INTRODUCTION
The COVID-19 pandemic, which will be referred to as pandemic throughout the rest of the paper, resulted in an unprecedented increase in the healthcare burden, negatively impacting both maternal and neonatal care.1 Although the rates of premature births did not increase during the first year of the pandemic, the indirect impact of the pandemic on infant development and access to care is currently unknown.2 Research studies designed to increase the knowledge-base for clinical rehabilitation services were impacted by the pandemic. In many cases, clinical research was completely arrested for a period of time followed by periods of no or limited in-person contact. The need for continuing clinical research studies assessing effects of intervention on the development of preterm infants was imperative.
This pandemic posed several challenges to parents whose infants were in the neonatal intensive care unit (NICU) such as prolonged separations from their infants due to social isolation, strict visitation rules limiting only one parent at a time, and fears of exposing their infant to COVID-19.3 These challenges likely continued once the infant was discharged to home. At home parents were juggling the pandemic’s impact on siblings such as supervising home education and providing their own childcare because out of home childcare facilities were closed.4 Some parents were working from home and others lost employment potentially leading to financial instability.4 Therefore, the combination of the pandemic and an increased risk of neurodevelopmental disorders predisposed preterm infants to long-term delays in physical, neuropsychological, and social emotional development.
Service delivery of rehabilitation therapies like physical therapy, occupational therapy, speech-language pathology, and early intervention services, provided under the Individuals with Disabilities Education Improvement Act was affected by the pandemic.5–7 These services were reduced or provided in an online format during the pandemic.5–7 In this switch from an in-person to telehealth delivery format, early intervention therapists experienced a decrease in caseload and service frequency in addition to a delayed initiation of telehealth visits at the start of the pandemic.8 Although the change to telehealth took time, examples of successful implementation of telehealth service delivery included intervention trials with children with autism and virtual assessment clinics for infants at high risk for neurodevelopmental disabilities.9,10
Given the need for research, as well as the longitudinal nature of developmental outcomes research, it was crucial that research teams use creative strategies to continue this important work. Limited enrollment or retention could limit the ability of a trial to be adequately powered before funding expires. Delays and suspension of the clinical trials, use of telehealth for intervention and assessment, and changes in frequency and dose of services all could impact the interpretation of study findings.7 The impact of protocol modifications and inequity in participation may have a lasting influence on research findings.11 The systemic bias toward higher rates of COVID-19 in people of color may lead to increased under-representation of that population in research contributing to a lack of data on diverse populations.12
The Efficacy of Motor and Cognitive Intervention for Infants Born Very Preterm (PI Dusing, SC, NICHD R01HD093624, Clinical trials registry. NCT02153736), also known as Supporting Play Exploration and Early Development Intervention (SPEEDI2) Trial, is a multi-site 3-arm randomized clinical trial designed to evaluate the efficacy of a physical therapy intervention for infants born preterm during the transition from NICU to home in the first months of life.13 Infant/parent dyads enroll and have their baseline assessment in the NICU when the infant is medically stable and between 35 and 42 weeks of gestation. Of those enrolled, one third immediately post baseline start SPEEDI, the experimental intervention, in the NICU, another third start SPEEDI 15 weeks post baseline, and the final third receives no study intervention. All groups continue routine care.13
This study provides an opportunity to evaluate the impact of the pandemic on research in 3 unique ways: i) enrollment was planned for 2.5 years starting in Feb 2019 resulting in 1 year of enrollment before the pandemic, ii) the clinical trial was deemed to be of high potential benefit by Human Subjects Review Board (HSRB) and thus continued throughout the pandemic with modifications to limit in-person interactions, and iii) all research staff completing assessment and intervention in the NICU were members of the clinical physical therapy staff of the NICU which was the primary reason the trial could continue to enroll as study coordinators were no longer allowed inside the NICU.
The purpose of this paper is to describe the impact of the pandemic on recruitment, retention, assessment and intervention completion of infants in a rehabilitation clinical trial during pre-pandemic, peak-pandemic, and late-pandemic periods. Because all post NICU intervention services were transitioned to telehealth for approximately 1 year, this study outlines various factors, including demographics, that influenced recruitment, retention, in-person and telehealth intervention and assessment completion.
METHODS
This analysis of prospectively collected data includes 63 infants enrolled in a clinical trial between February 6, 2019, and November 30, 2021. To identify the impact of the pandemic on recruitment and participation, the data were divided into the following time periods; pre-pandemic (February 2019 to March 12, 2020), peak-pandemic (March 13, 2020, to March 12, 2021) and late-pandemic (pre-omicron surge, March 12, 2021, to November 30, 2021) (Table 1). The following sections highlight the impact of each pandemic period sequentially on recruitment/retention, assessment, and intervention.
Table 1.
Participant Demographics and Enrollment
| Pandemic Time Periods | ||||
|---|---|---|---|---|
| Pre-Pandemic February 2019 to March 12 2020 (n= 29) | Peak-Pandemic March 13, 2020 to March 12, 2021 (n= 15) | Late-Pandemic March 13, 2021 to November 30, 2021 (n= 20) | ||
| Study Participant Demographics | Race White | 16/29 (55%) | 9/15 (60%) | 10/20 (50%) |
| Race Black | 12/29 (41%) | 3/15 (20%) | 5/20 (25%) | |
| Race (mixed race or did not report) | 1/29 (3%) | 3/15 (20%) | 5/20 (25%) | |
| Ethnicity (non-Hispanic) | 27/29 (93%) | 15/15 (100%) | 20/20 (100%) | |
| Insurance (Medicaid) | 21/29 (72%) | 5/15 (33%) | 10/20 (50%) | |
| Birth weight grams Means (SD) | 909.28 (251.33) | 886.25 (263.42) | 896.26 (126.59) | |
| Gestational age (weeks) | 26.07 (1.62) | 26.13 (1.26) | 26.42 (1.57) | |
| Length of stay (days) | 105.07 (35.14) | 104.81 (41.28) | 101.11 (34.44) | |
| Other children in the home | 17/29 (59%) | 5/16 (31%) | 8/18 (44%) | |
| Study Enrollment | Study enrollment rate | 29/61 (48%) | 15/58 (26%) | 16/41 (39%) |
| Study retention rate | 26/29 (90%) | 30/41 (73%) | 44/47 (94%) | |
| Cumulative enrollment (Usual care/Early/Late) | 29 (8/11/10) | 44 (15/16/13) | 63 (22/21/20) | |
| Number lost across all groups in study | 3 | 11 | 3 | |
| Withdrew entirely from study and loss to follow up | Withdrew prior to visit 3 (16 weeks) | 3 | 2 | 1 |
| Withdrew at or after visit 3 | 0 | 0 | 0 | |
| Lost to follow prior to visit 3 | 0 | 8 | 2 | |
| Withdrew or lost to follow up for subjects in intervention | SPEEDI Early | 3 | 3 | 0 |
| SPEEDI Late | 1 | 2 | 0 | |
Recruitment
Pre-pandemic, potential study participants were identified by screening the electronic medical record (EMR) using eligibility criteria. Each site’s Clinical Research Coordinator (CRC) contacted the site’s primary investigator to confirm eligibility, if needed, prior to contacting the infant’s parent. (Throughout this paper, “parent” is used to represent the infant’s primary caregiver(s) which could be the guardian(s) or the parent(s).) Infants who met inclusion criteria were introduced to the study via a flyer left at the infant’s bedside, followed by a phone call to discuss the study, and an in-person meeting between the CRC and parent in the NICU. During the in-person meeting, the study was fully explained, the informed consent form was reviewed and any questions by the parent were answered. Parents provided written informed consent prior to infant participation.
During peak- and late-pandemic periods, in-person meetings were limited. Following a rapid revision to the research protocol and necessary approvals, remote access to the EMR and phone or video conference was used for recruitment, consenting, and most parent communication. Research therapists assisted the CRC with placing flyers at the bedside and meeting with potential participants’ parents in-person if this was the parent’s preference. Obtaining consent was typically completed using a secure signature platform and a Zoom call; however, occasionally socially distanced in-person meetings were held to complete informed consent. Infants were considered to be enrolled in the study once the parent signed the consent form. Once an infant was enrolled in the study, the CRC remained a consistent point of contact with parents and used the parents’ preferred communication method of phone call, text messaging, and/or email to schedule visits, check in with the parent, or follow up with requests related to the study.
Study Retention
The research protocol described each infant engaged in the study for 2 years.13 As such, retention was an important factor to consider with the pandemic. The usual care group participated in assessment visits that are reflected in the data analysis of assessment visit completion rates and outcome measure completions rates. Assessment and intervention visits were categorized based on the pandemic period in which they occurred so the sample size is based on number of assessments, not the number of infants because infants could have intervention/assessment visits in multiple pandemic periods. Parents who were not retained for the study duration typically notified the team by phone of the need to withdraw or stopped responding to messages sent by the CRC and were lost to follow-up (Table 2). Our contact protocol included no more than 3 attempts with the parent’s preferred method, followed by 1 attempt using an alternative means such as a letter or email. After missing an assessment visit, parents were provided an opportunity to re-engage in the study for 2 sequential assessment visits. Thus, an infant was considered to be retained unless they withdrew or missed 2 consecutive assessment visits (Table 1).
Table 2.
Terms and Definitions
| Term | Definition |
|---|---|
| Study Enrollment Rate | Total number of infants whose parent consented to the infant’s participation in the study period divided by number of eligible infants in a time period |
| Cumulative Enrollment | Total enrollment during the specific period. (Usual care/SPEEDI- Early/SPEEDI-Late) |
| Withdrawal | Parent directly stating they no longer wanted the infant to participate in the study |
| Lost to Follow-up | Parent disengaged from the study despite multiple communication attempts with parent resulting in 2 consecutive missed assessment or intervention visits |
| Study Retention Rate | Total number of infants who had not withdrawn from the study or been lost to follow-up divided by number of infants enrolled. This could be calculated for the study as a whole or for each period by subtracting the number of infants lost to follow up or withdrew from previous time period from the denominator. |
| Missed Visit | Any visit described in the study protocol that was not completed. The reason for a missed visit was documented by study personnel if known. However, the rational was often multi-factorial during the pandemic periods. |
| Outcome Measure | This term is used to represent any individual tool used to determine if there was a change in the participants ability on that same tool over time, e.g., Test of Infant Motor Performance (TIMP) is an outcome measure. |
| Assessment Visit | This term is used to represent the visits with the participants in which multiple outcome measures are completed to evaluate change over time. |
| Assessment Visit Completion Rate | Total number of assessment visits completed during the study period divided by number of assessment visits scheduled based on the planned 5 visits per infant (baseline, visit 2, 3, 4, 5). Missed visits because of withdrawal or being temporarily lost to follow-up both result in reduced assessment completion rates. |
| Outcome Measure Completion Rate | This is the same as assessment visit completion rate, but applied at the level of the individual outcome measure. Since not all outcome measures could be completed at all planned visits, the denominator varied between outcome measures to reflect a lower denominator if the outcome measure could not be completed. Therefore, Outcome Measure Completion Rate was the total number of times a specific outcome measure was completed divided by the number of visits in which that measure was planned in the protocol. |
| Mode of Delivery | In-person, Telehealth, Hybrid |
| Missed Outcome Measure Rate | Total missed assessment measures divided by expected number of assessment measures. |
| Intervention Completion Rate | Total number of intervention visits completed during the study period divided by number of intervention visits scheduled. |
| Missed Intervention Visits Rate | Number of infants who missed one or more visits out of the visits scheduled in that time period. |
| Intervention Retention | Number of infants who did not drop out of the intervention either due to voluntary withdrawal or loss to follow-up. |
Assessment
Assessment visit time frames remained as planned per the protocol, with protocol deviations documented when individual outcome measures were not completed due to public health policy prohibiting non-essential in-person contact.13 Briefly, assessments were completed at 5 different time points: visit 1 (baseline) occurred between 35-42 weeks of gestation, visit 2 occurred 15 weeks post baseline, visit 3 occurred 30 weeks post baseline, visit 4 occurred at 12 months corrected age, and visit 5 occurred at 24 months chronological age. Parents completed a demographic survey at baseline and a description of community services at each assessment.13 The General Movement Assessment14 (GMA) and the Test of Infant Movement Performance15 (TIMP) were administered at visits 1 and 2 and the Bayley Scales of Infant and Toddler Development, 3rd edition16 (BSID-III), Gross Motor Function Measure-6617 item set version (GMFM-66-IS), Assessment of Problem-Solving in Play18 (APSP), and Hammersmith Infant Neurological Examination19 (HINE) were administered at visits 2 through 5. The video recording of a 5-minute free play session between the parent and infant was completed at visits 1 through 5.
During the pre-pandemic period, all outcome measures were completed in-person either in the NICU, research lab, clinical space, or parent home, at parent discretion. During the peak-pandemic period, outcome measures were administered either entirely via telehealth or using a hybrid model, depending on public health policy and research compliance officer. A visit, assessment or intervention, which was not completed at all or in which an outcome measure was not completed due to public health policy was reported as missed (Table 2). The hybrid model was introduced when pandemic restrictions allowed for time limited in-person visits. During hybrid visits, the TIMP, BSID-III, and HINE were completed in-person and a telehealth visit was used to complete remaining outcome measures. Prior to the telehealth assessment, the CRC collaborated with the parent to identify a preferred assessment time, determine appropriate space and supplies in the home with which to conduct the outcome measures and guide the parent through the Zoom platform, if needed. The CRC also mailed or delivered to the parent home, a box of fully sanitized manipulatives and a tripod for use during the telehealth assessment. On the day of the telehealth assessment, the CRC called the parent to answer any questions and remained present during the 60-90 minute Zoom-based telehealth visit. Outcome measures that could be completed via telehealth and were scored offline included the GMFM-66-IS, GMA, APSP, and the 5-minute free play session. The research therapist used an assessment guide developed by our team, including detailed scripting of instructions for the parent to position the child, objects, or camera, as needed, and to standardize the administration of outcome measures and video angles. After the telehealth visit, the CRC sent the parent a pre-paid shipping label to return the manipulatives/equipment or arranged a contactless pick-up from the parent’s home. Because there was no permitted in-person contact during the peak-pandemic period, outcome measures that could only be administered in-person (BSID-III, HINE and TIMP) were missed.
After 5 months of strict no contact visits, in-person contact was permitted with increasing duration until the entire visit could be in-person. Everyone except the infant wore a mask for the remaining peak-pandemic period. Visits 1, 2, and 3 were 3 months apart. If a visit or outcome measure was missed at any of those time points, it did not occur at a later time. However, if visit 4 or 5 was administered via telehealth, an in-person session was scheduled as soon as possible, to complete the BSID-III and HINE measures associated with the specific visit. In order to complete the in-person visit, parent and research therapist needed to have no signs/symptoms of COVID-19, a normal temperature and the parent needed to be comfortable with the team coming into their home, coming to the research lab, or bringing the infant to an outdoor location to administer the outcome measures. The time between the telehealth and in-person portion of the visit varied based on restrictions or parent availability.
During the late-pandemic period, the research board approved a return to all in-person research activities. Assessment visit location was based on parent comfort. Everyone except the infant continued wearing a mask during in-person assessment visits regardless of the location. If the parent desired social distancing, a hybrid model was used for assessment similarly to during the peak-pandemic period. If the parent did not want in-person contact, a telehealth assessment was used to administer the outcome measures and those that could only be administered in-person were missed.
Intervention
All infants participating in the study received standard care in the NICU and community. Following baseline assessment, the infants were randomized into 1 of 3 groups: usual care, SPEEDI-Early, or SPEEDI-Late.13 Infants in both SPEEDI intervention groups received 10 intervention visits plus 3 months of daily parent-provided intervention, delivered in 2 phases. Phase 1, included 5 visits over 3 weeks, focused on parent’s learning of their infant’s behavioral cues and interaction using a guided participation approach. Traditionally, and pre-pandemic, during Phase 1, the parents were provided with an iPad containing video demonstrations of SPEEDI principles and strategies. They watched each video with the interventionist and had unlimited access to them during this phase. The parent and intervention therapist would practice the intervention strategies together with infant and discuss the intervention principles using the videos. In Phase 2, parents provided 20 minutes per day of activities based using the strategies learned in Phase 1. They were also provided with an activity booklet with 3 stages of difficulty per activity. In both intervention groups, parents and interventionist worked together to identify the activity stage for which the infant was ready. For infants in the SPEEDI-Early group, Phase 1 began immediately after the baseline assessment, with the goal of completing this phase in the NICU and Phase 2 was completed at home during weeks 3-15 post baseline. For infants in the SPEEDI-Late group, all intervention visits were in the home. Phase 1 started at 15 weeks post-baseline after the second assessment visit and was followed by Phase 2 until 30 weeks post-baseline.13
During the pre-pandemic period, infants received all 10 intervention sessions (NICU or home) in-person regardless of the Phase. During the peak-pandemic period, intervention in the NICU was in-person and all home visits were conducted via telehealth. In addition to training interventionists in telehealth delivery, the study team created a secure website for use in 2 ways during the intervention. First, the videos used during Phase 1 were accessible on any web-enabled device, viewed together by the interventionist and parent over Zoom and available to view between sessions similar to that in the pre-pandemic period. The activity booklet was still provided in paper format in the NICU or mailed to those in the SPEEDI-Late group. Second, the website provided a demonstration of each activity and each stage with text and a narrative of what was being demonstrated during the video. While every attempt was made to schedule telehealth intervention visits at a convenient time for the parent, interventionists needed to be prepared to adapt in real time, while still meeting high study fidelity standards for adhering to the intervention key principles. During all telehealth intervention visits, the interventionist and parent determined the best delivery approach based on the real time internet connection speed, parent’s and infant’s needs, and stage of intervention. Ideally parents were able to demonstrate the activities they were working on with their infant over Zoom followed by a collaborative approach to trying new activities. Watching videos from the website together with the parent or referring the parent to a specific video helped them fully understand the concepts when the verbal description was not adequate. In the late-pandemic period, all sites were approved a return to all in-person intervention visits which were scheduled based on parent’s and staff’s comfort. Also, using parent preference for intervention delivery, a blend of delivery styles (all in-person, hybrid, and only telehealth) continued throughout the late-pandemic period for some infants.
Survey of Parents
The research team developed a survey to obtain parents’ perspectives on the telehealth and hybrid delivery styles based on modifications of the one used previously13. The purpose was to obtain from each group parent self-reported/perceived input about barriers and facilitators to their participation in research and to collect self-reported family characteristics in 2 pandemic periods, peak and late. The survey was electronically distributed to parents. 6teen parents, 5 in the peak-pandemic period and eleven in the late pandemic period, returned responses.
Analysis
Descriptive statistics were used to present a set of well-defined metrics including the frequency and percentages for the following parameters: i) study enrollment rate; ii) study retention rate; iii) assessment visit completion rate; iv) outcome measure completion rate; v) missed outcome measure rate; vi) intervention completion rate; vii) missed visits; and viii) intervention retention (Table 1). For both assessment and intervention, data are reported for the entire sample. However, the data are presented by pandemic period (Supplemental Digital Content 1), outcome measure (Supplemental Digital Content 2), and intervention group (Supplemental Digital Content 3).
RESULTS
The enrolled sample at the time of this analysis included 63 infants whose gestational age, birth weight, and length of stay in the NICU were all nearly equal across pandemic periods (Table 1). Data were collected at time of enrollment or NICU discharge (Table 2).
As a trend, the study enrollment rate was highest in the pre-pandemic period (48%), decreased in the peak-pandemic period (26%) and in the late-pandemic period started to increase (39%) towards pre-pandemic rates (Figure 1). A larger number of participants withdrew or were lost to follow up in the peak-pandemic period (10) than in the other 2 pandemic periods (Table 1, Figure 1).
Figure 1.
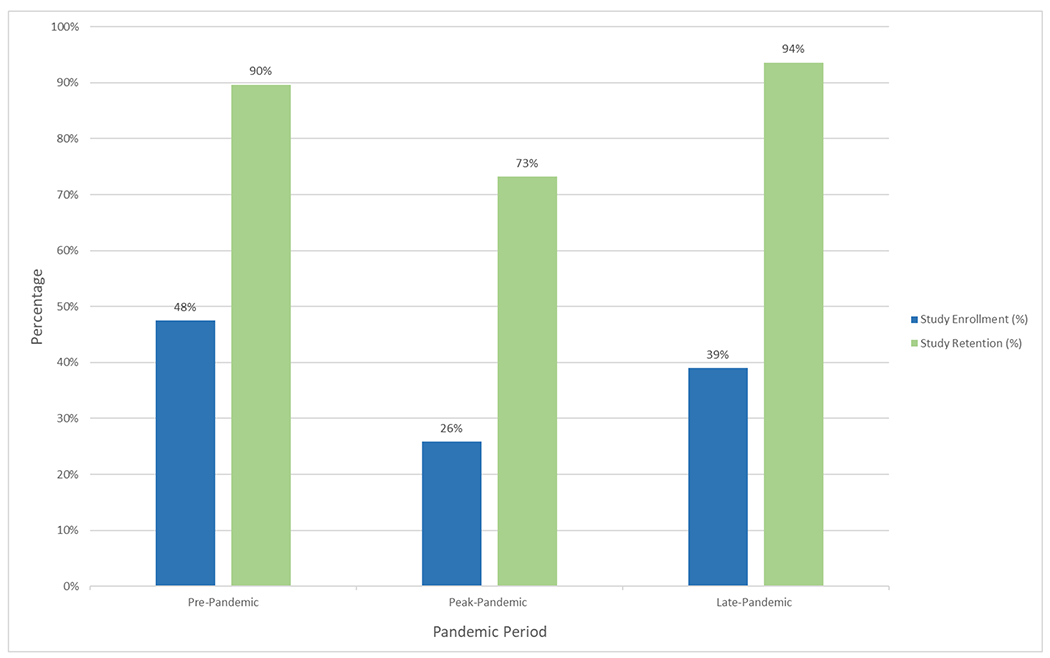
Study Enrollment and Retention Rates
Participant enrollment and retention rates across the 3 pandemic time periods.
Demographic characteristics did not appear to alter outcomes, however, changes over the study periods did influence the data. The study sample included over 30 percent of participants whose parents identified the infant’s race as Black. While there was a steady rate of participation of White infants across all time points of the pandemic (50-60% enrolled), the rate of enrollment of Black infants declined from the pre-pandemic period (41% enrolled), to peak-pandemic period (20% enrolled) and started to increase during the late-pandemic period (Table 1).
The sample was primarily non-Hispanic, limiting our ability to consider the impact of ethnicity. This may be related to the inclusion criteria of the study requiring parents to read and speak the English language. The highest proportion of parents had insurance during the pre-pandemic period which decreased during the peak-pandemic period (33%) and in the late-pandemic period (50%) this rate started to rise but did not reach pre-pandemic (72%) rates (Table 1). More parents living with children (siblings or other family members) in addition to the research participant consented to participate in the study in the pre-pandemic period compared to in the peak-pandemic (59%) or late-pandemic (31%) periods (Table 1).
Assessment completion
Nearly equal assessment visits were planned and completed during the 3 pandemic periods (Supplemental Digital Content 1). During the pre-pandemic period, 56 assessment visits were scheduled and 100% were completed in-person. During the peak-pandemic period, a little over half of the scheduled assessment visits were completed in-person (59%) and just under half were completed using telehealth (41%). During the late-pandemic period, nearly all of the scheduled assessment visits were completed in-person (92%) with only 2 completed using a hybrid model.
Assessment visits were further segregated by outcome measure type for each pandemic period (Figure 2, Supplemental Digital Content 2). Generally, those outcome measures that could be completed either in-person or via telehealth (GMA, APSP, and GMFM-66-IS) were completed using telehealth to limit contact time in hybrid visits. Because these outcome measures could be completed in either format their completion rates were consistent across time periods. Outcome measure completion rates for instruments that could be administered only via in-person delivery style (TIMP, BSID-III and HINE) declined in the peak-pandemic period and were higher before and after this time.
Figure 2.
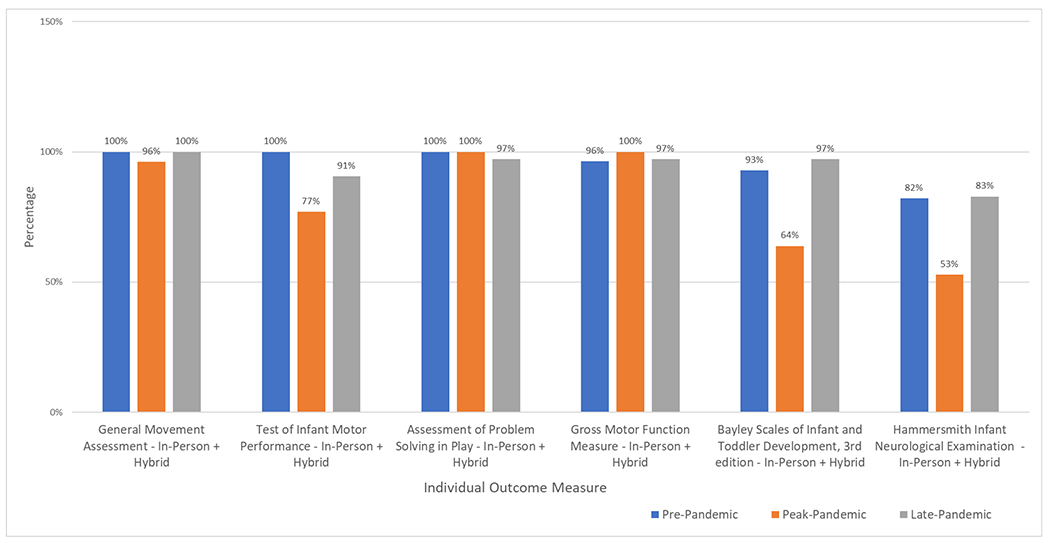
Comparison of Outcome Measure Completion Rates
Graphical representation of the individual outcome measure completion rates across the 3 pandemic periods.
Completion rates for GMA, TIMP and APSP were 100% of scheduled visits (Figure 2). The completion rates for the GMFM-66-IS, BSID-III and HINE were high and similar in comparison to one another in the pre- and late pandemic periods (Supplemental Digital Content 2). One GMFM-66-IS outcome measure was not completed because of a scheduling conflict on a second attempt after the participant was unable to participate during an in-person visit because of fatigue. A combined total of 7 BSID-III and HINE outcome measures were not completed due to subject fatigue (Supplemental Digital Content 2).
In the peak-pandemic period, one GMA was missed because of a technical upload issue, 5 TIMP outcome measures were missed at visit 2, 13 BSID-III and 17 HINE outcome measures were missed because in-person contact was prohibited. However, there were no missed outcome measures for APSP or GMFM-66-IS as both were completed during an in-person or telehealth visit. Therefore, a total of 36 outcome measures, which required in-person administration, were missed because in-person contact was prohibited at the visit time during this peak-pandemic period (Supplemental Digital Content 2).
In the late-pandemic period at a visit 2, 3 TIMP measures were missed because parents declined an in-person visit. Also missed in this same period, were one GMFM-66-IS and one BSID-III outcome measure and 6 HINE outcome measures either due to participant fatigue or scheduling issues. There were no missed outcome measures for the APSP (Supplemental Digital Content 2).
Intervention completion
Of the 63 extremely to very preterm infants enrolled, 25 were randomized to usual care, 22 to SPEEDI-Early and 20 to SPEEDI-Late. Of these 42 infants, 7 were actively receiving intervention at the time of this analysis and were excluded. Nine infants in intervention groups withdrew from the study, resulting in a total of 26 infants remaining for intervention analysis. Most infants (85%) completed all (100%) visits. Completion rates segregated based on intervention group and phase, pandemic period, and mode of delivery are described as follows:
SPEEDI-Early group (Supplemental Digital Content 3)
A total of 140 visits were scheduled for the infants in the SPEEDI-Early group across all pandemic periods. Of these 140 visits, nearly all were successfully completed. During the pre-pandemic period, 6 infants participated in the SPEEDI-Early group. 5 of the 6 infants were exclusively pre-pandemic participants while one infant completed the first 6 visits during the pre-pandemic period and the last 4 visits during the peak-pandemic period. Thus, a total of 56 visits (30 visits in Phase 1 and 26 visits in Phase 2) were scheduled to occur during the pre-pandemic period and all were completed in-person in either the NICU or home.
During the peak-pandemic period, 5 infants participated in the SPEEDI-Early group. Additionally, 1 infant completed the last 4 visits in the late-pandemic period. Thus, a total of 40 visits (20 visits in Phase 1, 20 visits in Phase 2) were scheduled in the peak-pandemic period and nearly all visits were completed. Half of the visits were completed in-person in the NICU. The remaining visits were completed via telehealth.
During the late-pandemic period, 5 infants participated in the SPEEDI-Early group. Of these 5, one infant completed the first 6 visits during the peak-pandemic period and the last 4 visits during the late-pandemic period. A total of 44 visits (20 visits in Phase 1, 24 visits in Phase 2) were scheduled during the late-pandemic period and nearly all were completed. Of these 42 visits, almost all visits were completed in-person; 6 were NICU visits and 35 were home visits. Only 1 visit was conducted via telehealth.
SPEEDI-Late group (Supplemental Digital Content 3)
A total of 120 visits were scheduled for the infants in the SPEEDI-Late group across pandemic periods. Of these 120 visits, all but 1 were successfully completed. During the pre-pandemic period, 6 infants participated in the SPEEDI-Late group. One infant completed the last 3 visits in Phase 2 in the peak-pandemic period. Thus, a total of 57 visits (30 visits in Phase 1 and 27 visits in Phase 2) were scheduled to occur during the pre-pandemic and almost all were completed. All visits during the pre-pandemic period were completed in-person at home. During the peak-pandemic period, 3 infants participated in the SPEEDI-Late group. Of these 3, 2 infants were exclusive peak-pandemic period participants, e.g., 1 infant completed the first 7 intervention visits during the pre-pandemic period and the last 3 visits were scheduled during the peak pandemic period. Thus, a total of 23 visits (10 visits in Phase 1, 13 visits in Phase 2) were scheduled during the peak-pandemic period and all visits were completed via telehealth. During the late-pandemic period, 4 infants participated in the SPEEDI-Late group. A total of 40 visits (20 visits in Phase 1, 20 visits in Phase 2) were scheduled and all were completed. Nearly all of those visits were completed in-person at home and only a few visits were conducted via telehealth.
Four infants (3 in SPEEDI-Early and 1 in SPEEDI-Late) missed 1 or more visits. For the SPEEDI-Early group, one participant missed a single visit because that visit coincided with the initiation of the peak-pandemic related lockdown. Two SPEEDI-Early participants missed 1 visit each during the late pandemic period due to their medical appointments running longer than expected and an inability to reschedule. For the SPEEDI-Late group, only 1 participant missed a single visit during the pre-pandemic period due to a non-study related leg injury.
As a general trend, intervention completion rates in both groups were minimally affected by the pandemic (Figure 3). In Phase 1 of the SPEEDI-Early group, in-person intervention remained constant. However, in Phase 2 in the peak-pandemic period, intervention flipped delivery styles and was delivered exclusively through telehealth and then returned to near pre-pandemic in-person rates in the late-pandemic period (Figure 3).
Figure 3.
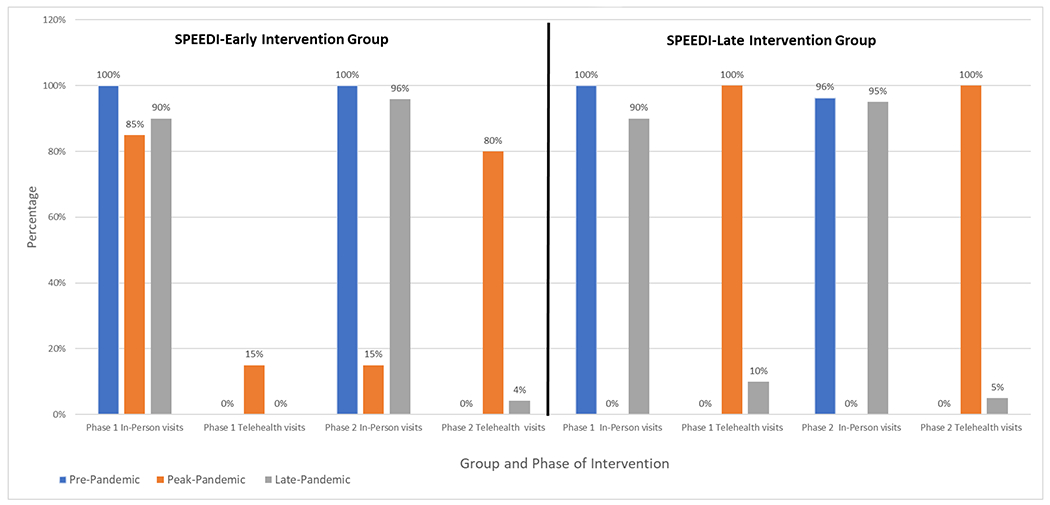
Intervention Completion Rates by Delivery Method
Graphical representation of the comparison of intervention completion rates by delivery methods across the 3 pandemic time periods.
In the SPEEDI-Late group, both phases had high intervention completion rates using in-person delivery style in pre-pandemic and late-pandemic periods. The delivery style of intervention during peak-pandemic switched to all telehealth and completion rates remained high (Figure 3).
Parent Survey Results (Supplemental Digital Content 4)
During peak-pandemic, 5 of 41 parents responded to the first survey in the peak-pandemic period. Of those, all parents reported being employed, several reported having an internet capable device, a few reported having siblings at home as well as reported having the COVID-19 illness, and one reported inconsistent internet connection issues. No parent reported a COVID-19 diagnosis impacted their participation in research. Of the 11 parents out of 47 who responded to the second survey in the late-pandemic period, many reported being employed, some reported presence of siblings, a few indicated they had an internet capable device and a few reported exposures to or having the COVID-19 illness. Two parents reported that the illness caused by COVID-19 impacted participation in research visits and 1 reported an inconsistent internet connection.
DISCUSSION
The results of this study demonstrate the clear impact of the pandemic on this clinical trial. It also illustrates the creativity of researchers, flexibility of parents, and ability to continue performing a rigorous high-risk infant study during a pandemic. Solutions used to preserve the integrity of the clinical trial were communication engagement with parents to keep them involved in the study, adapting data collection methods when in-person visits were not feasible and using outcome measures that could be adapted to a telehealth format.20 In a study of intervention for children with autism, researchers also successfully adapted their protocol when they changed to a telehealth delivery style.9
Demographically, those parents who self-reported their infant as Black, had the lowest participation rate in the peak-pandemic period. The pandemic disproportionately impacted the minority groups, with highest prevalence reported in Black and Latinx individuals.21 While high dropout rates occurred during the peak-pandemic period, successful continuation of the study into the late-pandemic period was demonstrated with a rebound in enrollment and maintenance of a high rate of assessment and intervention visit completion and participant retention. The elements for successfully continuing and reducing the impact of the pandemic on this rigorous clinical trial are related to the characteristics displayed by the parents and the study team during the peak-pandemic time period.
Parents faced multiple barriers to participation in a clinical trial during the pandemic period including: concerns about contracting the COVID-19 illness, reduced personal connection and in-person collaboration with the CRC due to NICU visitation policy restrictions, primary use of electronic remote communication methods, lack of internet reliability for telehealth visits, an additional burden of being guided through electronic submission of consent forms, administering assessment outcome measure items and intervention activities during telehealth visits and travelling to the clinical research site for in-person assessment visits while simultaneously balancing their household responsibilities of managing other siblings and employment. At least 1 qualitative study published during the pandemic reports that the presence of siblings at home, for online schooling and otherwise, impacted parents’ participation in therapy sessions due to time, energy, and internet speed limitations.12 The increased cognitive demands of setting up the telehealth session and battling “Zoom fatigue” may have overwhelmed parents or therapists leading to drop-outs.22 Despite these barriers, parents remained willing to engage with the CRC and study staff to complete most of the requirements. This may be because of the high value they placed on the study conducted with preterm infants. These dedicated parents are applauded for their ability to remain committed to a clinical trial and manage their other responsibilities during a time of extreme stress. Without the resiliency of the parents, the continuation of the study would have stagnated.
During the peak-pandemic period, the study team overcame barriers, including the inability to administer the outcome measures or intervention in-person, and reduced personal connection with parents. While ultimately successful, these strategies required substantial time, energy and financial resources to quickly and efficiently create and implement a time-sensitive transition to telehealth delivery for study recruitment/retention, assessment and intervention while maintaining high standards of reliability and fidelity. Some successful strategies that could be helpful in others’ research include 1) hiring clinical staff for inpatient outcome assessment and intervention whom can be cross trained to help with enrollment and consenting if the CRC is not present in the NICU, 2) using a multi-format approach to share information about the study with parents; start with a study brochure placed at infant bedside, follow up with Zoom or phone communication with a parent, and establish a parent’s preferred method of follow-up contact, 3) remaining available for and flexible with scheduling, 4) preparing and guiding parents through study visits including the use of homemade tripods, varied camera angles, and item administration and online platform administration, and 5) being prepared to train staff to implement an intervention with high fidelity in an alternative format. In this study, staff were flexible and quickly created guides, participated in intensive training for administering telehealth assessment visits and developed a web-based platform with study principles and activities for telehealth intervention sessions to standardize the delivery of either assessment or intervention visits. Recently published studies on the adoption of telehealth by pediatric physical therapists during the pandemic identify adequate training for tele-intervention administration as a facilitator for intervention success.22 These studies also highlight that currently only 14.6% of therapists report receiving formal training in telehealth services.22 Latest evidence suggests that early intervention models incorporating active parent engagement are more successful in telehealth intervention across the United States.22 In this study, parents were active participants which is similar to that of another recent intervention study conducted with older children.9 Other factors that supported the successful continuation of the study were the resilience and adaptability of the research staff who were able to adjust to changing conditions of access to both the infants and their parents.
Limitations:
The biggest limitation of the current study is the lack of a priori planning of sample sizes and analysis planning. However, this was impossible given the nature of the pandemic’s changing conditions. Each period was not the same length. The late-pandemic period was interrupted by the Omicron surge which we anticipated would alter that period’s outcomes. Therefore, the late-pandemic period was shortened. Statistical analysis rather than purely descriptive statistics could have been conducted. However, given the nature of the pandemic’s continuous evolution and the somewhat arbitrary dates used to describe the time periods, we decided not to use statistical methods designed for data analysis in prospective studies.
CONCLUSIONS
The dramatic impact the pandemic has had on the healthcare and education systems also impacted the research on which evidence-based practice decisions will be made for years to come. Researchers will need to fully disclose the implications of the pandemic for their research to avoid over-interpretation of study results. Likewise, journals, editors, and reviewers will need to consider publishing studies with less than ideal data to ensure that the pandemic does not stall the progress in the field of pediatric rehabilitation. Clinicians should be careful consumers of the literature and interpret results considering any pandemic related missing data.
Supplementary Material
Supplemental Digital Content 1. Assessment Visit Completion Rates and Delivery Method
This contains the information of the assessment completion rates and delivery method. pdf
Supplemental Digital Content 2. Assessment Completion Rate by Outcome Measure during Pandemic Periods
This table contains information on specific outcome measure completion rates across 3 pandemic time periods using in-person or hybrid delivery methods. Also included are the missed outcome measure rates for each outcome measure. Abbreviations include: GMA: General Movement Assessment; TIMP: Test of Infant Motor Performance; APSP: Assessment of Problem-Solving in Play; BSID-III: Bayley Scales of Infant and Toddler Development, 3rd Edition; HINE: Hammersmith Infant Neurological Examination. pdf
Supplemental Digital Content 3. Intervention Completion Rates for both SPEEDI-Early and SPEEDI-Late Groups
This table contains intervention completions rates categorized by In-person or telehealth delivery method for phase 1 and phase 2 of each intervention group over the 3 pandemic periods (pre-, peak-, and late-pandemic). pdf
Supplemental Digital Content 4. Parent Survey Results
This table contains parent results from the survey sent to them during the peak and late pandemic periods to determine impact of pandemic on parents. COVID: Coronavirus Disease 2019. Pdf
Grant Support
This study was supported by funding from the National Institutes of Health – PI Dusing, SC, NICHD RO1 HD093624, NCT02153736, UL1TR002649
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Shaaron E. Brown, Pediatric Physical Therapist, Department of Physical Therapy, Virginia Commonwealth University, PhD Student, Rehabilitation and Movement Science Program, Department of Physical Therapy, Richmond, Virginia, USA.
Jodi Darring, Clinical Research Coordinator, Developmental Pediatrics, University of Virginia Children’s, Charlottesville, Virginia, USA.
Meagan Miller, Clinical Research Coordinator, Department of Physical Therapy Virginia Commonwealth University, Richmond, VA, USA.
Ketaki Inamdar, Rehabilitation and Movement Science Program, Department of Physical Therapy, Virginia Commonwealth University, Richmond, Virginia, USA.
Arya Salgaonkar, Division of Biokinesiology and Physical Therapy, University of Southern California. Los Angeles, California, USA.
Jennifer C. Burnsed, Associate Professor of Pediatrics and Neurology, Division of Neonatology, University of Virginia, Charlottesville, Virginia, USA.
Richard D. Stevenson, Professor of Pediatrics, Division Head, Neuro-Developmental and Behavioral Pediatrics, University of Virginia School of Medicine, Charlottesville, Virginia, USA.
Mary S. Shall, Professor Emeritus, Department of Physical Therapy, Virginia Commonwealth University, Richmond, Virginia, USA.
Amy D. Harper, Associate Professor of Neurology, Department of Neurology, Virginia Commonwealth University, Richmond, Virginia, USA.
Karen D. Hendricks-Munoz, Interim Chair Department of Pediatrics, Interim Physician in Chief Children’s Hospital of Richmond, William Tate Graham Professor Chair, Division of Neonatal Medicine Department of Pediatrics, Children’s Hospital of Richmond of VCU, Virginia Commonwealth University School of Medicine, Interim Executive Director VCU Center on Health Disparities, Deputy Director VCU Center on Health Disparities, Virginia Commonwealth University, Richmond, Virginia, USA.
Leroy R. Thacker, School of Medicine, Dept of Biostatistics, Virginia Commonwealth University, Richmond, Virginia, USA.
Meg Hyde, Physical Therapist – Pediatric Acute Care, University of Virginia Children’s Hospital, Charlottesville, Virginia, USA.
Stacey C. Dusing, Sykes Family Chair of Pediatric Physical Therapy, Health and Development. Director of the Motor Development Lab. Division of Biokinesiology and Physical Therapy, University of Southern California, Los Angeles, California, USA.
REFERENCES
- 1.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 Infection: The INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8). doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Heal. 2021;9(6). doi: 10.1016/S2214-109X(21)00079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vance AJ, Malin KJ, Miller J, Shuman CJ, Moore TA, Benjamin A. Parents’ pandemic NICU experience in the United States: a qualitative study. BMC Pediatr. 2021;21(1):558. doi: 10.1186/s12887-021-03028-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey EM, McNeer E, McDonald MF, et al. Association of preterm Birth rate with COVID-19 statewide Stay-at-Home orders in Tennessee. JAMA Pediatr. 2021;175(6). doi: 10.1001/jamapediatrics.2020.6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dragoo KE. The individuals with disabilities education act (IDEA), part C: Early intervention for infants and toddlers with disabilities (updated). In: Key Congressional Reports for August 2019: Part III. ; 2020.
- 6.Galeano SPO, Maya ÁMS. Experiences of parents of preterm children hospitalized regarding restrictions to interact with their children imposed because of the COVID-19 pandemic. Investig y Educ en Enferm. 2021;39(2). doi: 10.17533/udea.iee.v39n2e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National center on birth defects and developmental disabilities C for DC and P. Cerebal palsy treatment services (IDEA). Published 2020. Accessed January 23, 2022. https://www.cdc.gov/ncbddd/cp/treatment.html
- 8.Roberts MY, Thornhill L, Lee J, et al. The impact of COVID-19 on Illinois early intervention services. Am J speech-language Pathol. 2022;31(2):974–981. doi: 10.1044/2021_AJSLP-21-00112 [DOI] [PubMed] [Google Scholar]
- 9.Cleffi C, Su W-C, Srinivasan S, Bhat A. Using telehealth to conduct family-entered, movement intervention research in children with autism spectrum disorder during the COVID-19 pandemic. Pediatr Phys Ther. 2022;34(2):246–251. doi: 10.1097/PEP.0000000000000872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maitre NL, Benninger KL, Neel ML, et al. Standardized neurodevelopmental surveillance of high-risk infants using telehealth: implementation study during COVID-19. Pediatr Qual Saf. 2021;6(4). doi: 10.1097/pq9.0000000000000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sholas MG. The actual and potential impact of the novel 2019 coronavirus on pediatric rehabilitation: A commentary and review of its effects and potential disparate influence on Black, Latinx and Native American marginalized populations in the United States. J Pediatr Rehabil Med. 2020;13(3). doi: 10.3233/PRM-200722 [DOI] [PubMed] [Google Scholar]
- 12.Hall JB, Woods ML, Luechtefeld JT. Pediatric physical therapy telehealth and COVID-19: Factors, facilitators, and barriers influencing effectiveness - A Survey Study. Pediatr Phys Ther. 2021;33(3). doi: 10.1097/PEP.0000000000000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dusing S, Burnsed J, Brown S, et al. Efficacy of Supporting Play Exploration and Early Development Intervention (SPEEDI) in the first months of life for infants born very preterm: 3-Arm randomized clinical trial protocol. Phys Ther. Published online 2020. doi: 10.1093/ptj/pzaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Einspieler C, Prechtl HFR. Prechtl’s Method on the Qualitative Assessment of General Movements in Preterm, Term, and Young Infants. Mac Keith Press; 2004. [DOI] [PubMed] [Google Scholar]
- 15.Campbell SK, Hedeker D. Validity of the Test of Infant Motor Performance for discriminating among infants with varying risk for poor motor outcome. J Pediatr. 2001;139(4):546–551. doi: 10.1067/mpd.2001.117581 [DOI] [PubMed] [Google Scholar]
- 16.Albers CA, Grieve AJ. Test Review: Bayley N. (2006). Bayley Scales of Infant and Toddler Development–Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoeduc Assess. 2007;25(2):180–190. doi: 10.1177/0734282906297199 [DOI] [Google Scholar]
- 17.Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ. Improved scaling of the Gross Motor Function Measure for Children with cerebral palsy: Evidence of reliability and validity. Phys Ther. 2000;80(9):873–885. doi: 10.1093/ptj/80.9.873 [DOI] [PubMed] [Google Scholar]
- 18.Molinini RM, Koziol NA, Tripathi T, et al. Measuring early problem-solving in young children with motor delays: A validation study. Phys Occup Ther Pediatr. 2021;41(4):390–409. doi: 10.1080/01942638.2020.1865501 [DOI] [PubMed] [Google Scholar]
- 19.Romeo DM, Ricci D, Brogna C, Mercuri E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol. 2016;58(3):240–245. doi: 10.1111/dmcn.12876 [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Newman AB. Preserving clinical trial integrity during the coronavirus pandemic. JAMA. 2020;323(21):2135–2136. doi: 10.1001/jama.2020.4689 [DOI] [PubMed] [Google Scholar]
- 21.Magesh S, John D, Li WT, et al. Disparities in COVID-19 Outcomes by race, ethnicity, and socioeconomic status: A systematic-review and meta-analysis. JAMA Netw Open. 2021;4(11). doi: 10.1001/jamane2rkopen.2021.34147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall JB, Luechtefeld JT, Woods ML. Adoption of telehealth by pediatric physical therapists during COVID-19: A survey study. Pediatr Phys Ther. 2021;33(4). doi: 10.1097/PEP.0000000000000817 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Assessment Visit Completion Rates and Delivery Method
This contains the information of the assessment completion rates and delivery method. pdf
Supplemental Digital Content 2. Assessment Completion Rate by Outcome Measure during Pandemic Periods
This table contains information on specific outcome measure completion rates across 3 pandemic time periods using in-person or hybrid delivery methods. Also included are the missed outcome measure rates for each outcome measure. Abbreviations include: GMA: General Movement Assessment; TIMP: Test of Infant Motor Performance; APSP: Assessment of Problem-Solving in Play; BSID-III: Bayley Scales of Infant and Toddler Development, 3rd Edition; HINE: Hammersmith Infant Neurological Examination. pdf
Supplemental Digital Content 3. Intervention Completion Rates for both SPEEDI-Early and SPEEDI-Late Groups
This table contains intervention completions rates categorized by In-person or telehealth delivery method for phase 1 and phase 2 of each intervention group over the 3 pandemic periods (pre-, peak-, and late-pandemic). pdf
Supplemental Digital Content 4. Parent Survey Results
This table contains parent results from the survey sent to them during the peak and late pandemic periods to determine impact of pandemic on parents. COVID: Coronavirus Disease 2019. Pdf


