Abstract
BACKGROUND:
Smooth muscle cell (SMC) phenotypic switching has been increasingly detected in aortic aneurysm and dissection (AAD) tissues. However, the diverse SMC phenotypes in AAD tissues and the mechanisms driving SMC phenotypic alterations remain to be identified.
METHODS:
We examined the transcriptomic and epigenomic dynamics of aortic SMC phenotypic changes in mice with angiotensin II–induced AAD by using single-cell RNA sequencing (scRNA-seq) and single-cell sequencing assay for transposase-accessible chromatin (scATAC-seq). SMC phenotypic alteration in aortas from patients with ascending thoracic aortic aneurysm and dissection (ATAAD) was examined by using scRNA-seq analysis.
RESULTS:
ScRNA-seq analysis revealed that aortic stress induced the transition of SMCs from a primary contractile phenotype to proliferative, extracellular matrix (ECM)-producing, and inflammatory phenotypes. Lineage tracing showed the complete transformation of SMCs to fibroblasts and macrophages. ScATAC-seq analysis indicated that these phenotypic alterations were controlled by chromatin remodeling marked by the reduced chromatin accessibility of contractile genes and the induced chromatin accessibility of genes involved in proliferation, ECM, and inflammation. IRF3, a pro-inflammatory transcription factor activated by cytosolic DNA, was identified as a key driver of the transition of aortic SMCs from a contractile phenotype to an inflammatory phenotype. In cultured SMCs, cytosolic DNA signaled through its sensor STING-TBK1 to activate IRF3, which bound and recruited EZH2 to contractile genes to induce repressive H3K27me3 modification and contractile gene suppression. In contrast, dsDNA-STING-IRF3 signaling induced inflammatory gene expression in SMCs. In Sting−/− mice, the aortic stress–induced transition of SMCs into an inflammatory phenotype was prevented, and SMC populations were preserved. Finally, profound SMC phenotypic alterations toward diverse directions were detected in human ATAAD tissues.
CONCLUSION:
Our study reveals the dynamic epigenetic induction of SMC phenotypic alterations in AAD. DNA damage and cytosolic leakage drive SMCs from a contractile phenotype to an inflammatory phenotype.
Keywords: aortic aneurysm, aortic dissection, smooth muscle cell, phenotypic switching, STING, IRF3
INTRODUCTION
Aortic smooth muscle cells (SMCs) are a dynamic population of cells that possess remarkable phenotypic plasticity. Under stress, SMCs lose their contractile phenotype and gain other phenotypes1–3 that resemble fibroblasts,4 inflammatory cells,1,5 and osteogenic cells.6 Increasing evidence suggests that SMC phenotypic alteration is a common feature of both thoracic and abdominal aortic aneurysms and dissections (AAD).5,7–15 However, the diverse SMC phenotypic alterations in AAD remain to be characterized, and the mechanisms that drive these changes remain to be identified.
The recent development of single-cell RNA sequencing (scRNA-seq) and single-cell sequencing for transposase-accessible chromatin (scATAC-seq) technology has provided unprecedented opportunities to explore the transcriptomic and epigenomic dynamics of cell phenotypic changes. By utilizing single-cell multiomic profiling and lineage tracing techniques, we detected diverse SMC populations in sporadic AAD tissues. During AAD development, SMCs transformed from contractile SMCs to proliferative SMCs, inflammatory SMCs, extracellular matrix (ECM)-producing SMCs, and dying SMCs, partially through epigenetic regulation and chromatin remodeling. IRF3 was identified as a key driver of SMC transition to a proinflammatory phenotype. Furthermore, cytosolic DNA accumulation via STING-IRF3-EZH2 signaling induced repressive H3K27me3 modification and suppressed the expression of contractile genes while inducing the expression of inflammatory genes. Finally, Sting deficiency in mice prevented aortic SMC phenotypic alterations, pointing to the STING-IRF3-EZH2 pathway as a critical signaling pathway that promotes SMC transition toward a proinflammatory direction.
METHODS
Data Availability
All detailed methods are presented in the Materials and Methods of the Supplemental Material online. All raw data and analytical methods are available from the corresponding author upon reasonable request. Sequencing data have been made publicly available at the Gene Expression Omnibus (GEO): The lineage-tracing mouse scRNA-seq data can be accessed at GSE233257, the mouse scATAC-seq data can be accessed at GSE214082, and the Sting-KO mouse scRNA-seq data can be accessed at GSE233625. The computer code used in this study is available upon request.
Murine Studies
For experiments designed to visualize specific cellular markers, ROSAmT/mG mice (strain #007676) and Myh11-icre/ERT2 mice (strain #019079) were obtained from the Jackson Laboratory and crossbred. Male pups with a Myh11Cre+ genotype were administered tamoxifen to induce the expression of Cre recombinase. This led to the replacement of inherent red fluorescence (RFP) with green EGFP (mG) fluorescence localized at the cell membrane. At 8 weeks of age, these mice underwent infusion with either saline or a low dose of angiotensin II (AngII; 1000 ng/kg/min) for 7 days, and the aorta was collected at the 7-day timepoint for further analysis.
Wild-type (WT) mice (C57BL/6J) as well as Sting-deficient mice (C57BL/6J-Tmem173gt/J, Stinggt/gt) were obtained from the Jackson Laboratory and crossbred. At 8 weeks of age, all mice, both WT and Sting-deficient, underwent osmotic pump implantation. They were then infused with either saline or AngII (2000 ng/kg/min) for 7 days. At the end of the infusion period, the mice were euthanized by CO2-asphyxiation, followed by perfusion with 10 mL of ice-cold phosphate-buffered saline (PBS) through the left ventricle. The mice were divided into experimental groups based on their genotypes, and randomization was performed for subsequent experiments. For mice selected for scRNA-seq analysis, ascending aortic tissue was collected after the infusion period, and cells were prepared for single-cell suspension according to the protocol described in the following section.
Human Tissue Study
Ascending aortic tissues from patients with sporadic ascending thoracic aortic aneurysm without dissection (ATAA; n=9, 6 men and 3 women), patients with acute ascending thoracic aortic dissection (ATAD; n=9, 6 men and 3 women), and donors or recipients of heart or lung transplants without aortic diseases (controls, n=8, 5 men and 3 women) were collected for scRNA-seq study. Aortic diseases related to heritable conditions, infection, aortitis, and trauma were excluded from the study. The protocol for collecting human tissue samples was approved by the Institutional Review Board at Baylor College of Medicine (Houston, TX). Written informed consent was provided by all participants before enrollment. All experiments conducted with human tissue samples were performed in accordance with the relevant guidelines and regulations.
ScRNA-seq
Single-cell suspensions were separately dispensed onto the Chromium Controller (10x Genomics, Pleasanton, CA); we targeted 10,000 cells per sample. Our scRNA-seq library was constructed by using the Chromium Single-Cell 3′ v3 Reagent Kit (10X Genomics). Cells were then mixed with barcoded primer-linked gel beads such that every cell was attached to a unique barcode, and every transcript from a given cell had a unique molecular identifier (UMI). cDNAs were subsequently pooled, truncated, and amplified to generate cDNA libraries that were sequenced by using a Next Generation Sequencer NovaSeq 6000 (Illumina, Inc., San Diego, CA) in a pair-end fashion to obtain greater than 20,000 reads per cell.
ScATAC-seq
Single-nuclei suspensions were prepared according to the manufacturer’s instructions (10X Genomics). We loaded nuclei and set a capture target of 10,000 nuclei per sample. According to the 10X Genomics scATAC-seq solution protocol, we prepared scATAC-seq libraries ready for sequencing. Using PE150 sequencing on an Illumina NovaSeq, we selected a target depth of 25,000 reads per nucleus and sequenced the scATAC-seq libraries.
Statistical Analysis
Quantitative data are presented as mean ± standard deviation or as the median and interquartile range, as appropriate. All data were analyzed with either SPSS statistical analysis software (SPSS Inc., Chicago, IL, USA) or R. To examine whether a particular class of genes had increased (or decreased) expression in scRNA-seq datasets, the Wilcoxon rank sum test was performed to compare the genes between two groups; p values were adjusted by Bonferroni correction using all genes in the dataset. Statistical analysis of quantitative PCR and chromatin immunoprecipitation (ChIP) assay data was performed using two-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparisons test where appropriate. The analysis was performed using GraphPad Prism V5.01, and p values <0.05 were considered significant.
RESULTS
ScRNA-seq Analysis of Dissected and Non-dissected Aortic Tissues in Angiotensin II Infusion AAD Model
To understand aortic SMC dynamics during AAD development, we performed the single-cell transcriptomic analysis of aortic tissues collected from Myh11-CreERT2+ mT/mG lineage-tracing mice that were infused with saline or AngII (1000 ng/kg/min) for 7 days (Figure 1A). To understand the differential molecular and cellular changes associated with disease severity in the AngII-infused group, aortas with intramural hematoma/dissection (dissection) and without intramural hematoma/dissection (non-dissection) were separately processed and analyzed (Figure S1A). For each of the three groups (control, non-dissection, and dissection), single-cell suspensions from three ascending thoracic aortas were pooled. SMCs (GFP+ cells) and non-SMCs (RFP+ cells) were further separated by using flow cytometry and individually processed for scRNA-seq (Figure S1B and S1C). In total, six samples were submitted for scRNA-seq analysis (Figure 1A).
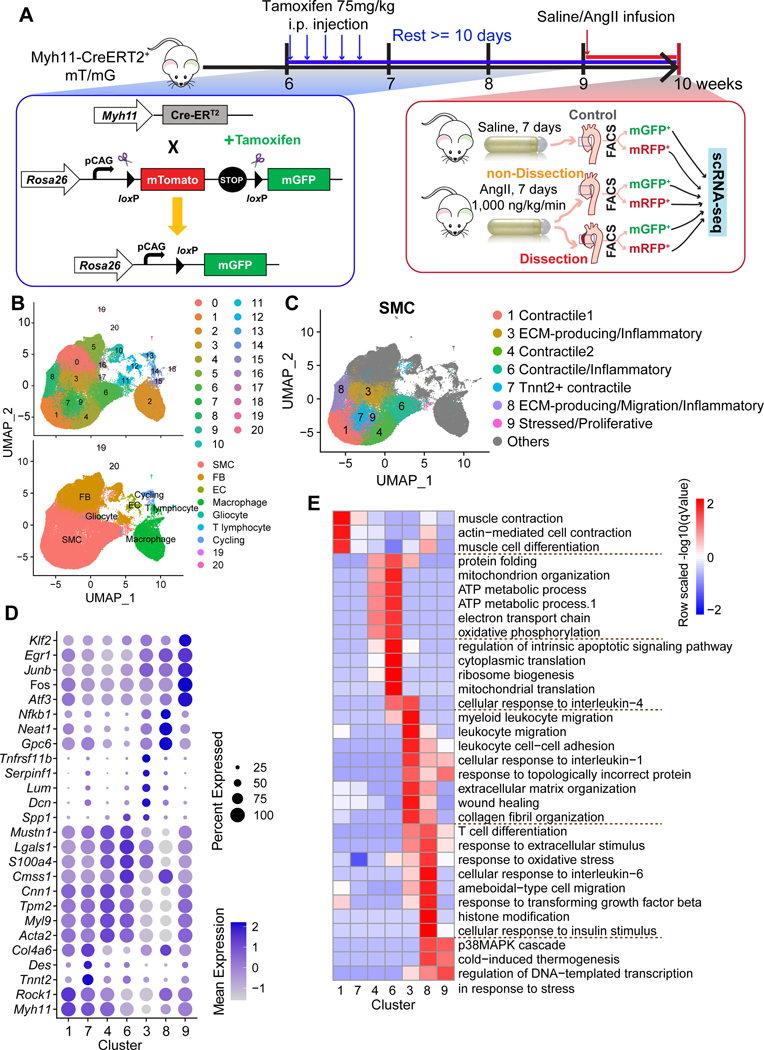
Figure 1. Single-cell analysis reveals the heterogeneity of cell populations in the aortic wall of SMC lineage-tracing mice.
A, Workflow for obtaining single-cell RNA-sequencing (scRNA-seq) data from Myh11-creERT2+ mT/mG lineage-tracing mice infused with saline (control mice) or angiotensin II (AngII-infused mice). The aortas were classified as control, non-dissection, and dissection. The cells were separated into GFP+ cells and RFP+ cells by flow cytometry. B, Two-dimensional uniform manifold approximation and projection (UMAP) plots showing all cells colored according to the identified 21 clusters. C, UMAP plot showing the subclusters of SMCs. A total of 7 clusters were identified. All other types of cells were colored in gray. D, Dot plot representing the conserved genes. E, Gene ontology (GO) analysis of the conserved genes for SMC subclusters.
Among the 98,585 qualified cells from all samples, 21 clusters were identified (Figure 1B and Figure S1D). According to the expression of cell type–specific marker genes,16 these clusters were defined as SMCs (Acta2, Myh11, Cnn1, Mylk, Myl9) (Figure S1D and S1E), endothelial cells (Pecam1, Vwf, Cldn5, Egfl7, Ptprb), fibroblasts (Col1a1, Col1a2, Col14a1, Serpinf1, Dcn), gliocytes/macrophages (C1qa, Apoe, Mrc1, Lyz2, Ccl6), T lymphocytes, and cycling cells (Stmn1, Ube2c, Cks2, Cenpa, Top2a).
Diverse SMC Populations in the Thoracic Aorta
Seven clusters were identified as SMCs (Figure 1C), representing 54% of all aortic cells. To define and characterize these SMC subclusters, we identified differentially expressed genes (DEGs) for each cluster (Figure 1D, Supplemental Excel File 1), and performed gene ontology (GO) analysis on cluster-specific DEGs to identify the overrepresented functions (Figure 1E, Supplemental Excel File 2).
Clusters 1, 4, and 7 were identified as contractile SMC clusters. Cluster 1 was defined as primary contractile SMCs. The results of DEG (Figure 1D), GO (Figure 1E), and top-conserved gene analyses (Supplemental Excel File 2) showed that Cluster 1 had a high level of genes involved in contraction without significant features of other functions. Cluster 4 was another contractile SMC cluster, with high expression of contractile genes such as Acta2, Myl9, and Tpm2 and enriched function in ATP production. Cluster 7 represents a unique SMC cluster in the ascending aorta that exhibits high levels of TNNt2 gene expression. In light of this distinct molecular signature, we have designated this cluster as the “TNNt2+ SMC cluster.” Immunofluorescence analysis confirmed the specific expression of Tnnt2 in the ascending part and curved region of the mouse aorta (Figure S1F). These findings imply a potential contribution of TNNt2 to regional specialization and functional diversity within the vasculature.
Cluster 3 was defined as ECM-producing/inflammatory SMCs, which exhibited enriched function in ECM organization and leukocyte-related functions. Cluster 6 was defined as contractile/inflammatory SMCs, which showed features of contractile as well as inflammatory pathways such as response to interleukin 4. Cluster 8 was defined as migration/inflammatory SMCs, which had features similar to those of Cluster 3 but also exhibited features in ameboidal-type cell migration and histone modification. Cluster 9 was defined as stressed/proliferative SMCs, with a high expression level of genes that function in proliferation (eg, Atf3,17 Fos,18 Dnaja1,19 Klf6,20 Irs2,21 Junb,22 Egr1,23 Btg2,24 Nr4a325). Of note, proliferative SMCs showed response to incorrect proteins (Figure 1E) and high expression of immediate early genes (eg, Fos, Fosb, and Junb) (Supplemental Excel File 2), which has been suggested to be a stress response to tissue processing.26
Aortic Stress–Induced SMC Phenotypic Transition From Contractile SMCs to ECM-producing SMCs and Inflammatory SMCs
After characterizing the features of aortic SMC clusters, we examined the dynamics of these clusters during AngII-induced AAD development. We observed that the primary contractile SMC cluster (Cluster 1) and the Tnnt2+ contractile SMC cluster (Cluster 7) were progressively smaller from control to non-dissection to dissection mice (Figure 2A and 2B). In contrast, compared with control mice, the ECM/inflammatory SMC cluster (Cluster 3) was increased in non-dissection mice and dissection mice, and the contractile/inflammatory SMC cluster (Cluster 6) was significantly increased in dissected aortas. Taken together, our data indicate that aortic stress promotes SMC transition from the contractile phenotype to the ECM-producing and inflammatory phenotypes.
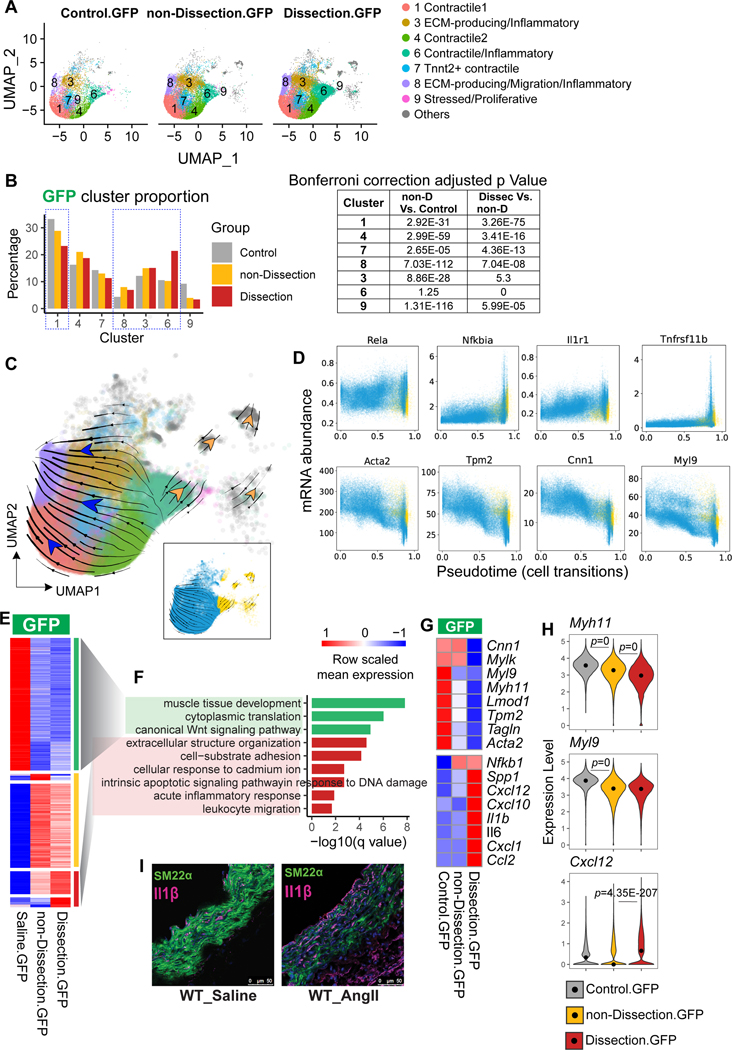
Figure 2. Decreased contractile smooth muscle cell (SMC) population and contractile gene expression and increased pro-inflammatory SMC population and inflammatory gene expression in the aorta of angiotensin II (AngII)-infused mice.
A, Uniform manifold approximation and projection (UMAP) plots representing the intercluster similarity in the aorta among control, non-dissection, and dissection aortas with GFP+ and RFP+ cells separately. B, A bar plot representing the percentage of different SMC subclusters in the GFP+ cells from aortas of control, non-dissection, and dissection mice. The chi-square test of goodness-of-fit was performed to compare the proportion between groups; the p values were adjusted by Bonferroni correction. C-D, RNA velocity analysis was performed to estimate the transition of aortic SMCs induced by aortic challenge. E, Heatmap showing the row-scaled mean expression of DEGs identified from non-dissection vs control, and from dissection vs non-dissection. DEGs were classified according to their expression profiles across 3 samples. F, The top biological process enriched from decreased DEGs (green bars) and increased DEGs (red bars) in GFP+ SMCs of AngII-infused mice. G, Heatmap showing the mean expression of contractile and inflammatory genes expression in GFP+ SMCs in control, non-dissection, and dissection samples. H, Violin plot showing the distribution of Myh11, Myl9, and Cxcl12 expression values in GFP+ SMCs of control, non-dissection, and dissection samples. Black dots in the violins indicate the median values. The Wilcoxon rank sum test was performed to compare the genes between two groups; p values were adjusted by Bonferroni correction using all genes in the dataset. I, Immunofluorescence analysis of mT/mG lineage tracing showing SM22α and IL-1β expression in the aortic tissue of WT mice infused with saline (left panel) or AngII (right panel).
Progressive Reduction of Contractile Genes and Induction of Inflammatory Genes During SMC Phenotypic Transition and Disease Development
To further understand the phenotypic transition of SMCs, we performed RNA velocity analysis using cellDancer27 (Figure 2C and 2D). The RNA velocity estimated the two directions of cell transition: from contractile toward migration/inflammatory phenotype, and from contractile/inflammatory toward other cell types (mostly macrophages) (Figure 2C). RNA velocity–predicted pseudotime was toward a proinflammatory and pro-death phenotype, with SMCs exhibiting the gradual loss of contractile gene expression and the gradual induction in the expression of genes involved in cell stress and the inflammatory response (Figure 2D).
We further examined the dynamics of gene expression in SMCs during aortic disease progression. By comparing genes in GFP+ SMCs between the non-dissection and control groups and between the dissection and non-dissection groups, we identified 760 DEGs that were classified into three types of expression patterns (Figure 2E). GO analysis showed that genes with functions in muscle tissue development and cytoplasmic translation gradually decreased (marked as green on the right side of heatmap) (Figure 2F) while genes with functions in ECM organization, inflammation, and apoptosis progressively increased from control to non-dissection to dissection (marked as red on the right side of heatmap) (Figure 2F). Further DEG analysis confirmed a progressive decrease in contractile gene expression during disease progression and an induction of inflammatory genes in dissection (Figure 2G and 2H). Immunofluorescence staining confirmed that SM22α expression was reduced and IL-1β expression was increased in SMCs after AngII infusion (Figure 2I).
Lineage-Tracing Analysis Detected Complete SMC Transformation to Fibroblasts and Macrophages Under Aortic Stress
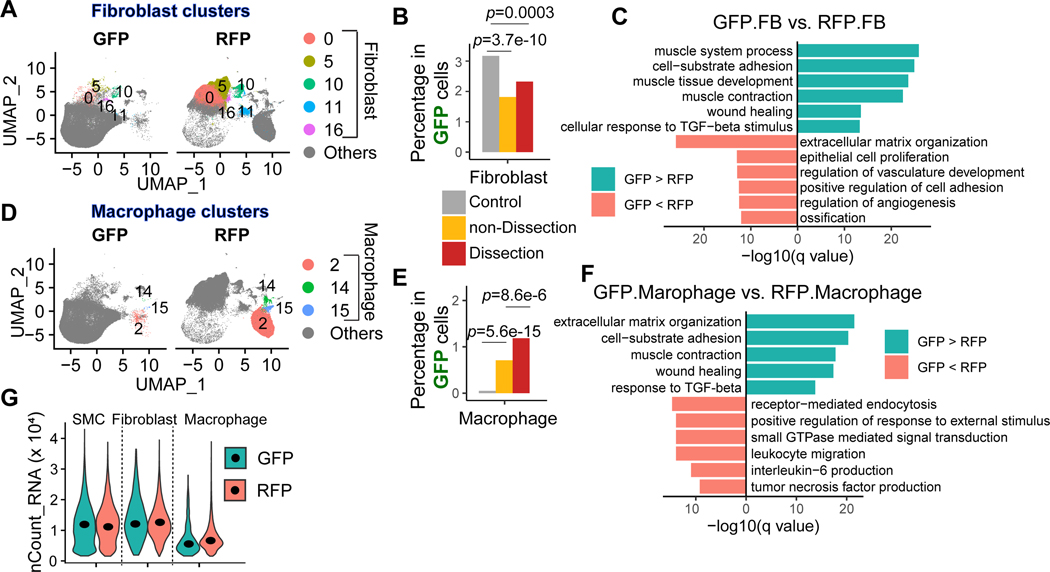
Taking advantage of the lineage-tracing system, we further examined whether SMCs can change their phenotypes to the extent that they completely transform into different types of cells. Indeed, some GFP+ cells with SMC origin were detected in fibroblast clusters (Figure 3A), suggesting that these SMCs transformed into fibroblasts. The proportion of GFP+ SMC-derived fibroblasts was lower in the aorta of AngII-infused mice compared with control mice (Figure 3B). Further DEG and GO analysis indicated that compared with regular RFP+ non-SMC lineage fibroblasts, the GFP+ SMC-derived fibroblasts exhibited higher expression of genes involved in adhesion, contraction, and wound healing and lower expression of genes involved in ECM organization and angiogenesis (Figure 3C). On the other hand, some GFP+ cells with SMC origin were detected in macrophage clusters (Figure 3D), suggesting that these SMCs transformed into macrophages. The proportion of SMC-derived macrophages was higher in the aorta of AngII-infused mice compared with control mice (Figure 3E). Furthermore, compared with regular RFP+ non-SMC lineage macrophages, the GFP+ SMC-derived macrophages exhibited higher expression of genes involved in ECM organization, adhesion, contraction, and wound healing and lower expression of genes involved in endocytosis, leukocyte migration, and cytokine production (Figure 3F). Of note, the existence of SMC-derived fibroblasts and macrophages was probably not due to technical reasons (eg, a doublet) because their count number per cell remained similar to or even lower than that of regular fibroblasts and macrophages (Figure 3G).
Figure 3. SMC lineage cells transformed to fibroblasts and macrophages.
A, Uniform manifold approximation and projection (UMAP) plots representing the fibroblast clusters in each sample. B, Proportion of fibroblasts in GFP+ cells in control, non-dissection, and dissection samples. C, A bar plot showing the top enriched biological process of significantly upregulated and downregulated genes in GFP+ fibroblasts compared with RFP+ fibroblasts. A one-tailed Fisher exact test was applied; all the mouse genes were set as background genes, and the p-value was adjusted by using the Benjamini-Hochberg method. The Fisher exact test was performed to compare the proportion between groups; the p values were adjusted by Bonferroni correction. D, Uniform manifold approximation and projection (UMAP) plots representing the macrophage clusters in each sample. E, Proportion of macrophages in GFP+ cells in control, non-dissection, and dissection samples. F, Bar plot showing the top enriched biological process of significantly upregulated and downregulated genes in GFP+ macrophages compared with RFP+ macrophages. A one-tailed Fisher exact test was applied; all the mouse genes were set as background genes, and the p-value was adjusted by using the Benjamini-Hochberg method. G, A violin plot showing the distribution of counts of RNA per cell in SMCs, fibroblasts, and macrophages that were GFP+ or RFP+.
ScATAC-seq Revealed the Chromatin Accessibility Landscape of Mouse Aortic SMCs
To understand the regulation of SMC phenotypic alterations in AAD, we examined genome-wide chromatin accessibility in aortic SMCs of AngII-infused mice by re-analyzing our previously published scATAC-seq data (Figure 4A). The scATAC-seq was performed on WT mice; we focused only on the SMC populations because of a lack of lineage-tracing information. ScATAC-seq analysis revealed the chromatin accessibility landscape of the whole genome in each cell. For each gene, we analyzed chromatin accessibility in the cis-regulatory elements (CREs) to calculate the gene activity score.28
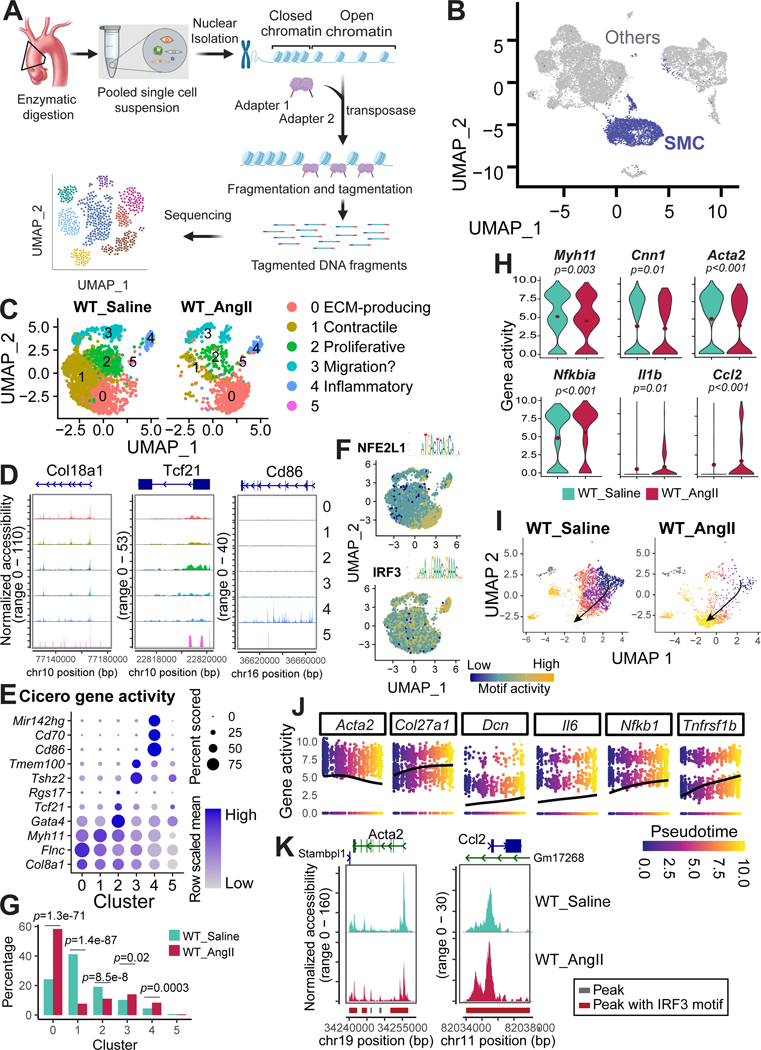
Figure 4. Decreased chromatin accessibility in contractile genes and increased chromatin accessibility in inflammatory genes in aortic smooth muscle cells (SMCs) of angiotensin II (AngII)-infused mice.
A, Workflow for obtaining and analyzing single-cell sequencing assay for transposase-accessible chromatin (scATAC-seq) data from the aortic tissues of saline-infused wild-type (WT) mice infused with saline or AngII-infused WT mice. B, Uniform manifold approximation and projection (UMAP) plot showing the SMCs identified from the scATAC-seq data. C, UMAP plot showing the SMC clusters identified by re-clustering all the SMCs. D, Chromatin accessibility of at locus of several marker genes in each SMC clusters. E, Dot plot of gene activity. The activity of genes was calculated by Cicero, and genes that were identified as markers by their activity are presented in the dot plot. F, Feature plot showing the motif activity of NFE2L1 and IRF3. G, Bar plot representing the percentage of different clusters in AngII-infused WT mice versus saline-infused WT mice. The Fisher exact test was performed to examine differences in the cluster proportion between groups; the p values were adjusted by Bonferroni correction. H, Violin plots showing the expression of contractile genes and inflammatory genes in control versus AngII-infused mice. I-J, Trajectory analysis illustrating the SMC transition from well-differentiated contractile SMCs in saline-infused mice to de-differentiated SMCs in AngII-infused mice. K, scATAC-seq analysis showing accessibility and peaks detected at the chromatin regions of a selected contractile gene (Acta2, left panel) and of a selected inflammatory gene (Ccl2, right panel). Peaks harboring Irf3 motifs were highlighted in red. Accessibility was shown for SMCs under different conditions.
We extracted SMCs in scATAC-seq data (Figure 4B, previously described29) and re-clustered the SMCs. Similar to the SMC subpopulations detected in the scRNA-seq data, six SMC clusters were identified in the scATAC-seq data (Figure 4C). The features of each of these SMC clusters were then determined from the chromatin accessibility of marker genes (Figure 4D), differentially activated genes (DAGs) (Figure 4E and Figure S2A and S2B), and activity of transcription factors’ motifs (Figure 4F and Figure S2C). Cluster 0 was defined as ECM-producing SMCs for higher gene activity of Col8a1 as well as higher motif activity of NEF2L1. Cluster 1 was defined as primary contractile SMCs due to higher gene activity of Myh11. Cluster 2 was defined as proliferative SMCs because it exhibited higher gene activity of Rgs17, Tcf21,30 and Gata431 (Figure 4D–3E). Cluster 3 exhibited specific higher gene activity of Teme100 and Tshz2, which can be defined as migrating SMCs. Cluster 4 was defined as inflammatory SMCs exhibiting higher gene activity of Cd86, Cd70, and Mir142hg (Figure 3D and 3E) and higher motif activity of IRF3 (Figure 3F). Cluster 5 will not be discussed as the number of cells in this cluster was too small for meaningful analysis (Figure 4C). Together, we identified similar SMC subpopulations in scRNA-seq and scATAC-seq datasets, suggesting that the diverse SMC subpopulations are programmed, at least partially, by chromatin remodeling via epigenetic mechanisms.
Aortic Stress Decreased the Chromatin Accessibility of Contractile Genes and Increased the Chromatin Accessibility of Inflammatory Genes in Aortic SMCs
Consistent with what we observed in our scRNA-seq analysis, we observed a reduction in the size of the contractile SMC cluster (Cluster 1) and an increase in the size of the ECM-producing SMC cluster (Cluster 0) and the inflammatory SMC cluster (Cluster 4) (Figure 4G) in AngII-infused mice, suggesting that SMC phenotypic alterations are controlled by chromatin remodeling. Additionally, the activity of contractile genes was downregulated, whereas the activity of inflammatory genes was upregulated in AngII-infused mice (Figure 4H).
Trajectory analysis of scATAC-seq data showed the transitioning of SMCs from a well-differentiated contractile phenotype in control mice toward a dedifferentiated phenotype in AngII-infused mice (Figure 4I). This transition in phenotype was associated with the gradual loss of activity/chromatin accessibility of SMC contractile genes and the gradual induction in the expression of genes involved in matrix organization, inflammation, and cell stress (Figure 4J).
When we further visualized genome track regulatory regions of SMC contractile genes (eg, Acta2) in SMCs, we observed less chromatin accessibility in these regions in AngII-infused mice than in control mice (Figure 4K and Figure S2B). In contrast, chromatin accessibility in the regulatory regions of inflammatory genes (eg, Ccl2, Il6, Nfkba, Cxcl10) in SMCs was relatively low in control mice and significantly higher in AngII-infused mice (Figure 4K and Figure S2D). Together, these data suggest that aortic challenge induces a SMC phenotypic switch from a contractile to inflammatory phenotype at the epigenetic level via chromatin remodeling.
IRF3 was Identified as a Key Transcription Factor Driving Chromatin Remodeling and Phenotypic Transition in Aortic SMCs
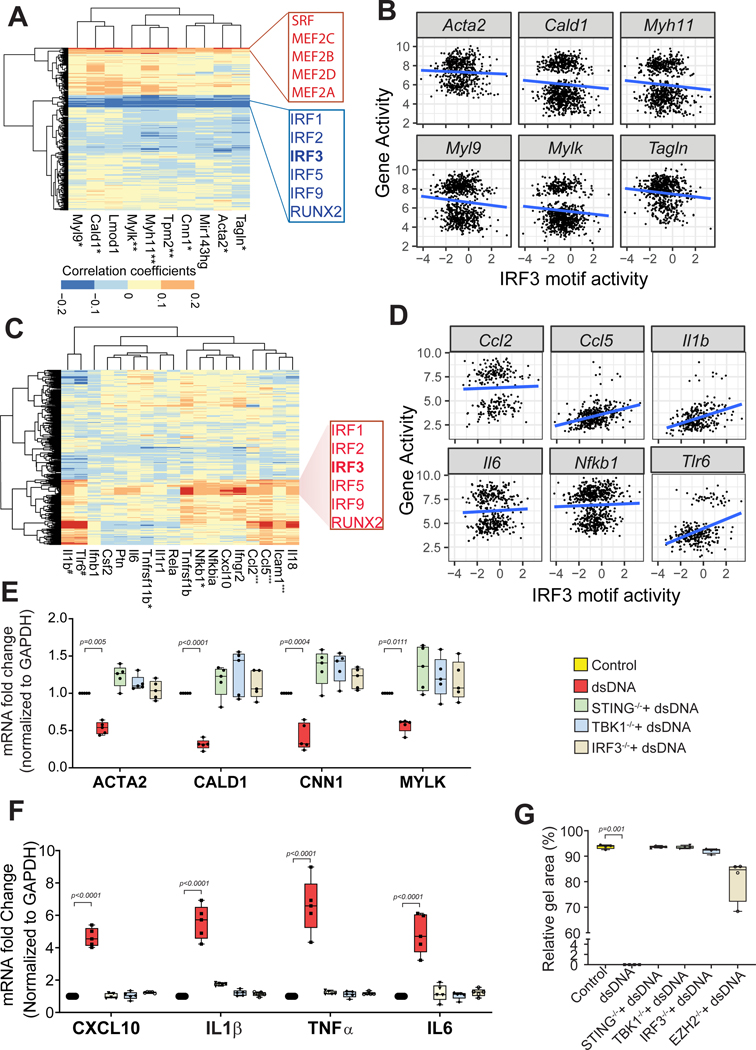
We next sought to identify the potential transcription factors (TFs) that drive the suppression of contractile gene expression in aortic SMCs. Using chromVAR,32 we calculated the activity of each TF-binding motif per cell. Correlation analysis was then performed between the activity of ten selected SMC contractile genes and the motif activity of TFs. The activity of SMC contractile genes was strongly associated with the motif activity of the MEF2 family and serum response factor (SRF) (Figure 5A), both of which are well-established master regulators of SMCs.33,34 This finding confirmed the reliability of our approach. Interestingly, the activity of SMC contractile genes was negatively associated with the motif activity of several TFs, including members of the proinflammatory IRF family of TFs such as IRF3 (Figure 5A). The negative association between the motif activity of IRF3 and the activity of contractile genes was confirmed by correlation analysis (Figure 5B), suggesting a role for IRF3 in inhibiting contractile gene expression. Indeed, we detected IRF3 motifs in the regulatory regions of Acta2 that showed reduced activity/chromatin accessibility in AngII-infused mice (Figure 4K).
Figure 5. Single-cell sequencing assay for transposase-accessible chromatin (scATAC-seq) and in vitro analysis data reveals IRF3 as a key transcription factor in the inhibition of smooth muscle cell (SMC) gene expression and the induction of inflammatory gene expression in aortic SMCs.
A, Heat map showing correlation coefficients between contractile genes and SMC-related transcription factor motif activity in mouse aortic SMCs. B, Representation of correlation between IRF3 motif activity and activity of SMC contractile genes. C, Heat map showing correlation coefficients between selected inflammatory genes and SMC-related transcription factor motif activity. D, Representation of correlation between IRF3 motif activity and activity of inflammatory genes. E, Quantitative PCR analysis from cultures of human VSMCs showing that treatment with dsDNA suppresses contractile gene expression in wild-type (WT) SMCs; this was partially reversed in STING−/−, TBK1−/−, and IRF3−/− SMCs. F, Treatment with dsDNA increased inflammatory gene expression in WT SMCs that was partially prevented in STING−/−, TBK1−/−, and IRF3−/− SMCs. G, dsDNA treatment compromised SMC contraction, which was restored in STING−/−, TBK1−/−, IRF3−/−, and EZH2−/− SMCs. dsDNA: double-stranded DNA, KO: knockout. *p≤0.05; **p≤0.01; ***p≤0.0001.
In contrast to the negative association between contractile gene activity and IRF motif activity, we observed a strong positive correlation between inflammatory gene activity and IRF motif activity, particularly for IRF3 (Figure 5C and 5D). Consistent with this, the IRF3 motif was detected in the peaks of inflammatory genes (eg, Ccl2) that showed increased expression in the aortic SMCs of AngII-infused mice (Figure 4K). Our findings indicate that the TF IRF3 potentially controls the suppression of contractile gene expression and the induction of inflammatory gene expression in aortic SMCs.
dsDNA-STING-TBK1-IRF3 Signaling Suppressed Contractile Gene Expression, Induced Inflammatory Gene Expression, and Compromised Contractile Function of Human SMCs In Vitro
We next investigated the direct role of IRF3 in contractile gene regulation. IRF3 is a target of cytosolic DNA and the proinflammatory cytosolic DNA–sensing STING pathway, which we have recently shown to play a critical role in AAD development.7 We therefore performed experiments in cultured human aortic SMCs to determine whether activation of the dsDNA-STING-TBK1-IRF3 pathway contributes to aortic SMC phenotypic alteration.
Using the CRISPR/Cas9 system, we generated stable lines of STING−/− SMCs, TBK1−/− SMCs, and IRF3−/− SMCs. WT, STING−/−, TBK1−/−, and IRF3−/− SMCs were stimulated with dsDNA (herring testis DNA, 2 μg/ml) for 24 hours. Treating WT SMCs with dsDNA suppressed contractile gene expression, but this was partially reversed in dsDNA-treated STING−/−, TBK1−/−, and IRF3−/− SMCs (Figure 5E and Figure S3A). In contrast, treating WT SMCs with dsDNA increased inflammatory gene expression, but this was partially prevented in dsDNA-treated STING−/−, TBK1−/−, and IRF3−/− SMCs (Figure 5F and Figure S3B). Furthermore, contraction analysis of SMCs embedded in collagen gel showed that contraction was compromised in WT SMCs treated with dsDNA but restored in STING−/−, TBK1−/−, and IRF3−/− SMCs (Figure 5G). These data suggest that the dsDNA-STING-TBK1-IRF3 signaling pathway plays a role in SMC contractile gene suppression, inflammatory gene induction, and contractile dysfunction in aortic SMCs.
dsDNA-STING-TBK1-IRF3 Signaling Suppressed Contractile Gene Expression by Recruiting EZH2 and Inducing Repressive H3K27me3 Modification and Chromatin Remodeling
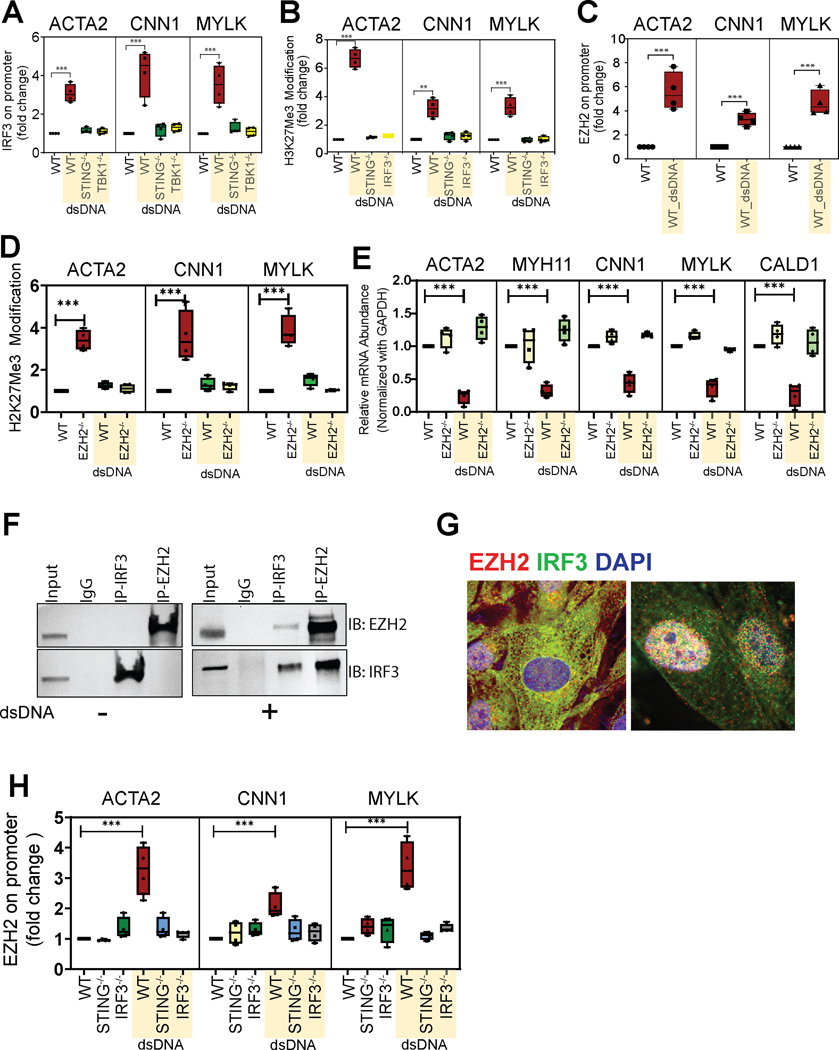
We further investigated the mechanisms underlying SMC contractile gene suppression induced by dsDNA-STING-TBK1-IRF3. To this end, we used a ChIP assay to examine whether IRF3 binds to the regulatory region of contractile genes. The DNA-IRF3 complex was immunoprecipitated from cultured SMCs with an anti-IRF3 antibody, and the regulatory regions of SMC contractile genes in the complexes were detected by PCR using primers with sequences flanking predicted IRF3 binding sites in these regions. Immunoprecipitation with anti-IgG antibody was used as a negative control. As shown in Figure 6A and Figure S4A, treating SMCs with dsDNA triggered the binding of IRF3 to the regulatory regions of ACTA2, CNN1, and MYLK, but this was prevented in STING−/− and TBK1−/− SMCs, suggesting that dsDNA-STING-TBK1 promotes IRF3 binding to the regulatory regions of SMC contractile genes. The binding of IRF3 to the regulatory regions of SMC contractile genes was further confirmed by performing the CUT&RUN assay (Figure S4B). dsDNA also induced the binding of IRF3 to the regulatory regions of inflammatory genes such as IL1β (Figure S4C).
Figure 6. Suppression of aortic smooth muscle cell (SMC) gene expression by IRF3 via the recruitment of EZH2 and the role of IRF3 in the induction of repressive chromatin remodeling.
Chromatin immunoprecipitation (ChIP) analysis in cultured human aortic SMCs confirms (A) the dsDNA-induced binding of IRF3 to the promoter region of selected contractile genes (ACTA2, CCN1, MYLK). Treatment with dsDNA increased H3K27me3 modification (B) and EZH2 binding (C) at the promoter of contractile genes in wild-type aortic SMCs but not in STING−/− or IRF3−/− SMCs. D, Silencing EZH2 reversed the dsDNA-induced H3K27me3 modification of selected contractile genes in aortic SMCs. E, Quantitative PCR analysis confirming that EZH2 depletion suppresses the expression of contractile genes. F, Results of a co-immunoprecipitation assay with endogenous proteins from cultured aortic SMCs confirming the EZH2-IRF3 interaction in response to dsDNA treatment. G, Immunofluorescence microscopy showing the colocalization of the individual components of the EZH2-IRF3 complex. H, ChIP assay confirmed that STING and IRF3 are necessary for the binding of EZH2 to the contractile gene promoter. dsDNA, double-stranded DNA. **p=0.0011; ***p<0.001.
We next studied how IRF3 regulates SMC contractile genes. Given that our scATAC-seq data indicated chromatin remodeling as a potential mechanism responsible for contractile gene suppression, we examined the involvement of histone modification with H3K27me3, a marker of repressive heterochromatin,35 in IRF3-induced contractile gene suppression. We found that treating WT SMCs with dsDNA increased the H3K27me3 modification of contractile genes (Figure 6B and Figure S4D), which was prevented in STING−/− and IRF3−/− SMCs, suggesting that dsDNA-STING-IRF3 signaling may suppress contractile gene expression by inducing repressive histone modification.
Histone methyltransferase EZH2 (enhancer of zeste homolog 2) is a catalytic subunit of PRC2 (polycomb repressive complex 2) that induces H3K27me3 modification and suppresses the expression of the target genes.36 We found that treating WT SMCs with dsDNA-induced EZH2 binding to contractile genes (Figure 6C). Additionally, the knockout of EZH2 reversed the dsDNA-induced H3K27me3 modification (Figure 6D) and suppression of contractile genes (Figure 6E), suggesting that EZH2 is involved in the repressive chromatin remodeling and suppression of contractile genes.
We next asked whether dsDNA-STING-IRF3 induced the H3K27me3 modification of contractile genes by regulating EZH2. Indeed, IRF3 interacted with EZH2 (Figure 6F and Figure S4E) and colocalized with EZH2 in the nuclei (Figure 6G and Figure S4F) of SMCs, and these observations were further enhanced by treating SMCs with dsDNA. Moreover, the dsDNA-induced binding of EZH2 to SMC contractile gene promoters (Figure 6H) was prevented in STING−/− and IRF3−/− SMCs. Together, these results suggest that dsDNA-STING-IRF3 suppresses contractile gene expression by recruiting EZH2 to contractile genes and subsequently inducing repressive chromatin remodeling.
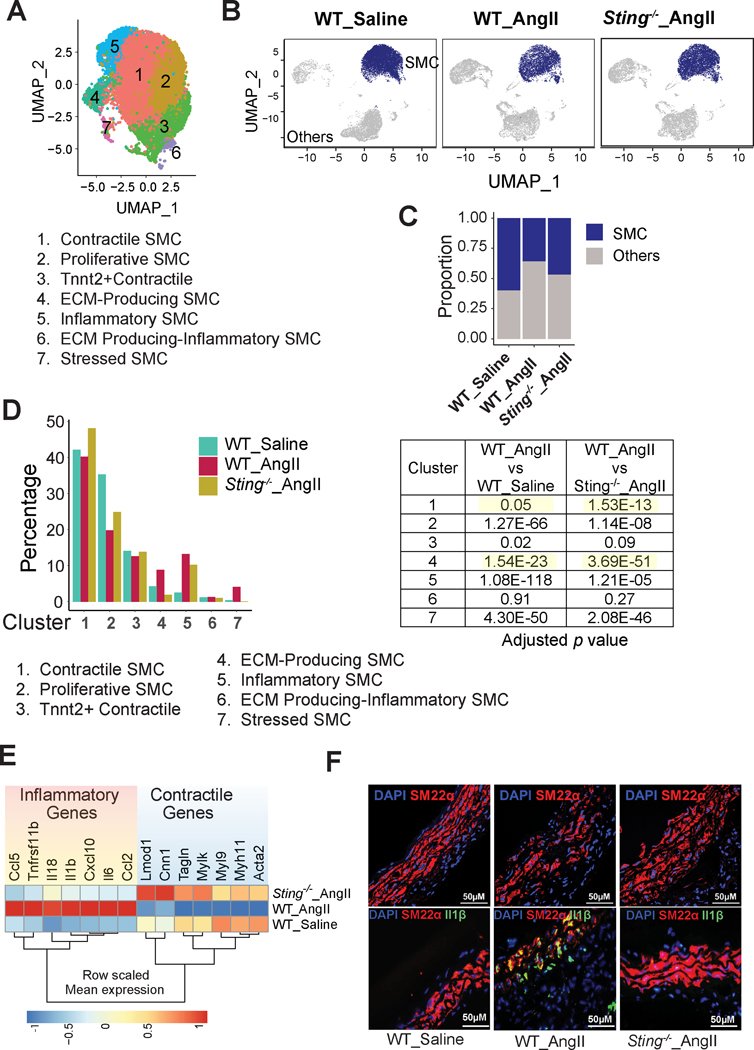
Sting Deficiency Reduced SMC Phenotypic Alterations and Preserved the SMC Population in AngII-infused Mice
To determine the role of STING in SMC phenotypic alteration during AAD development, we compared aortic SMC subpopulations and their gene expression profiles in AngII-infused WT mice and AngII-infused Sting-deficient (Sting−/−) mice. A total of 14 clusters were identified (Figure S5A), and four clusters were SMCs according to the expression of conserved contractile genes (Figure S5B). We extracted the SMCs and re-clustered them into seven clusters (Figure 7A). On the basis of DEGs (Figure S5C) and GO analysis of the DEGs (Figure S5D, Supplemental Excel File 3), we defined these SMC subclusters as contractile SMCs (Cluster 1), proliferating SMCs (Cluster 2), Tnnt2+ contractile SMCs (Cluster 3), ECM-producing SMCs (Cluster 4), inflammatory SMCs (Cluster 5), ECM-producing inflammatory SMCs (Cluster 6), and stressed SMCs (Cluster 7) (Figure 7A).
Figure 7. Reduced aortic smooth muscle cell (SMC) phenotypic alterations in Sting-deficient mice.
A, Uniform manifold approximation and projection (UMAP) plot representing the distribution of SMC subclusters. A total of 7 clusters were identified. B, UMAP showing a significant reduction in the size of the SMC population in angiotensin II (AngII)-infused wild-type (WT) mice that was partially prevented in AngII-infused Sting−/− mice. C, The proportion of SMC clusters in AngII-infused Sting−/− and in saline-infused mice. D, Bar diagram showing the percentage of the cluster population in aortic SMCs of the three different groups of mice. The Fisher exact test was performed to examine differences in the cluster proportion between groups. E, Heatmap representing the differential gene expression of contractile genes and inflammatory genes in aortic SMCs of the three different groups of mice. F, Immunofluorescence staining showing Sm22α and Il-1β expression in different groups of mice.
We found that AngII infusion decreased the size of the SMC population in WT mice and that this decrease was partially prevented in Sting−/− mice (Figure 7B and 7C). Additionally, aortic challenge reduced the size of the contractile and proliferating SMC clusters and increased the size of the inflammatory SMC cluster (Figure 7D). Importantly, these changes were partially prevented and the reduction in the size of the contractile SMC population was reversed in Sting−/− mice (Figure 7D).
Consistent with these findings, DEG analysis showed that AngII infusion inhibited the expression of contractile genes and increased the expression of inflammatory genes in the SMCs of WT mice but not in the SMCs of Sting−/− mice (Figure 7E). Furthermore, immunofluorescence staining confirmed the preservation of Sm22α37 and the reduction of the proinflammatory cytokine IL-1β38 in the SMCs of AngII-infused Sting−/− mice compared with AngII-infused WT mice (Figure 7F). We also re-analyzed public scRNA-seq data39 of thoracic aortas from mice that were treated with β-aminopropionitrile fumarate (BAPN) over a period of 7, 14, and 21 days. Similar to our findings, a consistent pattern of decreased expression of contractile genes and increased expression of inflammatory genes was detected in this model. The STING pathway was activated on day 21 after BAPN administration (Figure S5E), suggesting that the STING pathway may represent a common mechanism for driving proinflammatory phenotypic changes in SMCs of the aortic wall. Furthermore, to investigate the impact of STING−/− on the vascular phenotype and SMCs in the absence of AngII, we performed ScRNA-seq analysis of the aortic wall of Sting−/− mice and WT mice under basal condition (saline-infusion control). The data showed that, under basal conditions, the expression of inflammatory genes was comparable between Sting−/− mice and WT mice (Figure 5F). Interestingly, the expression of contractile genes was found to be higher in Sting−/− mice than in WT mice, suggesting that STING may inhibit the expression of contractile genes under basal condition. These data reinforce our findings and enhance our comprehension of the involvement of STING in SMCs. Together, these findings suggest that STING plays a role in promoting the transition of SMCs from a contractile phenotype to an inflammatory phenotype during AAD development.
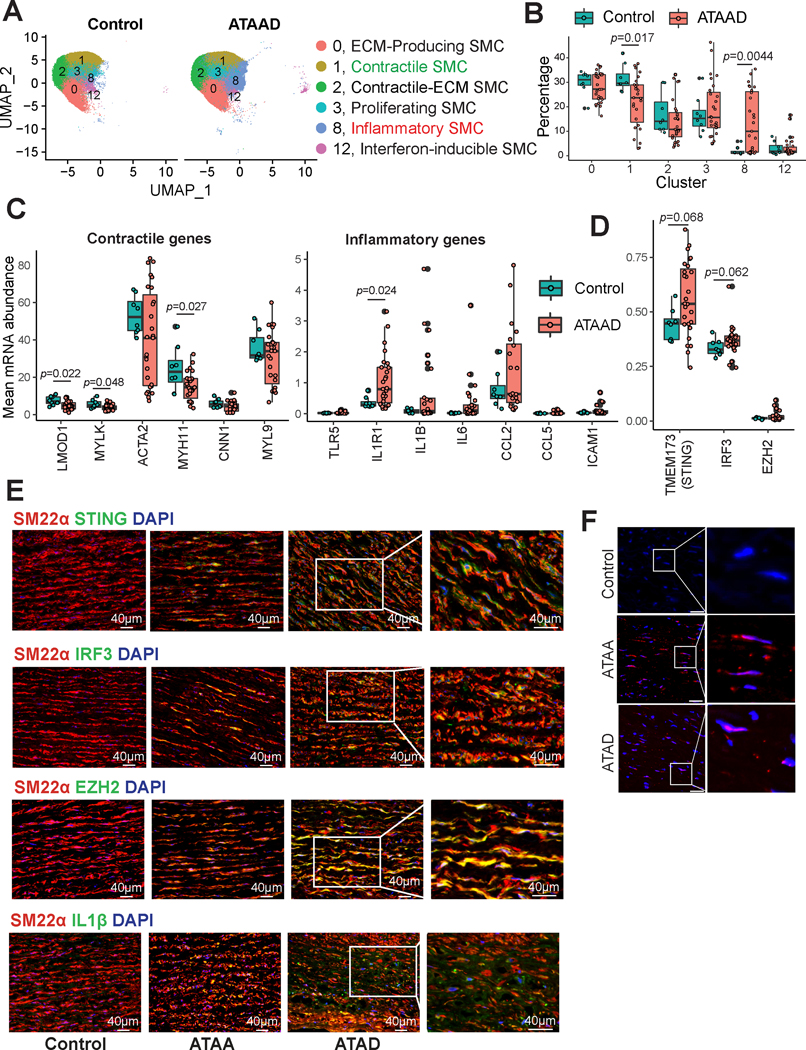
Significant Phenotypic Alterations and Activation of STING-IRF3-EZH2 in SMCs of Human ATAAD Tissues
We also examined SMC phenotypic alterations in aortic tissues from patients with ascending thoracic aortic aneurysm and dissection (ATAAD) using our human aortic scRNA-seq data (previously published40 and unpublished data). Diverse SMC populations were detected in human aortic tissues (Figure 8A), with similar subclusters as in mouse aortic tissues. We observed that the contractile SMC cluster was smaller and the inflammatory SMC cluster was larger in human ATAAD tissues than in control tissues (Figure 8B). Consistent with these findings, DEG analysis showed that, compared with control tissues, patient ATAAD tissues exhibited downregulation of contractile gene expression and upregulation of inflammatory gene expression (Figure 8C).
Figure 8. Single-cell RNA sequencing (scRNA-seq) analysis of human ascending thoracic aortic aneurysm and dissection (ATAAD) tissue revealing smooth muscle cell (SMC) phenotypic switching from a contractile to an inflammatory subtype.
A, Uniform manifold approximation and projection (UMAP) plot of SMC clusters identified from scRNA-seq analysis of patient ATAAD tissue and control tissue. B, Box plot representing the percent distribution of SMC clusters. The Wilcoxon rank sum test using the cluster percentage per sample was performed to compare the cluster proportion between ATAAD and control tissues. C, Boxplot showing the mean expression of contractile genes and inflammatory genes per sample in non-diseased controls versus patient ATAAD tissues. A Wilcoxon rank sum test using the mean expression value per sample per gene was performed to compare expression between ATAAD and control tissues. D, Boxplot showing the mean expression of STING, IRF3, and EZH2 per sample in non-diseased controls versus patient ATAAD tissues. A Wilcoxon rank sum test using the mean expression value per sample per gene was performed to compare expression between ATAAD and control tissues. E, Immunofluorescence staining showing SM22α, STING, IRF3 IL-1β, and EZH2 expression in patient ATAAD tissue versus non-diseased control tissue. F, Control, thoracic aortic aneurysm without dissection (ATAA), and acute ascending thoracic aortic dissection (ATAD) tissues were used to perform a proximity ligation assay followed by analysis by fluorescence microscopy. Scale bar, 40 μm.
We have previously detected profound cytosolic DNA accumulation and STING activation in human ATAAD.7 Here, we expanded upon the previous findings and examined the STING-IRF3-EZH2 axis in ATAAD. ScRNA-seq analysis (Figure 8D) showed a trend of elevated levels of STING, IRF3, and EZH2 in SMCs of the diseased aortas. Additionally, immunostaining analysis showed increased levels of STING, IRF3, and EZH2 (Figure 8E) in SMCs, suggesting the activation of this signaling. Immunofluorescence staining confirmed a lower level of SM22α and a higher level of inflammatory IL-1β in ATAA tissues than in control aortic tissues (Figure 8E). Furthermore, proximity ligation assay (PLA) showed that binding between IRF3 and EZH2 was entirely absent in control tissues but was frequent in ATAA and ATAD tissues (Figure 8F). Together, our data indicate significant SMC phenotypic switching from a contractile phenotype to a proinflammatory phenotype, and activation of the STING-IRF3-EZH2 pathway in human ATAAD tissues.
DISCUSSION
In this study, scRNA-seq analysis in a mouse model of sporadic AAD and in human thoracic AAD tissues revealed significant phenotypic switching from contractile SMCs to several other phenotypes, including a proinflammatory phenotype. ScATAC-seq analysis further indicated that SMC transformation was partially controlled by chromatin remodeling. In addition, IRF3 was identified as a top TF driving these changes. In cultured human aortic SMCs, IRF3 directly bound to the cis elements of SMC contractile genes and recruited EZH2, which induced the repressive H3K27me3 modification of contractile genes, leading to contractile gene suppression. Finally, in our mouse model of sporadic AAD, Sting deficiency prevented the SMC phenotypic switch to a proinflammatory phenotype and maintained the contractile phenotype.
It is crucial to acknowledge that the deficiency of Sting alone might not be adequate to fully restore the population size to the level observed in the control group. It is probable that there are other pathways or essential molecules that play a role in facilitating the transition of SMCs toward an inflammatory state. These supplementary factors might compensate for the absence of Sting’s functionality or contribute to the perpetuation of the inflammatory phenotype, even in the absence of Sting. As a result, Sting-knockout (Sting-KO) decreased the population size of the inflammatory SMCs triggered by AngII infusion, but the proportion of inflammatory SMCs remained higher in the aortas of Sting-KO subjects than in the unstressed aortas. Our findings reveal an epigenetic program that induces contractile gene suppression and SMC phenotypic changes and points to STING-IRF3-EZH2 as a critical signaling pathway that suppresses contractile gene expression.
SMC phenotypic alteration has been increasingly recognized as a common feature in both human and mouse thoracic aortic aneurysm or abdominal aortic aneurysm tissues.5,41–43 Two types of SMC phenotypes, the contractile and synthetic phenotypes, have been characterized in previous studies.43,44 Recent advances in single-cell techniques40 have enabled a much more granular characterization of the dynamic SMC phenotypes. From our scRNA-seq analysis, we identified seven distinct SMC clusters. Although these different clusters share many common features, each cluster has prominent features, suggesting that these SMC subpopulations may have specific functions and contribute to different aspects of aortic wall remodeling and function.
Further analysis showed that aortic challenge induced the phenotypic transition of SMCs from a well-differentiated contractile phenotype to several different phenotypes. Decreased expression of contractile genes and increased expression of inflammatory genes45,46 and ECM genes47 are common features of SMCs in AAD. The various phenotypic changes may have different effects on the aortic wall. For example, whereas the transition to pro-fibroblast and pro-progenitor/pro-proliferation phenotypes may enhance aortic repair and remodeling and strengthen the aortic wall, the transition to proinflammatory and pro-death phenotypes may promote aortic degeneration and dysfunction. Understanding the effect of these different SMC transitions on the aortic wall and the molecular mechanisms responsible for these different transitions is critical for developing effective strategies for maintaining aortic homeostasis.
The mechanisms underlying the repression of contractile gene expression in AAD are largely understudied and poorly understood. Our scATAC-seq analysis48 revealed a genome-wide chromatin accessibility landscape of genes in SMC populations and the dynamic changes that occurred after aortic challenge. We found that aortic stress induced by AngII infusion systemically reduced the chromatin accessibility of contractile genes, suggesting that the suppression of contractile gene expression was partially controlled at the epigenetic level. Interestingly, further analysis of the different peaks of contractile genes revealed a different pattern of changes. Whereas some peaks showed constant opening with minimal changes after aortic challenge, other peaks showed significant downregulation after aortic challenge, suggesting that these regions may be responsible for the repression of contractile genes. In contrast, we observed an induction in the chromatin accessibility of inflammatory genes in aortic SMCs. Most chromatin regions of inflammatory genes were closed in control mice but open in AngII-infused mice, suggesting that these regions may be responsible for the induction of inflammatory gene expression.
In our search for TFs that drive the suppression of contractile gene expression, we examined TF-binding motifs in contractile genes. The motif activity of IRFs showed a strong negative association with the activity/chromatin accessibility of contractile genes, indicating that IRFs are central TFs in contractile gene regulation. For example, multiple IRF motifs, including the IRF3 motif, were detected in the regions/peaks of contractile genes that were closed in AngII-infused mice, suggesting a role for IRF3 in the inducible suppression of contractile gene expression. By performing experiments in cultured human aortic SMCs, we confirmed the role of IRF3 in SMC gene suppression. IRF3 directly bound to promoters of contractile genes such as Acta2 and Mylk and suppressed their expression. In contrast, multiple IRF motifs, including the IRF3 motif, were detected in the peaks of inflammatory genes that were opened in AngII-infused mice. IRF3 motif activity was positively associated with the activity of inflammatory genes. In cultured human aortic SMCs, IRF3 bound to and induced the expression of inflammatory genes. Thus, we showed for the first time, to our knowledge, that the TF IRF3 promotes SMC phenotypic alterations by directly suppressing contractile gene expression and inducing inflammatory gene expression. Furthermore, we showed a chromatin accessibility signature and associated TFs that were significantly correlated with SMC phenotypic alteration, providing novel evidence of a chromatin organization–based prognostic paradigm for aortic aneurysms.
We also found that IRF3 suppresses contractile gene expression by inducing the chromatin remodeling of target genes. IRF3 bound to the key chromatin modifier EZH2, which induced and mediated H3K27me3 modification at the promoters of SMC-specific contractile genes. This in turn initiated an epigenetic off-state and resulted in the downregulation of SMC-specific contractile gene expression. An earlier study showed that inhibiting EZH2 improved aortic degeneration.49
One interesting question remains to be answered. We have shown that IRF3 binds to both contractile genes and inflammatory genes. However, the outcomes are different. Whereas IRF3 inhibits SMC contractile gene expression, it induces inflammatory gene expression. It is not clear whether IRF3 selectively recruits EZH2 to SMC contractile genes but not to inflammatory genes. If so, it remains to be determined what the mechanisms underlying this selection are and whether any additional binding proteins are involved in the selection process. Additionally, determining whether posttranslational modifications of IRFs and/or EZH2 are involved in this process warrants further investigation. Future studies will be needed to answer these questions. Furthermore, several other transcriptional factors and epigenetic factors such as ZEB250 DOT1L,51 BAF60c,52 and KLF453–55 have been shown to be involved in epigenetic control of SMC gene expression and SMC phenotypes via various mechanisms. It is interesting to ask whether IRF3 interacts with these factors, and whether these different factors coordinately control the direction(s) of SMC phenotypic transition.
We identified cytosolic DNA as a trigger in promoting SMC phenotypic alterations. Our previous study7 revealed the presence of cytosolic DNA in the SMCs of patients with AAD and the role of the cytosolic DNA sensor STING in sporadic AAD development. In the present study, we provided evidence supporting the importance of this mechanism in the epigenetic induction of SMC phenotypic alterations. In cultured human aortic SMCs, dsDNA-STING-TBK1 signaling activated IRF3, which promoted phenotypic alteration by triggering the induction of inflammatory gene expression and the EZH2-mediated epigenetic suppression of SMC contractile gene expression. Genetically silencing these pathways preserved the contractile phenotype and function of aortic SMCs. In mice, Sting deficiency prevented SMC phenotypic alterations and preserved the contractile phenotype of aortic SMCs. As nuclear and mitochondrial DNA damage and cytosolic DNA leakage are common features of stressed cells that can be induced by aging, metabolic disturbance, reactive oxygen species, and tissue inflammation,7,56,57 cytosolic DNA accumulation-STING-IRF3-EZH2 may represent an important trigger and mechanism for SMC phenotypic alterations and vascular disease development associated with these conditions.
In conclusion, our study reveals diverse SMC populations in the aortic wall and a dynamic epigenetic induction of aortic SMC phenotypic transitions during AAD formation. Our findings provide compelling evidence of the principle that DNA damage and cytosolic DNA release is an important trigger for SMC phenotypic change. Furthermore, cytosolic DNA, via STING-IRF3 signaling, induces chromatin remodeling that drives SMCs from a contractile phenotype to an inflammatory phenotype. These novel findings may lead to the development of a new therapeutic approach for aneurysm prevention. Several questions remain to be addressed, particularly related to the downstream effects of different directions of SMC phenotypic transition on aortic protection and destruction, as well as the specific molecular mechanisms that evoke the transition of SMCs to other phenotypes during AAD formation.
Supplementary Material
Clinical Perspective.
What Is New?
Aortic stress induces the transition of aortic smooth muscle cells (SMCs) from a primary contractile phenotype to diverse phenotypes including proliferative, extracellular matrix (ECM)-producing, inflammatory, and dying phenotypes.
These phenotypic alterations are controlled by chromatin remodeling in SMCs that reduces the chromatin accessibility of contractile genes and induces the chromatin accessibility of proliferation, ECM, inflammation, and cell death genes.
Pro-inflammatory transcription factor IRF3 is a key driver of the transition of SMCs from a contractile phenotype to an inflammatory phenotype.
Cytosolic DNA signals through its sensor STING to activate IRF3, which drives SMC phenotypic alteration by epigenetically repressing SMC contractile genes and inducing inflammatory genes.
What Are the Clinical Implications?
Cytosolic DNA, which commonly accumulates as a consequence of nuclear or mitochondrial DNA damage and leakage, is an important trigger for SMC phenotypic alteration and dysfunction in aortic aneurysms and dissections.
Targeting cytosolic DNA-sensing STING signaling may block SMC phenotypic alteration, preserve SMC functions, and prevent aortic aneurysm and dissection formation and progression.
ACKNOWLEDGMENTS
The authors wish to thank Nicole Stancel, PhD, ELS(D), of the Department of Scientific Publications at The Texas Heart Institute, for her editorial contributions. The graphic abstract was created using Figdraw and BioRender.
SOURCES OF FUNDING
This work was supported by NIH grants R01HL131980, R01HL143359, R01HL158157, and R01 HL159988 and by American Heart Association grant 18SFRN33960114. Transcriptome analyses were performed at the Department of Molecular and Human Genetics Functional Genomics Core and Single Cell Genomics Core at Baylor College of Medicine, which are partially supported by grants from the NIH (grants S10OD023469, S10OD018033, S10OD025240, CA125123, and 1P30ES030285) and the Cancer Prevention Research Institute of Texas (core grant RP170005). Dr. Yanming Li is supported by the Victor A. McKusick Fellowship Grant from The Marfan Foundation. Dr. Scott A. LeMaire’s work is supported in part by the Jimmy and Roberta Howell Professorship in Cardiovascular Surgery at Baylor College of Medicine.
Non-Standard Abbreviations and Acronyms
- AAD
aortic aneurysms and dissections
- ATAAD
ascending thoracic aortic aneurysm and dissection
- DEG
differentially expressed genes
- DAG
differentially activated genes
- ECM
extracellular matrix
- GO
gene ontology
- scRNA-seq
single-cell RNA sequencing
- SMC
smooth muscle cell
- TAAD
thoracic aortic aneurysm and dissection
- TF
transcription factor
- UMAP
uniform manifold approximation and projection
- WT
wild-type
Footnotes
DISCLOSURES
Dr. LeMaire serves as a consultant for Terumo Aortic and Cerus and serves as a principal investigator for clinical studies sponsored by Terumo Aortic and CytoSorbents.
Contributor Information
Abhijit Chakraborty, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA.
Yanming Li, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA.
Chen Zhang, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA.
Yang Li, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA.
Kimberly R. Rebello, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA.
Shengyu Li, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA; Center for Bioinformatics and Computational Biology, Houston Methodist Research Institute, Houston, TX, USA.
Samantha Xu, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA.
Hernan G. Vasquez, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA.
Lin Zhang, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA.
Wei Luo, Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA.
Guangyu Wang, Center for Bioinformatics and Computational Biology, Houston Methodist Research Institute, Houston, TX, USA.
Kaifu Chen, Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA.
Joseph S. Coselli, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA; Cardiovascular Research Institute, Baylor College of Medicine, Houston, Texas, USA.
Scott A. LeMaire, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Department of Cardiovascular Surgery, The Texas Heart Institute, Houston, Texas, USA; Cardiovascular Research Institute, Baylor College of Medicine, Houston, Texas, USA.
Ying H. Shen, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Cardiovascular Research Institute, Baylor College of Medicine, Houston, Texas, USA.
REFERENCES
- 1.Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. [DOI] [PubMed] [Google Scholar]
- 3.Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol (1985). 2005;98:2321–2327. [DOI] [PubMed] [Google Scholar]
- 4.Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK, et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med. 2019;25:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petsophonsakul P, Furmanik M, Forsythe R, Dweck M, Schurink GW, Natour E, Reutelingsperger C, Jacobs M, Mees B, Schurgers L. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2019;39:1351–1368. [DOI] [PubMed] [Google Scholar]
- 6.St Hilaire C, Liberman M, Miller JD, Early Career C. Bidirectional translation in cardiovascular calcification. Arterioscler Thromb Vasc Biol. 2016;36:e19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo W, Wang Y, Zhang L, Ren P, Zhang C, Li Y, Azares AR, Zhang M, Guo J, Ghaghada KB, et al. Critical role of cytosolic DNA and Its sensing adaptor STING in aortic degeneration, dissection, and rupture. Circulation. 2020;141:42–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedroza AJ, Dalal AR, Shad R, Yokoyama N, Nakamura K, Cheng P, Wirka RC, Mitchel O, Baiocchi M, Hiesinger W, et al. Embryologic origin influences smooth muscle cell phenotypic modulation signatures in murine Marfan syndrome aortic aneurysm. Arterioscler Thromb Vasc Biol. 2022:101161ATVBAHA122317381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G, Wang M, Caulk AW, Cilfone NA, Gujja S, Qin L, Chen PY, Chen Z, Yousef S, Jiao Y, et al. Chronic mTOR activation induces a degradative smooth muscle cell phenotype. J Clin Invest. 2020;130:1233–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clement M, Chappell J, Raffort J, Lareyre F, Vandestienne M, Taylor AL, Finigan A, Harrison J, Bennett MR, Bruneval P, et al. Vascular smooth muscle cell plasticity and autophagy in dissecting aortic aneurysms. Arterioscler Thromb Vasc Biol. 2019;39:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong L, He X, Si X, Wang H, Li B, Hu Y, Li M, Chen X, Liao W, Liao Y, et al. SM22alpha (smooth muscle 22alpha) prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching through suppressing reactive oxygen species/NF-kappaB (nuclear factor-kappaB). Arterioscler Thromb Vasc Biol. 2019;39:e10–e25. [DOI] [PubMed] [Google Scholar]
- 12.Nogi M, Satoh K, Sunamura S, Kikuchi N, Satoh T, Kurosawa R, Omura J, Elias-Al-Mamun M, Abdul Hai Siddique M, Numano K, et al. Small GTP-binding protein GDP dissociation stimulator prevents thoracic aortic aneurysm formation and rupture by phenotypic preservation of aortic smooth muscle cells. Circulation. 2018;138:2413–2433. [DOI] [PubMed] [Google Scholar]
- 13.Pedroza AJ, Tashima Y, Shad R, Cheng P, Wirka R, Churovich S, Nakamura K, Yokoyama N, Cui JZ, Iosef C, et al. Single-cell transcriptomic profiling of vascular smooth muscle cell phenotype modulation in Marfan syndrome aortic aneurysm. Arterioscler Thromb Vasc Biol. 2020;40:2195–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedroza AJ, Shad R, Dalal AR, Yokoyama N, Nakamura K, Hiesinger W, Fischbein MP. Acute induced pressure overload rapidly incites thoracic aortic aneurysmal smooth muscle cell phenotype. Hypertension. 2022;79:e86–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowther M, Goodall S, Jones JL, Bell PR, Thompson MM. Increased matrix metalloproteinase 2 expression in vascular smooth muscle cells cultured from abdominal aortic aneurysms. J Vasc Surg. 2000;32:575–583. [DOI] [PubMed] [Google Scholar]
- 16.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukasawa K, Park G, Iezaki T, Horie T, Kanayama T, Ozaki K, Onishi Y, Takahata Y, Yoneda Y, Takarada T, et al. ATF3 controls proliferation of osteoclast precursor and bone remodeling. Sci Rep. 2016;6:30918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Liu H, Wang Q, Zhou F, Liu Y, Zhang Y, Ding H, Yuan M, Li F, Chen Y. Involvement of c-Fos in cell proliferation, migration, and invasion in osteosarcoma cells accompanied by altered expression of Wnt2 and Fzd9. PloS One. 2017;12:e0180558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi L, He S, Cheng Z, Chen X, Ren X, Bai Y. DNAJA1 stabilizes EF1A1 to promote cell proliferation and metastasis of liver cancer mediated by miR-205–5p. J Oncol. 2022;2022:2292481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong LM, Yao L, Lu N, Dong YL, Zhang J, Wang YQ, Liu L, Zhang HL, Huang JG, Liao CG. Interaction of KLF6 and Sp1 regulates basigin-2 expression mediated proliferation, invasion and metastasis in hepatocellular carcinoma. Oncotarget. 2016;7:27975–27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei L, Han F, Cui Q, Liao W, Liu H, Guan G, Yang L. IRS2 depletion inhibits cell proliferation and decreases hormone secretion in mouse granulosa cells. J Reprod Dev. 2018;64:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piechaczyk M, Farras R. Regulation and function of JunB in cell proliferation. Biochem Soc Trans. 2008;36:864–867. [DOI] [PubMed] [Google Scholar]
- 23.Sun T, Tian H, Feng YG, Zhu YQ, Zhang WQ. Egr-1 promotes cell proliferation and invasion by increasing beta-catenin expression in gastric cancer. Dig Dis Sci. 2013;58:423–430. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Wang X. Lowly expressed LNC01136 fails to aid HIF-1alpha to induce BTG2 expression resulting in increased proliferation of retinal microvascular endothelial cells. Microvasc Res. 2022;141:104315. [DOI] [PubMed] [Google Scholar]
- 25.Alonso J, Galan M, Marti-Pamies I, Romero JM, Camacho M, Rodriguez C, Martinez-Gonzalez J. NOR-1/NR4A3 regulates the cellular inhibitor of apoptosis 2 (cIAP2) in vascular cells: role in the survival response to hypoxic stress. Sci Rep. 2016;6:34056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanlandewijck M, He L, Mae MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Lavina B, Gouveia L, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Zhang P, Chen W, Ye L, Brannan KW, Le NT, Abe JI, Cooke JP, Wang G. A relay velocity model infers cell-dependent RNA velocity. Nat Biotechnol. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corces MR, Buenrostro JD, Wu B, Greenside PG, Chan SM, Koenig JL, Snyder MP, Pritchard JK, Kundaje A, Greenleaf WJ, et al. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat Genet. 2016;48:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, Li Y, Chakraborty A, Li Y, Rebello KR, Ren P, Luo W, Zhang L, Lu HS, Cassis LA, et al. Aortic stress activates an adaptive program in thoracic aortic smooth muscle cells that maintains aortic strength and protects against aneurysm and dissection in mice. Arterioscler Thromb Vasc Biol. 2023;43:234–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagao M, Lyu Q, Zhao Q, Wirka RC, Bagga J, Nguyen T, Cheng P, Kim JB, Pjanic M, Miano JM, et al. Coronary disease-associated gene TCF21 inhibits smooth muscle cell differentiation by blocking the myocardin-serum response factor pathway. Circ Res. 2020;126:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, Liu J, Xiang M, Olson P, Guzzetta A, Zhang K, Moskowitz IP, Xie L. Gata4 potentiates second heart field proliferation and Hedgehog signaling for cardiac septation. Proc Natl Acad Sci U S A. 2017;114:E1422–E1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schep AN, Wu B, Buenrostro JD, Greenleaf WJ. chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat Methods. 2017;14:975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Firulli AB, Miano JM, Bi W, Johnson AD, Casscells W, Olson EN, Schwarz JJ. Myocyte enhancer binding factor-2 expression and activity in vascular smooth muscle cells. Association with the activated phenotype. Circ Res. 1996;78:196–204. [DOI] [PubMed] [Google Scholar]
- 34.Lee MY, Park C, Ha SE, Park PJ, Berent RM, Jorgensen BG, Corrigan RD, Grainger N, Blair PJ, Slivano OJ, et al. Serum response factor regulates smooth muscle contractility via myotonic dystrophy protein kinases and L-type calcium channels. PloS One. 2017;12:e0171262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavarone E, Barbieri CM, Pasini D. Dissecting the role of H3K27 acetylation and methylation in PRC2 mediated control of cellular identity. Nat Commun. 2019;10:1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan L, Yang Y, Li Q, Feng Y, Liu T, Guo W. Epigenetic regulation of cancer progression by EZH2: from biological insights to therapeutic potential. Biomark Res. 2018;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Miano JM, Cserjesi P, Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78:188–195. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Chen W, Zhu G, Yang H, Li W, Luo M, Shu C, Zhou Z. Single-cell RNA sequencing identifies an Il1rn(+)/Trem1(+) macrophage subpopulation as a cellular target for mitigating the progression of thoracic aortic aneurysm and dissection. Cell Discov. 2022;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Ren P, Dawson A, Vasquez HG, Ageedi W, Zhang C, Luo W, Chen R, Li Y, Kim S, et al. Single-cell transcriptome analysis reveals dynamic cell populations and differential gene expression patterns in control and aneurysmal human aortic tissue. Circulation. 2020;142:1374–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg. 2009;138:1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang P, Hou S, Chen J, Zhang J, Lin F, Ju R, Cheng X, Ma X, Song Y, Zhang Y, et al. Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm. Circ Res. 2016;118:388–399. [DOI] [PubMed] [Google Scholar]
- 43.Gurung R, Choong AM, Woo CC, Foo R, Sorokin V. Genetic and epigenetic mechanisms underlying vascular smooth muscle cell phenotypic modulation in abdominal aortic aneurysm. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. [DOI] [PubMed] [Google Scholar]
- 46.Cai D, Sun C, Zhang G, Que X, Fujise K, Weintraub NL, Chen SY. A novel mechanism underlying inflammatory smooth muscle phenotype in abdominal aortic aneurysm. Circ Res. 2021;129:e202–e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan J, Cai Y, Liu M, Li Z. Role of vascular smooth muscle cell phenotypic switching in plaque progression: A hybrid modeling study. J Theor Biol. 2021;526:110794. [DOI] [PubMed] [Google Scholar]
- 48.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lino Cardenas CL, Kessinger CW, MacDonald C, Jassar AS, Isselbacher EM, Jaffer FA, Lindsay ME. Inhibition of the methyltranferase EZH2 improves aortic performance in experimental thoracic aortic aneurysm. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng P, Wirka RC, Shoa Clarke L, Zhao Q, Kundu R, Nguyen T, Nair S, Sharma D, Kim HJ, Shi H, et al. ZEB2 shapes the epigenetic landscape of atherosclerosis. Circulation. 2022;145:469–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farina FM, Serio S, Hall IF, Zani S, Cassanmagnago GA, Climent M, Civilini E, Condorelli G, Quintavalle M, Elia L. The epigenetic enzyme DOT1L orchestrates vascular smooth muscle cell-monocyte crosstalk and protects against atherosclerosis via the NF-kappaB pathway. Eur Heart J. 2022;43:4562–4576. [DOI] [PubMed] [Google Scholar]
- 52.Zhao G, Zhao Y, Lu H, Chang Z, Liu H, Wang H, Liang W, Liu Y, Zhu T, Rom O, et al. BAF60c prevents abdominal aortic aneurysm formation through epigenetic control of vascular smooth muscle cell homeostasis. J Clin Invest. 2022;132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alencar GF, Owsiany KM, Karnewar S, Sukhavasi K, Mocci G, Nguyen AT, Williams CM, Shamsuzzaman S, Mokry M, Henderson CA, et al. Stem cell pluripotency genes Klf4 and Oct4 regulate complex SMC phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation. 2020;142:2045–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–9727. [DOI] [PubMed] [Google Scholar]
- 56.Mao Y, Luo W, Zhang L, Wu W, Yuan L, Xu H, Song J, Fujiwara K, Abe JI, LeMaire SA, et al. STING-IRF3 triggers endothelial inflammation in response to free fatty acid-induced mitochondrial damage in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2017;37:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan L, Mao Y, Luo W, Wu W, Xu H, Wang XL, Shen YH. Palmitic acid dysregulates the Hippo-YAP pathway and inhibits angiogenesis by inducing mitochondrial damage and activating the cytosolic DNA sensor cGAS-STING-IRF3 signaling mechanism. J Biol Chem. 2017;292:15002–15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, Satija R. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902 e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Risso D, Perraudeau F, Gribkova S, Dudoit S, Vert JP. A general and flexible method for signal extraction from single-cell RNA-seq data. Nat Commun. 2018;9:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollen AA, Nowakowski TJ, Shuga J, Wang X, Leyrat AA, Lui JH, Li N, Szpankowski L, Fowler B, Chen P, et al. Low-coverage single-cell mRNA sequencing reveals cellular heterogeneity and activated signaling pathways in developing cerebral cortex. Nat Biotechnol. 2014;32:1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng X, Tubbs A, Zhang C, Tang M, Sridharan S, Wang C, Jiang D, Su D, Zhang H, Chen Z, et al. ATR inhibition potentiates ionizing radiation-induced interferon response via cytosolic nucleic acid-sensing pathways. EMBO J. 2020;39:e104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.La Manno G, Soldatov R, Zeisel A, Braun E, Hochgerner H, Petukhov V, Lidschreiber K, Kastriti ME, Lonnerberg P, Furlan A, et al. RNA velocity of single cells. Nature. 2018;560:494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu X, Zhang Y, Martin-Rufino JD, Weng C, Hosseinzadeh S, Yang D, Pogson AN, Hein MY, Hoi Joseph Min K, Wang L, et al. Mapping transcriptomic vector fields of single cells. Cell. 2022;185:690–711 e645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All detailed methods are presented in the Materials and Methods of the Supplemental Material online. All raw data and analytical methods are available from the corresponding author upon reasonable request. Sequencing data have been made publicly available at the Gene Expression Omnibus (GEO): The lineage-tracing mouse scRNA-seq data can be accessed at GSE233257, the mouse scATAC-seq data can be accessed at GSE214082, and the Sting-KO mouse scRNA-seq data can be accessed at GSE233625. The computer code used in this study is available upon request.