0.43) | 20.01 |
| I2 | 95.01 | 79.01 | 93.09 | |||
Note. Report of the robust variance estimation meta-regression using single-behavior intervention as the reference (i.e., intercept). b = unstandardized coefficient; SE = standard error; df = degrees of freedom; p = p value; k = the number of effect sizes; average age = average age of the sample; percentages of females = percentages of female participants; expert facilitator = the intervention program was facilitated by experts (e.g., clinical psychologists); medical recruitment setting = participant recruited from medical settings; culturally appropriate = the intervention was culturally appropriate; clinical delivery setting = the intervention was delivered in clinical settings; I2 = proportion of variance due to between-group heterogeneity.
p < .10,
p < .05,
p < .01,
p < .001
Differential Effects of Multiple-Behavior Interventions Related to Intersectionality of Racial/Ethnic Minority and MSM Representation in the US
Finally, a distinct possibility for US samples is the differential effects of multiple-behavior intervention due to the intersection between state-level MSM and race/ethnicity. We thus examined whether MSM of different racial/ethnic minority backgrounds responded differently to multiple-behavior interventions. For each minority group, we regressed the effect size on MSM representation (i.e., the state-level estimated MSM percentages), racial/ethnic minority group representation, and their interaction. As shown in Table 9, the interaction between MSM and racial/ethnic minority groups were statistically nonsignificant, ps = .504 - .949, suggesting no differential effects among US MSM of different races/ethnicities in multiple-behavior interventions.
Table 9.
Differential Effects of Multiple-Behavior Interventions Related to Intersectionality of Racial/Ethnic Minority and MSM Representation in the US
| Variable | Minority group status | |||||
|---|---|---|---|---|---|---|
| Non-White representation | Black representation | Latinx representation | ||||
|
| ||||||
| b (SE) | df | b (SE) | df | b (SE) | df | |
| (Cluster = 98, k = 448) | (Cluster = 85, k = 364) | (Cluster = 80, k = 365) | ||||
| Intercept | 0.35 (0.54) | 11.37 | −0.4 (0.56) | 16.82 | −0.56 (0.38) | 20.17 |
| MSM representation | −9.99 (9.83) | 10.96 | −0.64 (9.5) | 15.29 | 3.15 (5.66) | 19.76 |
| Minority group status | −0.00 (0.01) | 11.36 | 0 (0.01) | 13.22 | −0.00 (0.01) | 9.02 |
| MSM representation × Minority group status | 0.08 (0.12) | 14.77 | 0.01 (0.15) | 14.27 | 0.02 (0.23) | 7.63 |
| Covariates: | ||||||
| Average age | 0.01* (0.00) | 25.96 | 0.01* (0.00) | 29.38 | 0.01* (0.00) | 39.24 |
| Percentages of females | 0.00 (0.00) | 35.50 | 0 .00 (0.00) | 32.44 | 0.00 (0.00) | 34.33 |
| Expert facilitator | 0.04 (0.07) | 48.20 | −0.01 (0.09) | 41.91 | 0.09 (0.09) | 38.61 |
| Medical recruitment setting | 0 (0.08) | 46.38 | −0.07 (0.1) | 43.04 | −0.09 (0.09) | 38.68 |
| Culturally appropriate | −0.02 (0.08) | 28.74 | 0.04 (0.09) | 28.54 | 0.09 (0.09) | 26.86 |
| Modified covariate for potential biases | 0.82† (0.44) | 20.78 | 1.22* (0.5) | 14.89 | 1.3* (0.53) | 15.40 |
| I2 | 89.42 | 90.77 | 89.39 | |||
Note. Report of the robust variance estimation meta-regression using single-behavior intervention as the reference (i.e., intercept). b = unstandardized coefficient; SE = standard error; df = degrees of freedom; p = p value; k = the number of effect sizes; average age = average age of the sample; percentages of females = percentages of female participants; expert facilitator = the intervention program was facilitated by experts (e.g., clinical psychologists); medical recruitment setting = participant recruited from medical settings; culturally appropriate = the intervention was culturally appropriate; clinical delivery setting = the intervention was delivered in clinical settings; I2 = proportion of variance due to between-group heterogeneity.
p < .10,
p < .05,
p < .01,
p < .001
Discussion
Overview of Main Findings
We combined a dataset consisting of 331 reports and 1364 effect sizes with open-source data about country development indices obtained from the World Bank, the United Nations Development Program, and the Institute for Health Metrics and Evaluation (University of Washington School of Medicine, 2023), together with the estimated percentages of MSM in the U.S. (Grey et al., 2016), to carry out a comprehensive meta-analysis of the efficacy of multiple behavioral program used in the area of HIV. The confirmatory factor analysis results provided meta-analytic evidence that outcomes about HIV/sexual risk, substance use, psychological wellbeing, and other syndemic group are interrelated, as suggested by the conceptualization of syndemics. Furthermore, even though multiple-behavior interventions and single-behavior interventions were more efficacious than passive controls, across the board, multiple-behavior interventions were not more efficacious than single-behavior interventions. Multiple-behavior interventions were more efficacious than single-behavior ones in countries that are more likely to experience syndemics.
To understand whether multiple-behavior interventions could be a response to syndemics in HIV, we assessed whether multiple-behavior interventions had the same or differential intervention efficacy across countries with different levels of economic development, and whether multiple-behavior interventions had greater efficacy for disadvantaged groups in the U.S. Results of the interactions showed that, in countries with lower development indices, multiple-behavior interventions had greater efficacy than single-behavior ones, whereas single-behavior interventions did not differ from controls. These effects were consistent for our four development indices, though the interaction with GDP per capita was marginally significant. These findings provide empirical evidence that multiple-behavior interventions could be an appropriate response to syndemics in HIV.
Within the U.S., results suggested largely no differential effects of multiple-behavior interventions related to the representation of disadvantaged groups, including racial/ethnic minorities, gay/bisexual MSM, and young adults, compared to single-behavior interventions. Similarly, results showed no differential effects of multiple-behavior interventions related to the intersection of MSM and racial/ethnic minority groups in the U.S.
Implications
Multiple-behavior interventions show great promise and are well poised to mitigate health disparities in countries with lower development indices compared to single-behavior interventions. For instance, Sub-Saharan African countries have the majority of both new HIV infections and people living with HIV (UNAIDS, 2014) in the world. One successful intervention to reduce HIV transmission targeted serostatus disclosure, alcohol use, condom use, antiretroviral adherence, and counseling all at the same time (Bachanas et al., 2016b). Less developed countries tend to have limited resources for health care and possibly co-occurring diseases and unhealthy conditions. Therefore, addressing multiple problems such as partner relationships, substance use, and HIV prevention is more practical (Tsai & Venkataramani, 2016).
The finding that multiple-behavior interventions were more efficacious in countries with lower development indices could provide insights for the design of context-sensitive multiple-behavior interventions. For example, facilitators could assess the syndemic conditions of different contexts or individuals and thus address unique needs (Mendenhall & Singer, 2020). As proposed by Gilbert et al. (2015), a screening protocol may inform the components that can be implemented to target specific syndemic conditions or regional vulnerabilities.
We also found that HIV/sexual risk, substance use, psychological wellbeing, and other syndemic factors are part of an overall syndemic factor (i.e., a second-order factor). However, as highlighted by Bhardwaja and Kohrt’s (2020), syndemic research has failed to characterize the relation between syndemic conditions or the strength of these relations. Future research should refine the syndemic framework to clarify processes and intervention priorities.
MSM, young adults, and Black and Latinx individuals in the U.S. were more likely to be diagnosed with HIV (CDC, 2019b), and Black and Latinx individuals and young adults of color were more likely to have a later HIV diagnosis and worse viral suppression than White adults of the same age (Chen et al., 2012; Schwarcz et al., 2006; Crepaz et al., 2018). Although 98 percent of the included records (i.e., 1342) had participants at risk or who experienced syndemic conditions, we found no differential effects of multiple-behavior interventions related to sexual or ethnic minority groups either internationally or in the U.S. However, the research reports have considerable amounts of missing data (e.g., percentages of Black individuals), which might prevent identifying effects. In addition, syndemic conditions interact with each other (Batchelder et al., 2019) but this meta-analysis did not have the power to estimate the effects of intersectionality. Future attempts at studying the impact of intersectionality will be well served by a greater accumulation of literature in the future (Earnshaw et al., 2015; Rice et al., 2018).
A final point regarding this meta-analysis is that it included all possible behavioral and clinical outcomes targeted in these studies – a total of 39 different outcomes across the studies in our dataset (see Table 2). Due to sample size, coverage limitations, and diversity in reported outcomes, we could not examine if specific mixtures of recommendations are more effective than others due to few exactly comparable studies for specific combinations of behaviors. As discussed before, a more granular approach that compares combinations of behaviors within specific samples could be important to the development of these interventions for under-resourced groups.
Limitations
Our work has several limitations due to the information availability and analyses we chose to conduct. We used general measures of income and development and relied on them to conclude about the resources of specific samples within those countries. Although the general pattern of resources available based on countries is likely correct, further studies could use more precise estimates for each sample. We could not use more precise measures in our analyses due to a lack of reporting of income and a lack of measures of socioeconomic resources of other types.
Likewise, our findings could depend on the fidelity with which interventions are applied. Although we estimated the impact of the intervention fidelity and found no significant moderation, only 20% of the included reports implemented the NIH Behavior Change Consortium’s (Ory et al., 2002) recommendations for intervention fidelity recommendations. As intervention fidelity checks become the norm (Bellg et al., 2004; Borrelli et al., 2005), we encourage researchers to thoroughly report on their measures to maximize it (Borrelli, 2011; Borrelli et al., 2005) and report fidelity checks in a standardized way. In this way, future meta-analyses will be able to include more detailed analyses of the impact of processes to maximize fidelity.
For the US analyses, demographic reporting had considerable missing data, in part because certain demographics are not recorded unless a sample was focused on a specific group (e.g., interventions only for Latinx youth). Probably for that reason, approximately 10 to 15 percent of the samples had missing values regarding Latinx percentages. Most likely, the missing information comes from low-percentage studies (i.e., those conducted with a 10-20 percent representation of a group). Future inclusion of more studies may therefore shed light on the role of race and ethnicity.
Finally, an inherent problem in all meta-analyses is aggregation across individuals and often across groups. Group membership is inherently an individual factor, and there is a distinct possibility that the individual patterns are different from the group patterns (e.g., Simpson’s Paradox; Simpson, 1951). These problems are difficult to solve other than conducting large randomized controlled trials that are powerful enough to examine the effects of conditions across multiple populations, such as those living in multiple counties. Unfortunately, this limitation could not be overcome in our synthesis because the overwhelming majority of included studies considered samples from single countries, and less than five percent of the included studies had a multinational sample. We highlight these concerns as an area for improvement in future intervention design. Of note, other characteristics such as identification as MSM and race/ethnicity, should be disaggregated whenever possible.
Conclusion
We examined whether multiple-behavior interventions were more efficacious in samples with lower access to resources and thus more likely to be affected by syndemics. We found that relative to single-behavior interventions, multiple-behavior interventions showed greater efficacy in countries with lower development indices, but not in countries with higher development indices. We also examined whether disadvantaged groups across all countries and within the U.S. had differential results from multiple-behavior interventions versus single-behavior interventions. Unlike an earlier analysis of single-recommendation interventions (Albarracín & Durantini, 2010), we found largely no differential effects of multiple-behavior interventions related to the representation of these groups and their intersections, compared to single-behavior interventions. Future research in this area may be well positioned to answer intersectionality questions, but as a whole, multiple-behavior interventions, in general, appear to be meeting the demands of the complex situations for which they were designed.
Supplementary Material
Public Health Impact Statement.
This study examines syndemics in HIV, defined as the synergistic epidemics of substance use, violence, diminished psychological wellbeing, and HIV among impacted populations, particularly those burdened by socioeconomic disadvantages. The meta-analytic results showed multiple- versus single-behavior interventions are differentially successful across samples with different levels of disadvantage. Specifically, multiple-behavior interventions, rather than single-behavior interventions, were more efficacious for samples from countries with lower log Gross Domestic Product (GDP), lower Human Development Index (HDI), and lower Healthcare Access and Quality (HAQ) Index. Similar but marginal significant results were also found for GDP per capita.
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Numbers R01MH094241 (D.A.) and R01MH114847 (D.A.), the National Institute on Drug Abuse of the National Institutes of Health under Award Number DP1 DA048570 (D.A.), and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers R01AI147487 (D.A. and M.S.C.) and P30AI045008 (Penn CFAR sub award; M.S.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was supported by the Science of Science Communication Endowment from the Annenberg Public Policy Center at the University of Pennsylvania.
Footnotes
We have no known conflict of interest to disclose.
Adherence ranges are both due to natural differences between countries and differences in how adherence is defined (e.g., total doses taken versus total doses taken at the correct time).
Reporting of Asian and Native American representation was too low to appropriately assess model convergence problems in both groups due to a lack of degrees of freedom.
Four syndemic categories were included in the model with the category “other syndemic” set as the reference group. Two factors (i.e., HIV/sexual risk and substance use), except psychological wellbeing, showed main effects on intervention efficacy for all development indices. Specifically, HIV/sexual risk outcomes, b = 0.41, SE = 0.22, p = .075, and substance use outcomes were associated with higher intervention efficacy than other syndemic outcomes, b = 0.52, SE = 0.22, p = .032.
References
- Albarracín D, & Durantini MR (2010). Are we going to close social gaps in HIV? Likely effects of behavioral HIV-prevention interventions on health disparities. Psychology, Health {&} Medicine, 15(6), 694–719. 10.1080/13548506.2010.498892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarracín D, Gillette JC, Earl AN, Glasman LR, Durantini MR, & Ho M-H (2005). A test of major assumptions about behavior change: A comprehensive look at the effects of passive and active HIV-prevention interventions since the beginning of the epidemic. Psychological Bulletin, 131(6), 856–897. 10.1037/0033-2909.13L6.856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarracín D, Sunderrajan A, & Dai W (2018). Action, inaction, and actionability: Definitions and implications for communications and interventions to change behaviors. In Nebraska Symposium on Motivation (Vol. 65, pp. 75–99). 10.1007/978-3-319-96920-6_3 [DOI] [Google Scholar]
- Albarracín D, Wang W, & McCulloch KC (2018). Action dominance: The performance effects of multiple action demands and the benefits of an inaction focus. Personality & Social Psychology Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarracín D, Wilson K, Chan M, pui S, Durantini M, & Sanchez F (2017). Action and inaction in multi-behaviour recommendations: a meta-analysis of lifestyle interventions. Health Psychology Review, 1–24. 10.1080/17437199.2017.1369140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro H, Larson MJ, Zhang A, Acevedo A, Dai J, & Marsumoro A (2007). Effects of trauma intervention on HIV sexual risk behaviors among women with co-occurring disorders in substance abuse treatment. Journal of Community Psychology, 35(7), 895–908. 10.1002/jcop.20188 [DOI] [Google Scholar]
- Amirkhanian YA, Kelly JA, Kabakchieva E, Kirsanova AV, Vassileva S, Takacs J, DiFranceisco WJ, McAuliffe TL, Khoursine RA, & Mocsonaki L (2005). A randomized social network HIV prevention trial with young men who have sex with men in Russia and Bulgaria. AIDS, 19(16), 1897–1905. 10.1097/01.aids.0000189867.74806.fb [DOI] [PubMed] [Google Scholar]
- Bachanas P, Kidder D, Medley A, Pals SL, Carpenter D, Howard A, Antelman G, DeLuca N, Muhenje O, Sheriff M, Somi G, Katuta F, Cherutich P, & Moore J (2016a). Delivering prevention interventions to people living with HIV in clinical care settings: Results of a cluster randomized trial in Kenya, Namibia, and Tanzania. AIDS and Behavior, 20(9), 2110–2118. 10.1007/s10461-016-1349-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachanas P, Kidder D, Medley A, Pals SL, Carpenter D, Howard A, Antelman G, DeLuca N, Muhenje O, Sheriff M, Somi G, Katuta F, Cherutich P, & Moore J (2016b). Delivering prevention interventions to people living with HIV in clinical care settings: Results of a cluster randomized trial in Kenya, Namibia, and Tanzania. AIDS and Behavior, 20(9), 2110–2118. 10.1007/s10461-016-1349-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett PG, Sorensen JL, Wong W, Haug NA, & Hall SM (2009). Effect of incentives for medication adherence on health care use and costs in methadone patients with HIV. Drug and Alcohol Dependence, 100(1–2), 115–121. 10.1016/j.drugalcdep.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelder AW, Choi K, Dale SK, Pierre-Louis C, Sweek EW, Ironson G, Safren SA, & O’Cleirigh C (2019). Effects of syndemic psychiatric diagnoses on health indicators in men who have sex with men. Health Psychology, 38(6), 509–517. 10.1037/hea0000724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman LJ, Braunstein S, Calderon Y, Chhabra R, Cutler B, Leider J, Rivera A, Sclafane J, Tsoi B, & Watnick D (2013). Barriers and facilitators of linkage to HIV primary care in New York City. Journal of Acquired Immune Deficiency Syndromes, 67(SUPPL. 1), S20–S26. 10.1097/QAI.0b013e3182a99cl9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M, Spitz E, Guillemin F, Ravaud J-F, Choquet M, Falissard B, Chau N, & Group L (2007). Associations of social and material deprivation with tobacco, alcohol, and psychotropic drug use, and gender: a population-based study. International Journal of Health Geographics, 6(1), 50. 10.1186/1476-072X-6-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckie TM (2012). A Systematic Review of Allostatic Load, Health, and Health Disparities. Biological Research For Nursing, 14(4), 311–346. 10.1177/1099800412455688 [DOI] [PubMed] [Google Scholar]
- Beer L, & Skarbinski J (2014). Adherence to antiretroviral therapy among HIV-infected adults in the United States. AIDS Education and Prevention, 26(6), 521–537. 10.1521/aeap.2014.26.6.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, Czajkowski S, & Treatment Fidelity Workgroup of the NIH Behavior Change Consortium. (2004). Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 23(5), 443–451. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, & Kohrt BA (2020). Syndemics of HIV with mental illness and other noncommunicable diseases. Current Opinion in HIV and AIDS. 10.1097/COH.0000000000000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonevski B, Randell M, Paul C, Chapman K, Twyman L, Bryant J, Brozek I, & Hughes C (2014). Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Medical Research Methodology, 14(1), 42. 10.1186/1471-2288-14-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvicini K. (2017). LGBT healthcare disparities: What progress have we made? Patient Education and Counseling, 100(12), 2357–2361. 10.1016/J.PEC.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, & Rothstein HR (2009a). Introduction to meta-analysis. In Introduction to Meta-Analysis. Wiley. 10.1002/9780470743386 [DOI] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, & Rothstein HR (2009b). Introduction to meta-analysis. In Introduction to Meta-Analysis. Wiley. 10.1002/9780470743386 [DOI] [Google Scholar]
- Borrelli B. (2011). The Assessment, Monitoring, and Enhancement of Treatment Fidelity In Public Health Clinical Trials. Journal of Public Health Dentistry, 71(s1), S52–S63. 10.1111/j.1752-7325.2011.00233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, Sepinwall D, Ernst D, Bellg AJ, Czajkowski S, Breger R, DeFrancesco C, Levesque C, Sharp DL, Ogedegbe G, Resnick B, & Orwig D (2005). A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. Journal of Consulting and Clinical Psychology, 73(5), 852–860. 10.1037/0022-006X.73.5.852 [DOI] [PubMed] [Google Scholar]
- Brookmeyer KA, Hogben M, & Kinsey J (2016). The role of behavioral counseling in sexually transmitted disease prevention program settings. In Sexually Transmitted Diseases (Vol. 43, Issue 2, pp. S102–S112). 10.1097/0LQ.0000000000000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2019a). CDC HIV prevention progress report, 2019. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of HIV/AIDS Prevention. https://www.cdc.gov/hiv/pdf/policies/progressreports/cdc-hiv-preventionprogressreport.pdf [Google Scholar]
- CDC. (2019b). CDC HIV prevention progress report, 2019. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of HIV/AIDS Prevention. https://www.cdc.gov/hiv/pdf/policies/progressreports/cdc-hiv-preventionprogressreport.pdf [Google Scholar]
- Chartier K, & Caetano R (2010). Ethnicity and health disparities in alcohol research. Alcohol Research and Health, 33(1–2), 152–160. [PMC free article] [PubMed] [Google Scholar]
- Chen NE, Gallant JE, & Page KR (2012). A Systematic Review of HIV/AIDS Survival and Delayed Diagnosis Among Hispanics in the United States. Journal of Immigrant and Minority Health, 14(1), 65–81. 10.1007/sl0903-011-9497-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates. [Google Scholar]
- Collins SE (2016). Associations between socioeconomic factors and alcohol outcomes. Alcohol Research : Current Reviews, 38(1), 83. /pmc/articles/PMC4872618/ [PMC free article] [PubMed] [Google Scholar]
- Crepaz N, Baack BN, Higa DH, & Mullins MM (2015). Effects of integrated interventions on transmission risk and care continuum outcomes in persons living with HIV: meta-analysis, 1996-2014. AIDS, 29(18), 2371–2383. 10.1097/QAD.0000000000000879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepaz N, Dong X, Wang X, Hernandez AL, & Hall HI (2018). Racial and Ethnic Disparities in Sustained Viral Suppression and Transmission Risk Potential Among Persons Receiving HIV Care — United States, 2014. MMWR Morbidity and Mortality Weekly Report, 67(4), 113–118. 10.15585/mmwr.mm6704a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Myers HF, Cole SW, & Irwin MR (2009). Mindfulness meditation training effects on CD4+ T lymphocytes in HIV-1 infected adults: A small randomized controlled trial. Brain, Behavior, and Immunity, 23(2), 184–188. 10.1016/j.bbi.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Palmer R, Sunderrajan A, Durantini M, Sánchez F, Glasman LR, Chen FX, & Albarracín D (2020). More behavioral recommendations produce more change: A meta-analysis of efficacy of multibehavior recommendations to reduce nonmedical substance use. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors, 34(7), 709–725. 10.1037/ADB0000586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco D, Verboeket SO, Underwood J, Bagkeris E, Wit FW, Mallon PWG, Winston A, Reiss P, Sabin CA, Babalis D, Boffito M, Burgess L, Mallon P, Post F, Sabin CA, Sachikonye M, Winston A, Anderson J, Asboe D, … van Oorspronk S. (2018). Patterns of Co-occurring Comorbidities in People Living With HIV. Open Forum Infectious Diseases, 5(11). 10.1093/ofid/ofy272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MR, Benning L, Weber KM, Sherman SG, Adedimeji A, Wilson TE, Cohen J, Plankey MW, Cohen MH, & Golub ET (2016). Physical and sexual violence predictors: 20 years of the women’s interagency HIV study cohort. American Journal of Preventive Medicine, 51(5), 731–742. 10.1016/j.amepre.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Jarlais DC, Sypsa V, Feelemyer J, Abagiu AO, Arendt V, Broz D, Chemtob D, Seguin-Devaux C, Duwve JM, Fitzgerald M, Goldberg DJ, Hatzakis A, Jipa RE, Katchman E, Keenan E, Khan I, Konrad S, McAuley A, Skinner S, & Wiessing L (2020). HIV outbreaks among people who inject drugs in Europe, North America, and Israel. The Lancet HIV, 7(6), e434–e442. 10.1016/S2352-3018(20)30082-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw VA, Smith LR, Cunningham CO, & Copenhaver MM (2015). Intersectionality of internalized HIV stigma and internalized substance use stigma: Implications for depressive symptoms. Journal of Health Psychology, 20(8), 1083–1089. 10.1177/1359105313507964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essig A, Kang S, & Sellers R (2015). The relationship between HIV infection rates and GDP per capita in African countries. In Econometric Analysis Undergraduate Research Papers. [Google Scholar]
- Förster J, Liberman N, & Higgins ET (2005). Accessibility from active and fulfilled goals. Journal of Experimental Social Psychology, 41(3), 220–239. 10.1016/jjesp.2004.06.009 [DOI] [Google Scholar]
- Freedland KE, Skala JA, Carney RM, Steinmeyer BC, & Rich MW (2021). Psychosocial Syndemics and Multimorbidity in Patients with Heart Failure. Journal of Psychiatry and Brain Science, 6, e210006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman PR, Hedges LV, & Olkin I (1986). Statistical Methods for Meta-Analysis. Biometrics, 42(2), 454. 10.2307/2531069 [DOI] [Google Scholar]
- Galea JT, Marhefka S, León SR, Rahill G, Cyrus E, Sánchez H, Zhang Z, & Brown B (2021). High levels of mild to moderate depression among men who have sex with men and transgender women in Lima, Peru: implications for integrated depression and HIV care. AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV. 10.1080/09540121.2021.1991877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CM, & Kushel MB (2022). Integrating mental health and substance use treatment with HIV care for people experiencing homelessness. The Lancet Psychiatry, 9(8), 606–608. 10.1016/S2215-0366(22)00228-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfein RS, Swartzendruber A, Ouellet LJ, Kapadia F, Hudson SM, Thiede H, Strathdee SA, Williams IT, Bailey SL, Hagan H, Golub ET, Kerndt P, Hanson DL, & Latka MH (2007). Methods to recruit and retain a cohort of young-adult injection drug users for the Third Collaborative Injection Drug Users Study/Drug Users Intervention Trial (CIDUS III/DUIT). Drug and Alcohol Dependence, 97(SUPPL. 1), S4–S17. 10.1016/j.drugalcdep.2007.05.007 [DOI] [PubMed] [Google Scholar]
- GBD 2019 HIV Collaborators. (2021). Global, regional, and national sex-specific burden and control of the HIV epidemic, 1990–2019, for 204 countries and territories: the Global Burden of Diseases Study 2019. Lancet HIV, 8, e633–e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L, Raj A, Hien D, Stockman J, Terlikbayeva A, & Wyatt G (2015). Targeting the SAVA (Substance Abuse, Violence and AIDS) Syndemic among Women and Girls: A Global Review of Epidemiology and Integrated Interventions HHS Public Access. J Acquir Immune Defic Syndr, 69(2), 118–127. 10.1097/QAI.0000000000000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn TR, Safren SA, Carrico AW, Mendez NA, Duthely LM, Dale SK, Jones DL, Feaster DJ, & Rodriguez AE (2019). High levels of syndemics and their association with adherence, viral non-suppression, and biobehavioral transmission risk in Miami, a U.S. city with an HIV/AIDS epidemic. AIDS and Behavior, 23(11), 2956–2965. 10.1007/S10461-019-02619-0/TABLES/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go VF, Frangakis C, Minh N. Le, Latkin C, T. V. Ha, Mo TT, Sripaipan T, Davis WW, Zelaya C, Vu PT, Celentano DD, & Quan VM (2015). Efficacy of a multi-level intervention to reduce injecting and sexual risk behaviors among HIV-infected people who inject drugs in Vietnam: A four-arm randomized controlled trial. PLoS ONE, 10(5), e0125909. 10.1371/journal.pone.0125909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey JA, Bernstein KT, Sullivan PS, Purcell DW, Chesson HW, Gift TL, & Rosenberg ES (2016). Estimating the population sizes of men who have sex with men in US states and counties using data from the American Community Survey. JMIR Public Health and Surveillance, 2(1), e14. 10.2196/publichealth.5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerras JM, Hoyos Miller J, Agustí C, Chanos S, Pichon F, Kuske M, Cigan B, Fuertes R, Stefanescu R, Ooms L, Casabona J, de la Fuente L, Belza MJ, Fernández-Balbuena S, Maté T, Fernández L, Platteau T, Slaeen P, Lixandru M, & Cosic M (2021). Association of sexualized drug use patterns with HIV/STI transmission risk in an internet sample of men who have sex with men from seven European countries. Archives of Sexual Behavior, 50(2), 461–477. 10.1007/S10508-020-01801-Z/TABLES/4 [DOI] [PubMed] [Google Scholar]
- Hedges L. v. (2007). Effect Sizes in Cluster-Randomized Designs. Journal of Educational and Behavioral Statistics, 32(4), 341–370. 10.3102/1076998606298043 [DOI] [Google Scholar]
- Hedges LV, Tipton E, & Johnson MC (2010). Robust variance estimation in meta-regression with dependent effect size estimates. Research Synthesis Methods, 1(1), 39–65. 10.1002/jrsm.5 [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, & Simoni JM (2009). Alcohol use and antiretroviral adherence: Review and meta-analysis. In Journal of Acquired Immune Deficiency Syndromes (Vol. 52, Issue 2, pp. 180–202). 10.1097/QAI.0b013e3181b18b6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.), 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, & White A (2014). New Research Findings Since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A Review. Journal of Studies on Alcohol and Drugs, 75(1), 158–169. 10.15288/jsad.2014.75.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudelson C, & Cluver L (2015). Factors associated with adherence to antiretroviral therapy among adolescents living with HIV/AIDS in low- and middle-income countries: A systematic review. AIDS Care, 27(7), 805–816. 10.1080/09540121.2015.1011073 [DOI] [PubMed] [Google Scholar]
- Huedo-Medina T, Sanchez-Meca J, Marin-Martinez F, & Botella J (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Part of the Psychology Commons Recommended Citation. Psycnet.Apa.Org [DOI] [PubMed] [Google Scholar]
- Hutton HE, Lesko CR, Li X, Thompson CB, Lau B, Napravnik S, Mayer KH, Mathews WC, McCaul ME, Crane HM, Fredericksen RJ, Cropsey KL, Saag M, Christopoulos K, & Chander G (2019). Alcohol use patterns and subsequent sexual behaviors among women, men who have sex with men and men who have sex with women engaged in routine HIV care in the United States. AIDS and Behavior, 23(6), 1634–1646. 10.1007/S10461-018-2337-5/TABLES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip EJ, Doroudgar S, Shah-Manek B, Barnett MJ, Tenerowicz MJ, Ortanez M, & Pope HG (2019). The CASTRO study: Unsafe sexual behaviors and illicit drug use among gay and bisexual men who use anabolic steroids. The American Journal on Addictions, 28(2), 101–110. 10.1111/AJAD.12865 [DOI] [PubMed] [Google Scholar]
- Jain JP, Santos G-M, Hao J, Leonard A, Miller AM, Cuca YP, & Dawson-Rose C (2022). The syndemic effects of adverse mental health conditions and polysubstance use on being at risk of clinical depression among marginally housed and homeless transitional age youth living in San Francisco, California. PLOS ONE, 17(3), e0265397. 10.1371/joumal.pone.0265397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC (2008). Co-occurrence of treatment nonadherence and continued HIV transmission risk behaviors: Implications for positive prevention interventions. In Psychosomatic Medicine (Vol. 70, Issue 5, pp. 593–597). 10.1097/PSY.0b013e3181773bce [DOI] [PubMed] [Google Scholar]
- Karlsson P, & Bergmark A (2015). Compared with what? An analysis of control-group types in Cochrane and Campbell reviews of psychosocial treatment efficacy with substance use disorders. Addiction, 110(3), 420–428. 10.1111/add.12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan D, & Barrington C (2022). HIV and mental health services for female sex workers. The Lancet HIV, 9(8), e528–e529. 10.1016/S2352-3018(22)00165-5 [DOI] [PubMed] [Google Scholar]
- Koblin BA, Chesney MA, Husnik MJ, Bozeman S, Celum CL, Buchbinder S, Mayer K, McKirnan D, Judson FN, Huang Y, & Coates TJ (2003). High-Risk Behaviors Among Men Who Have Sex With Men in 6 US Cities: Baseline Data From the EXPLORE Study. American Journal of Public Health, 93(6), 926–932. 10.2105/AJPH.93.6.926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, Barresi PJ, Coates TJ, Chesney MA, & Buchbinder S (2006). Risk factors for HIV infection among men who have sex with men. AIDS, 20(5), 731–739. 10.1097/01.aids.0000216374.61442.55 [DOI] [PubMed] [Google Scholar]
- Ladak F, Sodas E, Nolan S, Dong H, Kerr T, Wood E, Montaner J, & Milloy M-J (2019). Substance use patterns and HIV-1 RNA viral load rebound among HIV-positive illicit drug users in a Canadian setting. Antiviral Therapy, 24(1), 19–25. 10.3851/IMP3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin CA, Donnell D, Metzger D, Sherman S, Aramrattna A, Davis-Vogel A, Quan VM, Gandham S, Vongchak T, Perdue T, & Celentano DD (2009). The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Social Science & Medicine (1982), 68(4), 740–748. 10.1016/J.SOCSCIMED.2008.n.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi J, Pozniak A, Heath K, & Hill A (2018). The impact of HIV prevalence, conflict, corruption, and GDP/capita on treatment cascades: data from 137 countries. Journal of Virus Eradication, 4(2), 80–90. 10.1016/s2055-6640(20)30249-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou LX, Chen Y, Yu CH, Li YM, & Ye J (2014). National HIV/AIDS mortality, prevalence, and incidence rates are associated with the Human Development Index. American Journal of Infection Control, 42(10), 1044–1048. 10.1016/j.ajic.2014.06.029 [DOI] [PubMed] [Google Scholar]
- Mata J, Silva MN, Vieira PN, Carraça EV, Andrade AM, Coutinho SR, Sardinha LB, & Teixeira PJ (2009). Motivational “spill-over” during weight control: Increased self-determination and exercise intrinsic motivation predict eating self-regulation. Health Psychology, 28(6), 709–716. 10.1037/a0016764 [DOI] [PubMed] [Google Scholar]
- McComsey GA, Lingohr-Smith M, Rogers R, Lin J, & Donga P (2021). Real-world adherence to antiretroviral therapy among HIV-1 patients across the United States. Advances in Therapy, 38(9), 4961–4974. 10.1007/S12325-021-01883-8/FIGURES/5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall E, & Singer M (2020). What constitutes a syndemic? Methods, contexts, and framing from 2019. Current Opinion in HIV and AIDS, 15(4), 213–217. 10.1097/COH.0000000000000628 [DOI] [PubMed] [Google Scholar]
- Meyer JP, Springer SA, & Altice FL (2011). Substance abuse, violence, and HIV in women: A literature review of the syndemic. Journal of Women’s Health, 20(7), 991–1006. 10.1089/jwh.2010.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S, Atkins L, & West R (2014). The behaviour change wheel. In A guide to designing interventions (pp. 1003–1010). [Google Scholar]
- Morris CA, Vlassoff A, Bisset SA, Baker RL, Watson HTG, West CJ, & Wheeler M (2000). Continued selection of Romney sheep for resistance or susceptibility to nematode infection: Estimates of direct and correlated responses. Animal Science, 70(1), 17–27. 10.1017/S1357729800051560 [DOI] [Google Scholar]
- Mulia N, Ye Y, Zemore SE, & Greenfield TK (2008). Social Disadvantage, Stress, and Alcohol Use Among Black, Hispanic, and White Americans: Findings From the 2005 U.S. National Alcohol Survey. Journal of Studies on Alcohol and Drugs, 69(6), 824–833. 10.15288/jsad.2008.69.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöstlinger C, Platteau T, Bogner J, Buyze J, Dec-Pietrowska J, Dias S, Newbury-Helps J, Kocsis A, Mueller M, Rojas D, Stanekova D, Van Lankveld J, & Colebunders R (2016). Computer-assisted intervention for safer sex in HIV-positive men having sex with men: Findings of a European randomized multi-center trial. Journal of Acquired Immune Deficiency Syndromes, 71(3). 10.1097/QAI.0000000000000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operario D, Sun S, Bermudez AN, Masa R, Shangani S, van der Elst E, & Sanders E (2022). Integrating HIV and mental health interventions to address a global syndemic among men who have sex with men. The Lancet HIV, 9(8), e574–e584. 10.1016/S2352-3018(22)00076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH (2017). Depression and other common mental disorders: global health estimates. [Google Scholar]
- Ory MG, Jordan PJ, & Bazzarre T (2002). The Behavior Change Consortium: setting the stage for a new century of health behavior-change research. Health Education Research, 17(5), 500–511. 10.1093/her/17.5.500 [DOI] [PubMed] [Google Scholar]
- Outlaw AY, Phillips G, Hightow-Weidman LB, Fields SD, Hidalgo J, Halpern-Felsher B, & Green-Jones, and The Young MSM of C, M. (2011). Age of MSM Sexual Debut and Risk Factors: Results from a Multisite Study of Racial/Ethnic Minority YMSM Living with HIV. AIDS Patient Care and STDs, 25(S1), S23–S29. 10.1089/apc.2011.9879 [DOI] [PubMed] [Google Scholar]
- Pantalone DW, Nelson KM, Batchelder AW, Chiu C, Gunn HA, & Horvath KJ (2020). A Systematic Review and Meta-Analysis of Combination Behavioral Interventions Co-Targeting Psychosocial Syndemics and HIV-Related Health Behaviors for Sexual Minority Men. In Journal of Sex Research (Vol. 57, Issue 6). 10.1080/00224499.2020.1728514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks KA, Collins RL, & Derrick JL (2012). The influence of marijuana and alcohol use on condom use behavior: Findings from a sample of young adult female bar drinkers. Psychology of Addictive Behaviors, 26(4), 888–894. 10.1037/a0028166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Lelutiu-Weinberger C, Botsko M, & Golub SA (2014a). A randomized controlled trial utilizing motivational interviewing to reduce HIV risk and drug use in young gay and bisexual men. Journal of Consulting and Clinical Psychology, 82(1), 9–18. 10.1037/a0035311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Lelutiu-Weinberger C, Botsko M, & Golub SA (2014b). A randomized controlled trial utilizing motivational interviewing to reduce HIV risk and drug use in young gay and bisexual men. Journal of Consulting and Clinical Psychology, 82(1), 9–18. 10.1037/a0035311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitpitan EV, Semple SJ, Aarons GA, Palinkas LA, Chavarin CV, Mendoza DV, Magis-Rodriguez C, Staines H, & Patterson TL (2018). Factors associated with program effectiveness in the implementation of a sexual risk reduction intervention for female sex workers across Mexico: Results from a randomized trial. PLoS ONE, 13(9), e0201954. 10.1371/journal.pone.0201954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, De Walque D, MacKeen L, Haberer J, Kimaiyo S, Sidle J, Ngare D, & Bangsberg DR (2011). Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: A randomized controlled trial of text message reminders. AIDS, 25(6), 825. 10.1097/QAD.0b013e32834380cl [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J, Spring B, & Nigg C (2008). Multiple health behavior change research: An introduction and overview. Preventive Medicine, 46(3), 181–188. 10.1016/j.ypmed.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustejovsky JE (2022). Cluster-Robust (Sandwich) Variance Estimators with Small-Sample Corrections (0.5.8). CRAN. 10.1080/07350015.2016.1247004 [DOI] [Google Scholar]
- Pustejovsky JE, & Rodgers MA (2019). Testing for funnel plot asymmetry of standardized mean differences. Research Synthesis Methods, 10(1), 57–71. 10.1002/jrsm.1332 [DOI] [PubMed] [Google Scholar]
- Pustejovsky JE, & Tipton E (2022). Meta-analysis with Robust Variance Estimation: Expanding the Range of Working Models. Prevention Science, 23(3), 425–438. 10.1007/SI1121-021-01246-3 [DOI] [PubMed] [Google Scholar]
- Reece M, Herbenick D, Schick V, Sanders SA, Dodge B, & Fortenberry JD (2010a). Condom Use Rates in a National Probability Sample of Males and Females Ages 14 to 94 in the United States. The Journal of Sexual Medicine, 7, 266–276. 10.1111/j,1743-6109.2010.02017.x [DOI] [PubMed] [Google Scholar]
- Reece M, Herbenick D, Schick V, Sanders SA, Dodge B, & Fortenberry JD (2010b). Condom Use Rates in a National Probability Sample of Males and Females Ages 14 to 94 in the United States. The Journal of Sexual Medicine, 7, 266–276. 10.1111/j,1743-6109.2010.02017.x [DOI] [PubMed] [Google Scholar]
- Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, & Safren SA (2009). A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. In Topics in HIV medicine : a publication of the International AIDS Society, USA (Vol. 17, Issue 1, pp. 14–25). [PMC free article] [PubMed] [Google Scholar]
- Rice WS, Logie CH, Napoles TM, Walcott M, Batchelder AW, Kempf MC, Wingood GM, Konkle-Parker DJ, Turan B, Wilson TE, Johnson MO, Weiser SD, & Turan JM (2018). Perceptions of intersectional stigma among diverse women living with HIV in the United States. Social Science and Medicine, 208. 10.1016/j.socscimed.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers MA, & Pustejovsky JE (2020). Evaluating meta-analytic methods to detect selective reporting in the presence of dependent effect sizes. Psychological Methods, 26(2), 141–160. 10.1037/MET0000300 [DOI] [PubMed] [Google Scholar]
- Rodgers MA, & Pustejovsky JE (2021). Evaluating meta-analytic methods to detect selective reporting in the presence of dependent effect sizes. Psychological Methods, 26(2), 141–160. 10.1037/met0000300 [DOI] [PubMed] [Google Scholar]
- Rosenberg ES, Sullivan PS, DiNenno EA, Salazar LF, & Sanchez TH (2011). Number of casual male sexual partners and associated factors among men who have sex with men: Results from the National HIV Behavioral Surveillance system. BMC Public Health, 77(1), 189. 10.1186/1471-2458-11-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Swendeman D, & Chovnick G (2009). The Past, Present, and Future of HIV Prevention: Integrating Behavioral, Biomedical, and Structural Intervention Strategies for the Next Generation of HIV Prevention. Annual Review of Clinical Psychology, 5(1), 143–167. 10.1146/annurev.clinpsy.032408.153530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GM, Coffin PO, Vittinghoff E, DeMicco E, Das M, Matheson T, Raiford JL, Carry M, Colfax G, Herbst JH, & Dilley JW (2014). Substance use and drinking outcomes in Personalized Cognitive Counseling randomized trial for episodic substance-using men who have sex with men. Drug and Alcohol Dependence, 138(1), 234–239. 10.1016/j.drugalcdep.2014.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz S, Hsu L, Dilley J, Loeb N, Kimberly B, & Boyd S (2006). Late Diagnosis of HIV Infection Trends, Prevalence, and Change. JAIDS Journal of Acquired Immune Deficiency Syndromes, 43(4), 491–494. https://doi.org/doi: 10.1097/01.qai.0000243114.37035.de [DOI] [PubMed] [Google Scholar]
- Sheehan DM, Dawit R, Gbadamosi SO, Fennie KP, Li T, Gebrezgi M, Brock P, Ladner RA, & Trepka MJ (2020). Sustained HIV viral suppression among men who have sex with men in the Miami-Dade County Ryan White Program: The effect of demographic, psychosocial, provider and neighborhood factors. BMC Public Health, 20(1). 10.1186/S12889-020-8442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH (1951). The Interpretation of Interaction in Contingency Tables. Journal of the Royal Statistical Society: Series B (Methodological), 13(2), 238–241. 10.1111/j.2517-616L195Ltb00088.x [DOI] [Google Scholar]
- Singh S, Song R, Johnson AS, McCray E, & Hall HI (2018). HIV Incidence, HIV Prevalence, and Undiagnosed HIV Infections in Men Who Have Sex With Men, United States. Annals of Internal Medicine. 10.7326/m17-2082 [DOI] [PubMed] [Google Scholar]
- Skalski LM, Watt MH, MacFarlane JC, Proeschold-Bell R. J. ean, Stout JE, & Sikkema KJ (2015). Mental health and substance use among Patients in a North Carolina HIV Clinic. North Carolina Medical Journal, 76(3), 148–155. 10.18043/ncm.76.3.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall R, & Purcell DW (2000). Intertwining epidemics: A review of research on substance use among men who have sex with men and its connection to the AIDS epidemic. In AIDS and Behavior (Vol. 4, Issue 2, pp. 181–192). 10.1023/A:1009516608672 [DOI] [Google Scholar]
- Stappen V, van, Cardon G, Craemer M, de, Mavrogianni C, Usheva N, Kivelä J, Wikström K, Miquel-Etayo P, de, González-Gil EM, Radó AS, Nánási A, Iotova V, Manios Y, & Brondeel R (2021). The effect of a cluster-randomized controlled trial on lifestyle behaviors among families at risk for developing type 2 diabetes across Europe: the Feel4Diabetes-study. International Journal of Behavioral Nutrition and Physical Activity, 18(1). 10.1186/sl2966-021-01153-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Becker BJ, & Egger M (2006). The Funnel Plot. In Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments (pp. 73–98). 10.1002/0470870168.ch5 [DOI] [Google Scholar]
- Strathdee SA, Wechsberg WM, Kerrigan DL, & Patterson TL (2013). HIV Prevention Among Women in Low- and Middle-Income Countries: Intervening Upon Contexts of Heightened HIV Risk. Annual Review of Public Health, 34(1), 301–316. 10.1146/annurev-publhealth-031912-114411 [DOI] [PubMed] [Google Scholar]
- Sunderrajan A, White B, Durantini M, Sanchez F, Glasman L, & Albarracín D (2021a). Complex solutions for a complex problem: A meta-analysis of the efficacy of multiple-behavior interventions on change in outcomes related to HIV. Health Psychology, 40(9), 642–653. 10.1037/hea0001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderrajan A, White B, Durantini M, Sanchez F, Glasman L, & Albarracín D (2021b). Complex solutions for a complex problem: A meta-analysis of the efficacy of multiple-behavior interventions on change in outcomes related to HIV. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 40(9), 642–653. 10.1037/HEA0001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeting MJ, Sutton AJ, & Lambert PC (2004). What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Statistics in Medicine, 23(9), 1351–1375. 10.1002/sim.1761 [DOI] [PubMed] [Google Scholar]
- Tang AM, Bhatnagar T, Ramachandran R, Dong K, Skinner S, Kumar MS, & Wanke CA (2011). Malnutrition in a population of HIV-positive and HIV-negative drug users living in Chennai, South India. Drug and Alcohol Dependence, 118(1), 73–77. 10.1016/j.drugalcdep.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton E, & Pustejovsky JE (2015). Small-Sample Adjustments for Tests of Moderators and Model Fit Using Robust Variance Estimation in Meta-Regression. Journal of Educational and Behavioral Statistics, 40(6), 604–634. 10.3102/1076998615606099/ASSET/IMAGES/LARGE/10.3102_1076998615606099-FIG1.JPEG [DOI] [Google Scholar]
- Tsai AC (2018). Syndemics: A theory in search of data or data in search of a theory? Social Science & Medicine, 206, 117–122. 10.1016/j.socscimed.2018.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, & Burns BFO (2015). Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept. Social Science and Medicine, 139, 26–35. 10.1016/j.socscimed.2015.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, & Venkataramani AS (2016). Syndemics and health disparities: a methodological note. AIDS and Behavior, 20(2), 423. 10.1007/S10461-015-1260-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Washington School of Medicine. (2023). Institute for Health Metrics and Evaluation - Global Burden of Disease. Institute for Health Metrics and Evaluation. https://www.healthdata.org/ [Google Scholar]
- Valleroy LA, Mackellar DA, Karon JM, Rosen DH, McFarland W, Shehan DA, Stoyanoff SR, Lalota M, Celentano DD, Koblin BA, Thiede H, Katz MH, Torian LV, & Janssen RS (2000). HIV prevalence and associated risks in young men who have sex with men. Journal of the American Medical Association, 284(2), 198–204. 10.1001/jama.284.2.198 [DOI] [PubMed] [Google Scholar]
- Van der Elst EM, Mbogua J, Operario D, Mutua G, Kuo C, Mugo P, Kanungi J, Singh S, Haberer J, Priddy F, & Sanders EJ (2013). High Acceptability of HIV Pre-exposure Prophylaxis but Challenges in Adherence and Use: Qualitative Insights from a Phase I Trial of Intermittent and Daily PrEP in At-Risk Populations in Kenya. AIDS and Behavior, 17(6), 2162–2172. 10.1007/sl0461-012-0317-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W (2019). Package “metafor.” In CRAN. [Google Scholar]
- Wechsberg WM, Luseno WK, Lam WKK, Parry CDH, & Morojele NK (2006). Substance use, sexual risk, and violence: HIV prevention intervention with sex workers in Pretoria. AIDS and Behavior, 10(2), 521. 10.1007/sl0461-005-9036-8 [DOI] [PubMed] [Google Scholar]
- WHO. (2022a). World Health Organization: HIV/AIDS. WHO. https://www.who.int/health-topics/hiv-aids/#tab=tab_1 [Google Scholar]
- WHO. (2022b). World Health Organization: HIV/AIDS. WHO. https://www.who.int/health-topics/hiv-aids/#tab=tab_l [Google Scholar]
- Wilson K, Senay I, Durantini M, Sánchez F, Hennessy M, Spring B, & Albarracín D (2015). When it comes to lifestyle recommendations, more is sometimes less: A meta-analysis of theoretical assumptions underlying the effectiveness of interventions promoting multiple behavior domain change. Psychological Bulletin, 141(2), 474–509. 10.1037/a0038295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PA, Nanin J, Amesty S, Wallace S, Cherenack EM, & Fullilove R (2014). Using syndemic theory to understand vulnerability to HIV infection among Black and Latino men in New York City. Journal of Urban Health : Bulletin of the New York Academy of Medicine, 91(5), 983–998. 10.1007/sll524-014-9895-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf SH, & Braveman P (2011a). Where Health Disparities Begin: The Role Of Social And Economic Determinants—And Why Current Policies May Make Matters Worse. Health Affairs, 30(10), 1852–1859. 10.1377/hlthaff.2011.0685 [DOI] [PubMed] [Google Scholar]
- Woolf SH, & Braveman P (2011b). Where Health Disparities Begin: The Role Of Social And Economic Determinants—And Why Current Policies May Make Matters Worse. Health Affairs, 30(10), 1852–1859. 10.1377/hlthaff.2011.0685 [DOI] [PubMed] [Google Scholar]
- Zea MC, Barnett AP, Río-González AM, del, Parchem B, Pinho V, Le HN, & Poppen PJ (2022). Experiences of Violence and Mental Health Outcomes among Colombian Men who have Sex with Men (MSM) and Transgender Women. Journal of Interpersonal Violence, 37(13–14), NP11991–NP12013. 10.1177/0886260521997445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


