Abstract
The sympathetic nervous system is involved in the physiological pathogenesis of many different types of chronic pain. Sympathetic blocks can interrupt the reflex control system by intercepting the noxious afferent fibers accompanying autonomic nerves, resulting in changes in peripheral or central sensory processing. A lumbar sympathetic ganglion block (LSGB), as a treatment method, refers to the injection of nerve blockers into the corresponding lumbar sympathetic nerve segments, usually requiring imaging assistance (CT, X‐ray, ultrasound) to guide. At present, LSGB has been widely used in the clinical treatment of lower limb pain, such as neuropathic pain, lower limb ischemic pain, and so on. Its mechanism of action may be through inhibiting sympathetic nerve activity and dilating blood vessels, thereby alleviating pain and inhibiting stress response. However, there are few reports of LSGB during the perioperative period, especially in postoperative pain and gastrointestinal function. Therefore, by studying the literature about LSGB‐related studies, this article reviews the anatomy of the lumbar sympathetic nerve (LSN), with its clinical application and possible mechanism. We reviewed the analgesic effect of LSGB in patients with lower limb pain and postoperative pain and the potential application prospects in the recovery of gastrointestinal function, finally providing a reference for its clinical application.
Keywords: gastroenteric function, lower limb pain, lumbar sympathetic ganglion block, postoperative analgesia
At present, a lumbar sympathetic ganglion block (LSGB) has been widely used in the clinical treatment of lower limb pain, such as neuropathic pain, lower limb ischemic pain, and so on. Its mechanism of action may be through inhibiting sympathetic nerve activity and dilating blood vessels, thereby alleviating pain and inhibiting stress response. However, there are few reports of LSGB during the perioperative period, especially in postoperative pain and gastrointestinal function. Therefore, through the literature retrieval about LSGB‐related studies, this article reviews the anatomy of the lumbar sympathetic nerve, its clinical application, and possible mechanism, which elucidates the analgesic effect of LSGB in patients with lower limb pain and postoperative pain and the potential application prospects in the recovery of gastrointestinal function, finally providing a reference for its clinical application.
1. INTRODUCTION
The sympathetic nervous system (SNS) has a wide range of effects and plays a vital role in managing pain states and pathologies in the body. 1 , 2 Sympathetic nerves play a crucial signaling role in the pathological process of pain. Research has shown that efferent sympathetic fiber activity, in the periphery, can upregulate afferent nociceptive fiber pain signals. Mechanisms may include: on the one hand, norepinephrine, discharged by postganglionic sympathetic fibers, directly works on nociceptive fibers, which increases pain signals at any point along the nerve; on the other hand, SNS activity indirectly increases pain perception through interactions with other processes. 1 Sympathetic‐maintaining pain (SMP) can take place in varieties of pain syndromes, for example, complex regional pain syndrome (CRPS), 3 and pain syndromes with this characteristic are able to affect work, relationships, and mental health and even debilitating. 4 , 5 , 6 SNS‐driven pain signaling can be inhibited by blocking key sympathetic nerves or ganglia. 7 , 8
A lumbar sympathetic ganglion block (LSGB) is widespread in the diacrisis and therapy of SMP. 9 , 10 An LSGB refers to injecting drugs (local anesthetic drugs: lidocaine, ropivacaine, etc.) into the lumbar sympathetic ganglia of the corresponding segment to destroy the nerve conduction function, thereby achieving the method of treating certain diseases. LSGB technology has become increasingly popular in recent decades, and multitudinous diseases are treated with LSGB, including neuropathic pain (NP), vascular pain, and pain put down to spider bites, hyperhidrosis disease, erythematous extremity pain, and so on. 11 , 12 , 13 , 14 , 15
Although the clinical application of LSGB is becoming more and more popular, there are few reports on the application of LSGB during the perioperative period. Therefore, this article will focus on the relevant literature on LSGB for the therapeutic effect of lower limb pain and discuss its application prospect during the perioperative period and the potential application prospect in the recovery of gastrointestinal function, so as to provide experience for its clinical treatment.
2. ANATOMY OF THE LUMBAR SYMPATHETIC NERVE (LSN)
The lumbar sympathetic ganglion (LSG) is mostly located at the level of the corresponding vertebral body or between the upper and lower vertebral bodies, usually in the lateral nucleus of the lateral column of the gray matter of the spinal cord at lumbar 3, and their location varies greatly. The preganglionic fibers of the LSN (white communicating branches) originate mainly from the L2‐3 nerve roots. There is no white communicating branch between the sympathetic ganglia below the L3 spinal segment and the corresponding spinal nerves. These sympathetic nerve fibers pass down through the L2 sympathetic ganglion and then return to the lumbar nerve through the gray communicating branch to innervate the lower limbs, and the corresponding nerve areas include the buttocks, sciatic bones, and lower limbs. The densest parts of LSG are located in L2 and L3, and the L2 sympathetic ganglion is mainly located in the lower third (1/3) of the L2 vertebral body, between the L2‐3 intervertebral disc and the upper 1/3 of the L3 vertebral body; the position is relatively fixed. 16 Therefore, LSGB is most often performed in the lower 1/3 of L2 or the upper 1/3 of L3 (Figure 1). 16 , 17
Figure 1.
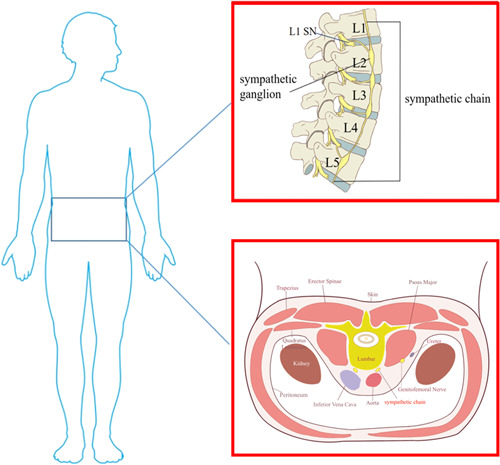
Anatomy of the lumbar sympathetic nerve. SN: sympathetic nerve. L1, L2, L3, L4, L5: lumbar 1, lumbar 2, lumbar 3, lumbar 4, lumbar 5. [Color figure can be viewed at wileyonlinelibrary.com]
3. MECHANISM AND EFFECTS OF LSGB
3.1. Denervation of sympathetic effects
LSGB makes sympathetic nerve fibrosis and produces “denervation of sympathetic effect,” which reduces the vascular tension of the lower limbs, relieves vascular smooth muscle spasm, increases the collateral circulation in the skin vascular bed and arteriovenous shunt, and makes the skin of the feet congested and pink color. Previous research has shown that the blockage of sympathetic nerves could reduce the release of plasma endothelin (ET) (which has a strong vasoconstrictor effect and can produce strong arterial spasm), making it and the calcitonin gene‐related peptide (CGRP) (reverse the vasoconstrictive effect of plasma ET, effectively alleviate vasospasm) to achieve dynamic balance. This mechanism can correct the imbalance of vasoactive substance metabolism, further improve lower limb tissue perfusion, promote the elimination of pain‐causing substances, and reduce ischemic pain. 18 , 19 , 20 High‐intensity focused ultrasound (HIFU)‐guided LSG therapy, on the one hand, expands the body's blood vessels, improves local blood supply, reduces inflammatory reactions, and participates in painful nerve endings; on the other hand, it promotes the increase of CGRP and substance P (SP) release from nerve endings, further through positive feedback to promote the release of beta‐endorphin (β‐EP) and terminate the vicious cycle of ischemic pain. At the same time, it can also reduce the SP level in the posterior horn of the spinal cord, and β‐EP gradually returns to a level close to normal. 21 As a pain transmitter, SP exerts a variety of biological effects, such as causing pain, lowering blood pressure, dilating blood vessels, and increasing capillary permeability. 22
3.2. Sympathetic‐sensory coupling effects
Studies have shown that the pathways of sensory afferent and sympathetic pathways are independent of each other under normal physiological conditions. 23 However, when peripheral nerves are injured, sympathetic‐sensory coupling occurs in the nerve injury area, tissue inflammation area, noninjured afferent nerve fibers, and dorsal root ganglia (DRG) related to the injury area. In this coupling, α2‐adrenergic receptors in the cell membrane of sensory neurons are upregulated, showing increased sensitivity to adrenergic receptor agonists and abnormal sensitization to post‐sympathetic ganglion fiber excitement, causing hyperalgesia, which triggers pain and spontaneous pain. Iwase et al. demonstrated that sympathetic nerve fibers sprouting in DRG are the main mechanism for the formation of sympathetic‐sensory coupling when peripheral nerves are injured. The sympathetic sprouting branch forms a net‐like structure around sensory neurons to form a connection between the two. 24 Evidence demonstrated that potassium channel blockers could increase the sprouting of sympathetic postganglionic fibers by promoting the abnormal discharge of sensory neuron terminals. 25 On the contrary, some studies found that injection of sodium channel blockers into injured nerves can inhibit this spontaneous discharge, reduce sympathetic sprouting, and relieve NP, while injection of normal nerves has no such effect. 25 , 26 Moreover, it has been proved that systemic application of triamcinolone acetonide can reduce the gemmation of sympathetic nerve fibers in DRG and reduce the release of cytokines, and it can also relieve pain. 27
4. APPLICATION OF LSGB IN LOWER LIMB PAIN
LSGB is clinically used for the treatment of a variety of diseases (Figure 2). Here, we focus on the application of LSGB in lower limb pain.
Figure 2.
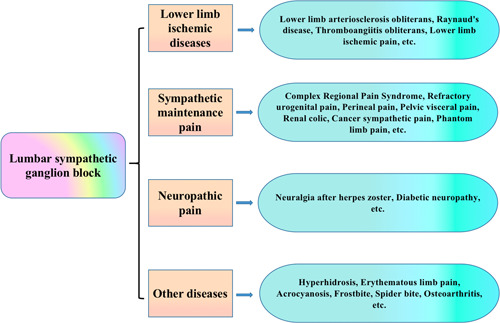
Application of the lumbar sympathetic ganglion block (LSGB) in diseases [Color figure can be viewed at wileyonlinelibrary.com]
4.1. Neuropathic pain
Sympathetic nerves exert a significant signal transmission role in the pathological process of pain, and severing the corresponding sympathetic nerves can result in the disappearance of NP and ischemic pain in the corresponding regions. 28 Blocking LSN function can achieve continuous vasodilation, improve local blood circulation and nutrient supply, eliminate allodynia, and relieve pain. CRPS is a typical sympathetic maintenance pain, and more and more pieces of evidence showed that patients can get significant pain relief after receiving LSGB. 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 In addition, postherpetic neuralgia is a common and intractable NP, and its incidence and prevalence gradually increase with age. The study found that patients with postherpetic neuralgia experienced a marked reduction in pain and improvement in quality of life after LSGB. 39 , 40 Moreover, diabetic peripheral neuropathy (DPN) and diabetic foot are one of the most common and serious complications of diabetes, respectively, often with severe pain and tissue destruction in the lower extremities, which cannot be ignored. More and more evidence showed that sympathetic nerve blockade for DPN can lastingly provide durable pain relief and increase skin temperature on the affected extremity. LSGB mainly dilates peripheral blood vessels of the lower limb through nerve block, increases blood flow, improves local microcirculation, promotes the establishment of collateral circulation, and plays an important role in preserving limbs. 41 , 42 Furthermore, the data show that the morbidity of phantom limb pain (PLP) in amputees ranged from 42% to 78%. Case reports find LSGB is safe and effective for reducing PLP. 43 , 44 , 45 Taken together, LSGB has a significant effect on NP relief (Table 1). 46 , 47
Table 1.
Application summary of the lumbar sympathetic ganglion block (LSGB) in neuropathic pain
| Reference | Type of disease | Assistant technology | Puncture location | Drug | Outcomes |
|---|---|---|---|---|---|
| [26] | CRPS | Fluoroscopy, ultrasound | L2, L3, L4 | Bupivacaine, lidocaine, ropivacaine, medications | 39% of respondents perform LSB at the L2, 53% at L3, the remaining at more than one single level (L2‐L4). |
| [27] | CRPS | No mention | No mention | No mention | Patient received pain relief after intense drugs, LSB, and physical treatment. |
| [28] | CRPS | Fluoroscopy | L2 | 1% lidocaine mixed with 100 UI of BTA, 20 ml | After 2 h, the patient was discharged without complaints of pain. |
| [29] | CRPS | Ultrasound | No mention | 1% lidocaine, 20 ml | After two LSGBs, pain scores are decreased and the girl could walk normally. |
| [30] | CRPS | Fluoroscopy | L2 and L3 level | 0.25% ropivacaine mixed with BTA 100 IU or BTB 5000 IU | The VAS scores of patients in the two groups were significantly decreased after LSB, and the LSB effect in the BTB group was longer than that in the BTA group. |
| [31] | CRPS | Ultrasound | Upper third of the L3 vertebra | 10 ml of 0.25% levobupivacaine | A successful case of a US‐guided LSGB without major complications. |
| [32] | CRPS | No mention | No mention | Lidocaine | The patient showed a marked relief of pain and sensitivity after trial LSB. |
| [33] | CRPS | Fluoroscopy | L3 | 0.25% levobupivacaine mixed with BTX‐B 5000 IU, 5 ml | Both pain intensity and LANSS score were observably decreased after LSB with BTX‐B; Besides, Skin color returned to normal, allergies and cold sensations disappeared. |
| [34] | CRPS | Fluoroscopy | L4 | 2% lidocaine and 0.5% bupivacaine (1:1), 15 ml | Patient's lower extremity temperatures were noted to be 24°C bilaterally at the feet and 27.8°C at the ankles. Her pain level was 0/10. |
| [35] | CRPS | CT | L3 | 0.5% bupivacaine, 4 ml, ethanol 96%, 2 ml | The patient's NRS score decreased from 7 to 4, and the skin temperature of the right foot increased significantly. |
| [36] | Intractable lower‐limb PHN | Fluoroscopy | L3 | 0.5% bupivacaine mixed with 40 mg of triamcinolone | LSB relieved the pain of two cases of lower extremity systemic drug‐resistant PHN, and NRS scores decreased by at least 50%. |
| [37] | Postherpetic neuralgia secondary to zoster | Full text is unavailable | Full text is unavailable | Full text is unavailable | After LSGB, patients who suffered postherpetic neuralgia secondary to zoster recieved pain relief and improvement in quality of life. |
| [38] | Refractory diabetic neuropathy | CT | Standard posterolateral approach | 1% lidocaine mixed with the iohexol mixture/lidocaine mixed with dexamethasone, 20 ml | After treatment, the VAS pain scores of each patient were dramatically reduced; the blood oxygen saturation, capillary refill time, and skin temperature were also markedly ameliorated in patients. |
| [39] | Refractory painful diabetic neuropathy | Fluoroscopy | L3 | 1% lidocaine mixed with 20 mg of Triamcinolone, 12 ml | The NRS scores were markedly decreased, and the temperature was strikingly increased after the treatment. |
| [40] | Postamputation pain | Fluoroscopy | L2 | 0.25% bupivacaine, 10 ml | Patients with PAP who received a single LSG could reduce both pain and PDI. |
| [43] | Anterior lumbar interbody fusion (ALIF) | Fluoroscopy | L3 | 0.36% ropivacaine, 10 ml | The patient's NRS decreased from 9 to 4 after treatment, and the ipsilateral plantar temperature increased by more than 2°C. |
| [44] | NP of the lower limb | Fluoroscopy | The lower 1/3 of the L2 or the upper 1/3 of the L3 | 1% lidocaine, 8–10 ml | PTT detection was an early objective index to judge the success of LSGB. |
Abbreviations: BTA/BTB, botulinum toxin type A/B; CRPS, complex regional pain syndrome; LANSS, leeds assessment of neuropathic symptoms and signs; LSB/LSGB, lumbar sympathetic block/lumbar sympathetic ganglion block; NP, neuropathic pain; NRS, numeric rating scales; PAP, postamputation pain; PDI, pain disability index; PHN, post herpetic neuralgia; PTT, pulse transit time.
4.2. Lower limb ischemic pain
Studies have found that around 20% of patients who suffered lower limb ischemic pain are not suitable for surgical intervention for various reasons. In these patients, LSGB can be used to reduce pain, improve the walking status and activities of daily living, and may delay or avoid amputation. The use of LSGB can destroy the innervation of sympathetic nerves on the blood vessels of the lower extremities, and the innervated blood vessels continue to expand to improve local blood circulation and nutrient supply, thereby reducing pain (Table 2). 47 , 48 , 49 , 50 , 51
Table 2.
Application summary of the lumbar sympathetic ganglion block (LSGB) in lower limb ischemic pain
| Reference | Type of disease | Assistant technology | Puncture location | Drug | Outcomes |
|---|---|---|---|---|---|
| [45] | Peripheral arterial disease | Fluoroscopy | L2, L3, and L4 | 1% lidocaine and radiographic dye (1:1), 2 ml | Increased BSSP after LSB was assessed by LSFG, indicating improved foot circulation. |
| [46] | Peripheral arterial disease | Ultrasound | L3 vertebral level | 0.2% ropivacaine with 15‐mcg clonidine, 20 ml | NRS dropped below 3 after ULSB. All patients had a 2°C rise in body temperature from baseline with high satisfaction. |
| [47] | Chronic obstructive arterial disease | Fluoroscopy | L2‐L3‐L4 | 0.25% bupivacaine with absolute alcohol, 3 ml | LSB is safe and effective for pain relief in patients with critical limb ischemia. |
| [48] | Livedo reticularis | Fluoroscopy | L3, L4 | Total phenol amount for both sides of <10 ml | The postoperative skin surface temperature increases. |
Abbreviations: BSSP, beat strength of skin perfusion; LSB, lumbar sympathetic block; LSFG, laser speckle flowgraphy; ULSB: ultrasound‐guided lumbar sympathetic block.
5. POTENTIAL COMPLICATIONS OF LSGB
The most common acute complications of LSGB are local hematoma and local pain (at the injection site), which usually resolve spontaneously within a few hours to a few days. In addition, some studies have reported dizziness, headache, and weakness or pain in the injected leg that is possibly due to damage to the genital femoral and lateral femoral cutaneous nerves. 38 , 52 , 53 , 54 Moreover, there are also some serious complications, such as accidentally stabbed into the subarachnoid space and epidural causing extensive blockade after injection and resulting in respiratory and circulatory disorders, nerve injury by repeated puncture, blood vessels or the lumbar intervertebral disc damage, intravascular or intralymphatic injection, infection, ureteral or renal injury, drug hypersensitivity reactions, and local anesthetic toxicity, although these are extremely rare. 55 , 56 , 57 , 58
6. ADVANTAGES OF ULTRASOUND APPLICATION IN LSGB
Because the LSN is adjacent to important large blood vessels, spinal cord, and nerves, this operation must be performed by a physician who is very familiar with the local anatomy and has experience in the treatment with the aid of positioning. LSGB is usually implemented with the assistance of fluoroscopy and computed tomography (CT). Nevertheless, when performing fluoroscopy and CT, the assistance of an equipped operating room or radiology department is required, and the level of radiation exposure is higher with repeated use. 59 Ultrasound‐guided techniques, introduced into pain treatment in the mid‐2000s, have been proven to be involved in a number of advantages, such as the ability to imagine the structures of soft tissues, watch diffusion patterns in real time for needle insertion, and drug injection with minimal radiation exposure. 60 , 61 , 62 Based on the ability of ultrasound‐guided LSGB to observe and identify tissue structures, the route of puncture needles, and the diffusion of local anesthetics in real‐time, it can improve the success rate of puncture, reduce complications, and avoid radiation damage, with obvious clinical advantages.
7. APPLICATION PROSPECT OF LSGB DURING THE PERIOPERATIVE PERIOD
LSN is adjacent to a number of vital organs and a large number of large blood vessels, and there is a risk of injury to the large vessels or puncture of the lumbar intervertebral discs by blind puncture. An ultrasound‐guided nerve block can directly observe the relationship between the needle and nerve and surrounding vascular tissues and can see the entire dynamic process of local diffusion after drug injection, which not only shortens the operation time and increases the success rate of the block, but also reduces the dosage of local anesthetics, the toxicity of the drug, and the risk of complications. Moreover, LSGB has a good application prospect during the perioperative period (Figure 3).
Figure 3.
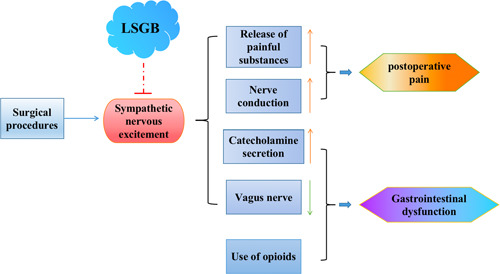
Application prospect of the lumbar sympathetic ganglion block (LSGB) during the perioperative period [Color figure can be viewed at wileyonlinelibrary.com]
7.1. LSGB application in perioperative analgesia
Chronic pain has been shown to be sympathetically related. 10 LSGB is widely used to diagnose and treat persistent sympathetic pain. 9 , 10 Under ultrasound guidance, a local anesthetic can be accurately injected, and blocking LSG can effectively block sensation in the corresponding area. Studies have reported that patients with cancer‐related pain in the back, abdomen, pelvis, or legs can have their pain reduced by LSGB. LSGB provides good analgesia after surgery. 63 With regard to perioperative pain management, LSGB can reduce residual limb pain and PLP during the perioperative period and help patients to recover psychologically after surgery. 43 The causes of pain after lumbar spine surgery are complex. Changes in the autonomic nervous system (ANS) have been reported to exert a vital role in the generation and maintenance of pain in patients who suffered from failed back surgery syndrome (FBSS). While LSGB has achieved good efficacy in pain relief of patients who suffered FBSS. 64 Severe sympathetic persistent pain can occur after anterior spinal surgery, and the application of LSGB can successfully relieve pain. 45
In recent years, LSGB has attracted the attention of anesthesiologists and microsurgeon in skin flap transplantation due to its good analgesic effect and unsympathetic vasoconstrictor effect, which can dilate blood vessels, improve blood supply to the lower limb, and promote the establishment of collateral circulation. 7 , 65 For lower limb flap transplantation, an intrathecal block is widely used. However, spinal anesthesia blocks the sympathetic activity and also blocks the motor function of the lower limb, which causes inconvenience for patients to move after surgery, increases the risk of venous thrombosis of the lower extremities, and is not conducive to rapid perioperative recovery. 66 , 67 Studies have shown that the introduction of LSGB for perioperative analgesia in lower limb free flap transplantation can reduce perioperative pain, increase the temperature of the transplanted flap, improve the blood supply of the transplanted flap, and promote the survival of the transplanted flap. In turn, it can improve sensory function recovery in patients with transplanted skin flaps, resulting in clinical benefits in patients undergoing lower limb free flap transplantation. 7 , 34 , 68
7.2. Application prospect of LSGB in the regulation of gastrointestinal function
It is well known that the stimulation of the surgical operation can increase the excitability of the sympathetic nerves, which will lead to a significant increase in the postoperative plasma catecholamine level. This in turn leads to a high degree of the mesenteric blood vessels and a reduction in the blood supply of the gastrointestinal tract, ultimately resulting in the long‐term inhibition of gastrointestinal motility. In addition, it can also reflexively inhibit the efferent fibers of the vagus nerve and weaken the motor function of the gastrointestinal tract. Meanwhile, because the inhibition of the vagus nerve blocks the body's cholinergic anti‐inflammatory pathway, the effect of inhibiting the secretion of inflammatory cytokines is weakened or disappeared, resulting in gastrointestinal dysfunction. 69 , 70 In addition, the use of opioids during anesthesia and postoperative analgesia inhibits bowel motility and delays the recovery of gastrointestinal function. Research has shown that postoperative ileus duration was positively correlated with perioperative opioid consumption. Moreover, different anesthesia methods have different effects on inhibiting postoperative gastrointestinal motility, among which general anesthesia has the greatest effect. 71 Studies have shown that the rehabilitation time of postoperative gastrointestinal function with abdominal surgery under general anesthesia combined with epidural anesthesia is significantly shorter than that in patients with traditional general anesthesia. 72
Importantly, LSGB in surgery can reduce the use of anesthetics, inhibit the fluctuation of hemodynamics, have a positive auxiliary effect on patients with cardiopulmonary insufficiency, and can effectively ameliorate the quality of analgesia for patients. LSGB can produce a good postoperative analgesic effect, reduce postoperative pain, effectively shorten the recovery time of patients, and improve patient satisfaction after the operation. In addition, the postganglionic fibers of LSN directly innervate internal organs, glands, and visceral blood vessels. 73 LSGB can inhibit sympathetic nerve activity, dilate blood vessels, and block the stress response caused by external stimuli, which is conducive to reducing postoperative gastric intestinal dysfunction. Thus, the above description indicates that LSGB has potential application value in the regulation of gastrointestinal function during the perioperative period. However, there is currently a lack of evidence for the study of gastrointestinal function in LSGB during the perioperative period. Therefore, this review is just theoretical speculation, and the feasibility still needs more clinical trials and data to prove.
8. CONCLUSION
Overall, LSGB is an effective treatment for patients with lower limb pain and postoperative pain and has great application prospects in the recovery of gastrointestinal function after surgery. Further clinical trials are needed to confirm its efficacy during the perioperative period.
AUTHOR CONTRIBUTIONS
Min‐Jian Geng conceived the study, wrote, and revised the manuscript; Jing‐Han Zhang and Yan‐Ping Deng collected the literature and summarized the data.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Zhang J‐H, Deng Y‐P, Geng M‐J. Efficacy of the lumbar sympathetic ganglion block in lower limb pain and its application prospects during the perioperative period. ibrain. 2022;8:442‐452. 10.1002/ibra.12069
DATA AVAILABILITY STATEMENT
The data contained in the article can be reasonably requested from the corresponding author.
REFERENCES
- 1. Baig S, Moon JY, Shankar H. Review of sympathetic blocks: anatomy, sonoanatomy, evidence, and techniques. Reg Anesth Pain Med. 2017;42(3):377‐391. 10.1097/AAP.0000000000000591 [DOI] [PubMed] [Google Scholar]
- 2. Phuphanich ME, Convery QW, Nanda U, Pangarkar S. Sympathetic blocks for sympathetic pain. Phys Med Rehabil Clin N Am. 2022;33(2):455‐474. 10.1016/j.pmr.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 3. Wie C, Gupta R, Maloney J, Pew S, Freeman J, Strand N. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. 10.1007/s11916-020-00904-5 [DOI] [PubMed] [Google Scholar]
- 4. Binder A, Baron R. Complex regional pain syndromes. In: McMahon S, Koltzenburg M, Tracey I, Turk D, editors., Wall & Melzack's textbook of pain. 6th ed. Elsevier; 2013:961‐977. [Google Scholar]
- 5. van Kleef M, Mekhail N, van Zundert J. Evidence‐based interventional pain medicine according to clinical diagnoses. Pain Pract. 2009;9(4):247‐251. 10.1111/j.1533-2500.2009.00297.x [DOI] [PubMed] [Google Scholar]
- 6. Wesselmann U, Burnett AL, Heinberg LJ. The urogenital and rectal pain syndromes. Pain. 1997;73(3):269‐294. 10.1016/S0304-3959(97)00076-6 [DOI] [PubMed] [Google Scholar]
- 7. Tran KM, Frank SM, Raja SN, El‐Rahmany HK, Kim LJ, Vu B. Lumbar sympathetic block for sympathetically maintained pain: changes in cutaneous temperatures and pain perception. Anesth Analg. 2000;90(6):1396‐1401. 10.1097/00000539-200006000-00025 [DOI] [PubMed] [Google Scholar]
- 8. Chen SS, Zhang JM. Progress in sympathetically mediated pathological pain. J Anesth Perioper Med. 2015;2(4):216‐225. 10.24015/JAPM.2015.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698‐703. [PubMed] [Google Scholar]
- 10. Rigaud J, Delavierre D, Sibert L, Labat JJ. Sympathetic nerve block in the management of chronic pelvic and perineal pain. Prog Urol. 2010;20(12):1124‐1131. 10.1016/j.purol.2010.08.047 [DOI] [PubMed] [Google Scholar]
- 11. Straube S, Derry S, Moore RA, Cole P. Cervico‐thoracic or lumbar sympathectomy for neuropathic pain and complex regional pain syndrome. Cochrane Database Syst Rev. 2013;2013(9):CD002918. 10.1002/14651858.CD002918.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rieger R. Management of plantar hyperhidrosis with endoscopic lumbar sympathectomy. Thorac Surg Clin. 2016;26(4):465‐469. 10.1016/j.thorsurg.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 13. Yi XB, AuBuchon J, Zeltwanger S, Kirby JP. Necrotic arachnidism and intractable pain from recluse spider bites treated with lumbar sympathetic block: a case report and review of literature. Clin J Pain. 2011;27(5):457‐460. 10.1097/AJP.0b013e31820b6424 [DOI] [PubMed] [Google Scholar]
- 14. Gupta A, Portonova B, Dadachanji C. Successful treatment of post thrombotic syndrome with sequential lumbar sympathetic block. Pain Physician. 2015;18(1):E65‐E69. [PubMed] [Google Scholar]
- 15. Wang WH, Zhang L, Dong GX, et al. Chemical lumbar sympathectomy in the treatment of recalcitrant erythromelalgia. J Vasc Surg. 2018;68(6):1897‐1905. 10.1016/j.jvs.2018.05.226 [DOI] [PubMed] [Google Scholar]
- 16. Umeda S, Arai T, Hatano Y. Cadaver anatomic analysis of the site for chemical lumbar sympathetomy. AnesthAnalg. 1987;66(7):643‐646. [PubMed] [Google Scholar]
- 17. An JW, Koh JC, Sun JM, et al. Clinical identification of the vertebral level at which the lumbar sympathetic ganglia aggregate. Korean J Pain. 2016;29(2):103‐109. 10.3344/kjp.2016.29.2.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coutinho E, Silva RDS, Wiggenhauser LM, et al. Thoracic bilateral sympathectomy attenuates oxidative stress and prevents ventricular remodelling in experimental pulmonary hypertension. Eur J Cardiothorac Surg. 2022;61(6):1337‐1345. 10.1093/ejcts/ezab549 [DOI] [PubMed] [Google Scholar]
- 19. Meens MJ, Mattheij NJ, Nelissen J, et al. Calcitonin gene‐related peptide terminates long‐lasting vasopressor responses to endothelin 1 in vivo. Hypertension. 2011;58(1):99‐106. 10.1161/HYPERTENSIONAHA.110.169128 [DOI] [PubMed] [Google Scholar]
- 20. Meens MJ, Mattheij NJ, van Loenen PB, et al. G‐protein βγ subunits in vasorelaxing and anti‐endothelinergic effects of calcitonin gene‐related peptide. Br J Pharmacol. 2012;166(1):297‐308. 10.1111/j.1476-5381.2011.01774.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tuzmen C, Campbell PG. Crosstalk between neuropeptides SP and CGRP in regulation of BMP2‐induced bone differentiation. Connect Tissue Res. 2018;59(suppl 1):81‐90. 10.1080/03008207.2017.1408604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen K, Zhang ZF, Liao MF, Yao WL, Wang J, Wang XR. Blocking PAR2 attenuates oxaliplatin‐induced neuropathic pain via TRPV1 and releases of substance P and CGRP in superficial dorsal horn of spinal cord. J Neurol Sci. 2015;352(1‐2):62‐67. 10.1016/j.jns.2015.03.029 [DOI] [PubMed] [Google Scholar]
- 23. Lovick TA. Autonomic control of sensory responsiveness. Proc Finn Dent Soc. 1989;85(4‐5):415‐419. [PubMed] [Google Scholar]
- 24. Iwase T, Takebayashi T, Tanimoto K, et al. Sympathectomy attenuates excitability of dorsal root ganglion neurons and pain behaviour in a lumbar radiculopathy model. Bone Joint Res. 2012;1(9):198‐204. 10.1302/2046-3758.19.2000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie W, Strong JA, Li H, Zhang JM. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol. 2007;97(1):492‐502. 10.1152/jn.00899.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y, Huo F. Inhibition of sympathetic sprouting in CCD rats by lacosamide. Eur J Pain. 2018;22(9):1641‐1650. 10.1002/ejp.1246 [DOI] [PubMed] [Google Scholar]
- 27. Li H, Xie W, Strong JA, Zhang JM. Systemic anti‐inflammatory corticosteroid reduces mechanical pain behavior, sympathetic sprouting, and elevation of proinflammatory cytokines in a rat model of neuropathic pain. Anesthesiology. 2007;107(3):469‐477. 10.1097/01.anes.0000278907.37774.8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pezzilli R. Antioxidatus for intractable pain in chronic pancreatitis patients. Is the end of the story? JOP. 2012;13(4):461‐463. 10.6092/1590-8577/979 [DOI] [PubMed] [Google Scholar]
- 29. Zhu X, Kohan LR, Morris JD, Hamill‐Ruth RJ. Sympathetic blocks for complex regional pain syndrome: a survey of pain physicians. Reg Anesth Pain Med. 2019. 10.1136/rapm-2019-100418 [DOI] [PubMed] [Google Scholar]
- 30. Roldan CJ, Lo TC, Huh B. Recurrence of complex regional pain syndrome after administration of adenosine. Pain Manag. 2019;9(3):233‐237. 10.2217/pmt-2018-0059 [DOI] [PubMed] [Google Scholar]
- 31. Carcamo CR. Dysarthria associated with lumbar sympathetic block using botulinum toxin. Pain Med. 2019;20(8):1634‐1635. 10.1093/pm/pnz007 [DOI] [PubMed] [Google Scholar]
- 32. Zyluk A, Puchalski P. Successful treatment of paediatric lower limb CRPS by continuous epidural anaesthesia: a report of 2 cases. Handchir Mikrochir Plast Chir. 2018;50(5):359‐362. 10.1055/a-0747-6127 [DOI] [PubMed] [Google Scholar]
- 33. Lee Y, Lee CJ, Choi E, Lee PB, Lee HJ, Nahm FS. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10(4):164. 10.3390/toxins10040164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moon JY, Choi JK, Shin JY, Chon SW, Dev S. A brief report on a technical description of ultrasound‐guided lumbar sympathetic block. Korean J Pain. 2017;30(1):66‐70. 10.3344/kjp.2017.30.1.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lederman A, Turk D, Howard A, Reddy S, Stern M. Case study: gluteal compartment syndrome as a cause of lumbosacral radiculoplexopathy and complex regional pain syndrome. J Rehabil Res Dev. 2016;53(4):483‐486. 10.1682/JRRD.2015.01.0007 [DOI] [PubMed] [Google Scholar]
- 36. Choi E, Cho CW, Kim HY, Lee PB, Nahm FS. Lumbar sympathetic block with botulinum toxin type B for complex regional pain syndrome: a case study. Pain Physician. 2015;18(5):E911‐E916. [PubMed] [Google Scholar]
- 37. Nagpal A, Eckmann M, Small S, Stevens S. Onset of spontaneous lower extremity pain after lumbar sympathetic block. Pain Physician. 2015;18(1):E89‐E91. [PubMed] [Google Scholar]
- 38. Pennekamp W, Krumova EK, Feigl GP, et al. Permanent lesion of the lateral femoral cutaneous nerve after low‐volume ethanol 96%application on the lumbar sympathetic chain. Pain Physician. 2013;16(4):391‐397. [PubMed] [Google Scholar]
- 39. Ozturk EC, Sencan S, Gunduz OH. Lumbar sympathetic block for intractable lower‐limb postherpetic neuralgia: report of two cases. Pain Pract. 2021;21(3):353‐356. 10.1111/papr.12958 [DOI] [PubMed] [Google Scholar]
- 40. Malec‐Milewska M, Sękowska A, Kolęda I, Horosz B, Guć M, Jastrzębski J. Sympathetic nerve blocks for the management of postherpetic neuralgia: 19 years of pain clinic experience. Anaesthesiol Intensive Ther. 2014;46(4):255‐261. 10.5603/AIT.a2014.0039 [DOI] [PubMed] [Google Scholar]
- 41. Sun H, He M, Pang J, Guo X, Huo Y, Ma J. Continuous lumbar sympathetic blockade enhances the effect of lumbar sympatholysis on refractory diabetic neuropathy: a randomized controlled trial. Diabetes Ther. 2020;11(11):2647‐2655. 10.1007/s13300-020-00918-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng J, Daftari A, Zhou L. Sympathetic blocks provided sustained pain relief in a patient with refractory painful diabetic neuropathy. Case Rep Anesthesiol. 2012;2012:285328. 10.1155/2012/285328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCormick ZL, Hendrix A, Dayanim D, Clay B, Kirsling A, Harden N. Lumbar sympathetic plexus block as a treatment for postamputation pain: methodology for a randomized controlled trial. Pain Med. 2018;19(12):2496‐2503. 10.1093/pm/pny041 [DOI] [PubMed] [Google Scholar]
- 44. Bornemann‐Cimenti H, Dorn C, Rumpold‐Seitlinger G. Early onset and treatment of phantom limb pain following surgical amputation. Pain Med. 2017;18(12):2510‐2512. 10.1093/pm/pnx111 [DOI] [PubMed] [Google Scholar]
- 45. Alexander CE, De Jesus O, Varacallo M. Lumbar Sympathetic Block. 2021 Aug 30. StatPearls. StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 46. Woo JH, Park HS. Successful treatment of severe sympathetically maintained pain following anterior spine surgery. J Korean Neurosurg Soc. 2014;56(1):66‐70. 10.3340/jkns.2014.56.1.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Joo EY, Kong YG, Lee J, Cho HS, Kim SH, Suh JH. Change in pulse transit time in the lower extremity after lumbar sympathetic ganglion block: an early indicator of successful block. J Int Med Res. 2017;45(1):203‐210. 10.1177/0300060516681398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanao‐Kanda M, Kanda H, Iida T, Kikuchi S, Azuma N. Clinical application of laser speckle flowgraphy to assess changes in blood flow to the foot after a lumbar sympathetic ganglion block: a case report. J Pain Res. 2021;14:1451‐1456. 10.2147/JPR.S305543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Punj J, Marada S. Ultrasound lumbar sympathetic block: out of plane approach with insulated stimulation needle: case series of three patients. Indian J Anaesth. 2020;64(2):148‐150. 10.4103/ija.IJA_686_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barreto Junior EPS, Nascimento JDS, de Castro APCR. Neurolitic block of the lumbar sympathetic chain improves chronic pain in a patient with critical lower limb ischemia. Braz J Anesthesiol. 2018;68(1):100‐103. 10.1016/j.bjan.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang WH, Zhang L, Li X, Zhao J, Zhuang JM, Dong GX. Chemical lumbar sympathectomy in the treatment of idiopathic livedo reticularis. J Vasc Surg. 2015;62(4):1018‐1022. 10.1016/j.jvs.2015.04.419 [DOI] [PubMed] [Google Scholar]
- 52. Feigl GC, Dreu M, Ulz H, Breschan C, Maier C, Likar R. Susceptibility of the genitofemoral and lateral femoral cutaneous nerves to complications from lumbar sympathetic blocks: is there a morphological reason? Br J Anaesth. 2014;112(6):1098‐1104. 10.1093/bja/aet552 [DOI] [PubMed] [Google Scholar]
- 53. Artuso JD, Stevens RA, Lineberry PJ. Post dural puncture headache after lumbar sympathetic block: a report of two cases. Reg Anesth. 1991;16(5):288‐291. [PubMed] [Google Scholar]
- 54. Joshi SM, Hehre FW. Peridural block complicating lumbar sympathetic block. Anesth Analg. 1977;56(6):873‐874. 10.1213/00000539-197711000-00029 [DOI] [PubMed] [Google Scholar]
- 55. Ranjan P, Kumar J, Chipde SS. Acute renal failure due to bilateral ureteric necrosis following percutaneous chemical lumbar sympathectomy. Indian J Nephrol. 2012;22(4):292‐294. 10.4103/0971-4065.101252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haynsworth RF, Noe CE, Fassy LR. Intralymphatic injection: another complication of lumbar sympathetic block. Anesthesiology. 1994;80(2):460‐462. [PubMed] [Google Scholar]
- 57. Egbert LD. Horner's syndrome; complication of lumbar sympathetic block. Anesthesiology. 1955;16(5):811‐812. 10.1097/00000542-195509000-00023 [DOI] [PubMed] [Google Scholar]
- 58. Samuelsson SM, Hoegstr‐OM S. Retroperiyoneal hematoma following lumbar sympathetic nerve block. Sven Lakartidn. 1964;61:2894‐2903. [PubMed] [Google Scholar]
- 59. Manchikanti L, Cash KA, Moss TL, Pampati V. Radiation exposure to the physician in interventional pain management. Pain Physician. 2002;5(4):385‐393. [PubMed] [Google Scholar]
- 60. Hurdle MF. Ultrasound‐guided spinal procedures for pain: a review. Phys Med Rehabil Clin N Am. 2016;27(3):673‐686. 10.1016/j.pmr.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 61. Jee H, Lee JH, Kim J, Park KD, Lee WY, Park Y. Ultrasoundguided selective nerve root block versus fluoroscopy‐guided transforaminal block for the treatment of radicular pain in the lower cervical spine: a randomized, blinded, controlled study. Skeletal Radiol. 2013;42(1):69‐78. 10.1007/s00256-012-1434-1 [DOI] [PubMed] [Google Scholar]
- 62. Narouze S. Ultrasound‐guided stellate ganglion block: safety and efficacy. Curr Pain Headache Rep. 2014;18(6):424. 10.1007/s11916-014-0424-5 [DOI] [PubMed] [Google Scholar]
- 63. Spiegel MA, Hingula L, Chen GH, Legler A, Puttanniah V, Gulati A. The use of L2 and L3 lumbar sympathetic blockade for cancer‐related pain, an experience and recommendation in the oncologic population. Pain Med. 2020;21(1):176‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rooney BA, Crown ED, Hulsebosch CE, McAdoo DJ. Preemptive analgesia with lidocaine prevents failed back surgery syndrome. Exp Neurol. 2007;204(2):589‐596. 10.1016/j.expneurol.2006.12.007 [DOI] [PubMed] [Google Scholar]
- 65. Fang F, Zou W, Zhang Z, Zhang Q, Xie Y. Patterns of sural nerve innervation of the sural artery with implication for reconstructive surgery. J Surg Res. 2017;220:261‐267. 10.1016/j.jss.2017.06.086 [DOI] [PubMed] [Google Scholar]
- 66. Erni D, Banic A, Signer C, Sigurdsson GH. Effects of epidural anaesthesia on microcirculatory blood flow in free flaps in patients under general anaesthesia. Eur J Anaesthesiol. 1999;16(10):692‐698. 10.1046/j.1365-2346.1999.00565.x [DOI] [PubMed] [Google Scholar]
- 67. Alam NH, Haeney JA, Platt AJ. Three episodes of gracilis free muscle transfer under epidural anaesthesia. J Plast Reconstr Aesthet Surg. 2006;59(12):1463‐1466. 10.1016/j.bjps.2005.12.021 [DOI] [PubMed] [Google Scholar]
- 68. Pekař M, Mazur M, Pekařová A, Kozák J, Foltys A. Lumbálna sympatektómia prehľad svetovej literatúry za posledných 15 rokov [lumbar sympathectomy literature review over the past 15 years]. Rozhl Chir. 2016;95(3):101‐106. [PubMed] [Google Scholar]
- 69. Mythen MG. Postoperative gastrointestinal tract dysfunction: an overview of causes and management strategies. Cleve Clin J Med. 2009;76(suppl 4):S66‐S71. [DOI] [PubMed] [Google Scholar]
- 70. Tan S, Wu G, Yu W, Li N. Research advance in causes of postoperative gastrointestinal dysfunction. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19(3):351‐355. [PubMed] [Google Scholar]
- 71. Chen ZQ, Cao LX. Current situation and prospect on post‐surgery assessment of peri‐operative gastrointestinal functions. Chin J Integr Trad Western Med. 2011;31(6):727‐731. [PubMed] [Google Scholar]
- 72. Han Q, Wu S, Chen H, Wang L, Zhang C. The choice of anesthesia for acute abdomen surgery patients and its influence on gastrointestinal function recovery. Am J Transl Res. 2021;13(8):9621‐9626. [PMC free article] [PubMed] [Google Scholar]
- 73. Minic Z, O'Leary DS, Scislo TJ. Nucleus tractus solitarii A(2a) adenosine receptors inhibit cardiopulmonary chemoreflex control of sympathetic outputs. Auton Neurosci. 2014;180:32‐42. 10.1016/j.autneu.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data contained in the article can be reasonably requested from the corresponding author.


