Abstract
INTRODUCTION.
Plasma tau phosphorylated at threonine 217 (P-tau217) and neurofilament light (NfL) have emerged as markers of Alzheimer’s disease (AD) pathology. Few studies have examined the role of sex in plasma biomarkers in sporadic AD, yielding mixed findings, and none in autosomal dominant AD.
METHODS.
We examined the effects of sex and age on plasma P-tau217 and NfL, and their association with cognitive performance in a cross-sectional study of 621 Presenilin-1 E280A mutation carriers and non-carriers.
RESULTS.
As plasma P-tau217 levels increase, cognitively unimpaired female carriers showed better cognitive performance than cognitively unimpaired male carriers. Yet, as disease progresses, female carriers had a greater plasma NfL increase than male carriers. There were no sex differences in the association between age and plasma biomarkers among non-carriers.
DISCUSSION.
Our findings suggest that, among PSEN1 mutation carriers, females had a greater rate of neurodegeneration than males, yet it did not predict cognitive performance.
Keywords: Alzheimer’s disease, Autosomal dominant Alzheimer’s disease, blood biomarkers, P-tau217, NfL, cognition, sex differences
Background
Blood biomarkers are gaining support as sensitive, non-invasive, and relatively inexpensive biomarkers of early Alzheimer’s disease (AD) pathology and neurodegeneration1. Among several soluble p-tau species, plasma tau phosphorylated at threonine 217 (P-tau217) has emerged as a marker of early tau pathology accumulation2,3 that predicts the clinical diagnosis of AD4-7. Similarly, plasma neurofilament light chain (NfL), a marker of axonal injury and neuronal degeneration, although not specific to AD, has been shown to be elevated in preclinical and clinical AD8,9, and is associated with markers of neurodegeneration (e.g., hippocampal atrophy or cortical thinning)10-12. Our group showed that Presenilin 1 (PSEN1) E280A mutation carriers, who are destined to develop early-onset dementia, have elevated levels of plasma P-tau2174 and NfL13, approximately 20 years before symptom onset. We also showed that, in carriers, higher levels of plasma P-tau217 and NfL were associated with worse memory performance4,14,15.
Several studies investigating sex differences in AD have shown that females may have greater neurodegeneration16,17 and tau pathology, as measured by cerebrospinal fluid,18 PET imaging,19,20 and postmortem neuropathology21,22. Moreover, studies show that females may exhibit a cognitive resilience to early accumulation of AD-related pathology and neurodegeneration at the initial stages of the disease23-25; however, as the disease progresses, females exhibit faster cognitive decline16,26 and progression to dementia22,27-29 than males. Despite prior evidence, few studies have examined the role of sex/gender on plasma biomarkers in sporadic AD, and none in autosomal dominant AD.
Findings from community and population-based AD studies found that levels of plasma P-tau217 did not differ between males and females2,5, while a recent study showed that higher plasma P-tau181 was associated with greater amyloid and entorhinal cortex tau accumulation, lower brain glucose metabolism, and faster cognitive decline in females, relative to males30. Research examining the effect of sex/gender on plasma biomarkers of neurodegeneration in AD cohort studies have also yielded mixed findings. Several studies have not found sex/gender differences in plasma NfL5,12,31 or total tau32,33. In contrast, other studies found higher levels of total tau in females31,34,35, whereas, a longitudinal study with healthy individuals with subjective concerns found higher levels of total tau in older males than younger males, but not in females31. Taken together, more research is needed to examine how sex/gender is associated with blood biomarkers and cognition across disease stages to further determine the usefulness of blood biomarkers in early detection, disease progression, and development of treatments in AD, and ultimately, to update current appropriate use recommendations36.
For this study, we used available cross-sectional blood-based biomarker samples to examine sex differences in the age-related trajectory of plasma P-tau217 and NfL levels in PSEN1 mutation carriers and age-matched non-carriers. We also examined the effect of sex on the associations between plasma biomarkers and cognitive performance among PSEN1 mutation carriers.
Methods
Participants & Procedures
A total of 621 participants were included in the study, including 259 cognitively unimpaired mutation carriers (mean age: 31, range 24-39; %male: 45.6), 106 cognitively impaired mutation carriers (mean age: 49, range 46-52; %male: 45.3), and 256 age- and sex-matched, cognitively unimpaired non-carriers from the same kindred (mean age: 34, range 25.3-42; %male: 39.5) who were enrolled from December 2013 to February 2017 in the Colombian Alzheimer Prevention Initiative (API) Registry37. In brief, this registry aims to locate, enroll, genotype, and perform medical and cognitive evaluations of PSEN1 E280A family members, and includes individuals with early-onset, potentially familial AD and healthy relatives, age 8 and older. Only those who were 18 years old or above were included in the present study.
Nearly all PSEN1 mutation carriers develop early-onset AD, with mild cognitive impairment (MCI) symptoms emerging at a median age of 44 years and dementia at 49 years38. Participants were considered cognitively unimpaired if they had a MMSE39 score ≥26 points, a functional assessment staging test (FAST)40 score ≤2, and no cognitive impairment on the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) battery41. Cognitive impairment was defined as a FAST score of ≥3 or MCI or dementia due to AD42,43. Clinical diagnoses were determined by an interdisciplinary team of dementia specialists (i.e., neurologists, neuropsychologists). Individuals with significant medical, psychiatric, or neurological disorders were excluded. Participants in the study reported their sex assigned at birth (i.e., male/female).
This study was approved by the institutional review board at the University of Antioquia, Colombia, including procedures undertaken outside the University of Antioquia. Informed written consent for participation and the use of data and samples was obtained from cognitively unimpaired adult participants, or from a legal representative (i.e., partner or offspring) of cognitively impaired participants. Participants included a representative sample from the region of Antioquia, Colombia. Participants were not excluded in the basis of sex, gender, race or ethnicity. Participants and investigators acquiring and analyzing data were blind to genetic status. All participants completed clinical and cognitive assessments, and blood samples were collected within 6 months of cognitive evaluations as part of the registry procedures.
Plasma Sampling
Plasma was collected in the morning (without fasting) at the University of Antioquia. Three aliquots of 1 mL were collected. Samples were centrifuged, stored at −80°C and shipped on dry ice for analysis. Plasma NfL concentration was measured at the Clinical Neurochemistry Laboratory, University of Gothenburg, Sweden, using the NF-Light kit on a Single molecule array (Simoa) HD-X Analyzer (Quanterix, Billerica, MA) according to the manufacturer’s instructions, as previously described44. Measurements were done by board-certified laboratory technicians. Calibrators were run in duplicates, and obvious outlier calibrator replicates were masked before curve fitting. Samples were diluted four-fold and run in singlicates. All measurements were made without information on any clinical data. The dynamic range of the assay was 1.9–1800 pg/mL. Two QC plasma samples were run in duplicates in the beginning and the end of each run. For the QC sample with a concentration of 10.8 pg/mL, repeatability was 4.8% and intermediate precision 6.2%, while for the QC sample with a concentration of 47.7 pg/mL, repeatability was 3.3% and intermediate precision 4.6%. One batch of reagents and one instrument was used to analyze all samples.
For P-tau217, concentrations of plasma P-tau217 were measured using immunoassays at Lilly Research Laboratories, using the MSD platform (Meso Scale Discovery), as previously described4. Biotinylated-IBA493 was used as a capture antibody and SULFO-TAG-4G10-E2 (anti-Tau) as the detector. The assay was calibrated using a recombinant tau (4R2N) protein that was phosphorylated in vitro using a reaction with glycogen synthase kinase-3 and characterized by mass spectrometry. Additional details of the plasma P-tau217 analysis are described in Palmqvist et al., 2020, Supplemental Material. Plasma samples from study participants were analyzed in duplicates with a mean intra-assay coefficient of variation (CV) of 13.9%. The mean inter-assay CVs of quality control samples were 3.4-5.5%. The lower limit of detection of the plasma P-tau217 assay was 0.48 pg/mL.
For genetic analyses, genomic DNA was extracted from blood by standard protocols, and PSEN1 E280A characterization was done at the University of Antioquia using methods described previously45. NfL analyses were supervised by co-authors Zetterberg and Blennow at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital (Sweden), and P-tau217 analyses by co-author Dage at Lilly Research Laboratories (USA).
Clinical & Cognitive Assessments
Clinical and cognitive assessments were undertaken at the University of Antioquia (Medellín, Colombia). Participants completed a battery of clinical and cognitive measures in Spanish, adapted by the Neurosciences Group of Antioquia (GNA) to characterize this Colombian population, including the MMSE, the Spanish CERAD battery, the Functional Assessment Staging Test, and the Geriatric Depression Scale46. Testing was done in Spanish by neuropsychologists or psychologists trained in neuropsychological assessment. We calculated the Alzheimer Prevention Initiative (API) global cognition composite score, which includes the MMSE (Orientation to Time), Boston Naming Test (15-item), Ravens Progressive Matrices (12-item), CERAD Word List (Delayed Recall), and Constructional Praxis (Copy). This cognitive composite score has been shown to track preclinical AD decline in autosomal dominant AD47. Clinical histories and neurological examinations were completed by neurologists or physicians trained in assessing neurodegenerative disorders.
Statistical Analyses
We compared demographic, clinical, neuroimaging, and cognitive data among males and females stratified by PSEN1 mutation status (i.e., carriers and non-carriers) using t-tests. Because plasma P-tau217 and NfL data were not normally distributed, plasma biomarker data was log transformed. To examine the age-related trajectory of plasma biomarkers, a proxy for disease progression in this kindred, we used multiple linear regression models to estimate the effect of age, sex, and the interaction between sex and age on P-tau217 and NfL levels, stratified by PSEN1 mutation carriers and non-carriers. Bivariate local polynomial regressions (LOESS) were used to characterize relationships between log-transformed plasma P-tau217 and NfL with age. We then used multiple linear regressions to examine whether sex modified the relationship between plasma biomarkers and cognitive performance among PSEN1 carriers. Models included plasma biomarkers – P-tau217 and NfL – as the independent variable, cognitive measures – API cognitive composite score and CERAD word list delayed recall – as dependent variables, sex as a covariate of interest, and the interaction terms between sex and plasma biomarkers (i.e., Sex*P-tau217 and Sex*NfL). Subsequent models were run controlling for age. To further understand the effect of age, a proxy for disease progression among PSEN1 mutation carriers, secondary analyses were conducted stratifying by disease stage (i.e., cognitively unimpaired carriers and cognitively impaired carriers). Analyses used a significance threshold of p < 0.05. Analyses were performed by a team of biostatisticians who were unblinded to genotype but had no role in the study design or data collection. Statistical analyses were done using R (version 4.0.2, The R Foundation) and SPSS version 24. Of note, a female non-carrier participant had NfL values >2 standard deviations above the non-carrier mean and was removed from the analyses.
Results
Sample Characteristics
Overall sample characteristics of male and female PSEN1 cognitively unimpaired carriers, impaired carriers and non-carriers are described in Table 1. There were no differences in age, years of education, MMSE score, API cognitive composite score, or CERAD word list delayed recall between male and female PSEN1 carriers and non-carriers, and the distribution of males and females did not differ between carriers and non-carriers. Plasma P-tau217 levels did not differ between males and females among cognitively unimpaired carriers, cognitively impaired carriers, or non-carriers. Compared to males, female carriers had lower NfL levels among cognitively unimpaired, but higher NfL levels among impaired carriers.
Table 1.
Demographic, clinical, cognitive, and biomarkers among male and female PSEN1 mutation carriers and non-carriers
| Non-carriers | Cognitively Unimpaired Carriers | Cognitively Impaired Carriers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | p a | Mean±SD | p b | Mean±SD | p c | ||||
| Males n = 101 |
Females n = 155 |
Male n = 118 |
Female n = 141 |
Male n = 48 |
Female n = 58 |
||||
| Age, years | 33.42±10.29 | 35.08±9.53 | .186 | 31.98±9.14 | 31.70±9.26 | .813 | 49.04±4.63 | 48.85±4.59 | .827 |
| Education, years | 8.13±4.72 | 8.46±4.32 | .566 | 7.90±4.43 | 8.77±4.24 | .109 | 4.85±3.78 | 5.36±3.66 | .485 |
| Body Mass Index, kg/m2 | 22.74±2.89 | 23.88±3.76 | .059 | 22.40±3.17 | 23.15±3.40 | .142 | 22.51±2.79 | 23.47±2.57 | .172 |
| MMSE score | 28.78±1.70 | 28.83±1.97 | .846 | 28.80±1.55 | 28.67±1.87 | .548 | 19.50±7.02 | 16.85±7.16 | .083 |
| API Cognitive Composite Score | 82.59±11.23 | 81.65±10.08 | .501 | 79.51±12.81 | 79.05±12.26 | .768 | 36.75±14.74 | 34.63±14.28 | .495 |
| CERAD Word List Delayed Recall | 6.30±1.88 | 6.40±2.00 | .708 | 5.59±2.11 | 5.98±2.04 | .136 | 0.76±1.37 | 0.88±1.45 | .697 |
| Plasma P-tau217, pg/ml | 1.92±1.46 | 1.85±1.45 | .696 | 4.69±3.95 | 4.42±5.78 | .662 | 16.14±6.81 | 17.42±7.20 | .352 |
| Plasma NfL, pg/ml | 7.33±3.98 | 6.57±2.91 | .082 | 9.74±6.34 | 7.97±4.45 | .009 | 24.92±12.11 | 33.32±24.12 | .023 |
Note. Abbreviations: MMSE, Mini-Mental Status Exam; CERAD, Consortium to Establish a Registry for AD; API, Alzheimer Prevention Initiative.
p-value as defined by an independent two sample t-test for male vs female non-carriers.
p-value as defined by an independent two sample t-test for male vs female cognitively unimpaired mutation carriers.
p-value as defined by an independent two sample t-test for male vs female symptomatic mutation carriers.
Effect of sex in the age trajectory of plasma biomarkers
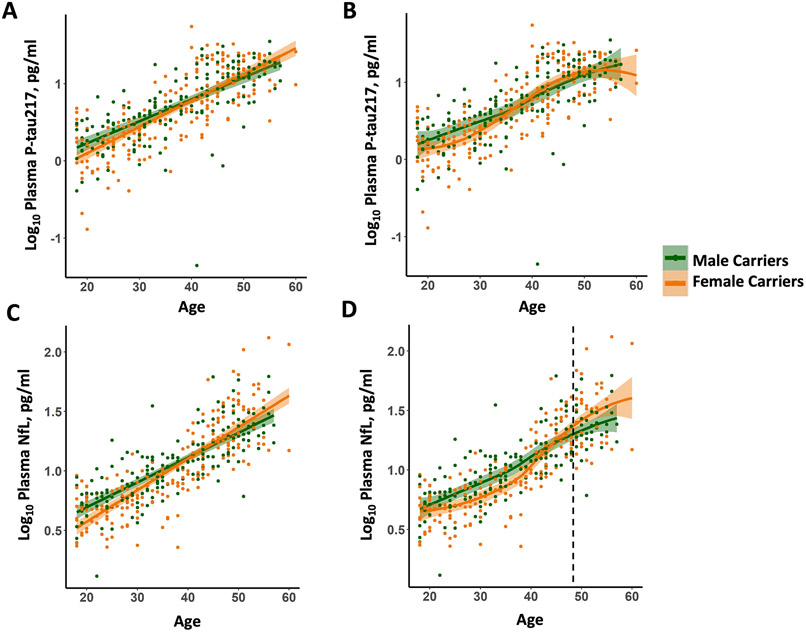
We examined the association between plasma biomarkers and age in PSEN1 mutation carriers. There were no sex differences in the association between age and plasma P-tau217 among PSEN1 mutation carriers; female carriers showed a trend towards higher P-tau217 levels than male carriers (Figure 1A & 1B; Age*Sex, β=1.885, p=.060). Female carriers showed a steeper slope for the association between age and plasma NfL than male carriers (Figure 1C; Age*Sex, β=2.999, p<.003). LOESS plots showed that younger female carriers have lower levels of plasma NfL, but then show a greater increase than male carriers (Figure 1D). Specifically, male and female carriers’ LOESS fit confidence band separated at age 48.45. There were no differences between non-carrier males and females in the age trajectory of plasma P-tau217 or NfL (Supplemental Figure 1; Age*Sex: P-tau217, β=−.319, p=.750; NfL, β=−.797, p=.426).
Figure 1. Age trajectories of plasma P-tau217 and NfL in male and female PSEN1 mutation carriers.
(A) Log10 Plasma P-tau217 as a function of age. (B) LOESS plot of Log10 Plasma P-tau217 as a function of age. (C) Log10 Plasma NfL as a function of age. (D) LOESS plot of Log10 Plasma NfL as a function of age. Dashed line represents the age at which male and female carriers LOESS fit confidence band separate (age of 48.45). Abbreviations: NfL, Neurofilament light chain; P-tau217, Plasma-measured tau phosphorylated at threonine 217. Orange represents female carriers and green represents male carriers.
Effect of sex in the association between P-tau217 & cognitive performance
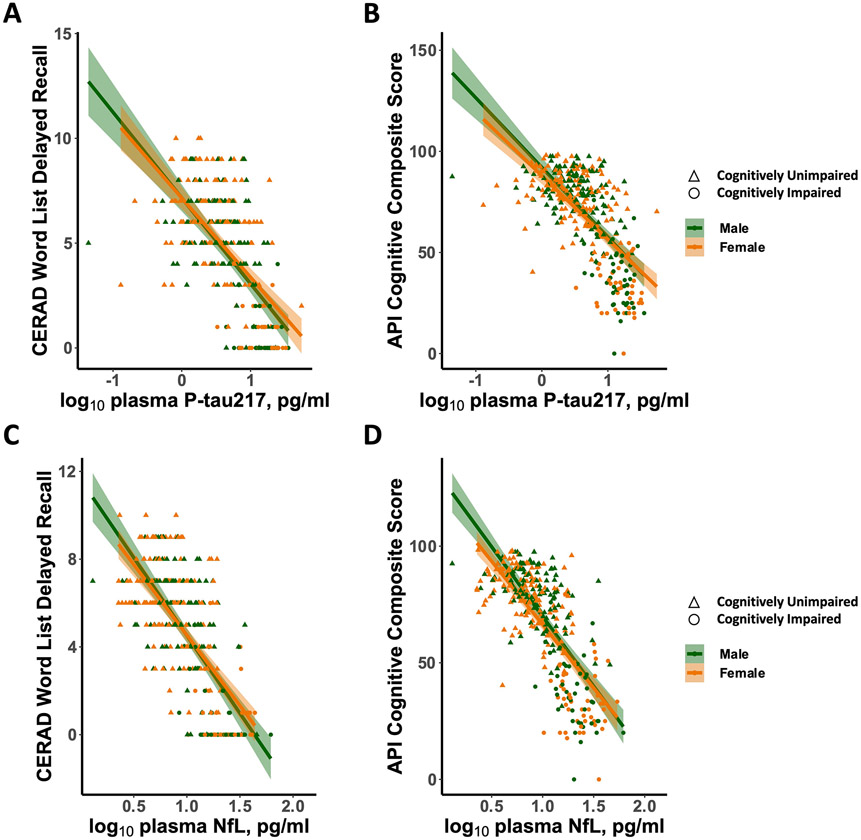
We examined the effect of sex on the association between plasma P-tau217 and cognition among PSEN1 mutation carriers. There was a significant interaction effect between sex and plasma P-tau217 in predicting cognitive performance, whereby as levels of P-tau217 increase, male carriers had worse API cognitive composite scores than female carriers (Table 2; Figure 2B; Sex*P-tau217: β=2.73, p=.007). This effect dissipated when controlling for age (Sex*P-tau217: β=1.537, p=.12507). Similarly, there was a significant interaction effect between sex and plasma P-tau217 in predicting memory performance, whereby as levels of P-tau217 increase, male carriers had worse CERAD word list delayed recall than female carriers (Figure 2A; Sex*P-tau217: β=2.093, p=.037). This effect dissipated when controlling for age (Sex*P-tau217: β=1.039, p=.299).
Table 2.
Regression estimates of the effect of sex on the relationship between plasma biomarkers and cognition among PSEN1 mutation carriers with and without age as a covariate.
| Model 1 | Model 2 (Adjusting for Age) | ||||
|---|---|---|---|---|---|
| Outcome Variable | Predictors | Standardized β | p-value | Standardized β | p-value |
| API Cognitive Composite Score | Sex | −2.09 | .037 | −1.524 | .129 |
| Plasma P-tau217 | −7.683 | <.001 | −4.357 | <.001 | |
| Sex*P-tau217 | 2.73 | .007 | 1.537 | .125 | |
| Age | . | . | −12.142 | <.001 | |
| CERAD Word List Delayed Recall | Sex | −.285 | .775 | .396 | .692 |
| Plasma P-tau217 | −6.909 | <.001 | −3.910 | <.001 | |
| Sex*P-tau217 | 2.093 | .037 | 1.039 | .299 | |
| Age | . | . | −9.923 | <.001 | |
| API Cognitive Composite Score | Sex | −1.492 | .137 | −1.030 | .304 |
| Plasma NfL | −6.846 | <.001 | −3.610 | <.001 | |
| Sex*NfL | .943 | .347 | .611 | .541 | |
| Age | . | . | −8.065 | <.001 | |
| CERAD Word List Delayed Recall | Sex | −.805 | .421 | −.404 | .686 |
| Plasma NfL | −6.075 | <.001 | −3.214 | .001 | |
| Sex*NfL | .858 | .391 | .624 | .533 | |
| Age | . | . | −6.957 | <.001 | |
Note. Abbreviations: PSEN1 status, PSEN1 Carriers/Non-carriers; P-tau217, Plasma P-tau217 pg/ml; NfL, Plasma NfL pg/ml; API, Alzheimer Prevention Initiative; CERAD, Consortium to Establish a Registry for AD. Bold text represents p-value <.05.
Figure 2. Associations between plasma P-tau217 and NfL and cognitive performance in male and female PSEN1 mutation carriers.
(A) CERAD word list delayed recall as a function of Log10 Plasma P-tau217. (B) API cognitive composite score as a function of Log10 Plasma P-tau217. (C) CERAD word list delayed recall as a function of Log10 Plasma NfL. (D) API cognitive composite score as a function of Log10 Plasma NfL. Abbreviations: NfL, Neurofilament light chain; P-tau217, Plasma-measured tau phosphorylated at threonine 217; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; API, Alzheimer Prevention Initiative. Orange represents female carriers, and green represents male carriers. Circles represent cognitively unimpaired carriers, and triangles represent cognitively impaired carriers.
To further understand the effect of age in the association between P-tau217 and cognition, given that age is a proxy for disease progression in PSEN1 mutation carriers, we conducted secondary analyses stratified by disease stage. Results showed that among cognitively unimpaired mutation carriers, as levels of P-tau217 increase, males had worse API cognitive composite scores (Table 3; Supplemental Figure 2; Sex*P-tau217: β=3.096, p=.002) and worse CERAD word list delayed recall (Sex*P-tau217: β=2.454, p=.015) than females. In contrast, among cognitively impaired carriers, the interaction effect between sex and plasma P-tau217 did not predict API cognitive composite scores (Sex*P-tau217: β=.271, p=.787) or CERAD word list delayed recall (Sex*P-tau217: β=−1.164, p=.870).
Table 3.
Regression estimates of the effect of sex on the relationship between plasma biomarkers and cognition among cognitively unimpaired and impaired PSEN1 mutation carriers.
| Cognitively Unimpaired Carriers | Cognitively Impaired Carriers | ||||
|---|---|---|---|---|---|
| Outcome Variable | Predictors | Standardized β | p-value | Standardized β | p-value |
| API Cognitive Composite Score | Sex | −2.553 | .011 | −.444 | .658 |
| Plasma P-tau217 | −4.541 | <.001 | −1.055 | .294 | |
| Sex*P-tau217 | 3.096 | .002 | .271 | .787 | |
| CERAD Word List Delayed Recall | Sex | −.741 | .459 | .415 | .679 |
| Plasma P-tau217 | −4.077 | <.001 | −.967 | .337 | |
| Sex*P-tau217 | 2.454 | .015 | −.164 | .870 | |
| API Cognitive Composite Score | Sex | −1.077 | .283 | −1.346 | .182 |
| Plasma NfL | −3.303 | .001 | −2.154 | .034 | |
| Sex*NfL | .610 | .543 | 1.314 | .192 | |
| CERAD Word List Delayed Recall | Sex | −.575 | .566 | −.640 | .524 |
| Plasma NfL | −2.917 | .004 | −1.571 | .120 | |
| Sex*NfL | .686 | .493 | .731 | .467 | |
Note. Abbreviations: PSEN1 status, PSEN1 Carriers/Non-carriers; P-tau217, Plasma P-tau217 pg/ml; NfL, Plasma NfL pg/ml; API, Alzheimer Prevention Initiative; CERAD, Consortium to Establish a Registry for AD. Bold text represents p-value <.05.
Effect of sex in the association between NfL & cognitive performance
We examined the effect of sex on the association between plasma NfL and cognition among all PSEN1 mutation carriers and found that the interaction effect between sex and NfL did not predict API cognitive composite scores (Figure 2D; Sex*NfL: β=0.943, p=.347; Sex*NfL, controlling for age: β=.611, p=.541) or CERAD word list delayed recall (Figure 2C; Sex*NfL: β=.858, p=.391; Sex*NfL, controlling for age: β=−.624, p=.533).
Secondary analyses stratified by disease stage showed that among cognitively unimpaired mutation carriers, the sex by NfL interaction did not predict API cognitive composite scores (Table 3; Sex*NfL: β=.610, p=.542) or CERAD word list delayed recall (Sex*NfL: β=.686, p=.493). Similarly, among impaired carriers, the sex by NfL interaction did not predict API cognitive composite scores (Sex*NfL: β=1.314, p=.192) or CERAD word list delayed recall (Sex*NfL: β=.731, p=.467).
Discussion
Plasma tau phosphorylated at threonine 217 (P-tau217) and neurofilament light (NfL) have been established as early markers of tau pathology2-6 and neurodegeneration8-11,48 that could be used as tools for diagnosis, disease monitoring, and clinical response to treatment49. Growing evidence has demonstrated sex differences in AD pathology and cognitive progression in sporadic50 and autosomal dominant AD51,52. While blood biomarkers hold promise for utility in AD research and clinical trials, few studies have specifically examined the role of sex in plasma biomarkers, yielding mixed findings. To address this gap, we leveraged cross-sectional blood-based biomarker samples to examine the effect of sex in the age-related trajectory of plasma P-tau217 and NfL, and their association with cognitive performance in PSEN1 E280A mutation carriers (PSEN1) and age-matched non-carriers. As nearly all PSEN1 mutation carriers develop mild cognitive impairment at a median age of 44 years and dementia at 49 years38, age in this sample is a proxy for disease progression, and we can estimate disease biomarker trajectories by modeling the effects of age.
Our results found a sex effect in the age-related trajectory of plasma NfL and found that female PSEN1 mutation carriers exhibited a significantly greater rate of plasma NfL increase than male carriers, starting around age 48. Previous studies have shown that NfL levels increase with age13; however, there were no sex differences in the association between age and NfL accumulation among non-carriers, suggesting that this effect may be disease-related. This study is the first to provide evidence that, among PSEN1 mutation carriers in preclinical and clinical stages of AD, females have a greater rate of neurodegeneration than males as disease progresses. Previous research examining plasma biomarkers of neurodegeneration had yielded mixed findings, with some showing higher levels of total tau in females15,19,20, whereas others did not find sex differences in NfL12,31 or total tau levels32,33. Our findings support previous work in sporadic AD showing faster hippocampal volume loss16 and greater brain glucose hypometabolism17 in females, compared to males, and help clarify previous inconsistencies in plasma biomarker studies. Assay differences may have contributed to prior mixed findings, as for instance, the total tau assay used in many studies (Simoa hTau Assay) is prone to variability due to differences in pre-analytical sample handling53. Moreover, most studies examined cross-sectional levels of plasma NfL and did not specifically examine the interaction between sex and plasma biomarkers, highlighting the importance of examining the effect of sex on biomarker trajectories across the disease spectrum.
The mechanisms underlying the faster neurodegeneration in female PSEN1 mutation carriers remain unknown. Several factors have been proposed to explain sex differences in sporadic and late-onset AD, including genetic factors54,55 (e.g., APOE4), inflammation56, cardiovascular disease57, and hormonal changes58. Notably, sex differences in the rate of NfL accumulation were observed starting around age 48, approximately 3 years before average menopause age59. However, perimenopausal changes begin 8–10 years before menopause, during which sex hormones fluctuate, followed by a decline in estrogen and progesterone60. Previous studies showed that reduced estrogen levels were associated with increased amyloid61,62 and tau burden63, and greater neurodegeneration64. More research is needed to investigate sex-specific mechanisms of risk and resilience to AD, including the role of sex steroid hormones, cardiovascular disease (and interactive effects)65 on AD biomarker trajectories and cognitive decline.
We also investigated the effect of sex in the age-related trajectory of plasma P-tau217 and found that there was no difference between male and female PSEN1 mutation carriers. Our results are consistent with previous cross-sectional studies showing that males and females did not differ in plasma P-tau217 levels2,5 in sporadic AD. Notably, these findings diverge from previous studies showing higher levels of tau pathology in females in cerebrospinal fluid18, PET imaging 19,66-69 and postmortem data21,22,70. There is a possibility that our study was underpowered to detect sex differences in the age-related accumulation of P-tau217, as female carriers showed a trend towards increased plasma P-tau217 levels, consistent with prior studies in sporadic AD using non-plasma biomarkers; however, this effect did not reach statistical significance. Furthermore, recent evidence suggests that plasma P-tau217 measures the earliest accumulation of both amyloid-beta and soluble hyperphosphorylated tau concentrations2 that occur even before insoluble tau aggregates can be detected using tau PET3,71,72, suggesting that sex differences previously shown in tau burden may appear in more advanced disease stages, as most individuals in our study were cognitively unimpaired. Alternatively, our findings may be specific to autosomal dominant AD. These findings warrant further investigation in other autosomal dominant and sporadic AD cohorts to clarify the effect of sex on plasma biomarkers, potential sex-specific confounding measurement factors73 (e.g., medical comorbidities), and underlying mechanisms and pathology clearance pathways74 that may explain differences between biomarker modalities and prior findings in sporadic AD. Of note, very few PSEN1 mutation carriers in our sample had medical comorbidities (e.g., history of cardiovascular, renal, or hepatic conditions), as expected given their young age of symptom onset.
We then examined the effect of sex on the relationship between plasma biomarkers and cognition in PSEN1 mutation carriers. Our findings show that, as P-tau217 levels increase, male carriers exhibited a steeper decline in global cognitive and memory performance than female carriers. Notably, this effect dissipated when adjusting for age. Given that in this kindred, age is a proxy for disease progression, we conducted secondary analyses stratified by disease stage. Results showed that the sex by P-tau217 interaction remained significant in predicting global cognitive and memory performance among cognitively unimpaired mutation carriers, but not among cognitively impaired mutation carriers, suggesting that this effect may be specific to early disease stages. In contrast, sex did not modify the relationship between NfL levels and cognitive performance, even though female carriers had a greater NfL increase with age, than male carriers. Taken together, these findings suggest a sex-specific cognitive resilience in female carriers to early accumulation of plasma P-tau2173, that may dissipate as disease progresses. Supporting this notion, previous work in sporadic AD showed that females may have greater cognitive resilience to early AD-pathology and neurodegeneration23-25, whereas the disease progresses, females exhibit faster cognitive decline16,20,26 and progression to dementia22,27-29 than males. Similarly, previous work from our group showed that among cognitively unimpaired PSEN1 mutation carriers and non-carriers, females may have greater cognitive resilience to AD-pathology and neurodegeneration than males51. It is important to note that our findings may be (at least to some extent) driven by female’s verbal memory advantage, as memory performance was assessed verbally, using the CERAD word list, which was also included in the API cognitive composite score to measure global cognitive performance. Our current findings expand on prior work by examining these associations using plasma biomarkers in a sample of cognitively unimpaired and impaired PSEN1 carriers. Future work as part of the Colombia-Boston Biomarker longitudinal study of autosomal dominant AD (COLBOS), will help clarify the effect of sex and AD pathology on cognitive decline across the disease spectrum and elucidate mechanisms of AD risk and resilience.
This study has some limitations. First, this is a cross-sectional, retrospective study that leveraged available blood biomarker data. However, this study includes a large sample of cognitively unimpaired and impaired individuals from a homogeneous cohort with a single PSEN1 mutation (E280A) who have a well-characterized clinical trajectory. As PSEN1 E280A mutation carriers are virtually destined to develop mild cognitive impairment starting at a median age of 44 years and dementia at 49 years38, age in this sample is a proxy for disease progression. Thus, cross-sectional assessments are analogous to the assessment of longitudinal trajectories of biomarkers and cognition. As mentioned, this study focused on plasma P-tau217 and NfL, however there are several other emerging blood biomarkers, including P-tau181, P-tau231, N-terminal fragment of tau (NT1), or glial fibrillary acidic protein (GFAP), among others. It is also worth noting that different assays were used to measure P-tau217 and NfL levels that may have contributed to our findings. In addition, this study did not examine plasma levels of sex hormones or reproductive health factors, or other data on other potential mechanisms. Further research examining sex differences in plasma P-tau217 and NfL, as well as other blood biomarkers, and its relation to cognitive trajectories is needed to elucidate mechanisms of risk and resilience in AD, including the role of sex hormones, reproductive and neuroendocrine health factors, or genetic factors. Lastly, replication of our results in independent cohorts will be required to determine generalizability to other at-risk groups for autosomal dominant AD and sporadic AD.
Conclusion
Findings from this cross-sectional study suggest that, among PSEN1 mutation carriers, as levels of plasma P-tau217 increase, cognitively unimpaired females showed better cognitive performance than cognitively unimpaired males. Yet, as disease progresses, female carriers had a greater plasma NfL increase than male carriers, indicative of faster rate of neurodegeneration. Our work highlights the need for further research examining sex/gender differences in blood biomarkers and their relations to cognitive decline to clarify AD pathophysiology and inform the use of blood biomarkers in clinical research, trials, and practice.
Supplementary Material
Acknowledgements
The authors thank the PSEN1 Colombian families for contributing their valuable time and effort, without which this study would not have been possible. We thank the research staff of the Group of Neuroscience of Antioquia for their help coordinating study visits for the Colombian API Registry.
Funding Sources
CVC is supported by the National Institute on Aging (K99AG073452). SL is supported by a grant from the Alzheimer’s Association (AARF-22-920754). YTQ is supported by grants from the National Institute on Aging (R01 AG054671, RF1AG077627], the Alzheimer’s Association, and Massachusetts General Hospital ECOR. OH is funded by the Swedish Research Council (2016-00906), the Knut and Alice Wallenberg foundation (2017-0383), the Marianne and Marcus Wallenberg foundation (2015.0125), the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation (AF-939932), the Swedish Brain Foundation (FO2021-0293), The Parkinson foundation of Sweden (1280/20), the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, the Skåne University Hospital Foundation (2020-O000028), Regionalt Forskningsstöd (2020-0314) and the Swedish federal government under the ALF agreement (2018-Projekt0279). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712 and #101053962), Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), and the UK Dementia Research Institute at UCL (UKDRI-1003). YS reports grants from NIH/NIBIB, The Alzheimer’s Association, The BrightFocus Foundation, NIH/NIA, State of Arizona, personal fees from Green Valley Pharmaceutical LLC, outside the submitted work. LRG reports grants from The Alzheimer’s Association and NIH/NIA. FL was supported by an Anonymous Foundation, and the Administrative Department of Science, Technology and Innovation (Colciencias Colombia;111565741185). ERM, FL, PT are principal investigators of the Alzheimer’s Prevention Initiative (API) Autosomal Dominant AD Trial, which is supported by NIA, philanthropy, Genentech, and Roche. KB is supported by the Swedish Research Council (#2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721 and #AF-968270), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986 and #ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the National Institute of Health (NIH), USA, (grant #1R01AG068398-01), and the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495). EMR reports grants from National Institute on Aging (R01 AG031581, P30 AG19610), Banner Alzheimer’s Foundation and the NOMIS Foundation during the conduct of the study. SRR was supported by NIA, NIH and Genentech/Roche.
Footnotes
Disclosures
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). JD is an inventor on patents or patent applications of Eli Lilly and Company relating to the assays, methods, reagents and / or compositions of matter used in this work. JD has served as a consultant for Karuna Therapeutics and received research support from ADx Neurosciences, Roche Diagnostics and Eli Lilly and Company. OH has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens. EMR reports receiving personal fees as a Scientific Advisor to Roche Diagnostics (travel expenses only), MagQ, Avid Radiopharmaceuticals and is a share-holding co-founder of ALZPath, outside the submitted work. In addition, he is the inventor of a patent issued to Banner Health, which involves the use of biomarker endpoints in at-risk persons to accelerate the evaluation of Alzheimer’s disease prevention therapies and is outside the submitted work. PT reports personal fees from Abbvie, AC Immune, Acadia, Auspex, Boehringer Ingelheim, Eisai, Genentech, GliaCure, Merck, Novo Nordisk, T3D Therapeutics, grants and AstraZeneca, grants and personal fees from Biogen, Roche, grants from Genentech, Novartis, and Arizona Department of Health Services, outside the submitted work. In addition, PT has a patent U.S. Patent # 11/632,747, “Biomarkers of Neurodegenerative disease.” KB has served as a consultant or at advisory boards for Abcam, Axon Neuroscience, BioArctic, Biogen, Lilly, MagQu, Novartis, Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. YTQ has served as consultant for Biogen. The other co-authors declare no competing interests.
Consent Statement
This study was approved by the institutional review board at the University of Antioquia, Colombia, including procedures undertaken outside the University of Antioquia. Informed written consent for participation and the use of data and samples was obtained from cognitively unimpaired adult participants, or from a legal representative (i.e., partner or offspring) of cognitively impaired participants. Participants included a representative sample from the region of Antioquia, Colombia. Participants were not excluded in the basis of gender, race or ethnicity.
References
- 1.Cullen NC, Leuzy A, Janelidze S, et al. Plasma biomarkers of Alzheimer’s disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nature communications. 2021;12(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mielke MM, Frank RD, Dage JL, et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA neurology. 2021;78(9):1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho-Tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA neurology. 2021;78(2):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. Jama. 2020;324(8):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer's disease biomarkers in a multi-ethnic, community study. Alzheimer's & Dementia. 2021;17(8):1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer's disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. The Lancet Neurology. 2021/September/01/ 2021;20(9):739–752. doi: 10.1016/S1474-4422(21)00214-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashton NJ, Janelidze S, Mattsson-Carlgren N, et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nature medicine. 2022:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattsson N, Andreasson U, Zetterberg H, Blennow K, Initiative AsDN. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA neurology. 2017;74(5):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedet AL, Leuzy A, Pascoal TA, et al. Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer’s disease. Brain. 2020;143(12):3793–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nature medicine. 2019;25(2):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weston PS, Poole T, Ryan NS, et al. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology. 2017;89(21):2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA neurology. 2019;76(7):791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quiroz YT, Zetterberg H, Reiman EM, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross-sectional and longitudinal cohort study. The Lancet Neurology. 2020;19(6):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzmán-Vélez E, Zetterberg H, Fox-Fuller JT, et al. Associations between plasma neurofilament light, in vivo brain pathology, and cognition in non-demented individuals with autosomal-dominant Alzheimer's disease. Alzheimer's & Dementia. 2021;17(5):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguillon D, Langella S, Chen Y, et al. Plasma p-tau217 predicts in vivo brain pathology and cognition in autosomal dominant Alzheimer's disease. Alzheimer's & Dementia. 2022:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koran MEI, Wagener M, Hohman TJ. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging and Behavior. 2017;11(1):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanan VK, Castillo AM, Knopman DS, et al. Association of Apolipoprotein E ε4, Educational Level, and Sex With Tau Deposition and Tau-Mediated Metabolic Dysfunction in Older Adults. JAMA Network Open. 2019;2(10):e1913909–e1913909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohman T, Dumitrescu L, Barnes L, et al. Sex-Specific Association of Apolipoprotein E with Cerebrospinal Fluid Levels of Tau. JAMA Neurology. 2018;75(8):989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley RF, Mormino EC, Rabin JS, et al. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured By Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurology. Feb 4 2019;76(5):542–551. doi: 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley RF, Scott MR, Jacobs HI, et al. Sex mediates relationships between regional tau pathology and cognitive decline. Annals of Neurology. 2020;88(5):921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathologica. 2018;136(6):887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Archives of General Psychiatry. 2005;62(6):685–691. [DOI] [PubMed] [Google Scholar]
- 23.Sundermann EE, Biegon A, Rubin LH, et al. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology. 2016;86(15):1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundermann EE, Maki PM, Reddy S, Bondi MW, Biegon A, Initiative AsDN. Women's higher brain metabolic rate compensates for early Alzheimer's pathology. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2020;12(1):e12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Digma LA, Madsen JR, Rissman RA, Jacobs DM, Brewer JB, Banks SJ. Women can bear a bigger burden: Ante-and post-mortem evidence for reserve in the face of tau. Brain Communications. 2020;2(1):fcaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckley RF, Mormino EC, Amariglio RE, et al. Sex, amyloid, and APOE epsilon4 and risk of cognitive decline in preclinical Alzheimer's disease: Findings from three well-characterized cohorts. Alzheimer's & Dementia. Sep 2018;14(9):1193–1203. doi: 10.1016/j.jalz.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin KA, Choudhury KR, Rathakrishnan BG, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimer's & dementia: translational research & clinical interventions. 2015;1(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer's disease: a meta analysis. Journal of clinical and experimental neuropsychology. 2012;34(9):989–998. [DOI] [PubMed] [Google Scholar]
- 29.Holland D, Desikan RS, Dale AM, McEvoy LK. Higher rates of decline for women and apolipoprotein E ε4 carriers. American Journal of Neuroradiology. 2013;34(12):2287–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsiknia AA, Edland SD, Sundermann EE, et al. Sex differences in plasma p-tau181 associations with Alzheimer’s disease biomarkers, cognitive decline, and clinical progression. Molecular Psychiatry. 2022/June/29 2022;27:4314–4322. doi: 10.1038/s41380-022-01675-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldacci F, Lista S, Manca ML, et al. Aging and sex impact plasma NFL and t-Tau trajectories in individuals at risk for Alzheimer’s disease: Biomarkers (non-neuroimaging)/plasma/serum/urine biomarkers. Alzheimer's & Dementia. 2020;16:e041792. [Google Scholar]
- 32.Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87(17):1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dage JL, Wennberg AM, Airey DC, et al. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimer's & Dementia. 2016;12(12):1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pase MP, Beiser AS, Himali JJ, et al. Assessment of plasma total tau level as a predictive biomarker for dementia and related endophenotypes. JAMA neurology. 2019;76(5):598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syrjanen JA, Campbell MR, Algeciras-Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimer's & Dementia. 2021;18:1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimer's & Dementia. 2022;18:2669–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rios-Romenets S, Lopez H, Lopez L, et al. The Colombian Alzheimer's Prevention Initiative (API) Registry. Alzheimer's & Dementia. 2017;13(5):602–605. [Google Scholar]
- 38.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer's disease: a retrospective cohort study. The Lancet Neurology. 2011;10(3):213–220. [DOI] [PubMed] [Google Scholar]
- 39.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. Nov 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 40.Reisberg B. Functional assessment staging (FAST). Psychopharmacology Bulletin. 1988;24(4):653–659. [PubMed] [Google Scholar]
- 41.Aguirre-Acevedo D, Gomez R, Moreno S, et al. Validity and reliability of the CERAD-Col neuropsychological battery. Revista de Neurologia. 2007;45(11):655–660. [PubMed] [Google Scholar]
- 42.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer's & Dementia 2011;7(3): 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKhann G, Knopman D, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2016;3:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lendon CL, Martinez A, Behrens IM, et al. E280A PS-1 mutation causes Alzheimer's disease but age of onset is not modified by ApoE alleles. Human Mutation. 1997;10(3):186–195. [DOI] [PubMed] [Google Scholar]
- 46.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 47.Ayutyanont N, Langbaum JB, Hendrix SB, et al. The Alzheimer’s prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer’s disease treatments in presenilin 1 E280A mutation carriers. The Journal of clinical psychiatry. 2014;75(6):652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bacioglu M, Maia LF, Preische O, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron. 2016;91(1):56–66. [DOI] [PubMed] [Google Scholar]
- 49.Teunissen CE, Verberk IM, Thijssen EH, et al. Blood-based biomarkers for Alzheimer's disease: towards clinical implementation. The Lancet Neurology. 2022;21(1):66–77. [DOI] [PubMed] [Google Scholar]
- 50.Mielke MM, Aggarwal NT, Vila-Castelar C, et al. Consideration of sex and gender in Alzheimer's disease and related disorders from a global perspective. Alzheimer's & Dementia. 2022:1–18. doi: 10.1002/alz.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vila-Castelar C, Tariot PN, Sink KM, et al. Sex differences in cognitive resilience in preclinical autosomal-dominant Alzheimer's disease carriers and non-carriers: Baseline findings from the API ADAD Colombia Trial. Alzheimer's & Dementia. 2022;18(11):2272–2282. doi: 10.1002/alz.12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vila-Castelar C, Guzmán-Vélez E, Pardilla-Delgado E, et al. Examining Sex Differences in Markers of Cognition and Neurodegeneration in Autosomal Dominant Alzheimer’s Disease: Preliminary Findings from the Colombian Alzheimer’s Prevention Initiative Biomarker Study. Journal of Alzheimer's Disease. 2020;77(4):1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verberk IM, Misdorp EO, Koelewijn J, et al. Characterization of pre-analytical sample handling effects on a panel of Alzheimer's disease–related blood-based biomarkers: Results from the Standardization of Alzheimer's Blood Biomarkers (SABB) working group. Alzheimer's & Dementia. 2021;18:1484–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurology. 2017;74(10):1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumitrescu L, Barnes LL, Thambisetty M, et al. Sex differences in the genetic predictors of Alzheimer’s pathology. Brain. 2019;142(9):2581–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher DW, Bennett DA, Dong H. Sexual dimorphism in predisposition to Alzheimer's disease. Neurobiology of Aging. 2018;70:308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocrine reviews. 2016;37(3):278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Frontiers in Neuroendocrinology. 2009;30(2):239–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Frontiers in Neuroendocrinology. 2014;35(1):8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gold EB. The timing of the age at which natural menopause occurs. Obstetrics and Gynecology Clinics. 2011;38(3):425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kantarci K, Lowe VJ, Lesnick TG, et al. Early postmenopausal transdermal 17β-estradiol therapy and amyloid-β deposition. Journal of Alzheimer's Disease. 2016;53(2):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schonknecht P, Pantel J, Klinga K, et al. Reduced cerebrospinal fluid estradiol levels are associated with increased b-amyloid levels in female patients with Alzheimer's disease. Neuroscience Letters. 2001;307(2):122–124. [DOI] [PubMed] [Google Scholar]
- 63.Buckley RF, O'Donnell A, McGrath ER, et al. Menopause status moderates sex differences in tau burden: a Framingham PET Study. Annals of Neurology. 2022;92:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mosconi L, Berti V, Quinn C, et al. Sex differences in Alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology. 2017;89(13):1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lohner V, Pehlivan G, Sanroma G, et al. The Relation Between Sex, Menopause, and White Matter Hyperintensities: The Rhineland Study. Neurology. 2022;99 (9):e935–e943. doi: 10.1212/WNL.0000000000200782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edwards L, La Joie R, Iaccarino L, et al. Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer's continuum: greater tau-PET retention in females. Neurobiology of Aging. 2021;105:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wisch JK, Meeker KL, Gordon BA, et al. Sex-related differences in tau positron emission tomography (PET) and the effects of hormone therapy (HT). Alzheimer disease and associated disorders. 2021;35(2):164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pereira JB, Harrison TM, La Joie R, Baker SL, Jagust WJ. Spatial patterns of tau deposition are associated with amyloid, ApoE, sex, and cognitive decline in older adults. European journal of nuclear medicine and molecular imaging. 2020;47(9):2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palta P, Rippon B, Tahmi M, et al. Sex differences in in vivo tau neuropathology in a multiethnic sample of late middle-aged adults. Neurobiology of Aging. 2021;103:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liesinger AM, Graff-Radford NR, Duara R, et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathologica. 2018;136(6):873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mattsson-Carlgren N, Andersson E, Janelidze S, et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Science Advances. 2020;6(16):eaaz2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mattsson-Carlgren N, Janelidze S, Bateman RJ, et al. Soluble P-tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau. EMBO molecular medicine. 2021;13(6):e14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pichet Binette A, Janelidze S, Cullen N, et al. Confounding factors of Alzheimer's disease plasma biomarkers and their impact on clinical performance. Alzheimer's & Dementia. 2022;19:1403–1414. doi: 10.1002/alz.12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stocker H, Beyer L, Trares K, et al. Association of Kidney Function With Development of Alzheimer Disease and Other Dementias and Dementia-Related Blood Biomarkers. JAMA Network Open. 2023;6(1):e2252387–e2252387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.