Abstract
Viewing metabolism through the lens of exercise biology has proven an accessible and practical strategy to gain new insights into local and systemic metabolic regulation. Recent methodological developments have advanced understanding of the central role of skeletal muscle in many exercise-associated health benefits and have uncovered the molecular underpinnings driving adaptive responses to training regimens. In this Review, we provide a contemporary view of the metabolic flexibility and functional plasticity of skeletal muscle in response to exercise. First, we provide background on the macrostructure and ultrastructure of skeletal muscle fibres, highlighting the current understanding of sarcomeric networks and mitochondrial subpopulations. Next, we discuss acute exercise skeletal muscle metabolism and the signalling, transcriptional and epigenetic regulation of adaptations to exercise training. We address knowledge gaps throughout and propose future directions for the field. This Review contextualizes recent research of skeletal muscle exercise metabolism, framing further advances and translation into practice.
Introduction
As a primary site of nutrient storage, energy use and locomotion, skeletal muscle is central to the impact of physical activity on human health. Periods of inactivity reduce skeletal muscle insulin sensitivity and oxidative capacity1, contributing towards impaired systemic metabolic flexibility2 and an increased risk for cardiometabolic disease3. Physical activity offers a degree of protection against the deleterious effects of sedentary behaviour on whole-body metabolism4: larger volumes4 and more vigorous types5 of physical activity, such as formal exercise, probably convey additional benefit.
Bouts of exercise rapidly sensitize skeletal muscle to hormones (Supplementary Table 1) and nutrients. A single (or ‘acute’) exercise session directly increases skeletal muscle transport of amino acids6 and glucose7. These effects appear somewhat specific to the contracted musculature, and they enhance postprandial muscle protein synthesis8 and insulin-stimulated glucose disposal9,10 in the recovery period after exercise. Consistent exercise training (over weeks, months and years) further augments skeletal muscle mass11–13, peripheral insulin-sensitivity12, maximal oxygen consumption () 12–15 and strength12,13. (ref. 16) and strength17 are well-known predictors of mortality, and training for their improvement through endurance and resistance exercise (Box 1) reduces mortality risk in a manner that is most effective when both training modalities are performed within the same exercise programme18.
Box 1. Different exercise (sub)types and training response heterogeneity in humans.
Exercise (sub)types
Exercise can be broadly classified as resistance, cardiorespiratory, balance and flexibility-based. Flexibility and balance are important aspects of physical fitness contributing to fall prevention in older individuals282. However, discussion herein is dedicated to iterations of resistance and cardiorespiratory exercise, which are the main focus of this Review.
Resistance exercise
Traditional resistance exercise entails repetitions of dynamic concentric (muscle-shortening) and eccentric (muscle-lengthening) contractions against external load and is an effective intervention to increase skeletal muscle mass and strength249,283. The total amount (volume), frequency and intensity of exercise are inherently linked training variables that impact adaptation and performance. For resistance exercise, volume is often reported as the number of times (or ‘sets’) a particular muscle group is trained per week and is the main stimulus for muscle accrual284. Twelve to 20 weekly sets is sufficient to maximize hypertrophy285, and the frequency of exercise can be adjusted to disperse training volumes according to personal preference286.
Intensity of resistance exercise is generally normalized to a percentage of the maximum load that an individual can lift (expressed as the percentage of 1 repetition maximum (RM))287 or how close an exercise set is taken to momentary muscular failure (in other words, the inability to complete the concentric portion of a movement)288. Equal gains in muscle mass can be made irrespective of load-intensity (<30% 1 RM to ≥80% 1 RM)287 when sets are taken proximal to failure288. Thus, increasing the number of repetitions against a fixed load or increasing the load lifted for a fixed number of repetitions are both viable progression strategies to promote muscle hypertrophy289. Conversely, absolute strength (1 RM)287, tendon stiffness290 and running economy (that is, the metabolic cost at a given velocity of submaximal running)254 are improved more by high-load resistance training. Collectively, this implies that implementing various loading strategies could represent the best approach for attaining the breadth of muscle-related adaptations to resistance training.
The stress imposed by a single bout of resistance exercise upregulates amino acid transporters and sensors in muscle at least in part through activating transcription factor 4 (ATF4)6. Synergizing with the mammalian target of rapamycin (mTOR) (see the section ‘Skeletal muscle responses to acute exercise’) (Fig. 3), this sensitizes muscle to amino acids for ≥24–48 h after resistance exercise6,8, and dietary protein intakes of ≥1.2–1.6 g per kg body mass daily modestly complement the hypertrophic response to resistance training291.
Endurance and high-intensity cardiorespiratory exercise
Cardiorespiratory exercise has numerous subtypes that are associated with training-induced improvements in maximal oxygen consumption ()292–294. Approaches used to standardize cardiorespiratory exercise are plentiful (reviewed in ref. 295) and most often include the allocation of intensity against fixed percentages of maximal power (), velocity (), heart rate (HRmax) or . However, the validity of normalizing cardiorespiratory exercise to maximal parameters has been questioned295,296, as this can produce dissimilar physiological and metabolic perturbations between individuals296. Moving forward, studies should strive to establish methods of standardization that provoke homeostatic disturbances consistent with distinct exercise intensity domains.
Endurance (also known as aerobic) exercise is typically considered to be a continuous bout of formal activity performed at low (<50%), moderate (~50–79%) or high intensities (≥80%) of (ref. 292). High-intensity cardiorespiratory exercise can be further divided into high-intensity interval exercise (HIE) and sprint interval exercise (SIE), both of which constitute several bursts of higher-intensity effort interspersed with periods of low-intensity active recovery. High-intensity intervals are conducted at ≥80% of , whereas sprint intervals are supramaximal or ‘all-out’292. Comparison of cardiorespiratory exercise suggests that training within the high-intensity range is more effective292,293 or equally as effective294 at increasing and requires less training volume than moderate intensities to do so294. HIE and SIE training also improve endothelial function to greater extents, whereas moderate intensities favour long-term glycaemic control (lower glycated haemoglobin A1c (HbA1c))292. Further intricacies are seen between interval training subtypes. Eight weeks of HIE training improved cardiac stroke volume, and endurance (3 km) performance297. By contrast, an equivalent period of SIE training potentiated anaerobic capacity and sprinting (300 m) performance297.
Training variables might also discretely regulate muscle mitochondria (see the section ‘Skeletal muscle adaptations to long-term exercise’). Hence, combinations of endurance exercise, HIE and SIE are recommended to maximize both health and performance benefits. Consistent with this, just 1–3 h per week of moderate or high-intensity cardiorespiratory exercise could lower mortality risk, and incorporating resistance exercise confers added protection18.
Interindividual variation in exercise adaptation
The magnitude of adaptation to resistance298 and endurance299,300 training differs substantially between individuals. In part, this probably stems from the aforementioned challenges regarding exercise standardization295,296, but also from genetic300 and environmental interactions that converge to produce the heterogenous molecular responses that are observed systemically19 and in muscle177 after a common exercise bout.
Interindividual difference in exercise trainability has led to the concept of ‘responders’ and ‘non-responders’. Increasing the volume of fixed-intensity exercise301,302 or the intensity of fixed-volume exercise302 can somewhat attenuate exercise non-response, with larger volumes of higher-intensity exercise perhaps doing so most effectively302. However, lower responders still require a greater training stimulus and time commitment to achieve results comparable to those of more-responsive trainees301,302.
As the field of sports genetics continues to grow and be refined, it should provide actionable knowledge for the efficient personalization of training programmes. In the meantime, it is important to emphasize that low responders for a given parameter, such as , often improve in other outcome measures299. Therefore, exercise remains beneficial for all who are able to partake.
Although the association between regular physical activity and health span has been realized since antiquity, recent methodological advances have allowed the field of exercise physiology to progress towards comprehensive systems-level profiling of the complex molecular interplay that occurs with exercise19,20. These advancements have enabled better mechanistic understanding of the cause-and-effect relationships underlying exercise adaptation and associated health benefits.
In this Review, we provide a contemporary summary of the role of skeletal muscle in response to exercise. First, we address the major cellular makeup of skeletal muscle, highlighting fibre type properties, and the importance of sarcomeric and mitochondrial networks. Next, we discuss skeletal muscle metabolism during acute exercise and the influence of select modifiers such as intensity and timing on this biology. Finally, we address underlying mechanisms of exercise-induced skeletal muscle adaptation and consider differences between training modalities that may facilitate distinct and complementary responses beneficial for human performance and health.
Skeletal muscle fibre types and subcellular characteristics
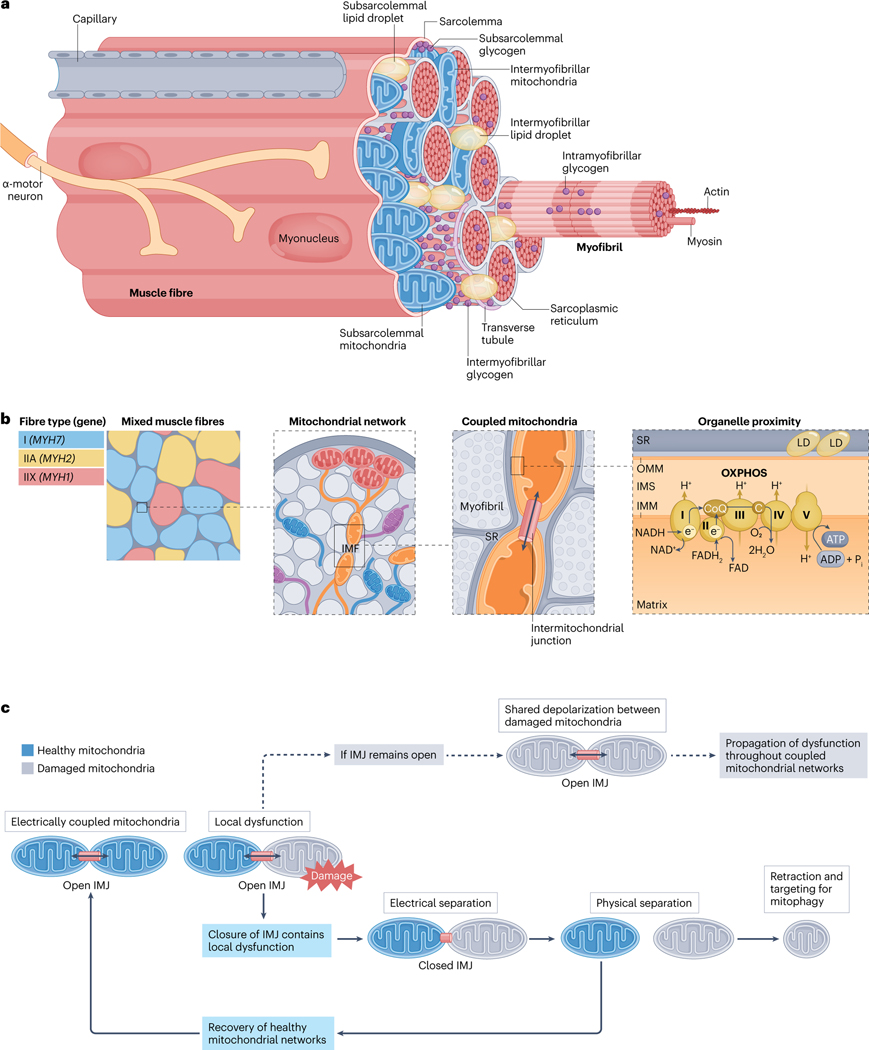
The human body contains >600 skeletal muscles mainly comprising long contractile cells called muscle fibres. The complex architecture of these fibres (Fig. 1a) provides clues into the intricacies of muscle function and homeostatic control. In this section we focus on the contractile and metabolic properties of different muscle fibre types and examine how these attributes are supported by interacting networks of sarcomeres and mitochondria.
Fig. 1 |. Skeletal muscle fibre ultrastructure.
a, Location of mitochondrial subpopulations and energy stores in muscle fibres. Skeletal muscle is composed of layers of connective tissue and fascicles (also known as muscle bundles). Fascicles contain organized arrangements of individual syncytial muscle fibres, each covered by an endomysium, or basal lamina, which is anchored to the fibre membrane (also known as the sarcolemma). Muscle stem cells, termed ‘satellite cells’, reside within this sarcolemma-basal lamina ‘niche’ (Supplementary Fig. 3). Specialized components, such as sodium/potassium pumps (Na+/K+-ATPase), triads (consisting of transverse tubule and sarcoplasmic reticulum (SR)) and proteins of the myofibrils (long arrangements of connected sarcomeres) enable fibre contraction through the process of excitation–contraction coupling and sliding filament theory (Supplementary Fig. 2). Free ATP in muscle is limited69,70, and fibres possess additional energy depots to maintain contractile activity, including creatine phosphate, glycogen and intramyocellular lipids (Box 3). Glycogen granules are nonuniformly distributed between intramyofibrillar, intermyofibrillar and subsarcolemmal pools35,267,268. Alternatively, intramyocellular lipids are stored in lipid droplets (LDs) found predominantly at central (intermyofibrillar) but also peripheral (subsarcolemmal) regions within healthy muscle fibres76,78. During submaximal54 and longer-duration high-intensity interval55 exercise most ATP in muscle is regenerated by mitochondrial oxidative phosphorylation (OXPHOS) (see the section ‘Acute exercise muscle metabolism’) (Fig. 2). b, Spatial distribution of the mitochondrial reticulum within muscle fibres. Human muscle comprises three main fibre types14,21,22,24,36, type I (marked by MYH7 expression), type IIA (with MYH2 expression) and type IIX (expressing MYH1). Differences in mitochondrial protein content14,21 and mitochondrial network configuration56,60 between fibre types directly impacts muscle metabolism and function. Muscle mitochondria form an interconnected reticulum56–60 that enables swift and efficient distribution of potential energy from subsarcolemmal (also known as peripheral) mitochondria to intermyofibrillar mitochondria (IMF), deep within the fibre56–58. The positioning of mitochondria in the intermyofibrillar space influences the structure of adjacent sarcomeres, resulting in variable cross-sectional areas and myofilament spacing at different regions across the sarcomere length53. The branching morphology of IMF also accommodates functional interactions with nearby cellular components, such as the sarcoplasmic reticulum and intermyofibrillar lipid droplets53,56,58. In oxidative mouse muscle, ~20% of all IMF are connected to lipid droplets, which may facilitate efficient ATP production and distribution56. c, Adjacent mitochondria form networks and share energy potential through the intermitochondrial junction (IMJ). Analogous to circuit breakers, intermitochondrial junctions split the reticulum into smaller subnetworks, permitting swift separation of defective mitochondria before their removal through mitophagy58. In this way, intermitochondrial junctions provide a dynamic layer of quality control, rapidly rewiring the mitochondrial reticulum through healthy network components to sustain muscle function58. C, cytochrome c; CoQ, coenzyme Q; IMM, inner mitochondrial membrane; IMS, intermembrane space; OMM, outer mitochondrial membrane.
Contractile, metabolic and myonuclear properties of muscle fibre types
Human muscles of the torso and limbs express three main fibre types, with slow-to-fast contractile properties in the following order: slow-oxidative myosin heavy chain (MyHC) type I (encoded by MYH7), fast oxidative-glycolytic (intermediate) MyHC type IIA (encoded by MYH2) and fast-glycolytic MyHC type IIX (formerly known as type IID; encoded by MYH1)21. Muscle fibres have classically been ‘typed’ according to metabolic — oxidative versus glycolytic enzyme — profiles22,23 and the predominant abundance22,24 or ATPase activity23 of MyHC isoforms. MyHC are the motor proteins of myofibril thick filaments and determine important aspects of muscle function, such as maximum shortening velocity25. Yet, the full extent of fibre type characteristics (Supplementary Fig. 1) depends upon matching the excitation–contraction coupling machinery and ATP provision to MyHC activity14,21. This coordinated expression is a product of -motor unit innervation and the transcriptional synchrony of resident myonuclei26,27.
During differentiation (myogenesis) (Supplementary Box 1), a combination of intracellular forces ‘squeeze’ centrally located myonuclei to the fibre periphery28, where they reside in a generally ordered pattern of ‘domains’ in mature muscle. Discrete populations of myonuclei serve specialized roles within fibres, including those governing the neuromuscular junction, myotendinous junction26,29,30 (the interface between muscle and tendon) and proprioceptive muscle spindles30. Likewise, the function of ‘body’26 or ‘canonical’29 myonuclei (constituting ≥90% of total myonuclei)26 is to direct fibre type specificity in muscle, and they can be identified in mouse muscle by their Myh isoform signature26,29,30. Myh-positive myonuclei from type I versus type II fibres possess unique chromatin accessibility and transcription factor motif enrichment profiles that are distinct from one another and from myonuclei in other cellular compartments, including the myotendinous junction26. This could underlie specific transcription factor-driven myonuclear programmes26,30 that are partially responsible for regionalized gene expression in muscle cells26,30,31 and for the co-expression of specific calcium ()-handling, sarcomeric and metabolic apparatus14,21,26,31 so that fibre type contractile (also known as ‘twitch’) and metabolic properties generally align (Supplementary Fig. 1b).
The myonuclei in fibres from endurance-trained younger and older individuals are more spherical, contain greater lamin A (LMNA) deposition and are stiffer and less deformable than myonuclei in untrained counterparts32. These structural and mechanical modifications may facilitate the transduction of cytoskeletal forces towards the nucleus and improve myonuclear resilience against contractile damage32. As such, exercise training-induced myonuclear remodelling could have important implications for muscle adaptation and integrity across the lifespan.
In general, human muscles often express a greater proportion of slower-twitch fibres than those of other species22,24, and human fibres are slower-contracting than orthologous fibre types in most mammals, including rats and mice24. Similarly, type IIA fibres are the most oxidative fibre type in rodent muscle, whereas type I fibres are most oxidative in humans22 (Supplementary Fig. 1). Such differences might contribute to the lack of conformity between the transcriptomes of human and mouse muscle after acute or chronic resistance exercise33 and should be considered when inferring human relevance from animal physiology. Moreover, the properties of muscle can vary markedly among individuals23,34, biological sexes23 (Supplementary Box 2), anatomical locations35 and during ageing34,36 (Box 2). For example, discrete spatial metabolomic differences within fibre types have been observed in mouse muscle37, and age-associated mitochondrial impairments may induce a glycolytic shift in human fibres without a corresponding change in MyHC36. Collectively, this cautions against the use of MyHC as a strict marker of metabolism and vice versa. Future inquiry should better define how covariates — including biological sex, social gender, biological versus chronological age, metabolic health and training status — interact to determine the full spectrum of muscle characteristics.
Box 2. Effects of ageing on skeletal muscle and the benefits of exercise.
The loss of total muscle mass303, fibre number34 and type II fibre size34,36,250 becomes most evident at ≥50 years of age34,303 and is inherently linked to perturbations in muscle metabolism. Mitochondrial content is lower in type I and type II fibres from older individuals than from younger individuals36, and diminished muscle oxidative capacity is linked to mobility decline among adults ≥60 years of age84,304. Glycolytic enzymes and chaperone proteins of myofibrillar quality control are also decreased in type II versus type I fibres from individuals >65 years of age36. Together with an age-dependent decline of MYH1 and MYH2 mRNA expression (encoding myosin heavy chain IIX isoform (MyHC-IIX) and MyHC-IIA, respectively)47, this disconnect between muscle metabolic and contractile apparatus could contribute towards the reduced type II fibre size34,36,250 that predominantly underlies detriments in muscle force and power with advanced age305 (reviewed in ref. 306). Older humans and aged mice can further accrue subsets of senescent muscle fibres307 and resident mononuclear cells (for example, satellite308, myeloid307–309 and fibroadipogenic progenitor307,308 cells). The number of senescent cells is typically low in resting muscle but increases after resistance exercise310 and injury308,309 irrespective of age. The defective clearance and potential accumulation of senescent cells during ageing308,309 might impair muscle regeneration308,309, hypertrophy (especially of fast-twitch glycolytic fibres)310, strength307,311 and maximal mitochondrial respiration311, in part through a Cdkn1a-driven transcriptional programme307,311.
Exercise can mitigate several aspects of muscle ageing and improves systemic insulin sensitivity12. Lifelong endurance exercisers have a greater density of muscle mitochondria312,313 and a more complex and connected mitochondrial reticulum313 relative to less-active elderly counterparts. This is coincident with higher protein levels of inner mitochondrial membrane fusion-factor optic atrophy 1 (OPA1)313. Additionally, resistance training was shown to offset age-related CpG-site methylations in the mitochondrial genome314, and long-term mixed-modality endurance-type exercise might attenuate methylation events in the promoter regions of important cytoskeletal, sarcomeric, glycolysis, glycogen synthesis and tricarboxylic acid cycle-related genes312. These epigenetic events could combine to preserve muscle contractile and metabolic integrity. Furthermore, exercise training-induced changes in plasma apelin (APLN) positively correlated with chair stand, balance and walking (Short Physical Performance Battery) test scores in elderly women and men315. This suggests that factors produced from exercising muscle help to mediate the protective effects of physical activity against age-related functional decline (Box 4). APLN released from muscle during exercise can act in an autocrine manner to augment satellite cell-mediated repair, mitochondrial abundance and muscle oxidative capacity315, and/or through paracrine mechanisms to stimulate endothelial cell expansion316 that could enhance hypertrophy of type II fibres248,250 (Supplementary Fig. 3).
Endurance61, high-intensity interval and concurrent12 training all increase maximal oxygen consumption () and muscle mitochondrial content in older individuals. Reciprocally, resistance training improves strength12,240,317 and fat-free mass12 but to a lesser extent than seen in young adults240. The attenuated muscle anabolic response with age could be due to blunted ribosomal biogenesis240, reduced activity of amino acid sensors (such as leucyl tRNA synthetase (LARS))318 and lower systemic production and local tissue sensitivity to hormones such as testosterone and insulin-like growth factor 1 (IGF1)240 (Supplementary Table 1). Progressive resistance training programmes can further benefit hip and femur bone mineral density in people ≥65 years of age317, and incorporating power-type resistance exercise (involving explosive concentric movements) may be superior for physical performance outcomes, including ‘get-up- and-go’ and chair stand tests319. Collectively, these findings emphasize the importance of maintaining a diverse physical activity profile across the lifespan.
Hybrid fibre types
Most canonical myonuclei within the same fibre display coordinated transcription of a single Myh isoform in mouse muscle26,27. However, a minority of fibres are hybrid26,27 (reviewed in ref. 38), containing myonuclei that express two or more Myh pre-mRNA genes in the same nucleus26,29 and/or in different nuclei across the fibre length26. Thus, the regional distribution of MyHC can vary between muscle biopsy sites39 and along a single fibre26,40.
In human vastus lateralis muscle, <10%22,41 to 40%38 of fibres can be hybrid types. ‘True’ non-transitioning or non-regenerating hybrid fibres have metabolic enzyme22 and single-fibre contractile (force-velocity producing)25,42 properties between those of their co-expressed MyHC isoforms, providing further functional nuance to the slow-oxidative to fast-glycolytic continuum in the following order of slowest and most oxidative to fastest and most glycolytic: type I → I/IIA → IIA → IIA/IIX → IIX22,25,42. In adult mice, hybrid fibres are most common in the slow-twitch soleus26,27, and the abundance of hybrid fibres is not altered by denervation in this muscle group26. By contrast, sciatic nerve transection or deletion of Six1 — a gene encoding the transcription factor homeobox protein SIX1 driving the fast-glycolytic phenotype in muscle43 — increases hybrid fibre content in the typically fast-twitch extensor digitorum longus26,27. Hence, anatomical position27, innervation26,27 and transcription factor profiles26 might coalesce to coordinate Myh expression in muscle.
For a given muscle group, a greater proportion of hybrid and pure type IIX fibres seems indicative of sedentary behaviour41. Alternatively, exercise tends to reduce hybrid fibre content41,44,45, promoting a shift away from type IIX fibres towards slower myosin types13,39,41. Consequently, pure type IIX fibres are rare in humans22,24,39,41 and account for <1% of the vastus lateralis fibre pool in healthy individuals40. As discussed in Supplementary Box 1, Myh expression in mouse muscle is regulated by competitive promoter–enhancer interactions27,46. Exercise epigenetically modifies chromatin accessibility (see the section ‘Skeletal muscle responses to acute exercise’), and resistance exercise increases MyHC-specific protein synthesis47. Yet, it is still unclear how established regulators of fibre type switching, such as peroxisome proliferator-activated receptor- coactivator (, also known as )48 and nuclear factor of activated T cells, cytoplasmic 1 (NFATc1)49, combine with Myh-promoter–enhancer dynamics to confer physical activity-dependent fibre type transitions. Further single-myonuclei RNA and chromatin profiling, alongside isolated fibre spatial transcriptomic and proteomic approaches, should extend the understanding of how gene expression and cellular phenotype are regulated among the many nuclei of a syncytial muscle fibre.
The myofibrillar matrix
The sarcomere is the basic contractile unit of skeletal muscle, and in-series assembly of sarcomeres forms myofibrils. Although myofibrils were initially viewed as single, tube-like structures organized in parallel within fibres, later evidence suggested that myofibrils instead form branching networks that could act in tandem with the cytoskeleton to facilitate lateral-force transmission50. Using focused ion beam-scanning electron microscopy, recent studies have built upon this hypothesis showing that myofibrils indeed form a nonlinear lattice of sarcomeres51–53 connected across the width and length of the muscle cell through three branching subtypes51 (Supplementary Fig. 2a). In mice, the frequency of sarcomere branching decreases during early-to-late postnatal development, but increases again in adult slow-oxidative soleus fibres51. In comparison to soleus muscle, fast-glycolytic gastrocnemius fibres exhibit a different branching morphology (preferring myofilament transfer over sarcomere splitting) and utilize fewer total sarcomeric connections51. Inducing sarcomere connections, such as through gene manipulation in Drosophila, reduces the myofibril cross-sectional area52. Thus, myofibrillar matrix assembly appears specific to functional demand, and its organization in fast-twitch muscle may support the greater size22 and power24 of these fibres.
The relevance of a nonlinear network of sarcomeres is implied by its structure — providing an elegant mechanism for both longitudinal and lateral force transmission from muscles to bones through tendons50,51. A linked configuration of myofibrils could also minimize the impact of localized sarcomere damage in muscle and thereby increase the robustness of the contractile machinery across an entire fibre. Further interrogation of the function and regulation of the myofibrillar matrix in health, disease and exercise adaptation will be enlightening.
Mitochondrial complexity in muscle
Sustained muscle contraction requires a continuous supply of ATP, which is mostly derived from mitochondrial oxidative phosphorylation (OXPHOS) during submaximal54 or longer-duration high-intensity exercise55 (see below; Fig. 2). Depending on fibre type, around 2–10%56 of muscle volume is filled by distinct subpopulations of subsarcolemmal (also known as peripheral) and intermyofibrillar mitochondria, differing in structure, function and localization56–59 (Fig. 1).
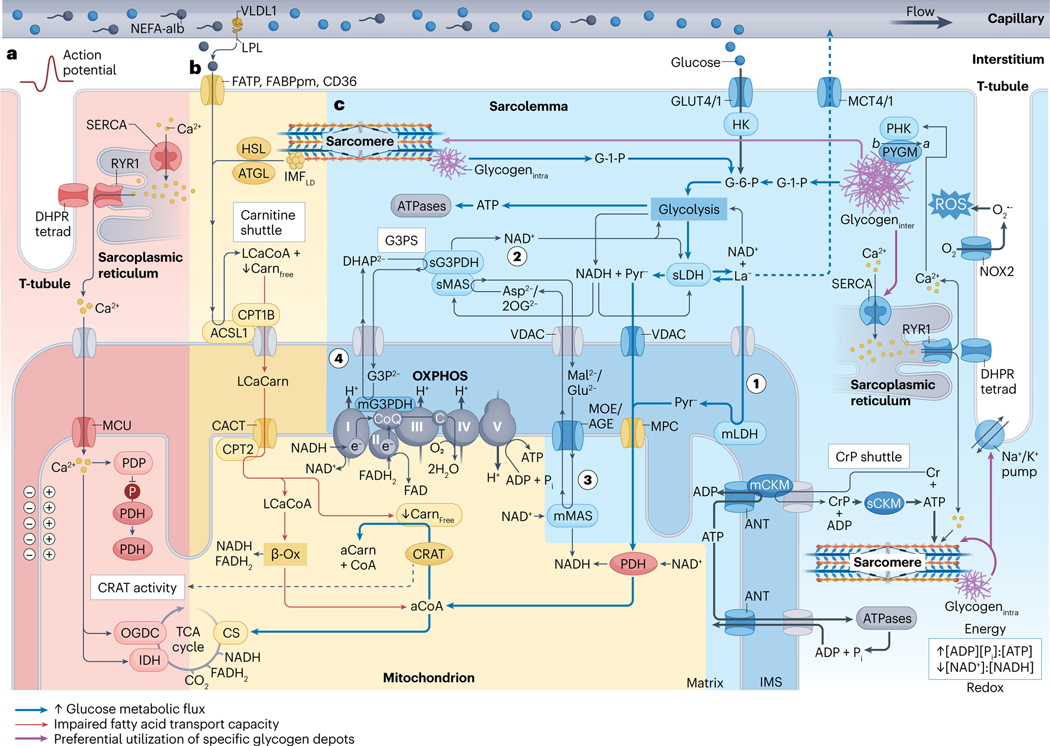
Fig. 2 |. Skeletal muscle metabolism during higher-intensity exercise.
a, Exercise-onset metabolic inertia (red area). Acetyl-carnitine (aCarn) abundance83–85 and the acetyl coenzyme A (aCoA)-producing capacities of carnitine acetyltransferase (CRAT)83,84 and pyruvate dehydrogenase (PDH)85 appear rate-limiting for oxidative adenosine triphosphate (ATP) regeneration at the onset of moderate-high intensity exercise. Contraction-induced calcium () transients promote mitochondrial uptake into the matrix space269 through the inner-membrane mitochondrial calcium uniporter (MCU) complex270,271. Increased matrix can upregulate PDH272 through activation of its phosphatase (PDP)273, and can upregulate isocitrate dehydrogenase (IDH)274 and 2-oxoglutarate dehydrogenase (OGDC)272,274,275 directly to fine-tune oxidative metabolism via stimulation of the tricarboxylic acid (TCA) cycle82. kinetics probably precede an allosteric86 rise in the [ADP][] to [ATP] and [creatine][] to [creatine phosphate] ratios in part because ADP and creatine (Cr) are buffered by the adenylate kinase (not shown) and creatine phosphate (CrP) shuttle reactions. Thus, synchrony between mechanisms of substrate provision, -feedforward and metabolite feedback regulation might underlie acute metabolic inertia. This could be particularly prominent in type II fibres, which have lower CRAT83 and MCU abundance21 and slower mitochondrial import rates276. Furthermore, metabolic inertia is more pronounced in metabolically compromised and older untrained adults, related to the lower CRAT activity and acetyl-carnitine content of muscle in these individuals84. b, Carbohydrates outcompete non-esterified fatty acids (NEFAs) for oxidation at higher intensities (yellow area). Muscle glucose uptake88 and carbohydrate utilization71,72,120 increases with exercise intensity. At workloads above maximum fat oxidation (>60–65% of maximal oxygen consumption ()) flux of pyruvate to acetyl-CoA progressively exceeds rates of TCA cycle entry at citrate synthase (CS), leading to depletion of the muscle free-carnitine (Carnfree) pool through CRAT-dependent acetylation to acetyl-carnitine83. After higher-intensity submaximal exercise, acetylation of the free-carnitine pool is greatest in type I fibres142. Insufficient free-carnitine availability would inhibit NEFA mitochondrial import at the first step of the carnitine shuttle — that is, carnitine palmitoyl transferase 1B (CPT1B) conjugation of carnitine to long-chain acyl-CoA (LCaCoA). Reduced fat oxidation is associated with diminished free-carnitine levels at ~70% of (ref. 72), whereas medium-chain NEFA metabolism bypasses carnitine shuttling and is maintained at higher submaximal workloads277. Therefore, free-carnitine levels appear rate-limiting for long-chain NEFA utilization at increasing exercise intensities. c, Lactate and pyruvate oxidation and NADH shuttles (blue area). Downstream of glycolysis, pyruvate (Pyr−) and/or lactate (La−) pass through voltage-dependent anion channels (VDAC), where lactate is converted to pyruvate by mitochondrial lactate dehydrogenase (mLDH) in the intermembrane space119 (step 1). Pyruvate then enters the mitochondrial matrix through the mitochondrial pyruvate carrier (MPC)81,119. The glycerol-3-phosphate (G3P2−) shuttle (G3PS) and malate (Mal2−)/aspartate (Asp2−) shuttle enables mitochondrial oxidation of lactate and pyruvate through compartmentalized redox shuttling. G3PS and MAS recycle extra-matrix nicotinamide adenine dinucleotide (NAD+) (step 2) and transport reducing power from glycolysis to the mitochondrial matrix. This occurs through reactions associated with Mal2− and Asp2− delivery into the matrix space81,119 (step 3) and G3P2− donation of electrons directly to coenzyme Q (CoQ) of the electron transport chain119 (step 4). As such, saturation of these shuttles increases lactate accumulation and upregulates the lactate-favouring LDHA isoform in vitro278. See Box 3 for discussion of CrP, intermyofibrillar and intramyofibrillar glycogen (glycogeninter and glycogenintra, respectively) and intermyofibrillar lipid droplet (IMFLD) stores, and section ‘Acute exercise muscle metabolism’ for details of their metabolism during acute exercise. -Ox, -oxidation; ACSL1, acyl-CoA synthetase long-chain family member 1; AGE, aspartate/glutamate exchanger; ANT, adenine nucleotide translocator; ATGL, adipose triacylglyceride lipase; denotes posttranslational modification of PYGM from its less-active form to the constitutively active form by PHK; C, cytochrome ; CACT, carnitine acylcarnitine translocase; mCKM mitochondrial creatine kinase muscle; sCKM, sarcoplasmic CKM; DHAP2−, dihydroxyacetone phosphate; DHPR tetrad, four dihydropyridine receptors associated with one ryanodine receptor 1 (RYR1); FABPpm, fatty acid binding protein plasma membrane; FAD, flavin AD; FADH2, reduced FAD; FATP, FA transporter protein; GLUT4/1; glucose transporters 4 and 1; G-1-P, glucose-1-phosphate; G-6-P, glucose-6-phosphate; HK, hexokinase; HSL, hormone-sensitive lipase; IMS, intermembrane space; LCaCarn, long-chain acyl carnitine; LPL, lipoprotein lipase; sLDH, sarcoplasmic LDH; mMAS, mitochondrial MAS; MCT4/1, monocarboxylate transporters 4 and 1; mG3PDH, mitochondrial glycerol-3-phosphate dehydrogenase; MOE, malate/2-oxoglutarate exchanger; NADH, reduced NAD; NEFA-alb, albumin-bound NEFA; NOX2, NAD phosphate oxidase 2; superoxide; 2OG2−, 2-oxoglutarate; OXPHOS, oxidative phosphorylation and associated respiratory complexes; PHK, muscle phosphorylase kinase; PYGM, glycogen phosphorylase muscle-associated; ROS, reactive oxygen species; SERCA, sarcoplasmic/endoplasmic reticulum -ATPase; sG3PDH, sarcoplasmic glycerol-3-phosphate dehydrogenase; sMAS, sarcoplasmic MAS; T-tubule, transverse tubule; VLDL1, very low density lipoprotein 1.
Subsarcolemmal mitochondria often cluster together in the sarcoplasmic space between myofibrils and the sarcolemma56–59, and they dedicate more of their volume to cristae (folds of the inner membrane) and matrix57,58. These globular mitochondria extend deep into the myofibrillar space and physically join with adjacent intermyofibrillar mitochondria through electron-dense intermitochondrial junctions57,58, forming the mitochondrial reticulum56–59 (Fig. 1b,c). Compared to subsarcolemmal mitochondria, intermyofibrillar mitochondria are more complex in morphology59 and physically interact with the myofibrillar matrix53,56,57, sarcoplasmic reticulum (SR) and intermyofibrillar lipid droplets53,56,58 (Fig. 1b).
In humans53,59,60 and mice53,56, intermyofibrillar mitochondrial (sub)networks are uniform, but their organization varies between muscle types56,60. In fast-glycolytic extensor digitorum longus fibres of mice, intermyofibrillar mitochondria wrap around the I-bands of sarcomeres, perpendicular to the contraction axis, whereas slow-oxidative soleus fibres contain larger subnetworks of connected intermyofibrillar mitochondria, arranged in grids that surround myosin like a cage56. The positioning of mitochondria in the intermyofibrillar space also directly impacts the structure of adjacent sarcomeres53. A greater proportion of mitochondria at the Z-disc reduces the cross-sectional area of sarcomeres in this region by bending (or ‘curving’) peripheral myosin filaments, causing heterogenous myosin–myosin spacing along the sarcomere length53. However, any detrimental impact on fibre contractility might be offset by the relative preservation of cross-sectional area and myofilament spacing towards the sarcomere centre (M-line)53 (the structure of an individual sarcomere is shown in Supplementary Fig. 2d).
Subsarcolemmal mitochondria are proximal to nutrient-delivering capillaries56–59 and have a greater abundance of proton-motive force driving electron transport chain (ETC) complex IV57. As such, this subpopulation is thought to specialize in membrane potential generation for subsequent transfer through the reticulum, into the intermyofibrillar mitochondrial network57. Here, intermyofibrillar mitochondria, possessing higher ATP synthase (ETC complex V) expression57 and higher surface area-to-volume ratios59, could utilize this potential energy to support rapid ATP production and diffusion to myofibrillar ATPases56. Hence, much like an electrical power grid, the mitochondrial reticulum may serve to efficiently disperse energy across the fibre56–58.
Within mitochondria, ETC complexes form higher-order structures termed ‘supercomplexes’. The maximal oxygen consumption of permeabilized fibres was positively correlated with the preferential redistribution of complexes III and IV into supercomplexes in the muscle of elderly individuals after 4 months of endurance training61. Alternatively, 6 weeks of high-intensity exercise improved muscle mitochondrial respiration and ETC enzymatic activity in the absence of supercomplex alterations in young adults15. Supercomplexes are clearly important structural features of the ETC, but whether they support exercise adaptation beyond their stoichiometric relationship with mitochondrial abundance is yet to be determined. Furthermore, the assembly and stability of supercomplexes depends on the integrity of mitochondrial cristae62,63, which can be regulated by SR stress-induced signalling based on eukaryotic translation initiation factor () kinase 3 (PERK, also known as EIF1AK3), and activating transcription factor 4 (ATF4) ()63. Endurance-trained athletes have greater cristae density in mitochondria of type I fibres64, and PERK and ATF4 proteins are increased in muscle ~48 h after acute resistance exercise65. However, cristae structure was unchanged after 10 weeks of moderate-intensity endurance training in sedentary individuals with obesity64. Thus, further study is required to distinguish the impact of exercise on mitochondrial cristae remodelling.
Moving forward, better understanding of mitochondrial networks, subpopulations and ETC configurations could benefit strategies to combat age-associated decline in muscle function (Box 2) and mitochondria-related diseases. Focus should be directed towards the heterogenous mitochondrial populations in human muscle, which are smaller than those in mice59.
Acute exercise metabolism in skeletal muscle
Relative to weight, the basal thermogenesis of muscle is lower than that of most other organs66 because myosin is maintained in disordered relaxed and super-relaxed states characterized by slow and extremely slow ATP kinetics, respectively67 (Supplementary Figs. 2b,c). Upon contraction, mechanosensing rapidly initiates myosin conformational change from relaxed to active68, and muscle ATP consumption increases dramatically during short-duration exhaustive exercise69,70. As free ATP in muscle (~20–25 mmol per kg dry mass) is only sufficient to sustain maximal exercise for <2 s (refs. 69,70), continued contractile activity requires ATP resynthesis from a combination of intramuscular energy stores (Fig. 1a and Box 3) and circulating substrates, such as glucose and non-esterified fatty acids (NEFAs)71,72 (Fig. 2).
Box 3. Energy depots in skeletal muscle.
Creatine phosphate
The total concentration of muscle creatine is ~120–130 mmol per kg dry mass in young adults320. Approximately 66–67% of this muscle creatine pool is creatine phosphate (CrP), with the rest existing in unphosphorylated form (free creatine)320. Resting CrP levels are ~10–15% higher in type II fibres, which rely on CrP to a greater extent than type I fibres75. CrP is the main ATP substrate of the phosphagen energy system through a reversible reaction catalysed by sarcoplasmic creatine kinase (CKM). At physiological pH during exercise (~6.5–7.0)54,55, CKM is bound to sarcomeres through association with myomesins (MYOM1 and MYOM2) at the M-line321 and phosphofructokinase (PFK) at actin filaments of the I-band322—coupling CKM to contraction and glycolysis. CrP hydrolysis peaks at the onset of maximum contraction but deteriorates within ~6 s (ref. 70), and stores can be ~75–90% depleted after roughly 30s of hard exercise70,75. The majority of CrP resynthesis is linked to aerobic metabolism through the ‘creatine phosphate shuttle’, whereby mitochondrial CKM re-phosphorylates free creatine to CrP in the intermembrane space, using ATP from oxidative phosphorylation323 (Fig. 2). Intramuscular creatine rarely reaches saturation by diet and de novo synthesis alone, and supplementing an additional ~3 g per day (~0.03 g per kg body mass daily) increases stores ~15% (to ~140 mmol per kg dry mass) in ~28 days, raising mostly free creatine324. Creatine supplementation appears safe for renal function325 and consistently improves strength performance326. The role of creatine in brain health is an emerging area of interest and creatine supplementation may improve the memory function of older adults327.
Glycogen
Muscle glycogen concentrations vary depending on nutritional state but are ~400–500 mmol per kg dry mass in the vastus lateralis of individuals following mixed macronutrient diets328. Glycogen granules are nonuniformly distributed between three specialized pools in muscle329: (1) the intermyofibrillar pool, found between myofibrils, close to mitochondria and the sarcoplasmic reticulum; (2) the subsarcolemmal pool, positioned just beneath the cell membrane; and (3) the intramyofibrillar pool, situated within the myofibril at the Z-line of the I-band (Fig. 1a). The intermyofibrillar pool is most abundant and accounts for ~77–84% of muscle glycogen, whereas intramyofibrillar and subsarcolemmal stores constitute ~8–15% and ~6–12%, respectively35,96,97,267,268. The relative concentration of each glycogen reserve appears unaffected by age267, type 2 diabetes (T2D)268 or anatomical location (triceps brachii compared to vastus lateralis)35, but may vary depending on physical activity status35,267,268 and fibre type35,97. Total glycogen content and utilization is typically higher in type II versus type I fibres75,97, and type II fibres have ~23% more glycogen in intramyofibrillar stores97.
Intramyofibrillar glycogen fuels myosin and sodium/potassium (Na+/K+)-ATPases329 and is preferentially mobilized during strenuous endurance35, high-intensity interval97,330 and resistance96 exercise. Myosin-ATPases also utilize intermyofibrillar glycogen, as does the sarcoplasmic/endoplasmic reticulum -ATPase (SERCA)329 (Fig. 2). Thus, sufficient muscle glycogen is important for many types of exercise and a critical threshold of ~250mmol per kg dry mass has been proposed330, below which self-perceived level of effort increases330 and sarcoplasmic reticulum function330,331, power output331 and repeated-sprint ability330 decline until glycogen stores are replenished330,331. Nevertheless, exercising with low glycogen availability (~100–300 mmol per kg dry mass) might augment signal transduction (for example, 5′-AMP-activated protein kinase (AMPK), p53) and gene expression (for example, PGC1A and TFAM)332 associated with oxidative metabolism and mitochondrial biogenesis (see the main text). This has led to the concept of ‘train low, compete high’ or ‘carbohydrate periodization’ (reviewed in ref. 332). Whether carbohydrate periodization leads to improved exercise performance over time awaits confirmation, as does delineation of the potential underlying factors (for example, glycogen content per se versus hypocaloric diets that cause weight loss333 and/or proximal glycogen-depleting exercise bouts that result in higher training volumes245).
Intramyocellular lipids
Intramyocellular lipids (IMCLs) are stored in the hydrophobic core of lipid droplet ellipsoids77 at peripheral (subsarcolemmal, SSLD) and central (intermyofibrillar, IMFLD) regions within fibres76–78,268.Women may have ~43% more individual lipid droplets in muscle, contributing to a greater (~84%) density of total IMCLs than in men334. Similarly, type I fibres utilize more IMCLs during moderate- intensity exercise130 and have ~2–3-fold higher IMCL contents and lipid droplet numbers than type II fibres76,78. Although IMCLs are mostly deposited (>85%) in IMFLD76–78, the relative distribution and characteristics of lipid droplet subpopulations (particularly in type II fibres) vary depending on training status, body composition and metabolic health78.
Endurance-trained athletes and adults with T2D have similar total IMCL concentrations in muscle78,335 despite markedly dissimilar insulin sensitivity profiles78,84,335. This apparent contradiction has been termed the ‘athlete paradox’335 but could be partly explained by contrasting lipid droplet properties77,78. Individuals with T2D have a greater number of extremely large SSLD in type II fibres77,78, which also possess lower subsarcolemmal mitochondrial contents77. This results in higher relative contributions of SSLD to the overall IMCL pool78 and fewer mitochondria-to-SSLD contacts77. Spatially, SSLD could interfere with insulin signalling and the T2D-SSLD phenotype negatively correlates with peripheral insulin sensitivity78. Conversely, endurance athletes have approximately twofold more IMCLs in type I fibres and an increased abundance of adipose triacylglyceride lipase (ATGL) and perilipin 5 (PLIN5)78 — proteins associated with lipid droplet turnover118. The higher type I fibre IMCL content of trained individuals is specifically stored in smaller IMFLD78, which are favourably depleted during prolonged endurance exercise76 (Fig. 2b).
An 8-week programme of high-intensity interval training reduced SSLD size, increased the subsarcolemmal mitochondria-to-lipid droplet ratio and redistributed IMCLs into small IMFLD in type II fibres77. Accordingly, lipid droplet profiles were similar between adults with normal weight, overweight or obesity, or T2D after the training intervention, regardless of baseline differences77. Therefore, consistent exercise may alleviate muscle insulin resistance somewhat through remodelling of lipid droplets77,78.
In this section we detail the metabolic responses that enable muscle to match the considerable demands of acute exercise. We also address how this substrate–energy pairing facilitates the integral role of muscle in exercise-mediated inter-organ communication (Box 4) and touch upon the interaction between exercise metabolism and biological rhythms.
Box 4. Select factors produced from skeletal muscle during exercise.
Enhanced blood flow during exercise not only improves delivery of nutrients and hormones (Supplementary Table 1) to muscle but also facilitates the release and transport of discrete factors from muscle20. These secreted molecules (known as exercise-induced ‘myokines’ or muscle-derived ‘exerkines’) can act in an autocrine, paracrine or endocrine fashion and are thought to promote many of the favourable adaptations associated with physical activity111.
Interleukin 6 (IL-6) is perhaps the prototypical example of an exercise-stimulated, muscle-secreted factor336. Elevations in circulating IL-6 with endurance-type exercise19,336,337 might contribute towards short-term energy allocation by transiently inhibiting inflammatory processes (for example, monocyte production of tumour necrosis factor (TNF)) while preferentially directing liberated non-esterified fatty acids (NEFAs) towards working muscle (reviewed in ref. 338). Accordingly, systemic pharmacological blockade of the IL-6 receptor promotes re-esterification and storage of NEFAs337. A ventromedial hypothalamic circuit of locally synthesized IL-6 controls NEFA oxidation after swimming exercise specifically in the soleus of mice, through sympathetic adrenoceptor modulation of 5′-AMP-activated protein kinase (AMPK)–acetyl-CoA carboxylase (ACC)339. The effects of centrally produced IL-6 preceded a rise in peripheral IL-6 concentrations, and whether IL-6 from muscle can activate this same neuromuscular axis is unclear339.
Lactate is an established signalling metabolite and its production is enhanced by glycolytic stressors. Elevated rates of muscle glycolysis during higher-intensity (for example, sprint and resistance) exercise increases plasma lactate to greater extents than moderate-intensity endurance exercise90. Muscle-derived lactate can initiate an adipose tissue-transforming growth factor () secretion axis, which mediates improvements in murine glucose tolerance after 11 days of voluntary wheel running340. Lactate exiting muscle can be further converted to -lactoyl-phenylalanine (Lac-Phe) in cells expressing CNDP2, such as immune cell populations (for example, macrophages and monocytes) or epithelial cell populations341. Intraperitoneal Lac-Phe delivery caused appetite suppression and weight loss in obese mice341. However, post-exercise concentrations of Lac-Phe in human plasma were orders of magnitude lower than those administered in the murine experimental model341. As such, the role of Lac-Phe in the hunger-suppressing effects ~0–3 h after high-intensity exercise342 warrants additional study.
Apelin (APLN)315 and succinate343 are other examples of molecules released from exercising muscle that promote crosstalk between muscle fibres and resident mononuclear cells. This retrograde signalling is particularly important for adaptive extracellular matrix remodelling and angiogenesis (Supplementary Fig. 3). Longer-duration high-intensity intervals55 and prolonged endurance exercise124 may increase circulating succinate more than resistance exercise does. Similarly, only endurance exercise lowered the [kynurenine (KYN)] to [kynurenic acid (KYNA)] ratio in plasma124. KYN is a neurotoxic metabolite and the systemic reduction in [KYN] to [KYNA] protects against stress-induced depressive-like symptoms in mice344. This occurs through a muscle peroxisome proliferator-activated receptor- coactivator () isoform 1 ()–peroxisome proliferator-activated receptor- ()–KYN aminotransferase (KAT) cascade that detoxifies KYN to KYNA344. KYNA subsequently released from muscle could influence energy homeostasis by activating G protein-coupled receptor 35 (GPR35) on adipocytes to stimulate lipid turnover345. Numerous other exercise-responsive secretory factors regulated by can reportedly signal from muscle (reviewed in ref. 223), including neurturin (NRTN)346. NRTN operates through both autocrine and paracrine mechanisms to coordinate slow-oxidative muscle fibre and slow-twitch motor neuron property transitions in mice, and its mRNA is upregulated in human vastus lateralis ~72 h after sprint interval exercise346.
Collectively, cell-to-cell and inter-organ communication appears to have an important role in the local and global effects of exercise. Although muscle is a mediator of this crosstalk, other metabolically active tissues (for example, the heart, liver, adipose, nervous, endocrine and immune systems) also contribute, as discussed in detail elsewhere (reviewed in ref. 111).
Muscle substrate utilization is exercise intensity-dependent
Muscle fibres are part of a functional ‘motor unit’ comprising an -motor neuron and the muscle fibres innervated by its axon. The force generated by a muscle depends on both the number of activated motor units — and thus fibres — and the rate at which motor units discharge action potentials once recruited (known as rate coding) (reviewed in ref. 73). Stimulation of motor units conforms to the size-orderly principle of recruitment, such that smaller units are activated first, followed sequentially by larger units as contraction intensifies73. Consequently, the higher power outputs achieved during progressively demanding physical activity relies on the stimulation of a greater number of motor units, a larger proportion of the muscle fibre pool, and therefore the recruitment of more type II fibres.
ATP consumption per unit of time is ~2.5–4-fold higher in type II fibres than in type I fibres74, and the maximal rate of ATP resynthesis is fastest through oxygen-independent (anaerobic) versus oxygen-dependent pathways, in the following order of descending speed: anaerobic phosphagen system (including adenylate kinase and creatine kinase (CKM)) and glycolysis reactions provide the fastest supply of ATP, followed by carbohydrate oxidation and finally NEFA oxidation. Human type II fibres are hence enriched with creatine phosphate (CrP) and glycogen energy depots75 and contain higher levels of adenylate kinase21, glycogenolysis and glycolysis metabolic machinery14,21. Conversely, type I fibres are more abundant in peroxisomes14, mitochondria14,21,76,77 and intramyocellular lipids (IMCLs)76–78, consistent with their slower ATP turnover74 (Supplementary Fig. 1b).
Together, this indicates that the contribution of specific energy systems and substrates to working muscle is mainly a function of the neuromuscular activation required to match the intensity of the exercise being performed. This understanding is envisaged within the ‘crossover concept’ (reviewed in ref. 79), which describes the larger relative contribution of NEFA oxidation towards whole-body energy expenditure at low-to-moderate exercise intensities, with an incremental and necessary ‘switch’ towards preferential (oxygen-dependent and independent) carbohydrate utilization during exercise at higher levels of mechanical effort71,72.
Metabolic inertia at the onset of exercise
Although the lower metabolic cost of rest72 and light exercise71 is mainly fuelled by the oxidation of circulating NEFAs, further upregulation of OXPHOS ATP provision is delayed at the onset of moderate-to-high intensity physical activity, and a larger oxygen-independent contribution fuels muscle in the first ≤30–60 s of exercise54,80. This is despite sufficient blood flow54,80 and adequate intramuscular oxygen levels for maximal mitochondrial respiration to occur (≥0.5–2 mmHg) (reviewed in ref. 81).
The lag in oxygen-dependent metabolism at the beginning of exercise may stem from a combination of linked temporal factors, including activation of the mitochondrial matrix enzymes pyruvate dehydrogenase (PDH), 2-oxoglutarate dehydrogenase (OGDC)82 and carnitine acetyltransferase (CRAT)83,84; the availability of tricarboxylic acid (TCA) cycle substrates (pyruvate and glutamate)82 and precursors (acetyl groups)83–85; and sarcoplasmic buffering of the interrelated allosteric metabolites creatine and ADP86 (Fig. 2a). Indeed, pharmaceutical activation of PDH increased muscle acetyl-carnitine availability and reduced the reliance on phosphagen and glycolytic energy pathways during acute ischaemic exercise85. The ability of previous sprinting intervals to prime OXPHOS in subsequent high-intensity work bouts could also occur through better coupling of oxidative substrate delivery to mitochondrial enzymatic activity, although carryover of residual fatigue probably plays an additional role in this scenario by impeding power output69,70.
Oxygen-independent exercise metabolism
During short-duration maximal efforts, accelerated muscle ATP demand is mostly met anaerobically, through rapid stimulation of the phosphagen and glycolytic energy systems69,70. The simplicity and proximity of the sarcoplasmic CKM reaction (Box 3) allows CrP to supply equimolar amounts of high-energy phosphate to ATPases within milliseconds87, which is essential for sprint performance69,70. CrP hydrolysis peaks at the onset of contraction but declines within less than 6 s, and CrP stores can be >90% depleted after around 30 s of intense exercise. 70 compared to only ~20% after 10 min of cycling at ~50% of (ref. 88).
The initial increase in glycogen breakdown is due to posttranslational modification of glycogen phosphorylase (PYGM) from a less-active form to the constitutively active form by phosphorylase kinase (PHK)70. Transients of and AMP are probable regulators of PHK and thus the switch to (reviewed in ref. 89). AMP can simultaneously increase activity to support maximal rates of glycogenolysis70, and the systemic rise of adrenaline during sprint intervals69 or endurance exercise71,90,91 may help to stabilize the conformation92. Downstream of glycogen and glucose, allosteric regulation and replenishment of nicotinamide adenine dinucleotide (NAD+) can stimulate the rate-limiting enzymes phosphofructokinase (PFK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively, to promote continued flow through glycolysis (Fig. 2c).
Average power output drops substantially over the final half of a 30-s all-out physical effort, corresponding to the pattern of PYGM activity (reversion from to ) and reduced substrate-level phosphorylation70. This muscular fatigue might be driven by alterations to the intracellular metabolic milieu. Above a critical threshold of ~15 mmol l−1 (ref. 93), inorganic phosphate can enter the SR and precipitate with , thereby impairing release and muscle contraction94. Additionally, owing to the high activity and near-equilibrium state of lactate dehydrogenase (LDH), the end product of glycolysis is always lactate95. After ~3.5–4 min of exhaustive cycling, muscle lactate reaches levels more than twofold higher than resting concentrations55, more than fourfold higher following acute resistance exercise96, and ≥8-fold97 to 30-fold69 higher after 10 rounds of short-duration sprinting. The accumulation of lactate (a strong anion) encourages dissociation of water to HO− + H+, and muscle pH can reach ≤6.5 during strenuous exercise bouts55. Lower intramuscular pH could diminish PYGM activity70, and H+ may act on group III (mainly mechanosensitive) and IV (mainly metabosensitive) muscle afferents in the interstitial space93. Once activated, these sensory neurons can feed back, potentially through inhibitory -aminobutyric acid type B () receptors98, to reduce motor cortex excitability and suppress motoneuronal output93. The severity of peripheral fatigue correlated with the extent of quadriceps activation during self-paced time-trial cycling99. However, mechanisms of exercise-induced peripheral and central fatigue are complex, and the major contributory factors are probably specific to modality, intensity and duration (reviewed in ref. 94).
Oxygen-dependent exercise metabolism
Reactive oxygen species production during exercise.
In contrast to maximal sprinting for ≤30 s (refs. 69,70), high-intensity cycling lasting ≥3 min derives >70% of energy from OXPHOS, with a post-exercise muscle metabolome enriched for pathways using pyruvate, long-chain NEFAs and amino acids such as alanine, arginine and glutamate55. This upregulation of OXPHOS reduces mitochondrial superoxide (H2O2) emission100,101 from ETC complexes I–III100 and thus mitochondria contribute minimally to reactive oxygen species (ROS) generation in contracting muscle102. Rather, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX), located in the sarcolemma and in transverse tubules (T-tubules)102 and the sarcoplasmic reticulum103, appear to be the predominant sources of ROS during contraction101,102. Specifically, NOX2 (ref. 101) and NOX4 (ref. 104) are indispensable for exercise-stimulated sarcoplasmic ROS production in mice.
ROS signalling is implicated in adaptations to endurance and resistance training (reviewed in ref. 105). High-intensity intervals augment NOX4 mRNA in human vastus lateralis ~3–4 h after exercise, and a retrograde NOX4–ROS-axis led to activation of transcriptional regulators — such as nuclear factor erythroid 2-related factor 2 (NRF2, also known as NFE2L2) and — required for mitochondrial biogenesis and the endogenous antioxidant defence response to exercise training in mouse muscle104 (Fig. 3). Accordingly, blunting oxidative stress through Nox4 deletion in mice104 or high-dose vitamin C and E intake in humans106 attenuates the insulin-sensitizing effects of endurance training. Still, any potential detriment of antioxidant (such as vitamin C and/or vitamin E) supplementation to gains in , lean body mass or human endurance and strength performance seems relatively minor107.
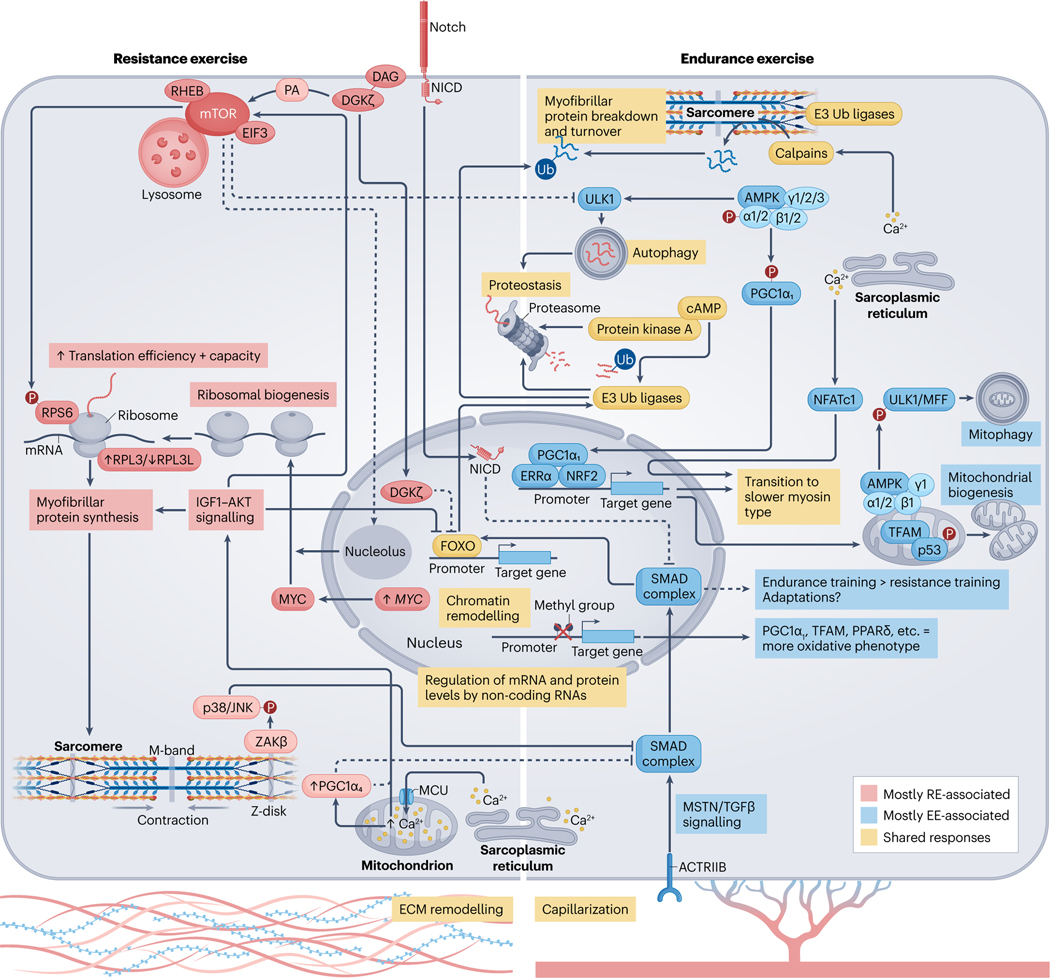
Fig. 3 |. Molecular responses to acute exercise and exercise training.
Exercise-induced alterations in circulating molecules19,124 and the intramuscular milieu55,101, together with mechanical tension178, initiates a temporal series of biochemical and molecular events that lead to muscle adaptation. Activation of signalling cascades promote substantial posttranslational modification of the muscle proteome90,170 and DNA accessibility198,199,230. Collectively, this drives transcription factor-dependent169 changes in gene expression167,183, alongside microRNAs204 and long-non-coding RNAs205 that are thought to ‘fine-tune’ the molecular responses to exercise. Endurance exercise (EE) and resistance exercise (RE) are often considered divergent stimuli, primarily driving oxidative versus hypertrophic muscle adaptations, respectively. However, common processes among exercise modalities can result in shared enrichment of signalling cascades90 and transcriptional networks183 in the post-exercise period. For example, coordinated proteolysis is detected following acute exercise, irrespective of exercise type90. This may require cAMP–protein kinase A (PKA)190,191 and ensures protein quality control and physiological muscle remodelling. 5′-AMP-activated protein kinase (AMPK) activity90 and the expression of total PGC1A mRNA183 are also increased after a single bout of either endurance or resistance exercise. AMPK phosphorylation activates peroxisome proliferator-activated receptor- coactivator isoform 1 ()279, and both AMPK and potentiate angiogenic factors260,280 and mitochondrial bioenergetics48,235,260,279 in muscle. After endurance exercise, a distinct pool of mitochondrial AMPK (composed of , , and isoforms) regulates mitophagy187–189 and promoter hypomethylation facilitates the transcription of peroxisome proliferator-activated receptor- () and mitochondrial transcription factor A (TFAM)230. Whether resistance exercise elicits these same effects is unclear. Despite similarities, there are more distinct than overlapping post-exercise responses between modalities90,183. Rapamycin-sensitive substrates of mammalian target of rapamycin (mTOR) complex 1 (mTORC1) are phosphorylated to a greater extent after resistance exercise90. Mechanical overload initiates translocation of the mTOR–lysosomal complex174 and diacylglycerol (DAG) kinase- ()173 to the sarcolemma. Here, mTOR colocalization with RAS homologue enriched in brain (RHEB) and eukaryotic initiation factor 3 (EIF3)174 and phosphatidic acid (PA) produced by 173 might coalesce to fully stimulate mTORC1 (ref. 175) and the translation of contraction-associated mRNAs. Acute attenuation of UNC-51-like autophagy activating kinase 1 (ULK1) autophagic signalling after resistance exercise65 and nuclear -mediated suppression of forkhead box protein O (FOXO)-dependent proteasomal degradation could also support muscle mass by moderating the breakdown of myofibrillar proteins173. At the sarcomere, contraction recruits to the Z-disc179 where it acts through JUN N-terminal kinase 1 (JNK1) and JNK2 (refs. 179,181), potentially alongside Notch182, to inhibit myostatin (MSTN)/transforming growth factor- () signalling. This represents one of many intracellular changes permitting resistance training-induced hypertrophy over endurance-like adaptations181. The upregulation of MYC with resistance exercise stimulates ribosomal biogenesis180,198, and the formation of a specialized pool of ribosomes with a high ratio of ribosomal protein large 3 (RPL3) to RPL3-like (RPL3L)238 may favour protein synthesis over translational fidelity. MYC expression is mTOR-independent but MYC cooperation with mTOR is necessary to successfully increase ribosomal content180, possibly requiring an mTOR-driven reorganization of nucleoli to aid rRNA transcription281. Divergence between endurance and resistance exercise at the transcriptomic level is magnified after a period of training183. Endurance training increases electron transport chain complex expression183, mitochondrial content and muscle oxidative capacity12. Conversely, growth-related pathways183, ribosomal abundance198 and muscle mass12 are augmented more by resistance training. This could be mediated in part by isoforms. Nuclear localization of and Ser15 phosphorylation of p53 are greater in resting muscle after high-intensity interval training239, which might help to preserve mitochondrial content and function257. By contrast, protein is unchanged after resistance training, whereas the isoform 4 () protein is preferentially enriched235. stimulates muscle hypertrophy and is associated with enhanced Igf1 expression229, insulin-like growth factor 1 (IGF1)–serine/threonine protein kinase (AKT) and mTORC1 signalling234 and downregulation of Mstn mRNA in mouse muscle229. However, unlike (ref. 280), does not coactivate oestrogen-related receptor- ()229 and has no effect on oxidative phosphorylation enzymes229,235. Appreciable overlap in the initial stages of exercise training probably underlies the degree of shared adaptation between endurance and resistance exercise (see the sections ‘Skeletal muscle responses to acute exercise’ and ‘Skeletal muscle adaptations to long-term exercise’). Depending on individual predisposition (Box 1), dedicated training of a certain exercise modality could amplify discrete differences in the adaptive response, resulting in distinct muscle adaptations and the development of specific phenotypes over time13. Evidence showing that combined endurance and resistance training can blunt muscle hypertrophy in humans is scarce184,185 but concurrent training could impede gains in explosive strength184. Still, a combined exercise regime may offer dual benefits for most individuals12. ACTRIIB, activin receptor type 2B; ECM, extracellular matrix; MFF, mitochondrial fission factor; MCU, mitochondrial calcium uniporter; NFATc1, nuclear factor of activated T cells, cytoplasmic 1; NICD, Notch intracellular domain; NRF2, nuclear factor erythroid 2-related factor 2; p38, p38 mitogen-activated protein kinase; RPS6, ribosomal protein S6; SMAD, mothers against decapentaplegic homologue; Ub, ubiquitin; , MAP3K20 isoform-.
Glucose uptake and carbohydrate oxidation in exercising muscle.
When workload71,72 and (to a lesser extent) duration71 of moderate-intensity exercise increases, so does the contribution of blood glucose towards total carbohydrate utilization, although energy provision from intramuscular glycogen is always higher71,72. Muscle glucose uptake reaches ~15-fold resting levels at the cessation of non-fatiguing exercise (~50% of ) and ~50-fold resting levels at the cessation of exhaustive endurance exercise (~100% of )88. This increased rate of blood glucose extraction is supported by greater hepatic glucose output108, a ≥5–10-fold rise in blood flow88, and enhanced perfusion of muscle capillaries109. As exercise intensifies, these circulatory and microvascular responses collectively maintain relatively constant plasma–to–interstitial glucose concentrations110 and enlarge the area available for nutrient exchange to occur109. Such events further serve to promote the release of discrete biologically active molecules from muscle20. As discussed in Box 4, many of these muscle-derived ‘exerkines’ or exercise-induced ‘myokines’ are implicated in aspects of local and systemic exercise adaptation (reviewed in ref. 111).
The exocytosis and fusion of glucose transporter 4 (GLUT4)-containing vesicles with the sarcolemmal membrane and T-tubules is essential for contraction-stimulated glucose transport into muscle112. During exercise, translocation of GLUT4 is regulated by a combination of , metabolic stimuli and mechanosensitive stimuli that converge on calcium/calmodulin-dependent protein kinase II (CaMKII), 5′-AMP-activated protein kinase (AMPK) and RAS-related C3 botulinum toxin substrate 1 (RAC1) (reviewed in ref. 113), with redundancy between pathways113,114. The role of RAC1 in contraction-induced glucose uptake might be mediated in part by downstream NOX2 activation101 but seems independent of (ref. 114) during submaximal treadmill running in mice. Indeed, the catalytic subunit of AMPK appears dispensable for exercise-stimulated glucose transport in vivo115,116. Instead plays a more notable role in post-exercise substrate metabolism117 and insulin sensitivity116 through the upregulation of pyruvate dehydrogenase kinase 4 (PDK4)117, the promotion of RAS-related protein RAB8A-perilipin 5 (PLIN5) lipid droplet–mitochondrial tethering118 and the phosphorylation of TBC1 domain family member 1 (TBC1D1)115 and TBC1D4 (also known as AS160)116.
For glucose to be oxidized in muscle, lactate — simultaneously produced from glycolysis and taken up through sarcolemmal monocarboxylate transporters (MCT1 and MCT4)81 — must first be converted to pyruvate by sarcoplasmic or mitochondrial LDH (reviewed in ref. 119) (Fig. 2c). Glucose oxidation is higher in men than in women (Supplementary Box 2) and rises with exercise intensity, dietary carbohydrate and muscle glycogen levels, and peri-exercise carbohydrate intake120. Intra-workout consumption121 or ‘mouth-rinsing’122 of exogenous carbohydrates can also benefit exercise performance. Whereas the ingestion of carbohydrates feasibly provides metabolizable substrate120 and spares liver glycogen stores during bouts of longer−duration exercise108, the potential ergogenic effect of mouth-rinsing is most probably achieved through oral receptor afferents that signal centrally to increase voluntary force production123.
Muscle lipid metabolism during exercise.
The post-exercise serum metabolome is distinct between exercise modalities. An acute bout of endurance or resistance exercise differentially regulates discrete subclusters of metabolites across a 3-h post-exercise period, despite similar trends for metabolome recovery over time124. Of note, amino acid, nucleotide and carbohydrate (for example, lactate and pyruvate) signatures are prominent after resistance exercise, compared to the lipid-derivative enrichment (for example, various acyl-carnitines and the ketone body -hydroxybutyrate) after endurance exercise124. This illustrates unique physiological challenges posed by specific interventions.
At intensities eliciting peak fat oxidation (~60–65% of ), the contribution of plasma NEFAs and IMCLs is ~1:1 and roughly equal to total carbohydrate utilization71,72. In mouse muscle, liberation of NEFAs from lipid droplets is almost entirely dependent125 on the redundant enzymes125,126 adipose triacylglyceride lipase (ATGL) and hormone-sensitive lipase (HSL). The recruitment of ATGL to PLIN5 at lipid droplets could be enhanced by AMPK-regulated assembly of the RAB8A–PLIN5 tethering complex118. Alternatively, exercise upregulates HSL in an intensity-dependent manner127 through additive contraction (–protein kinase C (PKC))128 and adrenaline (cAMP–PKA)91-mediated pathways that promote its translocation to lipid droplets129. These signalling events augment rates of muscle lipolysis, and IMCL contents of type I and type II fibres can be ~45% and ~20% depleted, respectively, after ~60–180 min of cycling at ~50–75% of (ref. 130). Muscle HSL activity is transient during acute moderate-intensity exercise91 and its downregulation — through negative feedback from AMPK91 or allosteric metabolites (for example, long-chain fatty acyl-CoA) — mirrors the greater relative contribution of circulating NEFAs towards whole-body energy expenditure71.
Circulating NEFA availability is increased during longer bouts of physical activity at low-to-moderate intensity71 due to greater release from adipose tissue and the improved affinity of triacylglycerol-rich lipoproteins for hydrolysis by lipoprotein lipases (LPL)131 anchored to the endothelial surface of interstitial capillaries. The utilization of plasma NEFAs is enhanced by contraction-induced sarcolemmal enrichment of long-chain fatty acid transporters (fatty acid translocase (FAT, also known as CD36), plasma membrane-associated fatty acid binding protein (FABPpm) and fatty acid transport protein 1 (FATP1) and FATP4)132, which have varying capacities for increasing NEFA uptake and oxidation in muscle133. Upon exercise, the exocytosis of CD36 (and possibly other transporters) seems independent of AMPK134 but could involve calcium/CaMK kinase (CaMKK)135 and MAPK/ERK kinase 1 (MEK1) and MEK2 signalling136. Furthermore, although FABPpm is structurally identical to mitochondrial aspartate aminotransferase (mAspAT), these proteins serve distinct functions within their respective subcellular compartments (that is, FABPpm-mediated transport of long-chain NEFAs across the sarcolemma versus mAspAT-based delivery of reducing equivalents into mitochondria)137.
Compared to the situation at rest, NEFAs entering exercising muscle are preferentially directed towards breakdown rather than to re-esterification and storage138. Before being used by downstream metabolic pathways, long-chain NEFAs must be activated by thioesterification to long-chain fatty acyl-CoA. Several fatty acid transport proteins possess intrinsic acyl-CoA synthetase activity139, but acyl-CoA synthetase long-chain family member 1 (ACSL1) is the predominant and critical ACSL isoform in muscle140. As the inner mitochondrial membrane is impermeable to long-chain NEFAs, long-chain fatty acyl-CoA molecules are delivered into the mitochondrial matrix through the ‘carnitine shuttle’, where they undergo subsequent -oxidation and OXPHOS.
Prolonged high-intensity exercise resulting in elevated rates of lactate production might directly impede IMCL lipolysis over time by downregulating cAMP–PKA signalling141. However, the glycolytic flux of higher-intensity exercise most probably outcompetes NEFAs for oxidation by depleting the muscle free-carnitine pool72,142. This limits the capacity of the carnitine shuttle and thus impairs the mitochondrial import of long-chain fatty acids (Fig. 2b). Longer exercise durations, lower intramuscular glycogen stores, higher dietary fat intakes and greater type I fibre abundance and aerobic fitness () levels can reduce carbohydrate reliance and increase fat utilization during physical activity120. Nevertheless, although these factors can delay the transition from predominant fat usage to predominant carbohydrate usage (referred to as the substrate ‘crossover point’), the biphasic pattern of NEFA oxidation with exercise intensity71,72 is ultimately maintained77,143.
Biological rhythms and skeletal muscle
Normal circadian fluctuations in behaviour and physiology — such as sleep–wake cycles, nutritional state, body temperature, cardiovascular function, and tissue production and sensitivity to hormones (Supplementary Table 1) — coincide to influence acute exercise capacity144 and response in a time-specific, tissue-specific manner20. Endogenously generated circadian rhythms are predominantly maintained by photic (light-dependent) cues relayed through the central pacemaker (hypothalamic suprachiasmatic nucleus)145 in concert with cell-autonomous clocks in peripheral tissues, including skeletal muscle (reviewed in ref. 146). Muscle clocks regulate transcriptional programmes that prepare the tissue for transitions between fasting and feeding147–149 and orchestrate 24-h rhythms in muscle glucose147,149, lipid148,149 and amino acid148 metabolism. Consequently, muscle clock disruption in mice impairs muscle insulin sensitivity147,150 and metabolic homeostasis147,148,150.
Hormonal, metabolic and temperature-dependent changes associated with exercise shift the central pacemaker151 and impact circadian clocks in cell types throughout the body152. In human vastus lateralis muscle, acute endurance153 and resistance153,154 exercise upregulates the core clock genes CRY1 and PER2, in part through -mediated activation of cAMP response element-binding protein (CREB)153. Reciprocally, physical activity patterns directly modulate the expression, phase and/or amplitude of ~15–20% of all rhythmic genes in mouse soleus and tibialis anterior muscles independently of the muscle clock155. This occurs through transcription factors such as NFATc1 that are stimulated by motoneuronal firing patterns and downstream dynamics155. Together, these studies provide a mechanistic and biochemical rationale for observed time-dependent variations in human athletic performance (reviewed in ref. 156) and the metabolic disturbances157,158 and increased risk of cardiovascular disease159 resulting from circadian misalignment.
A high-intensity exercise intervention in humans was shown to offset the detrimental impact of short-term sleep restriction on whole-body glucose tolerance and mitochondrial respiration in permeabilized muscle fibres160. Treadmill running exercise in mice also stimulated hypoxia-inducible factor- () and a broad range of muscle transcriptomic161 and metabolic20,161 responses in a time-dependent manner. Such findings have prompted researchers to begin investigating exercise as a therapy to promote circadian alignment and ameliorate metabolic disease. Preliminary human evidence indicates that training in the afternoon or evening might enhance the beneficial effects of exercise on aspects of insulin sensitivity162,163 and blood glucose control164. Furthermore, moderate-to-vigorous physical activity imparts greater risk reductions for cardiovascular disease and all-cause mortality when ≥50% of the total activity volume is undertaken after 11:00 h (ref. 165).
Although rapidly emerging, this branch of ‘chrono-therapeutics’ is still in its infancy, and precise protocols to elicit specific metabolic outcomes are only beginning to come to fruition20. Additionally, there is large population-level variation in human chronotypes166, and individual chronotype may interact with diurnal exercise timing to modify acute responses and longer-term adaptations. Each age group contains a normal distribution of early-to-late chronotypes, yet the average chronotype differs according to biological sex (Supplementary Box 2) and changes during ageing166 (Box 2). Individuals with later chronotypes could benefit from exercise-induced phase advances irrespective of exercise timing, whereas earlier chronotypes might suffer circadian misalignment with evening exercise151. Moving forward, chronotype will be an important covariate to consider in exercise biology, especially for the goal of making personalized therapeutic recommendations.
Skeletal muscle responses to acute exercise
The perturbation of systemic19,124 and local55 homeostasis posed by acute exercise triggers an integrated series of metabolic, hormonal, growth factor-related, inflammatory and mechanosensitive events. In this section we discuss how these stimuli converge to temporally regulate the acute post-exercise adaptive landscape in muscle — from signal transduction90 to gene expression167–169 (Fig. 3).
Post-exercise signalling
Sprint and resistance exercise impart stronger whole-body stress responses and more robustly impact the muscle phosphoproteome than continuous endurance exercise does, although there is similarity in the immediate post-exercise signature90. Over 400 phosphorylation sites on >200 proteins were commonly altered among these exercise modalities90, leading to a shared enrichment of canonical exercise regulatory kinases, including CaMK, AMPK, mammalian target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK), PKA and PKC90,170. Much of this post-exercise pathway convergence is conserved in contracted (exercised) rat and mouse muscle, despite little overlap at specific phosphorylation sites171.
Modality-driven divergence in the phosphoproteome becomes more apparent during the 3-h recovery period after exercise90 and may facilitate distinct muscle adaptations with endurance, sprint interval or resistance training. Phosphorylation of rapamycin-sensitive substrates of mTOR complex 1 (mTORC1) and p38 MAPK are potentiated after resistance exercise90. mTORC1 is a central regulator of muscle hypertrophy that critically permits mechanical overload-induced myofibrillar accretion172. In murine muscle, mTORC1 activity is in part mediated by mechanostimulated diacyl glycerol kinase- () production of phosphatidic acid173 and raptor-dependent translocation of the catalytic component of mTORC1 to the late endosomal and lysosomal system172. By contrast, mTOR in human muscle already associates with the lysosome, and resistance exercise does not further augment this interaction174. Thus, the contraction-induced migration of the mTOR–lysosomal complex to focal adhesions175 and microvasculature174 at the sarcolemma may be a more important step in humans174–176. Here, mTOR associates with RAS homologue enriched in brain (RHEB) and eukaryotic translation initiation factor 3 (EIF3)174, and activates downstream target ribosomal protein S6 (RPS6)175. The cooperation between mTOR–EIF3 (ref. 174) and Ser235 and Ser236 phosphorylation of RPS6 (ref. 175) at the fibre periphery was greater when a post-exercise protein and carbohydrate beverage was consumed, and this interplay could contribute towards the nutrient-sensitizing effects of resistance exercise on mTORC1 activation6,177.
Complementary contraction-responsive mechanisms act in tandem with mTORC1 to promote gains in muscle mass6,178–180. For example, sarcomere shortening recruits the -isoform of MAP3K20 (also known as ) to the Z-disc, resulting in downstream stimulatory phosphorylation of p38 MAPK and JUN N-terminal kinase 1 (JNK1) and JNK2 (ref. 179). Inhibition mediated by JNK1 and JNK2 (ref. 181) and/or Notch182-mediated inhibition of myostatin–suppressor of mothers against decapentaplegic (SMAD) signalling is a purported molecular ‘switch’ in muscle, promoting resistance-type over endurance-type adaptations by preventing SMAD complex nuclear translocation181. This suppression of the myostatin–transforming growth factor- () pathway might be distinct from the regulation of myostatin gene expression182, as both resistance and endurance training downregulate myostatin mRNA levels in human muscle183 despite contrasting hypertrophic phenotypes over time13.
Endurance training has been suggested to impair resistance training adaptations through AMPK-mediated blunting of mTORC1. Although this biochemical reaction is known to occur, exercise induction of both AMPK and mTORC1 signalling is consistent with meta-analyses indicating little, if any, interference effect of concurrent exercise on muscle hypertrophy184,185. Bidirectional AMPK–mTORC1 phosphorylation177 could coordinate simultaneous90 or temporally distinct177 post-exercise activity and downstream substrate specificity of these kinases.
Physiological proteome and mitochondrial remodelling with exercise requires the turnover of select proteins, and autophagic and proteasomal trafficking are integral to these processes. Indeed, genetic disruption of proteostasis results in the accumulation of damaged proteins and impairs force production in fast-twitch extensor digitorum longus and slow-twitch soleus mouse muscle186. Treadmill running exercise increases the activity of an AMPK complex composed of , , and isoforms located on the outer mitochondrial membrane (‘mitoAMPK’) that could spatially regulate quality control through mitophagy across the mitochondrial reticulum187. This pool of mitoAMPK is conserved in human muscle187 and may govern mitophagy through phosphorylation of downstream substrates such as UNC-51-like autophagy activating kinase 1 (ULK1)188 and mitochondrial fission factor (MFF)189. In humans, acute high-intensity190,191 and sprint interval90 exercise rapidly and transiently activates ubiquitin-proteasomal degradation in a cAMP–PKA-dependent manner190,191 that requires the proteasomal subunit PSD11 (ref. 190). cAMP can then upregulate enzymatic activity of E3 ubiquitin ligases for continued removal of damaged proteins in the post-exercise recovery period191 (Fig. 3). Coordinated proteolytic signalling is further detected 3 h after resistance exercise, as implied by co-regulated phosphorylation of proteins in the N-end rule, calpain regulation and ubiquitin receptor RAD23 pathways90.
Many exercise-responsive phospho-sites have yet to be functionally interrogated in muscle90,170,178. Additionally, there are numerous other protein modifications such as arginine methylation, ubiquitin-like modification, ribosylation and acylation that remain relatively understudied in the context of physical activity despite presumably coinciding to influence muscle phenotype. Deeper coverage of the posttranslational proteome combined with the use of subcellularly targeted biosensors187 should enhance the spatial understanding of how integrated post-exercise signalling governs unique exercise adaptations in muscle, and how these networks change with exercise training over time.
Epigenetic regulation of exercise adaptation
Histone modifications and DNA methylation.
Upstream signalling, together with an altered intramuscular metabolic milieu, can change the chromatin landscape in muscle and thus influence transcription factor-driven gene expression in response to exercise (reviewed in ref. 192). Epigenetic modifications on amino acids in histone tails or globular core domains render a permissive or repressive chromatin state depending on the type of modification and the residue and/or histone protein being targeted192. An acute bout of endurance exercise can increase H3K36 acetylation193, a mark of euchromatin, and promote CaMKII-dependent phosphorylation194,195 and nuclear exclusion of the transcriptionally repressive class II histone deacetylases (HDAC) HDAC4 and HDAC5 (ref. 193). High-intensity resistance exercise also transiently stimulates myonuclear H3S10 phosphorylation through p38 MAPK and mitogen and stress-activated protein kinase 1 (MSK1) in human muscle196. This same axis was necessary for the upregulation of myocardin-related transcription factor B (MRTFB)–serum response factor (SRF)-target genes and protein synthesis after in vivo contractions in mice196.
Post-exercise histone modifications coalesce with changes in DNA methylation, typically at the 5′ end of cytosine residues at CpG sites throughout the genome. DNA methylation in cis-acting regulatory regions is commonly associated with gene silencing, but some transcription factors, particularly of the homeodomain POU and NFAT families, favour methylated CpG sequences197. Acute exercise198–200 and exercise training199,201 induce both hypomethylation and hypermethylation of DNA in muscle, mostly within gene bodies, intergenic regions and enhancer regions201. The greatest number of CpG-site methylation changes and the highest percentage of mRNA transcripts with inversed DNA methylation patterns occurred 3 h after a single session of resistance exercise, irrespective of workload (80% or 30% of 1 RM)200. These changes began to revert towards baseline methylation levels by 6 h200, suggesting that the muscle methylome is dynamic and tightly regulated during recovery from exercise. Yet, select DNA hypomethylation and hypermethylation events are sustained after acute resistance exercise and resistance training, providing a plausible mechanism for a ‘muscle memory’ of previous exercise that facilitates future adaptation199. Modality-dependent DNA methylation signatures might also direct specific post-exercise transcriptional programmes in muscle, as acute bouts of resistance199 and sprinting-type202 exercise share enrichment for only 36 of the top 100 differentially methylated pathways.
Conceivably, exercise-induced metabolic fluxes, which stimulate glycolysis and OXPHOS, could alter acetyl and methyl group availability, cellular [NAD+] to [NADH] redox state and the activity of ‘writers’ and ‘erasers’ of epigenetic marks in muscle192. However, the current understanding of exercise-regulated DNA methylation and chromatin remodelling is incomplete. For example, lactate modifies (‘lactylates’) H3K18 in promoters and enhancers of genes related to tissue development and metabolism in human muscle203, but whether lactylation of histone lysine residues contributes to exercise adaptation remains to be ascertained.
Non-coding RNAs.
In addition to protein-coding genes, RNA polymerase II transcriptional activity produces microRNAs (miRNAs; ≤22 nucleotides) and long non-coding RNAs (lncRNAs; ≥200 nucleotides) that alter mRNA levels and protein abundance without influencing the genetic code. Many miRNAs204 and lncRNAs205 are exercise-responsive in muscle, and both classes seem involved in aspects of myogenesis. During resistance exercise, the downregulation of the muscle-enriched miRNA myomiR-1, through its contraction-mediated release in extracellular vesicles, may facilitate hypertrophy206. Once in circulation, myomiR-1 could act upon adipocytes to potentiate adrenergic signalling and lipolysis206. Still, muscle was not a major source of bloodborne extracellular vesicles upon resistance exercise in humans207 or treadmill running exercise in mice208. Rather, muscle-derived extracellular vesicles — loaded with myomiRs — preferentially accumulated in the interstitial space, and treatment with these vesicles promoted differentiation of myoblasts in vitro208. Therefore, extracellular vesicles originating from muscle might play a more prominent role in local cellular communication (Supplementary Fig. 3). Satellite cells may signal to muscle fibres209 and fibroadipogenic progenitor cells210 using extracellular vesicles containing myomiR-206. In mice, this was shown to regulate the levels of extracellular matrix (ECM)-related genes (for example, Mmp9) in muscle fibres209 and Rrbp1 in fibroadipogenic progenitor cells210 to coordinate physiological ECM remodelling during the early stages of hypertrophy. However, the ability of extracellular vesicles to deliver biologically active miRNA cargo into recipient cells remains contentious211 and their role in exercise adaptation warrants further interrogation.
Though the impact of lncRNAs in human muscle is less studied than that of miRNAs, lncRNAs respond to endurance, resistance, concurrent and high-intensity interval training with distinct differential expression profiles between modalities205. The lncRNA CYTOR is induced by endurance and resistance exercise in human muscle and can regulate fast-twitch fibre formation in rodents by sequestering TEA domain family member 1 (TEAD1) transcriptional activity212. Cytor mRNA is decreased upon ageing and overexpression of this lncRNA increased type IIA and type IIB fibre abundance, which was sufficient to improve grip strength and uphill running performance in aged mice212. RNAs putatively annotated as lncRNAs may also contain short open reading frames that encode for micropeptides (≤150 amino acids). The muscle-specific micropeptides dwarf open reading frame213 and myoregulin214 competitively interact with sarcoplasmic/endoplasmic reticulum -ATPase (SERCA) to positively and negatively affect SR kinetics and muscle contractility, respectively. Other classes of non-coding RNAs, including circular RNAs and tRNA fragments, await future study in the context of human muscle exercise adaptation.
mRNA transport and myonuclear propagation
The dense myofibrillar lattice in adult muscle fibres may impede diffusion of mRNAs expressed from myonuclei. Indeed, recent evidence suggests that mRNA distribution away from the perinuclear region is almost entirely dependent on microtubule networks in muscle, whereas diffusion has a minor role215,216. Transport might be reliant on motor proteins, reminiscent of cardiomyocytes that use kinesin-1-mediated transport of mRNA and ribosomes to direct protein translation to specific sites within the cell217. Microtubule-based trafficking of mRNA facilitates transcript distribution through the crowded sarcoplasmic space, but dispersion of mRNAs from progenitor myonuclei is still potentially confined to ≤50 μm (refs. 215,216). By contrast, the movement of proteins translated from transcripts derived from a specific myonucleus could be ≥5 times more far-reaching218.
The use of fluorescent reporters has shown that nuclear proteins can be detected in surrounding myonuclei and in those distant from the nucleus of origin218–220, a phenomenon termed ‘nuclear propagation’218. Smaller proteins (≤40 kDa) that freely diffuse across the nuclear pore complex can propagate to many myonuclei throughout a myotube in vitro218. Conversely, larger proteins (>40–60 kDa) are limited in their propagation potential, possibly due to increasing reliance on nuclear transport receptors to traverse the nuclear pore complex218. Protein properties (such as size) and myonuclear characteristics (such as shape or location within the muscle fibre) probably interact to control nuclear propagation. For example, myonuclei in ex vivo fibres differ in capacity for classical versus non-classical nuclear import, resulting in a gradient of import rates between myonuclei of the same fibre219.
The potential for myonuclear propagation casts further doubt on strict myonuclear domains in muscle and represents an additional layer of spatiotemporal control, combining fibre-wide communication with myonuclei-autonomous processes in response to adaptive cues. Furthermore, resident myonuclei may be capable of endoreplication in mouse muscle (resulting in polyploidy), which could support efficient DNA synthesis under heightened transcriptional demand221. Myonuclear propagation was detected after wheel-running exercise training220 and synergistic ablation-induced hypertrophy increased the total number (from ~0.7–1% to ~3%) of DNA-replicating myonuclei221 in murine muscle. The extent to which these processes occur under physiological conditions in humans remains unclear.
The post-exercise transcriptome
Considerable effort has been dedicated towards characterizing the transcriptional response of human muscle to exercise, and several key studies are highlighted in Supplementary Table 2. High-powered meta-analyses have curated much of this scientific endeavour to generate deeper insight into exercise adaptation167,183. Acute exercise initiates temporal clusters of gene expression167–169, including early induction of stress response genes, increased expression of muscle adaptation genes in early and middle stages following exercise167,169, and later167 or sustained168 increases in immune-related signatures. These transcriptional programmes are driven by combinations of different transcription factors167,169 and alternative transcription start-site usage169. Akin to the phosphoproteome90, a single bout of resistance exercise alters more transcripts than does moderate-intensity endurance exercise183. However, there is considerable overlap in pathway enrichments, and both training modalities upregulate stress response genes, kinase activity and metabolism-related processes, among others183.
NR4A3 (also known as NOR1)222 and PGC1A223 are genes commonly induced after endurance and resistance exercise183 and mainly promote oxidative muscle adaptations. NR4A3 and PGC1A have similar temporal expression patterns after exercise, peaking ~2–3 h into the recovery period167,183, suggesting common upstream regulation through CREB224,225. The nuclear receptor subfamily 4 group A (NR4A) family (including NR4A1 (also known as NUR77) and NR4A2 (also known as NURR1)) share >90% homology in their DNA-binding domains and can interact as monomers with a single NGFI-B response element226, or as homodimers and heterodimers (alongside other NR4A members) with the Nur response element227. NR4A1 is also exercise-responsive in muscle167,202 and may serve partial redundancy with NR4A3, as implied by the slow-oxidative phenotypes of mice expressing either Nr4a1 (ref. 228) or Nr4a3 (ref. 222) from skeletal muscle-specific promoters.
The family of coactivators consists of different isoforms, which are derived from alternative promoter use or splicing events (reviewed in ref. 223). Endurance exercise preferentially targets229 and hypomethylates230 the proximal promoter in muscle, increasing isoform 1 () expression. drives mitochondrial biogenesis and oxidative fibre properties48 at least in part through C-terminal domain RNA binding, which facilitates recruitment in transcriptionally active chromatin condensates231. A bout of endurance exercise also leads to promoter region hypomethylation of other key mitochondrial and metabolic genes, including TFAM and PPARD, with corresponding increases in mRNA expression230. The -mediated upregulation of lipid metabolism and suppression of glycolytic processes in muscle232 is largely dependent on protein–protein interaction with Krüppel-like factor 15 (KLF15)233.
Most isoforms have overlapping roles in muscle223. The truncated variant isoform 4 () differs in that it can be induced by intramitochondrial []234 and is transcribed from the alternative promoter after resistance exercise229,235 (Fig. 3). facilitates muscle hypertrophy229,234,235 and a –AMPK-dependent upregulation of glycolytic metabolism235, which could enhance the anaerobic capacity of muscle after resistance training. Incidentally, the accretion of muscle mass often coincides with a glycolytic switch that may support anabolism by increasing de novo synthesis of nucleotides and non-essential amino acids from intermediates in glucose metabolism (reviewed in ref. 236).
An acute bout of resistance exercise also stimulates MYC mRNA expression and the hypomethylation of enhancer and MYC-accessible intergenic regions of ribosomal DNA198. These events coalesce to augment pre-45S rRNA transcription and ribosomal biogenesis180,198. Whereas mTORC1 signalling promotes translational efficiency172,180 and directs ribosomal function through Ser235 and Ser236 phosphorylation of RPS6 (ref. 237), total ribosomal content180 and the altered composition of ribosomal proteins (for example, an increased ratio of RPL3 to RPL3L)238 determines translational capacity in muscle. Collectively, upregulation of the sarcoplasmic and mitochondrial protein synthetic machinery (such as mito-ribosomes) enables the beneficial proteome remodelling that occurs with high-intensity interval, resistance or combined exercise training12.
Skeletal muscle adaptations to long-term exercise
In this section, we contextualize the impact of consistent exercise (exercise training) on muscle phenotype, considering general and select adaptations to specific training interventions and the influence of muscle fibre type14. We then summarize how muscle-related adaptations can contribute to improvements in systemic measures of exercise performance and discuss whether altered molecular perturbations in muscle with exercise training33,239–241 reflect an attenuated or refined adaptive state.
Transcriptome, proteome and contractile property adaptations
Repeated exposure to specific exercise modalities results in shared and divergent alterations in muscle over time12,183 (Fig. 3). At the transcriptomic level, pathways related to ECM remodelling, angiogenesis and the TCA cycle are enriched in muscle after exercise training167. Whereas resistance exercise robustly effects ECM reorganization183,242 and growth-related pathways242, endurance training favourably impacts oxidative metabolic processes and the gene expression of mitochondrial complex subunits183. Crosstalk between the various cell populations in muscle appears particularly important for exercise-associated adaptations within the interstitial space (Supplementary Fig. 3).
Training-induced improvements in muscle respiration often coincide with expansion and modification of the mitochondrial proteome12,15,143 in type I and type II fibres14. Resistance training can augment the respiratory and ATP-producing capacity of mitochondria243, but the effects on mitochondrial phenotype are typically less pronounced than those observed after high-intensity interval12,15,143 and endurance14 training. The total volume of endurance exercise performed during a training intervention might be the strongest stimulus for mitochondrial biogenesis in muscle (reviewed in ref. 244). This could be related to the additive effects of proximal exercise bouts on the nuclear enrichment of transcriptional regulators of mitochondrial abundance (for example, and transcription factor EB (TFEB))245 and the upregulation of mito-ribosomes12,14,15 and mitochondrial protein synthesis12. During mitochondrial biogenesis, stoichiometry of the ETC is maintained through proportional translation of ETC complex subunits from the mitochondrial and nuclear genomes246. Increased expression of mitochondrial transit peptides and translocase proteins of the outer and inner mitochondrial membrane following endurance training14 facilitates the transport of nuclear-encoded precursor components into the mitochondrial matrix. However, in the early stages of mitochondrial remodelling, synthesis of fatty acid oxidation and TCA cycle enzymes may be prioritized over ETC machinery biogenesis, to enhance reducing equivalent NADH and production and electron delivery to OXPHOS15.
High-intensity interval training at ≥90% of maximum power output () most consistently increases normalized (mass-specific) mitochondrial respiration244, with intensities ≥100% (as in sprint interval training) doing so more efficiently per unit of time spent exercising244. The impact of intensity on mitochondrial respiration appears uncoupled from total training volume, suggesting that these variables drive complementary but somewhat distinct mitochondrial adaptations244. High-intensity interval training also induces lysine acetylation of TCA cycle and ETC proteins143, although whether such posttranslational modifications contribute towards intensity-dependent changes in mitochondrial function requires additional study.
After endurance training, some discrete differences can be seen in the adaptive response of type I and type II fibres14. Still, most of the commonly detected exercise-regulated proteins related to mitochondrial and glucose metabolism behaved similarly between fibre types14. For example, training upregulated ACSL1, malate/aspartate shuttle proteins, the mitochondrial CKM isoform CKMT2, PDH, CRAT and LDHB in both type I and type II fibres14. LDHB mRNA is increased after endurance247 and resistance235 training, potentially through coactivation of distal myocyte enhancer factor 2 (MEF2) and proximal oestrogen-related receptor (ERR) binding sites in the LDHB promoter247. LDHB preferentially catalyses the conversion of lactate to pyruvate and, together with enhanced mitochondrial density, could improve lactate clearance in trained muscle through higher rates of conversion to pyruvate and subsequent oxidation247 (Fig. 2c). Further refinement of substrate handling with programmed exercise is implied by greater expression of key proteins of metabolic pathways involving glycogen (for example, glycogen synthase)11, NAD+ (nicotinamide phosphoribosyltransferase) and branched-chain amino acid (branched-chain -ketoacid dehydrogenase kinase) metabolism, as well as ubiquinone biosynthesis (5-demethoxyubiquinone hydroxylase)143, after high-intensity interval training.
Muscle contractile properties are likewise affected by regular exercise in a somewhat training modality-dependent manner. Protein isoforms regulating SR release143 and myofibrillar sensitivity14,143 were altered after moderate-intensity endurance14 and high-intensity interval143 training, coincident with a reduction in fibre size-adjusted quadriceps peak-twitch torque and prolonged half-relaxation time11 — indicative of a slower muscle phenotype. Conversely, stretch-shortening cycle (ballistics or plyometrics) training increased contractile velocity, force production and thus peak power of single type I, type IIA and type IIA/X fibres42. Exercise modalities commonly decrease MYH1 mRNA183 and protein (MyHC-IIX)11,13 content in muscle. However, the upregulation of MYH7 gene expression183 and type I fibre abundance after endurance training44 can be in contrast to the effects of resistance training, which often downregulates MYH7 (ref. 183) with no corresponding change in the prevalence of type I fibres45. This is consistent with endurance44 and resistance45 exercise differentially shifting hybrid fibres towards pure type I and pure type IIA fibres, respectively (reviewed in ref. 38). Indeed, endurance training resulted in the modification of more proteins in type I than in type II fibres14, and long-term exposure to a particular exercise stimulus41 may largely explain the majority of type I or type II fibres detected in biopsies from endurance or strength athletes13.
Training-induced adaptations impact exercise performance
Together, exercise training adaptations in muscle contribute towards. improvements in gross performance measures such as (refs. 12,14,15), peak power output11,143, ballistic power42 and strength12,13. Endurance and resistance training increase muscle capillarization248–250 and endurance-type training enhances NEFA oxidation at a given exercise intensity77,120,143,251. These glycogen-sparing and metabolite-buffering (for example, lactate clearing)247 effects render muscle more robust to disturbances in metabolic homeostasis, delaying mechanisms of peripheral and central fatigue94 and raising exercise tolerance and capacity (that is, higher velocities and percentages of produced at ventilatory thresholds, and greater maximal running speeds or distances covered)251. Metabolomics analysis also implies that 4 weeks (13 bouts) of resistance exercise may suffice to modify the acute muscle metabolome252. Yet, the specific metabolic changes that occur with resistance training are not extensively characterized and warrant further interrogation.
High-intensity interval11,12 and endurance13 training both induce a degree of muscle hypertrophy, but gains in muscle mass and strength are less pronounced than seen with long-term resistance exercise13. Conversely, any beneficial effect of resistance training on appears limited to individuals with lower initial aerobic fitness levels (for example, a or ≤40 mL kg−1 min−1 in adults >60 and ~20–35 years of age, respectively) (reviewed in ref. 253) and thus high-intensity interval12 and endurance training13,248 typically increase to greater extents.
A period of aerobic pre-conditioning can potentiate the hypertrophic effects of resistance exercise248, in part by enhancing fibre capillary density248,250 (Supplementary Fig. 3). Alternatively, high-intensity resistance training (≥90% of 1 RM or ≤4 RM) can improve running economy (that is, reduce the metabolic or cost of running)254. These complementary adaptations further emphasize the utility of incorporating numerous exercise (sub)modalities into a sustainable routine for maximum performance12 and protective health benefits18.
Adaptive responses in muscle are modified with exercise training
The recurring stimulus of exercise training dampens select signalling239,240 and transcriptomic33,241 responses to an acute bout of physical activity. This is evident after just nine sessions of high-intensity interval exercise through a modest reduction in the number (~17%) and amplitude (~30%) of altered mRNA transcripts in human muscle compared to the first exercise bout, including glycolysis pathway and target genes241. The magnitude of change in the muscle transcriptome with exercise training becomes even clearer over 30 daily sessions of electrically induced mechanical overload in rat hindlimb muscle33. In particular, the directionality of altered genes contrasted substantially between exercise naive and trained muscle. At the 1-h sampling time point after exercise, ~70% of the (~2400) differentially expressed transcripts were upregulated on day 2 compared with ~83% (of ~3300 genes) downregulated on day 30 (ref. 33). This indicates that certain signalling mechanisms are sensitive to the modified intracellular environment after a period of regular exercise. In combination with genetic predisposition (Box 1), blunted post-exercise molecular responses probably converge to limit the adaptive potential of muscle, slowing progress with advanced training experience. Nevertheless, some alterations might reflect a refined (as opposed to impaired) response.
Pathways related to ribosomal biogenesis and protein synthesis are positively enriched in the early adaptive stages in exercised rat muscle33 but become downregulated in favour of metabolism-related processes once hypertrophy plateaus33. Rates of myofibrillar protein synthesis also correlate better with measures of muscle mass accrual after 8 weeks176 to 10 weeks255 of resistance training in humans, corresponding to the attenuation of exercise-induced muscle damage255. Furthermore, although 20 days (40 sessions) of high-frequency, high-intensity interval training reduced the post-exercise upregulation of nuclear and p53 phosphorylation at Ser15, resting levels of these same markers were greater in human muscle after the exercise intervention239. Phosphorylated p53 can translocate to the mitochondrial genome where it interacts with transcription factor A, mitochondrial (TFAM) to regulate mitochondrial transcription256 (Fig. 3). Muscle-specific Trp53-knockout mice have reduced basal mitochondrial contents and enzymatic activities but retain the capacity for exercise-stimulated mitochondrial biogenesis257. This suggests that p53 may be more important for the maintenance of muscle mitochondrial integrity than exercise adaptation.
Given these diverse modifications, longer-duration trials are needed to fully elucidate the modality-dependent temporal landscape of exercise adaptation in human muscle and to highlight specific changes in the acute exercise response with consistent training.
Future directions
The complex and intricate macrostructure to ultrastructure of skeletal muscle allows for a high degree of metabolic flexibility during exercise. Further study of the regulation and interaction of key cellular components, such as myofibrillar and mitochondrial networks53, should enable more comprehensive understanding of how muscles mount such a malleable response. The use of fluorescent metabolite258 and myofilament259 biosensors could aid this effort by elucidating real-time dynamics of muscle metabolism and contraction.
Current knowledge of exercise adaptation is heavily biased towards canonical regulators such as AMPK, PGC1s and mTORC1. Although these factors are integral to muscle remodelling with exercise, aspects of adaptation are maintained in the absence of and (ref. 260) and of and (ref. 261), and rapamycin-insensitive pathways also contribute towards muscle hypertrophy178,180. Most of the exercise-induced phosphoproteome remains relatively unexplored90,170,178, and its functional interrogation will help to identify redundancies in adaptative signalling and new players in the distinct and complementary effects of resistance, endurance and high-intensity exercise.
As exercise biology moves increasingly towards systems-level profiling, the multiplexing of new technologies could provide invaluable insight into the spatiotemporal control of gene expression262, translation263 and metabolism37 in discrete muscle cell populations. If performed in tandem with commensurate functional assays, this would enable causal association of specific cellular events with their phenotypic impact.
Exercise is an effective adjunct therapy for certain neurodegenerative264 and mental health265 disorders and offers protection against cardiometabolic disease266, sarcopenia (Box 2) and all-cause mortality18. Multicentre interventions including spectrums of chronological age, metabolic health, chronotype, ethnicity, biological sex and social gender will move the field closer to uncovering the dynamic changes that coordinate the benefits of exercise training and further define the role of muscle in the integrative exercise response. Knowledge derived from such approaches should not only inform personalized exercise prescription, but also reveal new molecular avenues of drug discovery for the improvement of human health.
Supplementary Material
Acknowledgements
The authors apologize to all colleagues whose work could not be included owing to space constraints. The authors thank M. Karlén for preparation of the artwork in the supplementary figures. K.A.M. was supported by the National Institutes of Health (NIH R00 AG063994). K.A.D. was supported by Deutsches Zentrum für Diabetesforschung (2020/21). J.R.Z. was supported by the Swedish Research Council (Vetenskapsrådet) (2015-00165), the Swedish Research Council for Sport Science (P2022-0013, P2023-0093) and the Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen (NNF18CC0034900).
Glossary
- Adrenoceptor
Transmembrane G-protein-coupled adrenergic receptors (GPCRs) that mediate the actions of the endogenous catecholamines adrenaline and noradrenaline. There are nine subtypes of adrenoceptors: , , , , , , , and . The and adrenoceptors regulate presynaptic neurotransmitter release from central adrenergic and peripheral sympathetic nerves
- Ergogenic
A performance-enhancing effect
- Hypertrophy
Typically refers to an increase in the cross-sectional area (or radial growth) of muscle fibres, resulting in gains of skeletal muscle mass in response to mechanical loading activities, such as resistance exercise
- Ketone body
A lipid-derived, water-soluble, organic compound produced in the liver that can be used as an alternative energy source by extra-hepatic tissues — predominantly the brain, but also heart and skeletal muscle
- Maximal oxygen consumption
(). The maximum volume of oxygen (ml kg−1 min−1) that can be inspired and utilized during exhaustive exercise, such that the value () plateaus despite increasing workloads. is a measure of aerobic or cardiorespiratory fitness and is commonly used to standardize exercise intensity for clinical trials (for example, x% of )
- Mitophagy
A specific form of lysosome-dependent catabolism (autophagy), through which damaged mitochondria are selectively removed. Mitophagy of the mitochondrial reticulum has an essential role in maintaining cellular energy homeostasis
- Muscle spindles
Structures embedded in most mammalian skeletal muscles that continuously relay proprioceptive information regarding muscle length and movement to the central nervous system. Muscle spindles consist of intrafusal muscle fibres enclosed within a capsule layer and are distinct from the extrafusal muscle fibres discussed in this Review
- Non-esterified fatty acids
(NEFAs). A metabolic substrate utilized by muscle at rest and in an intensity-dependent manner during exercise
- Peak-twitch torque
The force produced by muscle (through a moment arm) evoked by a single electrical stimulation from, for example, applied electrodes
- Phosphagen system
A rapid energy-producing pathway comprising the ATP regenerating adenylate kinase () and creatine kinase () reactions. Of these reactions, creatine kinase has a greater capacity for ATP resynthesis in muscle due to the availability of creatine phosphate stores
- Proprioceptive
Able to sense intrinsic information regarding bodily position and locomotion. The primary proprioceptive sensory organ of the body is the muscle spindle
- Proton-motive force
The proton electrochemical gradient in mitochondria consisting of an electrical charge gradient (also known as the ‘membrane potential’) and a pH gradient. The proton-motive force is generated by the proton-pumping action of respiratory complexes across the inner mitochondrial membrane and couples substrate oxidation to ATP generation
- Transverse tubules
(T-tubules). Invaginations in the sarcolemmal membrane that insert between myofibrils. T-tubules tightly associate with two terminal cisternae (calcium-releasing regions) of the sarcoplasmic reticulum, forming the ‘triads’, which are essential for excitation–contraction coupling
- Voluntary force production
The conscious or ‘voluntary’ production of muscle force (in other words, not triggered by exogenous electrical stimulation)
Footnotes
Additional information
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41580-023-00606-x.
Peer review information Nature Reviews Molecular Cell Biology thanks Kamala Sreekumaran Nair, who co-reviewed with Mark Pataky, Adam P Sharples and Brian Glancy for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
References
- 1.Bilet L. et al. One-leg inactivity induces a reduction in mitochondrial oxidative capacity, intramyocellular lipid accumulation and reduced insulin signalling upon lipid infusion: a human study with unilateral limb suspension. Diabetologia 63, 1211–1222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergouignan A. et al. Effect of contrasted levels of habitual physical activity on metabolic flexibility. J. Appl. Physiol 114, 371–379 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Kivimaki M. et al. Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. Brit. Med. J 365, l1495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang D. et al. Leisure-time physical activity and incident metabolic syndrome: a systematic review and dose-response meta-analysis of cohort studies. Metabolism 75, 36–44 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Millard LAC, Tilling K, Gaunt TR, Carslake D. & Lawlor DA Association of physical activity intensity and bout length with mortality: an observational study of 79,503 UK Biobank participants. PLoS Med. 18, e1003757 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Hulst G, Masschelein E. & De Bock K. Resistance exercise enhances long-term mTORC1 sensitivity to leucine. Mol. Metab 66, 101615 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodyear LJ et al. Skeletal muscle plasma membrane glucose transport and glucose transporters after exercise. J. Appl. Physiol 68, 193–198 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Burd NA et al. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J. Nutr 141, 568–573 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Sjoberg KA et al. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 66, 1501–1510 (2017). [DOI] [PubMed] [Google Scholar]
- 10.McConell GK et al. Insulin-induced membrane permeability to glucose in human muscles at rest and following exercise. J. Physiol 598, 303–315 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Hostrup M, Onslev J, Jacobson GA, Wilson R. & Bangsbo J. Chronic -adrenoceptor agonist treatment alters muscle proteome and functional adaptations induced by high intensity training in young men. J. Physiol 596, 231–252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robinson MM et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 25, 581–592 (2017). This study provides a thorough analysis of exercise adaptation at several -omics levels in human skeletal muscle.
- 13.Chapman MA et al. Skeletal muscle transcriptomic comparison between long-term trained and untrained men and women. Cell Rep. 31, 107808 (2020). [DOI] [PubMed] [Google Scholar]
- 14. Deshmukh AS et al. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat. Commun 12, 304 (2021). This study highlights notable shared and distinct adaptations in the proteomes of type I and type II fibres after a period of endurance training.
- 15. Granata C. et al. High-intensity training induces non-stoichiometric changes in the mitochondrial proteome of human skeletal muscle without reorganisation of respiratory chain content. Nat. Commun 12, 7056 (2021). This study interrogates the muscle mitochondrial proteome at multiple time points during a periodized high-intensity training intervention.
- 16.Laukkanen JA et al. Long-term change in cardiorespiratory fitness and all-cause mortality: a population-based follow-up study. Mayo Clin. Proc 91, 1183–1188 (2016). [DOI] [PubMed] [Google Scholar]
- 17.García-Hermoso A. et al. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: a systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil 99, 2100–2113.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Coleman CJ, McDonough DJ, Pope ZC & Pope CA Dose-response association of aerobic and muscle-strengthening physical activity with mortality: a national cohort study of 416 420 US adults. Br. J. Sports Med 10.1136/bjsports-2022-105519 (2022). [DOI] [PMC free article] [PubMed]
- 19. Contrepois K. et al. Molecular choreography of acute exercise. Cell 181, 1112–1130.e16 (2020). This study is a multi-omics analysis of human plasma and peripheral blood mononuclear cells at multiple time points across a 1-h recovery period immediately following a standardized exercise bout.
- 20. Sato S. et al. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 34, 329–345.e8 (2022). In this study, mass spectrometry-based metabolomics is used to characterize time-of-day differences in metabolic programming in several mouse tissues in response to an acute bout of exercise, including the net uptake and release of metabolites in hindlimb muscles and liver.
- 21. Murgia M. et al. Protein profile of fiber types in human skeletal muscle: a single-fiber proteomics study. Skelet. Muscle 11, 24 (2021). This study interrogates the proteome of single muscle fibres from young, healthy individuals and provides a useful resource for basal proteomic comparisons between type I and type II fibres.
- 22. Bloemberg D. & Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 7, e35273 (2012). This paper provides a valuable resource for myosin fibre type and enzymatic profile comparisons, and identifies notable differences between muscles and species.
- 23.Simoneau JA & Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am. J. Physiol 257, E567–E572 (1989). [DOI] [PubMed] [Google Scholar]
- 24.Pellegrino MA et al. Orthologous myosin isoforms and scaling of shortening velocity with body size in mouse, rat, rabbit and human muscles. J. Physiol 546, 677–689 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bottinelli R, Pellegrino MA, Canepari M, Rossi R. & Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J. Electromyogr. Kinesiol 9, 87–95 (1999). [DOI] [PubMed] [Google Scholar]
- 26. Dos Santos M. et al. Single-nucleus RNA-seq and FISH identify coordinated transcriptional activity in mammalian myofibers. Nat. Commun 11, 5102 (2020). This is one of the first studies to perform single myonuclear RNA-sequencing, revealing heterogeneity between myonuclei within a given muscle fibre.
- 27. Dos Santos M. et al. A fast myosin super enhancer dictates muscle fiber phenotype through competitive interactions with myosin genes. Nat. Commun 13, 1039 (2022). This study uses a ‘rainbow’ transgenic mouse model of the fast-type myosin locus to determine that competitive promoter–super enhancer interactions govern fast-twitch myosin isoform expression in skeletal muscle.
- 28.Roman W. et al. Myofibril contraction and crosslinking drive nuclear movement to the periphery of skeletal muscle. Nat. Cell Biol 19, 1189–1201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen Y. et al. Myonuclear transcriptional dynamics in response to exercise following satellite cell depletion. iScience 24, 102838 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim M. et al. Single-nucleus transcriptomics reveals functional compartmentalization in syncytial skeletal muscle cells. Nat. Commun 11, 6375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Ercole C. et al. Spatially resolved transcriptomics reveals innervation-responsive functional clusters in skeletal muscle. Cell Rep. 41, 111861 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Battey E. et al. Myonuclear alterations associated with exercise are independent of age in humans. J. Physiol 10.1113/JP284128 (2023). [DOI] [PubMed]
- 33.Viggars MR et al. Adaptation of the transcriptional response to resistance exercise over 4 weeks of daily training. FASEB J. 37, e22686 (2023). [DOI] [PubMed] [Google Scholar]
- 34. Lexell J, Taylor CC & Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci 84, 275–294 (1988). This classic study establishes fibre type differences in human skeletal muscle during healthy ageing, thereby providing insights on the aetiology of sarcopenia.
- 35.Nielsen J, Holmberg HC, Schroder HD, Saltin B. & Ortenblad N. Human skeletal muscle glycogen utilization in exhaustive exercise: role of subcellular localization and fibre type. J. Physiol 589, 2871–2885 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murgia M. et al. Single muscle fiber proteomics reveals fiber-type-specific features of human muscle aging. Cell Rep. 19, 2396–2409 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Luo L. et al. Spatial metabolomics reveals skeletal myofiber subtypes. Sci. Adv 9, eadd0455 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medler S. Mixing it up: the biological significance of hybrid skeletal muscle fibers. J. Exp. Biol 222, jeb200832 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Horwath O. et al. Variability in vastus lateralis fiber type distribution, fiber size, and myonuclear content along and between the legs. J. Appl. Physiol 131, 158–173 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Murach KA et al. Fiber typing human skeletal muscle with fluorescent immunohistochemistry. J. Appl. Physiol 127, 1632–1639 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bathgate KE et al. Muscle health and performance in monozygotic twins with 30 years of discordant exercise habits. Eur. J. Appl. Physiol 118, 2097–2110 (2018). This study of monozygotic twins suggests that fibre type plasticity in response to lifelong endurance training is greater than previously appreciated.
- 42.Malisoux L, Francaux M, Nielens H. & Theisen D. Stretch-shortening cycle exercises: an effective training paradigm to enhance power output of human single muscle fibers. J. Appl. Physiol 100, 771–779 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Sakakibara I, Santolini M, Ferry A, Hakim V. & Maire P. Six homeoproteins and a Iinc-RNA at the fast MYH locus lock fast myofiber terminal phenotype. PLoS Genet. 10, e1004386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trappe S. et al. Single muscle fiber adaptations with marathon training. J. Appl. Physiol 101, 721–727 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Williamson DL, Gallagher PM, Carroll CC, Raue U. & Trappe SW Reduction in hybrid single muscle fiber proportions with resistance training in humans. J. Appl. Physiol 91, 1955–1961 (2001). [DOI] [PubMed] [Google Scholar]
- 46. Long K. et al. Identification of enhancers responsible for the coordinated expression of myosin heavy chain isoforms in skeletal muscle. BMC Genomics 23, 519 (2022). This study, published shortly after Dos Santos et al. (2022), also identifies the fast-myosin super enhancer, as well as enhancers of slow-type myosin.
- 47.Balagopal P, Schimke JC, Ades P, Adey D. & Nair KS Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am. J. Physiol. Endocrinol. Metab 280, E203–E208 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Lin J. et al. Transcriptional co-activator PGC- drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Kuhnen G. et al. Genes whose gain or loss of function changes type 1, 2A, 2X, or 2B muscle fibre proportions in mice — a systematic review. Int. J. Mol. Sci 23, 12933 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Street SF Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J. Cell. Physiol 114, 346–364 (1983). [DOI] [PubMed] [Google Scholar]
- 51. Willingham TB, Kim Y, Lindberg E, Bleck CKE & Glancy B. The unified myofibrillar matrix for force generation in muscle. Nat. Commun 11, 3722 (2020). The microscopic analysis of muscle myofibrils in this study provides a new paradigm for the organization of muscle contractile apparatus.
- 52.Ajayi PT et al. Regulation of the evolutionarily conserved muscle myofibrillar matrix by cell type dependent and independent mechanisms. Nat. Commun 13, 2661 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katti P. et al. Mitochondrial network configuration influences sarcomere and myosin filament structure in striated muscles. Nat. Commun 13, 6058 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krustrup P, Ferguson RA, Kjaer M. & Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J. Physiol 549, 255–269 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zagatto AM et al. Impacts of high-intensity exercise on the metabolomics profile of human skeletal muscle tissue. Scand. J. Med. Sci. Sports 32, 402–413 (2022). This study is an analysis of the immediate post-exercise skeletal muscle metabolome in response to an exhaustive bout of high-intensity cycling.
- 56.Bleck CKE, Kim Y, Willingham TB & Glancy B. Subcellular connectomic analyses of energy networks in striated muscle. Nat. Commun 9, 5111 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Glancy B. et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523, 617–620 (2015). This work advances the understanding of how membrane potential is dispersed across the mitochondrial reticulum to facilitate efficient ATP distribution in muscle.
- 58.Glancy B. et al. Power grid protection of the muscle mitochondrial reticulum. Cell Rep. 19, 487–496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vincent AE et al. Quantitative 3D mapping of the human skeletal muscle mitochondrial network. Cell Rep. 26, 996–1009.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caffrey BJ et al. Semi-automated 3D segmentation of human skeletal muscle using focused ion beam-scanning electron microscopic images. J. Struct. Biol 207, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greggio C. et al. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab. 25, 301–311 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Cogliati S. et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balsa E. et al. ER and nutrient stress promote assembly of respiratory chain supercomplexes through the axis. Mol. Cell 74, 877–890.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nielsen J. et al. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J. Physiol 595, 2839–2847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hentila J. et al. Autophagy is induced by resistance exercise in young men, but unfolded protein response is induced regardless of age. Acta Physiol. 224, e13069 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Heymsfield SB et al. Human energy expenditure: advances in organ-tissue prediction models. Obes. Rev 19, 1177–1188 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phung LA, Foster AD, Miller MS, Lowe DA & Thomas DD Super-relaxed state of myosin in human skeletal muscle is fiber-type dependent. Am. J. Physiol. Cell Physiol. 319, C1158–C1162 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linari M. et al. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature 528, 276–279 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Gaitanos GC, Williams C, Boobis LH & Brooks S. Human muscle metabolism during intermittent maximal exercise. J. Appl. Physiol 75, 712–719 (1993). [DOI] [PubMed] [Google Scholar]
- 70.Parolin ML et al. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol 277, E890–E900 (1999). [DOI] [PubMed] [Google Scholar]
- 71.Romijn JA et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol 265, E380–E391 (1993). [DOI] [PubMed] [Google Scholar]
- 72.van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH & Wagenmakers AJ The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol 536, 295–304 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duchateau J. & Enoka RM Human motor unit recordings: origins and insight into the integrated motor system. Brain Res. 1409, 42–61 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Stienen GJ, Kiers JL, Bottinelli R. & Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J. Physiol 493, 299–307 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greenhaff PL et al. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J. Physiol 478, 149–155 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koh HE, Nielsen J, Saltin B, Holmberg HC & Ortenblad N. Pronounced limb and fibre type differences in subcellular lipid droplet content and distribution in elite skiers before and after exhaustive exercise. J. Physiol 595, 5781–5795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Almeida ME et al. Altered intramuscular network of lipid droplets and mitochondria in type 2 diabetes. Am. J. Physiol. Cell Physiol 324, C39–C57 (2023). [DOI] [PubMed] [Google Scholar]
- 78.Daemen S. et al. Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Mol. Metab 17, 71–81 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brooks GA & Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J. Appl. Physiol 76, 2253–2261 (1994). [DOI] [PubMed] [Google Scholar]
- 80.Wust RC et al. Kinetic control of oxygen consumption during contractions in self-perfused skeletal muscle. J. Physiol 589, 3995–4009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferguson BS et al. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol 118, 691–728 (2018). [DOI] [PubMed] [Google Scholar]
- 82.Wescott AP, Kao JPY, Lederer WJ & Boyman L. Voltage-energized calcium-sensitive ATP production by mitochondria. Nat. Metab 1, 975–984 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seiler SE et al. Carnitine acetyltransferase mitigates metabolic inertia and muscle fatigue during exercise. Cell Metab. 22, 65–76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mancilla RF et al. Skeletal muscle mitochondrial inertia is associated with carnitine acetyltransferase activity and physical function in humans. JCI Insight 8, e163855 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Timmons JA et al. Substrate availability limits human skeletal muscle oxidative ATP regeneration at the onset of ischemic exercise. J. Clin. Invest 101, 79–85 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walsh B. et al. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J. Physiol 537, 971–978 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roman BB, Meyer RA & Wiseman RW Phosphocreatine kinetics at the onset of contractions in skeletal muscle of MM creatine kinase knockout mice. Am. J. Physiol. Cell Physiol 283, C1776–C1783 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Katz A, Broberg S, Sahlin K. & Wahren J. Leg glucose uptake during maximal dynamic exercise in humans. Am. J. Physiol 251, E65–E70 (1986). [DOI] [PubMed] [Google Scholar]
- 89.Katz A. A century of exercise physiology: key concepts in regulation of glycogen metabolism in skeletal muscle. Eur. J. Appl. Physiol 122, 1751–1772 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Blazev R. et al. Phosphoproteomics of three exercise modalities identifies canonical signaling and C18ORF25 as an AMPK substrate regulating skeletal muscle function. Cell Metab. 34, 1561–1577 e1569 (2022). Building on work from Hoffman et al. (2015), this study compares the phosphoproteomic impact of an acute bout of endurance, resistance or sprinting exercise across a 3-h post-exercise recovery period.
- 91.Watt MJ et al. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab 290, E500–E508 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Richter EA, Ruderman NB, Gavras H, Belur ER & Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am. J. Physiol 242, E25–E32 (1982). [DOI] [PubMed] [Google Scholar]
- 93.Hureau TJ et al. On the role of skeletal muscle acidosis and inorganic phosphates as determinants of central and peripheral fatigue: a 31P-MRS study. J. Physiol 600, 3069–3081 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng AJ, Place N. & Westerblad H. Molecular basis for exercise-induced fatigue: the importance of strictly controlled cellular Ca(2+) handling. Cold Spring Harb. Perspect. Med 8, a029710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rogatzki MJ, Ferguson BS, Goodwin ML & Gladden LB Lactate is always the end product of glycolysis. Front. Neurosci 9, 22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hokken R. et al. Subcellular localization- and fibre type-dependent utilization of muscle glycogen during heavy resistance exercise in elite power and Olympic weightlifters. Acta Physiol. 231, e13561 (2021). [DOI] [PubMed] [Google Scholar]
- 97.Vigh-Larsen JF et al. Fibre type- and localisation-specific muscle glycogen utilisation during repeated high-intensity intermittent exercise. J. Physiol 600, 4713–4730 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sidhu SK et al. Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. J. Physiol 596, 4789–4801 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ducrocq GP & Blain GM Relationship between neuromuscular fatigue, muscle activation and the work done above the critical power during severe-intensity exercise. Exp. Physiol 107, 312–325 (2022). [DOI] [PubMed] [Google Scholar]
- 100.Goncalves RL, Quinlan CL, Perevoshchikova IV, Hey-Mogensen M. & Brand MD Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem 290, 209–227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henriquez-Olguin C. et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun 10, 4623 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakellariou GK et al. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal 18, 603–621 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun QA et al. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc. Natl Acad. Sci. USA 108, 16098–16103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xirouchaki CE et al. Skeletal muscle NOX4 is required for adaptive responses that prevent insulin resistance. Sci. Adv 7, eabl4988 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bouviere J. et al. Exercise-stimulated ROS sensitive signaling pathways in skeletal muscle. Antioxidants 10, 537 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ristow M. et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl Acad. Sci. USA 106, 8665–8670 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clifford T, Jeffries O, Stevenson EJ & Davies KAB The effects of vitamin C and E on exercise-induced physiological adaptations: a systematic review and Meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr 60, 3669–3679 (2020). [DOI] [PubMed] [Google Scholar]
- 108.Gonzalez JT et al. Ingestion of glucose or sucrose prevents liver but not muscle glycogen depletion during prolonged endurance-type exercise in trained cyclists. Am. J. Physiol. Endocrinol. Metab 309, E1032–E1039 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Vincent MA et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am. J. Physiol. Endocrinol. Metab 290, E1191–E1197 (2006). [DOI] [PubMed] [Google Scholar]
- 110.MacLean DA, Bangsbo J. & Saltin B. Muscle interstitial glucose and lactate levels during dynamic exercise in humans determined by microdialysis. J. Appl. Physiol 87, 1483–1490 (1999). [DOI] [PubMed] [Google Scholar]
- 111.Chow LS et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol 18, 273–289 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ryder JW et al. Postexercise glucose uptake and glycogen synthesis in skeletal muscle from GLUT4-deficient mice. FASEB J. 13, 2246–2256 (1999). [DOI] [PubMed] [Google Scholar]
- 113.Sylow L, Kleinert M, Richter EA & Jensen TE Exercise-stimulated glucose uptake — regulation and implications for glycaemic control. Nat. Rev. Endocrinol 13, 133–148 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Sylow L. et al. Rac1 and AMPK account for the majority of muscle glucose uptake stimulated by ex vivo contraction but not in vivo exercise. Diabetes 66, 1548–1559 (2017). [DOI] [PubMed] [Google Scholar]
- 115.Kjobsted R. et al. AMPK and TBC1D1 regulate muscle glucose uptake after, but not during, exercise and contraction. Diabetes 68, 1427–1440 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Kjobsted R. et al. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes 66, 598–612 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Fritzen AM et al. 5′-AMP activated protein kinase controls substrate metabolism during post-exercise recovery via regulation of pyruvate dehydrogenase kinase 4. J. Physiol 593, 4765–4780 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ouyang Q. et al. Rab8a as a mitochondrial receptor for lipid droplets in skeletal muscle. Dev. Cell 58, 289–305.e6 (2023). [DOI] [PubMed] [Google Scholar]
- 119.Glancy B. et al. Mitochondrial lactate metabolism: history and implications for exercise and disease. J. Physiol 599, 863–888 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rothschild JA, Kilding AE, Stewart T. & Plews DJ Factors influencing substrate oxidation during submaximal cycling: a modelling analysis. Sports Med. 52, 2775–2795 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.King A, Helms E, Zinn C. & Jukic I. The ergogenic effects of acute carbohydrate feeding on resistance exercise performance: a systematic review and meta-analysis. Sports Med. 52, 2691–2712 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hartley C, Carr A, Bowe SJ, Bredie WLP & Keast RSJ Maltodextrin-based carbohydrate oral rinsing and exercise performance: systematic review and meta-analysis. Sports Med. 52, 1833–1862 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gant N, Stinear CM & Byblow WD Carbohydrate in the mouth immediately facilitates motor output. Brain Res. 1350, 151–158 (2010). [DOI] [PubMed] [Google Scholar]
- 124.Morville T, Sahl RE, Moritz T, Helge JW & Clemmensen C. Plasma metabolome profiling of resistance exercise and endurance exercise in humans. Cell Rep. 33, 108554 (2020). [DOI] [PubMed] [Google Scholar]
- 125.Alsted TJ et al. Contraction-induced lipolysis is not impaired by inhibition of hormone-sensitive lipase in skeletal muscle. J. Physiol 591, 5141–5155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dube JJ et al. Adipose triglyceride lipase deletion from adipocytes, but not skeletal myocytes, impairs acute exercise performance in mice. Am. J. Physiol. Endocrinol. Metab 308, E879–E890 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Watt MJ, Heigenhauser GJ & Spriet LL Effects of dynamic exercise intensity on the activation of hormone-sensitive lipase in human skeletal muscle. J. Physiol 547, 301–308 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Donsmark M, Langfort J, Holm C, Ploug T. & Galbo H. Contractions induce phosphorylation of the AMPK site Ser565 in hormone-sensitive lipase in muscle. Biochem. Biophys. Res. Commun 316, 867–871 (2004). [DOI] [PubMed] [Google Scholar]
- 129.Prats C. et al. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. J. Lipid Res 47, 2392–2399 (2006). [DOI] [PubMed] [Google Scholar]
- 130.Stokie JR, Abbott G, Howlett KF, Hamilton DL & Shaw CS Intramuscular lipid utilization during exercise: a systematic review, meta-analysis, and meta-regression. J. Appl. Physiol 134, 581–592 (2023). [DOI] [PubMed] [Google Scholar]
- 131.Ghafouri K. et al. Moderate exercise increases affinity of large very low-density lipoproteins for hydrolysis by lipoprotein lipase. J. Clin. Endocrinol. Metab 100, 2205–2213 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Jain SS et al. Additive effects of insulin and muscle contraction on fatty acid transport and fatty acid transporters, FAT/CD36, FABPpm, FATP1, 4 and 6. FEBS Lett. 583, 2294–2300 (2009). [DOI] [PubMed] [Google Scholar]
- 133.Nickerson JG et al. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J. Biol. Chem 284, 16522–16530 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jeppesen J. et al. Contraction-induced skeletal muscle FAT/CD36 trafficking and FA uptake is AMPK independent. J. Lipid Res 52, 699–711 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abbott MJ, Edelman AM & Turcotte LP CaMKK is an upstream signal of AMP-activated protein kinase in regulation of substrate metabolism in contracting skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol 297, R1724–R1732 (2009). [DOI] [PubMed] [Google Scholar]
- 136.Turcotte LP, Raney MA & Todd MK ERK1/2 inhibition prevents contraction-induced increase in plasma membrane FAT/CD36 content and FA uptake in rodent muscle. Acta Physiol. Scand 184, 131–139 (2005). [DOI] [PubMed] [Google Scholar]
- 137.Holloway GP et al. Fatty acid binding protein facilitates sarcolemmal fatty acid transport but not mitochondrial oxidation in rat and human skeletal muscle. J. Physiol 582, 393–405 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sacchetti M, Saltin B, Osada T. & van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. J. Physiol 540, 387–395 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hall AM, Wiczer BM, Herrmann T, Stremmel W. & Bernlohr DA Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J. Biol. Chem 280, 11948–11954 (2005). [DOI] [PubMed] [Google Scholar]
- 140.Li LO et al. Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes 64, 23–35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen S, Zhou L, Sun J, Qu Y. & Chen M. The role of cAMP-PKA pathway in lactate-induced intramuscular triglyceride accumulation and mitochondria content increase in mice. Front. Physiol 12, 709135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Constantin-Teodosiu D, Howell S. & Greenhaff PL Carnitine metabolism in human muscle fiber types during submaximal dynamic exercise. J. Appl. Physiol 80, 1061–1064 (1996). [DOI] [PubMed] [Google Scholar]
- 143.Hostrup M. et al. High-intensity interval training remodels the proteome and acetylome of human skeletal muscle. eLife 11, e69802 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adamovich Y. et al. Clock proteins and training modify exercise capacity in a daytime-dependent manner. Proc. Natl Acad. Sci. USA 118, e2101115118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bano-Otalora B. et al. Bright daytime light enhances circadian amplitude in a diurnal mammal. Proc. Natl Acad. Sci. USA 118, e2100094118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martin RA, Viggars MR & Esser KA Metabolism and exercise: the skeletal muscle clock takes centre stage. Nat. Rev. Endocrinol 10.1038/s41574-023-00805-8 (2023). [DOI] [PubMed]
- 147.Dyar KA et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab 3, 29–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dyar KA et al. Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. PLoS Biol. 16, e2005886 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hodge BA et al. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet. Muscle 5, 17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harfmann BD et al. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 6, 12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thomas JM et al. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 5, e134270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun S. et al. A single-cell transcriptomic atlas of exercise-induced anti-inflammatory and geroprotective effects across the body. Innovation 4, 100380 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Small L. et al. Contraction influences Per2 gene expression in skeletal muscle through a calcium-dependent pathway. J. Physiol 598, 5739–5752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zambon AC et al. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol. 4, R61 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dyar KA et al. The calcineurin-NFAT pathway controls activity-dependent circadian gene expression in slow skeletal muscle. Mol. Metab 4, 823–833 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gabriel BM & Zierath JR Circadian rhythms and exercise — re-setting the clock in metabolic disease. Nat. Rev. Endocrinol 15, 197–206 (2019). [DOI] [PubMed] [Google Scholar]
- 157.Harmsen JF et al. Circadian misalignment disturbs the skeletal muscle lipidome in healthy young men. FASEB J. 35, e21611 (2021). [DOI] [PubMed] [Google Scholar]
- 158.Wefers J. et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl Acad. Sci. USA 115, 7789–7794 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morris CJ, Purvis TE, Hu K. & Scheer FA Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl Acad. Sci. USA 113, E1402–E1411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saner NJ et al. Exercise mitigates sleep-loss-induced changes in glucose tolerance, mitochondrial function, sarcoplasmic protein synthesis, and diurnal rhythms. Mol. Metab 43, 101110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sato S. et al. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab. 30, 92–110.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Mancilla R. et al. Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol. Rep 8, e14669 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.van der Velde J. et al. Timing of physical activity in relation to liver fat content and insulin resistance. Diabetologia 66, 461–471 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Savikj M. et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia 62, 233–237 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Feng H. et al. Associations of timing of physical activity with all-cause and cause-specific mortality in a prospective cohort study. Nat. Commun 14, 930 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fischer D, Lombardi DA, Marucci-Wellman H. & Roenneberg T. Chronotypes in the US — influence of age and sex. PLoS ONE 12, e0178782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amar D. et al. Time trajectories in the transcriptomic response to exercise — a meta-analysis. Nat. Commun 12, 3471 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pillon NJ et al. Distinctive exercise-induced inflammatory response and exerkine induction in skeletal muscle of people with type 2 diabetes. Sci. Adv 8, eabo3192 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Makhnovskii PA et al. Alternative transcription start sites contribute to acute-stress-induced transcriptome response in human skeletal muscle. Hum. Genomics 16, 24 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hoffman NJ et al. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 22, 922–935 (2015). This seminal study investigates the exercise-induced human muscle phosphoproteome.
- 171.Nelson ME et al. Phosphoproteomics reveals conserved exercise-stimulated signaling and AMPK regulation of store-operated calcium entry. EMBO J. 38, e102578 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.You JS et al. The role of raptor in the mechanical load-induced regulation of mTOR signaling, protein synthesis, and skeletal muscle hypertrophy. FASEB J. 33, 4021–4034 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.You JS et al. A -FoxO-ubiquitin proteolytic axis controls fiber size during skeletal muscle remodeling. Sci. Signal 11, eaao6847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Song Z. et al. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci. Rep 7, 5028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hodson N, Mazzulla M, Holowaty MNH, Kumbhare D. & Moore DR RPS6 phosphorylation occurs to a greater extent in the periphery of human skeletal muscle fibers, near focal adhesions, after anabolic stimuli. Am. J. Physiol. Cell Physiol 322, C94–C110 (2022). [DOI] [PubMed] [Google Scholar]
- 176.Abou Sawan S. et al. Trained integrated post-exercise myofibrillar protein synthesis rates correlate with hypertrophy in young males and females. Med. Sci. Sports Exerc 54, 953–964 (2022). [DOI] [PubMed] [Google Scholar]
- 177.Needham EJ et al. Personalized phosphoproteomics identifies functional signaling. Nat. Biotechnol 40, 576–584 (2022). [DOI] [PubMed] [Google Scholar]
- 178.Steinert ND et al. Mapping of the contraction-induced phosphoproteome identifies TRIM28 as a significant regulator of skeletal muscle size and function. Cell Rep. 34, 108796 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nordgaard C. et al. is activated by cellular compression and mediates contraction-induced MAP kinase signaling in skeletal muscle. EMBO J. 41, e111650 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.West DW et al. Acute resistance exercise activates rapamycin-sensitive and -insensitive mechanisms that control translational activity and capacity in skeletal muscle. J. Physiol 594, 453–468 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lessard SJ et al. JNK regulates muscle remodeling via myostatin/SMAD inhibition. Nat. Commun 9, 3030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.MacKenzie MG, Hamilton DL, Pepin M, Patton A. & Baar K. Inhibition of myostatin signaling through Notch activation following acute resistance exercise. PLoS ONE 8, e68743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Pillon NJ et al. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun 11, 470 (2020). This meta-analysis curates an extensive (and frequently updated) library of the skeletal muscle transcriptomic response to exercise across human demographics and exercise modalities.
- 184.Schumann M. et al. Compatibility of concurrent aerobic and strength training for skeletal muscle size and function: an updated systematic review and meta-analysis. Sports Med. 52, 601–612 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lundberg TR, Feuerbacher JF, Sunkeler M. & Schumann M. The effects of concurrent aerobic and strength training on muscle fiber hypertrophy: a systematic review and meta-analysis. Sports Med. 52, 2391–2403 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaiser MS et al. Dual roles of mTORC1-dependent activation of the ubiquitin-proteasome system in muscle proteostasis. Commun. Biol 5, 1141 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Drake JC et al. Mitochondria-localized AMPK responds to local energetics and contributes to exercise and energetic stress-induced mitophagy. Proc. Natl Acad. Sci. USA 118, e2025932118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Laker RC et al. AMPK phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun 8, 548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Toyama EQ et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351, 275–281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.VerPlank JJS, Lokireddy S, Zhao J. & Goldberg AL 26S Proteasomes are rapidly activated by diverse hormones and physiological states that raise cAMP and cause Rpn6 phosphorylation. Proc. Natl Acad. Sci. USA 116, 4228–4237 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Parker BL, Kiens B, Wojtaszewski JFP, Richter EA & James DE Quantification of exercise-regulated ubiquitin signaling in human skeletal muscle identifies protein modification cross talk via NEDDylation. FASEB J. 34, 5906–5916 (2020). [DOI] [PubMed] [Google Scholar]
- 192.Seaborne RA & Sharples AP The interplay between exercise metabolism, epigenetics, and skeletal muscle remodeling. Exerc. Sport Sci. Rev 48, 188–200 (2020). [DOI] [PubMed] [Google Scholar]
- 193.McGee SL, Fairlie E, Garnham AP & Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J. Physiol 587, 5951–5958 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Backs J, Song K, Bezprozvannaya S, Chang S. & Olson EN CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J. Clin. Invest 116, 1853–1864 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu X. et al. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J. Clin. Invest 116, 675–682 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Solagna F. et al. Exercise-dependent increases in protein synthesis are accompanied by chromatin modifications and increased MRTF-SRF signalling. Acta Physiol. 230, e13496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yin Y. et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 356, eaaj2239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Figueiredo VC et al. Genetic and epigenetic regulation of skeletal muscle ribosome biogenesis with exercise. J. Physiol 599, 3363–3384 (2021). [DOI] [PubMed] [Google Scholar]
- 199. Seaborne RA et al. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci. Rep 8, 1898 (2018). This comprehensive interrogation of the resistance exercise-induced methylome provides an epigenetic hypothesis for ‘muscle memory’.
- 200.Sexton CL et al. Skeletal muscle DNA methylation and mRNA responses to a bout of higher versus lower load resistance exercise in previously trained men. Cells 12, 263 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lindholm ME et al. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics 9, 1557–1569 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maasar MF et al. The comparative methylome and transcriptome after change of direction compared to straight line running exercise in human skeletal muscle. Front. Physiol 12, 619447 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Galle E. et al. H3K18 lactylation marks tissue-specific active enhancers. Genome Biol. 23, 207 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Massart J. et al. Endurance exercise training-responsive miR-19b-3p improves skeletal muscle glucose metabolism. Nat. Commun 12, 5948 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bonilauri B. & Dallagiovanna B. Long non-coding RNAs are differentially expressed after different exercise training programs. Front. Physiol 11, 567614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vechetti IJ Jr. et al. Mechanical overload-induced muscle-derived extracellular vesicles promote adipose tissue lipolysis. FASEB J. 35, e21644 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xhuti D, Nilsson MI, Manta K, Tarnopolsky MA & Nederveen JP Circulating exosome-like vesicle and skeletal muscle microRNAs are altered with age and resistance training. J. Physiol 10.1113/JP282663 (2023). [DOI] [PubMed]
- 208.Watanabe S. et al. Skeletal muscle releases extracellular vesicles with distinct protein and microRNA signatures that function in the muscle microenvironment. PNAS Nexus 1, pgac173 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Murach KA et al. Fusion-independent satellite cell communication to muscle fibers during load-induced hypertrophy. Function 1, zqaa009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ & Peterson CA Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20, 56–69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Albanese M. et al. MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet. 17, e1009951 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wohlwend M. et al. The exercise-induced long noncoding RNA CYTOR promotes fast-twitch myogenesis in aging. Sci. Transl Med. 13, eabc7367 (2021). [DOI] [PubMed]
- 213.Nelson BR et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351, 271–275 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Anderson DM et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 160, 595–606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pinheiro H. et al. mRNA distribution in skeletal muscle is associated with mRNA size. J. Cell Sci 134, jcs256388 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Denes LT, Kelley CP & Wang ET Microtubule-based transport is essential to distribute RNA and nascent protein in skeletal muscle. Nat. Commun 12, 6079 (2021). This study and Pinheiro et al. (2021) suggest that mRNAs are transported along microtubules in muscle, which has notable implications for the regulation of the ‘myonuclear domain’.
- 217.Scarborough EA et al. Microtubules orchestrate local translation to enable cardiac growth. Nat. Commun 12, 1547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Taylor-Weiner H. et al. Modeling the transport of nuclear proteins along single skeletal muscle cells. Proc. Natl Acad. Sci. USA 117, 2978–2986 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cutler AA, Jackson JB, Corbett AH & Pavlath GK Non-equivalence of nuclear import among nuclei in multinucleated skeletal muscle cells. J. Cell Sci 131, jcs207670 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Masschelein E. et al. Exercise promotes satellite cell contribution to myofibers in a load-dependent manner. Skelet. Muscle 10, 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Borowik AK et al. Skeletal muscle nuclei in mice are not post-mitotic. Function 4, zqac059 (2023). This study provides the first compelling evidence that myonuclei can synthesize DNA and that this process can be augmented by muscle mechanical overload.
- 222.Pearen MA et al. The nuclear receptor, Nor-1, markedly increases type II oxidative muscle fibers and resistance to fatigue. Mol. Endocrinol 26, 372–384 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Correia JC, Ferreira DM & Ruas JL Intercellular: local and systemic actions of skeletal muscle PGC-1s. Trends Endocrinol. Metab 26, 305–314 (2015). [DOI] [PubMed] [Google Scholar]
- 224.Goode JM et al. The nuclear receptor, Nor-1, induces the physiological responses associated with exercise. Mol. Endocrinol 30, 660–676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wu Z. et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC- transcription and mitochondrial biogenesis in muscle cells. Proc. Natl Acad. Sci. USA 103, 14379–14384 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wilson TE, Fahrner TJ, Johnston M. & Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science 252, 1296–1300 (1991). [DOI] [PubMed] [Google Scholar]
- 227.Maira M, Martens C, Philips A. & Drouin J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol. Cell Biol 19, 7549–7557 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chao LC et al. Skeletal muscle Nur77 expression enhances oxidative metabolism and substrate utilization. J. Lipid Res 53, 2610–2619 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ruas JL et al. A PGC- isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151, 1319–1331 (2012). This is the first report that PGC1A4 is transcribed from the alternative PGC1A promoter after resistance exercise and can stimulate muscle hypertroph.
- 230.Barres R. et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 15, 405–411 (2012). [DOI] [PubMed] [Google Scholar]
- 231.Perez-Schindler J. et al. RNA-bound PGC- controls gene expression in liquid-like nuclear condensates. Proc. Natl Acad. Sci. USA 118, e2105951118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fan W. et al. promotes running endurance by preserving glucose. Cell Metab. 25, 1186–1193.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fan L. et al. Transcription factors KLF15 and cooperatively orchestrate genome-wide regulation of lipid metabolism in skeletal muscle. J. Biol. Chem 298, 101926 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mammucari C. et al. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep. 10, 1269–1279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Koh JH et al. Enhancement of anaerobic glycolysis — a role of PGC- in resistance exercise. Nat. Commun 13, 2324 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wackerhage H. et al. Does a hypertrophying muscle fibre reprogramme its metabolism similar to a cancer cell? Sports Med. 52, 2569–2578 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bohlen J, Roiuk M. & Teleman AA Phosphorylation of ribosomal protein S6 differentially affects mRNA translation based on ORF length. Nucleic Acids Res. 49, 13062–13074 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chaillou T, Zhang X. & McCarthy JJ Expression of muscle-specific ribosomal protein L3-like impairs myotube growth. J. Cell. Physiol 231, 1894–1902 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Granata C, Oliveira RSF, Little JP & Bishop DJ Forty high-intensity interval training sessions blunt exercise-induced changes in the nuclear protein content of PGC- and p53 in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab 318, E224–E236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Brook MS et al. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J. Physiol 594, 7399–7417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Norrbom JM et al. A HIF-1 signature dominates the attenuation in the human skeletal muscle transcriptional response to high-intensity interval training. J. Appl. Physiol 132, 1448–1459 (2022). [DOI] [PubMed] [Google Scholar]
- 242.Stokes T. et al. Molecular transducers of human skeletal muscle remodeling under different loading states. Cell Rep. 32, 107980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Porter C, Reidy PT, Bhattarai N, Sidossis LS & Rasmussen BB Resistance exercise training alters mitochondrial function in human skeletal muscle. Med. Sci. Sports Exerc 47, 1922–1931 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Granata C, Jamnick NA & Bishop DJ Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med. 48, 1809–1828 (2018). [DOI] [PubMed] [Google Scholar]
- 245.Andrade-Souza VA et al. Exercise twice-a-day potentiates markers of mitochondrial biogenesis in men. FASEB J. 34, 1602–1619 (2020). [DOI] [PubMed] [Google Scholar]
- 246.Soto I. et al. Balanced mitochondrial and cytosolic translatomes underlie the biogenesis of human respiratory complexes. Genome Biol. 23, 170 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liang X. et al. Exercise inducible lactate dehydrogenase B regulates mitochondrial function in skeletal muscle. J. Biol. Chem 291, 25306–25318 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Thomas ACQ et al. Short-term aerobic conditioning prior to resistance training augments muscle hypertrophy and satellite cell content in healthy young men and women. FASEB J. 36, e22500 (2022). [DOI] [PubMed] [Google Scholar]
- 249.Binet ER et al. Sex-based comparisons of muscle cellular adaptations after 10 weeks of progressive resistance training in middle-aged adults. J. Appl. Physiol 134, 116–129 (2023). [DOI] [PubMed] [Google Scholar]
- 250.Verdijk LB, Snijders T, Holloway TM, Van Kranenburg J. & Van Loon LJC Resistance training increases skeletal muscle capillarization in healthy older men. Med. Sci. Sports Exerc 48, 2157–2164 (2016). [DOI] [PubMed] [Google Scholar]
- 251.Hetlelid KJ, Plews DJ, Herold E, Laursen PB & Seiler S. Rethinking the role of fat oxidation: substrate utilisation during high-intensity interval training in well-trained and recreationally trained runners. BMJ Open Sport Exerc. Med 1, e000047 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gehlert S. et al. Effects of acute and chronic resistance exercise on the skeletal muscle metabolome. Metabolites 12, 445 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ozaki H, Loenneke JP, Thiebaud RS & Abe T. Resistance training induced increase in VO2max in young and older subjects. Eur. Rev. Aging Phys. Act 10, 107–116 (2013). [Google Scholar]
- 254.Eihara Y. et al. Heavy resistance training versus plyometric training for improving running economy and running time trial performance: a systematic review and meta-analysis. Sports Med. Open 8, 138 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Damas F. et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J. Physiol 594, 5209–5222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Saleem A. & Hood DA Acute exercise induces tumour suppressor protein p53 translocation to the mitochondria and promotes a p53-Tfam-mitochondrial DNA complex in skeletal muscle. J. Physiol 591, 3625–3636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Beyfuss K, Erlich AT, Triolo M. & Hood DA The role of p53 in determining mitochondrial adaptations to endurance training in skeletal muscle. Sci. Rep 8, 14710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li X. et al. Ultrasensitive sensors reveal the spatiotemporal landscape of lactate metabolism in physiology and disease. Cell Metab. 35, 200–211.e9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Martin AA et al. Sarcomere dynamics revealed by a myofilament integrated FRET-based biosensor in live skeletal muscle fibers. Sci. Rep 12, 18116 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fentz J. et al. is essential for acute exercise-induced gene responses but not for exercise training-induced adaptations in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab 309, E900–E914 (2015). [DOI] [PubMed] [Google Scholar]
- 261.Ballmann C, Tang Y, Bush Z. & Rowe GC Adult expression of PGC- and in skeletal muscle is not required for endurance exercise-induced enhancement of exercise capacity. Am. J. Physiol. Endocrinol. Metab 311, E928–E938 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lu T, Ang CE & Zhuang X. Spatially resolved epigenomic profiling of single cells in complex tissues. Cell 185, 4448–4464.e17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gandin V. et al. Cap-dependent translation initiation monitored in living cells. Nat. Commun 13, 6558 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhen K. et al. A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson’s disease. NPJ Parkinsons Dis. 8, 146 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yu Q. et al. Comparative effectiveness of multiple exercise interventions in the treatment of mental health disorders: a systematic review and network meta-analysis. Sports Med. Open 8, 135 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Battista F. et al. Effect of exercise on cardiometabolic health of adults with overweight or obesity: focus on blood pressure, insulin resistance, and intrahepatic fat — a systematic review and meta-analysis. Obes. Rev 22, e13269 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nielsen J. et al. Subcellular localization-dependent decrements in skeletal muscle glycogen and mitochondria content following short-term disuse in young and old men. Am. J. Physiol. Endocrinol. Metab 299, E1053–E1060 (2010). [DOI] [PubMed] [Google Scholar]
- 268.Nielsen J. et al. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am. J. Physiol. Endocrinol. Metab 298, E706–E713 (2010). [DOI] [PubMed] [Google Scholar]
- 269.Yi J. et al. Mitochondrial calcium uptake regulates rapid calcium transients in skeletal muscle during excitation-contraction (E-C) coupling. J. Biol. Chem 286, 32436–32443 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.De Stefani D, Raffaello A, Teardo E, Szabo I. & Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Baughman JM et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011). This reference and De Stefani et al. (2011) co-identify the core component of the elusive mitochondrial calcium uniporter.
- 272.Denton RM, McCormack JG & Edgell NJ Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem. J 190, 107–117 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Turkan A, Hiromasa Y. & Roche TE Formation of a complex of the catalytic subunit of pyruvate dehydrogenase phosphatase isoform 1 (PDP1c) and the L2 domain forms a Ca2+ binding site and captures PDP1c as a monomer. Biochemistry 43, 15073–15085 (2004). [DOI] [PubMed] [Google Scholar]
- 274.Rutter GA & Denton RM The binding of Ca2+ ions to pig heart NAD+-isocitrate dehydrogenase and the 2-oxoglutarate dehydrogenase complex. Biochem. J 263, 453–462 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Denton RM, Pullen TJ, Armstrong CT, Heesom KJ & Rutter GA Calcium-insensitive splice variants of mammalian E1 subunit of 2-oxoglutarate dehydrogenase complex with tissue-specific patterns of expression. Biochem. J 473, 1165–1178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sembrowich WL, Quintinskie JJ & Li G. Calcium uptake in mitochondria from different skeletal muscle types. J. Appl. Physiol 59, 137–141 (1985). [DOI] [PubMed] [Google Scholar]
- 277.Sidossis LS, Gastaldelli A, Klein S. & Wolfe RR Regulation of plasma fatty acid oxidation during low- and high-intensity exercise. Am. J. Physiol 272, E1065–E1070 (1997). [DOI] [PubMed] [Google Scholar]
- 278.Wang Y. et al. Saturation of the mitochondrial NADH shuttles drives aerobic glycolysis in proliferating cells. Mol. Cell 82, 3270–3283.e9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jäger S, Handschin C, St-Pierre J. & Spiegelman BM AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-. Proc. Natl Acad. Sci. USA 104, 12017–12022 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Arany Z. et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-. Nature 451, 1008–1012 (2008). [DOI] [PubMed] [Google Scholar]
- 281.Miyake T. & McDermott JC Re-organization of nucleolar architecture in myogenic differentiation. J. Cell Sci 136, jcs260496 (2023). [DOI] [PubMed] [Google Scholar]
- 282.Emilio EJ, Hita-Contreras F, Jimenez-Lara PM, Latorre-Roman P. & Martinez-Amat A. The association of flexibility, balance, and lumbar strength with balance ability: risk of falls in older adults. J. Sports Sci. Med 13, 349–357 (2014). [PMC free article] [PubMed] [Google Scholar]
- 283.Roberts BM, Nuckols G. & Krieger JW Sex differences in resistance training: a systematic review and meta-analysis. J. Strength. Cond. Res 34, 1448–1460 (2020). [DOI] [PubMed] [Google Scholar]
- 284.Schoenfeld BJ, Ogborn D. & Krieger JW Dose-response relationship between weekly resistance training volume and increases in muscle mass: a systematic review and meta-analysis. J. Sports Sci 35, 1073–1082 (2017). [DOI] [PubMed] [Google Scholar]
- 285.Baz-Valle E, Balsalobre-Fernandez C, Alix-Fages C. & Santos-Concejero J. A systematic review of the effects of different resistance training volumes on muscle hypertrophy. J. Hum. Kinet 81, 199–210 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schoenfeld BJ, Grgic J. & Krieger J. How many times per week should a muscle be trained to maximize muscle hypertrophy? A systematic review and meta-analysis of studies examining the effects of resistance training frequency. J. Sports Sci 37, 1286–1295 (2019). [DOI] [PubMed] [Google Scholar]
- 287.Carvalho L. et al. Muscle hypertrophy and strength gains after resistance training with different volume-matched loads: a systematic review and meta-analysis. Appl. Physiol. Nutr. Metab 47, 357–368 (2022). [DOI] [PubMed] [Google Scholar]
- 288.Refalo MC, Helms ER, Trexler ET, Hamilton DL & Fyfe JJ Influence of resistance training proximity-to-failure on skeletal muscle hypertrophy: a systematic review with meta-analysis. Sports Med. 53, 649–665 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Plotkin D. et al. Progressive overload without progressing load? The effects of load or repetition progression on muscular adaptations. PeerJ 10, e14142 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Bohm S, Mersmann F. & Arampatzis A. Human tendon adaptation in response to mechanical loading: a systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med. Open 1, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Nunes EA et al. Systematic review and meta-analysis of protein intake to support muscle mass and function in healthy adults. J. Cachexia Sarcopenia Muscle 13, 795–810 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Mattioni Maturana F, Martus P, Zipfel S. & AM NI Effectiveness of HIIE versus MICT in improving cardiometabolic risk factors in health and disease: a meta-analysis. Med. Sci. Sports Exerc 53, 559–573 (2021). [DOI] [PubMed] [Google Scholar]
- 293.Milanovic Z, Sporis G. & Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 45, 1469–1481 (2015). [DOI] [PubMed] [Google Scholar]
- 294.Scribbans TD, Vecsey S, Hankinson PB, Foster WS & Gurd BJ The effect of training intensity on VO2max in young healthy adults: a meta-regression and meta-analysis. Int. J. Exerc. Sci 9, 230–247 (2016). [PMC free article] [PubMed] [Google Scholar]
- 295.Jamnick NA, Pettitt RW, Granata C, Pyne DB & Bishop DJ An examination and critique of current methods to determine exercise intensity. Sports Med. 50, 1729–1756 (2020). [DOI] [PubMed] [Google Scholar]
- 296.Iannetta D. et al. A critical evaluation of current methods for exercise prescription in women and men. Med. Sci. Sports Exerc 52, 466–473 (2020). [DOI] [PubMed] [Google Scholar]
- 297.Hov H. et al. Aerobic high-intensity intervals are superior to improve compared with sprint intervals in well-trained men. Scand. J. Med. Sci. Sports 33, 146–159 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Hubal MJ et al. Variability in muscle size and strength gain after unilateral resistance training. Med. Sci. Sports Exerc 37, 964–972 (2005). Similar to Bouchard et al. (1999), this study clearly illustrates interindividual heterogeniety in exercise adaptation to a given resistance training programme.
- 299.Vollaard NB et al. Systematic analysis of adaptations in aerobic capacity and submaximal energy metabolism provides a unique insight into determinants of human aerobic performance. J. Appl. Physiol 106, 1479–1486 (2009). [DOI] [PubMed] [Google Scholar]
- 300. Bouchard C. et al. Familial aggregation of VO2max response to exercise training: results from the HERITAGE Family Study. J. Appl. Physiol 87, 1003–1008 (1999). This is a seminal work on the contribution of genetics towards endurance training-induced changes in aerobic fitness.
- 301.Montero D. & Lundby C. Refuting the myth of non-response to exercise training: ‘non-responders’ do respond to higher dose of training. J. Physiol 595, 3377–3387 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ross R, de Lannoy L. & Stotz PJ Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin. Proc 90, 1506–1514 (2015). [DOI] [PubMed] [Google Scholar]
- 303.Janssen I, Heymsfield SB, Wang ZM & Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol 89, 81–88 (2000). [DOI] [PubMed] [Google Scholar]
- 304.Tian Q. et al. Muscle mitochondrial energetics predicts mobility decline in wellfunctioning older adults: the baltimore longitudinal study of aging. Aging Cell 21, e13552 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Alcazar J, Rodriguez-Lopez C, Delecluse C, Thomis M. & Van Roie E. Ten-year longitudinal changes in muscle power, force, and velocity in young, middle-aged, and older adults. J. Cachexia Sarcopenia Muscle 14, 1019–1032 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Grosicki GJ, Zepeda CS & Sundberg CW Single muscle fibre contractile function with ageing. J. Physiol 600, 5005–5026 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhang X. et al. Characterization of cellular senescence in aging skeletal muscle. Nat. Aging 2, 601–615 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Moiseeva V. et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature 613, 169–178 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Dungan CM et al. Deletion of SA -Gal+ cells using senolytics improves muscle regeneration in old mice. Aging Cell 21, e13528 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Dungan CM et al. Senolytic treatment rescues blunted muscle hypertrophy in old mice. Geroscience 44, 1925–1940 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Englund DA et al. p21 induces a senescence program and skeletal muscle dysfunction. Mol. Metab 67, 101652 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sailani MR et al. Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Sci. Rep 9, 3272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ringholm S. et al. Impact of aging and lifelong exercise training on mitochondrial function and network connectivity in human skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci 78, 373–383 (2023). [DOI] [PubMed] [Google Scholar]
- 314.Ruple BA et al. Resistance training rejuvenates the mitochondrial methylome in aged human skeletal muscle. FASEB J. 35, e21864 (2021). [DOI] [PubMed] [Google Scholar]
- 315.Vinel C. et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med 24, 1360–1371 (2018). [DOI] [PubMed] [Google Scholar]
- 316.Lee U. et al. A Tead1-Apelin axis directs paracrine communication from myogenic to endothelial cells in skeletal muscle. iScience 25, 104589 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.O’Bryan SJ et al. Progressive resistance training for concomitant increases in muscle strength and bone mineral density in older adults: a systematic review and meta-analysis. Sports Med. 52, 1939–1960 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.D’Hulst G. et al. PHD1 controls muscle mTORC1 in a hydroxylation-independent manner by stabilizing leucyl tRNA synthetase. Nat. Commun 11, 174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Balachandran AT et al. Comparison of power training vs traditional strength training on physical function in older adults: a systematic review and meta-analysis. JAMA Netw. Open 5, e2211623 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.McKenna MJ, Morton J, Selig SE & Snow RJ Creatine supplementation increases muscle total creatine but not maximal intermittent exercise performance. J. Appl. Physiol 87, 2244–2252 (1999). [DOI] [PubMed] [Google Scholar]
- 321.Hornemann T. et al. Muscle-type creatine kinase interacts with central domains of the M-band proteins myomesin and M-protein. J. Mol. Biol 332, 877–887 (2003). [DOI] [PubMed] [Google Scholar]
- 322.Kraft T, Hornemann T, Stolz M, Nier V. & Wallimann T. Coupling of creatine kinase to glycolytic enzymes at the sarcomeric I-band of skeletal muscle: a biochemical study in situ. J. Muscle Res. Cell Motil 21, 691–703 (2000). [DOI] [PubMed] [Google Scholar]
- 323.Perry CG et al. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J. Physiol 590, 5475–5486 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hultman E, Soderlund K, Timmons JA, Cederblad G. & Greenhaff PL Muscle creatine loading in men. J. Appl. Physiol 81, 232–237 (1996). [DOI] [PubMed] [Google Scholar]
- 325.de Souza ESA et al. Effects of creatine supplementation on renal function: a systematic review and meta-analysis. J. Ren. Nutr 29, 480–489 (2019). [DOI] [PubMed] [Google Scholar]
- 326.Lanhers C. et al. Creatine supplementation and lower limb strength performance: a systematic review and meta-analyses. Sports Med. 45, 1285–1294 (2015). [DOI] [PubMed] [Google Scholar]
- 327.Prokopidis K. et al. Effects of creatine supplementation on memory in healthy individuals: a systematic review and meta-analysis of randomized controlled trials. Nutr. Rev 81, 416–427 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Areta JL & Hopkins WG Skeletal muscle glycogen content at rest and during endurance exercise in humans: a meta-analysis. Sports Med. 48, 2091–2102 (2018). [DOI] [PubMed] [Google Scholar]
- 329. Nielsen J, Dubillot P, Stausholm MH & Ortenblad N. Specific ATPases drive compartmentalized glycogen utilization in rat skeletal muscle. J. Gen. Physiol 154, e202113071 (2022). This study finds that specific depots of glycogen fuel different ATPases to varying extents in skeletal muscle.
- 330.Vigh-Larsen JF et al. The role of muscle glycogen content and localization in high-intensity exercise performance: a placebo-controlled trial. Med. Sci. Sports Exerc 54, 2073–2086 (2022). [DOI] [PubMed] [Google Scholar]
- 331.Gejl KD et al. Muscle glycogen content modifies SR Ca2+ release rate in elite endurance athletes. Med. Sci. Sports Exerc 46, 496–505 (2014). [DOI] [PubMed] [Google Scholar]
- 332.Impey SG et al. Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 48, 1031–1048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Marquet LA et al. Enhanced endurance performance by periodization of carbohydrate intake: “sleep low” strategy. Med. Sci. Sports Exerc 48, 663–672 (2016). [DOI] [PubMed] [Google Scholar]
- 334.Tarnopolsky MA et al. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol 292, R1271–R1278 (2007). [DOI] [PubMed] [Google Scholar]
- 335.Goodpaster BH, He J, Watkins S. & Kelley DE Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab 86, 5755–5761 (2001). [DOI] [PubMed] [Google Scholar]
- 336.Steensberg A. et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol 529, 237–242 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Trinh B. et al. Blocking endogenous IL-6 impairs mobilization of free fatty acids during rest and exercise in lean and obese men. Cell Rep. Med 2, 100396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kistner TM, Pedersen BK & Lieberman DE Interleukin 6 as an energy allocator in muscle tissue. Nat. Metab 4, 170–179 (2022). [DOI] [PubMed] [Google Scholar]
- 339.Katashima CK et al. Evidence for a neuromuscular circuit involving hypothalamic interleukin-6 in the control of skeletal muscle metabolism. Sci. Adv 8, eabm7355 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Takahashi H. et al. TGF- is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab 1, 291–303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Li VL et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 606, 785–790 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Hu M, Nie J, Lei OK, Shi Q. & Kong Z. Acute effect of high-intensity interval training versus moderate-intensity continuous training on appetite perception: a systematic review and meta-analysis. Appetite 182, 106427 (2023). [DOI] [PubMed] [Google Scholar]
- 343.Reddy A. et al. pH-gated succinate secretion regulates muscle remodeling in response to exercise. Cell 183, 62–75.e17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Agudelo LZ et al. Skeletal muscle PGC- modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45 (2014). [DOI] [PubMed] [Google Scholar]
- 345.Agudelo LZ et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 27, 378–392.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 346.Correia JC et al. Muscle-secreted neurturin couples myofiber oxidative metabolism and slow motor neuron identity. Cell Metab. 33, 2215–2230.e8 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.