Abstract
Background:
Traumatic brain injury (TBI) is associated with significant morbidity, but the association of TBI with long-term stroke risk in diverse populations remains less clear. Our objective was to examine the long-term associations of TBI with stroke and to investigate potential differences by age, sex, race/ethnicity, and time since TBI diagnosis.
Methods:
Retrospective cohort study of U.S. military veterans aged 18+ years receiving healthcare in the VHA system between 10/1/2002 and 9/30/2019. Veterans with TBI were matched 1:1 to veterans without TBI on age, sex, race/ethnicity, and index date, yielding 306,796 veterans with TBI and 306,796 veterans without TBI included in the study. In primary analyses, Fine-Gray proportional hazards models adjusted for sociodemographics and medical/psychiatric comorbidities were used to estimate the association between TBI and stroke risk, accounting for the competing risk of mortality.
Results:
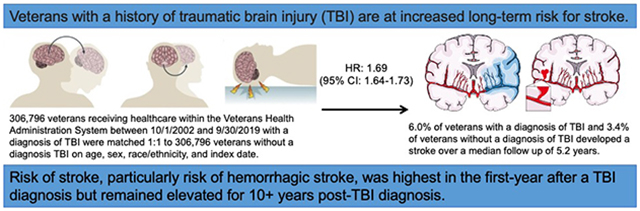
Participants were a mean age of 50 years, 9% were female, and 25% were of non-White race/ethnicity. Overall, 4.7% of veterans developed a stroke over a median follow-up of 5.2 years. Veterans with TBI had 1.69 times (95%CI=1.64-1.73) increased risk of any stroke (ischemic or hemorrhagic) compared to veterans without TBI. This increased risk was highest in the first-year post-TBI diagnosis (HR=2.16, 95%CI=2.03-2.29) but remained elevated for 10+ years. Similar patterns were observed for secondary outcomes, with associations of TBI with hemorrhagic stroke (HR=3.92, 95%CI=3.59-4.29) being stronger than with ischemic stroke (HR=1.56, 95%CI=1.52-1.61). Veterans with both mild (HR=1.47, 95%CI=1.43-1.52) and moderate/severe/penetrating injury (HR=2.02, 95%CI=1.96-2.09) had increased risk of stroke compared to veterans without TBI. Associations of TBI with stroke were stronger among older compared to younger individuals (p-interaction-by-age<0.001) and were weaker among Black veterans compared to other race/ethnicities (p-interaction-by-race<0.001).
Conclusions:
Veterans with prior TBI are at increased long-term risk for stroke, suggesting they may be an important population to target for primary stroke prevention measures.
Graphical Abstract
INTRODUCTION
Traumatic brain injury (TBI) is common and is associated with significant morbidity among survivors.1,2 Military veterans have a higher prevalence of lifetime history of TBI (up to 56%) compared to civilians and therefore represent an enriched population in which to study the long-term sequelae of TBI.3 Much of the prior research on TBI has focused on shorter-term injury recovery-related outcomes (i.e., functional outcomes occurring within 1 year of injury),4,5 with fewer studies focused on associations with longer-term neurologic consequences.
Traumatic cerebral microvascular injury is an increasingly recognized endophenotype of TBI and there is emerging evidence that resulting persistent microvascular dysfunction after TBI may lead to later neurologic disease, including dementia and stroke.6 Indeed, there have been several prior studies investigating associations of TBI with stroke risk.7-16 The majority of these prior studies are limited by racial/ethnic homogeneity9-13. Many of these prior studies also did not investigate associations of TBI with both ischemic and hemorrhagic stroke risk.7-16 The long-term risk of stroke after TBI remains unclear, particularly in populations comprised of diverse race/ethnicity groups. Information on these outcomes is important to better understand risk, burden, and possible mechanisms.
The overall objective of the present study was to examine the long-term associations of TBI and TBI severity with stroke risk (overall, and separately for ischemic and hemorrhagic stroke types) in a sample of U.S. military veterans receiving healthcare in the Veterans Health Administration (VHA) system. We additionally sought to investigate potential differences in associations of TBI with stroke risk by age, sex, race/ethnicity, and time since TBI diagnosis.
METHODS
Data Availability
The study data are derived from VHA electronic health records; please contact the authors for information regarding the process of accessing this data.
Study Design and Study Population
We conducted a retrospective cohort study of U.S. military veterans aged 18 years or older using data from two nationwide VHA system databases: 1) the inpatient and outpatient visits database (National Patient Care Databases) and 2) the Vital Status File. Of the 2,045,903 veterans with at least one VHA system visit between October 1, 2002 and September 30, 2019, we excluded 74,287 with history of stroke at their first VHA system visit and 171,149 with no follow-up visit in the VHA system, leaving 1,800,167 veterans (of whom 357,158 had a TBI diagnosis and 1,443,009 did not have a TBI diagnosis). We defined the index date for entry into follow-up as the first TBI diagnosis date for individuals who sustained a TBI. For individuals that did not have a TBI diagnosis, the index date for entry into follow-up was defined as a randomly selected healthcare encounter visit date occurring within 1 year of the matched individual’s index TBI diagnosis date. All eligible individuals were required to have had at least one visit in the VHA system within the 2 years prior to the index date in order to define prevalent medical comorbidities. We performed 1:1 matching of veterans with a TBI diagnosis to veterans without a TBI diagnosis on age (±3 years), sex (self-identified male versus female), race/ethnicity (self-identified non-Hispanic White versus non-Hispanic Black versus Hispanic versus other), and index date (±1 year), resulting in 306,796 veterans with a TBI diagnosis and 306,796 veterans without a TBI diagnosis included in our analytic population (Supplemental Figure 1).
Standard Protocol Approvals
This study was approved by the Institutional Review Boards at the University of California, San Francisco, the San Francisco Veterans Affairs Medical Center, and the US Army Medical Research and Materiel Command Human Research Protection Office. Informed consent was waived because de-identified archival data was used. This manuscript follows the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline.17
Traumatic Brain Injury
TBI diagnosis and TBI severity (mild versus moderate, severe, or penetrating injury) were defined using the Defense and Veterans Brain Injury Center list of International Classification of Disease, Ninth and Tenth Revisions (ICD-9 and ICD-10) Codes for TBI surveillance.18
Stroke
Incident stroke events were ICD-9 and ICD-10 code defined in accordance with the Department of Veterans Affairs Infrastructure for Clinical Intelligence project phenotype library definition.19 ICD codes used to define ischemic stroke and hemorrhagic stroke are shown in Supplemental Table 1. Our primary outcome was the composite outcome of any stroke (defined as ischemic stroke plus hemorrhagic stroke). Secondary outcomes considered ischemic stroke and hemorrhagic stroke separately.
Covariates
Information on age, sex, and race/ethnicity was collected from VHA inpatient or outpatient files. Zip codes and 2012 US Census data were used to categorize each veteran’s address into median annual income and education categories. Income was categorized as living in a zip code with median annual income <$25,930 (lowest tertile) versus ≥$25,930 (middle and highest tertiles). Education was categorized as ≤25% versus >25% of residents in zip code with bachelor’s degree or higher education. Current smoking, medical comorbidities (hypertension, hyperlipidemia, diabetes, atrial fibrillation, and coronary artery disease [comprised of myocardial infarction, cardiac arrest, coronary arteriosclerosis, or coronary artery bypass grafting procedure codes]), and psychiatric comorbidities (post-traumatic stress disorder and depression) were defined using ICD-9 and 10 codes from encounters occurring during the 2-year period prior to the index date.
Statistical Analyses
Baseline characteristics were presented by TBI status using means and standard deviations (SDs) or medians and 25th-75th percentiles for continuous variables and using numbers and percentages for categorical variables. Characteristics were compared between groups using standardized mean differences to allow for comparison of the magnitude of group differences between variables. A standardized mean difference of greater than 0.1 or less than −0.1 was considered a meaningful difference.20,21 We used Kaplan Meier analyses to calculate the cumulative incidence of stroke by TBI status. We first conducted Cox proportional hazards models to estimate the hazard ratios and 95% confidence intervals (CIs) for the associations of TBI with stroke risk using years since 30-days post-index date as the timescale and the end of follow up defined as the date of first stroke (defined as any stroke for primary analyses, and as either ischemic or hemorrhagic stroke in secondary analyses), date of death, or date of last VHA system visit occurring prior to September 30, 2019. A lag of 30-days post-index date was performed to reduce the possible influence of misdiagnosis of head injury as strokes in the immediate post-injury time-period. In our main analyses, we used Fine-Grey proportional hazards models to account for the competing risk of death.22 We used Schoenfeld residuals and complementary log-log plots to confirm that the proportional hazards assumption was met.23 Statistical models were adjusted for demographics, medical comorbidities and psychiatric comorbidities. We performed formal testing for multiplicative interaction by age group, sex, and race/ethnicity and present stratified results if there was evidence of interaction. In secondary analyses, we investigated the risk of stroke by TBI severity and by time since TBI diagnosis. In sensitivity analyses, we added index date as a covariate and performed analyses accounting for the variation between matched pairs24 (using a shared-frailty Cox proportional hazards model both without and with the use of methods described by Wolber et al.25,26 to account for the competing risk of mortality).
A two-sided p-value <0.05 was considered statistically significant. SAS version 9.4 and STATA/MP version 16.1 were used for all analyses.
RESULTS
Baseline characteristics of included veterans by TBI status are shown in Table 1. Overall participants were a mean age of 50 years, 9% were female, and 25% were of non-White race/ethnicity. Veterans with a TBI were more likely than veterans without a TBI to be current smokers (19% versus 13%), have hypertension (38% versus 31%), have hyperlipidemia (34% versus 29%), and have atrial fibrillation (4% versus 2%). Veterans with TBI were much more likely than veterans without TBI to have comorbid post-traumatic stress disorder (36% versus 10%) and depression (36% versus 16%).
Table 1.
Baseline Characteristics of U.S. Veterans With and Without TBI.
| Veterans Without TBI (n=306,796) |
Veterans With TBI (n=306,796) |
SMD | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 50.4 (17.6) | 50.2 (17.6) | −0.012 |
| Age category, n (%) | −0.010 | ||
| 18-44 years | 129,887 (42.3) | 130,909 (42.6) | |
| 45-64 years | 111,583 (36.3) | 111,742 (36.4) | |
| 65+ years | 65,326 (21.2) | 64,145 (20.9) | |
| Female, n (%) | 27,866 (9.0) | 27,866 (9.0) | −0.001 |
| Race/ethnicity, n (%) | <0.001 | ||
| Non-Hispanic White | 230,179 (75.0) | 230,179 (75.0) | |
| Non-Hispanic Black | 56,609 (18.4) | 56,609 (18.4) | |
| Hispanic | 9,925 (3.2) | 9,925 (3.2) | |
| Other* | 10,083 (3.2) | 10,083 (3.2) | |
| Median annual income in zip code <$25,930, n (%) | 97,708 (31.8) | 98,026 (31.9) | 0.004 |
| >25% of residents in zip code college educated, n (%) | 153,638 (50.0) | 155,741 (50.7) | 0.020 |
| Current smoking, n (%) | 38,456 (12.5) | 57,087 (18.6) | 0.168 |
| Index year, median (25th-75th percentile) | 2012 (2008-2015) | 2012 (2009-2015) | 0.006 |
| Follow-up time, median (25th-75th percentile) | 5.23 (2.35-8.78) | 5.18 (2.35-8.71) | −0.021 |
| Medical Comorbidities | |||
| Hypertension, n (%) | 94,700 (30.8) | 115,945 (37.7) | 0.145 |
| Hyperlipidemia, n (%) | 89,413 (29.1) | 105,547 (34.4) | 0.112 |
| Diabetes, n (%) | 36,557 (11.9) | 46,088 (15.0) | 0.090 |
| Atrial fibrillation, n (%) | 6,789 (2.2) | 13,256 (4.3) | 0.118 |
| Coronary artery disease, n (%) | 19,055 (6.2) | 24,836 (8.1) | 0.072 |
| Psychiatric Comorbidities | |||
| Post-traumatic stress disorder, n (%) | 32,479 (10.5) | 111,178 (36.2) | 0.635 |
| Depression, n (%) | 48,040 (15.6) | 111,669 (36.4) | 0.486 |
Abbreviations: SMD, standardized mean difference
Other race/ethnicities includes: American Indian or Alaska Native, Asian, and Native Hawaiian
A standardized mean difference of greater than 0.1 or less than −0.1 was considered a meaningful difference.
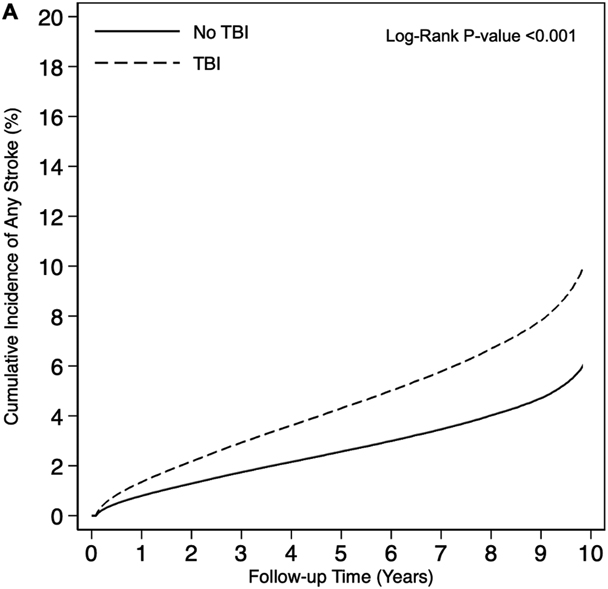
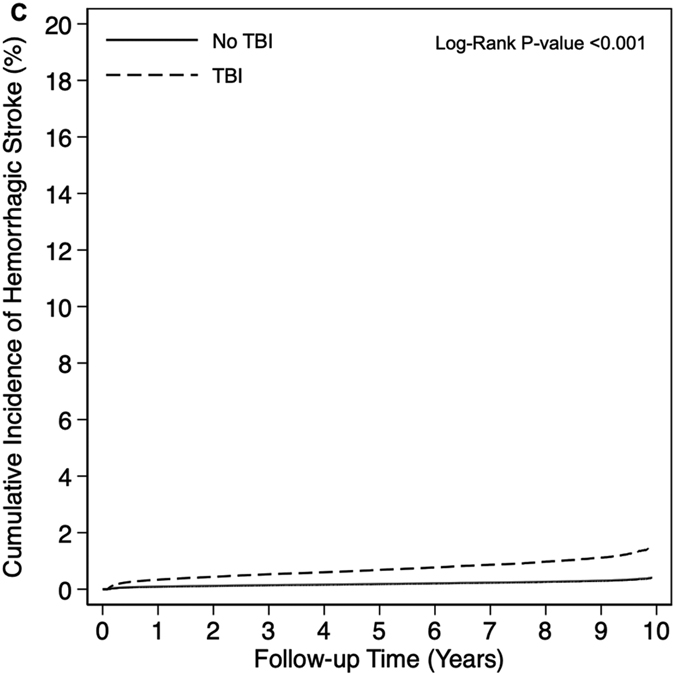
Overall, 4.7% of veterans developed a stroke over a median follow up of 5.2 years (25th percentile: 2.4 years, 75th percentile: 8.8 years). A total of 18,435 stroke events occurred over 1,787,238 person-years (PYs) of follow-up among veterans with TBI (unadjusted incidence rate [IR] per 1,000 PYs: 10.3, 95% CI: 10.0-10.7) compared to 10,297 stroke events occurring over 1,811,490 PYs of follow-up among veterans without TBI (unadjusted IR per 1,000 PYs: 5.7, 95% CI 5.4-6.0) (Table 2). Cumulative incidence was consistently increased among veterans with TBI compared to veterans without TBI for the primary outcome of any stroke as well as for the secondary individual outcomes of ischemic stroke and hemorrhagic stroke (all log-rank p-values <0.001) (Figure 1). Veterans with TBI had 1.80 times (95% CI: 1.76-1.85) increased risk of any stroke compared to veterans without TBI in adjusted Cox proportional hazards models. After accounting for the competing risk of death in adjusted Fine-Gray proportional hazards models, veterans with TBI had 1.69 times (95% CI: 1.64-1.73) increased risk of any stroke compared to veterans without TBI. Similar patterns were observed for the secondary individual outcomes of ischemic stroke and hemorrhagic stroke, with associations of TBI with hemorrhagic stroke being stronger than associations of TBI with ischemic stroke. In sensitivity analyses, adding index date as a covariate (Supplemental Table 2) and accounting for the variation between matched pairs (Supplemental Table 3), results were similar to our main statistical models.
Table 2.
Risk of Stroke by TBI Status.
| Veterans Without TBI | Veterans With TBI | |
|---|---|---|
| Any Stroke | ||
| No. Events/No. PYs | 10,297/1,811,490 | 18,435/1,787,238 |
| Unadjusted Incidence Rate per 1,000 PYs (95% CI) | 5.7 (5.4-6.0) | 10.3 (10.0-10.7) |
| Adjusted* Cox proportional hazards model, HR (95% CI) | 1 (Reference) | 1.80 (1.76-1.85) |
| Adjusted* Fine-Gray proportional hazards model, HR (95% CI) | 1 (Reference) | 1.69 (1.64-1.73)**† |
| Ischemic Stroke | ||
| No. Events/No. PYs | 9,924/1,812,455 | 16,645/1,795,283 |
| Unadjusted Incidence Rate per 1,000 PYs (95% CI) | 5.5 (5.2-5.7) | 9.3 (8.9-9.6) |
| Adjusted* Cox proportional hazards model, HR (95% CI) | 1 (Reference) | 1.68 (1.63-1.72) |
| Adjusted* Fine-Gray proportional hazards model, HR (95% CI) | 1 (Reference) | 1.56 (1.52-1.61)**† |
| Hemorrhagic Stroke | ||
| No. Events/No. PYs | 660/1,848,365 | 2,603/1,851,144 |
| Unadjusted Incidence Rate per 1,000 PYs (95% CI) | 0.4 (0.3-0.4) | 1.4 (1.3-1.5) |
| Adjusted* Cox proportional hazards model, HR (95% CI) | 1 (Reference) | 3.92 (3.59-4.29) |
| Adjusted* Fine-Gray proportional hazards model, HR (95% CI) | 1 (Reference) | 3.73 (3.40-4.08)**† |
Model adjusted for demographics (age, sex, race/ethnicity, income, education, and current smoking), medical comorbidities (hypertension, hyperlipidemia, diabetes, coronary artery disease, and atrial fibrillation) and psychiatric comorbidities (post-traumatic stress disorder and depression).
P-interaction by age <0.001
P-Interaction by race/ethnicity <0.001
Figure 1.
Cumulative Incidence of Stroke by Traumatic Brain Injury Status (A: Any Stroke, B: Ischemic Stroke, C: Hemorrhagic Stroke).
Associations of TBI with our primary composite outcome of any stroke were stronger among older individuals (aged 65+ years, adjusted HR: 1.94, 95% CI: 1.86-2.03) compared to younger individuals (aged 45-64 years, HR: 1.54, 95% CI: 1.49-1.60; aged 18-44 years, HR 1.68, 95% CI: 1.54, 1.83) (p-interaction-by-age <0.001) and were weaker among non-Hispanic Black individuals (HR: 1.42, 95% CI: 1.35-1.50) as compared to other race/ethnicities (non-Hispanic White individuals, HR: 1.74, 95% CI: 1.69-1.79; Hispanic individuals, HR: 1.84, 95% CI: 1.58-2.14; other race/ethnicity, HR: 1.73, 95% CI: 1.40-2.13) (p-interaction-by-race/ethnicity <0.001) (Table 2, Table 3). Similar patterns were seen by both age and race/ethnicity for associations of TBI with ischemic stroke and hemorrhagic stroke (all p-interaction <0.001). There was no evidence for effect modification by sex in the associations of TBI with stroke.
Table 3.
Adjusted* Risk of Stroke by TBI Status Stratified by Age and Race/Ethnicity.
| Veterans Without TBI | Veterans With TBI | |||
|---|---|---|---|---|
| No. Events/No. PYs | HR (95% CI) | No. Events/No. PYs | HR (95% CI) | |
| Any Stroke | ||||
| Stratified by Age | ||||
| Age 18-44 years | 1,002/733,018 | 1 (Reference) | 1,687/778,267 | 1.68 (1.54-1.83) |
| Age 45-64 years | 5,495/763,288 | 1 (Reference) | 9,043/744,928 | 1.54 (1.49-1.60) |
| Age 65+ years | 3,795/315,183 | 1 (Reference) | 7,696/264,044 | 1.94 (1.86-2.03) |
| Stratified by Race/Ethnicity | ||||
| Non-Hispanic White | 7,289/1,350,767 | 1 (Reference) | 13,760/1,324,665 | 1.74 (1.69-1.79) |
| Non-Hispanic Black | 2,518/352,689 | 1 (Reference) | 3,756/351,792 | 1.42 (1.35-1.50) |
| Hispanic | 309/55,856 | 1 (Reference) | 598/57,000 | 1.84 (1.58-2.14) |
| Other | 176/52,177 | 1 (Reference) | 312/53,781 | 1.73 (1.40-2.13) |
| Ischemic Stroke | ||||
| Stratified by Age | ||||
| Age 18-44 years | 930/733,201 | 1 (Reference) | 1,461/779,535 | 1.55 (1.42-1.70) |
| Age 45-64 years | 5,289/763,895 | 1 (Reference) | 8,221/749,174 | 1.45 (1.40-1.50) |
| Age 65+ years | 3,700/315,339 | 1 (Reference) | 6,957/266,574 | 1.78 (1.71-1.86) |
| Stratified by Race/Ethnicity | ||||
| Non-Hispanic White | 7,037/1,351,405 | 1 (Reference) | 12,344/1,330,957 | 1.60 (1.55-1.65) |
| Non-Hispanic Black | 2,420/352,959 | 1 (Reference) | 3,481/353,191 | 1.36 (1.29-1.44) |
| Hispanic | 296/55,890 | 1 (Reference) | 538/57,188 | 1.70 (1.45-1.98) |
| Other | 166/52,201 | 1 (Reference) | 276/53,947 | 1.55 (1.25-1.94) |
| Hemorrhagic Stroke | ||||
| Stratified by Age | ||||
| Age 18-44 years | 110/736,533 | 1 (Reference) | 292/784,821 | 2.70 (2.12-3.44) |
| Age 45-64 years | 385/784,829 | 1 (Reference) | 1,245/781,098 | 3.00 (2.65-3.38) |
| Age 65+ years | 165/328,003 | 1 (Reference) | 1,063/285,224 | 6.21 (5.21-7.40) |
| Stratified by Race/Ethnicity | ||||
| Non-Hispanic White | 420/1,376,949 | 1 (Reference) | 1,976/1,371,638 | 4.32 (3.86-4.83) |
| Non-Hispanic Black | 193/361,855 | 1 (Reference) | 476/365,711 | 2.37 (1.98-2.82) |
| Hispanic | 28/56,801 | 1 (Reference) | 99/58,978 | 3.27 (2.10-5.08) |
| Other | 19/52,760 | 1 (Reference) | 49/54,816 | 2.59 (1.45-4.63) |
Fine-Gray proportional hazards model adjusted for demographics (age, gender, race, income, education, and current smoking), medical comorbidities (hypertension, hyperlipidemia, diabetes, and atrial fibrillation) and psychiatric comorbidities (post-traumatic stress disorder and depression).
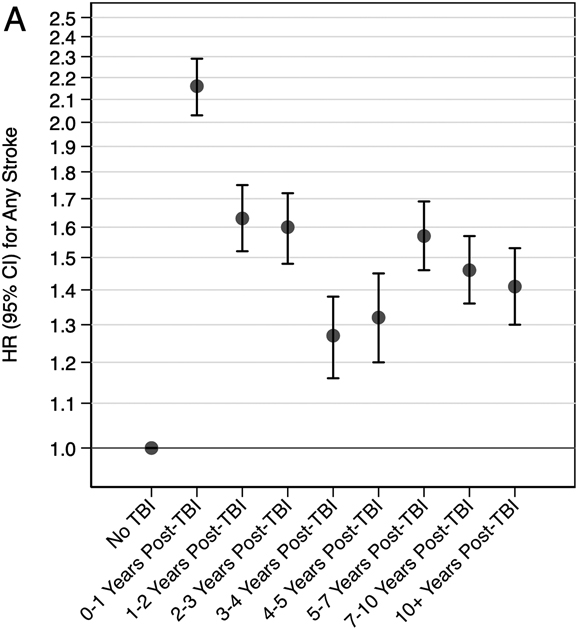
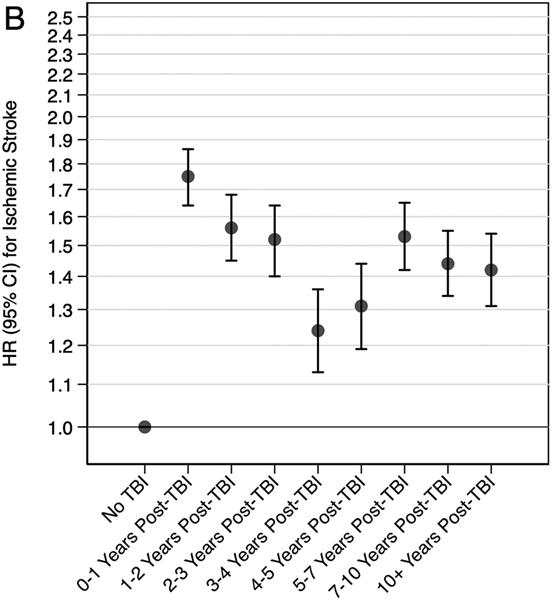
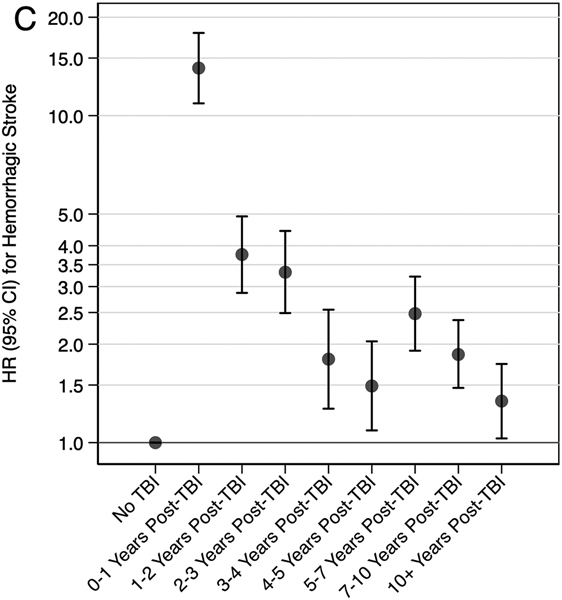
In secondary analyses, veterans with moderate, severe, or penetrating injury had 2.02 times (95% CI: 1.96-2.09) increased risk and veterans with mild injury had 1.47 times (95% CI: 1.43-1.52) increased risk of the composite outcome of any stroke compared to veterans without TBI (Table 4). Similar patterns were observed for associations of TBI severity with the secondary individual outcomes of ischemic stroke and hemorrhagic stroke. In secondary analyses stratified by time since TBI, the highest risk of the composite outcome of any stroke occurred in the first-year post injury (HR: 2.16, 95% CI: 2.03-2.29), but risk remained elevated for 10+ years (Figure 2). Similar patterns were observed for the secondary individual outcomes of ischemic stroke and hemorrhagic stroke.
Table 4.
Risk of Stroke by TBI Severity.
| Veterans Without TBI |
Veterans With Mild TBI |
Veterans With Moderate or Severe or Penetrating TBI |
|
|---|---|---|---|
| Any Stroke | |||
| No. Events/No. PYs | 10,297/1,811,490 | 6,744/791,250 | 8,767/607,738 |
| Unadjusted Incidence Rate per 1,000 PYs (95% CI) | 5.7 (5.4, 6.0) | 8.5 (8.1, 9.0) | 14.4 (13.7, 15.2) |
| Adjusted* Cox proportional hazards model, HR (95% CI) | 1 (Reference) | 1.59 (1.54-1.64) | 2.18 (2.11-2.24) |
| Adjusted* Fine-Gray proportional hazards model, HR (95% CI) | 1 (Reference) | 1.47 (1.43-1.52) | 2.02 (1.96-2.09) |
| Ischemic Stroke | |||
| No. Events/No. PYs | 9,924/1,812,455 | 6,323/792,827 | 7,585/613,534 |
| Unadjusted Incidence Rate per 1,000 PYs (95% CI) | 5.5 (5.2, 5.7) | 8.0 (7.5, 8.4) | 12.4 (11.7, 13.1) |
| Adjusted* Cox proportional hazards model, HR (95% CI) | 1 (Reference) | 1.55 (1.50-1.60) | 1.92 (1.7-1.99) |
| Adjusted* Fine-Gray proportional hazards model, HR (95% CI) | 1 (Reference) | 1.43 (1.38-1.48) | 1.78 (1.73-1.84) |
| Hemorrhagic Stroke | |||
| No. Events/No. PYs | 660/1,848,365 | 637/814,134 | 1,671/638,374 |
| Unadjusted Incidence Rate per 1,000 PYs (95% CI) | 0.4 (0.3, 0.4) | 0.8 (0.6, 0.9) | 2.6 (2.3, 2.9) |
| Adjusted* Cox proportional hazards model, HR (95% CI) | 1 (Reference) | 2.23 (1.99-2.50) | 6.45 (5.87-7.09) |
| Adjusted* Fine-Gray proportional hazards model, HR (95% CI) | 1 (Reference) | 2.11 (1.88-2.36) | 6.14 (5.58-6.76) |
Model adjusted for demographics (age, sex, race/ethnicity, income, education, and current smoking), medical comorbidities (hypertension, hyperlipidemia, diabetes, coronary artery disease, and atrial fibrillation) and psychiatric comorbidities (post-traumatic stress disorder and depression).
Figure 2. Adjusted* Risk of Stroke Stratified by Time Since TBI (A: Any Stroke, B: Ischemic Stroke, C: Hemorrhagic Stroke).
*Fine-Gray proportional hazards model adjusted for demographics (age, sex, race/ethnicity, income, education, and current smoking), medical comorbidities (hypertension, hyperlipidemia, diabetes, coronary artery disease, and atrial fibrillation) and psychiatric comorbidities (post-traumatic stress disorder and depression).
DISCUSSION
In this cohort of 613,592 U.S. military veterans with and without a diagnosed TBI receiving healthcare in the VHA system, TBI was consistently associated with long-term risk of stroke. We observed the strongest associations of TBI with hemorrhagic stroke and with stroke events occurring within the first year after TBI, but this risk remained elevated for 10+ years. Associations of TBI with stroke risk were stronger among older (i.e., aged 65+ years) individuals and were weaker among individuals of self-identified non-Hispanic Black race/ethnicity.
Our findings extend the prior literature7-16 on this topic in several important ways, including accounting for the competing risk of death in statistical models, a racially/ethnically diverse population of adults across the age-spectrum, and by the inclusion of a robust investigation into potential differences in observed associations by age, sex, race/ethnicity, and time since TBI diagnosis. Similar to several prior studies7,9,15 we found that the risk of hemorrhagic stroke was greater than the risk of ischemic stroke, particularly in the first year after TBI and particularly among individuals with greater TBI severity. In contrast to one prior study which found stronger associations of TBI with odds of ischemic stroke among younger (<50 years) compared to older (≥50 years)8 and one study which found no difference in associations of TBI with any stroke risk by age12, we found stronger associations of TBI with stroke risk among older (≥65 years) compared to younger (<65 years) veterans. Observed differences may be attributable to differences in study population, head injury severity/mechanism, or other factors; future studies including investigation for interaction by age in the association of TBI with stroke risk are warranted for clarification of which subgroups may be at higher risk for post-TBI strokes. Similar to a prior study, we found no difference in associations of TBI with stroke risk by sex.12 In contrast to prior studies which were largely performed in Taiwan,9-13 our population allowed for investigation into potential differences in the association of TBI with stroke risk by race/ethnicity. Indeed, we found that associations of TBI with stroke risk were weaker among Black veterans as compared to other race/ethnicity groups. Racial disparities in stroke incidence and mortality are well documented, with Black individuals having both higher incidence and mortality compared to White individuals27,28. Given the higher baseline risk of stroke in Black compared to in White individuals, it is possible that the additional contribution of TBI to stroke risk is less among Black as compared to among White individuals. Alternatively, the weaker associations observed among Black veterans in our study may be due to possible underdiagnosis of stroke using ICD codes, which may be related to differential access to health care and/or socioeconomic disadvantages among racial/ethnic minorities. Indeed, a greater proportion Black compared to White individuals lived in zip codes with lower median income and lower percentage of college educated residents, but our cohort included only veterans with access to healthcare, which may have mitigated some of these traditional barriers to care; additional research is needed on racial/ethnic differences in associations of TBI with neurological outcomes.
There are several potential mechanisms that may underly the observed association of TBI with stroke. Cerebral microvascular injury leading to persistent microvascular dysfunction after TBI is one mechanism hypothesized to link TBI with stroke and other neurologic sequelae including dementia.6,29 This hypothesis is supported by animal models showing increased vulnerability to cerebral ischemia after TBI via induced vascular dysfunction, which leads to worsened stroke outcomes secondary to impaired reperfusion.30 Trauma-induced coagulopathy (either hypocoagulable or hypercoagulable states) and blunt cerebrovascular injury (traumatic dissections) have also been hypothesized to contribute to stroke risk. 31,32 However, trauma-induced coagulopathy is most evident within 24 hours of injury and the risk remains for approximately 5 days post-injury and typically resolves within 14 days of injury.33 Similarly, the risk of stroke associated with blunt cerebrovascular injury is typically within the first 4 weeks post-injury34. Trauma-induced coagulopathy and blunt cerebrovascular injury are therefore unlikely mechanisms underlying the observed long-term associations in our cohort where we specifically excluded stroke events occurring within 30-days of TBI in order to decrease the possible influence of misdiagnosis of TBI-related sequelae as strokes in the acute post-injury time period. Alternatively, it is possible that individuals with TBI are at higher risk for stroke as a result of a higher prevalence of comorbid vascular risk factors due to disability and resultant decreased physical activity secondary to the TBI. Indeed, in our cohort, veterans with a TBI diagnosis had higher prevalence of hyperlipidemia, hypertension, and atrial fibrillation compared to veterans without a TBI diagnosis. However, even after accounting for these factors in our statistical models, the elevated risk of stroke associated with TBI remained. As we have shown, the risk for stroke changes over time, and it is possible that different mechanisms are responsible earlier versus later after TBI; more work is needed to elucidate mechanisms underlying the observed associations of TBI with stroke risk.
Our results should be interpreted in the context of study limitations and strengths. First, our study was performed in a cohort of U.S. military veterans receiving healthcare within the VHA system. Consequently, we do not capture either TBI or stroke events if veterans received care outside of the VHA system. The magnitude of bias attributable to non-captured events is unknown, however, among veterans receiving healthcare within the VHA system, the median number of primary care encounters per year is three.35 Therefore, the bias is more likely to be a delay in stroke diagnosis whereby a stroke event not treated at the VHA may be captured at the subsequent VHA primary care encounter rather than being an entirely missed stroke diagnosis. Veterans who receive non-VHA healthcare tend to be younger, have higher levels of education, and have alternative sources of healthcare coverage compared to veterans who receive healthcare within the VHA system,36,37 thus the generalizability of our results to populations beyond veterans receiving healthcare in the VHA system needs to be confirmed. Second, our TBI and stroke definitions are ICD-code based, and therefore we do not have specific details regarding history of prior remote TBIs, injury mechanism or TBI or stroke treatment. Further, our TBI definition captures all TBI diagnoses occurring within the VHA system between October 1, 2002 and September 30, 2019 and we are unable to determine if the TBI diagnosis is indicative of a prevalent or an incident injury. However, the ICD-code based definition for TBI is consistent with what is used by the Defense and Veterans Brain Injury Center for TBI surveillance18 and has been previously validated, with sensitivity of 70%, specificity of 82%, and positive predictive value of 85%.38 Similarly, the stroke definitions used are defined in accordance with the DaVINCI project phenotype library definition19 and the use of ICD codes to identify acute stroke events has been shown to have greater than 82% sensitivity, greater than 95% specificity, and greater than 81% positive predictive value.39 Since all of our TBI cases were identified via ICD codes, it is possible that our results may not generalize to more mild TBIs which do not necessitate medical care. Although we ascertained comorbidity status in the two years prior to the TBI index date, it is possible that some of the medical comorbidities herein may be a result of the TBI diagnosis, rather than prevalent at the time of TBI, particularly for individuals with milder TBIs who may have had a delayed diagnosis of TBI. It is also possible that acute TBI-related sequelae may have been misdiagnosed as strokes, but we implemented a lag of 30-days post-index date in order to reduce this possible influence of misdiagnosis of head-injury sequelae as strokes. We also reduced the possible influence of the competing risk of mortality by using Fine-Gray proportional hazards model in our main analyses. Additionally, our population was comprised of only 9% women and further study in populations with a greater proportion of women is warranted, but we were able to robustly investigate potential differences in associations between TBI and stroke risk across the age spectrum and by race/ethnicity group. Finally, the possibility of residual confounding remains due to the retrospective cohort design of our study. In particular, our study is subject to the possibility of residual confounding by socioeconomic status as a result of the coarseness of the education and income variables available.
In conclusion, this study provides strong inferential evidence supporting the association of TBI with long-term risk for stroke. Risk of stroke, particularly risk of hemorrhagic stroke and particularly among individuals with TBIs of greater injury severity, was highest in the first-year post-injury, but the risk remained elevated for 10+ years. This observed long-term increased risk of stroke after TBI suggests that scrupulous attention to vascular risk factor modification and to other primary stroke prevention strategies among individuals with prior TBI may be important.
Supplementary Material
SOURCES OF FUNDING
This work was supported by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense, through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium Award/W81XWH-18-PH/TBIRP-LIMBIC under Awards No. W81XWH-19-2-0067 and W81XWH-13-2-0095, and by the U.S. Department of Veterans Affairs Awards No. I01-CX002097, I01-CX002096, I01-HX003155, I01-RX003444, I01-RX003443, I01-RX003442, I01-CX001135, I01-CX001246, I01-RX001774, I01-RX 001135, I01-RX 002076, I01-RX001880, I01-RX002172, I01-RX002173, I01-RX002171, I01-RX002174, and I01-RX002170. The UCSF Population Based Research for Alzheimer's Innovation (UCSF Pop-BRAIN; Yaffe, PI) is supported by NIA R35AG071916. Dr. Schneider is supported by DoD grant W81XWH-21-1-0590.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- CI
Confidence interval
- HR
Hazard ratio
- ICD-9
International Classification of Disease, Ninth Revision
- ICD-10
International Classification of Disease, Tenth Revision
- IR
Incidence rate
- PYs
Person-years
- SD
Standard deviation
- TBI
Traumatic brain injury
- VHA
Veterans Health Administration
Footnotes
DISCLOSURES
Dr. Schneider reports serving as Assistant Associate Editor for the journal Neurology from the American Academy of Neurology. Dr. Gardner reports grants from United States-Israel Binational Science Foundation, U.S. Department of Defense, and National Institutes of Health; compensation from BrainBox Solutions Inc. for consultant services. Dr. Yaffe reports compensation from National Institute on Aging for data and safety monitoring services; compensation from Alpha Cognition for consultant services; service as Board Member for Alector; compensation from Dominantly Inherited Alzheimer Network Trials Unit for data and safety monitoring services; and compensation from Eli LIlly for data and safety monitoring services.
REFERENCES
- 1.Schneider ALC, Wang D, Ling G, Gottesman RF, Selvin E. Prevalence of Self-Reported Head Injury in the United States. N Engl J Med. 2018;379:1176–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider ALC, Wang D, Gottesman RF, Selvin E. Prevalence of Disability Associated With Head Injury With Loss of Consciousness in Adults in the United States: A Population-Based Study. Neurology. 2021;97:e124–e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornblith ES, Yaffe K, Langa KM, Gardner RC. Prevalence of Lifetime History of Traumatic Brain Injury among Older Male Veterans Compared with Civilians: A Nationally Representative Study. J Neurotrauma. 2020;37:2680–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCrea MA, Giacino JT, Barber J, Temkin NR, Nelson LD, Levin HS, Dikmen S, Stein M, Bodien YG, Boase K, et al. Functional Outcomes Over the First Year After Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study. JAMA Neurol. 2021;78:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson LD, Temkin NR, Dikmen S, Barber J, Giacino JT, Yuh E, Levin HS, McCrea MA, Stein MB, Mukherjee P, et al. Recovery After Mild Traumatic Brain Injury in Patients Presenting to US Level I Trauma Centers: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Study. JAMA Neurol. 2019;76:1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandsmark DK, Bashir A, Wellington CL, Diaz-Arrastia R. Cerebral Microvascular Injury: A Potentially Treatable Endophenotype of Traumatic Brain Injury-Induced Neurodegeneration. Neuron. 2019;103:367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrecht JS, Liu X, Smith GS, Baumgarten M, Rattinger GB, Gambert SR, Langenberg P, Zuckerman IH. Stroke incidence following traumatic brain injury in older adults. J Head Trauma Rehabil. 2015;30:E62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke JF, Stulc JL, Skolarus LE, Sears ED, Zahuranec DB, Morgenstern LB. Traumatic brain injury may be an independent risk factor for stroke. Neurology. 2013;81:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YH, Kang JH, Lin HC. Patients with traumatic brain injury: population-based study suggests increased risk of stroke. Stroke. 2011;42:2733–2739. [DOI] [PubMed] [Google Scholar]
- 10.Eric Nyam TT, Ho CH, Chio CC, Lim SW, Wang JJ, Chang CH, Kuo JR, Wang CC. Traumatic Brain Injury Increases the Risk of Major Adverse Cardiovascular and Cerebrovascular Events: A 13-Year, Population-Based Study. World Neurosurg. 2019;122:e740–e753. [DOI] [PubMed] [Google Scholar]
- 11.Lee YK, Lee CW, Huang MY, Hsu CY, Su YC. Increased risk of ischemic stroke in patients with mild traumatic brain injury: a nationwide cohort study. Scand J Trauma Resusc Emerg Med. 2014;22:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao CC, Chou YC, Yeh CC, Hu CJ, Chiu WT, Chen TL. Stroke risk and outcomes in patients with traumatic brain injury: 2 nationwide studies. Mayo Clin Proc. 2014;89:163–172. [DOI] [PubMed] [Google Scholar]
- 13.Liu SW, Huang LC, Chung WF, Chang HK, Wu JC, Chen LF, Chen YC, Huang WC, Cheng H, Lo SS. Increased Risk of Stroke in Patients of Concussion: A Nationwide Cohort Study. Int J Environ Res Public Health. 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris NA, Cool J, Merkler AE, Kamel H. Subarachnoid Hemorrhage and Long-Term Stroke Risk After Traumatic Brain Injury. Neurohospitalist. 2017;7:122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu D, Li W, Zhang S, Li R, Wang H, Chen B. Traumatic Brain Injury Is Associated With Both Hemorrhagic Stroke and Ischemic Stroke: A Systematic Review and Meta-Analysis. Front Neurosci. 2022;16:814684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart IJ, Amuan ME, Wang CP, Kennedy E, Kenney K, Werner JK, Carlson KF, Tate DF, Pogoda TK, Dismuke-Greer CE, et al. Association Between Traumatic Brain Injury and Subsequent Cardiovascular Disease Among Post-9/11-Era Veterans. JAMA Neurol. 2022;79:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 18.Traumatic Brain Injury (TBI): DoD Standard Surveillance Case Definition for TBI Adapted for Armed Forces Health Surveillence Branch (AFHSB) Use. https://health.mil/Reference-Center/Publications/2015/12/01/Traumatic-Brain-Injury. 2019. Accessed June 27.
- 19.DuVall SL, Matheny ME, Ibragimov IR, Oats TD, Tucker JN, South BR, Turano A, Saoudian H, Kangas C, Hofmann K, et al. A Tale of Two Databases: The DoD and VA Infrastructure for Clinical Intelligence (DaVINCI). Stud Health Technol Inform. 2019;264:1660–1661. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Kim HJ, Lonjon G, Zhu Y, written on behalf of AMEB-DCTCG. Balance diagnostics after propensity score matching. Ann Transl Med. 2019;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flury B, Riedwyl H. Standard Distance in Univariate and Multivariate Analysis. The American Statistician. 1986;40:249–251. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 24.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolbers M, Blanche P, Koller MT, Witteman JC, Gerds TA. Concordance for prognostic models with competing risks. Biostatistics. 2014;15:526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. [DOI] [PubMed] [Google Scholar]
- 27.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. [DOI] [PubMed] [Google Scholar]
- 29.Kenney K, Amyot F, Haber M, Pronger A, Bogoslovsky T, Moore C, Diaz-Arrastia R. Cerebral Vascular Injury in Traumatic Brain Injury. Exp Neurol. 2016;275 Pt 3:353–366. [DOI] [PubMed] [Google Scholar]
- 30.Weil ZM, Karelina K, Whitehead B, Velazquez-Cruz R, Oliverio R, Pinti M, Nwafor DC, Nicholson S, Fitzgerald JA, Hollander J, et al. Mild traumatic brain injury increases vulnerability to cerebral ischemia in mice. Exp Neurol. 2021;342:113765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, Schochl H, Hunt BJ, Sauaia A. Trauma-induced coagulopathy. Nat Rev Dis Primers. 2021;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumislawski JJ, Moore HB, Moore EE, Swope ML, Pieracci FM, Fox CJ, Campion EM, Lawless RA, Platnick KB, Sauaia A, et al. Not all in your head (and neck): Stroke after blunt cerebrovascular injury is associated with systemic hypercoagulability. J Trauma Acute Care Surg. 2019;87:1082–1087. [DOI] [PubMed] [Google Scholar]
- 33.Selby R, Geerts W, Ofosu FA, Craven S, Dewar L, Phillips A, Szalai JP. Hypercoagulability after trauma: hemostatic changes and relationship to venous thromboembolism. Thromb Res. 2009;124:281–287. [DOI] [PubMed] [Google Scholar]
- 34.Morris NA, Merkler AE, Gialdini G, Kamel H. Timing of Incident Stroke Risk After Cervical Artery Dissection Presenting Without Ischemia. Stroke. 2017;48:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed ST, Mahtta D, Rehman H, Akeroyd J, Al Rifai M, Rodriguez F, Jneid H, Nasir K, Samad Z, Alam M, et al. Association between frequency of primary care provider visits and evidence-based statin prescribing and statin adherence: Findings from the Veterans Affairs system. Am Heart J. 2020;221:9–18. [DOI] [PubMed] [Google Scholar]
- 36.Hanchate AD, Frakt AB, Kressin NR, Trivedi A, Linsky A, Abdulkerim H, Stolzmann KL, Mohr DC, Pizer SD. External Determinants of Veterans' Utilization of VA Health Care. Health Serv Res. 2018;53:4224–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller EA, Intrator O. Veterans use of non-VHA services: implications for policy and planning. Soc Work Public Health. 2012;27:379–391. [DOI] [PubMed] [Google Scholar]
- 38.Carlson KF, Barnes JE, Hagel EM, Taylor BC, Cifu DX, Sayer NA. Sensitivity and specificity of traumatic brain injury diagnosis codes in United States Department of Veterans Affairs administrative data. Brain Inj. 2013;27:640–650. [DOI] [PubMed] [Google Scholar]
- 39.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of Diagnostic Codes for Acute Stroke in Administrative Databases: A Systematic Review. PLoS One. 2015;10:e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study data are derived from VHA electronic health records; please contact the authors for information regarding the process of accessing this data.