Abstract
It is generally believed that immune activation can elicit pain through production of inflammatory mediators that can activate nociceptive sensory neurons. Emerging evidence suggests that immune activation may also contribute to the resolution of pain by producing distinct pro-resolution/anti-inflammatory mediators. Recent research into the connection between the immune and nervous systems has opened new avenues for immunotherapy in pain management. This review provides an overview of the most utilized forms of immunotherapies (e.g., biologics) and highlight their potential for immune and neuronal modulation in chronic pain. Specifically, we discuss pain-related immunotherapy mechanisms that target inflammatory cytokine pathways, the PD-L1/PD-1 pathway, and the cGAS/STING pathway. This review also highlights cell-based immunotherapies targeting macrophages, T cells, and mesenchymal stromal cells for chronic pain management.
Keywords: Immunotherapy, PD-1/PD-L1 pathway, cGAS/STING pathway, Pro-inflammatory cytokines, Cell therapy
1. Introduction
According to the revised definition of the International Association for the Study of Pain (IASP), pain is an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage (Raja, et al., 2020). Chronic pain is defined as pain that persists or recurs for more than 3 months and usually accompanied by central sensitization. Chronic pain includes but is not limited to chronic neuropathic pain following nerve injury, spinal cord injury, and stroke (Costigan, Scholz, & Woolf, 2009), chronic cancer-related pain, chronic musculoskeletal pain, and chronic headache or orofacial pain. While physiological pain or acute pain serves a protective purpose, chronic pain is detrimental to the quality of life of an individual. Chronic pain also places a substantial economic burden on health care resources with an annual cost of over $600 billion in the United States (Gereau, et al., 2014). Chronic pain can be characterized as spontaneous pain, as well as evoked pain such as hyperalgesia (increased painful response evoked by a noxious stimulus) and allodynia (painful reaction to a normally innocuous stimulus), with mechanical or tactile allodynia presenting as a cardinal symptom of chronic pain.
Pain is initiated by the activation of pain sensing neurons (nociceptors). Nociceptor neuron cell bodies are located in sensory ganglia in the peripheral nervous system (PNS), including the dorsal root ganglia (DRG) and trigeminal ganglia (TG). Primary sensory neurons project to the spinal cord and brain stem, and secondary order neurons project from these regions to the thalamus and other brain regions, which mediate the sensory and emotional aspects of pain (Kuner & Flor, 2017). Nociceptors express different types of transducer molecules, including transient receptor potential (TRP) ion channels (e.g., TRPV1, TRPM8) and piezo ion channels to detect heat pain, cold pain, mechanical pain, and chemical pain (Ji & Lee, 2021; Julius & Basbaum, 2001; Kim, Coste, Chadha, Cook, & Patapoutian, 2012), as well as pathogen-induced pain (Chiu, et al., 2013; Donnelly, Chen, & Ji, 2020). Furthermore, nociceptors can be sensitized by inflammatory mediators, in the process of peripheral sensitization, through the activation of protein kinases such as protein kinase A and C (PKA and PKC) and MAP kinases (MAPK) (Gold, Levine, & Correa, 1998; Ji, Gereau, Malcangio, & Strichartz, 2009; Reichling & Levine, 2009). Recent progress has revealed that the DRG and TG consist of different classes of primary sensory neurons and nociceptors in mice and humans (Tavares-Ferreira, et al., 2022; Usoskin, et al., 2015; Yang, et al., 2022). Preclinical studies have also identified specific neurocircuits for the regulation of mechanical and thermal pain (Duan, et al., 2014; Peirs & Seal, 2016; H. Wang, et al., 2022).
Central sensitization is characterized by synaptic plasticity (i.e. increased excitatory synaptic transmission and decreased inhibitory synaptic transmission) in CNS pain circuits and carries significant clinical implications. It has a critical role in the induction and maintenance of chronic pain and the spread of pain beyond the initial site of injury (Treede, Hoheisel, Wang, & Magerl, 2022; Woolf, 1983, 2011). Thus, blockade of nerve conduction and neurotransmission in the PNS and CNS by local anesthetics, ion channel blockers, opioids, and anticonvulsants are major pharmacological treatments for pain (Finnerup, Sindrup, & Jensen, 2010). However, these treatments usually provide only transient pain relief and have been unable to promote complete resolution from injury.
The immune system has gained increasing attention in its critical role in the induction and resolution of pain via interactions with the nervous system (Marchand, Perretti, & McMahon, 2005; Ren & Dubner, 2010; Talbot, Foster, & Woolf, 2016). In particular, non-neuronal cells, including but not limited to, immune cells, glial cells, keratinocytes, cancer cells, stem cells, as well as bacterial cells, can interact with nociceptors to modulate pain (Ji, Chamessian, & Zhang, 2016). These non-neuronal cells also regulate inflammation and neuroinflammation, a localized inflammation of the PNS and CNS, for the induction and resolution of pain (Ellis & Bennett, 2013; Ji, Xu, & Gao, 2014; Ji, Xu, Strichartz, & Serhan, 2011). Immunotherapy represents a wide variety of therapies using cytokines, vaccines, antibodies, and adoptive transfer of cells. In this review, we discuss different forms of immunotherapies that target pro-inflammatory cytokines, the PD-L1/PD-1 axis, the STING/IFN pathway, and immune cells/mesenchymal stem cells, with a specific focus on emerging therapies.
2. Immunotherapy targeting pro-inflammatory cytokines in chronic pain
Cytokines are small, secreted proteins that have specific effects on cellular interactions and communication. They can act on the cells that secrete them (autocrine action), on nearby cells (paracrine action), or on distant cells (endocrine action) (J. M. Zhang & An, 2007). Cytokines are categorized into two main groups, namely pro-inflammatory and anti-inflammatory. Pro-inflammatory cytokines include tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), interleukin-17 (IL-17), and granulocyte-macrophage colony-stimulating factor (GM-CSF). The expression of these cytokines is elevated in animal pain models (DeLeo, Colburn, & Rickman, 1997; Kalpachidou, Riehl, Schopf, Ucar, & Kress, 2022; Sommer & Kress, 2004). On the other hand, anti-inflammatory cytokines include interleukin 4 (IL-4), interleukin 10 (IL-10), and transforming growth factor beta (TGF-β), which have been shown to reduce pain when used as treatments in animal pain models (Dai, et al., 2019; Echeverry, et al., 2009; Ledeboer, et al., 2007). Current clinical immunotherapies are primarily focused on targeting pro-inflammatory cytokines (Table 1).
Table 1.
Overview of immunotherapies targeting pro-inflammatory cytokines in chronic pain related conditions.
| Cytokine | Principle | Immunotherapy | Chronic pain conditions |
Pain related positive and negative outcomes |
References |
|---|---|---|---|---|---|
| TNF-α | Anti-TNF-α | Infliximab Adalimumab Golimumab Certolizumab-pegol Etanercept Ozoralizumab | Rheumatoid arthritis; Psoriatic arthritis; Ankylosing spondylitis; | Certolizumab-pegol treatment (12 weeks) reduced pain VAS score in psoriatic arthritis patients. Ozoralizumab treatment (24 weeks) reduced pain in VAS score in rheumatoid arthritis patients. | (Esposito, et al., 2020; Gholami, Azizpoor, Aflaki, Rezaee, & Keshavarz, 2021; Oldfield & Plosker, 2009; Takeuchi, Kawanishi, Nakanishi, Yamasaki, & Tanaka, 2022) |
| IL-1β | Anti-IL-1β | Canakinumab | Rheumatoid arthritis; Systemic juvenile idiopathic arthritis; Recurrent Pericarditis; Traumatic brain injury/stroke; Covid-19; | In the withdrawal phase of canakinumab treatment (32 weeks), serious adverse arm pain occurred in 1 patient with systemic juvenile idiopathic arthritis out of 50 patients. Anakinra treatment (24 weeks) improved rheumatoid arthritis pain symptom. Rilonacept treatment (6 weeks) in recurrent pericarditis patients reduced average pericarditis pain. | (Fava, et al., 2022; Helmy, et al., 2014; Kyriazopoulou, et al., 2021; Maniscalco, et al., 2020; Orrock & Ilowite, 2016) |
| Anti-IL-1R | Anakinra Rilonacept | ||||
| IL-6 | Anti-IL-6 | Siltuximab Clazakizumab Olokizumab Sirukumab | Psoriatic arthritis; Rheumatoid arthritis; Juvenile idiopathic arthritis; Giant cell arteritis; Covid-19; | Tocilizumab treatment (24-52 weeks) improved pain VAS score in rheumatoid arthritis patients. Sarilumab treatment (2-52 weeks) showed improvements in pain VAS score in rheumatoid arthritis patients. | (Mease, et al., 2016; Tanaka, Narazaki, & Kishimoto, 2018; Vaidya, et al., 2020) |
| Anti-IL-6R | Tocilizumab Sarilumab | ||||
| IL-17 | Anti-IL-17A | Secukinumab Ixekizumab | Psoriatic arthritis; Ankylosing Spondylitis; | Secukinumab treatment (12-52 weeks) in patients with psoriatic arthritis showed greater improvements in the axial specific assessment of spinal pain. | (Baeten, et al., 2015; J. X. Huang, Lee, & Wei, 2020; Langley, et al., 2014; Lespessailles & Toumi, 2021) |
| GM-CSF | Anti-GM-CSF | Namilumab Otilimab | Rheumatoid arthritis; Psoriasis; Giant cell arteritis; Covid-19; | Namilumab treatment (12-16 weeks) in rheumatoid arthritis patients had improvements inpatient’s assessment of pain. Mavrilimumab treatment caused non-specific headache and neck pain in patients with giant cell arteritis. | (Crotti, Agape, Becciolini, Biggioggero, & Favalli, 2019) (Pourhoseingholi, Shojaee, & Ashtari, 2020) |
| Anti-GM-CSFR | Mavrilimumab |
2.1. TNF-α and its receptors
TNF-α is a transmembrane protein that can be transformed into a soluble form (sTNF-α) through cleavage by a metalloprotease called TNF-α-converting enzyme (TACE or ADAM17). Soluble TNF-α can then bind to its specific membrane-bound receptors TNFR1 (p55/p60) and TNFR2 (p75/p80) (Idriss & Naismith, 2000). Numerous studies have demonstrated a link between TNF-α and different types of pain (Schafers, et al., 2008; J. M. Zhang & An, 2007). TNF-α drives nociceptor sensitization by regulation of TRPV1 expression and sensitization of DRG neurons (Constantin, et al., 2008; X. H. He, et al., 2010; X. Jin & Gereau, 2006; B. Liu, Li, Brull, & Zhang, 2002; Park, et al., 2011; S. Wei, Qiu, Jin, Liu, & Hu, 2021). TNF-α further promotes neuropathic pain by inducing glial activation and central sensitization (Berta, et al., 2014; Bezzi, et al., 2001; Kawasaki, Zhang, Cheng, & Ji, 2008; Zhong, et al., 2010).
Anti-TNF-α-based immunotherapies, including IgG antibodies (Infliximab, Adalimumab and Golimumab), a PEGylated Fab fragment (Certolizumab-pegol), a fusion protein (Etanercept), and a nanobody (Ozoralizumab), have been approved by the FDA for the treatment of painful inflammatory disorders such as inflammatory bowel disease, rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis (Table 1). Several clinical studies have investigated the use of TNF-α inhibitors in the treatment of chronic pain conditions. A pilot study using subcutaneous etanercept (TNF inhibitor as a soluble receptor) to treat patients with acute severe sciatica showed improved pain scores compared to the control group (Genevay, Stingelin, & Gabay, 2004). An open-label study using infliximab also revealed promising results, with a significant reduction in pain intensity reported by patients with chronic low back pain (Grainger & Harrison, 2007). Overall, the prominent role of TNF-α in pain modulation and promising results from preclinical and clinical studies using TNF-α inhibitors suggest that this signaling pathway is a viable target for pain management. However, further research is needed to fully understand the mechanisms and timing underlying the effects of TNF-α inhibitors on pain and to determine their efficacy and safety in different pain conditions. It is noteworthy that the complications of anti-TNF-α-based immunotherapies is the small but significant risk of life threatening opportunistic infection (Ali, et al., 2013).
2.2. IL-1β and its receptors
IL-1β is a protein that belongs to the interleukin-1 family of cytokines. IL-1β exerts its effects through binding to its receptor, IL-1 receptor 1 (IL-1R1) (O'Neill & Dinarello, 2000). IL-1β has been implicated in the pathogenesis of chronic pain conditions such as rheumatoid arthritis, osteoarthritis, and neuropathic pain (Kirkham, 1991; Mailhot, et al., 2020). Studies on animals have demonstrated that exogenous IL-1β can induce both mechanical and thermal hyperalgesia, while treatment with anti-IL-1β inhibitors or antibodies can reduce pain symptoms (Milligan, et al., 2001; Sweitzer, Martin, & DeLeo, 2001; Thom, et al., 2019; T. Wei, Guo, Li, Kingery, & Clark, 2016; X. H. Wei, et al., 2012; Zelenka, Schafers, & Sommer, 2005). Similar to TNF-α, IL-1β was found to induce peripheral sensitization in DRG neurons and central sensitization in spinal cord and brain stem neurons (Binshtok, et al., 2008; W. Guo, et al., 2007; Kawasaki, Zhang, et al., 2008; Mailhot, et al., 2020; Sung, et al., 2017; M. H. Yi, et al., 2021).
IL-1β or IL-1 receptor (IL-1R)-based immunotherapies has been investigated as a potential treatment for chronic pain conditions associated with inflammation (Kalpachidou, et al., 2022) (Table 1). Current available immunotherapies targeting IL-1β or IL-1R include IL-1β antagonists (Canakinumab and Gevokizumab) and IL-1R antagonists (Anakinra and Rilonacept) (Goldbach-Mansky, et al., 2008). These antagonists are approved for the treatment of chronic pain-associated conditions, including rheumatoid arthritis and other autoinflammatory disorders (Aitken, et al., 2018; Goldbach-Mansky, et al., 2006; Ridker, et al., 2017). In clinical studies, canakinumab has been shown to reduce pain in patients with osteoarthritis, and anakinra was found to reduce pain and improve function in patients with complex regional pain syndrome (CRPS) (Helyes, et al., 2019; Ridker, et al., 2017). However, side effect of anti-IL-1β treatment (e.g., canakinumab) was also reported (Table 1) and more research is needed to fully understand the effectiveness and safety of IL-1β or IL-1R immunotherapy, as well as the timing and duration, for the treatment of chronic pain conditions.
2.3. IL-6 and its receptors
IL-6 is a member of the IL-6 family, which consists of polypeptide cytokines comprised of four–α-helix structures. The biological activity of IL-6 is mediated through its interaction with the IL-6 receptor (IL-6R). IL-6 can activate signaling pathways through two different modes of receptor activation: classical signaling and trans-signaling (Heinrich, et al., 2003). In classical signaling, IL-6 binds to the membrane-bound IL-6Rα subunit, leading to the recruitment and activation of gp130. This process results in the activation of downstream signaling pathways, including the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway and the MAPK pathway. In trans-signaling, IL-6 can also bind to a soluble form of the IL-6 receptor (sIL-6R), which is generated by alternative splicing or proteolytic cleavage of the membrane-bound IL-6Rα subunit. The sIL-6R can form a complex with IL-6, which can then bind to gp130 on cell membranes that do not express the membrane-bound IL-6Rα subunit. This allows IL-6 to activate downstream signaling pathways in a broader range of cells.
IL-6 and its receptor play a crucial role in the modulation of pain, especially in chronic pain conditions. IL-6 is produced by immune cells and other cell types in response to tissue damage, inflammation, or nerve injury and can activate signaling pathways that contribute to the development and maintenance of chronic pain. IL-6 drives peripheral sensitization via activation of JAK/STAT3, extracellular-signal regulated kinases (ERK, a MAPK family member), and protein translation signaling pathways, leading to subsequent upregulation of TRPV1 and voltage-gated sodium channels Nav1.7 and Nav1.8 or downregulation of potassium channel KCNA4 (Alvarez, Bogen, & Levine, 2019; Atmaramani, Black, de la Pena, Campbell, & Pancrazio, 2020; Dominguez, Rivat, Pommier, Mauborgne, & Pohl, 2008; Fang, et al., 2015; Kalpachidou, et al., 2022). IL-6 further regulates central sensitization in spinal cord pain circuits (Kawasaki, Zhang, et al., 2008).
Multiple immunotherapies have been developed to target IL-6 or IL-6R, including anti-IL-6 monoclonal antibodies (Siltuximab and Clazakizumab) and anti-IL-6R monoclonal antibodies (Tocilizumab and Sarilumab) (Table 1). These immunotherapies have been shown to be effective in the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, giant cell arteritis, multicentric Castleman disease, as well as COVID-19 (Kishimoto, 2005; Mease, et al., 2016; Potere, et al., 2021; Smolen, et al., 2008). Several preclinical studies have shown that blocking IL-6 or its receptor can alleviate pain in animal models of chronic pain. However, the results of clinical trials have been mixed. Some clinical trials have shown that blocking IL-6 or its receptor can reduce pain in patients with rheumatoid arthritis or chronic low back pain, while others failed to show significant benefits (Choy, et al., 2020; Genovese, et al., 2015; Yip & Yim, 2021). One challenge in developing IL-6 or IL-6R immunotherapy for pain is that IL-6 also plays an important role in normal immune function and its blockade can increase the risk of infections and other adverse effects (Gale, et al., 2019; Monemi, et al., 2016). Therefore, it is essential to carefully consider the potential risks and benefits of this approach before using it to treat chronic pain.
2.4. IL-17 and its receptors
IL-17 is a cytokine that is produced by various immune cells, including T-helper 17 (Th17) cells, gamma-delta T cells, and natural killer T cells. It plays an important role in the immune response to infections and in the development of autoimmune diseases. IL-17 signals through a receptor complex known as IL-17 receptor (IL-17R), which is expressed on a wide variety of cells, including epithelial cells, fibroblasts, and immune cells such as macrophages and neutrophils (Gu, Wu, & Li, 2013). IL-17RA is the primary signaling subunit and is required for most of the biological effects of IL-17. Upon IL-17 binding to its receptor, a signaling cascade is initiated, which ultimately leads to the activation of several downstream pathways, including the NF-κB and MAPK pathways. These pathways regulate the expression of various proinflammatory genes, such as cytokines, chemokines, and matrix metalloproteinases, which contribute to the immune response and tissue damage (Gaffen, 2009). Animal studies have shown that IL-17 is involved in the development and maintenance of neuropathic pain, inflammatory pain, and cancer pain (X. Jiang, et al., 2022). Exogenous IL-17 injection is sufficient to induce pain hypersensitivity in naïve animals, while blocking IL-17/IL-17R signaling decreased arthritic and neuropathic pain in animals with arthritis, nerve injury, and chemotherapy-induced neuropathy (H. Luo, et al., 2019; Luo, et al., 2021; Meng, et al., 2013; Richter, et al., 2012; Segond von Banchet, et al., 2013). Notably, the IL-23/IL-17A axis regulates mechanical pain via macrophage-nociceptor interaction in a sex-dependent manner (Luo, et al., 2021; Z. Tan, Lin, Wu, & Zhou, 2022). Nociceptive sensory neurons express IL-17R in rodents and humans and neuronal IL-17R was shown to regulate peripheral sensitization (Luo, et al., 2021; McNamee, et al., 2011; Richter, et al., 2012; Yang, et al., 2022).
Currently, two IL-17A antibodies, secukinumab and ixekizumab, are approved for the treatment of plaque psoriasis, ankylosing spondylitis and psoriatic arthritis (Table 1). Several preclinical studies have investigated the use of IL-17 and IL-17RA antagonists as potential treatments for chronic pain. Blocking IL-17RA with a monoclonal antibody reduced mechanical allodynia and thermal hyperalgesia in a rat model of neuropathic pain (Ultenius, Linderoth, Meyerson, & Wallin, 2006). It was also found that IL-17 neutralizing antibody reduced pain-like behaviors in a mouse model of osteoarthritis (Faust, et al., 2020). Clinical trials are currently in progress to investigate the efficacy of IL-17 and IL-17RA antagonists for the treatment of various chronic pain conditions, such as rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis (Mease, et al., 2017; van den Berg & Miossec, 2009). Results from these trials have shown promising effects on reducing pain and improving physical function (Jaller Char, Jaller, Waibel, Bhanusali, & Bhanusali, 2018).
2.5. GM-CSF and its receptor
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine that regulates the proliferation, differentiation, and activation of hematopoietic cells. The biological effects of GM-CSF are mediated through binding to its specific receptor, GM-CSFR. The binding of GM-CSF to GM-CSFR leads to the activation of downstream signaling pathways, including JAK2/STAT5, PI3K/Akt, Ras/MAPK, and NF-κB pathways, which can promote cell growth, differentiation, and inflammatory responses (Lehtonen, Matikainen, Miettinen, & Julkunen, 2002). The expression of GM-CSFR in DRG neurons was found to regulate nociceptor sensitization in mice, likely by phosphorylating the heat transducer ion channel TRPV1 through p38 MAPK and TrkA signaling (Donatien, et al., 2018; L. Zhao, et al., 2022). Treatment with GM-CSF led to the upregulation of TRPV1, sodium channels, and potassium channels, leading to peripheral sensitization (Tewari, et al., 2020; F. Zhang, et al., 2019).
Several monoclonal antibodies that target GM-CSF (Namilumab, Otilimab (MOR103)) or GM-CSFR (Mavrilimumab) are approved for treating chronic pain related conditions, including rheumatoid arthritis and multiple sclerosis (Behrens, et al., 2015; Nair, Edwards, & Moots, 2012; Taylor, et al., 2019) (Table 1). In mouse models of bone cancer, administration of a GM-CSF neutralizing antibody significantly reduced cancer pain behaviors and inhibited the upregulation of TRPV1 and Nav1.7 channels in DRG neurons (F. Zhang, et al., 2019). In a neuropathic pain model, treatment with a GM-CSFR antibody reduced mechanical allodynia and thermal hyperalgesia in mice (Nicol, et al., 2018). Treatment with a GM-CSF neutralizing antibody in a preclinical model of arthritis led to reductions in synovial inflammation and joint destruction and pain-related behaviors (Saleh, et al., 2018). Clinical trials are ongoing to evaluate the safety and efficacy of GM-CSF and GM-CSFR antagonists in the treatment of chronic pain. Preliminary results from a phase 2 clinical trial in patients with osteoarthritis have shown that treatment with a GM-CSF neutralizing antibody significantly reduced pain compared to placebo (Taylor, et al., 2019). However, Mavrilimumab was also shown to cause non-specific headache in some patients (Table 1).
In addtion to pro-inflammatory cytokines, matrix metalloproteases (MMPs) are extracellular matrix proteins with major roles in neuroinflammation and neurological diseases (Vandooren, Van Damme, & Opdenakker, 2014). MMP-9 is one of the best known members of the MMP family and is tighely regulated by pro-inflammatory cytokines such as TNF-α in peripheral neuropathy (Shubayev & Myers, 2000). MMP-9 was shown to drive neuropathic pain via activation of IL-1β and glial cells in the PNS and CNS (Kawasaki, Xu, et al., 2008). Through functional screening, a selective monoclonal antibody was developed against mouse/human MMP-9 and displays efficacy in attenuating neuropathic pain (Lopez, et al., 2019). Thus, MMP-9 monoclonal antiboids may offer new therpetutics for treating chronic pain, but the specfic pain conditions and timing of the treatment remain to be defined.
3. Immunotherapies targeting PD-L1/PD-1 pathway in chronic pain
The Programmed cell death 1 (PD-1) receptor, also named CD279, is a 288-amino acid protein first identified as an apoptosis molecule by Tasuku Honjo and colleagues at Kyoto University in 1992 (Ishida, Agata, Shibahara, & Honjo, 1992). In 1999, the team further discovered that PD-1 functions as a critical negative regulator of the immune response, contributing to self-tolerance of the immune cells, especially T cells. The Programmed cell death ligand 1 (PD-L1 or Pdcd1lg1, CD274, B7-H1) is a 290-amino acid protein first identified as a molecule with homology to B7-1 and B7-2 by Lieping Chen and colleagues at Mayo Clinic in 1999 (Dong, Zhu, Tamada, & Chen, 1999). In the year 2000 another research group led by Gordon Freeman at the Dana-Farber Cancer Institute collaborated with the Genetics Institute at Cambridge and found that this B7-1 like molecule was a ligand for PD-1, and named it PD-L1 (Freeman, et al., 2000).
3.1. PD-L1/PD-1 signaling in different cell types
PD-1 is a type I transmembrane protein consisting of an extracellular region, a transmembrane region, and a cytoplasmic region. The extracellular region contains an IgV-like domain and an IgC-like domain, which are connected by a hinge region. The IgV-like domain is composed of seven β-strands that fold into two antiparallel β-sheets and responsible for binding to its ligands, PD-L1 and PD-L2 (Zak, et al., 2015). Compared to PD-L1, PD-L2 has very limited expression (Latchman, et al., 2001; Lazar-Molnar, et al., 2008). The transmembrane domain is composed of a single α-helix that anchors the protein in the cell membrane. The cytoplasmic tail contains two tyrosine residues in its immune receptor tyrosine-based inhibitory motif (ITIM) and an immune receptor tyrosine-based switch motif (ITSM) that play a crucial role in PD-1 downstream signaling (X. Zhang, et al., 2004). PD-L1 is a type I transmembrane glycoprotein that is composed of an extracellular domain, a transmembrane domain, and a cytoplasmic domain (Perry, et al., 2019). The extracellular domain consists of two Ig-like domains, a V-like domain and a C-like domain, which are connected by a flexible hinge region (Lin, et al., 2008). The V-like domain, which is structurally similar to other B7 family members and mediates the interaction between PD-L1 and its receptor PD-1. The transmembrane domain anchors PD-L1 to the cell membrane while the cytoplasmic domain interacts with intracellular signaling pathways.
The PD-1 protein is primarily found on the surface of certain immune cells, such as activated T cells, B cells, natural killer cells, monocytes, and dendritic cells (Bally, Austin, & Boss, 2016). However, recent studies have identified the expression of PD-1 on neurons in several regions of the brain, including the hippocampus, cortex, thalamus, and hypothalamus (C. Jiang, et al., 2020; Zeisel, et al., 2015). The PD-L1 protein is expressed on a range of cells, including activated immune cells like T cells, B cells, dendritic cells, macrophages, and tumor cells, as well as neurons (G. Chen, et al., 2017; Dorand, et al., 2016).
In T cells, PD-1 activation initiates several downstream signaling cascades that reduce the secretion of inflammatory cytokines (IFN-γ, IL-2, TNF-α) and promote the secretion of immune inhibitory cytokines (IL-10 and IL-4) in order to inhibit T cell activation, proliferation, and survival (Riley, 2009). PD-1 activation inhibits T cell receptor (TCR)-mediated signaling by recruiting the tyrosine phosphatases SHP-1/2 (Src homology region 2 domain-containing phosphatase-1/2) to the immune synapse where TCRs are engaged with antigen-presenting cells (APCs) (Hui, et al., 2017; Patsoukis, et al., 2020; Plas, et al., 1996; Yokosuka, et al., 2012). PD-1 activation also inhibits the PI3K/Akt/mTOR and Ras/MAPK/Erk pathways, leading to decreased cytokine production and T cell proliferation (Patsoukis, et al., 2012; R. Zhao, et al., 2019). PD-1 signaling upregulates other immune inhibitory molecules, such as cytotoxic t-lymphocyte antigen-4 (CTLA-4) and lymphocyte-activation gene 3 (LAG-3) (Chocarro, et al., 2021; Ribas & Wolchok, 2018), which regulate the balance between immune activation and suppression. Finally, PD-1 signaling inhibits the activity of transcription factors, such as NFAT and AP-1, resulting in reduced cytokine production and T cell activation (Oestreich, Yoon, Ahmed, & Boss, 2008; G. Xiao, Deng, Liu, Ge, & Liu, 2012). Therefore, PD-1 plays a critical role in adaptive immune resistance that allows tumor cells to counteract endogenous anti-tumor activity. In this pathway, PD-L1 is often upregulated in tumor cells or non-transformed cells within the tumor microenvironment. When PD-L1 binds to PD-1 on activated T cells, it suppresses the function of cytotoxic T cells within the tumor microenvironment, thereby facilitating tumor growth and progression.
In macrophages and microglia, PD-1 activation inhibits macrophage/microglia function by recruiting phosphatases SHP-1/SHP-2 to ITIM and/or ITSM domains in the PD-1 tail. This recruitment leads to the inhibition of the PI3K/NF-κB signaling pathway (H. He, et al., 2018). Specifically, PD-1 signaling suppresses the activity of IFN-γ-activated M1 macrophages by reducing the phosphorylation of STAT1 and the secretion of interleukin-12 (IL-12). Furthermore, PD-1 signaling promotes the activity of IL-4-activated M2 macrophages by increasing STAT6 phosphorylation (Liang, et al., 2021; Yao, et al., 2014; J. Zhao, Roberts, Wang, Savage, & Ji, 2021).
Accumulating evidence indicates an active role of PD-1 in neurons in the PNS and CNS. Activation of PD-1 directly regulates neuronal excitability, synaptic transmission, and plasticity via the SHP-1/ERK signaling pathway and modification of ion channels, including sodium, calcium, and potassium channels (e.g., Kv4.2 channels), as well as NMDA/AMPA receptors and GABA receptors. Thus, the PD-L1/PD-1 axis may also serve as a neuronal checkpoint to regulate various functions of the nervous system, such as learning, memory, anesthesia, analgesia, and pain (C. Jiang, et al., 2020; J. Zhao, et al., 2021).
3.2. PD-L1/PD-1 axis in pain and opioid analgesia
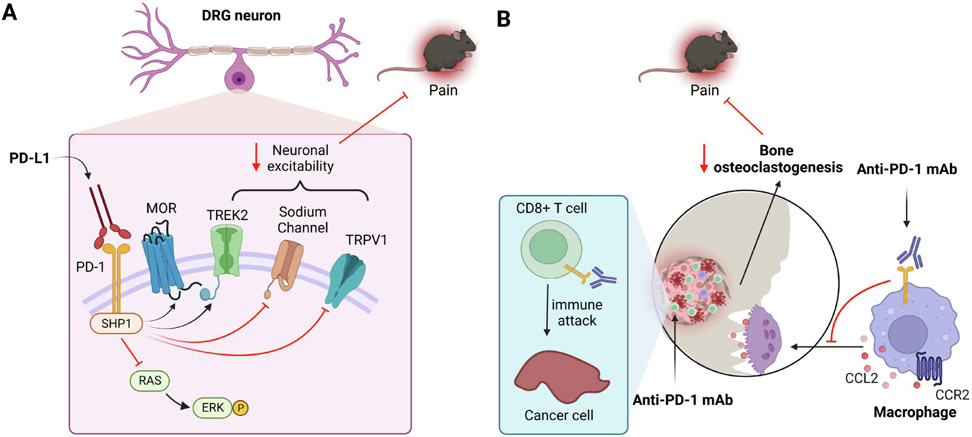
Preclinical and clinical studies suggest an important role of PD-L1 and PD-1 in regulating physiological and pathological pain (Table 2). Mice with a deficiency of PD-L1 (B7-H1) showed enhanced inflammation and neuropathic pain after nerve injury via immune modulation (Uceyler, et al., 2010a). The PD-L1/PD-1 axis also controls pain via neuromodulation. Pdcd1 global knockout (KO) mice exhibited increases in baseline pain sensitivity to thermal and mechanical stimuli compared to wild-type mice; and furthermore, mouse DRG neurons from KO mice showed increased excitability and increased action potential frequency (G. Chen, et al., 2017). Administration of PD-L1 via intraplantar or intrathecal (spinal) injection is sufficient to increase the pain threshold in non-injured naïve mice (G. Chen, et al., 2017). Additionally, in situ hybridization and immunohistochemistry revealed Pdcd1 transcript and PD-1 protein expression in the neuroaxis of pain, including primary sensory neurons and spinal cord dorsal horn neurons (C. Jiang, et al., 2020; B. L. Liu, Cao, Zhao, Liu, & Zhang, 2020; Livni, et al., 2022; Meerschaert, et al., 2022; Wanderley, et al., 2022; L. Zhao, et al., 2022). Moreover, primary sensory neurons, especially peptidergic neurons, express PD-L1 (G. Chen, et al., 2017; Deng, et al., 2023; Meerschaert, et al., 2022). Mechanistically, PD-L1 produces antinociception in mice through phosphorylation of SHP-1 in DRG sensory neurons (G. Chen, et al., 2017; B. L. Liu, et al., 2020; L. Zhao, et al., 2022). Activation of SHP-1 in DRG neurons results in increased ERK activation, decreased function of sodium channels (e.g. Nav1.7) and TRPV1, and increased potassium channel activity (TREK2) in DRG neurons (Figure 1), as well as decreased excitatory synaptic transmission in spinal cord neurons and increased GABAergic neurotransmission in CNS neurons (G. Chen, et al., 2017; C. Jiang, et al., 2020; B. L. Liu, et al., 2020; X. Xiao, et al., 2015). PD-L1 may also modulate nociception through direct interaction with TRPV1 (Meerschaert, et al., 2022). Interestingly, a PD-1 binding peptide, H20, identified by in silico modeling, elicited more potent pain inhibition than PD-L1 in several mouse models through induction of SHP-1 phosphorylation and inhibition of nociceptive neuron activity (L. Zhao, et al., 2022).
Table 2.
Overview of immunotherapy targeting the PD-L1/PD-1 pathway in pain.
| Pain conditions and treatments |
Species | Effects | Mechanisms | References |
|---|---|---|---|---|
| Neuropathic pain PD-L1 | Mouse | Reduces pain | Reduce DRG neurons excitability and EPSC in spinal cord neurons | (G. Chen, et al., 2017) |
| Neuropathic pain Anti-PD-L1 | Mouse | Increases pain | Incudes higher expression of TNF-α, IL-6, and CX3CR1 in peripheral nervous tissue | (Wanderley, et al., 2022) |
| Neuropathic pain PD-1 peptide agonist | Mouse | Reduces pain | Suppresses neuronal excitability in DRGs | (L. Zhao, et al., 2022) |
| Neuropathic pain Loss of PD-L1 in KO | Mouse | Increases pain | Increases TNFα and MCP-1 levels in sciatic nerve | (Uceyler, et al., 2010b) |
| Inflammatory pain PD-L1 | Mouse | Reduces pain | Reduces DRG neurons excitability and EPSC in spinal cord neurons | (G. Chen, et al., 2017) |
| Inflammatory pain PD-L1 | Rat | Reduces pain | EA upregulates PD-L1 expression in peptidergic DRG neurons | (Deng, et al., 2023) |
| Inflammatory pain Anti-PD-1/PD-L1 | Rat | Increases pain | Increases IL-6 and iNOS levels in DRG | (Deng, et al., 2023) |
| Inflammatory pain PD-1 peptide agonist | Mouse | Reduces pain | Suppresses neuronal excitability in DRG | (L. Zhao, et al., 2022) |
| Migraine PD-1 inhibitor | Mouse | Increases pain | Upregulates CGRP, IL-1β, TNF-α, IL-6 and IL-18 in TG | (Shi, et al., 2020) |
| Maternal pain PD-L1 | Mouse | Prevents pain | Increased PD-L1 level in late pregnancy and decreased PD-L1 level after delivery | (H. Tan, et al., 2021) |
| Bone cancer pain PD-L1 | Rat | Reduces pain | Reduces DRG neurons excitability and EPSC in spinal cord neurons | (G. Chen, et al., 2017) |
| Bone cancer pain PD-L1 | Mouse | Reduces pain | Downregulate TRPV1 in DRG neurons and decreases neuronal excitability | (B. L. Liu, et al., 2020) |
| Melanoma cancer pain Anti-PD-1/PD-L1 | Mouse | Unmasks pain | Increases spontaneous discharges in nerve fibers and induce spontaneous pain | (G. Chen, et al., 2017) |
| Bone cancer pain Anti-PD-1 | Mouse | Reduces pain | Suppresses osteoclastogenesis and protects against bone destruction and bone fracture | (K. Wang, et al., 2020) |
| Knee osteoarthritis PD-1 | Human | Unknown | Exercise relieves knee pain and increases serum PD-1 | (J. Liu, et al., 2019) |
| Enterocolitis Anti-PD-1 | Human | Induces pain | Increases abdominal pain | (Kurokawa, et al., 2021) |
| Epididymo-orchitis Anti-PD-1 | Human | Induces headache | Amplifies the inflammatory manifestations | (Quach, et al., 2019) |
Figure 1. Immunotherapy targeting the PD-L1/PD-1 pathway in chronic pain through neuronal and immune modulation.
(A) Neuronal modulation: Activation of PD-1 in DRG sensory neurons by PD-L1 causes phosphorylation of SHP-1, leading to the activation of mu opioid receptor (MOR) and potassium channel TREK2 and inactivation of sodium channels (e.g. Nav1.7) and TRPV1. SHP-1 activation also inhibits ERK phosphorylation. Through the PD-1/SHP1-mediated signaling, PD-L1 potently inhibits physiological and pathological pain. (B) Immune modulation: In bone cancer pain, activation of PD-1 by PD-L1 on osteoclasts can promote osteoclastogenesis in cancer-bearing bone marrow, leading to CCL2 secretion and CCR2 activation on osteoclasts and nociceptors. Anti-PD-1 treatment with nivolumab inhibits bone cancer pain through suppression of osteoclastogenesis and bone destruction and reduced CCR2 signaling in nociceptors.
PD-1 also regulates opioid analgesia and opioid-induced adverse effects such as antinociceptive tolerance and hyperalgesia (Z. Wang, et al., 2020). Opioids are a mainstay treatment for postoperative pain and cancer pain, but also have harmful side effects including addiction and respiratory inhibition. Opioids inhibit pain in part by suppressing Ca2+ channels in primary sensory neurons and inhibiting neurotransmitter release (Ballantyne & Mao, 2003; Corder, Castro, Bruchas, & Scherrer, 2018; P. W. Mantyh, Clohisy, Koltzenburg, & Hunt, 2002). It was found that PD-1 may interact with mu opioid receptors (MORs) in sensory neurons, suggesting a crosstalk between these pain inhibitory systems. Morphine’s inhibitory effects, including analgesia, suppression of Ca2+ currents in DRG neurons, and suppression of spinal cord synaptic transmission required PD-1 expression and reduced in Pdcd1 KO mice. Strikingly, PD-1 interaction with opioid receptors can be validated in nonhuman primates, as intrathecal morphine-induced antinociception is largely blocked by anti-PD-1 treatment (Z. Wang, et al., 2020).
PD-L1 and PD-1 levels are also regulated by the process causing the pain or hyperalgesia (Table 2). In female mice, PD-L1 levels were increased in late-pregnancy but decreased following delivery, and furthermore, PD-L1 is required for pregnancy-induced analgesia (H. Tan, et al., 2021). Electroacupuncture alleviated inflammatory pain via upregulation of the PD-L1/PD-1/SHP-1 pathway in DRG neurons of rats (Deng, et al., 2023). In patients with osteoarthritis, different exercise modalities (e.g., Tai Chi, Baduanjin, stationary cycling) relieved arthritic pain and increased serum PD-1 levels (J. Liu, et al., 2019). Further investigation is needed to test whether this upregulation serves as a biomarker or has causative role in pain. Taken together, these findings indicate that the PD-L1/PD-1 axis may act as a checkpoint to pain (Hirth, Gandla, & Kuner, 2017).
3.3. PD-1 and PD-L1 based immunotherapies and cancer pain
PD-1 immunotherapy involves the use of monoclonal antibodies that target the PD-1 receptor or its ligand, PD-L1, to restore the anti-tumor activity of T cells (Tumeh, et al., 2014). These antibodies bind to PD-1 (Nivolumab, Pembrolizumab, Cemiplimab, Sintilimab and Camrelizumab) or PD-L1 (Atezolizumab, Durvalumab, Avelumab and Cemiplimab) and block their interaction, which allows T cells to be re-activated and attack cancer cells (Bagchi, Yuan, & Engleman, 2021; Carlino, Larkin, & Long, 2021). PD-1/PD-L1 immunotherapies have shown remarkable clinical success in the treatment of various cancer types, including melanoma, non-small cell lung cancer, and bladder cancer, among others. However, only a portion of patients responds to PD-1 immunotherapy, and resistance mechanisms have been identified, including the upregulation of alternative immune checkpoint molecules and alterations in the tumor microenvironment. Ongoing research is focused on identifying biomarkers that can predict response to PD-1/PD-L1 immunotherapy as well as developing combination therapies that can enhance the effectiveness of PD-1 blockade (Chow, et al., 2019; Doroshow, et al., 2021; Dudley, Lin, Le, & Eshleman, 2016; M. Yi, et al., 2018).
PD-L1 was found to mask cancer pain in a mouse model of melanoma. Notably, hindpaw inoculation of melanoma resulted in robust tumor growth but did not elicit signs of cancer pain in 4 weeks (G. Chen, et al., 2017). Cancer pain in this model was masked by melanoma-induced PD-L1. Thus, blockade of PD-1/PD-L1 signaling evoked robust spontaneous pain with mice licking and flinching the melanoma-bearing hindpaw. In vivo recordings of mouse sciatic nerve showed that nivolumab treatment significantly increased the spontaneous firing of nerve fibers, suggesting that anti-PD-1 treatment can enhance pain sensitivity by increasing the excitability of primary afferent fibers. Interestingly, the increased pain sensitivity was observed only in the acute phase (first 3 hours) after PD-1/PD-L1 blockade, and not in the late phase (24 hours), suggesting that the pain inducing effect by nivolumab may be short-lived. This acute treatment did not change the levels of T cell markers (CD2, CD3), a macrophage marker (CD68), and inflammatory cytokine markers (TNF, IL-1β, IL-6, IFN-γ, CCL2), suggesting that PD-1 signaling masks pain via unconventional neuronal modulation (G. Chen, et al., 2017). It will be of great interest to investigate whether PD-L1 released from sensory neurons contributes to the development of melanoma. A recent study demonstrated that nociceptive neurons promote melanoma development via suppressing T cell immunity in mice (Balood, et al., 2022).
PD-L1/PD-1 signaling was also found to be involved in a mouse model of bone cancer pain, induced by femoral inoculation of Lewis Lung carcinoma cells. Bone cancer pain typically results from metastatic cancer cells reaching the bone, leading to the formation of osteolytic bone lesions and fractures (Andriessen, Donnelly, & Ji, 2021; P. Mantyh, 2013). PD-L1 can promote osteoclastogenesis in cancer-bearing bone marrow through activation of JNK (c-Jun N-terminal kinase, a MAPK family member) and subsequent secretion of the chemokine (C-C motif) ligand 2 (CCL2). Anti-PD-1 treatment was found to transiently increase bone cancer pain in the early phase of treatment due to neuronal modulation. However, in the late phase of treatment, Anti-PD1 treatment persistently reduced bone cancer pain through the suppression of osteoclastogenesis and protection from bone destruction (K. Wang, et al., 2020). Thus, the effects of anti-PD-1 treatment on bone cancer pain may depend on both neuromodulation and immunomodulation (Figure 1). Overall, immunotherapy targeting the PD-L1/PD-1 pathway has the potential to produce long-term benefits by preserving bone structure and alleviating bone cancer pain. However, further research is needed to fully understand the mechanisms underlying these effects and to optimize the ideal time window of the treatment (e.g. right before and during bone metastasis).
4. Immunotherapies targeting the cGAS-STING pathway in chronic pain
4.1. STING signaling in different cell types
Stimulator of interferon genes (STING) is also known as transmembrane protein 173 (TMEM173). It was first identified in 2008 as a critical mediator of innate immune responses to viral and bacterial infections (Ishikawa & Barber, 2008). STING is a transmembrane protein expressed in various cell types, including immune cells such as dendritic cells, macrophages, and T cells, as well as microglia, fibroblasts, epithelial cells, and neurons (Q. Chen, Sun, & Chen, 2016; Donnelly, et al., 2021a; M. Jin, et al., 2021; Mosallanejad & Kagan, 2022). The STING protein has four domains: an N-terminal transmembrane domain, a cytoplasmic domain, a linker domain, and a C-terminal domain. The N-terminal domain contains four tandem repeats of a conserved sequence motif called the STING domain. The cytoplasmic domain of STING contains a binding site for cyclic GMP-AMP (cGAMP), produced by activation of cGAMP synthase (cGAS), and the cytoplasmic C-terminal tail of STING interacts with downstream signaling molecules (H. Liu, et al., 2019; X. Zhang, Bai, & Chen, 2020). In addition to infections, STING is induced by DNA damage and cellular stress. In its inactive state, STING is primarily localized to the endoplasmic reticulum. Upon activation, STING translocates to the Golgi complex and further translocation to perinuclear vesicles is key for its function in the cGAS-STING pathway (W. Sun, et al., 2009). Upon binding to double-stranded DNA, the enzyme cGAS catalyzes the production of cGAMP, which functions as a second messenger and activates the STING pathway (Figure 2). Upon binding to cGAMP, STING undergoes a conformational change that activates the TANK-binding kinase 1 (TBK1) and the inhibitor of nuclear factor kappa-B kinase subunit epsilon (IKKε) (Gui, et al., 2019; C. Zhang, et al., 2019). TBK1 and IKKε then phosphorylate the transcription factor interferon regulatory factor 3 (IRF3), which triggers the expression of Type I interferons (IFN-I: IFN-α and IFN-β) that initiate an immune response to combat viral infections. Activation of the STING pathway also leads to the production of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) via the activation of nuclear factor-kappa B (NF-κB) (Q. Guo, et al., 2021; Messaoud-Nacer, et al., 2022; Yum, Li, Fang, & Chen, 2021). These cytokines play an essential role in the recruitment and activation of immune cells to the site of infection, aiding in the clearance of the invading pathogen. In addition to viral infections, STING has been implicated in autoimmune and neurodegenerative diseases (Fritsch, Kelly, & Pickrell, 2023).
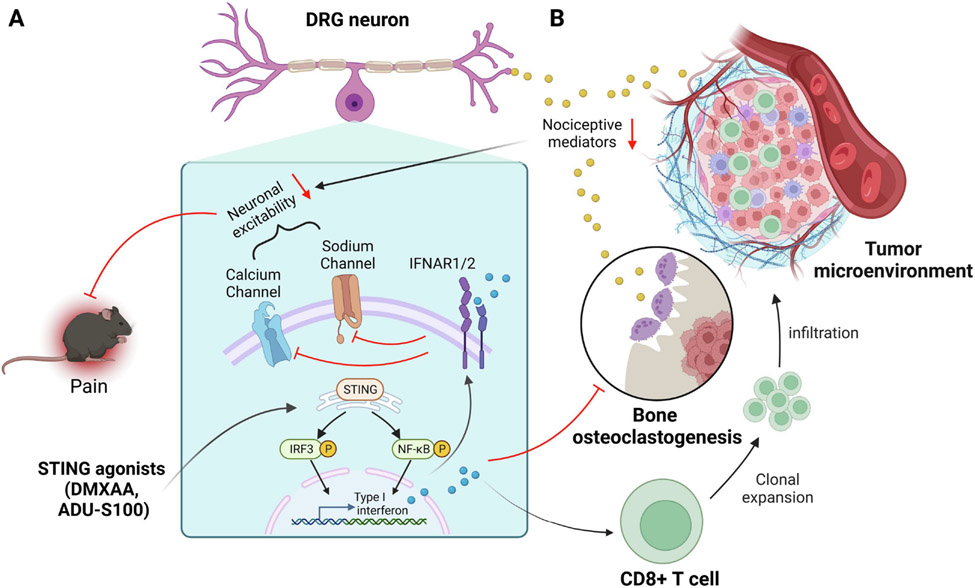
Figure 2. Immunotherapy targeting the STING pathway in chronic pain through neuronal and immune modulation.
(A) Neuronal modulation: Activation of the STING pathway by STING agonists DMXAA and ADU-S100 in DRG neurons and non-neuronal cells result in the production of Type I interferons (IFN-I, IFN-α/β). IFN-I activates its receptor IFNAR1/2 on sensory neurons to suppress the activity of calcium channels and sodium channels, leading to decreased neuronal excitability and pain. (B) Immune modulation: In bone cancer pain, IFN-α/β can also act on osteoclasts to suppress osteoclastogenesis. Treatment with STING agonists inhibits bone cancer pain through suppression of osteoclastogenesis and bone destruction, as well as activation of CD8+ T cells to reduce cancer burden.
4.2. STING signaling in acute and chronic pain
Increasing evidence points to a role of STING in regulating physiological and pathological pain. STING has been identified as a critical regulator of nociception through IFN-I signaling in DRG sensory neurons. Studies have shown that administration of synthetic (DMXAA and ADU-S100) or biological (cGAMP) STING agonists can increase mechanical pain thresholds in uninjured and neuropathic pain mice and produce analgesia in nonhuman primates. Conversely, mice lacking STING displayed enhanced pain hypersensitivity and heightened nociceptor excitability. Activation of STING drives the production of IFN-I family members IFN-α and IFN-β. Mice lacking IFN-I receptor (IFNAR1), including global (Ifnar1gt/gt) and sensory neuron-selective (Ifnar1fl/fl;Nav1.8-Cre) knockout mice, exhibited robust nociceptor hyperactivity. Mechanistically, IFN-I was found to suppress sodium channel activity in DRG neurons and EPSCs in spinal cord neurons (Donnelly, et al., 2021b; P. H. Tan, Ji, Yeh, & Ji, 2021). Therefore, STING signaling through IFN-I serves as a critical regulator of nociception and a promising target for immunotherapy to treat chronic pain.
As a pro-inflammatory signaling molecule, STING activation may also activate NF-κB signaling and produce pro-inflammatory cytokines and chemokines to trigger pro-nociceptive signaling. It was found that spared nerve injury (SNI) leads to a significant increase in double-stranded DNAs, which can activate the STING/TBK1/NF-κB pathway (J. Sun, et al., 2022). Intrathecal administration of STING antagonist C-176 in the early phase (1st week of nerve injury), but not at later time points can attenuate SNI-induced pain hypersensitivity, microglial activation, release of proinflammatory factors, and JAK2/STAT3 phosphorylation in the spinal cord dorsal horn (J. Sun, et al., 2022). However, the analgesic effect of C-176 is greatly reduced in the presence of recombinant IL-6 following SNI surgery, suggesting the involvement of this cytokine in the mechanism of action of C-176 (Wu, et al., 2022). Thus, the STING pathway may differentially regulate neuropathic pain via distinct signaling mechanisms (IFN-I vs. IL-6). Notably, at high doses, IFN-I may also trigger pronociceptive signaling (Barragan-Iglesias, et al., 2020; P. H. Tan, et al., 2021).
4.3. STING based immunotherapies
Tumor cells often have genomic instability that causes the release of endogenous DNA into the cytoplasm. The resulting cGAMP or DNA can activate the cGAS-STING pathway, leading to the production of IFN-Is, pro-inflammatory cytokines, and chemokines that attract immune cells such as T cells, natural killer cells, and dendritic cells to the tumor microenvironment (Corrales, et al., 2015; Li & Chen, 2018; Xia, Konno, Ahn, & Barber, 2016). This immune response can also stimulate the activation, maturation, and differentiation of T cells, leading to adaptive immune responses. Activation of the cGAS-STING pathway in cancer cells also triggers programmed cell death, or apoptosis, contributing to the elimination of cancer cells (Woo, Corrales, & Gajewski, 2015). Thus, the targeting of the cGAS-STING pathway has shown great potential for anti-tumor immunotherapy. Several clinical trials are currently underway to evaluate the effectiveness of cGAS-STING pathway-targeted immunotherapies for cancer treatment, such as STING agonists ADU-S100 (MIW815) (NCT02675439), MK-1454 (NCT03010176), GSK3745417 (NCT03843359), and E7766 (NCT04144140) (Chang, et al., 2022; K. C. Huang, et al., 2022; Le Naour, Zitvogel, Galluzzi, Vacchelli, & Kroemer, 2020; Messaoud-Nacer, et al., 2022). These trials aim to test the efficacy of these agents in patients with advanced solid tumors or lymphomas. These clinical trials will provide crucial insights into their effectiveness against cancer.
Activation of the STING pathway by synthetic agonists such as DMXAA and ADU-S100 has been shown to enhance anti-tumor immunity and promote bone formation in murine bone autoimmune disease models (Baum, et al., 2017; Corrales, et al., 2015). Additionally, STING activation can be used as a treatment for bone cancer pain with metastasis. STING-mediated IFN-I signaling directly controls bone cancer pain via neuromodulation, resulting in rapid pain inhibition by decreasing the neuronal excitability of mouse DRG neurons (K. Wang, et al., 2021). Moreover, STING-mediated IFN-I signaling also suppressed cancer-induced osteoclastogenesis, inhibiting osteoclast-mediated bone destruction that elicits severe pain (K. Wang, et al., 2020). Finally, in mouse bone cancer, systemic administration of STING activator DMXAA increased the proportion of CD8+ T cells in the bone marrow tumor microenvironment, inhibiting tumor growth. Thus, STING agonists can have immunomodulatory and neuromodulatory effects, which additively or synergistically suppress cancer pain (Figure 2). Studies have also shown that STING activation plays a critical role in driving bone cancer pain in mice through TBK1/NF-κB-mediated induction of IL-1β, IL-6, and TNF-α in the DRG, and STING inhibitor C-176 significantly alleviated hyperalgesia in the bone cancer pain model (Y. Zhang, et al., 2023). Overall, these findings suggest that STING may play different roles in regulating bone cancer pain through distinct regulations of gene expression (IFN-I vs. IL-1β, IL-6, and TNF-α) by STING activators and inhibitors under different conditions.
5. Cell-based immunotherapies in pain management
The immune system consists of innate and adaptive immune system. Increasing number of studies are pointing towards a potential role of immune cells in pain management. Notably, immune cells, such as T cells, macrophages, and neutrophils have been shown to be both pronociceptive and anti-nociceptive, contributing to the induction and resolution of pain in a context-dependent manner (Ji, et al., 2016).
5.1. Macrophages in the development and resolution of pain
It is well established that crosstalk between macrophages, including tissue-resident macrophages in DRGs, and sensory neurons contributes to the induction and maintenance of inflammatory and neuropathic pain (O. Chen, Donnelly, & Ji, 2019). Nerve injury and chemotherapy cause significant increases in macrophage numbers in DRGs. The causative role of macrophages in promoting pain was examined by pharmacological and genetic ablations of macrophages in neuropathic pain models. Ablation of DRG macrophages with clodronate reduced mechanical allodynia in a nerve injury model of neuropathic pain in mice (Yu, et al., 2020). In a mouse model of rheumatoid arthritis, CX3CR1 expressing monocytes/macrophages in the DRG contribute to arthritis pain (Oggero, et al., 2022). DRG macrophages were also shown to maintain osteoarthritis pain in mice (Raoof, et al., 2021). Macrophages drive chronic pain by producing various pro-inflammatory cytokines/chemokines, such as TNF-α, IL-6, IL-17, and CXCL1, which can interact with nociceptor neurons to modulate their activity (Luo, et al., 2021; X. Luo, et al., 2019; Oggero, et al., 2022). In addition to pathological pain, macrophages also play a role in physiological pain. Dermal macrophages were shown to regulate baseline pain sensitivity by modulating the production of nerve growth factor, which is required for setting normal pain sensitivity (Tanaka, et al., 2023).
Recent studies indicate that macrophages also regulate the resolution of inflammatory and neuropathic pain following inflammation and nerve injury. One study demonstrated that macrophages from the spinal cord of sham surgery animals had increased expression of the anti-inflammatory mediator CD163 and alleviated nociceptive hypersensitivity via the cytokine IL-10, compared to macrophages from animals with nerve injury. Furthermore, neuropathic pain symptoms (mechanical hypersensitivity) could be reversed by increasing macrophage CD163 expression (Niehaus, Taylor-Blake, Loo, Simon, & Zylka, 2021).
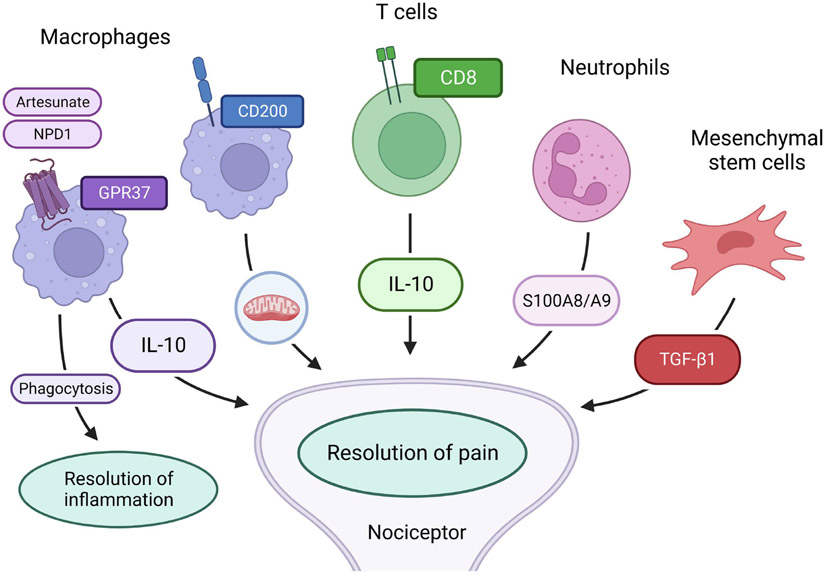
GPR37 was recently implicated as a potential receptor for neuroprotectin D1 (NPD1), a SPM derived from fish oil. GPR37 in macrophages contributes to the resolution of inflammatory pain and infection-induced pain (Q. Zhang, Bang, Chandra, & Ji, 2022). In GPR37 knockout mice, macrophages have deficits in phagocytic activity, a major function of macrophages for the resolution of inflammation. GPR37-deficient macrophages also displayed dysregulations of pro- or anti-inflammatory cytokines, switching to the pro-inflammatory M1 phenotype and delaying the resolution of zymosan-induced inflammatory pain (Bang, et al., 2018). A partial depletion of dermal macrophages delayed the resolution of zymosan-induced inflammatory pain, and conversely, adoptive transfer of wild-type macrophages facilitated inflammatory pain resolution (Bang, et al., 2018). Intriguingly, the anti-malarial drug artesunate can act as a ligand of GPR37. Activation of GPR37 by artesunate enhanced the resolution of bacterial infection-induced pain. Adoptive transfer of macrophages primed with GPR37 activators could protect against infections induced by listeria and parasites, providing a new mechanism of action of artesunate as an immunotherapy (Bang, et al., 2021; Q. Zhang, et al., 2022). Thus, specific targeting of macrophage-bound GPR37 with its agonists may serve as an immunotherapy to control pain and other inflammatory disorders (Figure 3).
Figure 3. Cell-based immunotherapies for the resolution of chronic pain.
Adoptive transfer of GPR37+ macrophages, CD8+ T cells, neutrophils, and mesenchymal stem/stromal cells can promote pain resolution via interactions with nociceptor through secretion of anti-inflammatory and anti-nociceptive mediators such as IL-10, TGF-β1, and S100A8/A9 or mitochondria transfer. Activation of macrophage GPR37 by neuroprotectin D1 (NPD1) or artisunate, an anti-malaria, can enhance macrophage phagocytosis of cell debris and pathogens to promote the resolution of inflammation and pain.
Recent research efforts also point to mitochondrial transfer from macrophages and neurons as a novel mechanism for pain resolution. Inflammation impairs mitochondrial function in neurons. Notably, M2-like macrophages infiltrate the DRG during the resolution phase of inflammatory pain resolution. This infiltration in the resolution phase is correlated with sensory neuron’s recovery of mitochondrial function (e.g., oxidative phosphorylation). Mechanistically, the resolution of inflammatory pain by mitochondrial transfer requires the expression of CD200 receptor by macrophages and CD200R-ligand iSec1 by sensory neurons (van der Vlist, et al., 2022).
5.2. T cells in the development and resolution of pain
Early studies evaluated the pronociceptive roles of T cells by comparing pain behaviors between wild-type mice and mice with different types of T cell deficiencies, including Rag1−/−, Rag2−/−, nude and SCID mice. Notably, there is no significant difference in baseline pain sensitivity between wild type and T cell-deficient mice (Cao & DeLeo, 2008; Luo, et al., 2021). In neuropathic pain models, T cells infiltrated the nervous system after nerve injury, mainly in damaged nerves and DRGs, and some in spinal cord, contributing to neuropathic pain (Costigan, Moss, et al., 2009; Singh, et al., 2022; Sorge, et al., 2015). It was also found that T cell ablation in nerve injury models resulted in reduced neuropathic pain symptoms (e.g., mechanical allodynia) (Costigan, Moss, et al., 2009; Kleinschnitz, et al., 2006). Mice lacking T cells (Rag1−/− or Rag2−/− KO mice) exhibited deficits in the development of neuropathic pain (Cao & DeLeo, 2008). In contrast, depletion of Foxp3+ Treg cells promotes neuropathic pain hypersensitivity, partially by increasing the concentration of RANTES, IL-2 and IL-5, and significant decreasing IL-12 and IFN-γ (Lees, Duffy, Perera, & Moalem-Taylor, 2015).
T cells have also been shown to regulate the resolution of chronic pain (Kavelaars & Heijnen, 2021). In paclitaxel or cisplatin-induced CIPN models, Rag1−/− or Rag2−/− mice developed neuropathic pain like wild-type mice, however, the resolution of neuropathic pain was prolonged in these T cell deficient mice. Importantly, reconstitution of CD8+ T cells was sufficient to resolve pain in these T cell-deficient CIPN mice by secretion of IL-10 (Krukowski, et al., 2016; Singh, et al., 2022) (Figure 3). A similar mechanism also applies to inflammatory pain, and the severity of complete Freund’s adjuvant (CFA)-induced mechanical allodynia was identical in wild-type and T cell-deficient mice, but the recovery of inflammatory pain was impaired in mutant mice (Laumet, Edralin, Dantzer, Heijnen, & Kavelaars, 2019; Petrovic, et al., 2019). Adoptive transfer of CD8+ T cells was shown to resolve neuropathic pain after chemotherapy (Kavelaars & Heijnen, 2021). Adoptive transfer of regulatory T cells (Tregs) via intrathecal administration alleviated neuropathic pain in mice (X. J. Liu, et al., 2014). One clinical study revealed that transfusion of immune cells in patients with advanced cancer is associated with reduced opioid consumption and pain intensity due to the activation of CD4+ T lymphocytes and increased production of endogenous opioids (X. Zhou, et al., 2020). Finally, in the recent human genome-wide association study, partitioned heritability identified enrichment for B and T cells specific genes that are associated with chronic postoperative pain (Parisien, et al., 2023). In both the plantar incision and the laparotomy mouse models, the prolonged duration of mechanical allodynia in Rag1−/− mice compared to wild-type mice was observed. Adoptive transfer of T cells did not significantly affect the time course of mechanical allodynia in Rag1−/− mice, however, B cell supplementation, or combined B and T supplementation, significantly shortened the duration of post-surgery allodynia (Parisien, et al., 2023).
5.3. Neutrophils in the development and resolution of pain.
Neutrophils make up the majority (~60%) of white blood cells in human and act as the body's first line of defense to eliminate infections by bacteria and fungi. During tissue injury and inflammation, neutrophils are the first responders, and neutrophil infiltration serves as a cardinal sign of acute inflammation. Following zymosan-induced inflammation, neutrophils were found to be accumulated in inflamed hind paw within hours (Bang, et al., 2018). Neutrophils contribute to the development of inflammatory, postoperative, and neuropathic pain (Ghasemlou, Chiu, Julien, & Woolf, 2015). Plantar incision causes CXCL1 upregulation leading to neutrophil accumulation and postoperative pain via activation of CXCR1/2 receptor. Additionally, depletion of neutrophils reduced immediate postsurgical pain in mice (Carreira, et al., 2013). Neutrophils release multiple pro-nociceptive enzymes, such as elastase, and inhibition of leuckocyte elastase was found to reduce neuropathic pain in rodents (Bali & Kuner, 2017). Interestingly, it was found that mice that are protected from neuropathic pain express SerpinA3N, a serine protease inhibitor secreted in response to nerve damage by DRG neurons that counteracts the induction of neuropathic pain (Vicuna, et al., 2015).
Recent research also demonstrated a critical role of neutrophils in the resolution of acute pain and prevention of pain chronification. Depletion of neutrophils delayed the resolution of CFA-induced inflammatory pain, and this inflammatory pain can further be extended for several months in animals treated with steroids (dexamethasone for 6 days) or nonsteroidal anti-inflammatory drugs (NSAIDs) at the acute pain stage. Conversely, adoptive transfer of neutrophils is sufficient to confer protection against the development of steroid-induced chronic pain in the CFA model (Parisien, et al., 2022). Mechanistically, neutrophils resolve pain by secretion of S100A8/A9 proteins. These are Ca2+ binding proteins of the S100 family and participate in inflammatory process (Figure 3). Finally, in patients with low back pain, transient upregulations of neutrophil-driven inflammatory responses is protective against the transition to chronic pain (Parisien, et al., 2022). Conversely, the adoptive transfer of neutrophils but not other blood cells from fibromyalgia patients created a state of prolonged hyperalgesia in naïve mice (Caxaria, et al., 2023), suggesting that neutrophils can be both beneficial and detrimental.
5.4. Mesenchymal stem cells (MSCs) in the resolution of pain
Regenerative pain medicine uses the body's own recovery mechanisms to heal pain (Buchheit, Huh, Maixner, Cheng, & Ji, 2020). Mesenchymal stem cells, also known as mesenchymal stromal cells (MSCs) or bone marrow stem cells (BMSCs) are present in nearly all organs in the perivascular space and are most prominent in the bone marrow with their ability to differentiate into other mesodermal cells, including cartilage, fat, muscle, and bone cells. Studies from many laboratory studies have shown analgesic efficacy of MSCs in different animal models of pain (Huh, Ji, & Chen, 2017). However, there is not clear evidence of tissue regeneration after MSC treatment. Instead, several lines of evidence indicate that MSCs resolve pain via neuroimmune modulation (Buchheit, et al., 2020).
Systemic or local injection of rat MSCs were shown to reverse long-lasting inflammatory pain in rats following a tendon injury (W. Guo, et al., 2011). This effect appears to depend on endogenous opioid production, as the opioid receptor antagonist naloxone can block the analgesia by MSCs in injured rats. Moreover, the same group found that the activation of monocytes is required for the analgesic actions of MSCs (W. Guo, et al., 2017). It was also found that a single intrathecal injection via lumbar puncture of 250,000 BMSCs resulted in long-term relief of neuropathic pain (mechanical allodynia and heat hyperalgesia) for more than six weeks in mouse models of neuropathic pain after nerve injury (G. Chen, Park, Xie, & Ji, 2015). Further analysis revealed migration of the intrathecally injected BMSCs to DRG and the spinal cord meninges, which are the membranes that cover the spinal cord and part of DRG and containing cerebrospinal fluid (CSF), via chemotaxic signaling mediated by the CXCL12/CXCR4 axis (G. Chen, et al., 2015). Importantly, BMSCs secrete the anti-inflammatory cytokine TGF-β1 to exert the antinociceptive actions in animal models of neuropathic pain. Intrathecal BMSCs potently reduced nerve injury-induced neuroinflammation in the spinal cord, by inhibiting the activation of microglia and astrocytes and the production of inflammatory cytokines and chemokines (G. Chen, et al., 2015) (Figure 3). MSC treatment also reversed anti-nociceptive tolerance and hyperalgesia induced by chronic opioid exposure (Hua, et al., 2016). Intra-articular injections of autologous MSCs alleviated pain in patients with osteoarthritis (Harrell, Markovic, Fellabaum, Arsenijevic, & Volarevic, 2019). Therefore, MSC transplantation has great potential to resolve chronic pain in patients as an immunotherapy.
6. Time-dependent contribution of immune pathways to pain states
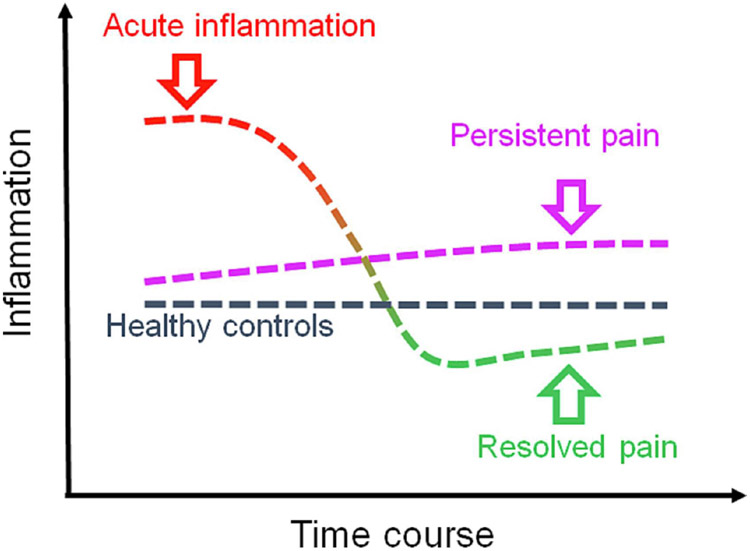
The contribution of inflammatory response to chronic pain progression or resolution appears to be a complex process involving multiple cell types and pathways. In normal pain resolution process, these components act concordantly in a time-dependent manner (Figure 4, “Resolved pain” trajectory). Strong acute inflammatory response appears to be necessary to launch subsequent reparative steps leading to pain resolution in these patients (Parisien, et al., 2022). This inflammatory response coincides with neutrophil and monocyte activation. In such patients, acute inflammatory response is followed by significant decrease of inflammation and pain resolution over time. In other patients (Figure 4, “Persistent pain” trajectory), the absence of strong acute inflammatory response leads to persistent low-grade inflammation which continues to maintain chronic pain. This persistent pain group is always present with a low-grade inflammatory state characteristic of chronic painful conditions.
Figure 4. Conceptual model of chronic pain resolution following acute inflammation.
The overall dynamics of the inflammatory response is critical for pain resolution, but this inflammatory response is not a simple increase or decrease over the disease progression. After transitory pain period, pain in some patients resolves, the “Resolved pain” group (red-to-green line), whereas for others the pain persists, the “persistent pain” group (purple line). During the first phase, the inflammatory response in the resolved pain group is stronger than that in the persistent pain group. Over time, the inflammatory response substantially lessened in patients with resolved pain.
This conceptual model implies the important role of acute inflammation phase and subsequent anti-inflammatory processes in chronic pain resolution. Immune therapy strategies should be tailored to facilitate the recovery process depending on the phase of inflammation. Reducing inflammation too early may delay pain resolution.
7. Conclusions and future directions
Immunotherapy is recognized as a recent medical breakthrough in cancer therapy and has been awarded a Nobel Prize in Physiology or Medicine (James Allison and Tasuku Honjo, 2018). Mounting evidence also highlights emerging immunotherapies in treating autoimmune and neurodegenerative diseases, including chronic pain. The mechanisms underlying the induction and resolution of acute and chronic pain are complex and context-dependent. Thus, the potential of immunotherapy for pain management should address the multi-faceted mechanisms of chronic pain (Junli Zhao, Roberts, Huh, & Ji, 2023). Both agonists and antagonists (e.g. PD-L1/PD-1 and STING) could be beneficial if we can identify specific pain conditions and time window of the treatment. Control of abnormal inflammation in patients with autoimmune conditions could be achieved by monoclonal antibodies targeting pro-inflammatory cytokines (Table 1). However, immune suppression by steroid and anti-inflammatory treatments can delay the resolution process for inflammation and pain (Parisien, et al., 2022). Some chronic pain may be associated with immune suppression, and immune activation may promote the resolution of pain. Immune activation could also be achieved by cell therapies, such as mesenchymal stromal cells, macrophages, and T cells, as well as cell-free blood products (Buchheit, et al., 2020; 2023; W. B. S Zhou, et al., 2023). Given the current crises of chronic pain and opioid use disorders (Volkow & Collins, 2017), ongoing and future research in connecting immunotherapy and pain and targeting neuroimmune interactions will hold great promise in the management and final resolution of chronic pain.
Acknowledgments
The figures are prepared with BioRender with license. This study was supported by Duke University Anesthesiology Research Funds and partially supported by NIH R01 grant DE17794 and DoD grants W81XWH2110885 and W81XWH2110752.
Abbreviations
- BMSC
bone marrow stromal/stem cells
- CCL2
(C-C motif) ligand 2
- CFA
complete Freund’s adjuvant
- cGAMP
cyclic GMP-AMP
- cGAS
cyclic GMP-AMP synthase
- CIPN
chemotherapy-induced peripheral neuropathy
- CRPS
complex regional pain syndrome
- CNS
central nervous system
- CTLA-4
cytotoxic t-lymphocyte antigen-4
- CSF
cerebrospinal fluid
- DRG
dorsal root ganglia
- ER
endoplasmic reticulum
- ERK
extracellular-signal regulated kinases
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IFN-I
type-I interferon
- IFN-α
interleukin-1 alpha
- IFN-β
interleukin-1 beta
- IFNAR1
interferon alpha and beta receptor subunit 1
- IL-1β
interleukin-1 beta
- IL-4
interleukin 4
- IL-6
interleukin-6
- IL-10
interleukin 10
- IL-12
interleukin 12
- IL-17
interleukin-17
- ITIM
immune receptor tyrosine-based inhibitory motif
- ITSM
immunoreceptor tyrosine-based switch motif
- LAG-3
lymphocyte-activation gene 3
- JAK
Janus kinase (JAK)
- JNK
c-Jun N-terminal kinase
- KO
knockout
- MAPK
mitogen-activated protein kinase
- MOR
mu opioid receptor
- MSC
mesenchymal stem cells
- NPD1
neuroprotectin D1
- PD-1
programed death protein 1
- PD-L1
programed death protein ligand 1
- PKA
protein kinase A
- PKC
protein kinase C
- PNS
peripheral nervous system
- sEPSC
spontaneous excitatory postsynaptic currents
- SHP-1/2
Src homology region 2 domain-containing phosphatase-1/2
- sIL-6R
soluble form of the IL-6 receptor
- SNI
spared nerve injury
- SPM
specialized pro-resolving mediator
- STAT
signal transducer and activator of transcription
- STING
stimulator of interferon gene
- TBK1
TANK-binding kinase 1
- TCR
T cell receptors
- TG
trigeminal ganglia
- TGF-β
transforming growth factor beta
- TMEM173
transmembrane protein 173
- TNF-α
tumor necrosis factor alpha
- TRPA1
transient receptor potential ion channel subtype A1
- TRPV1
transient receptor potential ion channel subtype V1
Footnotes
Declaration of Competing Interest
The authors have no competing financial interest in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitken D, Laslett LL, Pan F, Haugen IK, Otahal P, Bellamy N, Bird P, & Jones G (2018). A randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis - the HUMOR trial. Osteoarthritis Cartilage, 26, 880–887. [DOI] [PubMed] [Google Scholar]
- Ali T, Kaitha S, Mahmood S, Ftesi A, Stone J, & Bronze MS (2013). Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf, 5, 79–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Bogen O, & Levine JD (2019). Interleukin 6 decreases nociceptor expression of the potassium channel KV1.4 in a rat model of hand-arm vibration syndrome. Pain, 160, 1876–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriessen AS, Donnelly CR, & Ji RR (2021). Reciprocal interactions between osteoclasts and nociceptive sensory neurons in bone cancer pain. Pain Rep, 6, e867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmaramani RR, Black BJ, de la Pena JB, Campbell ZT, & Pancrazio JJ (2020). Conserved Expression of Nav1.7 and Nav1.8 Contribute to the Spontaneous and Thermally Evoked Excitability in IL-6 and NGF-Sensitized Adult Dorsal Root Ganglion Neurons In Vitro. Bioengineering (Basel), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, Deodhar A, Porter B, Martin R, Andersson M, Mpofu S, Richards HB, Group, M. S., & Group, M. S. (2015). Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med, 373, 2534–2548. [DOI] [PubMed] [Google Scholar]
- Bagchi S, Yuan R, & Engleman EG (2021). Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol, 16, 223–249. [DOI] [PubMed] [Google Scholar]
- Bali KK, & Kuner R (2017). Therapeutic potential for leukocyte elastase in chronic pain states harboring a neuropathic component. Pain, 158, 2243–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne JC, & Mao J (2003). Opioid therapy for chronic pain. N.Engl.J.Med, 349, 1943–1953. [DOI] [PubMed] [Google Scholar]
- Bally AP, Austin JW, & Boss JM (2016). Genetic and Epigenetic Regulation of PD-1 Expression. J Immunol, 196, 2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balood M, Ahmadi M, Eichwald T, Ahmadi A, Majdoubi A, Roversi K, Roversi K, Lucido CT, Restaino AC, Huang S, Ji L, Huang KC, Semerena E, Thomas SC, Trevino AE, Merrison H, Parrin A, Doyle B, Vermeer DW, Spanos WC, Williamson CS, Seehus CR, Foster SL, Dai H, Shu CJ, Rangachari M, Thibodeau J, S VDR, Drapkin R, Rafei M, Ghasemlou N, Vermeer PD, Woolf CJ, & Talbot S (2022). Nociceptor neurons affect cancer immunosurveillance. Nature, 611, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Donnelly CR, Luo X, Toro-Moreno M, Tao X, Wang Z, Chandra S, Bortsov AV, Derbyshire ER, & Ji RR (2021). Activation of GPR37 in macrophages confers protection against infection-induced sepsis and pain-like behaviour in mice. Nat Commun, 12, 1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, & Ji RR (2018). GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest, 128, 3568–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan-Iglesias P, Franco-Enzastiga U, Jeevakumar V, Shiers S, Wangzhou A, Granados-Soto V, Campbell ZT, Dussor G, & Price TJ (2020). Type I Interferons Act Directly on Nociceptors to Produce Pain Sensitization: Implications for Viral Infection-Induced Pain. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum R, Sharma S, Organ JM, Jakobs C, Hornung V, Burr DB, Marshak-Rothstein A, Fitzgerald KA, & Gravallese EM (2017). STING Contributes to Abnormal Bone Formation Induced by Deficiency of DNase II in Mice. Arthritis Rheumatol, 69, 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens F, Tak PP, Ostergaard M, Stoilov R, Wiland P, Huizinga TW, Berenfus VY, Vladeva S, Rech J, Rubbert-Roth A, Korkosz M, Rekalov D, Zupanets IA, Ejbjerg BJ, Geiseler J, Fresenius J, Korolkiewicz RP, Schottelius AJ, & Burkhardt H (2015). MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann Rheum Dis, 74, 1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lu N, Liu YC, & Ji RR (2014). Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J Clin.Invest, 124, 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, & Volterra A (2001). CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci, 4, 702–710. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, & Samad TA (2008). Nociceptors are interleukin-1beta sensors. J.Neurosci, 28, 14062–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheit T, Huh Y, Maixner W, Cheng J, & Ji RR (2020). Neuroimmune modulation of pain and regenerative pain medicine. J Clin Invest, 130, 2164–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheit T, Huh Y, Breglio A, Bang S, Xu J, Matsuoka Y, Guo R, Bortsov A, Reinecke J, Wehling P, Huang TJ, & Ji RR (2023). Intrathecal administration of conditioned serum from different species resolves Chemotherapy-Induced neuropathic pain in mice via secretory exosomes. Brain Behav Immun,111, 298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, & DeLeo JA (2008). CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol, 38, 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlino MS, Larkin J, & Long GV (2021). Immune checkpoint inhibitors in melanoma. Lancet, 398, 1002–1014. [DOI] [PubMed] [Google Scholar]
- Carreira EU, Carregaro V, Teixeira MM, Moriconi A, Aramini A, Verri WA Jr., Ferreira SH, Cunha FQ, & Cunha TM (2013). Neutrophils recruited by CXCR1/2 signalling mediate post-incisional pain. Eur J Pain, 17, 654–663. [DOI] [PubMed] [Google Scholar]
- Caxaria S, Bharde S, Fuller AM, Evans R, Thomas B, Celik P, Dell'Accio F, Yona S, Gilroy D, Voisin MB, Wood JN, & Sikandar S (2023). Neutrophils infiltrate sensory ganglia and mediate chronic widespread pain in fibromyalgia. Proc Natl Acad Sci U S A, 120, e2211631120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Altman MD, Lesburg CA, Perera SA, Piesvaux JA, Schroeder GK, Wyss DF, Cemerski S, Chen Y, DiNunzio E, Haidle AM, Ho T, Kariv I, Knemeyer I, Kopinja JE, Lacey BM, Laskey J, Lim J, Long BJ, Ma Y, Maddess ML, Pan BS, Presland JP, Spooner E, Steinhuebel D, Truong Q, Zhang Z, Fu J, Addona GH, Northrup AB, Parmee E, Tata JR, Bennett DJ, Cumming JN, Siu T, & Trotter BW (2022). Discovery of MK-1454: A Potent Cyclic Dinucleotide Stimulator of Interferon Genes Agonist for the Treatment of Cancer. J Med Chem, 65, 5675–5689. [DOI] [PubMed] [Google Scholar]
- Chen G, Kim YH, Li H, Luo H, Liu DL, Zhang ZJ, Lay M, Chang W, Zhang YQ, & Ji RR (2017). PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat Neurosci, 20, 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Park CK, Xie RG, & Ji RR (2015). Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin Invest, 125, 3226–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen O, Donnelly CR, & Ji RR (2019). Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol, 62, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Sun L, & Chen ZJ (2016). Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol, 17, 1142–1149. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck WJ, Hwang SW, Carroll MC, & Woolf CJ (2013). Bacteria activate sensory neurons that modulate pain and inflammation. Nature, 501, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocarro L, Blanco E, Zuazo M, Arasanz H, Bocanegra A, Fernandez-Rubio L, Morente P, Fernandez-Hinojal G, Echaide M, Garnica M, Ramos P, Vera R, Kochan G, & Escors D (2021). Understanding LAG-3 Signaling. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, Freeman GJ, Boland GM, & Luster AD (2019). Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity, 50, 1498–1512 e1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, & Kishimoto T (2020). Translating IL-6 biology into effective treatments. Nat Rev Rheumatol, 16, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann H, Ji RR, & Kress M (2008). Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J.Neurosci, 28, 5072–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, & Scherrer G (2018). Endogenous and Exogenous Opioids in Pain. Annu Rev Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW Jr., & Gajewski TF (2015). Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep, 11, 1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, & Fitzgerald M (2009). T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci, 29, 14415–14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, & Woolf CJ (2009). Neuropathic pain: a maladaptive response of the nervous system to damage. Annu.Rev.Neurosci, 32, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti C, Agape E, Becciolini A, Biggioggero M, & Favalli EG (2019). Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects. Drugs, 79, 1741–1755. [DOI] [PubMed] [Google Scholar]
- Dai WJ, Sun JL, Li C, Mao W, Huang YK, Zhao ZQ, Zhang YQ, & Lu N (2019). Involvement of Interleukin-10 in Analgesia of Electroacupuncture on Incision Pain. Evid Based Complement Alternat Med, 2019, 8413576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Colburn RW, & Rickman AJ (1997). Cytokine and growth factor immunohistochemical spinal profiles in two animal models of mononeuropathy. Brain Res., 759, 50–57. [DOI] [PubMed] [Google Scholar]
- Deng D, Xu F, Ma L, Zhang T, Wang Y, Huang S, Zhao W, & Chen X (2023). Electroacupuncture Alleviates CFA-Induced Inflammatory Pain via PD-L1/PD-1-SHP-1 Pathway. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Dominguez E, Rivat C, Pommier B, Mauborgne A, & Pohl M (2008). JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem, 107, 50–60. [DOI] [PubMed] [Google Scholar]
- Donatien P, Anand U, Yiangou Y, Sinisi M, Fox M, MacQuillan A, Quick T, Korchev YE, & Anand P (2018). Granulocyte-macrophage colony-stimulating factor receptor expression in clinical pain disorder tissues and role in neuronal sensitization. Pain Rep, 3, e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, & Chen L (1999). B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med, 5, 1365–1369. [DOI] [PubMed] [Google Scholar]
- Donnelly CR, Chen O, & Ji RR (2020). How Do Sensory Neurons Sense Danger Signals? Trends Neurosci, 43, 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly CR, Jiang C, Andriessen AS, Wang K, Wang Z, Ding H, Zhao J, Luo X, Lee MS, Lei YL, Maixner W, Ko MC, & Ji RR (2021a). STING controls nociception via type I interferon signalling in sensory neurons. Nature, 591, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly CR, Jiang C, Andriessen AS, Wang K, Wang Z, Ding H, Zhao J, Luo X, Lee MS, Lei YL, Maixner W, Ko MC, & Ji RR (2021b). STING controls nociception via type I interferon signalling in sensory neurons. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorand RD, Nthale J, Myers JT, Barkauskas DS, Avril S, Chirieleison SM, Pareek TK, Abbott DW, Stearns DS, Letterio JJ, Huang AY, & Petrosiute A (2016). Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science, 353, 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS, & Hirsch FR (2021). PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol, 18, 345–362. [DOI] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross SE, Lowell BB, Wang Y, Goulding M, & Ma Q (2014). Identification of spinal circuits transmitting and gating mechanical pain. Cell, 159, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley JC, Lin MT, Le DT, & Eshleman JR (2016). Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res, 22, 813–820. [DOI] [PubMed] [Google Scholar]
- Echeverry S, Shi XQ, Haw A, Liu H, Zhang ZW, & Zhang J (2009). Transforming growth factor-beta1 impairs neuropathic pain through pleiotropic effects. Mol.Pain, 5, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis A, & Bennett DL (2013). Neuroinflammation and the generation of neuropathic pain. Br.J Anaesth, 111, 26–37. [DOI] [PubMed] [Google Scholar]
- Esposito M, Carubbi F, Giunta A, Alunno A, Giacomelli R, & Fargnoli MC (2020). Certolizumab pegol for the treatment of psoriatic arthritis and plaque psoriasis. Expert Rev Clin Immunol, 16, 119–128. [DOI] [PubMed] [Google Scholar]
- Fang D, Kong LY, Cai J, Li S, Liu XD, Han JS, & Xing GG (2015). Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain, 156, 1124–1144. [DOI] [PubMed] [Google Scholar]
- Faust HJ, Zhang H, Han J, Wolf MT, Jeon OH, Sadtler K, Pena AN, Chung L, Maestas DR Jr., Tam AJ, Pardoll DM, Campisi J, Housseau F, Zhou D, Bingham CO 3rd, & Elisseeff JH (2020). IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J Clin Invest, 130, 5493–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava AM, Reyaldeen R, Lo Presti S, Goyal A, Akintoye E, Hughes D, & Klein AL (2022). Rilonacept for the treatment of recurrent pericarditis. Expert Opin Biol Ther, 22, 7–16. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Sindrup SH, & Jensen TS (2010). The evidence for pharmacological treatment of neuropathic pain. Pain, 150, 573–581. [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, & Honjo T (2000). Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med, 192, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch LE, Kelly C, & Pickrell AM (2023). The role of STING signaling in central nervous system infection and neuroinflammatory disease. WIREs Mech Dis, e1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL (2009). Structure and signalling in the IL-17 receptor family. Nat Rev Immunol, 9, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S, Trinh H, Tuckwell K, Collinson N, Stone JH, Sarsour K, Pei J, Best J, Birchwood C, & Mohan SV (2019). Adverse Events in Giant Cell Arteritis and Rheumatoid Arthritis Patient Populations: Analyses of Tocilizumab Clinical Trials and Claims Data. Rheumatol Ther, 6, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevay S, Stingelin S, & Gabay C (2004). Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis, 63, 1120–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese MC, Fleischmann R, Kivitz AJ, Rell-Bakalarska M, Martincova R, Fiore S, Rohane P, van Hoogstraten H, Garg A, Fan C, van Adelsberg J, Weinstein SP, Graham NM, Stahl N, Yancopoulos GD, Huizinga TW, & van der Heijde D (2015). Sarilumab Plus Methotrexate in Patients With Active Rheumatoid Arthritis and Inadequate Response to Methotrexate: Results of a Phase III Study. Arthritis Rheumatol, 67, 1424–1437. [DOI] [PubMed] [Google Scholar]
- Gereau R. W. t., Sluka KA, Maixner W, Savage SR, Price TJ, Murinson BB, Sullivan MD, & Fillingim RB (2014). A pain research agenda for the 21st century. J Pain, 15, 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemlou N, Chiu IM, Julien JP, & Woolf CJ (2015). CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A, 112, E6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami A, Azizpoor J, Aflaki E, Rezaee M, & Keshavarz K (2021). Cost-Effectiveness Analysis of Biopharmaceuticals for Treating Rheumatoid Arthritis: Infliximab, Adalimumab, and Etanercept. Biomed Res Int, 2021, 4450162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Levine JD, & Correa AM (1998). Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J.Neurosci, 18, 10345–10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O'Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesley RA, Hoffmann SC, Holland SM, Butman JA, & Kastner DL (2006). Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med, 355, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach-Mansky R, Shroff SD, Wilson M, Snyder C, Plehn S, Barham B, Pham TH, Pucino F, Wesley RA, Papadopoulos JH, Weinstein SP, Mellis SJ, & Kastner DL (2008). A pilot study to evaluate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (interleukin-1 Trap) in patients with familial cold autoinflammatory syndrome. Arthritis Rheum, 58, 2432–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger R, & Harrison AA (2007). Infliximab in the treatment of ankylosing spondylitis. Biologics, 1, 163–171. [PMC free article] [PubMed] [Google Scholar]
- Gu C, Wu L, & Li X (2013). IL-17 family: cytokines, receptors and signaling. Cytokine, 64, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui X, Yang H, Li T, Tan X, Shi P, Li M, Du F, & Chen ZJ (2019). Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature, 567, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Chen X, Chen J, Zheng G, Xie C, Wu H, Miao Z, Lin Y, Wang X, Gao W, Zheng X, Pan Z, Zhou Y, Wu Y, & Zhang X (2021). STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-kappaB signaling pathway. Cell Death Dis, 12, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Imai S, Yang JL, Zou S, Watanabe M, Chu YX, Mohammad Z, Xu H, Moudgil KD, Wei F, Dubner R, & Ren K (2017). In vivo immune interactions of multipotent stromal cells underlie their long-lasting pain-relieving effect. Sci Rep, 7, 10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, & Ren K (2007). Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J.Neurosci, 27, 6006–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang H, Zou S, Gu M, Watanabe M, Wei F, Dubner R, Huang GT, & Ren K (2011). Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells, 29, 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, & Volarevic V (2019). Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed Pharmacother, 109, 2318–2326. [DOI] [PubMed] [Google Scholar]
- He H, Zhou Y, Zhou Y, Zhuang J, He X, Wang S, & Lin W (2018). Dexmedetomidine Mitigates Microglia-Mediated Neuroinflammation through Upregulation of Programmed Cell Death Protein 1 in a Rat Spinal Cord Injury Model. J Neurotrauma, 35, 2591–2603. [DOI] [PubMed] [Google Scholar]
- He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, Wei XH, Li YY, Xin WJ, Qin ZH, & Liu XG (2010). TNF-alpha contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain, 151, 266–279. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, & Schaper F (2003). Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J, 374, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, & Hutchinson PJ (2014). Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab, 34, 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyes Z, Tekus V, Szentes N, Pohoczky K, Botz B, Kiss T, Kemeny A, Kornyei Z, Toth K, Lenart N, Abraham H, Pinteaux E, Francis S, Sensi S, Denes A, & Goebel A (2019). Transfer of complex regional pain syndrome to mice via human autoantibodies is mediated by interleukin-1-induced mechanisms. Proc Natl Acad Sci U S A, 116, 13067–13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth M, Gandla J, & Kuner R (2017). A checkpoint to pain. Nat Neurosci, 20, 897–899. [DOI] [PubMed] [Google Scholar]
- Hua Z, Liu L, Shen J, Cheng K, Liu A, Yang J, Wang L, Qu T, Yang H, Li Y, Wu H, Narouze J, Yin Y, & Cheng J (2016). Mesenchymal Stem Cells Reversed Morphine Tolerance and Opioid-induced Hyperalgesia. Sci Rep, 6, 32096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JX, Lee YH, & Wei JC (2020). Ixekizumab for the treatment of ankylosing spondylitis. Expert Rev Clin Immunol, 16, 745–750. [DOI] [PubMed] [Google Scholar]
- Huang KC, Chanda D, McGrath S, Dixit V, Zhang C, Wu J, Tendyke K, Yao H, Hukkanen R, Taylor N, Verbel D, Kim DS, Endo A, Noland TA, Chen Y, Matijevic M, Wang J, Hutz J, Sarwar N, Fang FG, & Bao X (2022). Pharmacologic Activation of STING in the Bladder Induces Potent Antitumor Immunity in Non-Muscle Invasive Murine Bladder Cancer. Mol Cancer Ther, 21, 914–924. [DOI] [PubMed] [Google Scholar]
- Huh Y, Ji RR, & Chen G (2017). Neuroinflammation, Bone Marrow Stem Cells, and Chronic Pain. Front Immunol, 8, 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, & Vale RD (2017). T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science, 355, 1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss HT, & Naismith JH (2000). TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech, 50, 184–195. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, & Honjo T (1992). Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J, 11, 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, & Barber GN (2008). STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature, 455, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaller Char JJ, Jaller JA, Waibel JS, Bhanusali DG, & Bhanusali N (2018). The Role of IL-17 in the Human Immune System and Its Blockage as a Treatment of Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis. J Drugs Dermatol, 17, 539–542. [PubMed] [Google Scholar]
- Ji RR, Chamessian A, & Zhang YQ (2016). Pain regulation by non-neuronal cells and inflammation. Science, 354, 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Gereau RW, Malcangio M, & Strichartz GR (2009). MAP kinase and pain. Brain Res.Rev, 60, 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, & Lee SY (2021). Molecular Sensors of Temperature, Pressure, and Pain with Special Focus on TRPV1, TRPM8, and PIEZO2 Ion Channels. Neurosci Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, & Gao YJ (2014). Emerging targets in neuroinflammation-driven chronic pain. Nat.Rev.Drug Discov, 13, 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Strichartz G, & Serhan CN (2011). Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Wang Z, Donnelly CR, Wang K, Andriessen AS, Tao X, Matsuda M, Zhao J, & Ji RR (2020). PD-1 Regulates GABAergic Neurotransmission and GABA-Mediated Analgesia and Anesthesia. iScience, 23, 101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhou R, Zhang Y, Zhu T, Li Q, & Zhang W (2022). Interleukin-17 as a potential therapeutic target for chronic pain. Front Immunol, 13, 999407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Shiwaku H, Tanaka H, Obita T, Ohuchi S, Yoshioka Y, Jin X, Kondo K, Fujita K, Homma H, Nakajima K, Mizuguchi M, & Okazawa H (2021). Tau activates microglia via the PQBP1-cGAS-STING pathway to promote brain inflammation. Nat Commun, 12, 6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, & Gereau RW (2006). Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J.Neurosci, 26, 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, & Basbaum AI (2001). Molecular mechanisms of nociception. Nature, 413, 203–210. [DOI] [PubMed] [Google Scholar]
- Kalpachidou T, Riehl L, Schopf CL, Ucar B, & Kress M (2022). Proinflammatory cytokines and their receptors as druggable targets to alleviate pathological pain. Pain, 163, S79–S98. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, & Heijnen CJ (2021). T Cells as Guardians of Pain Resolution. Trends Mol Med, 27, 302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, & Ji RR (2008). Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat.Med, 14, 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, & Ji RR (2008). Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J.Neurosci, 28, 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, & Patapoutian A (2012). The role of Drosophila Piezo in mechanical nociception. Nature, 483, 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham B (1991). Interleukin-1, immune activation pathways, and different mechanisms in osteoarthritis and rheumatoid arthritis. Ann Rheum Dis, 50, 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T (2005). Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol, 23, 1–21. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Hofstetter HH, Meuth SG, Braeuninger S, Sommer C, & Stoll G (2006). T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp Neurol, 200, 480–485. [DOI] [PubMed] [Google Scholar]
- Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, & Kavelaars A (2016). CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci, 36, 11074–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R, & Flor H (2017). Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci, 18, 113. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Kurokawa R, Hagiwara A, Gonoi W, Harayama S, Koizumi K, Yoshino K, Hishima T, Baba A, Ota Y, Abe O, & Takaki Y (2021). CT imaging findings of anti-PD-1 inhibitor-related enterocolitis. Abdom Radiol (NY), 46, 3033–3043. [DOI] [PubMed] [Google Scholar]
- Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, Kalomenidis I, Chrysos G, Angheben A, Kainis I, Alexiou Z, Castelli F, Serino FS, Tsilika M, Bakakos P, Nicastri E, Tzavara V, Kostis E, Dagna L, Koufargyris P, Dimakou K, Savvanis S, Tzatzagou G, Chini M, Cavalli G, Bassetti M, Katrini K, Kotsis V, Tsoukalas G, Selmi C, Bliziotis I, Samarkos M, Doumas M, Ktena S, Masgala A, Papanikolaou I, Kosmidou M, Myrodia DM, Argyraki A, Cardellino CS, Koliakou K, Katsigianni EI, Rapti V, Giannitsioti E, Cingolani A, Micha S, Akinosoglou K, Liatsis-Douvitsas O, Symbardi S, Gatselis N, Mouktaroudi M, Ippolito G, Florou E, Kotsaki A, Netea MG, Eugen-Olsen J, Kyprianou M, Panagopoulos P, Dalekos GN, & Giamarellos-Bourboulis EJ (2021). Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med, 27, 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C, Group, E. S., & Group, F. S. (2014). Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med, 371, 326–338. [DOI] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, & Freeman GJ (2001). PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol, 2, 261–268. [DOI] [PubMed] [Google Scholar]
- Laumet G, Edralin JD, Dantzer R, Heijnen CJ, & Kavelaars A (2019). Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain, 160, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar-Molnar E, Yan Q, Cao E, Ramagopal U, Nathenson SG, & Almo SC (2008). Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci U S A, 105, 10483–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Naour J, Zitvogel L, Galluzzi L, Vacchelli E, & Kroemer G (2020). Trial watch: STING agonists in cancer therapy. Oncoimmunology, 9, 1777624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, & Watkins LR (2007). Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun, 21, 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JG, Duffy SS, Perera CJ, & Moalem-Taylor G (2015). Depletion of Foxp3+ regulatory T cells increases severity of mechanical allodynia and significantly alters systemic cytokine levels following peripheral nerve injury. Cytokine, 71, 207–214. [DOI] [PubMed] [Google Scholar]
- Lehtonen A, Matikainen S, Miettinen M, & Julkunen I (2002). Granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced STAT5 activation and target-gene expression during human monocyte/macrophage differentiation. J Leukoc Biol, 71, 511–519. [PubMed] [Google Scholar]
- Lespessailles E, & Toumi H (2021). Ixekizumab in the treatment of psoriatic arthritis. Immunotherapy, 13, 19–33. [DOI] [PubMed] [Google Scholar]
- Li T, & Chen ZJ (2018). The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med, 215, 1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CL, Jiang H, Feng W, Liu H, Han L, Chen Y, Zhang Q, Zheng F, Lu CJ, & Dai Z (2021). Total Glucosides of Paeony Ameliorate Pristane-Induced Lupus Nephritis by Inducing PD-1 ligands(+) Macrophages via Activating IL-4/STAT6/PD-L2 Signaling. Front Immunol, 12, 683249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Tanaka Y, Iwasaki M, Gittis AG, Su HP, Mikami B, Okazaki T, Honjo T, Minato N, & Garboczi DN (2008). The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A, 105, 3011–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Li H, Brull SJ, & Zhang JM (2002). Increased sensitivity of sensory neurons to tumor necrosis factor alpha in rats with chronic compression of the lumbar ganglia. J Neurophysiol, 88, 1393–1399. [DOI] [PubMed] [Google Scholar]
- Liu BL, Cao QL, Zhao X, Liu HZ, & Zhang YQ (2020). Inhibition of TRPV1 by SHP-1 in nociceptive primary sensory neurons is critical in PD-L1 analgesia. JCI Insight, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Moura-Alves P, Pei G, Mollenkopf HJ, Hurwitz R, Wu X, Wang F, Liu S, Ma M, Fei Y, Zhu C, Koehler AB, Oberbeck-Mueller D, Hahnke K, Klemm M, Guhlich-Bornhof U, Ge B, Tuukkanen A, Kolbe M, Dorhoi A, & Kaufmann SH (2019). cGAS facilitates sensing of extracellular cyclic dinucleotides to activate innate immunity. EMBO Rep, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen L, Tu Y, Chen X, Hu K, Tu Y, Lin M, Xie G, Chen S, Huang J, Liu W, Wu J, Xiao T, Wilson G, Lang C, Park J, Tao J, & Kong J (2019). Different exercise modalities relieve pain syndrome in patients with knee osteoarthritis and modulate the dorsolateral prefrontal cortex: A multiple mode MRI study. Brain Behav Immun, 82, 253–263. [DOI] [PubMed] [Google Scholar]
- Liu XJ, Zhang Y, Liu T, Xu ZZ, Park CK, Berta T, Jiang D, & Ji RR (2014). Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livni L, Keating BA, Fiore NT, Lees JG, Goldstein D, & Moalem-Taylor G (2022). Effects of combined chemotherapy and anti-programmed cell death protein 1 treatment on peripheral neuropathy and neuroinflammation in mice. Pain, 163, 110–124. [DOI] [PubMed] [Google Scholar]
- Lopez T, Mustafa Z, Chen C, Lee KB, Ramirez A, Benitez C, Luo X, Ji RR, & Ge X (2019). Functional selection of protease inhibitory antibodies. Proc Natl Acad Sci U S A, 116, 16314–16319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Liu HZ, Zhang WW, Matsuda M, Lv N, Chen G, Xu ZZ, & Zhang YQ (2019). Interleukin-17 Regulates Neuron-Glial Communications, Synaptic Transmission, and Neuropathic Pain after Chemotherapy. Cell Rep, 29, 2384–2397 e2385. [DOI] [PubMed] [Google Scholar]
- Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH, Huh Y, Furutani K, He Q, Tao X, Ko MC, Bortsov A, Donnelly CR, Chen Y, Nackley A, Berta T, & Ji RR (2021). IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron, 109, 2691–2706 e2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Huh Y, Bang S, He Q, Zhang L, Matsuda M, & Ji RR (2019). Macrophage Toll-like Receptor 9 Contributes to Chemotherapy-Induced Neuropathic Pain in Male Mice. J Neurosci, 39, 6848–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhot B, Christin M, Tessandier N, Sotoudeh C, Bretheau F, Turmel R, Pellerin E, Wang F, Bories C, Joly-Beauparlant C, De Koninck Y, Droit A, Cicchetti F, Scherrer G, Boilard E, Sharif-Naeini R, & Lacroix S (2020). Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J Exp Med, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco V, Abu-Rumeileh S, Mastrolia MV, Marrani E, Maccora I, Pagnini I, & Simonini G (2020). The off-label use of anakinra in pediatric systemic autoinflammatory diseases. Ther Adv Musculoskelet Dis, 12, 1759720X20959575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P. (2013). Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain, 154 Suppl 1, S54–S62. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Clohisy DR, Koltzenburg M, & Hunt SP (2002). Molecular mechanisms of cancer pain. Nat.Rev.Cancer, 2, 201–209. [DOI] [PubMed] [Google Scholar]
- Marchand F, Perretti M, & McMahon SB (2005). Role of the immune system in chronic pain. Nat.Rev.Neurosci, 6, 521–532. [DOI] [PubMed] [Google Scholar]
- McNamee KE, Alzabin S, Hughes JP, Anand P, Feldmann M, Williams RO, & Inglis JJ (2011). IL-17 induces hyperalgesia via TNF-dependent neutrophil infiltration. Pain, 152, 1838–1845. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Gottlieb AB, Berman A, Drescher E, Xing J, Wong R, & Banerjee S (2016). The Efficacy and Safety of Clazakizumab, an Anti-Interleukin-6 Monoclonal Antibody, in a Phase IIb Study of Adults With Active Psoriatic Arthritis. Arthritis Rheumatol, 68, 2163–2173. [DOI] [PubMed] [Google Scholar]
- Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, Lin CY, Braun DK, Lee CH, Gladman DD, & Group, S.-P. S. (2017). Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis, 76, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerschaert KA, Edwards BS, Epouhe AY, Jefferson B, Friedman R, Babyok OL, Moy JK, Kehinde F, Liu C, Workman CJ, Vignali DAA, Albers KM, Koerber HR, Gold MS, Davis BM, Scheff NN, & Saloman JL (2022). Neuronally expressed PDL1, not PD1, suppresses acute nociception. Brain Behav Immun, 106, 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Zhang Y, Lao L, Saito R, Li A, Backman CM, Berman BM, Ren K, Wei PK, & Zhang RX (2013). Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain, 154, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoud-Nacer Y, Culerier E, Rose S, Maillet I, Rouxel N, Briault S, Ryffel B, Quesniaux VFJ, & Togbe D (2022). STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS). Cell Death Dis, 13, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, & Watkins LR (2001). Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J.Neurosci, 21, 2808–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monemi S, Berber E, Sarsour K, Wang J, Lampl K, Bharucha K, & Pethoe-Schramm A (2016). Incidence of Gastrointestinal Perforations in Patients with Rheumatoid Arthritis Treated with Tocilizumab from Clinical Trial, Postmarketing, and Real-World Data Sources. Rheumatol Ther, 3, 337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosallanejad K, & Kagan JC (2022). Control of innate immunity by the cGAS-STING pathway. Immunol Cell Biol, 100, 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair JR, Edwards SW, & Moots RJ (2012). Mavrilimumab, a human monoclonal GM-CSF receptor-alpha antibody for the management of rheumatoid arthritis: a novel approach to therapy. Expert Opin Biol Ther, 12, 1661–1668. [DOI] [PubMed] [Google Scholar]
- Nicol LSC, Thornton P, Hatcher JP, Glover CP, Webster CI, Burrell M, Hammett K, Jones CA, Sleeman MA, Billinton A, & Chessell I (2018). Central inhibition of granulocyte-macrophage colony-stimulating factor is analgesic in experimental neuropathic pain. Pain, 159, 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus JK, Taylor-Blake B, Loo L, Simon JM, & Zylka MJ (2021). Spinal macrophages resolve nociceptive hypersensitivity after peripheral injury. Neuron, 109, 1274–1282 e1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, & Dinarello CA (2000). The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today, 21, 206–209. [DOI] [PubMed] [Google Scholar]
- Oestreich KJ, Yoon H, Ahmed R, & Boss JM (2008). NFATc1 regulates PD-1 expression upon T cell activation. J Immunol, 181, 4832–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oggero S, Cecconello C, Silva R, Zeboudj L, Sideris-Lampretsas G, Perretti M, & Malcangio M (2022). Dorsal root ganglia CX3CR1 expressing monocytes/macrophages contribute to arthritis pain. Brain Behav Immun, 106, 289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield V, & Plosker GL (2009). Golimumab: in the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. BioDrugs, 23, 125–135. [DOI] [PubMed] [Google Scholar]
- Orrock JE, & Ilowite NT (2016). Canakinumab for the treatment of active systemic juvenile idiopathic arthritis. Expert Rev Clin Pharmacol, 9, 1015–1024. [DOI] [PubMed] [Google Scholar]
- Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, Grant AV, Huising J, Verma V, Meloto CB, Silva JR, Dutra GGS, Markova T, Dang H, Tessier PA, Slade GD, Nackley AG, Ghasemlou N, Mogil JS, Allegri M, & Diatchenko L (2022). Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Science Translational Medicine, 14, eabj9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisien M, van Reij RRI, Khoury S, Koseli E, Karaky M, van den Hoogen NJ, Peng G, Allegri M, de Gregori M, Chelly JE, Rakel BA, Aasvang EK, Kehlet H, Buhre W, Bryant CD, Damaj MI, King IL, Mogil JS, Joosten EAJ, & Diatchenko L (2023). Genome-wide association study suggests a critical contribution of the adaptive immune system to chronic post-surgical pain. medRxiv. [Google Scholar]
- Park CK, Lu N, Xu ZZ, Liu T, Serhan CN, & Ji RR (2011). Resolving TRPV1- and TNF-alpha-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci, 31, 15072–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoukis N, Brown J, Petkova V, Liu F, Li L, & Boussiotis VA (2012). Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal, 5, ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoukis N, Duke-Cohan JS, Chaudhri A, Aksoylar HI, Wang Q, Council A, Berg A, Freeman GJ, & Boussiotis VA (2020). Interaction of SHP-2 SH2 domains with PD-1 ITSM induces PD-1 dimerization and SHP-2 activation. Commun Biol, 3, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, & Seal RP (2016). Neural circuits for pain: Recent advances and current views. Science, 354, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E, Mills JJ, Zhao B, Wang F, Sun Q, Christov PP, Tarr JC, Rietz TA, Olejniczak ET, Lee T, & Fesik S (2019). Fragment-based screening of programmed death ligand 1 (PD-L1). Bioorg Med Chem Lett, 29, 786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic J, Silva JR, Bannerman CA, Segal JP, Marshall AS, Haird CM, Gilron I, & Ghasemlou N (2019). gammadelta T Cells Modulate Myeloid Cell Recruitment but Not Pain During Peripheral Inflammation. Front Immunol, 10, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, Chan AC, & Thomas ML (1996). Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science, 272, 1173–1176. [DOI] [PubMed] [Google Scholar]
- Potere N, Batticciotto A, Vecchie A, Porreca E, Cappelli A, Abbate A, Dentali F, & Bonaventura A (2021). The role of IL-6 and IL-6 blockade in COVID-19. Expert Rev Clin Immunol, 17, 601–618. [DOI] [PubMed] [Google Scholar]
- Pourhoseingholi MA, Shojaee S, & Ashtari S (2020). Mavrilimumab for severe COVID-19. Lancet Rheumatol, 2, e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach HT, Robbins CJ, Balko JM, Chiu CY, Miller S, Wilson MR, Nelson GE, & Johnson DB (2019). Severe Epididymo-Orchitis and Encephalitis Complicating Anti-PD-1 Therapy. Oncologist, 24, 872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, & Vader K (2020). The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain, 161, 1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoof R, Martin Gil C, Lafeber F, de Visser H, Prado J, Versteeg S, Pascha MN, Heinemans ALP, Adolfs Y, Pasterkamp J, Wood JN, Mastbergen SC, & Eijkelkamp N (2021). Dorsal Root Ganglia Macrophages Maintain Osteoarthritis Pain. J Neurosci, 41, 8249–8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, & Levine JD (2009). Critical role of nociceptor plasticity in chronic pain. Trends Neurosci, 32, 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, & Dubner R (2010). Interactions between the immune and nervous systems in pain. Nat.Med, 16, 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, & Wolchok JD (2018). Cancer immunotherapy using checkpoint blockade. Science, 359, 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter F, Natura G, Ebbinghaus M, von Banchet GS, Hensellek S, Konig C, Brauer R, & Schaible HG (2012). Interleukin-17 sensitizes joint nociceptors to mechanical stimuli and contributes to arthritic pain through neuronal interleukin-17 receptors in rodents. Arthritis Rheum, 64, 4125–4134. [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, & Group, C. T. (2017). Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet, 390, 1833–1842. [DOI] [PubMed] [Google Scholar]
- Riley JL (2009). PD-1 signaling in primary T cells. Immunol Rev, 229, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh R, Lee MC, Khiew SH, Louis C, Fleetwood AJ, Achuthan A, Forster I, Cook AD, & Hamilton JA (2018). CSF-1 in Inflammatory and Arthritic Pain Development. J Immunol, 201, 2042–2053. [DOI] [PubMed] [Google Scholar]
- Schafers M, Sommer C, Geis C, Hagenacker T, Vandenabeele P, & Sorkin LS (2008). Selective stimulation of either tumor necrosis factor receptor differentially induces pain behavior in vivo and ectopic activity in sensory neurons in vitro. Neuroscience, 157, 414–423. [DOI] [PubMed] [Google Scholar]
- Segond von Banchet G, Boettger MK, Konig C, Iwakura Y, Brauer R, & Schaible HG (2013). Neuronal IL-17 receptor upregulates TRPV4 but not TRPV1 receptors in DRG neurons and mediates mechanical but not thermal hyperalgesia. Mol Cell Neurosci, 52, 152–160. [DOI] [PubMed] [Google Scholar]
- Shi S, Han Y, Wang D, Guo P, Wang J, Ren T, & Wang W (2020). PD-L1 and PD-1 expressed in trigeminal ganglia may inhibit pain in an acute migraine model. Cephalalgia, 40, 288–298. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, & Myers RR (2000). Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res, 855, 83–89. [DOI] [PubMed] [Google Scholar]
- Singh SK, Krukowski K, Laumet GO, Weis D, Alexander JF, Heijnen CJ, & Kavelaars A (2022). CD8+ T cell-derived IL-13 increases macrophage IL-10 to resolve neuropathic pain. JCI Insight, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R, & Investigators O (2008). Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet, 371, 987–997. [DOI] [PubMed] [Google Scholar]
- Sommer C, & Kress M (2004). Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci.Lett, 361, 184–187. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, & Mogil JS (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat.Neurosci, 18, 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhou YQ, Xu BY, Li JY, Zhang LQ, Li DY, Zhang S, Wu JY, Gao SJ, Ye DW, & Mei W (2022). STING/NF-kappaB/IL-6-Mediated Inflammation in Microglia Contributes to Spared Nerve Injury (SNI)-Induced Pain Initiation. J Neuroimmune Pharmacol, 17, 453–469. [DOI] [PubMed] [Google Scholar]
- Sun W, Li Y, Chen L, Chen H, You F, Zhou X, Zhou Y, Zhai Z, Chen D, & Jiang Z (2009). ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci U S A, 106, 8653–8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CS, Wen ZH, Feng CW, Chen CH, Huang SY, Chen NF, Chen WF, & Wong CS (2017). Potentiation of spinal glutamatergic response in the neuron-glia interactions underlies the intrathecal IL-1beta-induced thermal hyperalgesia in rats. CNS Neurosci Ther, 23, 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer S, Martin D, & DeLeo JA (2001). Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience, 103, 529–539. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Kawanishi M, Nakanishi M, Yamasaki H, & Tanaka Y (2022). Phase II/III Results of a Trial of Anti-Tumor Necrosis Factor Multivalent NANOBODY Compound Ozoralizumab in Patients With Rheumatoid Arthritis. Arthritis Rheumatol, 74, 1776–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot S, Foster SL, & Woolf CJ (2016). Neuroimmunity: Physiology and Pathology. Annu Rev Immunol, 34, 421–447. [DOI] [PubMed] [Google Scholar]
- Tan H, Ding Z, Zhang C, Yan J, Yang Y, & Li P (2021). The Programmed Cell Death Ligand-1/Programmed Cell Death-1 Pathway Mediates Pregnancy-Induced Analgesia via Regulating Spinal Inflammatory Cytokines. Anesth Analg, 133, 1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PH, Ji J, Yeh CC, & Ji RR (2021). Interferons in Pain and Infections: Emerging Roles in Neuro-Immune and Neuro-Glial Interactions. Front Immunol, 12, 783725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z, Lin ZJ, Wu LJ, & Zhou LJ (2022). The Macrophage IL-23/IL-17A Pathway: A New Neuro-Immune Mechanism in Female Mechanical Pain. Neurosci Bull, 38, 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M, & Kishimoto T (2018). Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Okuda H, Isonishi A, Terada Y, Kitabatake M, Shinjo T, Nishimura K, Takemura S, Furue H, Ito T, Tatsumi K, & Wanaka A (2023). Dermal macrophages set pain sensitivity by modulating the amount of tissue NGF through an SNX25-Nrf2 pathway. Nat Immunol, 24, 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares-Ferreira D, Shiers S, Ray PR, Wangzhou A, Jeevakumar V, Sankaranarayanan I, Cervantes AM, Reese JC, Chamessian A, Copits BA, Dougherty PM, Gereau RW, Burton MD, Dussor G, & Price TJ (2022). Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Science Translational Medicine, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Saurigny D, Vencovsky J, Takeuchi T, Nakamura T, Matsievskaia G, Hunt B, Wagner T, Souberbielle B, & Group, N. S. (2019). Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: a randomized, controlled trial. Arthritis Res Ther, 21, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari D, Cook AD, Lee MC, Christensen AD, Croxford A, Becher B, Poole D, Rajasekhar P, Bunnett N, Smith JE, Hamilton JA, & McMahon SB (2020). Granulocyte-Macrophage Colony Stimulating Factor As an Indirect Mediator of Nociceptor Activation and Pain. J Neurosci, 40, 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom G, Tian MM, Hatcher JP, Rodrigo N, Burrell M, Gurrell I, Vitalis TZ, Abraham T, Jefferies WA, Webster CI, & Gabathuler R (2019). A peptide derived from melanotransferrin delivers a protein-based interleukin 1 receptor antagonist across the BBB and ameliorates neuropathic pain in a preclinical model. J Cereb Blood Flow Metab, 39, 2074–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Hoheisel U, Wang D, & Magerl W (2022). Central sensitization: clinical utility of a physiological concept for the International Statistical Classification of Diseases and Related Health Problems and for nociplastic pain. Pain, 163, S99–S107. [DOI] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, & Ribas A (2014). PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature, 515, 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uceyler N, Gobel K, Meuth SG, Ortler S, Stoll G, Sommer C, Wiendl H, & Kleinschnitz C (2010a). Deficiency of the negative immune regulator B7-H1 enhances inflammation and neuropathic pain after chronic constriction injury of mouse sciatic nerve. Exp.Neurol, 222, 153–160. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Gobel K, Meuth SG, Ortler S, Stoll G, Sommer C, Wiendl H, & Kleinschnitz C (2010b). Deficiency of the negative immune regulator B7-H1 enhances inflammation and neuropathic pain after chronic constriction injury of mouse sciatic nerve. Exp Neurol, 222, 153–160. [DOI] [PubMed] [Google Scholar]
- Ultenius C, Linderoth B, Meyerson BA, & Wallin J (2006). Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett, 399, 85–90. [DOI] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, & Ernfors P (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat.Neurosci, 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Vaidya G, Czer LSC, Kobashigawa J, Kittleson M, Patel J, Chang D, Kransdorf E, Shikhare A, Tran H, Vo A, Ammerman N, Huang E, Zabner R, & Jordan S (2020). Successful Treatment of Severe COVID-19 Pneumonia With Clazakizumab in a Heart Transplant Recipient: A Case Report. Transplant Proc, 52, 2711–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg WB, & Miossec P (2009). IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol, 5, 549–553. [DOI] [PubMed] [Google Scholar]
- van der Vlist M, Raoof R, Willemen H, Prado J, Versteeg S, Martin Gil C, Vos M, Lokhorst RE, Pasterkamp RJ, Kojima T, Karasuyama H, Khoury-Hanold W, Meyaard L, & Eijkelkamp N (2022). Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron, 110, 613–626 e619. [DOI] [PubMed] [Google Scholar]
- Vandooren J, Van Damme J, & Opdenakker G (2014). On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog Brain Res, 214, 193–206. [DOI] [PubMed] [Google Scholar]
- Vicuna L, Strochlic DE, Latremoliere A, Bali KK, Simonetti M, Husainie D, Prokosch S, Riva P, Griffin RS, Njoo C, Gehrig S, Mall MA, Arnold B, Devor M, Woolf CJ, Liberles SD, Costigan M, & Kuner R (2015). The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med, 21, 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, & Collins FS (2017). The Role of Science in Addressing the Opioid Crisis. N Engl J Med, 377, 391–394. [DOI] [PubMed] [Google Scholar]
- Wanderley CWS, Maganin AGM, Adjafre B, Mendes AS, Silva CEA, Quadros AU, Luiz JPM, Silva CMS, Silva NR, Oliveira FFB, Gomes FIF, Restrepo JLJ, Speck-Hernandez CA, Turaca F, Silva GVL, Pigatto GR, Nakaya HI, Mota JM, Barroso-Sousa R, Alves-Filho JC, Cunha TM, & Cunha FQ (2022). PD-1/PD-L1 Inhibition Enhances Chemotherapy-Induced Neuropathic Pain by Suppressing Neuroimmune Antinociceptive Signaling. Cancer Immunol Res, 10, 1299–1308. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen W, Dong Z, Xing G, Cui W, Yao L, Zou WJ, Robinson HL, Bian Y, Liu Z, Zhao K, Luo B, Gao N, Zhang H, Ren X, Yu Z, Meixiong J, Xiong WC, & Mei L (2022). A novel spinal neuron connection for heat sensation. Neuron, 110, 2315–2333 e2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Donnelly CR, Jiang C, Liao Y, Luo X, Tao X, Bang S, McGinnis A, Lee M, Hilton MJ, & Ji RR (2021). STING suppresses bone cancer pain via immune and neuronal modulation. Nat Commun, 12, 4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Gu Y, Liao Y, Bang S, Donnelly CR, Chen O, Tao X, Mirando AJ, Hilton MJ, & Ji RR (2020). PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J Clin Invest, 130, 3603–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jiang C, He Q, Matsuda M, Han Q, Wang K, Bang S, Ding H, Ko MC, & Ji RR (2020). Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Science Translational Medicine, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Qiu CY, Jin Y, Liu TT, & Hu WP (2021). TNF-alpha acutely enhances acid-sensing ion channel currents in rat dorsal root ganglion neurons via a p38 MAPK pathway. J Neuroinflammation, 18, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Guo TZ, Li WW, Kingery WS, & Clark JD (2016). Acute versus chronic phase mechanisms in a rat model of CRPS. J Neuroinflammation, 13, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XH, Yang T, Wu Q, Xin WJ, Wu JL, Wang YQ, Zang Y, Wang J, Li YY, & Liu XG (2012). Peri-sciatic administration of recombinant rat IL-1beta induces mechanical allodynia by activation of src-family kinases in spinal microglia in rats. Exp Neurol, 234, 389–397. [DOI] [PubMed] [Google Scholar]
- Woo SR, Corrales L, & Gajewski TF (2015). The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol, 36, 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (1983). Evidence for a central component of post-injury pain hypersensitivity. Nature, 306, 686–688. [DOI] [PubMed] [Google Scholar]
- Woolf CJ (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain, 152, S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zhang X, Wang S, Li T, Hao Q, Li S, Yao W, & Sun R (2022). Pharmacological inhibition of the cGAS-STING signaling pathway suppresses microglial M1-polarization in the spinal cord and attenuates neuropathic pain. Neuropharmacology, 217, 109206. [DOI] [PubMed] [Google Scholar]
- Xia T, Konno H, Ahn J, & Barber GN (2016). Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates With Tumorigenesis. Cell Rep, 14, 282–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Deng A, Liu H, Ge G, & Liu X (2012). Activator protein 1 suppresses antitumor T-cell function via the induction of programmed death 1. Proc Natl Acad Sci U S A, 109, 15419–15424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Zhao XT, Xu LC, Yue LP, Liu FY, Cai J, Liao FF, Kong JG, Xing GG, Yi M, & Wan Y (2015). Shp-1 dephosphorylates TRPV1 in dorsal root ganglion neurons and alleviates CFA-induced inflammatory pain in rats. Pain, 156, 597–608. [DOI] [PubMed] [Google Scholar]
- Yang L, Xu M, Bhuiyan SA, Li J, Zhao J, Cohrs RJ, Susterich JT, Signorelli S, Green U, Stone JR, Levy D, Lennerz JK, & Renthal W (2022). Human and mouse trigeminal ganglia cell atlas implicates multiple cell types in migraine. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao A, Liu F, Chen K, Tang L, Liu L, Zhang K, Yu C, Bian G, Guo H, Zheng J, Cheng P, Ju G, & Wang J (2014). Programmed death 1 deficiency induces the polarization of macrophages/microglia to the M1 phenotype after spinal cord injury in mice. Neurotherapeutics, 11, 636–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, & Wu K (2018). Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer, 17, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi MH, Liu YU, Umpierre AD, Chen T, Ying Y, Zheng J, Dheer A, Bosco DB, Dong H, & Wu LJ (2021). Optogenetic activation of spinal microglia triggers chronic pain in mice. PLoS Biol, 19, e3001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip RML, & Yim CW (2021). Role of Interleukin 6 Inhibitors in the Management of Rheumatoid Arthritis. J Clin Rheumatol, 27, e516–e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, & Saito T (2012). Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med, 209, 1201–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, & Basbaum AI (2020). Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun, 11, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yum S, Li M, Fang Y, & Chen ZJ (2021). TBK1 recruitment to STING activates both IRF3 and NF-kappaB that mediate immune defense against tumors and viral infections. Proc Natl Acad Sci U S A, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak KM, Kitel R, Przetocka S, Golik P, Guzik K, Musielak B, Domling A, Dubin G, & Holak TA (2015). Structure of the Complex of Human Programmed Death 1, PD-1, and Its Ligand PD-L1. Structure, 23, 2341–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, & Linnarsson S (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science, 347, 1138–1142. [DOI] [PubMed] [Google Scholar]
- Zelenka M, Schafers M, & Sommer C (2005). Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain, 116, 257–263. [DOI] [PubMed] [Google Scholar]
- Zhang C, Shang G, Gui X, Zhang X, Bai XC, & Chen ZJ (2019). Structural basis of STING binding with and phosphorylation by TBK1. Nature, 567, 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang Y, Liu Y, Han H, Zhang D, Fan X, Du X, Gamper N, & Zhang H (2019). Transcriptional Regulation of Voltage-Gated Sodium Channels Contributes to GM-CSF-Induced Pain. J Neurosci, 39, 5222–5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, & An J (2007). Cytokines, inflammation, and pain. Int Anesthesiol Clin, 45, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Bang S, Chandra S, & Ji RR (2022). Inflammation and Infection in Pain and the Role of GPR37. Int J Mol Sci, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bai XC, & Chen ZJ (2020). Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity, 53, 43–53. [DOI] [PubMed] [Google Scholar]
- Zhang X, Schwartz JC, Guo X, Bhatia S, Cao E, Lorenz M, Cammer M, Chen L, Zhang ZY, Edidin MA, Nathenson SG, & Almo SC (2004). Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity, 20, 337–347. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang W, Gong Z, Peng Y, Li X, Zhang Z, Zhang X, You X, & Wu J (2023). Activation of the STING pathway induces peripheral sensitization via neuroinflammation in a rat model of bone cancer pain. Inflamm Res, 72, 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Roberts A, Huh Y, & Ji R-R (2023). Immunotherapy and Pain. In Ji R-R, Cheng J & Ji J (Eds.), Neuroimmune Interactions in Pain : Mechanisms and Therapeutics (pp. 223–245). Cham: Springer International Publishing. [Google Scholar]
- Zhao J, Roberts A, Wang Z, Savage J, & Ji RR (2021). Emerging Role of PD-1 in the Central Nervous System and Brain Diseases. Neurosci Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Luo H, Ma Y, Zhu S, Wu Y, Lu M, Yao X, Liu X, & Chen G (2022). An analgesic peptide H-20 attenuates chronic pain via the PD-1 pathway with few adverse effects. Proc Natl Acad Sci U S A, 119, e2204114119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Song Y, Wang Y, Huang Y, Li Z, Cui Y, Yi M, Xia L, Zhuang W, Wu X, & Zhou Y (2019). PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif, 52, e12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Zhou LJ, Ren WJ, Xin WJ, Li YY, Zhang T, & Liu XG (2010). The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: the role of tumor necrosis factor-alpha. Brain Behav.Immun, 24, 874–880. [DOI] [PubMed] [Google Scholar]
- Zhou WBS, Shi XQ, Liu Y, Tran SD, Beaudry F, & Zhang J (2023). Unbiased proteomic analysis detects painful systemic inflammatory profile in the serum of nerve-injured mice. Pain, 164, e77–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Qiao G, Ren J, Wang X, Wang S, Zhu S, Yuan Y, Morse MA, Hobeika A, & Lyerly HK (2020). Adoptive immunotherapy with autologous T-cell infusions reduces opioid requirements in advanced cancer patients. Pain, 161, 127–134. [DOI] [PubMed] [Google Scholar]