Abstract
After two decades of study of lipid antigens that activate CD1-restricted T cells, new studies show how autoreactive αβ TCRs can directly recognize the outer surface of CD1 proteins in ways that are lipid agnostic. Most recently, this lipid agnosticism has turned to negativity, with the discovery of natural CD1 ligands that dominantly negatively block autoreactive αβ TCR binding to CD1a and CD1d. This review highlights basic differences between positive and negative regulation of cellular systems. We outline strategies to discover lipid inhibitors of CD1-reactive T cells, whose roles in vivo are becoming clear, especially in CD1-mediated skin disease.
Keywords: T cells, CD1, Autoimmunity, Autoreactivity, MHC, Antigen Complexes
The discovery of CD1 recognition by αβ T cells broadened immunologists’ views by showing that lipid antigens exist [1,2] and represent natural targets for T cell response. Based on the discovery of glycolipid [3,4] antigens, early structural studies of CD1-lipid complexes [5] and glycolipid-loaded CD1 tetramers [6–8], the field coalesced around a unitary model of lipid antigen display. This now widely accepted ‘corecognition’ model emphasizes that TCRs bind both CD1 and lipid antigen, where specific interactions of T cell receptors (TCR) with the hydrophilic head group of the antigen lead to T cell activation (Figure 1). Accordingly, CD1-reactive T cells are thought of as an ‘off until on system,’ where the default interaction between CD1 and TCR is failure to activate, and T cells are positively regulated by lipid antigens that appear in rare situations. This corecognition model, along with activating antigens, has dominated thinking in the CD1 system since the 1990s. However, our current opinion in immunology is that this model is fundamentally incomplete (Table 1).
Figure 1.
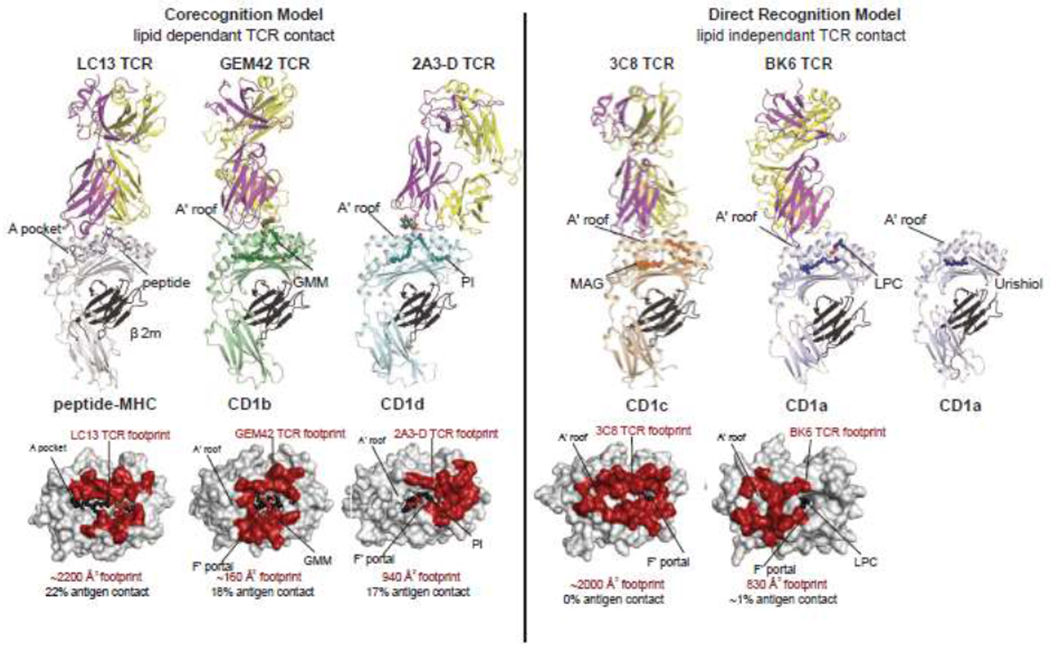
Differing modes of TCR contact with CD1 establish the older corecognition and newly proposed direct recognition models.
Table 1.
Lipids play differing positive and negative regulatory roles in the older corecognition model and the newly proposed direct recognition model, respectively.
| Corecognition Model | Direct Recognition Model | |
|---|---|---|
| TCR Target | CD1 and lipid | CD1 |
| TCR Epitope | periportal CD1 residues and hydrophilic cap of lipid | A’ roof and periportal residues of CD1 |
| Activating Ligands | amphipathic lipids protrude for TCR contact | small lipids reside inside CD1 |
| Abundance of Ligand | low | high |
| Mechanism of Activation | epitope discrimination | absence of TCR interference |
| Non-activating Lipids | amphiphathic lipid protrudes but fails to bind the TCR | dominant negative blockade of TCR binding to the A’ roof |
| Default Outcome of CD1-TCR Contact | non-activation (unless rare antigen present) | activation (unless blocker is dominant) |
| Ligand-based Therapy | activation-based adjuvancy or immunization | inhibition of autoimmune response |
We are not proposing that immunologists forget about lipid antigens, but instead to recognize a separate ‘on until off’ system that is based on two general discoveries playing out since 2014. The first is lipid-independent direct recognition of CD1, which broadly occurs in the human CD1a [9,10] and CD1c systems [11]. The second pillar of this new model is the recent discovery of naturally occurring lipid ligands for CD1a [12] and CD1d proteins [13,14], which can dominantly negatively regulate CD1-TCR interactions. For direct recognition the key event is not the appearance of a rare antigen that suddenly leads to activation, but instead involves autoreactive TCRs with intrinsic recognition of the CD1 protein itself. Low level baseline recognition can be modulated upward or downward, like a rheostat, by activating ligands with small inside CD1 versus larger lipids that block TCR approach to CD1. Because immunologists usually think of MHC and CD1 ligands as activators, consideration of the larger ramifications of a dominantly negatively regulated system requires a kind of unfamiliar ‘reverse’ thinking. As summarized in Table 1, ligand frequency, default outcomes of CD1-TCR interaction, and mechanisms of T cell selection and maintenance may be different in systems regulated by inhibitors rather than antigens.
CD1 and Lipid Corecognition
Two models of glycolipid antigen recognition by T cells were simultaneously reported in 1997: α-galactosyl ceramide (αGalCer) presentation by CD1d [4] and glucose monomycolate (GMM) presentation by CD1b [3,15]. Functional studies of T cell clones demonstrated recognition patterns that required the hexose sugar present in the glycolipid antigen, which was explained through crystal structures of αβTCR-lipid-CD1 complexes [5,16]. The corecognition model takes its name from the fact that the TCR binds extensively with CD1 and lipid, which is summarized quantitatively in Figure 1 by measures of TCR footprint size and percent contribution of lipids to TCR epitopes. For GMM and αGalCer, TCRs contact the antigens’ carbohydrate as it protrudes through a structure known as the F’ portal (Figure 1), as well as periportal residues on CD1b or CD1d. These dual interactions regulate in vivo T cell populations, as shown through CD1d-aGalCer [6–8] or CD1b-GMM tetramers [15,17,18], which could enumerate and capture polylconal T cells with reactivity to CD1 and glycolipid. With the subsequent discovery of many other amphipathic glycolipids, phospholipids, or sulfated lipids that present their hydrophilic moieties to TCRs [19], the CD1 corecognition model, like MHC-peptide corecognition, became widely accepted as the main or sole basis of physiological control of T cells.
Lipid Independent Recognition of CD1
Two key features of the corecognition model are the presence of a hydrophilic chemical epitope (often a sugar) in the antigen and precise TCR affinity for this kind of epitope (Table 1). Surprisingly, De Jong and Cheng discovered CD1a-mediated lipid stimulants in skin that lacked both features [20]. They found clonal TCR-mediated cross-reactivity to recombinant CD1a presenting highly hydrophobic, but structurally unrelated molecules: squalene, triacylglycerol, free fatty acid and wax ester lipids. The lack of hydrophilic epitopes, the small molecular size and TCR promiscuity all pointed to a theoretical mechanism whereby such molecules may not be directly recognized. Instead small ‘headless’ lipids might nest inside CD1a to free up the outer surface of CD1a for TCR recognition (Table 1). The following year a ternary crystal structure of the BK6 TCR recognizing CD1a-lysophosphatidycholine (LPC) complex revealed that 99 percent of the TCR footprint was contributed by CD1a (Figure 1) [10]. Subsequently, the CD1c-autoreactive αβ TCR 3C8 was co-crystallized with CD1c presenting a small hydrophobic molecule that likewise lacked a large or rigid hydrophilic determinant, monoacylglycerol (Figure 1) (MAG) [11].
If an antigen is defined as a molecule that directly contacts a clonotypic immune receptor, then LPC and MAG are not antigens. However, in activation assays their presence is often required, and MAG-treated CD1c tetramers capture T cells with functional CD1c autoreactivity [11]. The original squalene, triacylglycerol, wax ester and fatty acid antigens have not been co-crystalized within CD1a, so their sequestered nature is still presumptive. However, other studies confirmed the interior sequestration of small hydrophobic T cell stimulants, farnesol [21] and urushiol [22], the poison ivy antigen. In CD1a-farnesol complexes, more than half of the cleft remained without any visible ligand bound, and both ligands were positioned at a distance from the presumed TCR contact on the A’ roof (Figure 1, right). Collectively, LPC, MAG, farnesol and urushiol show that small ligands next inside CD1a and CD1c, establishing a new mode of direct reactivity, whereby TCRs to contact CD1 proteins themselves.
Lipid ligands are present inside CD1, but their role is limited to ‘absence of interference’ (Table 1). Most evidence for direct reactivity is for CD1a and CD1c, and several studies show that CD1a, compared to other human CD1 isoforms, more frequently serves as the target of autoreactive T cells in human blood [23,24] and skin [12,25]. Whether direct TCR reactivity to small sequestered lipids occurs for CD1b and CD1d is not yet known. However, important work from Mallevaey and Gapin showed that an NKT TCR can recognize CD1d with minimal contribution of antigen [26]. CD1b TCRs can have a channel that allows promiscuous recognition of diverse self-phospholipids independently of the particular phosphoglycerol headgroup [18,27]. These studies provide examples of TCRs that are less dependent on or less specific for lipids.
CD1-endo tetramers
CD1 directly reactive T cells were discovered as clones [9,10,28]. Until recently there has been little information about their frequency in blood and tissues or clear evidence that CD1 directly reactive T cells constitute biologically active T cell populations in vivo. This situation has now changed based on experiments in which the predictions of the direct recognition model were used to implement an unusual CD1 tetramer approach. In a paper entitled ‘Phenotypic Analysis of Antigen specific Lymphocytes,’ Altman and Davis reported that fluorescent MHC I tetramers can enumerate T cells recognizing a peptide antigen of interest [29]. In contrast, the direct recognition model predicts that the complement of diverse endogenous lipids bound to a pool of CD1a proteins allows direct detection of CD1+ cells. Thus, rather than loading CD1a or CD1c tetramers with a defined lipid, CD1 tetramers carrying diverse endogenous lipids from mammalian cells (CD1-endo) were used to count and isolate CD1a and CD1c autoreactive T cells [11,12,25]. On a practical level, this approach obviates the need to know the identities of immunodominant lipid antigens to study CD1-reactive T cells. These experiments showed not only that directly CD1a- and CD1c-reactive T cells exist as populations in human blood and skin, but also allows their enumeration in a one-step experiment. Although CD36 serves as a non-TCR ligand of CD1c [30], false positive CD1 tetramer staining can be efficiently blocked with anti-CD36. Overall, CD1-endo tetramer+ T cells are now well validated to capture T cells with bona fide functional responses to CD1c or CD1a. Further by treating tetramers with small hydrophobic antigens, such T cells can show brighter tetramer staining [11,12,25].
Roughly matching precursor frequencies previously determined with laborious RT-PCR and limiting dilution assays [23,24], CD1a-endo tetramer+ cells are in the range of 0.01 to 1 % of blood T cells [12,25], which is a high frequency for naive TCRs recognizing a single molecular determinant without disease or antigen-driven expansion. One recent [25] and one ongoing study (Clark, Van Rhijn, Moody, unpublished, 2023) found that skin T cells assayed after 3-week culture are ~1 % CD1a-endo tetramer+. Further, five skin donors have shown very high staining rates with > 10% CD1a tetramer+ T cells. Going beyond clonal analyses, these measurements show that CD1a autoreactive T cells are an abundant component of the human αβ T cell repertoire. Several studies now suggest a non-redundant, in vivo role of CD1a autoreactive T cells in human transgenic disease models [22,31], setting the stage for ex vivo studies in human autoimmune disease using CD1a-endo tetramers. For example, recent work by Dejong, demonstrates response to CD1a and phosphatidylglycerol antigens released by symbionts in the human skin microbiome that are associated with atopic dermatitis (Monnot, Nature Immunology in press, 2023 - the complete reference is in annotations and full cite will be available before publication of this review).
Regulating autoimmunity
For MHC and CD1, the ‘off until on’ corecognition provides a straightforward explanation for the avoidance of autoreactivity: activating antigens may be absent in any given situation. However, with increasing evidence for ‘on until off’ T cells that recognize CD1 in the absence of added antigen, basic questions arise about negative immune regulation. Unlike MHC I, CD1 is not ubiquitously expressed, so one possibility is the physical separation of T cells and CD1-expressing cells [20]. A second general idea is that autoreactivity is common, but it leads to low level activation IL-22 and other epithelial or T cell maintenance signals, rather than overt immunopathology [23]. A third possibility is that CD1 captures lipids that block T cell response. The bar is high for evidence needed to support a working model of negative regulation of natural response via blockers [20]. Negative regulation in vivo requires that lipid blockers occur naturally in cells, occupy a substantial fraction of CD1 proteins on any given cell, and dominantly negatively block response to CD1 by many types of TCRs. Therefore, a potentially important advance in CD1 immunology is the clear identification of natural blocking ligands in the CD1a and CD1d systems that meet all three criteria.
Sulfatide inhibitors
While most studies emphasize T cell activation by lipids, De Jong’s studies of CD1a autoreactive T cells found that both sulfatide and sphingomyelin (SM) can inhibit T cell response. For sulfatide, crystallography demonstrated that the sulfate moiety disrupts the TCR recognition surface of CD1a through interactions with three residues in the A’ roof located near the F’ portal [10]. These studies provide proof of principle for blockade by a natural self ligand. Since CD1 proteins are non-polymorphic and the affected A’ roof and periportal regions are common TCR binding sites, sulfatides might now be developed as pharmacological reagents to inhibit CD1a-specific T cell responses in autoimmunity. However, as a candidate physiological blocker, sulfatides are rare in cells [32]. Also, our first efforts to elute natural ligands suggest that sulfatides are uncommon in CD1a eluents [12]. If a substantial fraction of CD1a proteins on a given cell are not occupied with sulfatide, this molecule acting alone, could not likely cause negative T cell regulation in physiological states.
Sphingomyelin fine structures
However, SMs are more common in cells [33], and three studies have recently demonstrated strong negative effects on CD1d- and CD1a-reactive T cells. Melum and Blumberg hypothesized that sphngomyelinase controls CD1d-mediated NKT response [13]. Taking broad advantage of in vivo analyses that are possible in mice, they found decreased selection and function of NKT cells in sphingomyelinase deficient animals. Although enzyme depletion could affect many activating or inhibitory lipids, a proximal and major change was the accumulation of SM in cells. Mechanistic studies, including CD1d plate bound activation studies, identified SM, as being sufficient to block CD1d mediated NKT cell activation.
Separately, Cotton and Cheng used an unbiased approach to measure which lipids are selectively captured by CD1a from human cells [12]. Unlike prior approaches to antigen discovery, this approach used mass spectrometry rather than activation assays, so it was possible to identify both activating and inhibitory lipids. Lipid eluents of CD1a showed ‘overcapture’ of SMs, especially unsaturated fatty acids with very long chains (C24:1), rather than the more common self SMs with C16 or C18 fatty acids. CD1a tetramer studies demonstrated strong blockade of polyclonal binding responses among unrelated human donors. Crystal structures of CD1a-SM demonstrated that the longer chains found in blockers allowed the charged choline headgroup to protrude more extensively (~6 angstrom) from the cleft (Figure 2), where it might sterically hinder the approach of any TCR.
Figure. 2.
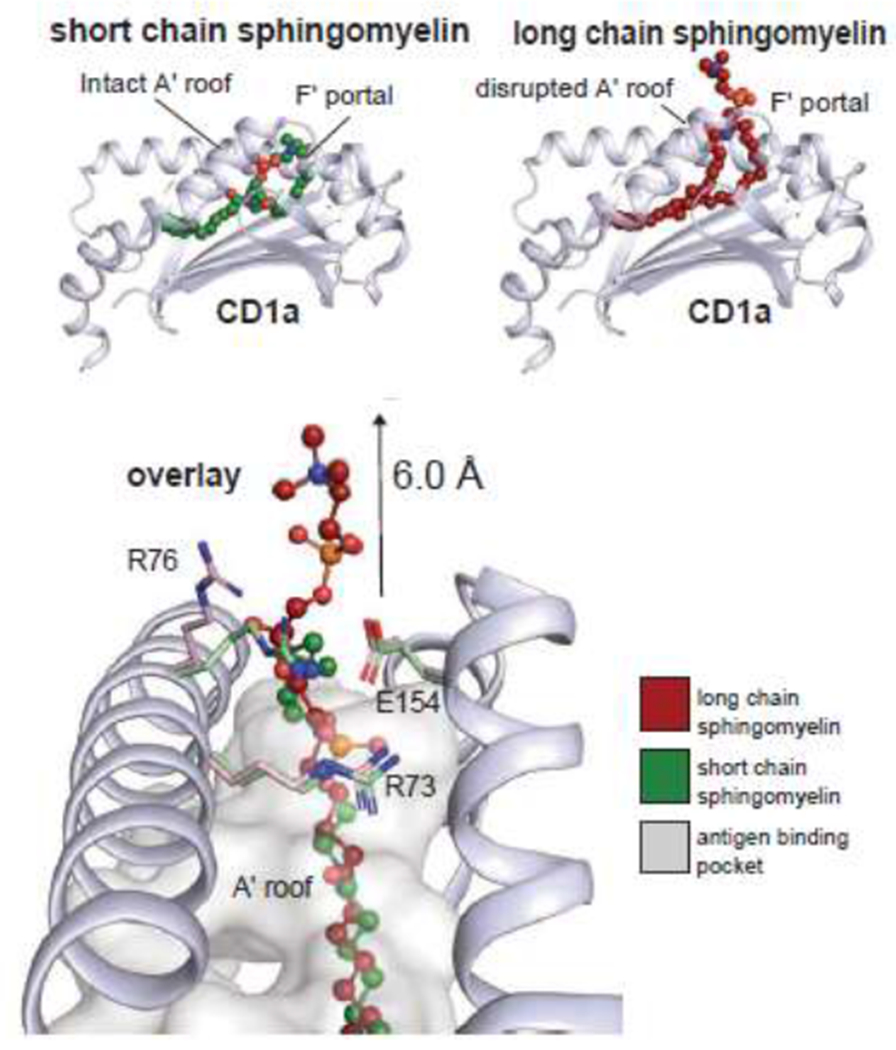
Sphingomyelins with short alkyl chains allow the choline headgroup to reside inside CD1a, but longer chain lipids shift the ligand so that the choline headgroup extends ~6 angstroms above the CD1 roof. Long chain sphingomyelins with blocking properties are selectively captured by CD1a in human cells.
Whereas these two studies independently identified SM blockers, CD1a and CD1d have differing antigen binding clefts, and the CD1d studies did not focus on the chain length of captured lipids. However, recent work by Rudolph and Zeissig now shows that CD1d can overcapture long chain C42 SMs and that such blockers are abundant in cells [14]. Thus, all three studies show remarkably convergent findings, emphasizing long chain length as a mechanism of favored lipid capture to generate complexes that optimally inhibit T cell response. Unlike sulfatides, SMs are typically the most abundant sphingolipid in cells. Based on these strong overcapture patterns, SMs likely occupy a sizable minority of CD1a or CD1d proteins in any cell, plausibly creating a baseline situation in which autoreactivity could dampened at steady state. Since overproduction or exogenous addition of long chain sphingomyelins provides strong negative regulation of polyclonal T cell response in vivo [13] and ex vivo [12], the emerging model is that CD1 specific TCRs frequently exist that do not require any given antigen. Instead they respond, or not, to CD1-expressing cells based on the relative balance of activating and blocking ligands.
Looking forward
Looking ahead, study of autoimmune skin disease remains a priority based on the demonstrated pro-inflammatory effects of human CD1a transgenesis in mouse skin [22,31], the high precursor frequencies of CD1a autoreactive cells [12,25]. Human skin provides ready access to T cells and blister fluids [34], and there is increasing evidence for a role of CD1a in human skin disease. For example, Ogg’s group showed increased numbers or effector functions of CD1a autoreactive T cells in psoriasis [35,36], atopic dermatitis [37] and contact dermatitis [37]. Conversely, antibody [22,31] or lipid blockers of CD1a function [12] can reduce T cell response or skin inflammation in vivo.
Further, the known ‘headless’ ligands for CD1a and CD1c are plausibly related to T cell mediated skin disease. LPC is generated by house dust mite, bee and wasp phospholipases that represent a major cause of atopic dermatitis [34,38]. Farnesol itself, related polyprenols and urushiol are contact dermatitis antigens [21,22]. Balsam of Peru, which contains small benzene-based hydrophobe presented by CD1a, is a common additive in commercial skin creams, where it causes of contact dermatitis [21]. Finally, the CD1a-dependent immune activator, squalene, is a major adjuvant that is widely used in anti-viral vaccines [9]. Augmenting traditional immunological assays, CD1-endo tetramers now provide a one-step means of estimating the numbers of CD1 autoreactive T cells in any individual, organ or disease state.
Conclusion
The central message of this review is that αβ TCRs can take differing approaches to CD1 proteins that either do or do not involve contact with carried lipids. Recently, γδ TCRs have been shown to bind or recognize all four types of human CD1 proteins, providing a new facet of CD1 biology. Whereas early studies of CD1d [39,40] and CD1c [41] suggested that γδ TCRs recognized carried lipids, CD1b [42] and CD1a [43] show lipid-independent recognition, similar to patterns for αβ T cells that are discussed here. Moreover unlike the upright, end-to-end approach of αβ TCRs (Figure 1), the first known γδ TCR-CD1a structure shows a wildly divergent approach, where it contacts the side of the CD1a and β2-microglobulin [43]. Thus, the new area of CD1-γδTCR interactions likely holds additional biological surprises as well.
Acknowledgements
We acknowledge funding support from the Wellcome Trust Collaborative Award, the NIH (AR048632, AI049313), an NHMRC Investigator Grant, an Australian Research Council Grant and the Australian Research Council DECRA Fellowship (DE210101031).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The website requests a conflict of interest, but the form cannot be found in the invitation, the COI website or FAQ on the Elsevier link. We expect that COI will require a more complete interest disclosure. Please provide the forms to the authors. We have declared the following competing interests in the cover letter.
The authors declare possible perceived conflicts based on consultation for Pfizer, EnaraBio and based on Brigham and Women’s pending patents.
References
- 1.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA: Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature 1989, 341:447–450. [DOI] [PubMed] [Google Scholar]
- 2.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB: Recognition of a lipid antigen by CD1-restricted alpha-beta T cells. Nature 1994, 372:691–694. [DOI] [PubMed] [Google Scholar]
- 3.Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, et al. : Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 1997, 278:283–286. [DOI] [PubMed] [Google Scholar]
- 4.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. : CD1d-restricted and TCR-mediated activation of Valpha14 NKT cells by glycosylceramides. Science 1997, 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 5.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, et al. : CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 2007, 448:44–49. [DOI] [PubMed] [Google Scholar]
- 6.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A: In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J.Exp.Med 2000, 191:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M: Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J.Exp.Med 2000, 192:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karadimitris A, Gadola S, Altamirano M, Brown D, Woolfson A, Klenerman P, Chen JL, Koezuka Y, Roberts IA, Price DA, et al. : Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc.Natl.Acad.Sci.U.S.A 2001, 98:3294–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong A, Cheng TY, Huang S, Gras S, Birkinshaw RW, Kasmar AG, Van Rhijn I, Pena-Cruz V, Ruan DT, Altman JD, et al. : CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat Immunol 2014, 15:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birkinshaw RW, Pellicci DG, Cheng TY, Keller AN, Sandoval-Romero M, Gras S, de Jong A, Uldrich AP, Moody DB, Godfrey DI, et al. : alphabeta T cell antigen receptor recognition of CD1a presenting self lipid ligands. Nature Immunology 2015, 16:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wun KS, Reijneveld JF, Cheng TY, Ladell K, Uldrich AP, Le Nours J, Miners KL, McLaren JE, Grant EJ, Haigh OL, et al. : T cell autoreactivity directed toward CD1c itself rather than toward carried self lipids. Nature Immunology 2018, 19:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotton RN, Wegrecki M, Cheng TY, Chen YL, Veerapen N, Le Nours J, Orgill DP, Pomahac B, Talbot SG, Willis R, et al. : CD1a selectively captures endogenous cellular lipids that broadly block T cell response. J Exp Med 2021, 218:e20202699. [DOI] [PMC free article] [PubMed] [Google Scholar]; Highly recommendedThis work uses and unbiased molecular screen to detect CD1a capture of sphingomyelin blockers of T cell response in cells. Lipids with blocking effect require long chain length, where the molecular mechanism of that longer alkyl chains increase binding to CD1a and protrusion of the choline head group.
- 13.Melum E, Jiang X, Baker KD, Macedo MF, Fritsch J, Dowds CM, Wang J, Pharo A, Kaser A, Tan C, et al. : Control of CD1d-restricted antigen presentation and inflammation by sphingomyelin. Nature Immunology 2019, 20:1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]; Highly recommendedThis paper describes the knockout of sphingomyelinase in mice, which leads overexpression of sphingomyelin, which binds CD1d and inhibits NKT cell appearance in organs. This work identifies a dominant negative effect of a CD1d presented blocking lipid in vivo.
- 14.Rudolph M, Wang Y, Simolka T, Huc-Claustre E, Dai L, Grotenbreg G, Besra GS, Shevchenko A, Shevchenko A, Zeissig S: Sortase A-Cleavable CD1d Identifies Sphingomyelins as Major Class of CD1d-Associated Lipids. Front Immunol 2022, 13:897873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez K, Iwany SK, Suliman S, Reijneveld JF, Ocampo TA, Jimenez J, Calderon R, Lecca L, Murray MB, Moody DB, et al. : CD1b Tetramers Broadly Detect T Cells That Correlate With Mycobacterial Exposure but Not Tuberculosis Disease State. Front Immunol 2020, 11:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gras S, Van Rhijn I, Shahine A, Le Nours J: Molecular recognition of microbial lipid-based antigens by T cells. Cell Mol Life Sci 2018, 75:1623–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasmar AG, van Rhijn I, Cheng TY, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, et al. : CD1b tetramers bind alpha-beta T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. The Journal of Experimental Medicine 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de Jager W, et al. : A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nature immunology 2013, 14:706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salio M, Silk JD, Jones EY, Cerundolo V: Biology of CD1- and MR1-restricted T cells. Annual Reviews Immunology 2014, 32:323–366. [DOI] [PubMed] [Google Scholar]
- 20.de Jong A: Activation of human T cells by CD1 and self-lipids. Immunol Rev 2015, 267:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolai S, Wegrecki M, Cheng TY, Bourgeois EA, Cotton RN, Mayfield JA, Monnot GC, Le Nours J, Van Rhijn I, Rossjohn J, et al. : Human T cell response to CD1a and contact dermatitis allergens in botanical extracts and commercial skin care products. Sci Immunol 2020, 5:eaax5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, Hu Y, Yongqing T, Kim J, Hughes VA, Le Nours J, Marquez EA, Purcell AW, Wan Q, Sugita M, et al. : CD1a on Langerhans cells controls inflammatory skin disease. Nat Immunol 2016, 17:1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jong A, Pena-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB: CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nature immunology 2010, 11:1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, Mori L, De Libero G, Dellabona P, Casorati G: High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. European journal of immunology 2011, 41:602–610. [DOI] [PubMed] [Google Scholar]
- 25.Cotton RN, Cheng TY, Wegrecki M, Le Nours J, Orgill DP, Pomahac B, Talbot SG, Willis RA, Altman JD, de Jong A, et al. : Human skin is colonized by T cells that recognize CD1a independently of lipid. J Clin Invest 2021, 131:e140706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, Patel O, Vivian JP, Matsuda JL, McCluskey J, et al. : A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity 2011, 34:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahine A, Reinink P, Reijneveld JF, Gras S, Holzheimer M, Cheng TY, Minnaard AJ, Altman JD, Lenz S, Prandi J, et al. : A T-cell receptor escape channel allows broad T-cell response to CD1b and membrane phospholipids. Nature Communications 2019, 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porcelli S, Morita CT, Brenner MB: CD1b restricts the response of human CD4 - 8 - T lymphoyctes to a microbial antigen. Nature 1992, 360:593–597. [DOI] [PubMed] [Google Scholar]
- 29.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM: Phenotypic analysis of antigen-specific T lymphocytes. Science 1996, 274:94–96. [PubMed] [Google Scholar]
- 30.Gherardin NA, Redmond SJ, McWilliam HEG, Almeida CF, Gourley KHA, Seneviratna R, Li S, De Rose R, Ross FJ, Nguyen-Robertson CV, et al. : CD36 family members are TCR-independent ligands for CD1 antigen-presenting molecules. Sci Immunol 2021, 6:eabg4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardman CS, Chen YL, Wegrecki M, Ng SW, Murren R, Mangat D, Silva JP, Munro R, Chan WY, O’Dowd V, et al. : CD1a promotes systemic manifestations of skin inflammation. Nat Commun 2022, 13:7535. [DOI] [PMC free article] [PubMed] [Google Scholar]; RecommendedThis recent work implicates CD1a in systemic autoimmune inflammation and advances antibody therapy via mapping of anti-CD1a to the TCR contact surfaces of CD1a that are discussed here.
- 32.van Meer G: Cellular lipidomics. EMBO Journal 2005, 24:3159–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH Jr., Murphy RC, Raetz CR, Russell DW, Seyama Y, Shaw W, et al. : A comprehensive classification system for lipids. J.Lipid Res 2005, 46:839–862. [DOI] [PubMed] [Google Scholar]
- 34.Bourgeois EA, Subramaniam S, Cheng TY, De Jong A, Layre E, Ly D, Salimi M, Legaspi A, Modlin RL, Salio M, et al. : Bee venom processes human skin lipids for presentation by CD1a. J Exp Med 2015, 212:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung KL, Jarrett R, Subramaniam S, Salimi M, Gutowska-Owsiak D, Chen YL, Hardman C, Xue L, Cerundolo V, Ogg G: Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med 2016, 213:2399–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh R, Chen YL, Ng SW, Cain D, Etherington R, Hardman C, Ogg G: Phospholipase activity of acyloxyacyl hydrolase induces IL-22-producing CD1a-autoreactive T cells in individuals with psoriasis. Eur J Immunol 2022, 52:511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarrett R, Salio M, Lloyd-Lavery A, Subramaniam S, Bourgeois E, Archer C, Cheung KL, Hardman C, Chandler D, Salimi M, et al. : Filaggrin inhibits generation of CD1a neolipid antigens by house dust mite-derived phospholipase. Sci Transl Med 2016, 8:325ra318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramaniam S, Aslam A, Misbah SA, Salio M, Cerundolo V, Moody DB, Ogg G: Elevated and cross-responsive CD1a-reactive T cells in bee and wasp venom allergic individuals. Eur J Immunol 2016, 46:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, Anderson B, Scharf L, Kung JE, Sibener LV, et al. : Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity 2013, 39:1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, Patel O, Beddoe T, Gras S, Rossjohn J, et al. : CD1d-lipid antigen recognition by the gammadelta TCR. Nat Immunol 2013, 14:1137–1145. [DOI] [PubMed] [Google Scholar]
- 41.Roy S, Ly D, Castro CD, Li NS, Hawk AJ, Altman JD, Meredith SC, Piccirilli JA, Moody DB, Adams EJ: Molecular Analysis of Lipid-Reactive Vdelta1 gammadelta T Cells Identified by CD1c Tetramers. J Immunol 2016, 196:1933–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reijneveld JF, Ocampo TA, Shahine A, Gully BS, Vantourout P, Hayday AC, Rossjohn J, Moody DB, Van Rhijn I: Human gammadelta T cells recognize CD1b by two distinct mechanisms. Proc Natl Acad Sci U S A 2020, 117:22944–22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wegrecki M, Ocampo TA, Gunasinghe SD, von Borstel A, Tin SY, Reijneveld JF, Cao TP, Gully BS, Le Nours J, Moody DB, et al. : Atypical sideways recognition of CD1a by autoreactive gammadelta T cell receptors. Nat Commun 2022, 13:3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnot GC, Wegrecki M, Cheng T, Chen Y, Sallee B, Chakravarthy R, Karantza IM, Tin SY, Khaleel AE, Monga I, et al. Staphylococcal phosphatidylglycerol antigens activate human T cells via CD1a, Nature Immunology, in press, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]; RecommendedDe Jong’s group identified phosphatidylgycerol antigens for CD1a-autoreactive T cells produced by bacteria related to atopic dermatitis. The antigen is foreign, but is produced by skin symbionts that can be considered part of the skin microbiome.


