Abstract
Lipids are essential components of brain structure and shown to affect brain function. Previous studies have shown that aging men undergo greater brain atrophy than women, but whether the associations between lipids and brain atrophy differ by sex is unclear. We examined sex differences in the associations between circulating lipids by liquid chromatography-tandem mass spectrometry and the progression of MRI-derived brain atrophy index SPARE-BA over an average of 4.7 (SD=2.3) years in 214 men and 261 women aged 60 or older who were initially cognitively normal using multivariable linear regression, adjusted for age, race, education, and baseline SPARE-BA. We found significant sex interactions for beta-oxidation rate, short-chain acylcarnitines, long-chain ceramides, and very long-chain triglycerides. Lower beta-oxidation rate and short-chain acylcarnitines in women and higher long-chain ceramides and very long-chain triglycerides in men were associated with faster increases in SPARE-BA (accelerated brain aging). Circulating lipid profiles of accelerated brain aging are sex-specific and vary by lipid classes and structure. Mechanisms underlying these sex-specific lipid profiles of brain aging warrant further investigation.
Keywords: lipids, metabolomics, brain aging, plasma, sex difference
1. Introduction
Lipids are a group of complex compounds with diverse functions in cellular physiology and pathophysiology. The biological functions of lipids are highly variable depending on their structure, length of the carbon chain, chemical modifications such as oxidation, and other factors (Kihara 2012, Schonfeld and Wojtczak 2016, Wattenberg 2018). Lipids are building blocks for several important structures in the brain, such as cell membranes and myelin sheaths, and may affect brain function through neuronal signaling and trafficking of microvesicles. Understanding molecular mechanisms underlying the role of lipids in the central nervous system (CNS) is an area of intense investigation (Montesinos et al. 2020, Poljak et al. 2020, Ooi et al. 2021), with some data suggesting that short-chain fatty acids, mainly produced by the microbiota, affect the gut-brain axis and brain function, and long-chain fatty acids, the main components of cell membranes and metabolic intermediates, serve as an energy source for the brain and other organs. Recent data have suggested that very long-chain fatty acids may induce neuronal damage through mitochondrial dysfunction and lead to neurodegeneration (Zarrouk et al. 2012). Mitochondrial ATP synthase and cardiolipin have been hypothesized as key lipoxidative targets in neurodegeneration (Jove et al. 2019).
In the CNS, some fatty acids are synthesized de novo, while essential fatty acids are derived from circulation. Existing evidence supports the notion that fatty acids can cross the blood-brain barrier to enter the CNS, although the mechanism of transport remains unclear. Several observational studies have examined the relationship between circulating lipids and brain structure in aging, but the findings are somewhat mixed and mostly from cross-sectional studies. For instance, some studies found higher omega-3 polyunsaturated fatty acids assessed in plasma or dietary intake were associated with preserved brain structure in late life, but others did not observe a relationship (Macaron et al. 2021, Tokuda et al. 2022). Some studies examined associations of clinical lipid markers, such as low-density and high-density lipoprotein cholesterol, with brain structure, but there is a lack of understanding of lipid profiles that can positively or negatively affect brain aging (Moazzami et al. 2020). In studying the relationships of lipid profiles with brain aging, it is important to examine sex differences. In fact, some longitudinal data have shown that men had greater brain volume loss during aging compared to women (McCarrey et al. 2016, Armstrong et al. 2019). A deeper understanding of sex differences in comprehensive lipid profiles of brain aging will provide insights into the mechanisms of sex differences in brain aging.
In this study, we aimed to examine sex differences in the associations of circulating lipid profiles with changes in brain aging patterns over time in a sample of well-characterized community-dwelling older adults. We hypothesize that there would be sex differences in the associations of lipid profiles with the progression of brain aging. We also hypothesize that sex differences would vary based on the length of the carbon chain.
2. Methods
2.1. Study Population
Participants were drawn from the Baltimore Longitudinal Study of Aging (BLSA), an ongoing population study that began in 1958. We identified 214 men and 261 women aged 60 or older who were initially cognitively normal and had baseline plasma metabolomics data by Biocrates p500 and repeated measures of a brain aging index based on brain MRI over an average of 4.7 (SD=2.3) years of follow-up. During the follow-up, 25 men and 12 women developed mild cognitive impairment or dementia. In this study, data were collected between June 30, 2008, and March 9, 2020. Information on sex (i.e., men, women) was collected by self-report from the participants using a questionnaire at an in-person interview during the BLSA visit. Participants answered this question orally and the answer was recorded by the staff.
The BLSA protocol was approved by the Institutional Review Board of the National Institutes of Health. All participants provided written informed consent at each BLSA visit.
2.2. Lipids
Blood was collected at the National Institute on Aging Clinical Research Unit, Medstar Harbor Hospital in Baltimore, MD, following a standardized protocol (Yamaguchi et al. 2021). The collection of EDTA plasma in the BLSA is consistent with guidelines for biomarker studies (Tuck et al. 2009). Before sample collection, participants were not allowed to smoke, exercise, or take medications. Blood samples were drawn from the antecubital vein between 07:00 and 08:00 AM after an overnight fast, then stored immediately at 4 °C, centrifuged within 4 hours, and immediately aliquoted and frozen at −80 °C.
Plasma lipid metabolites were measured using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Metabolites were extracted and concentrations were measured using the MxP Quant 500 kit (Biocrates Life Sciences, AG, Innsbruck, Austria) following the manufacturer’s protocol for a 5500 QTAP (Sciex, Framingham, MA, USA) (Yamaguchi et al. 2021).
Because we are interested in lipid structure, we grouped lipid metabolites based on biochemical nature of the molecules, especially chain length (Ng et al. 2021), for six lipid classes (i.e., acylcarnitines, ceramides, glycoceramides, glycolipids and cholesterol esters, sphingomyelins, and triglycerides) (Supplementary Table 1). Using specific short-chain acylcarnitines, we also computed an index of the beta-oxidation rate, which was a catabolic process in which fatty acids are degraded into prokaryotes in the cytosol and acetyl-CoA in the mitochondria which further enters the citric acid cycle. The beta-oxidation rate was calculated as the ratio of the sum of acetylcarnitine (C2) and propionyl carnitine (C3) by carnitine (C0) (Bartlett and Eaton 2004, Houten and Wanders 2010, Ottas et al. 2017). Short-chain lipids were defined as having less than 6 carbons, medium-chain between 6 and 10 carbons, long-chain between 14 and 20 carbons, and very long-chain 22 or more carbons.
2.3. Brain aging index
The brain aging index was derived from brain MRI and was estimated using supervised machine learning models. An index score was computed for each participant, namely the Spatial Patterns of Atrophy for Recognition of Brain Aging (SPARE-BA) score (Habes et al. 2021). Imaging features involved in SPARE-BA calculation included volumes of 145 anatomical regions of interest (ROI) that were automatically segmented on the T1-weighted MRI image using MUSE, a multi-atlas segmentation method (Doshi et al. 2016). The SPARE-BA score was positively associated with chronological age (Supplementary Figure 1). A higher SPARE-BA score indicates greater brain atrophy and a greater increase in SPARE-BA over time indicates accelerated progression of age-related brain atrophy. SPARE-BA score was shown to be associated with cognitive function and neurodegeneration (Bashyam et al. 2020, Habes et al. 2021). In this study, we computed the annual rate of change (i.e., slope) of SPARE-BA index during follow-up using simple linear regression as the outcome measure.
2.4. Diagnoses of mild cognitive impairment and dementia
In the BLSA, diagnoses of dementia and AD have continued to follow the Diagnostic and Statistical Manuel, the third edition, revised (DSM-III-R) and the National Institute of Neurological and Communication Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria, respectively (Tian et al. 2022, Tian et al. 2022).
2.5. Other measures of interest
We explored other measures of interest, including sex hormones and mitochondrial function. In an additional analysis, we adjusted for sex hormones, including total testosterone (TT), ammonium sulfate precipitation–measured bioavailable testosterone (mBT), estradiol, and hormone–binding glycoprotein (SHBG). Assessments of these sex hormones were described previously (Fabbri et al. 2016). TT levels were measured using high-performance liquid chromatography-tandem mass spectrometry (Esoterix part of LabCorp, Calabasas Hills, CA, certified by the Centers for Disease Control and Prevention’s Hormone Standardization Project [CDC-HoSt Program]). mBT levels were measured using a technique in which SHBG-bound steroids were separated from albumin-bound and free steroids using ammonium sulfate (Esoterix part of LabCorp). SHBG levels were measured using an immunoradiometric assay (Esoterix part of LabCorp). Because the initial findings suggested sex differences in mitochondrial function may play a role, we compared skeletal muscle mitochondrial function between men and women, measured as kPCr by MR spectroscopy (Tian et al. 2022). Because the assessment of skeletal muscle mitochondria function began in 2012, few participants had available data concurrent with plasma lipids. Thus, we chose to compare sex differences at participants’ most recent visits. We also explored cognitive outcomes, including memory, attention/processing speed, executive function, and visuoperceptual speed. Memory was measured using the California Verbal Learning Test (CVLT) immediate recall test (Delis et al. 1987). Attention/processing speed was measured using the Trail Making Test (TMT) part A (Reitan 1992). Executive function was measured using the TMT part B (Reitan 1992). Visuoperceptual speed was measured using the Digit Symbol Substitution Test (DSST) (Wechsler 1981).
2.6. Data Availability Statement
Data from the BLSA will be available upon request by proposal submission through the BLSA website (blsa.nih.gov). All requests are reviewed by the BLSA Data Sharing Proposal Review Committee and are also subject to approval from the NIH Institutional Review Board.
2.7. Statistical analysis
Sex differences in participants’ demographic variables were examined using independent t-tests for continuous variables and the chi-square tests for categorical variables. Sex differences in SPARE-BA index and lipid variables were examined using linear regression controlling for age.
Lipid variables were initially pre-processed by excluding metabolites with more than 30% missing values (below the limit of detection). For the remaining metabolites, we imputed the missing values using half of the minimum quantifiable value. Then, we performed normalization using the log2 transformation. Of 638 metabolites potentially assessed by the MxP Quant 500 kit, 468 metabolites were included in this analysis. Composite scores of lipid variables were computed as sums of log2-transformed values (Ng et al. 2021). To examine whether the associations of lipids with rates of change in the SPARE-BA index differed by sex, we tested the “sex-by-lipid” interaction in multivariable linear regression adjusted for baseline age, race, years of education, and baseline SPARE-BA index score. For lipid variables that showed significant interactions with sex, we further examined sex-stratified associations of each lipid variable with rates of change in the SPARE-BA index. We also tested the strength of the associations by further adjusting for waist circumference, Apolipoprotein E (APOE) ε4 carrier status, and medication use for high cholesterol as sensitivity analyses. Lipid variables and rates of change in the SPARE-BA index were standardized to Z scores due to different scales.
We performed several sets of exploratory analyses to further understand the results. (1) To understand whether sex hormones affect sex-specific associations, we additionally adjusted for estradiol, testosterone, and SHBG in a subset of the participants. (2) Because mitochondria play a key role in lipids utilization, we explored sex differences in skeletal muscle mitochondrial function in a subset of the participants. (3) To understand whether the associations with SPARE-BA scores also existed at the cognitive function level, we explored sex differences in associations with cognitive changes. We first computed annual rates of change of cognitive measures of interest using simple linear regression. We then used multivariable linear regression models to test the “sex-lipid” interaction with cognitive changes as outcomes. Models were adjusted for the same demographic covariates above and baseline cognitive performance.
In this exploratory analysis, significance was set at p<0.05 considering each lipid variable as separate predictor. We additionally corrected for multiple testing using FDR as sensitivity analysis. All analyses were performed using SAS v6.4 (Cary, NC).
3. Results
Participants’ characteristics are presented in Table 1. There was no significant sex difference in age or APOE ε4 carrier status. Men had more years of education and greater waist circumference than women. After controlling for age, men showed a higher beta-oxidation rate and higher levels of long-chain and short-chain acylcarnitines, compared to women. Men had lower concentrations of ceramides, glycoceramides, glycolipids and cholesterol esters, and sphingomyelins compared to women. There were no significant sex differences in very long-chain and long-chain triglycerides.
Table 1.
Participants’ baseline characteristics
| Men (N=214) | Women (N=261) | Sex difference | |||
|---|---|---|---|---|---|
| Mean (SD) or N (%) as noted | Range | Mean (SD) or N (%) | Range | p-value | |
| Demographics | |||||
| Age, years | 75.2 (8) | 60–94 | 73.9 (8.2) | 60–98 | 0.086 |
| Race and ethnicity, n (%) | |||||
| Chinese | 2 (0.9) | - | 4 (1.5) | - | - |
| Black | 34 (16) | - | 78 (30) | - | - |
| Filipino | 0 (0) | - | 3 (1.2) | - | - |
| Japanese | 0 (0) | - | 2 (0.8) | - | - |
| Other Asian or Other Pacific Islander | 3 (1.4) | - | 6 (2.3) | - | - |
| Other Non-White | 4 (1.9) | - | 0 (0) | - | - |
| White | 171 (80) | - | 167 (64) | - | - |
| Not Classifiable | 0 (0) | - | 1 (0.4) | - | - |
| Education, years | 18.3 (3.1) | 7–32 | 17.3 (2.7) | 12–24 | <0.001 |
| Waist circumference, cm | 95.7 (9.7) | 73–146.5 | 83.1 (11.1) | 56.4–111.2 | <0.001 |
| Brain Aging measures | |||||
| SPARE-BA score, unitless | 73.6 (10.8) | 46.2–99.7 | 72.6 (9.9) | 48.4–97.9 | 0.697 |
| SPARE-BA Slope (annual rate of change) | 1.3 (1.1) | −2.3–5.7 | 1.1 (0.9) | −2–4.6 | 0.052 |
| Number of brain MRI visits per participant | 3.4 (1.5) | 2–10 | 3.5 (1.4) | 2–11 | 0.787 |
| Follow-up, years | 4.7 (2.4) | 1–11 | 4.8 (2.3) | 1–10 | 0.512 |
| Other measures of interest | |||||
| Apolipoprotein E ε4 carrier status, n (%) | 44 (21) (n=207) | - | 67 (26) | - | 0.276 |
| Subsequent cognitive impairment/dementia | 36 (17%) | - | 24 (9%) | - | 0.017 |
| Total cholesterol, mg/dL | 171 (32) | 88–255 | 193 (35) | 102–373 | <0.001 |
| High-density lipoprotein, mg/dL | 55 (14) | 23 – 100 | 69 (16) | 34 – 128 | <0.001 |
| Low-density lipoprotein, mg/dL | 96 (29) | 23 – 182 | 106 (31) | 38 – 276 | <0.001 |
| Triglycerides, mg/dL | 100 (54) | 33 – 497 | 92 (40) | 34 – 357 | 0.075 |
| Medication use for high cholesterol, n (%) | 90 (42) | - | 101 (39) | - | 0.510 |
| Estradiol, ng/dL | 2.5 (0.8) (n=204) | 0.5–5.3 | 0.7 (0.6) (n=230) | 0.1 – 4.4 | <0.001 |
| Total testosterone, ng/dL | 489.1 (185.0) (n=204) | 11.0–1192.0 | 22.8 (13.6) (n=239) | 3.4–97.0 | <0.001 |
| Bioavailable testosterone, ng/dL | 112 (46) (n=203) | 8.8–310 | 3.7 (2.8) (n=224) | 0.7 – 18 | <0.001 |
| Sex hormone-binding glycoprotein, nmol/L | 58.4 (24.4) (n=203) | 14.0–139.4 | 74.9 (38.4) (n=241) | 13.3–334.7 | <0.001 |
| Lipid variables | |||||
| Acylcarnitines: Beta-oxidation rate | 0.13 (1.00) | −2.71 – 2.77 | −0.10 (0.99) | −3.48 – 2.99 | 0.013 |
| Acylcarnitines: Long-chain | 0.11 (0.98) | −3.76 – 2.11 | −0.09 (1.01) | −3.76 – 1.92 | 0.050 |
| Acylcarnitines: Medium-chain | 0.06 (0.88) | −2.79 – 2.30 | −0.05 (1.09) | −2.79 – 2.18 | 0.327 |
| Acylcarnitines: Short-chain | 0.12 (0.94) | −2.86 – 2.77 | −0.10 (1.04) | −4.03 – 3.01 | 0.023 |
| Ceramides: Very Long-chain | −0.26 (1.01) | −2.96 – 2.34 | 0.21 (0.94) | −2.44 – 2.96 | <0.001 |
| Ceramides: Long-chain | −0.31 (1.00) | −2.98 – 2.23 | 0.26 (0.93) | −2.72 – 2.66 | <0.001 |
| Glycoceremides: Very Long-chain | −0.23 (1.02) | −2.84 – 2.20 | 0.19 (0.94) | −2.40 – 2.88 | <0.001 |
| Glycoceremides: Long-chain | −0.28 (1.05) | −3.31 – 2.50 | 0.23 (0.89) | −2.21 – 3.17 | <0.001 |
| Glycerolipids & Cholesteryl Esters:Very Long-chain | −0.31 (1.01) | −3.24 – 2.27 | 0.25 (0.92) | −1.90 – 2.49 | <0.001 |
| Glycerolipids & Cholesteryl Esters:Long-chain | −0.27 (0.96) | −3.26 – 1.81 | 0.22 (0.98) | −3.18 – 2.79 | <0.001 |
| Sphingomyelins: Very Long-chain | −0.40 (0.99) | −3.24 – 2.35 | 0.33 (0.89) | −1.86 – 2.90 | <0.001 |
| Sphingomyelins: Long-chain | −0.51 (0.92) | −3.05 – 2.11 | 0.42 (0.86) | −2.51 – 3.70 | <0.001 |
| Triglycerides: Very Long-chain | −0.01 (1.07) | −4.15 – 2.86 | 0.01 (0.94) | −3.60 – 2.62 | 0.890 |
| Triglycerides: Long-chain | 0.01 (1.07) | −2.88 – 3.16 | −0.01 (0.95) | −2.84 – 2.82 | 0.763 |
Note: p-values were based on t-tests for continuous variables and chi-square tests for categorical variables. Sex differences in SPARE-BA index and lipid variables were adjusted for age. Lipid variables were based on log-transformed values and standardized to Z scores. The bold number indicates significance at a two-tailed p<0.05.
After adjustment, there were significant interactions between four lipid variables and sex in models predicting the rate of change in SPARE-BA score (Table 2). The four lipid variables were beta-oxidation rate, short-chain acylcarnitines, long-chain ceramides, and very long-chain triglycerides (Table 2). These significant interactions indicated that the associations between lipids and the rate of change in SPARE-BA score were different by sex. After stratification by sex, we found that lower beta oxidation rate and lower concentrations of short-chain acylcarnitines were associated with a faster rate of increase in SPARE-BA in women, and not in men (Table 2; Supplementary Figure 2–3). Higher concentrations of long-chain ceramides and very long-chain triglycerides were associated with a faster rate of increase in SPARE-BA in men, but not in women (Table 2; Supplementary Figure 2–3). In addition, the interaction between long-chain triglycerides and sex showed a p-value of 0.085 (Table 2). Higher concentrations of long-chain triglycerides were associated with a faster rate of increase in SPARE-BA in men (Table 2; Supplementary Figure 2–3). Additional adjustment for waist circumference, APOE ε4 carrier status, or medication use for high cholesterol did not substantially alter these sex-specific associations (Supplementary Table 2).
Table 2.
Sex-by-lipid interaction and sex-specific associations of lipid variables with change in SPARE-BA index score
| Interaction (n=475) | Men (n=214) | Women (n=261) | |||||
|---|---|---|---|---|---|---|---|
| Lipid class | Variables | β (95% CI) | p-value | β (95% CI) | P-value | β (95% CI) | P-value |
| Acylcarnitines | Beta-oxidation rate | 0.181 (0.0002, 0.361) | 0.0497 | 0.057 (0.091, 0.205) | 0.450 | −0.128 (0.239, 0.018) | 0.023 |
| Long-chain | 0.040 (−0.141, 0.222) | 0.663 | 0.024 (0.127, 0.175) | 0.757 | −0.020 (0.132, 0.092) | 0.724 | |
| Medium-chain | 0.099 | 0.298 | 0.045 | 0.595 | −0.059 | 0.263 | |
| (−0.088, 0.287) | (0.122, 0.212) | (0.162, 0.044) | |||||
| Short-chain | 0.196 (0.012, 0.379) | 0.036 | 0.068 (0.088, 0.225) | 0.392 | −0.128 (0.234, 0.022) | 0.018 | |
| Ceramides | Very Long-chain | 0.041 (−0.143, 0.225) | 0.662 | 0.112 (0.034, 0.259) | 0.132 | 0.067 (0.054, 0.188) | 0.275 |
| Long-chain | 0.196 (0.010, 0.381) | 0.039 | 0.207 (0.061, 0.352) | 0.006 | 0.002 (0.120, 0.124) | 0.971 | |
| Glycosylceramides | Very Long-chain | 0.044 (−0.139, 0.228) | 0.634 | 0.044 (0.101, 0.190) | 0.551 | 0.019 (0.101, 0.140) | 0.753 |
| Long-chain | 0.083 (−0.102, 0.269) | 0.379 | 0.053 (0.088, 0.193) | 0.458 | −0.010 (0.137, 0.117) | 0.877 | |
| Glycerolipids & Cholesteryl Esters | Very Long-chain | 0.108 (−0.079, 0.294) | 0.257 | 0.009 (0.137, 0.156) | 0.901 | −0.085 (0.206, 0.037) | 0.172 |
| Long-chain | 0.113 (−0.074, 0.300) | 0.237 | 0.112 (0.042, 0.265) | 0.153 | −0.004 (0.124, 0.116) | 0.946 | |
| Sphingomyelins | Very Long-chain | −0.046 (−0.238, 0.146) | 0.637 | −0.039 (0.188, 0.110) | 0.611 | −0.002 (0.127, 0.123) | 0.971 |
| Long-chain | 0.053 (−0.059, 0.353) | 0.607 | 0.030 (0.128, 0.189) | 0.707 | −0.024 (0.154, 0.106) | 0.716 | |
| Triglycerides | Very Long-chain | 0.190 (0.011, 0.369) | 0.037 | 0.149 (0.011, 0.286) | 0.035 | −0.061 (0.181, 0.060) | 0.324 |
| Long-chain | 0.157 (−0.022, 0.337) | 0.085 | 0.153 (0.015, 0.291) | 0.030 | −0.028 | 0.650 | |
Note: Bold number indicates significant associations at two-tailed p<0.05. All models were adjusted for baseline age, race, education, and baseline SPARE-BA score. The reported beta is equivalent to Cohen’s d effect size (per standard deviation change in the independent variable).The interaction effects did not survive FDR-adjusted p<0.05.
In men, the observed associations of long-chain ceramides and very long-chain triglycerides with SPARE-BA slopes remained significant after additional adjustments for sex hormones (all p<0.05; Supplementary Table 2). In women, the additional adjustment for sex hormones did not substantially affect the association of beta-oxidation rate but attenuated the association of short-chain acylcarnitines (Supplementary Table 2).
We compared sex differences in a subset of the participants who had skeletal muscle mitochondrial function at their most recent visit (mean ± SD: 0.0207±0.0057 in 118 men; 0.0186±0.0039 in 148 women). After adjustment for age, race, and extent of PCr depletion during exercise, kPCr was significantly higher in men compared to women (p=0.005).
In the exploratory analyses of cognitive outcomes, we found significant “sex-lipid” interactions of long-chain triglycerides in models predicting annual changes in DSST and TMT-A (β (95% CI), p-value: −0.199 (−0.377, −0.021), 0.028, and 0.195 (0.023, 0.368), 0.027, respectively). After sex stratification, higher long-chain triglycerides were associated with a greater rate of decline in DSST and a greater rate of increase in TMT-A in men (both p<0.05), but not in women (both p>0.05). We also found a significant “sex-lipid” interaction of very long-chain triglycerides in models predicting annual changes in TMT-A (β (95% CI), p-value: 0.193 (0.020, 0.365), 0.029). The association of higher very long-chain triglycerides and a greater rate of increase in TMT-A in men showed p-value of 0.070 in men and 0.258 in women. These interaction effects were not significant after FDR correction.
4. Discussion
In this sample of community-dwelling older adults who were cognitively normal at baseline, men and women showed different patterns of lipid profiles predicting the progression of brain atrophy over time. These sex-specific associations varied in the lipid groups and structures based on the carbon chain length. Specifically, lower levels of short-chain acylcarnitines were associated with accelerated brain aging in women, whereas higher levels of long-chain ceramides and very long-chain triglycerides were associated with accelerated brain aging in men. We extend prior research on associations of circulating lipids with brain structure in several aspects. First, we investigated sex differences in these associations. Second, we used longitudinal assessments of the start-of-the-art MRI-derived brain aging index by machine learning. We examined lipid profiles based on the length of the carbon chain related to lipid functions.
Mechanisms underlying these sex-specific lipid profiles of brain aging are not fully understood. These differential lipid profiles of brain aging may be related to where lipid metabolism takes place at the cellular level. Because acylcarnitines are transported from the inner membrane of the mitochondria into the matrix (Tonazzi et al. 2015), and lipid metabolism such as ceramides and triglycerides occurs predominantly outside of the mitochondria, perhaps impaired mitochondrial function plays a key role in sex-specific brain aging (Figure 1).
Figure 1. Hypothesized mechanisms of the role of lipids in brain aging.
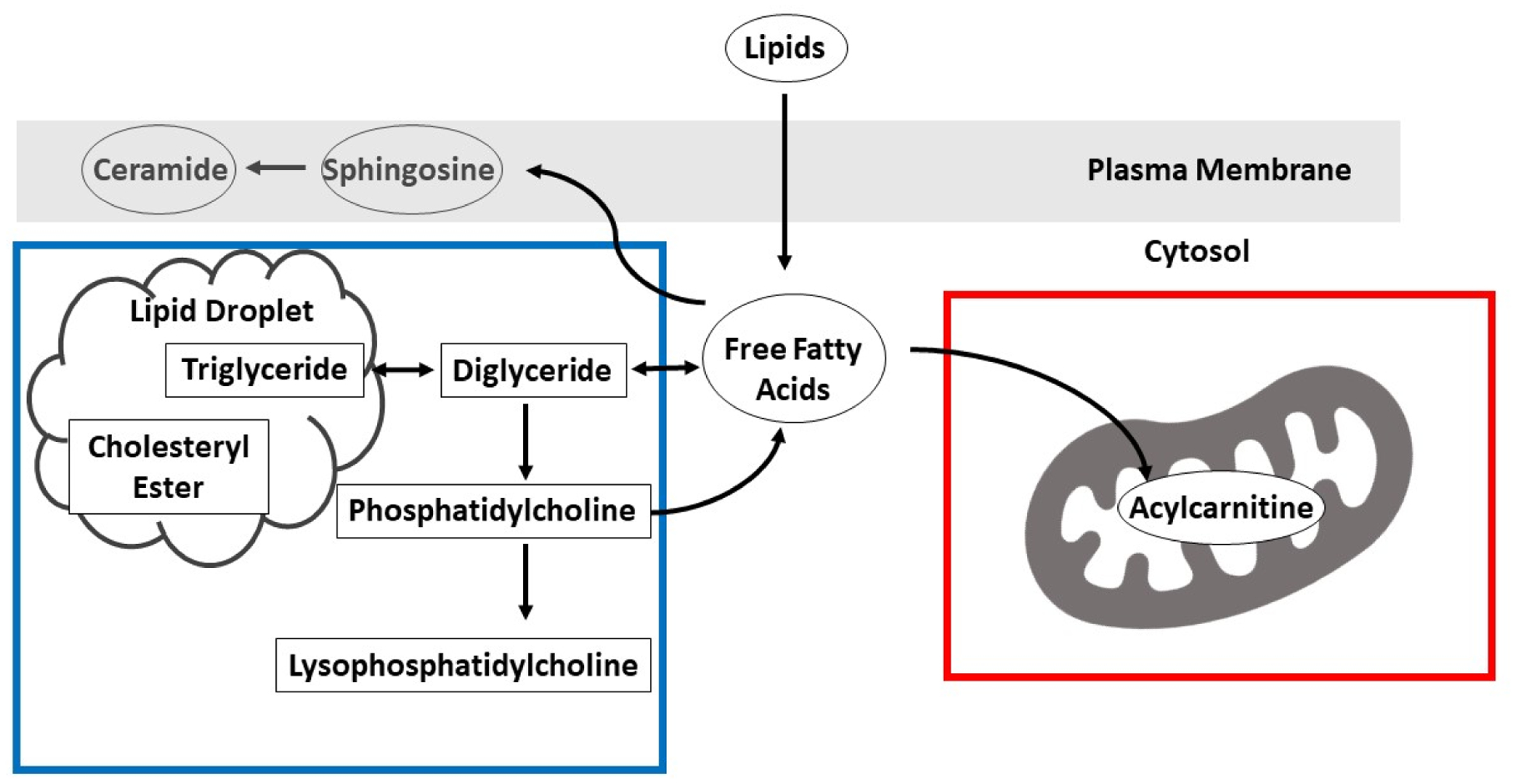
Lower levels of short-chain acylcarnitines were associated with accelerated brain aging in women. Lower acylcarnitine levels suggest lower mitochondrial function, as acylcarnitines are transported from the inner mitochondrial membrane into the matrix and can influence oxidative phosphorylation (red box). In contrast, high levels of long-chain ceramides and very long-chain triglycerides were associated with accelerated brain aging in men. Long-chain ceramides can permeabilize and damage mitochondria, leading to a decrease in electron transport chain activity. Thus, in both cases, the adverse effect on the brain may be explained by mitochondrial dysfunction, but with different mechanisms in men and women.
The observed association of acylcarnitines with brain aging in women but not in men may be due to lower levels of acylcarnitines and mitochondrial function in women than men. Recent data have shown that intramuscular short-chain acylcarnitines are lower in older women, but not in men (van der Hoek et al. 2020). In our study, the additional analysis of sex differences in skeletal muscle mitochondrial function in a subset of participants showed lower mitochondrial function in women than men. Mitochondria are thought to be a central target for sex differences in various pathologies. Mitochondrial function is regulated by sex hormones and plays a key role in lipids utilization (Ventura-Clapier et al. 2017). The biosynthesis of membrane lipids is also needed for mitochondrial biogenesis and mitophagy because lipids are essential structural components and the main fuel for energy production. In addition, the oxidation of lipids within mitochondrial is the main mechanism that triggers an inflammatory response (Walker et al. 2022). Other possible explanations for the observed sex differences may include sex hormones (Marrocco and McEwen 2016) and exposure to cardiovascular risk and obesity (Wang et al. 2011). Sex hormones, including estradiol, slightly attenuated the observed associations in short-chain acylcarnitines in this sample of women aged 60 or older. This may suggest that even in older age, sex hormones may still play a role in the lipid profiles of female brain aging. Some data have suggested that estradiol decreases carnitine levels in women, although mechanisms are unclear (Takiyama and Matsumoto 1998). There is also a connection between estrogen and mitochondrial function. Estrogen increases mitochondrial respiration efficiency by increasing oxidative phosphorylation and decreasing ATPase activity. Estradiol translation through estrogen receptor β into mitochondria regulates mitochondrial calcium sequestration (Nilsen and Brinton 2004). It may be speculated that mitochondrial dysfunction contributes to brain aging in women through lipid metabolism and the regulation of sex hormones.
The associations of long-chain ceramides and long-chain and very-long triglycerides with the progression of brain aging in men could indicate lipid metabolism occurring predominantly outside of the mitochondria may contribute to male brain aging. Our findings are in line with previous studies. In one postmortem study of adults aged 65 or older, ceramides, along with sphingomyelin, and sulfate content, were strongly associated with age in the hippocampus of men, and not in women (Couttas et al. 2018). Peroxisome proliferator-activated receptor α (PPAR α) is a known transcription factor and metabolic regulator of the catabolism of fatty acids. It is involved in lipids metabolism, inflammatory response as well as amyloid precursor protein, and it plays a key role in neuronal function by governing synaptic plasticity. PPAR α shows sex differences where males had higher levels than females. Studies have also shown that PPARα agnostic treatment is neuroprotective only in males, not in females (Dotson et al. 2016, Saez-Orellana et al. 2020). Sex differences are suggested in PPAR α involved prevention and treatment for brain function. The observed associations in men are not affected by sex hormones, waist circumference, or APOE ε4 carrier status.
In the exploration of cognitive outcomes, we observed similar sex differences in the association of ceramides and triglycerides with the progression of brain aging and these findings further confirmed the observed sex differences in lipid profiles of MRI-derived brain aging. Long-chain ceramides and triglycerides in particular are related to greater declines in attention/processing speed and visuoperceptual speed in men but not in women. We did not observe sex differences in the association with memory decline. Future studies of larger samples with longer follow-ups are warranted to confirm these cognitive findings.
This study has several strengths. First, the study population is well-characterized community-dwelling older adults, allowing us to study brain aging without overt neurological diseases. Second, the assessment of brain aging patterns is state-of-the-art, derived from brain MRIs using a machine learning approach based on a large consortium. Third, the exploratory analyses of sex hormones and mitochondrial function provide new insights into mechanisms underlying sex differences in lipid profiles of brain aging. Fourth, the quantification of structure-based lipid groups highlights the importance of the length of the carbon chain in lipid metabolism. This study has limitations. First, participants in this study are volunteers and tend to be healthier than the general aging population. Second, data on mitochondrial function are only available to a subset of the participants at their most recent visit, which may be underpowered to detect sex differences. The observed interaction effects between lipid measures and sex are somewhat modest and need to be validated in other cohorts.
In conclusion, circulating lipid profiles of accelerated brain aging are sex-specific. These sex-specific associations varied by lipid classes and the length of the carbon chain. Short-chain acylcarnitines in women and long-chain ceramides and very long-chain triglycerides in men predict future accelerated brain aging. Mechanisms underlying these sex-specific lipid profiles of brain aging warrant further investigation.
Supplementary Material
Highlights.
Circulating lipid profiles of accelerated brain aging are sex-specific.
These associations vary by lipid classes and the length of the carbon chains.
Mitochondrial dysfunction may play a role in these sex-specific associations.
Acknowledgment:
This study was supported in part by the Intramural Research Program of the National Institute on Aging, NIH, Baltimore, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Credit author statement:
Qu Tian: Conceptualization, Investigation, Methodology, Formal analysis, Software, Writing- Original draft preparation
Brendan A. Mitchell: Investigation, Methodology, Formal analysis, Software, Writing- Original draft preparation
Guray Erus, Christos Davatzikos: Methodology, Writing- Reviewing and Editing
Ruin Moaddel: Investigation, Methodology, Writing- Reviewing and Editing
Susan M. Resnick: Supervision, Methodology, Writing- Reviewing and Editing
Luigi Ferrucci: Conceptualization, Supervision, Methodology, Writing- Reviewing and Editing
References:
- Armstrong NM, An Y, Beason-Held L, Doshi J, Erus G, Ferrucci L, Davatzikos C and Resnick SM (2019). “Sex differences in brain aging and predictors of neurodegeneration in cognitively healthy older adults.” Neurobiol Aging 81: 146–156. 10.1016/j.neurobiolaging.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett K and Eaton S (2004). “Mitochondrial beta-oxidation.” Eur J Biochem 271(3): 462–469. 10.1046/j.1432-1033.2003.03947.x [DOI] [PubMed] [Google Scholar]
- Bashyam VM, Erus G, Doshi J, Habes M, Nasrallah I, Truelove-Hill M, Srinivasan D, Mamourian L, Pomponio R, Fan Y, Launer LJ, Masters CL, Maruff P, Zhuo C, Volzke H, Johnson SC, Fripp J, Koutsouleris N, Satterthwaite TD, Wolf D, Gur RE, Gur RC, Morris J, Albert MS, Grabe HJ, Resnick S, Bryan RN, Wolk DA, Shou H and Davatzikos C (2020). “MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide.” Brain 143(7): 2312–2324. 10.1093/brain/awaa160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couttas TA, Kain N, Tran C, Chatterton Z, Kwok JB and Don AS (2018). “Age-Dependent Changes to Sphingolipid Balance in the Human Hippocampus are Gender-Specific and May Sensitize to Neurodegeneration.” J Alzheimers Dis 63(2): 503–514. 10.3233/JAD-171054 [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E and Ober B (1987). California Verbal Learning Test, Research edition. New York, NY, Psychological Corporation [Google Scholar]
- Doshi J, Erus G, Ou Y, Resnick SM, Gur RC, Gur RE, Satterthwaite TD, Furth S, Davatzikos C and Alzheimer’s Neuroimaging I (2016). “MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection.” Neuroimage 127: 186–195. 10.1016/j.neuroimage.2015.11.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Wang J, Chen Y, Manning D, Nguyen H, Saugstad JA and Offner H (2016). “Sex differences and the role of PPAR alpha in experimental stroke.” Metab Brain Dis 31(3): 539–547. 10.1007/s11011-015-9766-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri E, An Y, Gonzalez-Freire M, Zoli M, Maggio M, Studenski SA, Egan JM, Chia CW and Ferrucci L (2016). “Bioavailable Testosterone Linearly Declines Over A Wide Age Spectrum in Men and Women From The Baltimore Longitudinal Study of Aging.” J Gerontol A Biol Sci Med Sci 71(9): 1202–1209. 10.1093/gerona/glw021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habes M, Pomponio R, Shou H, Doshi J, Mamourian E, Erus G, Nasrallah I, Launer LJ, Rashid T, Bilgel M, Fan Y, Toledo JB, Yaffe K, Sotiras A, Srinivasan D, Espeland M, Masters C, Maruff P, Fripp J, Volzk H, Johnson SC, Morris JC, Albert MS, Miller MI, Bryan RN, Grabe HJ, Resnick SM, Wolk DA, Davatzikos C, t. P. A. D. c. t. A. iStaging consortium and C. s. the (2021). “The Brain Chart of Aging: Machine-learning analytics reveals links between brain aging, white matter disease, amyloid burden, and cognition in the iSTAGING consortium of 10,216 harmonized MR scans.” Alzheimers Dement 17(1): 89–102. 10.1002/alz.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houten SM and Wanders RJ (2010). “A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation.” J Inherit Metab Dis 33(5): 469–477. 10.1007/s10545-010-9061-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove M, Pradas I, Dominguez-Gonzalez M, Ferrer I and Pamplona R (2019). “Lipids and lipoxidation in human brain aging. Mitochondrial ATP-synthase as a key lipoxidation target.” Redox Biol 23: 101082. 10.1016/j.redox.2018.101082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A (2012). “Very long-chain fatty acids: elongation, physiology and related disorders.” J Biochem 152(5): 387–395. 10.1093/jb/mvs105 [DOI] [PubMed] [Google Scholar]
- Macaron T, Giudici KV, Bowman GL, Sinclair A, Stephan E, Vellas B and de Souto Barreto P (2021). “Associations of Omega-3 fatty acids with brain morphology and volume in cognitively healthy older adults: A narrative review.” Ageing Res Rev 67: 101300. 10.1016/j.arr.2021.101300 [DOI] [PubMed] [Google Scholar]
- Marrocco J and McEwen BS (2016). “Sex in the brain: hormones and sex differences.” Dialogues Clin Neurosci 18(4): 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L and Resnick SM (2016). “Sex differences in cognitive trajectories in clinically normal older adults.” Psychol Aging 31(2): 166–175. 10.1037/pag0000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazzami K, Power MC, Gottesman R, Mosley T, Lutsey PL, Jack CR Jr., Hoogeveen RC, West N, Knopman DS and Alonso A (2020). “Association of mid-life serum lipid levels with late-life brain volumes: The atherosclerosis risk in communities neurocognitive study (ARICNCS).” Neuroimage 223: 117324. 10.1016/j.neuroimage.2020.117324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Guardia-Laguarta C and Area-Gomez E (2020). “The fat brain.” Curr Opin Clin Nutr Metab Care 23(2): 68–75. 10.1097/MCO.0000000000000634 [DOI] [PubMed] [Google Scholar]
- Ng TKS, Kovalik JP, Ching J, Chan AW and Matchar DB (2021). “Novel metabolomics markers are associated with pre-clinical decline in hand grip strength in community-dwelling older adults.” Mech Ageing Dev 193: 111405. 10.1016/j.mad.2020.111405 [DOI] [PubMed] [Google Scholar]
- Nilsen J and Brinton RD (2004). “Mitochondria as therapeutic targets of estrogen action in the central nervous system.” Curr Drug Targets CNS Neurol Disord 3(4): 297–313. 10.2174/1568007043337193 [DOI] [PubMed] [Google Scholar]
- Ooi KM, Vacy K and Boon WC (2021). “Fatty acids and beyond: Age and Alzheimer’s disease related changes in lipids reveal the neuro-nutraceutical potential of lipids in cognition.” Neurochem Int 149: 105143. 10.1016/j.neuint.2021.105143 [DOI] [PubMed] [Google Scholar]
- Ottas A, Fishman D, Okas TL, Pussa T, Toomik P, Martson A, Kingo K and Soomets U (2017). “Blood serum metabolome of atopic dermatitis: Altered energy cycle and the markers of systemic inflammation.” PLoS One 12(11): e0188580. 10.1371/journal.pone.0188580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak A, Nady B, Matthew Wai Kin W, Liu Y, Housseini M and Perminder Singh S (2020). Lipids, brain ageing, dementia, and lipidomics. Diagnosis and Management in Dementia: 183–205. 10.1016/b978-0-12-815854-8.00012-4 [DOI] [Google Scholar]
- Reitan RM (1992). Trail Making Test: Manual for administration and scoring. Tucson, AZ, Reitan Neuropsychology Laboratory [Google Scholar]
- Saez-Orellana F, Octave JN and Pierrot N (2020). “Alzheimer’s Disease, a Lipid Story: Involvement of Peroxisome Proliferator-Activated Receptor alpha.” Cells 9(5). 10.3390/cells9051215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld P and Wojtczak L (2016). “Short- and medium-chain fatty acids in energy metabolism: the cellular perspective.” J Lipid Res 57(6): 943–954. 10.1194/jlr.R067629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiyama N and Matsumoto K (1998). “Age-and sex-related differences of serum carnitine in a Japanese population.” J Am Coll Nutr 17(1): 71–74. 10.1080/07315724.1998.10720458 [DOI] [PubMed] [Google Scholar]
- Tian Q, Bilgel M, Moghekar AR, Ferrucci L and Resnick SM (2022). “Olfaction, Cognitive Impairment, and PET Biomarkers in Community-Dwelling Older Adults.” J Alzheimers Dis. 10.3233/JAD-210636 [DOI] [PubMed] [Google Scholar]
- Tian Q, Bilgel M, Moghekar AR, Ferrucci L and Resnick SM (2022). “Olfaction, Cognitive Impairment, and PET Biomarkers in Community-Dwelling Older Adults.” J Alzheimers Dis 86(3): 1275–1285. 10.3233/JAD-210636 [DOI] [PubMed] [Google Scholar]
- Tian Q, Mitchell BA, Zampino M and Ferrucci L (2022). “Longitudinal associations between blood lysophosphatidylcholines and skeletal muscle mitochondrial function.” Geroscience. 10.1007/s11357-022-00548-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H, Horikawa C, Nishita Y, Nakamura A, Kato T, Kaneda Y, Obata H, Rogi T, Nakai M, Shimokata H and Otsuka R (2022). “The association between long-chain polyunsaturated fatty acid intake and changes in brain volumes among older community-dwelling Japanese people.” Neurobiol Aging 117: 179–188. 10.1016/j.neurobiolaging.2022.05.008 [DOI] [PubMed] [Google Scholar]
- Tonazzi A, Giangregorio N, Console L and Indiveri C (2015). “Mitochondrial carnitine/acylcarnitine translocase: insights in structure/ function relationships. Basis for drug therapy and side effects prediction.” Mini Rev Med Chem 15(5): 396–405. 10.2174/138955751505150408142032 [DOI] [PubMed] [Google Scholar]
- Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S, Wang W and Brenner DE (2009). “Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group.” J Proteome Res 8(1): 113–117. 10.1021/pr800545q [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek MD, Nieuwenhuizen AG, Kuda O, Bos P, Paluchova V, Verschuren L, van den Hoek AM, Kleemann R, Veeger N, van der Leij FR and Keijer J (2020). “Intramuscular short-chain acylcarnitines in elderly people are decreased in (pre-)frail females, but not in males.” FASEB J 34(9): 11658–11671. 10.1096/fj.202000493R [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V and Garnier A (2017). “Mitochondria: a central target for sex differences in pathologies.” Clin Sci (Lond) 131(9): 803–822. 10.1042/CS20160485 [DOI] [PubMed] [Google Scholar]
- Walker KA, Basisty N, Wilson DM 3rd and Ferrucci L (2022). “Connecting aging biology and inflammation in the omics era.” J Clin Invest 132(14). 10.1172/JCI158448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Magkos F and Mittendorfer B (2011). “Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones.” J Clin Endocrinol Metab 96(4): 885–893. 10.1210/jc.2010-2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg BW (2018). “The long and the short of ceramides.” J Biol Chem 293(25): 9922–9923. 10.1074/jbc.H118.003522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1981). Wechsler Adult Intelligence Scale-Revised (Vol. 1). New York, NY, Psychological Corporation. [Google Scholar]
- Yamaguchi Y, Zampino M, Moaddel R, Chen TK, Tian Q, Ferrucci L and Semba RD (2021). “Plasma metabolites associated with chronic kidney disease and renal function in adults from the Baltimore Longitudinal Study of Aging.” Metabolomics 17(1): 9. 10.1007/s11306-020-01762-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrouk A, Vejux A, Nury T, El Hajj HI, Haddad M, Cherkaoui-Malki M, Riedinger JM, Hammami M and Lizard G (2012). “Induction of mitochondrial changes associated with oxidative stress on very long chain fatty acids (C22:0, C24:0, or C26:0)-treated human neuronal cells (SK-NB-E).” Oxid Med Cell Longev 2012: 623257. 10.1155/2012/623257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the BLSA will be available upon request by proposal submission through the BLSA website (blsa.nih.gov). All requests are reviewed by the BLSA Data Sharing Proposal Review Committee and are also subject to approval from the NIH Institutional Review Board.


